Abstract
Background
There is still ongoing debate about the benefits of mini-thoracotomy (MTH) approach in mitral valve surgery in comparison with complete sternotomy (STER). This study aims to update the current evidence with mortality as primary end point.
Methods
The MEDLINE and EMBASE databases were searched through June 2022. Two randomized studies and 16 propensity score matched studies published from 2011 to 2022 were included with a total of 12,997 patients operated on from 2005 (MTH: 6467, STER: 6530). Data regarding early mortality, stroke, reoperation for bleeding, new renal failure, new onset of atrial fibrillation, need of blood transfusion, prolonged ventilation, wound infection, time-related outcomes (cross clamp time, cardiopulmonary bypass time, ventilation time, length of intensive care unit stay, length of hospital stay), midterm mortality and reoperation, and costs were extracted and submitted to a meta-analysis using weighted random effects modeling.
Results
The incidence of early mortality, stroke, reoperation for bleeding and prolonged ventilation were similar, all in the absence of heterogeneity. However, the sub-group analysis showed a significant OR in favor of MTH when robotic enhancement was used. New renal failure (OR 1.67, 95% CI 1.06–2.62, p = 0.03), new onset of atrial fibrillation (OR 1.31, 95% CI 1.15–1.51, p = 0.001) and the need of blood transfusion (OR 1.77, 95% CI 1.39–2.27, p = 0.001) were significantly lower in MTH group. Regarding time-related outcomes, there was evidence for important heterogeneity of treatment effect among the studies. Operative times were longer in MTH: differences in means were 20.7 min for cross clamp time (95% CI 14.9–26.4, p = 0.001), 36.8 min for CPB time (95% CI 29.8–43.9, p = 0.001) and 37.7 min for total operative time (95% CI 19.6–55.8, p < 0.001). There was no significant difference in ventilation duration; however, the differences in means showed significantly shorter ICU stay and hospital stay after MTH compared to STER: − 0.6 days (95% CI − 1.1/− 0.21, p = 0.001) and − 1.88 days (95% CI − 2.72/− 1.05, p = 0.001) respectively, leading to a significant lower hospital cost after MTH compared to STER with difference in means − 4528 US$ (95% CI − 8725/− 326, p = 0.03).
The mid-term mortality was significantly higher after STER compared to MTH: OR = 1.50, 1.09–2.308 (95% CI), p = 0.01; the rate of mid-term reoperation was reported similar in MTH and STER: OR = 0.76, 0.50–1.15 (95% CI), p = 0.19.
Conclusions
The present meta-analysis confirms that the MTH approach for mitral valve disease remains associated with prolonged operative times, but it is beneficial in terms of reduced postoperative complications (renal failure, atrial fibrillation, blood transfusion, wound infection), length of stay in ICU and in hospitalization, with finally a reduction in global cost. MTH approach appears associated with a significant reduction of postoperative mortality that must be confirmed by large randomized study.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13019-023-02229-x.
Keywords: Mitral valve surgery, Minimally invasive surgery, Minithoracotomy, Sternotomy, Meta-analysis
Introduction
Minimally invasive mitral valve surgery with mini-thoracotomy approach (MTH) has been introduced 25 years ago [1]. Previous meta-analyses [2–5] failed to detect any positive impact of MTH on the occurrence of postoperative major adverse cardiac events in comparison with classic sternotomy approach (STER). Nowadays, mini-thoracotomy is established as a new standard for mitral valve surgery and the surgical community is far from the learning curve with this minimally invasive technique; the proponents arguing its utility for treating even the most complex mitral valve disease without any additional risk of potential complications despite prolonged operation times.
The aim of this meta-analysis based only on recent comparative series published from 2010 and including patients operated after 2005, was to investigate the early and late performance of MTH versus STER in mitral valve surgery and to detect any more substantial benefit and less drawbacks that could be expected with larger experience, larger expertise and more standardized techniques in minimally invasive approach, over time. Mortality as primary end point and major complications were the main interest; in addition procedure-related and resource-related outcomes were assessed.
Methods
The meta-analysis was performed in accordance with PRISMA and MOOSE guidelines [6, 7]. Databases were searched for articles meeting our inclusion criteria and published by June 2022: PubMed/MEDLINE, Cochrane Controlled Trials Register (CENTRAL/CCTR), EMBASE, Google Scholar, Clinical Trials.gov. Search terms were “minimally invasive mitral”, “mitral minithoracotomy”, “less invasive mitral”, “robotic mitral”, “endoscopic mitral”, “totally endoscopic mitral”, robotically assisted mitral”, “mitral sternotomy”, and variants and combinations of these keywords.
Inclusion criteria
Randomized controlled trials or propensity-score matched nonrandomized observational studies, comparing mitral valve surgery (repair or replacement) via a right lateral minithoracotomy (with or without robotic support) versus sternotomy (through a complete median sternotomy) were included.
Exclusion criteria
Studies published before 2010 and studies including surgery performed before 2005 were excluded. Studies including mainly redo surgical procedures were excluded.
End-points
End points were defined as early mortality, stroke, reoperation for bleeding, new renal failure, new onset of atrial fibrillation, need of blood transfusion, prolonged ventilation, wound infection, time-related outcomes: cross clamp time, cardiopulmonary bypass time (CPB), ventilation time, length of intensive care unit (ICU) stay, length of hospital stay, midterm mortality and reoperation, and costs.
Study quality appraisal
Study quality of the included studies was assessed using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool [8]. Using this tools, seven domains of bias were assessed and each study was then classified as either low, moderate, serious or critical risk. Quality appraisal was undertaken independently by two reviewers (AA & OJ).
Data analysis
Baseline characteristics were checked by two independent reviewers in each selected study to assess the balance in randomization or matching and the associated risk of bias. For studies reporting interquartile ranges, the mean and standard deviation were estimated according to appropriate formula [9]. Funnel plot was used to evaluate publication bias statistically analyzed by Egger’s test. The χ2 test and I2 test were used to assess study heterogeneity; if heterogeneity was significant (I2 > 75%), the analysis used a random effects model. Odds ratio (OR) with 95% confidence interval (CI) were calculated for discrete data. For continuous data, differences in means with 95% CI were considered. p-values < 0.05 were considered statistically significant. The OR and differences in means were combined across the studies using a weighted random effects model. A sub-group analysis regarding robotic-enhancement was added. Forest plots of log-OR were used to represent the synthesis of the results when appropriate. The analysis and data modelling were performed with the IBM-SPSS statistics software version 28.0 (IBM-SPSS Inv, Armonk, NY).
Results
Study selection
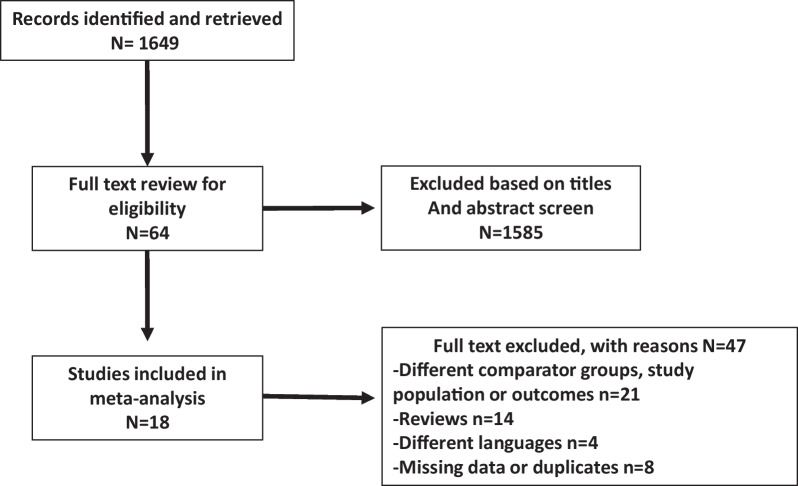
A total of 1649 citations were identified, of which 64 studies were potentially relevant and retrieved for full review. Eighteen articles included studies that met our eligibility criteria for the comparison of MTH versus STER (Fig. 1). Two studies [11, 24] were prospectively randomized; the other 16 studies [10, 12–23, 25–27] were nonrandomized, retrospective and propensity score matched. The quality of the included nonrandomized studies, which was assessed using the ROBINS-I tool, was deemed to be low risk of bias in six studies, moderate in six studies and serious in four (Additional file 1). Four studies were multicentric, nine unicentric and five from database (Table 1). A robotic-enhancement for MTH was used in 6 studies [22–27].
Fig. 1.
Flow chart of the study
Table 1.
Study characteristics of relevant articles identified for meta-analysis
| Author | Date of publication | Study period | Origin of series | Propensity matched (PM) or Randomized (Rand) | Sternotomy approach (N) | Mini-thoracotomy approach (N) | Robotic Enhancement (Y/N) |
|---|---|---|---|---|---|---|---|
| Grossi et al. [10] | 2014 | 2007–2011 | Database | PM | 367 | 367 | N |
| Nasso et al. [11] | 2014 | 2008–2013 | Multicentric | Rand | 80 | 80 | N |
| Nishi et al. [12] | 2015 | 2008–2012 | Database | PM | 750 | 750 | N |
| Downs et al. [13] | 2016 | 2011–2014 | Database | PM | 355 | 355 | N |
| Hawkins et al. [14] | 2018 | 2011–2016 | Unicentric | PM | 74 | 74 | N |
| Wang Q et al. [15] | 2018 | 2012–2015 | Unicentric | PM | 67 | 67 | N |
| Grant et al. [16] | 2019 | 2008–2016 | Multicentric | PM | 639 | 639 | N |
| Liu et al. [17] | 2019 | 2012–2015 | Unicentric | PM | 202 | 202 | N |
| Paparella et al. [18] | 2020 | 2011–2017 | Multicentric | PM | 1493 | 1493 | N |
| Cetinkaya et al. [19] | 2021 | 2005–2015 | Unicentric | PM | 422 | 422 | N |
| Pojar et al. [20] | 2021 | 2012–2018 | Unicentric | PM | 225 | 158 | N |
| Olsthoorn et al. [21] | 2022 | 2013–2018 | Multicentric | PM | 718 | 718 | N |
| Mihaljevic et al. [22] | 2011 | 2006–2009 | Unicentric | PM | 106 | 106 | Y |
| Suri et al. [23] | 2011 | 2007–2010 | Unicentric | PM | 95 | 95 | Y |
| Iyigun et al. [24] | 2017 | 2013–2015 | Unicentric | Rand | 29 | 33 | Y |
| Hawkins et al. [25] | 2018 | 2011–2016 | Database | PM | 314 | 314 | Y |
| Wang A et al. [26] | 2018 | 2011–2014 | Database | PM | 503 | 503 | Y |
| Coyan et al. [27] | 2018 | 2013–2015 | Unicentric | PM | 91 | 91 | Y |
Baseline characteristics
A total of 12,997 patients operated on from 2005 (MTH: 6467, STER: 6530) were included from studies published from 2011 to 2022. The baseline characteristics of patients are summarized in Table 2, by study, regardless the surgical technique performed, to identify the population treated. Globally the populations were homogenous: a young population (mean age 59 years), mainly in functional class 1 or 2, with a preserved left ventricular (LV) function (mean LV ejection fraction from 56 to 65%), and a low incidence of cerebro-vascular event or coronary artery disease. Wang A et al. [26] reported an older population with mean age 71 years, but without other significant risk factors. These characteristics defined a population with a low risk for mitral valve surgery as it was confirmed by risk scores when they were available (Table 3). The mitral surgery performed was mainly mitral repair. In only two studies [17, 24], the rate of valve replacement was > 30%, but well balanced in randomization or propensity score matching. An associated tricuspid valve repair was frequent in 3 studies [15, 17, 20] and an associated atrial fibrillation surgery was reported higher than 20% in 5 studies (Table 3); both associated procedures represented a moderate risk of bias because they were well balanced in propensity score matching.
Table 2.
Summary of baseline characteristics in patient populations
| Author | Age | Male Gender (%) | NYHA class 3–4 (%) | Hypertension (%) | Coronary Disease (%) | Cerebro-vascular events (%) | Atrial fibrillation (%) | LV impairment (%) | LVEF (%) |
|---|---|---|---|---|---|---|---|---|---|
| Grossi et al. [10] | 65* | 56.1 | NI | NI | NI | NI | NI | NI | NI |
| Nasso et al. [11] | 54.1 ± 10.5 | 56.8 | 26.8 | NI | NI | NI | NI | 26.8 | NI |
| Nishi et al. [12] | 55.5 ± 12.6 | 59.8 | 11.3 | 41.6 | NI | 2.8 | NI | 11 | NI |
| Downs et al. [13] | 58.1 ± 13.5 | 60.6 | 19.4 | 56.8 | NI | NI | NI | NI | 57.9 ± 9.6 |
| Hawkins et al. [14] | 61.6 ± 13.8 | 57.4 | NI | 60.1 | 14.1 | 10.1 | 39.9 | NI | 60 |
| Wang Q et al. [15] | 51 ± 12 | 51.5 | 26.9 | 22.4 | NI | 2.2 | 39.5 | NI | 55.9 ± 12 |
| Grant et al. [16] | 62.8 ± 12.6 | 66.4 | 47.6 | 45.6 | 5.9 | 2.7 | 33.6 | 17.5 | NI |
| Liu et al. [17] | 50.7 ± 11.5 | 34.6 | 26.9 | 6.9 | 2.2 | 6.7 | 49.2 | NI | 62.8 ± 7.9 |
| Paparella et al. [18] | 66.5 ± 12 | 48.9 | NI | 62 | 6.5 | 1 | 29.2 | 26.6 | NI |
| Cetinkaya et al. [19] | 64.1 ± 12.7 | 54.7 | 84.8 | 49.5 | 7.7 | 5.2 | 33.4 | NI | 57.5* |
| Pojar et al. [20] | 65.1 ± 10.1 | 41.5 | 43.3 | 73 | NI | 8.1 | 44.3 | NI | 58.4 ± 10.7 |
| Olsthoorn et al. [21] | 63.6 ± 12 | 57 | NI | NI | NI | NI | NI | 14.6 | NI |
| Mihaljevic et al. [22] | 61 ± 11 | 75 | 20 | 46 | NI | 1.8 | 7 | NI | NI |
| Suri et al. [23] | 55.3 ± 12.6 | 78.4 | 10 | 32.1 | 1.6 | 0.5 | 4.7 | NI | 65.3 ± 6.2 |
| Iyigun et al. [24] | 49.9 ± 13.7 | 32.2 | NI | NI | NI | NI | NI | NI | NI |
| Hawkins et al. [25] | 61* | 58.1 | NI | 63.2 | 14.9 | 6.2 | 10.8 | NI | 60* |
| Wang A et al. [26] | 71 ± 5 | 61 | 44.9 | 68.2 | 7.4 | 3.4 | 14.4 | NI | 59 ± 8 |
| Coyan et al. [27] | 62* | 56 | 32 | 57 | NI | 10 | 26 | NI | 59* |
NYHA, New York heart association; LV, left ventricle; LVEF, left ventricular ejection fraction; NI, not indicated
*Median
Table 3.
Summary of risk scores, surgical techniques performed and follow-up in patient populations
| Author | Risk score | MV Repair (%) | MV replacement (%) | Associated TV repair (%) | Associated AF surgery (%) | Follow-up (years) |
|---|---|---|---|---|---|---|
| Grossi et al. [10] | NI | 100 | 0 | NI | NI | NI |
| Nasso et al. [11] | NI | 100 | 0 | NI | NI | 3.2 ± 1.4 |
| Nishi et al. [12] | NI | 100 | 0 | NI | NI | NI |
| Downs et al. [13] | 1.75 ± 3.9a | 77.9 | 22.1 | NI | 27.3 | NI |
| Hawkins et al. [14] | NI | 73.3 | 26.7 | 9.4 | 37.8 | NI |
| Wang Q et al. [15] | 3.17 ± 1.2b | 100 | 0 | 70.1 | 23.9 | 2.8* |
| Grant et al. [16] | 5.4 ± 5.7c | 84.1 | 15.9 | 8.05 | 17.1 | 3.7* |
| Liu et al. [17] | 1.34 ± 0.67d | 0 | 100 | 93.8 | NI | 2.2 ± 1.1 |
| Paparella et al. [18] | 2.6 ± 2.96d | 65.2 | 34.8 | 13.7 | NI | NI |
| Cetinkaya et al. [19] | 7.6 ± 10.3c | 86.75 | 13.25 | 16.65 | 26.4 | 3 |
| Pojar et al. [20] | 2.78 ± 2.4d | 83.8 | 16.2 | 49.3 | 46.7 | 3.6 ± 2.1 |
| Olsthoorn et al. [21] | 3b* | 78.6 | 21.4 | 10.7 | 14.1 | 3.2 ± 2 |
| Mihaljevic et al. [22] | NI | 100 | 0 | NI | NI | NI |
| Suri et al. [23] | NI | 100 | 0 | NI | NI | NI |
| Iyigun et al. [24] | NI | 32.2 | 64.8 | 12.9 | NI | NI |
| Hawkins et al. [25] | 0.6a* | 82.9 | 17.1 | NI | NI | NI |
| Wang A et al. [26] | 2 ± 2a | 94.4 | 5.6 | NI | NI | 1.8 ± 1.2 |
| Coyan et al. [27] | 0.8a* | 83.5 | 16.5 | NI | NI | NI |
MV Mitral valve, TV Tricuspid valve, AF Atrial fibrillation, NI Not indicated
*Median
aSTS PROM score
bEuroscore
clogistic Euroscore
dEuroscore 2
Mortality
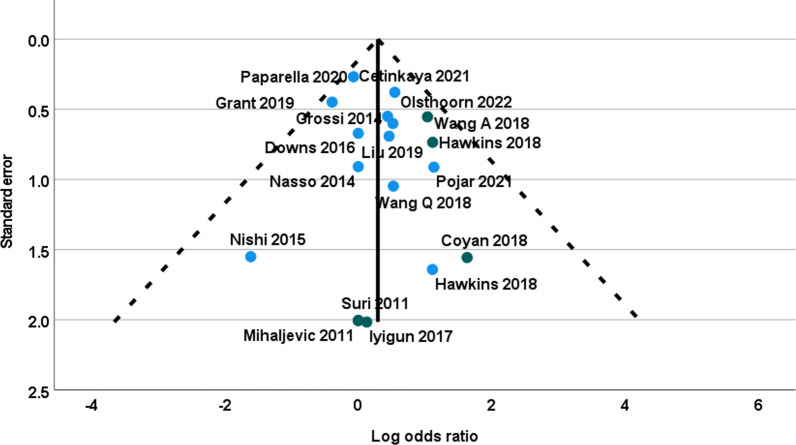
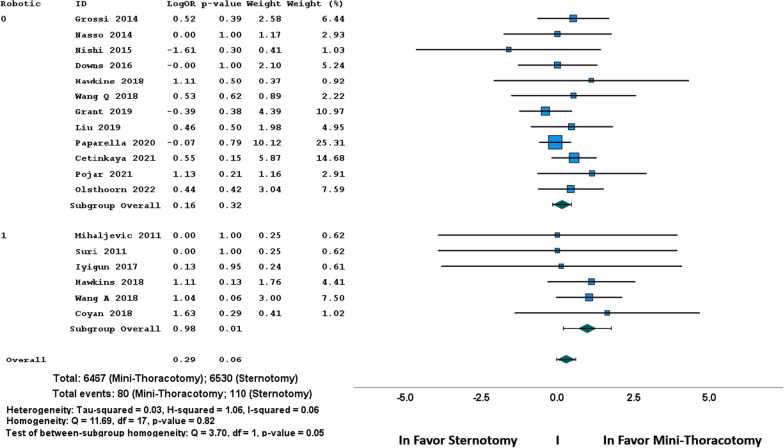
Early mortality was described in all the 18 studies; it was 1.48% and it was significantly lower in patients treated with MTH than in patients treated with STER (1.23% vs 1.63% respectively, χ2 = 4.51, p = 0.033). There was no heterogeneity among the studies (Fig. 2) and the funnel plot showed no asymmetry (Fig. 3). However, the overall OR of early mortality showed no difference between MTH and STER: OR = 1.37, 0.96–1.92 (95% CI), p = 0.06 (Table 4). The sub-group analysis showed a significant OR in favor of MTH when robotic enhancement was used (Fig. 2, Table 4).
Fig. 2.
Funnel plot of early mortality (Log odds ratio)
Fig. 3.
Forest plot of early mortality (Odds ratio)
Table 4.
Summary of postoperative complications
| Complications | N | Sample size/Events | Statistics | Heterogeneity | Sub-group homogeneity | Publication bias |
|||
|---|---|---|---|---|---|---|---|---|---|
| MiniTh (n) | Sternotomy (n) | OR | 95% CI p-value |
χ2 test p-value |
I2 test (%) |
Q test p-value |
Eggers test p-value |
||
| Mortality | 18 |
6467 80 |
6530 110 |
1.37 |
0.96–1.92 0.06 |
0.76 | 8.6 | 0.05 | 0.850 |
| Stroke | 16 |
6367 68 |
6434 85 |
1.23 |
0.89–1.70 0.21 |
0.99 | 0 | 0.69 | 0.159 |
| Reoperation for bleeding | 14 |
5565 192 |
5632 180 |
0.91 |
0.73–1.12 0.38 |
0.41 | 0 | 0.43 | 0.765 |
| Renal failure | 14 |
5881 132 |
5948 202 |
1.67 |
1.06–2.62 0.03 |
0.04 | 46 | 0.81 | 0.276 |
| Atrial fibrillation | 14 |
5348 973 |
5415 1232 |
1.31 |
1.15–1.51 0.001 |
0.20 | 37 | 0.74 | 0.108 |
| Blood transfusion | 12 |
4756 1155 |
4823 1557 |
1.77 |
1.39–2.27 0.001 |
0.001 | 81 | 0.86 | 0.642 |
| Prolonged ventilation | 10 |
3140 96 |
3207 120 |
1.22 |
0.80–1.88 0.36 |
0.07 | 47 | 0.31 | 0.987 |
| Wound infection | 10 |
4415 38 |
4482 75 |
1.86 |
1.17–2.96 0.01 |
0.72 | 11 | 0.73 | 0.978 |
| Mid-term mortality | 8 |
2519 107 |
2555 164 |
1.50 |
1.09–2.08 0.01 |
0.51 | 24 | 0.72 | 0.174 |
| Mid-term reoperation | 7 |
2059 56 |
2106 45 |
0.76 |
0.50–1.15 0.19 |
0.65 | 0 | 0.41 | 0.266 |
N Number of series, MiniTh Mini-thoracotomy, CI Confidence interval, OR Odds ratio
Stroke
Total stroke rate was 1.2% in 16 studies (NA: 15, 24) and 12,801 patients, without difference between groups. The overall OR of stroke was 1.23 without difference between MTH and STER (95% CI 0.89–1.70, p = 0.21). There was no heterogeneity and sub-group analysis according to the robotic enhancement did not differ (Table 4).
Other complications
All other complications are summarized in Table 4. Reoperation for bleeding and prolonged ventilation were observed without difference between groups, and with a non-significant overall OR. New renal failure, new onset of atrial fibrillation and the need of blood transfusion were significantly lower in MTH group than in STER group with significant ORs. In 14 studies (NA: 10, 11, 22, 24) and 11,829 patients, overall OR of new renal failure was 1.67 (95% CI 1.06–2.62, p = 0.03). In 14 studies (NA: 10, 11, 16, 24) and 10,763 patients, overall OR of new onset of atrial fibrillation was 1.31 (95% CI 1.15–1.51, p = 0.001). In 12 studies (NA: 10, 11, 19, 24, 27) and 9579 patients, overall OR of blood transfusion requirement was 1.77 (95% CI 1.39–2.21, p = 0.001). The occurrence of wound infection was lower in MTH approach and overall OR was 1.86 (95% CI 1.17–2.96, p = 0.01). There was significant heterogeneity among the studies in new renal failure and blood transfusion with moderate disparity, as observed in funnel and forest plots (Additional file 2). The sub-group analysis according to the robotic enhancement did not differ in results (Table 4).
Time related outcomes
Time related data are reported in Table 5. Cross clamp time and CPB time were significantly longer after MTH approach compared to STER approach; 16 studies (NA: 8) reported the data of 10,827 patients. The overall differences in means according to random-effects model were 20.7 min for cross clamp time (95% CI 14.9–26.4, p = 0.001) and 36.8 min for CPB time (95% CI 29.8–43.9, p = 0.001); a significant publication bias was detected for both criteria, it was related to the overstatement of the difference in one series with robotic enhancement (Additional file 2) and it did not justify the withdrawal of the series [15].
Table 5.
Summary of time-related outcomes and costs
| Outcomes | N | Sample size | Statistics | Heterogeneity | Sub-group homogeneity | Publication bias |
|||
|---|---|---|---|---|---|---|---|---|---|
| MiniTh (n) | Sternotomy (n) | Diff. in means | 95% CI p-value |
χ2 test p-value |
I2 test (%) |
Q test p-value |
Eggers test p-value |
||
| Cross-Clamp time, min | 16 | 5382 | 5445 | 20.7 |
14.9/26.4 0.001 |
0.001 | 96 | 0.87 | 0.001 |
| CPB time, min | 16 | 5382 | 5445 | 36.8 |
29.8/43.9 0.001 |
0.001 | 95 | 0.67 | 0.001 |
| Total operative time, min | 9 | 2173 | 2240 | 37.7 |
19.6/55.8 0.001 |
0.001 | 97 | 0.001 | 0.193 |
| Ventilation time, hrs | 8 | 1453 | 1520 | − 4.6 |
− 10.6/1.4 0.13 |
0.001 | 99 | 0.001 | 0.469 |
| ICU stay, days | 14 | 4637 | 4700 | − 0.6 |
− 1.1/− 0.21 0.001 |
0.001 | 98 | 0.48 | 0.473 |
| Hospital stay, days | 17 | 6100 | 6163 | − 1.88 |
− 2.72/− 1.05 0.001 |
0.001 | 97 | 0.97 | 0.458 |
| Total cost (USD) | 5 | 1045 | 1112 | − 4525 |
− 8725/− 326 0.03 |
0.001 | 92 | 0.13 | 0.222 |
N Number of series, MiniTh Mini-thoracotomy, Diff Difference, CI Confidence interval, CPB Cardio-pulmonary bypass, ICU Intensive care unit, USD US dollar
The overall differences in means showed a significantly longer total operative time in MTH compared to STER: 37.7 min (95% CI 19.6–55.8, p = 0.001), and robotic enhancement made the difference greater implying a significant sub-group heterogeneity (Table 4, Additional file 2). There was no significant difference in ventilation duration. However, the overall differences in means showed a significantly shorter ICU stay after MTH compared to STER: − 0.6 days (95% CI − 1.1/− 0.21, p = 0.001), in 14 studies (NA: 10, 16, 22) and 9337 patients, and a significantly shorter hospital stay after MTH compared to STER: − 1.88 days (95% CI − 2.72/− 1.05, p = 0.001), in 17 studies (NA: 10) and 12,263 patients. There were evidences for important heterogeneity of treatment effect in time-related criteria among the studies (Table 4), but the disparities were always in the same side as observed in funnel and forest plots (Additional file 2) and there were well compensated by the random-effects model used.
Inhospital cost
Total cost of both procedures was reported in 5 studies [10, 13, 14, 20, 27] and 2157 patients (Table 5). The overall difference in means showed a significant lower cost after MTH compared to STER: − 4528 US$ (95% CI − 8725/− 326, p = 0.03). The sub-group analysis according to the robotic enhancement did not differ in results (Table 5).
Long-term outcomes
Mid-term mortality was reported in 8 studies [11, 15–17, 19–21, 26] and 5074 patients within 3-year mean follow-up, from 1.8 to 3.6 years (Table 3). There was no evidence of heterogeneity of treatment effect among the studies (Additional file 2). The overall OR of mid-term mortality showed a significant higher rate after STER compared to MTH: OR = 1.50, 1.09–2.08 (95% CI), p = 0.01 (Table 4). The sub-group analysis according to the robotic enhancement did not differ in results.
Rate of mid-term reoperation was reported in 7 studies [11, 15–17, 20, 21, 26] and 4165 patients within a 2.8-year mean follow-up, from 1.8 to 3.6 years (Table 3). There was no difference between MTH and STER groups (Table 4): overall OR was 0.76 (95% CI 0.50–1.15, p = 0.19).
Discussion
The benefits of minimally invasive approach via mini-thoracotomy in mitral valve surgery remains controversial, when compared to conventional approach via a sternotomy. It is currently unclear whether the potential benefits of MTH outweigh its disadvantages or drawbacks. Previous meta-analyses were mainly based on historical series, including learning curve, regardless the evolution of the surgical technique itself and the improvement of the tools dedicated to a minimally invasive environment; the main differences between the two approaches were found for procedure and resource related outcomes [2–5]. These outcomes are often used to argue for or against one or the other procedure. Nowadays, mini-thoracotomy approach is established as a new standard for mitral valve surgery with dedicated tools and techniques [28]. The aim of this meta-analysis based only on recent randomized or matched series published from 2010 and including patients operated after 2005, was to identify substantial benefits on perioperative outcomes when standardized MTH approach and mitral valve operation were performed.
In line with previous reports [4, 5], we observed no difference regarding early mortality and major postoperative complications as stroke, reoperation for bleeding, or prolonged ventilation. However, a trend towards a lower early mortality in MTH approach was observed with a significant difference in basic tests (p = 0.03) that was not confirmed in the weighted random effects model analysis (p = 0.06). Interestingly, the sub-group analysis showed a significant OR in favor of MTH when robotic enhancement was used (p = 0.01); this result is mainly related to two database series [25, 26] and must be carefully interpreted (Figs. 2 and 3); it has been reported by Williams ML et al. [29] in a previous meta-analysis based on the same series and it needs further confirmation. The benefits of MTH appear limited in a significant lower rate of renal failure, of new onset of atrial fibrillation and a significant lower requirement of blood transfusion. Despite having longer operative times (clamp time, CPB time and total operations time), MTH was associated with significant shorter lengths of ICU and hospital stay, and finally a significant reduction in the mean hospitalization cost. These results in time-related outcomes are consistent and possibly correlated together: lower incidence of renal failure, of atrial fibrillation and blood transfusion may contribute to lower lengths of stay and costs.
Interestingly, some drawbacks of the minimally invasive approach previously reported [2, 3] have been less observed and reported in this meta-analysis; there is no more an additional risk of stroke or vascular complication in MTH approach compared with conventional STER, probably thanks to the standardization of the technique that made this approach safer [30, 31].
According to this meta-analysis, the benefit impact of MTH in comparison of sternotomy remains limited. Consequently, in one hand the advantages of MTH are not strong enough to convince surgeons to change their practice and in the other hand the benefits were observed in a low risk population and are too limited to contribute to extending the indications to a higher risk population for whom longer operative times represent an obvious potential risk of increased complications [32, 33]. That remains the dilemma of minimally invasive approach in mitral valve surgery, more than 20 years after its introduction. The robotic-enhancement of the technique that is becoming a new standard [34] does not contribute to solve the problem; the sub-group analysis done in our study was not able to identify a difference in results, except for early mortality with the limitations mentioned previously, in line with previous report [29, 35].
However, indications for mitral valve surgery are recommended at the early stage of the disease, especially in mitral regurgitation [36, 37], representing a low risk population, more often asymptomatic or pauci-symptomatic patients who are demanding mini surgical access; that contributes to the diffusion of MTH approach. Moreover, if the tendency to a lower mortality is confirmed in the next future it could contribute to a better adoption rate of the MTH approach as well.
The meta-analysis reported by Moscarelli et al. [38] showed that the inclusion of high risk patients has not compromised the expected results of mini-access and it could be time to explore the advantages of MTH approach in high risk populations for mitral valve surgery. Possibly, the multicentric randomized controlled trial in process in UK (UK mini mitral) could modify the debate in a next future [39].
In this meta-analysis the midterm mortality was reported significantly lower in MTH approach; this result is based on eight studies with risk of bias due to confounding moderate in two and severe in three and it must be considered carefully. However, we can speculate that a lower rate of renal failure, atrial fibrillation, wound infection and even blood transfusion could have an impact on the midterm mortality that was reported within 3 year mean follow-up in studies. The midterm rate of reoperation was reported low and similar in both groups, confirming the durability of the results with both approaches [40].
Limitations
The present study had several limitations. Different pathologies and techniques were reported and may have increased the level of clinical heterogeneity among studies; however, it was part of the selection criteria to verify if this heterogeneity had been well balanced in randomization or propensity score matching. Nevertheless, it was not possible to analyze the heterogeneity of the techniques of repair among the series. It was a choice in the design of the study to consider MTH as the concept of mini-approach and robotic- or video-assistance as tools; however a sub-group analysis was included to detect any specific impact of robotic enhancement. Femoral cannulation was a criteria of inclusion, however, the techniques of cross clamp were variable (transthoracic or endoaortic). Nowadays, both technique are equivalent after some learning curve [30, 31]. Regarding the analysis of the outcomes, the completeness of the series was not enough to pool the events in a “MACE index”, in reference with STS complications, and they were reported separately. The statistical heterogeneity in outcome was moderate, mainly reported in time-related outcomes and it was counterbalanced by using weighted random effects model. Finally forest and funnel plots for significant OR were reported in Additional file 2 to illustrate the possible bias across studies and results. Definition of early mortality and follow-up for midterm results were changing according to studies and it led to being careful in the interpretation of the results regarding early and midterm mortality, regardless risk of bias due to confounding that has been reported above.
Conclusion
The present meta-analysis confirmed that the MTH approach for mitral valve disease has remained associated with prolonged operative times but it was beneficial in terms of reduced postoperative complications (renal failure, atrial fibrillation, blood transfusion, wound infection), length of stay in ICU and in hospitalization, with finally a reduction in global cost. This limited impact may explain that in daily practice, MTH approach remain performed mainly in low risk patient to avoid any additional risk related to longer operative times in patient with a more severe profile related to cardiac or non-cardiac risk factors. However, this meta-analysis detected that MTH approach could be associated with a significant reduction of postoperative early and midterm mortality that must be confirmed by large randomized study but it may open the way to a new era demonstrating that benefits of MTH outweigh its drawbacks. Finally, in this meta-analysis there was no evidence of any additional benefit from robotic enhancement in MTH approach for mitral valve surgery; but that needs to be analyzed in a dedicated randomized study.
Supplementary Information
Additional file 1. Risk of bias in non-randomized studies according to ROBINS-I tool.
Additional file 2. Forest plots of the criteria analyzed with respective funnel plots if significant Odds ratio.
Acknowledgements
Not applicable.
Author contributions
AA: Concept/design, Data collection, data interpretation, MJ: Data analysis/interpretation, statistics, SA: Data collection, data interpretation. AE: Critical revision of the article, OJ: Concept/design, data interpretation, Critical revision of the article, All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The data sets used and analysed during the current study are available from the corresponding author.
Declarations
Ethical approval and consent to participate.
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Casselman FP, Slycke SV, Dom H, Lambrechts DL, Vermeulen Y, Vanermen H. Endoscopic mitral valve repair: feasible, reproducible, and durable. J Thorac Cardiovasc Surg. 2003;125:273–282. doi: 10.1067/mtc.2003.19. [DOI] [PubMed] [Google Scholar]
- 2.Modi P, Hassan A, Chitwood WR. Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardio-thorac Surg. 2008;34:943–952. doi: 10.1016/j.ejcts.2008.07.057. [DOI] [PubMed] [Google Scholar]
- 3.Cheng DCH, Martin J, Lal A, Diegeler A, Folliguet TA, Nifong W, et al. Minimally invasive versus conventional open mitral valve surgery. A meta-analysis and systematic review. Innovations. 2011;6:84–103. doi: 10.1097/imi.0b013e3182167feb. [DOI] [PubMed] [Google Scholar]
- 4.Sundermann SH, Sromicki J, Biefer HRC, Seifert B, Holubec T, et al. Mitral valve surgery: right lateral minithoracotomy or sternotomy ? A systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2014;148:1989–1995. doi: 10.1016/j.jtcvs.2014.01.046. [DOI] [PubMed] [Google Scholar]
- 5.Moscarelli M, Fattouch K, Gaudino M, Nasso G, Paparella D, Punjabi P, et al. Minimal access versus sternotomy for complex valve repair: a meta-analysis. Ann Thorac Surg. 2020;109:737–744. doi: 10.1016/j.athoracsur.2019.07.034. [DOI] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG, Grouppara TP. Preferred reporting item for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epideniol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 8.Sterne JAC, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grossi EA, Goldman S, Wolfe A, Mehall J, Smith JM, Ailawadi G, et al. Minithoracotomy for mitral valve repair improves inpatient and postdischarge economic savings. J Thorac Cardiovasc surg. 2014;148:2818–2822. doi: 10.1016/j.jtcvs.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 11.Nasso G, Bonifazi R, Romano V, Bartolomucci F, Rosano G, Massari F, et al. Three-year results of repaired Barlow mitral valves via right minithoracotomy versus median sternotomy in a randomized trial. Cardiology. 2014;128:97–105. doi: 10.1159/000357263. [DOI] [PubMed] [Google Scholar]
- 12.Nishi H, Miyata H, Motomura N, Toda K, Miyagawa S, Sawa Y, et al. Propensity-matched analysis of minimally invasive mitral valve repair using a nationwide surgical database. Surg today. 2015;45:1144–1152. doi: 10.1007/s00595-015-1210-7. [DOI] [PubMed] [Google Scholar]
- 13.Downs EA, Johnston LE, Lapar DJ, Ghanta RK, Kron IL, Speir AM, et al. Minimally invasive mitral valve surgery provides excellent outcomes without increased cost: a multi-institutional analysis. Ann Thorac Surg. 2016;102:14–21. doi: 10.1016/j.athoracsur.2016.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawkins RB, Mehaffey JH, Kessel SM, Dahl JJ, Kron IL, Kern JA, et al. Minimally invasive mitral valve surgery is associated with excellent resource utilization, cost, and outcomes. J Thorac Cardiovasc surg. 2018;156:611–616. doi: 10.1016/j.jtcvs.2018.03.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, Xi W, Gao Y, Shen H, Min J, Yang J, et al. Short-term outcomes of minimally invasive mitral valve repair: a propensity-matched comparison. Interact CardioVasc Thorac surg. 2018;26:805–812. doi: 10.1093/icvts/ivx402. [DOI] [PubMed] [Google Scholar]
- 16.Grant SW, Hickey GL, Modi P, Hunter S, Akowuah E, Zacharias J. Propensity-matched analysis of minimally invasive approach versus sternotomy for mitral valve surgery. Heart. 2019;105:783–789. doi: 10.1136/heartjnl-2018-314049. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Chen B, Zhang YY, Fang LZ, Xie B, Huang HL, et al. Mitral valve replacement via minimally invasive totally thoracoscopic surgery versus traditional median sternotomy: a propensity score matched comparative study. Ann Transl Med. 2019;7:341. doi: 10.21037/atm.2019.07.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paparella D, Fattouch K, Moscarelli M, Santarpino G, Nasso G, Guida P, et al. Current trends in mitral valve surgery: a multicenter national comparison between full-sternotomy and minimally-invasive approach. Int J Cardiol. 2020;306:147–151. doi: 10.1016/j.ijcard.2019.11.137. [DOI] [PubMed] [Google Scholar]
- 19.Cetinkaya A, Geier A, Bramlage K, Hein S, Bramlage P, Schonburg M, et al. Long-term results after mitral valve surgery using minimally invasive versus sternotomy approach: a propensity matched comparison of a large single-center series. BMC Cardiovasc Disord. 2021;21:314. doi: 10.1186/s12872-021-02121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pojar M, Karalko M, Dergel M, Vojacek J. Minimally invasive or sternotomy approach in mitral valve surgery: a propensity-matched comparison. J Cardiothorac Surg. 2021;16:228. doi: 10.1186/s13019-021-01578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olsthoorn JR, Heuts S, Houterman S, Maessen JG, Sardari P. Effect of minimally invasive mitral valve surgery compared to sternotomy on short- and long-term outcomes: a retrospective multicenter interventional cohort study based on Netherland heart registration. Eur J Cardiothorac Surg. 2022;61:1099–1106. doi: 10.1093/ejcts/ezab507. [DOI] [PubMed] [Google Scholar]
- 22.Mihaljevic T, Jarrett CM, Gillinov M, Williams SJ, Devilliers PA, Stewart WJ, et al. Robotic repair of posterior mitral valve prolapse versus conventional approaches: potential realized. J Thorac Cardiovasc surg. 2011;141:72–80. doi: 10.1016/j.jtcvs.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Suri RM, Burkhart HM, Daly RC, Dearani JA, Park SJ, Sundt TM, et al. Robotic mitral valve repair for all prolapse subsets using techniques identical to open valvuloplasty: establishing the benchmark against which percutaneous interventions should be judged. J Thorac Cardiovasc surg. 2011;142:970–979. doi: 10.1016/j.jtcvs.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 24.Iyigun T, Kaya M, Gulbeyaz SO, Fistikci N, Uyanik G, Yilmaz B, et al. Patient body image, self-esteem, and cosmetic results of minimally invasive robotic cardiac surgery. Inter J Surg. 2017;39:88–94. doi: 10.1016/j.ijsu.2017.01.105. [DOI] [PubMed] [Google Scholar]
- 25.Hawkins RB, Mehaffey HJ, Mullen MM, Nifong W, Chitwood WR, Katz MR, et al. A propensity matched analysis of robotic, minimally invasive and conventional mitral valve surgery. Heart. 2018;104:1970–1975. doi: 10.1136/heartjnl-2018-313129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang A, Brennan LM, Zhang S, Jung SH, Yerokun B, Cox ML, et al. Robotic mitral valve repair in older individuals: an analysis of the society of thoracic surgeons database. Ann Thorac Surg. 2018;106:1388–1395. doi: 10.1016/j.athoracsur.2018.05.074. [DOI] [PubMed] [Google Scholar]
- 27.Coyan G, Wei L, Althouse A, Roberts HG, Schauble D, Murashita T, et al. Robotic mitral valve operations by experienced surgeons are cost-neutral and durable at 1 year. J Thorac Cardiovasc surg. 2018;156:1040–1047. doi: 10.1016/j.jtcvs.2018.03.147. [DOI] [PubMed] [Google Scholar]
- 28.Kempfert J, Kofler M, Falk V, Sundermann S. Minimally invasive endoscopic mitral valve repair-the new gold standard for degenerative mitral valve disease. Eur J Cardiothorac Surg. 2022;61:645–646. doi: 10.1093/ejcts/ezab568. [DOI] [PubMed] [Google Scholar]
- 29.Williams ML, Hwang B, Huang L, Wilson-Smith A, Brookes J, Eranki A, et al. Robotic versus conventional sternotomy mitral valve surgery: a systematic review and meta-analysis. Ann cardiothorac Surg. 2022;11:490–503. doi: 10.21037/acs-2022-rmvs-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rival PM, Moore THM, McAleenan A, Hamilton H, Du Toit Z, Akowuah E, et al. Transthoracic clamp versus endoaortic balloon occlusion in minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2019;56:643–653. doi: 10.1093/ejcts/ezy489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kesavuori R, Raivio P, Jokinen JJ, Sahlman A, Teittinen K, Vento A. Early experience with robotic mitral valve repair with intra-aortic occlusion. J Thorac Cardiovasc Surg. 2018;155:1463–1471. doi: 10.1016/j.jtcvs.2017.10.076. [DOI] [PubMed] [Google Scholar]
- 32.Almutairi N, Al Shamry A, Ashafy S, Jegaden O. Comparison between minimally invasive approach and conventional sternotomy in mitral valve surgery: critical analysis of a daily practice. EC Cardiology. 2018;5:11. [Google Scholar]
- 33.Van Praet KM, Kofler M, Hirsch S, Akansel S, Hommel M, Sundermann SH, et al. Factors associated with an unsuccessful fast-track couse following minimally invasive surgical mitral valve repair. Eur J Cardiothorac Surg. 2022 doi: 10.1093/ejcts/eazc451. [DOI] [PubMed] [Google Scholar]
- 34.Bonatti J, Crailsheim I, Grabenwoger M, Winkler B. Minimally invasive and robotic mitral valve surgery: methods and outcomes in a 20-year review. Innovations. 2021;16:317–326. doi: 10.1177/15569845211012389. [DOI] [PubMed] [Google Scholar]
- 35.Chemtob RA, Wierup P, Mick SL, Javorski MJ, Burns DJP, Blackstone EH, et al. A conservative screening algorithm to determine candidacy for robotic mitral valve surgery. J Thorac Cardiovasc surg. 2022;164:1080–1087. doi: 10.1016/j.jtcvs.2020.12.036. [DOI] [PubMed] [Google Scholar]
- 36.Enriquez-Sarano M, Suri RM, Clavel MA, Mantovani F, Michelena HI, Pislaru S, et al. Is there an outcome penalty to guideline-based indications for valvular surgery? Early and long-term analysis of patients with organic mitral regurgitation. J Thorac Cardiovasc surg. 2015;150:50–58. doi: 10.1016/j.jtcvs.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Beyersdorf F, Vahanian A, Milojevic M, Praz F, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. 2021;60:727–800. doi: 10.1093/ejcts/ezab389. [DOI] [PubMed] [Google Scholar]
- 38.Moscarelli M, Fattouch K, Casula C, Speziale G, Lancelloti P, Athanasiou T. What is the role of minimally invasive mitral valve surgery in high risk patients ? A meta-analysis of observational studies. Ann Thorac Surg. 2016;101:981–989. doi: 10.1016/j.athoracsur.2015.08.050. [DOI] [PubMed] [Google Scholar]
- 39.Maier RH, Kasim AS, Zacharias J, Vale L, Graham R, Walker A, et al. Minimally invasive versus conventional sternotomy for mitral valve repair: protocol for a multicenter randomized controlled trial (UK mini mitral) BMJ Open. 2021;11:e047676. doi: 10.1136/bmjopen-2020-047676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonaros N, Hoefer D, Oezpeker C, Gollmann-Tepekoylu C, Holfeld J, Dumfarth J, et al. Predictors of safety and success in minimally invasive surgery for degenerative mitral disease. Eur J Cardiothorac Surg. 2022;61:637–644. doi: 10.1093/ejcts/ezab438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Risk of bias in non-randomized studies according to ROBINS-I tool.
Additional file 2. Forest plots of the criteria analyzed with respective funnel plots if significant Odds ratio.
Data Availability Statement
The data sets used and analysed during the current study are available from the corresponding author.