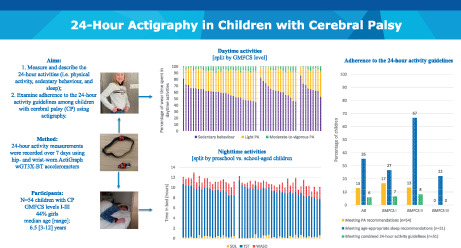
Abstract
Aim
To measure and describe the 24‐hour activities (i.e. physical activity, sedentary behavior, and sleep) and to examine adherence to the 24‐hour activity guidelines among children with cerebral palsy (CP) using actigraphy.
Method
Children's 24‐hour activities were recorded over 7 days using hip‐ and wrist‐worn ActiGraph wGT3X‐BT accelerometers.
Results
In total, 362 days and 340 nights from 54 children with CP (Gross Motor Function Classification System [GMFCS] levels I–III; 44% females; median age [range] 6 years 6 months [3–12 years]) were included. Mean (SD) daily wear time was 746.2 (48.9) minutes, of which children spent on average 33.8% in light physical activity (251.6 [58.7] minutes per day), 5.2% in moderate‐to‐vigorous physical activity (38.5 [20.1] minutes per day), and the remaining 61.1% being sedentary (456.1 [80.4] minutes per day). Physical activity decreased while sedentary behavior increased with increasing GMFCS level. In total, 13% of all children met the physical activity recommendations, and 35% met the age‐appropriate sleep duration recommendation. The proportion of children meeting the combined 24‐hour guidelines for physical activity and sleep was low (5.9%), especially in those classified in GMFCS level III (0%).
Interpretation
The observed low 24‐hour guideline adherence rates emphasize the importance of considering the entire continuum of movement behaviors in the care of children with CP, in efforts to promote healthy lifestyle behaviors and prevent negative health outcomes.
Visual abstract of the adherence to the 24‐hour activity guidelines.
Abbreviations
- LPA
light physical activity
- MVPA
moderate‐to‐vigorous physical activity
- SOL
sleep onset latency
- TIB
time in bed
- TST
total sleep time
- WASO
wakefulness after sleep onset
What this paper adds
Physical activity decreases while sedentary behavior increases with increasing Gross Motor Function Classification System level in children with cerebral palsy (CP).
Only 13% of children with CP meet the daily physical activity recommendation.
Only 35% of children with CP meet the age‐appropriate sleep duration recommendation.
The proportion of children meeting the CP‐specific 24‐hour activity guidelines is low.
Physical activity, sedentary behavior, and sleep, collectively known as movement behaviors, are modifiable lifestyle factors affecting child health and well‐being. In the past decade, accumulating evidence has highlighted the independent health benefits of high physical activity levels (especially moderate‐to‐vigorous physical activity [MVPA]), low sedentary behavior, and sufficient hours of good quality sleep among children. 1 More recently, the collective importance of these behaviors has been recognized under the concept that ‘the whole day matters’, which has led to the development of integrated 24‐hour movement guidelines for children. 2 Good health outcomes may be attained by optimizing the balance between physical activity, sedentary behavior, and sleep for the individual child. Although the health benefits of meeting the 24‐hour movement guidelines are well established for typically developing children, 2 , 3 little is known about the 24‐hour activities of children with physical disabilities.
Cerebral palsy (CP) is a common cause of physical disability in childhood. Children with CP demonstrate reduced physical activity levels and engage in significantly more sedentary time than their typically developing peers. 4 , 5 In addition, children with CP are more likely to have sleep problems than typically developing children, and include difficulties falling asleep, frequent night awakenings, pain or discomfort in bed, and waking up too early. 6 Along with reduced sleep quality, a large proportion (41%) of children with CP aged 4 to 18 years were reported to have a sleep duration of less than 8 hours per night, 7 compared with the recommended 8 to 13 hours of sleep per day for preschool‐aged children up to teenagers, 8 suggesting that these children may not be getting enough sleep. Given that sleep is an important determinant for health‐related quality of life in children with CP, 9 together with the high prevalence of inactive lifestyles, and the concomitant risk of chronic health conditions in adults with CP, 10 it is likely that this population stands to benefit from meeting the 24‐hour movement guidelines throughout childhood and adolescence. It is therefore no surprise that the 24‐hour activity guideline approach has been adopted for children with CP in a recently published clinical practice guide 11 to promote healthy lifestyle behaviors and prevent negative health outcomes in this vulnerable population.
Studies reporting on 24‐hour movement behaviors and guideline adherence in pediatric populations with heterogeneous conditions have predominantly relied on subjective measures such as surveys or questionnaires. 12 , 13 , 14 In children with CP, the few studies that have measured physical activity and sedentary behavior objectively either did not include sleep as part of the 24‐hour activities 5 , 15 or assessed sleep using parental reports, 16 which can be unreliable and introduce different sources of reporting bias. 17 For example, subjective reports have been shown to consistently overestimate sleep duration. 18 In contrast, actigraphy provides a device‐based measure of activity frequency, duration, and intensity, and is increasingly used to assess physical activity and sedentary behavior in children. 19 Actigraphy is also recognized as a valid measure to quantify periods of sleep and wakefulness. 17 Nevertheless, no studies have objectively examined the combined 24‐hour activities and guideline adherence in children with CP using solely device‐based measures. Therefore, the aims of the present study were to (1) measure and describe the 24‐hour activities (i.e. physical activity, sedentary behavior, and sleep) and (2) examine adherence to the 24‐hour activity guidelines for physical activity and sleep among a sample of children with CP using actigraphy. Additionally, group comparisons were explored across the levels of functional ability (Gross Motor Function Classification System [GMFCS] levels I–III) and by age groups (preschool and school‐age).
METHOD
A multicenter, cross‐sectional, observational study was conducted. The study was approved by the Medical Ethics Research Committee of the University Medical Center Utrecht (file number 19–630), the Netherlands.
Participants
Children with CP were recruited from the outpatient clinics of five rehabilitation settings (three pediatric rehabilitation centers, a school for special education, and the rehabilitation department of a children's hospital) in the Netherlands. Inclusion criteria were children (1) diagnosed with CP; (2) classified in GMFCS levels I to III (i.e. able to walk independently with or without an assistive device), since actigraphy has been validated only in ambulatory children with CP; and (3) between the ages of 3 and 12 years. This age range was chosen to allow us to describe adherence to the international, age‐appropriate sleep duration recommendations for preschool (3–5 years) and school‐aged (6–12 years) children. 8 Parents of children who met the inclusion criteria were informed of the study by their healthcare professional. All parents and children aged 12 years provided written consent/assent before participation in the study. Consenting families were briefed by telephone and received a box with study materials (accelerometers, activity logbooks, and a sleep diary) along with instructions. Measurements took place in the child's home environment, while the researcher was available to address any questions or issues by telephone or e‐mail. Upon completion of the study week, parents returned the study materials.
24‐hour activity measurements
Device
Habitual physical activity, sedentary behavior, and sleep were measured using the ActiGraph wGT3X‐BT triaxial accelerometer (ActiGraph Corporation, Pensacola, FL, USA) (Figure 1). At the beginning of each data collection session, the device was initialized to collect data at a resolution of 30 Hz using the Actilife software version 6.13.4 (ActiGraph Corporation, Pensacola, FL, USA). Although more challenging than in typically developing children (e.g. because of hypersensitivity), accelerometry has been validated for the measurement of sedentary time and physical activity in ambulatory children and adolescents with CP, 20 and has also been shown to be a feasible and acceptable measure of activity in this population. 21
FIGURE 1.

The ActiGraph wGT3X‐BT triaxial accelerometers (upper left) worn around a child's wrist using a watch strap during the night (upper right), and worn around the hip using an elastic belt (lower left) covered by clothing (lower right) during the day.
Data collection
Participants were instructed to wear the accelerometer for 24 hours per day over 7 consecutive days. A 7‐day period was selected to ensure that the measured activities, accounting for missing days/nights due to technical failures or unforeseen circumstances, was representative of habitual physical activity and sleep measures. 22 Typical accelerometer placement site for physical activity measurements is the hip as it allows detection of the body's acceleration and deceleration during locomotion, while wrist placement is generally used for sleep assessment to optimize the recording of small movements that occur at the distal extremities when the individual is in supine position. 17 Therefore, in this study, participants were instructed to wear the accelerometer over the right hip, at the mid‐axillary line, using an elastic belt during waking hours, and to exchange accelerometer placement upon bedtime to the non‐dominant wrist using a watch strap (Figure 1); after getting out of bed the next morning, accelerometer placement was changed back to the hip, etc. Parents and teachers/educators were asked to record the times that the accelerometer was worn and removed in an activity log, and to record naps and overnight bed times in a sleep diary. Accelerometers were to be removed during water‐based activities, and all times and reasons for non‐wear were recorded in the activity log. Upon completion of the study week, accelerometers and diaries were obtained from the participants and data were extracted and processed for further analyses. Data were collected between January and December 2020 during non‐holiday periods. Owing to the COVID‐19 pandemic, data collection was paused during the school lockdown period from mid‐March to mid‐April 2020.
Data validation and processing
Raw accelerometer data were downloaded and converted into 15‐second epochs for hip‐worn physical activity and sedentary behavior data, while sleep data were downloaded into 60‐second epochs. Data were visually inspected for any technical malfunctions or spurious counts. All analyses were completed in Actilife software (ActiGraph Corporation, Pensacola, FL, USA).
Physical activity and sedentary behavior
Activity data from the hip‐worn devices were cleaned using a semi‐automated protocol, whereby any 5 minutes of zero counts were flagged and verified. Only non‐wear periods identified in the participant logbook were excluded. The average vertical axis activity counts and combined triaxial average vector magnitude activity counts were extracted. Activity counts were normalized to wear time to account for differences in device wear time among participants, and reported as counts per minute of wear time. Next, activity by intensity was calculated, and included sedentary behavior, light physical activity (LPA), and MVPA, as defined by the cut‐points of Evenson et al. (using the vertical axis counts). 23 These cut‐points have been validated in ambulatory children with CP, 20 and have since then been used in several studies among children, young people, and adolescents with CP. 5 , 15 , 21 To support comparisons across studies, we also determined time spent in sedentary behavior, LPA, and MVPA using the CP‐specific cut‐points developed by Trost et al. 24 Activities by intensity are presented as percentages of total wear time. Only data from participants who met the minimum wear time of at least 6 hours a day on at least 4 days with at least 1 weekend day (Saturday or Sunday) were included in the analyses. The estimated reliability coefficient for 4 days of wear in ambulatory children with CP was 0.73 (95% confidence interval 0.58–0.83), and an average of 6 days of wear would achieve reliability of 0.8. 25
Sleep
Data from the wrist‐worn devices were manually and visually inspected against the sleep diary records containing the child's bedtimes (in bed, lights off, out of bed). The difference between lights off and out‐of‐bed times (or between in‐bed and out‐of‐bed times, in those cases when lights were turned off after sleep onset), as noted in the sleep diary, were used to calculate total time in bed (TIB) and subsequently used to define the start and end of the sleep analysis period. Note that TIB includes time out of bed during night‐time awakenings, a manifestation of sleep discontinuity. Within the identified TIB, the algorithm of Sadeh et al., 26 the most commonly used algorithm for sleep–wake determination for children, 17 was used to calculate sleep onset latency (SOL; the time between lights off and the first epoch scored as sleep), total sleep time (TST; the sum of all periods scored as sleep), and wakefulness after sleep onset (WASO; the sum of all periods scored as awake after sleep onset); these outcomes are reported in average daily hours. Sleep efficiency was calculated as the ratio of TST to TIB (as a percentage). A night was excluded when the difference between sleep times identified by the algorithm deviated more than 10% from the bed times entered in the sleep diary. To our knowledge, there are no established recommendations for the number of nights for sleep data; however, two existing studies in younger children (3–7 years of age) 27 and adolescents (11–16 years) 28 suggest that 4 to 7 days of wear are needed to achieve a minimum reliability of 0.7 for sleep outcomes derived from the Sadeh algorithm. Therefore we applied the same minimum number of nights for sleep analyses as for the analyses of physical activity. Specifically, only participants with valid sleep measures for at least 4 nights with at least 1 weekend night (i.e. Friday to Saturday, or Saturday to Sunday) were included in the analyses.
Criteria for 24‐hour activity guideline adherence
To describe guideline adherence, the 24‐hour activity guidelines for children with CP 11 were applied, from which the following operational definitions were derived and applied in our study.
Physical activity
Children were classified as meeting the physical activity recommendations if their average daily MVPA was at least 60 minutes per day.
Sedentary behavior
For sedentary behavior, the guidelines recommend (1) ‘no more than 2 hours per day of recreational screen time’ and (2) ‘limit sitting for extended periods’. 11 Because no measures for screen time were included in this study to assess the first recommendation, and there is no specification on the length of the sedentary period in the second, we could not quantify participants' adherence to the sedentary behavior component of the guidelines. Therefore, the sedentary behavior component is not included in the 24‐hour guideline adherence but rather reported descriptively as the proportion of wear time (percentage) and total time (minutes per day) spent in sedentary behavior, as outlined in the section on ‘Daytime activities'.
Sleep
Children were classified as meeting the sleep duration recommendations if their average daily TST was between 10 and 13 hours per night for preschool children (aged 3–5 years) and 9 to 11 hours per night for school‐aged children (aged 6–12 years).
Statistics
Data were analyzed using IBM SPSS Statistics version 26 (IBM Corp., Armonk, NY, USA). Descriptive statistics were calculated and variables were visually inspected for normality on the basis of their boxplots and quantile–quantile plots. Additionally, Shapiro–Wilk tests were performed to test the assumption for normality, and age was the only variable not normally distributed and was therefore reported as median. Next, day and night values were averaged per child, normalized to wear days and daily wear times, and analyzed for all children with CP and across subgroups for GMFCS level (I–III) and by age group (preschool and school‐age). Levene's tests were conducted to assess the homogeneity of variances of accelerometer variables across (sub)groups. As none of the variables had significant Levene's test results, we were confident to proceed with parametric statistics. Between‐group differences were assessed using analysis of covariance, with GMFCS level and age group as fixed factors, and sex, age, or GMFCS level entered as covariates (depending on the group comparisons). Tukey's post hoc tests were performed between subgroups (GMFCS level) with Bonferroni corrections for multiple pairwise comparisons. To describe 24‐hour guideline adherence, the proportions of children meeting the individual and combined recommendations for physical activity and sleep were calculated, and group differences were assessed using Fisher's exact test. The level of significance was set at p < 0.05.
RESULTS
Study group characteristics
In total, 60 children with CP participated in this study. Six children were excluded because no actigraphy data were collected owing to hypersensitivity (n = 4), unwillingness of the child to wear an accelerometer on their body (n = 1), or unknown reasons (n = 1). Therefore, the final study sample consisted of 54 children (median age 6 years 6 months), of whom 30 (55.6%) were male (median age 7 years) and 24 (44.4%) were female (median age 6 years); 38.9% were categorized into the preschool age group (3–5 years) and 61.1% into the school‐aged (6–12 years) group. Most (88.9%) of the children presented with a spastic CP and just over half of the sample (55.6%) were classified in GMFCS level I. Detailed characteristics of the total study sample, and by GMFCS level, are presented in Table 1. No statistically significant age differences were observed across sex (Mann–Whitney U test, p = 0.243) and GMFCS level (Kruskal–Wallis test, p = 0.064). Similarly, the two age groups did not differ with respect to sex (Fisher's exact test, p = 0.263) or GMFCS level (Fisher's exact test, p = 0.052), and the proportion of males/females was not different across GMFCS levels (Fisher's exact test, p = 0.347).
TABLE 1.
Characteristics of the total study sample and by Gross Motor Function Classification System (GMFCS) level
| All children n = 54 | GMFCS level I n = 30 | GMFCS level II n = 15 | GMFCS level III n = 9 | p | |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Female | 24 (44.4) | 11 (36.7) | 9 (60) | 4 (44.4) | 0.347 |
| Male | 30 (55.6) | 19 (63.3) | 6 (40) | 5 (55.6) | |
| Age, years:months | |||||
| Range | 3–12 | 3–12 | 5–11 | 3–8 | |
| Median | 6:6 | 6 | 7 | 4 | 0.064 |
| Age group, n (%) | |||||
| Preschool (3–5 years) | 21 (38.9) | 14 (46.7) | 2 (13.3) | 5 (55.6) | 0.052 |
| School (6–12 years) | 33 (61.1) | 16 (53.3) | 13 (86.7) | 4 (44.4) | |
| CP subtype, n (%) | |||||
| Spastic | 48 (88.9) | 26 (86.7) | 14 (93.3) | 8 (88.9) | 0.884 |
| Dyskinetic | 2 (3.7) | 2 (6.7) | — | — | |
| Mixed | 3 (5.6) | 1 (3.3) | 1 (6.7) | 1 (11.1) | |
| Unclassified/unknown | 1 (1.9) | 1 (3.3) | — | — | |
| Affected side, n (%) | |||||
| Unilateral | 34 (63) | 23 (76.7) | 10 (66.7) | 1 (11.1) | 0.002 a |
| Bilateral | 20 (37) | 7 (23.3) | 5 (33.3) | 8 (88.9) |
Affected side was not evenly distributed across different levels of GMFCS. CP, cerebral palsy.
Daytime activities: physical activity and sedentary behavior
For daytime activities (i.e. sedentary behavior, LPA, and MVPA), out of the total 367 days measured, 5 (1.4%) days (deriving from four children) were excluded from physical activity analysis owing to non‐wear (sickness, n = 1, hip monitor not worn tightly around the waist, n = 1, not meeting the minimum daily wear time of ≥6 hours, n = 3). The final sample with valid physical activity data comprised 54 children, from which a total of 362 days were included in the daytime analyses. Children had a mean (SD) of 6.7 (0.7) valid wear days (26% wore the device for 4 to 6 days; 72% had 7 days of wear time; 2% wore the device between 8 and 9 days) with a mean (SD) daily wear time of 746.2 (48.9) minutes per day (12.4 [0.8] hours per day) (Table 2).
TABLE 2.
Accelerometer‐based data for physical activity, sedentary behavior, and sleep, for all children and by Gross Motor Function Classification System (GMFCS) level
| All children | GMFCS level I | GMFCS level II | GMFCS level III | |
|---|---|---|---|---|
| Physical activity data, a mean (SD) | n = 54 | n = 30 | n = 15 | n = 9 |
| Daily wear time (minutes per day) | 746.2 (48.9) | 750.4 (50.2) | 756.6 (42.3) | 715.1 (47.4) |
| Sedentary behavior (% of wear time) | 61.1 (9.6) | 58.8 (8.7) | 62.1 (10.6) | 66.9 (9.2) |
| Light physical activity (% of wear time) | 33.8 (7.8) | 35.3 (7.5) | 33.2 (8.3) | 29.7 (7.2) |
| Moderate‐to‐vigorous physical activity (% of wear time) | 5.2 (2.7) | 5.9 (2.3) | 4.7 (3.1) | 3.5 (2.7) |
| Total moderate‐to‐vigorous physical activity (minutes per day) | 38.5 (20.1) | 44.3 (17.3) | 35.4 (22.8) | 24.2 (17.7) |
| Sleep data, b mean (SD) | n = 51 | n = 30 | n = 12 | n = 9 |
| Time in bed, hours | 11.0 (0.8) | 10.9 (0.8) | 10.9 (0.6) | 11.4 (0.8) |
| Total sleep time, hours | 9.0 (0.8) | 8.9 (0.8) | 9.1 (0.9) | 9.4 (0.9) |
| Sleep onset latency, hours | 0.3 (0.2) | 0.3 (0.2) | 0.3 (0.2) | 0.3 (0.3) |
| Wakefulness after sleep onset, hours | 1.6 (0.6) | 1.6 (0.6) | 1.5 (0.7) | 1.7 (0.8) |
| Sleep efficiency (%) | 82.4 (6.0) | 82.0 (5.6) | 83.4 (6.9) | 82.8 (6.6) |
Derived from the hip‐worn device.
Derived from the wrist‐worn device.
To gain a better understanding of how children with CP accumulate their daytime activities, the average daily time spent in sedentary behavior, LPA, and MVPA was calculated and normalized to actual wear time. Figure 2 shows the proportion (percentage) of time spent in each of the different physical activity intensities for all individual children clustered by GMFCS level, sorted from highest to lowest percentage of sedentary behavior (Figure 2a). Subgroup data are shown across GMFCS level (Figure 2b) and age group (Figure 2c).
FIGURE 2.
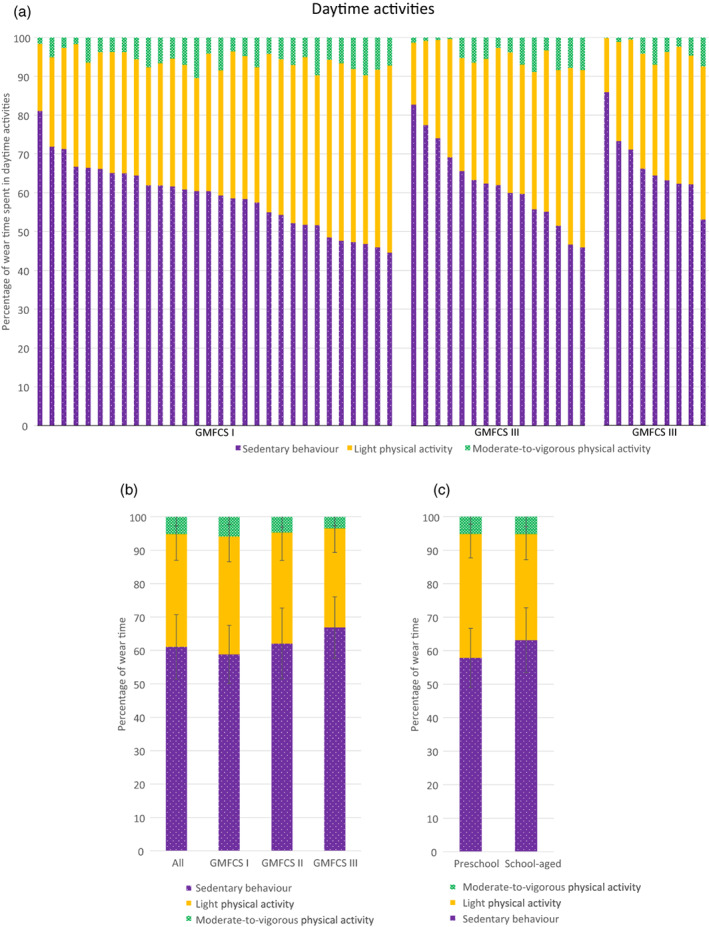
Normalized wear time spent in sedentary behavior, light physical activity, and moderate‐to‐vigorous physical activity for (a) all individual participants clustered by Gross Motor Function Classification System (GMFCS) level and sorted from highest to lowest sedentary behavior, and subgroup data across (b) GMFCS level and (c) age group.
On average, children spent 33.8% of their total wear time engaging in LPA (mean [SD], 251.6 [58.7] minutes per day), 5.2% (38.5 [20.1] minutes per day) in MVPA, and the remaining 61.1% of their time (456.1 [80.4] minutes per day) being sedentary.
GMFCS level
Average daily wear time was equal across the different GMFCS levels (Table 2). After controlling for sex and age, a significant effect of GMFCS level was found on the percentage of wear time spent in sedentary behavior (F 2,49 = 6.1, p < 0.01), LPA (F 2,49 = 5.8, p < 0.01), and MVPA (F 2,49 = 3.4, p < 0.05). Despite the significant effect for GMFCS level, post hoc tests revealed no significant differences between individual GMFCS levels after correcting for multiple pairwise comparisons. Sedentary time tended to increase with increasing GMFCS level from I (58.8%; 441.9 [74.9] minutes per day) to III (66.9%; 478.0 [69.0] minutes per day), while the opposite pattern was found for time spent in MVPA, which tended to decrease from level I (5.9%; 44.3 [17.2] minutes per day) to level III (3.5%; 24.2 [17.8] minutes per day) (Table 1). The covariate age was significantly related to the percentage of wear time spent in sedentary behavior (F 1,49 = 18.6, p < 0.001) and LPA (F 1,49 = 25.6, p < 0.001) but not on MVPA (p > 0.05).
Age group
Average daily wear time was significantly higher in the school‐aged group (762.9 [46.8] minutes per day) than the preschool‐aged group (720 [40.9] minutes per day; t 52 = −3.4, p = 0.001). Sedentary time in preschool‐aged children (415.5 [60.3] minutes per day) was roughly 1 hour less than that of school‐aged children (482.0 [81.6] minutes per day), while time spent in MVPA was similar (preschool: 37.0 [16.0] minutes per day; school‐aged: 39.4 [16.0] minutes per day). After controlling for GMFCS level and sex, a significant effect of age group was found on the percentage of wear time spent in LPA (F 1,50 = 5.4, p < 0.05), with preschool‐aged children accumulating significantly more time in LPA (37.0%; 267.6 [58.5] minutes per day) than school‐aged children (31.2%; 241.5 [57.4] minutes per day). The covariate GMFCS level was significantly related to the percentage of time spent in sedentary behavior (F 1,50 = 4.7, p < 0.05) and MVPA (F 1,50 = 6.1, p < 0.05).
supporting
Figure S1 shows the count‐based physical activity parameters for the vertical axis and the combined triaxial vector magnitude, both normalized to wear time, for all children and by GMFCS level (Figure S1a) and age group (Figure S1b). Figure S2 shows the percentage of wear time spent in different physical activity intensities using the GMFCS‐specific cut‐points by Trost et al. 24 to support comparisons across studies.
Night‐time activities: sleep and wakefulness
From the total of 54 children, three did not meet the minimum number of valid monitoring nights (≥4 of which 1 weekend night) and were excluded from the sleep analysis. Of the remaining children, a total of 418 nights were recorded, of which 78 (deriving from 24 children) were excluded owing to absence of actigraphy data (n = 52), sickness (n = 4), missing sleep diary data to confirm bed times (n = 6), greater than 10% difference between actigraphy data and bed times stated in the sleep diary (n = 7), accelerometer fell off during the night (n = 1), accelerometer was worn around the ankle (n = 1), or unknown reasons (n = 7). Therefore, the final sample with valid sleep data comprised 51 children, from which a total of 340 nights were included in the sleep analysis. On average, children had 6.7 (0.8) monitored nights (61% had 7 nights of monitoring; 33% wore the device for 5 or 6 nights, and 6% wore the device for 8 or 9 nights). The total time children spent in bed (TIB, in hours) is shown in Figure 3, represented by the total length of the bars. The sleep algorithm further divided TIB into time spent trying to fall asleep (SOL), time asleep (TST), or time being awake (WASO), represented by the different colors. Data are presented for all individual children with valid sleep data (n = 51) clustered by age group in Figure 3a, as well as for subgroups across GMFCS level (Figure 3b) and age group (Figure 3c).
FIGURE 3.
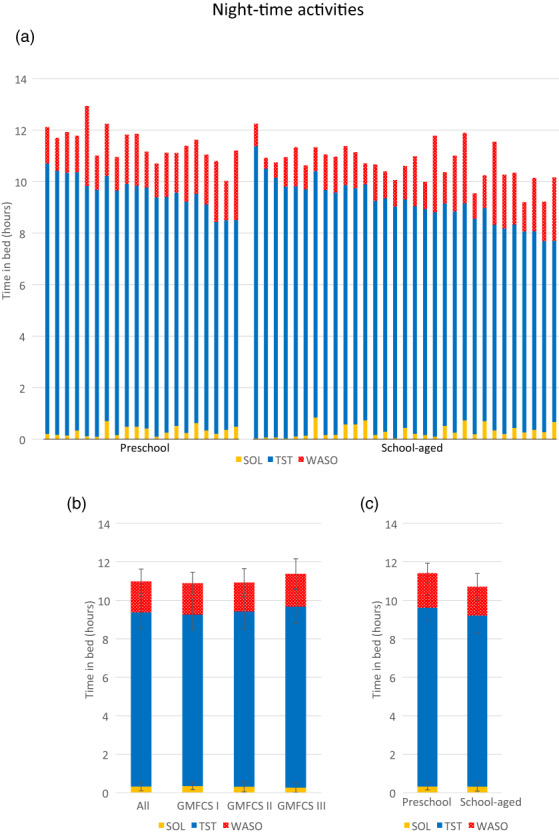
Total time in bed (hours) spent in sleep onset latency (SOL), total sleep time (TST), and wakefulness after sleep onset (WASO) for (a) all individual participants sorted by age group, and subgroup data across (b) Gross Motor Function Classification System (GMFCS) level and (c) age group.
On average, children spent 11.0 (0.2) hours in bed (TIB), of which 9.0 (0.9) hours was asleep (TST), 1.6 (0.6) hours awake (WASO), with a sleep latency (SOL) of 0.3 (0.2) hours (Table 2). Most (78.4%) of the children had an average SOL of less than 30 minutes. The sleep efficiency of the total study sample was 82.4% (6.0), with 21 (41.2%) children having an average sleep efficiency above 85%.
GMFCS level
No significant differences were observed between any of the sleep parameters for GMFCS level, after adjusting for age and sex (Table 2 and Figure 3b).
Age group
After controlling for GMFCS level and sex, a significant effect of age group was found on TIB (F 1,47 = 13.7, p = 0.001), with preschool‐aged children spending more hours in bed (mean 11.4 [0.6] hours) on average than school‐aged children (mean 10.7 [0.7] hours) (Figure 3c). Despite these differences, no differences were found between age groups on average TST and SOL (both p > 0.05). Specifically, preschool‐aged children spent on average 9.3 (0.7) hours asleep compared with 8.9 (0.9) hours for school‐aged children. Preschool‐aged children trended towards spending a greater amount of time (108 [30.5] minutes) awake (i.e. WASO) than school‐aged children (89.6 [41.5] minutes), but this difference failed to reach significance (F 1,47 = 3.6, p = 0.06).
Adherence to the 24‐hour activity guidelines
Figure 4 shows the proportion of children meeting individual components (i.e. the physical activity recommendations and age‐appropriate sleep recommendations) as well as the combined 24‐hour activity guidelines for children with CP, 11 organized by GMFCS level (Figure 4a) and age group (Figure 4b).
FIGURE 4.
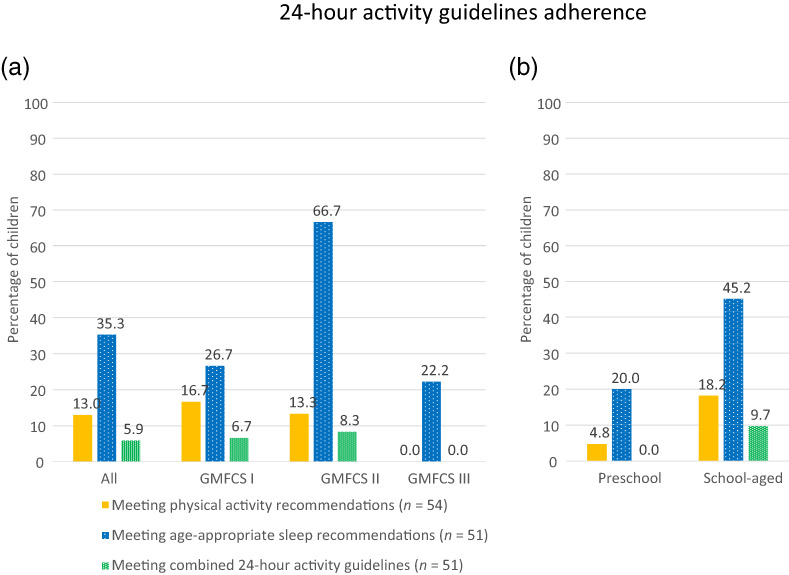
Adherence to individual components of the 24‐hour activity guidelines and adherence to the combined 24‐hour activity guidelines for children with cerebral palsy for (a) all children and by Gross Motor Function Classification System (GMFCS) level and (b) by age group.
Of all children with CP, looking at the individual components of the 24‐hour activity guidelines, a small proportion (13%) met the physical activity recommendations, and one‐third (35.3%) met the age‐appropriate sleep recommendations. In all cases, not meeting the sleep recommendations was due to shortened sleep duration (i.e. too little sleep rather than too much sleep). More than half (56.9%) of all children with CP did not meet the combined recommendations for both physical activity and sleep, while only three (5.9%) children met both the physical activity and sleep recommendations (Figure 4a).
GMFCS level
Significant group differences were observed between GMFCS levels for the proportion of children who did not meet either the physical activity or age‐appropriate sleep recommendations (p < 0.05; Fisher's exact test), and the proportion of children meeting the sleep recommendations alone (p < 0.05; Fisher's exact test, Figure 4a). Children classified in GMFCS level II showed the highest adherence rates for meeting the age‐appropriate sleep recommendations. GMFCS level III showed the highest proportion of children who did not comply with the physical activity or sleep recommendations (77.8%), and the lowest number of children meeting the physical activity recommendations alone (0%), meeting the sleep recommendations alone (22.2%), and meeting the combined 24‐hour activity guidelines (0%).
Age group
With regard to age groups, a higher proportion of preschool‐aged children (75%) failed to meet both the physical activity recommendations as well as the sleep recommendations, compared with 45.2% of school‐aged children (p < 0.05; Fisher's exact test). Of the younger group, a mere 4.8% met the physical activity recommendations, one in five (20%) met the age‐appropriate sleep duration recommendations, and none of them complied with the combined 24‐hour activity guidelines in their entirety (Figure 4b). Of the school‐aged children, 18.2% met the physical activity recommendations, 45.2% met the recommended hours of sleep, and 9.7% complied with the combined 24‐hour activity guidelines.
DISCUSSION
This study describes the 24‐hour activity behaviors (i.e. physical activity, sedentary behavior, and sleep) of ambulatory children with CP (GMFCS levels I–III) aged 3 to 12 years, and their adherence to the 24‐hour activity guidelines for physical activity and sleep, using device‐based measures. We found that the proportion of children with CP meeting the 24‐hour activity guidelines is low, with an overall adherence rate of 5.9%. The vast majority (87%) of ambulatory children with CP did not engage in sufficient MVPA to meet the physical activity recommendations, and two‐thirds (64.7%) did not meet the age‐appropriate recommended hours of sleep. On the basis of these findings, it is clear that awareness, knowledge, and/or adoption of the 24‐hour activity guidelines in pediatric rehabilitation is needed to optimize health outcomes and well‐being in individuals with CP over the life course.
Our findings support a body of literature demonstrating high levels of physical inactivity among children with CP, with decreasing amounts of physical activity with increasing GMFCS level. 15 , 21 The 24‐hour activity guidelines recommend 60 minutes of MVPA per day for children with CP, 11 which was achieved by 13% of the children in our study. These findings are consistent with a recent study reporting a 19% physical activity guideline compliance rate among a mixed sample of children with one or more (neuro)developmental disability. 29 Notably, results from the Dutch Report Card+ showed that the percentages of children meeting the physical activity guidelines were similar for children with (26%) and without (29%) disabilities, 30 suggesting that the MVPA recommendations are often not met across pediatric populations, including typically developing children. Similar to what has been observed in typically developing children, 31 we found physical activity to be the behavior least complied with across all subgroups. This is especially notable given that physical activity, specifically MVPA, is most consistently associated with desirable health indicators in school‐aged children and young people. 2 , 32 For young persons with CP, the consequences of low levels of physical activity may be even more devastating. For example, higher‐functioning children with CP are at increased risk to lose ambulatory skills in adulthood, which has been attributed to both chronic inactivity and secondary complications common among adults with CP such as pain, fatigue, loss of strength, balance, and reduced physical fitness. 33 Our findings emphasize the continued need for more efforts towards stimulating physical activity in children with CP, for example by creating opportunities for them to acquire more active lifestyles and by increasing awareness among parents of the importance of exercise as a health‐promoting behavior with long‐term implications for health maintenance.
Time during a 24‐hour period can only be spent engaging in one activity behavior at a time (i.e. if time spent in one activity is increased, there is less time available in the day for the remaining activity domains 11 ). Therefore, in addition to focusing on increasing physical activity, part of achieving a more active lifestyle includes reducing sedentary time. Children in our study spent on average 61% of their time engaging in sedentary behavior. This proportion is lower than the previously reported 79% sedentary behavior in ambulatory children with CP aged 8 to 17 years, 5 which could be explained by our much younger sample of children. This suggestion is supported by our finding showing that the preschool‐aged children engaged in significantly more LPA (at the expense of sedentary behavior) than school‐aged children. In this context, it is important to realize that too much sedentary time is distinct from too little physical activity. Reducing and/or breaking up sedentary time (i.e. replacing sedentary behavior with LPA) may be easier for some to achieve than increasing higher intensity physical activity levels, and can have substantial (additional) health benefits. Given that both physical activity and sedentary behavior are modifiable behaviors known to track from early childhood into adulthood, 34 they represent viable targets of intervention for children with CP. For those with high levels of inactivity, aiming to improve behavior in one domain can have a positive effect on the activity composition of the entire 24‐hour period.
Our study further adds to the literature by adopting a 24‐hour lens, specifically integrating measures of sleep, which has not been considered in earlier studies in children with CP. We found that only one‐third of the children in our study met the age‐appropriate sleep duration recommendations. This is much lower than reported in a previous study, 35 in which more than 81% of the preschool‐aged and 92% of the school‐aged children with CP adhered to the sleep recommendations. In a recent study, 36 the proportion of children who were typically developing and meeting sleep recommendations was 50%. However, it should be noted that these studies used subjective tools (parent‐reported questionnaires) to measure sleep duration. While self‐/parent‐reported sleep outcomes can provide important insights into sleep perception, these measures can overestimate sleep duration, making it difficult to accurately compare findings across studies. 18 It is also important to note that actigraphy measures movement (i.e. accelerations) and not sleep, per se. Thus, activity during the night scored as wakefulness may also reflect body movements in bed (e.g. spasms, restlessness, or other types of involuntary/unconscious movement) while the child is sleeping, which may be more pronounced in children with CP. We recommend using sleep diaries as an important adjunct to accelerometer‐based sleep measures.
As the 24‐hour activity guidelines for children with CP were adapted from those originally developed for typically developing children, the question remains to what extent these guidelines are applicable for children with physical or other disabilities. Nevertheless, there is evidence for a dose–response pattern between the number of recommendations achieved within the 24‐hour guidelines and the odds of better health outcomes for children with and without disabilities. 3 , 14 None of the 24‐hour activity behaviors are independent of each other, and there are indications of a mutually beneficial relationship between the different behaviors. For example, in children with autism spectrum disorder, improved sleep outcomes (i.e. fewer wake minutes and higher sleep efficiency) were associated with more minutes of MVPA the following day, 37 while increased physical activity was favorably associated with restoring sleep in a non‐clinical sample of adolescents. 38 Future research is needed to explore the complex bidirectional relationship between the 24‐hour activities in children with CP and the health outcomes associated with meeting the individual and combined 24‐hour activity guidelines. Nonetheless, increasing adherence to the guidelines is likely to be clinically beneficial in a population that is at risk of various secondary health complications. Given the physical challenges that children with CP and their parents face daily, tailored interventions aimed at improving one (or more) of the 24‐hour activity behaviors in children with CP should be considered as a viable, initial target towards long‐term health benefits.
A strength of this study is that the 24‐hour activities in children with CP were measured using device‐based measures for both physical activity and sleep over a period of 1 week. Although actigraphy is more challenging in children with CP than in typically developing children, our relatively high adherence rates (only 5 out of 60 children did not tolerate wearing the device) are consistent with previous research, and suggest that actigraphy is feasible and acceptable in ambulatory children with CP. 20 , 21
Our findings must be interpreted in the light of some limitations. First, validation of accelerometry in children and young people with CP remains incomplete. The cut‐points for activity intensity classification have only been validated for ambulatory school‐age children with CP. This precludes younger children (<4 years old) and those with CP classified in GMFCS levels IV and V from participating in these studies, thereby limiting the generalizability of our findings. In the current study, we included four children younger than 4 years old, and decided to apply the same cut‐points throughout the entire study sample to allow comparisons of daytime activities without mixing up multiple cut‐points for different age groups. From the sleep perspective, while the algorithm of Sadeh et al. is the most commonly used for sleep–wake determination for children who are typically developing, it has yet to be validated in children with CP. Further studies are warranted to expand existing validations, and to establish reliable and valid device‐based measures of sleep in CP, as this would allow the evaluation of 24‐hour activities in a wider range of participants using a single device and single wear location.
Second, for the younger children who still needed daytime naps, parents were asked to record nap times in the activity logbook and take off the device, so as not to confuse sleep for sedentary time during physical activity analysis. As nap times were based on subjective reports, and could not be confirmed using the accelerometer, it should be noted that naps were not accounted for in the TST of the children. This could have caused an underestimation of the TST, and therefore could have affected the proportion of children meeting the sleep duration recommendations. However, naps were only reported in a relatively small number (9%, all preschool‐aged) of children.
Third, with regard to physical activity, as participants were instructed to remove the accelerometer during water‐based activities, this could have led to an underestimation of MVPA in children who participated in swimming (as part of therapy or leisure activity). According to the logbooks, hip‐worn devices were taken off for swimming on a total of 19 days from a total of 15 children across all GMFCS levels (I: n = 9; II: n = 4; III: n = 2), resulting in a total 1450 minutes (average 76 minutes per swimming episode) that were not accounted for in the physical activity analysis.
Fourth, as the study group of children with CP was further split by GMFCS level and age group to allow subgroup analyses, this resulted in subgroups with a relatively small sample size, especially for the group of children classified in GMFCS level III (n = 9).
Fifth, we did not assess screen time as a marker of sedentary behavior, and, as such, could not assess adherence to the sedentary behavior recommendations. Children with neurodevelopmental disorders may have more difficulties disengaging from screen devices, and their average screen use duration has been reported to be 4 hours per day. 40 Also, children with CP not only engage in more sedentary time than their typically developing peers, but their sedentary behavior is also characterized by less frequent breaks. 5 This suggests that the overall 24‐hour guideline adherence rate may in fact be even lower than the 5.9% reported in the present study, if the sedentary behavior component were to be taken into account as well.
Lastly, information about comorbidities (e.g. epilepsy, intellectual disability) was not obtained from the children's medical files, and these may have potentially influenced our outcomes (e.g. excess nighttime wakefulness), especially in the higher GMFCS level III.
CONCLUSION
In conclusion, the results of this study contribute to our understanding of the 24‐hour activities among children with CP, and draw attention to the importance of considering the entire continuum of movement behaviors (i.e. physical activity, sedentary behavior, and sleep) into the (rehabilitative) care for this population. The very low guideline adherence rates emphasize the need for tailored intervention strategies aimed at improving the activity behaviors throughout a 24‐hour period, including increased physical activity, limited sitting, and sufficient hours of good quality sleep. Promoting healthy lifestyle behaviors in (early) childhood has the potential to provide children a strong foundation for lifelong physical and mental health, and this may be especially critical for more vulnerable populations such as children with CP.
Funding information
JKF Kinderfonds and Kinderrevalidatiefonds Adriaanstichting, the Netherlands.
Supporting information
Figure S1: Count‐based physical activity parameters.
Figure S2: Daytime activities using the GMFCS‐specific cut‐points by Trost.
ACKNOWLEDGMENTS
We are grateful to the children and their parents for participating in this study. We thank the healthcare professionals from the rehabilitation settings for their assistance in the recruitment of participants, in particular Sanne van der Vossen (De Hoogstraat Revalidatie), Rick van de Ven (Tolbrug/Atlent, Jeroen Bosch Ziekenhuis), and Mirjam Jeurissen (Rijndam Revalidatie). We also acknowledge Wibe van Grunderbeek for assisting with writing macros. The authors have stated that they had no interests which might be perceived as posing a conflict or bias.
Hulst RY, Gorter JW, Obeid J, Voorman JM, van Rijssen IM, Gerritsen A, et al. Accelerometer‐measured physical activity, sedentary behavior, and sleep in children with cerebral palsy and their adherence to the 24‐hour activity guidelines. Dev Med Child Neurol. 2023;65(3):393–405. 10.1111/dmcn.15338
This original article is commented on by Sit on pages 305–306 of this issue.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Poitras VJ, Gray CE, Borghese MM, Carson V, Chaput JP, Janssen I, et al. Systematic review of the relationships between objectively measured physical activity and health indicators in school‐aged children and youth [Internet]. Vol. 41, Applied Physiology, Nutrition and Metabolism. Canadian Science Publishing; 2016. [cited 2021 Jan 27]. p. S197–239. Available from: 10.1139/apnm-2015-0663. [DOI] [PubMed] [Google Scholar]
- 2. Rollo S, Antsygina O, Tremblay MS. The whole day matters: Understanding 24‐hour movement guideline adherence and relationships with health indicators across the lifespan. J Sport Heal Sci. 2020;9(6):493–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Janssen I, Roberts KC, Thompson W. Is adherence to the Canadian 24‐Hour movement behaviour guidelines for children and youth associated with improved indicators of physical, mental, and social health? Appl Physiol Nutr Metab [Internet]. 2017. [cited 2021 Mar 26];42(7):725–31. Available from: 10.1139/apnm-2016-0681 [DOI] [PubMed] [Google Scholar]
- 4. Verschuren O, Peterson MD, Balemans ACJ, Hurvitz EA. Exercise and physical activity recommendations for people with cerebral palsy. Dev Med Child Neurol [Internet]. 2016;58(8):798–808. Available from: 10.1111/dmcn.13053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Obeid J, Balemans ACJ, Noorduyn SG, Gorter JW, Timmons BW. Objectively measured sedentary time in youth with cerebral palsy compared with age‐, sex‐, and season‐matched youth who are developing typically: An explorative study. Phys Ther. 2014;94(8):1163–7. [DOI] [PubMed] [Google Scholar]
- 6. Hulst RY, Gorter JW, Voorman JM, Kolk E, Van Der Vossen S, Visser‐Meily JMA, et al. Sleep problems in children with cerebral palsy and their parents. Dev Med Child Neurol [Internet]. 2021. Nov 1 [cited 2021 Oct 28];63(11):1344–50. Available from: 10.1111/dmcn.14920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atmawidjaja RW, Wong SW, Yang WW, Ong LC. Sleep disturbances in Malaysian children with cerebral palsy. Dev Med Child Neurol. 2014;56(7):681–5. [DOI] [PubMed] [Google Scholar]
- 8. Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National sleep foundation's sleep time duration recommendations: Methodology and results summary. Sleep Heal [Internet]. 2015;1(1):40–3. Available from: 10.1016/j.sleh.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 9. Horwood L, Li P, Mok E, Oskoui M, Shevell M, Constantin E. Health‐related quality of life in Canadian children with cerebral palsy: what role does sleep play? Sleep Med [Internet]. 2019;54:213–22. Available from: 10.1016/j.sleep.2018.10.022 [DOI] [PubMed] [Google Scholar]
- 10. Peterson MD, Ryan JM, Hurvitz EA, Mahmoudi E. Chronic conditions in adults with cerebral palsy. JAMA ‐ J Am Med Assoc. 2015;314(21):2303–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verschuren O, Hulst RY, Voorman J, Pillen S, Luitwieler N, Dudink J, et al. 24‐hour activity for children with cerebral palsy: a clinical practice guide. Dev Med Child Neurol [Internet]. 2021. Jan 27 [cited 2021 Feb 3];63(1):54–9. Available from: 10.1111/dmcn.14654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Healy S, Foley J, Haegele JA. Physical Activity, Screen Time, and Sleep Duration Among Youth With Chronic Health Conditions in the United States. Am J Heal Promot. 2020;34(5):505–11. [DOI] [PubMed] [Google Scholar]
- 13. Arbour‐Nicitopoulos KP, Bassett‐Gunter RL, Leo J, Sharma R, Olds T, Latimer‐Cheung AE, et al. A cross‐sectional examination of the 24‐hour movement behaviours in Canadian youth with physical and sensory disabilities. Disabil Health J [Internet]. 2021;14(1):100980. Available from: 10.1016/j.dhjo.2020.100980 [DOI] [PubMed] [Google Scholar]
- 14. DMY Brown, PG McPhee, Kwan MY, Timmons BW. Implications of Disability Severity on 24‐Hour Movement Guideline Adherence Among Children With Neurodevelopmental Disorders in the United States. J Phys Act Heal [Internet]. 2021. Sep 21 [cited 2021 Oct 22];1(aop);18:1–7. Available from: https://journals.humankinetics.com/view/journals/jpah/aop/article‐10.1123‐jpah.2021‐0282/article‐10.1123‐jpah.2021‐0282.xml [DOI] [PubMed] [Google Scholar]
- 15. Mitchell LE, Ziviani J, Boyd RN. Habitual physical activity of independently ambulant children and adolescents with cerebral palsy: Are they doing enough? Phys Ther. 2015;95(2):202–11. [DOI] [PubMed] [Google Scholar]
- 16. Smit DJM, Zwinkels M, Takken T, Hulst RY, de Groot JF, Lankhorst K, et al. Sleep quantity and its relation with physical activity in children with cerebral palsy; insights using actigraphy. J Paediatr Child Health [Internet]. 2020. Jul 6 [cited 2020 Jul 6];56(10):1618‐22. Available from: 10.1111/jpc.15055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quante M, Kaplan ER, Rueschman M, Cailler M, Buxton OM, Redline S. Practical considerations in using accelerometers to assess physical activity, sedentary behavior, and sleep. Sleep Heal [Internet]. 2015;1(4):275–84. Available from: 10.1016/j.sleh.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 18. Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self‐reported and measured sleep duration: How similar are they? Epidemiology [Internet]. 2008. Nov [cited 2021 May 14];19(6):838–45. Available from: /pmc/articles/PMC2785092/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Santos R, Zhang Z, Pereira JR, Sousa‐Sá E, Cliff DP, Okely AD. Compliance with the Australian 24‐hour movement guidelines for the early years: Associations with weight status. BMC Public Health. 2017;17(Suppl 5):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clanchy KM, Tweedy SM, Boyd RN, Trost SG. Validity of accelerometry in ambulatory children and adolescents with cerebral palsy. Eur J Appl Physiol [Internet]. 2011. Dec 26 [cited 2019 Aug 16];111(12):2951–9. Available from: 10.1007/s00421-011-1915-2 [DOI] [PubMed] [Google Scholar]
- 21. Gorter JW, Noorduyn SG, Obeid J, Timmons BW. Accelerometry: A Feasible Method to Quantify Physical Activity in Ambulatory and Nonambulatory Adolescents with Cerebral Palsy. Int J Pediatr [Internet]. 2012. [cited 2021 Feb 8];2012:1–6. Available from: http://www.hindawi.com/journals/ijpedi/2012/329284/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trost SG, Pate RR, Freedson PS, Sallis JF, Taylor WC. Using objective physical activity measures with youth: How many days of monitoring are needed? Med Sci Sports Exerc. 2000;32(2):426–31. [DOI] [PubMed] [Google Scholar]
- 23. Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG. Calibration of two objective measures of physical activity for children. J Sports Sci [Internet]. 2008. Dec 15 [cited 2021 Feb 8];26(14):1557–65. Available from: 10.1080/02640410802334196 [DOI] [PubMed] [Google Scholar]
- 24. Trost SG, Fragala‐Pinkham M, Lennon N, O'Neil ME. Decision Trees for Detection of Activity Intensity in Youth with Cerebral Palsy. Med Sci Sports Exerc [Internet]. 2016. May 1 [cited 2022 May 18];48(5):958–66. Available from: https://pubmed.ncbi.nlm.nih.gov/26673127/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mitchell LE, Ziviani J, Boyd RN. Variability in measuring physical activity in children with cerebral palsy. Med Sci Sports Exerc [Internet]. 2015. Jan 1 [cited 2021 Nov 9];47(1):194–200. Available from: https://pubmed.ncbi.nlm.nih.gov/24824775/ [DOI] [PubMed] [Google Scholar]
- 26. Sadeh A, Sharkey KM, Carskadon MA. Activity‐based sleep‐wake identification: An empirical test of methodological issues. Sleep [Internet]. 1994. May 1 [cited 2021 May 17];17(3):201–7. Available from: https://academic.oup.com/sleep/article/17/3/201/2749453 [DOI] [PubMed] [Google Scholar]
- 27. Taylor RW, Williams SM, Farmer VL, Taylor BJ. The stability of sleep patterns in children 3 to 7 years of age. J Pediatr [Internet]. 2015. Mar 1 [cited 2022 May 20];166(3):697‐702.e1. Available from: https://pubmed.ncbi.nlm.nih.gov/25524316/ [DOI] [PubMed] [Google Scholar]
- 28. Acebo C, Sadeh A, Seifer R, Tzischinsky O, Wolfson AR, Hafer A, et al. Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep [Internet]. 1999. Feb 1 [cited 2022 May 20];22(1):95–103. Available from: https://pubmed.ncbi.nlm.nih.gov/9989370/ [DOI] [PubMed] [Google Scholar]
- 29. Case L, Ross S, Yun J. Physical activity guideline compliance among a national sample of children with various developmental disabilities. Disabil Health J. 2020. Apr 1;13(2):100881. [DOI] [PubMed] [Google Scholar]
- 30. Burghard M, de Jong NB, Vlieger S, Takken T. 2017. Dutch Report Card+: Results from the first physical activity report card plus for Dutch youth with a chronic disease or disability. Front Pediatr [Internet]. 2018 Apr 30 [cited 2021 Jun 21];6:122. Available from: www.frontiersin.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Craemer M, McGregor D, Androutsos O, Manios Y, Cardon G. Compliance with 24‐h movement behaviour guidelines among belgian pre‐school children: The toybox‐study. Int J Environ Res Public Health. 2018;15(10):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saunders TJ, Gray CE, Poitras VJ, Chaput JP, Janssen I, Katzmarzyk PT, et al. Combinations of physical activity, sedentary behaviour and sleep: Relationships with health indicators in school‐aged children and youth. Appl Physiol Nutr Metab. 2016;41(6):S283–93. [DOI] [PubMed] [Google Scholar]
- 33. TURK MA. Health, mortality, and wellness issues in adults with cerebral palsy. Dev Med Child Neurol [Internet]. 2009 [cited 2021 Aug 13];51(SUPPL. 4):24–9. Available from: 10.1111/j.1469-8749.2009.03429.x [DOI] [PubMed] [Google Scholar]
- 34. Telama R, Yang X, Leskinen E, Kankaanpää A, Hirvensalo M, Tammelin T, et al. Tracking of physical activity from early childhood through youth into adulthood. Med Sci Sports Exerc. 2014;46(5):955–62. [DOI] [PubMed] [Google Scholar]
- 35. Horwood L, Mok E, Li P, Oskoui M, Shevell M, Constantin E. Prevalence of sleep problems and sleep‐related characteristics in preschool‐ and school‐aged children with cerebral palsy. Sleep Med [Internet]. 2018;50:1–6. Available from: 10.1016/j.sleep.2018.05.008 [DOI] [PubMed] [Google Scholar]
- 36. Hansen J, Hanewinkel R, Galimov A. Physical activity, screen time, and sleep: do German children and adolescents meet the movement guidelines? Eur J Pediatr [Internet]. 2022. May 1 [cited 2022 May 20];181(5):1985. Available from: /pmc/articles/PMC8811591/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Benson S, Bender AM, Wickenheiser H, Naylor A, Clarke M, Samuels CH, et al. Differences in sleep patterns, sleepiness, and physical activity levels between young adults with autism spectrum disorder and typically developing controls. Dev Neurorehabil [Internet]. 2019;22(3):164–73. Available from: 10.1080/17518423.2018.1501777 [DOI] [PubMed] [Google Scholar]
- 38. Lang C, Brand S, Feldmeth AK, Holsboer‐Trachsler E, Pühse U, Gerber M. Increased self‐reported and objectively assessed physical activity predict sleep quality among adolescents. Physiol Behav. 2013. Aug 15;120:46–53. [DOI] [PubMed] [Google Scholar]
- 39. Trost S, Hinkley T, Cliff D. Comparison of Accelerometer Cut Points for Predicting Activity Intensity in Youth Related papers Comparison Of Acceleromet er Cut‐Point s For Predict ing Physical Act ivit y Int ensit y In Yout h St ewart Trost Predict ive Validit y and Classificat ion Accuracy of Act iGraph Energy Expendit ure Equat ions and Cut‐Po … . 2011. [cited 2022 Apr 15]; Available from: www.acsm‐msse.org
- 40. Lin J, Magiati I, Chiong SHR, Singhal S, Riard N, Ng IHX, et al. The Relationship among Screen Use, Sleep, and Emotional/Behavioral Difficulties in Preschool Children with Neurodevelopmental Disorders. J Dev Behav Pediatr [Internet]. 2019. Sep 1 [cited 2021 Aug 16];40(7):519–29. Available from: https://journals.lww.com/jrnldbp/Fulltext/2019/09000/The_Relationship_Among_Screen_Use,_Sleep,_and.4.aspx [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Count‐based physical activity parameters.
Figure S2: Daytime activities using the GMFCS‐specific cut‐points by Trost.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


