PURPOSE
In patients with diffuse low-grade glioma (LGG), the extent of surgical tumor resection (EOR) has a controversial role, in part because a randomized clinical trial with different levels of EOR is not feasible.
METHODS
In a 20-year retrospective cohort of 392 patients with IDH-mutant grade 2 glioma, we analyzed the combined effects of volumetric EOR and molecular and clinical factors on overall survival (OS) and progression-free survival by recursive partitioning analysis. The OS results were validated in two external cohorts (n = 365). Propensity score analysis of the combined cohorts (n = 757) was used to mimic a randomized clinical trial with varying levels of EOR.
RESULTS
Recursive partitioning analysis identified three survival risk groups. Median OS was shortest in two subsets of patients with astrocytoma: those with postoperative tumor volume (TV) > 4.6 mL and those with preoperative TV > 43.1 mL and postoperative TV ≤ 4.6 mL. Intermediate OS was seen in patients with astrocytoma who had chemotherapy with preoperative TV ≤ 43.1 mL and postoperative TV ≤ 4.6 mL in addition to oligodendroglioma patients with either preoperative TV > 43.1 mL and residual TV ≤ 4.6 mL or postoperative residual volume > 4.6 mL. Longest OS was seen in astrocytoma patients with preoperative TV ≤ 43.1 mL and postoperative TV ≤ 4.6 mL who received no chemotherapy and oligodendroglioma patients with preoperative TV ≤ 43.1 mL and postoperative TV ≤ 4.6 mL. EOR ≥ 75% improved survival outcomes, as shown by propensity score analysis.
CONCLUSION
Across both subtypes of LGG, EOR beginning at 75% improves OS while beginning at 80% improves progression-free survival. Nonetheless, maximal resection with preservation of neurological function remains the treatment goal. Our findings have implications for surgical strategies for LGGs, particularly oligodendroglioma.
INTRODUCTION
Over the past decade, the WHO has reclassified diffuse gliomas into separate clinical diagnoses on the basis of tumor histology and molecular characteristics.1-6 These tumors include astrocytoma IDH-mutant (astrocytomas) and oligodendroglioma IDH-mutant 1p19q codeleted (oligodendroglioma), both with distinct clinical trajectories.1,3,4,6,7 Despite their relatively slow growth, low-grade gliomas (LGGs) are locally invasive and prone to malignant transformation.8,9
CONTEXT
Key Objective
The notion that extensive resection of diffuse low-grade gliomas is associated with longer survival has been challenged by recent molecular subclassification in which certain patient subgroups experience more favorable prognosis and longer survival. This study examines the interactive effects of molecular and clinical variables on survival in adults with WHO grade 2 oligodendroglioma and astrocytoma.
Knowledge Generated
A multicenter multinational cohort of 757 patients was used to establish overall, progression-free, and malignant transformation–free survival risk groups. The protective effects of greater extent of glioma resection and smaller volume of residual tumor were established. Propensity score analysis was used to mimic a randomized clinical trial to estimate an extent of resection threshold.
Relevance (I.K. Mellinghoff)
-
Surgery plays an important role in the initial treatment of gliomas. This study confirms that more complete tumor resection (≥ 75%) improves long-term outcomes of patients with IDH-mutant CNS WHO grade 2 tumors and suggests that resection beyond the imaging-defined tumor margins may improve outcomes in some patients.*
*Relevance section written by JCO Associate Editor Ingo K. Mellinghoff, MD.
The notion that more extensive tumor resection is associated with longer survival was established as the standard of care before the WHO's reclassification of gliomas in 2016.10-13 Some studies continue to support this notion for all LGGs.14-18 However, others propose that the benefit is limited to certain molecular subgroups.19-21 In particular, complete surgical resection for patients with oligodendroglioma may not offer a survival advantage given their relatively favorable prognosis and better response to chemoradiation.20-22 These discrepancies have created controversy and confusion among both providers and patients. However, a randomized controlled clinical trial of different levels of cytoreduction would be not feasible because of lack of equipoise by physicians and patients.
In this study, we tested two hypotheses that overall survival (OS) is longer after more extensive resection than after subtotal resection, regardless of tumor subgroup and that resection beyond the imaging-defined tumor margins influences survival outcomes. Our analysis focused on adults in whom a WHO grade 2 oligodendroglioma or astrocytoma was initially diagnosed between 1998 and 2017. First, we analyzed whether the extent of resection (EOR) was associated with OS in a large single-institution cohort and verified the findings in independent patient cohorts from the United States and Europe. Next, we examined associations between OS and resection beyond imaging-defined tumor margins. We also assessed oncological and clinical factors associated with malignant and nonmalignant progression. Last, we used propensity score analysis across the three cohorts to mimic a randomized clinical trial to estimate the influence of EOR on OS, progression-free survival (PFS), and malignant transformation–free survival (MTFS).
METHODS
In this retrospective study, we modeled survival risk in a development cohort of patients with newly diagnosed WHO grade 2 astrocytoma IDH-mutant and oligodendroglioma IDH-mutant 1p19q codeleted on the basis of WHO 2021 diagnostic criteria and validated the findings in two external cohorts.1,3,4 The clinical characteristics of the patients are summarized in Table 1 and the Data Supplement (online only; Fig 1A). Additional details on patients, tumor classification and imaging, and clinical data collection are given in the Data Supplement.23,24 The study was approved by the University of California, San Francisco, Institutional Review Board.
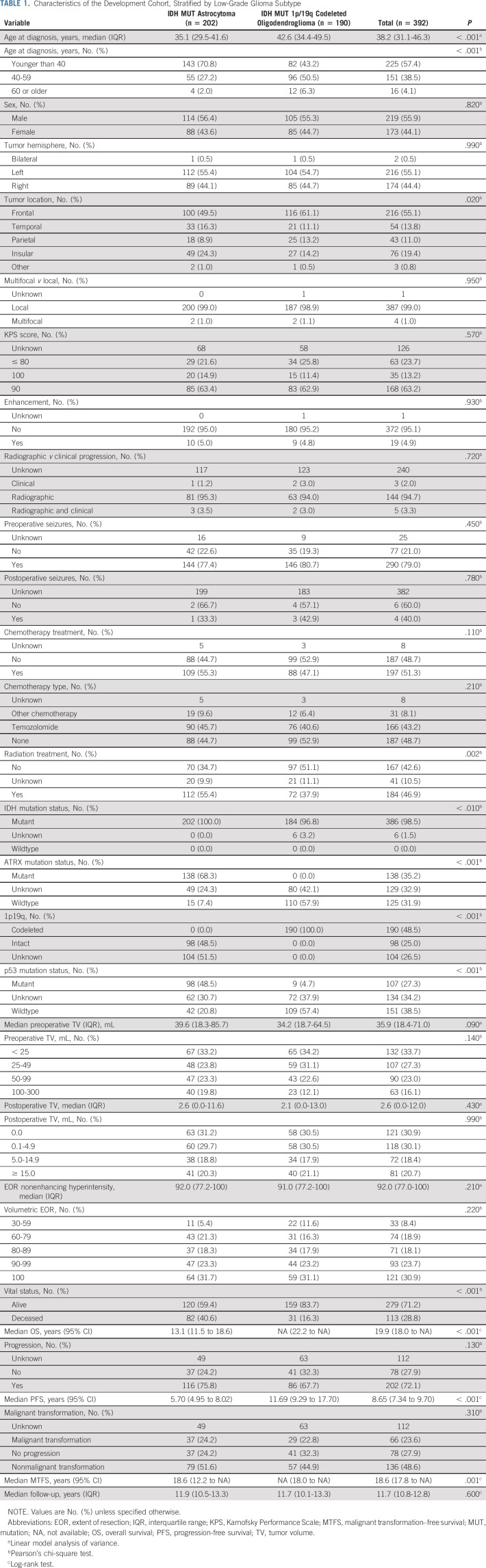
TABLE 1.
Characteristics of the Development Cohort, Stratified by Low-Grade Glioma Subtype
FIG 1.
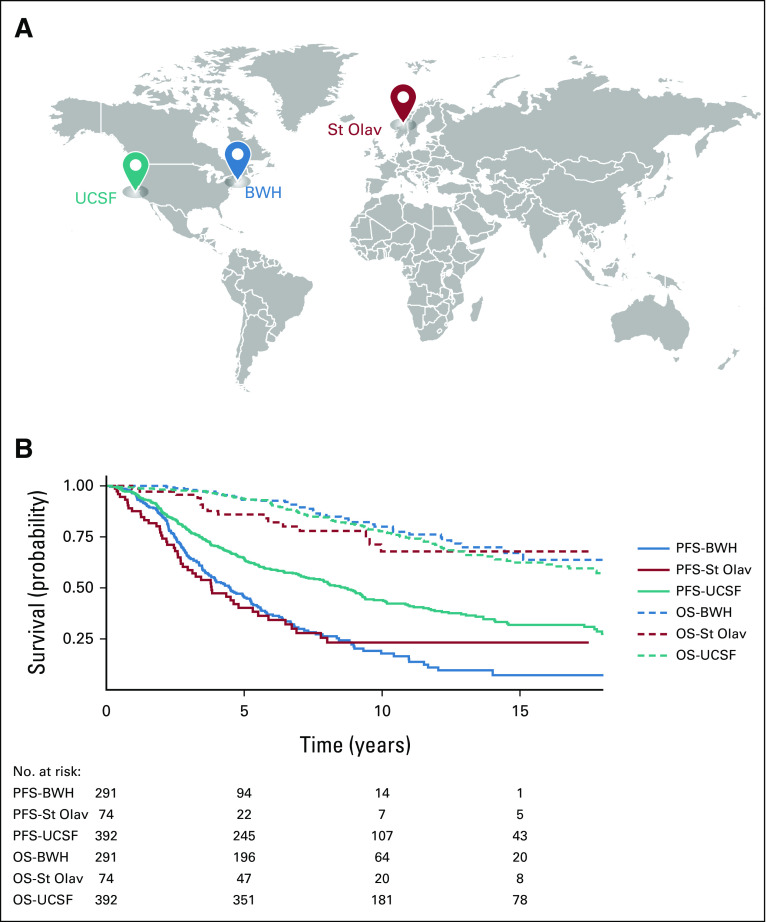
(A) World map indicating multicenter–multinational study cohorts. (B) Kaplan-Meier curves for OS and PFS across development and external validation cohorts. OS was similar across all three sites, but PFS differed across the sites. BWH, Brigham and Women's Hospital, Boston, MA; OS, overall survival; PFS, progression-free survival; St Olav, St Olavs University Hospital, Trondheim, Norway; UCSF, University of California, San Francisco, CA.
Details of analytic methods are summarized in the Methods in the Data Supplement. In brief, patient demographics and tumor characteristics were summarized with descriptive statistics. Differences in continuous and categorical variables between cohorts were analyzed by t test and chi-square test, respectively. OS was defined as the time from surgery (or biopsy if before surgery) until death or last contact date. Median follow-up was estimated with the reverse Kaplan-Meier method. PFS was defined as the time between surgery (or biopsy) and tumor progression (or death) on the basis of Neuro-Oncology assessment and RANO criteria.25 MTFS was defined as the time from first surgery (or biopsy) to malignant transformation to grade 3 or higher (or death). Patients who did not have progression or malignant transformation were censored at the time of loss to follow-up or last follow-up date. Cox proportional hazard (Cox-PH) models were used to evaluate the associations of potential risk factors with survival. Recursive partitioning analysis (RPA) with the partDSA algorithm26,27 was performed to identify survival risk groups in a multivariate setting using all known prognostic variables. Median survival times and hazard ratios (HRs) were determined with the Kaplan-Meier method and Cox-PH models, respectively. The log-rank test was used to compare curves unless assumptions were violated; then, the Tarone-Ware test was applied. Assumptions for Cox-PH models were also verified. The final RPA selected from the development cohort was validated in the external validation cohorts. To estimate the effects of EOR and volume of residual (VOR) on OS, PFS, and MTFS, we used propensity score matching to mimic a randomized trial and remove potential confounding effects between survival outcomes and EOR cutoff values.28 Matching was based on age at diagnosis, LGG subtype, chemotherapy, radiation, preoperative tumor volume (TV), and tumor location. For each EOR cutoff value and corresponding matched data set, HRs were estimated from a multivariate Cox-PH model with all matching variables included. All analyses were performed with R v.4.0.29
RESULTS
Patient demographics, clinical characteristics, and survival are shown in Table 1. As of the last data collection, 113 (28.8%) of the 392 patients in the development cohort had died. The median follow-up was 11.7 years (95% CI, 10.8 to 12.8); 181 patients were followed for > 10 years. The median OS was 19.9 years (95% CI, 18.0 to not available [NA]). Progression was identified in 202 patients (72.1%) and malignant transformation in 66 (23.6%). The median PFS was 8.65 years (95% CI, 7.3 to 9.7). The median MTFS was 18.6 years (95% CI, 17.8 to NA). OS, PFS, and MTFS were longer in patients with oligodendroglioma, where their OS has not reached the median yet (95% CI, 22.2 to NA); the median PFS was 11.7 years (95% CI, 9.3 to 17.7), and MTFS had not reached the median yet (95% CI, 18 to NA). The median OS for patients with astrocytoma was 13.1 years (95% CI, 11.5 to 18.6); the median PFS was 5.7 years (95% CI, 5.0 to 8.0), and the median MTFS was 18.6 years (95% CI, 12.2 to NA).
The validation cohorts were similar to the development cohort in age at diagnosis and sex (Data Supplement). OS was comparable in the three cohorts; however, PFS and MTFS differed (Fig 1B, Data Supplement).
OS Risk Groups in Development and Validation Cohorts
RPA identified three distinct survival risk groups in the development cohort (P < .001 by log-rank test; Figs 2A and 2B, Data Supplement). The groups were based on postoperative (residual) TV with a cutoff of 4.6 mL, preoperative TV with a cutoff of 43.1 mL, LGG subtype, and whether a patient received chemotherapy. For ease of discussion, we refer to residual TV ≤ 4.6 mL as smaller and > 4.6 mL as larger. A similar distinction is made for preoperative TV with the corresponding cutoff.
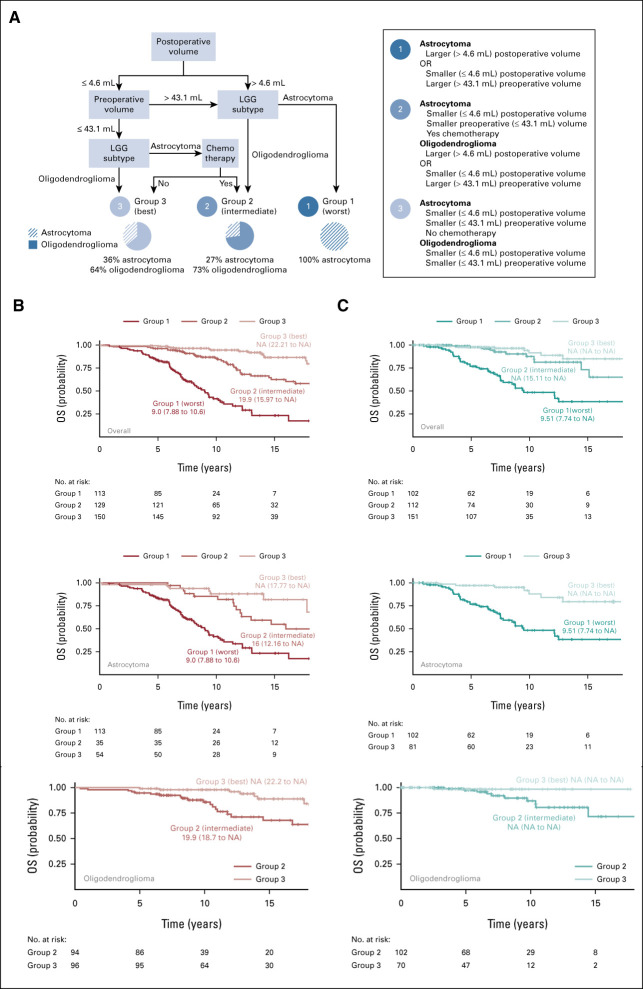
FIG 2.
OS outcomes for development cohort and external validation. (A) RPA for the development cohort identified three risk groups on the basis of postoperative TV, preoperative TV, LGG subtype, and chemotherapy. Groups are denoted by number. Group 1 had the worst survival and consisted of the astrocytoma patients with residual tumor > 4.6 mL or astrocytoma patients with preoperative TV > 43.1 mL and residual tumor ≤ 4.6 mL. Group 2 had intermediate survival and consisted of a combination of two subgroups: (1) oligodendroglioma patients with residual tumor > 4.6 mL or oligodendroglioma patients with preoperative TV > 43.1 mL and residual tumor ≤ 4.6 mL and (2) patients with astrocytoma who had chemotherapy with preoperative TV ≤ 43.1 mL and residual tumor ≤ 4.6 mL. Group 3 had the best survival and consisted of the combination of two subgroups: (1) oligodendroglioma patients with preoperative TV ≤ 43.1 mL and residual tumor ≤ 4.6 mL and (2) patients with astrocytoma who had no chemotherapy with preoperative TV ≤ 43.1 mL and residual tumor ≤ 4.6 mL. (A) Overall Survival RPA Tree (UCSF). (B) Kaplan-Meier curves for OS (UCSF) by the three risk groups delineated in (A) for all patients (overall) in the development cohort, patients with astrocytoma, and patients with oligodendroglioma, respectively. (C) Kaplan-Meier curves for OS (BWH and St Olav) by the three risk groups delineated in (A) for all patients in the external validation, patients with astrocytoma, and patients with oligodendroglioma, respectively. Hazard ratios and CIs for Figure 2 are included in the Data Supplement. BWH, Brigham and Women's Hospital; LGG, low-grade glioma; NA, not available; OS, overall survival; RPA, recursive partitioning analysis; St Olav, St Olavs University Hospital; TV, tumor volume; UCSF, University of California, San Francisco.
In group 1, OS was shortest in astrocytoma patients with larger postoperative TV and astrocytoma patients with larger preoperative TV plus smaller residual TV (group 1; n = 113; median OS, 9.0 years; 95% CI, 7.9 to 10.6). In group 2, OS was intermediate for subsets of both LGG subtypes (group 2; n = 129; median OS, 19.9 years; 95% CI, 16 to NA). Group 2 includes astrocytoma patients treated with chemotherapy with smaller preoperative and residual TV. Group 2 also includes oligodendroglioma patients with either larger preoperative and smaller residual TV or just larger residual TV. In group 3, OS was longest and the median was not reached (group 3; n = 150; median OS, NA; 95% CI, 22.2 to NA). Group 3 includes oligodendroglioma patients with smaller preoperative and residual TV. Group 3 also includes patients with astrocytoma who had not received chemotherapy with smaller preoperative and residual TV. The best and intermediate survival groups (groups 3 and 2, respectively) had similar survival until 7 years when it diverged (Fig 2B, overall). In a univariate Cox-PH model, the HR for best versus intermediate survival group is 0.40 (95% CI, 0.2 to 0.7; P = .001).
In Figure 2B, the OS risk groups are also stratified by LGG subtype. Patients with astrocytoma had a similar median OS to the overall cohort (group 1: median, 9 years [95% CI, 7.9 to 10.6]; group 2: median, 16 years [95% CI, 12.2 to NA]; group 3: median, NA [95% CI, 17.8 to NA]; group 1 v 2 v 3: P < .001 by log-rank test). The association between OS risk, LGG subtype, and chemotherapy revealed that patients with group 2 astrocytoma (ie, treated with chemotherapy) had larger preoperative and residual TV than patients with group 3 astrocytoma (ie, those not treated with chemotherapy), representing a provider treatment bias (Data Supplement). Patients with oligodendroglioma were not included in risk group 1 (ie, those with shortest survival). Patients with oligodendroglioma in group 2 experienced a median survival of 19.9 years (95% CI, 18.7 to NA) while those in group 3 (the longest survival) did not reach a median (95% CI, 22.2 to NA; group 2 v 3: P = .002 by log-rank test). The OS model was corroborated by the external validation cohorts (Fig 2C). Risk group 1 in the external cohort had a median OS of 9.5 years (95% CI, 7.7 to NA) while the median OS was not reached for risk groups 2 or 3 (P < .001 by Tarone-Ware test). Importantly, external cohorts stratified by LGG subtype confirmed the risk stratifications with the exception of intermediate risk group 2 astrocytoma because of early censoring (Data Supplement). HR and CIs for all comparisons in Figure 2 are provided in the Data Supplement.
Survival Benefit of Gross Total Resection+ Is Preserved Across LGG Subtypes
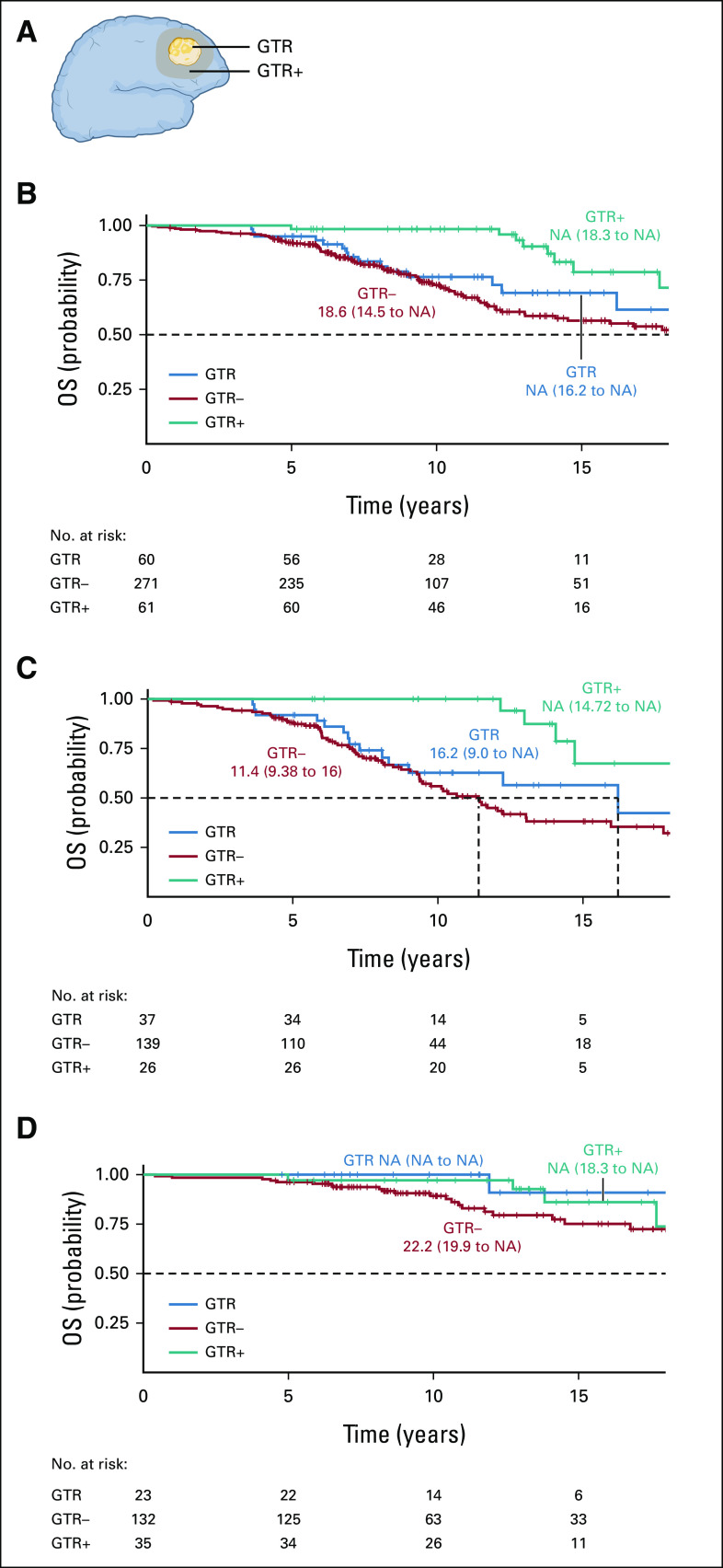
As smaller postoperative TV was associated with longer OS for both LGG subtypes (Fig 2A), we explored the effects of gross total resection (GTR)+ over GTR and GTR– (Fig 3A). GTR+ was most prevalent in patients with small preoperative TVs (Data Supplement). In the development cohort, OS was longest in patients with GTR+ (median OS, NA; 95% CI, 18.3 to NA) and GTR+ was significantly different than GTR and GTR– (P = .001 and P = .0004, respectively; both by log-rank test). There was no notable difference between GTR and GTR– until 10 years (GTR median OS, NA; 95% CI, 16.2 to NA; GTR– median OS, 18.6 years; 95% CI, 14.5 to NA; P < .001 by Tarone-Ware test; Fig 3B). Next, we determined whether the survival advantage of GTR+ persisted within subtype. In patients with astrocytoma, the survival curves are quite similar to those in the overall cohort. OS was longer after GTR+ (median OS, NA [95% CI, 14.7 to NA]) compared with GTR (median OS, 16.2 years [95% CI, 9 to NA]) and GTR– (median OS, 11.4 years [95% CI, 9.4 to 16]; P < .001 by Tarone-Ware test; Fig 3C). For patients with oligodendroglioma, the median OS was longer after GTR+ (median OS, NA [95% CI, 18.3 to NA]) and GTR (median OS, NA [95% CI, NA to NA]) compared with GTR– (median OS, 22.2 years [95% CI, 19.9 to NA]; GTR/GTR+ v GTR–: P = .04 by Tarone-Ware test). Interestingly, there were no statistical differences in survival between GTR and GTR+ (GTR v GTR+: P = .47 by Tarone-Ware test; Fig 3D). Finally, we assessed survival models with and without controlling for preoperative TV. In patients with astrocytoma, with GTR+ versus GTR– and GTR versus GTR– with and without controlling for preoperative TV both were significant predictors of OS (P = .015 and P = .001, respectively). For patients with oligodendroglioma, GTR+/GTR versus GTR– was not a significant predictor when controlling for preoperative TV (P = .32) but trended significant without preoperative TV (P = .065; Data Supplement).
FIG 3.
GTR and GTR+ in the development cohort. (A) Schematic of GTR, GTR+ (resection beyond the imaging-defined tumor margin), and GTR– (resection ≤ 100%). (B) Kaplan-Meier curves for all OS UCSF patients stratified by GTR status. OS was longer in patients with GTR+ (P < .001 by Tarone-Ware test). (C) Kaplan-Meier curves stratified by GTR status in UCSF patients with astrocytoma (P < .001 by Tarone-Ware test). (D) Kaplan-Meier curves stratified by GTR status in UCSF patients with oligodendroglioma (P = .11 by Tarone-Ware test). GTR, gross total resection; NA, not available; OS, overall survival; UCSF, University of California, San Francisco.
PFS and MTFS in the Development and Validation Cohorts
Next, we sought to understand the interactive effects of clinical and treatment variables on tumor progression, a nearly universal characteristic of diffuse, LGGs. Since PFS and MTFS differed in the cohorts (Fig 1B and Data Supplement), we combined the data from all three cohorts. Almost identical to our OS model in Figure 2A, the RPA identified three PFS risk groups on the basis of postoperative TVs and LGG tumor subtypes (Fig 4A). Kaplan-Meier curves were generated for each risk group (P < .0001 by log-rank test, Fig 4B). PFS was shortest in astrocytoma patients with the largest postoperative TVs (> 32.7 mL; group 1, n = 29; median PFS, 1.8 years; 95% CI, 1.1 to 2.6); intermediate in astrocytoma patients with moderate postoperative TV (between 1.2 and 32.7 mL; group 2, n = 218; median PFS, 3.99 years; 95% CI, 3.3 to 4.8); and longest in the combination of the astrocytoma patients with small residual TV (≤ 1.2 mL) with all oligodendroglioma patients (group 3, n = 510; median PFS, 8.1 years; 95% CI, 6.9 to 9.3).
FIG 4.
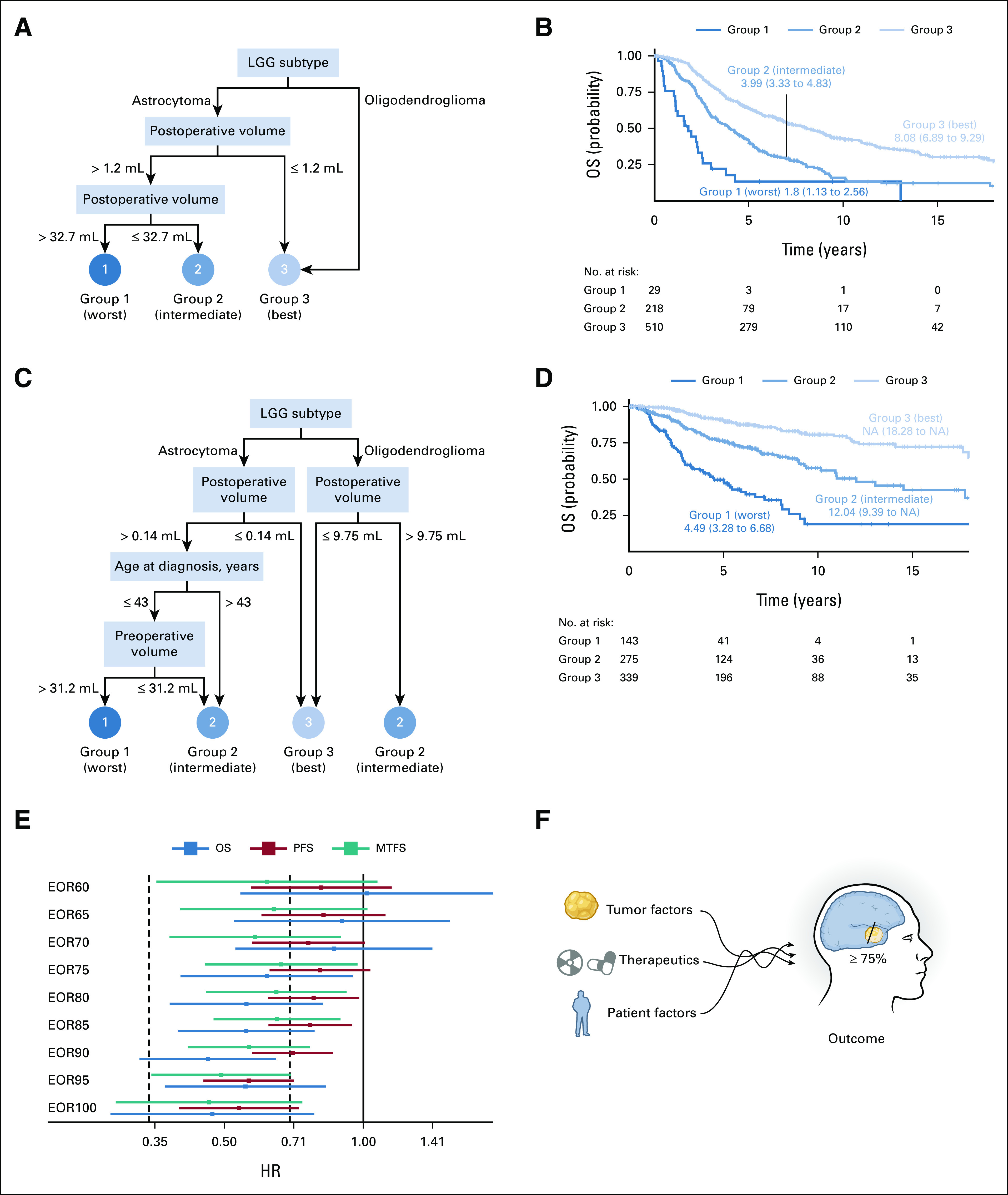
RPA of PFS and MTFS and corresponding Kaplan-Meier survival curves. (A) RPA identified three PFS risk groups on the basis of postoperative TV and LGG subtype. Group 1 patients (n = 29) had the worst PFS and included those patients with astrocytoma and residual tumor > 32.7 mL. Group 2 patients (n = 218) had better PFS and included the astrocytoma patients with residual tumor between 1.2 and 32.7 mL. Group 3 patients (n = 510) had the best PFS and included a combination of two subgroups: (1) all patients with oligodendroglioma and (2) astrocytoma patients with residual tumor ≤ 1.2 mL. (B) Kaplan-Meier curves for the three PFS risk groups identified in (A) (P < .001 by log-rank test). (C) RPA identified three MTFS risk groups on the basis of postoperative TV, LGG subtype, age at diagnosis, and preoperative TV. Group 1 patients (n = 143) were patients with astrocytoma age younger than 43 years with preoperative TV > 31.2 mL and residual TV > 0.14 mL and had the poorest MTFS. Group 2 patients (n = 275) had better MTFS and was the combination of three subgroups: (1) oligodendroglioma patients with residual TV > 9.75 mL, (2) patients with astrocytoma age older than 43 years with residual TV > 0.14 mL, and (3) patients with astrocytoma age younger than 43 years with preoperative TV ≤ 31.2 mL and residual TV > 0.14 mL. Group 3 patients (n = 339) had the best MTFS and included both (1) the astrocytoma patients with residual TV ≤ 0.14 mL and (2) oligodendroglioma patients with residual TV ≤ 9.75 mL. (D) Kaplan-Meier curves for the three MTFS risk groups identified in (C; P < .0001 by log-rank test). (E) Forest plot of HRs determined by propensity score analysis (UCSF + BWH + St Olavs). (F) The interactive effects of molecular (tumor), therapeutic, and patient factors indicates that EOR ≥ 75% confers a survival benefit. BWH, Brigham Women’s Hospital; EOR, extent of resection; HR, hazard ratio; LGG, low-grade glioma; OS, overall survival; MTFS, malignant transformation–free survival; NA, not available; PFS, progression-free survival; RPA, recursive partitioning analysis; St Olavs, St Olavs University Hospital; TV, tumor volume; UCSF, University of California, San Francisco.
For MTFS, RPA identified three risk groups on the basis of postoperative TV, LGG subtype, preoperative TV, and age at diagnosis (Fig 4C). MTFS was shortest in patients with astrocytoma age younger than 43 years at diagnosis with larger preoperative TV (> 31.2 mL) and larger residual TV (> 0.14 mL; group 1, n = 143; median MTFS, 4.5 years; 95% CI, 3.3 to 6.7). MTFS was intermediate in oligodendroglioma patients with larger residual tumor > 9.75 mL and those patients with astrocytoma age older than 43 years with larger residual TV (> 0.14 mL) or those patients with astrocytoma age younger than 43 years with smaller preoperative TV (≤ 31.2 mL) and larger residual TV (> 0.14 mL; group 2, n = 275; median MTFS, 12 years; 95% CI, 9.4 to NA). MTFS was longest in oligodendroglioma patients with smaller residual TV (≤ 9.75 mL) and astrocytoma patients with smaller residual TV (≤ 0.14 mL; group 3, n = 339; median MTFS, NA; 95% CI, 18.3 to NA). Kaplan-Meier curves for the three MTFS risk groups (P < .0001 by log-rank test) are shown in Figure 4D and MTFS separated by institution in the Data Supplement.
To mimic a randomized control trial and get robust power to predict the influence of EOR, we used propensity score matching at EOR cutoffs between 60% and 100% with all three cohorts combined (n = 757). Patients were matched for age at diagnosis, diffuse LGG subtype, chemotherapy, radiation, preoperative tumor volume (TV), and tumor location (Data Supplement). As EOR increased, the HR decreased (Fig 4E), and by 75%, the effect of EOR was significant (CI for HR did not include 1) and protective (HR < 1; Data Supplement). EOR had a similar effect on PFS at a threshold of 80% and MTFS at a threshold of 70%. We performed the same propensity score matching for VOR at cutoffs between 1 and 10 mL. VOR below 10 mL was significant for OS and MPFS (Data Supplement).
DISCUSSION
In this study, we confirmed two hypotheses that OS is longer after more extensive resection than after subtotal resection of LGG regardless of subtype and that resection beyond the imaging-defined tumor margins improves survival outcomes with greatest benefit seen in patients with astrocytoma. The findings of all four presented analyses reinforce the importance of maximal EOR and smaller residual TV regardless of LGG subtype.
In the first analysis, we investigated the combined effects of volumetric EOR and molecular and clinical factors on OS in the development cohort (median follow-up, 11.7 years; median OS, 19.9 years). We delineated three risk groups on the basis of an interaction between preoperative and postoperative TVs, chemotherapy use, and LGG subtype. OS was longest in oligodendroglioma patients with smaller pre-operative and residual TVs as well as in patients with astrocytoma who had not received chemotherapy who also had smaller preoperative and residual TVs (Fig 2A). OS was shortest in astrocytoma patients with larger postoperative TV and astrocytoma patients with larger preoperative TV plus smaller residual TV. These findings persisted when risk groups were stratified by LGG subtype (Fig 2B). Interestingly, patients with astrocytoma in the intermediate and longest survival risk groups (groups 2 and 3, respectively) experienced similar survival outcomes with divergent outcomes after 7 years. Comparisons of these two risk groups determined that intermediate risk (group 2) patients who received chemotherapy had larger gliomas, greater residual TV, and less EOR when compared with risk group 3 patients who did not receive chemotherapy (Data Supplement). European Organisation for Research and Treatment of Cancer defined LGG risk was greater for patients in group 2 when compared with those in risk group 3.30 Thus, these results suggest that the use of chemotherapy represents a provider treatment bias with the early introduction of chemotherapy for patients presumed to be at a higher than average risk (Data Supplement).8,31-34
In the second analysis, we determined whether GTR+ provided a survival advantage. We found that median OS after GTR+ was longer when compared with GTR and GTR– (Figs 3B-3D). However, the relative benefit of EOR beyond the imaging defined tumor margin appears greatest for patients with astrocytoma given the demonstrated survival advantage of GTR+ compared with GTR and GTR– in this LGG subtype. This important distinction sits in contrast to patients with oligodendroglioma tumors in which GTR and GTR+ demonstrated similar beneficial survival outcomes when compared with patients receiving GTR–.
Current imaging techniques cannot define the margins of brain tumors accurately and precisely, so margin surgery has never been considered the standard of care. Furthermore, few tools exist to identify and quantify tumor burden quickly and reliably during surgery. Intraoperative magnetic resonance imaging and fluorescent labeling of tumors have been helpful but are often limited to investigational use at high-volume tertiary care centers.35,36 In the setting of glioblastoma, EOR outside the classically defined contrast-enhancing glioma margins offered a survival advantage in a large subset of patients.24 EOR beyond the imaging-defined tumor margin has been investigated in LGG, but those studies were done before WHO tumor subclassification or included small, single-institution series with a median follow-up of 5-6 years.15,37-41
In the third analysis, we investigated the interactive effects of molecular, clinical, and treatment variable on tumor progression, as LGGs are highly likely to progress. Analysis of the three cohorts combined identified PFS risk groups on the basis of postoperative TVs and LGG subtype (Fig 4A). The interaction was similar to that seen in the OS model (Fig 2A). Three MTFS risk groups on the basis of preoperative and postoperative TV, LGG subgroup, and age at diagnosis were also identified (Fig 4C).
The interactions between LGG subtype and clinical and therapeutic factors such as EOR have been a topic of great interest to the cancer community. However, a randomized trial of EOR would not be feasible because of the perceived lack of equipoise. Therefore, in the fourth analysis, we sought to mimic such a trial by propensity score matching analysis using the 757 patients in the combined three cohorts. This analysis provided convincing evidence that EOR ≥ 75% improves OS; however, the EOR threshold to alter the natural history of the disease by PFS and MTFS differs (EOR ≥ 80% and ≥ 70%, respectively).
In the analyses presented, we were able to address the concern that the survival benefit associated with LGG subtype may be attributable to the extent of resectability. We found no significant associations between TV and tumor subtype or EOR and tumor subtype (Table 1). In most cases, decisions about EOR are made without knowledge of the LGG subclassification. A presurgical biopsy of patients with presumed LGG would be costly, add risk, and delay treatment. However, advances in biomedical imaging and liquid biopsies are increasing diagnostic accuracy and may be clinically useful in the future.42-44
This study has several limitations. This retrospective cohort involves patients from three large tertiary referral centers across the United States and Europe. However, cohort size, median follow-up, and PFS varied across institution. As clinical practice varied across all sites treatment characteristics such as use of chemoradiation and EOR were not completely uniform. Furthermore, this analysis was focused on surgically resectable LGGs, as determined by the treating neurosurgeon. Therefore, patients with multifocal, diffuse disease in which biopsy might be indicated are not included in this analysis. Since slow-growing tumors may be treated multiple times over many years, there may be several interactions between factors over time. It is important to note that GTR and GTR+ occur predominantly in patients with smaller preoperative TVs (Data Supplement). When controlling for preoperative TVs, GTR+ was associated with a survival benefit for patients with astrocytoma; however, the same was not true for patients with oligodendroglioma. It remains unknown whether the apparent survival benefit is driven by lead time bias, extent of tumor resection, smaller preoperative TVs, or a combination. Furthermore, although glioma resection outside of the imaging defined tumor margins portends a survival advantage, these data do not offer a GTR+ EOR threshold and the volume of resection and interactions between tissue removal, neurological function, and OS are beyond the scope of this work. As a randomized clinical trial of EOR is not feasible, we used propensity matched scoring instead. This analysis, which considered prognostic covariates that would be used to stratify patients, demonstrated an EOR threshold; however, clinicians must consider the long-term effects of treatment on function, as well as survival.
ACKNOWLEDGMENT
The authors wish to acknowledge Ann Griffin, Joseph McGuire, and the UCSF Cancer Registry for providing updated survival data. The authors also wish to thank Sabrina Lin.
Shawn L. Hervey-Jumper
Consulting or Advisory Role: Gilmartin Capital
Open Payments Link: https://openpaymentsdata.cms.gov/physician/95897
Andrew Gogos
Honoraria: BrainLab
Travel, Accommodations, Expenses: Synaptive Medical
Javier Villanueva-Meyer
Consulting or Advisory Role: GE Healthcare
Research Funding: GE Healthcare
Nancy Ann Oberheim Bush
Consulting or Advisory Role: Gerson Lehrman Group
Jennie W. Taylor
Research Funding: Bristol Myers Squibb, AbbVie, Agios, Navio Theragnostics (Inst)
Nicholas Butowski
Stock and Other Ownership Interests: Cordance
Consulting or Advisory Role: VBL Therapeutics, DelMar Pharmaceuticals, QED Therapeutics, Karyopharm Therapeutics, VBI Vaccines, Lynx Group, Plus Therapeutics, Cellinta, Tocagen, KIYATEC, Gan & Lee, Sagimet Biosciences
Research Funding: AbbVie (Inst), Bristol Myers Squibb (Inst), Tocagen (Inst), Merck, Medicenna, Five Prime Therapeutics, Amgen (Inst), Orbus Therapeutics, Ipsen, Arbor Pharmaceuticals (Inst), EpicentRx, Deciphera (Inst), BeiGene (Inst), Oncoceutics (Inst), Istari (Inst), KIYATEC (Inst), BioMimetix (Inst)
Jennifer Clarke
Consulting or Advisory Role: Agios (Inst), Servier (Inst)
Research Funding: Agios (Inst), Merck (Inst), Servier (Inst)
Susan Chang
Consulting or Advisory Role: AstraZeneca
Michael McDermott
Honoraria: Insightec
Consulting or Advisory Role: Stryker
Patents, Royalties, Other Intellectual Property: Royalties from Limitorr CSF reservoir sales excluding my institution; manufacturer Integra Lifesciences; patent rights signed over to University of California many years ago
No other potential conflicts of interest were reported.
DISCLAIMER
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
PRIOR PRESENTATION
Presented at Society for Neuro-Oncology annual meeting, Austin, TX, November 19-20, 2020.
SUPPORT
Work at University of California, San Francisco, was supported by the National Institutes of Health (Grants Nos. 5P50CA097257-19, 5R01CA207360-05, 5K08NS110919-03, and R25CA112355), the loglio Collective, the National Brain Tumor Foundation, the Stanley D. Lewis and Virginia S. Lewis Endowed Chair in Brain Tumor Research, and the Robert Magnin Newman Endowed Chair in Neuro-oncology and by donations from families and friends of John Berardi, Helen Glaser, Elvera Olsen, Raymond E. Cooper, Resonance Philanthropies, and William Martinusen. Resources were provided by the UCSF Brain Tumor SPORE Biorepository [National Institutes of Health 5P50CA097257-18]. Supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant UL1 RR024131. The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; the Centers for Disease Control and Prevention's (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP006344; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute, Cancer Registry of Greater California.
M.S.B. and A.M.M. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Shawn L. Hervey-Jumper, Andrew Egladyous, Susan Chang, Timothy Smith, Mitchel S. Berger, Annette M. Molinaro
Financial support: Mitchel S. Berger
Administrative support: Lucie McCoy, Terri Rice
Provision of study materials or patients: Anny Shai, Terri Rice, Margaret Wrensch, John K. Wiencke, Nancy Ann Oberheim Bush, Jennie W. Taylor, Nicholas Butowski, Jennifer Clarke, Susan Chang, Edward Chang, Manish Aghi, Philip Theodosopoulos, Michael McDermott
Collection and assembly of data: Shawn L. Hervey-Jumper, Yalan Zhang, Joanna J. Phillips, Ramin A. Morshed, Jacob S. Young, Marisa Lafontaine, Tracy Luks, Simon Ammanuel, Sofia Kakaizada, Andrew Egladyous, Andrew Gogos, Anny Shai, Terri Rice, Jason Crane, Margaret Wrensch, John K. Wiencke, Mariza Daras, Nancy Ann Oberheim Bush, Jennie W. Taylor, Nicholas Butowski, Jennifer Clarke, Susan Chang, Edward Chang, Manish Aghi, Philip Theodosopoulos, Michael McDermott, Asgeir S. Jakola, Vasileios K. Kavouridis, Noah Nawabi, Ole Solheim, Timothy Smith, Annette M. Molinaro
Data analysis and interpretation: Shawn L. Hervey-Jumper, Yalan Zhang, Ramin A. Morshed, Jacob S. Young, Lucie McCoy, Andrew Egladyous, Javier Villanueva-Meyer, Gayathri Warrier, Vasileios K. Kavouridis, Ole Solheim, Timothy Smith, Mitchel S. Berger, Annette M. Molinaro
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Interactive Effects of Molecular, Therapeutic, and Patient Factors on Outcome of Diffuse Low-Grade Glioma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Shawn L. Hervey-Jumper
Consulting or Advisory Role: Gilmartin Capital
Open Payments Link: https://openpaymentsdata.cms.gov/physician/95897
Andrew Gogos
Honoraria: BrainLab
Travel, Accommodations, Expenses: Synaptive Medical
Javier Villanueva-Meyer
Consulting or Advisory Role: GE Healthcare
Research Funding: GE Healthcare
Nancy Ann Oberheim Bush
Consulting or Advisory Role: Gerson Lehrman Group
Jennie W. Taylor
Research Funding: Bristol Myers Squibb, AbbVie, Agios, Navio Theragnostics (Inst)
Nicholas Butowski
Stock and Other Ownership Interests: Cordance
Consulting or Advisory Role: VBL Therapeutics, DelMar Pharmaceuticals, QED Therapeutics, Karyopharm Therapeutics, VBI Vaccines, Lynx Group, Plus Therapeutics, Cellinta, Tocagen, KIYATEC, Gan & Lee, Sagimet Biosciences
Research Funding: AbbVie (Inst), Bristol Myers Squibb (Inst), Tocagen (Inst), Merck, Medicenna, Five Prime Therapeutics, Amgen (Inst), Orbus Therapeutics, Ipsen, Arbor Pharmaceuticals (Inst), EpicentRx, Deciphera (Inst), BeiGene (Inst), Oncoceutics (Inst), Istari (Inst), KIYATEC (Inst), BioMimetix (Inst)
Jennifer Clarke
Consulting or Advisory Role: Agios (Inst), Servier (Inst)
Research Funding: Agios (Inst), Merck (Inst), Servier (Inst)
Susan Chang
Consulting or Advisory Role: AstraZeneca
Michael McDermott
Honoraria: Insightec
Consulting or Advisory Role: Stryker
Patents, Royalties, Other Intellectual Property: Royalties from Limitorr CSF reservoir sales excluding my institution; manufacturer Integra Lifesciences; patent rights signed over to University of California many years ago
No other potential conflicts of interest were reported.
REFERENCES
- 1.Louis DN, Wiestler OD, Cavenee WK: World Health Organization Histological Classification of Tumours of the Central Nervous System (ed 4 revised). Lyon, France, International Agency for Research on Cancer, 2016 [Google Scholar]
- 2.Brat DJ, Verhaak RG, Aldape KD, et al. : Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 372:2481-2498, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis DN, Perry A, Wesseling P, et al. : The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol 23:1231-1251, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weller M, van den Bent M, Preusser M, et al. : EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 18:170-186, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckel-Passow JE, Lachance DH, Molinaro AM, et al. : Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 372:2499-2508, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molinaro AM, Taylor JW, Wiencke JK, et al. : Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol 15:405-417, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louis DN, Giannini C, Capper D, et al. : cIMPACT-NOW update 2: Diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant. Acta Neuropathol 135:639-642, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Chang EF, Smith JS, Chang SM, et al. : Preoperative prognostic classification system for hemispheric low-grade gliomas in adults. J Neurosurg 109:817-824, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Chaichana KL, McGirt MJ, Laterra J, et al. : Recurrence and malignant degeneration after resection of adult hemispheric low-grade gliomas. J Neurosurg 112:10-17, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Jakola AS, Myrmel KS, Kloster R, et al. : Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA 308:1881-1888, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Capelle L, Fontaine D, Mandonnet E, et al. : Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization grade II gliomas: A series of 1097 cases: Clinical article. J Neurosurg 118:1157-1168, 2013 [DOI] [PubMed] [Google Scholar]
- 12.McGirt MJ, Chaichana KL, Attenello FJ, et al. : Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery 63:700-707, 2008; author reply 707-708 [DOI] [PubMed] [Google Scholar]
- 13.Aghi MK, Nahed BV, Sloan AE, et al. : The role of surgery in the management of patients with diffuse low grade glioma: A systematic review and evidence-based clinical practice guideline. J Neurooncol 125:503-530, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Kavouridis VK, Boaro A, Dorr J, et al. : Contemporary assessment of extent of resection in molecularly defined categories of diffuse low-grade glioma: A volumetric analysis. J Neurosurg 1-11, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wijnenga MMJ, French PJ, Dubbink HJ, et al. : The impact of surgery in molecularly defined low-grade glioma: An integrated clinical, radiological, and molecular analysis. Neuro Oncol 20:103-112, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harary M, Kavouridis VK, Torre M, et al. : Predictors and early survival outcomes of maximal resection in WHO grade II 1p/19q-codeleted oligodendrogliomas. Neuro Oncol 22:369-380, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordier D, Gozé C, Schädelin S, et al. : A better surgical resectability of WHO grade II gliomas is independent of favorable molecular markers. J Neurooncol 121:185-193, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Jakola AS, Pedersen LK, Skjulsvik AJ, et al. : The impact of resection in IDH-mutant WHO grade 2 gliomas: A retrospective population-based parallel cohort study. J Neurosurg:1-8, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tom MC, Cahill DP, Buckner JC, et al. : Management for different glioma subtypes: Are all low-grade gliomas created equal? Am Soc Clin Oncol Ed Book 39:133-145, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Ding X, Wang Z, Chen D, et al. : The prognostic value of maximal surgical resection is attenuated in oligodendroglioma subgroups of adult diffuse glioma: A multicenter retrospective study. J Neurooncol 140:591-603, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Alattar AA, Brandel MG, Hirshman BR, et al. : Oligodendroglioma resection: A Surveillance, Epidemiology, and End Results (SEER) analysis. J Neurosurg 128:1076-1083, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Delev D, Heiland DH, Franco P, et al. : Surgical management of lower-grade glioma in the spotlight of the 2016 WHO classification system. J Neurooncol 141:223-233, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Fedorov A, Beichel R, Kalpathy-Cramer J, et al. : 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 30:1323-1341, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molinaro AM, Hervey-Jumper S, Morshed RA, et al. : Association of maximal extent of resection of contrast-enhanced and non-contrast-enhanced tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol 6:495-503, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen PY, Chang SM, Van den Bent MJ, et al. : Response assessment in neuro-oncology clinical trials. J Clin Oncol 35:2439-2449, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molinaro AM, Lostritto K, van der Laan M: partDSA: Deletion/substitution/addition algorithm for partitioning the covariate space in prediction. Bioinformatics 26:1357-1363, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lostritto K, Strawderman RL, Molinaro AM: A partitioning deletion/substitution/addition algorithm for creating survival risk groups. Biometrics 68:1146-1156, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Yao XI, Wang X, Speicher PJ, et al. : Reporting and guidelines in propensity score analysis: A systematic review of cancer and cancer surgical studies. J Natl Cancer Inst 109:djw323, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.R: A Language and Environment for Statistical Computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing, 2020. http://www.r-project.org/
- 30.Pignatti F, van den Bent M, Curran D, et al. : Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol 20:2076-2084, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Stupp R, Janzer RC, Hegi ME, et al. : Prognostic factors for low-grade gliomas. Semin Oncol 30:23-28, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Shaw E, Arusell R, Scheithauer B, et al. : Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: Initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol 20:2267-2276, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Franceschi E, Mura A, Lamberti G, et al. : Concordance between RTOG and EORTC prognostic criteria in low-grade gliomas. Future Oncol 15:2595-2601, 2019 [DOI] [PubMed] [Google Scholar]
- 34.Chang EF, Clark A, Jensen RL, et al. : Multiinstitutional validation of the University of California at San Francisco Low-Grade Glioma Prognostic Scoring System. Clinical article. J Neurosurg 111:203-210, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Claus EB, Horlacher A, Hsu L, et al. : Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer 103:1227-1233, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Pekmezci M, Morshed RA, Chunduru P, et al. : Detection of glioma infiltration at the tumor margin using quantitative stimulated Raman scattering histology. Sci Rep 11:12162, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pallud J, Varlet P, Devaux B, et al. : Diffuse low-grade oligodendrogliomas extend beyond MRI-defined abnormalities. Neurology 74:1724-1731, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Yordanova YN, Moritz-Gasser S, Duffau H: Awake surgery for WHO grade II gliomas within “noneloquent” areas in the left dominant hemisphere: Toward a “supratotal” resection. Clinical article. J Neurosurg 115:232-239, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Duffau H: Long-term outcomes after supratotal resection of diffuse low-grade gliomas: A consecutive series with 11-year follow-up. Acta Neurochir (Wien) 158:51-58, 2016 [DOI] [PubMed] [Google Scholar]
- 40.Albuquerque LAF, Almeida JP, de Macêdo Filho LJM, et al. : Extent of resection in diffuse low-grade gliomas and the role of tumor molecular signature-a systematic review of the literature. Neurosurg Rev 44:1371-1389, 2021 [DOI] [PubMed] [Google Scholar]
- 41.Rossi M, Gay L, Ambrogi F, et al. : Association of supratotal resection with progression-free survival, malignant transformation, and overall survival in lower-grade gliomas. Neuro Oncol 23:812-826, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villanueva-Meyer JE, Wood MD, Choi BS, et al. : MRI features and IDH mutational status of grade II diffuse gliomas: Impact on diagnosis and prognosis. AJR Am J Roentgenol 210:621-628, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Connolly ID, Li Y, Pan W, et al. : A pilot study on the use of cerebrospinal fluid cell-free DNA in intramedullary spinal ependymoma. J Neurooncol 135:29-36, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nassiri F, Chakravarthy A, Feng S, et al. : Detection and discrimination of intracranial tumors using plasma cell-free DNA methylomes. Nat Med 26:1044-1047, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]