Abstract
Objectives
To explore the effects of preoperative high‐intensity interval training (HIIT) compared to usual care on tumour natural killer (NK)‐cell infiltration in men with localised prostate cancer (PCa), as NK‐cell infiltration has been proposed as one of the key mechanisms whereby exercise can modulate human tumours.
Patients and Methods
A total of 30 patients with localised PCa undergoing radical prostatectomy (RP) were randomised (2:1) to either preoperative aerobic HIIT four‐times weekly (EX; n = 20) or usual care (CON; n = 10) from time of inclusion until scheduled surgery. Tumour NK‐cell infiltration was assessed by immunohistochemistry (CD56+) in diagnostic core needle biopsies and corresponding prostatic tissue from the RP. Changes in cardiorespiratory fitness, body composition, blood biochemistry, and health‐related quality of life were also evaluated.
Results
The change in tumour NK‐cell infiltration did not differ between the EX and CON groups (between‐group difference: −0.09 cells/mm2, 95% confidence interval [CI] –1.85 to 1.66; P = 0.913) in the intention‐to‐treat analysis. The total number of exercise sessions varied considerably from four to 30 sessions. The per‐protocol analysis showed a significant increase in tumour NK‐cell infiltration of 1.60 cells/mm2 (95% CI 0.59 to 2.62; P = 0.004) in the EX group. Further, the total number of training sessions was positively correlated with the change in NK‐cell infiltration (r = 0.526, P = 0.021), peak oxygen uptake (r = 0.514, P = 0.035) and peak power output (r = 0.506, P = 0.038).
Conclusion
Preoperative HIIT did not result in between‐group differences in tumour NK‐cell infiltration. Per‐protocol and exploratory analyses demonstrate an enhanced NK‐cell infiltration in PCa. Future studies are needed to test the capability of exercise to increase tumour immune cell infiltration.
Keywords: exercise, prostate cancer, NK cells, immune cells, preoperative, high‐intensity exercise training, #PCSM, #ProstateCancer, #uroonc
Abbreviations
- CON
control group
- EX
exercise group
- FACT‐P
Functional Assessment of Cancer Treatment‐Prostate
- HIIT
high‐intensity interval training
- hs‐CRP
high‐sensitivity C‐reactive protein
- IL
interleukin
- NK
natural killer
- PCa
prostate cancer
- PROM
patient‐reported outcome measure
- RCT
randomised controlled trial
- RP
radical prostatectomy
- VO2peak
peak oxygen uptake
- W peak
peak power output
Introduction
Physical activity and exercise behaviour have been demonstrated to be inversely associated with clinical cancer outcomes, including disease recurrence, cancer‐specific, and overall mortality [1, 2]. Moreover, preclinical studies have shown that exercise training can reduce tumour growth in rodents [3, 4], and early explorative clinical reports support that exercise training may delay disease progression and improve survival [5]. In concert, early data suggest that exercise training constitutes a targeted anti‐cancer treatment strategy [1], but the mechanisms underpinning this anti‐cancer effect remain ambiguous.
In a seminal experiment, our group was among the first to demonstrate a possible biological mechanism through which voluntary wheel running can reduce tumour growth in mice [6]. Specifically, we showed that wheel running led to mobilisation of adrenaline‐sensitive natural killer (NK) cells, which were redistributed into the tumours, in part, by an interleukin 6 (IL‐6)‐dependent mechanism leading to a suppression of tumour growth. This finding warrants further interest as it is well established that acute exercise drives a significant increase in circulating immune cells, especially NK cells, in a dose‐dependent manner (exercise intensity and/or volume) in humans [7, 8, 9].
Therefore, a critical step in the further exploration of exercise as a targeted anti‐cancer strategy is to elucidate if tumoral redistribution of NK cells in response to exercise can be demonstrated in patients with cancer. Due to the need for rapid removal of local tumours in most operable patients, exercise‐conditioned tumour tissue is difficult to obtain. To this end, radical prostatectomy (RP) in patients with localised prostate cancer (PCa) serves as an enticing setting, as these patients do not receive neoadjuvant treatment, and surgery can be safely postponed for short periods due to the slow‐growing nature of the disease [10].
Accordingly, the present randomised controlled trial (RCT) was designed to investigate the effect of high‐intensity aerobic exercise training on tumour NK‐cell infiltration in a human setting, utilising the preoperative window in patients with early‐stage PCa. First, this study examined the effects of high‐intensity aerobic exercise training four‐times weekly in men with localised PCa before scheduled RP on tumour NK‐cell infiltration. Second, we explored the effects of preoperative exercise training on physiological and patient‐reported outcomes.
Patients and Methods
This was a prospective, RCT based at the Centre for Physical Activity Research at Rigshospitalet, Copenhagen, Denmark. The study was conducted from November 2016 to December 2019 and was approved by the local Ethics Committee of the Capital Region of Denmark (H‐16034670) and preregistered at www.clinicaltrials.gov (NCT02954783). All participants provided signed informed consent before any study‐related procedures were performed.
Patients and Procedures
The eligibility criteria were: men with histologically verified localised adenocarcinoma of the prostate scheduled for RP; age >18 years; no other known malignancy requiring active treatment; Eastern Cooperative Oncology Group (ECOG) performance status score of <1; no ongoing treatment with β‐adrenergic receptor antagonists; no physical disabilities precluding physical testing and/or exercise; ability to read and understand Danish.
Patients were recruited from Departments of Urology at Rigshospitalet and Herlev Hospital, Copenhagen, Denmark. Patients were assessed at baseline (at diagnosis) and follow‐up (shortly before RP) for cardiopulmonary fitness, body composition, evaluation of fasting blood biochemistry, health‐related quality of life, and anxiety and depression. Participants were randomised to either a high‐intensity interval training (HIIT; EX) group or a control (CON) group following baseline assessment. The random allocation sequence was computer‐generated and based on block randomisation using a block size of three and an allocation ratio of 2:1. Allocation concealment was ensured by an on‐line clinical trial software (easytrial.net) that kept the allocation sequence unavailable for the investigators and participants. Analyses of NK‐cell infiltration and blood biochemistry were performed blinded to group allocation.
Exercise Group
The intervention consisted of four sessions of supervised HIIT each week. The exercise intervention ranged from 2 to 8 weeks, depending on scheduled RP. A light warm‐up was followed by 20–25 min of aerobic HIIT on a stationary bicycle ergometer. The HIIT consisted of 4–6 cycles of high‐intensity intervals for 1 min at 100–120% of peak power output (W peak), followed by 3 min of active recovery at 30% of W peak. The exercise programme consisted of four periods comprising week 1 (period 1), week 2 (period 2), weeks 3 + 4 (period 3), and weeks 5–8 (period 4). The number of cycles and intensity increased during each period. The exercise intervention is described in full in Table S1.
Control Group
Participants in the control group were instructed to maintain their everyday lifestyle, including physical activity, during their participation in the study.
Outcome Measures
Natural killer‐cell infiltration
The immunohistochemical analysis was performed on formalin‐fixed, paraffin‐embedded prostatic tissue from diagnostic core needle biopsies and corresponding tissue from RP for pre‐ and post‐measures. The core needle biopsy containing the highest percentage of tumour tissue was chosen together with the corresponding focus in the tissue from the RP. Immunohistochemistry was performed using standard methods (Table S2). Briefly, 3‐μm thick tissue sections were immunostained using the CD56 antibody (Roche Diagnostics International AG, Rotkreuz, Switzerland) following the manufacturer's instructions. Tissue sections were pre‐treated in PT Link (Agilent Technologies, CA, USA) using a high pH/low pH target retrieval solution (Dako DM828). The staining took place using the Ready‐To‐Use (RTU) format on the DakoLink 48 (Agilent Technologies) utilising the EnVision Flex+ detection kit (K8002). The incubation time was 20 min. Sections were counterstained with haematoxylin.
Stained slides were digitalised using the Hamamatsu Nano ZoomerXR at a magnification equivalent to ×20. The NK‐cell quantification was performed manually using the Hamamatsu NDP.view V.2.6.13 viewing software at ×40 and determined as the number of NK cells (cells/mm2) in the whole tumour area in core needle biopsies and tissue from RP, respectively. In healthy tissue, NK‐cell quantification was performed in the same manner as for the tumour tissue but in a randomly chosen area with the same size as the tumour area. NK‐cell quantification is described in detail in Table S2.
Physiological outcomes
Cardiorespiratory fitness was assessed as peak oxygen uptake (VO2peak) during an incremental test to volitional exhaustion performed on a stationary bike (LC4, Monark Exercise AB, Vansbro, Sweden) at baseline and follow‐up. Patients performed a 3 min warm‐up at 70‐watt load followed by an increase of 20 watts every minute until exhaustion. Gas exchange parameters and heart rate were measured throughout the test using on‐line measurement equipment (Quark CPET System; COSMED, Rome, Italy). A full description of the VO2peak test can be found in Table S3.
Height was measured to the nearest 0.1 cm using the Holtain stadiometer (Holtain Ltd, Crymych, UK). Body weight was measured with an electronic scale to the nearest 0.1 kg. Body composition comprising fat mass, lean soft tissue, and bone mineral density was assessed at baseline and follow‐up using whole‐body dual‐energy X‐ray absorptiometry scans (Lunar Prodigy, Lunar Corporation, Madison, WI, USA).
Fasting blood samples at baseline and follow‐up were collected for analyses of serum concentration of cholesterols (total, high‐density lipoprotein, and low‐density lipoprotein), triglycerides, glucose, insulin, C‐peptide, haemoglobin A1c (HbA1c), and PSA. TNF‐α, IL‐6, high‐sensitivity C‐reactive protein (hs‐CRP), and IL‐1 receptor antagonist (IL‐1Ra) were measured using Meso Scale Discovery's V‐PLEX Proinflammatory Panel 1 Human Kit and Human CRP Kit and V‐PLEX Human IL‐1RA Kit (Meso Scale Discovery), respectively.
Patient‐Reported Outcome Measures (PROMs)
Health‐related quality of life and anxiety and depression were assessed by the Functional Assessment of Cancer Treatment‐Prostate (FACT‐P) questionnaire, including all its domains, and the Hospital Anxiety and Depression Scale (HADS) questionnaire [11, 12, 13], respectively.
Statistical Analyses
The present study aimed to include 30 patients. To our knowledge, tumoral NK‐cell infiltration in response to exercise has not previously been investigated in humans. Therefore, it was not possible to determine the expected effect size, and thus no formal power calculations could be made.
The primary analyses were conducted using a linear mixed‐model with study outcomes as the dependent variable, with time (baseline/follow‐up), and group (EX/CON), and an interaction between them as fixed effects and a random effect of the subject. Raw baseline and follow‐up data were expressed as mean or median for all outcomes with corresponding SD or interquartile range (IQR), respectively. The estimated between‐group difference and within‐group changes were obtained from the mixed‐model analysis. To improve compliance of the linear mixed models, some outcome variables were analysed on a log‐scale with results back‐transformed and reported as relative ratios, i.e., a back‐transformed estimate of 1.20 corresponds to a median relative change/difference of 20%.
Analyses of the pre‐specified outcomes were performed both as intention‐to‐treat and per‐protocol analyses. Per‐protocol analysis was defined as attendance of ≥75% of the total training sessions from week 1 to 5. One study participant was excluded from the per‐protocol and the exploratory analysis, as he did not perform the HIIT exercise. Deviations from the pre‐registered protocol are described in Table S4. To explore a potential correlation between changes in NK‐cell infiltration and different variables, we used Pearson's bivariate correlation or Spearman's rho correlation analyses depending on normal distribution of the data. Analyses were performed using R (The R Foundation for Statistical Computing, Vienna, Austria) and the package ‘lme4’ [14]. The statistical significance level was set at 0.05.
Results
Participants and Training Adherence
A total of 104 patients were screened for eligibility (Fig. 1), and 30 patients were enrolled and randomised (Table 1). The total number of training sessions ranged from four to 30 sessions with a median of 16.0 (Fig. S1). The median number of days from the last training session until RP was 5 days. Overall, 55% (11/20) of the participants attended ≥75% of the exercise sessions during a minimum of 5 weeks. There was a 100% adherence to the prescribed exercise in all attended sessions for all participants, i.e., all sessions were performed with no reductions in intensity or time and thus exercise volume. No adverse events related to exercise were observed.
Fig. 1.
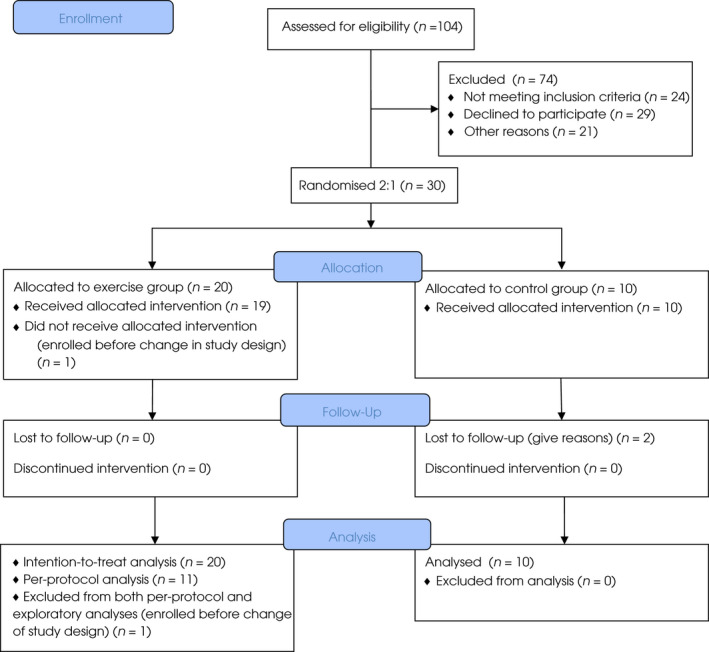
Study flow diagram.
Table 1.
Baseline characteristics.
| CON group (n = 10) | EX group (n = 20) | |
|---|---|---|
| Age, years, median (IQR) | 68 (61; 70) | 63 (57; 67) |
| Gleason score, n/N (%) | ||
| <7 | 2/10 | 6/20 (30) |
| 7 | 6/10 | 12/20 (60) |
| >7 | 2/10 | 2/20 (10) |
| PSA level, μg/L, median (IQR) | 9.1 (8.4; 18.0) | 7.4 (5.4; 13.0) |
| Body composition | ||
| BMI, kg/m2, median (IQR) | 27 (24; 30) | 27 (25; 29) |
| Total fat percentage, mean (SD) | 28.5 (6.7) | 29.3 (6.1) |
| Total fat mass, kg, mean (SD) | 23.6 (8.0) | 24.4 (6.1) |
| Android fat mass, kg, mean (SD) | 2.8 (1.2) | 2.8 (0.9) |
| Gynoid fat mass, kg, mean (SD) | 3.6 (1.0) | 3.6 (0.8) |
| Lean body mass, kg, mean (SD) | 57.3 (6.4) | 58.3 (5.9) |
| Smoker, n (%) | ||
| Never | 5/10 | 9/20 (45) |
| Previous | 3/10 | 9/20 (45) |
| Current | 2/10 | 2/20 (10) |
| Alcohol intake (units/week), n (%) | ||
| ≤14 | 9/10 | 16/20 (80) |
| >14 | 1/10 | 4/20 (20) |
| Physical activity level (min MVPA/week), n (%) | ||
| <150 min | 3/10 | 2/20 (10) |
| ≥150 min | 7/10 | 18/20 (90) |
| Cardiorespiratory fitness* | ||
| VO2peak, L/min, mean (SD) | 2.62 (0.59) | 2.89 (0.50) |
| VO2peak, mL/min/kg, mean (SD) | 31.4 (8.4) | 33.6 (6.3) |
| NK cell infiltration, mean (SD) | ||
| NK cells/mm2, tumour tissue | 1.05 (1.37) | 1.51 (1.85) |
| NK cells/mm2, healthy tissue | 4.15 (4.50) | 2.37 (3.33) |
Values are presented as mean (SD), median (IQR [Q1; Q3]), or n/N (%).
BMI, body mass index; MVPA, moderate to vigorous physical activity.
Cardiorespiratory fitness data are based on cardiorespiratory tests meeting two of the following test criteria: respiratory exchange ratio (RER) ≥1.10, rate of perceived exertion (RPE) ≥18, and a VO2 plateau (EX, n = 18; CON, n = 8).
Natural Killer‐Cell Infiltration
The change in tumour NK‐cell infiltration from baseline to follow‐up did not differ between groups, neither with the intention‐to‐treat nor the per‐protocol analysis (Fig. 2a–d and Table 2). In the per‐protocol analysis, we observed a significant within‐group increase in tumour NK cells in the EX group.
Fig. 2.
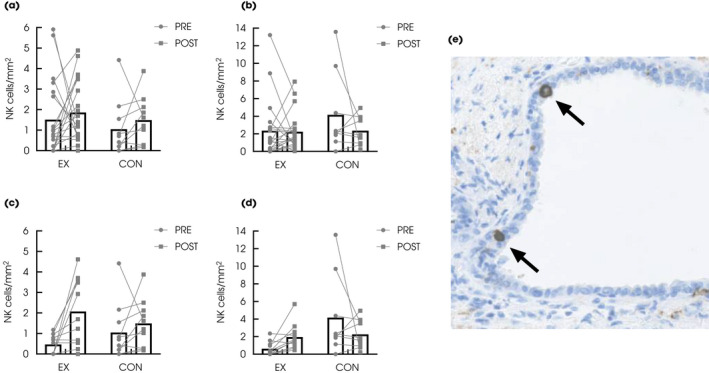
Intention‐to‐treat analysis showing NK‐cell infiltration in (a) tumour and (b) healthy prostatic tissue pre‐ and post‐intervention in the EX (n = 20) and CON (n = 10) group. Per‐protocol analysis of NK‐cell infiltration in (c) tumour and (d) healthy prostatic tissue pre‐ and post‐intervention in the EX (n = 11) and CON (n = 10) group. (e) CD56+ NK cells (arrows) in prostatic tissue (original magnification ×40). Data are presented as mean bars with individual data points. *The analyses in the healthy tissue in the CON group are based on n = 9 at baseline due to lack of normal tissue in core needle biopsy.
Table 2.
NK‐cell infiltration.
| Intention‐to‐treat | Baseline | Follow‐up | Within‐Group change | Between‐Group difference | ||
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Change (95% CI) | P | Difference (95% CI) | P | |
| NK cells tumour, cells/mm2 | ||||||
| EX | 1.51 (1.86) | 1.86 (1.50) | 0.35 (−0.67 to 1.36) | 0.489 | −0.09 (−1.85 to 1.66) | 0.913 |
| CON | 1.05 (1.37) | 1.49 (1.17) | 0.44 (−0.99 to 1.88) | 0.534 | ||
| NK cells healthy, cells/mm2 * | ||||||
| EX | 2.37 (3.33) | 2.24 (2.12) | −0.13 (−1.87 to 1.62) | 0.884 | 1.75 (−1.34 to 4.84) | 0.255 |
| CON | 4.15 (4.50) | 2.26 (1.59) | −1.88 (−4.43 to 0.67) | 0.142 | ||
| Per‐protocol | Mean (SD) | Mean (SD) | Change (95% CI) | P | Difference (95% CI) | P |
|---|---|---|---|---|---|---|
| NK cells tumour, cells/mm2 | ||||||
| EX | 0.47 (0.48) | 2.07 (1.65) | 1.60 (0.59 to 2.62) | 0.004 | 1.16 (−0.31 to 2.63) | 0.114 |
| CON | 1.05 (1.37) | 1.49 (1.17) | 0.44 (−0.62 to 1.50) | 0.396 | ||
| NK cells healthy, cells/mm2 * | ||||||
| EX | 0.62 (0.78) | 1.95 (1.48) | 1.33 (−0.82 to 3.48) | 0.210 | 3.23 (0.07 to 6.38) | 0.046 |
| CON | 4.15 (4.50) | 2.26 (1.59) | −1.90 (−4.21 to 0.42) | 0.102 | ||
Means (SDs) are based on all available data for EX and CON at baseline and follow‐up with intention‐to‐treat (EX, n = 20 and CON, n = 10) and per‐protocol (EX, n = 11, and CON, n = 10) analysis. The mean differences are estimated means from the mixed models and therefore, within‐group change may not reflect the numerical difference between baseline and follow‐up.
Bold values statistically significant at P < 0.05.
The analyses in healthy tissue are based on CON, n = 9 at baseline due to lack of normal tissue in core needle biopsy.
We observed a significant between‐group difference in NK‐cell infiltration with the per‐protocol analysis in the healthy prostatic tissue but not with the intention‐to‐treat analysis (Table 2).
Physiological Outcomes
All results related to physiological outcomes are presented in Table 3, Table S5 and Table S7. We found no difference between the EX or CON group in terms of absolute (L/min) or relative (mL/min/kg) VO2peak with neither the intention‐to‐treat nor per‐protocol analysis. We found a small reduction in diastolic blood pressure and maximum heart rate in the EX group compared to the CON group. For body composition outcomes, we found no differences between the EX or CON group. The blood biochemistry analyses revealed a within‐group reduction in PSA level with the intention‐to‐treat analysis in the EX group, but no significant changes were observed in the CON group. No between‐group changes were observed for PSA.
Table 3.
Physiological outcomes.
| Intention‐to‐treat | Baseline | Follow‐up | Within‐Group change | Between‐Group difference |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Change (95% CI) | Difference (95% CI) | |
| VO2peak, mL/min/kg* | ||||
| EX | 34.0 (6.4) | 35.2 (6.7) | 0.8 (−0.8 to 2.3) | −0.4 (−3.6 to 1.9) |
| CON | 31.4 (8.4) | 32.6 (8.6) | 1.2 (−1.1 to 3.5) | |
| VO2peak, L/min* | ||||
| EX | 2.89 (0.50) | 2.99 (0.51) | 0.06 (−0.06 to 0.19) | −0.02 (−0.24 to 0.20) |
| CON | 2.62 (0.59) | 2.70 (0.64) | 0.08 (−0.10 to 0.27) | |
| Total fat mass, kg | ||||
| EX | 24.4 (6.1) | 23.4 (5.9) | −1.0 (−1.8 to −0.1) | 0.5 (−1.1 to 2.0) |
| CON | 23.6 (8.0) | 22.5 (8.8) | −1.5 (−2.7 to −0.2) | |
| Lean mass, kg | ||||
| EX | 58.3 (5.9) | 59.3 (5.8) | 1.0 (0.4 to 1.6) | 0.2 (−1.0 to 1.4) |
| CON | 57.3 (6.4) | 58.5 (7.3) | 0.8 (−0.2 to 1.8) | |
| Lymphocytes, 109/L | ||||
| EX | 1.55 (0.52) | 1.64 (0.59) | 0.09 (−0.03 to 0.21) | 0.01 (−0.21 to 0.23) |
| CON | 1.58 (0.44) | 1.69 (0.57) | 0.08 (−0.11 to 0.26) | |
| hs‐CRP, mg/L † | ||||
| EX | 3.14 (5.29) | 3.52 (7.89) | 0.82 (0.54 to 1.24) | 0.75 (0.35 to 1.64) |
| CON | 5.13 (11.17) | 1.68 (2.06) | 1.08 (0.56 to 2.09) | |
| IL‐6, pg/mL | ||||
| EX | 0.65 (0.43) | 0.59 (0.50) | −0.06 (−0.19 to 0.07) | −0.09 (−0.33 to 0.15) |
| CON | 4.09 (11.10) | 0.60 (0.21) | 0.03 (−0.17 to 0.23) | |
| TNF‐α, pg/mL | ||||
| EX | 2.68 (0.69) | 2.60 (0.51) | −0.08 (−0.25 to 0.09) | −0.12 (−0.44 to 0.21) |
| CON | 2.56 (0.74) | 2.45 (0.63) | 0.04 (−0.24 to 0.31) | |
| PSA, μg/L † , ‡ | ||||
| EX | 11.82 (11.79) | 10.69 (10.62) | 0.881 (0.80 to 0.97) | 0.889 (0.75 to 1.06) |
| CON | 15.71 (13.91) | 16.31 (13.69) | 0.992 (0.86 to 1.15) | |
Means (SD) are based on all available data for EX and CON at baseline and follow‐up (intention‐to‐treat analysis EX, n = 20 at baseline and follow‐up; CON, baseline n = 10 and follow‐up n = 8). The mean differences are estimated means from the mixed models and therefore, within‐group change may not reflect the numerical difference between baseline and follow‐up.
VO2peak data are based on cardiorespiratory tests meeting two of the following test criteria: respiratory exchange ratio (RER) ≥1.10, rate of perceived exertion (RPE) ≥18, and a VO2 plateau (EX, baseline n = 18, follow‐up n = 17; CON, baseline and follow‐up n = 8).
PSA and hs‐CRP are analysed on log‐transformed data and estimates for within‐group changes and between‐group differences are back‐transformed and reported as median relative changes/differences with back‐transformed 95% CIs.
PSA follow‐up analysis is based on n = 19, due to missing data.
Patient‐Reported Outcome Measures
Data for PROMs are presented in Table S6 and S8. No between‐group changes were observed for the health‐related quality of life for neither the total FACT‐P score nor any subdomains. Similarly, we did not observe any between‐group changes regarding anxiety or depression, neither with the intention‐to‐treat nor per‐protocol analysis.
Exploratory Analyses
Post hoc analyses in the EX group revealed a positive correlation between the change in tumour NK‐cell infiltration and the total number of training sessions (Fig. 3a). Similarly, we observed a significant positive correlation between the total number of training sessions and the change in absolute VO2peak (L/min) and W peak (Fig. 3b,c).
Fig. 3.
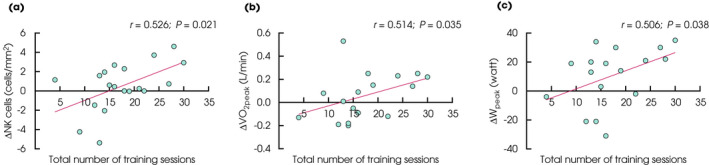
Correlations between the total number of training sessions and change in (a) tumour NK‐cell infiltration (n = 19), (b) VO2peak (L O2/min) (n = 17), and (C) W peak (n = 17).
Discussion
Investigation of the potential effects of physical exercise training on tumour biology has been a focus of significant interest over the last 20 years [1]. Preclinical studies have demonstrated a direct effect of exercise interventions on tumour growth and incidence [3]. Our earlier discovery showed that wheel running in mice led to the release and redistribution of adrenaline‐sensitive NK cells, which infiltrated tumours and subsequently led to suppression of tumour growth, in part, by an IL‐6‐dependent mechanism [6]. Yet, there is a lack of translational evidence to a patient setting. Here, we present the first study specifically designed to explore the direct effects of exercise on tumour immune cell infiltration in a human setting.
The main finding of the present study was that a preoperative HIIT intervention did not increase tumour NK‐cell infiltration, indicating that it was not possible or feasible to significantly modify tumour NK‐cell profile within the preoperative window. However, the duration of the intervention varied substantially, resulting in large differences in exercise dose, with a total number of training sessions ranging from four to 30. These large differences in exercise dose are also highlighted by the lack of changes in expected exercise adaptations, e.g., VO2peak (on group level), and should be considered when interpreting the findings of the present study.
To investigate the potential impact of the varying length of the intervention, we performed a per‐protocol analysis, including the participants in the EX group attending a minimum of 75% of total training sessions in 5 weeks. This analysis showed a significant within‐group increase of 1.60 cells/mm2 (95% CI 0.59 to 2.62; P = 0.004) in tumour NK cells, and a between‐group difference in NK‐cell infiltration in healthy prostatic tissue. With both the intention‐to‐treat and per‐protocol analyses there was a high inter‐individual variability between participants. Also, we found a positive correlation between the number of training sessions and tumour NK‐cell infiltration (r = 0.526, P = 0.021). These findings suggest that exercise training may impact tumour NK‐cell infiltration, but this likely requires a considerable exercise dose (intensity and volume).
To date, there have only been few studies reporting the effects of exercise training on tumour biology in a human setting [1, 15, 16, 17]. One of the first explorative studies investigating the direct effect of aerobic exercise on different host‐ and tumour‐related pathways was performed in women with early‐stage breast cancer during adjuvant chemotherapy [18]. The study demonstrated an increased exercise capacity (VO2peak) and change in various cytokines and angiogenic factors in conjunction with alterations in tumour gene expression in the exercise group compared with chemotherapy alone. Another important window‐of‐opportunity trial studying the effect of exercise on several biological outcomes in women with newly‐diagnosed breast cancer awaiting surgery did not show an effect of exercise on the expression of the proliferative marker Ki‐67, which was the main outcome of the study [19]. However, pathways related to NK cell‐mediated cytotoxicity were found to be upregulated in breast tumour tissue in the exercise group only, providing some of the first evidence that exercise could directly affect human breast cancer.
Hence, studying the direct effects of exercise on human tumours proves to be challenging. In the present study with patients with localised PCa, the special treatment trajectory provided a unique opportunity to collect tumour tissue following participation in an exercise study, making this patient group an ideal model for investigating our hypothesis. However, given the pragmatic study design, combined with the multifocal nature of PCa with several potential tumour foci, complicating tissue selection for NK‐cell quantification, it is evident that assessment of tumour NK‐cell infiltration by exercise in humans is arduous.
The present study was designed as a proof‐of‐concept trial to examine if preoperative HIIT could modify tumour NK‐cell infiltration. The clinical implications of the findings, therefore, by nature, remain uncertain. Given that the degree of tumour immune cell infiltration has been recognised as a strong predictor of clinical outcome in several cancers [20, 21], and that exercise activates NK‐cell cytotoxicity, together with the findings of the present study indicating a dose–response relationship between exercise and tumour NK‐cell infiltration, it could be speculated that this could have positive implications on treatment efficacy by enhancing the cytotoxic potential of anti‐cancer therapies. Thus, exercise potentially could play a synergistic role in the treatment trajectory of patients with cancer together with conventional anti‐cancer treatments.
The present study has acknowledgeable limitations. First, our data are based on a small sample size. No data are available to perform power calculations, and the sample size is therefore pragmatic, making our study prone to type 2 errors. Second, due to recruitment difficulties, changes were made in the study design resulting in substantial variations in the length of the exercise intervention period. Third, contamination could have affected our results, i.e., increased physical activity in the CON group. Lastly, the participants in both groups demonstrated high cardiorespiratory fitness levels at baseline, likely reflecting an active lifestyle of the participants. We did not control for training status; thus, if tumour NK‐cell infiltration occurs in response to repeated bouts of exercise, the inclusion of already active participants could have affected our results. Lastly, the present study focused on NK cells, but other immune cells are also of interest in the context of exercise and anti‐cancer immunity [22].
In summary, supervised HIIT in patients with early‐stage PCa scheduled for RP did not result in increased tumour NK‐cell infiltration compared with usual care. For the participants fulfilling the per‐protocol criteria there was an effect of exercise on tumour NK‐cell infiltration. These findings are novel and provide pivotal translational data linking novel discoveries in animal models to a clinical setting. The findings of the present studies should be used to power future studies testing the capability of exercise to increase tumour immune cell infiltration.
Funding
This study was funded by TrygFonden and Lundbeckfonden.
Conflict of Interest
The authors declare no conflict of interests.
Author Contributions
Study concept, design, and protocol writing: Sissal Sigmundsdóttir Djurhuus, Jesper Frank Christensen, Bente Klarlund Pedersen, Klaus Brasso; Funding acquisition: Bente Klarlund Pedersen, Jesper Frank Christensen; Data collection and acquisition: Sissal Sigmundsdóttir Djurhuus, Simon Nørskov Thomsen, Sabrina Wielsøe, Thomas Hasselager, Birgitte Grønkær Toft; Patient recruitment: Klaus Brasso, Martin Andreas Røder, Sissal Sigmundsdóttir Djurhuus, Peter Busch Østergren, Henrik Jakobsen; Statistical analysis: Sissal Sigmundsdóttir Djurhuus, Casper Simonsen; Data analysis and interpretation: Sissal Sigmundsdóttir Djurhuus, Birgitte Grønkær Toft, Jesper Frank Christensen, Casper Simonsen, Bente Klarlund Pedersen, Klaus Brasso; Manuscript preparation: Sissal Sigmundsdóttir Djurhuus, Jesper Frank Christensen; Critical review and edit of the final version of the manuscript: all authors; Project supervision: Jesper Frank Christensen, Casper Simonsen, Birgitte Grønkær Toft, Bente Klarlund Pedersen, Klaus Brasso.
Ethics Approval and Consent for Participation
The study was approved by the local Ethics Committee of the Capital Region of Denmark and conducted in accordance with the Declaration of Helsinki. All participants provided signed informed consent before any study‐related procedures were performed.
Supporting information
Figure S1 Schematic overview of training adherence, individual exercise attendance, and missed sessions for each participant in the EX group, including exercise intensity and progression throughout the intervention period.
Table S1 Exercise intervention.
Table S2 Immunohistochemistry and NK‐cell quantification.
Table S3 Cardiorespiratory fitness assessment.
Table S4 Deviations from pre‐registered protocol.
Table S5 Physiological outcomes (intention‐to‐treat analysis).
Table S6 Patient‐reported outcome measures (intention‐to‐treat analysis).
Table S7 Physiological outcomes (per‐protocol analysis).
Table S8 Patient‐reported outcome measures (per‐protocol analysis).
Acknowledgements
The authors thank all clinicians from the recruiting departments at Rigshospitalet and Herlev and Gentofte Hospital for their help with patient recruitment. We also thank Anna Sundberg, Sarah Thorsen‐Streit, Stine Mørup, Mark Lyngbæk, and Anita Herrstedt for their assistance with test assessments and medical screening of participants. Anne Kirstine Lundby, Lene Foged, Anne Jørgensen, and Ida Holm are acknowledged for their technical assistance. Niels Bo Hansen and Nadine Hammouda from the Pathology Department at Rigshospitalet are acknowledged for their excellent assistance during the study. Finally, we wish to extend our deepest gratitude and appreciation to former CFAS Group Leader Dr Pernille Hojman, who sadly passed away during the study. She was invaluable to the research of our group and played a pivotal role in the conception of this study.
Registration number: NCT02954783 (www.clinicaltrials.gov).
References
- 1. Christensen JF, Simonsen CHP. Exercise training in cancercontrol and treatment. Compr Physiol 2018; 9: 165–205 [DOI] [PubMed] [Google Scholar]
- 2. Mctiernan A, Friedenreich CM, Katzmarzyk PT, et al. Physical activity in cancer prevention and survival: a systematic review. Vol. 51, Medicine and Science in Sports and Exercise. 2019. pp. 1252–61. [DOI] [PMC free article] [PubMed]
- 3. Ashcraft KA, Peace RM, Betof AS, Dewhirst MW, Jones LW. Efficacy and mechanisms of aerobic exercise on cancer initiation, progression, and metastasis: a critical systematic review of in vivo preclinical data. Cancer Res 2016; 76: 4032–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pedersen L, Christensen JF, Hojman P. Effects of exercise on tumor physiology and metabolism. Cancer J 2015; 21: 111–6 [DOI] [PubMed] [Google Scholar]
- 5. Courneya KS, Segal RJ, McKenzie DC et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med Sci Sports Exerc 2014; 46: 1744–51 [DOI] [PubMed] [Google Scholar]
- 6. Pedersen L, Idorn M, Olofsson GH et al. Voluntary running suppresses tumor growth through epinephrine‐ and IL‐6‐dependent NK cell mobilization and redistribution. Cell Metab 2016; 23: 554–62 [DOI] [PubMed] [Google Scholar]
- 7. Pedersen BK, Tvede N, Hansen FR et al. Modulation of natural killer cell activity in peripheral blood by physical exercise. Scand J Immunol 1988; 27: 673–8 [DOI] [PubMed] [Google Scholar]
- 8. Gabriel H, Urhausen A, Kindermann W. Mobilization of circulating leucocyte and lymphocyte subpopulations during and after short, anaerobic exercise. Eur J Appl Physiol Occup Physiol 1992; 65: 164–70 [DOI] [PubMed] [Google Scholar]
- 9. Idorn M, Hojman P. Exercise‐dependent regulation of NK cells in cancer protection. Trends Mol Med 2016; 22: 565–77 [DOI] [PubMed] [Google Scholar]
- 10. Fossati N, Rossi MS, Cucchiara V et al. Evaluating the effect of time from prostate cancer diagnosis to radical prostatectomy on cancer control: can surgery be postponed safely? Urol Oncol Semin Orig Investig 2017; 35: 150e9–15. Available at: 10.1016/j.urolonc.2016.11.010. Accessed 20 June 2021 [DOI] [PubMed] [Google Scholar]
- 11. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–70 [DOI] [PubMed] [Google Scholar]
- 12. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale. J Psychosom Res 2002; 52: 69–77 [DOI] [PubMed] [Google Scholar]
- 13. Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy‐prostate instrument. Urology 1997; 50: 920–8 [DOI] [PubMed] [Google Scholar]
- 14. Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed‐effects models using lme4. J Stat Softw 2015; 67: 48 [Google Scholar]
- 15. Hvid T, Lindegaard B, Winding K et al. Effect of a 2‐year home‐based endurance training intervention on physiological function and PSA doubling time in prostate cancer patients. Cancer Causes Control 2016; 27: 165–74 [DOI] [PubMed] [Google Scholar]
- 16. Hojan K, Kwiatkowska‐Borowczyk E, Leporowska E, Milecki P. Inflammation, cardiometabolic markers, and functional changes in men with prostate cancer. A randomized controlled trial of a 12 month exercise program. Polish Arch Intern Med 2017; 127: 25–35 [DOI] [PubMed] [Google Scholar]
- 17. Chiarotto JA, Akbarali R, Bellotti L, Dranitsaris G. A structured group exercise program for patients with metastatic cancer receiving chemotherapy and CTNNB1 (beta‐catenin) as a biomarker of exercise efficacy. Cancer Manag Res 2017; 9: 495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones LW, Fels DR, West M et al. Modulation of circulating angiogenic factors and tumor biology by aerobic training in breast cancer patients receiving neoadjuvant chemotherapy. Cancer Prev Res 2013; 6: 925–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ligibel JA, Dillon D, Giobbie‐Hurder A et al. Impact of a pre‐operative exercise intervention on breast cancer proliferation and gene expression: results from the pre‐operative health and body (PreHAB) study. Clin Cancer Res 2019; 25: 5398–406 [DOI] [PubMed] [Google Scholar]
- 20. Zhang L, Conejo‐Garcia JR, Katsaros D et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348: 203–13 [DOI] [PubMed] [Google Scholar]
- 21. Galon J, Costes A, Sanchez‐Cabo F et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science (80‐) 2006; 313: 1960–4 [DOI] [PubMed] [Google Scholar]
- 22. Fiuza‐Luces C, Valenzuela PL, Castillo‐García A, Lucia A. Exercise benefits meet cancer immunosurveillance: Implications for immunotherapy. Trends Cancer 2021; 7: 91–3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Schematic overview of training adherence, individual exercise attendance, and missed sessions for each participant in the EX group, including exercise intensity and progression throughout the intervention period.
Table S1 Exercise intervention.
Table S2 Immunohistochemistry and NK‐cell quantification.
Table S3 Cardiorespiratory fitness assessment.
Table S4 Deviations from pre‐registered protocol.
Table S5 Physiological outcomes (intention‐to‐treat analysis).
Table S6 Patient‐reported outcome measures (intention‐to‐treat analysis).
Table S7 Physiological outcomes (per‐protocol analysis).
Table S8 Patient‐reported outcome measures (per‐protocol analysis).


