Abstract
Human diseases, particularly infectious diseases and cancers, pose unprecedented challenges to public health security and the global economy. The development and distribution of novel prophylactic and therapeutic vaccines are the prioritized countermeasures of human disease. Among all vaccine platforms, viral vector vaccines offer distinguished advantages and represent prominent choices for pathogens that have hampered control efforts based on conventional vaccine approaches. Currently, viral vector vaccines remain one of the best strategies for induction of robust humoral and cellular immunity against human diseases. Numerous viruses of different families and origins, including vesicular stomatitis virus, rabies virus, parainfluenza virus, measles virus, Newcastle disease virus, influenza virus, adenovirus and poxvirus, are deemed to be prominent viral vectors that differ in structural characteristics, design strategy, antigen presentation capability, immunogenicity and protective efficacy. This review summarized the overall profile of the design strategies, progress in advance and steps taken to address barriers to the deployment of these viral vector vaccines, simultaneously highlighting their potential for mucosal delivery, therapeutic application in cancer as well as other key aspects concerning the rational application of these viral vector vaccines. Appropriate and accurate technological advances in viral vector vaccines would consolidate their position as a leading approach to accelerate breakthroughs in novel vaccines and facilitate a rapid response to public health emergencies.
Subject terms: Vaccines, Infectious diseases
Introduction
The outbreak of infectious diseases and the occurrence of cancers cause a huge impact on humans throughout history. Hemorrhagic fever, including Ebola, Marburg, and Lassa fever, cause fatality rates of up to 50%.1–3 In addition, there have been three waves of beta coronavirus emergence since 2003, of which coronavirus disease 2019 (COVID-19) has caused billions of confirmed cases and millions of deaths since 2019.4–6 Globally, an estimated 19.3 million new cancer cases and almost 10.0 million cancer deaths occur every year,7 which pose as the leading health threat.
For infectious diseases, vaccination and establishment of herd immunity are of primary importance. Among all vaccine technologies, recombinant viral vectors represent promising vaccine platforms due to their ability to express heterologous antigens and induction of cellular immune responses and humoral immune responses without exogenous adjuvants. Viral vector vaccines consist of viral particles whose genomes have been modified to contain one or more foreign genes encoding the targeted antigens. The rationale for using viruses to deliver the ‘vaccine gene’ is in several folds. Viral vectored vaccines are safe and induce both arm of innate and adaptive immune responses without involvement of the complete hazardous pathogen.8 Moreover, viral vectors have intrinsic adjuvant properties due to the expression of diverse pathogen-associated molecular patterns (PAMPs) and the activation of innate immunity.9 In addition, viral vectors can be engineered to deliver antigens to specific cells or tissues. Similarly, they can be rendered replication-competent or replication-deficient to increase their safety and reduce reactogenicity. Notably, the viral vector vaccine can recapitulate the natural infection process of specific pathogens, thus triggering classical acute inflammation and immune detection through the natural production of PAMPs, enabling mucosal delivery and induction of local-mucosal and systemic immunity. Several viral vector-based prophylactic vaccines have entered Phase III clinical trials or have been approved.10–15 In the field of cancers, viral vectors are ideal oncolytic viruses (OVs) since they can trigger cellular immunity and could be armed, shielded and targeting tumor cells. The release of tumorassociated antigens (TAAs) could activate and regulate the anti-tumor immune response. Several OV preparations have been approved for marketing, which present promising directions for immunotherapy of tumors.
Nevertheless, the systematic and comparative review of these viral vectors is less well established. Moreover, the generality and individuality of these viral vectors are not fully elucidated. In this review, the general overview of vesicular stomatitis virus (VSV), rabies virus (RABV), parainfluenza virus (PIV), measles virus (MeV), Newcastle disease virus (NDV), influenza virus (IFV), adenovirus (AdV), and poxvirus vector vaccines was summarized in terms of their application to life-threatening infectious diseases as well as immunotherapy for cancer. The characteristics, merits and limitations of these viral vectors were analyzed and presented in depth. Taken together, these issues would compel the acceleration and approval of novel viral vector vaccines confronting human health threats.
Structure and design strategies for viral vectors
Nonsegmented negative‐strand RNA viruses (NNSVs) as vaccine vectors
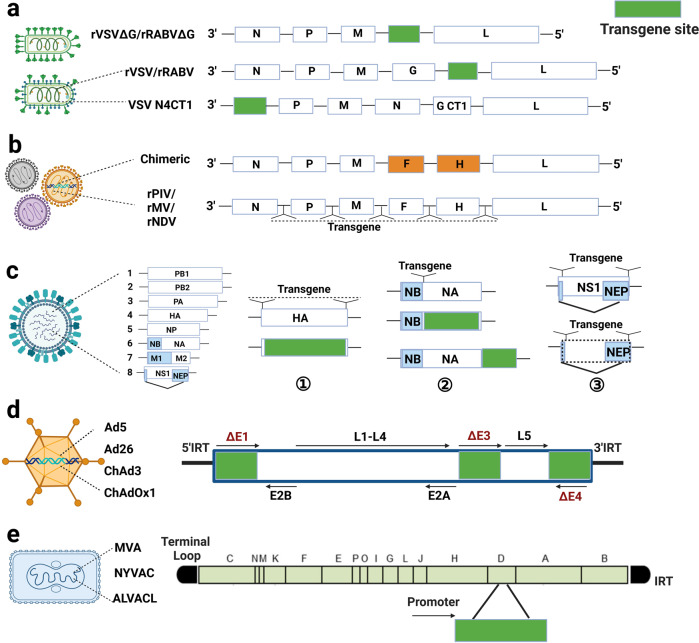
VSV and RABV are enveloped NNSVs belonging to Rhabdoviridae. Rhabdoviridae is composed of five structural proteins including nuclear protein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and RNA-dependent RNA polymerase (L).16,17 PIV, MeV and NDV belong to Paramyxoviridae. Their nucleotide genome contains six structural genes including N, P, M, fusion glycoprotein (F), hemagglutinin glycoprotein (H), and L.18,19 Both surface envelope glycoproteins are responsible for host cell binding and invasion. The rescue and operation of these NNSVs were accomplished through reverse genetics approaches of negative single strand RNA. In 1994, RABV was the first to be rescued from cloned cDNA, which marking a major milestone in the field of NNSVs.20 The virus was rescued from a cloned cDNA that contains the full genome sequence in the positive‐sense orientation flanked by a T7 promoter and hepatitis delta virus ribozyme. Subsequently, the reverse genetic system of other NNSVs was established, which enables the reconstruction of the full-length genome.21–30 For these NNSV vectors, there are two major strategies for foreign gene delivery. (1) Delete the glycoprotein gene of the viral vector and replace it with a targeted gene (NNSVΔG or NNSVΔF) (Fig. 1a, b).31 (2) Involving an additional transcriptional unit for foreign antigen while retain the vector glycoprotein gene in the full-length genome (rNSSV) (Fig. 1a, b).32–34 Foreign genes could be inserted at different gene junctions of the genome as an additional expression cassette.
Fig. 1.
Design strategies for Vesicular stomatitis virus (VSV), rabies virus (RABV), parainfluenza virus (PIV), measles virus (MeV), Newcastle disease virus (NDV), influenza virus (IFV), Adenovirus (AdV) and poxvirus for vaccine platforms. a rVSVΔG/rRABVΔG, in which the glycoprotein (G) of the vector is replaced by a foreign gene; rVSV/rRABV, an additional transcription unit is involved between G and L of the genome. N4CT1, involves an additional transcriptional unit at the 3′ end of the genome, translocation of N gene and truncation of the VSV G cytoplasmic tail. b Chimeric paramyxovirus vector, in which the fusion and hemagglutinin glycoprotein of paramyxovirus is replaced by those of other paramyxoviruses; recombinant paramyxovirus vectors, which involve an additional transcription unit for foreign genes. c Manipulation of the genome of IFV based on HA, NA, and NS. c1. Inserting foreign gene based on HA: the foreign gene is inserted into the receptor binding site of the HA head or the N-terminal of HA; insert the foreign gene in place of HA while retaining packaging sequences. c2. Inserting foreign gene based on NA: the foreign gene is inserted into the stem of NA; preserve the non-coding sequences and adjacent coding regions of NA for transgene in place of NA coding sequence; involves an additional transcription unit at the 5′ end of NA. c3. Inserting foreign gene based on NS: insert transgene after the 125th amino acid of NS; retain NS1 and NEP, and introduce 2 A self-cutting site at the end of NS1; insert transgene in place of NS1. d Genome of AdV, E1, E3 and (or) E4 regions are designed for transgene. e Genome of poxvirus, the D transcription units could be replaced by the transgene of choice under the promoter. (Created in BioRender)
In the NNSVΔG/NNSVΔF design strategy, the targeted glycoprotein could be displayed on the surface of the recombinant virus. Accordingly, the cell and tissue tropism of the recombinant virus is largely depended on foreign glycoproteins. In cases that the target glycoprotein was similar in the molecular size and function of the vector glycoprotein, NNSVΔG/NNSVΔF design strategy rendered the recombinant virus ideal for biological growth properties and minimization of anti-vector immunity.35,36 Although recombination of large foreign genes is achievable, the growth titer of recombinant virus is relatively low. For example, rVSVΔG-SARS-CoV-2-S and rVSVΔG-CCHFV-G represented an upmost growth titer of about 106 TCID50.37,38 To overcome this issue, truncation of the cytoplasmic tail (CT) region of the foreign gene and screening of the optimum cell line for virus culturing are alternative measures. In contrast, a higher growth titer could be achieved in viral vectors that carries an additional transcriptional unit for the external gene.32,39 Foreign genes other than glycoprotein can also be incorporated into recombinant viruses. In some cases, transmembrane (TM) and CT domains of the foreign gene should be replaced by those of the glycoprotein of the viral vector to maximize the incorporation of the foreign protein into the virion and optimize immunogenicity.40 Of particularly note, transcriptional translation decreased from 3′ to 5′ end of the genome.41 For example, the polar mechanism of VSV transcription results in a gradient of mRNA abundance that is highest at the 3′ end of the genome and decreases toward the 5′ end, following the order of N > P > M > G > L, thus the expression level of specific antigens was correlated with the insertion position. An ideal insertion site for the foreign gene should balance virus replication and foreign gene expression and contain an optimized arrangement of gene junction sequences before and after the exotic gene.42–44
Segmented RNA (IFVs) as vaccine vectors
IFV is an enveloped, segmented RNA virus belonging to the Orthomyxoviridae family.45 IFV is classified into four genera according to nucleoprotein (NP): influenza A, B, C, and D. Of which influenza A virus (IAV) and influenza B virus (IBV) viruses are of public health relevance due to their potential to cause severe disease in humans. IAV and IBV carry 8 segments of single-stranded, negative-sense RNA that encode at least 8 proteins: polymerase basic 1 (PB1), polymerase basic 2 (PB2), polymerase acidic (PA), hemagglutinin (HA, surface glycoprotein), NP, neuraminidase (NA, surface glycoprotein), NB (surface glycoprotein), matrix protein 1 (M1 and M2), non-structural protein (NS1 and NEP). Based on reverse genetic approaches of IFV,46–48 multiple segments of IFV were manipulated for transgene, including HA, NA, NS1, etc. (Fig. 1c), chimeric construction between IAV and IBV was also reported.49–56
When a foreign gene was inserted into the receptor binding site of HA head or the N-terminal of HA, the function of IFV HA was not affected, thus complete replication ability retained. In the case of the construction of replication-defective recombinant virus, only the packaging sequences of the 3′ and 5′ ends of HA were retained, and the coding region of HA was replaced by foreign sequences. This replication-defective virus could replicate in Madin-Darby canine kidney (MDCK) cell lines that stably express HA protein.
For NA stem, only 28–41 amino acid insertion is permissive. Inserting foreign sequences into the NA stem would affect the virulence of the virus.57,58 Another strategy concerning the NA fusion proteins was prepared by preserving the non-coding sequences and adjacent coding regions of NA. In this strategy, IFV was mostly replication defective, which required the addition of exogenous NA enzymes. There is also a strategy that involved an additional transcription unit at the 5′ end of IFV NA, which maintained the complete structure and function of NA. Approximately 680 bp foreign gene fragments were allowed.59 Overall, ~1.5 kb of the foreign gene was permissive to be incorporated into the IFV NA segment.60
The nonstructural protein 1 (NS1) and nuclear export protein (NEP) are encoded by the NS gene of IFV, which can tolerate 250 amino acids insertion. NS1 protein of IFV is a virulence element which could inhibit the interferon production and lead to the escape of the IFV to the initial immune response.61 The deletion of NS1 gene weakened the virulence of the virus significantly, which has been applied to the development of IFV-vectored vaccine.62 NEP works in regulating the IFV ribonucleoprotein complex and virus nucleation. NS is not involved in virion formation, thus NS protein change does not alter the antigenicity of IFV.63 There are three methods to construct chimeric IFV vector vaccines based on NS segments. The first construction method is to establish a bicistronic reading frame, that is, inserted a start-stop reading frame (UAAUG) after the 125th amino acid.63 The second construction method retained NS1 and NEP, and introduced 2 A self-cutting site at the end of NS1.64 Finally, in the case of NS1 deletion constructs, the replication ability of the recombinant virus in MDCK cells was significantly weakened. To address this issue, mutations in M gene A14U enhanced replication of NS1-deleted viruses in MDCK cells.65 Indeed, NS1 gene deletion may not merely act as an attenuation strategy, but exhibit more potent and long-lasting immunity compared to cold-adapted IFV by activating multidimensional immune responses.
Adenoviruses as vaccine vectors
AdVs are non-enveloped dsDNA viruses belonging to Adenoviridae.66 AdVs are of wide host origin and can be divided into various serotypes. Their double-stranded linear genome ranges from 26 kb to 45 kb, a size that is amenable to manipulation.67 AdVs have transition from tools for gene replacement therapy to bona fide vaccine delivery vehicles. They are attractive vaccine vectors as they simulaneously induce both innate and adaptive immune responses in mammalian hosts. AdV-based vectors can be rendered replication-competent or replication-defective via the manipulation of early 1 (E1) region or part of it.68 In addition, the early 3 (E3) gene could be deleted to enlarge the capacity for transgene insertion since the E3 gene is dispensable for virus replication. Consequently, E1 or E3 deleted regions are expression cassettes for transgene expression (Fig. 1d). AdV vectors are well established, easy to operated, amenable to rapid, inexpensive manufacturing and cold chain-free storage. AdVs of human, simian and avian origin are involved in vaccine vectors.
Poxviruses as vaccine vectors
Poxvirus is the largest enveloped DNA virus. In the 1980s, smallpox was successfully eradicated by vaccination with the vaccinia virus (VACV). During the same period, VACA was applied as a transgenic expression vector.69,70 The passage of parental VACA resulted in random mutations and deletions, which contributed to the reduced pathogenicity of VACV. The third generation poxvirus vectors include Listeria clone 16m8 (LC16m8), Dairen I strain (Dis), M65, M101, modified vaccinia virus Ankara (MVA) as well as several attenuated fowlpox viruses.71
MVA is highly attenuated by passaging 570 generations on chicken embryos. Due to the blocking in virus assembly, MVA doesn’t produce infectious progeny while maintains robust DNA replication and antigen expression ability in most mammalian cells.72–74 Thereinto, MVA-572, MVA-I721 and MVA-BN share 100% identical nucleotide sequence in coding regions while exhibit significantly different phenotypes. Among them, MVA-BN shows better safety and immunogenicity than other two strains.75 MVA is an excellent third-generation smallpox vaccine that has been vaccinated by more than 120,000 people in Germany.71,76–79 MVA-VLP HIV vaccine candidate has shown excellent safety in clinical trials of 500 people, including immunocompromised individuals and HIV patients.80,81 Recombinant MVA is genetically stable, easily modified, safe and shows good immunogenicity even under the preexisting anti-vector immunity, especially when used in combination with other viral vector vaccines, such as AdV vector vaccine.82–85 These characteristics make MVA a promising vaccine vector. In addition to MVA, other poxviruses are used as vectors including Canarypox virus (ALVACL), C16m8 deriving from the Lister strain as well as New York attenuated vaccinia virus NYVAC (Fig. 1e). Comparison of viral vectors was summarized in Table 1.
Table 1.
Comparison of viral vectors
| Vector | Type of virus(kb) | Genome size(kb) | Genome type | Cargo capacity(kb) | Predominant immune response | Administration route | Strengths | Weaknesses | References |
|---|---|---|---|---|---|---|---|---|---|
| Vesicular stomatitis virus | Enveloped, RNA | ~11 | Single stranded, negative‐sense, nonsegmented | ~6 | Humoral and cellular immune response | IM, IN, or OR | No concerns of virulence reversion, residual virulence or virus recombination; small and easily manipulated genome; stable expression of foreign genes; rapid replication and high growth titer | Safety concerns | 514 |
| Rabies virus | Enveloped, RNA | ~12 | Single stranded, negative‐sense, nonsegmented | ~6.5 | Humoral response in dominant | IM or OR | Small and easily manipulated genome; design as inactivated bivalent vaccines | A potential risk for reversion to virulence; less well immunogenicity than VSV vector | 515,516 |
| Parainfluenza virus | Enveloped, RNA | ~15 | Single-stranded negative-sense, nonsegmented | ~4 | Humoral, cellular and mucosal immune response | IM, IN, or OR | Ideal for paediatric and respiratory diseases; safe; genomic stability | Anti-vector immunity; Safety concerns | 44 |
| Measles virus | Enveloped, RNA | ~16 | Single-stranded negative-sense, nonsegmented | ~6 | Humoral, cellular and mucosal immune response | IM, IP or SC | Licensed live-attenuated measles vaccines are effective and safe; lack of genomic integration in the host; established manufacturing infrastructure | Limited challenge models; low viral titers | 378,517–519 |
| Newcastle disease virus | Enveloped, RNA | ~15 | Single-stranded negative-sense, nonsegmented | ~4 | Humoral and cellular immune response | IM,IN | High growth titers; lack of genomic integration in the host; host restriction; no pre-existing antibody to NDV in the human | Less well immunogenic than other paramyxovirus vector-based vaccines | 210 |
| Lentivirus | Enveloped, RNA | ~9.2 | Single-stranded positive-sense, nonsegmented | ~4 | Humoral and cellular immune response | IM,IN | Low anti-vector immunity; less integration into the host genome; Durable immune responses | Safety concerns; potential batch to batch variation in manufacturing | 8 |
| Influenza virus | Enveloped, RNA | ~13.5(total), 0.89–2.3 kb per each segment | Single stranded, negative‐sense, segmented | <1.5 | Humoral and cellular immune response | IM, IN | A broad host range; easily manipulated genome; highly attenuated; established manufacturing infrastructure | Limited transgene ability; genetic reassortment; safety concerns | 520–522 |
| Adenovirus | Non-enveloped, DNA | 26–45 | Double-stranded, nonsegmented | ~7.5 | Humoral and cellular immune response | IM, IN, or OR | Well-established; high transduction efficiencies; relative large capacities for transgenes; high titer of production | Anti-vector immunity | 523 |
| Poxvirus | Enveloped, DNA | 130–300 | Double-stranded, nonsegmented | ~25 | Low/moderate antibodies response and strong cellular immune response | IM | Packing flexibility of the genome; without genomic integration in the host; expressing VLPs | Existence of the viral immunomodulatory genes | 8,524 |
IM intramuscular, IN intranasal, OR oral, IP intraperitoneal, SC subcutaneous, VLPs virus like particles, VSV Vesicular stomatitis virus
Application of viral vector vaccines in human disease
Vesicular stomatitis virus vector
A single dose of VSV-vectored vaccine is potent in inducing long-lasting protection
In most cases, VSV vectored vaccines are designed as a single dose regime. For viral hemorrhagic fever, a single dose of VSV vectored vaccine induced long-lasting protection. Representatively, rVSVΔG-ZEBOV, a recombinant EBOV vaccine candidate in which VSV G gene was replaced with the G gene of Zaire Ebola virus (ZEBOV) for the rescue of recombinant virus. A single intramuscular (IM) dose vaccination of rVSVΔG-ZEBOV fully protected mice and non-human primates (NHPs) against the lethal challenge of EBOV.86–91 Animals with delayed activation of innate responses succumbed to challenge.92 In Guinea ring vaccination, a single dose vaccination of 2 × 107 PFU of rVSVΔG-ZEBOV showed good safety and immunogenicity in volunteers. rVSVΔG-ZEBOV offered substantial protection against EBOV disease, with an overall protective efficacy of 100%.93 After vaccination, antibodies appeared on day 14, peaked around day 28, and were detectable within 2 years.94–96 rVSVΔG-ZEBOV has been approved by the European Medicines Agency (EMA) and has been licensed for emergency use.10,97,98
Similarly, a single dose vaccination of rVSVΔG vectored vaccine expressing the glycoprotein of other haemorrhagic fever viruses like Marburg virus (MARV), Lassa virus (LASV) and Crimean-Congo hemorrhagic fever virus (CCHFV) protected NHPs completely.38,87,99,100 For MARV vaccine candidate, a recombinant VSV-based virus expressing MARV (Musoke strain) GP (rVSVΔG-MARV-GP) showed cross-protection against MARV Angola and Ravn strain in NHPs.101 rVSVΔG-MARV-GP vaccinated cynomolgus monkeys were challenged ~14 months after vaccination, no clinical signs of disease were observed in vaccinated animals. In outbred guinea pigs, a single dose of VSV-based recombinant virus expressing LASV GP (rVSVΔG/LASV-GPC) induced rapid and long-term protection.102 Protection rates at 25 days, 6 months and 1 year post vaccination were 83%, 87% and 71%, respectively. For CCHFV, a single dose of rVSVΔG-vectored vaccine expressing CCHFV glycoprotein precursor (GPC) showed good tolerability and achieved 100% protection against the lethal challenge of CCHFV in mice.38
American Hantavirus Cardiopulmonary Syndrome (HCPS) is caused by Andes virus (ANDV) and Sinobrei virus (SNV). Prescott, J. et al. constructed a rVSVΔG-vectored vaccine rVSVΔG/ANDVGPC in which the GP of VSV was replaced by ANDV GPC.103 A single IM dose vaccination of rVSVΔG/ANDVGPC induced high titers of NAbs and achieved sterile immunity in hamsters. The post-challenge protective efficacy was 100%. In another study, the vaccine was effective against ANDV infection 6 months after inoculation in hamsters whilst no protective efficacy was observed 1 year after inoculation. Warner, BM et al. constructed two live vector vaccines, rVSVΔG/SNVGPC and rVSVΔG/ANDVGPC, which expressed GPC of SNV and ADNV, respectively.104 Both rVSVΔG/SNVGPC and rVSVΔG/ANDVGPC induced a cross-reactive immune response and played a protective role in Syrian hamsters.
Similarly, a single does vaccination of VSV-based vaccine expressing surface glycoprotein of other pathogenetic viruses, such as Nipah virus (NiV),105–107 Zika virus (ZIKV),108–111 severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1)112,113 and Middle East respiratory syndrome coronavirus (MERS-CoV),114 proved to be immunogenic and protective in preclinical animal models. The above studies emphasized that VSV vectored vaccines could completely protect against a large part of pathogens post a single IM dose of injection, and the immune response is durable, which represents the prominent feature of VSV vector vaccine.
Multivalent VSV-vectored vaccines protect animals from lethal challenges of multiple pathogens
Multivalent vaccines are of great significance in areas where multiple severe pathogens overlap, such as West Africa. According to previous research, rVSVΔG strategy exhibited weakened neurovirulence and experienced lower anti-vector immunity.115–120 Moreover, rVSV vectored vaccines expressing different foreign proteins could be inoculated simultaneously without interference of post-challenge protection of all targeted pathogens.121 These results enlightened the potential of VSV vectored vaccines for multivalent administration. In a preclinical study, a single dose vaccination of a recombinant bivalent vaccine VSVΔG/DUAL expressing ZEBOV and ANDV glycoproteins achieved sterile immunity to ZEBOV and ANDV in hamsters.122 Geisbert, T. W. et al. conducted a multivalent vaccine involving Sudan Ebola virus (SUDV), ZEBOV, Cote d’Ivoire Ebola virus (CIEBOV) and MARV.123 Cynomolgus monkeys were vaccinated with the multivalent vaccine consisting of equal doses of VSVΔG/SUDV GP, VSVΔG/ZEBOV GP and VSV ΔG/MARV GP. When challenged with the above four filoviruses, all vaccinated macaques survived. Likewise, the tetravalent VSV-vectored vaccine expressing antigens from LASV, EBOV, MARV and SUDV achieved 100% protection against the four hemorrhagic fever viruses including LASV, EBOV, MARV and SUDV after two doses.124 NAbs to the glycoproteins of the four filoviruses were detected in all vaccinated animals, while cell-mediated immune responses to glycoproteins were also detected in most vaccinated cynomolgus monkeys. rVSV-N4CT1 vector was also applied in trivalent vaccine development against EBOV, SUDV, and MARV.124 Although the trivalent vaccine exhibited decreased immunogenicity compared to the monovalent vaccine, the protective effect remained at 100%. The above results suggest that VSV-based monovalent vaccine are applicable. Representative VSV vector-based vaccines for human disease were summarized in Table 2.
Table 2.
Vaccine candidates based on vesicular stomatitis virus vector
| Pathogen | Design strategy | Stage | Results | Advantages | Overall concerns | Reference |
|---|---|---|---|---|---|---|
| Ebola virus | rVSVΔG-EBOV GP | Phase III | 100% protection | Postexposure, long-term, and cross protection; single dose regimen | Safety concerns, adverse effect | 93,525,526 |
| Marburg virus | rVSVΔG-MARV GP | NHPs | 100% protection | Sterile immunity; single-dose | Safety concerns | 87,99,101,527–529 |
| Lassa virus | rVSVΔG-LASV GPC | NHPs | 100% protection | Long-term, cross-protection; multivalent; single-dose | Safety concerns | 100,121,530 |
| CCHFV | rVSVΔG-CCHFV GPC | Mice | 100% protection | Stronger immunogenicity than RABV-based CCHFV vaccine candidates | Safety concerns | 38,125 |
| Andes virus | rVSVΔG-ANDV GP | Hamsters | 100% protection | Postexposure protection; cross-protection; sterile immunity | Safety concerns | 103,104 |
| SARS-CoV | rVSV-S/rVSVΔG-S | Mice | / | Long-term antibody response | Safety concerns | 112 |
| MERS-CoV | rVSVΔG-S | NHPs | / | Long-term antibody response | Safety concerns | 114 |
| SARS-CoV-2 | rVSVΔG-S | Phase I | / | Reduce viral load; mucosal delivery | Poor immunogenicity post IM vaccination | 332 |
| Nipah virus | rVSVΔG-NIV F/G/F + G | NHPs | 100% protection | Single round replication | \ | 107 |
| Hendra virus | rVSV-HEV G | Mice | / | More immunogenic than RABV vector-based vaccine candidate | Safety concerns | 126 |
| Zika virus | rVSV-prM-E-NS1 | Mice | 100% protection | MTase-defective, co-expression of prM and E, higher levels of Th2 and Th17 cytokine responses | Safety concerns | 111,531,532 |
CCHFV Crimean Congo hemorrhagic fever virus, SARS-CoV severe acute respiratory syndrome coronavirus, MERS-CoV Middle East respiratory syndrome coronavirus, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, NHPs nonhuman primates, IM intramuscular, RABV rabies virus, MTase methyltransferase, prM membrane precursor, E envelope
Rabies virus vector
Inactivated RABV-vectored vaccines combined with adjuvant confer full protection and trigger long-lasting immune responses
Although live RABV could be attenuated through genetically engineered strategies, a live recombinant RABV is unlikely to be approved due to safety concerns. Simultaneously, attenuated and replication-defective RABV vector vaccines were less immunogenic compared to VSV vectored vaccines expressing homologous antigen.125–127 Alternatively, inactivated RABV-vectored vaccines were safe and immunogenic, which represented a reasonable choice.128–130
For viral hemorrhagic fever, replication-competent and replication-defective vaccine candidates expressing ZEBOV GP were generated based on RABV BNSP333 vector.131 ZEBOV GP proteins could be efficiently incorporated into virions. Immunization with a live or inactivated vaccine candidate induced humoral immunity and conferred protection against both lethal RABV and EBOV challenges in mouse models. Further evaluation in NHPs showed that the replication-competent vaccine conferred 100% protection against EBOV infection, while the replication-defective or inactivated vaccine provided only 50% protection.132 Improvements were made to overcome the unsatisfactory protective efficacy of the inactivated vaccine by increasing the amount of GP incorporation into RABV virions through GP codon optimization.133 After that, two or three doses of BNSP333-coZGP (FILORAB1) adjuvanted with GLA-SE induced robust ZEBOV GP-specific IgG, NAbs and provided 100% protection after the lethal challenge of EBOV in NHPs.134
Meanwhile, SUDV and MARV vaccines have been developed based on the same strategy. FILORAB3 is a MARV vaccine expressing a codon-optimized GP of MARV Angola strain based on the RABV BSNP333 vector.128 Inactivated FILORAB3 adjuvant with Toll-like receptor 4 (TLR-4) agonist (GLA-SE) induced potent MARV GP-specific IgG antibodies. Interestingly, mice in the live FILORAB3 vaccination group succumbed to lethal challenge, while a single dose of inactivated FILORAB3 adjuvanted with GLA-SE conferred full protection. NK cell-dependent antibody-mediated cellular cytotoxicity (ADCC) played a critical role in immune protection in mice, which was consistent with the protective mechanism of RABV-vectored LASV vaccine.129 RABV vector has also been widely utilized to in vaccine development for genome-segmented pathogens, such as LASV and Rift Valley fever virus (RVFV). LASSARAB was a bivalent vaccine candidate that expressed codon-optimized LASV GPC based on BNSP333.129 Inactivated LASSARAB adjuvanted by GLA-SE induced long-lasting humoral responses to LASV and RABV in mice and guinea pigs. LASSARAB fully protected guinea pigs and mice against the LASV challenge mainly through non-NAbs-mediated ADCC and antibody-dependent cell-mediated phagocytosis (ADCP). Our group expressed codon-optimized RVFV eGn glycoprotein based on the RABV SRV9 strain, termed rSRV9-eGn.135,136 Inactivated rSRV9-eGn combined with poly (I:C) and ISA201VG adjuvant induced cellular immune response and RVFV-specific IgG antibodies. Moreover, rSRV9-eGn immunized mice produced memory T cell-dominant proliferating T cells.
Inactivated RABV-vectored vaccines also exhibit efficacy in emerging beta coronavirus.137,138 Full-length S protein incorporation into the RABV vector reduced the growth titers of recombinant virus.139 Thus BNSP333-S1 was constructed, which contains the MERS-CoV S1 domain that fused with the C-terminus of RABV G protein.139–144 Inactivated BNSP333-S1 induced high levels of NAbs in mice and conferred complete protection against the fatal challenge of MERS-CoV. In our previous study, a parallel comparison was conducted between recombinant RABV SRV9 vectored vaccine candidate expressing MERS-CoV S1 protein fragment and Gram-positive enhancer matrix (GEM) particles displaying MERS-CoV receptor binding domain (RBD) protein.145 The RABV vector-based vaccine induced remarkably earlier antibody response and higher levels of cellular immunity, while the GEM particle vector-based vaccine induced a higher antibody response, even at a low dose of 1 µg. This study described a platform-dependent manner of MERS vaccines. CORAVAX is an inactivated RABV SADB19 vectored COVID-19 vaccine candidate expressing S1 of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S).146–148 A single dose of CORAVAX vaccine induced high levels of SARS-CoV-2 and RABV NAbs, yet two doses were required for complete viral clearance in the nasal turbinate. CORAVAX was highly effective and conferred protection against hamster model post SARS-CoV-2 challenge. TLR4 agonist (AddaVax) was determined to have the greatest potential according to quality antibody titers. Pre-existing RABV immunity showed no significant impact on the immune response. Antigen-specific serum antibody titers and long-lived antibody-secreting cells in the spleen and bone marrow lasted over 1-year post CORAVAX immunization.149 Human clinical trials of CORAVAX are ongoing. Our group developed inactivated recombinant viral vector vaccines based on the RABV SRV9 strain, which chimerically expressed RBD or S1 of SARS-CoV-2 in the additional transcriptional unit of RABV genome.150 Combined with poly(I:C) and ISA 201VG adjuvant, three dose of inactivated recombinant viruses (SRV-nCoV-RBD or SRV-nCoV-S1) induced durable NAbs against SARS-CoV-2 and RABV. Notably, inactivated SRV-nCoV-RBD induced earlier and well-maintained antibody production than SRV-nCoV-S1. In further evaluations, inactivated SRV-nCoV-RBD induced NAbs against both SARS-CoV-2 and RABV in cats and dogs, with a relatively broad-spectrum cross-neutralization capability against SARS-CoV-2 variants of concern (VOCs).
For encephalitis viruses, a recombinant NIV vaccine expressing NiV G was constructed based on BNSP333, termed NIPARAB. After intranasal (IN) inoculation with live NIPARAB, mice showed no clinical signs of disease.130 Although mice intramuscularly inoculated with a single dose of live NIPARAB or two doses of inactivated NIPARAB produced NAbs and NIV-G-specific binding antibodies, a higher antibody level was only observed in the inactivated vaccine group. Of note, anti-NIV G-specific immune serum had cross-reactivity against Hendra virus (HEV), another paramyxovirus that causes fatal encephalitis. Parallel comparisons between VSV and RABV-based HEV vaccine were conducted126 Codon optimization increased the incorporation of HEV G into the RABV BNSP333 vector by 2–3 times, while it had no influence on the VSV-vectored vaccine candidate compared to those expressing the original antigen sequence. Surprisingly, both vaccine candidates were safe and induced high levels of HEV G-specific antibodies in mice. Three doses of inactivated vaccines induced higher levels of HEV G-specific IgG and NAbs than that of a single dose of live vaccine. Under the same conditions, the VSV-vectored live vaccine induced higher HEV G-specific antibodies and NAbs than the RABV vector live vaccine, which might be due to the rapid replication ability of VSV. Overall, considering the biosafety issue and the lower immunogenetics of RABV compared to VSV-based vaccines, inactivated form seems to be a more attractive direction. Representative RABV vector-based vaccines for human disease were summarized in Table 3.
Table 3.
Vaccine candidates based on rabies virus vector
| Pathogens | Design strategy | Stage | Results | Advantages | Overall concerns | Reference |
|---|---|---|---|---|---|---|
| Ebola virus | BNSP333-GP | NHPs | 100% protection | \ | Poor NAbs; safety concern | 131,132,458,533 |
| INACBNSP333-GP | NHPs | 50% protection | Safe | Poor NAbs | 133 | |
| INACBNSP333 co (EBOV + SUDV + MARV) GP | NHPs | 100% protection | Safe; immunogenic; high titer of NAbs | \ | 134 | |
| rERAG333E-(EBOV + SUDV) GP | Dogs | NAbs and specific Abs | Long-term protection (1 year); oral delivery | Safety concern | 335,336 | |
| Marburg virus | INACBNSP333-coGPC | Mice | 100% protection | Safe | Poor NAbs | 128 |
| Lassa virus | BNSP333-coGPC | Guinea pigs | 40% protection | \ | Poor binding IgGs | 129 |
| BNSPΔG-coGPC | Mice | \ | \ | Poor binding IgGs | ||
| INACBNSP333-coGPC | Guinea pigs | 80% protection | Safe | No NAbs | ||
| RVFV | rSRV9-eGn | Mice | \ | Safe | Poor NAbs | 135,136 |
| MERS-CoV | INACBNSP333-S1 | Mice | 100% protection | High titer of NAbs; safe | \ | 139 |
| RVΔP-S1 | Mice | NAbs | Safe | \ | 534 | |
| INACrSRV9-S1 | Mice | \ | Earlier humoral and cellular immunity | \ | 145 | |
| SARS-CoV | pSPBN-333-S | Mice | Binding Abs and NAbs | \ | \ | 137 |
| SARS-CoV-2 | BNSP333-S1 | Golden hamsters | NAbs and reduced virus load | Single dose; safe; long-lasting immune response | \ | 146,147,149 |
| rSRV9-RBD/S1 | Mice, cats and dogs | NAbs against SARS-CoV-2 and RABV | Long-lasting antibody response (4 months); broad-spectrum immune response | \ | 150 | |
| Nipah virus | INACBNSP333-G | Mice | G-specific Abs and NAbs | Cross-protection | \ | 130 |
| BNSP333-G | Mice | G-specific Abs and NAbs | \ | \ | ||
| rERAG333E-G/F | Mice and Pigs | G/F-specific Abs and NAbs | Oral delivery | \ | 338 | |
| Hendra virus | BNSP333-coG | Mice | G-specific Abs and NAbs | \ | Poor G-specific Abs | 126 |
| INACBNSP333-coG | Mice | G-specific Abs and NAbs | More immunogenic than RABV vector-based live vaccines | \ |
RVFV Rift Valley fever virus, MERS-CoV Middle East respiratory syndrome coronavirus, SARS-CoV severe acute respiratory syndrome coronavirus, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, NHPs nonhuman primates, Abs antibodies, NAbs neutralizing antibodies
Parainfluenza virus vector
A single IN dose vaccination of PIV vectored vaccines provide complete protection against respiratory diseases
Parainfluenza virus is a potential viral vector for its safety, genomic stability and abilities to be cultured in multiple cell lines.44 Multiple serotypes of PIV are involved in viral vector, including PIV1, 2, 3 and 5. In addition, B/HPIV3 is a chimeric Bovine/human PIV consisting of bovine PIV3 (BPIV3) strain Kansas in which BPIV3 HN and F glycoproteins have been replaced by those of human PIV3 strain JS.151,152 The BPIV3 backbone provides the host range restriction of replication in humans, which was well tolerated and immunogenic in young children.152,153 Till now, no evidence of enhanced pathogenicity has been confirmed in PIV vectored vaccines.154,155 PIVs are paediatric pathogens targeting respiratory epithelium, which made them attractive for developing vaccines that induce mucosal immune responses.156–158
A replication-defective COVID-19 vaccine has been developed based on human parainfluenza virus type 2 (hPIV2) vector BC-PIV, which expressed the full-length prefusion-stabilized S protein of SARS-CoV-2, termed BC-PIV/S-2PM.159,160 Massive S proteins were incorporated on the viral surface. A single IN dose vaccination with BC-PIV/S-2PM induced high levels of S-specific IgG and mucosal IgA antibodies in mice and protected hamsters against SARS-CoV-2 infection. Booster vaccinations were needed to confer complete protection on hamsters. Several replication-competent PIV vectored COVID-19 vaccines were also developed. CVXGA1 is a recombinant PIV5-vectored vaccine expressing S protein from SARS-CoV-2 WA1.161 Native configuration of the S protein was generated to maximize protective immune responses.162,163 A single IN dose of CVXGA1 induced viral-specific NAbs and provided 100% protection in K18-hACE2 mice and blocked contact transmission to cohoused naive ferrets. When CVXGA1 was administered as a booster following two doses of a COVID-19 mRNA vaccine, PIV5-vectored vaccines generate higher levels of cross-reactive NAbs compared to three doses of COVID-19 mRNA vaccine.164 These results indicate that CVXGA1 could serve as a booster vaccine against emerging variants. CVXGA1 is currently under Phase I clinical trial in the United States (NCT04954287). B/HPIV3 based COVID-19 was also constructed by expressing the native or prefusion-stabilized S protein (S-2P).39 Prefusion stabilization increased the expression of S proteins by B/HPIV3 in vitro. In hamsters, a single IN dose of B/HPIV3/S-2P induced 12-fold higher NAbs titers and significant higher SARS-CoV-2-specific IgA and IgG compared to B/HPIV3/S. Post SARS-CoV-2 challenge, B/HPIV3/S-2P provided better protection than B/HPIV3/S. Further, optimized version of B/HPIV3/S-2P, which involves another 4 proline mutations to consolidate the prefusion-stabilized S protein (B/HPIV3/S-6P) was evaluated in rhesus macaques.165 A single IN/intratracheal(IT) dose of B/HPIV3/S-6P induced strong S-specific airway mucosal IgA, IgG responses as well as high levels of peripheral S-specific antibodies, which efficiently neutralized SARS-CoV-2 VOCs, but the ability to neutralize Omicron sub-lineages was weakened. Furthermore, B/HPIV3/S-6P induced robust systemic and pulmonary S-specific CD4+ and CD8+ T cell responses, including tissue-resident memory cells in the lungs. B/HPIV3/S-6P vaccination effectively inhibited and eliminated viral proliferation in the upper and lower respiratory tract of immunized macaques. Natural attenuated human parainfluenza virus type 3 (HPIV3) vector-based COVID-19 vaccine was also proved to be effective166,167 In a same manner, PIV5 or B/HPIV3 vectored SARS-CoV-1 and MERS-CoV vaccines were immunogenic by a single IN dose of administration in preclinical.159,168–170
Human respiratory syncytial virus (RSV) is the leading viral agent of severe acute respiratory infections in infants and young children worldwide.171 Thus far, there is no licensed RSV vaccine. PIV-based RSV vaccines were constructed by expressing RSV-F protein from an additional transcription unit.40,172–178 In recombinant B/HPIV3, F protein of RSV was engineered for prefusion conformation, of which TM and CT domains were replaced of HPIV3 F to increase incorporation in vector virion.179 Booster with rB/HPIV3-RSV-pre-F resulted in significantly higher RSV NAbs than booster with live attenuated RSV vaccine in both hamsters and African green monkeys. PIV-based RSV vaccine provided a greater antigenic load of RSV F and increased immunogenicity compared to attenuated RSV. However, additional attenuation might make the construct over-attenuated in humans such that immunogenicity might be suboptimal.175,179 For these reasons, rHPIV3 JS was developed as a new generation vector to be available when rB/HPIV3-RSV-F was over-attenuated. Encouragingly, bivalent HPIV3/HRSV vaccine candidate was well tolerated in children >2 months of age, and optimized versions are in further clinical development as pediatric vaccines.153,159,172,175 Two RSV vaccines were constructed based on PIV5 expressing glycoproteins F (PIV5/F) and G (PIV5/G), respectively.180–182 PIV5/F was more immunogenic and provided better protection than PIV5/G in animal models. PIV5/F enhanced NAb responses in RSV-post exposed African green monkeys. These studies indicate that PIV5/F is a promising single-dose IN vaccine for RSV‐naive and RSV‐exposed individuals. In addition, PIV5‐based RSV vaccines could be administered subcutaneously, which provides a favorable route of vaccination for infants who may suffer from nasal congestion due to IN inoculation.
Based on the PIV platform, several IFV vaccine candidates were constructed by incorporating HA or NP of IAV H5N1 into recombinant PIV virions.170,183–185 A single IN dose inoculation of recombinant virus bearing HA of IAV induced sterile immunity and protected animals from homologus challenge of IFV. Compared with HA, NP of IAV seemed to be more conserved, but it was less immunogenic. This issue could be addressed by selection of appropriate locations for foreign gene delivery within the PIV genome. After that, a single IN inoculation of PIV vectored vaccine bearing NA of IFV provided broad protection against IFV. These results suggested that NP could be further investigated as a broad-spectrum antigen for IFV.
PIV vectored EBOV vaccines in development
Based on the HPIV3 vector, two EBOV vaccine candidates were constructed by inserting the GP gene alone or together with the NP protein gene of EBOV into the genome of HPIV3. After a single IN inoculation of the above vaccine candidates, guinea pigs were 100% protected from EBOV challenge in both vaccine groups.186 In rhesus monkeys, a single dose immunization with any construct expressing GP was moderately immunogenic against EBOV and protected 88% of animals against severe hemorrhagic fever and death caused by EBOV. Two doses vaccination were highly immunogenic, and all of the animals survived the challenge and were free of signs of disease and detectable challenge virus. The immune responses of PIV-based EBOV vaccines were equivalent to the AdV vector vaccine, but lower than that of the VSV vectored vaccine. Virus-specific binding antibody titer was directly related to protective efficacy. The incorporation of NP protein contribute little to the protective efficacy.187 Preexisting anti-vector immunity could affect replication of HPIV3, but had limited effect on the antigen expression and immunogenicity. The antibody titer against GP protein was only slightly lower in the group with pre-existing HPIV3 antibody than their counterparts. After the second immunization, antibody titers reached the equivalent level between two groups.188,189
Bukreyev et al. tried to remove HN and F protein from HPIV3 and replace its function with GP protein from EBOV. They successfully packaged the HPIV3 vectored EBOV vaccine without HN and F protein. The vaccine retained immunogenicity and completely protected guinea pigs against the lethal challenge of EBOV. Most importantly, the vaccine escaped pre-existing HPIV3 immunity.36 Deletion of HN and F protein resulted in a higher expression levels of Ebola GP protein. Meanwhile, the attenuation of the viral vector was also accomplished.
Equally, an attenuated recombinant human parainfluenza virus type 1 (rHPIV1) expressing the membrane-anchored form of EBOV GP was reported as an IN-delivered EBOV vaccine.190 GP was codon-optimized and expressed either as a full-length protein or as an engineered chimeric form in which its TM and CT domains (TMCT) were replaced by those of HPIV1 F protein to enhance packaging into the vector particle and immunogenicity. The GP gene was inserted either preceding the N gene (pre-N) or between the N and P genes (N-P) of rHPIV1 bearing a stabilized attenuating mutation in the P/C gene (CΔ170). These constructs grew to high titers and stably expressed EBOC GP. In addition, recombinant viruses were attenuated, which replicated at low titers over several days, in the respiratory tract of African green monkeys. Two doses of candidates expressing GP from the pre-N position elicited higher NAbs than N-P viruses, and unmodified GP induced much higher levels of NAbs than its TMCT counterpart. The unmodified EBOV GP was packaged into the HPIV1 particle, and the TMCT modification did not increase packaging or immunogenicity, but rather reduced the stability of GP expression during in vivo replication. This study indicated that TMCT replacement did not always enhance ectopic protein incorporation and the immunogenicity of the vaccine, which was determined by attribute of specific pathogen. Representative PIV vector-based vaccines for human disease were summarized in Table 4.
Table 4.
Vaccines based on parainfluenza virus vector
| Pathogens | Design strategy | Stage | Results | Advantages | Overall concerns | Reference |
|---|---|---|---|---|---|---|
| SARS-CoV | B/HPIV3-S | Hamsters and NHPs | Protected from disease and detectable viral replication | Single dose | \ | 159,168 |
| SARS-CoV-2 | hPIV2-prS | Mice and hamsters | Protected from disease and detectable viral replication | Single dose; massive spike proteins incorporation; mucosal immunity | Two doses needed to complete protection in nasal turbinates | 160 |
| PIV5-S(CVXGA1) | Mice and ferrets | 100% protection or protected from the contact transmission | Single dose; broad spectrum; well-maintained NAbs; mucosal immunity; tissue-resident memory cells | \ | 161,164 | |
| B/HPIV3-prS | Hamsters and NHPs | Protected from disease and detectable viral replication | Single dose; broad spectrum neutralizing; mucosal immunity; spike proteins incorporation | \ | 39,165 | |
| HPIV3-S/S1/RBD | Hamsters | Protected from disease and detectable viral replication | Single dose; HPIV3-S was selected as the best construct in terms of immune response; safe | \ | 166,167 | |
| MERS-CoV | PIV5-S | Mice | 100% protection | Single dose | \ | 169,170 |
| RSV | B/HPIV3-F | Phase I | Immunogenicity and well-tolerated | Single dose; safe; applicable to infants and children; bivalent | \ | 153,159,172,175,179,535 |
| PIV5-F/G | Mice, cotton rats and NHPs | Protected from disease and detectable viral replication | Single dose; PIV5-F was selected; applicable for RSV-exposed persons | Pre‐fusion RSV‐F do not enhance immune response | 180–182 | |
| IFV | PIV5-HA/NP | Mice | 67–100% protection | Single dose; broad spectrum; optimized insertion site was selected; | Incomplete protection of NA as immunogen | 170,183–185 |
| Ebola virus | HPIV3-GP/GP + NP | Guinea pigs and NHPs | 100% protection | Single dose; limited effect about pre-existing immunity | Immune response lower than VSV vectored vaccine | .137,186–189 |
| hPIV2-GP | Mice | NAbs | Low pathogenicity and recurrent infections of parental hPIV2 | \ | 27,536 | |
| Rabies virus | PIV5- G | Mice | 50–100% protection | Single dose; protective immune responses via IN, IM, and OR immunization | \ | 339 |
SARS-CoV severe acute respiratory syndrome coronavirus, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, MERS-CoV Middle East respiratory syndrome coronavirus, RSV respiratory syncytial virus, IFV influenza virus, NHPs nonhuman primates, NAbs neutralizing antibodies, IM intramuscular, IN intranasal, OR oral, VSV vesicular stomatitis virus
Measles virus vector
Live attenuated measles virus (MeV) vaccine was one of the most effective and safe human vaccines in clinical.191 Accordingly, the manufacturing industry of MeV vaccines is mature enough. Given the significant success of the MeV vaccine, this virus was considered a backbone for viral vectored vaccines against other diseases. Among them, MeV strain Schwarz and Moraten were frequently applied backbones. Notable progress has been made in MeV-based vaccines.
MeV-based vaccines expressing distinct forms of antigens provide protection against respiratory diseases
Homologous prime-boost immunization with replication-competent rMeVs expressing the S glycoprotein of MERS-CoV, either in its full-length, truncated or soluble variant, induced robust levels of both rMeV- and MERS-CoV NAbs and T cells in MeV susceptible mice.192 Post challenge with MERS-CoV, viral loads in the lungs of vaccinated mice were significantly reduced, coinciding with reduced pathological alterations in the lung, suggesting that rMeV-MERS vaccines confer full protection against MERS-CoV infection. The expression of the soluble version of S by MeV did not enhance NAb titers and slightly impaired replication in contrast to MeV expressing full-length MERS-S. These results indicated that the soluble structure of the S protein hampered the assembly of the recombinant virus. In a same manner, rMeV expressing codon-optimized S glycoprotein (S) SARS-CoV is immunogenic in mice.193
Several attempts have been made to develop MeV-based COVID-19 vaccines. These preclinical candidates were constructed by harboring membrane-anchored wild-type S protein, the pre-fusion stabilized S protein (S-2P) or secreted form of S-2P with a self-trimerizing “foldon” domain. Both of them were claimed to be effective in animal models.194–196 Besides, the new version was also designed to encode prefusion-stabilized, trimerized SARS-CoV-2 S glycoproteins displayed on a dodecahedral miniferritin scaffold. Surface glycoproteins of MeV were modified to bypass anti-measles antibodies. The optimized version of the MeV-based COVID-19 vaccine induced a high titer of NAbs in mice. These antigen-engineering strategies may also be applicable to measles-based vaccines for other emerging beta coronaviruses.197 Unfortunately, immunogenicity was insufficient after a single IM dose of MeV-based COVID-19 vaccine expressing a pre-fusion stabilized SARS-CoV-2 S protein (V591) in Phase I/II clinical trials, especially in measles-immunized individuals.198,199 Currently the relationship between low immunogenicity and anti-vector immunity is not clear. Most importantly, IP inoculations were conducted in animal models, while IM inoculations were applied in clinical trials, which may help explain the conflicting results between preclinical and clinical trials.
Apart from the above strategy that involved another transcription unit to co-express the foreign antigen, a chimeric version of MeV was also constructed, in which the CT and TM domains of MeV F and H was maintained, while ectodomains of MeV F and H were substituted by RSV F and G, correspondingly.200 The chimeric MeV/RSV induced NAbs against RSV in cotton rats and significantly reduced viral loads after challenge. The ectodomain replacement strategy may be similarly practicable for other paramyxoviruses, done under critical monitor since the change of entry receptor tropism.
MeV-based vaccines for vector-borne diseases
West Nile virus (WNV) is an arthropod-borne flavivirus that causes numerous cases of human encephalitis. MeV-based vaccine candidate (MeVSchw-sE) was constructed by expressing envelope glycoprotein from WNV. An IP dose inoculation with MeVSchw-sE induced both high levels of specific anti-WNV NAbs and protection from lethal challenge of WNV in mice and squirrel monkeys.201,202
Chikungunya virus (CHIKV) is a mosquito-borne alphavirus that causes severe polyarthralgia. rMeV expressing CHIKV capsid and envelope structural proteins resulted in the formulation of virus-like particles (MeV-CHIKV). MeV-CHIKV elicited broad spectrum and high titers of CHIKV antibodies as well as cellular immune responses. All mice survived the lethal challenge of CHIKV post a single IP dose of immunization.203 Passive transfer of immune sera conferred protection to naïve mice, highlighting the essential role of humoral immune response in protecting CHIKV. The final preclinical evaluation of MeV-CHIKV was performed in cynomolgus macaques. Homologous prime-boost vaccination with MeV-CHIKV protected macaques from abnormal clinical signs, viremia, blood cell indicators, cytokine changes upon challenge with CHIKV.204
This Schwarz strain-based rMeV encoding CHIKV VLPs has undergone Phase I/II clinical trials. MeV-CHIKV was well tolerated and immunogenic despite pre-existing anti-MeV immunity, with immunity persisted up to 6 months.205,206 Two doses are required for 100% seroconversion rates. Moreover, the vaccine boost at 6 months appeared to increase NAb titres to a greater extent. MeV-based Lassa fever vaccines were constructed by expressing GPC, GPC + NP or GPC + Z proteins of LASV, respectively. In cynomolgus monkeys, MeV-GPC + NP was determined as the optimal schedule after a single subcutaneous (SC) dose of vaccination in terms of immune response and post-challenge protective efficacy.207 Further evaluation confirmed that a single SC dose of MeV-GPC + NP protected cynomolgus monkeys from both homologous (Josiah, lineage IV) and heterologous (lineage II and lineage VII) strains of LASV. One year post a single dose of MeV-GPC + NP vaccination, 100% of monkeys were protected from homologous lethal challenge. These studies suggested that MeV-GPC + NP confer long-term and broad-spectrum protection against LASV.208 Currently, the Phase I clinical trial of MeV-GPC + NP is ongoing (NCT04055454).
Given the ideal results of a VSV-based vaccine co-expressing prM and E protein of ZIKV, a recombinant MeV encoding ZIKV prM and soluble E proteins (MV-Zika-sE) was constructed. Mice were inoculated with two doses of MV-Zika-sE via IP injection. MV-Zika-sE vaccinated mice were protected from weight loss and plasma viremia.209 There has also been attempts to screen a panel of MeV-based vaccine constructs expressing ZIKV-E, NS1, or both. Although MeV-E2 provided a 100% survival rate in mice, complete viral clearance was not achieved. NS1 was required to provide full protection. Representative MeV vector-based vaccines for human disease were summarized in Table 5.
Table 5.
Vaccines based on measles virus vector
| Pathogens | Design strategy | Stage | Results | Advantages | Overall concerns | Reference |
|---|---|---|---|---|---|---|
| SARS-CoV | rMeV-coS | Mice | Immunogenic | \ | \ | 193 |
| SARS-CoV-2 | MeV-S/S-2P/secreted S-2P/self-trimerizing S displayed on miniferritin | Mice and hamsters | Immunogenic and protected animals from disease and detectable viral replication | Safe, less influenced by anti-vector immunity | Lack of convenient animal model; contradictory results in preclinical and clinical trials | 194–197 |
| Phase I/II | Well-tolerated but less well immunogenic | Safe | Reconsidering of delivery route or design strategy | 198,199 | ||
| MERS-CoV | MeV-S/S(truncated)/S(soluble) | Mice | Immunogenic and protected animals from disease and detectable viral replication | Vaccinated animals were fully protected | Soluble version of S impaired replication of rMeV | 192 |
| RSV | MeV/RSV | Cotton rats | Immunogenicity and reduce virus load in respiratory tract | Chimeric version of MeV whose ectodomains of F and H were substituted by the RSV F and G, while CT and TM domain were maintained | Changing of cell tropism should be monitored | 200 |
| CHIKV | MeV-CHIKV capsid+envelope | Mice and cynomolgus macaques | Immunogenic and protected animals from disease | Formulation of virus-like particles; broad-spectrum NAbs; highlight the role of humoral immune response in protection | / | 203,204 |
| Phase I/II | Well-tolerated and immunogenic | Less influenced by anti-measles antibodies; immune response persisted up to 6 months | / | 205,206 | ||
| WNV | MeV-envelope | Mice and squirrel monkeys | Immunogenic and protected animals from lethal challenges | Single dose regime | / | 201,202 |
| LASV | MeV-GPC/GPC + NP/GPC + Z | Cynomolgus monkeys | Immunogenic and protected animals from disease | MeV-GPC + NP was determined as the optimal schedule; broad spectrum and long-term protection for 1 year | MeV-based vaccine expressing VLPs doesn’t always work | 207,208 |
| ZIKV | MeV-prM+E | Mice | Immunogenic and protected animals from weight loss and viremia | / | NS1 is needed for fully protection | 209,537 |
SARS-CoV severe acute respiratory syndrome coronavirus, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, MERS-CoV Middle East respiratory syndrome coronavirus, RSV respiratory syncytial virus, CHIKV Chikungunya virus, WNV West Nile virus, LASV lassa fever virus, ZIKV zika virus, S-2P pre-fusion stabilized spike protein with two proline mutations. nonhuman primates, MeV measles virus, S spike, E envelope protein, GPC glycoprotein precursor, NP Nucleoprotein, Z zinc finger protein, F fusion protein, H hemagglutinin glycoprotein, CT cytosolic tail, TM Transmembrane, NAbs neutralizing antibodies, NS1 non-structure protein 1
Newcastle disease virus vector
Newcastle disease virus (NDV) is another highly contagious paramyxovirus that could cause varying disease severity in avians but behaves strict host restrictions.210 In mammals, NDV triggered interferon responses, which restricted the replication of NDV and simultaneously posed an adjuvant effect on adaptive immunity.211,212 Low‐virulence NDV strains, such as LaSota and B1, are widely used as live attenuated vaccines for lethal NDVs and engineered for veterinary and human vaccines.
NDV-based SARS-CoV vaccine was developed by expressing the S protein of SARS-CoV from an added transcriptional unit.213 After two IN doses vaccination, African green monkeys developed high titers of NAbs against SARS-CoV. Post a high-dose challenge of SARS-CoV, viral titer in lung tissue was significantly reduced compared to control animals.
NDV vectored COVID-19 vaccine has been constructed and evaluated in preclinical and clinical. Previous antigen-engineering strategy re-occurred in NDV-based COVID-19 vaccines, including stabilizing S protein by the introduction of 6 prolines and adding TM and CT domains of NDV fusion protein to enhance the expression of S protein on the surface of the viral particles.165,179 Representatively, rNDV‐S was constructed by expressing S protein of SARS-CoV-2 based on NDV vector. In mice, rNDV‐S induced both humoral and cellular immunity through IM immunization, while no NAbs were detected despite a higher S‐specific T‐cell response induced by IN injection.214,215 Similarly, rNDV‐S was less immunogenicity through solely IN inoculation compared to IM inoculation in pigs despite the combination of the two delivery routes inducing strong NAbs. Interestingly, live rNDV‐S via IN inoculation induced antibody response and protective efficacy comparable to IM inoculation in hamsters.216 These proof-of-concept studies illustrated the animal model-dependent manner of the rNDV‐S vaccine, emphasizing the need for clarification of animal models that accurately reflect the status in human beings post vaccination.217
Inactivated rNDV‐S was evaluated in Phase I clinical trials, which proved safe and immunogenic.218,219 Indeed, this vaccine candidate could be inexpensive and scalable in manufacturing. However, inactivated NDV-based vaccines seem to be less attractive than novel protein vaccines for COVID-19.220–222 Live rNDV‐S was also evaluated in prime-boost regimens via IM, IN, or IN followed by IM routes in Phase I clinical trial. Live rNDV‐S was safe and well tolerated. IM inoculation and IN followed by IM administration were proved to be immunogenic.223 Superficially, preclinical evaluation of rNDV‐S in pigs seems better reflect clinical outcomes in humans. However, complicated issues should be addressed as inequality exists in these IN delivery routes. For IN inoculation, humans and pigs were given by nasal sprayer device, while hamsters were given under anesthesia, which enable the deeply distributed of rNDV‐S and represented more likely those in aerosol inhalation vaccines.216 Therefore, clinical trial should be designed and handled carefully, in case that delicate divisions in the delivery route exist.
Bivalent rNDV vaccines have been developed by targeting both NDV and highly pathogenic avian influenza (HPAI) by expressing chimeric HA from IFV. These vaccine candidates could provide cross-protection between different IFV lineages.224,225 Similar in NDV-based COVID-19 vaccine, inactivated rNDV was more immunogenic through IM inoculation than that of IN inoculation.225,226 This could be explained by the mucosal tropism of live NDV, while inactivated NDV display more antigen proteins and benefit from adjuvant effect. Currently NDV-based IFV vaccine is used as veterinary vaccines in Mexico.
Given that NDV is a potent inducer of interferon production and dendritic cell maturation, a recombinant NDV expressing RSV fusion glycoprotein was administered to BALB/c mice. A single IN dose of vaccination protected animals from the RSV challenge.227 Further evaluation of cotton rats showed that vaccination also protected them from RSV challenge and induced long-lived NAbs up to 6 months.228
To compare with the PIV3-based EBOV vaccine, the same team developed an NDV-based EBOV vaccine expressing EBOV GP, termed NDV/GP. Following one IN plus IT dose inoculation with NDV/GP, EBOV-specific binding antibodies and NAbs were undetectable or low compared to those induced by HPIV3/GP in rhesus monkeys. Boosting vaccination led to a substantial increase in serum IgG ELISA titers, yet remained lower than those induced by a second dose of HPIV3/GP. In contrast, secretory IgA titers in the respiratory tract and NAbs were equal to those induced after the second dose of HPIV3/GP. These results suggested that NDV-based EBOV vaccine was equivalent to or slightly less immunogenic than PIV3-based EBOV vaccine, particularly in the single-dose regimen.229 To overcome the anti-vector immunity of Ad5, rNDV was generated by expressing the GP protein of the EBOV and was combined with AdV-5-MakGP as a heterologous prime-boost strategy.230 This strategy exhibited more-potent EBOV GP-specific antibodies and cellular immune responses than those received the same vaccine twice in mice. These results suggest that the AdV-5 prime-NDV boost regimen is more effective in stimulating EBOV-specific immunity than the homologous regimen. Representative NDV vector-based vaccines for human disease were summarized in Table 6.
Table 6.
Vaccines based on Newcastle disease virus vector
| Pathogens | Design strategy | Stage | Results | Advantages | Overall concerns | Reference |
|---|---|---|---|---|---|---|
| SARS-CoV | rNDV-S | Mice | Immunogenic and reduced virus load | \ | \ | 213 |
| SARS-CoV-2 | rNDV-S/S-6P/ | Mice, hamsters and pigs | Immunogenic and protected animals from disease and detectable viral replication | Pre-fusion stabilized; both live and inactivated forms are available; IN plus IM inoculation | Poor immunogenicity through IN inoculation | 165,179,214,215 |
| Phase I | Well-tolerated and immunogenic | Both live and inactivated rNDV are safe and immunogenic; IN prime-IM boost strategy | 218,219,223 | |||
| IFV | rNDV-HA | Avian | Immunogenic and protected animals from lethal challenge | Bivalent; cross-protection | \ | 224,225,229 |
| RSV | rNDV-F | Mice and cotton rats | Immunogenicity and protected animals from challenge | Single dose; long-lasting NAbs response (6 months) | \ | 227,228 |
| EBOV | rNDV-GP | Cynomolgus macaques | Immunogenic | Comparable or slightly lower immunogenic than HPIV3/GP | Poor immunogenicity through a single IN dose inoculation | 229 |
| rNDV+Ad5-GP | Immunogenic | Heterologous prime-boost strategy | \ | 230 | ||
| RVFV | rNDV-GnGc | Mice and lambs | Immunogenic and protected animals from challenge | \ | \ | 538 |
| NIV | rNDV-F/G | Mice and pigs | Immunogenic | Co-immunization with rNDV-F and rNDV-G; long-lasting immune response | \ | 539 |
SARS-CoV severe acute respiratory syndrome coronavirus, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, IFV Influenza virus, RSV respiratory syncytial virus, EBOV Ebola virus, RVFV rift valley virus, NIV Nipah virus, rNDV recombinant new castle disease virus, S spike, S-6P pre-fusion stabilized spike protein with 6 proline mutations, HA hemagglutinin glycoprotein, F fusion protein, GP glycoprotein, GnGc glycoprotein of rift valley virus, NAbs neutralizing antibodies, NS1 non-structure protein, IM intramuscular, IN intranasal, HPIV3/GP human parainfluenza virus type III vector-based EBOV vaccine expressing EBOV GP
Influenza virus vector
IN-delivered IFV-based COVID-19 vaccine is potent when standing alone or as booster vaccines
The existing influenza vaccines production infrastructure is highly optimized and capable of delivering more than a billion doses per year.231 To combat another respiratory disease, COVID-19, multiple vaccines based on IFV vectors have been developed. scPR8-RBD-M2 was designated as a single-round replication IFV-based COVID-19 vaccine. Chimeric gene was utilized to encode 2 A peptide-based bicistronic protein cassette of the SARS-CoV-2 RBD and IFA M2. The C-terminus of the RBD was linked to the cytoplasmic domain of IFV HA to anchor the RBD to the surface of producing cells and the virus envelope. Cellular, humoral and mucosal immune responses to RBD can be produced in mice with two doses of IN immunization. Vaccination-induced antibodies represented broad-spectrum neutralizing activity against SARS-CoV-2 variants.232 HA protein provided by MDCK-HA cells may lead to instability of the inserted gene maintenance. To address this issue, a vaccine cocktail that contained mixed antigens/epitopes of interest could be generated to circumvent such limitations. Chaparian et al. inserted SARS-CoV-2-RBD into IFV A/Puerto Rico/8/1934 (H1N1) HA, vaccination with this combination vaccine elicited NAbs and provided protection against the lethal challenge of both SARS-CoV-2 and IAV in mice.233,234
More recently, a live-attenuated SARS-CoV-2 vaccine was manufactured based on a cold-adapted IFV strain without NS1, in which the RBD gene of SARS-CoV-2 was inserted by gene reassortment, termed CA4-dNS1-nCoV-RBD (dNS1-RBD).62,235 In preclinical studies, dNS1-RBD induced rapid, long-term, broad-spectrum protection against SARS-CoV-2 challenge in hamsters by inducing strong innate and adaptive local immune responses in the respiratory tract, despite weaker responses in the circulation, which might be attributable to innate immune response in the nasal epithelium and local cross-variant specific T-cell immune response.235 Lung-resident memory RBD-specific CD4+ and CD8+ T cells could be induced by vaccination, and the T-cell immune response produced in lung tissue was about 26-time stronger than that in peripheral mononuclear cells (PBMCs) in mice immunized with a single dose. Moreover, such cellular immunity is relatively unimpaired for most SARS-CoV-2 VOCs, especially for the latest Omicron variant. In addition, this vaccine also provides cross-protection against IFV H1N1 and H5N1. In Phase I/II clinical trials, dNS1-RBD was administered by IN inoculation in healthy adults.14 dNS1-RBD was well tolerated in adults, less than 20% of vaccine-related adverse reactions were observed, no serious adverse event was noted. In the Phase I/II trial, specific T-cell immune responses, seroconversion for RBD-specific IgG and positive conversion for RBD-specific s-IgA were observed at 44%, 10% and 12%, respectively, in vaccine recipients 1 month after the second dose. Overall, T-cell, humoral and mucosal immune responses to SARS-CoV-2 were weak in vaccine recipients. This study provided evidence of cross-contamination caused by aerosols of the IN vaccine produced during administration, which could help pave the way for the clinical development of other IN vaccines in the future. Although the probability of vaccine strain transmission through close contact with a vaccinated person is believed to be very low.236 This issue should be properly addressed by the assessment of viral shedding and specific immune responses in vaccinators, probability of environmental infection. Phase III clinical trials of dNS1-RBD are ongoing (ChiCTR2100051391). Notably, broad-spectrum efficacy against Omicron has been achieved. The overall protective efficacy of dNS1-RBD against hospitalizing of COVID-19 was 100%. For people without immunization history, the absolute protective effect of dNS1-RBD at 3 months after immunization was 55%. For people with immunization history, the absolute protective efficacy of nasal spray COVID-19 vaccine within 6 months after booster immunization was 82% (unpublished data). On December 2, 2022, dNS1-RBD was approved for emergency use in China.
IFV-vectored vaccines for other pathogens
Cold-adapted, live-attenuated influenza vaccine (CAIV; FluMist, AstraZeneca, London UK) was licensed as a safe and effective vaccine by the US Food & Drug Administration in 2003 and is approved for use in people aged 2–49 years.237 In a human challenge trial of FluMist, a low antibody response was not directly associated with low protective efficacy.238 Among 103 adults aged 18–45 years who received a single dose, the seropositive rates of haemagglutination-inhibiting antibodies for IAV/H1N1, IAV/H3N2, and IBV/Harbin were 23%, 33%, and 3%, and the response rates of IgA antibodies in nasal wash were 14%, 32%, and 18%, respectively. Encouragingly, the virus challenge results indicated that the protective effects of FluMist for A/H1N1, A/H3N2, and B/Harbin were 80%, 78%, and 100%, respectively, which were higher than those of IM vaccine candidates (60%, 67%, and 100%) that inducing higher seroresponse rates (91%, 76%, and 76%). Likewise, the PIV5/G vaccine did not produce detectable levels of NAbs in cotton rats but still provided protection against RSV challenge.180 The above results suggested that immune responses other than peripheral antibody responses may provide benefits of protection against these respiratory diseases.
Our group constructed an H5N1 chimeric IAV/B vaccine based on a cold-adapted (ca) IBV B/Vienna/1/99 backbone.239 Modified HA of H5N1 was inserted while the packaging signals of HA of IBV were retained. The recombinant virus maintained a temperature-sensitive and cold-adapted phenotype. The H5N1 vaccine was attenuated in mice. Systemic humoral and cellular immunity and local mucosal IgA were induced. Two-dose IN vaccination of the chimeric H5N1 vaccine candidate conferred full protection against the lethal challenge of IFV H5N1 in mice. In 2021, a conserved extracellular domain of IFV ion channel protein M2 (M2e) (4 × M2e) was inserted into the N terminal of A/Switzer land/9715293/ 2013 (H3N2) HA. Intranasally inoculation of this vaccine induced antibodies and T cell immune response in mice, thus achieving protection against H1N1, H3N2, H5N1, H7N9 and H9N2 viruses.240 Representative IFV vector-based vaccines for human disease were summarized in Table 7.
Table 7.
Vaccines based on influenza virus vector
| Pathogens | Design strategy | Stage | Results | Advantages | Overall concerns | Reference |
|---|---|---|---|---|---|---|
| SARS-CoV-2 | HA-RBD-M2 | Mice | Protected from the disease and detectable viral replication | Broad spectrum neutralizing activity; local and systematic immunity | Instability of the inserted gene maintenance | 232 |
| HA-RBD | Mice | Protected from the disease and detectable viral replication | Protect against both SARS-CoV-2 and IAV | Instability of the inserted gene maintenance | 233 | |
| CA4-dNS1-nCoV-RBD (dNS1-RBD) | Hamsters | Protected from the disease and detectable viral replication | Rapid, long term, and broad-spectrum protection; innate and adaptive local immune responses | Weaker responses in circulation | 235 | |
| Phase I/II | Well tolerated | <20% vaccine-related adverse reactions | T-cell, humoral and mucosal immune responses against SARS-CoV-2 were weak in recipients; cross-contamination | 14 | ||
| Phase III | 100% protection against hospitalization | 55% and 82% protection for people without/with immunization history | \ | Unpublished | ||
| IFV | Live attenuated (FluMist) | Phase III | 78–100% protection | Low level of NAbs but provide effectively protection | \ | 237,238 |
| Chimeric IBV-HA(IAV) | Mice | 100% protection | Cold adaption; attenuated; systemic and local immune response | Poor binding IgGs | 239 | |
| RSV | HA-F243-294 | Mice | Protected from the disease and detectable viral replication | Single dose; no ADE effect | Poor NAbs | 540 |
| WNV | NA-DIII | Mice | Humoral and cellular immunity | \ | \ | 541 |
SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, IFV influenza virus, RSV respiratory syncytial virus, WNV West Nile virus, IBV influenza B virus, IAV influenza A virus, NAbs neutralizing antibodies, ADE antibody-dependent enhancement
Adenovirus vector
Homologus or heterologous primer-boost of AdV based vaccines provide protection against viral haemorrhagic fever
Adenovirus type 5 (Ad5) is the most frequently applied adenovirus vector, which is well established and easily accessible. In preclinical trials, cynomolgus macaques were boosted with the replication-defective Ad5-vectored vaccine candidate Ad5-EBOV encoding EBOV GP after initial immunization with the DNA vaccine. These animals generated vigorous cellular and humoral immunity and received full cross-protection.241,242 Passive transfer of polyclonal antibodies from vaccinated animals to naive macaques failed to confer protection against the lethal challenge of EBOV, while depletion of CD8+ cells in vivo abrogated protection for NHPs.243 These results indicated that CD8+ T cells play a major role in rAd5-EBOV induced immune protection against EBOV infection. In Phase I clinical trial, Ad5-EBOV was safe and immunogenic.244 However, humoral responses were impacted by pre-existing anti-vector immunity. Likewise, a single IM dose of Ad5-MakGP, a recombinant Ad5 expressing the GP of EBOV Makona strain, provided sterile immunity and 100% protection for NHPs.245 In Phase I clinical trial, Ad5-MakGP showed good safety and immunogenicity. Dose-dependent magnitude of immune response was observed. Both the EBOV-specific antibody response and T-cell response were blunted by the presence of anti-vector immunity, particularly in the low-dose group.246,247 One homologous booster immunization with Ad5-MakGP at month 6 after primary immunization stimulated a stronger humoral immune response. One year after booster immunization, a 100% positive rate of GP antibody remained to be detected.248 According to clinical outcomes in Phase II clinical trials of Ad5-MakGP in Sierra Leone, when the vaccination dose was increased to 8 × 1010 viral particles, adverse reactions to vaccination were acceptable and the incidence rate was even lower than in Phase I clinical trial.249 Whereas, the duration of EBOV-specific antibodies in African participants was shorter than in Chinese participants, also seen in clinical trials of rVSV-ZEBOV in Africa and Europe.95 In Phase I/II clinical trials, the rVSV-ZEBOV + Ad5-EBOV prime-boost regime induced a robust immune response.250 Heterologous prime-boosting strategy could quickly awaken immune memory and induce a stronger immune response, simultaneously alleviating the influence of anti-vector immunity. Russia approved the registration of this vaccine in December 2015.
Adenovirus type 26 (Ad26) is another promising vaccine vector with lower seroprevalence than Ad5. Ad26-based EBOV vaccine was also constructed. A single IM dose vaccination of Ad26-ZEBOV vaccine candidate expressing ZEBOV GP conferred partial protection in NHPs. Subsequently boosted immunization with Adenovirus type 35 (Ad35)-ZEBOV significantly increased humoral and cellular response and conferred complete protection.251
Regarding high-quality and magnitude of immune responses induced by recombinant Chimpanzee Adenoviruses (ChAdVs), they were equally applied as viral vectors.252–254 ChAdVs-based vaccine aroused comparable humoral and cellular immune responses to human AdV vectors.255–257 Chimpanzee adenoviruses type 3 (ChAd3)-vectored bivalent vaccine (cAd3-EBO) encoding GPs of EBOV and SUDV induced superior humoral and cellular responses and conferred uniform protection against EBOV challenge for macaques compared to chimpanzee adenoviruses type 63 (ChAd63) and (MVA) vectored vaccines.258 Boosted cAd3-EBO with MVA-vectored vaccine generated long-lasting protection against lethal challenge in NHPs.258 Acute protection was strongly associated with antibody responses, while long-term protection required the generation of both effector and memory CD8+ T-cell response and cytokines. In Phase I clinical trial, cAd3-EBO was safe and induced dose-dependent immune responses.257 ChAd3-based monovalent vaccine (ChAd3-EBO-Z) encoding the GP of ZEBOV was also constructed. In a Phase I clinical trial, antibodies induced by ChAd3-EBO-Z were slightly lower than those induced by rVSV-ZEBOV.259 When ChAd3-EBO-Z was boosted with MVA-EBO-Z, virus-specific antibodies and CD8+ T cells were increased by 12 and 5 times, respectively. Virus-specific antibody responses in participants primed with ChAd3-EBO-Z remained positive 6 months post immunization but were significantly lower than those who received MVA-EBO-Z booster.259 Other Phase I trials validated the safety and immunogenicity of ChAd3-EBO-Z.260,261 In addition, prime-boost strategy involving ChAd3-EBO-Z and MVA-BN-Filo (MVA-vectored vaccine candidate expressing ZEBOV GP, SUDV GP and MARV-Musoke GP) conferred long-lasting protection.260 Immune responses were largely maintained through 12 months.262,263
A DNA prime-Ad5 boost strategy was also conducted on MARV. Based on DNA or Ad5 platform, vaccine candidates DNA-MARV-GP and rAd5-MARV-GP were constructed by expressing EBOV GP. In NHPs, the protective efficacy of heterologous DNA-MARV-GP/rAd5-MARV-GP prime-boost strategy, single-dose rAd5-MARV-GP regimen, and DNA-MARV-GP homologous prime-boost strategy were compared.264 All three programs prevented the lethal challenge of EBOV in NHPs. A single-dose inoculation of rAd5-MARV-GP induced humoral and cellular responses comparable to those induced by two doses of DNA vaccine. Vaccine regimens containing rAd5-MARV-GP, either alone or as a booster, exhibited CD8+ T-cell dominant cellular responses. The dominance of the CD8+ T-cell subset was positively associated with a low frequency of clinical signs, suggesting that both the magnitude and functional phenotype of CD8+ T cells determined the vaccine efficacy against MARV infection.264
Maruyama et al. developed an Ad5-vectored vaccine candidate expressing LASV-GPC, termed Ad5-LASV. Two IM doses of Ad5-LASV provided complete protection for guinea pigs. All vaccinated animals produced anti-GP antibodies, while only 37.5% produced NAbs. No detectable viruses were observed in vaccinated guinea pigs post lethal LASV challenge.265 Zivcec et al. constructed an Ad5-based vaccine candidate Ad-CCHFV-N which the nucleocapsid protein of CCHFV.266 Ad-CCHFV-N induced anti-N humoral immune response. Single dose vaccination with Ad-CCHFV-N provided 30% protection for IFNAR−/− mice against the lethal CCHFV challenge, while the prime-boost regimen increased protection efficacy to 78%. This study demonstrated the feasibility of genetically conserved N protein as a protective antigen against CCHFV.
ChAdOx1 is a replication-deficient chimpanzee adenovirus vector that is phylogenetically classified as Human adenovirus E.267 Warimwe et al. constructed a replication-deficient chimpanzee adenovirus vectored RVF vaccine termed ChAdOx1-GnGc, which encodes Gn and Gc of RVFV.268 ChAdOx1-GnGc induced potent RVFV-specific NAbs and CD8+ T-cell response in mice.269 A single dose of each vaccine candidate protected mice from lethal RVFV challenge. Meanwhile, two commercially available adjuvants, Matrix-M™ and AddaVax™, were demonstrated to significantly enhance the RVFV-specific neutralizing response induced by ChAdOx1-GnGc. A single dose vaccination of ChAdOx1-GnGc elicited robust NAb comparable to the licensed livestock vaccine Smithburn and conferred full protection against challenge in the most susceptible natural target species sheep, goats and cattle.270
Remarkable progresses achieved in AdV-based COVID-19 vaccines
During the pandemic of COVID-19, vaccine candidate Ad5-nCoV was designed to deliver the S protein of SARS-CoV-2. In Phase I/II clinical trial, a single IM dose vaccination of Ad5-nCoV was tolerable and immunogenic in healthy adults, binding antibodies, NAbs and SARS-CoV-2 specific T-cell responses were induced. However, preexisting anti-vector immunity compromised seroconversion of SARS-CoV-2 NAbs and reduced post-vaccination T-cell responses.271,272 The results of the Phase III clinical trial suggested that 14 or 28 days after a single IM dose injection of the vaccine, the overall protective efficacy was 68.83% and 65.28%, respectively. The protective efficacy against severe illness 14 or 28 dpi was 95.47% and 90.07%, respectively.11 On February 5, 2021, the conditional listing application for Ad5-nCoV was approved.
Harvard Medical School constructed an Ad26-vectored COVID-19 vaccine termed Ad26.COV2.S, which contained the wild-type SARS-CoV-2 leader sequence, the full-length membrane-bound S with a mutation in the furin cleavage site and two proline stabilizing mutations.273 A single IM dose of Ad26.COV2.S induced robust NAbs and provided complete or near-complete protection in bronchoalveolar lavage and nasal swabs following the SARS-CoV-2 challenge in NHPs. Russia developed and tested the single-dose rAd26 vector-based COVID-19 vaccine (Sputnik Light) in a Phase I clinical trial, which showed a good safety profile and induced a strong humoral and cellular immune responses in both seronegative and seropositive participants.274 According to the efficacy and safety analysis of single-dose Ad26.COV2.S in Phase III clinical trial, Ad26.COV2.S provided 52.9% protection against moderate to severe critical COVID-19, the protection sustained for 6 months.12
The University of Oxford developed a ChAdOx1-vectored COVID-19 vaccine encoding the codon-optimized full-length S gene, termed ChAdOx1-S.275 In rhesus macaques, ChAdOx1-S induced certain levels of immune response, but the protective efficacy was not ideal.276 The prime-boost regimen significantly enhanced antibody and T-cell responses in pigs but not mice compared to the single-dose group. In Phase I/II and II/III clinical trials, ChAdOx1 nCoV-19 was tolerated, humoral and cellular immune responses were observed in most volunteers, comparable T-cell responses were induced in HIV-infected individuals.277–279 Antibody and protective efficacy lasted at least 3 months. The overall efficacy of two-dose vaccinations was 70.4%.13 However, the Phase III clinical trial was once called off due to a case report of transverse myelitis. What’s more, vaccination associated-thrombus is possible.280 These safety issues of ChAdOx1 require further evaluation.
Heterologous prime-boost strategy is an effective countermeasure to alleviate anti-vector immunity. Russia developed rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccines, termed Sputnik V.281 Compared to the single-dose strategy, the heterologous rAd26 and rAd5 vector-based COVID-19 vaccine induced significantly stronger humoral and cellular immune responses in participants. In Phase I/II studies, Sputnik V induced pronounced humoral responses in all participants, with a 100% seroconversion. In the Phase III trial involved almost 20,000 subjects, a 91.6% protective efficacy was reported.15 Representative AdV vector-based vaccines for human disease were summarized in Table 8.
Table 8.
Vaccine candidates based on adenovirus vectors
| Pathogens | Design strategy | Stage | Results | Advantages | Overall concerns | Reference |
|---|---|---|---|---|---|---|
| Ebola virus | Ad5-(Zaire + SUDV) GP | Phase I | Safe and immunogenic | Bivalent | Anti-vector immunity | 244 |
| Ad5-Zaire (Makona) GP | Phase II | Safe and immunogenic | Sterile immunity | Anti-vector immunity | 245–247,249 | |
| rVSV-GP + Ad5-GP | Phase II | Safe and immunogenic | Alleviated anti-vector immunity | \ | 250 | |
| Ad26-ZaireGP + MVA-BN-Filo | Phase I | Safe and immunogenic | Antibodies persisted to 1 year | \ | 285,286 | |
| ChAd3-(Zaire + SUDV) GP | Phase I | Safe and immunogenic | Bivalent | \ | 257 | |
| ChAd3-Zaire GP | Phase III/IIa | Safe and immunogenic | Antibodies persisted to 1 year | \ | 261,262 | |
| ChAd3-ZaireGP + MVA-BN-Filo | Phase I | Safe and immunogenic | Durable in immune response | \ | 259,260 | |
| Marburg virus | Ad5-GP | NHPs | 100% protection | More immunogenic than DNA vaccine | Anti-vector immunity | 264 |
| Lassa virus | Ad5-GPC | NHPs | 100% protection | \ | Anti-vector immunity | |
| Guinea pigs | 100% protection | \ | Poor NAbs | 265 | ||
| RVFV | ChAdO×1-Gn+Gc | Ruminants | 100% protection | \ | \ | 268,270 |
| CCHFV | Ad5-N | Mice | S-specific Abs and NAbs | \ | \ | 266 |
| SARS-CoV-2 | Ad5-S | Phase III | 57.5% protection | Tolerable, safe in elder people, | Anti-vector immunity | 11 |
| Ad26-S | Phase III | 52.9% protection | / | Adverse effect | 12 | |
| Ad5-S + Ad26-S | Phase III | 91.6% protection | Alleviate anti-vector immunity | \ | .15 | |
| ChAdOx1-S | Phase III | 70.4% protection | / | Adverse effect | 13 | |
| MERS-CoV | Ad5-S1/F/CD40 L | Mice | 100% protection | Optimized immunogenicity | \ | 542–544 |
| ChAdO×1-S + MVA-S | Mice | 100% protection | Long-term protection (40 week) | \ | 545 | |
| Hantavirus | Ad5-N/GN/GC | Syrian hamsters | 100% protection | Nearly sterile protection | Poor NAbs | 546 |
RVFV Rift Valley fever virus, CCHFV Crimean Congo hemorrhagic fever virus, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, MERS-CoV Middle East respiratory syndrome coronavirus, NHPs nonhuman primates, Abs antibodies, NAbs neutralizing antibodies
Poxvirus vector
Heterologous prime-boost strategy combining poxvirus-vectored vaccines with other vaccine platforms
There have been several attempts to improve the immunogenicity of poxvirus-vectored vaccines, including the involvement of stronger promoters, deletion of genes responsible for immune regulation, and replacement of MVA181R/182 R with the anti-apoptotic gene B13R. Unfortunately, these attempts failed to achieve a breakthrough.282–284 Protective efficacy poxvirus-vectored vaccines could be ameliorated through a prime-boost strategy with other viral-vectored vaccine candidates. In a randomized clinical trial, a prime-boost regimen was conducted involving AD26-ZEBOV and MVA-BN-Filo.285 No serious vaccine-related adverse events were observed during vaccination and 8-month follow-up. The seroconversion rate of Ad26-ZEBOV recipients was higher than that of the MVA-BN-Filo group after primary vaccination. All vaccine recipients had detectable virus specific-IgG after booster with alternative vaccine and 8-month follow-up. Primed with Ad26-ZEBOV and boosted with MVA-BN-Filo elicited more vigorous cellular and humoral immune responses, which sustained for up to 1 year.286 The Ad26-ZEBOV + MVA-BN-Filo regimen was approved by the EMA on July 1, 2020.
Based on ChAdOx1 and MVA, a cross-filovirus immunogen was constructed based on conserved regions of the filovirus N, M and L protein. Protection of mice against Ebola and MARV was elicited by this vaccine candidate.287 In the absence of GP-specific antibodies and NAbs, ChAdOx1-MVA vectored prime-boost strategy elicited T cell immunity and conferred full protection, further demonstrating the prominent efficacy of heterologous prime-boost strategy.
For pathogens of complicated or multiple immunogen-dominant proteins, prime-boost strategy expressing different antigens is a promising strategy. Guillaume et al. developed a VACV-vectored vaccine expressing NiV glycoprotein G and fusion protein F, respectively, termed VV-NiV.G or VV-NiV.F.288 Two doses of VV-NiV.G or VV-NiV.F or a combined two-dose regimen induced binding antibodies and relatively low titers of NAbs in hamsters and provided 100% post-challenge protection. Moreover, the passive transfer experiment of immune serum proved that antibodies play an important role in the process of immune protection. Likewise, ALVAC-G and ALVAC-F were constructed using the canarypox virus (ALVAC)-vector.289 Although all protocols achieved full protection, pigs vaccinated with ALVAC-F showed low NAbs and a small amount of virus shedding, which was consistent with VSV-vectored NIV vaccine.107 Pigs vaccinated with both antigens (ALVAC-F/G) developed moderate neutralizing titers against HeV. The combined use of ALVAC-G and ALVAC-F induced the highest levels of NAbs and antigen-specific antibodies, which were likely to achieve sterile immunity. Virus shedding in pigs was also effectively blocked, indicating great significance in cutting off the NiV transmission chain from pigs to humans.
In Phase III clinical trials, ALVAC vectored HIV vaccine ALVAC-HIV was used alongside a recombinant glycoprotein 120 subunit vaccine. However, the protective efficacy was controversial.290–293
Expressing VLPs based on poxvirus vector
VLPs could mimic natural pathogens and render native presentation of antigens.294–296 Co-expression of VP40 and GP protein in EBOV resulted in the formation of EBOV-VLP and provided effective protection against challenge.297–300 Schweneker et al. developed MVA-BN-EBOV-VLP, in which VP40 and GP of EBOV Mayinga strain and NP of Tai forest virus Ebola were co-expressed based on MVA-BN vector.301 Human cells infected with MVA-BN-EBOV-VLP produced a large number of EBOV VLPs while poxvirus membrane protein B5 was excluded. MVA-BN-EBOV-VLP vaccinated mice produced EBOV GP-specific cellular and humoral immune responses quantitatively comparable to those of MVA-BN-EBOV-GP. Co-expression of GP and VP40 similarly led to the production of VLPs.302 However, no obvious advantage was observed in MVA-BN-EBOV-VLP vaccine candidates compared to MVA-BN-EBOV-GP in terms of immune response. Moreover, although full protection was achieved with 1 or 2 doses of MVA-VLPs vaccination, low transient viremia was detected in some vaccinated guinea pigs and NHPs.303,304 To protect against multiple pathogenic EBOV species, Karnail et al. developed a bivalent spherical Ebola VLP vaccine that incorporates GPs from ZEBOV and SUDV. Vaccination of rhesus macaques with bivalent VLPs generated strong humoral and cellular immune responses.305 The incorporation of both EBOV GP and SUDV GP significantly extended the breadth of both NAbs and ADCC responses compared to those of EBOV GP alone.
GEO-LM01, an MVA-vectored vaccine expressing LASV GPC and Z protein, could produce VLPs of LASV.306 A single IM dose of GEO-LM01 induced high levels of CD4+ and CD8+ T cells and provided 100% protection against LASV ML29 in mice.306,307 Co-expressing of ZIKV structural proteins PrM and E based on MVA resulting in the assembly of ZIKV VLPs. MVA-ZIKV VLPs induced potent NAbs and cellular immunity dominated by CD8 + T cell responses in mice.308 In addition, a single dose of MVA-ZIKV significantly reduced the viremia in susceptible immunocompromised IFNAR−/− mice challenged with ZIKV.
Other poxvirus vectored vaccines in clinical
MVA-based CCHFV, MERS-CoV and SARS-CoV-2 vaccines have been constructed and moved into clinical trials.
A single dose of an MVA-vectored vaccine candidate expressing MERS-CoV S protein (MVA-MERS-S) induced high levels of NAbs in the mouse model. The immune response was dose-dependent. Histopathological analysis of lung and bronchial tubes showed that MVA-MERS-S limited replication of MERS-CoV in the lower respiratory tract of animal models.309–311 MERS-CoV is largely fueled by introductions from dromedary camels, thus evaluation of MVA-MERS-S was conducted in camels. After two doses of vaccination, MVA-MERS-S induced both systemic and local immunity in dromedary camels.312 The excretion of infectious virus and viral RNA was significantly reduced when challenged with MERS-CoV after two-dose immunizations. The protective effect was related to the presence of NAbs. In addition, vaccinated serum has cross-neutralizing activity against camelpox virus. The Phase I clinical trial showed that two doses of MVA-MERS-S inoculation induced antigen-specific antibodies and T cell responses in a dose-escalation manner. No serious adverse events were observed.313 The third dose of MVA-MERS-S boosted at 12 ± 4 months induced persistent MERS-CoV-S-specific B cells and antibodies for 2 years after the latest boost.314
MVA-SARS-CoV-2-S was constructed by expressing the full-length SARS-CoV-2 S protein based on the MVA vector. After two doses of vaccination, binding IgG antibodies and NAbs against SARS-CoV-2 were induced in mice, golden hamsters and rhesus macaques. After the SARS-CoV-2 challenge, vaccinated animals showed a significant reduction in viral loads, lung pathology, and free from symptomatic.315–320 IM, IN and IP delivery routes were all conducted and proved effective. However, point-to-point comparison and the delivery route-dependent manner of MVA-S in identical animal models were not performed. Therefore, it is difficult to determine the optimal delivery route. Representative Poxvirus vector-based vaccines for human disease were summarized in Table 9. Lentiviral vectors (LV), originally derived from human immunodeficiency virus (HIV), are ideal vaccine platforms due to their highly immunogenic and persistent immune responses even after a single dose immunization. The production of LVs involves the co-transfection of transfer vector, the envelope and packaging plasmids in appropriate cell lines. Pending that, the replication and integration of LVs can be abrogated by manipulation of the long terminal repeats (LTRs), the packaging signal, and the integrase gene.321 Apart from HIV, single-dose LV-based vaccines achieve progress in Zika and SARS-CoV-2 in pre-clinical studies, which have proven to be immunogenic, durable in immune response, and protective in challenge models.322–324 The Long-term immunity could be attributed to persistent transcriptions of LV in vivo. As reported, transgene expression could be detected in immunized mice and NHPs for 3–6-months post-injection or even longer.324,325 At cell level, LVs stay as an episomal form and produce the encoded protein for the lifetime of the cell, which raised the safety concerns about potential insertional mutagenesis with integrating vectors.326 Similar to the VSV/RABV ΔG strategy discussed above, anti-vector immunity of LVs can be coupled with a VSV GP serotype exchange strategy, even other envelope glycoproteins.324,327,328
Table 9.
Vaccine candidates based on poxvirus vector
| Virus | Design strategy | Stage | Results | Advantages | Overall concerns | Reference |
|---|---|---|---|---|---|---|
| Ebola virus | MVA-VP40 + GP + NP | Mice | Cellular and humoral immunity | Formulate VLPs | \ | 301 |
| MVA-GP + VP40 | Mice | 50–80% protection | Formulate VLPs | \ | 302 | |
| Lassa virus | NYBH-GP1 + GP2 + NP | NHPs | 90% protection | \ | Low Abs, safety concerns | 547 |
| NYBH-GPC | NHPs | 88% protection | \ | Low Abs, safety concerns | ||
| MVA-NP | Mice and guinea pigs | 100% protection | \ | \ | 548 | |
| MVA-GPC + Z | Mice | 100% protection | Formulate VLPs | Low Abs | 306 | |
| Rift Valley Fever Virus | VACV-vCOGnGc | NHPs | Immunogenic | NAbs higher than vCOGnGcγ | \ | 549 |
| VACV-vCOGnGcγ | NHPs | Immunogenic | Nearly sterile immunity | No protection efficacy in IFNAR-/-mice | ||
| rMVA-Gn/Gc | Mice | 100% protection | \ | No protective effect | 550,551 | |
| Lambs | Reduced virus shedding and viremia | \ | Low effective | |||
| CCHFV | MVA-GP | Mice | 100% protection | \ | Do not reduce virus load | 552 |
| MVA-NP | Mice | Failed protection | \ | \ | 553 | |
| SARS-CoV-2 | MVA-S | NHPs | Protects from infection | Multiple immunation routes | \ | 320 |
| MERS-CoV | MVA-S | Camels | Reduced virus load and viral RNA | Cross-neutralize camelpox virus | Existence of viremia | 312 |
| Phase I | Humoral and cellular responses | Safe, well-tolerated | \ | 554 | ||
| Zika virus | MVA-PrM E | Mice | Reduced virus replication | Cross-neutralization | Relatively low NAbs | 308 |
| Nipah virus | VV-G/F/G + F | Hamsters | 100% protection | \ | \ | 288 |
| ALVAC-G/F/G + F | Pigs | 100% protection | Inhibition of virus shedding | \ | 289 |
CCHFV Crimean Congo hemorrhagic fever virus, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, MERS-CoV Middle East respiratory syndrome coronavirus, VLPs virus like particles, NAbs neutralizing antibodies, Abs antibodies, NHPs nonhuman primates
Mucosal delivery of viral vectored vaccines induces local and peripheral immune responses
Mucosal delivery and triggering both local and systematic immune responses are extraordinary features of viral vector vaccines. Generally, mucosal delivery routes for vaccines include IN, IT, oral (OR), aerosol inhalation, etc. Intranasally or orally delivered VSV-vectored vaccines have been investigated. When rVSVΔG/ZEBOVGP vaccine was given via either IN, OR, or IM routes, NHPs were 100% protected against the lethal challenge of ZEBOV. The IN immunization of recombinant vaccines appeared to be more immunogenic than that of IM immunization.88 For COVID-19, immunization with rVSVΔG-S induced significantly higher NAbs and better post-challenge protection against SARS-CoV-2 through IN immunization than that of IM immunization in golden hamsters and NHPs.329–331 In human clinical trials, the antibody response following single IM dose administration of rVSV-SARS-CoV-2 was not ideal.331,332 This disappointing result may be related to the choice of vaccine delivery route. For respiratory disease, the VSV-vectored replication-competent COVID-19 vaccine could better simulate the natural infection process of SARS-CoV-2 through the respiratory tract, thus eliciting robust and protective immune responses. Tissue tropism and the expression and abundance of angiotensin-converting enzyme 2 (ACE2), the receptor of SARS-CoV-2, may help elucidate the delivery route-dependent manner of rVSV-SARS-CoV-2 in human and animal models. ACE2 is broadly distributed in the upper respiratory tract and lungs, while the distribution of ACE2 is low in skeletal muscle.333,334 Challenge studies in SARS-CoV-2 suspect animal models supported this hypothesis.329,330 Analogous results have been presented in the preclinical study of rVSV vector MERS vaccines.114
RABV vector was engaged as an OR-delivered bivalent rabies vaccine, known as rERAG333E, which contained a G333E mutation in the G protein of the RABV ERA strain.335 Subsequently, recombinant viruses rERAG333E/ZGP and rERAG333E/SGP expressing the glycoprotein of ZEBOV or SUDV were rescued.336 Both vaccines induced viral-specific NAbs and binding antibody responses in mice. However, rERAG 333E/ZGP induced lower ZEBOV NAbs than VSV-vectored Ebola vaccine either by OR or IM route.337 All rERAG333E/ZGP immunized dogs via OR route developed persistent NAbs against ZEBOV despite the pre-existence of anti-RABV immunity. Further, rERAG333E/NiVG and rERAG333E/NiVF were constructed based on rERAG333E vector expressing either attachment glycoprotein (NIV-G) or fusion glycoprotein (NIV-F) of NIV Malaysia strain.338 After OR immunization in mice and pigs, both rERAG333E/NiVG and rERAG333E/NiVF induced RABV and NIV NAbs and high levels of NIV-G or NIV-F specific Ig G. This study provided a safe and convenient OR vaccine for NiV for the first time. Therefore, live rERAG333E is a potential OR-delivered bivalent vaccine for free-roaming animals in endemic areas of RABV and other pathogens.
Mucosal vaccines based on PIV and NDV have been summarized in the second part of this review. These IN-delivered vaccines offer unique advantages for pediatric diseases and respiratory diseases.165 In addition, the preliminary application has been conducted in viral hemorrhagic fever, which appears to be less well effective than those for respiratory disease.186–189 Interestingly, PIV5‐G expressing RABV G induced protective immune responses via IN, IM, and OR immunization against lethal RABV challenge in mice, which was present as an efficacious paramyxovirus‐vectored OR rabies vaccine. It aligns with a needle‐free vaccination strategy to protect stray dogs and wild animals from rabies.339 Currently, mucosal-delivered IFV-vectored vaccines are limited to respiratory diseases. The limited size allowed for ectopic gene expression and concerns about stability hinder the rational application of IFV in other infectious diseases.
AdV vector vaccines are compatible with mucosal delivery due to distinctive tissue tropism, involving the upper or lower respiratory tract, gastrointestinal tract, or conjunctiva.340,341 Compared to IM immunization, IN administration of Ad5-EBOV provided complete protection of mice, guinea pigs, and NHPs from lethal challenge equally. Moreover, IN administration could bypass pre-existing immunity to Ad5 vector.342–345 In hamsters, the Ad5-vectored COVID-19 vaccine delivered orally or intranasally reduced disease severity and transmission.346 Heterologous boost immunization with an aerosolized Ad5-nCoV after two-dose priming with an inactivated COVID-19 vaccine is safe and highly immunogenic, and NAbs were significantly higher than that of homologous prime-boost strategy.347–349 ChAdOx1 nCoV-19 was also tested by IN-delivered in clinical trials. Mucosal responses to IN vaccination were detectable only in a minority of participants, which were largely lower than seen after SARS-CoV-2 infection. Systemic responses to IN vaccination were typically weaker than after IM vaccination with ChAdOx1 nCoV-19. Of interest, mucosal antibody was detectable in participants who received an IM dose of mRNA vaccine after IN vaccination.350 Vaxart developed an OR tablet COVID-19 vaccine consisting of an Ad5 vector expressing the SARS-CoV-2 S and N genes and RNA adjuvant. In Phase I clinical trials, the vaccine was well tolerated.351 Vaccine recipients exhibited an increase in mucosal secretory IgA that persisted up to 360 days. Nevertheless, no serum NAbs were observed. The protective efficacy against COVID-19 needs further investigation. The above results showed that mucosal vaccine may be an important supplemental pools for novel COVID-19 vaccines.352 Likewise, a single dose of ChAdOx1-S expressing MERS-CoV S protein immunization via both IN and IM routes induced a strong immune response and conferred protection against lethal challenge in lethal transgenic BALB/c mouse model.353
In the above proof-of-concept studies, IN or OR delivery of VSV-based vaccines performed well in viral haemorrhagic fever and beta coronavirus. IN delivery route was even more immunogenic than IM delivery. This may be owing to the rapid replication of VSV and abundant expression of antigens, simultaneously associated with the complicated pulmonary immune environment, which enabled massive antigen presentation and triggered both local and systematic immune responses. RABV vector was engaged as an OR-delivered rabies vaccine, which induced a long-lasting immune response. However, other mucosal delivery routes for RABV-based vaccines have not been fully investigated. PIV, NDV, and IFV-vectored vaccines were largely designed as IN-delivered regimens, particularly in respiratory disease. These single-dose vaccines were immunogenic and protective. Similar to those seen in VSV-based vaccines, IN administration of AdV-based EBOV vaccine provided better protection compared to IM immunization. This phenomenon further confirmed the potential of IN inoculation. However, as has been discussed, these viral vector-based vaccines were designed and delivered through different regimens and targeted separate antigens, rendering parallel comparison of mucosal delivery inapplicable. To our knowledge, potential of mucosal delivery may be tightly related to with the replication ability of the recombinant virus. In the near future, mucosal delivery of these viral vector vaccines warrants further investigation. Particularly, the correlation between mucosal immunity and protective efficacy should be clearly defined.
Application of viral vectors as therapeutic vaccines against cancer
Immunotherapy is an effective therapeutic approach in cancer, of which oncolytic virotherapy is an important branch of tumor immunotherapy.354 Briefly, oncolytic viruses (OVs) are naturally occurring or genetically engineered to preferentially replicat and selectively kill tumor cells, release TAAs, and stimulate the anti-tumor immune response through foreign gene delivery. Currently, four OV therapies have been approved worldwide, including Rigvir, Oncorine, Imlygic and Delytact, designed for melanoma, nasopharyngeal carcinoma, melanoma and glioma, respectively. Compared to prophylactic vaccines for infectious diseases, therapeutic cancer vaccines are more challenging as their activity may be hindered by consolidated immunosuppressive complicated tumor microenvironment (TME) and low immunogenicity of autologous TAAs. There are several aspects concerning the targeting and oncolysis process of OVs, including the surface receptors of the tumor cell, tumor-associated signaling pathways, TME, and anti-tumor immune cells (Fig. 2).355–357 Modification and manipulation of the genome of OVs enable the efficient targeting and killing of tumor cells, provided that the right tumor antigens are selected, in particular so-called tumor neoantigens.358
Fig. 2.
Mechanism for targeting and oncolysis of OVs. Oncolytic viruses (OVs) target dysfunctional signaling pathways. (1) Targeting the Wnt signaling pathway, recombinant adenovirus express lung cancer suppressor-1 (TSLC1), acts by downregulating the transcriptional activity of Tcf4/β catenin and inhibiting the expression of CyclinD1 and C-myc, thereby killing Wnt abnormally activated liver cancer cells. (2) E1A(922-947) deleted AdVs replicate and lyse tumor cells. OVs targeting for the adaptation or improvement of the tumor microenvironment (TME) of the tumor. (3) VSV replicates under hypoxic conditions with increased phosphorylated subunits of eIF-2α and inhibits the translation of host cell proteins through the dephosphorylation of translation initiation factor eIF-4E. (4) The Hypoxia inducible factors (HIFs) promoter was applied in AdV to the delivery target genes under hypoxia. (5) VSV naturally targets vascular endothelial cells and inhibits the growth of tumor cells. (6) Expression of vascular growth factor inhibitors (sFlt-1) and anti-angiogenic cytokine (IL-24) by OVs to inhibit tumor angiogenesis. OVs Targeting immunosuppressive TME. (7) OVs were designed to express a variety of cytokines, chemotactic factors, immune checkpoint inhibitors (ICIs) and neoantigens. (8) OVs simultaneously expressing cytokines (GM-CSF) and ICIs (iPD-L1). (9) Neoantigens encoding OVs and heterologous prime-boost strategy (AdV+MVA) (10) Activation of immune cells for tumor lysis by OVs. (11) Combine tumor-specific promoter-derived transcriptional targeting with transductional targeting (through viral capsid incorporation of anti-human carcinoembryonic antigen single variable domains). (12) Combined OV therapy with chemotherapy, radiotherapy and other immunotherapies, etc. (Created in BioRender)
Targeting tumor-associated signaling pathways
The occurrence and progression of tumors are closely related to dysregulated intracellular signaling pathways. The Wnt signaling pathway is abnormally activated in tumors, mainly due to truncation mutation of colorectal adenomatous polyposis coli, render the formulation of stable β-Catenin, followed by the entry of β-Catenin into the nucleus, binding to Tcf/Lef family transcription factors and activate the cyclin D, C-myc and other Wnt target genes, which lead to tumorigenesis.359 RB-E2F is another signaling pathway concerning tumors.360 In normal cells, Retinoblastoma tumor suppressor protein (RB) inhibits E2F activity by recruiting histone deacetylation. When RB is dysfunctional, E2F releases and recruits transcriptional activators to promote the occurrence and progression of tumors. IFN signaling pathway is associated with antiviral immunity, simultaneously associated with tumors. IFN binds to the interferon receptor (IFN-R) and induces the expression of protein kinase R (PKR). When the dsRNA of virus binds to PKR, the activated PKR leads to the phosphorylation of elF-2α, which inhibits the protein synthesis, thus inhibiting the replication of the virus in cells.361,362 Post-entry of OVs, the cellular IFN response is a key determinant of oncolysis sensitivity. Gene expression signature has been devised to predict the outcome of oncolytic virus treatment designating constitutive IFN pathway activation.363
Genetic-engineered OVs are designated to act on these dysfunctional signal pathways. Targeting the Wnt signaling pathway, recombinant adenovirus Ad.wnt-E1A(Δ24bp)-TSLC1 expressing lung cancer suppressor-1 was constructed.364 Ad.wnt-E1A(Δ24bp)-TSLC1 could target and kill cells with abnormal activated Wnt signaling pathway, while showing no obvious killing effect on normal cells. Further study validated that TSLC1 down regulated the transcriptional activity of Tcf4/β catenin and inhibited the expression of CyclinD1 and C-myc, thereby killing Wnt abnormally activated liver cancer cells, which were further confirmed in the mouse xenograft tumor model of human hepatocellular carcinoma SMMC-7721.365 In the genome of AdV, 922–947 bp of E1A is the binding region of RB family. Thus, 922–947 bp of E1A was deleted to construct recombinant adenovirus dl922-947. Post infection with RB deficient tumor cells, dl922-947 can replicate and lyse tumor cells.366 What’s more, dl922-947 can effectively inhibit the occurrence of tumor metastasis in the xenotransplantation model of breast cancer. As reviewed, NS1 protein is a virulence factor of IAV, which can resist PKR mediated antiviral response in the IFN signaling pathway. NS1 deleted IAV cannot replicate in normal cells, while activated Ras can dephosphorylate PKR in tumor cells, thus NS1 deleted IAV can replicate in Ras activated tumor cells.367 Bergmann et al. knocked out the NS1 fragment of IAV to construct delNS1. Then delNS1 was tested in normal cells and normal cells expressing N-ras gene respectively. The results showed that delNS1 selectively replicated in normal cells expressing N-ras gene, which verified IAV as an effective OV targeting IFN signaling pathways.368 Therefore, targeting the abnormally activated signaling pathway can improve the targeting of OVs.
Targeting the adaptation or improvement of the TME
Hypoxia, neoangiogenesis, and immunosuppressive state are microenvironmental issues that determine the initiation, progression, and metastasis of tumors.369 Accordingly, OVs work by improving the hypoxic environment, inhibiting neoangiogenesis, and modulating the immunosuppressive state of the tumor, which correspondingly inhibit the proliferation and spread of tumor cells. Besides, the most common mechanism for tumor targeting of OVs is the handling of replication-associated genes, that is, inactivation of viral genes whose function is not required for replication in cancer cells, but is essential for virus replication in healthy tissues.
Coincidentally, VSV is more effective in mRNA production under hypoxia conditions.356 VSV can overcome increased phosphorylated subunits of eIF-2α under hypoxic conditions at the late stage of infection and inhibition of viral protein synthesis at the initial stage of infection. Meanwhile, VSV infection can inhibit host cell protein translation through the dephosphorylation translation initiation factor eIF-4E, which inhibits the growth and proliferation of tumor cells. Later, replication and cytopathic effects of VSV were confirmed in vitro and in vivo. Hypoxia inducible factors (HIFs) promoters can also be applied to target gene delivery under hypoxia. As the main molecule in the tumor hypoxia environment, HIFs are activated under hypoxia.370 Hypoxia inducible factor-1 (HIF-1) initiates transcriptional response under hypoxia conditions by directly binding to hypoxia responsive element (HRE). Therefore, HIF-1 and HRE genes are targets of tumor cells in a hypoxic environment. HYPR-Ad#1 is a modified AdV in which the E1A gene was under the HIF-1 promoter. HYPR-Ad#1 can only replicate in hypoxic tumor cells that show HIF-1 activation. After infection with HYPR-Ad#1 in HIF-1 activated brain tumor cells, E1A was overexpressed, and more than 90% of cells showed significant cytopathic effect (CPE). The above results indicate that HYPRAd#1 can be replicated in tumor cells activated by hypoxia and HIFs.371
Tumor angiogenesis plays a key role in the growth and metastasis of invasive tumors.371 Thus, inhibition of tumor angiogenesis through natural targeted killing effect or indirectly expressing vascular growth factor inhibitor may help inhibit tumor angiogenesis. VSV can naturally target vascular endothelial cells. Infection with VSV can effectively inhibit the growth of tumor cells. Previous research has shown that wild-type VSV specifically infected endothelial cells (ECs) in tumor tissues and showed anti-tumor effect.372 In addition, expression of vascular growth factor inhibitors by OVs could improve their ability to inhibit tumor angiogenesis, thereby inhibiting tumor growth and metastasis. ZD55-sflt-1 is an oncolytic AdV expressing soluble vascular endothelial growth factor receptor inhibitor sFlt-1. It showed an inhibitory effect on tumor angiogenesis in the animal model of human colorectal cancer.373 Specifically, IL-24 is an effective anti-angiogenic cytokine, which can inhibit angiogenesis and induce apoptosis of tumor cells.374 IL-24 gene was inserted into AdV, termed HE1B55D-RGD-IL-24.375 On this basis, the additional anti-angiogenic arrested fragment was inserted into AdV to construct HE1B55D-RGD. After administration of these two oncolytic adenoviruses in the nude mouse melanoma transplant tumor model, vascular endothelial growth factor (VEGF) and transforming growth factor β were inhibited, which led to anti-angiogenic activity.
Oncolytic efficacy could be further activated by tumor-secreted matrix metalloproteinases (MMP). Originally, MeV fusion protein encompasses a furin cleavage site and depends on intracellular proteases to process proteins and activate particles. Thus, the replacement the furin cleavage site with sequences recognized by matrix metalloproteinases or the urokinase-type plasminogen activator increased tumor specificity, that is, recombinant MVs expressing the modified F proteins spread only in cells secreting MMP. These studies emphasized the conjunction between the targeting and particle activation of OVs under the tumor microenvironment.376,377
Targeting the immunosuppressive TME
Cancer cells adapt to tightly restrict anti-tumor immunity by expressing multiple inhibitory ligands, which serve the so-called ‘immune checkpoint’ molecules for immune cells, thus delivering inhibitory signals that block T cell activation and survival.378 To regulate the immunosuppressive state and enhance anti-tumor immunity, OVs were designed by expressing a variety of cytokines, chemotactic factors and immune checkpoint inhibitors (ICIs) and acting jointly with chimeric antigen receptor T cells (CAR-T).379,380 Besides, neoantigens are supposed to be promising tumor antigens for cancer vaccination with no self-tolerance but the potential to induce robust and selective T cell responses. The discovery and use of neoantigens depends on new technologies such as next-generation sequencing. The definition of tumor-specific neoantigens together with the approval of effective ICIs, contributes to the clinical development of novel vector-based cancer vaccines.
OVs expressing cytokines such as IL-12, IL-13, granulocyte-macrophage colony-stimulating factor (GM-CSF), chemotactic factor CCL19, and CD40L have been proved to effectively recruit and activate antigen presenting cells (APCs) and CD4+T cells and CD8+T cells and reduce the expression of TGF-β and VEGF, thus alleviating the immunosuppressive.381–386 Notably, interleukin-13 receptor α2 (IL-13Rα2) was overexpressed in 80% of glioblastoma multiforme (GBM) tumors. To retarget GBM via IL-13Rα2, MeV-GFP-HAA-IL-13 was generated by displaying human IL-13. MV-GFP-HAA-IL-13 exhibited potent syncytia formation in IL-13Rα2 overexpressing glioma lines. In vivo treatment of MV-GFP-HAA-IL-13 significantly prolonged survival in orthotopically implanted GBM12 xenograft mice. Neurotoxicity was not observed post administration of MV-GFP-HAA-IL-13 in the central nervous system in mice.386 Vaccinia virus VV-IPDL1/GM was constructed by simultaneously expressing GM-CSF and programmed death-ligand inhibitor (iPD-L1). In tumor cells, iPD-L1 binds to programmed death-ligand (PD-L1), thus restoring anti-tumor cell immunity. Intratumoral administration with VV-IPDL1/GM in B16-F10 mouse melanoma model may promote the maturation of tumor infiltrating dendritic cells (DCs) and the activation of tumor-specific T cells. VV-IPDL1/GM provides a more effective targeted therapy regimen for targeted tumor therapy, particularly for patients with resistance to PD1/PDL1 blocking therapy.387
Moving away from the use of canonical immune mediators, the clearance of immunosuppressive molecules from the TME has also been considered. An oncolytic VACA was engineered to express the prostaglandin-inactivating enzyme hydroxyprostaglandin dehydrogenase 15-(NAD) (HPGD). Expression of HPGD selectively depleted Treg and myeloid derived suppressive cells (MDSCs) populations, thereby enhancing the antitumor immune response by upregulation of Th1-associated chemokines.388 Similarly, metabolic reprogramming of TME has been achieved by engineering oncolytic VACA to express leptin. Leptin, in the context of OV-induced immune infiltration, improved mitochondrial oxidative phosphorylation (OXPHOS) in tumor infiltrating lymphocytes, preventing metabolic exhaustion of CD8 + T cells and thus enhancing the therapeutic efficacy and antitumor memory development.389,390
MY-NEOVAX, an oncolytic adenoviral platform encoding up to 50 patients’ specific neoantigens, has been developed. MY-NEOVAX therapy proved effective in improving survival in two patients with last-line treatment refractory colorectal cancer and high-grade neuroendocrine carcinoma.391 Based on AdVs derived from non-human Great Apes, the potency and efficacy of a novel Great Ape Adenoviral (GAd) encoding multiple neoantigens was investigated.392 Prophylactic or early therapeutic vaccination with GAd efficiently control tumor growth in mice. In contrast, combining the vaccine with checkpoint inhibitors is required to eradicate large tumors. Abundance of activated tumor infiltrating T cells with a more diverse TCR repertoire in animals treated with GAd and anti-PD1 compared to anti-PD1. This study suggests that vaccination effectiveness in the presence of a high tumor burden correlates with the breadth of neoantigens-specific T cells and requires concomitant reversal of tumor suppression through checkpoint blockade. Heterologous prime-boosting strategies have also been applied in tumor vaccines to overcome the anti-vector immunity. Leoni et al. has developed a neoantigen-based prime-boost vaccine for the treatment of microsatellite instability (MSI) tumors.393 Neoantigens (FSP) were selected and cloned into non-human GAd and MVA vectors to generate a virus-vectored vaccine, referred to as Nous-209. In mice, Nous-209 was potent to induce broad FSP-specific CD8+ and CD4+ T-cell responses. Moreover, FSP was processed in vitro by human APCs and subsequently human CD8+ T cells were activated. Nous-209 encodes many neoantigens who shared across MSI tumors, which induces the optimal breadth of immune responses, which may achieve clinical benefits to treat and prevent MSI tumors. Another heterologous prime/boost regimen based on non-replicating AdVs combined with oncolytic Maraba virus MG1, both expressing MAGE-A3, has been shown to induce the expansion and long-term persistence of TAA-specific immune response in Macaca.394 These results indicated that the heterologous prime-boost strategy was equally applicable in cancer therapy.
Transcriptional and transductional double targeting
In theory, targeting tumor cells via surface receptors based on the natural tropism of OVs or genetically modified OVs is applicable. However, this receptor-based strategy is not suitable for OVs with extensive receptors and cell tropism, which hinders the precise killing of tumors. By engineering TAA-specific ligands (or antibodies) on virus particles, retargeting OVs to tumor-specific antigens was achieved.395,396 The full replication activity of OVs was maintained without compromising their safety profile.
To further improved the precision of targeting, attempts have been made to combine tumor-specific promoter-derived transcriptional targeting with transductional targeting (through viral capsid incorporation of antihuman carcinoembryonic antigen single variable domains).397 The results showed that employment of a single variable domain genetically incorporated into an AdV fiber increased specificity of infection and efficacy of replication of single variable domain-targeted oncolytic AdV. Double targeting, both transcriptional and transductional, is a promising means of improving the therapeutic index for these advanced generation conditionally replicative AdVs. This re-targeting strategy provides selectivity for OVs to tumor cells, simultaneously enabling the de-targeting of the virus from its natural receptor. This approach is considered suitable for systemic delivery as it minimizes virus replication in healthy tissues.394
Activation of immune cells
MeV naturally and preferentially replicates in malignant cells, which facilitates antitumor immunity and tumor lysis.398–401 To improve the cytotoxic activity against tumor cells directed by MeV-activated NK cells, oncolytic MeV vaccines encoding both CD16A on NK cells and carcinoembryonic antigen (CEA) as a model tumor antigen was developed, termed bispecific killer engagers (MV-BiKE).402 MV-BiKE mediated the secretion of functional BiKE from infected tumor cells. In colorectal or pancreatic cancer cells, MV-BiKE specified the anti-tumor cytotoxicity by NK cells and mediated expression of effector cytokines and degranulation. Viral vector vaccine-harnessed NK cells as anti-tumor effectors were proved in this proof-of-concept study. Analogously, OVs can also increase myeloid and plasmacytoid DCs-mediated cytotoxicity, modulate macrophages toward an antitumor phenotype and activate neutrophils, leading to secretion of related cytokines.403,404
NDV has been reported as OV in clinical. Pediatric high-grade glioma was treated with the oncolytic viral MTH-68/H, an attenuated strain of NDV, combined with oral valproic acid. The above treatment resulted in a far-reaching regression of thalamic glioma despite second neurosurgical intervention were required subsequently. This study documents the oncolytic effect of NDV directed toward virus presence and replication in neoplastic cells.405 In Phase I/II trial in patients with GBM, oncolytic NDV was well tolerated when high doses were applied and responsible through intravenous delivery.406
Advanced OVs in clinical and approved OVs
Herpes simplex virus type 1 (HSV-1) is a distinguished OV work through intratumoral replication and induction of antitumor immune responses.407 Talimogene laherparepvec (T-VEC, Imlygic) is an HSV-1 based OVs for unresectable stage IIIB–IV melanoma.408 Underwent preclinical evaluation in cell lines and animal models,409–411 T-VEC was extensively evaluated in clinical trials. In the Phase I clinical trial, HSV-1 was engineered to express GM-CSF, another mechanism for enhancing local and systemic anti-tumor immunity was aroused by recruitment and maturation of dendritic cells.412 Further, the efficacy of T-VEC was evaluated in Phase II clinical trials.413 In 50 participants with advanced-stage melanomas, 10 patients had a complete response (CR) and 3 patients had a partial response (PR) following a median of six injections of T-VEC, the overall response rate (ORR) was 26%. The overall survival rate was 58% at 1 year and 52% at 24 months, these evidences proved the systemic effectiveness of T-VEC. T-VEC ameliorated durable response in patients with advanced melanoma in Phase III clinical trials. The durable response rate was significantly higher in T-VEC treatment group (16.3%; 95% CI, 12.1–20.5%) compared with GM-CSF treatment group (2.1%; 95% CI, 0–4.5%); odds ratio, 8.9; P < 0.001). The ORR and overall survival rate were also superior in T-VEC treatment group, with most pronounced therapeutic efficacy in patients with stage IIIB, IIIC, or IV melanoma. T-VEC remains the only widely approved OV therapy, which has been optimized during the clinical application process.414
G207 is the second-generation oncolytic HSV-1 involves deletions in the γ34.5 gene and inactivation of the ICP6 gene.415 These modifications diminished pathogenicity and promoted anti-tumor properties.416 Besides direct oncolytic activity, G207 was proven to strengthen anti-tumor immunity in a mouse tumor model.417 In patients with malignant glioma, G207 was safe when applied pre-and post-tumor resection and in combination with other tumor therapy.418–421 During the evolution process, the deletion of alpha47 gene and overlapping of the promoter region of US11 from the second-generation oncolytic HSV-1,415 enhanced the tumor-specific replication capability and cytopathic effect in tumor cells.407,422,423 This third-generation HSV-1, namely G47∆, was significantly more efficacious in vivo than its parent G207 at inhibiting tumors while maintained safety profile. In Phase I/II clinical trials, G47Δ was administered for up to six doses or two doses within 2 weeks in patients with residual or recurrent glioblastoma. G47Δ treatment was associated with improved survival rate and median overall survival. Overall, response and stable disease in patients were observed during the follow-up.424,425 Biopsies revealed that the TEM was improved by increased numbers of tumor-infiltrating lymphocytes. G47∆ (Delytact) has been approved in Japan for glioblastoma.
H101 is an E1B-55 kDa gene-deleted replication-selective AdV, which has been approved as an OV in China (Oncorine). In a Phase III randomized clinical trial, H101 was applied for the treatment of nasopharyngeal carcinoma. H101 in combination with chemotherapy achieved an ORR of 72.7%, compared to that of 40.3% with chemotherapy alone.426 Overall, H101 was safe and effective in patients with squamous cell cancer. Similarly, AdV-based OVs, DNX-2401, has been tested in clinical trials for recurrent malignant glioma, which resulted in potent responses and long-term survival, which may owe to direct oncolytic effects of the virus, followed by elicitation of an immune-mediated anti-glioma response.427,428 Nadofaragene firadenovec (rAd-IFNa/Syn3) is a replication-deficient rAdV that delivers human interferon alfa-2b cDNA into the bladder epithelium, which was indicated for Bacillus Calmette-Guérin-unresponsive non-muscle-invasive bladder cancer.429 Post-treatment with Nadofaragene firadenovec, 53.4% (55/103) of patients with carcinoma in situ had a complete response within 3 months of the first dose and this response was maintained in 45.5% (25/55) of patients at 12 months. The above results suggested that nadofaragene firadenovec was efficacious and provided favorable benefits.
ECHO-7 (Rigvir), an approved echovirus for stage I–II melanoma, decreased the risk of disease progression with ECHO-7 relative to other experimental immunotherapies.430
Concerns and prospects of OVs
Natural viruses are applied as the first generation of oncolytic virotherapy. Although they achieved some efficacy in the treatment of solid tumors and a small number of metastatic tumors, they suffered from defects such as poor targeting, side effects, inability to elicit effective tumor immunity, and the ability to be administered intratumorally only. Further, improved tumor targetability, reduced toxic side effects and boosted antitumor immunity through the insertion of cytokines, etc. have been pursued the treatment of refractory solid tumors and metastases.431 Although oncolytic virotherapy has made tremendous progress, there are also many obstacles in the therapeutic process. Beyond these issues appeared in prophylactic viral vector vaccines, like anti-OV immunity, the transgene stably etc.,380 both the route of administration of OVs and the choice of clinical patients will be difficult during the development of OVs. Although some of the above issues could be addressed by equivalent solutions in prophylactic viral vector vaccines, additional countermeasures are needed.
To further improve the therapeutic efficacy of OVs, remarkable breakthroughs have been made in combination therapy with oncolytic virotherapy T-VEC, anti-PD-1 antibody and chemotherapies.432 In the treatment of melanoma, T-VEC combined with PD-1 antibodies resulted in a tumor objective response rate of up to 62%, with a complete response rate of 33%.433 For patients with resistance to antibody therapy, treatment with Oncorine together with antibody drugs provides additional benefits. The patient experienced symptomatic improvement and achieved stable disease despite partial necrosis of lung tissue.434 These findings suggest that oncolytic virotherapy combined with tumor-associated antibodies and chemotherapy drugs may perform better by altering the tumor microenvironment.
Nonetheless, the correlation between the immune response induced by OVs and antitumor efficacy is largely unknown, either innate or adaptive anti-tumor immunity. Clinical knowledge of the combination of OV and CPI will help to better understand how to optimize the use of these viruses for cancer therapy. Of importance, the in-depth understanding and accurate harness of the underlying biology and pharmacology of OVs may enable the systemic administration of OVs and broaden their range of application in cancer.
Several key aspects of viral vector vaccines
Trigger immune responses in a delivery route-dependent manner
The clarification of immune response triggered in delivery routes-dependent manner is of significance to vaccine design and delivery route selection for specific pathogens. The potential mechanism of antigen presentation and immune response induction post-IM vaccination of the viral vector vaccine was illustrated in Fig. 3 (Recombinant AdV as an example).435 Virus entry into muscle cells is mediated by receptor and ligand recognition. Then viruses are absorbed through endocytosis. In cytoplasm, the virus escapes from the endosome, partially disassembles capsids and enters the nucleus through the microtubule network. Transcription of the target genes is conducted in the nucleus, then translation and post-translational modification of the antigen protein are completed in the endoplasmic reticulum and golgi apparatus, respectively. The capture of antigen proteins by APCs resulted in MHC class I presentation and MHC class II presentation to CD8+ T cells and CD4+ T cells, respectively. CD8+ T cells mediate cytolysis of infected cells under the regulation of cytokines. Meanwhile, stimulated B cells differentiate into memory B cells and plasma cells. Plasma cells produce NAbs and binding antibodies. These antibodies are involved in virus neutralization and Fc mediated function, including ADCC and ADCP, etc.
Fig. 3.
Antigen presentation mechanisms of intramuscular-delivered viral vector vaccine. (1) Construction and vaccination of a recombinant viral vector vaccine. (2) Viral vector vaccine is taken up by muscle cells through endocytosis. (3) Endosome escape from viral vector vaccine. (4) Partially disassembled virus capsid traffic to the nucleus, initiating the transcription process. (5) Protein translation and post-translational modification in endoplasmic reticulum and golgi apparatus. (6) Antigen proteins are presented to antigen presenting cells. The antigen can be loaded onto MHC class I for direct presentation to CD8+ T cells, and loaded onto MHC class II for direct presentation to CD4+ T cells. (7) T cells lyse infected cells under the mediation of cytokines. (8) Stimulated B cells differentiate into memory B cells and antibody-releasing plasma cells. (9) Antigen proteins are recognized by antibodies, including those capable of Fc-mediated effector function, including antibody-mediated cellular cytotoxicity and antibody-dependent cell-mediated phagocytosis. (10) Neutralizing antibodies neutralize pathogens and block their entry. (Created in BioRender)
In the unique circumstances of the respiratory tract, innate and adaptive immune responses are tightly regulated and in continual flux for careful balance between pathogen clearance, immune modulation, and tissue repair.436 Compared to IM delivery, IN delivery of recombinant viral vector vaccine induces both local and peripheral immune response (Fig. 4) (rVSV as an example). After vaccination and virus entry into the mucosa, secretory immunoglobulins (sIgA and sIgM) are produced by subepithelial plasma cells. They provide antigen-specific targeting of foreign antigens parallel to their innate immune counterparts. Simultaneously, innate immune cells are recruited. Some of them process and pass the antigen to APCs, mainly DCs. Activated DC traffic to drain lymph nodes (LNs). In the T cell zone, DCs train naive T cells and lead to clonal expansion. Then, antigen recognition induces effector expression by T cell activating B cell. Activated B cells enter the germinal center (GC), undergo expansions, leading to long-lived memory B cells and high-affinity plasma cells.436
Fig. 4.
The local and peripheral immune response induced by intranasal-delivered viral vector vaccine. (1) Intranasal delivery of the viral vector vaccine and entry of the recombinant virus. (2) Local secretory immunoglobulin A produced by subepithelial plasma cells. (3) Antigen is recognized and processed by innate immune cells and antigen presenting cells. (4) Immune cell recruitment, including neutrophils, natural killer cells, and monocytes. (5) Activated dendritic cells traffic to draining lymph nodes via afferent lymphatics to prime adaptive responses. In lymph node T cell zones, externally derived antigens are presented on class II MHC, prompting CD4+ T cell training, while internally derived antigens are processed and presented on class I MHC to CD8+ T cells. (6) APCs promote maturation and expansion of naive CD4+ and CD8+ T cells. CD8+ cytotoxic T cells and subsets of CD4+ T helper cells traffic back to the site of infection. (7) Activated B cells undergo expansion and somatic hypermutation of the B cell receptor (BCR), resulting in BCR specificity that strongly binds to peptides maintained on the surface of follicular dendritic cells (FDCs). B cells cycle through iterative rounds of expansion/somatic hypermutation and affinity selection, resulting in the selection of high-affinity BCRs. (8) Interactions with T follicular helper (Tfh) cells lead to B cell differentiation and class-switching to long-lived memory B cells and high-affinity plasma cells, which traffic to sites of infection or maintained as long-lived memory populations. (Created in BioRender)
Several vaccine candidates have shown potent profile post OR vaccination.88,337,338,347,348,351,437 Nevertheless, limited data on the interaction between OR-delivered recombinant virus vector vaccine and the complicated oral gastrointestinal (GI) environment rendered the mechanism unknown in terms of virus entry and establishment of humoral and cellular immune responses at both systemic and mucosal sites. Current evidence shows that tonsils & adenoids as well as Peyer’s patch in the small intestine are potential sites for induction of post-vaccination immune response (Fig. 5).438,439 Notably, Peyer’s patches are core sites for immune response stimulation. This process involves antigens uptake by M cells, transport and release of antigens, and activation of T cells. However, due to the harsh chemical conditions in the stomach and intestine, it seems difficult for viral vector vaccines to reach the small intestines without the package of specific material. In the near future, antigen presentation mechanisms of viral vector vaccine post OR inoculation warrant further investigation.
Fig. 5.
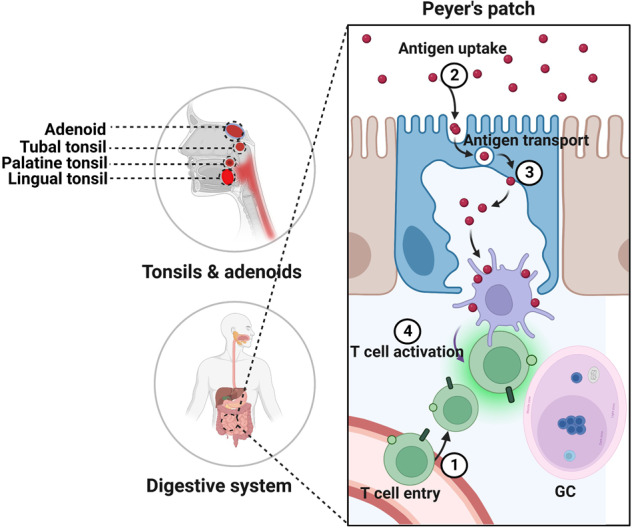
Potential sites for induction of immune response induced by oral-delivered viral vector vaccine. In the oral cavity, viral vector vaccines enter through oral lymphoid tissue, including adenoid, tubal tonsil, palatine tonsil, and lingual tonsil, etc. In the small intestine, Peyer’s patch is the core site for immune response (1) T cells enter Peyer’s patch from blood vessels. (2) M cells take up antigens by endocytosis and phagocytosis. (3) Antigens are transported across the M cell in vessels and released at the basal surface. (4) T cells in the Peyer’s patch encounter antigens and are activated by dendritic cells. (Created in BioRender)
Choice of viral vector platforms and balancing safety and immunogenicity
Safety and immunogenicity are key components of a promising vaccine. In most cases, a delicate balance should be achieved depending on the given condition. For urgent large-scale vaccination against lethal haemorrhagic fever with a high fatality rate, rVSV-vector replication-competent vaccine is a reasonable choice since its characterization of a single-dose regime, robust immunogenicity and rapid immune response. For frequently emerging respiratory diseases such as influenza and COVID-19, PIV, IFV and NDV-vectored vaccines provide eligible options for IN-delivered, single-dose vaccine or booster vaccines. For medical workers with corresponding medical conditions, multi-dose inoculation of RABV-based inactivated vaccines or heterologous prime-boost regimen based on AdV and poxvirus vectors combine immunogenicity and durability while minimizing anti-vector immunity. The heterologous prime-boost regimes could maximize the benefits and circumvent the limitations of those seen in specific single vectors. In the near future, clearer definitions of the general and distinctive characteristics of these viral vectors are needed to further support the selection of viral vectors.
According to the limited study about the point-to-point comparison of these NNSV vector vaccines, VSV vector vaccines appear to be more immunogenic and effective after a single dose of IM inoculation than RABV or PIV and DNA virus vector vaccines. The above phenomenon can be attributed to the robust replication dynamics of VSV. As a live vector, PIV is considered to be a relatively safe vaccine vector, which is advantageous over other NNSV vectors developed from which encounter issues with virus reversion, residual virulence, etc. In particular, the safety of the PIV vector vaccine has been assessed in children over 2 months, which represents an ideal platform for pediatric diseases.
Indeed, replication-competent viral vector vaccines provide additional benefits for mucosal delivery and the duration of immune response. In contrast, strong replication ability may be followed by a higher risk of adverse effects, especially in immunocompromised individuals like pregnant women, infants and the elderly. Therefore, an attenuation strategy is needed to address the biosafety issue.440 Ideally, attenuation of the viral vector vaccine should maintain immunogenicity. For VSV, the second generation rVSV design strategy was implemented, termed N4CT1, which involves an additional transcriptional unit at the 3′ end of the genome, translocation of the N gene to the fourth transcriptional unit, and truncation of the VSV G CT domain (Fig. 1a).124 N4CT1 is attenuated by changing the gene location of specific proteins in the genome, which has been verified in human clinical trials. This strategy may also apply to other NNSVs. Besides, viral mRNA cap (methyltransferase, MTase) activity is an excellent target for the development of live attenuated viral vector vaccines, as the viral mRNA cap is essential for mRNA stability, protein translation, and innate immune evasion.441–444 Deficiency of MTase has been shown to completely attenuated in both immunocompetent and immunocompromised mice while the immunogenicity was not dampened. In addition, utilizing a temperature-sensitive assembly-defective mutation of L111A and combining it with an M51R mutation in the M protein of rVSV significantly reduced the pathogenicity of the virus while maintaining highly effective virus production.32,445 These strategies can be stand alone or combination to improve the safety of replication-competent viral vector vaccines. In RABV, previous efforts in the attenuation strategy were directed towards the deletion of pathogenic genes (G/P/M).446–454 Nevertheless, these strategies rendered recombinant virus replication-defective, thus compromising post-challenge protection effectiveness, making them less attractive. Instead, the attenuation strategy was conducted based on SADB19, a vaccine strain licensed in Europe for wild animal vaccination.455–457 RABV SADB19 involving an R333E mutation in G protein was proved to be significantly attenuated in neurovirulence.458 Similarly seen in PIV vector vaccines, over-attenuation may lead to suboptimal efficacy in humans.175,179 Consequently, pending the attenuation of viral vectors, immunogenicity should be timely regarded and maintained.
Currently, there have been three generations of AdV vectors. The first generation AdV vector lacks E1 or E3 genes. This type of vector can cause strong inflammatory response and immune responses. In the second generation AdV vector, the E2A or E4 gene was further deleted, resulting in a weaker immune response, but improved capacity and safety. The third generation AdV vector lost all or most of the AdV genes, retaining only inverted terminal repeat (ITR) and packaging signal sequences. The cellular immune response caused by the third generation AdV vector is further reduced. The evolution of AdV vector is also a balancing process between safety and immunogenicity.
To sum up, the selection of viral vectors, replication-competent, single-round replication or inactivated, is a balance between safety and immunogenicity, and depends on the properties of given pathogens and the target population.
Mucosal delivery is a prominent feature of viral vectored vaccines
Due to the intrinsic adjuvant properties and active mucosal infection, viral vector vaccines could be delivered via mucosal routes and offer several distinguished advantages. (1) Beyond the systematic immune response, local mucosal immune response induced by mucosal vaccines would serve the first line of defense against foreign pathogens, which is supposed to block virus entry and provid broader heterosubtypic protection.459–467 As has been reviewed, humoral immune responses in PBMCs are not always the exclusive indicator for evaluating a mucosal vaccine.180,238 For instance, the secretory immunoglobulin A (SIgA) in the nasal cavity can last for about 9 months after natural infection with SARS-CoV-2, whereas injectable vaccines are effective in producing and enhancing antibodies in the blood, and can prevent serious diseases, but have little impact on nasal IgA levels.468–470 (2) Local CD8+ T cells and SIgA exhibit broader spectrum effects than NAbs, which would be particularly essential for frequently mutated pathogens like IFV and SARS-CoV-2.471,472 (3) Mucosal immunity could alleviate the impact of anti-vector immunity on viral vector vaccines to some extent.342 (4) These mucosal immunization routes are more convenient and acceptable than injectable vaccines, especially for needle-fearing populations, which would contribute to the full establishment of herd immunity. Simultaneously, for diseases of animal origin, mucosal-delivered vaccines are convenient and practicable. IN or inhalation inoculation could achieve large-scale immunization in huge animal groups whilst OR inoculation facilitates the full distribution of vaccines in wildlife habitats. (5) In cases that most people worldwide have received at least two doses of injectable COVID-19 vaccines, either mRNA vaccines or inactivated vaccines, boosting with mucosal-delivered viral vector vaccines would consolidate the systemic immune response and offer additional mucosal immune response.347 (6) Mucosal vaccines may help fill gaps in traditional vaccines.473,474 For example, results from numerous clinical trials of licensed SARS-CoV-2 vaccines have shown lower efficacy in older adults than in younger adults.11,475,476 While dNS1-RBD, an IN-delivered COVID-19 vaccine, was well tolerated in all participants aged 18–86 years, and immunogenicity in older adults (aged ≥60 years) was similar to that in younger participants. (7) For those mucosal-associated pathogens that transmit through the respiratory or digestive tract, viral vector vaccines could maximize the recapitulation of the natural infection process of specific pathogens. Consequently, provide a comprehensive immune response and protection. (8) The respiratory tract and digestive tract are not completely separated. For example, Ad5nCoV is administrated by aerosol inhalation through the oral cavity, which is then fully distributed in the respiratory tract, mainly in the lungs.347 That is, the IN-delivered vaccine could be converted into an oral-respiratory aerosol inhalation vaccine, as the inhalation vaccine provides better immune response and protection than nasal spray vaccines.477
Nevertheless, the relationship between local mucosal immunity and protective efficacy is not yet well established, especially in human clinical trials. Translational gaps between animals and humans should be noted. Promising results in preclinical animal studies may not necessarily predict safety and efficacy in humans. The human immune system is more sophisticated, and the local environment of the human nasal or respiratory tract is likely to have been exposed to a variety of pathogens prior to trial participation, whereas that of an animal raised in a controlled laboratory environment is likely to be naive to such exposures, which may affect immune responses to vaccination. This gap between species should be further explored. Besides, for the evaluation of those replication competent viral vector vaccines assembling solely foreign glycoprotein, the animal model should strictly reflect the actual situation in humans in terms of receptor-ligand recognition, pre or post exposure, and composition of the immune system.329,330,478
More recently, novel vaccine technologies such as mRNA vaccines and protein subunit vaccines attract attention and are also involved in mucosal vaccine platforms.479 For mRNA vaccines, intranasally administered COVID-19 mRNA vaccines systemically induced S-specific binding antibodies and NAbs comparable to IM inoculation group.480 Correspondingly, IN vaccination exhibited protective efficacy against challenge of SARS-CoV-2 in hamsters. Nevertheless, secretory IgA in the turbinate and alveolar lavage fluid was not detected in this study, thus the local immune response and activation of tissue resident T or B cells were uncertain. For the protein subunit vaccine, a vaccine strategy called “prime and S” was noted,352 which was conducted by boosting IM-delivered COVID-19 mRNA vaccines with IN-delivered S protein vaccines. Robust resident memory B and T cell responses and IgA were induced in the respiratory mucosa of mice. Actually, this strategy aroused mucosal immunity by protein vaccine on the condition that existing immunity was generated by primary vaccination, which elicited mucosal immune memory in the respiratory tract. In theory, all vaccine approaches could be conducted likewise the “prime and S”. Overall, preclinical data concerning mucosal vaccines are limited for mRNA vaccines and protein subunit vaccines. To a large extent, local mucosal immunity induced by solely mRNA vaccines is uncertain. Pre-existing immunity is required for mucosal delivery of protein subunit vaccines. In contrast, viral vector platforms were well established in mucosal vaccines, which induced both local and systemic immune responses ignoring the immune status. Importantly, innate myeloid cells, such as monocytes/macrophages, can produce vigorous responses following subsequent encounters, so called “natural immune memory” or “trained immunity”, which may provide support for viral vector vaccine in mucosal delivery.481–483 As has been reported, respiratory virus infection simulated alveolar macrophage memory and produced trained immunity, fosterting a sustained response to a secondary challenge, this process may even acquire help from effector CD8 T cells.484–486
Duration of immune response
Ideally, long-lasting protective efficacy would facilitate the eradication of pathogens and ease the medical and economic burden, especially for developing countries. Single dose IM-delivered VSV vectored vaccine has shown potential. As reviewed, 100% and 89% of participants remained seropositive at 2 years after a single high or low dose of rVSV-ZEBOV vaccination, respectively. NAbs were less durable, with seropositivity falling from 64–71% at 28 days to 27–31% at 6 months.94 Likewise, VSV vector vaccine achieved a long-lasting immune response in other hemorrhagic fever viruses.101,102 VSVΔG/LASVGPC induced rapid and long-term immunity to LASV. Post a single IM dose vaccination in guinea pigs, the protection rate was 100%, 87%, 83% and 71% on day 14, day 25 day 6 months and day 1 year, respectively.102 Further, the persistence of IN or OR- delivered VSV vector vaccine should be assessed.
In general, RABV-vector vaccines are designed as inactivated or OR delivered, while PIV vector vaccine are IN delivered. Diversity in immunization programs hindered the point-to-point comparison between these viral vectors. Mice orally inoculated with a single dose of rERAG333E produced strong and one year-long NAbs to RABV. 100% of vaccinated animals were protected from challenge of RABV at 12 months after immunization. Dogs who received one or two OR vaccinations with rERAG333E generated a strong protective NAbs response lasting for over 3 years, and moderate saliva RABV-specific IgA was also detected.335 In the case of PIV-vectored COVID-19 vaccines, the duration of the immune response after one or two IN doses of CVXGA vaccination in hamsters was measured and compared with those of two doses of COVID-19 mRNA vaccines. At day 36 post vaccination, 2X mRNA induced the upmost level of anti-S ELISA titers, 2X CVXGA1-immunized hamsters induced higher anti-S titers than 1X CVXGA1 immunized hamsters. Interestingly, anti-S titers on day 108 were comparable for all three vaccination groups. Compared to mRNA vaccines, anti-S ELISA titers and NAb titers in CVXGA1 vaccination groups were well maintained. The animal challenge study confirmed this phenomenon.164 When hamsters were challenged at 9 months post vaccination, CVXGA1 immunized hamsters were well protected than mRNA vaccine. The live-attenuated MeV vaccine has also been proven to elicit long-lasting B-cell and T-cell responses, with a reported measles-specific antibody half-life of more than 200 years.487 Duration in protective immune response could be attributed to the prolonged replication and spread of MeV in lymphoid tissue.488
Long-lasting protective efficacy was also observed in IFV-vectored COVID-19 vaccines. For example, dNS1-RBD induced a protective immune response lasting at least one year in hamster models.235 The above results indicate that the replication-competent viral vector vaccine exhibits an excellent profile in the persistence of protective immunity against multiple pathogens despite different delivery routes.
In contrast, AdV and poxvirus vector vaccines are largely designed as single-round replication or replication-defective constructs. Although the single-dose regimen of these vaccines has been tested in human clinical trials or approved. Less well immune persistence was observed when standing alone or applied as a single-dose regime compared to those replication-competent viral vector constructs.95,248 Representatively, in the Phase II clinical trial of ChAd3-EBO-Z and rVSV∆G-ZEBOV-GP in Liberia, seroconversion of the ChAd3-EBO-Z vaccination group was 63.5% at 1 year post a single IN dose vaccination, which was lower than the 79.5% rVSV∆G-ZEBOV-GP vaccination group.262 Generally, a prime-boost strategy was conducted to prolong the persistence of the immune response.262,285,286
Overcome the anti-vector immunity
Preexisting anti-vector immunity is a common problem faced by all viral vector vaccines, especially in AdV vectored-vaccines.251,252,489–491 Relatively, NNSVs and were less dampened by anti-vector immunity due to their single dose regimen, low serum positive rate, or replacement of surface glycoproteins.155,193,205,206,492,493 However, Serum positive rates of AdV are prevalent worldwide, ranging from 58.4 to 90%.342,494–502 Current solutions include increasing doses, selecting vectors with low seropositivity, chenge of delivery route and heterologous prime-boost strategy. The above solutions reduced the impact of anti-vector immunity to some extent. To fundamentally overcome this issue, the novel solution involves an immune escape strategy that deletes or modifies relevant regions, sequences, or epitopes of the viral vector targeted by pre-existing immunity. For example, the major determinants of AdV neutralization are in the fiber and hypervariable regions (HVRs) of Hexon protein, while replacing of seven short hypervariable regions on the surface of Ad5 hexon protein with corresponding HVRs of rare AdV serotype Ad48 successfully bypassed anti-Ad5 immunity.503 In the future, the determination of the dominant site of anti-vector immunity and corresponding genome modification-based immune escape strategies should be conducted to address the issue of anti vector immunity.
Advantages, limitations, and potential entry points
NNSV vectors share several advantages. The RNA of NNSV is not likely to integrate into the host genome and thus recombination rarely occurs.35,504 Besides, NNSV can quickly grow to high titers and propagate in appropriate cell lines, facilitating large-scale production. Meanwhile, the genome of NNSV is simple and easy-operated, thus the insertion of one or more foreign antigens and the rescue of recombinant virus is convenient. The NNSV genome harboring a foreign gene is relatively stable. It does not have issues with genome recombination and loss of foreign genes as frequently happens with positive-stand RNA virus genomes.505,506 Generally, low seropositive rate was reported in NNSV, and replacement of glycoprotein could further alleviate anti-vector immunity. Regarding and steps taken to address the paramount challenge of these NNSV vectors, the biosafety issue, was reviewed in part 3.2.
There are 18 subtypes of IFV HA and 11 subtypes of NA. By replacing HA and NA, chimeric viruses can be rescued through reverse genetics. Currently, the IFV vaccine production platform is highly optimized, permitting large-scale manufacturing.231 Nevertheless, capacity limitations may hinder the full application of IFV, as the length of foreign gene insertion is limited to about 1.5 kb nucleotides or less. What’s more, transgene stability of IFV-vectored vaccine should be improved. Recently, A/PR/8/1934 (H1N1) (PR8) and A/WSN/33 (H1N1) (WSN) are the most frequently used IFV skeletons. Although both of them are of low pathogenicity and can be handled in biosafety level two (BSL-2) laboratories, IN immunization may cause reassortment with circulating strains, leaving safety concerns. Changing the delivery route may avoid the reassortment. Additional studies concerning the potential mechanism to overcome species-specific restriction of IFV are needed to address the issue of reassortment in influenza.507
AdVs are well established viral vectors that have been fully evaluated in human clinical trials. These single-round replicated recombinant viruses are safe and well tolerated in humans. Subsequently, the immunogenicity and duration of these vaccines warrant further optimization.
Poxvirus vectors have some unique properties.378 (1) Lack of genomic integration in the host due to their cytoplasmic replication. (2) Low prevalence of anti-vector immunit. (3) Acceptable safety profiles in humans, particularly for ALVAC and MVA, their inability to replicate in mammalian cells further underlies their improved safety profile. (4) Established procedures for the large-scale production of clinical grade material. Compared to NNSV or AdV vectors, poxvirus appears to be less immunogenic in the application of prophylactic vaccines against viral haemorrhagic fever or beta coronavirus, as clinical trials of poxvirus vector vaccines are largely combined with other vaccine platforms. Seeking to optimize the poxvirus vector, several strategies have been implemented, including heterologous prime/boost protocols, use of co-stimulatory molecules, deletion of viral immunomodulatory genes still present in the poxvirus genome, enhancement of virus promoter strength, enhancement of vector replication capacity, optimizing expression of foreign heterologous sequences, and the combined use of adjuvants.508
Conclusion and perspective
In response to acute public health events, viral vector vaccine platforms facilitate a timely response. Notably, viral-vectored COVID-19 vaccines achieved remarkable progress in human clinical trials and have been approved in a short period of time during the pandemic of COVID-19. Although potent immune response and protective efficacy have been conferred, intrinsic properties and extraordinary superiorities of these viral-vectored vaccines have not been fully exploited, especially mucosal delivery and mucosal immunity. Also seen in therapeutic cancer vaccines, advances in viral vector vaccines rely on improved understanding of viral biology and updated insights into reciprocal interactions between viruses and the host immune system.509,510
In the near future, a better understanding of the similarities and individualities of these viral vectors would push the revolutionary advances. Typically, NNSV is a large group of viral vectors that share collective viral biology characteristics and confronting homologous obstacles. Indeed, some progress has been made owing to the comprehensive knowledge of these NNSVs, including reverse genetic approaches, polarized transcription mechanism, chimeric strategy that retained the TMCT origin for foreign antigen incorporation, as well as the attenuation modification. Particularly important, the trained immunity induced by respiratory virus offer substantial benefits for antimicrobial infections and anti-tumor activity.511–513 Nevertheless, far more aspects should be taken into consideration under the in-depth master of similarities between viral vectors. Correspondingly, issues like anti-vector immunity, and safety concerns could be addressed in a same manner. Further, the individualities of these viral vectors should be clearly elucidated depending on the targeted pathogens or neoplasm. In this process, interdisciplinary cooperations, structural biology, artificial intelligence and gene editing, etc. may provide additional support.
As has been exhaustively reviewed, VSV and MeV are distinguished viral vectors and of the potential for mucosal delivery and induction of durable local and systematic immune response. Nevertheless, VSV and MeV-based COVID-19 initiated by Merck, V590332 and V591,198,199 received disappointed responses in Phase I clinical trials despite promising results in preclinical studies (Table 10). This could be attributed to the less well connection and coordination between preclinical and clinical trials, specifically, the suboptimal selection of delivery route. Ideally, animal models should accurately and comprehensively reflect the immune status and post-vaccination response in human beings, conversely, outcomes from inappropriate animal models would mislead the experimental design of the clinical trial, ultimately determining the final direction. For VSV and MeV, their potential for mucosal delivery were largely unexplored, particular in clinical trials. Consequently, the essential attributes of these NSSVs warrant further investigation in human clinical trials on the basis that convincing approaches achieved in preclinical trials. Overall, appropriate and accurate technological advances, sufficient exploration in potential, and tightly connection between preclinical and clinical studies would consolidate the position of viral vector vaccines and to compel the acceleration and approval of novel viral vector vaccines.
Table 10.
Viral vectored vaccines in clinical trials
| Vector | Pathogens | Developer | Constructs (name) | Reported status | Results | Clinical trials registry |
|---|---|---|---|---|---|---|
| VSV | EBOV | Merck | VSV-ZEBOV-G(Ervebo) | Phase III | An overall protective efficacy of 100% | PACTR201503001057193 |
| Profectus | N4CT1-GP1 | Phase I | Safe and immunogenic | NCT02718469 | ||
| SARS-CoV-2 | Merck | rVSVΔG-S (V590) | Phase I | Poor immunogenicity | NCT04569786 | |
| PIV | RSV | AstraZeneca | B/HPIV3-F | Phase I | Safe and immunogenic in infants and children | |
| MeV | CHIKV |
Rostock University |
MV-CHIKV VLP | Phase II | Well-tolerated; immunogenic; persistent in immune response | NCT02861586 |
| SARS-CoV-2 | Merck | MeV-S (V591) | Phase I/II | Well tolerated but insufficient immunogenicity | NCT04498247 | |
| NDV | SARS-CoV-2 | Mahidol University | NDV-S(NDV-HXP-S) | Phase I | Safe and immunogenic | |
| Cancer | Israel | NDV-HUJ | Phase I/II | Good tolerability and encouraging responses | \ | |
| IFV | SARS-CoV-2 | Wantai BioPharm | dNS1-RBD | Phase III | 55% and 82% protection for people without/with immunization history | ChiCTR2100051391 |
| IFV | AstraZeneca | FluMist | \ | 78–100% protection against different IFV strains | \ | |
| AdV | EBOV | CanSino | Ad5-Makona GP | Phase II | Safe and highly immunogenic; 8 × 1010 viral particles was validated as the optimal dose | PACTR201509001259869 |
| Russia | GamEvac-Combi | Phase II | 100% seroconversion rate; robust immune response | 0373100043215000055 | ||
| NIH | ChAd3-EBO-Z | Phase III/II | Immune responses largely maintained through 12 months | NCT02344407 | ||
| SARS-CoV-2 | CanSino | Ad5-S (Convidecia) | Phase III/IV | 57.5% efficacy against symptomatic; heterologous boosting with Convidecia following with inactivated COVID-19 vaccine is safe and more immunogenic than homologous prime boost | ||
| Ad5-S (Convidecia Air) | Phase III | Heterogenous boost with inactivated vaccine induced better immune response than homologous prime boost | NCT05043259 | |||
| Janssen | Ad26-S (Jcovden) | Phase III | 52.9% protection against symptomatic infection | NCT04505722 | ||
| Gamaleya | Ad5 + Ad26-S (Sputnik V) | Phase III | 91.6% overall efficacy | NCT04530396 | ||
| Ad26-S (Sputnik Light) | Phase I | Safe and immunogenic | NCT04741061 | |||
| AstraZeneca | ChAdOx1-S (Vaxzevria) | Phase III | 70.4% overall efficacy | NCT04324606, NCT04444674 | ||
| Vaxzevria (i.n.) | Phase I | Tolerate, mucosal and systemic response | NCT04871737 | |||
| Vaxart | Ad5-S + N(oral tablet) | Phase I | Safe and generated mucosal immune responses | NCT04563702 | ||
| MERS-CoV | Saudi Arabia | ChAdOx1 (MERS002) | Phase I | Safe and immunogenic | NCT04170829 | |
| RABV | Oxford | ChAdOx2 RabG | Phase I | Safety, tolerate and immunogenic | NCT04162600 | |
| Cancer | Spain | DNX-2401 | Phase I | Dramatic responses with long-term survival in gliomas | NCT00805376 | |
| Sunway | Oncorine (H101) | Phase III | FDA approved for head and neck neoplasms | \ | ||
| FerGene | Nadofaragene firadenovec | Phase III | Efficacious and favorable benefit | NCT02773849 | ||
| Pox virus | EBOV | Janssen | Ad26-ZaireGP+MVA-BN-Filo | Phase I | Well tolerated; highly immunogenic; long-lasting antibodies duration (1 year) | NCT02376426 |
| GlaxoSmithKline | ChAd3-ZaireGP+MVA-BN-Filo | Phase I | Safe and immunogenic | |||
| MERS-CoV | Germany | MVA- S | Phase I | Safe and immunogenic | NCT03615911 | |
| HIV | USA | ALVAC-HIV + protein vaccine | Phase III | Controversial efficacy | ||
| Smallpox | Bavarian Nordic | MVA | Phase III | Safe, seroconversion rate over 90.8% | NCT01913353 | |
| HSV-1 | Cancer | University of T okyo | HSV-1 | Phase II | Survival benefit and safety profile | NCT02457845 UMIN000015995 |
| Amgen | HSV-1 | Phase III | Well tolerated, longer durable response rate and longer survival | NCT00769704 |
VSV vesicular stomatitis virus, PIV parainfluenza virus, MeV measles virus, NDV Newcastle disease virus, IFV influenza virus, AdV adenovirus, EBOV Ebola virus, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, RSV respiratory syncytial virus, MERS-CoV Middle East respiratory syndrome coronavirus, RABV rabies virus, HIV Human immunodeficiency virus, HSV-1 Herpesvirus type I
Author contributions
F.Y., S.Y., and X.X. designed the research; S.W., L.L., W.W., and N.F. read and analyzed the papers; S.W., T.W., and Y.Z. participated in the discussion; S.W., B.L., and F.Y. wrote and revised the paper. All authors have read and approved the paper.
Funding
F.Y. declares grants from the National Key Research and Development Program of China (Research and Development of COVID-19 Vaccine for Animals, grant number: 2022YFC0867900).
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Shen Wang, Bo Liang, Weiqi Wang, Ling Li
Contributor Information
Feihu Yan, Email: yanfh1990@163.com.
Songtao Yang, Email: yst62041@163.com.
Xianzhu Xia, Email: xiaxzh@cae.cn.
References
- 1.Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011;377:849–862. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borio L, et al. Hemorrhagic fever viruses as biological weapons: medical and public health management. Jama. 2002;287:2391–2405. doi: 10.1001/jama.287.18.2391. [DOI] [PubMed] [Google Scholar]
- 3.Paessler S, Walker DH. Pathogenesis of the viral hemorrhagic fevers. Annu Rev. Pathol. 2013;8:411–440. doi: 10.1146/annurev-pathol-020712-164041. [DOI] [PubMed] [Google Scholar]
- 4.Zhu N, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peiris JSM, Yuen KY, Osterhaus ADME, Stöhr K. The Severe Acute Respiratory Syndrome. N. Engl. J. Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 6.Zaki AM, et al. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 7.Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 8.Travieso T, et al. The use of viral vectors in vaccine development. NPJ Vaccines. 2022;7:75. doi: 10.1038/s41541-022-00503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ewer KJ, et al. Viral vectors as vaccine platforms: from immunogenicity to impact. Curr. Opin. Immunol. 2016;41:47–54. doi: 10.1016/j.coi.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Callaway E. ‘Make Ebola a thing of the past’: first vaccine against deadly virus approved. Nature. 2019;575:425–426. doi: 10.1038/d41586-019-03490-8. [DOI] [PubMed] [Google Scholar]
- 11.Halperin SA, et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet. 2022;399:237–248. doi: 10.1016/S0140-6736(21)02753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadoff J, et al. Final Analysis of Efficacy and Safety of Single-Dose Ad26.COV2.S. N. Engl. J. Med. 2022;386:847–860. doi: 10.1056/NEJMoa2117608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voysey M, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu F, et al. Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Respir. Med. 2022;10:749–760. doi: 10.1016/S2213-2600(22)00131-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logunov DY, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomme EA, et al. Immune clearance of attenuated rabies virus results in neuronal survival with altered gene expression. PLoS Pathog. 2012;8:e1002971. doi: 10.1371/journal.ppat.1002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D CG, P HB, F OJ. The structural proteins of rabies virus and evidence for their synthesis from separate monocistronic RNA species. J. Gen. Virol. 1980;49:161–180. doi: 10.1099/0022-1317-49-1-161. [DOI] [PubMed] [Google Scholar]
- 18.Paterson RG, Harris TJ, Lamb RA. Analysis and gene assignment of mRNAs of a paramyxovirus, simian virus 5. Virology. 1984;138:310–323. doi: 10.1016/0042-6822(84)90354-4. [DOI] [PubMed] [Google Scholar]
- 19.Shioda T, Shibuta H. [Structure of paramyxovirus genome] Uirusu. 1984;34:99–108. doi: 10.2222/jsv.34.99. [DOI] [PubMed] [Google Scholar]
- 20.Conzelmann KK, Schnell M. Rescue of synthetic genomic RNA analogs of rabies virus by plasmid-encoded proteins. J. Virol. 1994;68:713–719. doi: 10.1128/jvi.68.2.713-719.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawson ND, Stillman EA, Whitt MA, Rose JK. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl Acad. Sci. USA. 1995;92:4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose NF, et al. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell. 2001;106:539–549. doi: 10.1016/S0092-8674(01)00482-2. [DOI] [PubMed] [Google Scholar]
- 23.Schnell MJ, Buonocore L, Whitt MA, Rose JK. The minimal conserved transcription stop-start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J. Virol. 1996;70:2318–2323. doi: 10.1128/jvi.70.4.2318-2323.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He B, Paterson RG, Ward CD, Lamb RA. Recovery of infectious SV5 from cloned DNA and expression of a foreign gene. Virology. 1997;237:249–260. doi: 10.1006/viro.1997.8801. [DOI] [PubMed] [Google Scholar]
- 25.J SM, T M, K CK. Infectious rabies viruses from cloned cDNA. EMBO J. 1994;13:4195–4203. doi: 10.1002/j.1460-2075.1994.tb06739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durbin AP, et al. Human parainfluenza virus type 3 (PIV3) expressing the hemagglutinin protein of measles virus provides a potential method for immunization against measles virus and PIV3 in early infancy. J. Virol. 2000;74:6821–6831. doi: 10.1128/JVI.74.15.6821-6831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawano M, et al. Recovery of infectious human parainfluenza type 2 virus from cDNA clones and properties of the defective virus without V-specific cysteine-rich domain. Virology. 2001;284:99–112. doi: 10.1006/viro.2001.0864. [DOI] [PubMed] [Google Scholar]
- 28.Radecke F, et al. Rescue of measles viruses from cloned DNA. EMBO J. 1995;14:5773–5784. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Billeter MA, Naim HY, Udem SA. Reverse genetics of measles virus and resulting multivalent recombinant vaccines: applications of recombinant measles viruses. Curr. Top. Microbiol. Immunol. 2009;329:129–162. doi: 10.1007/978-3-540-70523-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnamurthy S, Huang Z, Samal SK. Recovery of a virulent strain of newcastle disease virus from cloned cDNA: expression of a foreign gene results in growth retardation and attenuation. Virology. 2000;278:168–182. doi: 10.1006/viro.2000.0618. [DOI] [PubMed] [Google Scholar]
- 31.Garbutt M, et al. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J. Virol. 2004;78:5458–5465. doi: 10.1128/JVI.78.10.5458-5465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu M, et al. A Methyltransferase-Defective Vesicular Stomatitis Virus-Based SARS-CoV-2 Vaccine Candidate Provides Complete Protection against SARS-CoV-2 Infection in Hamsters. J. Virol. 2021;95:e0059221. doi: 10.1128/JVI.00592-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.T M, et al. Highly stable expression of a foreign gene from rabies virus vectors. Proc. Natl Acad. Sci. USA. 1996;93:7310–7314. doi: 10.1073/pnas.93.14.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGettigan JP, et al. Functional human immunodeficiency virus type 1 (HIV-1) Gag-Pol or HIV-1 Gag-Pol and env expressed from a single rhabdovirus-based vaccine vector genome. J. Virol. 2003;77:10889–10899. doi: 10.1128/JVI.77.20.10889-10899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geisbert TW, Feldmann H. Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg virus infections. J. Infect. Dis. 2011;204:1075–1081. doi: 10.1093/infdis/jir349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bukreyev A, et al. Chimeric human parainfluenza virus bearing the Ebola virus glycoprotein as the sole surface protein is immunogenic and highly protective against Ebola virus challenge. Virology. 2009;383:348–361. doi: 10.1016/j.virol.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Case JB, et al. Neutralizing antibody and soluble ACE2 inhibition of a replication-competent VSV-SARS-CoV-2 and a clinical isolate of SARS-CoV-2. Cell Host Microbe. 2020;28:475–485.e475. doi: 10.1016/j.chom.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez SE, et al. Vesicular Stomatitis Virus-Based Vaccine Protects Mice against Crimean-Congo Hemorrhagic Fever. Sci. Rep. 2019;9:7755. doi: 10.1038/s41598-019-44210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, et al. A single intranasal dose of a live-attenuated parainfluenza virus-vectored SARS-CoV-2 vaccine is protective in hamsters. Proc. Natl Acad. Sci. USA. 2021;118:e2109744118. doi: 10.1073/pnas.2109744118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang B, et al. Packaging and Prefusion Stabilization Separately and Additively Increase the Quantity and Quality of Respiratory Syncytial Virus (RSV)-Neutralizing Antibodies Induced by an RSV Fusion Protein Expressed by a Parainfluenza Virus Vector. J. Virol. 2016;90:10022–10038. doi: 10.1128/JVI.01196-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iverson LE, Rose JK. Localized attenuation and discontinuous synthesis during vesicular stomatitis virus transcription. Cell. 1981;23:477–484. doi: 10.1016/0092-8674(81)90143-4. [DOI] [PubMed] [Google Scholar]
- 42.Carnero E, et al. Optimization of human immunodeficiency virus gag expression by newcastle disease virus vectors for the induction of potent immune responses. J. Virol. 2009;83:584–597. doi: 10.1128/JVI.01443-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakaya T, et al. Recombinant Newcastle disease virus as a vaccine vector. J. Virol. 2001;75:11868–11873. doi: 10.1128/JVI.75.23.11868-11873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Z. Parainfluenza virus 5-vectored vaccines against human and animal infectious diseases. Rev. Med. Virol. 2018;28:e1965. doi: 10.1002/rmv.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ritchey MB, Palese P, Kilbourne ED. RNAs of influenza A, B, and C viruses. J. Virol. 1976;18:738–744. doi: 10.1128/jvi.18.2.738-744.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson D, Cadman A, Zurcher T, Barclay WS. A reverse genetics approach for recovery of recombinant influenza B viruses entirely from cDNA. J. Virol. 2002;76:11744–11747. doi: 10.1128/JVI.76.22.11744-11747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoffmann E, et al. Rescue of influenza B virus from eight plasmids. Proc. Natl Acad. Sci. USA. 2002;99:11411–11416. doi: 10.1073/pnas.172393399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffmann E, Webster RG. Unidirectional RNA polymerase I-polymerase II transcription system for the generation of influenza A virus from eight plasmids. J. Gen. Virol. 2000;81:2843–2847. doi: 10.1099/0022-1317-81-12-2843. [DOI] [PubMed] [Google Scholar]
- 49.Moser MJ, et al. Single-replication BM2SR vaccine provides sterilizing immunity and cross-lineage influenza B virus protection in mice. Vaccine. 2019;37:4533–4542. doi: 10.1016/j.vaccine.2019.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wan Z, et al. Alternative Strategy for a Quadrivalent Live Attenuated Influenza Virus Vaccine. J. Virol. 2018;92:e01025–01018. doi: 10.1128/JVI.01025-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santos JJS, et al. Development of an Alternative Modified Live Influenza B Virus Vaccine. J. Virol. 2017;91:e00056–00017. doi: 10.1128/JVI.00056-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ermler ME, et al. Chimeric Hemagglutinin Constructs Induce Broad Protection against Influenza B Virus Challenge in the Mouse Model. J. Virol. 2017;91:e00286–00217. doi: 10.1128/JVI.00286-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stech J, et al. Influenza B virus with modified hemagglutinin cleavage site as a novel attenuated live vaccine. J. Infect. Dis. 2011;204:1483–1490. doi: 10.1093/infdis/jir613. [DOI] [PubMed] [Google Scholar]
- 54.Wressnigg N, et al. Development of a live-attenuated influenza B DeltaNS1 intranasal vaccine candidate. Vaccine. 2009;27:2851–2857. doi: 10.1016/j.vaccine.2009.02.087. [DOI] [PubMed] [Google Scholar]
- 55.Hai R, et al. Influenza B virus NS1-truncated mutants: live-attenuated vaccine approach. J. Virol. 2008;82:10580–10590. doi: 10.1128/JVI.01213-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li SQ, et al. Influenza A virus transfectants with chimeric hemagglutinins containing epitopes from different subtypes. J. Virol. 1992;66:399–404. doi: 10.1128/jvi.66.1.399-404.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castrucci MR, Bilsel P, Kawaoka Y. Attenuation of influenza A virus by insertion of a foreign epitope into the neuraminidase. J. Virol. 1992;66:4647–4653. doi: 10.1128/jvi.66.8.4647-4653.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takizawa N, et al. Induction of immune responses to a human immunodeficiency virus type 1 epitope by novel chimeric influenza viruses. Drug Disco. Ther. 2009;3:252–259. [PubMed] [Google Scholar]
- 59.Vieira Machado A, et al. Recombinant influenza A viruses harboring optimized dicistronic NA segment with an extended native 5’ terminal sequence: induction of heterospecific B and T cell responses in mice. Virology. 2006;345:73–87. doi: 10.1016/j.virol.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 60.Watanabe T, et al. Exploitation of nucleic acid packaging signals to generate a novel influenza virus-based vector stably expressing two foreign genes. J. Virol. 2003;77:10575–10583. doi: 10.1128/JVI.77.19.10575-10583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.García-Sastre A, et al. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology. 1998;252:324–330. doi: 10.1006/viro.1998.9508. [DOI] [PubMed] [Google Scholar]
- 62.Wang P, et al. Generation of DelNS1 Influenza Viruses: a Strategy for Optimizing Live Attenuated Influenza Vaccines. mBio. 2019;10:e02180–02119. doi: 10.1128/mBio.02180-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kittel C, et al. Rescue of influenza virus expressing GFP from the NS1 reading frame. Virology. 2004;324:67–73. doi: 10.1016/j.virol.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 64.Hock K, et al. Oncolytic influenza A virus expressing interleukin-15 decreases tumor growth in vivo. Surgery. 2017;161:735–746. doi: 10.1016/j.surg.2016.08.045. [DOI] [PubMed] [Google Scholar]
- 65.Zheng M, et al. An A14U Substitution in the 3’ Noncoding Region of the M Segment of Viral RNA Supports Replication of Influenza Virus with an NS1 Deletion by Modulating Alternative Splicing of M Segment mRNAs. J. Virol. 2015;89:10273–10285. doi: 10.1128/JVI.00919-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Norrby E, et al. Adenoviridae. Intervirology. 1976;7:117–125. doi: 10.1159/000149945. [DOI] [PubMed] [Google Scholar]
- 67.Davison AJ, Benko M, Harrach B. Genetic content and evolution of adenoviruses. J. Gen. Virol. 2003;84:2895–2908. doi: 10.1099/vir.0.19497-0. [DOI] [PubMed] [Google Scholar]
- 68.Vemula SV, Mittal SK. Production of adenovirus vectors and their use as a delivery system for influenza vaccines. Expert Opin. Biol. Ther. 2010;10:1469–1487. doi: 10.1517/14712598.2010.519332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mackett M, Smith GL, Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc. Natl Acad. Sci. USA. 1982;79:7415–7419. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Panicali D, Paoletti E. Construction of Poxviruses as Cloning Vectors: Insertion of the Thymidine Kinase Gene from Herpes Simplex Virus into the DNA of Infectious Vaccinia Virus. Proc. Natl Acad. Sci. USA. 1982;79:4927–4931. doi: 10.1073/pnas.79.16.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sanchez-Sampedro L, et al. The evolution of poxvirus vaccines. Viruses. 2015;7:1726–1803. doi: 10.3390/v7041726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.A M, E M. [Changes in the vaccinia virus through continuing passages in chick embryo fibroblast cultures] Zentralblatt fur Bakteriologie, Parasitenkd., Infektionskrankheiten und Hyg. 1. Abt. Medizinisch-hygienische Bakteriologie, Virusforsch. und Parasitologie. Originale. 1964;195:24–35. [PubMed] [Google Scholar]
- 73.Mayr A, et al. [The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism (author’s transl)] Zentralbl Bakteriol. B. 1978;167:375–390. [PubMed] [Google Scholar]
- 74.Sutter G, Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl Acad. Sci. USA. 1992;89:10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suter M, et al. Modified vaccinia Ankara strains with identical coding sequences actually represent complex mixtures of viruses that determine the biological properties of each strain. Vaccine. 2009;27:7442–7450. doi: 10.1016/j.vaccine.2009.05.095. [DOI] [PubMed] [Google Scholar]
- 76.von Krempelhuber A, et al. A randomized, double-blind, dose-finding Phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE. Vaccine. 2010;28:1209–1216. doi: 10.1016/j.vaccine.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hochstein-Mintzel V, Huber H-C, Stickl H. Virulence and immunogenicity of a modified vaccinia virus (strain MVA)(author’s transl) Z. Immunitatsforsch Exp. Klin. Immunol. 1972;144:104–156. [PubMed] [Google Scholar]
- 78.Mayr A, et al. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism (author’s transl) Zentralbl Bakteriol. B. 1978;167:375–390. [PubMed] [Google Scholar]
- 79.Pittman PR, et al. Phase 3 Efficacy Trial of Modified Vaccinia Ankara as a Vaccine against Smallpox. N. Engl. J. Med. 2019;381:1897–1908. doi: 10.1056/NEJMoa1817307. [DOI] [PubMed] [Google Scholar]
- 80.Goepfert PA, et al. Specificity and 6-month durability of immune responses induced by DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J. Infect. Dis. 2014;210:99–110. doi: 10.1093/infdis/jiu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goepfert PA, et al. Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J. Infect. Dis. 2011;203:610–619. doi: 10.1093/infdis/jiq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.G CM, W CM. Recombinant MVA vaccines: dispelling the myths. Vaccine. 2013;31:4247–4251. doi: 10.1016/j.vaccine.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 83.Zhou Y, Sullivan NJ. Immunology and evolvement of the adenovirus prime, MVA boost Ebola virus vaccine. Curr. Opin. Immunol. 2015;35:131–136. doi: 10.1016/j.coi.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 84.Stittelaar KJ, et al. Safety of modified vaccinia virus Ankara (MVA) in immune-suppressed macaques. Vaccine. 2001;19:3700–3709. doi: 10.1016/S0264-410X(01)00075-5. [DOI] [PubMed] [Google Scholar]
- 85.Blanchard TJ, Alcami A, Andrea P, Smith GL. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J. Gen. Virol. 1998;79:1159–1167. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]
- 86.Jones SM, et al. Assessment of a vesicular stomatitis virus-based vaccine by use of the mouse model of Ebola virus hemorrhagic fever. J. Infect. Dis. 2007;196:S404–S412. doi: 10.1086/520591. [DOI] [PubMed] [Google Scholar]
- 87.Jones SM, et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat. Med. 2005;11:786–790. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- 88.Qiu X, et al. Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong ebola GP-specific immune responses. PLoS ONE. 2009;4:e5547. doi: 10.1371/journal.pone.0005547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Geisbert TW, et al. Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses. Vaccine. 2008;26:6894–6900. doi: 10.1016/j.vaccine.2008.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huttner A, et al. A dose-dependent plasma signature of the safety and immunogenicity of the rVSV-Ebola vaccine in Europe and Africa. Sci. Transl. Med. 2017;9:eaaj1701. doi: 10.1126/scitranslmed.aaj1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marzi A, et al. Efficacy of Vesicular Stomatitis Virus-Ebola Virus Postexposure Treatment in Rhesus Macaques Infected With Ebola Virus Makona. J. Infect. Dis. 2016;214:S360–S366. doi: 10.1093/infdis/jiw218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Menicucci AR, et al. Antiviral Innate Responses Induced by VSV-EBOV Vaccination Contribute to Rapid Protection. mBio. 2019;10:e00597–00519. doi: 10.1128/mBio.00597-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Henao-Restrepo AM, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!) Lancet. 2017;389:505–518. doi: 10.1016/S0140-6736(16)32621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huttner A, et al. Determinants of antibody persistence across doses and continents after single-dose rVSV-ZEBOV vaccination for Ebola virus disease: an observational cohort study. Lancet Infect. Dis. 2018;18:738–748. doi: 10.1016/S1473-3099(18)30165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Agnandji ST, et al. Phase 1 Trials of rVSV Ebola Vaccine in Africa and Europe. N. Engl. J. Med. 2016;374:1647–1660. doi: 10.1056/NEJMoa1502924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Regules JA, et al. A Recombinant Vesicular Stomatitis Virus Ebola Vaccine. N. Engl. J. Med. 2017;376:330–341. doi: 10.1056/NEJMoa1414216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haug CJ. Keeping Your Cool - Doing Ebola Research during an Emergency. N. Engl. J. Med. 2018;378:2353–2355. doi: 10.1056/NEJMp1806978. [DOI] [PubMed] [Google Scholar]
- 98.Marzi A, Mire CE. Current Ebola Virus Vaccine Progress. BioDrugs. 2019;33:9–14. doi: 10.1007/s40259-018-0329-7. [DOI] [PubMed] [Google Scholar]
- 99.Daddario-DiCaprio KM, et al. Cross-protection against Marburg virus strains by using a live, attenuated recombinant vaccine. J. Virol. 2006;80:9659–9666. doi: 10.1128/JVI.00959-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Geisbert TW, et al. Development of a new vaccine for the prevention of Lassa fever. PLoS Med. 2005;2:e183. doi: 10.1371/journal.pmed.0020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mire CE, et al. Durability of a vesicular stomatitis virus-based marburg virus vaccine in nonhuman primates. PLoS ONE. 2014;9:e94355. doi: 10.1371/journal.pone.0094355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stein DR, et al. A recombinant vesicular stomatitis-based Lassa fever vaccine elicits rapid and long-term protection from lethal Lassa virus infection in guinea pigs. NPJ Vaccines. 2019;4:8. doi: 10.1038/s41541-019-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Prescott J, DeBuysscher BL, Brown KS, Feldmann H. Long-term single-dose efficacy of a vesicular stomatitis virus-based Andes virus vaccine in Syrian hamsters. Viruses. 2014;6:516–523. doi: 10.3390/v6020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Warner BM, et al. Vesicular Stomatitis Virus-Based Vaccines Provide Cross-Protection against Andes and Sin Nombre Viruses. Viruses. 2019;11:645. doi: 10.3390/v11070645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chattopadhyay A, Rose JK. Complementing defective viruses that express separate paramyxovirus glycoproteins provide a new vaccine vector approach. J. Virol. 2011;85:2004–2011. doi: 10.1128/JVI.01852-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Geisbert TW, et al. Development of an acute and highly pathogenic nonhuman primate model of Nipah virus infection. PLoS ONE. 2010;5:e10690. doi: 10.1371/journal.pone.0010690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mire CE, et al. Use of Single-Injection Recombinant Vesicular Stomatitis Virus Vaccine to Protect Nonhuman Primates Against Lethal Nipah Virus Disease. Emerg. Infect. Dis. 2019;25:1144–1152. doi: 10.3201/eid2506.181620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mlakar J, et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 109.van der Eijk AA, et al. Miscarriage Associated with Zika Virus Infection. N. Engl. J. Med. 2016;375:1002–1004. doi: 10.1056/NEJMc1605898. [DOI] [PubMed] [Google Scholar]
- 110.Betancourt D, et al. Cutting Edge: Innate Immune Augmenting Vesicular Stomatitis Virus Expressing Zika Virus Proteins Confers Protective Immunity. J. Immunol. 2017;198:3023–3028. doi: 10.4049/jimmunol.1602180. [DOI] [PubMed] [Google Scholar]
- 111.Emanuel J, et al. A VSV-based Zika virus vaccine protects mice from lethal challenge. Sci. Rep. 2018;8:11043. doi: 10.1038/s41598-018-29401-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kapadia SU, et al. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology. 2005;340:174–182. doi: 10.1016/j.virol.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shan D, et al. Immunogenicity of a recombinant VSV-Vectored SARS-CoV vaccine induced robust immunity in rhesus monkeys after single-dose immunization. Virol. Sin. 2022;37:248–255. doi: 10.1016/j.virs.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu R, et al. A recombinant VSV-vectored MERS-CoV vaccine induces neutralizing antibody and T cell responses in rhesus monkeys after single dose immunization. Antivir. Res. 2018;150:30–38. doi: 10.1016/j.antiviral.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wollmann G, et al. Lassa-vesicular stomatitis chimeric virus safely destroys brain tumors. J. Virol. 2015;89:6711–6724. doi: 10.1128/JVI.00709-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Muik A, et al. Re-engineering vesicular stomatitis virus to abrogate neurotoxicity, circumvent humoral immunity, and enhance oncolytic potency. Cancer Res. 2014;74:3567–3578. doi: 10.1158/0008-5472.CAN-13-3306. [DOI] [PubMed] [Google Scholar]
- 117.Beier KT, et al. Anterograde or retrograde transsynaptic labeling of CNS neurons with vesicular stomatitis virus vectors. Proc. Natl Acad. Sci. USA. 2011;108:15414–15419. doi: 10.1073/pnas.1110854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Beier KT, et al. Vesicular stomatitis virus with the rabies virus glycoprotein directs retrograde transsynaptic transport among neurons in vivo. Front. Neural Circuits. 2013;7:11. doi: 10.3389/fncir.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mire CE, et al. Recombinant vesicular stomatitis virus vaccine vectors expressing filovirus glycoproteins lack neurovirulence in nonhuman primates. PLoS Negl. Trop. Dis. 2012;6:e1567. doi: 10.1371/journal.pntd.0001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rose NF, Roberts A, Buonocore L, Rose JK. Glycoprotein exchange vectors based on vesicular stomatitis virus allow effective boosting and generation of neutralizing antibodies to a primary isolate of human immunodeficiency virus type 1. J. Virol. 2000;74:10903–10910. doi: 10.1128/JVI.74.23.10903-10910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marzi A, et al. Vesicular stomatitis virus-based vaccines against Lassa and Ebola viruses. Emerg. Infect. Dis. 2015;21:305–307. doi: 10.3201/eid2102.141649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tsuda Y, et al. Protective efficacy of a bivalent recombinant vesicular stomatitis virus vaccine in the Syrian hamster model of lethal Ebola virus infection. J. Infect. Dis. 2011;204:S1090–S1097. doi: 10.1093/infdis/jir379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Geisbert TW, et al. Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of ebola virus. J. Virol. 2009;83:7296–7304. doi: 10.1128/JVI.00561-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cross RW, et al. Quadrivalent VesiculoVax vaccine protects nonhuman primates from viral-induced hemorrhagic fever and death. J. Clin. Investig. 2019;130:539–551. doi: 10.1172/JCI131958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Q, et al. GEM-PA-Based Subunit Vaccines of Crimean Congo Hemorrhagic Fever Induces Systemic Immune Responses in Mice. Viruses. 2022;14:1664. doi: 10.3390/v14081664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kurup D, et al. Rhabdovirus-based vaccine platforms against henipaviruses. J. Virol. 2015;89:144–154. doi: 10.1128/JVI.02308-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Takayama-Ito M, et al. Replication-incompetent rabies virus vector harboring glycoprotein gene of lymphocytic choriomeningitis virus (LCMV) protects mice from LCMV challenge. PLoS Negl. Trop. Dis. 2018;12:e0006398. doi: 10.1371/journal.pntd.0006398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Keshwara R, et al. A Recombinant Rabies Virus Expressing the Marburg Virus Glycoprotein Is Dependent upon Antibody-Mediated Cellular Cytotoxicity for Protection against Marburg Virus Disease in a Murine Model. J. Virol. 2019;93:e01865–01818. doi: 10.1128/JVI.01865-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abreu-Mota T, et al. Non-neutralizing antibodies elicited by recombinant Lassa–Rabies vaccine are critical for protection against Lassa fever. Nat. Commun. 2018;9:4223. doi: 10.1038/s41467-018-06741-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Keshwara R, et al. Rabies-based vaccine induces potent immune responses against Nipah virus. NPJ Vaccines. 2019;4:15–15. doi: 10.1038/s41541-019-0109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Blaney JE, et al. Inactivated or live-attenuated bivalent vaccines that confer protection against rabies and Ebola viruses. J. Virol. 2011;85:10605–10616. doi: 10.1128/JVI.00558-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Blaney JE, et al. Antibody quality and protection from lethal Ebola virus challenge in nonhuman primates immunized with rabies virus based bivalent vaccine. PLoS Pathog. 2013;9:e1003389. doi: 10.1371/journal.ppat.1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Willet M, et al. Preclinical Development of Inactivated Rabies Virus-Based Polyvalent Vaccine Against Rabies and Filoviruses. J. Infect. Dis. 2015;212:S414–S424. doi: 10.1093/infdis/jiv251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Johnson RF, et al. An Inactivated Rabies Virus-Based Ebola Vaccine, FILORAB1, Adjuvanted With Glucopyranosyl Lipid A in Stable Emulsion Confers Complete Protection in Nonhuman Primate Challenge Models. J. Infect. Dis. 2016;214:S342–s354. doi: 10.1093/infdis/jiw231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang H, et al. Using rabies virus vaccine strain SRV9 as viral vector to express exogenous gene. Virus Genes. 2015;50:299–302. doi: 10.1007/s11262-014-1160-y. [DOI] [PubMed] [Google Scholar]
- 136.Zhang S, et al. Genetically modified rabies virus vector-based rift valley fever virus vaccine is safe and induces efficacious immune responses in mice. Viruses. 2019;11:919. doi: 10.3390/v11100919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Faber M, et al. A single immunization with a rhabdovirus-based vector expressing severe acute respiratory syndrome coronavirus (SARS-CoV) S protein results in the production of high levels of SARS-CoV-neutralizing antibodies. J. Gen. Virol. 2005;86:1435–1440. doi: 10.1099/vir.0.80844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tai W, et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020;17:613–620. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wirblich C, et al. One-Health: a Safe, Efficient, Dual-Use Vaccine for Humans and Animals against Middle East Respiratory Syndrome Coronavirus and Rabies Virus. J. Virol. 2017;91:e02040–02016. doi: 10.1128/JVI.02040-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Du L, et al. MERS-CoV spike protein: a key target for antivirals. Expert Opin. Ther. Targets. 2017;21:131–143. doi: 10.1080/14728222.2017.1271415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cuiqing M, et al. Intranasal vaccination with recombinant receptor-binding domain of MERS-CoV spike protein induces much stronger local mucosal immune responses than subcutaneous immunization: Implication for designing novel mucosal MERS vaccines. Vaccine. 2014;32:2100–2108. doi: 10.1016/j.vaccine.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stalin RV, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lanying D, et al. A truncated receptor-binding domain of MERS-CoV spike protein potently inhibits MERS-CoV infection and induces strong neutralizing antibody responses: implication for developing therapeutics and vaccines. PloS ONE. 2013;8:e0278474. doi: 10.1371/journal.pone.0081587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Du L, et al. Identification of a receptor-binding domain in the S protein of the novel human coronavirus Middle East respiratory syndrome coronavirus as an essential target for vaccine development. J. Virol. 2013;87:9939–9942. doi: 10.1128/JVI.01048-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li E, et al. Characterization of the Immune Response of MERS-CoV Vaccine Candidates Derived from Two Different Vectors in Mice. Viruses. 2020;12:125. doi: 10.3390/v12010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kurup D, et al. A Single Dose of the Deactivated Rabies-Virus Vectored COVID-19 Vaccine, CORAVAX, Is Highly Efficacious and Alleviates Lung Inflammation in the Hamster Model. Viruses. 2022;14:1126. doi: 10.3390/v14061126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kurup D, et al. Inactivated rabies virus vectored SARS-CoV-2 vaccine prevents disease in a Syrian hamster model. PLoS Pathog. 2021;17:e1009383. doi: 10.1371/journal.ppat.1009383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kurup D, Wirblich C, Ramage H, Schnell MJ. Rabies virus-based COVID-19 vaccine CORAVAX™ induces high levels of neutralizing antibodies against SARS-CoV-2. NPJ Vaccines. 2020;5:98. doi: 10.1038/s41541-020-00248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yankowski C, Wirblich C, Kurup D, Schnell MJ. Inactivated rabies-vectored SARS-CoV-2 vaccine provides long-term immune response unaffected by vector immunity. NPJ Vaccines. 2022;7:110. doi: 10.1038/s41541-022-00532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang H, et al. An inactivated recombinant rabies virus chimerically expressed RBD induces humoral and cellular immunity against SARS-CoV-2 and RABV. Virol. Sin. 2022;S1995-820X:00212–00217. doi: 10.1016/j.virs.2022.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schmidt AC, et al. Bovine parainfluenza virus type 3 (BPIV3) fusion and hemagglutinin-neuraminidase glycoproteins make an important contribution to the restricted replication of BPIV3 in primates. J. Virol. 2000;74:8922–8929. doi: 10.1128/JVI.74.19.8922-8929.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Karron RA, et al. Evaluation of two chimeric bovine-human parainfluenza virus type 3 vaccines in infants and young children. Vaccine. 2012;30:3975–3981. doi: 10.1016/j.vaccine.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bernstein DI, et al. Phase 1 study of the safety and immunogenicity of a live, attenuated respiratory syncytial virus and parainfluenza virus type 3 vaccine in seronegative children. Pediatr. Infect. Dis. J. 2012;31:109–114. doi: 10.1097/INF.0b013e31823386f1. [DOI] [PubMed] [Google Scholar]
- 154.Tompkins SM, et al. Recombinant parainfluenza virus 5 (PIV5) expressing the influenza A virus hemagglutinin provides immunity in mice to influenza A virus challenge. Virology. 2007;362:139–150. doi: 10.1016/j.virol.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Capraro GA, Johnson JB, Kock ND, Parks GD. Virus growth and antibody responses following respiratory tract infection of ferrets and mice with WT and P/V mutants of the paramyxovirus Simian Virus 5. Virology. 2008;376:416–428. doi: 10.1016/j.virol.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang L, et al. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J. Virol. 2005;79:1113–1124. doi: 10.1128/JVI.79.2.1113-1124.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Howard LM, et al. Parainfluenza Virus Types 1-3 Infections Among Children and Adults Hospitalized With Community-acquired Pneumonia. Clin. Infect. Dis. 2021;73:e4433–e4443. doi: 10.1093/cid/ciaa973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.DeGroote NP, et al. Human parainfluenza virus circulation, United States, 2011–2019. J. Clin. Virol. 2020;124:104261. doi: 10.1016/j.jcv.2020.104261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Buchholz UJ, et al. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc. Natl Acad. Sci. USA. 2004;101:9804–9809. doi: 10.1073/pnas.0403492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ohtsuka J, et al. Non-propagative human parainfluenza virus type 2 nasal vaccine robustly protects the upper and lower airways against SARS-CoV-2. iScience. 2021;24:103379. doi: 10.1016/j.isci.2021.103379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.An D, et al. Protection of K18-hACE2 mice and ferrets against SARS-CoV-2 challenge by a single-dose mucosal immunization with a parainfluenza virus 5-based COVID-19 vaccine. Sci. Adv. 2021;7:eabi5246. doi: 10.1126/sciadv.abi5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wrapp D, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Corbett KS, et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020;383:1544–1555. doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Beavis, A. C. et al. Efficacy of Parainfluenza Virus 5 (PIV5)-vectored Intranasal COVID-19 Vaccine as a Single Dose Vaccine and as a Booster against SARS-CoV-2 Variants. bioRxiv[preprint], (2022).
- 165.Le Nouën C, et al. Intranasal pediatric parainfluenza virus-vectored SARS-CoV-2 vaccine is protective in monkeys. Cell. 2022;185:4811–4825.e4817. doi: 10.1016/j.cell.2022.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ilinykh PA, et al. A single intranasal dose of human parainfluenza virus type 3-vectored vaccine induces effective antibody and memory T cell response in the lungs and protects hamsters against SARS-CoV-2. NPJ Vaccines. 2022;7:47. doi: 10.1038/s41541-022-00471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Clements ML, et al. Evaluation of bovine, cold-adapted human, and wild-type human parainfluenza type 3 viruses in adult volunteers and in chimpanzees. J. Clin. Microbiol. 1991;29:1175–1182. doi: 10.1128/jcm.29.6.1175-1182.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bukreyev A, et al. Mucosal immunisation of African green monkeys (Cercopithecus aethiops) with an attenuated parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet. 2004;363:2122–2127. doi: 10.1016/S0140-6736(04)16501-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li K, et al. Single-Dose, Intranasal Immunization with Recombinant Parainfluenza Virus 5 Expressing Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Spike Protein Protects Mice from Fatal MERS-CoV Infection. mBio. 2020;11:e00554–00520. doi: 10.1128/mBio.00554-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li Z, et al. Single-dose vaccination of a recombinant parainfluenza virus 5 expressing NP from H5N1 virus provides broad immunity against influenza A viruses. J. Virol. 2013;87:5985–5993. doi: 10.1128/JVI.00120-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Miller EK, et al. Viral etiologies of infant bronchiolitis, croup and upper respiratory illness during 4 consecutive years. Pediatr. Infect. Dis. J. 2013;32:950–955. doi: 10.1097/INF.0b013e31829b7e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liang B, et al. Enhanced Neutralizing Antibody Response Induced by Respiratory Syncytial Virus Prefusion F Protein Expressed by a Vaccine Candidate. J. Virol. 2015;89:9499–9510. doi: 10.1128/JVI.01373-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liang B, et al. Chimeric bovine/human parainfluenza virus type 3 expressing respiratory syncytial virus (RSV) F glycoprotein: effect of insert position on expression, replication, immunogenicity, stability, and protection against RSV infection. J. Virol. 2014;88:4237–4250. doi: 10.1128/JVI.03481-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mackow N, et al. Attenuated Human Parainfluenza Virus Type 1 (HPIV1) Expressing the Fusion Glycoprotein of Human Respiratory Syncytial Virus (RSV) as a Bivalent HPIV1/RSV Vaccine. J. Virol. 2015;89:10319–10332. doi: 10.1128/JVI.01380-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liang B, et al. Improved Prefusion Stability, Optimized Codon Usage, and Augmented Virion Packaging Enhance the Immunogenicity of Respiratory Syncytial Virus Fusion Protein in a Vectored-Vaccine Candidate. J. Virol. 2017;91:e00189–00117. doi: 10.1128/JVI.00189-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu X, et al. Attenuated Human Parainfluenza Virus Type 1 Expressing the Respiratory Syncytial Virus (RSV) Fusion (F) Glycoprotein from an Added Gene: Effects of Prefusion Stabilization and Packaging of RSV F. J. Virol. 2017;91:e01101–e01117. doi: 10.1128/JVI.01101-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schmidt AC, McAuliffe JM, Murphy BR, Collins PL. Recombinant bovine/human parainfluenza virus type 3 (B/HPIV3) expressing the respiratory syncytial virus (RSV) G and F proteins can be used to achieve simultaneous mucosal immunization against RSV and HPIV3. J. Virol. 2001;75:4594–4603. doi: 10.1128/JVI.75.10.4594-4603.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liang B, et al. Effects of Alterations to the CX3C Motif and Secreted Form of Human Respiratory Syncytial Virus (RSV) G Protein on Immune Responses to a Parainfluenza Virus Vector Expressing the RSV G Protein. J. Virol. 2019;93:e02043–02018. doi: 10.1128/JVI.02043-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liang B, et al. A Parainfluenza Virus Vector Expressing the Respiratory Syncytial Virus (RSV) Prefusion F Protein Is More Effective than RSV for Boosting a Primary Immunization with RSV. J. Virol. 2020;95:e01512–e01520. doi: 10.1128/JVI.01512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang D, et al. A Single-Dose Recombinant Parainfluenza Virus 5-Vectored Vaccine Expressing Respiratory Syncytial Virus (RSV) F or G Protein Protected Cotton Rats and African Green Monkeys from RSV Challenge. J. Virol. 2017;91:e00066–00017. doi: 10.1128/JVI.00066-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Phan SI, et al. A respiratory syncytial virus (RSV) vaccine based on parainfluenza virus 5 (PIV5) Vaccine. 2014;32:3050–3057. doi: 10.1016/j.vaccine.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Phan SI, et al. Parainfluenza Virus 5 Expressing Wild-Type or Prefusion Respiratory Syncytial Virus (RSV) Fusion Protein Protects Mice and Cotton Rats from RSV Challenge. J. Virol. 2017;91:e00560–00517. doi: 10.1128/JVI.00560-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li Z, et al. Efficacy of parainfluenza virus 5 mutants expressing hemagglutinin from H5N1 influenza A virus in mice. J. Virol. 2013;87:9604–9609. doi: 10.1128/JVI.01289-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li Z, et al. Recombinant parainfluenza virus 5 expressing hemagglutinin of influenza A virus H5N1 protected mice against lethal highly pathogenic avian influenza virus H5N1 challenge. J. Virol. 2013;87:354–362. doi: 10.1128/JVI.02321-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mooney AJ, et al. Vaccination with Recombinant Parainfluenza Virus 5 Expressing Neuraminidase Protects against Homologous and Heterologous Influenza Virus Challenge. J. Virol. 2017;91:e01579–01517. doi: 10.1128/JVI.01579-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bukreyev A, et al. A single intranasal inoculation with a paramyxovirus-vectored vaccine protects guinea pigs against a lethal-dose Ebola virus challenge. J. Virol. 2006;80:2267–2279. doi: 10.1128/JVI.80.5.2267-2279.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bukreyev A, et al. Successful topical respiratory tract immunization of primates against Ebola virus. J. Virol. 2007;81:6379–6388. doi: 10.1128/JVI.00105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yang L, et al. A paramyxovirus-vectored intranasal vaccine against Ebola virus is immunogenic in vector-immune animals. Virology. 2008;377:255–264. doi: 10.1016/j.virol.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bukreyev AA, et al. Mucosal parainfluenza virus-vectored vaccine against Ebola virus replicates in the respiratory tract of vector-immune monkeys and is immunogenic. Virology. 2010;399:290–298. doi: 10.1016/j.virol.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lingemann M, et al. Attenuated Human Parainfluenza Virus Type 1 Expressing Ebola Virus Glycoprotein GP Administered Intranasally Is Immunogenic in African Green Monkeys. J. Virol. 2017;91:e02469–02416. doi: 10.1128/JVI.02469-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hilleman MR. Current overview of the pathogenesis and prophylaxis of measles with focus on practical implications. Vaccine. 2001;20:651–665. doi: 10.1016/S0264-410X(01)00384-X. [DOI] [PubMed] [Google Scholar]
- 192.Malczyk AH, et al. A Highly Immunogenic and Protective Middle East Respiratory Syndrome Coronavirus Vaccine Based on a Recombinant Measles Virus Vaccine Platform. J. Virol. 2015;89:11654–11667. doi: 10.1128/JVI.01815-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liniger M, et al. Induction of neutralising antibodies and cellular immune responses against SARS coronavirus by recombinant measles viruses. Vaccine. 2008;26:2164–2174. doi: 10.1016/j.vaccine.2008.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hörner C, et al. A highly immunogenic and effective measles virus-based Th1-biased COVID-19 vaccine. Proc. Natl Acad. Sci. USA. 2020;117:32657–32666. doi: 10.1073/pnas.2014468117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Frantz PN, et al. A live measles-vectored COVID-19 vaccine induces strong immunity and protection from SARS-CoV-2 challenge in mice and hamsters. Nat. Commun. 2021;12:6277. doi: 10.1038/s41467-021-26506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lu M, et al. A safe and highly efficacious measles virus-based vaccine expressing SARS-CoV-2 stabilized prefusion spike. Proc. Natl Acad. Sci. USA. 2021;118:e2026153118. doi: 10.1073/pnas.2026153118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Muñoz-Alía, M. et al. Surface-modified measles vaccines encoding oligomeric, fusion-stabilized SARS-CoV-2 spike glycoproteins bypass measles seropositivity, boosting neutralizing antibody responses to omicron and historical variants. bioRxiv[Preprint], (2022). [DOI] [PMC free article] [PubMed]
- 198.Vanhoutte F, et al. Safety and immunogenicity of the measles vector-based SARS-CoV-2 vaccine candidate, V591, in adults: results from a phase 1/2 randomised, double-blind, placebo-controlled, dose-ranging trial. EBioMedicine. 2022;75:103811. doi: 10.1016/j.ebiom.2021.103811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Launay O, et al. Safety and immunogenicity of a measles-vectored SARS-CoV-2 vaccine candidate, V591 / TMV-083, in healthy adults: results of a randomized, placebo-controlled Phase I study. EBioMedicine. 2022;75:103810. doi: 10.1016/j.ebiom.2021.103810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sawada A, Ito T, Yamaji Y, Nakayama T. Chimeric Measles Virus (MV/RSV), Having Ectodomains of Respiratory Syncytial Virus (RSV) F and G Proteins Instead of Measles Envelope Proteins, Induced Protective Antibodies against RSV. Vaccines (Basel) 2021;9:156. doi: 10.3390/vaccines9020156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Desprès P, et al. Live measles vaccine expressing the secreted form of the West Nile virus envelope glycoprotein protects against West Nile virus encephalitis. J. Infect. Dis. 2005;191:207–214. doi: 10.1086/426824. [DOI] [PubMed] [Google Scholar]
- 202.Brandler S, et al. Measles vaccine expressing the secreted form of West Nile virus envelope glycoprotein induces protective immunity in squirrel monkeys, a new model of West Nile virus infection. J. Infect. Dis. 2012;206:212–219. doi: 10.1093/infdis/jis328. [DOI] [PubMed] [Google Scholar]
- 203.Brandler S, et al. A recombinant measles vaccine expressing chikungunya virus-like particles is strongly immunogenic and protects mice from lethal challenge with chikungunya virus. Vaccine. 2013;31:3718–3725. doi: 10.1016/j.vaccine.2013.05.086. [DOI] [PubMed] [Google Scholar]
- 204.Rossi SL, et al. Immunogenicity and Efficacy of a Measles Virus-Vectored Chikungunya Vaccine in Nonhuman Primates. J. Infect. Dis. 2019;220:735–742. doi: 10.1093/infdis/jiz202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ramsauer K, et al. Immunogenicity, safety, and tolerability of a recombinant measles-virus-based chikungunya vaccine: a randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial. Lancet Infect. Dis. 2015;15:519–527. doi: 10.1016/S1473-3099(15)70043-5. [DOI] [PubMed] [Google Scholar]
- 206.Reisinger EC, et al. Immunogenicity, safety, and tolerability of the measles-vectored chikungunya virus vaccine MV-CHIK: a double-blind, randomised, placebo-controlled and active-controlled phase 2 trial. Lancet. 2019;392:2718–2727. doi: 10.1016/S0140-6736(18)32488-7. [DOI] [PubMed] [Google Scholar]
- 207.Mateo M, et al. Vaccines inducing immunity to Lassa virus glycoprotein and nucleoprotein protect macaques after a single shot. Sci. Transl. Med. 2019;11:eaaw3163. doi: 10.1126/scitranslmed.aaw3163. [DOI] [PubMed] [Google Scholar]
- 208.Mateo M, et al. A single-shot Lassa vaccine induces long-term immunity and protects cynomolgus monkeys against heterologous strains. Sci. Transl. Med. 2021;13:eabf6348. doi: 10.1126/scitranslmed.abf6348. [DOI] [PubMed] [Google Scholar]
- 209.Nürnberger C, et al. A Measles Virus-Based Vaccine Candidate Mediates Protection against Zika Virus in an Allogeneic Mouse Pregnancy Model. J. Virol. 2019;93:e01485–01418. doi: 10.1128/JVI.01485-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ganar K, Das M, Sinha S, Kumar S. Newcastle disease virus: current status and our understanding. Virus Res. 2014;184:71–81. doi: 10.1016/j.virusres.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Park MS, et al. Newcastle disease virus (NDV)-based assay demonstrates interferon-antagonist activity for the NDV V protein and the Nipah virus V, W, and C proteins. J. Virol. 2003;77:1501–1511. doi: 10.1128/JVI.77.2.1501-1511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Park MS, et al. Newcastle disease virus V protein is a determinant of host range restriction. J. Virol. 2003;77:9522–9532. doi: 10.1128/JVI.77.17.9522-9532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.DiNapoli JM, et al. Newcastle disease virus, a host range-restricted virus, as a vaccine vector for intranasal immunization against emerging pathogens. Proc. Natl Acad. Sci. USA. 2007;104:9788–9793. doi: 10.1073/pnas.0703584104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.He L, et al. Development of SARS-CoV-2 animal vaccines using a stable and efficient NDV expression system. J. Med. Virol. 2023;95:e28237. doi: 10.1002/jmv.28237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sun W, et al. Newcastle disease virus (NDV) expressing the spike protein of SARS-CoV-2 as a live virus vaccine candidate. EBioMedicine. 2020;62:103132. doi: 10.1016/j.ebiom.2020.103132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sun W, et al. A Newcastle disease virus expressing a stabilized spike protein of SARS-CoV-2 induces protective immune responses. Nat. Commun. 2021;12:6197. doi: 10.1038/s41467-021-26499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lara-Puente JH, et al. Safety and Immunogenicity of a Newcastle Disease Virus Vector-Based SARS-CoV-2 Vaccine Candidate, AVX/COVID-12-HEXAPRO (Patria), in Pigs. mBio. 2021;12:e0190821. doi: 10.1128/mBio.01908-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pitisuttithum P, et al. Safety and immunogenicity of an inactivated recombinant Newcastle disease virus vaccine expressing SARS-CoV-2 spike: Interim results of a randomised, placebo-controlled, phase 1 trial. EClinicalMedicine. 2022;45:101323. doi: 10.1016/j.eclinm.2022.101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Duc Dang A, et al. Safety and immunogenicity of an egg-based inactivated Newcastle disease virus vaccine expressing SARS-CoV-2 spike: Interim results of a randomized, placebo-controlled, phase 1/2 trial in Vietnam. Vaccine. 2022;40:3621–3632. doi: 10.1016/j.vaccine.2022.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yang S, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect. Dis. 2021;21:1107–1119. doi: 10.1016/S1473-3099(21)00127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Smolenov I, et al. Impact of previous exposure to SARS-CoV-2 and of S-Trimer (SCB-2019) COVID-19 vaccination on the risk of reinfection: a randomised, double-blinded, placebo-controlled, phase 2 and 3 trial. Lancet Infect. Dis. 2022;22:990–1001. doi: 10.1016/S1473-3099(22)00144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sun W, et al. A Newcastle Disease Virus (NDV) Expressing a Membrane-Anchored Spike as a Cost-Effective Inactivated SARS-CoV-2 Vaccine. Vaccines (Basel) 2020;8:771. doi: 10.3390/vaccines8040771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ponce-de-León, S. et al. Safety and immunogenicity of a live recombinant Newcastle disease virus-based COVID-19 vaccine (Patria) administered via the intramuscular or intranasal route: Interim results of a non-randomized open label phase I trial in Mexico. medRxiv[Preprint], (2022).
- 224.Nagy A, et al. Recombinant Newcastle disease virus expressing H9 HA protects chickens against heterologous avian influenza H9N2 virus challenge. Vaccine. 2016;34:2537–2545. doi: 10.1016/j.vaccine.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ma J, et al. Newcastle disease virus-based H5 influenza vaccine protects chickens from lethal challenge with a highly pathogenic H5N2 avian influenza virus. NPJ Vaccines. 2017;2:33. doi: 10.1038/s41541-017-0034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Liu Q, et al. Newcastle Disease Virus-Vectored H7 and H5 Live Vaccines Protect Chickens from Challenge with H7N9 or H5N1 Avian Influenza Viruses. J. Virol. 2015;89:7401–7408. doi: 10.1128/JVI.00031-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Martinez-Sobrido L, et al. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector. J. Virol. 2006;80:1130–1139. doi: 10.1128/JVI.80.3.1130-1139.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Grieves JL, et al. A viral-vectored RSV vaccine induces long-lived humoral immunity in cotton rats. Vaccine. 2018;36:3842–3852. doi: 10.1016/j.vaccine.2018.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.DiNapoli JM, et al. Respiratory tract immunization of non-human primates with a Newcastle disease virus-vectored vaccine candidate against Ebola virus elicits a neutralizing antibody response. Vaccine. 2010;29:17–25. doi: 10.1016/j.vaccine.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhao W, et al. Heterologous prime-boost regimens with HAdV-5 and NDV vectors elicit stronger immune responses to Ebola virus than homologous regimens in mice. Arch. Virol. 2021;166:3333–3341. doi: 10.1007/s00705-021-05234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sparrow E, et al. Global production capacity of seasonal and pandemic influenza vaccines in 2019. Vaccine. 2021;39:512–520. doi: 10.1016/j.vaccine.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Koonpaew S, et al. A Single-Cycle Influenza A Virus-Based SARS-CoV-2 Vaccine Elicits Potent Immune Responses in a Mouse Model. Vaccines (Basel) 2021;9:850. doi: 10.3390/vaccines9080850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chaparian, R. R. et al. Influenza viral particles harboring the SARS-CoV-2 spike RBD as a combination respiratory disease vaccine. bioRxiv[Preprint], 2021.2004.2030.441968, (2021).
- 234.Loes AN, et al. Attenuated Influenza Virions Expressing the SARS-CoV-2 Receptor-Binding Domain Induce Neutralizing Antibodies in Mice. Viruses. 2020;12:987. doi: 10.3390/v12090987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Chen J, et al. A live attenuated virus-based intranasal COVID-19 vaccine provides rapid, prolonged, and broad protection against SARS-CoV-2. Sci. Bull. (Beijing) 2022;67:1372–1387. doi: 10.1016/j.scib.2022.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Vesikari T, et al. A randomized, double-blind study of the safety, transmissibility and phenotypic and genotypic stability of cold-adapted influenza virus vaccine. Pediatr. Infect. Dis. J. 2006;25:590–595. doi: 10.1097/01.inf.0000220229.51531.47. [DOI] [PubMed] [Google Scholar]
- 237.Grohskopf LA, et al. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021-22 Influenza Season. MMWR Recomm. Rep. 2021;70:1–28. doi: 10.15585/mmwr.rr7005a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Treanor JJ, et al. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine. 1999;18:899–906. doi: 10.1016/S0264-410X(99)00334-5. [DOI] [PubMed] [Google Scholar]
- 239.Sun W, et al. Safety, Immunogenicity, and Protective Efficacy of an H5N1 Chimeric Cold-Adapted Attenuated Virus Vaccine in a Mouse Model. Viruses. 2021;13:2420. doi: 10.3390/v13122420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Park BR, et al. Broad cross protection by recombinant live attenuated influenza H3N2 seasonal virus expressing conserved M2 extracellular domain in a chimeric hemagglutinin. Sci. Rep. 2021;11:4151. doi: 10.1038/s41598-021-83704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sullivan NJ, et al. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408:605–609. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- 242.Hensley LE, et al. Demonstration of cross-protective vaccine immunity against an emerging pathogenic Ebolavirus Species. PLoS Pathog. 2010;6:e1000904. doi: 10.1371/journal.ppat.1000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sullivan NJ, et al. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat. Med. 2011;17:1128–1131. doi: 10.1038/nm.2447. [DOI] [PubMed] [Google Scholar]
- 244.Ledgerwood JE, et al. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine. 2010;29:304–313. doi: 10.1016/j.vaccine.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 245.Wu S, et al. An Adenovirus Vaccine Expressing Ebola Virus Variant Makona Glycoprotein Is Efficacious in Guinea Pigs and Nonhuman Primates. J. Infect. Dis. 2016;214:S326–S332. doi: 10.1093/infdis/jiw250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhu FC, et al. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet. 2015;385:2272–2279. doi: 10.1016/S0140-6736(15)60553-0. [DOI] [PubMed] [Google Scholar]
- 247.Lihua W, et al. Open-label phase I clinical trial of Ad5-EBOV in Africans in China. Hum. Vaccin Immunother. 2017;13:2078–2085. doi: 10.1080/21645515.2017.1342021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Li JX, et al. Immunity duration of a recombinant adenovirus type-5 vector-based Ebola vaccine and a homologous prime-boost immunisation in healthy adults in China: final report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Glob. Health. 2017;5:e324–e334. doi: 10.1016/S2214-109X(16)30367-9. [DOI] [PubMed] [Google Scholar]
- 249.Zhu F-C, et al. Safety and immunogenicity of a recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in Sierra Leone: a single-centre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2017;389:621–628. doi: 10.1016/S0140-6736(16)32617-4. [DOI] [PubMed] [Google Scholar]
- 250.Dolzhikova IV, et al. Safety and immunogenicity of GamEvac-Combi, a heterologous VSV- and Ad5-vectored Ebola vaccine: An open phase I/II trial in healthy adults in Russia. Hum. Vaccin Immunother. 2017;13:613–620. doi: 10.1080/21645515.2016.1238535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Geisbert TW, et al. Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against ebolavirus challenge. J. Virol. 2011;85:4222–4233. doi: 10.1128/JVI.02407-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Xiang Z, et al. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg. Infect. Dis. 2006;12:1596–1599. doi: 10.3201/eid1210.060078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ersching J, et al. Neutralizing antibodies to human and simian adenoviruses in humans and New-World monkeys. Virology. 2010;407:1–6. doi: 10.1016/j.virol.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 254.Stefania C, et al. Development of chimpanzee adenoviruses as vaccine vectors: challenges and successes emerging from clinical trials. Expert Rev. Vaccines. 2013;12:379–393. doi: 10.1586/erv.13.15. [DOI] [PubMed] [Google Scholar]
- 255.Xiang Z, et al. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J. Virol. 2002;76:2667–2675. doi: 10.1128/JVI.76.6.2667-2675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Quinn KM, et al. Comparative analysis of the magnitude, quality, phenotype, and protective capacity of simian immunodeficiency virus gag-specific CD8+ T cells following human-, simian-, and chimpanzee-derived recombinant adenoviral vector immunization. J. Immunol. 2013;190:2720–2735. doi: 10.4049/jimmunol.1202861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ledgerwood JE, et al. Chimpanzee Adenovirus Vector Ebola Vaccine. N. Engl. J. Med. 2017;376:928–938. doi: 10.1056/NEJMoa1410863. [DOI] [PubMed] [Google Scholar]
- 258.Stanley DA, et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat. Med. 2014;20:1126–1129. doi: 10.1038/nm.3702. [DOI] [PubMed] [Google Scholar]
- 259.Ewer K, et al. A Monovalent Chimpanzee Adenovirus Ebola Vaccine Boosted with MVA. N. Engl. J. Med. 2016;374:1635–1646. doi: 10.1056/NEJMoa1411627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Tapia MD, et al. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2016;16:31–42. doi: 10.1016/S1473-3099(15)00362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Santis OD, et al. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect. Dis. 2016;16:311–320. doi: 10.1016/S1473-3099(15)00486-7. [DOI] [PubMed] [Google Scholar]
- 262.Kennedy SB, et al. Phase 2 Placebo-Controlled Trial of Two Vaccines to Prevent Ebola in Liberia. N. Engl. J. Med. 2017;377:1438–1447. doi: 10.1056/NEJMoa1614067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Agua-Agum J, et al. Ebola virus disease among children in West Africa. N. Engl. J. Med. 2015;372:1274–1277. doi: 10.1056/NEJMc1415318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Geisbert TW, et al. Vector choice determines immunogenicity and potency of genetic vaccines against Angola Marburg virus in nonhuman primates. J. Virol. 2010;84:10386–10394. doi: 10.1128/JVI.00594-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Maruyama J, et al. Adenoviral vector-based vaccine is fully protective against lethal Lassa fever challenge in Hartley guinea pigs. Vaccine. 2019;37:6824–6831. doi: 10.1016/j.vaccine.2019.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zivcec M, et al. Nucleocapsid protein-based vaccine provides protection in mice against lethal Crimean-Congo hemorrhagic fever virus challenge. PLoS Negl. Trop. Dis. 2018;12:e0006628. doi: 10.1371/journal.pntd.0006628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Dicks MD, et al. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS ONE. 2012;7:e40385. doi: 10.1371/journal.pone.0040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Warimwe GM, et al. Immunogenicity and efficacy of a chimpanzee adenovirus-vectored Rift Valley fever vaccine in mice. Virol. J. 2013;10:349. doi: 10.1186/1743-422X-10-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Holman DH, et al. A complex adenovirus-vectored vaccine against Rift Valley fever virus protects mice against lethal infection in the presence of preexisting vector immunity. Clin. Vaccin. Immunol. 2009;16:1624–1632. doi: 10.1128/CVI.00182-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Warimwe GM, et al. Chimpanzee Adenovirus Vaccine Provides Multispecies Protection against Rift Valley Fever. Sci. Rep. 2016;6:20617. doi: 10.1038/srep20617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zhu F-C, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zhu F-C, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Mercado NB, et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;590:E25. doi: 10.1038/s41586-020-03100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Tukhvatulin AI, et al. An open, non-randomised, phase 1/2 trial on the safety, tolerability, and immunogenicity of single-dose vaccine “Sputnik Light” for prevention of coronavirus infection in healthy adults. Lancet Reg. Health Eur. 2021;11:100241. doi: 10.1016/j.lanepe.2021.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Lambe T, et al. ChAdOx1 nCoV-19 protection against SARS-CoV-2 in rhesus macaque and ferret challenge models. Commun. Biol. 2021;4:915. doi: 10.1038/s42003-021-02443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Graham SP, et al. Evaluation of the immunogenicity of prime-boost vaccination with the replication-deficient viral vectored COVID-19 vaccine candidate ChAdOx1 nCoV-19. NPJ Vaccines. 2020;5:69–69. doi: 10.1038/s41541-020-00221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Voysey M, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Jenkin D, et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. Med. 2020;27:279–288. doi: 10.1038/s41591-020-01179-4. [DOI] [PubMed] [Google Scholar]
- 279.McMahon WC, et al. T-cell responses induced by ChAdOx1 nCoV-19 (AZD1222) vaccine to wild-type severe acute respiratory syndrome coronavirus 2 among people with and without HIV in South Africa. AIDS. 2023;37:105–112. doi: 10.1097/QAD.0000000000003414. [DOI] [PubMed] [Google Scholar]
- 280.Monagle P, et al. Vaccine-induced immune thrombosis and thrombocytopenia syndrome following adenovirus-vectored severe acute respiratory syndrome coronavirus 2 vaccination: a novel hypothesis regarding mechanisms and implications for future vaccine development. Immunol. Cell Biol. 2021;99:1006–1010. doi: 10.1111/imcb.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Logunov DY, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Falivene J, et al. Improving the MVA vaccine potential by deleting the viral gene coding for the IL-18 binding protein. PLoS ONE. 2017;7:e32220. doi: 10.1371/journal.pone.0032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Davison AJ, Moss B. New vaccinia virus recombination plasmids incorporating a synthetic late promoter for high level expression of foreign proteins. Nucleic Acids Res. 1990;18:4285–4286. doi: 10.1093/nar/18.14.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.S CL, et al. Novel Modified Vaccinia Virus Ankara Vector Expressing Anti-apoptotic Gene B13R Delays Apoptosis and Enhances Humoral Responses. J. Virol. 2019;93:e01648–01618. doi: 10.1128/JVI.01648-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Milligan ID, et al. Safety and Immunogenicity of Novel Adenovirus Type 26- and Modified Vaccinia Ankara-Vectored Ebola Vaccines: A Randomized Clinical Trial. JAMA. 2016;315:1610–1623. doi: 10.1001/jama.2016.4218. [DOI] [PubMed] [Google Scholar]
- 286.Mutua G, et al. Safety and Immunogenicity of a 2-Dose Heterologous Vaccine Regimen With Ad26.ZEBOV and MVA-BN-Filo Ebola Vaccines: 12-Month Data From a Phase 1 Randomized Clinical Trial in Nairobi, Kenya. J. Infect. Dis. 2019;220:57–67. doi: 10.1093/infdis/jiz071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Rahim MN, et al. Complete protection of the BALB/c and C57BL/6J mice against Ebola and Marburg virus lethal challenges by pan-filovirus T-cell epigraph vaccine. PLoS Pathog. 2019;15:e1007564. doi: 10.1371/journal.ppat.1007564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Guillaume V, et al. Nipah virus: vaccination and passive protection studies in a hamster model. J. Virol. 2004;78:834–840. doi: 10.1128/JVI.78.2.834-840.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Weingartl HM, et al. Recombinant nipah virus vaccines protect pigs against challenge. J. Virol. 2006;80:7929–7938. doi: 10.1128/JVI.00263-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Robb ML, et al. Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: a post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect. Dis. 2012;12:531–537. doi: 10.1016/S1473-3099(12)70088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Rerks-Ngarm S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 292.Pitisuttithum P, et al. Late boosting of the RV144 regimen with AIDSVAX B/E and ALVAC-HIV in HIV-uninfected Thai volunteers: a double-blind, randomised controlled trial. Lancet HIV. 2020;7:e238–e248. doi: 10.1016/S2352-3018(19)30406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Gray GE, et al. Vaccine Efficacy of ALVAC-HIV and Bivalent Subtype C gp120-MF59 in Adults. N. Engl. J. Med. 2021;384:1089–1100. doi: 10.1056/NEJMoa2031499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Frietze KM, Peabody DS, Chackerian B. Engineering virus-like particles as vaccine platforms. Curr. Opin. Virol. 2016;18:44–49. doi: 10.1016/j.coviro.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Naskalska A, Pyrć K. Virus Like Particles as Immunogens and Universal Nanocarriers. Pol. J. Microbiol. 2015;64:3–13. doi: 10.33073/pjm-2015-001. [DOI] [PubMed] [Google Scholar]
- 296.Rynda-Apple A, Patterson DP, Douglas T. Virus-like particles as antigenic nanomaterials for inducing protective immune responses in the lung. Nanomed. (Lond.) 2014;9:1857–1868. doi: 10.2217/nnm.14.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Warfield KL, et al. Role of natural killer cells in innate protection against lethal ebola virus infection. J. Exp. Med. 2004;200:169–179. doi: 10.1084/jem.20032141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Warfield KL, et al. Ebola virus-like particles protect from lethal Ebola virus infection. Proc. Natl Acad. Sci. USA. 2003;100:15889–15894. doi: 10.1073/pnas.2237038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Noda T, et al. Ebola virus VP40 drives the formation of virus-like filamentous particles along with GP. J. Virol. 2002;76:4855–4865. doi: 10.1128/JVI.76.10.4855-4865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Jasenosky LD, Neumann G, Lukashevich I, Kawaoka Y. Ebola virus VP40-induced particle formation and association with the lipid bilayer. J. Virol. 2001;75:5205–5214. doi: 10.1128/JVI.75.11.5205-5214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Schweneker M, et al. Recombinant Modified Vaccinia Virus Ankara Generating Ebola Virus-Like Particles. J. Virol. 2017;91:e00343–00317. doi: 10.1128/JVI.00343-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Lazaro-Frias A, et al. Distinct Immunogenicity and Efficacy of Poxvirus-Based Vaccine Candidates against Ebola Virus Expressing GP and VP40 Proteins. J. Virol. 2018;92:e00363–00318. doi: 10.1128/JVI.00363-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Malherbe DC, et al. A single immunization with a modified vaccinia Ankara vectored vaccine producing Sudan virus-like particles protects from lethal infection. NPJ Vaccines. 2022;7:83. doi: 10.1038/s41541-022-00512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Warfield KL, et al. Homologous and heterologous protection of nonhuman primates by Ebola and Sudan virus-like particles. PLoS ONE. 2015;10:e0118881. doi: 10.1371/journal.pone.0118881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Singh K, et al. A Bivalent, Spherical Virus-Like Particle Vaccine Enhances Breadth of Immune Responses against Pathogenic Ebola Viruses in Rhesus Macaques. J. Virol. 2020;94:e01884–01819. doi: 10.1128/JVI.01884-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Salvato MS, et al. A Single Dose of Modified Vaccinia Ankara Expressing Lassa Virus-like Particles Protects Mice from Lethal Intra-cerebral Virus Challenge. Pathogens. 2019;8:133. doi: 10.3390/pathogens8030133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Goicochea MA, et al. Evaluation of Lassa virus vaccine immunogenicity in a CBA/J-ML29 mouse model. Vaccine. 2012;30:1445–1452. doi: 10.1016/j.vaccine.2011.12.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Patricia P, et al. A Vaccine Based on a Modified Vaccinia Virus Ankara Vector Expressing Zika Virus Structural Proteins Controls Zika Virus Replication in Mice. Sci. Rep. 2018;8:17385. doi: 10.1038/s41598-018-35724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Langenmayer MC, et al. Distribution and absence of generalized lesions in mice following single dose intramuscular inoculation of the vaccine candidate MVA-MERS-S. Biologicals. 2018;54:58–62. doi: 10.1016/j.biologicals.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Song F, et al. Middle East respiratory syndrome coronavirus spike protein delivered by modified vaccinia virus Ankara efficiently induces virus-neutralizing antibodies. J. Virol. 2013;87:11950–11954. doi: 10.1128/JVI.01672-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Volz A, et al. Protective Efficacy of Recombinant Modified Vaccinia Virus Ankara Delivering Middle East Respiratory Syndrome Coronavirus Spike Glycoprotein. J. Virol. 2015;89:8651–8656. doi: 10.1128/JVI.00614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Haagmans BL, et al. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science. 2016;351:77–81. doi: 10.1126/science.aad1283. [DOI] [PubMed] [Google Scholar]
- 313.Folegatti PM, et al. Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect. Dis. 2020;20:816–826. doi: 10.1016/S1473-3099(20)30160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Weskamm LM, et al. Persistence of MERS-CoV-spike-specific B cells and antibodies after late third immunization with the MVA-MERS-S vaccine. Cell Rep. Med. 2022;3:100685. doi: 10.1016/j.xcrm.2022.100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Boudewijns R, et al. MVA-CoV2-S Vaccine Candidate Neutralizes Distinct Variants of Concern and Protects Against SARS-CoV-2 Infection in Hamsters. Front. Immunol. 2022;13:845969. doi: 10.3389/fimmu.2022.845969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Mooij P, et al. Poxvirus MVA Expressing SARS-CoV-2 S Protein Induces Robust Immunity and Protects Rhesus Macaques From SARS-CoV-2. Front. Immunol. 2022;13:845887. doi: 10.3389/fimmu.2022.845887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.García-Arriaza J, et al. COVID-19 vaccine candidates based on modified vaccinia virus Ankara expressing the SARS-CoV-2 spike induce robust T- and B-cell immune responses and full efficacy in mice. J. Virol. 2021;95:e02260–02220. doi: 10.1128/JVI.02260-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Pérez P, et al. A Single Dose of an MVA Vaccine Expressing a Prefusion-Stabilized SARS-CoV-2 Spike Protein Neutralizes Variants of Concern and Protects Mice From a Lethal SARS-CoV-2 Infection. Front. Immunol. 2021;12:824728. doi: 10.3389/fimmu.2021.824728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Bosnjak B, et al. Intranasal Delivery of MVA Vector Vaccine Induces Effective Pulmonary Immunity Against SARS-CoV-2 in Rodents. Front. Immunol. 2021;12:772240. doi: 10.3389/fimmu.2021.772240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Routhu NK, et al. A modified vaccinia Ankara vector-based vaccine protects macaques from SARS-CoV-2 infection, immune pathology, and dysfunction in the lungs. Immunity. 2021;54:542–556.e549. doi: 10.1016/j.immuni.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Powers AD, et al. Lentiviral Vector Production from a Stable Packaging Cell Line Using a Packed Bed Bioreactor. Mol. Ther. Methods Clin. Dev. 2020;19:1–13. doi: 10.1016/j.omtm.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Ku MW, et al. Intranasal vaccination with a lentiviral vector protects against SARS-CoV-2 in preclinical animal models. Cell Host Microbe. 2021;29:236–249.e236. doi: 10.1016/j.chom.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Ku MW, et al. A Single Dose of NILV-Based Vaccine Provides Rapid and Durable Protection against Zika Virus. Mol. Ther. 2020;28:1772–1782. doi: 10.1016/j.ymthe.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Blasi M, et al. IDLV-HIV-1 Env vaccination in non-human primates induces affinity maturation of antigen-specific memory B cells. Commun. Biol. 2018;1:134. doi: 10.1038/s42003-018-0131-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Lin YY, et al. Skeletal Muscle Is an Antigen Reservoir in Integrase-Defective Lentiviral Vector-Induced Long-Term Immunity. Mol. Ther. Methods Clin. Dev. 2020;17:532–544. doi: 10.1016/j.omtm.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Gaspar HB, et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet. 2004;364:2181–2187. doi: 10.1016/S0140-6736(04)17590-9. [DOI] [PubMed] [Google Scholar]
- 327.Blasi M, et al. Therapeutic vaccination with IDLV-SIV-Gag results in durable viremia control in chronically SHIV-infected macaques. NPJ Vaccines. 2020;5:36. doi: 10.1038/s41541-020-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Blasi M, et al. Immunogenicity, safety, and efficacy of sequential immunizations with an SIV-based IDLV expressing CH505 Envs. NPJ Vaccines. 2020;5:107. doi: 10.1038/s41541-020-00252-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Li H, et al. Enhanced protective immunity against SARS-CoV-2 elicited by a VSV vector expressing a chimeric spike protein. Signal Transduct. Target Ther. 2021;6:389. doi: 10.1038/s41392-021-00797-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Wang S, et al. Characterization of Immune Response Diversity in Rodents Vaccinated with a Vesicular Stomatitis Virus Vectored COVID-19 Vaccine. Viruses. 2022;14:1127. doi: 10.3390/v14061127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Espeseth AS, et al. Preclinical immunogenicity and efficacy of a candidate COVID-19 vaccine based on a vesicular stomatitis virus-SARS-CoV-2 chimera. EBioMedicine. 2022;82:104203. doi: 10.1016/j.ebiom.2022.104203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Robbins JA, et al. Safety and immunogenicity of intramuscular, single-dose V590 (rVSV-SARS-CoV-2 Vaccine) in healthy adults: Results from a phase 1 randomised, double-blind, placebo-controlled, dose-ranging trial. eBioMedicine. 2022;82:104138. doi: 10.1016/j.ebiom.2022.104138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Li YW, Zhou W, Yang L, You R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharm. Res. 2020;157:104833. doi: 10.1016/j.phrs.2020.104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Hou YJ, et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell. 2020;182:429–446. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Shuai L, et al. Genetically modified rabies virus ERA strain is safe and induces long-lasting protective immune response in dogs after oral vaccination. Antivir. Res. 2015;121:9–15. doi: 10.1016/j.antiviral.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 336.Shuai L, et al. Genetically modified rabies virus-vectored Ebola virus disease vaccines are safe and induce efficacious immune responses in mice and dogs. Antivir. Res. 2017;146:36–44. doi: 10.1016/j.antiviral.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 337.Dahlke C, et al. Dose-dependent T-cell Dynamics and Cytokine Cascade Following rVSV-ZEBOV Immunization. EBioMedicine. 2017;19:107–118. doi: 10.1016/j.ebiom.2017.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Shuai L, et al. Immune responses in mice and pigs after oral vaccination with rabies virus vectored Nipah disease vaccines. Vet. Microbiol. 2020;241:108549. doi: 10.1016/j.vetmic.2019.108549. [DOI] [PubMed] [Google Scholar]
- 339.Chen Z, et al. A novel rabies vaccine based on a recombinant parainfluenza virus 5 expressing rabies virus glycoprotein. J. Virol. 2013;87:2986–2993. doi: 10.1128/JVI.02886-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Rafie K, et al. The structure of enteric human adenovirus 41-A leading cause of diarrhea in children. Sci. Adv. 2021;7:eabe0974. doi: 10.1126/sciadv.abe0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Torres JM, et al. Tropism of human adenovirus type 5-based vectors in swine and their ability to protect against transmissible gastroenteritis coronavirus. J. Virol. 1996;70:3770–3780. doi: 10.1128/jvi.70.6.3770-3780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Richardson JS, Pillet S, Bello AJ, Kobinger GP. Airway delivery of an adenovirus-based Ebola virus vaccine bypasses existing immunity to homologous adenovirus in nonhuman primates. J. Virol. 2013;87:3668–3677. doi: 10.1128/JVI.02864-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Patel A, et al. Mucosal delivery of adenovirus-based vaccine protects against Ebola virus infection in mice. J. Infect. Dis. 2007;196:S413–S420. doi: 10.1086/520603. [DOI] [PubMed] [Google Scholar]
- 344.Choi JH, et al. A single sublingual dose of an adenovirus-based vaccine protects against lethal Ebola challenge in mice and guinea pigs. Mol. Pharm. 2012;9:156–167. doi: 10.1021/mp200392g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Wong G, et al. Intranasal immunization with an adenovirus vaccine protects guinea pigs from Ebola virus transmission by infected animals. Antivir. Res. 2015;116:17–19. doi: 10.1016/j.antiviral.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 346.Langel SN, et al. Adenovirus type 5 SARS-CoV-2 vaccines delivered orally or intranasally reduced disease severity and transmission in a hamster model. Sci. Transl. Med. 2022;14:eabn6868. doi: 10.1126/scitranslmed.abn6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Li JX, et al. Safety and immunogenicity of heterologous boost immunisation with an orally administered aerosolised Ad5-nCoV after two-dose priming with an inactivated SARS-CoV-2 vaccine in Chinese adults: a randomised, open-label, single-centre trial. Lancet Respir. Med. 2022;10:739–748. doi: 10.1016/S2213-2600(22)00087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Li J, et al. Heterologous AD5-nCOV plus CoronaVac versus homologous CoronaVac vaccination: a randomized phase 4 trial. Nat. Med. 2022;28:401–409. doi: 10.1038/s41591-021-01677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Jin L, et al. Antibody persistence and safety after heterologous boosting with orally aerosolised Ad5-nCoV in individuals primed with two-dose CoronaVac previously: 12-month analyses of a randomized controlled trial. Emerg. Microbes Infect. 2023;12:2155251. doi: 10.1080/22221751.2022.2155251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Madhavan M, et al. Tolerability and immunogenicity of an intranasally-administered adenovirus-vectored COVID-19 vaccine: An open-label partially-randomised ascending dose phase I trial. EBioMedicine. 2022;85:104298. doi: 10.1016/j.ebiom.2022.104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Johnson, S. et al. SARS-CoV-2 oral tablet vaccination induces neutralizing mucosal IgA in a phase 1 open label trial. medRxiv[Preprint], 2022.2007.2016.22277601, (2022).
- 352.Mao T, et al. Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science. 2022;378:eabo2523. doi: 10.1126/science.abo2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Munster VJ, et al. Protective efficacy of a novel simian adenovirus vaccine against lethal MERS-CoV challenge in a transgenic human DPP4 mouse model. NPJ Vaccines. 2017;2:28. doi: 10.1038/s41541-017-0029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 355.Vergadi E, et al. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J. Immunol. 2017;198:1006–1014. doi: 10.4049/jimmunol.1601515. [DOI] [PubMed] [Google Scholar]
- 356.Connor JH, Naczki C, Koumenis C, Lyles DS. Replication and cytopathic effect of oncolytic vesicular stomatitis virus in hypoxic tumor cells in vitro and in vivo. J. Virol. 2004;78:8960–8970. doi: 10.1128/JVI.78.17.8960-8970.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Elvington M, Liszewski MK, Atkinson JP. CD46 and Oncologic Interactions: Friendly Fire against. Cancer Antibodies (Basel) 2020;9:59. doi: 10.3390/antib9040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Gros A, et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat. Med. 2016;22:433–438. doi: 10.1038/nm.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Zhong Z, Yu J, Virshup DM, Madan B. Wnts and the hallmarks of cancer. Cancer Metastasis Rev. 2020;39:625–645. doi: 10.1007/s10555-020-09887-6. [DOI] [PubMed] [Google Scholar]
- 360.Johnson J, et al. Targeting the RB-E2F pathway in breast cancer. Oncogene. 2016;35:4829–4835. doi: 10.1038/onc.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Raftery N, Stevenson NJ. Advances in anti-viral immune defence: revealing the importance of the IFN JAK/STAT pathway. Cell Mol. Life Sci. 2017;74:2525–2535. doi: 10.1007/s00018-017-2520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Kesterson SP, et al. Effect of the Viral Hemorrhagic Septicemia Virus Nonvirion Protein on Translation via PERK-eIF2α Pathway. Viruses. 2020;12:499. doi: 10.3390/v12050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Kurokawa C, et al. Constitutive Interferon Pathway Activation in Tumors as an Efficacy Determinant Following Oncolytic Virotherapy. J. Natl Cancer Inst. 2018;110:1123–1132. doi: 10.1093/jnci/djy033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Zhang J, et al. A novel oncolytic adenovirus targeting Wnt signaling effectively inhibits cancer-stem like cell growth via metastasis, apoptosis and autophagy in HCC models. Biochem. Biophys. Res. Commun. 2017;491:469–477. doi: 10.1016/j.bbrc.2017.07.041. [DOI] [PubMed] [Google Scholar]
- 365.Wang Y, et al. An oncolytic adenovirus delivering TSLC1 inhibits Wnt signaling pathway and tumor growth in SMMC-7721 xenograft mice model. Acta Biochim. Biophys. Sin. (Shanghai) 2021;53:766–774. doi: 10.1093/abbs/gmab048. [DOI] [PubMed] [Google Scholar]
- 366.Heise C, et al. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat. Med. 2000;6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 367.Hale BG, Randall RE, Ortín J, Jackson D. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 2008;89:2359–2376. doi: 10.1099/vir.0.2008/004606-0. [DOI] [PubMed] [Google Scholar]
- 368.Bergmann M, et al. A genetically engineered influenza A virus with ras-dependent oncolytic properties. Cancer Res. 2001;61:8188–8193. [PubMed] [Google Scholar]
- 369.Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–68. doi: 10.1016/j.canlet.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 370.Joseph JP, Harishankar MK, Pillai AA, Devi A. Hypoxia induced EMT: A review on the mechanism of tumor progression and metastasis in OSCC. Oral. Oncol. 2018;80:23–32. doi: 10.1016/j.oraloncology.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 371.Post DE, Van Meir EG. A novel hypoxia-inducible factor (HIF) activated oncolytic adenovirus for cancer therapy. Oncogene. 2003;22:2065–2072. doi: 10.1038/sj.onc.1206464. [DOI] [PubMed] [Google Scholar]
- 372.Breitbach CJ, et al. Targeting tumor vasculature with an oncolytic virus. Mol. Ther. 2011;19:886–894. doi: 10.1038/mt.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Zhang Z, et al. Suppression of tumor growth by oncolytic adenovirus-mediated delivery of an antiangiogenic gene, soluble Flt-1. Mol. Ther. 2005;11:553–562. doi: 10.1016/j.ymthe.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 374.Persaud L, et al. IL-24 Promotes Apoptosis through cAMP-Dependent PKA Pathways in Human Breast Cancer Cells. Int J. Mol. Sci. 2018;19:3561. doi: 10.3390/ijms19113561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Chai L, et al. A novel conditionally replicating adenoviral vector with dual expression of IL-24 and arresten inserted in E1 and the region between E4 and fiber for improved melanoma therapy. Cancer Gene Ther. 2012;19:247–254. doi: 10.1038/cgt.2011.84. [DOI] [PubMed] [Google Scholar]
- 376.Springfeld C, et al. Oncolytic efficacy and enhanced safety of measles virus activated by tumor-secreted matrix metalloproteinases. Cancer Res. 2006;66:7694–7700. doi: 10.1158/0008-5472.CAN-06-0538. [DOI] [PubMed] [Google Scholar]
- 377.Mühlebach MD, et al. Liver cancer protease activity profiles support therapeutic options with matrix metalloproteinase-activatable oncolytic measles virus. Cancer Res. 2010;70:7620–7629. doi: 10.1158/0008-5472.CAN-09-4650. [DOI] [PubMed] [Google Scholar]
- 378.Sasso E, et al. New viral vectors for infectious diseases and cancer. Semin. Immunol. 2020;50:101430. doi: 10.1016/j.smim.2020.101430. [DOI] [PubMed] [Google Scholar]
- 379.Ludgate CM. Optimizing cancer treatments to induce an acute immune response: radiation Abscopal effects, PAMPs, and DAMPs. Clin. Cancer Res. 2012;18:4522–4525. doi: 10.1158/1078-0432.CCR-12-1175. [DOI] [PubMed] [Google Scholar]
- 380.Zheng M, Huang J, Tong A, Yang H. Oncolytic Viruses for Cancer Therapy: Barriers and Recent Advances. Mol. Ther. Oncolytics. 2019;15:234–247. doi: 10.1016/j.omto.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Ge Y, et al. Oncolytic vaccinia virus delivering tethered IL-12 enhances antitumor effects with improved safety. J. Immunother. Cancer. 2020;8:e000710. doi: 10.1136/jitc-2020-000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Guo ZS, et al. Vaccinia virus-mediated cancer immunotherapy: cancer vaccines and oncolytics. J. Immunother. Cancer. 2019;7:6. doi: 10.1186/s40425-018-0495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Li J, et al. Expression of CCL19 from oncolytic vaccinia enhances immunotherapeutic potential while maintaining oncolytic activity. Neoplasia. 2012;14:1115–1121. doi: 10.1593/neo.121272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Pesonen S, et al. Oncolytic immunotherapy of advanced solid tumors with a CD40L-expressing replicating adenovirus: assessment of safety and immunologic responses in patients. Cancer Res. 2012;72:1621–1631. doi: 10.1158/0008-5472.CAN-11-3001. [DOI] [PubMed] [Google Scholar]
- 385.Ylösmäki E, et al. Characterization of a novel OX40 ligand and CD40 ligand-expressing oncolytic adenovirus used in the PeptiCRAd cancer vaccine platform. Mol. Ther. Oncolytics. 2021;20:459–469. doi: 10.1016/j.omto.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Allen C, et al. Interleukin-13 Displaying Retargeted Oncolytic Measles Virus Strains Have Significant Activity Against Gliomas With Improved Specificity. Mol. Ther. 2008;16:1556–1564. doi: 10.1038/mt.2008.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Wang G, et al. An engineered oncolytic virus expressing PD-L1 inhibitors activates tumor neoantigen-specific T cell responses. Nat. Commun. 2020;11:1395. doi: 10.1038/s41467-020-15229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Hou W, Sampath P, Rojas JJ, Thorne SH. Oncolytic Virus-Mediated Targeting of PGE2 in the Tumor Alters the Immune Status and Sensitizes Established and Resistant Tumors to Immunotherapy. Cancer Cell. 2016;30:108–119. doi: 10.1016/j.ccell.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Rivadeneira DB, et al. Oncolytic Viruses Engineered to Enforce Leptin Expression Reprogram Tumor-Infiltrating T Cell Metabolism and Promote Tumor Clearance. Immunity. 2019;51:548–560.e544. doi: 10.1016/j.immuni.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Kroemer G, Zitvogel L. Leptin-Producing Oncolytic Virus Makes Tumor-Infiltrating T Cells Fit, Not Fat. Immunity. 2019;51:423–425. doi: 10.1016/j.immuni.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 391.Bouvet M, et al. Extended treatment with MY-NEOVAX, personalized neoantigen-enhanced oncolytic viruses, for two end-stage cancer patients. Oxf. Med. Case Rep. 2019;2019:461–463. doi: 10.1093/omcr/omz105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.D’Alise AM, et al. Adenoviral vaccine targeting multiple neoantigens as strategy to eradicate large tumors combined with checkpoint blockade. Nat. Commun. 2019;10:2688. doi: 10.1038/s41467-019-10594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Leoni G, et al. A Genetic Vaccine Encoding Shared Cancer Neoantigens to Treat Tumors with Microsatellite Instability. Cancer Res. 2020;80:3972–3982. doi: 10.1158/0008-5472.CAN-20-1072. [DOI] [PubMed] [Google Scholar]
- 394.De Lucia M, et al. Retargeted and Multi-cytokine-Armed Herpes Virus Is a Potent Cancer Endovaccine for Local and Systemic Anti-tumor Treatment. Mol. Ther. Oncolytics. 2020;19:253–264. doi: 10.1016/j.omto.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Peng KW, et al. Oncolytic measles viruses displaying a single-chain antibody against CD38, a myeloma cell marker. Blood. 2003;101:2557–2562. doi: 10.1182/blood-2002-07-2195. [DOI] [PubMed] [Google Scholar]
- 396.Bucheit AD, et al. An oncolytic measles virus engineered to enter cells through the CD20 antigen. Mol. Ther. 2003;7:62–72. doi: 10.1016/S1525-0016(02)00033-3. [DOI] [PubMed] [Google Scholar]
- 397.van Erp EA, Kaliberova LN, Kaliberov SA, Curiel DT. Retargeted oncolytic adenovirus displaying a single variable domain of camelid heavy-chain-only antibody in a fiber protein. Mol. Ther. Oncolytics. 2015;2:15001. doi: 10.1038/mto.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Grote D, Cattaneo R, Fielding AK. Neutrophils contribute to the measles virus-induced antitumor effect: enhancement by granulocyte macrophage colony-stimulating factor expression. Cancer Res. 2003;63:6463–6468. [PubMed] [Google Scholar]
- 399.Peng KW, et al. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002;62:4656–4662. [PubMed] [Google Scholar]
- 400.Phuong LK, et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63:2462–2469. [PubMed] [Google Scholar]
- 401.Anderson BD, Nakamura T, Russell SJ, Peng KW. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64:4919–4926. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- 402.Floerchinger A, et al. A vector-encoded bispecific killer engager to harness virus-activated NK cells as anti-tumor effectors. Cell Death Dis. 2023;14:104. doi: 10.1038/s41419-023-05624-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Tan DQ, et al. Macrophage response to oncolytic paramyxoviruses potentiates virus-mediated tumor cell killing. Eur. J. Immunol. 2016;46:919–928. doi: 10.1002/eji.201545915. [DOI] [PubMed] [Google Scholar]
- 404.Dey A, et al. The Role of Neutrophils in Measles Virus-mediated Oncolysis Differs Between B-cell Malignancies and Is Not Always Enhanced by GCSF. Mol. Ther. 2016;24:184–192. doi: 10.1038/mt.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Wagner S, et al. Combined treatment of pediatric high-grade glioma with the oncolytic viral strain MTH-68/H and oral valproic acid. Apmis. 2006;114:731–743. doi: 10.1111/j.1600-0463.2006.apm_516.x. [DOI] [PubMed] [Google Scholar]
- 406.Freeman AI, et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol. Ther. 2006;13:221–228. doi: 10.1016/j.ymthe.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 407.Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc. Natl Acad. Sci. USA. 2001;98:6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Andtbacka RH, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 409.Postow MA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Hu JC, et al. A novel HSV-1 virus, JS1/34.5-/47-, purges contaminating breast cancer cells from bone marrow. Clin. Cancer Res. 2006;12:6853–6862. doi: 10.1158/1078-0432.CCR-06-1228. [DOI] [PubMed] [Google Scholar]
- 411.Bommareddy PK, et al. MEK inhibition enhances oncolytic virus immunotherapy through increased tumor cell killing and T cell activation. Sci. Transl. Med. 2018;10:eaau0417. doi: 10.1126/scitranslmed.aau0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Hu JC, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin. Cancer Res. 2006;12:6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 413.Senzer NN, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J. Clin. Oncol. 2009;27:5763–5771. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- 414.Shalhout SZ, Miller DM, Emerick KS, Kaufman HL. Therapy with oncolytic viruses: progress and challenges. Nat. Rev. Clin. Oncol. 2023;20:160–177. doi: 10.1038/s41571-022-00719-w. [DOI] [PubMed] [Google Scholar]
- 415.Mineta T, et al. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat. Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 416.Liu BL, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10:292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 417.Todo T, et al. Systemic antitumor immunity in experimental brain tumor therapy using a multimutated, replication-competent herpes simplex virus. Hum. Gene Ther. 1999;10:2741–2755. doi: 10.1089/10430349950016483. [DOI] [PubMed] [Google Scholar]
- 418.Markert JM, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 419.Markert JM, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol. Ther. 2009;17:199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Markert JM, et al. A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol. Ther. 2014;22:1048–1055. doi: 10.1038/mt.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Friedman GK, et al. Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N. Engl. J. Med. 2021;384:1613–1622. doi: 10.1056/NEJMoa2024947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Ma W, He H, Wang H. Oncolytic herpes simplex virus and immunotherapy. BMC Immunol. 2018;19:40. doi: 10.1186/s12865-018-0281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Carson J, Haddad D, Bressman M, Fong Y. Oncolytic herpes simplex virus 1 (HSV-1) vectors: increasing treatment efficacy and range through strategic virus design. Drugs Future. 2010;35:183–195. doi: 10.1358/dof.2010.35.3.1470166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Todo T, et al. A phase I/II study of triple-mutated oncolytic herpes virus G47∆ in patients with progressive glioblastoma. Nat. Commun. 2022;13:4119. doi: 10.1038/s41467-022-31262-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Todo T, et al. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: a phase 2 trial. Nat. Med. 2022;28:1630–1639. doi: 10.1038/s41591-022-01897-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Xia ZJ, et al. [Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus] Ai Zheng. 2004;23:1666–1670. [PubMed] [Google Scholar]
- 427.Lang FF, et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018;36:1419–1427. doi: 10.1200/JCO.2017.75.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Gállego Pérez-Larraya J, et al. Oncolytic DNX-2401 Virus for Pediatric Diffuse Intrinsic Pontine Glioma. N. Engl. J. Med. 2022;386:2471–2481. doi: 10.1056/NEJMoa2202028. [DOI] [PubMed] [Google Scholar]
- 429.Boorjian SA, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2021;22:107–117. doi: 10.1016/S1470-2045(20)30540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Alberts P, et al. The advent of oncolytic virotherapy in oncology: The Rigvir® story. Eur. J. Pharm. 2018;837:117–126. doi: 10.1016/j.ejphar.2018.08.042. [DOI] [PubMed] [Google Scholar]
- 431.Ylösmäki E, Cerullo V. Design and application of oncolytic viruses for cancer immunotherapy. Curr. Opin. Biotechnol. 2020;65:25–36. doi: 10.1016/j.copbio.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 432.Lal G, Rajala MS. Combination of Oncolytic Measles Virus Armed With BNiP3, a Pro-apoptotic Gene and Paclitaxel Induces Breast Cancer Cell Death. Front. Oncol. 2018;8:676. doi: 10.3389/fonc.2018.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Ribas A, et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell. 2017;170:1109–1119.e1110. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Zhang QN, et al. Recombinant human adenovirus type 5 (Oncorine) reverses resistance to immune checkpoint inhibitor in a patient with recurrent non-small cell lung cancer: A case report. Thorac. Cancer. 2021;12:1617–1619. doi: 10.1111/1759-7714.13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Coughlan L, Kremer EJ, Shayakhmetov DM. Adenovirus-based vaccines-a platform for pandemic preparedness against emerging viral pathogens. Mol. Ther. 2022;30:1822–1849. doi: 10.1016/j.ymthe.2022.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Mettelman RC, Allen EK, Thomas PG. Mucosal immune responses to infection and vaccination in the respiratory tract. Immunity. 2022;55:749–780. doi: 10.1016/j.immuni.2022.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Thippeshappa R, et al. Oral Immunization with Recombinant Vaccinia Virus Prime and Intramuscular Protein Boost Provides Protection against Intrarectal Simian-Human Immunodeficiency Virus Challenge in Macaques. Clin. Vaccin. Immunol. 2015;23:204–212. doi: 10.1128/CVI.00597-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.van Kempen MJ, Rijkers GT, Van Cauwenberge PB. The immune response in adenoids and tonsils. Int. Arch. Allergy Immunol. 2000;122:8–19. doi: 10.1159/000024354. [DOI] [PubMed] [Google Scholar]
- 439.te Kamp V, et al. Responsiveness of various reservoir species to oral rabies vaccination correlates with differences in vaccine uptake of mucosa associated lymphoid tissues. Sci. Rep. 2020;10:2919. doi: 10.1038/s41598-020-59719-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.van den Pol AN, et al. Chikungunya, Influenza, Nipah, and Semliki Forest Chimeric Viruses with Vesicular Stomatitis Virus: Actions in the Brain. J. Virol. 2017;91:e02154–02116. doi: 10.1128/JVI.02154-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Li J, Wang JT, Whelan SP. A unique strategy for mRNA cap methylation used by vesicular stomatitis virus. Proc. Natl Acad. Sci. USA. 2006;103:8493–8498. doi: 10.1073/pnas.0509821103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Ma Y, et al. mRNA cap methylation influences pathogenesis of vesicular stomatitis virus in vivo. J. Virol. 2014;88:2913–2926. doi: 10.1128/JVI.03420-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Daffis S, et al. 2’-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature. 2010;468:452–456. doi: 10.1038/nature09489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Li J, Fontaine-Rodriguez EC, Whelan SP. Amino acid residues within conserved domain VI of the vesicular stomatitis virus large polymerase protein essential for mRNA cap methyltransferase activity. J. Virol. 2005;79:13373–13384. doi: 10.1128/JVI.79.21.13373-13384.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Kim GN, et al. Creation of matrix protein gene variants of two serotypes of vesicular stomatitis virus as prime-boost vaccine vectors. J. Virol. 2015;89:6338–6351. doi: 10.1128/JVI.00222-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Shoji Y, et al. Generation and characterization of P gene-deficient rabies virus. Virology. 2004;318:295–305. doi: 10.1016/j.virol.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 447.Morimoto K, Shoji Y, Inoue S. Characterization of P gene-deficient rabies virus: propagation, pathogenicity and antigenicity. Virus Res. 2005;111:61–67. doi: 10.1016/j.virusres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 448.Cenna J, et al. Immune modulating effect by a phosphoprotein-deleted rabies virus vaccine vector expressing two copies of the rabies virus glycoprotein gene. Vaccine. 2008;26:6405–6414. doi: 10.1016/j.vaccine.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Naoto I, et al. Characterization of M gene-deficient rabies virus with advantages of effective immunization and safety as a vaccine strain. Microbiol. Immunol. 2005;49:971–979. doi: 10.1111/j.1348-0421.2005.tb03692.x. [DOI] [PubMed] [Google Scholar]
- 450.Cenna J, et al. Replication-deficient rabies virus-based vaccines are safe and immunogenic in mice and nonhuman primates. J. Infect. Dis. 2009;200:1251–1260. doi: 10.1086/605949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Luo J, et al. A recombinant rabies virus carrying GFP between N and P affects viral transcription in vitro. Virus Genes. 2016;52:379–387. doi: 10.1007/s11262-016-1313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Huang Y, et al. Development of a reverse genetics system for a human rabies virus vaccine strain employed in China. Virus Res. 2010;149:28–35. doi: 10.1016/j.virusres.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 453.Wu X, Rupprecht CE. Glycoprotein gene relocation in rabies virus. Virus Res. 2008;131:95–99. doi: 10.1016/j.virusres.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 454.Mebatsion T, Konig M, Conzelmann KK. Budding of rabies virus particles in the absence of the spike glycoprotein. Cell. 1996;84:941–951. doi: 10.1016/S0092-8674(00)81072-7. [DOI] [PubMed] [Google Scholar]
- 455.Vos A, et al. An update on safety studies of SAD B19 rabies virus vaccine in target and non-target species. Epidemiol. Infect. 1999;123:165–175. doi: 10.1017/S0950268899002666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.McGettigan JP, et al. Second-generation rabies virus-based vaccine vectors expressing human immunodeficiency virus type 1 gag have greatly reduced pathogenicity but are highly immunogenic. J. Virol. 2003;77:237–244. doi: 10.1128/JVI.77.1.237-244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Mebatsion T. Extensive attenuation of rabies virus by simultaneously modifying the dynein light chain binding site in the P protein and replacing Arg333 in the G protein. J. Virol. 2001;75:11496–11502. doi: 10.1128/JVI.75.23.11496-11502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Papaneri AB, et al. A replication-deficient rabies virus vaccine expressing Ebola virus glycoprotein is highly attenuated for neurovirulence. Virology. 2012;434:18–26. doi: 10.1016/j.virol.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Zens KD, Chen JK, Farber DL. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight. 2016;1:e85832. doi: 10.1172/jci.insight.85832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Lau YF, Santos C, Torres-Vélez FJ, Subbarao K. The magnitude of local immunity in the lungs of mice induced by live attenuated influenza vaccines is determined by local viral replication and induction of cytokines. J. Virol. 2011;85:76–85. doi: 10.1128/JVI.01564-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.van Doremalen N, et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models. Sci. Transl. Med. 2021;13:eabh0755. doi: 10.1126/scitranslmed.abh0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Bricker TL, et al. A single intranasal or intramuscular immunization with chimpanzee adenovirus-vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters. Cell Rep. 2021;36:109400. doi: 10.1016/j.celrep.2021.109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Tioni MF, et al. Mucosal administration of a live attenuated recombinant COVID-19 vaccine protects nonhuman primates from SARS-CoV-2. NPJ Vaccines. 2022;7:85. doi: 10.1038/s41541-022-00509-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.King RG, et al. Single-Dose Intranasal Administration of AdCOVID Elicits Systemic and Mucosal Immunity against SARS-CoV-2 and Fully Protects Mice from Lethal Challenge. Vaccines (Basel) 2021;9:881. doi: 10.3390/vaccines9080881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Hassan AO, et al. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell. 2020;183:169–184.e113. doi: 10.1016/j.cell.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Ambrose CS, Wu X, Jones T, Mallory RM. The role of nasal IgA in children vaccinated with live attenuated influenza vaccine. Vaccine. 2012;30:6794–6801. doi: 10.1016/j.vaccine.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 467.Barría MI, et al. Localized mucosal response to intranasal live attenuated influenza vaccine in adults. J. Infect. Dis. 2013;207:115–124. doi: 10.1093/infdis/jis641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Corbett KS, et al. Immune correlates of protection by mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. Science. 2021;373:eabj0299. doi: 10.1126/science.abj0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Liew F, et al. SARS-CoV-2-specific nasal IgA wanes 9 months after hospitalisation with COVID-19 and is not induced by subsequent vaccination. eBioMedicine. 2023;87:104402. doi: 10.1016/j.ebiom.2022.104402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Oh JE, et al. Intranasal priming induces local lung-resident B cell populations that secrete protective mucosal antiviral IgA. Sci. Immunol. 2021;6:eabj5129. doi: 10.1126/sciimmunol.abj5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Wang Z, et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci. Transl. Med. 2021;13:eabf1555. doi: 10.1126/scitranslmed.abf1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Debisarun PA, et al. Induction of trained immunity by influenza vaccination - impact on COVID-19. PLoS Pathog. 2021;17:e1009928. doi: 10.1371/journal.ppat.1009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Tenforde MW, et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years - United States, January-March 2021. MMWR Morb. Mortal. Wkly Rep. 2021;70:674–679. doi: 10.15585/mmwr.mm7018e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Lund FE, Randall TD. Scent of a vaccine. Science. 2021;373:397–399. doi: 10.1126/science.abg9857. [DOI] [PubMed] [Google Scholar]
- 475.Al Kaabi N, et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA. 2021;326:35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Polack FP, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Xu F, et al. Safety, mucosal and systemic immunopotency of an aerosolized adenovirus-vectored vaccine against SARS-CoV-2 in rhesus macaques. Emerg. Microbes Infect. 2022;11:438–441. doi: 10.1080/22221751.2022.2030199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Ohtsuka J, et al. A versatile platform technology for recombinant vaccines using non-propagative human parainfluenza virus type 2 vector. Sci. Rep. 2019;9:12901. doi: 10.1038/s41598-019-49579-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Mouro V, Fischer A. Dealing with a mucosal viral pandemic: lessons from COVID-19 vaccines. Mucosal Immunol. 2022;15:584–594. doi: 10.1038/s41385-022-00517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Vaca, G. B. et al. Intranasal mRNA-LNP vaccination protects hamsters from SARS-CoV-2 infection. bioRxiv[Preprint], 2023.2001.2011.523616, (2023). [DOI] [PMC free article] [PubMed]
- 481.Mitroulis I, et al. Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell. 2018;172:147–161.e112. doi: 10.1016/j.cell.2017.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Dai H, et al. PIRs mediate innate myeloid cell memory to nonself MHC molecules. Science. 2020;368:1122–1127. doi: 10.1126/science.aax4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Jeyanathan M, et al. Parenteral BCG vaccine induces lung-resident memory macrophages and trained immunity via the gut-lung axis. Nat. Immunol. 2022;23:1687–1702. doi: 10.1038/s41590-022-01354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Yao Y, et al. Induction of Autonomous Memory Alveolar Macrophages Requires T Cell Help and Is Critical to Trained Immunity. Cell. 2018;175:1634–1650.e1617. doi: 10.1016/j.cell.2018.09.042. [DOI] [PubMed] [Google Scholar]
- 485.Kopf M, Schneider C, Nobs SP. The development and function of lung-resident macrophages and dendritic cells. Nat. Immunol. 2015;16:36–44. doi: 10.1038/ni.3052. [DOI] [PubMed] [Google Scholar]
- 486.Aegerter H, et al. Influenza-induced monocyte-derived alveolar macrophages confer prolonged antibacterial protection. Nat. Immunol. 2020;21:145–157. doi: 10.1038/s41590-019-0568-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 488.Lin WW, et al. A durable protective immune response to wild-type measles virus infection of macaques is due to viral replication and spread in lymphoid tissues. Sci. Transl. Med. 2020;12:eaax7799. doi: 10.1126/scitranslmed.aax7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Paris R, et al. Adenovirus type 4 and 7 vaccination or adenovirus type 4 respiratory infection elicits minimal cross-reactive antibody responses to nonhuman adenovirus vaccine vectors. Clin. Vaccin. Immunol. 2014;21:783–786. doi: 10.1128/CVI.00011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Sumida SM, et al. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J. Immunol. 2005;174:7179–7185. doi: 10.4049/jimmunol.174.11.7179. [DOI] [PubMed] [Google Scholar]
- 491.Wilson JM. Lessons learned from the gene therapy trial for ornithine transcarbamylase deficiency. Mol. Genet Metab. 2009;96:151–157. doi: 10.1016/j.ymgme.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 492.Chen Z, et al. Evaluating a parainfluenza virus 5-based vaccine in a host with pre-existing immunity against parainfluenza virus 5. PLoS ONE. 2012;7:e50144. doi: 10.1371/journal.pone.0050144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Lorin C, et al. A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV. J. Virol. 2004;78:146–157. doi: 10.1128/JVI.78.1.146-157.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Wang X, et al. Neutralizing antibody responses to enterovirus and adenovirus in healthy adults in China. Emerg. Microbes Infect. 2014;3:e30. doi: 10.1038/emi.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Mast TC, et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: Correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2010;28:950–957. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 496.Ye X, et al. Seroprevalence of Neutralizing Antibodies to Human Adenovirus Type 4 and 7 in Healthy Populations From Southern China. Front. Microbiol. 2018;9:3040. doi: 10.3389/fmicb.2018.03040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Alonso-Padilla J, et al. Development of Novel Adenoviral Vectors to Overcome Challenges Observed With HAdV-5-based Constructs. Mol. Ther. 2016;24:6–16. doi: 10.1038/mt.2015.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Guo J, Mondal M, Zhou D. Development of novel vaccine vectors: Chimpanzee adenoviral vectors. Hum. Vaccin. Immunother. 2018;14:1679–1685. doi: 10.1080/21645515.2017.1419108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Richardson JS, et al. Enhanced protection against Ebola virus mediated by an improved adenovirus-based vaccine. PLoS ONE. 2009;4:e5308. doi: 10.1371/journal.pone.0005308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Choi JH, et al. A Single Dose Respiratory Recombinant Adenovirus-Based Vaccine Provides Long-Term Protection for Non-Human Primates from Lethal Ebola Infection. Mol. Pharm. 2015;12:2712–2731. doi: 10.1021/mp500646d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Xingui T, et al. Characterization of a replication-competent vector encoding DsRed based on a human adenovirus type 4 a-like strain. Virus Res. 2019;270:197662. doi: 10.1016/j.virusres.2019.197662. [DOI] [PubMed] [Google Scholar]
- 502.Lemckert AAC, et al. Generation of a novel replication-incompetent adenoviral vector derived from human adenovirus type 49: manufacture on PER.C6 cells, tropism and immunogenicity. J. Gen. Virol. 2006;87:2891–2899. doi: 10.1099/vir.0.82079-0. [DOI] [PubMed] [Google Scholar]
- 503.Roberts DM, et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–243. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- 504.Clarke DK, et al. Live virus vaccines based on a vesicular stomatitis virus (VSV) backbone: Standardized template with key considerations for a risk/benefit assessment. Vaccine. 2016;34:6597–6609. doi: 10.1016/j.vaccine.2016.06.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Chen Z, et al. Construction and characterization of a full-length cDNA infectious clone of emerging porcine Senecavirus A. Virology. 2016;497:111–124. doi: 10.1016/j.virol.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 506.Fang Y, et al. A full-length cDNA infectious clone of North American type 1 porcine reproductive and respiratory syndrome virus: expression of green fluorescent protein in the Nsp2 region. J. Virol. 2006;80:11447–11455. doi: 10.1128/JVI.01032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Mehle A, Dugan VG, Taubenberger JK, Doudna JA. Reassortment and mutation of the avian influenza virus polymerase PA subunit overcome species barriers. J. Virol. 2012;86:1750–1757. doi: 10.1128/JVI.06203-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.García-Arriaza J, Esteban M. Enhancing poxvirus vectors vaccine immunogenicity. Hum. Vaccin. Immunother. 2014;10:2235–2244. doi: 10.4161/hv.28974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Liu MA. Immunologic basis of vaccine vectors. Immunity. 2010;33:504–515. doi: 10.1016/j.immuni.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 510.Draper SJ, Heeney JL. Viruses as vaccine vectors for infectious diseases and cancer. Nat. Rev. Microbiol. 2010;8:62–73. doi: 10.1038/nrmicro2240. [DOI] [PubMed] [Google Scholar]
- 511.Kalafati L, et al. Innate Immune Training of Granulopoiesis Promotes Anti-tumor Activity. Cell. 2020;183:771–785.e712. doi: 10.1016/j.cell.2020.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Casanova-Acebes M, et al. Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells. Nature. 2021;595:578–584. doi: 10.1038/s41586-021-03651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Wang T, et al. Influenza-trained mucosal-resident alveolar macrophages confer long-term antitumor immunity in the lungs. Nat. Immunol. 2023;24:423–438. doi: 10.1038/s41590-023-01428-x. [DOI] [PubMed] [Google Scholar]
- 514.Fathi A, Dahlke C, Addo MM. Recombinant vesicular stomatitis virus vector vaccines for WHO blueprint priority pathogens. Hum. Vaccin. Immunother. 2019;15:2269–2285. doi: 10.1080/21645515.2019.1649532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Scher G, Schnell MJ. Rhabdoviruses as vectors for vaccines and therapeutics. Curr. Opin. Virol. 2020;44:169–182. doi: 10.1016/j.coviro.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Gomme EA, Wanjalla CN, Wirblich C, Schnell MJ. Rabies virus as a research tool and viral vaccine vector. Adv. Virus Res. 2011;79:139–164. doi: 10.1016/B978-0-12-387040-7.00009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Griffin DE. Measles Vaccine. Viral Immunol. 2018;31:86–95. doi: 10.1089/vim.2017.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm. Rep. 2013;62:1–34. [PubMed] [Google Scholar]
- 519.Ueda S. Development of measles vaccines in Japan. Vaccine. 2009;27:3230–3231. doi: 10.1016/j.vaccine.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 520.Gerlach T, Elbahesh H, Saletti G, Rimmelzwaan GF. Recombinant influenza A viruses as vaccine vectors. Expert Rev. Vaccines. 2019;18:379–392. doi: 10.1080/14760584.2019.1582338. [DOI] [PubMed] [Google Scholar]
- 521.McDonald SM, Nelson MI, Turner PE, Patton JT. Reassortment in segmented RNA viruses: mechanisms and outcomes. Nat. Rev. Microbiol. 2016;14:448–460. doi: 10.1038/nrmicro.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Su S, et al. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Sakurai F, Tachibana M, Mizuguchi H. Adenovirus vector-based vaccine for infectious diseases. Drug Metab. Pharmacokinet. 2022;42:100432. doi: 10.1016/j.dmpk.2021.100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Garcia-Arriaza J, et al. A novel poxvirus-based vaccine, MVA-CHIKV, is highly immunogenic and protects mice against chikungunya infection. J. Virol. 2014;88:3527–3547. doi: 10.1128/JVI.03418-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Halperin SA, et al. Immunogenicity, Lot Consistency, and Extended Safety of rVSVDeltaG-ZEBOV-GP Vaccine: A Phase 3 Randomized, Double-Blind, Placebo-Controlled Study in Healthy Adults. J. Infect. Dis. 2019;220:1127–1135. doi: 10.1093/infdis/jiz241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Samai M, et al. The Sierra Leone Trial to Introduce a Vaccine Against Ebola: An Evaluation of rVSVG-ZEBOV-GP Vaccine Tolerability and Safety During the West Africa Ebola Outbreak. J. Infect. Dis. 2018;217:S6–S15. doi: 10.1093/infdis/jiy020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Woolsey C, et al. Postexposure Efficacy of Recombinant Vesicular Stomatitis Virus Vectors Against High and Low Doses of Marburg Virus Variant Angola in Nonhuman Primates. J. Infect. Dis. 2018;218:S582–S587. doi: 10.1093/infdis/jiy293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Geisbert TW, et al. Postexposure treatment of Marburg virus infection. Emerg. Infect. Dis. 2010;16:1119–1122. doi: 10.3201/eid1607.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Daddario-DiCaprio KM, et al. Postexposure protection against Marburg haemorrhagic fever with recombinant vesicular stomatitis virus vectors in non-human primates: an efficacy assessment. Lancet. 2006;367:1399–1404. doi: 10.1016/S0140-6736(06)68546-2. [DOI] [PubMed] [Google Scholar]
- 530.Safronetz D, et al. A recombinant vesicular stomatitis virus-based Lassa fever vaccine protects guinea pigs and macaques against challenge with geographically and genetically distinct Lassa viruses. PLoS Negl. Trop. Dis. 2015;9:e0003736. doi: 10.1371/journal.pntd.0003736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Li A, et al. A Zika virus vaccine expressing premembrane-envelope-NS1 polyprotein. Nat. Commun. 2018;9:3067. doi: 10.1038/s41467-018-05276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Shi X, et al. A Vesicular Stomatitis Virus-Based Vaccine Carrying Zika Virus Capsid Protein Protects Mice from Viral Infection. Virol. Sin. 2019;34:106–110. doi: 10.1007/s12250-019-00083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.B PA, et al. Further characterization of the immune response in mice to inactivated and live rabies vaccines expressing Ebola virus glycoprotein. Vaccine. 2012;30:6136–6141. doi: 10.1016/j.vaccine.2012.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Kato H, et al. Development of a recombinant replication-deficient rabies virus-based bivalent-vaccine against MERS-CoV and rabies virus and its humoral immunogenicity in mice. PloS ONE. 2019;14:e0223684–e0223684. doi: 10.1371/journal.pone.0223684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Gomez M, et al. Phase-I study MEDI-534, of a live, attenuated intranasal vaccine against respiratory syncytial virus and parainfluenza-3 virus in seropositive children. Pediatr. Infect. Dis. J. 2009;28:655–658. doi: 10.1097/INF.0b013e318199c3b1. [DOI] [PubMed] [Google Scholar]
- 536.Hara K, et al. Human parainfluenza virus type 2 vector induces dendritic cell maturation without viral RNA replication/transcription. Hum. Gene Ther. 2013;24:683–691. doi: 10.1089/hum.2013.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Kurup D, et al. Measles-based Zika vaccine induces long-term immunity and requires NS1 antibodies to protect the female reproductive tract. NPJ Vaccines. 2022;7:43. doi: 10.1038/s41541-022-00464-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Kortekaas J, et al. Rift Valley fever virus immunity provided by a paramyxovirus vaccine vector. Vaccine. 2010;28:4394–4401. doi: 10.1016/j.vaccine.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 539.Kong D, et al. Newcastle disease virus-vectored Nipah encephalitis vaccines induce B and T cell responses in mice and long-lasting neutralizing antibodies in pigs. Virology. 2012;432:327–335. doi: 10.1016/j.virol.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 540.Lee YN, et al. Recombinant influenza virus expressing a fusion protein neutralizing epitope of respiratory syncytial virus (RSV) confers protection without vaccine-enhanced RSV disease. Antivir. Res. 2015;115:1–8. doi: 10.1016/j.antiviral.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Martina BE, et al. A recombinant influenza A virus expressing domain III of West Nile virus induces protective immune responses against influenza and West Nile virus. PLoS ONE. 2011;6:e18995. doi: 10.1371/journal.pone.0018995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Hashem AM, et al. A Highly Immunogenic, Protective, and Safe Adenovirus-Based Vaccine Expressing Middle East Respiratory Syndrome Coronavirus S1-CD40L Fusion Protein in a Transgenic Human Dipeptidyl Peptidase 4 Mouse Model. J. Infect. Dis. 2019;220:1558–1567. doi: 10.1093/infdis/jiz137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Guo X, et al. Systemic and mucosal immunity in mice elicited by a single immunization with human adenovirus type 5 or 41 vector-based vaccines carrying the spike protein of Middle East respiratory syndrome coronavirus. Immunology. 2015;145:476–484. doi: 10.1111/imm.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Kim E, et al. Immunogenicity of an adenoviral-based Middle East Respiratory Syndrome coronavirus vaccine in BALB/c mice. Vaccine. 2014;32:5975–5982. doi: 10.1016/j.vaccine.2014.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Jia W, et al. Single intranasal immunization with chimpanzee adenovirus-based vaccine induces sustained and protective immunity against MERS-CoV infection. Emerg. Microbes Infect. 2019;8:760–772. doi: 10.1080/22221751.2019.1620083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Safronetz D, et al. Adenovirus vectors expressing hantavirus proteins protect hamsters against lethal challenge with andes virus. J. Virol. 2009;83:7285–7295. doi: 10.1128/JVI.00373-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Fisher-Hoch SP, Hutwagner L, Brown B, McCormick JB. Effective vaccine for lassa fever. J. Virol. 2000;74:6777–6783. doi: 10.1128/JVI.74.15.6777-6783.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Kennedy EM, et al. A vaccine based on recombinant modified Vaccinia Ankara containing the nucleoprotein from Lassa virus protects against disease progression in a guinea pig model. Vaccine. 2019;37:5404–5413. doi: 10.1016/j.vaccine.2019.07.023. [DOI] [PubMed] [Google Scholar]
- 549.Papin JF, et al. Recombinant Rift Valley fever vaccines induce protective levels of antibody in baboons and resistance to lethal challenge in mice. Proc. Natl Acad. Sci. USA. 2011;108:14926–14931. doi: 10.1073/pnas.1112149108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Busquets N, et al. Efficacy assessment of an MVA vectored Rift Valley Fever vaccine in lambs. Antivir. Res. 2014;108:165–172. doi: 10.1016/j.antiviral.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 551.Lopez-Gil E, et al. A single immunization with MVA expressing GnGc glycoproteins promotes epitope-specific CD8+-T cell activation and protects immune-competent mice against a lethal RVFV infection. PLoS Negl. Trop. Dis. 2013;7:e2309. doi: 10.1371/journal.pntd.0002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Buttigieg KR, et al. A novel vaccine against Crimean-Congo Haemorrhagic Fever protects 100% of animals against lethal challenge in a mouse model. PLoS ONE. 2014;9:e91516. doi: 10.1371/journal.pone.0091516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Dowall SD, et al. A Crimean-Congo hemorrhagic fever (CCHF) viral vaccine expressing nucleoprotein is immunogenic but fails to confer protection against lethal disease. Hum. Vaccin. Immunother. 2016;12:519–527. doi: 10.1080/21645515.2015.1078045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Koch T, et al. Safety and immunogenicity of a modified vaccinia virus Ankara vector vaccine candidate for Middle East respiratory syndrome: an open-label, phase 1 trial. Lancet Infect. Dis. 2020;20:827–838. doi: 10.1016/S1473-3099(20)30248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]