Abstract
This review examines putative, yet likely critical evolutionary pressures contributing to human skin pigmentation and subsequently, depigmentation phenotypes. To achieve this, it provides a synthesis of ideas that frame contemporary thinking, without limiting the narrative to pigmentation genes alone. It examines how geography and hence the quality and quantity of UV exposure, pigmentation genes, diet‐related genes, vitamins, anti‐oxidant nutrients, and cultural practices intersect and interact to facilitate the evolution of human skin color. The article has a strong focus on the vitamin D‐folate evolutionary model, with updates on the latest biophysical research findings to support this paradigm. This model is examined within a broad canvas that takes human expansion out of Africa and genetic architecture into account. A thorough discourse on the biology of melanization is provided (includes relationship to BH4 and DNA damage repair), with the relevance of this to the UV sensitivity of folate and UV photosynthesis of vitamin D explained in detail, including the relevance of these vitamins to reproductive success. It explores whether we might be able to predict vitamin‐related gene polymorphisms that pivot metabolism to the prevailing UVR exposome within the vitamin D‐folate evolutionary hypothesis context. This is discussed in terms of a primary adaptive phenotype (pigmentation/depigmentation), a secondary adaptive phenotype (flexible metabolic phenotype based on vitamin‐related gene polymorphism profile), and a tertiary adaptive strategy (dietary anti‐oxidants to support the secondary adaptive phenotype). Finally, alternative evolutionary models for pigmentation are discussed, as are challenges to future research in this area.
Keywords: evolution, folate, gene polymorphisms, skin pigmentation, vitamin D
Early humans were subjected to two opposing UVR/pigmentation clines; the first (folate driven) involved melanization as one moves closer to the equator (ancestral phenotype). The second (vitamin D driven) involved depigmentation following early human expansion out of Africa and changing cultural practices (agricultural development). This second cline, therefore, drives depigmentation from the equator to the pole.

1. INTRODUCTION
Human skin color is one of the most visible human phenotypes, universally recognized as a highly variable attribute. Despite this diversity, our complete understanding of melanogenesis biochemistry means skin color as an evolved trait is often thought of as a relatively straightforward human phenotype to study (Quillen et al., 2018). In truth, the reality is more complex, and one has to consider a gamut of relevant evolutionary pressures acting on disparate global populations. Indeed, we now know that skin color is highly polygenic, with complex epistatic and stochastic (i.e., dietary and environmental/UV) interactions underpinning the direction of evolutionary change. A combination of natural selection, drift, bottlenecks, and admixture are considered to be critical. Furthermore, relevant alleles are often population specific and effects highly pleiotropic in nature (Quillen et al., 2018). In a Darwinian sense, there is no finality to the dynamic phenomenon of evolving human skin color, merely a series of questions that when answered will help us to better explain the nature of this fascinating dynamic.
Of course, ideas themselves are always evolving, and the aim of this article is to demonstrate contemporary thought without limiting the narrative to genes alone. Recent research shows that the quality and quantity of ultraviolet radiation (UVR) exposure, a plethora of pigmentation genes, diet‐related genes, vitamins, antioxidant nutrients, and cultural practices intersect and interact to influence the natural selection of human skin color. Indeed, diet as an evolutionary pressure has shaped human biology through our lineage and across geography, resonating strongly with contemporary ideas on Darwinian Medicine (Lucock et al., 2014). Darwinian or Evolutionary Medicine is a concept gaining significant traction across medical disciplines (Berlim & Abeche, 2001; Pennisi, 2011; Román‐Franco, 2009), and one that is likely impacted by changes in vitamin biology caused by the maladaptation of pigmentation phenotype to specific UV environments. This idea is explored later.
This article will have a strong focus on the “vitamin D‐folate hypothesis” as a core component within the evolution of skin phototype (Jablonski & Chaplin, 2000, 2010); recent biophysical research findings have helped to support and extend this enduring paradigm (Lucock et al., 2021), and this will be discussed within a broad canvas that takes in population history/dispersal and genetic architecture. As a brief introduction to this concept, folate and vitamin D are structurally and metabolically very discrete entities. However, they are linked in that both are photosensitive. Vitamin D is a prohormone that is photosynthesized in the skin by UV‐B energy (this is optimal <310 nm with peak synthesis around 297 nm). By contrast, reduced folate vitamers are photodegraded by both UV‐B and longer UV‐A wavelengths (Lucock et al., 2018; Lucock, 2021). Branda and Eaton (1978) first proposed natural selection for melanisation to protect folate. Their study was carried out in the 1970s and provides the first evidence for the vitamin D‐folate hypothesis, although they did not refer specifically to this hypothesis. They were concerned only with the effects of simulated sunlight on folate, alluding at the end of their paper to the importance of skin pigmentation in protecting folate, but they did not discuss vitamin D. The first mention of skin pigmentation being the product of selection on pathways affecting folate and vitamin D was by Jablonski and Chaplin (2000). More recently, Jablonski and Chaplin (Jablonski & Chaplin, 2010) have developed and advanced the vitamin D‐folate hypothesis as a paradigm to partially explain how the evolution of human skin pigmentation phenotype fits with the prevailing UVR regime (they describe human skin pigmentation as the product of two clines, which were produced by the dual selective pressures of photoprotection and vitamin D photosynthesis), an idea that has been further strengthened by a series of recent studies which add support to this construct (Batai et al., 2021; Jones et al., 2016; Jones, Lucock, et al., 2018; Jones, Veysey, et al., 2018; Lucock et al., 2017; Lucock, 2021). The theory is straightforward: Folate deficiency impacts reproductive success, as does vitamin D deficiency. Variation in human skin pigmentation phenotype evolved as an adaptation to the prevailing UVR regime. UV photolysis of folate in the dermal vasculature provided a strong selection pressure for a more highly melanized, darker skin at tropical latitudes where Homo sapiens first evolved. However, following early dispersals out of Africa to more northerly latitudes, less skin pigmentation was needed to facilitate adequate vitamin D photosynthesis. Ultimately, early humans were subjected to two opposing UVR/pigmentation clines; the first (folate driven) involved melanisation as one moves closer to the equator and the geographic origin of our species (making this the ancestral phenotype). The second (vitamin D driven) involved loss of pigmentation following early human expansion out of Africa. This second cline, therefore, drives depigmentation from the equator to the pole (Jablonski & Chaplin, 2010). Figure 1 provides a schematic view of this generalized model.
FIGURE 1.
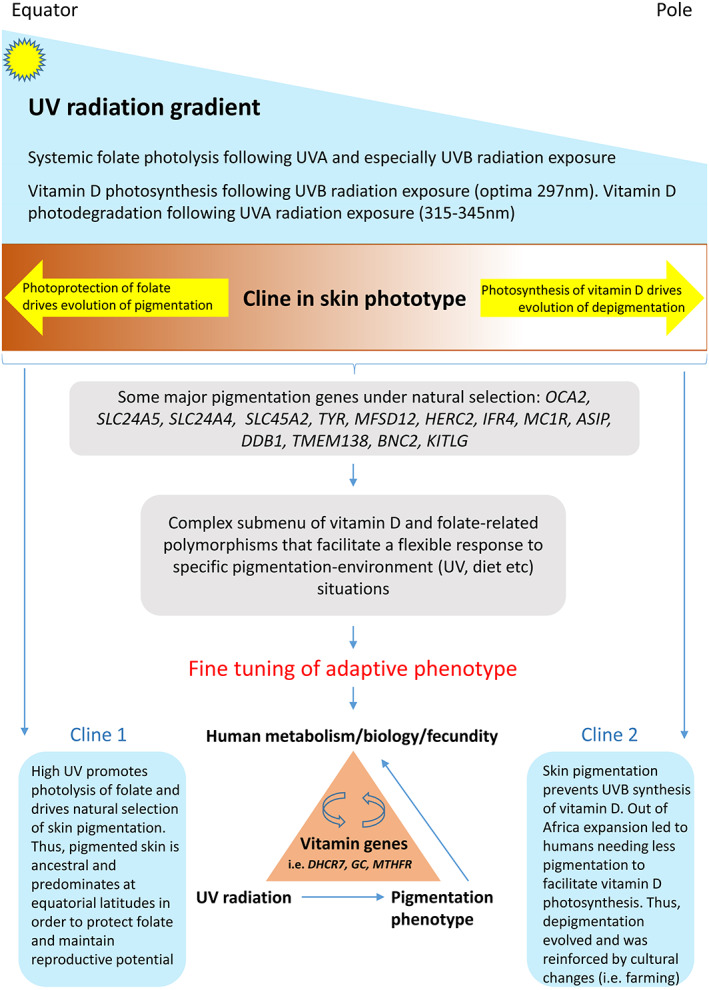
Early humans were subjected to two opposing UVR/pigmentation clines; the first (folate driven) involved melanization as one moves closer to the equator (ancestral phenotype). The second (vitamin D driven) involved depigmentation following early human expansion out of Africa and changing cultural practices (agricultural development). This second cline therefore drives depigmentation from equator to pole. This figure provides a simplified schematic view of this generalized model
This elegant, and ostensibly simple adaptive trait, is maintained by natural selection involving diet, UVR and a complex and variable nexus of key pigmentation and diet‐related gene polymorphisms (Deng & Xu, 2017; Graf et al., 2005; Lao et al., 2007; Lucock et al., 2021; Quillen & Shriver, 2011; Rees, 2004; Rocha, 2020; Sturm, 2009; Sturm & Duffy, 2012). It modulates the availability/adequacy of folate and vitamin D for maintenance of reproductive efficiency, and provides a sensitive target for natural selection to shape phenotypic variation in skin phototype via photo‐responsive reciprocity in these two critical vitamins. A deeper metabolic rationale to underpin this phenomenon is given later, but it is worth mentioning that recent research has extended this evolutionary model; this addendum adds the need for folate (and hence tetrahydrobiopterin [BH4]) in the synthesis of eumelanin (black/brown pigment) and pheomelanin (yellow/red pigment), it also considers relevant UVR‐folate‐gene and UVR‐folate‐antioxidant interactions. Again, these aspects, and the critical role of folate in offsetting UV‐DNA‐related damage are dealt with in more detail later (Lucock et al., 2021).
A comprehensive understanding of the biology of skin color is emerging. Skin, hair, and eye color are highly variable across populations, and unsurprisingly, a large number of genes act on the melanosome to grade the biosynthesis of melanin (Sturm & Duffy, 2012). It has been shown that polygenicity in skin color is higher in Africa than on any other continent and is thought to be amongst the most rapidly evolving of evolutionary traits, being one of the strongest examples of positive selection in human biology (Martin et al., 2017). There are around 170 genes implicated in defining skin pigmentation phenotype across model organisms, but only around 15 genes have been linked to human skin color (Martin et al., 2017). Unfortunately, early work has led to a bias in favor of genetic research on European/European derived populations, and this hid the true global complexity in genetic architecture that underscores skin color. Indeed, loci relevant to European pigmentation variation may be neither relevant to, nor as numerous as in populations outside of Europe (Crawford et al., 2017; Martin et al., 2017; Norton, 2020; Quillen et al., 2018). Graves Jr (2021) has put this into perspective; he estimates that 5.43 billion individuals on Earth are dark skinned compared to only 3.6 billion fair skinned individuals—his arguments on what the “normal’ means in biology are truly insightful.
Despite this previous observation, it is important to note that a European bias in pigmentation genetics work was driven by attempts to urgently understand the relationship between MC1R polymorphisms and skin cancer susceptibility, especially amongst recent European migrants to Australia (Sturm et al., 2001; Sturm, 2002; Sturm, Duffy, Box, Chen, et al., 2003; Sturm, Duffy, Box, Newton, et al., 2003).
While this article will focus primarily on the “vitamin D‐folate hypothesis” as a significant driver of skin pigmentation/depigmentation, other evolutionary models have been postulated and these include the following: “the skin mutagenesis hypothesis,”, “the skin barrier hypothesis,” and the “metabolic conservation hypothesis” (Jones, Lucock, et al., 2018). The relative merits of these models will be discussed later.
One of the major objectives of this article is to recognize that while pigmentation itself represents the primary adaptive phenotype, secondary adaptive phenotypes exist in the genetics and biochemistry that regulates folate‐dependent one‐carbon metabolism and vitamin D biology. The interplay between primary and secondary adaptive phenotypes, diet, environment and geography will be discussed in terms of evolutionary significance.
2. MAPPING HUMAN EXPANSION OUT OF AFRICA, INCLUDING IDEAS ON PIGMENTATION PHENOTYPE
The emergence of the earliest hominins possessing most anatomical traits associated with H. sapiens has been traced back to Jebel Irhoud, Morocco, around 300,000 years BP (Hublin et al., 2017). However, it is clear that anatomical modernity evolved piecemeal over several tens of thousands of years, as did the cultural evolution of fully articulate speech and language, modern tool types, and the ability to control fire. Some of the earliest subsequent evidence for anatomically modern humans comes from Ethiopian fossils dated to around 150,000–190,000 years BP (McDougall et al., 2005; White et al., 2003). In accord with the fossil evidence, phylogenetic data based on human mitochondrial DNA analysis also places the origin of H. sapiens in Africa. Indeed, Africans have the greatest genetic diversity among all extant human populations (Rosenberg et al., 2002). This and further data support a large, sub‐divided African population structure throughout human evolution, with the deepest split in sub‐Saharan Africa. There is also evidence of significant admixture in the region (Nielsen et al., 2017). Evidence for introgression exists, although the degree of archaic admixture in Africa is still an open question (Nielsen et al., 2017). This rich diversity is supported by genome‐wide microsatellite DNA variation in 3000 African subjects demonstrating 14 ancestral population groupings. These clusters showed a broad corollary with language, culture and geography (Tishkoff et al., 2009).
Recent data on the genomic architecture of skin pigmentation in Africans reflects a parallel complexity with intricate, polygenic traits. These loci vary across populations according to different local evolutionary pressures (Martin et al., 2017). This is supported by Crawford and colleagues (2017) who examined ethnically diverse African genomes, identifying pigmentation critical gene variants that repair DNA damage and underpin melanocyte biology (DDB1/TMEM138). They show that both light and dark pigmentation alleles evolved prior to the emergence of modern humans, and that dark pigmentation in Africans is identical (by descent) in both South Asian and Australo‐Melanesian populations (Crawford et al., 2017).
The dispersal of modern humans out of Africa left a strong genetic signature in all non‐African populations, which includes a reduced level of genetic diversity and increased linkage disequilibrium (Nielsen et al., 2017; Ramachandran et al., 2005).
As small human populations dispersed out of high UV environments in Africa into the continent's geographic extremes and into Eurasia, they encountered a wide range of habitats, eventually including arctic tundra, low lying savannas and extreme high elevations such as the Tibetan Plateau, where they were subjected to strong local selection pressures. Natural selection conferred upon us the ability to survive in extremes of temperature, under the hypoxia of altitude, or in the presence of pathogens. Indeed, the expansion of our ancestors into a myriad of challenging environments allowed us to acquire a veritable smorgasbord of polymorphisms that help us not just to survive, but to thrive. Selected examples include variants that permit us to live on high starch and fat diets, digest milk lactose, influence height (i.e., pygmies in rainforests), confer adaptations in the spleen size to permit longer diving times in Bajau sea nomads, and the example most germane to the present thesis, adaptation of skin to the prevailing UV regime based on latitude, altitude, environment, and culture (Jablonski, 2021; Lucock et al., 2014).
Outside Africa, modern human fossils occur in the Middle‐East around 100,000 years BP (Grün et al., 2005) and in China by 80,000 years BP (Liu et al., 2015). Despite what are considered to be some of the oldest fossil remains outside Africa excavated at Skhul and Qafzeh in Israel, dated to 90,000–120,000 years BP, any further evidence of H. sapiens living outside of Africa largely vanishes about 90,000 years BP, only to emerge much later. Only then, tens of thousands of years on are our African Ancestors' diaspora ready to dramatically expand across the planet. There is debate on the likely routes of passage; a northern journey would have taken our species through the Levant up into Europe. However, more recent and tenable ideas suggest an early and extensive occupation of southeast Asia and Australasia, with movements into more northerly latitudes and then west into what is now Europe occurring later. In this Asia first model, human fossil remains have been found in Borneo and Lake Mungo in Australia at 40,000–46,000 years BP. In Eastern Europe, fossil remains from Romania have been dated at 40,000 years BP, while in Western Europe the oldest skeletal remains are marginally younger at 36,000 to 37,000 years BP. The Americas were believed to be colonized far later at around 16,000 years BP, however, recent evidence of human footprints from New Mexico suggests settlement may have occurred even earlier, between 21,000 and 23,000 years BP (Bennett et al., 2021).
During these remarkable dispersals, our skin underwent a genetically driven change in pigmentation phenotype, from its ancestral dark pigmented state in Africa where we first evolved, to lighter tones at higher latitudes under the selective influence of a variety of environmental and cultural factors, particularly, but not exclusively the prevailing UV regime. Of course, as discussed below, selection pressures can operate on pigmentation in opposing directions. Recent findings muddy the waters for any simple theory. After H. sapiens left Africa in their major expansion out of the continent, their skin did not lighten immediately; several polymorphisms in pigmentation genes were acted on by natural selection tens of thousands of years after we emerged from Africa. A sweep shared by Europeans and East Asians at KITLG occurred approximately 30,000 years BP, after the out‐of‐Africa dispersal. Even more relevant, selective sweeps for European‐specific alleles in three genes—TYRP1, SLC24A5, and SLC45A2 occurred far later, within the last 11,000–19,000 years, long after the first dispersals of modern humans into Europe (Beleza et al., 2013). Furthermore, the genome of a 7000 year old Mesolithic hunter from the La Braña‐Arintero site in León, Spain was found to carry ancestral alleles in several skin pigmentation genes, suggesting that the light skin of modern Europeans was far from ubiquitous, even in relatively recent Mesolithic times (Olalde et al., 2014). Wilde and colleagues (2014) also examined how European skin pigmentation altered over time. They examined three pigmentation‐related genes (TYR, SLC45A2, and HERC2) from 48 ancient Ukrainian skeletons (4000–5500 years BP). The variant allele frequency rates in these genes which affect skin, hair, and eye color were compared to 60 modern‐day Ukrainians and almost 250 modern genomes from surrounding regions. Neutrality was rejected for all alleles examined, with estimates of selection ranging from around 2%–10% per generation, demonstrating that strong selection favored lighter skin, hair, and eye pigmentation in European populations over the last 5000 years. Africans have none of these lighter gene variants (Wilde et al., 2014). It is interesting to compare different strongly selected genetic traits. Selection for a lighter skin is similar to that seen for lactose digestion and protection against Plasmodium falciparum malaria.
Ultimately, in Western Eurasia, deep ancestral selection for light skin was driven by a relatively small pool of gene polymorphisms that associate with contemporary phenotypic variation. Ju and Mathieson (2021) examined more than 1000 ancient West Eurasian genomes covering 40,000 years BP. They showed that the evolution of light skin was driven by frequency shifts in a relatively small fraction of gene variants that are linked to pigmentation today—one of the most notable large effect variants was at SLC24A5 (rs2675345).
The big question has to be why did natural selection for lighter skin, hair and eye pigmentation act so long after humans moved away from Africa with its harsh UV regime? The most probable explanation resides in diet, and specifically the availability of vitamin D. As hunter gatherers, our diet was rich in vitamin D (particularly from fish and meat), but after the development of farming practices, grains became a major dietary component, leading to a deficit in vitamin D status. Given the significance of vitamin D in maintaining reproductive efficiency (see later), natural selection will have favored a loss in pigmentation to allow for improved vitamin D photosynthesis in our skin. So the agricultural revolution that first emerged in the Levant may have triggered this out of Africa late onset natural selection of skin depigmentation. It is worth bearing in mind that initially, coastal dispersion of early humans into Europe may have permitted retention of ancestral pigmentation alleles because reduced vitamin D photosynthesis in the skin (due to a high melanin content) was mitigated by marine and plant based sources of the vitamin, including fungi and lichens (Chaplin & Jablonski, 2013; Jablonski, 2021; Lucock et al., 2021).
These genes also influence eye color (Jablonski, 2021; Sturm & Duffy, 2012); in particular, OCA2‐HERC2 is strongly associated with blue iris color in Europeans and has been linked to sexual selection (Laeng et al., 2007). This may also be a direct counter‐measure to a short winter photoperiod at high latitudes; blue eyes increase intraocular light scattering and thereby suppress melatonin release from the pineal gland. This may be an adaptive trait to reduce/prevent depression, a condition linked to short day length (Higuchi et al., 2007; Lucock et al., 2021).
Like SLC45A2, the SLC24A5 gene is important in regulation of melanogenesis and hence an important loci in determining skin pigmentation phenotype. It has a large‐effect variant that was introduced into Western Europe via migrating Neolithic farming populations, and continued to be under selection post‐admixture (Ju & Mathieson, 2021). Although the SLC24A5 light skin allele is now almost fixed in Europe, it is interestingly also associated with a lighter skin phototype in Africans, achieving high frequencies in Afro‐Asiatic groups in East Africa (28%–50%), and some Khoisan groups from South Africa (33%–53%) (Crawford et al., 2017; Martin et al., 2017; Pagani et al., 2012; Rocha, 2020). It is believed that the SLC24A5 variant arrived in East Africa via the Middle East around 3000–9000 years BP (Crawford et al., 2017; Pagani et al., 2012), and was carried into South Africa by migrating East African agriculturalists around 2000 years ago, and once there was favored by natural selection, with admixture promoting the evolution of complex mixed pigmentation genotypes and a wide gamut of moderately pigmented phenotypes (Jablonski, 2021).
Of course, while repeated episodes of depigmentation have occurred in our recent evolutionary history, so too have repeated episodes of repigmentation, including the evolution of markedly enhanced tanning abilities, as populations dispersed out of, and then back into, regions of high seasonal UVB (Quillen et al., 2012, 2017, 2018).
Before ending this section, it is worth emphasizing how important genetic bottlenecks are in defining natural selection. The relevance of this was first stressed by Henn and colleagues (Henn et al., 2012). The random effects of population bottlenecks will have had particularly profound consequences for the evolution of human skin pigmentation because the pool of potential gene variants available for selection varied with every dispersal and were further compounded in cases of sequential dispersals. An example of the latter would be where humans dispersed into northeastern Asia and then into North America. Recent research into skin pigmentation genetics indicate that the number of genetic variants (loci/SNPs) that impact variability in skin phototype is quite large, and includes; genes that control up‐ and down‐regulation of melanin biosynthesis and the packaging of melanin in melanosomes, by various pathways. When selection for increased or decreased skin pigmentation was strong, it acted on whatever suite of variants was available at that place, and at that time, to produce a set of variants that would confer a selective advantage. Thus, natural selection could only occur on the limited number of variants that were available in any particular “genetic grab bag” produced by the bottleneck.
While the primary focus of this article is to achieve the most up‐to‐date contemporary synthesis on aspects of the vitamin D‐folate hypothesis as a model for the evolution of skin pigmentation, it is none‐the‐less important to also provide a good background on human dispersals with an integration of how this fits with what we know about pigmentation genes. Out of necessity, this is limited, but for readers who want to delve deeper into this, the following key papers/reviews should prove useful (Crawford et al., 2017; Deng & Xu, 2017; Feng et al., 2021; Henn et al., 2012; Jablonski, 2021; Martin et al., 2017; Nielsen et al., 2017; Pavan & Sturm, 2019; Quillen & Shriver, 2011; Quillen et al., 2018; Rocha, 2020).
3. THE BIOLOGY OF SKIN PIGMENTATION
3.1. Genes
The effect of UVR on the biochemistry of melanogenesis (enzymes and signaling proteins) is profoundly underpinned by genetics (Pavan & Sturm, 2019). Table 1 describes a selection of some of the most relevant gene polymorphisms linked to hair, skin, and eye pigmentation phenotype. The table identifies the expression product and its function along with specific biologically important gene variants. Figure 2 shows how some of these genes/proteins are integrated into the biochemistry of melanogenesis within the melanocyte and melanosome.
TABLE 1.
A selection of some of the most relevant gene polymorphisms linked to hair, skin and eye pigmentation phenotype
| Pigmentation related gene | Expression product | Function | Selected variants | Relevant to the vitamin D‐folate hypothesis |
|---|---|---|---|---|
| TYR | Tyrosinase enzyme | Melanogenesis | rs1042602 | ? |
| GMR5 | Glutamate metabotropic receptor 5 | Tyrosine expression | rs10831496 | ? |
| IRF4 | Interferon regulatory factor 4 | Tyrosine expression | rs12203592 | YES |
| HERC2 | HECT and RLD domain containing E3 ubiquitin ligase 2 | Regulation of OCA2 expression and hence melanogenesis |
rs12913832 rs4932620 rs649721 |
YES |
| OCA2 | Oculocutaneous albinism 2 (melanocyte‐specific transporter protein/pink‐eyed dilution protein homolog) | Melanogenesis |
rs1800404 rs1800414 rs74653330 rs7495174 |
? |
| APBA2 | Amyloid beta A4 precursor protein‐binding family A member 2 | Potential regulation of OCA2 expression and hence melanogenesis | rs442881 | ? |
| SLC45A2 | Solute carrier family 45 member 2 (membrane associated transporter) | Melanogenesis | rs16891982 | ? |
| SLC24A4 | Membrane transporter (NCKX4) | Na+/Ca2+, K+ exchanger. Eye color | rs12896399 | ? |
| SLC24A5 | Solute carrier family 24 member 5 (NCKX5) | K+ dependent Na+/Ca2+ exchanger (melanogenesis) | rs1426654 | ? |
| MC1R | Melanocortin 1 receptor (G protein‐coupled receptor) | Eumelanin synthesis |
rs1805007 rs42687448 |
? |
| KITLG | KIT Ligand | Melanocyte migration |
rs642742 rs12821256 |
? |
| MFSD12 | Major facilitator superfamily domain containing 12 | Transporter mediating import of cysteine into melanosome, regulating pheomelanin synthesis |
rs10424065 rs2240751 rs2240751 |
? |
| DDB1/TMEM138 | Damage specific DNA binding protein 1/Transmembrane protein 138 | UVR exposure response and DNA damage repair |
rs2513329 rs7948623 rs11230664 |
? |
| TYRP1 | Tyrosinase‐related protein 1 | Melanogenesis | rs115075138 | ? |
| ASIP | Agouti‐signaling protein | Interacts with MC1R to determine whether melanocyte synthesizes pheomelanin or eumelanin |
rs6058017 rs6059655 rs4911414 |
? |
| BNC2 | Zinc finger protein basonuclin‐2 | Potential regulated expression of pigmentation genes | rs10756819 | ? |
| MITF | Melanocyte inducing transcription factor | Controls development and function of melanocytes | rs149617956 | ? |
FIGURE 2.
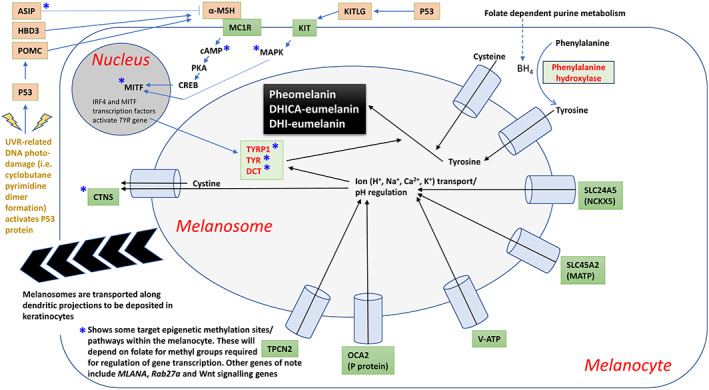
Simplified schematic showing some of the genes/proteins/ionic transport that are integrated into the biochemistry of melanogenesis within the melanocyte and melanosome. Details are fully elaborated upon in the text
3.2. Biochemistry
Melanin is derived from tyrosine and is synthesized in epidermal melanosomes and subsequently translocated to surrounding keratinocytes via transport along dendritic projections. It is a darkly pigmented macromolecular biopolymer that protects from the deleterious effects of UVR. Greater melanisation is also associated with protection against systemic oxidative stress (Shih et al., 2020).
The regulation of melanogenesis is dependent on both ion transport and precursor substrates: Figures 2 and 3 show how BH4‐dependent phenylalanine hydroxylase (PAH) converts phenylalanine into tyrosine, a process located in the cytoplasm of the melanocyte. Tyrosine is then actively transported into the melanosome, where it is converted to L‐DOPA by tyrosine hydroxylase (isoform 1). L‐DOPA then activates tyrosinase (TYR) initiating melanogenesis. Tyrosinase activity is regulated by BH4 by the formation of a 1:1 inhibitory complex with tyrosinase. This can be reversed by α‐ and β‐melanocyte‐stimulating hormone (MSH), rendering tyrosinase active once again. Inteferon regulatory factor 4 (IRF4) cooperates with the melanocyte master regulator (MITF) to activate tyrosinase. Interestingly, it has recently been shown that the IRF4 pigmentation gene interacts with UV to influence folate levels (Lucock et al., 2021).
FIGURE 3.
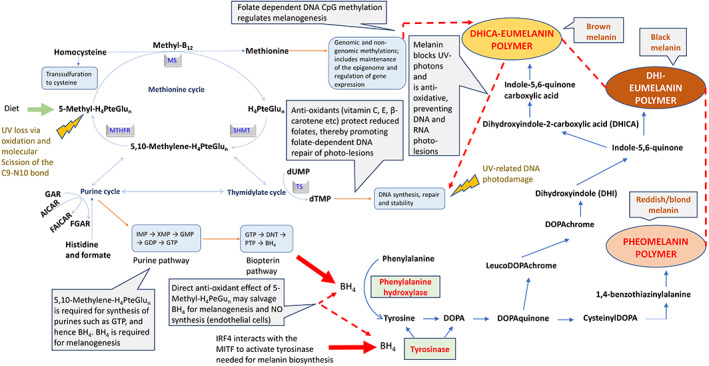
The figure shows the complex relationship between folate dependent one‐carbon transfers into dTMP (DNA), purines (GTP and hence BH4) and methionine (methyl groups, including genomic CpG groups) and the contribution these make to melanogenesis. It also shows how anti‐oxidant vitamins interact to protect labile folates from UVR, and details the biochemistry related to the formation of the three forms of melanin (pheomelanin, DHICA‐eumelanin and DHI‐eumelanin—in order of increasing darkness). A working hypothesis is that folate is required for BH4 and hence melanin production. Therefore, a low folate equates to lower BH4 and hence reduced melanin biosynthesis. This reduction in melanin leads to even more UVR‐related folate loss and hence ever greater DNA damage. This propagates a progressive negative spiral in folate status since the low folate that does exist will be redirected to DNA repair, and demonstrates how dietary antioxidant status is likely relevant in this scenario for protecting reduced and highly labile folates from UV‐related oxidation. Folate gene and pigmentation gene interactions are also likely important in modulating this progressive negative spiral in folate status
In addition to the provision of key substrates, ion transport into the melanosome is also a critical component within melanin biosynthesis. TYR is pH sensitive, and Na+, K+, Ca2+, Cl−, and H+ transport are coupled to the membrane‐associated transporter protein (MATP), K+‐dependent Na+/Ca2+ exchanger 5 (NCKX5), P protein, two‐pore segment channel 2 (TPC2), and ATP (V‐ATP), respectively. In addition, cysteine is actively translocated into the melanosome, with cystine, a negative regulator, removed from the melanosome by cystinosin (CTNS). This regulatory mechanism for ion transport in the melanosome is shown in Figure 2. It is the MATP gene, SLC45A2 described earlier, which is responsible for very recent depigmentation in Europeans (Beleza et al., 2013; Wilde et al., 2014).
It is believed that the default biochemical pathway is for pheomelanin (red/yellow pigment) synthesis, a process that continues until cysteine within the melanosome is consumed (cysteine and DOPAquinone are conjugated to form cysteinylDOPA). At this point, eumelanin synthesis begins (DHI‐eumelanin = black pigment; DHICA‐eumelanin = brown pigment). Silver locus protein homolog provides the matrix in which these melanin compounds are polymerized. (Pavan & Sturm, 2019). The full biochemical pathway for the synthesis of all three melanin polymers is given in Figure 3.
The varying proportion of these three forms of melanin contribute to the wide range of variation in skin color exhibited across the human species. In terms of geometry, the melanosomes provide an umbrella‐like cover over the nucleus of the keratinocyte. This provides protection against UV radiation induced damage (i.e., DNA lesions and folate degradation). Figures 2 and 3 also show how the melanocyte responds to UVR initiated DNA damage, particularly cyclobutane pyrimidine dimer formation, which can activate the p53 protein. P53 activation leads to the generation of pro‐opiomelanocortin (POMC) and KITLG (KIT ligand). POMC forms α‐MSH, which binds to the melanocortin 1 receptor (MC1R), initiating cAMP‐protein kinase activity. This cAMP‐responsive element binding protein (CREB) pathway leads to activation of MITF. Additionally, KITLG binds the KIT receptor on the surface of the melanocyte, leading to initiation of mitogen‐activated protein kinase (MAPK), which also interacts with MITF. Furthermore, activation of MCIR can be inhibited by the agouti signaling protein (ASIP). Ultimately, granules containing melanin migrate to the cell membrane and are transported into the keratinocyte cells.
4. PIGMENTATION AND THE NEED TO PROTECT FOLATE
4.1. Biochemistry and genetics
Folate in our contemporary diet is a mixture of natural 5‐methyltetrahydrofolate (5‐methyl‐H4PteGlun) and the synthetic analog, pteroylmonoglutamic acid (PteGlu). However, ancestral humans will only have been exposed to 5‐methyl‐H4PteGlun. An oral dose of around 266–400 μg PteGlu is efficiently converted into 5‐methyl‐H4PteGlu (Kelly et al., 1997; Lucock et al., 1989). Despite this, scientists remain concerned about potential risks of being exposed to excessive PteGlu, including negative UV‐related effects (Choi et al., 2014). The normal transport form of the vitamin is monoglutamyl 5‐methyl‐H4PteGlu. Once this vitamer is transported out of the blood plasma into the cell, it must be demethylated and converted into a polyglutamate coenzyme. Polyglutamylation is essential before it can be used as a cofactor for folate‐dependent one‐carbon metabolism. The key biochemical roles of folate are biosynthesis of methionine (i.e., genomic CpG methylation/gene expression), purine (i.e., impacts GTP and hence BH4 production), pyrimidine (i.e., dTMP and hence DNA synthesis), and the interconversion of serine and glycine. It is also required for the catabolism of histidine (Lucock, 2000; Shane, 2008) (Selected aspects of folate‐dependent one‐carbon metabolism are given in Figure 4).
FIGURE 4.
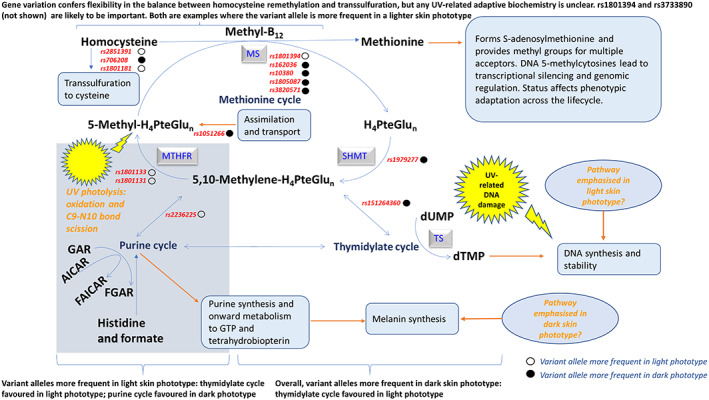
Selected aspects of folate‐dependent one‐carbon metabolism designed to explore putative metabolic consequences of gene variants potentially relevant to skin pigmentation phenotype and the evolutionary model. Publicly available data on gene frequency from several sources have been mapped to the metabolic pathway for folate. Indicative mapping of gene rsid to metabolism suggests the effect of variant alleles on folate dependent one‐carbon transfer tends to support the evolutionary model; i.e. based on disposition of polymorphisms, the thymidylate cycle seems favored in a light phototype and the purine cycle favored in a dark phototype. This supports the need for DNA repair in light skin and melanin synthesis in dark skin. The effect of variant alleles on methylation is less obvious, and may reflect biochemical flexibility at this critical locus. This secondary adaptive metabolic phenotype supports the primary pigmentation phenotype. The collective effect is a higher survival index tailored to specific environments (UVR regimes). Diet (antioxidant vitamins), is a tertiary factor that is also supportive of the secondary adaptive phenotype, especially in populations with a light skin and high UVR
The biochemistry that is most closely linked to phenotype is likely to involve de novo pyrimidine, purine and methionine biosynthesis. That is, DNA‐thymidylate (dTMP), GTP, and CpG methylation, respectively (Lucock, 2021; Sohn et al., 2009). If folate is in short supply (diet, UVR loss, genetic polymorphism), DNA fragility occurs, leading to uracil being misincorporated into the primary DNA base sequence in place of thymine (Blount et al., 1997). In addition to this, 5‐methyl‐H4PteGlun dependent de novo methionine biosynthesis provides methyl groups for the methylome, underpinning gene expression. Therefore, since half of our methionine requirement is met via this route (Mosharov et al., 2000; Lucock, 2006), folate depletion may impact regulated gene expression (Friso et al., 2017). Figure 2 identifies some of the pigmentation specific, target epigenetic methylation sites/pathways found within the melanocyte (Zhou et al., 2021). Reduced folate coenzymes also contribute to the de novo biosynthesis of purine nucleotides. Carbon‐8 and carbon‐2 positions of the purine ring are derived from 10‐formyltetrahydrofolate (10‐formyl‐H4PteGlun) via reactions catalyzed by glycinamide ribonucleotide transformylase and 5‐amino‐4‐imidazolecarboxamide ribonucleotide transformylase, converting glycinamide ribonucleotide (GAR) into formyl‐glycinamide ribonucleotide (FGAR) and aminomidazole carboxamide ribonucleotide (AICAR) into formyl‐aminoimidazole carboxyamide ribonucleotide (FAICAR), respectively (Shane, 2008). Folate depletion would impact purine synthesis and onward metabolism to GTP and hence BH4 (Schallreuter et al., 2008; Shi et al., 2004). Given that BH4 is critical for melanogenesis, 5‐methyl‐H4PteGlu is UVR sensitive, and that UVR‐related DNA damage is dependent on folate for DNA repair, folate and skin pigmentation share an obvious nexus. However, this nexus receives far less attention than research linking this part of metabolism to neural tube defects (NTD) and a raft of other early developmental and late‐life degenerative disorders, including several cancers and vascular disease (Lucock & Yates, 2009; Selhub, 2002). Consequently, it is important to consider the UV exposome, along with dietary and genetic factors when considering folate biology and pigmentation phenotype (Lucock, 2021). Indeed, folate genes are highly polymorphic, with gene clusters working in tight synergy. For example, one specific gene cluster encodes serine hydroxymethyltransferase (SHMT1), thymidylate synthase (TS) and dihydrofolate reductase (DHFR), and is responsible for utilization of 5,10 methylene‐H4PteGlu for dTMP/DNA biosynthesis. 5,10‐methylenetetrahydrofolate reductase (MTHFR) provides an alternative fate for 5,10 methylene‐H4PteGlu, namely reduction to 5‐methyl‐H4PteGlu and utilization for the conversion of homocysteine into methionine, a process that also requires vitamin B12. This pathway nexus provides a bifurcation in the fate of one‐carbon units, with gene variation (i.e. rs1801133) shifting the probability of which route one‐carbon units partition into. There is little doubt that diet, folate status, UV exposure and other factors (discussed later) could also easily modulate the favored metabolic route.
As discussed earlier, the role of UVR‐related folate loss in the evolution of skin pigmentation is a mature hypothesis. It has been established that UV exposure can reduce red cell and plasma folate status. Erythrocyte levels are considered to be the best general measure of overall folate status, and the effect of UVR on erythrocyte folate is clearly influenced by the MTHFR C677T genotype (rs1801133) (Lucock et al., 2017). A large cohort of 649 subjects were examined for cumulative UV irradiance over 42 and 120 days prior to blood collection. The UV irradiance was found to be significantly negatively associated with erythrocyte folate status. When data were stratified by MTHFR C677T genotype, the relationship between UV irradiance and red cell folate remained significant only in carriers of the polymorphic T allele. This provides the strongest evidence to date that UV irradiance arriving at the Earth's surface reduces systemic folate status (Lucock et al., 2017). Many other reports now outline the UVR sensitivity of folate. One of the most significant ones, which is also highly supportive of in vivo UV loss of systemic folate, and represents the only other population based study, was conducted by Borradale and colleagues. (Borradale et al., 2014). These researchers showed that 3 weeks of solar UV exposure diminished the effectiveness of PteGlu supplements in raising serum vitamin levels in a population of young females of reproductive age. As with the previous population study (Lucock et al., 2017), this cohort lived in a geographic region (Eastern Australia) with extreme UV exposure (largely maladapted skin phenotype). However, unlike the former study, this one was relatively small with only 45 participants. It should be noted that serum folate does not reflect overall folate status as well as erythrocyte folate values do. In both these population studies (Borradale et al., 2014; Lucock et al., 2017), the observed decline in systemic folate needs to be considered alongside the possibility that a diminished folate status might also reflect an increased requirement for the vitamin to maintain DNA repair processes (Duthie, 1999) in the face of UV induced genomic damage; 1 day's sun exposure results in up to 105 UV‐propagated photo‐lesions per individual skin cell (Hoeijmakers, 2009). Despite this, a direct effect of UVR on folate is also likely; HPLC evidence from in vitro studies unambiguously demonstrate how UV‐B light at 312 nm degrades the monoglutamate form of 5‐methyl‐H4PteGlu into oxidized 5‐methyl‐H2PteGlu (Lucock et al., 2003), with eventual loss of all vitamin activity via scission of the C9–N10 bond linking pteridine and aminobenzoyl glutamate moieties. These findings have been further validated by an ex vivo study showing that longer UV‐A as well as UV‐B wavelengths of light can degrade the natural monoglutamyl 5‐methyl‐H4PteGlu blood vitamer (Hasoun et al., 2015). From a pigmentation perspective, UV‐A wavelengths can penetrate deep under the skin to reach the dermal circulation, making this a likely wavelength trigger for folate driven evolutionary change in melanin density/skin phototype.
It is not possible to examine folate biochemistry without giving consideration to vitamin B12, since “active reduced folate” and de novo methionine for maintaining genomic methylation patterns is dependent upon B12’s cofactor role at the methionine synthase/methionine synthase reductase locus (Figure 4). Indeed, a 2014 study suggests vitamin B12 deficiency is associated with geographic latitude (and hence solar radiation) within an older population living in Chile (Cabrera et al., 2014); vitamin B12 deficiency was associated with higher solar radiation and living closer to the Equator.
It has also been suggested that another B‐vitamin coenzyme may be relevant to folate UV‐photolysis; Vitamin B2 (riboflavin) is light sensitive itself, and has been postulated to act as a photosensitizer (Cardoso et al., 2012), enhancing the UV‐dependent degradation of folate vitamers. However, recent research shows that riboflavin, does not sensitize erythrocyte folate to UVR, and actually affords anti‐oxidative protection toward folate (Lucock et al., 2021).
One of the more interesting UVR‐folate‐related findings may provide another link with BH4 synthesis and function. Wolf and colleagues recently uncovered a novel effect of UV light on the vascular system mediated by folate (Wolf et al., 2018). UV‐B irradiance attenuates nitric oxide (NO)‐mediated vasodilation within the cutaneous microvasculature by degrading 5‐methyl‐H4PteGlu, a vitamin thought to be required for NO synthesis. BH4 is also an important cofactor for NO synthesis and both folate and biopterin cofactors may have a degree of functional overlap/interaction. The authors suggest this UV‐folate effect may be via a direct and/or reactive oxygen species‐induced loss of 5‐methyl‐H4PteGlu. Interestingly, this UV‐B effect on the vascular system was independent of skin phototype.
4.2. The argument for natural selection driven by folate depletion
It is now firmly established that an inadequate folate status (for whatever reason‐dietary, genetic, UVR related) adversely impacts the reproductive success of both males and females. The impact of low folate on female embryonic biology is well documented; the teratogenic effects of a low folate lead to NTDs such as spina bifida and anencephaly (Smithells et al., 1983; MRC Vitamin Study Research Group, 1991) and have been the driver of modern folate fortification programs. However, congenital malformations induced by inadequate folate are also believed to have been instrumental in the natural selection of dark skin at low latitudes to protect folate for reproductive success and the maintenance of fertility (Jablonski & Chaplin, 2010; Tamura & Picciano, 2006). Similarly, in males, a diminished sperm count and motility also occurs due to low folate status (Boxmeer et al., 2009). It is also of note that homocysteine is considered to be an embryotoxic thiol, and that the most effective mechanism to lower homocysteine is increased intake of folic acid (Lucock, 2006).
Ultimately, the critical nature of folic acid in the elaboration and synthesis of DNA, and associated cellular division during embryogenesis (and spermatogenesis) provides a developmental timeline that is particularly vulnerable around 4 weeks post‐conception, when the embryo's neural tube closes. A vitamin shortage at this time increases risk of malformation. Eumelanin concentrated in keratinocytes in the epidermal stratum basale would/does protect folate from UVR degradation, improving fertility and buffering the stochastic nature of dietary folate (Jablonski, 2021). This pigmentation barrier is considered to be most effective at shielding against UVB, with less influence on longer, more penetrative UVA wavelengths. This is beneficial because UVB is deleterious to DNA and also exerts a greater effect on folate loss than at longer wavelengths. It has recently been shown that UVB and low UVA (near UVB) wavelengths cause the greatest loss of erythrocyte folate, and that this is enhanced in a lighter skin phototype (IRF4 TT genotype—rs12203592) (Lucock et al., 2021). With this in mind, and despite the previous data being derived from an older population, it is worth noting that an individual's maximal level of constitutive pigmentation occurs early in life (late teens to early 20s) when humans enter their period of optimal fertility and hence presumably, peak folate requirement (Jablonski & Chaplin, 2010; Robins, 1991).
Clearly, nutrient–gene interactions are likely to be important and influence this well‐established evolutionary model of skin pigmentation. An evidence‐based extension of the prevailing evolutionary model that integrates metabolism genes and diet is detailed later.
5. DEPIGMENTATION AND THE NEED TO PHOTOSYNTHESISE VITAMIN D
5.1. Biochemistry and genetics
Vitamin D photosynthesis uses short UVB wavelengths (<310 nm peaking around 297 nm) and involves the conversion of 7‐dehydrocholesterol into previtamin D3 within the skin epidermis. Previtamin D3 subsequently photo‐converts into the inactive metabolites, lumisterol or tachysterol, or it undergoes a temperature‐dependent isomerization to form vitamin D3 (cholecalciferol/calciol). This vitamer is then metabolized in the liver by calciol 25‐hydroxylase (CYP2R1 gene) to form the main plasma and storage form of the vitamin—calcidiol [25(OH)D3]. Calcidiol is subsequently converted into the biologically active form of the vitamin, calcitriol [1,25(OH2)D3] in the kidney via the action of calcidiol 1‐hydroxylase (CYP27B1 gene) (Martin et al., 2014; Webb & Holick, 1988). Calcidiol and calcitriol vitamers can also be redirected to form inactive metabolites (24, 25‐dihydroxyvitamin D and 1α,24R,25‐trihydroxyvitamin D, respectively) by calcidiol 24‐hydroxylase (CYP24A1 gene), depending on metabolic need, particularly in regard to the maintenance of calcium balance (see Figure 5).
FIGURE 5.
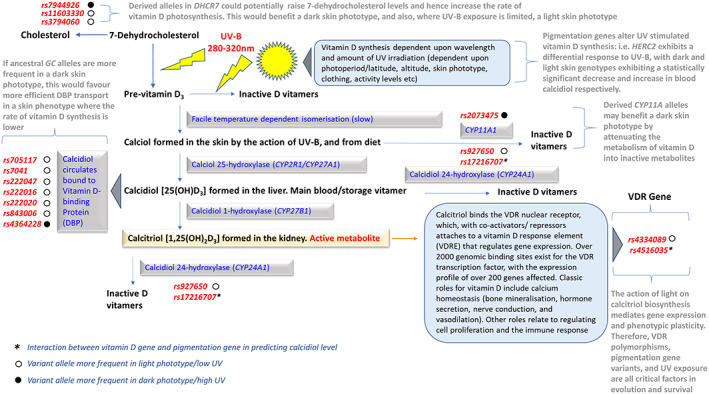
Selected aspects of vitamin D metabolism designed to explore putative metabolic consequences of gene variants potentially relevant to skin pigmentation phenotype and the evolutionary model. Details are fully elaborated upon in the text, but might be particularly relevant for GC, DHCR7 and CYP11A1 gene variants. In some cases, they may have evolved to provide adaptive plasticity to differing skin phototypes i.e. DHCR7 rs11603330 and rs3794060. This secondary adaptive metabolic/genetic phenotype supports the primary pigmentation phenotype. The collective effect is a higher survival index tailored to specific environments (UVR regimes)
Ultimately, the sunlight‐dependent vitamin D endocrine system underpins calcium regulation, fostering a stable sub‐micromolar level of free intracellular calcium. In turn, calcium exhibits a reciprocal homeostatic effect on production of the active calcitriol vitamer. This is a complex biochemistry, with several feedback mechanisms in play to regulate this critical nexus, and hence maintain calcium levels within physiological norms. Briefly, major feedback steps operate at the level of the 1‐ and 24‐hydroxylase enzymes. The first control point involves calcitriol slowing its own synthesis by inducing the 24‐hydroxylase while repressing the 1‐hydroxlase enzyme. In both these cases, metabolic inhibition occurs due to altered gene expression (CYP27B1 and CYP24A1 respectively). The second control point involves a drop in blood calcium, which initiates the secretion of parathyroid hormone, promoting 1‐hydroxylase activity, but inhibiting 24‐hydroxylase activity. This phenomenon is counteracted by rising calcium and calcitriol levels, which conspire to repress parathyroid hormone synthesis. The third control point is less overt; calcium directly inhibits the 1‐hydroxylase enzyme (Jeon & Shin, 2018; Lucock et al., 2015, 2018).
Classic hormonal signaling via the vitamin D receptor (VDR) is thought to have first evolved to regulate calcium flux, equilibrium, storage, and signaling within nerve and muscle tissue. It mediates actions that augment phenotypic plasticity by modulating gene expression, and hence phenotypic outcomes in response to UVR originated cues. The VDR is a member of the nuclear receptor superfamily of steroids. (Beckett et al., 2016). When activated by D vitamers, the nuclear receptor has high tissue specificity and regulates calcium and phosphorus homeostasis. Additionally, the VDR underpins the growth and differentiation of many cell types found in VDR‐dependent target tissues (Bouillon et al., 2008). VDR action influences gene expression, including those that modify and remodel chromatin, and hence can alter the DNA methylome. Interestingly, the VDR gene is itself methylated at key CpG islands; providing a mechanism by which light transduces/signals biological outcomes (Fetahu et al., 2014). To render perspective on the magnitude of vitamin D activity in human biology, the VDR potentiates gene expression at the single gene and complex gene‐network level. Clearly, solar exposure (and diet) along with genetic and epigenetic mechanisms interact to alter gene expression in a way that has extremely wide pleiotropic effects (Pike & Meyer, 2012). This point is well illustrated by the fact that 2276 genomic loci are occupied by the VDR with 229 genes exhibiting altered expression profiles in response to vitamin D. Of even greater interest is the fact that more than 4000 protein coding mRNAs within adipose tissue and white blood cells show seasonally derived expression profiles that are different between the Northern and Southern hemisphere with implications for immunity and physiology (Dopico et al., 2015). A more recent 2020 study on vitamin D signaling showed that CD14 was the most significantly regulated vitamin D target gene, while the most induced vitamin D target gene was CYP24A1 (Hanel et al., 2020). In human studies, the same group (Neme et al., 2019) noted 702 genes with significantly altered expression patterns following a 2 mg vitamin D bolus. In THP‐1 cells, ChIP‐seq detected 11,657 VDR binding sites (Neme et al., 2017), although only 510 were occupied under all conditions. In addition to these persistent binding sites, 2109 transient VDR‐binding sites were found to exist. The majority of binding sites (9038) were only observed after 24 h ligand stimulation. Clearly, the spatiotemporal VDR transcription factor binding within vitamin D target tissues elicits an epigenome and transcriptome‐wide response. Relevant tissues include the plasma membrane, nucleus, cytosol, cytoskeleton and endoplasmic reticulum (Hanel et al., 2020). The effects of vitamin D pleiotropy are, therefore, manifold and help adapt the human phenome according to a narrow wave band of UV‐B radiation and a limited number of dietary food sources.
5.2. The argument for natural selection driven by vitamin D depletion
Recent (Ca. 5000 years BP) selection for a lighter skin pigmentation in Europe to cope with a reduced intake of vitamin D has already been discussed in the context of human agricultural development. However, there are some key aspects of the evolutionary mechanism responsible for this change that have, as yet, not been mentioned, and which relate to the vitamin's biochemistry, particularly calcium homeostasis. Although vitamin D is a hormone, it is also a vitamin, with an important deficiency syndrome—rickets, in which there is insufficient calcium for normal bone formation. As with folate, inadequate vitamin D during pregnancy can affect fertility by several vitamin D‐calcium‐related mechanisms. Elevated follicular fluid and blood vitamin D (calcidiol) predict the success of in vitro fertilization (Ozkan et al., 2010). In addition, during pregnancy there is a four‐ to five‐fold increase in vitamin D to satisfy the calcium requirement for fetal skeletal development. However, one of the most relevant aspects driving a strong selection pressure against reproductive success due to vitamin D inadequacy occurs because of the likelihood of maternal pelvis malformation in the face of suboptimal calcium homeostasis. This is a serious skeletal deformation that prevents childbirth because the width of the birth canal becomes restricted, and is a form of rickets that additionally increases maternal mortality (Holick, 2012).
Historically, this vitamin deficiency disease has been widespread. Rickets has been identified in early archeological samples and Neolithic materials as occurring at a rate of 1%–2.7%, which is a reasonably high selective value (Chaplin & Jablonski, 2009; Littleton, 1991; Mays et al., 2006; Robins, 1991). Written accounts of rickets can be found in literature from many early cultures (Chaplin & Jablonski, 2009). Even today, rickets is not uncommon in developing nations, and following industrialization the incidence of rickets reached 80% in children under the age of two (Shaw, 2003) and 33% in the general population in studies prior to 1935 (Vieth, 2001).
Readers who want to gain a comprehensive perspective on vitamin D and the evolution of depigmentation should read Chaplin & Jablonski, 2009, which is both thorough and wide reaching in its analysis and critique of the literature.
6. INTEGRATING THE LATEST IDEAS ON THE VITAMIN D‐FOLATE HYPOTHESIS INTO AN EXTENDED MODEL FOR THE EVOLUTION OF SKIN PIGMENTATION
Although the vitamin D‐folate hypothesis endures as an explanation for the evolution of skin pigmentation, recent research has extended this model to incorporate a multiplex of critical factors (environmental and genetic) that conspire to adapt human skin phototype to a variable UVR regime (Lucock et al., 2021). Total ozone mapping spectrometer (TOMS) satellite data were used to examine surface UV‐irradiance in a large Australian study. Twenty‐two vitamin D‐ and folate‐related gene polymorphisms, along with two pigmentation gene variants (IRF4‐rs12203592/HERC2‐rs12913832) were examined as was calcidiol and erythrocyte folate.
This paper provides several findings that help clarify the evolutionary model. UVR and pigmentation genes interacted to alter blood vitamin levels; the light skin IRF4‐TT genotype showed the highest folate loss while light skin HERC2‐GG genotype had the highest vitamin D3 synthesis. These findings were based on both TOMS and seasonal data analysis.
It was interesting that, as might be predicted on an a priori basis, UV‐wavelength exhibited a dose–response relationship with respect to folate loss in individuals with the light skin IRF4‐TT genotype (305 > 310 > 324 > 380 nm). Clearly, UVB is more deleterious than UVA with respect to folate loss. Statistically significant vitamin D3 photosynthesis only occurred in individuals with the light skin HERC2‐GG genotype and was maximal at 305 nm.
One of the most novel, and unexpected findings was that three dietary antioxidant vitamins (vitamins C, E, and β‐carotene) interacted with UVR and pigmentation genes to prevent oxidative loss of highly labile reduced erythrocyte folate vitamers. The greatest benefit was to individuals with the light skin IRF4‐TT genotype. It was particularly interesting that the putative photosensitizer, riboflavin, did not sensitize erythrocyte folate to UVR degradation, and actually afforded protection. It had previously been thought that UVR folate loss could potentially arise due to oxidation of folates by radical species generated by UVR reacting with endogenous photosensitizers, particularly riboflavin (vitamin B2) (Steindal et al., 2008). However, riboflavin actually protects native reduced folate stores from UVR loss, possibly via independent antioxidant action, or through its role within the glutathione redox cycle (Ashoori & Saedisomeolia, 2014).
Although one might predict a varied genetic contribution to the model, including epistasis, nothing had previously been reported. However, four genes (5xSNPs) were found to modify blood vitamin levels when stratified by pigmentation genotype (Lucock et al., 2021). These genes were MTHFR‐rs1801133/rs1801131, TS‐rs34489327, CYP24A1‐rs17216707, and VDR‐ApaI‐rs7975232.
The study also closed in on a genotype–phenotype corollary that was highly supportive of the model; Individuals with the lightest IRF4‐TT/darkest HERC2‐AA genotype combination (greatest folate loss/lowest vitamin D3 synthesis) had a 0% occurrence. By contrast, the opposing, commonest compound genotype (darkest IRF4‐CC/lightest HERC2‐GG) had a 39% occurrence. This latter compound genotype permits the least folate loss, and greatest synthesis of vitamin D3.
Collectively, the above findings (Lucock et al., 2021) provide new biophysical evidence to support and extend the vitamin D‐folate hypothesis for the evolution of skin pigmentation.
Given that a link exists between folate and melanin (see Figure 3), the same authors have suggested a broadening of the vitamin D‐folate hypothesis of skin pigmentation to incorporate this link. The new working hypothesis is that a low folate (for whatever reason, but in the current context due to high UVR) limits BH4, and hence melanin biosynthesis. This scenario would lead to an even greater loss of folate due to UVR photolysis, and hence propagate significant DNA damage and a reduced capacity for DNA repair. In other words, it sets up a progressive negative spiral in folate status, which in an evolutionary context would have led to reduced reproductive fitness.
Given that vitamin and pigmentation genes interact to tailor biochemistry to the prevailing UV exposome, it is interesting to consider the above findings within a Darwinian medicine context. Because of the central role for folate and vitamin D in the pathology of several diseases (Lucock & Yates, 2009), broad questions about the link between UVR and folate/vitamin D and health are inevitable. Do pigmentation or vitamin‐related genes impact disease etiology? Does the intensity and wavelength of UVR influence disease etiology? If so, can high risk environment‐metabolism profiles be identified and disease mitigation implemented? This is one area where understanding evolutionary biology may pay real dividends in contemporary healthcare.
6.1. Can we predict how vitamin‐related gene polymorphisms might pivot metabolism to the prevailing UVR exposome within the vitamin D‐folate hypothesis model for the evolution of skin pigmentation based on recent literature?
It is interesting to examine the geographic (i.e. latitudinal/UVR) occurrence of folate and vitamin D‐related polymorphisms, and see whether any putative functional metabolic consequences of these gene variants might have relevance to skin pigmentation phenotype and the evolutionary model. To examine this, publicly available data on gene frequency from several sources and recent studies (i.e. Beckett et al., 2017; Jones et al., 2016; Jones, Veysey, et al., 2018; Jones et al., 2020; Lucock et al., 2015; 2021; ALFRED –Allele Frequency Database; 1000 Genomes Database] have been mapped to the metabolic pathway for each vitamin. Figures 4 and 5 show this mapping, and help expand the model by considering the breadth of secondary effects relevant to the evolutionary model. Although findings can only be considered indicative, mapping of rsid to metabolism in Figure 4 suggests the effect of variant alleles on folate dependent one‐carbon transfer tends to support the evolutionary model; that is, based on the disposition of polymorphisms, the thymidylate cycle seems to be favored in a light phototype and the purine cycle favored in a dark phototype. This supports the need for DNA repair in light skin and melanin synthesis in dark skin (Lucock et al., 2021). The effect of variant alleles on methylation is less clear and may reflect a need for biochemical flexibility at this critical locus.
A similar story holds for the mapping of rsid to vitamin D metabolism (Figure 5), suggesting a potentially good fit to the evolutionary model. Ancestral GC alleles (gene for vitamin D binding protein needed for vitamin transport) are more frequent in a dark skin phototype, which would enhance vitamin D transport in a skin phenotype where photosynthesis of vitamin D is much lower. Indeed, data suggests that as a population's UVR exposure increases (likely darker skin phototype), so the frequency of the GC variant decreases. This is true for 6 of the 7 GC variants examined (Jones et al., 2020) (Figure 5).
A particularly interesting mapping is given by DHCR7. Derived alleles in DHCR7 have the potential to raise precursor 7‐dehydrocholesterol levels, and hence increase the rate of vitamin D3 photosynthesis, which would be beneficial for individuals with a dark skin phototype. Figure 5 shows this mapping (rs7944926) would be consistent with the evolutionary model. However, in areas where the relevant wavelengths of UV‐B are limited (i.e. high latitudes), a light skin phototype would be beneficial. This potentially maps DHCR7 rs11603330 and rs3794060 to the evolutionary model (Figure 5).
Similarly, derived CYP11A alleles may potentially benefit individuals with a dark skin phototype by slowing up the metabolism of vitamin D into inactive metabolites.
The effects of UVR on allelic variation in HERC2 (rs12913832) and IRF4 (rs12203592) and hence vitamin D3 and folate, respectively, have been discussed earlier.
Given the above mapping of several vitamin and pigmentation gene polymorphisms to metabolism, and their fit to the evolutionary model, I suggest that the vitamin D‐folate evolutionary model of skin pigmentation can be broken down into:
A. Primary adaptive phenotype—pigmentation (highest evolutionary significance).
B. Secondary adaptive phenotype—biochemical/metabolic (supportive, although flexible and adaptive in its own right).
C. Tertiary adaptive strategy—Dietary support; role of dietary antioxidants to counter UVR‐related oxidative damage (supportive of the secondary adaptive phenotype).
The secondary adaptive phenotype supports the primary one. The collective effect is a very high survival index tailored to specific environments (UVR regimes). Dietary factors are also supportive of the secondary adaptive phenotype, especially in populations with a light skin and high UVR exposure. This seems especially true for folate and is consistent with a major conclusion made by Lucock et al. (2021)—“Folate metabolism is challenged most in light skin phototypes in high UV regimes. IRF4 genotype, MTHFR genotype, and folate act together in a contemporary population, and hence, historically, may have acted to drive the evolution of skin pigmentation phenotype as an adaptive mechanism to the prevailing UV exposome.”
Figure 6 shows the relationship between UVR, pigmentation phenotype, folate, and vitamin D–related reproduction efficiency and evolutionary fitness. It also adds in what we now know about antioxidant vitamins and pigmentation genes, as well as folate (BH4)‐related melanin synthesis and DNA repair.
FIGURE 6.
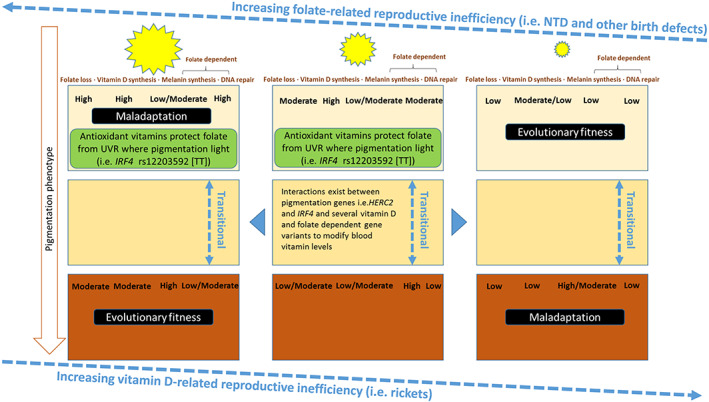
Figure shows the relationship between UVR, pigmentation phenotype, folate and vitamin D–related reproduction efficiency, and evolutionary fitness. It also shows what we now know about antioxidant vitamins and pigmentation genes, as well as folate (BH4)‐related melanin synthesis and DNA repair
7. ALTERNATE HYPOTHESES
A variety of ideas have been postulated to explain the evolutionary basis of skin pigmentation. Patrice Jones and colleagues recently integrated these into a review that explores the vitamin D‐folate hypothesis, as well as alternative and even complementary theories such as the “skin mutagenesis hypothesis,” “the skin barrier hypothesis,” and the “metabolic conservation hypothesis” (Jones, Lucock, et al., 2018). Although this review has a major focus on the “vitamin D‐folate hypothesis of skin pigmentation,” aspects of the other theories may have merit. In particular, “the metabolic conservation hypothesis” implies that evolutionary depigmentation of ancestral humans resulted from the need to redirect resources away from melanin production to other metabolic processes designed to combat the stress of a colder climate (Elias & Williams, 2016; Jones, Lucock, et al., 2018). Nevertheless, most of the alternative theories have now been significantly challenged or disproven (Jablonski & Chaplin, 2017).
The idea that less melanization facilitates vitamin D photosynthesis in the skin has been challenged. Bogh et al. (2010) found no significant correlations between skin pigmentation and calcidiol levels after UVB exposure. Hakim et al. (2016) also found no differences in photosynthesis of calcidiol in different skin phototypes. More recently, Young et al. (2020), by comparing vitamin D production in extreme skin types II and VI, concluded that the inhibitory effect of melanin on vitamin D3 synthesis is small. Although Batai et al. (2021) found an association between pigmentation‐related gene variants (Genetic Score) and vitamin D deficiency, there was no association between melanin Index and serum calcidiol. Although such findings are equivocal as far as supporting the vitamin D‐folate hypothesis is concerned, other recent findings are strongly supportive. Clearly, the relationship between skin phototype and vitamin D photosynthesis requires further investigation, taking the fullest account possible of the complexity of factors involved. Below, in the future directions section, I suggest how this might be achieved.
The most recent critique of the vitamin D‐folate evolutionary model is presented in a review by Hanel and Carlberg (2020a). These authors assess recent archeogenomic data for changing skin, hair, and eye color in European populations over the last 10,000 years. They also look at allele frequency in vitamin D transport, metabolism and signaling genes. The authors argue that variation in vitamin D–related allele frequencies allows for an alternate mechanism for adapting to the UVR regime at northern latitudes. That is to say, ancestral European populations may have dealt with a scarcity in vitamin D by developing a higher sensitivity (avidity) toward vitamin D3 vitamers, rather than simply evolving a lighter skin. However, as alluded to earlier, considerable genetic/evolutionary evidence links depigmentation to the expansion of agriculture and hence loss of vitamin D that would otherwise have been supplied by hunter‐gatherers (Ju & Mathieson, 2021; Wilde et al., 2014). The authors suggest that the introduction of high vitamin D responsiveness (polymorphisms) was an essential trait that permitted early Europeans to survive long dark winters without suffering vitamin D deficiency (Hanel & Carlberg, 2020b). These are interesting ideas, and are not inconsistent with the suggestion above that a secondary adaptive biochemical/metabolic phenotype supports the primary adaptive pigmentation phenotype. They also align with our group's findings on vitamin D genes (Jones et al., 2020) and folate genes (Jones, Lucock, et al., 2018; Jones, Veysey, et al., 2018). In the latter case, we have suggested that folate genotypes are selected to maintain homeostasis in the folate system under differing UVR conditions, as well as being a target for selection.
Ultimately, the evolutionary model is simply more complex than we previously thought, but recent studies and ideas all help shed light on the mechanisms and implications involved.
8. FUTURE DIRECTION AND CHALLENGES
Although hard evidence is now beginning to emerge that strongly supports the vitamin D‐folate evolutionary model (Lucock et al., 2021), there is still much to understand, in particular the secondary adaptive biochemical/metabolic phenotype that supports the primary adaptive pigmentation phenotype. Furthermore, the latter study by Lucock and colleagues examines a population that for the most part, exhibits an environmentally “maladapted” pigmentation phenotype since it gathers data from individuals of mostly European ancestry living in a very high UVR regime in Australia. Although this may appear to be a weakness, these polar extremes of generally lighter pigmentation phototype and extreme UVR help amplify any vitamin‐gene–environment interactions. Thus, in many ways, this is a valid and useful approach. Despite this, without doubt, future studies should critically examine the same and expanded parameters in a variety of diverse populations whose skin phototype is matched to the prevailing UVR regime, and whose skin color is accurately measured. The study should also look at individuals in their late teens to early 20s, when humans enter optimal fertility and have their maximal level of constitutive pigmentation. Only then can conclusions related to the evolutionary biology of skin, and the role of vitamins and UVR be fully characterized. Additional analysis should look beyond IFR4 and HERC2 pigmentation genotypes to examine the full gamut of relevant pigmentation genes, as different pigment‐gene variant combinations lead to different coloration phenotypes (Jablonski & Chaplin, 2017; Jablonski, 2021)—see Table 1.
Cultural factors are also potentially very important and are often overlooked as is the contribution from sexual selection for skin and eye color (Jablonski, 2021; Sturm & Duffy, 2012). Assessments such as the time spent outdoors and clothing worn are also likely to be germane in future studies. Thorough dietary information also needs to be captured given the latest data pointing to the importance of anti‐oxidants. However, for the best interpretation, blood measurements of anti‐oxidant vitamins and other antioxidant metabolites along with biopterin, markers of DNA damage and associated/relevant gene variants should also be included to provide the widest metabolomics/genomics library possible (see Figure 3). Studies of this depth and magnitude are extremely difficult and expensive to conduct, but are possible. They are justified given the evolutionary significance of skin pigmentation and its importance to human biology. It also seems likely that this biology will have great relevance to human health given the critical nature of vitamin D and folate to so many diseases.
Within any future modeling, one also needs to examine the fusion of epistasis, pleiotropy and many aspects of culture in shaping the geography of skin color. Without doubt, the vitamin D‐folate evolutionary model is far more complex than text books suggest.
One of the biggest challenges is to elucidate how recent dispersals to locations with different UVR regimes, and resulting population admixture contribute to the model—what, if any, are the clinical and natural selection ramifications? Although we do not have all the answers, we can be certain that in the long term, the human genome has adequate reserve capacity (2.97 × 106 polymorphic loci [1 per 300 nucleotides]) to accommodate phenotypic variation to cope with the full repertoire of environmental selection pressures.
9. SUMMARY
Human skin pigmentation provides an effective shield to damaging UVR. The prevailing evolutionary model suggests this melanin barrier evolved at equatorial latitudes to protect UV‐labile folate, which is a prerequisite for reproductive success (needed for DNA, purine and methionine biosynthesis). Similarly, when dark skinned humans migrated out of Africa, and eventually established agricultural cultures, a shortage of both dietary and photosynthesized vitamin D led to the evolution of depigmentation. This happened fairly recently within Europe and is probably also linked to reproductive success (vitamin D deficiency leads to rickets and promotes skeletal malformations, including of the pelvis, which potentially impacts childbirth). Recent evidence strongly supports the vitamin D‐folate evolutionary model, although there is still much to learn.
AUTHOR CONTRIBUTIONS
Mark D. Lucock: Conceptualization (lead); visualization (lead); writing – original draft (lead); writing – review and editing (lead).
CONFLICT OF INTEREST
The author declares no potential conflict of interest.
ACKNOWLEDGMENTS
The author wishes to thank the important contribution made by so many PhD scholars and academic colleagues over the years. Notably, Patrice Jones, Zoe Yates, Emma Beckett, Charlotte Martin, Martin Veysey, John Furst, Nina Jablonski, George Chaplin, Manohar Garg, Robert Leeming, and many others stretching back several decades to the point at which I was first introduced to folic acid through the enlightened research of Professor Richard W Smithells. His seminal work led to the establishment of a clear link between folic acid deficiency and neural tube defects. Since the 1980s, vitamins, particularly folic acid have remained my main research interest through to, and beyond, my own retirement in 2019. Thanks to everyone, including many not named here who have shared their knowledge, inspired me, and supported my varied research endeavors. I would also like to thank the reviewers and editorial board members of the American Journal of Biological Anthropology who provided an extremely useful, constructive and supportive critique of my original submission. Open access publishing facilitated by The University of Newcastle, as part of the Wiley ‐ The University of Newcastle agreement via the Council of Australian University Librarians.
Lucock, M. D. (2023). The evolution of human skin pigmentation: A changing medley of vitamins, genetic variability, and UV radiation during human expansion. American Journal of Biological Anthropology, 180(2), 252–271. 10.1002/ajpa.24564
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no data sets were generated or analyzed during the current study.
REFERENCES
- Ashoori, M. , & Saedisomeolia, A. (2014). Riboflavin (vitamin B₂) and oxidative stress: A review. British Journal of Nutrition, 111(11), 1985–1991. 10.1017/S0007114514000178 [DOI] [PubMed] [Google Scholar]
- Batai, K. , Cui, Z. , Arora, A. , Shah‐Williams, E. , Hernandez, W. , Ruden, M. , Hollowell, C. M. P. , Hooker, S. E. , Bathina, M. , Murphy, A. B. , Bonilla, C. , & Kittles, R. A. (2021). Genetic loci associated with skin pigmentation in African Americans and their effects on vitamin D deficiency. PLoS Genetics, 17(2), e1009319. 10.1371/journal.pgen.1009319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett, E. L. , Duesing, K. , Martin, C. , Jones, P. , Furst, J. , King, K. , Niblett, S. , Yates, Z. , Veysey, M. , & Lucock, M. (2016). Relationship between methylation status of vitamin D‐related genes, vitamin D levels, and methyl‐donor biochemistry. Journal of Nutrition & Intermediary Metabolism, 6, 8–15. 10.1016/j.jnim.2016.04.010 [DOI] [Google Scholar]
- Beckett, E. L. , Jones, P. , Veysey, M. , Duesing, K. , Martin, C. , Furst, J. , Yates, Z. , Jablonski, N. G. , Chaplin, G. , & Lucock, M. (2017). VDR gene methylation as a molecular adaption to light exposure: Historic, recent and genetic influences. American Journal of Human Biology, 29(5). 10.1002/ajhb.23010 [DOI] [PubMed] [Google Scholar]
- Beleza, S. , Santos, A. M. , McEvoy, B. , Alves, I. , Martinho, C. , Cameron, E. , Shriver, M. D. , Parra, E. J. , & Rocha, J. (2013). The timing of pigmentation lightening in Europeans. Molecular Biology and Evolution, 30(1), 24–35. 10.1093/molbev/mss207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, M. R. , Bustos, D. , Pigati, J. S. , Springer, K. B. , Urban, T. M. , Holliday, V. T. , Reynolds, S. C. , Budka, M. , Honke, J. , Hudson, A. M. , Fenerty, B. , Connelly, C. , Martinez, P. J. , Santucci, V. L. , & Odess, D. (2021). Evidence of humans in North America during the last glacial maximum. Science, 373(6562), 1528–1531. 10.1126/science.abg7586 [DOI] [PubMed] [Google Scholar]
- Berlim, M. T. & Abeche, A. M. (2001). Evolutionary approach to medicine. South Med J, 94, 26–32. [PubMed] [Google Scholar]
- Blount, B. C. , Mack, M. M. , Wehr, C. M. , MacGregor, J. T. , Hiatt, R. A. , Wang, G. , Wickramasinghe, S. N. , Everson, R. B. , & Ames, B. N. (1997). Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: Implications for cancer and neuronal damage. Proceedings of the National Academy of Sciences of the United States of America, 94, 3290–3295. 10.1073/pnas.94.7.3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogh, M. K. , Schmedes, A. V. , Philipsen, P. A. , Thieden, E. , & Wulf, H. C. (2010). Vitamin D production after UVB exposure depends on baseline vitamin D and total cholesterol but not on skin pigmentation. The Journal of Investigative Dermatology, 130(2), 546–553. 10.1038/jid.2009.323 [DOI] [PubMed] [Google Scholar]
- Borradale, D. , Isenring, E. , Hacker, E. , & Kimlin, M. G. (2014). Exposure to solar ultraviolet radiation is associated with a decreased folate status in women of childbearing age. Journal of Photochemistry and Photobiology B, 131, 90–95. 10.1016/j.jphotobiol.2014.01.002 [DOI] [PubMed] [Google Scholar]
- Bouillon, R. , Carmeliet, G. , Verlinden, L. , van Etten, E. , Verstuyf, A. , Luderer, H. F. , Lieben, L. , Mathieu, C. , & Demay, M. (2008). Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocrine Reviews, 29(6), 726–776. 10.1210/er.2008-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxmeer, J. C. , Smit, M. , Utomo, E. , Romijn, J. C. , Eijkemans, M. J. , Lindemans, J. , Laven, J. S. , Macklon, N. S. , Steegers, E. A. , & Steegers‐Theunissen, R. P. (2009). Low folate in seminal plasma is associated with increased sperm DNA damage. Fertility and Sterility, 92(2), 548–556. 10.1016/j.fertnstert.2008.06.010 [DOI] [PubMed] [Google Scholar]
- Branda, R. F. & Eaton, J. W. (1978). Skin color and nutrient photolysis: an evolutionary hypothesis. Science, 201(4356), 625–626. 10.1126/science.675247. [DOI] [PubMed] [Google Scholar]
- Cabrera, S. , Benavente, D. , Alvo, M. , de Pablo, P. , & Ferro, C. J. (2014). Vitamin B12 deficiency is associated with geographical latitude and solar radiation in the older population. Journal of Photochemistry and Photobiology B, 140, 8–13. 10.1016/j.jphotobiol.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Cardoso, D. R. , Libardi, S. H. , & Skibsted, L. H. (2012). Riboflavin as a photosensitizer. Effects on human health and food quality. Food & Function, 3(5), 487–502. 10.1039/c2fo10246c [DOI] [PubMed] [Google Scholar]
- Chaplin, G. , & Jablonski, N. G. (2009). Vitamin D and the evolution of human depigmentation. American Journal of Physical Anthropology, 139(4), 451–461. 10.1002/ajpa.21079 [DOI] [PubMed] [Google Scholar]
- Chaplin, G. , & Jablonski, N. G. (2013). The human environment and the vitamin D compromise: Scotland as a case study in human biocultural adaptation and disease susceptibility. Human Biology, 85(4), 529–552. 10.3378/027.085.0402 [DOI] [PubMed] [Google Scholar]
- Choi, J. H. , Yates, Z. , Veysey, M. , Heo, Y. R. , & Lucock, M. (2014). Contemporary issues surrounding folic acid fortification initiatives. Preventive Nutrition and Food Science, 19(4), 247–260. 10.3746/pnf.2014.19.4.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford, N. G. , Kelly, D. E. , Hansen, M. E. B. , Beltrame, M. H. , Fan, S. , Bowman, S. L. , Jewett, E. , Ranciaro, A. , Thompson, S. , Lo, Y. , Pfeifer, S. P. , Jensen, J. D. , Campbell, M. C. , Beggs, W. , Hormozdiari, F. , Mpoloka, S. W. , Mokone, G. G. , Nyambo, T. , Meskel, D. W. , … Tishkoff, S. A. (2017). Loci associated with skin pigmentation identified in African populations. Science, 358(6365):eaan8433. 10.1126/science.aan8433. Erratum 2020: Science, 367 (6475). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, L. , & Xu, S. (2017). Adaptation of human skin color in various populations. Hereditas, 155(1), 1. 10.1186/s41065-017-0036-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopico, X. C. , Evangelou, M. , Ferreira, R. C. , Guo, H. , Pekalski, M. L. , Smyth, D. J. , Cooper, N. , Burren, O. S. , Fulford, A. J. , Hennig, B. J. , Prentice, A. M. , Ziegler, A. G. , Bonifacio, E. , Wallace, C. , & Todd, J. A. (2015). Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nature Communications, 6(7000). 10.1038/ncomms8000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie, S. J. (1999). Folic acid deficiency and cancer: Mechanisms of DNA instability. British Medical Bulletin, 55(3), 578–592. 10.1258/0007142991902646 [DOI] [PubMed] [Google Scholar]
- Elias, P. M. , & Williams, M. L. (2016). Basis for the gain and subsequent dilution of epidermal pigmentation during human evolution: The barrier and metabolic conservation hypotheses revisited. American Journal of Physical Anthropology, 161(2), 189–207. 10.1002/ajpa.23030 [DOI] [PubMed] [Google Scholar]
- Feng, Y. , McQuillan, M. A. , & Tishkoff, S. A. (2021). Evolutionary genetics of skin pigmentation in African populations. Human Molecular Genetics, 30(R1), R88–R97. 10.1093/hmg/ddab007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetahu, I. S. , Höbaus, J. , & Kállay, E. (2014). Vitamin D and the epigenome. Frontiers in Physiology, 5(164). 10.3389/fphys.2014.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friso, S. , Udali, S. , De Santis, D. , & Choi, S. W. (2017). One‐carbon metabolism and epigenetics. Molecular Aspects of Medicine, 54, 28–36. 10.1016/j.mam.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Graf, J. , Hodgson, R. , & van Daal, A. (2005). Single nucleotide polymorphisms in the MATP gene are associated with normal human pigmentation variation. Human Mutation, 25(3), 278–284. 10.1002/humu.20143 [DOI] [PubMed] [Google Scholar]
- Graves, J. L., Jr. (2021). Human biological variation and the "normal". American Journal of Human Biology, 33(5), e23658. 10.1002/ajhb.23658 [DOI] [PubMed] [Google Scholar]
- Grün, R. , Stringer, C. , McDermott, F. , Nathan, R. , Porat, N. , Robertson, S. , Taylor, L. , Mortimer, G. , Eggins, S. , & McCulloch, M. (2005). U‐series and ESR analyses of bones and teeth relating to the human burials from Skhul. Journal of Human Evolution, 49(3), 316–334. 10.1016/j.jhevol.2005.04.006 [DOI] [PubMed] [Google Scholar]
- Hakim, O. A. , Hart, K. , McCabe, P. , Berry, J. , Francesca, R. , Rhodes, L. E. , Spyrou, N. , Alfuraih, A. , & Lanham‐New, S. (2016). Vitamin D production in UKCaucasian and south Asian women following UVR exposure. The Journal of Steroid Biochemistry and Molecular Biology, 164, 223–229. 10.1016/j.jsbmb.2016.03.025 [DOI] [PubMed] [Google Scholar]
- Hanel, A. , & Carlberg, C. (2020a). Skin colour and vitamin D: An update. Experimental Dermatology, 29(9), 864–875. 10.1111/exd.14142 [DOI] [PubMed] [Google Scholar]
- Hanel, A. , & Carlberg, C. (2020b). Vitamin D and evolution: Pharmacologic implications. Biochemical Pharmacology, 173(113595), 113595. 10.1016/j.bcp.2019.07.024 [DOI] [PubMed] [Google Scholar]
- Hanel, A. , Malmberg, H. R. , & Carlberg, C. (2020). Genome‐wide effects of chromatin on vitamin D signaling. Journal of Molecular Endocrinology, 64(4), R45–R56. 10.1530/JME-19-0246 [DOI] [PubMed] [Google Scholar]
- Hasoun, L. Z. , Bailey, S. W. , Outlaw, K. K. , & Ayling, J. E. (2015). Rearrangement and depletion of folate in human skin by ultraviolet radiation. British Journal of Dermatology, 173(4), 1087–1090. 10.1111/bjd.13885 [DOI] [PubMed] [Google Scholar]
- Henn, B. M. , Cavalli‐Sforza, L. L. , & Feldman, M. W. (2012). The great human expansion. Proceedings of the National Academy of Sciences USA, 109(44), 17758–17764. 10.1073/pnas.1212380109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi, S. , Motohashi, Y. , Ishibashi, K. , & Maeda, T. (2007). Influence of eye colors of Caucasians and Asians on suppression of melatonin secretion by light. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 292(6), R2352–R2356. 10.1152/ajpregu.00355.2006 [DOI] [PubMed] [Google Scholar]
- Hoeijmakers, J. H. (2009). DNA damage, aging, and cancer. New England Journal of Medicine, 361(15), 1475‐1485. 10.1056/NEJMra0804615. Erratum in: New England Journal of Medicine, 361 (19), 1914. [DOI] [PubMed] [Google Scholar]
- Holick, M. F. (2012). The D‐lightful vitamin D for child health. Journal of Parenteral and Enteral Nutrition., 36(1), 9S–19S. 10.1177/0148607111430189 [DOI] [PubMed] [Google Scholar]
- Hublin, J.‐J. , Ben‐Ncer, A. , Bailey, S. E. , Freidline, S. E. , Neubauer, S. , Skinner, M. M. , Bergmann, I. , Le Cabes, A. , Benazzi, S. , Harvati, K. , & Gunz, P. (2017). New fossils from Jebel Irhoud, Morocco and the pan‐African origin of Homo sapiens. Nature, 546(7657), 289–292. 10.1038/nature22336 [DOI] [PubMed] [Google Scholar]
- Jablonski, N. G. (2021). The evolution of human skin pigmentation involved the interactions of genetic, environmental, and cultural variables. Pigment Cell and Melanoma Research, 34(4), 707–729. 10.1111/pcmr.12976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski, N. G. , & Chaplin, G. (2000). The evolution of human skin coloration. Journal of Human Evolution, 39(1), 57–106. 10.1006/jhev.2000.0403 [DOI] [PubMed] [Google Scholar]
- Jablonski, N. G. , & Chaplin, G. (2010). Colloquium paper: Human skin pigmentation as an adaptation to UV radiation. Proceedings of the National Academy of Sciences U S A, 107(2), 8962–8968. 10.1073/pnas.0914628107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski, N. G. , & Chaplin, G. (2017). The colours of humanity: The evolution of pigmentation in the human lineage. Philosophical Transactions of the Royal Society B, 372(1724), 20160349. 10.1098/rstb.2016.0349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon, S. M. , & Shin, E. A. (2018). Exploring vitamin D metabolism and function in cancer. Experimental & Molecular Medicine, 50(4), 1–14. 10.1038/s12276-018-0038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, P. , Beckett, E. , Yates, Z. , Veysey, M. , & Lucock, M. (2016). Converging evolutionary, environmental and clinical ideas on folate metabolism. Exploratory Research and Hypothesis in Medicine, 1(3), 34–41. 10.14218/ERHM.2016.00003b [DOI] [Google Scholar]
- Jones, P. , Lucock, M. , Chaplin, G. , Jablonski, N. G. , Veysey, M. , Scarlett, C. , & Beckett, E. (2020). Distribution of variants in multiple vitamin D‐related loci (DHCR7/NADSYN1, GC, CYP2R1, CYP11A1, CYP24A1, VDR, RXRα and RXRγ) vary between European, east‐Asian and sub‐Saharan African‐ancestry populations. Genes & Nutrition, 15(1), 5. 10.1186/s12263-020-00663-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, P. , Lucock, M. , Veysey, M. , & Beckett, E. (2018). The vitamin D‐folate hypothesis as an evolutionary model for skin pigmentation: An update and integration of current ideas. Nutrients, 10(5), 554. 10.3390/nu10050554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, P. , Lucock,M., Veysey, M. , Jablonski, N. , Chaplin, G. , & Beckett, E. (2018). Frequency of folate‐related polymorphisms varies by skin pigmentation. American Journal of Human Biology, 30(2). 10.1002/ajhb.23079, e23079. [DOI] [PubMed] [Google Scholar]
- Ju, D. , & Mathieson, I. (2021). The evolution of skin pigmentation‐associated variation in West Eurasia. Proceedings of the National Academy of Sciences USA, 118(1), e2009227118. 10.1073/pnas.2009227118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, P. , McPartlin, J. , Goggins, M. , Weir, D. G. , & Scott, J. M. (1997). Unmetabolized folic acid in serum: Acute studies in subjects consuming fortified food and supplements. American Journal of Clinical Nutrition, 65(6), 1790–1795. 10.1093/ajcn/65.6.1790 [DOI] [PubMed] [Google Scholar]
- Laeng, B. , Mathisen, R. , & Johnsen, J. A. (2007). Why do blue‐eyed men prefer women with the same eye colour. Behavioral Ecology and Sociobiology, 61, 371–384. 10.1007/s00265-006-0266-1 [DOI] [Google Scholar]
- Lao, O. , de Gruijter, J. M. , van Duijn, K. , Navarro, A. , & Kayser, M. (2007). Signatures of positive selection in genes associated with human skin pigmentation as revealed from analyses of single nucleotide polymorphisms. Annals of Human Genetics, 71(3), 354–369. 10.1111/j.1469-1809.2006.00341.x [DOI] [PubMed] [Google Scholar]
- Littleton, J. (1991). Vitamin D deficiency rickets: Prevalence in early societies. In Bruce N. W. (Ed.), Living with civilisation: Proceedings of the Australasian Society for Human Biology (pp. 15–21). Centre for Human Biology, Australian National University. [Google Scholar]
- Liu, W. , Martinón‐Torres, M. , Cai, Y. J. , Xing, S. , Tong, H. W. , Pei, S. W. , Sier, M. J. , Wu, X. H. , Edwards, R. L. , Cheng, H. , Li, Y. Y. , Yang, X. X. , de Castro, J. M. , & Wu, X. J. (2015). The earliest unequivocally modern humans in southern China. Nature, 526(7575), 696–699. 10.1038/nature15696 [DOI] [PubMed] [Google Scholar]
- Lucock, M. (2000). Folic acid: Nutritional biochemistry, molecular biology, and role in disease processes. Molecular Genetics & Metabolism, 71(1–2), 121–138. 10.1006/mgme.2000.3027 [DOI] [PubMed] [Google Scholar]
- Lucock, M. (2021). Vitamin‐related phenotypic adaptation to exposomal factors: The folate‐vitamin D‐exposome triad. Molecular Aspects of Medicine, 100944. 10.1016/j.mam.2021.100944 [DOI] [PubMed] [Google Scholar]
- Lucock, M. , Beckett, E. , Martin, C. , Jones, P. , Furst, J. , Yates, Z. , Jablonski, N. G. , Chaplin, G. , & Veysey, M. (2017). UV‐associated decline in systemic folate: Implications for human nutrigenetics, health, and evolutionary processes. American Journal of Human Biology, 29(2). 10.1002/ajhb.22929 [DOI] [PubMed] [Google Scholar]
- Lucock, M. , Jones, P. , Martin, C. , Beckett, E. , Yates, Z. , Furst, J. , & Veysey, M. (2015). Vitamin D: Beyond metabolism. Journal of Evidence‐Based Complementary and Alternative Medicine, 20(4), 310–322. 10.1177/2156587215580491 [DOI] [PubMed] [Google Scholar]
- Lucock, M. , Jones, P. , Martin, C. , Yates, Z. , Veysey, M. , Furst, J. , & Beckett, E. (2018). Photobiology of vitamins. Nutrition Reviews, 76(7), 512–525. 10.1093/nutrit/nuy013 [DOI] [PubMed] [Google Scholar]
- Lucock, M. , & Yates, Z. (2009). Folic acid fortification: A double‐edged sword. Current Opinion in Clinical Nutrition & Metabolic Care, 12(6), 555–564. 10.1097/MCO.0b013e32833192bc [DOI] [PubMed] [Google Scholar]
- Lucock, M. D. (2006). Synergy of genes and nutrients: The case of homocysteine. Current Opinion in Clinical Nutrition & Metabolic Care, 9(6), 748–756. 10.1097/01.mco.0000247468.18790.1e [DOI] [PubMed] [Google Scholar]
- Lucock, M. D. , Jones, P. R. , Veysey, M. , Thota, R. , Garg, M. , Furst, J. , Martin, C. , Yates, Z. , Scarlett, C. J. , Jablonski, N. G. , Chaplin, G. , & Beckett, E. L. (2021). Biophysical evidence to support and extend the vitamin D‐folate hypothesis as a paradigm for the evolution of human skin pigmentation. American Journal of Human Biology, e23667, e23667. 10.1002/ajhb.23667 [DOI] [PubMed] [Google Scholar]
- Lucock, M. D. , Martin, C. E. , Yates, Z. R. , & Veysey, M. (2014). Diet and our genetic legacy in the recent anthropocene: A Darwinian perspective to nutritional health. Journal of Evidence‐Based Complementary and Alternative Medicine, 19(1), 68–83. 10.1177/2156587213503345 [DOI] [PubMed] [Google Scholar]
- Lucock, M. D. , Wild, J. , Smithells, R. W. , & Hartley, R. (1989). In vivo characterization of the absorption and biotransformation of pteroylmonoglutamic acid in man: A model for future studies. Biochemical Medicine & Metabolic Biology, 42(1), 30–42. 10.1016/0885-4505(89)90038-8 [DOI] [PubMed] [Google Scholar]
- Lucock, M. D. , Yates, Z. , Glanville, T. , Leeming, R. J. , Simpson, N. A. , & Daskalakis, I. (2003). A critical role for B‐vitamin nutrition in human developmental and evolutionary biology. Nutrition Research, 23, 1463–1475. 10.1016/S0271-5317(03)00156-8 [DOI] [Google Scholar]
- Martin, A. R. , Lin, M. , Granka, J. M. , Myrick, J. W. , Liu, X. , Sockell, A. , Atkinson, E. G. , Werely, C. J. , Möller, M. , Sandhu, M. S. , Kingsley, D. M. , Hoal, E. G. , Liu, X. , Daly, M. J. , Feldman, M. W. , Gignoux, C. R. , Bustamante, C. D. , & Henn, B. M. (2017). An unexpectedly complex architecture for skin pigmentation in Africans. Cell, 171(6), 1340–1353.e14. 10.1016/j.cell.2017.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, C. E. , Veysey, M. , Yates, Z. R. , & Lucock, M. (2014). Vitamin D: Genetics, environment & health. Journal of Food and Nutritional Disorders, 3(5). 10.4172/2324-9323.1000155 [DOI] [Google Scholar]
- Mays, S. , Brickley, M. , & Ives, R. (2006). Skeletal manifestations of rickets in infants and young children in a historic population from England. American Journal of Physical Anthropology, 129(3), 362–374. 10.1002/ajpa.20292 [DOI] [PubMed] [Google Scholar]
- McDougall, I. , Brown, F. H. , & Fleagle, J. G. (2005). Stratigraphic placement and age of modern humans from Kibish, Ethiopia. Nature, 433(7027), 733–736. 10.1038/nature03258 [DOI] [PubMed] [Google Scholar]
- Mosharov, E. , Cranford, M. R. , & Banerjee, R. (2000). The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry, 39(42), 13005–13011. 10.1021/bi001088w [DOI] [PubMed] [Google Scholar]
- MRC Vitamin Study Research Group . (1991). MRC vitamin study research group prevention of neural tube defects: Results of the Medical Research Council vitamin study. Lancet, 338(8760), 131–137. [PubMed] [Google Scholar]
- Neme, A. , Seuter, S. , & Carlberg, C. (2017). Selective regulation of biological processes by vitamin D based on the spatio‐temporal cistrome of its receptor. Biochimica Biophysica Acta Gene Regulatory Mechanisms, 1860(9), 952–961. 10.1016/j.bbagrm.2017.07.002 [DOI] [PubMed] [Google Scholar]
- Neme, A. , Seuter, S. , Malinen, M. , Nurmi, T. , Tuomainen, T. P. , Virtanen, J. K. , & Carlberg, C. (2019). In vivo transcriptome changes of human white blood cells in response to vitamin D. Journal of Steroid Biochemistry and Molecular Biology, 188, 71–76. 10.1016/j.jsbmb.2018.11.019 [DOI] [PubMed] [Google Scholar]
- Nielsen, R. , Akey, J. M. , Jakobsson, M. , Pritchard, J. K. , Tishkoff, S. , & Willerslev, E. (2017). Tracing the peopling of the world through genomics. Nature, 541(7637), 302–310. 10.1038/nature21347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton, H. L. (2020). The color of normal: How a Eurocentric focus erases pigmentation complexity. American Journal of Human Biology, Early View, 33, e23554. 10.1002/ajhb.23554 [DOI] [PubMed] [Google Scholar]
- Olalde, I. , Allentoft, M. E. , Sánchez‐Quinto, F. , Santpere, G. , Chiang, C. W. , DeGiorgio, M. , Prado‐Martinez, J. , Rodríguez, J. A. , Rasmussen, S. , Quilez, J. , Ramírez, O. , Marigorta, U. M. , Fernández‐Callejo, M. , Prada, M. E. , Encinas, J. M. , Nielsen, R. , Netea, M. G. , Novembre, J. , Sturm, R. A. , … Lalueza‐Fox, C. (2014). Derived immune and ancestral pigmentation alleles in a 7,000‐year‐old Mesolithic European. Nature, 507(7491), 225–228. 10.1038/nature12960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkan, S. , Jindal, S. , Greenseid, K. , Shu, J. , Zeitlian, G. , Hickmon, C. , & Pal, L. (2010). Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertility and Sterility, 94(4), 1314–1319. 10.1016/j.fertnstert.2009.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani, L. , Kivisild, T. , Tarekegn, A. , Ekong, R. , Plaster, C. , Gallego Romero, I. , Ayub, Q. , Mehdi, S. Q. , Thomas, M. G. , Luiselli, D. , Bekele, E. , Bradman, N. , Balding, D. J. , & Tyler‐Smith, C. (2012). Ethiopian genetic diversity reveals linguistic stratification and complex influences on the Ethiopian gene pool. American Journal of Human Genetics, 91(1), 83–96. 10.1016/j.ajhg.2012.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan, W. J. , & Sturm, R. A. (2019). The genetics of human skin and hair pigmentation. Annual Review of Genomics and Human Genetics, 20, 41–72. 10.1146/annurev-genom-083118-015230 [DOI] [PubMed] [Google Scholar]
- Pennisi, E. (2011). Evolution. Darwinian medicine's drawn‐out dawn. Science, 334(6062), 1486–1487. 10.1126/science.334.6062.1486 [DOI] [PubMed] [Google Scholar]
- Pike, J. W. , & Meyer, M. B. (2012). The vitamin D receptor: New paradigms for the regulation of gene expression by 1,25‐dihydroxyvitamin D3. Rheumatic Disease Clinics of North America, 38(1), 13–27. 10.1016/j.rdc.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillen, E. E. , Bauchet, M. , Bigham, A. W. , Delgado‐Burbano, M. E. , Faust, F. X. , Klimentidis, Y. C. , Mao, X. , Stoneking, M. , & Shriver, M. D. (2012). OPRM1 and EGFR contribute to skin pigmentation differences between indigenous Americans and Europeans. Human Genetics, 131(7), 1073–1080. 10.1007/s00439-011-1135-1 [DOI] [PubMed] [Google Scholar]
- Quillen, E. E. , Foster, J. , Sheldrake, A. , Jablonski, N. G. , & Shriver, M. D. (2017). The role of FZD6 in the evolution of tanning response in the Americas. American Journal of Physical Anthropology, 162(S64), 323–423. 10.1002/ajpa.23210 [DOI] [Google Scholar]
- Quillen, E. E. , Norton, H. L. , Parra, E. J. , Lona‐Durazo, F. , Ang, K. C. , Illiescu, F. M. , Pearson, L. N. , Shriver, M. D. , Lasisi, T. , Gokcumen, O. , Starr, I. , Lin, Y. L. , Martin, A. R. , & Jablonski, N. G. (2018). Shades of complexity: New perspectives on the evolution and genetic architecture of human skin. American Journal of Physical Anthropology, 168(67), 4–26. 10.1002/ajpa.23737 [DOI] [PubMed] [Google Scholar]
- Quillen, E. E. , & Shriver, M. D. (2011). Unpacking human evolution to find the genetic determinants of human skin pigmentation. Journal of Investigative Dermatology, 131(1), 5–7. 10.1038/skinbio.2011.3 [DOI] [PubMed] [Google Scholar]
- Ramachandran, S. , Deshpande, O. , Roseman, C. C. , Rosenberg, N. A. , Feldman, M. W. , & Cavalli‐Sforza, L. L. (2005). Support from the relationship of genetic and geographic distance in human populations for a serial founder effect originating in Africa. Proceedings of the National Academy of Sciences USA, 102(44), 15942–15947. 10.1073/pnas.0507611102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees, J. L. (2004). The genetics of sun sensitivity in humans. American Journal of Human Genetics, 75(5), 739–751. 10.1086/425285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins, A. H. (1991). Biological perspectives on human pigmentation. Cambridge University Press. [Google Scholar]
- Rocha, J. (2020). The evolutionary history of human skin pigmentation. Journal of Molecular Evolution, 88(1), 77–87. 10.1007/s00239-019-09902-7 [DOI] [PubMed] [Google Scholar]
- Román‐Franco, A. A. (2009). Medicine in the 21st century: Towards a Darwinian medical epistemology. Puerto Rico Health Sciences Journal, 28(4), 345–351. [PubMed] [Google Scholar]
- Rosenberg, N. A. , Pritchard, J. K. , Weber, J. L. , Cann, H. M. , Kidd, K. K. , Zhivotovsky, L. A. , & Feldman, M. W. (2002). Genetic structure of human populations. Science, 298(5602), 2381–2385. 10.1126/science.1078311 [DOI] [PubMed] [Google Scholar]
- Schallreuter, K. U. , Kothari, S. , Chavan, B. , & Spencer, J. D. (2008). Regulation of melanogenesis—controversies and new concepts. Experimental Dermatology, 17(5), 95–404. 10.1111/j.1600-0625.2007.00675.x [DOI] [PubMed] [Google Scholar]
- Selhub, J. (2002). Folate, vitamin B12 and vitamin B6 and one carbon metabolism. Journal of Nutrition, Health & Aging, 6(1), 39–42. [PubMed] [Google Scholar]
- Shane, B. (2008). Folate and vitamin B12 metabolism: Overview and interaction with riboflavin, vitamin B6, and polymorphisms. Food and Nutrition Bulletin, 29(2), S5–S16. 10.1177/15648265080292S103 [DOI] [PubMed] [Google Scholar]
- Shaw, N. J. (2003). Vitamin D deficiency rickets. In Hochberg Z. E. (Ed.), Vitamin D and rickets (pp. 93–104). Karger. [DOI] [PubMed] [Google Scholar]
- Shi, W. , Meininger, C. J. , Haynes, T. E. , Hatakeyama, K. , & Wu, G. (2004). Regulation of tetrahydrobiopterin synthesis and bioavailability in endothelial cells. Cell Biochemistry and Biophysics, 41(3), 415–434. 10.1385/CBB:41:3:415 [DOI] [PubMed] [Google Scholar]
- Shih, B. B. , Farrar, M. D. , Vail, A. , Allan, D. , Chao, M. R. , Hu, C. W. , Jones, G. D. D. , Cooke, M. S. , & Rhodes, L. E. (2020). Influence of skin melanisation and ultraviolet radiation on biomarkers of systemic oxidative stress. Free Radical Biology & Medicine, 160, 40–46. 10.1016/j.freeradbiomed.2020.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithells, R. W. , Nevin, N. C. , Seller, M. J. , Sheppard, S. , Harris, R. , Read, A. P. , Fielding, D. W. , Walker, S. , Schorah, C. J. , & Wild, J. (1983). Further experience of vitamin supplementation for prevention of neural tube defect recurrences. Lancet, 1(8332), 1027–1031. 10.1016/s0140-6736(83)92654-5 [DOI] [PubMed] [Google Scholar]
- Sohn, K. J. , Jang, H. , Campan, M. , Weisenberger, D. J. , Dickhout, J. , Wang, Y. C. , Cho, R. C. , Yates, Z. , Lucock, M. , Chiang, E. P. , Austin, R. C. , Choi, S. W. , Laird, P. W. , & Kim, Y. I. (2009). The methylenetetrahydrofolate reductase C677T mutation induces cell‐specific changes in genomic DNA methylation and uracil misincorporation: A possible molecular basis for the site‐specific cancer risk modification. International Journal of Cancer, 124(9), 1999–2005. 10.1002/ijc.24003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steindal, A. H. , Tam, T. T. , Lu, X. Y. , Juzeniene, A. , & Moan, J. (2008). 5‐Methyltetrahydrofolate is photosensitive in the presence of riboflavin. Photochemical & Photobiological Sciences, 7(7), 814–818. 10.1039/b718907a [DOI] [PubMed] [Google Scholar]
- Sturm, R. A. (2002). Skin colour and skin cancer ‐ MC1R, the genetic link. Melanoma Research, 12(5), 405–416. 10.1097/00008390-200209000-00001 [DOI] [PubMed] [Google Scholar]
- Sturm, R. A. (2009). Molecular genetics of human pigmentation diversity. Human Molecular Genetics, 18(1), R9–R17. 10.1093/hmg/ddp003 [DOI] [PubMed] [Google Scholar]
- Sturm, R. A. , & Duffy, D. L. (2012). Human pigmentation genes under environmental selection. Genome Biology, 13(9), 248. 10.1186/gb-2012-13-9-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm, R. A. , Duffy, D. L. , Box, N. F. , Chen, W. , Smit, D. J. , Brown, D. L. , Stow, J. L. , Leonard, J. H. , & Martin, N. G. (2003). The role of melanocortin‐1 receptor polymorphism in skin cancer risk phenotypes. Pigment Cell Research, 16(3), 266–272. 10.1034/j.1600-0749.2003a.00041.x [DOI] [PubMed] [Google Scholar]
- Sturm, R. A. , Duffy, D. L. , Box, N. F. , Newton, R. A. , Shepherd, A. G. , Chen, W. , Marks, L. H. , Leonard, J. H. , & Martin, N. G. (2003). Genetic association and cellular function of MC1R variant alleles in human pigmentation. Annals of the New York Academy of Sciences, 994, 348–358. 10.1111/j.1749-6632.2003b.tb03199.x [DOI] [PubMed] [Google Scholar]
- Sturm, R. A. , Teasdale, R. D. , & Fox, N. F. (2001). Human pigmentation genes: Identification, structure and consequences of polymorphic variation. Gene, 277(1–2), 49–62. 10.1016/S0378-1119(01)00694-1 [DOI] [PubMed] [Google Scholar]
- Tamura, T. , & Picciano, M. F. (2006). Folate and human reproduction. American Journal of Clinical Nutrition, 83(5), 993–1016. 10.1093/ajcn/83.5.993 [DOI] [PubMed] [Google Scholar]
- Tishkoff, S. A. , Reed, F. A. , Friedlaender, F. R. , Ehret, C. , Ranciaro, A. , Froment, A. , Hirbo, J. B. , Awomoyi, A. A. , Bodo, J. M. , Doumbo, O. , Ibrahim, M. , Juma, A. T. , Kotze, M. J. , Lema, G. , Moore, J. H. , Mortensen, H. , Nyambo, T. B. , Omar, S. A. , Powell, K. , … Williams, S. M. (2009). The genetic structure and history of Africans and African Americans. Science, 324(5930), 1035–1044. 10.1126/science.1172257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieth, R. (2001). Would prehistoric human 25‐hydroxyvitamin D concentrations be beneficial, and how much vitamin D do we need to ensure desirable nutritional targets? In Burckhardt P., Dawson‐Hughes B., & Heaney R. P. (Eds.), Nutritional aspects of osteoporosis (pp. 173–195). Academic Press. [Google Scholar]
- Webb, A. R. , & Holick, M. F. (1988). The role of sunlight in the cutaneous production of vitamin D3. Annual Review of Nutrition, 8, 375–399. 10.1146/annurev.nu.08.070188.002111 [DOI] [PubMed] [Google Scholar]
- White, T. D. , Asfaw, B. , DeGusta, D. , Gilbert, H. , Richards, G. D. , Suwa, G. , & Howell, F. C. (2003). Pleistocene Homo sapiens from middle awash, Ethiopia. Nature, 423(6941), 742–747. 10.1038/nature01669 [DOI] [PubMed] [Google Scholar]
- Wilde, S. , Timpson, A. , Kirsanow, K. , Kaiser, E. , Kayser, M. , Unterländer, M. , Hollfelder, N. , Potekhina, I. D. , Schier, W. , Thomas, M. G. , & Burger, J. (2014). Direct evidence for positive selection of skin, hair, and eye pigmentation in Europeans during the last 5,000 y. Proceedings of the National Academy of Sciences USA, 111(13), 4832–4837. 10.1073/pnas.1316513111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, S. T. , Stanhewicz, A. E. , Jablonski, N. G. , & Kenney, W. L. (2018). Acute ultraviolet radiation exposure attenuates nitric oxide‐mediated vasodilation in the cutaneous microvasculature of healthy humans. Journal of Applied Physiology, 125(4), 1232–1237. 10.1152/japplphysiol.00501.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, A. R. , Morgan, K. A. , Ho, T. W. , Ojimba, N. , Harrison, G. I. , Lawrence, K. P. , Jakharia‐Shah, N. , Wulf, H. C. , Cruickshank, J. K. , & Philipsen, P. A. (2020). Melanin has a small inhibitory effect on cutaneous vitamin D synthesis: A comparison of extreme phenotypes. The Journal of Investigative Dermatology, 140(7), 1418–1426.e1. 10.1016/j.jid.2019.11.019 [DOI] [PubMed] [Google Scholar]
- Zhou, S. , Zeng, H. , Huang, J. , Lei, L. , Tong, X. , Li, S. , Zhou, Y. , Guo, H. , Khan, M. , Luo, L. , Xiao, R. , Chen, J. , & Zeng, Q. (2021). Epigenetic regulation of melanogenesis. Ageing Research Reviews, 69, 101349. 10.1016/j.arr.2021.101349 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no data sets were generated or analyzed during the current study.


