Abstract
Introduction
The mandibular profile undergoes progressive wasting with aging, and the deepening of nasolabial folds (NLFs) has a leading role. Hyaluronic acid (HA) efficiently controls tissue hydration and permeability to small and large molecules. NLFs are an acknowledged HA target; at the same time, another class of agents, PN‐HPT® (Polynucleotides Highly Purified Technology), enjoy growing acknowledgement in aesthetic medicine. This exploratory, prospective study probed the rationale of sequentially associating PN‐HPT® as a first priming agent acting in the skin followed by HA dermal filler injections for correcting moderate to severe NLFs.
Methods
Following strict inclusion and exclusion criteria, the authors screened Caucasian ambulatory women aged 40–65 with moderate to severe NLFs and randomly selected two NLFs for each enrolled woman. Due to the purely explorative nature of the study, the authors initially planned to enroll no >10 women. According to a split‐face design, the selected right‐side NLFs received 4 ml of PN‐HPT® intradermally in the initial priming phase (“NLF Rx group”); the selected left‐side NLFs received 4 ml of saline (placebo) (“NLF Lx group”). After 3 and 6 weeks, all patients received 2 ml of subdermal cross‐linked HA over both NLF areas (4 ml overall). The total study follow‐up was 6 months after the first injection, with objective assessments, based on the qualitative and quantitative Antera 3D® and Vectra H2® skin imaging technologies, after 6 weeks and 3 and 6 months.
Results
Because of the favorable early outcomes, the authors let enrollment progress between January and June 2020 up to a total of 20 women and 40 NLFs. All treated women completed the six‐month follow‐up without reporting side effects, even clinically minor. The Antera 3D® device demonstrated that wrinkles and skin texture significantly improved in the NLF Rx after 6 weeks (monotherapy phase) and 3 and 6 months (PN‐HPT® priming + HA phase) compared with baseline. HA levels, measured with the quantitative Vectra H2® assessment technology in the right NLFs, were significantly higher than contralaterally at both 3 and 6 months.
Conclusions
Although conceived only as an exploratory investigation, the study confirmed that PN‐HPT® monotherapy might be a valuable and effective option to rapidly improve the skin dermis texture and quality in individuals with moderate to severe NLFs. Acting as a priming agent in the skin, PN‐HPT® prolong the clinical efficacy of cross‐linked HA. Well‐designed trials in larger treatment groups will hopefully confirm these early promising results.
Keywords: hyaluronic acid, nasolabial folds, PN‐HPT®, polynucleotides highly purified technology
1. INTRODUCTION
Facial aging modifications involve all skin layers. 1 , 2 , 3 The dermal atrophy and depletion of deep fat and sub‐epidermal elastin fiber network together contribute to the progressive wasting of the mandibular profile and the deepening of nasolabial folds (NLFs). 4 , 5
Hyaluronic acid (HA) is crucial for skin hydration and permeability, and exogenous HA helpfully contributes to maintaining the mechanical properties and immune function of the skin. 6 , 7 , 8 , 9 When injected alone or with additional molecules such as polynucleotides, antioxidants, and glycosaminoglycans precursors, exogenous HA consistently associates with an improved wound healing process and better tissue regeneration after burns or ulcers in vitro. 10
Polynucleotides Highly Purified Technology (PN‐HPT®) are DNA polymers extracted from male salmon trout gonads thanks to advanced purification and high‐temperature sterilization procedures. PN‐HPT® have been used in aesthetic medicine in monotherapy, in combination with HA, or associated with CO2 laser for treating stretch marks. 11 , 12 , 13 PN‐HPT® showed valuable in several clinical studies for treating lower limb venous ulcers, postmenopausal atrophic labia majora, and knee osteoarthritis by intra‐articular infiltration. 14 , 15 , 16
In this pilot study, the authors explored the value, so far untested, of sequential PN‐HPT® and HA dermal filler injections for correction of moderate to severe NLFs.
2. METHODS
The authors performed their study according to the ethical principles of the Declaration of Helsinki and the Good Clinical Practice principles.
2.1. Study population
The original study protocol planned to enlist, under full insurance coverage, a small exploratory cohort of no >10 subjects among the women who attended the authors' private ambulatory practice in Milan and met the inclusion criteria. Before treatment, the women had to sign an informed consent form, stop using cosmetic and sunscreen creams and make‐up, and avoid sun exposure for 4 weeks before and during the study period.
The inclusion criteria were: Caucasian ethnicity, age between 40 and 65 years old and severe NLFs (scores between seven and nine, validated nine‐score NLF Severity Scale or NLFSS) (Table 1). 17 Exclusion criteria were male gender, smoking habit, any anti‐wrinkle or volumizing facial treatment over the last 12 months, current clinically severe or autoimmune disease, active inflammation or infection involving the injection areas, tendency to develop hypertrophic scars or keloids, current antiplatelet or anticoagulant therapy, and alcohol abuse.
TABLE 1.
Nine‐point nasolabial folds severity scale (NLFSS)
| Wrinkle descriptors | Score |
|---|---|
| Very shallow or lines yet visible wrinkle | 1 |
| Just visible wrinkle, like hazy crease | 2 |
| Visible wrinkle, like light clear crease | 3 |
| Clearly visible wrinkle | 4 |
| Clearly visible wrinkle and well‐defined edges | 5 |
| Moderately deep wrinkle | 6 |
| Deep wrinkle and carven edges | 7 |
| Deep and prominent wrinkle with furrow | 8 |
| Redundant folds | 9 |
2.2. Study design
The split‐face design followed by the authors called for the random selection of two NLFs over the right‐side hemiface and two NLFs over the left‐side hemiface. The cumulated right‐side NLFs of all women (“NLF Rx group”) were the active group—treated with 2 ml of intradermal PN‐HPT® formulated as a Class III CE 0373 medical device (Plinest®, Mastelli S.r.l., Sanremo, Italy) as monotherapy and priming agent before hyaluronic acid as a consolidator. The cumulated left‐side NLFs of all women (“NLF Lx group”), the control group, received 2 ml of normal saline (placebo). Treatment technique over the two split‐face sides: injection of 10 micro‐drops (0.2 ml at each injection point) delivered with 30G, 8‐mm needles without further manipulation, including touching, of the treated area. No medication was planned and needed after treatment.
After 3 weeks, the protocol prescribed repeating the same PN‐HPT® priming or placebo treatment schedule. In the end, the two NLFs in the NLF Rx group received an overall 4 ml of intradermal PN‐HPT®; the two NLFs in the NLF Lx group received an overall 4 ml of intradermal saline.
In the third session, after three more weeks, all women received subdermally 2 ml of cross‐linked HA formulated as a Class III CE 0373 medical device (Triplest® n. 3‐Volume, Mastelli S.r.l.)—1 ml over the right NLF area and 1 ml over the left NLF area, delivered with a 25G, and 38‐mm cannula and the retrograde technique. Once again, no medication after treatment was required, and the women were asked not to touch the treated area.
After three more weeks, the women repeated the treatment schedule in the fourth treatment session—2 ml of subdermal cross‐linked HA over the right NLF area and 2 ml of subdermal cross‐linked HA over the left NLF area. Figure 1 summarizes the timing of split‐face treatments and assessments.
FIGURE 1.
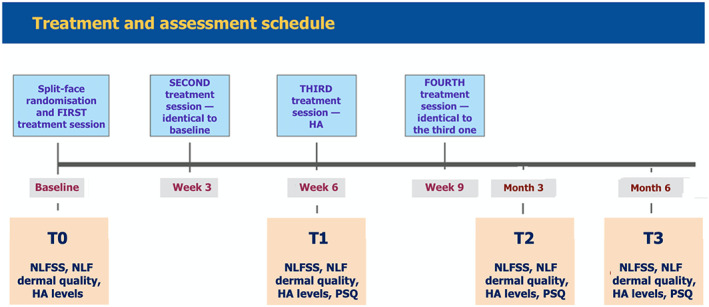
Schedule of split‐face exploratory treatments and assessments from baseline screening and randomization of candidate women and first treatment session (T0) up to the last assessment visit after six months (T3)
2.3. Efficacy assessments
The authors took digital photographs of treated nasolabial areas in a unique center, with women in standard positions under steady lighting, using the same camera (Nikon Corporation, Tokyo, Japan; D7100, 12.0 megapixels, AF‐5 micro‐Nikkor 60 mm, close‐up 4D–62 mm + Nital Macro Lighting Spider). The Antera 3D® imaging device, already validated for the qualitative and quantitative analysis of skin texture and wrinkles (Miravex Limited, Dublin, Ireland), also allowed to estimate the hemoglobin (Hb) levels in NLF dermal vessels and their variations over time as a proxy of changes in tissue vascularity and microcirculation efficiency. 18 , 19 The Vectra H2® software (Canfield Scientific, Parsippany, NJ, USA), providing optimal resolution, focus distance, and three‐dimensional biomechanical evaluation, allowed to quantify the soft tissue changes over the study period and provided quantitative information about the degree of lift, compression, and stretch. 20
Finally, at the assessment visits, the women filled an anonymous 10‐score patient's satisfaction questionnaire (PSQ) to assess their satisfaction when allowed to compare the photographs of their nasolabial areas taken before and after treatment (Table 2). The impromptu questionnaire, designed by the authors and their academic research groups and currently undergoing validation studies, is no novelty as already used in a 2021 study in subjects with acne scars. 21
TABLE 2.
Descriptors of the impromptu patient satisfaction questionnaire (PSQ) developed by the authors and their academic research groups and used in the study
| PSQ descriptor | Score |
|---|---|
| I look worst then before | 0 |
| I can not see any difference before and after—My relatives do not notice any difference | 1 |
| I can see minimal difference before and after—My relatives do not notice any difference | 2 |
| I can see moderate difference before and after—My relatives notice a minimal difference | 4 |
| I can see moderate difference before and after—My relatives notice a moderate difference | 6 |
| I can see good difference before and after—My relatives notice a moderate difference | 8 |
| Beyond my expectation—All my relatives notice a great improvement | 10 |
The exploratory study protocol prescribed a series of repeated assessments after 6 weeks (T1) and after three (T2) and 6 months (T3):
2.3.1. Primary efficacy endpoint and timing
Quality of NLF dermis changes (wrinkles, texture, and mean Hb dermal levels; Antera 3D® assessment) at T1 after PN‐HPT® injection vs placebo.
2.3.2. Secondary efficacy endpoints and timing
NLF dermal quality: assessed quantitatively (Antera® 3D technology) at T1 (direct PN‐HPT® monotherapy effect) and T2 and T3 in the NLF Rx group after PN‐HPT® priming and the NLF Lx group, which received the saline placebo, after treatment with subdermal cross‐linked HA. Assessment by an independent physician with the NLF Severity Score (NLFSS); comparison vs baseline and NLF Lx group.
Direct PN‐HPT® monotherapy and priming effect in the dermis of NLF areas in terms of length of the voluminising effect by the dermal filler gel (quantitative assessment with the Vectra H2® technology) at T1, T2, and T3.
Women's PSQ responses, assessed at T1 (PN‐HPT® monotherapy response) and T2 and T3 compared with the first six‐week visit (T1).
2.4. Safety assessment
Assessment of local side effects such as swelling and erythema; women questioned for minor or significant adverse events they experienced before each treatment session.
2.5. Statistical analysis
Software: Statistical Package for the Social Sciences (SPSS, Chicago, IL, USA), version 13.0. Descriptive data were tabulated as means ± standard deviations (SD). Although variances were homogeneous, the low number of cohort women and outcome data suggested a non‐parametric approach (Levene's test). The analysis thus applied the general linear model for repeated measures (Kruskal–Wallis test for independent samples or non‐parametric one‐way ANOVA) to assess how the treatments influenced the follow‐up curves. After detecting significant effects of treatments, pairwise post‐hoc Sidak multiple comparisons identified the exact time points of divergence of the curves during the follow‐up period. Qualitative variables (scores) were expressed as frequencies and compared with the χ 2 and Fisher's exact tests. Significance threshold set at p‐values < 0.05.
3. RESULTS
Due to uniformly favorable early outcomes, the authors let the enrollment progress, between January and June 2020, beyond the initially planned maximum of 10 women. To avoid compromising the study's purely exploratory probing nature, the authors stopped enrollment at 20 women and 40 NLFs (average age 49.7 ± 5.82 years, range 45–60 years). Table 3 reports the enlisted women's baseline demographics and NLF profile: all women completed the study without reporting any clinically significant local or systemic side effects.
TABLE 3.
Baseline demographics of enlisted women (upper table) and comparison of baseline clinical homogeneity of the right‐ and left‐side NLF groups (lower table)
| Baseline demographics of enlisted women | |
|---|---|
| Screened and enrolled subjects | 20 + 20 |
| Overall female individuals | 20 |
| Overall Caucasian ethnicity | 20 |
| Overall mean Age | 49.7 ± 5.82 |
| Four‐type Fitzpatrick skin type | 3.05 ± 0.83 |
| Individual parameter | NLF Rx group | NLF Lx group |
|---|---|---|
| NLFSS scores | 7.6 ± 0.60 | 7.7 ± 0.73 |
| Wrinkles severity | 36.1 ± 1.76 | 35.3 ± 1.39 |
| Texture | 29.1 ± 0.50 | 29.3 ± 1.30 |
| Mean hemoglobin levels in NLF dermis | 129.2 ± 10.50 | 123.0 ± 10.30 |
3.1. Antera 3D® qualitative and quantitative outcomes and women's satisfaction vs baseline
3.1.1. NLF Rx group (PN‐HPT® monotherapy and preliminary priming + HA)
The Antera 3D® assessment showed significant improvements in wrinkle presentation and skin texture at T1, T2, and T3 (p < 0.05 at all assessment points versus baseline). The mean dermal NLF hemoglobin levels significantly improved at T2 and T3 (p < 0.05 at all assessment points vs baseline), but not after 6 weeks. The cohort women expressed high and moderate satisfaction (PSQ self‐assessment) at T2 and T3, respectively (Table 4).
TABLE 4.
NLF Rx group—mean skin quality outcomes, mean hemoglobin (Hb) levels in the NLF dermis, and mean PSQ scores after 6 weeks (T1—PN‐HPT® vs saline placebo monotherapy outcome) and 3 and 6 months (T2 and T3, respectively—PN‐HPT® priming + HA consolidation); outcomes in red and Italics: p < 0.05 vs baseline
| NLF Rx Group | Baseline | Six weeks | Three months | Six months |
|---|---|---|---|---|
| Wrinkles | 36.1 ± 1.76 | 27.6 ± 2.47 | 24.0 ± 1.00 | 26.5 ± 1.10 |
| Texture | 29.1 ± 0.50 | 22.2 ± 1.62 | 16.1 ± 2.19 | 18.9 ± 1.41 |
| Mean NLF Hb levels | 129.2 ± 10.50 | 137.7 ± 12.00 | 152.0 ± 13.20 | 160.0 ± 12.20 |
| PSQ score | // | 1.65 ± 0.49 | 7.6 ± 0.50 | 4.3 ± 0.67 |
3.1.2. NLF Lx group (saline control + HA)
Based on Antera 3D® outcomes, skin texture, wrinkle presentation, and mean dermal NLF hemoglobin levels failed to improve at T1 and T3; only while skin texture and wrinkles showed a significant improvement (p < 0.05 vs baseline for both parameters) after 3 months (T2) vs baseline. The cohort women stated a moderate satisfaction at T2 (Table 5).
TABLE 5.
NLF Lx group—mean skin quality outcomes, mean hemoglobin (Hb) levels in the NLF dermis, and mean PSQ scores after 6 weeks (T1—PN‐HPT® vs saline placebo monotherapy outcome) and 3 and 6 months (T2 and T3, respectively—PN‐HPT® priming + HA consolidation); outcomes in red and Italics: p < 0.05 vs baseline
| NLF Lx Group | Baseline | Six weeks | Three months | Six months |
|---|---|---|---|---|
| Wrinkles | 35.3 ± 1.39 | 33.7 ± 2.25 | 26.2 ± 1.00 | 31.5 ± 2.76 |
| Texture | 29.3 ± 1.39 | 28.4 ± 1.96 | 19.2 ± 1.83 | 27.1 ± 1.13 |
| Mean NLF Hb levels | 123.0 ± 10.30 | 119.0 ± 10.70 | 130.9 ± 10.40 | 132.4 ± 8.12 |
| PSQ score | // | 1.5 ± 0.50 | 6.6 ± 0.60 | 3.4 ± 0.60 |
3.2. Vectra H2® HA tissue levels and subjectively assessed NLF presentation severity (NLFSS)
The quantitative Vectra H2® assessment demonstrated significantly higher HA tissue levels in the treated right‐side NLF area compared with the left‐side NLF area over the longer term (T2 and T3; p < 0.05 vs baseline at both time points), but not after 6 weeks (Table 6). Analogously, the clinical presentation of right‐side NLFs appeared significantly less severe compared with left‐side NLFs at T2 and T3 (p < 0.05 vs baseline at both time points) according to the independently assessed NLFSS (Figure 2).
TABLE 6.
Comparison of mean cross‐linked hyaluronic acid (HA) levels in treated right‐ and left‐side NLFs after 6 weeks (T1—PN‐HPT® vs saline placebo monotherapy outcome) and 3 and 6 months (T2 and T3, respectively—PN‐HPT® priming + HA consolidation); outcomes in red and Italics: p < 0.05 vs left‐side NLFs
| Tissue HA over follow‐up | Right‐side NLFs | Left‐side NLFs |
|---|---|---|
| 6 weeks | 174.1 ± 9.25 | 150.1 ± 9.25 |
| 3 months | 103.4 ± 9.25 | 69.1 ± 6.80 |
| 6 months | 26.8 ± 2.63 | 12.3 ± 2.00 |
FIGURE 2.
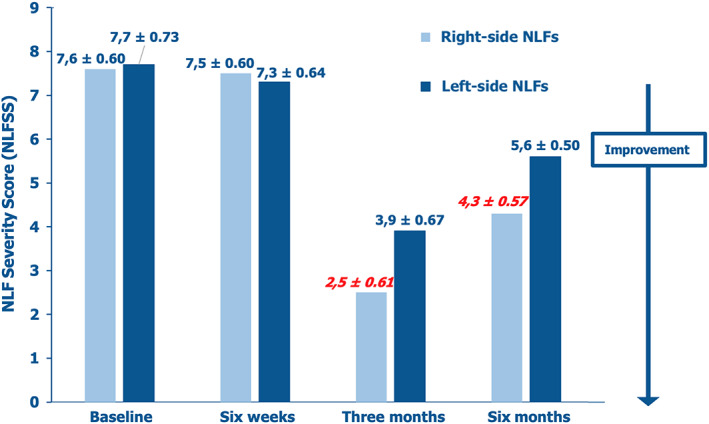
Comparison of NLF severity scores (NLFSS) levels in treated right‐ and left‐side NLFs at baseline (T0) and after 6 weeks (T1—PN‐HPT® vs saline placebo monotherapy) and 3 and 6 months (T2 and T3, respectively—PN‐HPT® priming + HA consolidation); outcomes in red and Italics: p < 0.05 vs baseline
3.3. Comparison of objective and subjective outcomes between right‐side and left‐side NLFs
The Antera 3D® scoring demonstrated that wrinkles improved significantly more after 6 weeks in the right‐side NLFs treated with PN‐HPT® monotherapy (from 36.1 ± 1.76 to 27.6 ± 2.47) compared with the control left‐side NLFs treated with saline (from 35.3 ± 1.39 to 33.7 ± 2.25; p < 0.05 in favor of PN‐HPT® monotherapy). Concomitantly, the skin texture improved significantly more in the right‐side NLFs compared with the control left‐side NLFs both after 6 weeks (from 29.1 ± 0.50 to 22.2 ± 1.62 vs 29.3 ± 1.39 to 28.4 ± 1.96; p < 0.05 in favor of PN‐HPT® monotherapy) and 6 months (18.9 ± 1.41 vs 27.1 ± 1.13; p < 0.05 in favor of PN‐HPT® skin priming and HA consolidation).
Conversely, the mean dermal NLF hemoglobin levels as a proxy of efficient dermal microcirculation significantly improved in the treated right‐side NLFs compared with left‐side NLFs only after 6 months (160.0 ± 12.00 vs 132.4 ± 8.12; p < 0.05 in favor of PN‐HPT® priming and HA consolidation). The HA persistence was longer, with significantly higher levels at T2 and T3, in the right‐side NLFs exposed to PN‐HPT® priming before HA consolidation vs contralateral NLFs. As highlighted in Figure 1, the NLFSS variations over the long term were similar, improving more significantly in the right‐side NLFs exposed to PN‐HPT® priming before HA consolidation than left‐side controls treated only with HA at T2 and T3.
The PSQ comparison revealed a satisfaction improvement at three and 6 months compared with the first assessment visit, but no differences between the two split‐face treatment groups (Table 7). Figures 3 and 4 illustrate the two examples of NLF outcomes over the first 3 months of the study: the more severe NLFs tend to show especially impressive medium‐term aesthetic improvements.
TABLE 7.
Comparative mean PSQ score, right‐side vs, left‐side NLFs, after 6 weeks (PN‐HPT® vs saline placebo monotherapy) and 3 and 6 months (PN‐HPT® priming + HA consolidation); no significance
| PSQ score | Right‐side NLFs | Left‐side NLFs |
|---|---|---|
| 6 weeks | 1.6 ± 0.49 | 1.5 ± 0.50 |
| 3 months | 7.6 ± 0.49 | 6.6 ± 0.60 |
| 6 months | 4.3 ± 0.67 | 3.4 ± 0.60 |
FIGURE 3.
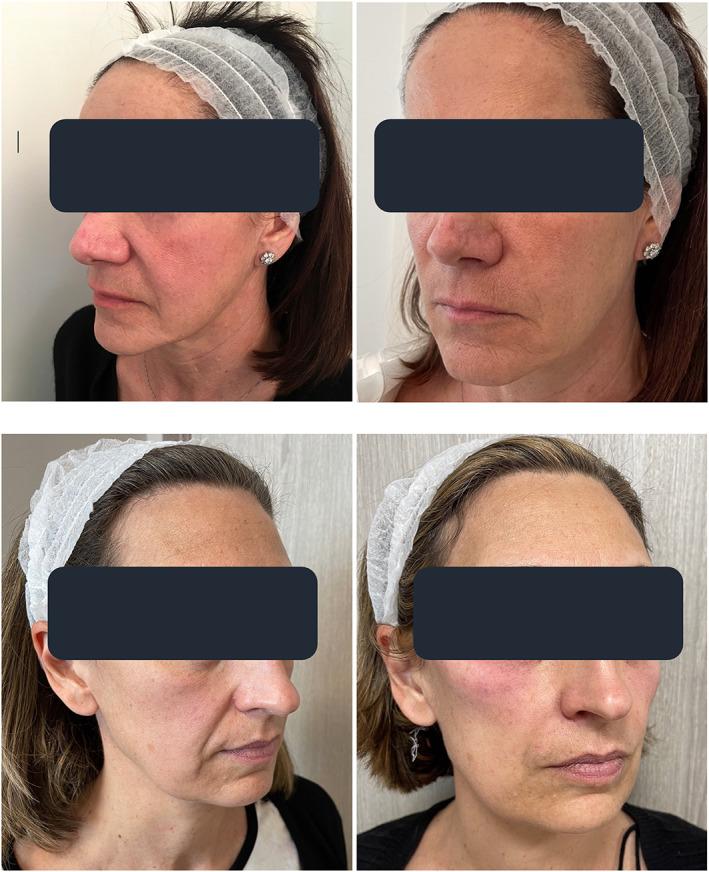
Moderately but increasingly deep NLFs in two mid‐50s women at baseline (T0, photographs on the left) and outcomes after 3 months of sequential PN‐HPT® + cross‐linked HA treatment (T2, photographs on the right)
FIGURE 4.
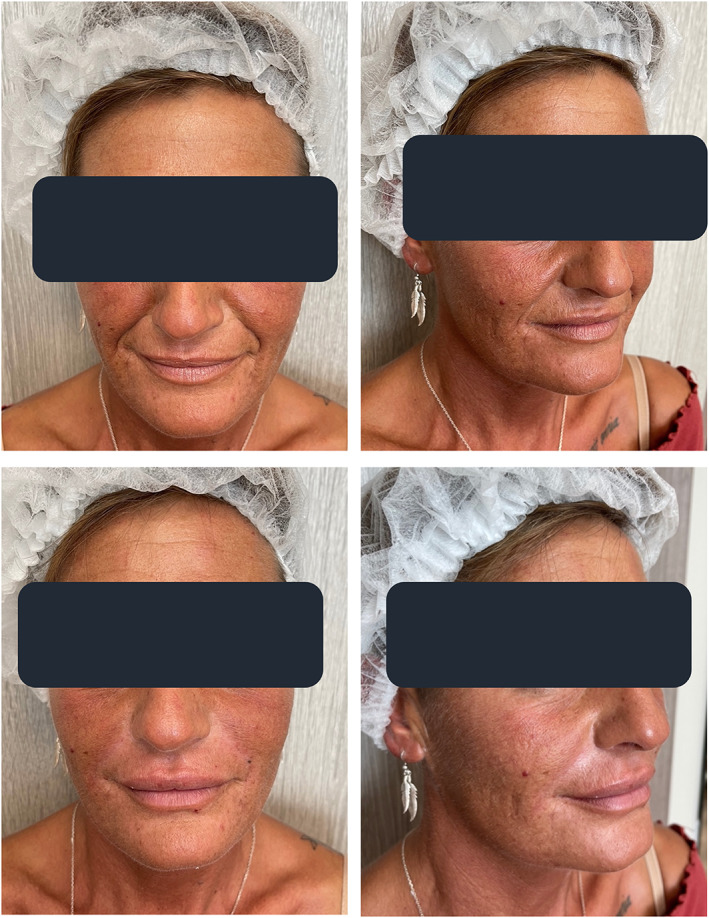
Aesthetically severe NLFs in a mid‐60s woman at baseline (T0, upper photographs) and after 3 months of either sequential PN‐HPT® + cross‐linked HA treatment (eight‐side NLFs) (T2, lower photographs)
4. DISCUSSION
Aesthetic medicine specialists have resorted to HA dermal supplementations since the early 1980s for correcting wrinkles and the first and most evident sign of facial aging—NLFs. 22 , 23 , 24 , 25 Unsurprisingly, many dermal HA‐based medical devices, mainly cross‐linked, have become available to aesthetic medicine specialists over the years. Cross‐linking modifies the molecular structure of natural HA, improving its biophysical properties—elasticity, rigidity, and stability. 26 , 27
PN‐HPT® are a mixture of DNA polynucleotides of different lengths. Numerous studies have shown that PN‐HPT® injections are helpful across several medical specialities 10 , 11 , 12 , 13 , 14 , 15 ; a recent expert report extensively discussed the PN‐HPT® benefits, as monotherapy or combined with other agents, in aesthetic medicine. 13 Such benefits include the enhanced deposition of the collagen and elastin fibers and restoration of collagen and elastin networks associated with exposure to PN‐HPT®. 13 In a prospective randomized study about the treatment of atrophic acne scars compared with placebo, the authors too showed that PN‐HPT® monotherapy is safe and effective. 21
The exploratory study aimed to explore the value of PN‐HPT® monotherapy in NLF treatment and the possible aesthetic benefits in the skin dermis of combining PN‐HPT® and HA in sequence. The working hypothesis was that PN‐HPT® might act as a priming agent in the dermis of NLF areas to prepare the biological substrate to the consolidating action of later administered HA, thus enhancing and extending the HA clinical efficacy over time. To fulfill those goals, the authors tested a sequential treatment of PN‐HPT® and HA on NLFs with a split‐face design and compared the PN‐HPT® group with a homogeneous control group at 6 weeks, 3 and 6 months. They only included Caucasian women to avoid ethnicity bias.
Dermal quality, quantitatively assessed with the help of the Antera 3D® imaging device, confirmed that the wrinkle appearance and texture improve consistently with PN‐HPT® monotherapy in the right‐side, PN‐HPT®‐treated NLFs after 6 weeks compared with saline placebo. However, tentative and no more than exploratory, those Antera 3D® observations confirmed the profile of rapid PN‐HPT® benefits in monotherapy on the NLF structure the authors had already reported in acne scars. 27
Regarding the value of the PN‐HPT® priming concept preliminary to HA consolidation, outcomes are contradictory, possibly due to low numbers, although the overall outcomes seem to confirm the working hypothesis. At least tentatively, PN‐HPT® seem to act as a preliminary priming agent to prepare the NLF dermis for HA consolidation and prolong the HA action over time. While the Antera® 3D scores for wrinkle appearance and NLF texture did not improve in right‐side NLFs after three and 6 months vs the monotherapy phase (T1) and contralateral control NLFs, the self‐assessed NLF Severity Score were significantly lower for right‐side NLFs over all the priming phase at both T2 and T3. Moreover, the women who underwent the PN‐HPT® + HA treatment perceived a significantly superior subjective aesthetic benefit after three and 6 months despite the lack of microstructure differences in right‐side and left‐side NLFs, compared with women treated with HA alone.
The preliminary environmental modulation by PN‐HPT® in the NLF dermis might also be the basis of the protracted effect developed by HA in the active right‐side NLFs compared with the contralateral NLFs—Vectra H2® analysis showed significantly higher mean HA levels in right‐side NLFs at both 3 and 6 months. The mean dermal NLF hemoglobin levels as a proxy for dermal blood perfusion and, indirectly, recovery of dermal eutrophic health also appeared significantly improved after 6 months in the active vs control NLF areas—possibly a marker of a slow improvement in the NLF dermis related to a protracted dermal restructuring action.
Of course, any comment about the actual value of differences in dermal NLF hemoglobin levels must be cautious: the Antera 3D® technology allows to estimate the hemoglobin concentrations in tissues, 18 , 19 but it does not discriminate between oxygenated and de‐oxygenated tissue hemoglobin and does not discriminate the effects of post‐injection tissue irritation on hemoglobin NLF levels. Although quantitative Antera 3D® analysis showed statistically different differential increases in NLF dermal hemoglobin, any comment about their relation with NLF dermal microcirculation and aesthetic benefits can only be tentative and circumspect. More sensitive methods—for example, videomicroscopy for direct in vivo viewing of microcirculation and assessing of total vascular and functional capillary densities—will strengthen the demonstrative value of future well‐designed studies. 28
The authors failed to archive the high‐definition Antera 3D® and Vectra® images; they only registered the quantitative Antera 3D® and Vectra® outcomes—a failure of preliminary planning and another weak point of the study. Once again, the reason was the purely probing nature of the study, at least initially: the authors did not foresee the surprising differences they observed. Any future well‐designed investigation will not neglect the all‐important iconographical documentation.
Summarizing, with all the limitations of a purely exploratory study, PN‐HPT® appear rapidly effective as monotherapy on the NLF structure as revealed by the Antera® 3D analysis, although these benefits do not seem to translate in a subjectively perceived, overall aesthetic benefit. Conversely, PN‐HPT® priming + HA seem to have only marginal benefits compared with HA monotherapy over many months in terms of NLF dermal microstructure, but the subjectively expressed macro‐aesthetic benefits are significant and protracted over the whole priming period. PSQ results agreed with clinical evaluations—all HA‐treated women were satisfied after 3 months, but only the women in the NLF Rx group were still moderately satisfied after 6 months. No minor or significant adverse events occurred during the study period, suggesting that both PN‐HPT® and HA are safe as NLF treatments.
PN‐HPT® must not be confused with another class of polydeoxyribonucleotide derivatives, synthetically described with the acronym PDRN, which are biochemically and clinically different from PN‐HPT®. The main PDRN indication, available on prescription for intramuscular administration, is treating torpid wounds and diabetic foot ulcers. 29 , 30 Conversely, PN‐HPT® are only available as Class‐III medical devices for topical use and intradermal and intra‐articular administration. They fill intradermal spaces, provide hydration, viscoelasticity, and plump up tissues, as discussed in a recent consensus report in aesthetic medicine 13 and several PN‐HPT® clinical studies. 31 , 32 , 33
Of course, only further clinical studies with larger cohorts and more comprehensive ranges of materials (HA, calcium hydroxyapatite) will confirm the working hypothesis that PN‐HPT® work as a dermal primer.
The small number of enlisted women and NLFs and the resulting low statistical power are limiting factors, yet they were a deliberate choice of the authors to preserve the exploratory study identity. The split‐face randomization design is a strong point, but a double‐blind design would have conferred more strength to the exploratory outcomes. The impromptu PSQ scale, although of academic origin and highly discriminatory, strictly speaking, is not validated for NLF studies. Analogously, the authors resorted to a highly discriminatory, nine‐point NLFSS scale validated only in Han ethnicity women instead of the conventional, five‐point, investigator‐assessed NLFSS validated for Caucasian women. 34 Once again, the reason that justified the authors’ decision was the exploratory nature of their investigation—small comparative cohorts vs high discriminatory power: the authors deemed it a good compromise.
5. CONCLUSIONS
This randomized, prospective clinical study highlights the role of intradermal PN‐HPT® in monotherapy to rapidly improve the dermal quality in treating moderate to severe NLFs with consistent, subjectively expressed aesthetic benefits. Administered in a preliminary priming phase, PN‐HPT® prolong the clinical efficacy of HA administered as a consolidator of aesthetic benefits.
AUTHOR CONTRIBUTIONS
Antonino and Francesco Araco sought and got informed consent from the women subjects seeking aesthetic treatment for their moderate to severe nasolabial folds and enrolled in the study; Mauro Raichi was responsible for statistical analyses. All women were aware of the benefits and risks they could reasonably expect from the PN‐HPT™ and hyaluronic acid aesthetic procedures. The authors confirm they are accountable for the manuscript's accuracy and integrity, including all comments on outcomes, and are responsible for its submission to the Journal of Cosmetic Dermatology. The authors confirm they adhered to the ethical policies of the journal, as noted on the journal's author guidelines page.
CONFLICTS OF INTEREST STATEMENT
The manuscript's authors state they have no conflict of interest related to the study, they received no funds, and they have no paid or unpaid relations with industry manufacturers, publishers, or other companies in some way related to their study.
PHOTOGRAPH CONSENT STATEMENT
All photographs belong to the authors, who permitted their submission with the patients' agreement after making them unrecognizable with black bars.
ETHICAL APPROVAL
The authors performed the study in agreement with the Declaration of Helsinki. Participating women could reasonably expect no unusual risk other than those associated with routine ambulatory aesthetic medicine procedures for nasolabial folds. Furthermore, the study protocol excluded any unusual practice different from PN‐HPT™ and hyaluronate infiltrations—long‐established and extensively documented aesthetic medicine procedures. According to accepted conventions, these considerations allowed waiving the requirement for formal approval by an Ethical Committee.
ACKNOWLEDGEMENTS
Mastelli S.r.l., Sanremo, Italy, is the patent holder of the polynucleotides HPT™ technology and the gel formulations of injectable polynucleotides used in this exploratory study. The authors acknowledge the contribution of Mastelli S.r.l. for supporting the publication costs.
Araco A, Araco F, Raichi M. Clinical efficacy and safety of polynucleotides highly purified technology (PN‐HPT®) and cross‐linked hyaluronic acid for moderate to severe nasolabial folds: A prospective, randomized, exploratory study. J Cosmet Dermatol. 2023;22:146‐155. doi: 10.1111/jocd.15064
Funding information
The authors declare their study was spontaneous and born of scientific curiosity and published literature. Support with the article processing charges required by the Journal of Cosmetic Dermatology will be the only funding provided by the corporate sponsor
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Guida S, Pellacani G, Ciardo S, Longo C. Reflectance confocal microscopy of aging skin and skin cancer. Dermatol Pract Concept. 2021;11:e2021068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guida S, Ciardo S, De Pace B, et al. Atrophic and hypertrophic skin photoaging and melanocortin‐1 receptor (MC1R): the missing link. J Am Acad Dermatol. 2021;84:187‐190. [DOI] [PubMed] [Google Scholar]
- 3. Guida S, Ciardo S, De Pace B, et al. The influence of MC1R on dermal morphological features of photo‐exposed skin in women revealed by reflectance confocal microscopy and optical coherence tomography. Exp Dermatol. 2019;28:1321‐1327. [DOI] [PubMed] [Google Scholar]
- 4. Gierloff M, Stohring C, Buder T, et al. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconstr Surg. 2012;129:263‐273. [DOI] [PubMed] [Google Scholar]
- 5. Le Louarn C, Buthiau D, Buis J. Structural aging: the facial recurve concept. Aesthet Plast Surg. 2007;31:213‐218. [DOI] [PubMed] [Google Scholar]
- 6. Beasley KL, Weiss MA, Weiss RA. Hyaluronic acid fillers: a comprehensive review. Facial Plast Surg. 2009;25:86‐94. [DOI] [PubMed] [Google Scholar]
- 7. Dicker KT, Gurski LA, Pradhan‐Bhatt S, Witt RL, Farach‐Carson MC, Jia X. Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater. 2014;10:1558‐1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vigetti D, Karousou E, Viola M, Deleonibus S, de Luca G, Passi A. Hyaluronan: biosynthesis and signaling. Biochim Biophys Acta. 2014;1840:2452‐2459. [DOI] [PubMed] [Google Scholar]
- 9. Galimberti MG, Guida S, Pellacani G, Bencini PL. Hyaluronic acid filler for skin rejuvenation: the role of diet on outcomes. A pilot study. Dermatol Ther. 2018;31:e12646. [DOI] [PubMed] [Google Scholar]
- 10. Hiramoto K, Kobayashi H, Yamate Y, Ishii M, Sato EF. Intercellular pathway through hyaluronic acid in UVB‐induced inflammation. Exp Dermatol. 2012;21:911‐914. [DOI] [PubMed] [Google Scholar]
- 11. Cavallini M, Papagni M. Long‐chain polynucleotides gel and skin biorevitalization. J Plastic Dermatol. 2007;3:27‐32. [Google Scholar]
- 12. Matera G, Dodici N, Raichi M. Improving on laser: biorevitalization of stretch marks, the polynucleotides infiltrations combined with CO2 laser option. Aesthetic Med. 2020;6:19‐26. [Google Scholar]
- 13. Cavallini M, Bartoletti E. Members of the polynucleotides HPT® priming board, Italian College of the Aesthetic Medicine Scientific Societies (SIME, AGORÀ, SIES). Consensus report on the use of PN‐HPT® (polynucleotide highly purified technology) in aesthetic medicine. J Cosmet Dermatol. 2021;20:922‐928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Caridi G, Massara M, Acri I, et al. Trophic effects of polynucleotides and hyaluronic acid in the healing of venous ulcers of the lower limbs: a clinical study. Int Wound J. 2016;13:754‐758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Palmieri IP, Raichi M. Biorevitalization of postmenopausal labia majora, the polynucleotide/hyaluronic acid option. Obstet Gynecol Rep. 2019;3:2‐5. [Google Scholar]
- 16. Giarratana LS, Marelli BM. A randomized, double‐blind clinical trial on the treatment of knee osteoarthritis: the efficacy of polynucleotides compared to standard hyaluronan viscosupplementation. Knee. 2014;21:661‐668. [DOI] [PubMed] [Google Scholar]
- 17. Jiechen Z, H W. Classification of facial wrinkles among Chinese women. J Biomed Res. 2017;31:108‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guida S, Pellacani G, Bencini PL. Picosecond laser treatment of atrophic and hypertrophic surgical scars: in vivo monitoring of results by means of 3D imaging and reflectance confocal microscopy. Skin Res Technol. 2019;25:896‐902. [DOI] [PubMed] [Google Scholar]
- 19. Jang SI, Kim EJ, Park H, et al. A quantitative evaluation method using processed optical images and analysis of age‐dependent changes on nasolabial lines. Skin Res Technol. 2015;21:201‐206. [DOI] [PubMed] [Google Scholar]
- 20. Tanizaki H, Tanioka M, Yamashita Y, Hayashi N. Quantitative evaluation of atrophic acne scars using 3D image analysis with reflected LED light. Skin Res Technol. 2020;26:20‐24. [DOI] [PubMed] [Google Scholar]
- 21. Araco A, Araco F. Preliminary prospective and randomized study of highly purified polynucleotide versus placebo in treatment of moderate to severe acne scars. Aesthet Surg J. 2021;41:NP866‐NP874. [DOI] [PubMed] [Google Scholar]
- 22. Baumann L. Skin aging and its treatment. J Pathol. 2007;211:241‐251. [DOI] [PubMed] [Google Scholar]
- 23. Ezure T, Amano S. Involvement of upper cheek sagging in nasolabial fold formation. Skin Res Technol. 2012;18:259‐264. [DOI] [PubMed] [Google Scholar]
- 24. Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermato‐Endocrinol. 2012;4:253‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ascher B, Bayerl C, Brun P, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of severe nasolabial lines—6‐month interim results of a randomized, evaluator‐blinded, intra‐individual comparison study. J Cosmet Dermatol. 2011;10:94‐98. [DOI] [PubMed] [Google Scholar]
- 26. Croce MA, Dyne K, Boraldi F, et al. Hyaluronan affects protein and collagen synthesis by in vitro human skin fibroblasts. Tissue Cell. 2001;33:326‐331. [DOI] [PubMed] [Google Scholar]
- 27. La Gatta A, Schiraldi C, Papa A, De Rosa M. Comparative analysis of commercial dermal fillers based on cross‐linked hyaluronan: physical characterization and in vitro enzymatic degradation. Polym Degrad Stab. 2011;96:630‐636. [Google Scholar]
- 28. De Backer D, Hollenberg S, et al. How to evaluate the microcirculation: report of a round table conference. Crit Care. 2007;11:R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galeano M, Pallio G, Irrera N, et al. Polydeoxyribonucleotide: a promising biological platform to accelerate impaired skin wound healing. Pharmaceuticals (Basel). 2021;14:1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Polito F, Bitto A, Galeano M, et al. Polydeoxyribonucleotide restores blood flow in an experimental model of ischemic skin flaps. J Vasc Surg. 2012;55:479‐488. [DOI] [PubMed] [Google Scholar]
- 31. Massirone A. Polynucleotides highly purified technology and the face skin, a story of innovative skin priming. Aesth Med. 2021;7:35‐40. [Google Scholar]
- 32. Bartoletti E, Cavallini M, Maioli L, et al. Introduction to polynucleotides highly purified technology. Aesth Med. 2020;6:43‐47. [Google Scholar]
- 33. Brandi C, Cuomo R, Nisi G, Grimaldi L, D'Aniello C. Face rejuvenation: a new combined protocol for biorevitalization. Acta Biomed. 2018;89:400‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang J, Hou W, Feng S, Chen X, Wang H. Classification of facial wrinkles among Chinese women. J Biomed Res. 2017;31:108‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


