Abstract
Drug-resistant variants of herpes simplex viruses (HSV) have been reported that are not effectively treated with first-line antiviral agents. The objective of this study was to evaluate available literature on the possible efficacy of second-line treatments in HSV and the use of second-line treatments in HSV strains that are resistant to first-line treatments. Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, a final search was conducted in six databases on November 5, 2021 for all relevant literature using terms related to antiviral resistance, herpes, and HSV. Eligible manuscripts were required to report the presence of an existing or proposed second-line treatment for HSV-1, HSV-2, or varicella zoster virus (VZV); have full-text English-language access; and potentially reduce the rate of antiviral resistance.
Following screening, 137 articles were included in qualitative synthesis. Of the included studies, articles that examined the relationship between viral resistance to first-line treatments and potential second-line treatments in HSV were included. The Cochrane risk-of-bias tool for randomized trials was used to assess risk of bias. Due to the heterogeneity of study designs, a meta-analysis of the studies was not performed. The dates in which accepted studies were published spanned from 2015-2021. In terms of sample characteristics, the majority (72.26%) of studies used Vero cells. When looking at the viruses on which the interventions were tested, the majority (84.67%) used HSV-1, with (34.31%) of these studies reporting testing on resistant HSV strains. Regarding the effectiveness of the proposed interventions, 91.97% were effective as potential managements for resistant strains of HSV. Of the papers reviewed, nectin in 2.19% of the reviews had efficacy as a second-line treatments in HSV, amenamevir in 2.19%, methanol extract in 2.19%, monoclonal antibodies in 1.46%, arbidol in 1.46%, siRNA swarms in 1.46%, Cucumis melo sulfated pectin in 1.46%, and components from Olea europeae in 1.46%. In addition to this griffithsin in 1.46% was effective, Morus alba L. in 1.46%, using nucleosides in 1.46%, botryosphaeran in 1.46%, monoterpenes in 1.46%, almond skin extracts in 1.46%, bortezomib in 1.46%, flavonoid compounds in 1.46%, andessential oils were effective in 1.46%, but not effective in 0.73%.
The available literature reviewed consistently supports the existence and potentiality of second-line treatments for HSV strains that are resistant to first-line treatments. Immunocompromised patients have been noted to be the population most often affected by drug-resistant variants of HSV. Subsequently, we found that HSV infections in this patient population are challenging to manage clinically effectively. The goal of this systematic review is to provide additional information to patients on the potentiality of second-line treatment in HSV strains resistant to first-line treatments, especially those who are immunocompromised. All patients, whether they are immunocompromised or not, deserve to have their infections clinically managed in a manner supported by comprehensive research. This review provides necessary information about treatment options for patients with resistant HSV infections and their providers.
Keywords: vzv, hsv-2, anti-hsv, second line drugs, resistant hsv, experimental pharmacology, general obgyn, antiviral resistance, direct anti-viral agents, hsv-1
Introduction and background
Although antiviral agents, including acyclovir, ganciclovir, and foscarnet, hold a vital role in the clinical management of herpes virus infections, drug-resistant variants of herpes simplex viruses (HSV) have been reported that are not effectively treated with these drugs [1,2]. Immunocompromised patients have been reported to be the primary population to present with viral strains that have mutations conferring resistance [1,3]. The primary goal of this systematic review is to evaluate the available literature on the possible efficacy of second-line treatments in HSV strains that are resistant to first-line treatments.
Review
Material and methods
Following prospectively registered protocol and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [4], a final search was conducted in six databases including PubMed via PubMed.gov, Embase via Embase.com, the Cochrane Library via Wiley, Web of Science, Clinicaltrials.gov, and ScienceDirect on November 5, 2021, for all relevant literature using terms including antiviral resistance, herpes virus, and HSV. Two authors independently reviewed studies for adherence to criteria, conducted risk assessments, and extracted results.
Eligibility Criteria
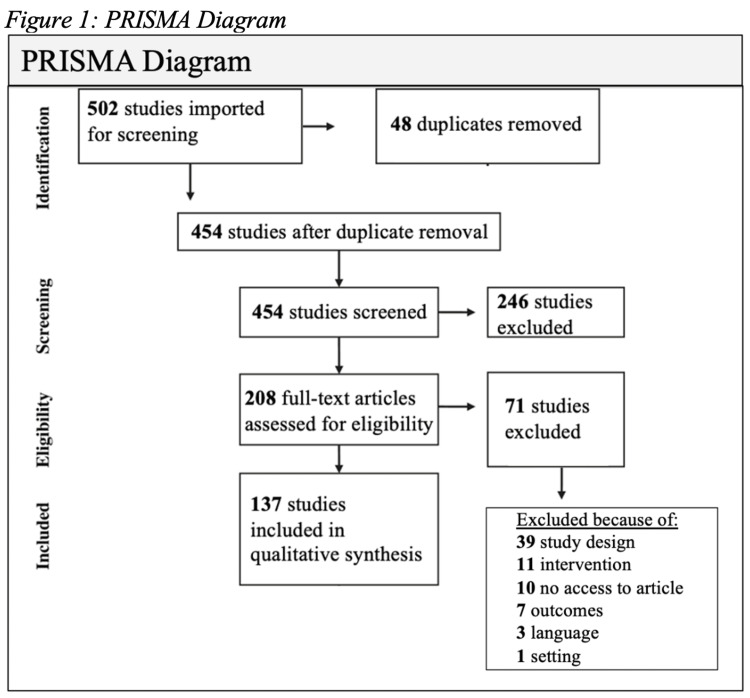
Eligible manuscripts were required to report the presence of an existing or proposed second-line treatment for HSV-1, HSV-2, or varicella zoster virus (VZV) and have full-text English-language access. Types of studies that were included within the search encompassed randomized controlled trials, research studies or articles, and research reports including alternative grey literature (Figure 1). Systematic reviews, clinical practice guidelines, and case reports were excluded. There were no limitations applied to this study that involved gender and age. The Cochrane risk-of-bias tool for randomized trials was used to assess the risk of bias. The study team conducted a database search of six databases on November 5, 2021.This search did not have severe limitations and was broadly inclusive in terms of types of articles and antiviral approaches. A broad search was conducted that included key terminology such as “antiviral resistance”, “herpes virus”, and “HSV” and was limited to English language studies published after 2015.
Figure 1. PRISMA diagram.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Study Selection
Utilizing the Covidence systematic review manager, two individual reviewers independently analyzed each publication for inclusion or exclusion criteria. Discrepancies were resolved through discussion by the entire review team including two subject matter experts.
Data Extraction
Seven reviewers independently analyzed manuscripts in a two-part methodology that consisted of a first pass and second pass so that each article was reviewed twice by two individual reviewers. In both the first and second pass, each reviewer extracted standardized information in accordance with the study objective. This process reported details such as a confirmation of intervention existence as well as details including intervention description, if the intervention was tested on HSV-1, HSV-2, and/or VZV and if said intervention was tested on a resistant strain of the virus.
Reviewers also outlined the methodology of the study as well as extracted what mutation conferring resistance was present (if any) and efficacy of the intervention. Reviewers stated the type of study in each article, sample size, sample characteristics, and if the intervention was recommended for inclusion or exclusion. Discrepancies were resolved through discussion by the entire review team including two subject matter experts. The main outcomes evaluated were the efficacy of a novel intervention against HSV-1, HSV-2, and VZV and the efficacy of these antivirals against drug-resistant variants of herpes simplex viruses. Efficacy was primarily determined by a decline in viral load, severity of infection, and/or reported interference with viral replication.
Assessment of Risk of Bias
The Cochrane Risk of Bias Tool for randomized trials was used to assess risk of bias including hidden allocations for personnel and participants [5]. There was also an evaluation of the generation of sequences in addition to outcome blindness, outcome data that was incomplete, reporting that was deemed selective, and additional sources of bias. The reporting, statistical results, and conflicts of interest, including funding, were also analyzed in terms of efficacy for drug-resistant variants of herpes simplex viruses for each proposed intervention.
Independently, two reviewers analyzed each of the 137 included studies, classifying them as ‘high’, ‘low’, or ‘unclear’ risk of bias for each criterion. A third reviewer resolved discrepancies in quality assessment. For each domain, the reasoning for the decision was detailed. The full-text manuscripts for individual studies were utilized as a summary of the reason for the decision. Among other domains included in this quality assessment, ‘other sources of bias’ were not included in this quality assessment.
Data Synthesis
Due to the heterogeneity of study designs, a meta-analysis of the studies was excluded. Pertinent study details were instead narrated.
Results
Study Selection
The initial literature search yielded 597 references. After duplicate removal, 454 articles underwent title and abstract screening. This yielded 208 articles eligible for full-text review that resulted in 137 articles that were included in qualitative synthesis.
Data Extraction
Of the included studies, articles that examined the relationship between viral resistance to first-line treatments and potential second-line treatments in HSV were included. A meta-analysis of the studies was excluded because of the nature of the study.
Synthesis of Results
The dates in which accepted studies were published spanned from 2015-2021. Within this time frame, there were two (1.46%) studies in 2015 [6,7], 15 (10.95%) studies in 2016 [1,3,8-20], 19 (13.87%) studies in 2017 [21-39], 27 (19.71%) studies in 2018 [40-64], 23 (16.79%) studies in 2019 [2,65-86], 24 (17.52%) studies in 2020 [87-110], and 27 (19.70%) studies in 2021 [111-137]. Regarding the type of studies reviewed, 121 (88.32%) studies included in vitro elements [2,3,6-12,14-20,23-26,28-42,44-47,49-56,58-64,66,68-72,74-77,79,80,82-87,89-118,120,122-133,135-139] and 23 (16.79%) in vivo [2,13,15,18,22,25,27,28,37,43,48,56,62,67,73,78,81,100,106,108-110,134,137]. Sample size was extremely variable and unable to be compared due to the differences in study designs. In terms of sample characteristics, 99 (72.26%) studies used Vero cells [2,3,6,8-10,14-20,23,25,26,28,30-34,36,39-42,44-47,50,51,53-56,58-64,66-70,72,74,76,77,79,82,83,85-87,89-95,97-108,111-113,115-118,120,122,123,125-130,132,133,135,137,138], 41 (29.93%) used human cell lines [7,11,12,16,19,24,29,31,36,38,47,49,52-56,61,62,67,69,71,75,78,80,83,84,96,99,100,102,104,109,110,112,114, 115,124,130,135,136], and 12 (8.76%) used various other animal cells (not Vero) [7,19,35,37,64, 78,86,89,109,126,131,139]. Of the 23 in vivo studies, mice and guinea pigs were the most common model [2,13,15,18,22,25,27,28,37,43,56,62,64,73,81,100,106,108-110,134,137].
Regarding which viruses the interventions were tested on, 113 (82.48%) studies used HSV-1 [1-3,6-16,19,21-27,29-31,33-35,37,38,40-44,46-49,51,53-56,60-65,67-74,76-79,82,83,85-99,103-123,125-134,136-138], 61 (44.53%) studies used HSV-2 [7-11,14-20,25,27,28,30-33,35-37,39,44-53,55,58,59,61, 66,69,73,75,81,83,85,89,91,93,100,102,104,105, 108-110,118,124,129,135-138], 7 (5.11%) used VZV [21,25,38,43,48,60,121] and 47 (34.31%) of these studies [1,2,6,9,11,13-15,19,20,27,30,31,33,35,38,39, 42,47,49,61,67,69,76,83,89-91,93,96,98,104,106,110,113,115,116,120,121, 123,126,128,130,131,134,136,137] reported testing on resistant HSV strains.
When determining the effectiveness of the proposed interventions, five (3.65%) were shown not to be effective [84,107,126,131,139], three (2.19%) were somewhat effective [21,46,53], and 128 (93.43%) were effective as potential managements for resistant strains of HSV [1-3,6-20,22-45,47-52,54-56,58-83,85-106,108-125,127-130,132-138].
Of the papers reviewed, three (2.19%) papers displayed that nectin had efficacy as a second-line treatments in HSV [17,28,73], three (2.19%) papers showed efficacy using amenamevir [21,43,95], three (2.19%) using methanol extract were effective in various cell line [24,32,39], two (1.46%) using monoclonal antibodies [27,46], two (1.46%) with arbidol [75,81], two (1.46%) with siRNA swarms [22,98], two (1.46%) using Cucumis melo sulfated pectin were effective in Vero cells [10,123], two (1.46%) using components from Olea europeae were effective in Vero cells [125,130]. In addition to this, two (1.46%) with griffithsin (GRFT) were effective in various cell lines [45,80], two (1.46%) with Morus alba L. (compounds from mulberry root bark extract) were effective in Vero cells [85,103], two (1.46%) using nucleosides were effective in Vero cells [41,76], two (1.46%) with botryosphaeran were effective in Vero cells [74,128], two (1.46%) monoterpenes were effective in Vero cells [34,92], two (1.46%) with almond skin extracts were effective in Vero cells [26,68], two (1.46%) using bortezomib were effective in HCL and Vero cells [83,99], two (1.46%) using various flavonoid compounds [25,97], and two (1.46%) papers using essential oils were effective in Vero cells [31,34], but one (0.73%) showed that essential oils were not effective [131].
Main characteristics of studies included in the systematic review is shown in Table 1.
Table 1. Main characteristics of studies included in the systematic review.
| Main characteristics of studies included in the systematic review | |||||||||||||
| First author | Title | Year | Type of study | Sample characteristics | Intervention | Intervention description | Tested on HSV-1? | Tested on HSV-2? | Tested on VZV? | Tested on resistant strain(s)? | Mutation conferring resistance | Efficacy? | Citation |
| Agostinho | Cucumis melo pectin as potential candidate to control herpes simplex virus infection | 2021 | In-vitro | Vero cells | Y | Cucumis melo sulfated pectin | Y | N | N | Y | ACV resistant | Y | [123] |
| Al-Salahi | Molecular docking study and antiviral evaluation of2-thioxo-benzo[g]quinazolin-4(3H)-one derivatives | 2016 | In-vitro | Vero cells | Y | Cucumis melo sulfated pectin | Y | Y | N | N | N/A | Y | [10] |
| Alvarez | Cetylpyridinium chloride blocks herpes simplex virus replication in gingival fibroblasts | 2020 | In-vitro | Epithelial cells, primary human gingival fibroblasts, Vero cells | Y | Cetylpyridinium chloride (CPC) | Y | Y | N | Y | ACV resistant | Y | [104] |
| Andrei | The Anti-Human Immunodeficiency Virus Drug Tenofovir, a Reverse Transcriptase Inhibitor, Also Targets the Herpes Simplex Virus DNA Polymerase | 2018 | In-vitro | Human embryonic lung (HEL) fibroblasts | Y | HSV-1 and HSV-2 mutants that are resistance to tenofovir and PMEO-DAPy were retested with PMEO-DAPy | Y | Y | N | Y | Tenofovir and PMEO-DAPy–resistant | Y | [49] |
| Andronova | Study of Antiherpetic Efficiency of Phosphite of Acycloguanosine Able to Overcome the Barrier of Resistance to Acyclovir | 2016 | In-vivo | Male white mice, male Agouti guinea pigs | Y | Phosphite of acycloguanosine | Y | N | N | Y | ACV resistant HSV-1 | Y | [13] |
| Arunkumar | Study on antiviral activities, drug-likeness and molecular docking of bioactive compounds of Punica granatum L. to Herpes simplex virus - 2(HSV-2) | 2018 | In-vitro | Human Epidermoid larynx carcinoma cell line | Y | Punica granatum fruit | N | Y | N | N | N/A | Y | [52] |
| Awad | Synthesis and Evaluation of Some Uracil Nucleosides as Promising Anti-Herpes Simplex Virus 1 Agents | 2021 | In-vitro | Vero cells | Y | In-vitro cyclic and acyclic nucleosies that incorporated 6-substituted- pyrimidine moieties | Y | N | N | N | N/A | Y | [111] |
| Barboza | In vitro effects of bufotenine against RNA and DNA viruses | 2021 | In-vitro | N/A | Y | Bufotenine, an alkaloid that can be found in plant extracts and skin secretions of amphibians | Y | N/A | N | Y | ACV resistant HSV-1 | N | [126] |
| Bauer | Antibody-based immunotherapy of acyclovir resistant ocular herpes simplex virus infections | 2017 | In-vivo | BALB/c mice | Y | Humanized monoclonal antibody (mAb) hu2c that targeted the HSV-1/2 glycoprotein B | Y | Y | N | Y | ACV resistant | Y | [27] |
| Ben-Amor | Phytochemical Characterization of Olea europea Leaf Extracts and Assessment of Their Anti-Microbial and Anti-HSV-1 Activity | 2021 | In-vitro | Vero cells | Y | Leaf extracts obtained from Olea europea L. var. sativa (OESA) and Olea europea var. sylvestris (OESY) from Tunisia | Y | N | N | N | N/A | Y | [125] |
| Benassi-Zanqueta | Evaluation of anti-HSV-1 activity and toxicity of hydroethanolic extractof Tanacetum parthenium (L.) Sch.Bip. (Asteraceae) | 2018 | In-vitro | N/A | Y | Crude extract of aerial parts of Tanacetum parthenium (L.) Sch.Bip. (Asteraceae), Liquid chromatography-mass spectrometry | Y | N | N | N | N/A | Y | [64] |
| Benzekri | Anti HSV-2 activity of Peganum harmala (L.) and isolation of the active compound | 2017 | In-vitro | Vero cells | Y | Methanol Seeds extract, know as alled Peganum harmala | N | Y | N | N | N/A | Y | [32] |
| Bereczki | Synthesis of Antiviral Perfluoroalkyl Derivatives of Teicoplanin and Vancomycin | 2020 | In-vitro | N/A | Y | Teichoplanins, a glycopeptide antibiotic derivative bearing perfluroroalkyl side chains | Y | Y | N | Y | TK mutation | Y | [89] |
| Bhutta | Peptide Inhibitor of Complement C1, RLS-0071, Reduces Zosteriform Spread of Herpes Simplex Virus Type 1 Skin Infection and Promotes Survival in Infected Mice | 2021 | In-vivo | BALB/cJ Mice | Y | RLS-0071, also known as peptide inhibitor of complement C1 (PIC1) | Y | N | N | Y | ACV resistant HSV-1 | Y | [134] |
| Bhutta | Ginkgolic Acid Inhibits Herpes Simplex Virus Type 1 Skin Infection and Prevents Zosteriform Spread in Mice | 2021 | In-vitro | Vero cells | Y | Ginkgolic acid | Y | N | N | Y | ACV resistant HSV-1 | Y | [120] |
| Bisignano | Almond Skin Extracts Abrogate HSV-1 Replication by Blocking Virus Binding to the Cell | 2017 | In-vitro, experimental study | Vero cells | Y | Extracts with the prevalent compounds quercetin, epicatechin and catechin | Y | N | N | N | N/A | Y | [26] |
| Bonvicini | Hemidesmus indicus (L.) R. Br. extract inhibits the early step of herpes simplex type 1 and type 2 replication | 2018 | In-vitro, experimental study | Vero cells | Y | A hydroalcoholic extract from Hemidesmus indicus root | Y | Y | N | N | N/A | Y | [44] |
| Brenner | The Molecular Tweezer CLR01 Inhibits Antibody-Resistant Cell-to-Cell Spread of Human Cytomegalovirus | 2021 | In-vitro, experimental study | Human foreskin fibroblasts (HFF) | Y | CLR01 | Y | Y | N | Y | Multi-resistant HSV-2 | Y | [136] |
| Brezáni | Anti-Infectivity against Herpes Simplex Virus and Selected Microbes and Anti-Inflammatory Activities of Compounds Isolated from Eucalyptus globulus Labill | 2018 | In-vitro | Vero cells | Y | 12 pure compounds and one mixture of two constitutional isomers from the leaves and twigs of E. globulus. E. Golulus from the Centrum of Medicinal Plants of the Medical Faculty of Masaryk University in Brno | Y | Y | N | N | N/A | Y | [138] |
| Cagno | In vitro anti-herpes simplex virus-2 activity of Salvia desoleana Atzei & V. Picci essential oil | 2017 | In-vitro | Vero cells, epithelial cell lines Hep-2 | Y | S. desoleana EO, fractions and main components: linalyl acetate, alpha terpinyl acetate, and germacrene D | Y | Y | N | Y | ACV resistant HSV-2 | Y | [31] |
| Castillo | Anti-herpetic Activity of Macrocystis pyrifera and Durvillaea antarctica Algae Extracts Against HSV-1 and HSV-2 | 2020 | In-vitro, in-vivo | Human cervix epithelial cell line (HeLa cells), primary human gingival fibroblasts, a mouse model | Y | Aqueous extracts obtained from two brown macroalgae, namely Macrocystis pyrifera and Durvillaea antarctica | Y | Y | N | Y | ACV resistant HSV-1 | Y | [110] |
| Chen | Targeting Aryl Hydrocarbon Receptor Signaling Enhances Type I Interferon-Independent Resistance to Herpes Simplex Virus | 2021 | In-vitro | Human monocytic THP-1 cells, human foreskin fibroblast 1 (HFF-1) cells, Vero cells | Y | Aryl hydrocarbon receptor (AHR) signaling | Y | N | N | N | N/A | Y | [112] |
| Crameri | MxB is an interferon-induced restriction factor of human herpesviruses | 2018 | In-vitro | Glioblastoma cells, human lung adenocarcinoma cells, vero cells, HEK-293 cells, and HeLa cells | Y | MxB, protein coded for/released in response to activation of the IFN system | Y | Y | N | N | N/A | PY | [53] |
| Criscuolo | Synergy evaluation of anti-Herpes Simplex Virus type 1 and 2 compounds acting on different steps of virus life cycle | 2018 | In-vitro | Vero cells | Y | Pairing viral DNA inhibitors + human IgG mAb | Y | Y | N | N | N/A | PY | [46] |
| Čulenová | Multiple In vitro biological effects of phenolic compounds from Morus alba root bark | 2019 | In-vitro | Vero cells | Y | Morus alba L. (compounds from mulberry root bark extract) | Y | Y | N | N | N/A | Y | [85] |
| D'Aiuto | R430: A potent inhibitor of DNA and RNA viruses | 2018 | In-vitro | Vero cells and human induced pluripotent stem cells (hiPSC-neurons) | Y | Transdihydrolycoricidine (R430), a lycorane-type alkaloid derivative | Y | Y | N | Y | ACV resistant | Y | [47] |
| Dai | Antiviral Effect of Retro-2.1 against Herpes Simplex Virus Type 2 In Vitro | 2018 | In-vitro | Vero cells | Y | Retro-2.1, an optimized, more potent derivative of Retro-2cyc | N | Y | N | N | N/A | Y | [50] |
| Dai | Antiviral Effects of ABMA against Herpes Simplex Virus Type 2 In Vitro and In Vivo | 2018 | In-vitro | Vero cells | Y | The small molecule ABMA [1-adamantyl (5-bromo-2-methoxybenzyl) amine], acting on host-endosomal trafficking | N | Y | N | N | N/A | Y | [58] |
| Deback | Antiviral effects of Cacicol (®), a heparan sulfate biomimetic for corneal regeneration therapy, for herpes simplex virus type-1 and varicella zoster virus infection | 2018 | In-vitro | Vero cells | Y | Cacicol a poly-carboxymethylglucose sulfate solution that is a regernating matrix therapy agent intended for wound healing | Y | N | Y | N | N/A | Y | [60] |
| Derby | Griffithsin carrageenan fast dissolving inserts prevent SHIV HSV-2 and HPV infections in vivo | 2018 | In-vitro | Vero cells | Y | GRFT Gel for vaginal use | N | Y | N | N | N/A | Y | [45] |
| Deschamps | Discovery of Small-Molecule Inhibitors Targeting the E3 Ubiquitin Ligase Activity of the Herpes Simplex Virus 1 ICP0 Protein Using an In Vitro High-Throughput Screening Assay | 2019 | In-vitro | Vero cells | Y | ICP0, a promiscuous transactivator that enables viral gene expression by disrupting DNA repressor complexes and blocking antiviral responses | Y | N | N | N | N/A | Y | [82] |
| Ding | T-type calcium channels blockers inhibit HSV-2 infection at the late stage of genome replication | 2020 | In-vitro | Vero cells, HeLa Cells | Y | T-type calcium channel blockers | N | Y | N | N | N/A | Y | [102] |
| Ding | Cellular Signaling Analysis shows antiviral, ribavirin-mediated ribosomal signaling modulation | 2019 | In-vitro | Vero cells | Y | Ribavirin-mediated ribosomal signaling modulation, interferons, and S6 kinase inhibitor SL010 | Y | N | N | N | N/A | Y | [77] |
| Donalisio | The traditional use of Vachellia nilotica for sexually transmitted diseases is substantiated by the antiviral activity of its bark extract against sexually transmitted viruses | 2017 | In-vitro | Vero cells | Y | V. nilotica chloroform, methanolic and water bark extracts | N | Y | N | Y | ACV resistant HSV-2 | Y | [39] |
| Dong | The Natural Compound Homoharringtonine Presents Broad Antiviral Activity In Vitro and In Vivo | 2018 | In-vitro, in-vivo | Specific Pathogen-free Chicken Embryo, Vero cells, HEK293Y cells, HeLa cells | Y | Homoharringtonine (HHT) | Y | N | N | N | N/A | Y | [62] |
| Du | The antiviral activity of arbidol hydrochloride against herpes simplex virus type II (HSV-2) in a mouse model of vaginitis | 2019 | In-vivo | Vaginitis animals model | Y | Arbidol (ARB) | N | Y | N | N | N/A | Y | [81] |
| El-Haddad | Brincidofovir (CMX-001) for refractory and resistant CMV and HSV infections in immunocompromised cancer patients: A single-center experience | 2016 | Clinical trial | 4 cancer patients with resistant to CMV or HSV infections | Y | Brincidofovir under emergency IND application | Y | N | N | Y | ACV resistant HSV | Y | [1] |
| El-Shiekh | Novel Antiviral and Antibacterial Activities of Hibiscus schizopetalus | 2020 | In-vitro | N/A | Y | 70% ethanolic extract (Et-E) of the aerial parts of the Hibiscus schizopetalus (Dyer) Hook.f. (Malvaceae), an ornamental plant | N | N | N | N | N/A | Y | [101] |
| Eletskaya | Enzymatic synthesis of novel purine nucleosides bearing a chiral benzoxazine fragment | 2019 | In-vitro | N/A | Y | A series of ribo- and deoxyribonucleosides | Y | N | N | Y | Acyclovir-resistant strain of HSV-1 | Y | [76] |
| Elias | In Vitro Analysis of the Antioxidant and Antiviral Activity of Embelin against Herpes Simplex Virus-1 | 2021 | In-vitro | Vero cells | Y | Embelin | Y | N | N | N | N/A | Y | [122] |
| Fujimoto | Accumulation of a soluble form of human nectin-2 is required for exerting the resistance against herpes simplex virus type 2 infection in transfected cells | 2016 | In-vitro | Vero cells | Y | A soluble form of human nectin-2 (hNectin-2Ig), transfected cells expressing the entire ectodomain of nectin-2 fused to the Fc portion of human IgG | N | Y | N | N | N/A | Y | [17] |
| Fujimoto | Evaluation of the antiviral potential of the soluble forms of glycoprotein D receptors on ocular herpes caused by HSV-1 and HSV-2 infections in a transgenic mouse model | 2019 | In-vivo | Mice | Y | Transgenic mouse serum containing nectin-1Ig | Y | Y | N | N | N/A | Y | [73] |
| Fujimoto | Comparison of the antiviral potential among soluble forms of herpes simplex virus type-2 glycoprotein D receptors, herpes virus entry mediator A, nectin-1 and nectin-2, in transgenic mice | 2017 | In-vitro, in-vivo | Mice | Y | Soluble forms of HVEM, nectin-1 and nectin-2 | N | Y | N | N | N/A | Y | [28] |
| García-Serradilla | Drug repurposing for new, efficient, broad spectrum antivirals | 2019 | Data Analysis | N/A | Y | Repurposed antiviral drug with different mechanisms of action: digoxin, sunitinib, chloroquine, cyclosporine A and silver nanoparticles in addition to combination therapies with more than one drug | PY | N | N | N | N/A | Y | [65] |
| Ghaffari | Inhibition of herpes simplex virus type 1 infection by Sambucus ebulus extract in vitro | 2021 | In-vitro, experimental | Vero cells | Y | Extracts from S. ebulus | Y | N | N | N | N/A | Y | [132] |
| Ghosh | Ficus religiosa L. bark extracts inhibit infection by herpes simplex virus type 2 in vitro | 2016 | In-vitro, experimental | Vero cells | Y | F. religiosa extract | N | Y | N | Y | ACV resistant | Y | [20] |
| González-García | Antimicrobial Activity of Cyclic-Monomeric and Dimeric Derivatives of the Snail-Derived Peptide Cm-p5 against Viral and Multidrug-Resistant Bacterial Strains | 2021 | In-vitro, experimental | Vero cells, HEK293T cells | Y | Cm-p5 is a snail-derived antimicrobial peptide | N | Y | N | N | N/A | Y | [135] |
| Greeley | Acyclovir, cidofovir, and amenamevir have additive antiviral effects on herpes simplex virus TYPE 1 | 2020 | In-vitro | Vero cells | Y | (DOE) function in Minitab analyzed the drug-drug interactions of the combination of acyclovir, cidofovir, and amenamevir | Y | N | N | N | N/A | Y | [95] |
| Hopkins | In Vitro and In Vivo Activity, Tolerability, and Mechanism of Action ofBX795 as an Antiviral against Herpes Simplex Virus 2 Genital Infection | 2020 | In-vitro, in-vivo | BX795, 8-week-old C57BL/6 female mice | Y | BX795 | N | Y | N | N | N/A | Y | [100] |
| Hou | Antiviral activity of PHA767491 against human herpes simplex virus invitro and in vivo | 2017 | In-vitro, in-vivo | L929 cells, 8-week-old RIP3 KO mice | Y | More than 1000 compounds for some antiviral drugs were screened by using the model in which HSV-1 directly induced necrosis of L929 | Y | Y | N | N | N/A | Y | [37] |
| Houston | Potentiated virucidal activity of pomegranate rind extract (PRE) and punicalagin against Herpes simplex virus (HSV) when co-administered with zinc (II) ions, and antiviral activity of PRE against HSV and aciclovir-resistant HSV | 2017 | In-vitro | Vero cells | Y | Pomegranate rind extract (PRE) was used in conjunction with zinc (II) salts | Y | Y | N | Y | ACV-resistant HSV-2 | Y | [30] |
| Huang | Antiviral activity of mitoxantrone dihydrochloride against human herpes simplex virus mediated by suppression of the viral immediate early genes | 2019 | In-vitro | Mouse fibroblast cells (L929), Vero cells | Y | Mitoxantrone dihydrochloride (MD) | Y | N | N | N | N/A | Y | [86] |
| Hutterer | Inhibitors of dual-specificity tyrosine phosphorylation-regulated kinases(DYRK) exert a strong anti-herpes viral activity | 2017 | In-vitro | Human foreskin fibroblasts (HFFs), Vero cells | Y | Novel benzohydrofurane derivatives that target DYRK activity | Y | N | Y | Y | GCV- resistant strain | Y | [38] |
| Ibáñez | Pharmacological Induction of Heme Oxygenase-1 Impairs Nuclear Accumulation of Herpes Simplex Virus Capsids upon Infection | 2017 | In-vitro | Vero cells, HeLa cells | Y | Modulating heme oxygenase-1 (HO-1) | N | Y | N | N | N/A | Y | [36] |
| Ireland | Synthetic α-Hydroxytropolones Inhibit Replication of Wild-Type and Acyclovir-Resistant Herpes Simplex Viruses | 2016 | In-vitro | Vero cells | Y | Hydroxytropolone pharmacophore | Y | Y | N | Y | (TK)-deficient mutant of HSV-1 and HSV-2 | Y | [9] |
| Ishimaru | MG132 exerts anti-viral activity against HSV-1 by overcoming virus-mediated suppression of the ERK signaling pathway | 2020 | In-vitro | Vero cells, HepG2, H1299, ME180, MCF7, HeLa cells | Y | Protease inhibitors (TLCK, TPCK, E64, bortezomib, or MG132) | Y | N | N | N | N/A | Y | [99] |
| Jaishankar | An off-target effect of BX795 blocks herpes simplex virus type 1 infection of the eye | 2018 | In-vitro, in-vivo, ex-vivo | Human corneal epithelial (HCE) cells, Mouse model, porcine and human cornea organ culture | Y | BX795 and its potential synergism with trifluridine (TFT) | Y | N | N | N | N/A | Y | [56] |
| Jin | Pentagalloylglucose Blocks the Nuclear Transport and the Process of Nucleocapsid Egress to Inhibit HSV-1 Infection | 2015 | In-vitro | Vero cells | Y | Pentagalloylglucose (PGG)-induced inhibition of nuclear transport and nucleocapsid egress | Y | N | N | Y | A TK mutant from HSV-1 and two ACV-resistant clinical HSV-1 strains | Y | [6] |
| Jones | Modified cyclodextrins as broad-spectrum antivirals | 2020 | In-vitro | Vero cells | Y | Cyclodextrins modified with mercaptoundecane sulfonic acids | Y | Y | N | Y | ACV resistant HSV-2 | Y | [93] |
| Kalke | Herpes Simplex Virus Type 1 Clinical Isolates Respond to UL29-TargetedsiRNA Swarm Treatment Independent of Their Acyclovir Sensitivity | 2020 | In-vitro | Vero cells | Y | Enzymatically synthesized siRNA swarms | Y | N | N | Y | ACV resistant HSV-1 | Y | [98] |
| Kannan | Anti-herpes virus activity of the carnivorous botanical, Sarracenia purpurea | 2020 | In-vitro | Vero cells | Y | S. purpurea extract | Y | N | N | N | N/A | Y | [87] |
| Karpov | [A Plasmid-Expressed CRISPR/Cas9 System Suppresses Replication of HSV Type I in a Vero Cell Culture] | 2019 | In-vitro | Vero cells | Y | Genome editing via prokaryotic plasmid CRISPR/Cas9 | Y | N | N | N | N/A | Y | [79] |
| Katsumata | Antiviral efficacy of the helicase-primase inhibitor amenamevir in murinemodels of severe herpesvirus infection | 2018 | In-vivo | Mice | Y | Amenamevir, a helicase-primase inhibitor | Y | N | Y | N | N/A | Y | [43] |
| Kaushik | Antiviral potential and mode of action of Indigofera heterantha against HSV-2 by targeting the early stages of infection | 2016 | In-vitro, in-vivo | Mice and plaque reduction assays | Y | Extract of roots of the plant Indigofera heterantha | N | Y | N | N | N/A | Y | [18] |
| Kim | Quercus acuta Thunb. (Fagaceae) and Its Component, Isoquercitrin, InhibitHSV-1 Replication by Suppressing Virus-Induced ROS Production and NF-κB Activation | 2021 | In-vitro | Vero cells | Y | Quercus acuta Thunb (Fagaceae) (QA) extract | Y | N | N | N | N/A | Y | [127] |
| Kim | Mori ramulus and its Major Component Morusin Inhibit Herpes Simplex Virus Type 1 Replication and the Virus-Induced Reactive Oxygen Species | 2020 | In-vitro | Vero cells | Y | Mori ramulus (the young twig of Morus alba L.) | Y | N | N | N | N/A | Y | [103] |
| Kongyingyoes | 3,19-isopropylideneandrographolide suppresses early gene expression of drug-resistant and wild type herpes simplex viruses | 2016 | In-vitro | Vero cells | Y | A diterpenoid lactone, 3,19-isopropylideneandrographolide (IPAD) compound isolated from Andrographis | Y | Y | N | Y | ACV-resistant and (TK) deficient | Y | [14] |
| Kumar | Inhibition of herpes simplex virus-1 infection by MBZM-N-IBT: in silico and in vitro studies | 2021 | In-vitro | Vero cells | Y | MBZM-N-IBT impact against HSV-1 | Y | N | N | N | N/A | Y | [133] |
| Labrunie | UL23, UL30, and UL5 characterization of HSV1 clinical strains isolated from hematology department patients | 2019 | In-vitro | N/A | N | Genetic variants | N | N | N | N | N/A | N | [84] |
| Le-Trilling | Broad and potent antiviral activity of the NAE inhibitor MLN4924 | 2016 | In-vitro | N/A | Y | NAE inhibitor MLN4924 | Y | Y | N | Y | ACV, CDV and PFA resistant HSV-1 | Y | [19] |
| Lebrun | Varicella-Zoster Virus ORF9p Binding to Cellular Adaptor Protein Complex 1Is Important for Viral Infectivity | 2018 | In-vitro | Yeast cells | N | ORF9p proteins | N | N | N | N | N/A | N | [139] |
| Lee | Efficacy of brincidofovir as prophylaxis against HSV and VZV in hematopoietic cell transplant recipients | 2018 | In-vivo | 2710 patient-days | Y | Brincidofovir a lipid conjugate of cidofovir | Y | Y | Y | N | N/A | Y | [48] |
| Lei | Preparation of a monoPEGylated derivative of cyanovirin-N and its virucidal effect on acyclovir-resistant strains of herpes simplex virus type 1 | 2019 | In-vivo | N/A | Y | Cyanovirin-N (CV-N) more specifically LCV-N as the most potent of three compounds | Y | N | N | Y | ACV resistant | Y | [2] |
| Li | Amentoflavone Inhibits HSV-1 and ACV-Resistant Strain Infection by Suppressing Viral Early Infection | 2019 | In-vivo | N/A | Y | Amentoflavone, a naturally occurring biflavonoid | Y | N | N | Y | ACV resistant | Y | [67] |
| Li | Anti-herpes simplex virus type 1 activity of Houttuynoid A, a flavonoidfrom Houttuynia cordata Thunb | 2017 | In-vitro, in-vivo | Mice | Y | Houttuynia A cordata Thunb. water extract, a new type of flavonoid isolated from H. cordata | Y | Y | Y | N | N/A | Y | [25] |
| Liu | Antiviral activities of Janus-type nucleosides and their related oxime-intermediates | 2018 | In-vitro | Vero cells | Y | Janus-type nucleosides combining the natural genetic alphabets into a singular nucleoside structural unit | Y | N | N | N | N/A | Y | [41] |
| Liu | Harringtonine Inhibits Herpes Simplex Virus Type 1 Infection by Reducing Herpes Virus Entry Mediator Expression | 2021 | In-vitro | Vero cells | Y | Harringtonine | Y | N | N | Y | (TK) mutation in HSV-1 | Y | [116] |
| Lopes | Sulfonated and Carboxymethylated β-Glucan Derivatives with Inhibitory Activity against Herpes and Dengue Viruses | 2021 | In-vitro | Vero cells | Y | (1→3)(1→6)-β-D-glucan, botryosphaeran, similar to an anionic polysaccharide | Y | N | N | Y | ACV resistant | Y | [128] |
| Luganini | Effective deploying of a novel DHODH inhibitor against herpes simplex type1 and type 2 replication | 2021 | In-vitro | Vero cells | Y | MEDS433 a pyrimidine synth inhibitor | Y | Y | N | N | N/A | Y | [129] |
| Ma | Herpes simplex virus type 1 (HSV-1) specific T-cell generation fromHLA-A1- and HLA-A2-positive donors for adoptive immunotherapy | 2016 | In-vitro | Peripheral blood mononuclear cells from HLA-A1 and HLA-A2 HSV-seropositive hereditary hemochromatosis donors | Y | HSV-1-specific T cells | Y | N | N | N | N/A | Y | [12] |
| Ma | Assessment of a new arbidol derivative against herpes simplex virus II inhuman cervical epithelial cells and in BALB/c mice | 2019 | In-vitro | HCE cells | Y | Arbidol derivative (ARD) | N | Y | N | N | N/A | Y | [75] |
| Maizel | Study of the Extremely-Tolerant Brevibacterium linens AE038-8 with Antiviral Activity Against Herpes Simplex Virus Type 1 | 2021 | In-vitro | N/A | Y | B. linens AE038-8 | Y | N | N | Y | ACV resistant | Y | [113] |
| Mandalari | Simulated human digestion of N1-aryl-2-arylthioacetamidobenzimidazoles and their activity against Herpes-simplex virus 1 in vitro | 2019 | In-vitro | N/A | Y | NAAB-496 and NAAB-503 | Y | N | N | N | N/A | Y | [72] |
| Marcocci | The Amphibian Antimicrobial Peptide Temporin B Inhibits In Vitro Herpes Simplex Virus 1 Infection | 2018 | In-vitro | Vero cells, human epithelial cells | Y | Temporin B (TB) | Y | N | N | N | N/A | Y | [54] |
| Marino-Merlo | Anti-herpes simplex virus 1 and immunomodulatory activities of a poly-γ-glutamic acid from Bacillus horneckiae strain APA of shallow vent origin | 2017 | In-vitro | HEp-2 cells, U937 cells, | Y | Poly-γ-glutamic acid (γ-PGA-APA) | Y | N | N | N | N/A | Y | [29] |
| Mello | Perillyl alcohol and perillic acid exert efficient action upon HSV-1maturation and release of infective virus | 2020 | In-vitro | Vero cells | Y | Monoterpenes perillyl alcohol (POH) and perillic acid (PA) | Y | N | N | N | N/A | Y | [92] |
| Mishra | Herbal Gel Formulation Developed for Anti-Human Immunodeficiency Virus(HIV)-1 Activity Also Inhibits In Vitro HSV-2 Infection | 2018 | In-vitro | Vero cells | Y | Polyherbal gel formulation (aqueous gel formulation comprising of 50% ethanolic extracts prepared from stem bark of Acacia catechu, leaves of Lagerstroemia speciosa, and fruits of Terminalia chebula & Phyllanthus emblica) | N | Y | N | N | N/A | Y | [59] |
| Mohammed | Synthesis and anti-HSV activity of tricyclic penciclovir and hydroxybutyl guanine derivatives | 2019 | In-vitro | Human embryonic lung (HEL) cell, Vero cells, HeLa cells, MDCK cells | Y | Novel tricyclic derivatives | Y | Y | N | Y | ACV resistant, (TK-) | Y | [69] |
| Monjo | Photodynamic Inactivation of Herpes Simplex Viruses | 2018 | In-vitro | HeLa, HEK293A, Vero cells | Y | Orthoquin in sub-cytotoxic doses | Y | Y | N | N | N/A | Y | [55] |
| Moshaverinia | Evaluation of the effect of hydro alcoholic extract of cinnamon on herpes simplex virus-1 | 2020 | In-vitro | N/A | Y | Hydroalcoholic extract of cinnamon | Y | N | N | N | N/A | Y | [94] |
| Musarra-Pizzo | The Antimicrobial and Antiviral Activity of Polyphenols from Almond(Prunus dulcis L.) Skin | 2019 | In-vitro | Vero cells | Y | Natural almond skin (NS MIX) | Y | N | N | N | N/A | Y | [68] |
| Novoa | Antiviral Activity of Myticin C Peptide from Mussel: an Ancient Defense against Herpesviruses | 2016 | In-vitro | Vero cells | Y | Myticin C Peptide | Y | Y | N | N | N/A | Y | [8] |
| Paavilainen | Topical treatment of herpes simplex virus infection with enzymatically created siRNA swarm | 2017 | In-vivo | BALB/c mice | Y | Treated with a swarm of enzymatically created, Dicer-substrate small interfering RNA (siRNA) molecules that targeted the HSV gene UL29 | Y | N | N | N | N/A | Y | [22] |
| Parsania | Antiviral screening of four plant extracts against acyclovir resistant herpes simplex virus type-1 | 2017 | In-vitro | N/A | Y | Methanolic extract of four plants | Y | N | N | N | N/A | Y | [24] |
| PiresdeMello | Aminomethylnaphthoquinones and HSV-1: in vitro and in silico evaluations of potential antivirals | 2016 | In-vitro | Vero cells | Y | Three 2-aminomethyl-3-hydroxy-1,4-naphthoquinones | Y | N | N | N | N/A | Y | [3] |
| Pradhan | Herpes simplex virus virucidal activity of MST-312 and epigallocatechin gallate | 2018 | In-vitro | N/A | Y | MST-312 | Y | N | N | N/A | N/A | Y | [63] |
| Praena | Amidic derivatives of valproic acid, valpromide and valnoctamide, inhibitHSV-1 infection in oligodendrocytes | 2019 | In-vivo | Glial cells | Y | Two amidic derivatives of valproic acid (VPA) - valpromide (VPD) and valnoctamide (VCD) | Y | N | N | N | N/A | Y | [78] |
| Pujol | Polyhydroxylated sulfated steroids derived from 5α-cholestanes as antiviral agents against herpes simplex virus | 2016 | In-vitro | Human cells lines, vero cells | Y | Twelve polyhydroxylated sulfated steroids synthesized from a 5α-cholestane skeleton with different substitutions in C-2, C-3 and C-6 | Y | Y | N | N | N/A | Y | [16] |
| Quenelle | Efficacy of pritelivir and acyclovir in the treatment of herpes simplex virus infections in a mouse model of herpes simplex encephalitis | 2017 | In-vitro | Mice | Y | Pritelivir, a helicase-primase inhibitor, has excellent in vitro and in vivo activity against human herpes simplex virus (HSV). Mice lethally infected with HSV type 1 or 2, including acyclovir-resistant strains, were treated 72 h after infection for 7 days with pritelivir or acyclovir. | Y | Y | N | Y | ACV resistant | Y | [35] |
| Rechenchoski | Mangiferin: A promising natural xanthone from Mangifera indica for the control of acyclovir - resistant herpes simplex virus 1 infection | 2020 | In-vitro, in-vivo | Vero cells | Y | M. Indica (Mangiferin; a mango extract) | Y | N | N | Y | ACV-resistant HSV-1 | Y | [106] |
| Rittà | Antiviral Activity of a Arisaema Tortuosum Leaf Extract and Some of its Constituents against Herpes Simplex Virus Type 2 | 2020 | In-vitro | Vero cells | Y | Arisaema tortuosum, a plant medicine from India | Y | Y | N | Y | Acyclovir-resistant HSV-2 | Y | [91] |
| Ruzsics | A Novel, Broad-Acting Peptide Inhibitor of Double-Stranded DNA Virus Gene Expression and Replication | 2020 | In-vitro | Vero cells | Y | A novel peptide called TAT-I24 | Y | N | N | Y | ACV resistant | Y | [90] |
| Sacchelli | Botryosphaeran and sulfonated derivatives as novel antiviral agents for herpes simplex and dengue fever | 2019 | In-vitro | Vero cells | Y | Botryosphaeran, a fungal exocellular (1 → 3)(1 → 6)-β-D glucan devoid of sulfate groups | Y | N | N | N | N/A | Y | [74] |
| SadeghEhdaei | Cellular miR-101-1 Reduces Efficiently the Replication of HSV-1 in HeLa Cells | 2021 | In-vitro | HeLa cells | Y | Hsa-miR-101-1 | Y | N | N | N | N/A | Y | [114] |
| Sanchez | Development and evaluation of a host-targeted antiviral that abrogates herpes simplex virus replication through modulation of arginine-associated metabolic pathways | 2016 | In-vitro | Primary human corneal epithelial cells (HCEC) | Y | A pegylated recombinant human Arginase I (peg-ArgI) | Y | Y | N | Y | Polymerase (PAAr5) or thymidine kinase (tkLTRZ1; tkG7dG.2) genes | Y | [11] |
| Sasaki | In vitro and in vivo antiherpetic effects of(1R,2R)-1-(5'-methylful-3'-yl)propane-1,2,3-triol | 2016 | In-vitro, in-vivo | Female BALB/c mice 5–6 weeks old | Y | MFPT | Y | Y | N | Y | ACV resistant HSV-1 | Y | [15] |
| Schneider | Early Steps in Herpes Simplex Virus Infection Blocked by a Proteasome Inhibitor | 2019 | In-vitro | Vero cells, human foreskin fibroblasts | Y | Bortezomib and many of its property against HSV | Y | Y | N | Y | ACV resistant | Y | [83] |
| Shabani | Inhibition of herpes simplex virus type 1 replication by novelhsa-miR-7704 in vitro | 2019 | In-vitro | HeLa cells | Y | A novel miRNA (hsa-miR-7704), expressed in macrophages | Y | N | N | N | N/A | Y | [71] |
| Shan | Viral UL8 Is Involved in the Antiviral Activity of Oleanolic Acid AgainstHSV-1 Infection | 2021 | In-vitro | Vero cells, Human immortalized keratinocyte cell line (HaCaT) | Y | Oleanolic acid, a pentacyclic triterpenoid widely existing in natural product | Y | N | N | Y | TK mutant from HSV-1 and two clinical ACV-resistant HSV-1 strains | Y | [130] |
| Shao | Poly(dA:dT) Suppresses HSV-2 Infection of Human Cervical Epithelial Cells Through RIG-I Activation | 2021 | In-vitro | Human endocervical epithelia (End1) cells | Y | Poly (dA:dT) treatment of End1/E6E7 cells | N | Y | N | N | N/A | Y | [124] |
| Sharifi-Rad | Susceptibility of herpes simplex virus type 1 to monoterpenes thymol, carvacrol, p-cymene and essential oils of Sinapis arvensis L., Lallemantia royleana Benth. and Pulicaria vulgaris Gaertn | 2017 | In-vitro | Vero cells | Y | Three monoterpenes (thymol, carvacrol and p-cymene) and three essential oils | Y | N | N | N | N/A | Y | [34] |
| Sharifi-Rad | Antiviral activity of Veronica persica Poir. on herpes virus infection | 2018 | In-vitro | Vero cells | Y | Veronica persica Poir extract | Y | Y | N | N | N/A | Y | [51] |
| Shiraki | Helicase-primase inhibitor amenamevir for herpesvirus infection: Towards practical application for treating herpes zoster | 2017 | N/A | N/A | Y | Helicase-primase inhibitors (HPIs) inhibit the progression of the replication fork ( initial step in DNA synthesis to separate the double strand into two single strands). The HPIs amenamevir and pritelivir have a novel mechanism of action, once-daily administration with nonrenal excretory characteristics, and clinical efficacy for genital herpes. | Y | N | Y | N | N/A | PY | [21] |
| Shiraki | Amenamevir, a Helicase-Primase Inhibitor, for the Optimal Treatment of Herpes Zoster | 2021 | N/A | N/A | Y | Amenamevir and synergism with acyclovir. | Y | N | Y | Y | Amenamevir-resistant viruses with changes in the helicase and primase of amenamevir-resistant HSV mutants | Y | [121] |
| Spengler | Antiviral, Antimicrobial and Antibiofilm Activity of Selenoesters and Selenoanhydrides | 2019 | In-vitro | Vero cells | Y | Selenoesters and selenium isostere | N | Y | N | N | N/A | Y | [66] |
| Stegman | Volatile Acid-Solvent Evaporation (VASE): Molecularly Homogeneous Distribution of Acyclovir in a Bioerodable Polymer Matrix for Long-Term Treatment of Herpes Simplex Virus-1 Infections | 2018 | In-vitro | Vero cells | Y | Bioerodable polymer polycaprolactone | Y | N | N | N | N/A | Y | [40] |
| Suryawanshi | Bacterial Pigment Prodigiosin Demonstrates a Unique Antiherpes virus Activity That Is Mediated through Inhibition of Prosurvival Signal Transducers | 2020 | In-vitro, ex-vivo, in-vivo | Human corneal epithelial (HCE) cells, HeLa cells, C57BL/6 mice, porcine corneal model, whole pig eyes | Y | Prodigiosin (PG) | Y | Y | N | N | N/A | Y | [109] |
| Tavakoli | Inhibition of herpes simplex virus type 1 by copper oxide nanoparticles | 2019 | In-vitro | Vero cells | Y | Copper oxide nanoparticles (CuO-NPs) on HSV-1 infection | Y | N | N | N | N/A | Y | [70] |
| Tintori | Rhodanine derivatives as potent anti-HIV and anti-HSV microbicides | 2018 | In-vitro | Vero cells, human CD4+ lymphocytes | Y | Rhodanine derivatives | Y | Y | N | Y | ACV resistant HSV-2 | Y | [61] |
| Toscani | Synthesis and Biological Evaluation of Amidinourea Derivatives against Herpes Simplex Viruses | 2021 | In-vitro | Vero cells | Y | Amidinourea analogues of moroxydine | Y | Y | N | N | N/A | Y | [118] |
| Toulabi | The efficacy of olive leaf extract on healing herpes simplex virus labialis: A randomized double-blind study | 2021 | Randomized double-blind clinical trial | 66 human patients diagnosed with HSV-1 | Y | Comparison of 2% OLE cream or 5% acyclovir cream five times a day for six days | Y | N | N | N | N/A | Y | [119] |
| Tyo | pH-responsive delivery of Griffithsin from electrospun fibers | 2019 | In-vitro | Vaginal keratinocyte, endocervical, and ectocervical cells, TZM-bl cell | Y | H-responsive fibers comprised of poly(lactic-co-glycolic acid) (PLGA) or methoxypolyethylene glycol-b-PLGA (mPEG-PLGA) with varying ratios of poly(n-butyl acrylate-co-acrylic acid) (PBA-co-PAA), to selectively release griffithsin (GRFT) under pH-conditions that mimic semen introduction | N | N | N | N | N/A | Y | [80] |
| Uhlig | Helicase primase inhibitors (HPIs) are efficacious for therapy of human herpes simplex virus (HSV) disease in an infection mouse model | 2021 | In-vitro, in-vivo | Female BALB/c mice (8 weeks old), Vero cells | Y | Diverse racemates of the sulfonimidoyl thiazole amide class compounds | Y | Y | N | Y | ACV-resistant HSV-1 and HSV-2 | Y | [137] |
| Urbancikova | Efficacy of Pleuran (β-Glucan from Pleurotus ostreatus) in the Managementof Herpes Simplex Virus Type 1 Infection | 2020 | Clinical trial | 90 human patients over 6years with herpes simplex facialis/labialis | Y | β-glucanpleuran (insolubleβ-1,3/1,6-D-glucan isolated from Pleurotus ostreatus) based supplements | Y | N | N | N | N/A | Y | [88] |
| Vanheule | Basic chemokine-derived glycosaminoglycan binding peptides exert antiviral properties against dengue virus serotype 2, herpes simplex virus-1 and respiratory syncytial virus | 2015 | In-vitro | Chinese Hamster ovary, human embryonic lung and human cervical carcinoma (HeLa) cells | Y | COOH-terminal peptides of CXCL9 and CXCL12γ for their affinity to GAGs and KD values | Y | Y | N | N | N/A | Y | [7] |
| Viegas | Antiviral activity of 1,4-disubstituted-1,2,3-triazoles against HSV-1 invitro and effects of amino acid changes in drug-resistant α and βherpesviruses DNA polymerase | 2020 | In-vitro | Human fibroblast cells | Y | Triazole compounds | Y | N | N | Y | ACV resistant HSV-1 | Y | [96] |
| VilasBoas | Linear antimicrobial peptides with activity against herpes simplex virus 1and Aichi virus | 2017 | In-vitro | N/A | Y | Various antimicrobial peptides | Y | N | N | N | N/A | Y | [23] |
| Vilhelmova-Ilieva | Antiviral Activity of Rosa damascena Mill. and Rosa alba L. Essential Oils against the Multiplication of Herpes Simplex Virus Type 1 Strains Sensitive and Resistant to Acyclovir | 2021 | In-vitro | Madin-Darby bovine kidney (MDBK) cells | Y | Rosa damascena Mill. and Rosa alba L. essential oils | Y | N | N | Y | ACV resistant | N | [131] |
| Wang | Guanidine modifications enhance the anti-herpes simplex virus activity of(E,E)-4,6-bis(styryl)-pyrimidine derivatives in vitro and in vivo | 2020 | In-vitro, in-vivo | Vero cells | Y | Guanidine-modified (E,E)-4,6-bis(styryl)-pyrimidine (BS-pyrimidine) derivative compound 5d | Y | Y | N | N | N/A | Y | [108] |
| Wang | Anti-HSV-1 activity of Aspergilli peptide D, a cyclic pentapepetide isolated from fungus Aspergillus sp. SCSIO 41501 | 2020 | In-vitro | N/A | Y | Aspergillipeptide D | Y | N | N | N | N/A | N | [107] |
| Whitley | Clinical management of herpes simplex virus infections: past, present, and future | 2018 | N/A | N/A | N | N/A | N | N | N | N | N/A | N/A | [57] |
| Wright | Inhibition of Herpes Simplex Viruses, Types 1 and 2, by Ginsenoside20(S)-Rg3 | 2020 | In-vitro | Vero cells | Y | Ginsenosides derived from Panax ginseng | Y | Y | N | N | N/A | Y | [105] |
| Ye | Lupeol impairs herpes simplex virus type 1 replication by inhibiting the promoter activity of the viral immediate early gene α0 | 2021 | In-vitro | N/A | Y | Lupeol, a triterpenoid compound | Y | N | N | Y | ACV resistant | Y | [115] |
| Zhang | NSC23766 and Ehop016 Suppress Herpes Simplex Virus-1 Replication by Inhibiting Rac1 Activity | 2021 | In-vitro | Vero cells | Y | Ras-related C3 botulinum toxin substrate 1 Rac1 as a target using Rac1-specific inhibitors, titled NSC23766 and Ehop016 | Y | N | N | N | N/A | Y | [117] |
| Zhou | Anti-HSV-1 effect of dihydromyricetin from Ampelopsis grossedentata via the TLR9-dependent anti-inflammatory pathway | 2020 | In-vitro, experimental study | Vero cells | Y | A flavonoid compound dihydromyricetin (DHM) from Ampelopsis grossedentata | Y | N | N | N | N/A | Y | [97] |
| Zígolo | Chemoenzymatic synthesis of new derivatives of glycyrrhetinic acid with antiviral activity. Molecular docking study | 2018 | In-vitro, experimental study | Vero cells | Y | Synthesized GA derivative, 4d (N-(3-acetylglycyrrhetinoyl)-2-amino-1-propanol) | Y | N | N | Y | ACV resistant HSV-1 | Y | [42] |
| Zinser | A new promising candidate to overcome drug resistant herpes simplex virus infections | 2017 | In-vitro, experimental study | Vero cells | Y | Synthesized SC95377 | Y | Y | N | Y | ACV and multi-resistant resistant HSV-1 and HSV-2 | Y | [33] |
Assessment of Risk of Bias
Quality assessment using the Cochrane Risk of Bias Tool for randomized trials is shown in Table 2. All studies did not report sufficient information to assess other sources of bias, so this area of judgement was excluded from Table 2. Within the domains assessed in the Cochrane Risk of Bias Tool, the ‘Blinding of Outcome Assessment” domain had the greatest number of studies (25) rated as “high risk” of bias (Table 2).
Table 2. Cochrane risk of bias assessment.
| Cochrane Risk of Bias Assessment | |||||||
| Author | Title | Random Sequence Generation | Allocation Concealment | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Citation |
| Agostinho | Cucumis melo pectin as potential candidate to control herpes simplex virus infection | Low | Unclear | Unclear | Low | Unclear | [123] |
| Al-Salahi | Molecular docking study and antiviral evaluation of2-thioxo-benzo[g]quinazolin-4(3H)-one derivatives | Low | Unclear | Unclear | Low | Unclear | [10] |
| Alvarez | Cetylpyridinium chloride blocks herpes simplex virus replication in gingival fibroblasts | Low | Unclear | Unclear | Low | Unclear | [104] |
| Andrei | The Anti-Human Immunodeficiency Virus Drug Tenofovir, a Reverse Transcriptase Inhibitor, Also Targets the Herpes Simplex Virus DNA Polymerase | Low | Unclear | Unclear | Low | Unclear | [49] |
| Andronova | Study of Antiherpetic Efficiency of Phosphite of Acycloguanosine Able to Overcome the Barrier of Resistance to Acyclovir | Unclear | Unclear | Unclear | Unclear | Unclear | [13] |
| Arunkumar | Study on antiviral activities, drug-likeness and molecular docking of bioactive compounds of Punica granatum L. to Herpes simplex virus - 2(HSV-2) | Low | Unclear | Unclear | Low | Unclear | [52] |
| Awad | Synthesis and Evaluation of Some Uracil Nucleosides as Promising Anti-Herpes Simplex Virus 1 Agents | Low | Unclear | Unclear | Low | Unclear | [111] |
| Barboza | In vitro effects of bufotenine against RNA and DNA viruses | Low | Unclear | Unclear | Low | Unclear | [126] |
| Bauer | Antibody-based immunotherapy of aciclovir resistant ocular herpes simplex virus infections | Unclear | Unclear | Unclear | Unclear | Unclear | [27] |
| Ben-Amor | Phytochemical Characterization of Olea europea Leaf Extracts and Assessment of Their Anti-Microbial and Anti-HSV-1 Activity | Low | Unclear | Unclear | Low | Unclear | [125] |
| Benassi-Zanqueta | Evaluation of anti-HSV-1 activity and toxicity of hydroethanolic extract of Tanacetum parthenium (L.) Sch.Bip. (Asteraceae) | Low | Unclear | Unclear | Low | Unclear | [64] |
| Benzekri | Anti HSV-2 activity of Peganum harmala (L.) and isolation of the active compound | Low | Unclear | Unclear | Low | Unclear | [32] |
| Bereczki | Synthesis of Antiviral Perfluoroalkyl Derivatives of Teicoplanin and Vancomycin | Low | Unclear | Unclear | Low | Unclear | [89] |
| Bhutta | Peptide Inhibitor of Complement C1, RLS-0071, Reduces Zosteriform Spread of Herpes Simplex Virus Type 1 Skin Infection and Promotes Survival in Infected Mice | Unclear | Low | Unclear | Low | Unclear | [134] |
| Bhutta | Ginkgolic Acid Inhibits Herpes Simplex Virus Type 1 Skin Infection and Prevents Zosteriform Spread in Mice | Unclear | Low | Unclear | Low | Unclear | [120] |
| Bisignano | Almond Skin Extracts Abrogate HSV-1 Replication by Blocking Virus Binding to the Cell | Low | Unclear | Unclear | Low | Unclear | [26] |
| Bonvicini | Hemidesmus indicus (L.) R. Br. extract inhibits the early step of herpes simplex type 1 and type 2 replication | Low | Unclear | Unclear | Low | Unclear | [44] |
| Brenner | The Molecular Tweezer CLR01 Inhibits Antibody-Resistant Cell-to-Cell Spread of Human Cytomegalovirus | Low | Low | Low | Low | Unclear | [136] |
| Brezáni | Anti-Infectivity against Herpes Simplex Virus and Selected Microbes and Anti-Inflammatory Activities of Compounds Isolated from Eucalyptusglobulus Labill | Low | Unclear | Unclear | Low | Unclear | [138] |
| Cagno | In vitro anti-herpes simplex virus-2 activity of Salvia desoleana Atzei &V. Picci essential oil | Low | Unclear | Unclear | Low | Unclear | [31] |
| Castillo | Anti-herpetic Activity of Macrocystis pyrifera and Durvillaea antarctica Algae Extracts Against HSV-1 and HSV-2 | Low | Low | High | Low | Unclear | [110] |
| Chen | Targeting Aryl Hydrocarbon Receptor Signaling Enhances Type I Interferon-Independent Resistance to Herpes Simplex Virus | Low | Unclear | Unclear | Low | Unclear | [112] |
| Crameri | MxB is an interferon-induced restriction factor of human herpes viruses | Low | Low | Low | Low | Unclear | [53] |
| Criscuolo | Synergy evaluation of anti-Herpes Simplex Virus type 1 and 2 compounds acting on different steps of virus life cycle | Low | Low | Unclear | HIgh | Low | [46] |
| Čulenová | Multiple In vitro biological effects of phenolic compounds from Morus albaroot bark | Low | Low | Unclear | Unclear | Unclear | [85] |
| D'Aiuto | R430: A potent inhibitor of DNA and RNA viruses | Low | Unclear | Unclear | Low | Unclear | [47] |
| Dai | Antiviral Effect of Retro-2.1 against Herpes Simplex Virus Type 2 In Vitro | Low | Unclear | Unclear | Low | Unclear | [50] |
| Dai | Antiviral Effects of ABMA against Herpes Simplex Virus Type 2 In Vitro and In Vivo | Low | Unclear | Unclear | Unclear | Unclear | [58] |
| Deback | Antiviral effects of Cacicol(®), a heparan sulfate biomimetic for corneal regeneration therapy, for herpes simplex virus type-1 and varicella zoster virus infection | Low | Unclear | Unclear | Low | Unclear | [60] |
| Derby | Griffithsin carrageenan fast dissolving inserts prevent SHIV HSV-2 and HPV infections in vivo | Low | Low | Unclear | Unclear | Unclear | [45] |
| Deschamps | Discovery of Small-Molecule Inhibitors Targeting the E3 Ubiquitin Ligase Activity of the Herpes Simplex Virus 1 ICP0 Protein Using an In Vitro High-Throughput Screening Assay | Unclear | Low | Low | Unclear | Unclear | [82] |
| Ding | T-type calcium channels blockers inhibit HSV-2 infection at the late stage of genome replication | Unclear | Low | Unclear | Low | Unclear | [102] |
| Ding | Cellular Signaling Analysis shows antiviral, ribavirin-mediated ribosomal signaling modulation | Low | High | Unclear | Low | Unclear | [77] |
| Donalisio | The traditional use of Vachellia nilotica for sexually transmitted diseases is substantiated by the antiviral activity of its bark extract against sexually transmitted viruses | Low | Unclear | N/A | Low | Unclear | [39] |
| Dong | The Natural Compound Homoharringtonine Presents Broad Antiviral Activity In Vitro and In Vivo | Unclear | Low | N/A | Low | Low | [62] |
| Du | The antiviral activity of arbidol hydrochloride against herpes simplex virus type II (HSV-2) in a mouse model of vaginitis | Unclear | Low | Low | Unclear | Unclear | [81] |
| El-Haddad | Brincidofovir (CMX-001) for refractory and resistant CMV and HSV infections in immunocompromised cancer patients: A single-center experience | Unclear | Low | Unclear | N/A | Low | [1] |
| El-Shiekh | Novel Antiviral and Antibacterial Activities of Hibiscus schizopetalus | Low | Unclear | Unclear | N/A | Unclear | [101] |
| Eletskaya | Enzymatic synthesis of novel purine nucleosides bearing a chiral benzoxazine fragment | Unclear | High | Low | Unclear | N/A | [76] |
| Elias | In Vitro Analysis of the Antioxidant and Antiviral Activity of Embelin against Herpes Simplex Virus-1 | Low | Unclear | Unclear | Low | High | [122] |
| Fujimoto | Accumulation of a soluble form of human nectin-2 is required for exerting the resistance against herpes simplex virus type 2 infection in transfected cells | Unclear | Unclear | Unclear | Low | N/A | [17] |
| Fujimoto | Evaluation of the antiviral potential of the soluble forms of glycoprotein D receptors on ocular herpes caused by HSV-1 and HSV-2 infections in a transgenic mouse model | Low | Low | High | N/A | Unclear | [73] |
| Fujimoto | Comparison of the antiviral potential among soluble forms of herpes simplex virus type-2 glycoprotein D receptors, herpes virus entry mediator A, nectin-1 and nectin-2, in transgenic mice | Low | Low | High | Unclear | N/A | [28] |
| García-Serradilla | Drug repurposing for new, efficient, broad-spectrum antivirals | Low | Unclear | High | Unclear | N/A | [65] |
| Ghaffari | Inhibition of herpes simplex virus type 1 infection by Sambucus ebulus extract in vitro | Low | Unclear | Low | Unclear | Unclear | [132] |
| Ghosh | Ficus religiosa L. bark extracts inhibit infection by herpes simplex virus type 2 in vitro | Low | Unclear | Unclear | Low | Low | [20] |
| González-García | Antimicrobial Activity of Cyclic-Monomeric and Dimeric Derivatives of the Snail-Derived Peptide Cm-p5 against Viral and Multidrug-Resistant Bacterial Strains | Unclear | Low | Unclear | Unclear | Low | [135] |
| Greeley | Acyclovir, cidofovir, and amenamevir have additive antiviral effects on herpes simplex virus TYPE 1 | Unclear | Low | Low | Unclear | Unclear | [95] |
| Hopkins | In Vitro and In Vivo Activity, Tolerability, and Mechanism of Action of BX795 as an Antiviral against Herpes Simplex Virus 2 Genital Infection | Unclear | Low | Unclear | Unclear | Low | [100] |
| Hou | Antiviral activity of PHA767491 against human herpes simplex virus in vitro and in vivo | Low | N/A | Unclear | Low | Low | [37] |
| Houston | Potentiated virucidal activity of pomegranate rind extract (PRE) and punicalagin against Herpes simplex virus (HSV) when co-administered with zinc (II) ions, and antiviral activity of PRE against HSV and acyclovir-resistant HSV | Unclear | Low | Low | Unclear | Unclear | [30] |
| Huang | Antiviral activity of mitoxantrone dihydrochloride against human herpes simplex virus mediated by suppression of the viral immediate early genes | Low | Low | Unclear | N/A | Low | [86] |
| Hutterer | Inhibitors of dual-specificity tyrosine phosphorylation-regulated kinases (DYRK) exert a strong anti-herpes viral activity | Unclear | Low | Low | Unclear | Low | [38] |
| Ibáñez | Pharmacological Induction of Heme Oxygenase-1 Impairs Nuclear Accumulation of Herpes Simplex Virus Capsids upon Infection | N/A | Low | High | Unclear | Low | [36] |
| Ireland | Synthetic α-Hydroxytropolones Inhibit Replication of Wild-Type and Acyclovir-Resistant Herpes Simplex Viruses | Unclear | Low | Unclear | Low | Unclear | [9] |
| Ishimaru | MG132 exerts anti-viral activity against HSV-1 by overcoming virus-mediated suppression of the ERK signaling pathway | Low | Low | N/A | Unclear | Low | [99] |
| Jaishankar | An off-target effect of BX795 blocks herpes simplex virus type 1 infection of the eye | Low | N/A | Unclear | Low | Unclear | [56] |
| Jin | Pentagalloyl glucose Blocks the Nuclear Transport and the Process of Nucleocapsid Egress to Inhibit HSV-1 Infection | Low | Unclear | Unclear | Low | N/A | [6] |
| Jones | Modified cyclodextrins as broad-spectrum antivirals | Low | Low | Unclear | Unclear | Unclear | [93] |
| Kalke | Herpes Simplex Virus Type 1 Clinical Isolates Respond to UL29-Targeted siRNA Swarm Treatment Independent of Their Acyclovir Sensitivity | Low | Low | Unclear | Low | Unclear | [98] |
| Kannan | Anti-herpes virus activity of the carnivorous botanical, Sarraceniapurpurea | N/A | Low | Low | Unclear | N/A | [87] |
| Karpov | [A Plasmid-Expressed CRISPR/Cas9 System Suppresses Replication of HSV Type I in a Vero Cell Culture] | High | Low | Unclear | Unclear | Low | [79] |
| Katsumata | Antiviral efficacy of the helicase-primase inhibitor amenamevir in murine models of severe herpesvirus infection | Low | Unclear | Unclear | Unclear | Low | [43] |
| Kaushik | Antiviral potential and mode of action of Indigofera heterantha against HSV-2 by targeting the early stages of infection | Low | Unclear | Unclear | Unclear | Low | [18] |
| Kim | Quercus acuta Thunb. (Fagaceae) and Its Component, Isoquercitrin, InhibitHSV-1 Replication by Suppressing Virus-Induced ROS Production and NF-κB Activation | Low | Unclear | Unclear | Unclear | Low | [127] |
| Kim | Mori ramulus and its Major Component Morusin Inhibit Herpes Simplex Virus Type 1 Replication and the Virus-Induced Reactive Oxygen Species | Low | Unclear | Unclear | Unclear | Low | [103] |
| Kongyingyoes | 3,19-isopropylideneandrographolide suppresses early gene expression of drug-resistant and wild type herpes simplex viruses | Low | Unclear | Unclear | Unclear | Low | [14] |
| Kumar | Inhibition of herpes simplex virus-1 infection by MBZM-N-IBT: in silico and in vitro studies | Low | Unclear | Unclear | Unclear | Low | [133] |
| Labrunie | UL23, UL30, and UL5 characterization of HSV1 clinical strains isolated from hematology department patients | Low | Unclear | Unclear | Unclear | Low | [84] |
| Le-Trilling | Broad and potent antiviral activity of the NAE inhibitor MLN4924 | Low | Unclear | Unclear | Unclear | Low | [19] |
| Lebrun | Varicella-Zoster Virus ORF9p Binding to Cellular Adaptor Protein Complex 1 Is Important for Viral Infectivity | Low | Unclear | Low | Unclear | Low | [139] |
| Lee | Efficacy of brincidofovir as prophylaxis against HSV and VZV in hematopoietic cell transplant recipients | High | High | High | Unclear | High | [48] |
| Lei | Preparation of a monoPEGylated derivative of cyanovirin-N and its virucidal effect on acyclovir-resistant strains of herpes simplex virus type 1 | Low | High | High | Unclear | Low | [2] |
| Li | Amentoflavone Inhibits HSV-1 and ACV-Resistant Strain Infection by Suppressing Viral Early Infection | Low | Unclear | Unclear | Unclear | Low | [67] |
| Li | Anti-herpes simplex virus type 1 activity of Houttuynoid A, a flavonoid from Houttuynia cordata Thunb | Low | Low | Unclear | Unclear | Low | [25] |
| Liu | Antiviral activities of Janus-type nucleosides and their related oxime-intermediates | Low | Unclear | Unclear | Unclear | Low | [41] |
| Liu | Harringtonine Inhibits Herpes Simplex Virus Type 1 Infection by Reducing Herpes Virus Entry Mediator Expression | Low | Unclear | Unclear | Unclear | Low | [116] |
| Lopes | Sulfonated and Carboxymethylated β-Glucan Derivatives with Inhibitory Activity against Herpes and Dengue Viruses | Low | Unclear | Unclear | Unclear | Low | [128] |
| Luganini | Effective deploying of a novel DHODH inhibitor against herpes simplex type 1 and type 2 replication | Low | Unclear | Unclear | Unclear | Low | [129] |
| Ma | Herpes simplex virus type 1 (HSV-1) specific T-cell generation from HLA-A1- and HLA-A2-positive donors for adoptive immunotherapy | Low | Unclear | Unclear | Unclear | Low | [12] |
| Ma | Assessment of a new arbidol derivative against herpes simplex virus II in human cervical epithelial cells and in BALB/c mice | Low | Low | High | Unclear | Low | [75] |
| Maizel | Study of the Extremely-Tolerant Brevibacterium linens AE038-8 with Antiviral Activity Against Herpes Simplex Virus Type 1 | Low | Unclear | Unclear | Unclear | Low | [113] |
| Mandalari | Simulated human digestion of N1-aryl-2-arylthioacetamidobenzimidazoles and their activity against Herpes-simplex virus 1 in vitro | Low | Unclear | Unclear | Unclear | Low | [72] |
| Marcocci | The Amphibian Antimicrobial Peptide Temporin B Inhibits In Vitro Herpes Simplex Virus 1 Infection | Low | Unclear | Unclear | Unclear | Low | [54] |
| Marino-Merlo | Anti-herpes simplex virus 1 and immunomodulatory activities of a poly-γ-glutamic acid from Bacillus horneckiae strain APA of shallow vent origin | Low | Unclear | Unclear | Unclear | Low | [29] |
| Mello | Perillyl alcohol and perillic acid exert efficient action upon HSV-1maturation and release of infective virus | Low | Unclear | Unclear | Unclear | Low | [92] |
| Mishra | Herbal Gel Formulation Developed for Anti-Human Immunodeficiency Virus (HIV)-1 Activity Also Inhibits In Vitro HSV-2 Infection | Low | Unclear | Unclear | Unclear | Low | [59] |
| Mohammed | Synthesis and anti-HSV activity of tricyclic penciclovir and hydroxybutylguanine derivatives | Low | Unclear | Unclear | Unclear | Low | [69] |
| Monjo | Photodynamic Inactivation of Herpes Simplex Viruses | Low | Unclear | Unclear | Unclear | Low | [55] |
| Moshaverinia | Evaluation of the effect of hydro alcoholic extract of cinnamon on herpes simplex virus 1 | Low | Unclear | Unclear | Unclear | Low | [94] |
| Musarra-Pizzo | The Antimicrobial and Antiviral Activity of Polyphenols from Almond (Prunus dulcis L.) Skin | Low | Unclear | Unclear | Unclear | Low | [68] |
| Novoa | Antiviral Activity of Myticin C Peptide from Mussel: an Ancient Defense against Herpes viruses | Low | Unclear | Unclear | Unclear | Low | [8] |
| Paavilainen | Topical treatment of herpes simplex virus infection with enzymatically created siRNA swarm | Low | Low | High | High | Low | [22] |
| Parsania | Antiviral screening of four plant extracts against acyclovir resistant herpes simplex virus type-1 | Low | Unclear | High | Unclear | Low | [24] |
| PiresdeMello | Aminomethylnaphthoquinones and HSV-1: in vitro and in silico evaluations of potential antivirals | Low | Unclear | High | Unclear | Low | [3] |
| Pradhan | Herpes simplex virus virucidal activity of MST-312 and epigallocatechingallate | Low | Unclear | High | Unclear | Low | [63] |
| Praena | Amidic derivatives of valproic acid, valpromide and valnoctamide, inhibitHSV-1 infection in oligodendrocytes | Low | Unclear | High | Unclear | Low | [78] |
| Pujol | Polyhydroxylated sulfated steroids derived from 5α-cholestanes as antiviral agents against herpes simplex virus | Low | Unclear | Unclear | Unclear | Low | [16] |
| Quenelle | Efficacy of pritelivir and acyclovir in the treatment of herpes simplex virus infections in a mouse model of herpes simplex encephalitis | Low | Low | High | Unclear | Low | [35] |
| Rechenchoski | Mangiferin: A promising natural xanthone from Mangifera indica for the control of acyclovir - resistant herpes simplex virus 1 infection | Low | Low | High | Unclear | Low | [106] |
| Rittà | Antiviral Activity of a Arisaema Tortuosum Leaf Extract and Some of its Constituents against Herpes Simplex Virus Type 2 | Low | Unclear | High | Unclear | Low | [91] |
| Ruzsics | A Novel, Broad-Acting Peptide Inhibitor of Double-Stranded DNA Virus Gene Expression and Replication | Low | Low | High | Low | Unclear | [90] |
| Sacchelli | Botryosphaeran and sulfonated derivatives as novel antiviral agents for herpes simplex and dengue fever | Low | Low | High | Low | Unclear | [74] |
| SadeghEhdaei | Cellular miR-101-1 Reduces Efficiently the Replication of HSV-1 in HeLaCells | Low | Low | High | Unclear | Unclear | [114] |
| Sanchez | Development and evaluation of a host-targeted antiviral that abrogatesherpes simplex virus replication through modulation of arginine-associated metabolic pathways | Low | Unclear | High | Unclear | Unclear | [11] |
| Sasaki | In vitro and in vivo antiherpetic effects of(1R,2R)-1-(5'-methylful-3'-yl)propane-1,2,3-triol | Low | Unclear | Unclear | Low | Unclear | [15] |
| Schneider | Early Steps in Herpes Simplex Virus Infection Blocked by a Proteasome Inhibitor | Low | Low | Low | Unclear | Unclear | [83] |
| Shabani | Inhibition of herpes simplex virus type 1 replication by novel hsa-miR-7704 in vitro | Low | Unclear | Unclear | Unclear | Unclear | [71] |
| Shan | Viral UL8 Is Involved in the Antiviral Activity of Oleanolic Acid Against HSV-1 Infection | Low | Unclear | Unclear | Low | Unclear | [130] |
| Shao | Poly(dA:dT) Suppresses HSV-2 Infection of Human Cervical Epithelial Cells Through RIG-I Activation | Unclear | Unclear | Unclear | Unclear | Unclear | [124] |
| Sharifi-Rad | Susceptibility of herpes simplex virus type 1 to monoterpenes thymol, carvacrol, p-cymene and essential oils of Sinapis arvensis L., Lallemantiaroyleana Benth. and Pulicaria vulgaris Gaertn | Low | Unclear | Unclear | Low | Unclear | [34] |
| Sharifi-Rad | Antiviral activity of Veronica persica Poir. on herpes virus infection | Unclear | Unclear | Unclear | Unclear | Unclear | [51] |
| Shiraki | Helicase-primase inhibitor amenamevir for herpesvirus infection: Towards practical application for treating herpes zoster | Unclear | Unclear | Unclear | Unclear | Unclear | [21] |
| Shiraki | Amenamevir, a Helicase-Primase Inhibitor, for the Optimal Treatment of Herpes Zoster | Low | Unclear | Low | Low | Unclear | [121] |
| Spengler | Antiviral, Antimicrobial and Antibiofilm Activity of Selenoesters and Selenoanhydrides | Low | Unclear | Unclear | Low | Unclear | [66] |
| Stegman | Volatile Acid-Solvent Evaporation (VASE): Molecularly Homogeneous Distribution of Acyclovir in a Bioerodable Polymer Matrix for Long-Term Treatment of Herpes Simplex Virus-1 Infections | Low | Unclear | Unclear | Low | Unclear | [40] |
| Suryawanshi | Bacterial Pigment Prodigiosin Demonstrates a Unique Anti herpesvirus Activity That Is Mediated through Inhibition of Pro survival Signal Transducers | Low | Unclear | Unclear | Low | Unclear | [109] |
| Tavakoli | Inhibition of herpes simplex virus type 1 by copper oxide nanoparticles | Low | Unclear | High | Unclear | Unclear | [70] |
| Tintori | Rhodanine derivatives as potent anti-HIV and anti-HSV microbicides | Low | Unclear | Low | Low | Unclear | [61] |
| Toscani | Synthesis and Biological Evaluation of Amidinourea Derivatives against Herpes Simplex Viruses | Low | Low | Low | Low | Unclear | [118] |
| Toulabi | The efficacy of olive leaf extract on healing herpes simplex virus labialis: A randomized double-blind study | Low | Unclear | Unclear | Low | Low | [119] |
| Tyo | pH-responsive delivery of Griffithsin from electrospun fibers | Low | Unclear | Unclear | Low | Unclear | [80] |
| Uhlig | Helicase primase inhibitors (HPIs) are efficacious for therapy of human herpes simplex virus (HSV) disease in an infection mouse model | Low | Low | Low | Low | Unclear | [137] |
| Urbancikova | Efficacy of Pleuran (β-Glucan from Pleurotus ostreatus) in the Management of Herpes Simplex Virus Type 1 Infection | Low | Unclear | Unclear | Low | Unclear | [88] |
| Vanheule | Basic chemokine-derived glycosaminoglycan binding peptides exert antiviral properties against dengue virus serotype 2, herpes simplex virus-1 and respiratory syncytial virus | Low | Unclear | Unclear | Low | Unclear | [7] |
| Viegas | Antiviral activity of 1,4-disubstituted-1,2,3-triazoles against HSV-1 in vitro and effects of amino acid changes in drug-resistant α and β herpes viruses DNA polymerase | Low | High | High | Unclear | Unclear | [96] |
| VilasBoas | Linear antimicrobial peptides with activity against herpes simplex virus 1 and Aichi virus | Unclear | High | High | Unclear | Unclear | [23] |
| Vilhelmova-Ilieva | Antiviral Activity of Rosa damascena Mill. and Rosa alba L. Essential Oils against the Multiplication of Herpes Simplex Virus Type 1 Strains Sensitive and Resistant to Acyclovir | Unclear | High | High | Low | Low | [131] |
| Wang | Guanidine modifications enhance the anti-herpes simplex virus activity of (E,E)-4,6-bis(styryl)-pyrimidine derivatives in vitro and in vivo | Low | Unclear | Unclear | Low | Unclear | [108] |
| Wang | Anti-HSV-1 activity of Aspergilli peptide D, a cyclic pentapepetide isolated from fungus Aspergillus sp. SCSIO 41501 | Low | Unclear | Unclear | Low | Unclear | [107] |
| Whitley | Clinical management of herpes simplex virus infections: past, present, and future | N/A | N/A | N/A | N/A | N/A | [57] |
| Wright | Inhibition of Herpes Simplex Viruses, Types 1 and 2, by Ginsenoside 20(S)-Rg3 | Low | Unclear | Unclear | Low | Low | [105] |
| Ye | Lupeol impairs herpes simplex virus type 1 replication by inhibiting the promoter activity of the viral immediate early gene α0 | Low | Unclear | Unclear | Low | Unclear | [115] |
| Zhang | NSC23766 and Ehop016 Suppress Herpes Simplex Virus-1 Replication by Inhibiting Rac1 Activity | Low | Unclear | Unclear | Low | Unclear | [117] |
| Zhou | Anti-HSV-1 effect of dihydromyricetin from Ampelopsis grossedentata via the TLR9-dependent anti-inflammatory pathway | Low | Unclear | Unclear | Low | Unclear | [97] |
| Zígolo | Chemoenzymatic synthesis of new derivatives of glycyrrhetinic acid with antiviral activity. Molecular docking study | Low | Unclear | Unclear | Low | Unclear | [42] |
| Zinser | A new promising candidate to overcome drug resistant herpes simplex virus infections | Low | Unclear | High | Low | Unclear | [33] |
Principal Findings
The available literature reviewed consistently supports the existence and potentiality of second-line treatments for HSV strains that are resistant to first-line treatments. We have shown that a majority of the studies reviewed have efficacy as potential managements for resistant strains of HSV [1-3,6-20,22-45,47-52,54-56,58-83,85-106,108-125,127-130,132-138]. The predominance of studies utilized Vero cells [2,3,6,8-10,14-20,23,25,26,28,30-34,36,39-42,44-47,50,51,53-56,58-64,66-70,72,74,76,77,79,82,83,85-87,89-95,97-108,111-113,115-118,120,122,123,125-130,132,133,135,137,138] to test their treatments on. Lastly the most commonly tested treatments that displayed efficacy as second-line treatments in HSV included nectin [17,28,73], amenamevir [21,43,95] and methanol extracts [24,32,39].
Comparison with Existing Literature
This systematic review provides additional information to patients on the potentiality of second-line treatment in HSV strains resistant to first-line treatments. The existing literature comparing medications as treatments for HSV primarily include acyclovir, ganciclovir, and foscarnet [1,2]. There has also been research that compares various plant extracts [24,110]. However, there is a paucity of research that compares greater than 100 second-line HSV interventions for efficacy, as our study has done.
Strengths and limitations
The primary strength of this study is the utilization of the most well-renowned methodology when conducting a systematic review. This included using a prospective protocol created to answer a specific research question, the risk of bias assessment applied to each article, and the summarized table of findings. An additional strength is the relatively large quantity of studies included within this review.
Limitations were that the literature search included language restrictions to only English articles. The search also included interventions for HSV-1, HSV-2, and VZV, and these differences were not considered when assessing efficacy of interventions. Various methodologies were utilized in studies meeting criteria and so the relationship of sample size and efficacy was not able to be successfully analyzed. Another limitation is that the relationship of severity of HSV infections and efficacy of the intervention was not studied.
Conclusions
Immunocompromised patients have been noted to be the population most affected by drug-resistant variants of HSV. Subsequently, we found that HSV infections within this patient population are challenging to manage clinically effectively. This systematic review provides additional information to patients on the potentiality of second-line treatment in HSV strains resistant to first-line treatments, especially for immunocompromised patients. This review is important as all patients, whether immunocompromised or not, deserve to have their infections clinically managed in a manner supported by comprehensive research. This review provides the necessary information about treatment options for patients with resistant HSV infections and their providers.
Acknowledgments
This material is the result of research supported with resources by the University of the Incarnate Word School of Osteopathic Medicine Office of Research and Innovation.
The authors have declared that no competing interests exist.
References
- 1.Brincidofovir (CMX-001) for refractory and resistant CMV and HSV infections in immunocompromised cancer patients: a single-center experience. El-Haddad D, El Chaer F, Vanichanan J, et al. Antiviral Res. 2016;134:58–62. doi: 10.1016/j.antiviral.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 2.Preparation of a monoPEGylated derivative of cyanovirin-N and its virucidal effect on acyclovir-resistant strains of herpes simplex virus type 1. Lei Y, Chen W, Liang H, et al. Arch Virol. 2019;164:1259–1269. doi: 10.1007/s00705-018-04118-4. [DOI] [PubMed] [Google Scholar]
- 3.Aminomethylnaphthoquinones and HSV-1: in vitro and in silico evaluations of potential antivirals. Pires de Mello CP, Sardoux NS, Terra L, et al. Antivir Ther. 2016;21:507–515. doi: 10.3851/IMP3039. [DOI] [PubMed] [Google Scholar]
- 4.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Moher D, Liberati A, Tetzlaff J, Altman DG. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 5.The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Higgins JP, Altman DG, Gøtzsche PC, et al. BMJ. 2011;343:0. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pentagalloylglucose blocks the nuclear transport and the process of nucleocapsid egress to inhibit HSV-1 infection. Jin F, Ma K, Chen M, Zou M, Wu Y, Li F, Wang Y. Jpn J Infect Dis. 2016;69:135–142. doi: 10.7883/yoken.JJID.2015.137. [DOI] [PubMed] [Google Scholar]
- 7.Basic chemokine-derived glycosaminoglycan binding peptides exert antiviral properties against dengue virus serotype 2, herpes simplex virus-1 and respiratory syncytial virus. Vanheule V, Vervaeke P, Mortier A, et al. Biochem Pharmacol. 2016;100:73–85. doi: 10.1016/j.bcp.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Antiviral activity of myticin c peptide from mussel: an ancient defense against herpesviruses. Novoa B, Romero A, Álvarez ÁL, et al. J Virol. 2016;90:7692–7702. doi: 10.1128/JVI.00591-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Synthetic α-hydroxytropolones inhibit replication of wild-type and acyclovir-resistant herpes simplex viruses. Ireland PJ, Tavis JE, D'Erasmo MP, et al. Antimicrob Agents Chemother. 2016;60:2140–2149. doi: 10.1128/AAC.02675-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molecular docking study and antiviral evaluation of 2-thioxo-benzo[g]quinazolin-4(3H)-one derivatives. Al-Salahi R, Abuelizz HA, Ghabbour HA, El-Dib R, Marzouk M. Chem Cent J. 2016;10:21. doi: 10.1186/s13065-016-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Development and evaluation of a host-targeted antiviral that abrogates herpes simplex virus replication through modulation of arginine-associated metabolic pathways. Sanchez MD, Ochoa AC, Foster TP. Antiviral Res. 2016;132:13–25. doi: 10.1016/j.antiviral.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herpes simplex virus type 1 (HSV-1) specific T-cell generation from HLA-A1- and HLA-A2-positive donors for adoptive immunotherapy. Ma CK, Clancy L, Deo S, Blyth E, Micklethwaite KP, Gottlieb DJ. Cytotherapy. 2017;19:107–118. doi: 10.1016/j.jcyt.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Study of antiherpetic efficiency of phosphite of acycloguanosine able to over come the barrier of resistance to acyclovir. Andronova VL, Jasko MV, Kukhanova MK, Galegov GA, Skoblov YS, Kochetkov SN. https://www.ncbi.nlm.nih.gov/pubmed/27099786https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4837573. Acta Naturae. 2016;8:74–81. [PMC free article] [PubMed] [Google Scholar]
- 14.3,19-Isopropylideneandrographolide suppresses early gene expression of drug-resistant and wild type herpes simplex viruses. Kongyingyoes B, Priengprom T, Pientong C, Aromdee C, Suebsasana S, Ekalaksananan T. Antiviral Res. 2016;132:281–286. doi: 10.1016/j.antiviral.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 15.In vitro and in vivo antiherpetic effects of (1R,2R)-1-(5'-methylful-3'-yl)propane-1,2,3-triol. Sasaki K, Hayashi K, Matsuya Y, Sugimoto K, Lee JB, Kurosaki F, Hayashi T. J Nat Med. 2016;70:217–224. doi: 10.1007/s11418-016-0964-6. [DOI] [PubMed] [Google Scholar]
- 16.Polyhydroxylated sulfated steroids derived from 5α-cholestanes as antiviral agents against herpes simplex virus. Pujol CA, Sepúlveda CS, Richmond V, Maier MS, Damonte EB. Arch Virol. 2016;161:1993–1999. doi: 10.1007/s00705-016-2867-y. [DOI] [PubMed] [Google Scholar]
- 17.Accumulation of a soluble form of human nectin-2 is required for exerting the resistance against herpes simplex virus type 2 infection in transfected cells. Fujimoto Y, Ozaki K, Iwamori N, Takakuwa H, Ono E. Acta Virol. 2016;60:41–48. doi: 10.4149/av_2016_01_41. [DOI] [PubMed] [Google Scholar]
- 18.Antiviral potential and mode of action of Indigofera heterantha against HSV-2 by targeting the early stages of infection. Kaushik NK, Guha R, Thomas BM. Antivir Ther. 2017;22:381–391. doi: 10.3851/IMP3118. [DOI] [PubMed] [Google Scholar]
- 19.Broad and potent antiviral activity of the NAE inhibitor MLN4924. Le-Trilling VT, Megger DA, Katschinski B, et al. Sci Rep. 2016;6:19977. doi: 10.1038/srep19977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ficus religiosa L. bark extracts inhibit infection by herpes simplex virus type 2 in vitro. Ghosh M, Civra A, Rittà M, et al. Arch Virol. 2016;161:3509–3514. doi: 10.1007/s00705-016-3032-3. [DOI] [PubMed] [Google Scholar]
- 21.Helicase-primase inhibitor amenamevir for herpesvirus infection: towards practical application for treating herpes zoster. Shiraki K. Drugs Today (Barc) 2017;53:573–584. doi: 10.1358/dot.2017.53.11.2724803. [DOI] [PubMed] [Google Scholar]
- 22.Topical treatment of herpes simplex virus infection with enzymatically created siRNA swarm. Paavilainen H, Lehtinen J, Romanovskaya A, Nygårdas M, Bamford DH, Poranen MM, Hukkanen V. Antivir Ther. 2017;22:631–637. doi: 10.3851/IMP3153. [DOI] [PubMed] [Google Scholar]
- 23.Linear antimicrobial peptides with activity against herpes simplex virus 1 and Aichi virus. Vilas Boas LC, de Lima LM, Migliolo L, Mendes GD, de Jesus MG, Franco OL, Silva PA. Biopolymers. 2017;108 doi: 10.1002/bip.22871. [DOI] [PubMed] [Google Scholar]
- 24.Antiviral screening of four plant extracts against acyclovir resistant herpes simplex virus type-1. Parsania M, Rezaee MB, Monavari SH, Jaimand K, Mousavi-Jazayeri SM, Razazian M, Nadjarha MH. https://www.ncbi.nlm.nih.gov/pubmed/29043989. Pak J Pharm Sci. 2017;30:1407–1411. [PubMed] [Google Scholar]
- 25.Anti-herpes simplex virus type 1 activity of Houttuynoid A, a flavonoid from Houttuynia cordata Thunb. Li T, Liu L, Wu H, et al. Antiviral Res. 2017;144:273–280. doi: 10.1016/j.antiviral.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Almond skin extracts abrogate HSV-1 replication by blocking virus binding to the cell. Bisignano C, Mandalari G, Smeriglio A, Trombetta D, Pizzo MM, Pennisi R, Sciortino MT. Viruses. 2017;9 doi: 10.3390/v9070178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antibody-based immunotherapy of aciclovir resistant ocular herpes simplex virus infections. Bauer D, Keller J, Alt M, et al. Virology. 2017;512:194–200. doi: 10.1016/j.virol.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 28.Comparison of the antiviral potential among soluble forms of herpes simplex virus type-2 glycoprotein D receptors, herpes virus entry mediator A, nectin-1 and nectin-2, in transgenic mice. Fujimoto Y, Tomioka Y, Ozaki K, et al. J Gen Virol. 2017;98:1815–1822. doi: 10.1099/jgv.0.000804. [DOI] [PubMed] [Google Scholar]
- 29.Anti-herpes simplex virus 1 and immunomodulatory activities of a poly-γ- glutamic acid from Bacillus horneckiae strain APA of shallow vent origin. Marino-Merlo F, Papaianni E, Maugeri TL, et al. Appl Microbiol Biotechnol. 2017;101:7487–7496. doi: 10.1007/s00253-017-8472-5. [DOI] [PubMed] [Google Scholar]
- 30.Potentiated virucidal activity of pomegranate rind extract (PRE) and punicalagin against herpes simplex virus (HSV) when co-administered with zinc (II) ions, and antiviral activity of PRE against HSV and aciclovir-resistant HSV. Houston DM, Bugert JJ, Denyer SP, Heard CM. PLoS One. 2017;12:0. doi: 10.1371/journal.pone.0179291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.In vitro anti-herpes simplex virus-2 activity of Salvia desoleana Atzei & V. Picci essential oil. Cagno V, Sgorbini B, Sanna C, et al. PLoS One. 2017;12:0. doi: 10.1371/journal.pone.0172322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anti HSV-2 activity of Peganum harmala (L.) and isolation of the active compound. Benzekri R, Bouslama L, Papetti A, Hammami M, Smaoui A, Limam F. Microb Pathog. 2018;114:291–298. doi: 10.1016/j.micpath.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 33.A new promising candidate to overcome drug resistant herpes simplex virus infections. Zinser E, Krawczyk A, Mühl-Zürbes P, et al. Antiviral Res. 2018;149:202–210. doi: 10.1016/j.antiviral.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Susceptibility of herpes simplex virus type 1 to monoterpenes thymol, carvacrol, p-cymene and essential oils of Sinapis arvensis L., Lallemantia royleana Benth. and Pulicaria vulgaris Gaertn. Sharifi-Rad J, Salehi B, Schnitzler P, et al. Cell Mol Biol (Noisy-le-grand) 2017;63:42–47. doi: 10.14715/cmb/2017.63.8.10. [DOI] [PubMed] [Google Scholar]
- 35.Efficacy of pritelivir and acyclovir in the treatment of herpes simplex virus infections in a mouse model of herpes simplex encephalitis. Quenelle DC, Birkmann A, Goldner T, et al. Antiviral Res. 2018;149:1–6. doi: 10.1016/j.antiviral.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pharmacological induction of heme oxygenase-1 impairs nuclear accumulation of herpes simplex virus capsids upon infection. Ibáñez FJ, Farías MA, Retamal-Díaz A, Espinoza JA, Kalergis AM, González PA. Front Microbiol. 2017;8:2108. doi: 10.3389/fmicb.2017.02108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antiviral activity of PHA767491 against human herpes simplex virus in vitro and in vivo. Hou J, Zhang Z, Huang Q, et al. BMC Infect Dis. 2017;17:217. doi: 10.1186/s12879-017-2305-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inhibitors of dual-specificity tyrosine phosphorylation-regulated kinases (DYRK) exert a strong anti-herpesviral activity. Hutterer C, Milbradt J, Hamilton S, et al. Antiviral Res. 2017;143:113–121. doi: 10.1016/j.antiviral.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 39.The traditional use of Vachellia nilotica for sexually transmitted diseases is substantiated by the antiviral activity of its bark extract against sexually transmitted viruses. Donalisio M, Cagno V, Civra A, et al. J Ethnopharmacol. 2018;213:403–408. doi: 10.1016/j.jep.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 40.Volatile acid-solvent evaporation (VASE): molecularly homogeneous distribution of acyclovir in a bioerodable polymer matrix for long-term treatment of herpes simplex virus-1 infections. Stegman JR, Badin JK, Biles KA, et al. J Drug Deliv. 2018;2018:6161230. doi: 10.1155/2018/6161230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antiviral activities of Janus-type nucleosides and their related oxime-intermediates. Liu J, Zhao H, Zhou X, He Y, Chen Q. Bioorg Med Chem. 2019;27:2332–2339. doi: 10.1016/j.bmc.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 42.Chemoenzymatic synthesis of new derivatives of glycyrrhetinic acid with antiviral activity. Molecular docking study. Zígolo MA, Salinas M, Alché L, Baldessari A, Liñares GG. Bioorg Chem. 2018;78:210–219. doi: 10.1016/j.bioorg.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 43.Antiviral efficacy of the helicase-primase inhibitor amenamevir in murine models of severe herpesvirus infection. Katsumata K, Chono K, Suzuki H. Biochem Pharmacol. 2018;158:201–206. doi: 10.1016/j.bcp.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 44.Hemidesmus indicus (L.) R. Br. extract inhibits the early step of herpes simplex type 1 and type 2 replication. Bonvicini F, Lianza M, Mandrone M, Poli F, Gentilomi GA, Antognoni F. https://www.ncbi.nlm.nih.gov/pubmed/29874387. New Microbiol. 2018;41:187–194. [PubMed] [Google Scholar]
- 45.Griffithsin carrageenan fast dissolving inserts prevent SHIV HSV-2 and HPV infections in vivo. Derby N, Lal M, Aravantinou M, et al. Nat Commun. 2018;9:3881. doi: 10.1038/s41467-018-06349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Synergy evaluation of anti-herpes simplex virus type 1 and 2 compounds acting on different steps of virus life cycle. Criscuolo E, Clementi N, Mancini N, Burioni R, Miduri M, Castelli M, Clementi M. Antiviral Res. 2018;151:71–77. doi: 10.1016/j.antiviral.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 47.R430: a potent inhibitor of DNA and RNA viruses. D'Aiuto L, McNulty J, Hartline C, et al. Sci Rep. 2018;8:16662. doi: 10.1038/s41598-018-33904-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Efficacy of brincidofovir as prophylaxis against HSV and VZV in hematopoietic cell transplant recipients. Lee YJ, Neofytos D, Kim SJ, et al. Transpl Infect Dis. 2018;20:0. doi: 10.1111/tid.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The anti-human immunodeficiency virus drug tenofovir, a reverse transcriptase inhibitor, also targets the herpes simplex virus DNA polymerase. Andrei G, Gillemot S, Topalis D, Snoeck R. J Infect Dis. 2018;217:790–801. doi: 10.1093/infdis/jix605. [DOI] [PubMed] [Google Scholar]
- 50.Antiviral effect of retro-2.1 against herpes simplex virus type 2 in vitro. Dai W, Wu Y, Bi J, et al. J Microbiol Biotechnol. 2018;28:849–859. doi: 10.4014/jmb.1712.12052. [DOI] [PubMed] [Google Scholar]
- 51.Antiviral activity of Veronica persica Poir. on herpes virus infection. Sharifi-Rad J, Iriti M, Setzer WN, Sharifi-Rad M, Roointan A, Salehi B. https://www.ncbi.nlm.nih.gov/pubmed/29981678. Cell Mol Biol (Noisy-le-grand) 2018;64:11–17. [PubMed] [Google Scholar]
- 52.Study on antiviral activities, drug-likeness and molecular docking of bioactive compounds of Punica granatum L. to Herpes simplex virus - 2 (HSV-2) Arunkumar J, Rajarajan S. Microb Pathog. 2018;118:301–309. doi: 10.1016/j.micpath.2018.03.052. [DOI] [PubMed] [Google Scholar]
- 53.MxB is an interferon-induced restriction factor of human herpesviruses. Crameri M, Bauer M, Caduff N, et al. Nat Commun. 2018;9:1980. doi: 10.1038/s41467-018-04379-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.The amphibian antimicrobial peptide Temporin b inhibits in vitro herpes simplex virus 1 infection. Marcocci ME, Amatore D, Villa S, et al. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.02367-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Photodynamic inactivation of herpes simplex viruses. Monjo AL, Pringle ES, Thornbury M, et al. Viruses. 2018;10 doi: 10.3390/v10100532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.An off-target effect of BX795 blocks herpes simplex virus type 1 infection of the eye. Jaishankar D, Yakoub AM, Yadavalli T, et al. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aan5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clinical management of herpes simplex virus infections: past, present, and future. Whitley R, Baines J. F1000Res. 2018;7 doi: 10.12688/f1000research.16157.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Antiviral effects of ABMA against herpes simplex virus type 2 in vitro and in vivo. Dai W, Wu Y, Bi J, et al. Viruses. 2018;10 doi: 10.3390/v10030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herbal gel formulation developed for anti-human immunodeficiency virus (HIV)-1 activity also inhibits in vitro HSV-2 infection. Mishra NN, Kesharwani A, Agarwal A, Polachira SK, Nair R, Gupta SK. Viruses. 2018;10 doi: 10.3390/v10110580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Antiviral effects of Cacicol®, a heparan sulfate biomimetic for corneal regeneration therapy, for herpes simplex virus type-1 and varicella zoster virus infection. Deback C, Rousseau A, Breckler M, et al. Antivir Ther. 2018;23:665–675. doi: 10.3851/IMP3254. [DOI] [PubMed] [Google Scholar]
- 61.Rhodanine derivatives as potent anti-HIV and anti-HSV microbicides. Tintori C, Iovenitti G, Ceresola ER, et al. PLoS One. 2018;13:0. doi: 10.1371/journal.pone.0198478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.The natural compound homoharringtonine presents broad antiviral activity in vitro and in vivo. Dong HJ, Wang ZH, Meng W, Li CC, Hu YX, Zhou L, Wang XJ. Viruses. 2018;10 doi: 10.3390/v10110601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herpes simplex virus virucidal activity of MST-312 and epigallocatechin gallate. Pradhan P, Nguyen ML. Virus Res. 2018;249:93–98. doi: 10.1016/j.virusres.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 64.Evaluation of anti-HSV-1 activity and toxicity of hydroethanolic extract of Tanacetum parthenium (L.) Sch.Bip. (Asteraceae) Benassi-Zanqueta É, Marques CF, Valone LM, et al. Phytomedicine. 2019;55:249–254. doi: 10.1016/j.phymed.2018.06.040. [DOI] [PubMed] [Google Scholar]
- 65.Drug repurposing for new, efficient, broad spectrum antivirals. García-Serradilla M, Risco C, Pacheco B. Virus Res. 2019;264:22–31. doi: 10.1016/j.virusres.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Antiviral, antimicrobial and antibiofilm activity of selenoesters and selenoanhydrides. Spengler G, Kincses A, Mosolygó T, et al. Molecules. 2019;24 doi: 10.3390/molecules24234264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amentoflavone inhibits HSV-1 and ACV-resistant strain infection by suppressing viral early infection. Li F, Song X, Su G, et al. Viruses. 2019;11 doi: 10.3390/v11050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.The antimicrobial and antiviral activity of polyphenols from almond (Prunus dulcis L.) skin. Musarra-Pizzo M, Ginestra G, Smeriglio A, Pennisi R, Sciortino MT, Mandalari G. Nutrients. 2019;11 doi: 10.3390/nu11102355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Synthesis and anti-HSV activity of tricyclic penciclovir and hydroxybutylguanine derivatives. Mohammed AF, Andrei G, Hayallah AM, Abdel-Moty SG, Snoeck R, Simons C. Bioorg Med Chem. 2019;27:1023–1033. doi: 10.1016/j.bmc.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Inhibition of herpes simplex virus type 1 by copper oxide nanoparticles. Tavakoli A, Hashemzadeh MS. J Virol Methods. 2020;275:113688. doi: 10.1016/j.jviromet.2019.113688. [DOI] [PubMed] [Google Scholar]
- 71.Inhibition of herpes simplex virus type 1 replication by novel hsa-miR-7704 in vitro. Shabani M, Nasr Esfahani B, Sadegh Ehdaei B, Moghim S, Mirzaei A, Sharifi M, Mouhebat L. Res Pharm Sci. 2019;14:167–174. doi: 10.4103/1735-5362.253364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simulated human digestion of N1-aryl-2-arylthioacetamidobenzimidazoles and their activity against Herpes-simplex virus 1 in vitro. Mandalari G, Bisignano C, Smeriglio A, et al. PLoS One. 2019;14:0. doi: 10.1371/journal.pone.0216384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Evaluation of the antiviral potential of the soluble forms of glycoprotein D receptors on ocular herpes caused by HSV-1 and HSV-2 infections in a transgenic mouse model. Fujimoto Y, Hikita SI, Takeda K, et al. J Med Virol. 2019;91:820–828. doi: 10.1002/jmv.25384. [DOI] [PubMed] [Google Scholar]
- 74.Botryosphaeran and sulfonated derivatives as novel antiviral agents for herpes simplex and dengue fever. Sacchelli BA, Faccin-Galhardi LC, Ito VY, Lopes JL, Dekker RF, Barbosa-Dekker AM, Orsato A. Int J Biol Macromol. 2019;138:334–339. doi: 10.1016/j.ijbiomac.2019.07.084. [DOI] [PubMed] [Google Scholar]
- 75.Assessment of a new arbidol derivative against herpes simplex virus II in human cervical epithelial cells and in BALB/c mice. Ma N, Shen M, Chen T, et al. Biomed Pharmacother. 2019;118:109359. doi: 10.1016/j.biopha.2019.109359. [DOI] [PubMed] [Google Scholar]
- 76.Enzymatic synthesis of novel purine nucleosides bearing a chiral benzoxazine fragment. Eletskaya BZ, Gruzdev DA, Krasnov VP, et al. Chem Biol Drug Des. 2019;93:605–616. doi: 10.1111/cbdd.13458. [DOI] [PubMed] [Google Scholar]
- 77.Cellular signaling analysis shows antiviral, ribavirin-mediated ribosomal signaling modulation. Ding X, Krutzik PO, Ghaffari AA, et al. Antiviral Res. 2019;171:104598. doi: 10.1016/j.antiviral.2019.104598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amidic derivatives of valproic acid, valpromide and valnoctamide, inhibit HSV-1 infection in oligodendrocytes. Praena B, Bello-Morales R, de Castro F, López-Guerrero JA. Antiviral Res. 2019;168:91–99. doi: 10.1016/j.antiviral.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 79.A Plasmid-Expressed CRISPR/Cas9 System Suppresses Replication of HSV Type I in a Vero Cell Culture (Article in Russian) Karpov DS, Karpov VL, Klimova RR, Demidova NA, Kushch AA. Mol Biol (Mosk) 2019;53:91–100. doi: 10.1134/S0026898419010051. [DOI] [PubMed] [Google Scholar]
- 80.pH-responsive delivery of Griffithsin from electrospun fibers. Tyo KM, Duan J, Kollipara P, Dela Cerna MV, Lee D, Palmer KE, Steinbach-Rankins JM. Eur J Pharm Biopharm. 2019;138:64–74. doi: 10.1016/j.ejpb.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 81.The antiviral activity of arbidol hydrochloride against herpes simplex virus type II (HSV-2) in a mouse model of vaginitis. Du Q, Gu Z, Leneva I, Jiang H, Li R, Deng L, Yang Z. Int Immunopharmacol. 2019;68:58–67. doi: 10.1016/j.intimp.2018.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Discovery of small-molecule inhibitors targeting the E3 ubiquitin ligase activity of the herpes simplex virus 1 ICP0 protein using an in vitro high-throughput screening assay. Deschamps T, Waisner H, Dogrammatzis C, et al. J Virol. 2019;93 doi: 10.1128/JVI.00619-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Early steps in herpes simplex virus infection blocked by a proteasome inhibitor. Schneider SM, Pritchard SM, Wudiri GA, Trammell CE, Nicola AV. mBio. 2019;10 doi: 10.1128/mBio.00732-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.UL23, UL30, and UL5 characterization of HSV1 clinical strains isolated from hematology department patients. Labrunie T, Ducastelle S, Domenech C, Ader F, Morfin F, Frobert E. Antiviral Res. 2019;168:114–120. doi: 10.1016/j.antiviral.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 85.Multiple In vitro biological effects of phenolic compounds from Morus alba root bark. Čulenová M, Sychrová A, Hassan ST, et al. J Ethnopharmacol. 2020;248:112296. doi: 10.1016/j.jep.2019.112296. [DOI] [PubMed] [Google Scholar]
- 86.Antiviral activity of mitoxantrone dihydrochloride against human herpes simplex virus mediated by suppression of the viral immediate early genes. Huang Q, Hou J, Yang P, et al. BMC Microbiol. 2019;19:274. doi: 10.1186/s12866-019-1639-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anti-herpes virus activity of the carnivorous botanical, Sarracenia purpurea. Kannan L, Kumar A, Kumar A, Jacobs B, Langland J. Sci Rep. 2020;10:18953. doi: 10.1038/s41598-020-76151-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Efficacy of pleuran (β-glucan from Pleurotus ostreatus) in the management of herpes simplex virus type 1 infection. Urbancikova I, Hudackova D, Majtan J, Rennerova Z, Banovcin P, Jesenak M. Evid Based Complement Alternat Med. 2020;2020:8562309. doi: 10.1155/2020/8562309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Synthesis of antiviral perfluoroalkyl derivatives of teicoplanin and vancomycin. Bereczki I, Csávás M, Szűcs Z, et al. ChemMedChem. 2020;15:1661–1671. doi: 10.1002/cmdc.202000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.A novel, broad-acting peptide inhibitor of double-stranded dna virus gene expression and replication. Ruzsics Z, Hoffmann K, Riedl A, et al. Front Microbiol. 2020;11:601555. doi: 10.3389/fmicb.2020.601555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Antiviral activity of a Arisaema tortuosum leaf extract and some of its constituents against herpes simplex virus type 2. Rittà M, Marengo A, Civra A, et al. Planta Med. 2020;86:267–275. doi: 10.1055/a-1087-8303. [DOI] [PubMed] [Google Scholar]
- 92.Perillyl alcohol and perillic acid exert efficient action upon HSV-1 maturation and release of infective virus. Mello CP, Quirico-Santos T, Amorim LF, Silva VG, Fragel LM, Bloom DC, Paixão IP. Antivir Ther. 2020;25:1–11. doi: 10.3851/IMP3315. [DOI] [PubMed] [Google Scholar]
- 93.Modified cyclodextrins as broad-spectrum antivirals. Jones ST, Cagno V, Janeček M, et al. Sci Adv. 2020;6:0. doi: 10.1126/sciadv.aax9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Evaluation of the effect of hydro alcoholic extract of cinnamon on herpes simplex virus-1. Moshaverinia M, Rastegarfar M, Moattari A, Lavaee F. https://www.ncbi.nlm.nih.gov/pubmed/32435433https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7224267. Dent Res J (Isfahan) 2020;17:114–119. [PMC free article] [PubMed] [Google Scholar]
- 95.Acyclovir, cidofovir, and amenamevir have additive antiviral effects on herpes simplex virus type 1. Greeley ZW, Giannasca NJ, Porter MJ, Margulies BJ. Antiviral Res. 2020;176:104754. doi: 10.1016/j.antiviral.2020.104754. [DOI] [PubMed] [Google Scholar]
- 96.Antiviral activity of 1,4-disubstituted-1,2,3-triazoles against HSV-1 in vitro. Viegas DJ, da Silva VD, Buarque CD, Bloom DC, Abreu PA. Antivir Ther. 2020;25:399–410. doi: 10.3851/IMP3387. [DOI] [PubMed] [Google Scholar]
- 97.Anti-HSV-1 effect of dihydromyricetin from Ampelopsis grossedentata via the TLR9-dependent anti-inflammatory pathway. Zhou HY, Gao SQ, Gong YS, et al. J Glob Antimicrob Resist. 2020;23:370–376. doi: 10.1016/j.jgar.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 98.Herpes simplex virus type 1 clinical isolates respond to UL29-targeted siRNA swarm treatment independent of their acyclovir sensitivity. Kalke K, Lehtinen J, Gnjatovic J, et al. Viruses. 2020;12 doi: 10.3390/v12121434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.MG132 exerts anti-viral activity against HSV-1 by overcoming virus-mediated suppression of the ERK signaling pathway. Ishimaru H, Hosokawa K, Sugimoto A, Tanaka R, Watanabe T, Fujimuro M. Sci Rep. 2020;10:6671. doi: 10.1038/s41598-020-63438-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.In vitro and in vivo activity, tolerability, and mechanism of action of BX795 as an antiviral against herpes simplex virus 2 genital infection. Hopkins J, Yadavalli T, Suryawanshi R, et al. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.00245-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Novel antiviral and antibacterial activities of Hibiscus schizopetalus. El-Shiekh RA, Abdelmohsen UR, Ashour HM, Ashour RM. Antibiotics (Basel) 2020;9 doi: 10.3390/antibiotics9110756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.T-type calcium channels blockers inhibit HSV-2 infection at the late stage of genome replication. Ding L, Jiang P, Xu X, et al. Eur J Pharmacol. 2021;892:173782. doi: 10.1016/j.ejphar.2020.173782. [DOI] [PubMed] [Google Scholar]
- 103.Mori ramulus and its major component morusin inhibit herpes simplex virus type 1 replication and the virus-induced reactive oxygen species. Kim TI, Kwon EB, Oh YC, Go Y, Choi JG. Am J Chin Med. 2021;49:163–179. doi: 10.1142/S0192415X21500099. [DOI] [PubMed] [Google Scholar]
- 104.Cetylpyridinium chloride blocks herpes simplex virus replication in gingival fibroblasts. Alvarez DM, Duarte LF, Corrales N, Smith PC, González PA. Antiviral Res. 2020;179:104818. doi: 10.1016/j.antiviral.2020.104818. [DOI] [PubMed] [Google Scholar]
- 105.Inhibition of herpes simplex viruses, types 1 and 2, by ginsenoside 20(S)-Rg3. Wright S, Altman E. J Microbiol Biotechnol. 2020;30:101–108. doi: 10.4014/jmb.1908.08047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mangiferin: a promising natural xanthone from Mangifera indica for the control of acyclovir - resistant herpes simplex virus 1 infection. Rechenchoski DZ, Agostinho KF, Faccin-Galhardi LC, et al. Bioorg Med Chem. 2020;28:115304. doi: 10.1016/j.bmc.2020.115304. [DOI] [PubMed] [Google Scholar]
- 107.Anti-HSV-1 activity of aspergillipeptide D, a cyclic pentapepetide isolated from fungus Aspergillus sp. SCSIO 41501. Wang Z, Jia J, Wang L, et al. Virol J. 2020;17:41. doi: 10.1186/s12985-020-01315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guanidine modifications enhance the anti-herpes simplex virus activity of (E,E)-4,6-bis(styryl)-pyrimidine derivatives in vitro and in vivo. Wang W, Xu C, Zhang J, et al. Br J Pharmacol. 2020;177:1568–1588. doi: 10.1111/bph.14918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bacterial pigment prodigiosin demonstrates a unique antiherpesvirus activity that is mediated through inhibition of prosurvival signal transducers. Suryawanshi RK, Koujah L, Patil CD, et al. J Virol. 2020;94 doi: 10.1128/JVI.00251-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Anti-herpetic activity of Macrocystis pyrifera and Durvillaea antarctica algae extracts against HSV-1 and HSV-2. Castillo E, Duarte LF, Corrales N, et al. Front Microbiol. 2020;11:2006. doi: 10.3389/fmicb.2020.02006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Synthesis and evaluation of some uracil nucleosides as promising anti-herpes simplex virus 1 agents. Awad SM, Ali SM, Mansour YE, Fatahala SS. Molecules. 2021;26 doi: 10.3390/molecules26102988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Targeting aryl hydrocarbon receptor signaling enhances type i interferon-independent resistance to herpes simplex virus. Chen J, Liang J, Xu H, et al. Microbiol Spectr. 2021;9:0. doi: 10.1128/Spectrum.00473-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Study of the extremely-tolerant brevibacterium linens AE038-8 with antiviral activity against herpes simplex virus type 1. Maizel D, Salinas FM, Solórzano I, Raiger Iustman L, Ferrero MA, Mauas PJ, Alché LE. Curr Microbiol. 2021;78:688–695. doi: 10.1007/s00284-020-02316-5. [DOI] [PubMed] [Google Scholar]
- 114.Cellular miR-101-1 reduces efficiently the replication of HSV-1 in HeLa cells. Sadegh Ehdaei B, Pirouzmand A, Shabani M, Mirzaei A, Moghim S. Intervirology. 2021;64:88–95. doi: 10.1159/000512956. [DOI] [PubMed] [Google Scholar]
- 115.Lupeol impairs herpes simplex virus type 1 replication by inhibiting the promoter activity of the viral immediate early gene α0. Ye J, Wang Z, Jia J, et al. Acta Virol. 2021;65:254–263. doi: 10.4149/av_2021_302. [DOI] [PubMed] [Google Scholar]
- 116.Harringtonine inhibits herpes simplex virus type 1 infection by reducing herpes virus entry mediator expression. Liu Y, You Q, Zhang F, Chen D, Huang Z, Wu Z. Front Microbiol. 2021;12:722748. doi: 10.3389/fmicb.2021.722748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.NSC23766 and Ehop016 suppress herpes simplex virus-1 replication by inhibiting Rac1 activity. Zhang F, Liu Y, You Q, et al. Biol Pharm Bull. 2021;44:1263–1271. doi: 10.1248/bpb.b21-00054. [DOI] [PubMed] [Google Scholar]
- 118.Synthesis and biological evaluation of amidinourea derivatives against herpes simplex viruses. Toscani A, Denaro R, Pacheco SF, Biolatti M, Anselmi S, Dell'Oste V, Castagnolo D. Molecules. 2021;26 doi: 10.3390/molecules26164927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.The efficacy of olive leaf extract on healing herpes simplex virus labialis: a randomized double-blind study. Toulabi T, Delfan B, Rashidipour M, Yarahmadi S, Ravanshad F, Javanbakht A, Almasian M. Explore (NY) 2022;18:287–292. doi: 10.1016/j.explore.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 120.Ginkgolic acid inhibits herpes simplex virus type 1 skin infection and prevents zosteriform spread in mice. Bhutta MS, Shechter O, Gallo ES, Martin SD, Jones E, Doncel GF, Borenstein R. Viruses. 2021;13 doi: 10.3390/v13010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Amenamevir, a helicase-primase inhibitor, for the optimal treatment of herpes zoster. Shiraki K, Yasumoto S, Toyama N, Fukuda H. Viruses. 2021;13 doi: 10.3390/v13081547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.In vitro analysis of the antioxidant and antiviral activity of embelin against herpes simplex virus-1. Elias T, Lee LH, Rossi M, Caruso F, Adams SD. Microorganisms. 2021;9 doi: 10.3390/microorganisms9020434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cucumis melo pectin as potential candidate to control herpes simplex virus infection. Agostinho KF, Rechenchoski DZ, Faccin-Galhardi LC, et al. FEMS Microbiol Lett. 2021;368 doi: 10.1093/femsle/fnab013. [DOI] [PubMed] [Google Scholar]
- 124.Poly(dA:dT) suppresses HSV-2 infection of human cervical epithelial cells through RIG-I activation. Shao DD, Meng FZ, Liu Y, et al. Front Immunol. 2020;11:598884. doi: 10.3389/fimmu.2020.598884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Phytochemical characterization of Olea europea leaf extracts and assessment of their anti-microbial and anti-HSV-1 activity. Ben-Amor I, Musarra-Pizzo M, Smeriglio A, et al. Viruses. 2021;13 doi: 10.3390/v13061085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.In vitro effects of bufotenine against RNA and DNA viruses. Barboza CM, Pimenta DC, Vigerelli H, et al. Braz J Microbiol. 2021;52:2475–2482. doi: 10.1007/s42770-021-00612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Quercus acuta Thunb. (Fagaceae) and its component, isoquercitrin, inhibit HSV-1 replication by suppressing virus-induced ROS production and NF-κB activation. Kim B, Kim YS, Hwang YH, et al. Antioxidants (Basel) 2021;10 doi: 10.3390/antiox10101638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sulfonated and carboxymethylated β-glucan derivatives with inhibitory activity against herpes and dengue viruses. Lopes JL, Quinteiro VS, Wouk J, et al. Int J Mol Sci. 2021;22 doi: 10.3390/ijms222011013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Effective deploying of a novel DHODH inhibitor against herpes simplex type 1 and type 2 replication. Luganini A, Sibille G, Mognetti B, et al. Antiviral Res. 2021;189:105057. doi: 10.1016/j.antiviral.2021.105057. [DOI] [PubMed] [Google Scholar]
- 130.Viral UL8 is involved in the antiviral activity of oleanolic acid against HSV-1 infection. Shan T, Ye J, Jia J, et al. Front Microbiol. 2021;12:689607. doi: 10.3389/fmicb.2021.689607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Antiviral activity of Rosa damascena Mill. and Rosa alba L. essential oils against the multiplication of herpes simplex virus type 1 strains sensitive and resistant to acyclovir. Vilhelmova-Ilieva N, Dobreva A, Doynovska R, Krastev D, Mileva M. Biology (Basel) 2021;10 doi: 10.3390/biology10080746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Inhibition of herpes simplex virus type 1 infection by Sambucus ebulus extract in vitro. Ghaffari H, Ataei-Pirkooh A, Mirghazanfari SM, Barati M. Med J Islam Repub Iran. 2021;35:9. doi: 10.47176/mjiri.35.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Inhibition of herpes simplex virus-1 infection by MBZM-N-IBT: in silico and in vitro studies. Kumar A, De S, Moharana AK, et al. Virol J. 2021;18:103. doi: 10.1186/s12985-021-01581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peptide inhibitor of complement C1, RLS-0071, reduces zosteriform spread of herpes simplex virus type 1 skin infection and promotes survival in infected mice. Bhutta MS, Sausen DG, Reed KM, et al. Viruses. 2021;13 doi: 10.3390/v13081422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Antimicrobial activity of cyclic-monomeric and dimeric derivatives of the snail-derived peptide Cm-p5 against viral and multidrug-resistant bacterial strains. González-García M, Morales-Vicente F, Pico ED, et al. Biomolecules. 2021;11 doi: 10.3390/biom11050745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.The molecular tweezer CLR01 inhibits antibody-resistant cell-to-cell spread of human cytomegalovirus. Brenner S, Braun B, Read C, et al. Viruses. 2021;13 doi: 10.3390/v13091685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Helicase primase inhibitors (HPIs) are efficacious for therapy of human herpes simplex virus (HSV) disease in an infection mouse model. Uhlig N, Donner AK, Gege C, Lange F, Kleymann G, Grunwald T. Antiviral Res. 2021;195:105190. doi: 10.1016/j.antiviral.2021.105190. [DOI] [PubMed] [Google Scholar]
- 138.Anti-infectivity against herpes simplex virus and selected microbes and anti-inflammatory activities of compounds isolated from Eucalyptus globulus Labill. Brezáni V, Leláková V, Hassan ST, et al. Viruses. 2018;10 doi: 10.3390/v10070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Varicella-zoster virus orf9p binding to cellular adaptor protein complex 1 is important for viral infectivity. Lebrun M, Lambert J, Riva L, et al. J Virol. 2018;92 doi: 10.1128/JVI.00295-18. [DOI] [PMC free article] [PubMed] [Google Scholar]