Abstract
Background and Aims
Treatment indications for chronic hepatitis B (CHB) differ among recommendations by European Association for the Study of the Liver (EASL), American Association for the Study of Liver Diseases (AASLD) and World Health Organization (WHO). We aimed to assess treatment eligibility and linkage to therapy at a large tertiary care center.
Methods
All CHB patients who were evaluated for treatment at the Vienna General Hospital between January 2010 and December 2020 were retrospectively included. Clinical, virological, and long‐term treatment efficacy data were analyzed.
Results
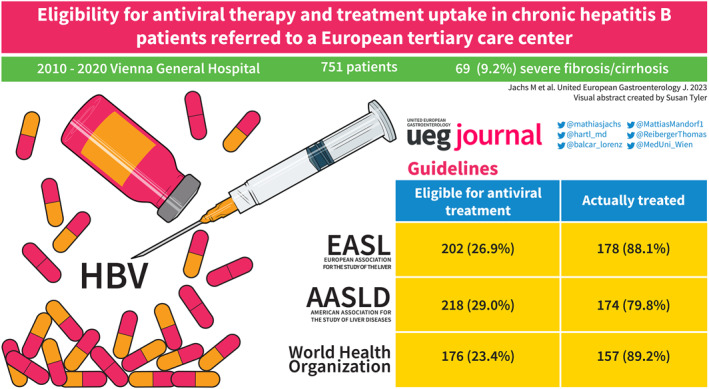
A total of 751 CHB patients were included (53.3% male; median age: 39.5 years; HBeAg‐positive: 10.8%). The median Hepatitis B Virus (HBV)‐DNA and HBsAg levels were 569 (68–11,750) IU/mL and 3467.65 (620.05–11,935.43) IU/mL, respectively. Overall, 9.2% of patients had severe fibrosis/cirrhosis, and 5.7% were coinfected with hepatitis D virus (HDV), which was highly prevalent in cirrhosis. According to the recent EASL nomenclature, 3.2% of patients had HBeAg‐positive chronic infection, 7.6% had HBeAg‐positive chronic hepatitis, 58.9% had HBeAg‐negative chronic infection, and 30.4% had HBeAg‐negative chronic hepatitis. At the time of evaluation, 36.4% had HBV‐DNA >2000 IU/mL, and 37.3% showed alanine aminotransferase >40 U/L. Ultimately, 26.9% (EASL), 29.0% (AASLD) and 23.4% (WHO) met the treatment criteria. Treatment was initiated in most patients, mainly with tenofovir (61.8%) or entecavir (34.9%). Treatment efficiently suppressed HBV‐DNA in all patients; however, HBsAg loss was observed only in 2.8% at 5 years of therapy.
Conclusions
Severe fibrosis/cirrhosis was found in 9.2% of CHB patients at presentation, and 23.4%–29.5% met current treatment recommendations with a high treatment uptake of 79.8%–89.2% among eligible patients.
Keywords: chronic hepatitis B, entecavir, hepatitis B infection, hepatitis D coinfection, linkage to care, liver cirrhosis, tenofovir, treatment uptake
Key Summary.
Established knowledge on this subject
Treatment indications in patients with chronic hepatitis B slightly differ between guidelines published by major organizations (European Association for the Study of the Liver, American Association for the Study of Liver Diseases, World Health Organization).
Limited data exists on the treatment eligibility among patients referred to a tertiary care center.
We retrospectively evaluated treatment eligibility and uptake among all patients referred to our center between 2010 and 2020.
What are the significant and/or new findings of this study?
Among the 751 patients evaluated, 9.2% had severe fibrosis or cirrhosis and 5.7% were anti‐hepatitis D virus positive.
At evaluation, 36.4% had Hepatitis B Virus‐DNA >2000 IU/m and 37.3% had alanine aminotransferase >40 U/L.
Treatment indications were met by 23.4%–29.0% depending on the applied criteria.
INTRODUCTION
Up to 290 million people worldwide are living with chronic hepatitis B (CHB). 1 , 2 A recent Austrian report estimates the nationwide prevalence at 42,000 people, 3 corresponding to roughly 0.5% of the overall population, which is in line with reports from neighboring low‐endemic central European countries. 4 , 5
Despite extensive research efforts, a (functional) cure for the disease is not yet available. 6 However, well tolerated and highly effective oral antiviral medications can suppress viral replication and thus can protect most patients from deleterious outcomes, that is, progression to cirrhosis, hepatocellular carcinoma, and liver‐related death. 7
High viral load, evidence for moderate to severe inflammatory activity, and evidence for the presence of severe fibrosis or cirrhosis indicate antiviral treatment as per the most recent recommendations of the European Association for the Study of the Liver (EASL), 8 the American Association for the Study of Liver Diseases (AASLD), 9 and the World Health Organization (WHO). 10 Depending on the setting (community care vs. specialized centers), population and region, a proportion of 12%–25% seems to be eligible for antiviral treatment according to these different guidelines, as shown by a recent meta‐analysis. 11 However, this seminal analysis revealed a lack of studies reporting the proportion of treatment eligibility among CHB patients, and an underreporting of data on linkage to therapy among those patients. To date, no specific data for treatment eligibility among Austrian CHB patients are available, and only limited data exist on treatment eligibility in Europe according to the 2017 EASL guidelines. 11 , 12 In addition, no European study has reported on treatment eligibility according to EASL criteria in comparison to other recommendations (AASLD, WHO) that might be applied to guide the treatment of CHB in Europe and North America, but also in settings that allow only for essential evaluation. Furthermore, the EASL introduced a new nomenclature for the natural history stages of CHB in the most recent guideline; however, few data exist on its applicability in clinical practice. 8 Lastly, seminal research has suggested a potential diagnostic role of quantitative HBsAg in distinguishing stages of HBeAg‐negative chronic hepatitis; 13 however, these notions lack validation in cohorts stratified according to the latest classification.
Therefore, this study was designed to evaluate (i) the proportion of CHB patients who were evaluated for antiviral treatment, (ii) the real‐life applicability of recently introduced natural history stages as proposed by the EASL and the diagnostic role of quantitative HBsAg levels in HBeAg‐negative CHB, and (iii) treatment uptake among CHB patients eligible as per different guidelines, and (iv) the long‐term outcome of CHB patients linked to antivirals at an Austrian tertiary care center.
METHODS
Patient cohort and study design
We retrospectively included all consecutive patients who were diagnosed with CHB at our center or referred for treatment evaluation with an established CHB diagnosis between January 2010 and December 2020. CHB was diagnosed by repeated positive HBsAg tests at least 6 months apart.
Patients who (i) were younger than 18 years, (ii) had a history of liver transplantation, (iii) were coinfected with human immunodeficiency virus (HIV) and/or hepatitis C virus (HCV), or (iv) were not treatment‐naïve were excluded.
Next, we evaluated the proportion of patients who underwent a basic laboratory evaluation to assess their need for antiviral therapy, including the assessment of alanine aminotransferase (ALT), viral load, that is, Hepatitis B Virus (HBV)‐DNA levels, and HBeAg status on the same day. Additional laboratory values were obtained longitudinally from an automated inquiry into our central laboratory's database, and data on overall patient and treatment characteristics and clinically relevant parameters were gathered by a thorough manual search of all available medical records.
Laboratory tests
Routine laboratory tests to assess ALT, aspartate transaminase (AST), international normalized ratio (INR), and other baseline parameters were performed by the ISO‐certified Department of Laboratory Medicine of the Medical University of Vienna. AST to platelet ratio index and Fibrosis‐4 score were calculated according to published formulas.
Qualitative HBsAg and HBeAg status were determined using commercially available chemiluminescent immunoassays. Hepatitis B Virus (HBV)‐DNA was quantified using a real‐time PCR assay (COBAS® AmpliPrep/COBAS® TaqMan® version 2, Roche Diagnostics) with a lower limit of linear quantification of 20 IU/mL and an upper limit of linear quantification of 1.7 × 108 IU/mL. The quantitative assessment of HBsAg was conducted applying the Abbott ARCHITECT® assay (Abbott Diagnostics) with a lower limit of linear quantification of 0.05 IU/mL and an upper limit of linear quantification of 250 IU/mL. Samples were diluted according to the manual of the manufacturer, and if results were >250 IU/mL, samples were retested at a higher dilution. The presence of hepatitis D virus (HDV) antibodies was investigated using a commercially available chemiluminescent assay. If antibodies against HDV were detected, viremia was evaluated by an HDV‐RNA‐PCR assay with a lower limit of linear quantification of 100 copies/mL developed in‐house by the Center for Virology of the Medical University of Vienna, that is, the Austrian reference center for HDV diagnostics, as previously described. 14
Assessment of liver fibrosis by transient elastography
Liver stiffness measurements (LSM) were obtained by vibration‐controlled transient elastography (TE) using the FibroScan® system (Echosens) as previously described. 15 The decision to refer to TE was made at the discretion of the treating physician.
Severe fibrosis or cirrhosis was diagnosed if LSM yielded ≥9 kPa in patients with normal ALT, or LSM ≥12 kPa in patients with ALT ≤5 × upper limit of normal (ULN), histologically proven F3/F4 fibrosis, or clinical signs of cirrhosis (varices, ascites, encephalopathy). These cut‐offs, which were derived from a meta‐analysis on LSM in CHB patients, 16 were chosen in accordance with the EASL clinical practice guideline on non‐invasive tests published in 2015 15 (CHB‐specific cut‐offs were not updated in the guidelines 2021 update 17 ) as no cut‐off for ruling‐in cirrhosis was proposed in the EASL CHB guideline published in 2017. 8
Assessment of treatment eligibility and treatment initiation
The eligibility for treatment was evaluated according to the latest editions of EASL, 8 AASLD 9 and WHO 10 guidelines based on HBeAg status, HBV‐DNA and ALT levels, and fibrosis staging according to liver biopsy or TE. A summary of the treatment indications considered for this analysis is given in Table 1. Treatment eligibility was evaluated cross‐sectionally, that is, based on all available data available/gathered at the date of CHB diagnosis or first referral to our center.
TABLE 1.
Treatment indications for chronic hepatitis B according to the most recent recommendations of the EASL, AASLD, and WHO.
| Organization | Grade | HBeAg | HBV‐DNA (IU/mL) | ALT a | Fibrosis | Other/comment |
|---|---|---|---|---|---|---|
| EASL (CPG 2017) | Strong | (+) or (−) | >20,000 | >2 × ULN | Any | |
| Strong | (+) or (−) | >2000 | >ULN | ≥F2 | HBV‐DNA + one other criteria | |
| Strong | (+) or (−) | Detectable | Any | Any | Cirrhosis | |
| Weak | (+) | >20,000 | ≤ULN | Any | Age: >30 years | |
| Weak b | (+) or (−) | Any | Any | Any | Family history of HCC | |
| AASLD (CPG 2018) | ‐ | (+) | >20,000 | >2 × ULN | Any | |
| ‐ | (+) | >20,000 | >ULN | ≥F2 | ||
| ‐ | (−) | >2000 | >2 × ULN | Any | ||
| ‐ | (−) | >2000 | >ULN | ≥F2 | HBV‐DNA + one other criteria | |
| (+) or (−) | Detectable | Any | Any | Cirrhosis | ||
| WHO (CPG 2015) | Strong | (+) or (−) | Any | Any | Any | Cirrhosis |
| Strong | (+) or (−) | Any | Any | Any | APRI >2 | |
| Strong | (+) or (−) | >20,000 | >ULN | Any | Particularly if age >30 years |
Abbreviations: AASLD, American Association for the Study of Liver Disease; ALT, alanine aminotransferase; APRI, AST to platelet ratio index; EASL, European Association for the Study of the Liver; HBV, Hepatitis B Virus; ULN, upper limit of normal; WHO, World Health Organization.
ULN not specified in the EASL guidelines, local ULN specified at 40 IU/L for both sexes (also applied for WHO treatment rules). ULN of 35 IU/L (males) and 25 IU/L (females) recommended as per AASLD guideline to guide management decisions.
Indication could not be considered in this retrospective analysis.
Long‐term outcome
In the subgroup of patients in whom antiviral therapy was initiated, all available longitudinal HBV‐DNA, ALT, qualitative HBeAg and quantitative HBsAg values were gathered. Values were obtained at intervals of 1 year after treatment initiation. Values closest to the respective dates, that is, baseline plus one, two, three, etc., years, were considered for the analysis; however, values outside a range of plus/minus 3 months were excluded.
Statistical analysis
Statistical analysis was conducted using R 4.0.2 (R Core Team, R Foundation for Statistical Computing) and GraphPad Prism 8 (GraphPad Software). Group comparisons of categorical variables were performed using χ 2 test Fisher's exact test. For unpaired comparisons of continuous variables, Mann‐Whitney U test was applied. Categorical variables are presented as the number (percentage) of patients with a certain characteristic, while continuous variables are presented as median (25th–75th percentile). Correlations were investigated by calculating Spearman's ρ correlation coefficient.
Data on long‐term treatment efficacy are presented as median (95% confidence interval) of the respective parameters at a distinct timepoint (baseline and years one to five after treatment initiation). If patients missed a visit, the last known value was carried forward. Long‐term efficacy data were gathered for the first 5 years after treatment initiation. Patients who dropped out of routine surveillance were censored at the last clinical visit for which laboratory values were available. Moreover, long‐term data on the outcome in patients with severe fibrosis/cirrhosis who were linked to treatment were collected. First, hepatic decompensation (in patients compensated at baseline/treatment initiation), liver transplantation, and liver‐related and non‐liver related death during follow‐up were recorded and analyzed to estimate the cumulative incidence of adverse hepatic outcomes during follow‐up.
A two‐sided p‐value ≤0.05 was considered statistically significant.
RESULTS
Overall characteristics of the study population
After applying all in‐ and exclusion criteria, 817 patients were included in the final study population, as shown in Figure 1. Among those patients, 66 (8.1%) were incompletely evaluated for treatment, that is, lacked ALT, HBV‐DNA and/or HBeAg status information. The remaining 751 (91.9%) patients were considered for further analyses. No significant dynamics in HBV incidence over earlier years of the study period were observed as shown in Figure S1, but a decreasing incidence was noted in 2019 and 2020, most likely owing to restrictions to healthcare access that were put in place due to the global SARS‐CoV‐2 pandemic. 18 A slight majority of patients were male (53.3%), and the median age was 39.5 (30.6–52.8) years. Liver biopsy and LSM were conducted in 42 and 352 patients, respectively. A total of 69 (9.2%) patients were diagnosed with severe fibrosis or cirrhosis. Further patient characteristics and a comparison between the cirrhotic and non‐cirrhotic subcohort of patients are given in Table 2.
FIGURE 1.
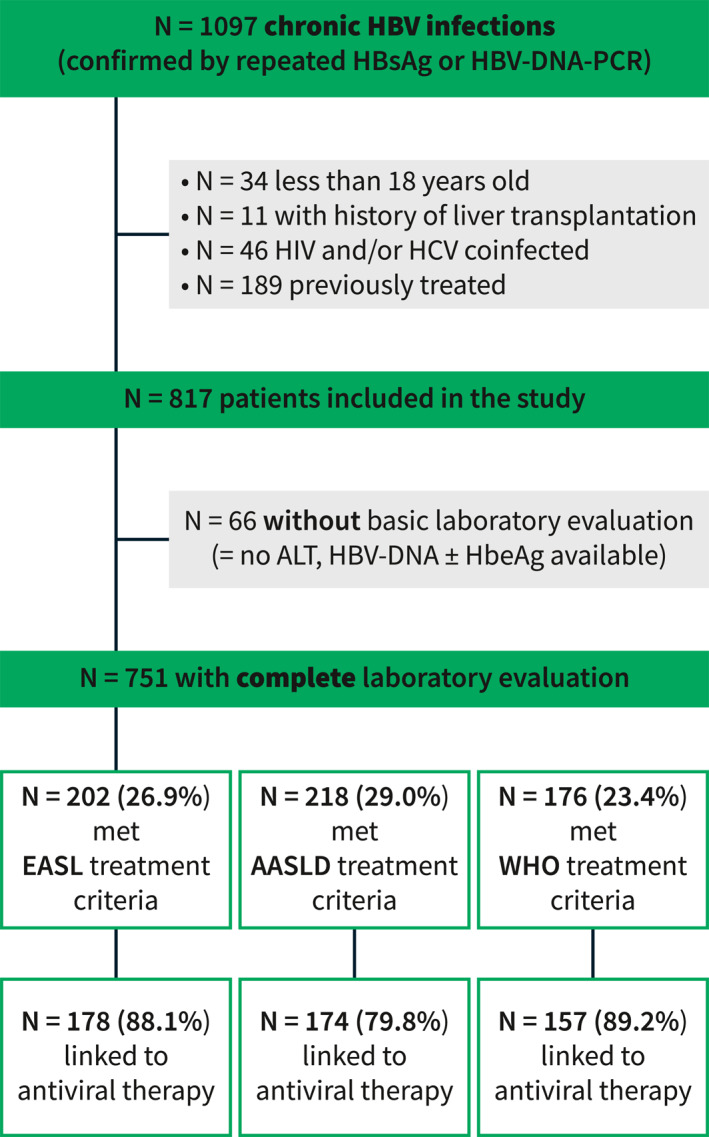
Study flowchart. Fibrosis staging via biopsy or transient elastography missing in n = 54 non‐cirrhotic patients with HBV‐DNA >2000 IU/mL but ALT ≤ ULN, in whom the presence of moderate fibrosis could have changed treatment eligibility. ALT, alanine aminotransferase; HBV, Hepatitis B Virus; ULN, upper limit of normal.
TABLE 2.
Characteristics of the overall final study cohort and differences in baseline characteristics according to the presence or absence of severe fibrosis or cirrhosis.
| Patient characteristics | Overall cohort n = 751 | No cirrhosis n = 682 | Severe fibrosis/cirrhosis N = 69 | p‐Value |
|---|---|---|---|---|
| Sex, male (%) | 400 (53.3) | 350 (51.3) | 50 (72.5) | 0.001 |
| Age, years | 39.5 (30.6–52.8) | 38.6 (29.6–52.6) | 49.1 (39.8–56.0) | <0.001 |
| HBeAg‐positive, n (%) | 81 (10.8) | 72 (10.6) | 9 (13.0) | 0.667 |
| Natural history stage at inclusion, a n (%) | <0.001 | |||
| HBeAg (+) chronic infection | 24 (3.2) | 24 (3.5) | 0 (0.0) | |
| HBeAg (+) chronic hepatitis | 57 (7.6) | 48 (7.0) | 9 (13.0) | |
| HBeAg (−) chronic infection | 442 (58.9) | 442 (64.8) | 0 (0.0) | |
| HBeAg (−) chronic hepatitis | 228 (30.4) | 168 (24.6) | 60 (87.0) | |
| HDV coinfection, c n (%) | 43 (5.7) | 20 (2.9) | 23 (33.3) | <0.001 |
| HDV‐PCR (+) | 25 (58.1%) | 8 (40.0) | 17 (73.9) | 0.046 b |
| HDV‐PCR (−) | 12 (27.9%) | 8 (40.0) | 4 (17.4) | |
| HDV‐PCR not ordered | 6 (14.0%) | 4 (20.0) | 2 (8.7) | |
| HBV‐DNA, IU/mL | 569 (68–11,750) | 567 (72–8555) | 1570 (32–97,900) | 0.520 |
| <2000 | 478 (63.6) | 441 (64.7) | 37 (53.6) | 0.017 |
| 2000–20,000 | 103 (13.7) | 88 (12.9) | 7 (10.1) | |
| ≥20,000 | 170 (22.6) | 153 (22.4) | 25 (36.2) | |
| HBsAg quant., d IU/mL | 3467.65 (620.05–11,935.43) | 3444.51 (567.59–12,172.17) | 4014.18 (1571.56–10,990.00) | 0.313 |
| <1000 | 175 (29.3) | 161 (30.4) | 14 (20.3) | 0.208 |
| 1000–10,000 | 253 (42.1) | 219 (41.3) | 34 (49.3) | |
| ≥10,000 | 171 (28.6) | 150 (28.3) | 21 (30.4) | |
| ALT, U/L | 31.00 (21–53) | 29 (20–48) | 55 (34–95) | <0.001 |
| ≤40 | 471 (62.7) | 448 (65.7) | 23 (33.3) | <0.001 |
| 41–80 | 149 (19.8) | 125 (18.3) | 24 (34.8) | |
| >80 | 131 (17.4) | 109 (16.0) | 22 (31.9) | |
| AST, U/L | 27 (21–38) | 26 (21–35) | 50 (29–87) | <0.001 |
| Gamma‐glutamyltransferase, U/L | 22 (14–38) | 21 (14–36) | 48 (30–136) | <0.001 |
| Alkaline phosphatase, U/L | 68 (55–86) | 67 (54–83) | 84 (69–106) | <0.001 |
| Bilirubin, mg/dL | 0.51 (0.36–0.75) | 0.49 (0.35–0.72) | 0.78 (0.51–1.32) | <0.001 |
| Platelets, G/L | 216 (177–259) | 220 (183–261) | 157 (107–212) | <0.001 |
| INR | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | 1.1 (1.0–1.3) | <0.001 |
| FIB‐4 | 0.92 (0.63–1.40) | 0.87 (0.60–1.28) | 2.31 (1.24–3.70) | <0.001 |
| <1.25 | 517 (68.8) | 499 (73.2) | 19 (27.5) | |
| 1.25–3.25 | 192 (25.6) | 165 (24.2) | 27 (39.1) | |
| >3.25 | 42 (5.6) | 18 (2.6) | 23 (33.3) | |
| APRI | 0.32 (0.23–0.48) | 0.31 (0.22–0.44) | 1.09 (0.37–1.99) | <0.001 |
| Severe fibrosis/cirrhosis, e n (%) | 69 (9.2) | 0 (0.0) | 69 (100.0) | N/A |
| EASL treatment indication, n (%) | 202 (26.9) | 133 (19.5) | 69 (100.0) | <0.001 |
| Proportion treated, n (%) | 178 (88.1) | 114 (85.7) | 64 (92.8) | |
| AASLD treatment indication, n (%) | 218 (29.0) | 149 (21.8) | 69 (100.0) | <0.001 |
| Proportion treated, n (%) | 174 (79.8) | 110 (73.8) | 64 (92.8) | |
| WHO treatment indication, n (%) | 176 (23.4) | 96 (14.1) | 69 (100.0) | <0.001 |
| Proportion treated, n (%) | 157 (89.2) | 89 (92.7) | 64 (92.8) |
Note: Values in bold indicate p‐Values below the threshold for statistical significance (< 0.05).
Abbreviations: AASLD, American Association for the Study of Liver Disease; ALT, alanine aminotransferase; APRI, AST to platelet ratio index; AST, aspartate transaminase; EASL, European Association for the Study of the Liver; FIB‐4, Fibrosis‐4; HBV, Hepatitis B Virus; HDV, hepatitis D virus; LSM, liver stiffness measurements; ULN, upper limit of normal; WHO, World Health Organization.
As defined in the most recent EASL CPG. 8
p‐value referring to χ 2 test among patients with available HDV‐RNA‐PCR test.
Presence of HDV coinfection was investigated in 69.8% of patients seen in 2019–2020.
Available in n = 599.
Defined as either (i) LSM >9 kPa if ALT ≤ ULN, (ii) LSM >12 kPa if ALT ≤5 × ULN, (iii) F3/F4 fibrosis in histology, or (iv) clinical signs of cirrhosis (varices or ascites/encephalopathy). 8
Virological characteristics and natural history stage of chronic hepatitis B infection
HBeAg was detected in 81 (10.8%) patients, who tended to be younger (33.7 [26.4–45.3] vs. 41.0 [31.1–53.3] years; p = 0.001) and showed significantly higher HBV‐DNA (57,500,000 [574,000–170,000,000] vs. 404 (49–3440) IU/mL; p < 0.001), quantitative HBsAg (22,967.83 [6352.44–66,093.82] vs. 2835.94 [470.83–9400.32] IU/mL; p < 0.001), and ALT (76 [34–93] vs. 30 [20–48] U/L; p < 0.001) levels as compared to HBeAg‐negative patients. Overall, 7.5% showed undetectable HBV‐DNA at baseline, while 36.4% showed HBV‐DNA ≥2000 IU/mL. The baseline median level of quantitative HBsAg was 3467.65 (620.05–11,935.43) IU/mL. ALT elevations, that is, values above 40 U/L, were present in 37.3% of patients, and ALT values >2 × ULN were recorded in 17.4%.
When stratifying the cohort according to the natural history classification recently proposed by the EASL, 24 (3.2%) had HBeAg‐positive chronic infection, 57 (7.6%) had HBeAg‐positive chronic hepatitis, and 442 (58.9%) and 228 (30.4%) had HBeAg‐negative chronic infection and HBeAg‐negative chronic hepatitis, respectively.
While quantitative HbsAg levels showed correlations of weak (HBeAg‐negative cohort: Spearman's ρ: 0.208; p < 0.001) to moderate (HBeAg‐positive cohort: Spearman's ρ: 0.503; p < 0.001) strength with HBV‐DNA levels (overall cohort: Spearman's ρ: 0.375; p < 0.001), quantitative HBsAg levels conferred no diagnostic value for distinguishing HBeAg‐negative patients with chronic infection from those with chronic hepatitis (area under the receiver operating characteristics curve: 0.593 [95% confidence interval: 0.544–0.641]), as shown in Figure 2.
FIGURE 2.
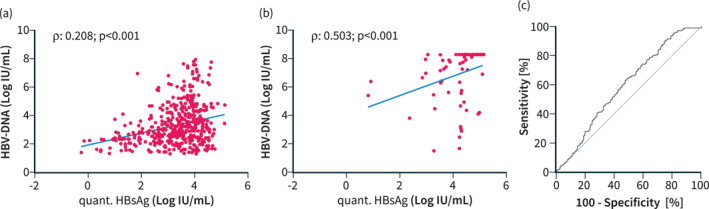
The correlation between HBV‐DNA and quantitative HBsAg values in (a) HBeAg‐negative patients, (b) HBeAg‐positive patients, and (c) receiver operated characteristic curve showing the diagnostic value of quantitative HBsAg level for HBeAg‐negative chronic infection or hepatitis. HBV, Hepatitis B Virus.
Viral coinfections
Viral coinfections were rare among our CHB cohort: 31 (2.8%) and 37 (3.3%) of the initially 1097 CHB patients, who were initially screened for inclusion, were living with immunodeficiency virus, or had antibodies against HCV, respectively. Among the patients with CHB who underwent a complete diagnostic workup, HDV‐coinfection, defined by the presence of anti‐HDV antibodies, was diagnosed in 43 (5.7%) patients. Importantly, HDV‐coinfection was highly prevalent among cirrhotic patients in our cohort (n = 23/69 [33.3%] vs. n = 20/682 [2.9%]; p < 0.001). HDV‐RNA‐PCR was ordered in 37 (86.0%) of seropositive patients, and viremia was detected in 25 (67.6%) of tested patients.
Antiviral treatment eligibility
Considering all the latest treatment indications proposed by EASL, AASLD and WHO that are summarized in Table 1, 202/751 (26.9%), 218/751 (29.0%) and 176/751 (23.4%) were found eligible based on age, HBV‐DNA, ALT, HBeAg status, and fibrosis staging. Treatment was initiated in 80%–90% of patients, depending on the guidelines used to evaluate eligibility.
Most patients meeting the EASL treatment criteria had HBV‐DNA levels above 2000 IU/mL (81.7%) or even above 20,000 IU/mL (69.5%). Almost all the eligible patients who had therapy‐naïve HBV‐DNA <2000 IU/mL (18.3%) had severe fibrosis or cirrhosis. Overall, ALT was elevated in 81.7%, surpassing the threshold of 2 × ULN in 51.2%.
Treatment uptake and long‐term treatment efficacy
Among the 178 patients who were eligible according to EASL criteria and started treatment, 62.8% received tenofovir disoproxile fumarate (TDF) or tenefovir alafenamide (TAF), while 34.9% were prescribed entecavir (ETV), and only a few were started on other nucleos(t)ide analogs or interferon, as shown in Table 3. Patients who did not meet traditional treatment criteria were treated at the discretion of the treating physician or remained under surveillance. Treatment was well tolerated in all patients. ALT and AST decreased swiftly and sustainably, especially in patients without cirrhosis at baseline, while HBV‐DNA was effectively suppressed in all patients and no viral breakthroughs were observed during long‐term follow‐up, as shown in Figure 3. However, as shown in Figure 4, quantitative HBsAg levels were not impacted by long‐term antiviral treatment, and only 2.8% cleared HBsAg within 5 years after treatment initiation. Meanwhile, 28% of HBeAg‐positive patients at baseline cleared HBeAg within 5 years.
TABLE 3.
Baseline characteristics of patients who fulfilled a treatment indication as per EASL CPG 2017 recommendations and were linked to antiviral therapy.
| Patient characteristics | Patients linked to antiviral therapy n = 178 |
|---|---|
| Sex, male (%) | 127 (71.3) |
| Age, years | 39.1 (31.9–52.5) |
| HBeAg‐positive, n (%) | 61 (34.3) |
| Natural history stage at inclusion, a n (%) | |
| HBeAg (+) chronic infection | 9 (5.1) |
| HBeAg (+) chronic hepatitis | 52 (29.2) |
| HBeAg (−) chronic infection | 3 (1.7) |
| HBeAg (−) chronic hepatitis | 114 (64.0) |
| HDV coinfection, n (%) | 27 (15.2) |
| HBV‐DNA, IU/mL | 236,000 (12,950–22,150,000) |
| <2000 | 33 (18.5) |
| 2000–20,000 | 17 (9.6) |
| ≥20,000 | 128 (71.9) |
| HBsAg quant., b IU/mL | 7428.45 (2936.54–21,275.96) |
| <1000 | 23 (13.1) |
| 1000–10,000 | 80 (45.7) |
| ≥10,000 | 72 (41.1) |
| ALT, U/L | 81 (48–112) |
| ≤40 | 30 (16.9) |
| 40–80 | 46 (25.8) |
| >80 | 102 (57.3) |
| AST, U/L | 43 (31–77) |
| Gamma‐glutamyltransferas, U/L | 35 (22–68) |
| Alkaline phosphatase, U/L | 72 (57–98) |
| Bilirubin, mg/dL | 0.63 (0.44–1.01) |
| Platelets, G/L | 196 (152–242) |
| INR | 1.1 (1.0–1.2) |
| FIB‐4 | 1.20 (0.67–2.43) |
| <1.25 | 94 (52.8) |
| 1.25–3.25 | 54 (30.3) |
| >3.25 | 30 (16.9) |
| APRI | 0.60 (0.38–1.22) |
| Liver stiffness, b kPa | 8.9 (6.3–12.9) |
| Moderate/severe disease activity, c n (%) | 127 (71.3) |
| Severe fibrosis/cirrhosis, d n (%) | 64 (36.0) |
| HCC, n (%) | 3 (1.7) |
| Therapy, n (%) | |
| TDF | 102 (57.3) |
| ETV | 62 (34.9) |
| TAF | 8 (4.5) |
| Other NA | 2 (1.1) |
| Interferon | 4 (2.2) |
Abbreviations: AASLD, American Association for the Study of Liver Disease; ALT, alanine aminotransferase; APRI, AST to platelet ratio index; AST, aspartate transaminase; EASL, European Association for the Study of the Liver; ETV, entecavir; FIB‐4, Fibrosis‐4; HBV, Hepatitis B Virus; HDV, hepatitis D virus; LSM, liver stiffness measurements; TDF, tenofovir disoproxile fumarate; ULN, upper limit of normal; WHO, World Health Organization.
As defined in the most recent EASL CPG. 8
Available in n = 137 (75.3%).
Defined as ≥F2 in LSM (>7.5 kPa).
Defined as either (i) LSM >9 kPa if ALT ≤ ULN, (ii) LSM >12 kPa if ALT ≤5 × ULN, (iii) F3/F4 fibrosis in histology, or (iv) clinical signs of cirrhosis (varices or ascites/encephalopathy). 8
FIGURE 3.
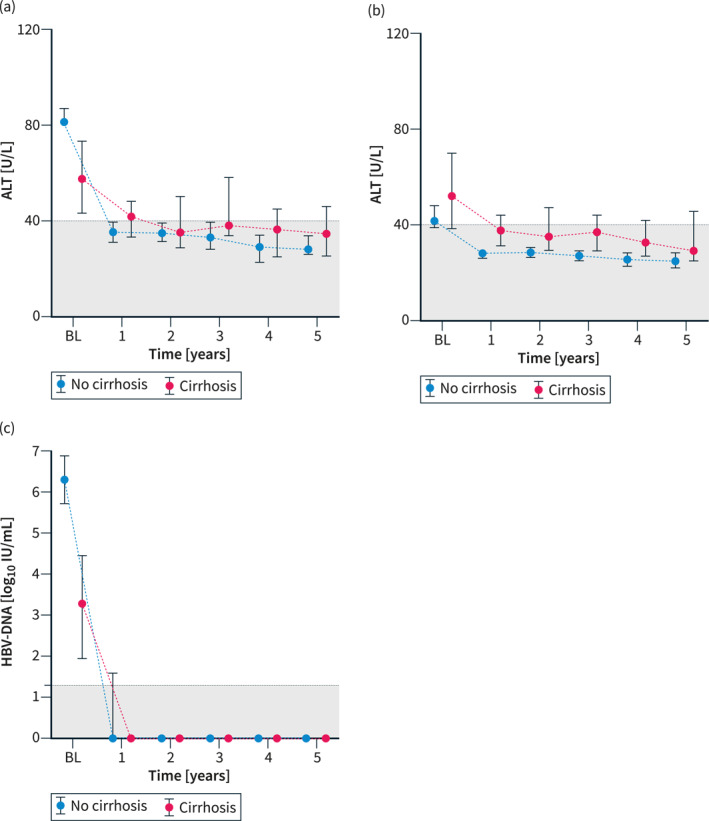
The depiction of the evolution of the median (95% confidence interval) of (a) ALT, (b) AST, (c) HBV‐DNA within 5 years in patients fulfilling (EASL) treatment indications who could successfully be linked to antiviral treatment, as stratified by the baseline absence or presence of severe fibrosis/cirrhosis, defined by LSM ≥9 kPa in patients with normal ALT, or LSM ≥12 kPa in patients with ALT ≤5 × ULN, histologically proven F3/F4 fibrosis, or clinical signs of cirrhosis (varices, ascites, encephalopathy). ALT, alanine aminotransferase; AST, aspartate transaminase; EASL, European Association for the Study of the Liver; HBV, Hepatitis B Virus; LSM, liver stiffness measurements; ULN, upper limit of normal.
FIGURE 4.
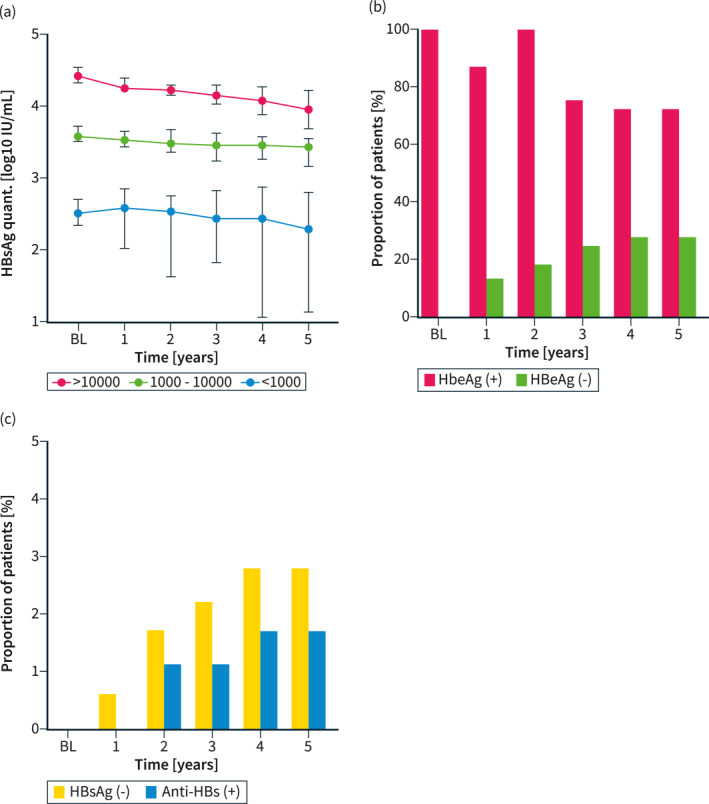
The impact of antiviral therapy initiation (as per EASL treatment indications, n = 178) on quantitative HBsAg, as stratified by baseline levels, is shown in panel (a), while the cumulative incidences of HBeAg seroconversion (in patients who were HBeAg‐positive at baseline, n = 61) and HBsAg seroconversion (n = 178) are shown as panels (b) and (c), respectively. EASL, European Association for the Study of the Liver.
Among the 64 patients with severe fibrosis/cirrhosis at treatment initiation, nine (14.1%) had already developed decompensated disease at baseline, as evidenced by a history of variceal bleeding, ascites, and/or hepatic encephalopathy. Overall, patients were followed for a median of 5.1 (2.7–7.4) years. Among patients who had compensated disease at baseline (n = 55), eight developed hepatic decompensation that is, variceal bleeding, ascites or hepatic encephalopathy, during follow‐up, while one patient underwent liver transplantation, and two patients died of non‐liver‐related causes, corresponding to cumulative incidences of first hepatic decompensation of 5.5%, 9.7% and 9.7% at 1, 3, and 5 years of follow‐up, respectively. In the overall cohort of patients with severe fibrosis/cirrhosis, liver‐related death occurred in 12 patients, three patients died of non‐liver related causes, and two patients underwent transplantation. The cumulative incidences of liver‐related death were 3.2%, 10.2%, and 16.3% at 1, 3, and 5 years of follow‐up, respectively.
DISCUSSION
In our large cohort of treatment naïve CHB patients managed at our tertiary care center in Austria, we demonstrate that, based on basic laboratory and virological parameters and depending on the applied treatment guidance, a percentage of approximately 20%–30% were eligible for antiviral therapy. One out of three patients showed at least moderately elevated ALT and/or HBV‐DNA values, and, in accordance with previous reports, almost one out of 10 patients had already progressed to severe fibrosis/cirrhosis at the time of diagnosis/referral. 11
At baseline, HBeAg was detected in one out of 10 patients, a frequency similar to that of a cohort of Caucasian voluntary blood donors collected in Austria approximately 40 years ago. 19 In contrast to that study, the CHB cohort at our center currently consists of approximately 80% immigrants from Turkey, Southeastern and Eastern Europe, Asia, and Africa (unpublished data); however, the median age of our current ethnically diverse cohort lies approximately 6 years higher than the median age of the previously described Caucasian cohort. Thus, the high rate of HBeAg negativity mirrors the natural history of the disease in aging and evolving Central‐European population. Accordingly, HBeAg‐negative chronic infection (previously known as the “inactive carrier” phase) and HBeAg‐negative chronic hepatitis were the most frequently observed CHB phenotypes in our study. Interestingly, and in conflict with earlier studies, HBsAg levels yielded no relevant diagnostic value for the distinction between HBeAg‐negative disease stages. This might be explained by the fact that previous studies have focused on smaller subcohorts of CHB patients, for example, genotype D patients, 20 , 21 or a ratio of HBsAg to HBV‐DNA levels. 20
When comparing treatment eligibility criteria proposed by the different major stakeholders (EASL, AASLD, WHO), the AASLD criteria deemed slightly more patients eligible for treatment, as compared to EASL criteria, which was expected due to the lower (and sex‐specific) ALT‐ULN applied for treatment guidance in the AASLD guideline. Overall, however, the criteria classified similar proportions of patients as candidates for treatment. Interestingly, the WHO criteria, focusing more on readily available parameters and less on (non‐invasive) fibrosis staging by biopsy and/or TE, classified only a slightly lower rate of patients as treatment eligible. Notably, the investigated treatment criteria might change in the future, as recent notions have supported the idea of expanding treatment indications in selected cohorts, especially in older patients and those with subclinical evidence for active disease, 22 owing to an observed higher risk for adverse hepatic outcomes, although historically CHB without abnormal biochemical evidence has been associated with a rather benign disease trajectory. 19
While coinfection with HIV and/or HCV was rarely present in the cohort of >1000 patients initially screened for inclusion, a surprisingly high HDV coinfection (seropositivity) rate of almost 6% was recorded in the cohort finally assessed for treatment eligibility, even though screening was not universally conducted, and based on data from later years of the study period, an alarming proportion of 30% of patients were never tested in our cohort. Notably, HDV coinfection was present in one out of three cirrhotic CHB patients in our cohort. Corroborating previous reports from our and other European centers, two out of three anti‐HDV seropositive patients had detectable HDV‐RNA. 23 , 24 Given the availability of novel treatment options against HDV 25 and the high prevalence of HDV coinfection in the overall cohort, installing universal anti‐HDV antibody testing in every CHB patient seems warranted.
Lastly, we investigated treatment uptake rates and long‐term efficacy of antiviral therapy in eligible patients in whom treatment was eventually initiated. Approximately nine out of 10 patients who were eligible for treatment according to EASL recommendations could be linked to treatment, while only a small number of patients did not receive or refused therapy. HBsAg levels remained stable during long‐term follow‐up, and only a few patients achieved HBsAg seroconversion. Stopping chronic antiviral treatment in selected patients with low HBsAg levels to instigate seroconversion should thus be considered under close monitoring. 26 Nonetheless, to date, most patients on chronic antiviral treatment require indefinite treatment. However, many promising compounds targeting “functional cure” are currently under development. 6
Our retrospective study has some limitations that need to be addressed. First, its monocentric design might have introduced selection/referral bias. However, our center is the largest specialized hepatitis treatment clinic in Austria, and thus, our cohort might well be representative of other cohorts treated at European academic institutions. Along these lines, Patients with advanced disease and cirrhosis were likely overrepresented in our cohort, as compared to community care settings, where a significantly lower treatment eligibility rate can be expected. 27 , 28 In addition, data on the ethnicity and HBV‐genotype of patients were not gathered in our study, which would have been appreciated in the face of an ever‐evolving European HBV scenario. Still, the overall patient characteristics of our cohort strongly resemble those of other (current) European cohorts, 11 , 12 and ethnicity and genotype per se do not impact treatment eligibility. Second, TE was not universally performed in all patients. This can be explained by the fact that during earlier years of the study period, TE was mostly applied for the evaluation of patients with chronic hepatitis C at our center. The availability of liver stiffness values in the entire cohort could have provided relevant information, especially in patients with normal ALT, low HBV‐DNA levels and/or without clinical signs of cirrhosis. On the other hand, reassuringly, the proportion of patients with cirrhosis within our cohort corresponds well to previous reports from academic centers. 1 Nonetheless, we recommend the universal application of TE in all patients with CHB. Along these lines, data on metabolic comorbidities, 29 the presence of concomitant non‐alcoholic fatty liver disease, 30 and lifestyle‐related risk factors 31 for disease progression were missing in our cohort, which might have influenced the management of some patients. Thus, the true treatment eligibility among our cohort might have been slightly underestimated. Third, treatment eligibility was assessed cross‐sectionally at diagnosis/referral, thereby neglecting potential changes in HBV‐DNA, ALT, and/or fibrosis over time. Lastly, our study did not investigate the potential value of novel virological biomarkers of disease activity, for example, hepatitis B core‐related antigen (HBcrAG), HBV covalently closed circular DNA (cccDNA), or HBV‐RNA. However, in the absence of specific recommendations in clinical guidelines, those biomarkers have limited value in clinical practice and should not impact the treatment decision.
In conclusion, in our large cohort of CHB patients evaluated for treatment at a tertiary care center in a low‐endemic European country, up to 25%–30% of patients met the EASL/AASLD/WHO criteria. HDV coinfection is highly prevalent, especially in HBV patients with cirrhosis, and universal screening for HDV is essential to enable linkage to novel therapeutic options. Treatment targeted at HBV was initiated in most eligible patients, and was safe and effective, although HBsAg levels were not influenced by currently available treatment, and HBsAg loss or seroconversion was rarely achieved.
AUTHOR CONTRIBUTIONS
All authors contributed to conceptualization (Mathias Jachs and Thomas Reiberger), data curation (all authors), formal analysis and visualization (Mathias Jachs), writing of the original draft (Mathias Jachs and Thomas Reiberger), reviewing and editing (all authors), and supervision (Thomas Reiberger).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest regarding this study. Outside the submitted work, the authors declare the following potential conflicts of interest: M.J. has served as a speaker for Gilead. R. St. served as a speaker and/or consultant and/or advisory board member for Abbott Diagnostics, Qiagen and received grants/research support from Qiagen. M.M. served as a speaker and/or consultant and/or advisory board member for AbbVie, Gilead, Collective Acumen, and W. L. Gore & Associates and received travel support from AbbVie and Gilead. M.T. served as a speaker and/or consultant and/or advisory board member for Albireo, BiomX, Boehringer Ingelheim, Bristol‐Myers Squibb, Falk, Genfit, Gilead, Intercept, Janssen, MSD, Novartis, Phenex, Regulus, and Shire and received travel support from AbbVie, Falk, Gilead, and Intercept, as well as grants/research support from Albireo, Cymabay, Falk, Gilead, Intercept, MSD, and Takeda. Moreover, he is a co‐inventor of patents on the medical use of 24‐norursodeoxycholic acid. P.F. received an unrestricted research grant from Gilead, was a member of the safety review Committee for MyrPharma, speaking honoraria from Gilead and Abbvie, and consulting/advisory board fee from Vivaraxx. P.M. served as a speaker and/or consultant and/or advisory board member for MSD, AbbVie, Intercept and Gilead, and received travel support from Gilead and AbbVie. T.R. served as a speaker and/or consultant and/or advisory board member for AbbVie, Bayer, Boehringer Ingelheim, Gilead, Intercept, MSD, Siemens, and W. L. Gore & Associates and received grants/research support from AbbVie, Boehringer Ingelheim, Gilead, MSD, Philips, and W. L. Gore & Associates, as well as travel support from Boehringer Ingelheim and Gilead. R. Sa., A.S., A.D., R.T., and L.H. have nothing to disclose.
ETHICS APPROVAL
This retrospective study was conducted in accordance with the Declaration of Helsinki and approved by the institutional review board of the Medical University of Vienna (No. 1515/2020).
Supporting information
Figure S1
Supporting Information S1
ACKNOWLEDGMENTS
No funding specific to this study was received.
Jachs M, Sauberer R, Stiegler A, Dechêne A, Tazreiter R, Hartl L, et al. Eligibility for antiviral therapy and treatment uptake in chronic hepatitis B patients referred to a European tertiary care center. United European Gastroenterol J. 2023;11(3):293–304. 10.1002/ueg2.12376
DATA AVAILABILITY STATEMENT
Data will be made available upon reasonable request.
REFERENCES
- 1. Razavi‐Shearer D, Gamkrelidze I, Nguyen MH, Chen DS, Van Damme P, Abbas Z, et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383–403. [DOI] [PubMed] [Google Scholar]
- 2. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015;386(10003):1546‐55, 10.1016/s0140-6736(15)61412-x [DOI] [PubMed] [Google Scholar]
- 3. BMASGK . HIV/AIDS, hepatitis B und C in Österreich. Vienna; 2019. [Google Scholar]
- 4. Sperle I, Steffen G, Leendertz SA, Sarma N, Beermann S, Thamm R, et al. Prevalence of hepatitis B, C, and D in Germany: results from a scoping review. Front Public Health. 2020;8:424. 10.3389/fpubh.2020.00424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fretz R, Negro F, Bruggmann P, Lavanchy D, De G, Pache I, et al. Hepatitis B and C in Switzerland ‐ healthcare provider initiated testing for chronic hepatitis B and C infection. Swiss Med Wkly. 2013;143:w13793. 10.4414/smw.2013.13793 [DOI] [PubMed] [Google Scholar]
- 6. Wong GLH, Gane E, Lok ASF. How to achieve functional cure of HBV: stopping NUCs, adding interferon or new drug development? J Hepatol. 2022;76(6):1249–62. 10.1016/j.jhep.2021.11.024 [DOI] [PubMed] [Google Scholar]
- 7. Lok ASF, McMahon BJ, Brown RS, Wong JB, Ahmed AT, Farah W, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta‐analysis. Hepatology. 2016;63(1):284–306. 10.1002/hep.28280 [DOI] [PubMed] [Google Scholar]
- 8. Lampertico P, Agarwal K, Berg T, Buti M, Janssen HL, Papatheodoridis G, et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–98. 10.1016/j.jhep.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 9. Terrault NA, Lok ASF, McMahon BJ, Chang K, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–99. 10.1002/hep.29800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. WHO guidelines approved by the guidelines review committee. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. Geneva: World Health Organization, 2015. [PubMed] [Google Scholar]
- 11. Tan M, Bhadoria AS, Cui F, Tan A, Van Holten J, Easterbrook P, et al. Estimating the proportion of people with chronic hepatitis B virus infection eligible for hepatitis B antiviral treatment worldwide: a systematic review and meta‐analysis. Lancet Gastroenterol & Hepatol. 2021;6(2):106–19. 10.1016/s2468-1253(20)30307-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chevaliez S, Roudot‐Thoraval F, Brouard C, Gordien E, Zoulim F, Brichler S, et al. Clinical and virological features of chronic hepatitis B in the French national surveillance program, 2008 ‐ 2012: a cross‐sectional study. JHEP Rep 4(12), 2022;4, 10.1016/j.jhepr.2022.100593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martinot‐Peignoux M, Lapalus M, Asselah T, Marcellin P. The role of HBsAg quantification for monitoring natural history and treatment outcome. Liver Int. 2013;33(Suppl 1):125–32. 10.1111/liv.12075 [DOI] [PubMed] [Google Scholar]
- 14. Le Gal F, Gordien E, Affolabi D, Hanslik T, Alloui C, Dény P, et al. Quantification of hepatitis delta virus RNA in serum by consensus real‐time PCR indicates different patterns of virological response to interferon therapy in chronically infected patients. J Clin Microbiol. 2005;43(5):2363–9. 10.1128/jcm.43.5.2363-2369.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. EASL‐ALEH Clinical Practice Guidelines: non‐invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 2015;63:237‐64. [DOI] [PubMed] [Google Scholar]
- 16. Chon YE, Choi EH, Song KJ, Park JY, Kim DY, Han KH, et al. Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: a meta‐analysis. PLoS One. 2012;7(9):e44930. 10.1371/journal.pone.0044930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berzigotti A, Tsochatzis E, Boursier J, Castera L, Cazzagon N, Friedrich‐Rust M, et al. EASL Clinical Practice Guidelines on non‐invasive tests for evaluation of liver disease severity and prognosis ‐ 2021 update. J Hepatol. 2021;75(3):659–89. 10.1016/j.jhep.2021.05.025 [DOI] [PubMed] [Google Scholar]
- 18. Hartl L, Semmler G, Hofer BS, Schirwani N, Jachs M, Simbrunner B, et al. COVID‐19‐related downscaling of in‐hospital liver care decreased patient satisfaction and increased liver‐related mortality. Hepatol Commun. 2021;5(10):1660–75. 10.1002/hep4.1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dragosics B, Ferenci P, Hitchman E, Denk H. Long‐term follow‐up study of asymptomatic HBsAg‐positive voluntary blood donors in Austria: a clinical and histologic evaluation of 242 cases. Hepatology 1987;7(2):302‐6, 10.1002/hep.1840070215 [DOI] [PubMed] [Google Scholar]
- 20. Brunetto MR, Oliveri F, Colombatto P, Moriconi F, Ciccorossi P, Coco B, et al. Hepatitis B surface antigen serum levels help to distinguish active from inactive hepatitis B virus genotype D carriers. Gastroenterology. 2010;139(2):483–90. 10.1053/j.gastro.2010.04.052 [DOI] [PubMed] [Google Scholar]
- 21. Jaroszewicz J, Calle Serrano B, Wursthorn K, Deterding K, Schlue J, Raupach R, et al. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)‐infection: a European perspective. J Hepatol 2010;52(4):514‐22, 10.1016/j.jhep.2010.01.014 [DOI] [PubMed] [Google Scholar]
- 22. Jeng W.‐J, Lok AS. Should treatment indications for chronic hepatitis B Be expanded? Clin Gastroenterol Hepatol. 2021;19(10):2006–14. 10.1016/j.cgh.2020.04.091 [DOI] [PubMed] [Google Scholar]
- 23. Jachs M, Binter T, Schmidbauer C, Hartl L, Strasser M, Laferl H, et al. Hepatitis D virus (HDV) prevalence in Austria is low but causes considerable morbidity due to fast progression to cirrhosis. United Eur Gastroenterol J. 2021;9(10):1119–27. 10.1002/ueg2.12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kamal H, Westman G, Falconer K, Duberg A, Weiland O, Haverinen S, et al. Long‐term study of hepatitis delta virus infection at secondary care centers: the impact of viremia on liver‐related outcomes. Hepatology. 2020;72(4):1177–90. 10.1002/hep.31214 [DOI] [PubMed] [Google Scholar]
- 25. Jachs M, Schwarz C, Panzer M, Binter T, Aberle SW, Hartl L, et al. Response‐guided long‐term treatment of chronic hepatitis D patients with bulevirtide‐results of a “real world” study. Aliment Pharmacol Ther. 2022;56(1):144–54. 10.1111/apt.16945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berg T, Simon KG, Mauss S, Schott E, Heyne R, Klass DM, et al. Long‐term response after stopping tenofovir disoproxil fumarate in non‐cirrhotic HBeAg‐negative patients ‐ FINITE study. J Hepatol. 2017;67(5):918–24. 10.1016/j.jhep.2017.07.012 [DOI] [PubMed] [Google Scholar]
- 27. Ye Q, Kam LY, Yeo YH, Dang N, Huang DQ, Cheung R, et al. Substantial gaps in evaluation and treatment of patients with hepatitis B in the US. J Hepatol. 2022;76(1):63–74. 10.1016/j.jhep.2021.08.019 [DOI] [PubMed] [Google Scholar]
- 28. Kam LY, Huang DQ, Tobias AF, Poon K, Henry L, Kwo P, et al. Impact of advanced practice providers on characteristics and quality of care of patients with chronic hepatitis B. Alimentary Pharmacology & Therapeutics; 2022. [DOI] [PubMed] [Google Scholar]
- 29. Mak L.‐Y, Hui RW.‐H, Lee C.‐H, Mao X, Cheung K, Wong DK, et al. Glycemic burden and the risk of adverse hepatic outcomes in patients with chronic hepatitis B with type 2 diabetes. Hepatology. 2023;77:606–8. 10.1002/hep.32716 [DOI] [PubMed] [Google Scholar]
- 30. Zhang J, Lin S, Jiang D, Li M, Chen Y, Li J, et al. Chronic hepatitis B and non‐alcoholic fatty liver disease: conspirators or competitors? Liver Int. 2020;40(3):496–508. 10.1111/liv.14369 [DOI] [PubMed] [Google Scholar]
- 31. Jachs M, Tillmann HL. It's not all about the virus—on the importance of modifiable lifestyle factors in the development of hepatocellular carcinoma in chronic hepatitis B. Hepatology. 2022;77(2):352–4. 10.1002/hep.32798 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Supporting Information S1
Data Availability Statement
Data will be made available upon reasonable request.


