Abstract
Obesity is an epidemic that has led to a rise in the incidence of many comorbidities: among others, reduced fertility is often under‐evaluated in clinical practice. The mechanisms underlying the link between reduced fertility and obesity are numerous, with insulin resistance, hyperglycaemia and the frequent coexistence of polycystic ovary syndrome being the most acknowledged. However, several other factors concur, such as gut microbiome alterations, low‐grade chronic inflammation and oxidative stress. Not only do women with obesity take longer to conceive, but in vitro fertilization (IVF) is also less likely to succeed. We herein provide an updated state‐of‐the‐art regarding the molecular bases of what we could define as dysmetabolic infertility, focusing on the clinical aspects, as well as possible treatment.
Keywords: assisted reproductive technology, body mass index, in vitro fertilization, miscarriage, overweight, polycystic ovary syndrome, pregnancy
1. INTRODUCTION
Obesity is a global epidemic with a rising prevalence worldwide. 1 , 2 Lifestyle factors, such as reduced physical activity as well as high‐sugar and high‐fat intake, may lead to a positive energy balance, resulting in weight gain. This has led to an increase in the incidence of many complications of weight excess. type 2 diabetes, obstructive sleep apnoea syndrome, cardiovascular and liver disease are well‐acknowledged. However, many others are emerging and currently investigated, and infertility is among these. Infertility is defined as the failure to achieve pregnancy within 12 months of unprotected intercourse. 3 It is one of the emerging complications of obesity, and even though it affects about 15% of couples, it is often under‐evaluated in clinical practice. 4 Women with obesity frequently experience irregular menstrual cycles with anovulation and/or endometrial pathology, 5 with polycystic ovary syndrome (PCOS) frequently coexisting, 6 and time to conception is longer than that of women with normal weight even when menstrual cycles are regular. 7 The mechanisms underlying the link between what we could define as dysmetabolic infertility and obesity are numerous, with insulin resistance (IR) and PCOS, on different sides of the same medal, being the most acknowledged. However, several other factors are currently being proposed. We herein provide an updated state‐of‐the‐art review regarding these factors, focusing on in vitro fertilization (IVF) outcomes, whose recourse is more and more frequent among patients suffering from dysmetabolic infertility.
2. HORMONAL AND MOLECULAR BASIS OF DYSMETABOLIC INFERTILITY
Obesity is a chronic disease characterized by adipose tissue (AT) expansion. This is mainly due to the hypertrophy of adipose cells storing triglycerides. 8 Such alterations lead to AT dysfunction, which is associated with AT hypoxia, inflammation, as well as endoplasmic reticulum and oxidative stress. 8 AT acts as an endocrine organ secreting bioactive signalling products called adipokines (i.e. leptin, chemerin, monocyte‐chemotactic‐protein‐1 and retinol‐binding‐protein‐4). 9 Signals deriving from a dysfunctional AT affect glucose and lipid metabolism, food intake, inflammation and insulin sensitivity through autocrine, paracrine and endocrine mechanisms. 9 Altogether, this, in turn, may lead to dysmetabolic infertility, which has been classically identified with PCOS, whereas multiple additional mechanisms make the picture far more complex: hormonal dysregulation including IR and hyperandrogenism, along with chronic inflammation and oxidative stress, and the altered gut‐brain axis, all seem to play significant roles (Figure 1).
Figure 1.
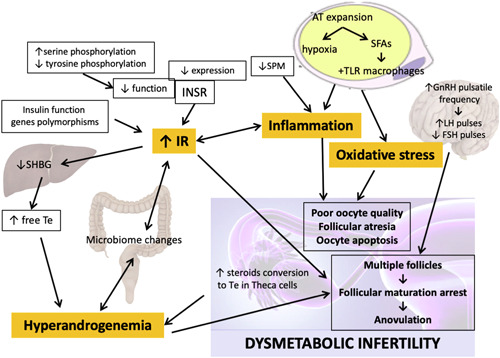
Mechanisms underlying dysmetabolic infertility. The main culprits of dysmetabolic infertility are depicted in orange: Insulin resistance, inflammation, oxidative stress and hyperandrogenemia. These are finely interconnected and no single one is causing the other, but they altogether contribute in causing polycystic ovary syndrome and, more generally, infertility. AT, adipose tissue; FSH, Follicle stimulating hormone; GnRH, Gonadotropin releasing hormone; INSR, insulin receptor, IR, insulin resistance; LH, Luteinizing Hormone; SFAs, saturated fatty acids; SHBG, Sex Hormone, Binding Globulin; SPM, proresolving lipid mediators; Te, Testosterone; TLR, Toll like receptor. [Color figure can be viewed at wileyonlinelibrary.com]
2.1. Hormonal dysregulation
Alterations in the hypothalamic−pituitary−ovarian axis, as well as glucose and lipid metabolism signalling pathways, contribute to the pathogenesis of PCOS. 10 IR is a fundamental aspect of PCOS, as nearly 60%−70% of women with the syndrome exhibit it. 11 Several mechanisms might explain the cellular defects that cause IR among women with PCOS. Dunaif et al. described that fibroblasts derived from the skin of women with PCOS showed reduced tyrosine‐ and increased serine‐phosphorylation of the insulin receptor (INSR), with activating and inhibiting effects, respectively. 12 Belani et al. reported a reduced expression of the INSR beta subunit and phosphatidylinositol 3‐kinase (PI3K) as well as a downregulation in the phosphorylation of Akt in luteinized granulosa cells derived from women with IR and PCOS compared to both women without PCOS and women with PCOS but not IR. 13
Polymorphisms of genes involved in insulin action have been associated with PCOS 14 : a polymorphism in a variable number of tandem repeats located in the 5′ untranslated region of the insulin gene is associated with PCOS, 15 and studies have evaluated the association between INSR polymorphisms and infertility in women with obesity and PCOS. 16 Moreover, women exhibiting INSR gene polymorphisms are characterized by hyperandrogenism and IR. 17
Cells derived from the ovarian theca of women with PCOS have been reported to be more efficient in converting precursors into testosterone compared to control. 18 The levels of luteinizing hormone (LH) and follicle‐stimulating hormone (FSH) are increased and decreased, respectively, due to the observed increased gonadotrophin‐releasing hormone (GnRH) pulse frequency. 17 It is not clear whether granulosa cells of women with PCOS produce more or less anti‐Mullerian hormone (AMH), whose primary role is to inhibit excessive follicular maturation. 19 However, due to the increased number of preantral and small antral follicles in this syndrome, all contributing to the circulating AMH, its serum concentration is approximately three times higher in women with PCOS. 20 Hyperinsulinemia also suppresses sex hormone‐binding globulin (SHBG) synthesis in the liver, leading to increased free plasma androgens contributing to clinical hyperandrogenemia. 5
Hyperinsulinemia leads to excessive androgen secretion, contributing to greater ovarian sensitivity to LH, along with an intrinsic theca cell phenotype, that is independent of LH receptor status. 10 Also, granulosa cell dysfunction due to FSH inhibition probably contributes to androgen excess; in turn, hyperandrogenism activates the growth of small antral follicles but impairs ovulation by inhibiting the growth of the dominant follicle. 21
Hyperinsulinemia, a result of tissue selective IR as that seen in skeletal muscle, is paradoxically associated with insulin sensitivity in the ovarian tissue, meaning that insulin excess leads to androgen excess. This paradoxical insulin effect could be explained as a result of incomplete desensitization of ovarian responsiveness to LH, thus premature luteinization happens. 22 Furthermore, in women with PCOS, adiponectin levels in AT were found to be reduced, and this could play a role in the development IR‐dependent hyperandrogenism. 23
About two‐thirds of PCOS cases have typical functional ovarian hyperandrogenism, in one‐third of PCOS it is functionally atypical, and a small number are due to isolated functional andrenal hyperandrogenism or overlap between functional ovarian and adrenal androgen excess. 10 The adrenal excess in PCOS and its clinical severity are in correlation with adrenal volume increase, that is almost 50%. 24 Obesity could be a very powerful contributing factor, or even the only cause of androgen excess. This could be the explanation of the fact that even in the absence of ovarian androgen excess, the adrenal or even peripheral androgen excess and IR are the reasons of anovulation and polycystic ovarian morphology. Finally, women with PCOS seem to have increased leptin levels positively correlated with IR, body mass index (BMI), and central obesity indices, and circulating ghrelin is associated with testosterone levels. 25
Beyond insulin and androgens, several other hormonal derangements are observed in women with obesity presenting for evaluation at infertility clinics. Excess weight may be associated with hypothalamo–pituitary axis dysfunctions, with or without anatomical pituitary alterations. 26 Furthermore, hyperglycemia and diabetes are two important co‐risk factors for reproductive dysfunction. 27 A possible mechanism by which hyperglycemia could induce fertility impairment is the advanced glycation end products accumulation. 28 Moreover, the generation of reactive oxygen species secondary to high glucose levels may damage the developing yolk sac, and epigenetic changes possibly affecting embryogenesis have also been suggested. Certainly, the main mechanism underlying the correlation between diabetes and infertility lies in the hyperinsulinemia and IR discussed previously. 29
2.2. Chronic low‐grade inflammation and oxidative stress
Chronic low‐grade inflammation and oxidative stress are closely associated with infertility itself. These conditions are capable of affecting the reproductive system and the oocytes, as well as the systemic pregnancy environment. 30 Preclinical evidence also suggests that inflammation is closely associated with ovarian ageing and thus age‐related infertility. 31
It is well known that the immune and metabolic systems are strictly interconnected: AT hypoxia triggers chronic low‐grade inflammation 32 ; a rise in leptin, resistin and other proinflammatory adipokines, coupled with a decrease in the antiinflammatory adipokine adiponectin, are associated with the onset of metabolic derangements, and most importantly IR. 33 The molecular mechanisms underlying these processes are not fully understood. The release of cytokines and the insulin signalling impairment may at least partially derive from saturated fatty acids initiating inflammation through toll‐like receptor activation in AT resident macrophages. 34 If proinflammatory mediators are crucial in determining low‐grade chronic inflammation, insufficient proresolutive mechanisms are also responsible. One of the pathways involved in inflammation modulation has specialized proresolving lipid mediators (SPM) as main characters, including molecules derived from eicosapentaenoic acid (EPA), omega‐2, and docosahexaenoic acid (DHA). SPMs biosynthesis is severely affected in the inflamed AT further worsening systemic inflammation. 35
It was suggested that obesity is also associated with Ni accumulation and subsequent sensitization, and we previously reported that those who are sensitized also develop worse metabolic disruption and increased inflammation. 36 , 37 Two studies have evaluated circulating Ni levels in women with PCOS, finding them increased compared to control subjects, 38 , 39 whereas no study is available to date investigating infertility in relation to Ni sensitivity or circulating levels.
The association between PCOS and chronic low‐grade inflammation is well established, as well as that between obesity and chronic low‐grade inflammation. Many studies have reported an elevation of inflammatory markers, such as CRP, interleukin‐8 (IL‐8), MCP‐1, interleukin‐18 (IL‐18), IL‐6 and tumor necrosis factor‐α in women with PCOS. 40 If the presence of inflammation in PCOS is closely related to IR and obesity, androgen excess also plays an important role in what can be defined as a vicious circle, where ovarian inflammation stimulates steroidogenesis, further fuelling hyperandrogenism, inflammation and IR. 41 Previously mentioned SPMs deriving from ω3‐poly‐unsaturated fatty acids (PUFAs) have an influence on granulosa cells of PCOS women, metabolic and endocrine parameters, beyond obesity and IR, through downregulation of proinflammatory chemokines and cytokines and adaptation of immune response with beneficial effects on plasma testosterone levels, HOMA‐index, and SHBG levels. 42 A recent study also reported that white blood count (WBCs) are increased in patients with PCOS and that there is a positive correlation between these and circulating androgens, further supporting the thesis that hyperandrogenism is implicated in the pathogenesis of low‐grade inflammation. 43
Oxidative stress contributes to female infertility, causing oocyte and granulosa cell apoptosis as well as atresia of follicles, and this aspect may be relevant in PCOS, too. 44 In fact, women with PCOS undergoing IVF compared to women with tubal block were shown to have higher follicular fluid mean reactive oxygen species levels and lower number of oocytes with spindle visualization, reflecting poor oocyte quality. 44 Notably, the follicular fluid of higher quality oocytes and embryos shows better glutathione glutathione‐dependent antioxidant system functions, suggesting that oxidative stress may be both a cause and consequence of poor oocyte quality. 45
2.3. The gut‐brain axis and the microbiome
The gut‐brain axis bidirectionally connects the central and enteric nervous systems through a network that involves the hypothalamus−pituitary−adrenal axis. The interactions are often mediated by the gut microbiota via immune, neuroendocrine and vagal pathways. 46 At least 20 gastrointestinal hormones are involved in the gut‐brain interactions, like ghrelin, glucagon‐like peptide 1 (GLP‐1), peptide YY (PYY) and cholecystokinin, and they have a key role, as anorexigenic hormones, in reducing appetite, enhancing satiety, delaying gastric emptying time, promoting insulin secretion and proliferation of pancreatic islet β cells. 47 It was previously reported that women with PCOS show lower gut hormones such as ghrelin, PYY and serotonin, 48 as well as lower GLP‐1 levels. 49
Interestingly, hyperandrogenism, often present in PCOS, seems to be linked to the gut‐brain axis: Sherman et al. showed that high levels of prenatal androgen exposure cause dysbiosis in the infant, as well as long term alterations that lead to a higher risk of developing PCOS. 50 Torres et al. reported that the microbiome composition is correlated with clinical and biochemical hyperandrogenism. 51 Chu et al. 52 found a correlation between some gram‐negative bacteria and serum testosterone, LH and AMH. Moreover, some gut hormones were shown to be negatively correlated with testosterone levels in women with PCOS. 48 The gut microbiota is confirmed to be connected with IR, one of the critical factors in PCOS, in both animal and human studies, 53 because it is responsible for the secretion of gut‐brain peptides and the regulation of inflammation pathways. 54
The gut‐brain axis seems to be involved in immune regulation as well. More than 70% of the immune cells of the body are stored in the intestinal lymphoid tissues. 55 According to Qin et al. patients with PCOS and infertility have higher circulating T helper 1 cells and lower Th2 levels compared to control, 56 an imbalance that has been linked to impaired oocyte quality and ovulation disorders, resulting in poor clinical outcomes. Disorders in the gut microbiota significantly correlate with imbalances in the Th1/Th2 ratio. 57 Interestingly, PCOS was also associated with increased circulating gut damage and inflammation markers, such as calprotectin, zonulin, and lipopolysaccharide. 58
Lately, several studies have used cutting edge technology to identify and quantify intestinal bacteria. 48 , 51 , 58 , 59 Interestingly, patients with PCOS and/or obesity were reported to have a gut microbiome characterized by a reduction in overall species richness and composition (alpha and beta diversity, respectively) compared to healthy individuals. This was previously proved in mouse models 60 and then confirmed in humans by Lindheim et al. 58 and Torres et al. 51 However, Qi et al. 59 reported no difference in alpha diversity, but a significant beta diversity decreases in women with PCOS.
The microbiome of women with PCOS has specific peculiarities at phylum, family and genus levels. 60 At a phyla level, women with PCOS show a decrease in Tenericutes and Bacteroidetes. At a family level, they show reduced Prevotellaceae and, more generally, bifidobacteria and lactobacilli, capable of boosting the immune system and improving nutrient absorption. 61 At the genus level, these women exhibit a lower abundance of Roseburia, Anaerococci, Ruminococci and Odoribacteria, 51 as well as Faecalibacterium, Bifidobacterium and Blautia. 62 This change in the microbiome composition is likely the one leading to the detrimental decrease in short‐chain fatty acids (SCFAs) observed in women with PCOS, 51 , 62 whose production promote insulin, PYY and serotonin secretion and protect intestinal barrier integrity. 63 Always at a genus level, women with PCOS show a significant increase in Bacteroides, Escherichia and Shigella, gram‐negative bacteria. 48 Gram‐negative bacteria have a role in inducing inflammation, IR and obesity, because they produce lipopolysaccharides. 64 Specifically, the microbiome of women with PCOS was shown to be rich in Bacteroides vulgatus, 59 and its transplantation in wild‐type mice caused increased inflammation, IR, and infertility. 59 These findings were confirmed by Chu et al. who also found a correlation with BMI. 52
3. CLINICAL ASPECTS OF DYSMETABOLIC INFERTILITY
Pregnancy itself carries higher morbidity rates in the presence of obesity than in women with normal weight, and IVF seems to be less safe in women with dysmetabolic infertility. Interestingly, when it comes to IVF outcomes in women with PCOS, they tend to perform better than women with no PCOS, whereas women with obesity and dysmetabolic infertility have poorer outcomes compared to normal‐weight women. This should be kept in mind while counselling these women both at a general practitioner level and infertility clinics. 65 Similar to the several mechanisms underlying the link between fertility and obesity, the clinical aspects of dysmetabolic infertility are multifaceted, and they are summarized below, with a specific focus on IVF outcomes.
3.1. Spontaneous pregnancy
According to a prospective study enroling over 7000 women, those with obesity take almost double the time to conceive independent of age, with those with regular menstrual cycles still incurring in longer times to conception compared to women with normal weight. 7 Morbid obesity is associated with an increase of 6 times the likelihood of having a conception time of over 1 year compared to normal weight; similarly, 40% of women with PCOS experience a time to conception of over 12 months, and this is enhanced when obesity coexists. 66
3.2. Miscarriage risk
Patients with PCOS show a higher risk of spontaneous miscarriage, ranging from 30% to 50% in the first trimester. 67 Hyperinsulinism and obesity, common metabolic comorbidities found in PCOS patients, are known to decrease implantation rates, 68 as well as contribute to early and recurrent pregnancy loss. Baseline intrinsic IR found in lean women with PCOS gets superimposed if these patients gain weight, and it seems further exacerbated by entry into pregnancy. 69 A possible explanation could be that plasminogen activator inhibitor (PAI) activity is increased in women with PCOS compared to healthy controls; IR and hyperinsulinism impact fertility and pregnancy by increasing androgen and PAI levels and decreasing uterus vascularization. 70
The correlation between hereditary thrombophilia and the higher risk of recurrent pregnancy loss has been well‐established; in this regard, patients with PCOS could exhibit more frequently homozygosity for methylenetetrahydrofolate reductase gene mutations, 71 activated protein C resistance ratio (APC R) and factor V Leiden, which were found to be associated with recurrent pregnancy loss. Although a higher prevalence of APC was found in women with previous miscarriages, a study by Atiomo et al. showed that the prevalence in patients with PCOS was the same compared to the general population. 72 Further, in a recent meta‐analysis by Cavalcante et al. no association between recurrent miscarriage and inherited thrombophilia in patients with PCOS was found; however, this could be due to the small number of research papers included in the study. 73
3.3. IVF outcomes
3.3.1. Oocytes
PCOS is associated with an increase in ovarian reserve markers, including AMH levels and antral follicle count, which is reflected in an increased number of oocytes retrieved after ovarian stimulation; however, the quality of these oocytes is deficient, resulting in low fertilization and implantation rates. 74 High endogenous androgen levels in PCOS, different from what can occur following supplementation with androgens (i.e., dehydroepiandrosterone) during ovarian stimulation, 75 impairs folliculogenesis, and the result is a premature arrest of oocytes development with impaired competence for future fertilization. 76 Qiao et al. found a higher retrieval of immature oocytes in women with PCOS, leading to poor fertilization and lower cleavage. 77
Similar findings are also reported in women with excess weight, such as a significantly reduced number of mature or good quality oocytes in overweight women compared with normal‐weight control women, independent of PCOS. 76 This may at least partially be due to the absence of adjustment by patient body weight of both FSH and human chorionic gonadotropin preparations, leading to a relatively reduced dose of stimulation. 78 Furthermore, high leptin levels in the follicular fluid, related to high serum levels of leptin in women with obesity, affect steroidogenic activity in granulosa cells, with a subsequent decrease in oestrogen and progesterone production. 79
3.3.2. Clinical pregnancy rate
A clinical pregnancy is defined as the presence of a gestational sac on transvaginal ultrasonography. 80 A recent meta‐analysis showed that the clinical pregnancy rate per started cycle is similar in PCOS and non‐PCOS women. 81 However, studies were heterogeneous in the cause of infertility in control patients, fresh or frozen embryo transfer, and confounding factors. After adjustment for confounding factors, the results showed an increased clinical pregnancy rate per started cycle in women with PCOS compared to non‐PCOS patients. 81 To this regard, Li et al. showed a cumulative live birth rate (CLBR) significantly higher in the PCOS group (60.3%), compared to age‐matched controls (47.5%) 82 ; similar results were observed considering a clinical pregnancy rate expressed per embryo transfer.
Interestingly, when women with obesity were investigated, the clinical pregnancy rate was significantly reduced compared to those with normal weight, especially when PCOS and obesity coexisted. 83
3.3.3. Live birth rate
The live birth rate per cycle is the number of cycles resulting in live births compared with all cycles started. 81 Several studies showed a higher live birth rate in PCOS women than in women with causes of infertility other than PCOS. 84 However, Kalra et al. did not find significant differences after 40 years of age. 84 Even if live birth is the principal clinical outcome of IVF treatment, CLBR represents an important parameter for the efficiency of IVF. In this regard, retrieving more than 10 oocytes in PCOS patients leads to no significant improvement of this parameter. 85 Furthermore, no significant difference seems to exist concerning multiple pregnancy rates and ectopic pregnancy rates in women with PCOS. 86
Interestingly, a recent meta‐analysis reported a reduced live birth rate in women with obesity undergoing IVF compared to women with normal weight, and the treatment outcome was poorer when PCOS was also associated. 87
3.3.4. Miscarriage and biochemical pregnancy rate
The miscarriage rate is the ratio of spontaneous abortions to clinical pregnancies. 88 Several studies reported that PCOS patients showed a higher miscarriage rate than non‐PCOS patients 82 , 84 except for women with a tubal factor, 89 similar to what observed in women with obesity compared to those with normal weight. 90 According to these results, two recent studies evaluating preimplantation genetic testing ICSI cycles and euploid blastocyst transfers highlighted an increased risk of miscarriage in case of overweight and obesity. 91 Going in the same direction, Bu et al. found that PCOS significantly increased the spontaneous miscarriage rate, but only in patients younger than 35 years old. 92 Interestingly, Liu et al. 93 reported that PCOS patients had a higher biochemical loss, defined as a positive pregnancy test with a low concentration of serum beta‐human chorionic gonadotrophin, followed by a rapid fall compared to women without PCOS, but the clinical miscarriage rate was no higher. However, in this study, the biochemical loss rate varied significantly from as low as 7.9% to 46.7%, depending on the number of embryos transferred and whether fresh or frozen‐thawed embryos were transferred.
Furthermore, considering donor oocyte IVF and embryo transfer, an association between obesity and adverse reproductive outcomes were observed to demonstrate a possible effect of obesity also on the endometrium and implantation 94 ; however these data have not been confirmed by others. 95
3.3.5. Ovarian hyperstimulation syndrome (OHSS)
Women with PCOS presented a higher risk of exaggerated response and OHSS. OHSS is more frequent in young patients, and it is characterized by cystic enlargements of the ovaries and an increased capillarity permeability that can induce an extra‐vascular fluid shift. 96 A meta‐analysis conducted by Sha et al. highlighted a higher OHSS rate in women with PCOS than non‐PCOS, with an OR of 4.96. 81 Interestingly, the rate of OHSS is reduced in women with obesity and PCOS, and the prognosis is better if this complication occurs. 97
3.4. Obstetrical outcomes
Regarding pregnancy‐related complications, the prevalence of gestational diabetes, high blood pressure in pregnancy and large for gestational age is higher in PCOS women. 98 , 99 It could be because PCOS women are more frequently with overweight/obesity or affected by IR. However, preterm birth, small for gestational age and congenital malformations rates were similar to those of women without PCOS. 98 , 99
Conversely, women with obesity also experience obstetrical outcomes such as preterm birth, stillbirth, congenital abnormalities, gestational diabetes mellitus and pregnancy‐induced hypertension more frequently compared to normal weight. 100 Finally, obesity multiplies the maternal death risk by 1.6 in overweight women and more than three times in pregnant women with severe obesity. 101
4. TREATMENT OF DYSMETABOLIC INFERTILITY
4.1. Weight loss
Weight loss, through caloric restriction and/or increased exercise, exerts beneficial effects by reducing adiposity, androgen and insulin levels. These conservative therapeutic interventions, as well as surgical approaches, likely ameliorate female fertility by improving hormonal profiles, ovulation and oocyte quality, 102 leading to improved pregnancy rates. 103 Even though increased fertility results from weight reduction after bariatric surgery, some evidence indicates adverse effects of bariatric surgery on fertility. 102 Moreover, once a woman undergoes bariatric surgery it is recommended not to seek pregnancy for the subsequent 18 months, 104 possibly representing a limitation in those where age‐related infertility might be an issue.
As the final desired outcome of the infertility treatment is pregnancy, considering the obesity‐related maternal morbidity and mortality, weight loss should be the primary therapeutic approach in every woman with overweight and obesity seeking pregnancy.
The use of antiobesity drugs is being discussed. A significant number of studies are available on metformin in women trying to conceive, especially in women with PCOS. Its use is mostly related to its insulin sensitizing effect, more than for its role in weight loss. 105 Metformin and Orlistat appear to have a comparable beneficial effect on weight loss and ovulation rates in women with PCOS and obesity, 106 even if their use prior IVF‐ET does not seem to improve live birth rate. 107 The use of GLP‐1 RA is also being studied. Liraglutide, in monotherapy or in combination with metformin, does show beneficial effects on weight loss in women with PCOS. 108 Few data are available on the safety profile of these drugs during pregnancy, so they are not recommended in pregnant women. However, their use in preconception intervention for weight loss does show promising results.
Several studies suggest that the use of nutritional interventions well known to reduce the obesity‐related low‐grade chronic inflammation, such as a very low carbohydrate diet (VLCKD), is also capable of reversing androgen excess and the ovulatory function in women with PCOS, 109 likely thanks to both weight loss and the inflammation reduction obtained. VLCKDs are safe antiinflammatory regimens, and a pilot study showed that in the presence of overweight or obesity in association with PCOS they could improve body weight, hormonal profiles and fertility, 110 although it should be kept in mind that this diet might represent a risk if not discontinued upon pregnancy onset. 111
4.2. Food supplements
Food supplements have shown some promising evidence. 112 Myo‐inositol acts as an insulin sensitizer exerting a beneficial effect on intracellular metabolic processes such as glucose metabolism and reducing oxidative stress and is thought to be a reasonably effective treatment for PCOS women. Myo‐inositol improves, in fact, oocyte quality and the fertilization rate in women with PCOS 113 by increasing intracellular Ca2+, and by exerting antioxidant effects, along with those of methionine and alpha‐lipoprotein, to improve the embryo and oocyte quality. 113 Thus, myo‐inositol increases the metaphase II oocytes rate comparing to total oocytes rate, followed by higher fertilization and good quality embryo rates in PCOS patients undergoing assisted reproduction. 113 In this regard, inositol may improve ovulation. 114 Hyperinsulinemia realizes its damaging effect on oocyte quality, accelerating the conversion of myo‐inositol to d‐chiro‐inositol (DCI), thus leading to myo‐inositol deficiency and DCI overproduction milieu. 115 Furthermore, a study conducted on patients with obesity and type 1 diabetes showed that DCI could improve glucose control reducing IR. 116
The importance of the role of the gut‐brain axis in the pathophysiology of PCOS is also related to the role it could play in its treatment. At the moment, lifestyle changes are the first step in the treatment, and many studies evaluated how the gut microbiota relates to changes in diet and physical exercise. 117 Synbiotics, prebiotics and probiotics could represent a new treatment option. Probiotics supplementation in women with PCOS appears to be associated with lower levels of glucose, insulin, HOMA‐IR, triglycerides, and cholesterol. 118 A recent study evaluated the use of Bifidobacterium Lactis V9 as probiotic in women with PCOS, and the intervention proved an increased growth of SCFA producing microorganisms. The study also showed higher PYY and ghrelin levels that caused a change in the production of sexual hormones through the gut‐brain axis. 62 Faecal microbiota transplantation was evaluated in rodent models of PCOS. Guo et al. 119 observed an improvement in oestrous cycles and ovarian morphologies and a normalization in the androgen biosynthesis. This finding suggests FMT as a possible new treatment option for PCOS. Beneficial effects of ω3‐PUFAs including the protective effect and reduction in proinflammatory eicosanoids and concomitant increase in proresolving mediators have been demonstrated. 42 Based on the results of a meta‐analysis, including nine trials with 591 women with PCOS, omega‐3 fatty acid supplementation may be recommended for the treatment of PCOS with IR and dyslipidemia. 120
4.3. Pharmacotherapy
When anovulatory infertility occurs in women with PCOS and/or obesity, lifestyle changes are strongly suggested. 121 In fact, well‐balanced glycemic status in patients with obesity and/or PCOS is strictly related to lifestyle changes; in this regard, educational therapy represents an important approach. 122 , 123
Clomiphene citrate (CC) is a selective oestrogen receptor modulator, and along with letrozole, is the most common medication used for the treatment of subfertility in PCOS anovulatory women. It acts stimulating pituitary gonadotropins secretion through GnRH release, but some adverse effects such as negative changes in the endometrium and cervical mucus might impair the implantation after successful induction of ovulation. 124 These adverse effects lead to a smaller pregnancy rate, approximately 18%, considering the high ovulation rate. 125 The first‐line treatment suggested by guidelines is letrozole, 121 which is considered a better alternative for ovulation induction, including higher pregnancy rates, shorter time to pregnancy and less chances of multiple pregnancy, 126 , 127 but the combination of CC and letrozole seems to be even superior to letrozole alone. 128 Opposite to CC, letrozole has no antioestrogenic effects on the endometrium, it has a short half‐life and causes a late follicular rise in circulating oestrogens following endometrial development, leading to better pregnancy rates, shorter FSH window with subsequent mono‐ovulation. 124 It has been suggested that in the case of CC resistance, in 20%−25% of patients, letrozole and gonadotropins could be more effective options. 129 Women with PCOS tend to have a high prevalence of IR in the majority of cases, but higher CC resistance is likely, too. 130 Current evidence supports no difference in miscarriage rates between letrozole and CC. 131
Despite insulin sensitizers not being recommended as first‐line therapy for infertility, 132 metformin seems to play a role. In fact, in the presence of IR, metformin may improve the ovulatory performance. 114 , 133 Metformin as an insulin sensitizer effectively decreases circulating androgens, improving oocyte quality compared with placebo. 134 It has multiple beneficial effects on metabolic parameters and menstrual disorders in PCOS, and it may reduce the chances of developing an OHSS when used before or during controlled ovarian stimulation for IVF. 135 Metformin improves live birth, clinical pregnancy and ovulation rates with no evidence of a difference in the miscarriage rates compared to placebo. 131 Furthermore, enoxaparin and metformin were shown to reduce pregnancy loss in women with PCOS and thrombophilia and/or hypo fibrinolysis with a history of one or more previous spontaneous abortions. 136 It is well known that obesity per se exacerbates metabolic balance and fertility, and is a very important cause of infertility, often associated with PCOS, IR and hyperinsulinemia additionally exacerbating androgen excess resulting in follicular atresia. CC and metformin are less effective than letrozole and metformin is less effective than CC in women with obesity. 131 In women with PCOS and moderate obesity who are resistant to CC, the use of letrozole over CC in combination with metformin was superior. 137 In women with PCOS and obesity, CC was shown to be superior to metformin for the outcomes of live birth, clinical pregnancy, and ovulation, with insufficient evidence for a difference in multiple pregnancy or miscarriage, whereas the simultaneous use of CC and metformin was superior in ovulation, pregnancy and live birth outcomes. 131
Moreover, sitagliptin treatment may help in women with PCOS and IR to enhance the number of mature oocytes and embryos and to reduce the number of immature oocytes, likely due to a decreased apoptosis in granulosa cells leading to improved quality of oocytes. 138 Gonadotrophins are usually proposed as a second‐line therapy, at an initial dose of 37.5–50 IU/day, and subsequent increasing by 50% every 5–7 days. 139 If ovulation induction treatment fails, third‐line treatment is represented by IVF. 121 , 139
4.4. IVF protocols adjustments
As recommended by the latest European Society of Human Reproduction and Embryology guidelines, to limit the risk of incurring into OHSS upon stimulation of women with PCOS, a GnRH antagonist protocol is recommended to shorten the duration of stimulation and reduce the total gonadotrophin dose, as well as low dose stimulation considering AMH and antral follicle count. 140 Finally, a GnRH agonist trigger and freezing of embryos make the IVF treatment safer and with better outcomes. 121
Recently developed technique in vitro maturation has been suggested as a possible solution to the increased immature oocytes retrieval observed in women with PCOS undergoing IVF. It implies the in vitro culture of immature oocytes until the metaphase II stage, when the oocyte is considered completely mature. 141 Immature oocytes from under‐stimulated PCOS ovaries subjected to in vitro maturation have shown marked improvement in oocyte maturation rates along with increased pregnancy rates. 76
5. CONCLUSIONS
Dysmetabolic infertility truly represents a clash of two pandemics in an era where women seeking pregnancy are often heavier and older than in the past. Beyond a longer time to conceive, a smaller chance of IVF success is also acknowledged. Several treatments may have a beneficial impact, both at a lifestyle level as well as at a pharmacotherapy level. Furthermore, IVF techniques may be adjusted to improve the outcomes. A deeper understanding of the less established mechanistic links between obesity and infertility is warranted to develop better ad hoc treatment: focusing on the role of the brain‐gut axis and chronic inflammation on fertility outcomes may be beneficial, as well as conducting controlled studies investigating the efficacy of novel treatments.
AUTHOR CONTRIBUTIONS
Sanja Medenica, Rossella Mazzilli and Mikiko Watanabe conception and design of the study. Maria Elena Spoltore, Paulina Ormazabal, Ljiljana V. Marina and Antoan Stefan Sojat acquisition of data. Sanja Medenica, Maria Elena Spoltore, Paulina Ormazabal, Ljiljana V. Marina, Antoan Stefan Sojat and Rossella Mazzilli and Mikiko Watanabe draughting the article. Antongiulio Faggiano, Lucio Gnessi, Rossella Mazzilli and Mikiko Watanabe Revising the article critically for important intellectual content. All authors: Final approval of the version to be submitted.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENT
Open Access Funding provided by Universita degli Studi di Roma La Sapienza within the CRUI‐CARE Agreement.
Medenica S, Spoltore ME, Ormazabal P, et al. Female infertility in the era of obesity: the clash of two pandemics or inevitable consequence? Clin Endocrinol (Oxf). 2023;98:141‐152. 10.1111/cen.14785
Rossella Mazzilli and Mikiko Watanabe contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Collaboration NCDRF . Trends in adult body‐mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population‐based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377‐1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Watanabe M, Risi R, De Giorgi F, et al. Obesity treatment within the Italian national healthcare system tertiary care centers: what can we learn? Eat Weight Disord. 2021;26(3):771‐778. [DOI] [PubMed] [Google Scholar]
- 3. Infertility Workup for the Women's Health Specialist: ACOG Committee Opinion, Number 781. Obstet Gynecol. 2019;133(6):e377‐e384. [DOI] [PubMed] [Google Scholar]
- 4. Practice Committee of the American Society for Reproductive Medicine . Electronic address aao, Practice Committee of the American Society for Reproductive M. Obesity and reproduction: a committee opinion. Fertil Steril. 2021;116(5):1266‐1285. [DOI] [PubMed] [Google Scholar]
- 5. Silvestris E, de Pergola G, Rosania R, Loverro G. Obesity as disruptor of the female fertility. Reprod Biol Endocrinol. 2018;16(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCartney CR, Marshall JC. CLINICAL PRACTICE. Polycystic Ovary Syndrome. N Engl J Med. 2016;375(1):54‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22(2):414‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nono Nankam PA, Nguelefack TB, Goedecke JH, Bluher M. Contribution of adipose tissue oxidative stress to obesity‐associated diabetes risk and ethnic differences: focus on women of African ancestry. Antioxidants (Basel). 2021;10(4):622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bluher M. Clinical relevance of adipokines. Diabetes Metab J. 2012;36(5):317‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of pcos as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37(5):467‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Unluhizarci K, Karaca Z, Kelestimur F. Role of insulin and insulin resistance in androgen excess disorders. World J Diabetes. 2021;12(5):616‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dunaif A, Xia J, Book CB, Schenker E, Tang Z. Excessive insulin receptor serine phosphorylation in cultured fibroblasts and in skeletal muscle. A potential mechanism for insulin resistance in the polycystic ovary syndrome. J Clin Invest. 1995;96(2):801‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Belani M, Deo A, Shah P, Banker M, Singal P, Gupta S. Differential insulin and steroidogenic signaling in insulin resistant and non‐insulin resistant human luteinized granulosa cells‐A study in PCOS patients. J Steroid Biochem Mol Biol. 2018;178:283‐292. [DOI] [PubMed] [Google Scholar]
- 14. Khan MJ, Ullah A, Basit S. Genetic basis of polycystic ovary syndrome (PCOS): current perspectives. Appl Clin Genet. 2019;12:249‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Waterworth DM, Bennett ST, Gharani N, et al. Linkage and association of insulin gene VNTR regulatory polymorphism with polycystic ovary syndrome. Lancet. 1997;349(9057):986‐990. [DOI] [PubMed] [Google Scholar]
- 16. Sorbara LR, Tang Z, Cama A, et al. Absence of insulin receptor gene mutations in three insulin‐resistant women with the polycystic ovary syndrome. Metabolism. 1994;43(12):1568‐1574. [DOI] [PubMed] [Google Scholar]
- 17. Witchel SF, Oberfield SE, Pena AS. Polycystic ovary syndrome: pathophysiology, presentation, and treatment with emphasis on adolescent girls. J Endocr Soc. 2019;3(8):1545‐1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nelson VL, Qin KN, Rosenfield RL, et al. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86(12):5925‐5933. [DOI] [PubMed] [Google Scholar]
- 19. Bhide P, Dilgil M, Gudi A, Shah A, Akwaa C, Homburg R. Each small antral follicle in ovaries of women with polycystic ovary syndrome produces more antimullerian hormone than its counterpart in a normal ovary: an observational cross‐sectional study. Fertil Steril. 2015;103(2):537‐541. [DOI] [PubMed] [Google Scholar]
- 20. Wachs DS, Coffler MS, Malcom PJ, Chang RJ. Serum anti‐mullerian hormone concentrations are not altered by acute administration of follicle stimulating hormone in polycystic ovary syndrome and normal women. J Clin Endocrinol Metab. 2007;92(5):1871‐1874. [DOI] [PubMed] [Google Scholar]
- 21. Walters KA, Allan CM, Handelsman DJ. Androgen actions and the ovary. Biol Reprod. 2008;78(3):380‐389. [DOI] [PubMed] [Google Scholar]
- 22. Poretsky L, Seto‐Young D, Shrestha A, et al. Phosphatidyl‐inositol‐3 kinase‐independent insulin action pathway(s) in the human ovary. J Clin Endocrinol Metab. 2001;86(7):3115‐3119. [DOI] [PubMed] [Google Scholar]
- 23. Li S, Huang X, Zhong H, et al. Low circulating adiponectin levels in women with polycystic ovary syndrome: an updated meta‐analysis. Tumour Biol. 2014;35(5):3961‐3973. [DOI] [PubMed] [Google Scholar]
- 24. Unlu E, Unlu BS, Yildiz Y, et al. Adrenal gland volume assessed by magnetic resonance imaging in women with polycystic ovary syndrome. Diagn Interv Imaging. 2016;97(1):57‐63. [DOI] [PubMed] [Google Scholar]
- 25. Houjeghani S, Pourghassem Gargari B, Farzadi L. Serum leptin and ghrelin levels in women with polycystic ovary syndrome: correlation with anthropometric, metabolic, and endocrine parameters. Int J Fertil Steril. 2012;6(2):117‐126. [PMC free article] [PubMed] [Google Scholar]
- 26. Tozzi R, Moramarco A, Watanabe M, et al. Case report: pituitary morphology and function are preserved in female patients with idiopathic intracranial hypertension under pharmacological treatment. Front Endocrinol (Lausanne). 2020;11:613054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thong EP, Codner E, Laven JSE, Teede H. Diabetes: a metabolic and reproductive disorder in women. Lancet Diabetes Endocrinol. 2020;8(2):134‐149. [DOI] [PubMed] [Google Scholar]
- 28. Zhu JL, Cai YQ, Long SL, Chen Z, Mo ZC. The role of advanced glycation end products in human infertility. Life Sci. 2020;255:117830. [DOI] [PubMed] [Google Scholar]
- 29. Nandi A, Poretsky L. Diabetes and the female reproductive system. Endocrinol Metab Clin North Am. 2013;42(4):915‐946. [DOI] [PubMed] [Google Scholar]
- 30. Robker RL, Wu LL, Yang X. Inflammatory pathways linking obesity and ovarian dysfunction. J Reprod Immunol. 2011;88(2):142‐148. [DOI] [PubMed] [Google Scholar]
- 31. Navarro‐Pando JM, Alcocer‐Gómez E, Castejón‐Vega B, et al. Inhibition of the NLRP3 inflammasome prevents ovarian aging. Sci Adv. 2021;7(1):eabc7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93(1):1‐21. [DOI] [PubMed] [Google Scholar]
- 33. Arkan MC, Hevener AL, Greten FR, et al. IKK‐beta links inflammation to obesity‐induced insulin resistance. Nat Med. 2005;11(2):191‐198. [DOI] [PubMed] [Google Scholar]
- 34. Yazici D, Sezer H. Insulin Resistance, Obesity and Lipotoxicity. Adv Exp Med Biol. 2017;960:277‐304. [DOI] [PubMed] [Google Scholar]
- 35. Claria J, Nguyen BT, Madenci AL, Ozaki CK, Serhan CN. Diversity of lipid mediators in human adipose tissue depots. Am J Physiol Cell Physiol. 2013;304(12):C1141‐C1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Watanabe M, Masieri S, Costantini D, et al. Overweight and obese patients with nickel allergy have a worse metabolic profile compared to weight matched non‐allergic individuals. PLoS One. 2018;13(8):e0202683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Risi R, Masieri S, Poggiogalle E, et al. Nickel sensitivity is associated with GH‐IGF1 axis impairment and pituitary abnormalities on MRI in overweight and obese subjects. Int J Mol Sci. 2020;21(24):9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gunkov S, Tatarchuk T, Zhminko P, Regeda S. [Effect of manganese and nickel on prolactin levels in women with polycystic ovary syndrome]. Georgian Med News. 2019;00(289):21‐25. [PubMed] [Google Scholar]
- 39. Zheng G, Wang L, Guo Z, et al. Association of serum heavy metals and trace element concentrations with reproductive hormone levels and polycystic ovary syndrome in a chinese population. Biol Trace Elem Res. 2015;167(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 40. Duleba AJ, Dokras A. Is PCOS an inflammatory process? Fertil Steril. 2012;97(1):7‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wild RA, Carmina E, Diamanti‐Kandarakis E, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE‐PCOS) Society. J Clin Endocrinol Metab. 2010;95(5):2038‐2049. [DOI] [PubMed] [Google Scholar]
- 42. Regidor PA, Mueller A, Sailer M, Gonzalez Santos F, Rizo JM, Egea FM. Chronic inflammation in PCOS: the potential benefits of specialized pro‐resolving lipid mediators (SPMs) in the improvement of the resolutive response. Int J Mol Sci. 2020;22(1):384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rudnicka E, Kunicki M, Suchta K, Machura P, Grymowicz M, Smolarczyk R. Inflammatory markers in women with polycystic ovary syndrome. BioMed Res Int. 2020;2020:4092470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rajani S, Chattopadhyay R, Goswami SK, Ghosh S, Sharma S, Chakravarty B. Assessment of oocyte quality in polycystic ovarian syndrome and endometriosis by spindle imaging and reactive oxygen species levels in follicular fluid and its relationship with IVF‐ET outcome. J Hum Reprod Sci. 2012;5(2):187‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zal F, Ahmadi P, Davari M, et al. Glutathione‐dependent enzymes in the follicular fluid of the first‐retrieved oocyte and their impact on oocyte and embryos in polycystic ovary syndrome: a cross‐sectional study. Int J Reprod Biomed. 2020;18(6):415‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mayer EA. Gut feelings: the emerging biology of gut‐brain communication. Nat Rev Neurosci. 2011;12(8):453‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Org E, Mehrabian M, Parks BW, et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016;7(4):313‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu R, Zhang C, Shi Y, et al. Dysbiosis of gut microbiota associated with clinical parameters in polycystic ovary syndrome. Front Microbiol. 2017;8:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Saydam BO, Yildiz BO. Gut‐brain axis and metabolism in polycystic ovary syndrome. Curr Pharm Des. 2016;22(36):5572‐5587. [DOI] [PubMed] [Google Scholar]
- 50. Sherman SB, Sarsour N, Salehi M, et al. Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis. Gut Microbes. 2018;9(5):400‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Torres PJ, Siakowska M, Banaszewska B, et al. Gut microbial diversity in women with polycystic ovary syndrome correlates with hyperandrogenism. J Clin Endocrinol Metab. 2018;103(4):1502‐1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chu W, Han Q, Xu J, et al. Metagenomic analysis identified microbiome alterations and pathological association between intestinal microbiota and polycystic ovary syndrome. Fertil Steril. 2020;113(6):1286‐1298 e1284. [DOI] [PubMed] [Google Scholar]
- 53. Hartstra AV, Bouter KE, Backhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38(1):159‐165. [DOI] [PubMed] [Google Scholar]
- 54. Yurtdas G, Akdevelioglu Y. A new approach to polycystic ovary syndrome: the gut microbiota. J Am Coll Nutr. 2020;39(4):371‐382. [DOI] [PubMed] [Google Scholar]
- 55. Riaz T, Sollid LM, Olsen I, de Souza GA. Quantitative proteomics of gut‐derived Th1 and Th1/Th17 clones reveal the presence of CD28+ NKG2D‐ Th1 cytotoxic CD4+ T cells. Mol Cell Proteomics. 2016;15(3):1007‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Qin L, Xu W, Li X, et al. Differential expression profile of immunological cytokines in local ovary in patients with polycystic ovarian syndrome: analysis by flow cytometry. Eur J Obstet Gynecol Reprod Biol. 2016;197:136‐141. [DOI] [PubMed] [Google Scholar]
- 57. Kim CH, Park J, Kim M. Gut microbiota‐derived short‐chain fatty acids, T cells, and inflammation. Immune Netw. 2014;14(6):277‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lindheim L, Bashir M, Münzker J, et al. Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with polycystic ovary syndrome (PCOS): a pilot study. PLoS One. 2017;12(1):e0168390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Qi X, Yun C, Sun L, et al. Gut microbiota‐bile acid‐interleukin‐22 axis orchestrates polycystic ovary syndrome. Nat Med. 2019;25(8):1225‐1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kelley ST, Skarra DV, Rivera AJ, Thackray VG. The gut microbiome is altered in a letrozole‐induced mouse model of polycystic ovary syndrome. PLoS One. 2016;11(1):e0146509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zeng B, Lai Z, Sun L, et al. Structural and functional profiles of the gut microbial community in polycystic ovary syndrome with insulin resistance (IR‐PCOS): a pilot study. Res Microbiol. 2019;170(1):43‐52. [DOI] [PubMed] [Google Scholar]
- 62. Zhang J, Sun Z, Jiang S, et al. Probiotic bifidobacterium lactis V9 regulates the secretion of sex hormones in polycystic ovary syndrome patients through the gut‐brain axis. mSystems. 2019;4(2):e00017‐e00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823‐1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761‐1772. [DOI] [PubMed] [Google Scholar]
- 65. Garolla A, Pizzol D, Carosso AR, et al. Practical clinical and diagnostic pathway for the investigation of the infertile couple. Front Endocrinol (Lausanne). 2020;11:591837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Joham AE, Palomba S, Hart R. Polycystic ovary syndrome, obesity, and pregnancy. Semin Reprod Med. 2016;34(2):93‐101. [DOI] [PubMed] [Google Scholar]
- 67. Balen AH, Tan SL, MacDougall J, Jacobs HS. Miscarriage rates following in‐vitro fertilization are increased in women with polycystic ovaries and reduced by pituitary desensitization with buserelin. Hum Reprod. 1993;8(6):959‐964. [DOI] [PubMed] [Google Scholar]
- 68. Zhao B, Koon D, Bethin KE. Identification of transcription factors at the site of implantation in the later stages of murine pregnancy. Reproduction. 2006;131(3):561‐571. [DOI] [PubMed] [Google Scholar]
- 69. Kamalanathan S, Sahoo JP, Sathyapalan T. Pregnancy in polycystic ovary syndrome. Indian J Endocrinol Metab. 2013;17(1):37‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vujović S, Ivovic M, Tančić‐Gajić M, et al. Endometrium receptivity in premature ovarian insufficiency—how to improve fertility rate and predict diseases? Gynecol Endocrinol. 2018;34(12):1011‐1015. [DOI] [PubMed] [Google Scholar]
- 71. Arruda VR, von Zuben PM, Chiaparini LC, Annichino‐Bizzacchi JM, Costa FF. The mutation Ala677—>Val in the methylene tetrahydrofolate reductase gene: a risk factor for arterial disease and venous thrombosis. Thromb Haemost. 1997;77(5):818‐821. [PubMed] [Google Scholar]
- 72. Atiomo WU, Condon J, Adekanmi O, Friend J, Wilkin TJ, Prentice AG. Are women with polycystic ovary syndrome resistant to activated protein C? Fertil Steril. 2000;74(6):1229‐1232. [DOI] [PubMed] [Google Scholar]
- 73. Cavalcante MB, Sarno M, Cavalcante C, Araujo Junior E, Barini R. Coagulation biomarkers in women with recurrent miscarriage and polycystic ovarian syndrome: systematic review and meta‐analysis. Geburtshilfe Frauenheilkd. 2019;79(7):697‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Heijnen EM, Eijkemans MJ, Hughes EG, Laven JS, Macklon NS, Fauser BC. A meta‐analysis of outcomes of conventional IVF in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12(1):13‐21. [DOI] [PubMed] [Google Scholar]
- 75. Zhang Y, Zhang C, Shu J, et al. Adjuvant treatment strategies in ovarian stimulation for poor responders undergoing IVF: a systematic review and network meta‐analysis. Hum Reprod Update. 2020;26(2):247‐263. [DOI] [PubMed] [Google Scholar]
- 76. Marquard KL, Stephens SM, Jungheim ES, et al. Polycystic ovary syndrome and maternal obesity affect oocyte size in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril. 2011;95(6):2146‐2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Qiao J, Feng HL. Extra‐ and intra‐ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update. 2011;17(1):17‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Abbara A, Patel A, Hunjan T, et al. FSH requirements for follicle growth during controlled ovarian stimulation. Front Endocrinol (Lausanne). 2019;10:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity's impact. Fertil Steril. 2017;107(4):840‐847. [DOI] [PubMed] [Google Scholar]
- 80. Murugan VA, Murphy BO, Dupuis C, Goldstein A, Kim YH. Role of ultrasound in the evaluation of first‐trimester pregnancies in the acute setting. Ultrasonography. 2020;39(2):178‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sha T, Wang X, Cheng W, Yan Y. A meta‐analysis of pregnancy‐related outcomes and complications in women with polycystic ovary syndrome undergoing IVF. Reprod Biomed Online. 2019;39(2):281‐293. [DOI] [PubMed] [Google Scholar]
- 82. Li HW, Lee VC, Lau EY, Yeung WS, Ho PC, Ng EH. Cumulative live‐birth rate in women with polycystic ovary syndrome or isolated polycystic ovaries undergoing in‐vitro fertilisation treatment. J Assist Reprod Genet. 2014;31(2):205‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jungheim ES, Lanzendorf SE, Odem RR, Moley KH, Chang AS, Ratts VS. Morbid obesity is associated with lower clinical pregnancy rates after in vitro fertilization in women with polycystic ovary syndrome. Fertil Steril. 2009;92(1):256‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kalra SK, Ratcliffe SJ, Dokras A. Is the fertile window extended in women with polycystic ovary syndrome? Utilizing the Society for Assisted Reproductive Technology registry to assess the impact of reproductive aging on live‐birth rate. Fertil Steril. 2013;100(1):208‐213. [DOI] [PubMed] [Google Scholar]
- 85. Chen YH, Wang Q, Zhang YN, Han X, Li DH, Zhang CL. Cumulative live birth and surplus embryo incidence after frozen‐thaw cycles in PCOS: how many oocytes do we need? J Assist Reprod Genet. 2017;34(9):1153‐1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang J, Wei Y, Diao F, et al. The association between polycystic ovary syndrome and ectopic pregnancy after in vitro fertilization and embryo transfer. Am J Obstet Gynecol. 2013;209(2):139 .e131‐139. [DOI] [PubMed] [Google Scholar]
- 87. Sermondade N, Huberlant S, Bourhis‐Lefebvre V, et al. Female obesity is negatively associated with live birth rate following IVF: a systematic review and meta‐analysis. Hum Reprod Update. 2019;25(4):439‐451. [DOI] [PubMed] [Google Scholar]
- 88. Toulis KA, Goulis DG, Venetis CA, et al. Risk of spontaneous miscarriage in euthyroid women with thyroid autoimmunity undergoing IVF: a meta‐analysis. Eur J Endocrinol. 2010;162(4):643‐652. [DOI] [PubMed] [Google Scholar]
- 89. Zhong YP, Ying Y, Wu HT, et al. Comparison of endocrine profile and in vitro fertilization outcome in patients with PCOS, ovulatory PCO, or normal ovaries. Int J Endocrinol. 2012;2012:492803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng‐Ntim E, El‐Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta‐analysis. Reprod Biomed Online. 2011;23(4):421‐439. [DOI] [PubMed] [Google Scholar]
- 91. Fabozzi G, Cimadomo D, Allori M, et al. Maternal body mass index associates with blastocyst euploidy and live birth rates: the tip of an iceberg? Reprod Biomed Online. 2021;43(4):645‐654. [DOI] [PubMed] [Google Scholar]
- 92. Bu Z, Hu L, Su Y, Guo Y, Zhai J, Sun YP. Factors related to early spontaneous miscarriage during IVF/ICSI treatment: an analysis of 21,485 clinical pregnancies. Reprod Biomed Online. 2020;40(2):201‐206. [DOI] [PubMed] [Google Scholar]
- 93. Liu L, Tong X, Jiang L, Li TC, Zhou F, Zhang S. A comparison of the miscarriage rate between women with and without polycystic ovarian syndrome undergoing IVF treatment. Eur J Obstet Gynecol Reprod Biol. 2014;176:178‐182. [DOI] [PubMed] [Google Scholar]
- 94. DeUgarte DA, DeUgarte CM, Sahakian V. Surrogate obesity negatively impacts pregnancy rates in third‐party reproduction. Fertil Steril. 2010;93(3):1008‐1010. [DOI] [PubMed] [Google Scholar]
- 95. Jungheim ES, Schon SB, Schulte MB, DeUgarte DA, Fowler SA, Tuuli MG. IVF outcomes in obese donor oocyte recipients: a systematic review and meta‐analysis. Hum Reprod. 2013;28(10):2720‐2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ubaldi FM, Cimadomo D, Vaiarelli A, et al. Advanced maternal age in IVF: still a challenge? The present and the future of its treatment. Front Endocrinol (Lausanne). 2019;10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sun B, Ma Y, Li L, et al. Factors associated with ovarian hyperstimulation syndrome (OHSS) severity in women with polycystic ovary syndrome undergoing IVF/ICSI. Front Endocrinol (Lausanne). 2020;11:615957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sterling L, Liu J, Okun N, Sakhuja A, Sierra S, Greenblatt E. Pregnancy outcomes in women with polycystic ovary syndrome undergoing in vitro fertilization. Fertil Steril. 2016;105(3):791‐797. [DOI] [PubMed] [Google Scholar]
- 99. Han AR, Kim HO, Cha SW, et al. Adverse pregnancy outcomes with assisted reproductive technology in non‐obese women with polycystic ovary syndrome: a case‐control study. Clin Exp Reprod Med. 2011;38(2):103‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev. 2015;16(8):621‐638. [DOI] [PubMed] [Google Scholar]
- 101. Saucedo M, Esteves‐Pereira AP, Pencolé L, et al. Understanding maternal mortality in women with obesity and the role of care they receive: a national case‐control study. Int J Obes (Lond). 2021;45(1):258‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Slopien R, Horst N, Jaremek JD, Chinniah D, Spaczynski R. The impact of surgical treatment of obesity on the female fertility. Gynecol Endocrinol. 2019;35(2):100‐102. [DOI] [PubMed] [Google Scholar]
- 103. Best D, Avenell A, Bhattacharya S. How effective are weight‐loss interventions for improving fertility in women and men who are overweight or obese? A systematic review and meta‐analysis of the evidence. Hum Reprod Update. 2017;23(6):681‐705. [DOI] [PubMed] [Google Scholar]
- 104. Wax JR, Lucas FL, Lamont M, Pinette MG, Cartin A, Blackstone J. Maternal and newborn outcomes in planned home birth vs planned hospital births: a metaanalysis. Am J Obstet Gynecol. 2010;203(3):243 .e1‐8. [DOI] [PubMed] [Google Scholar]
- 105. Morley LC, Tang T, Yasmin E, Norman RJ, Balen AH. Insulin‐sensitising drugs (metformin, rosiglitazone, pioglitazone, D‐chiro‐inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 2017;11:CD003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kumar P, Arora S. Orlistat in polycystic ovarian syndrome reduces weight with improvement in lipid profile and pregnancy rates. J Hum Reprod Sci. 2014;7(4):255‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wang Z, Zhao J, Ma X, et al. Effect of orlistat on live birth rate in overweight or obese women undergoing IVF‐ET: a randomized clinical trial. J Clin Endocrinol Metab. 2021;106(9):e3533‐e3545. [DOI] [PubMed] [Google Scholar]
- 108. Cena H, Chiovato L, Nappi RE. Obesity, polycystic ovary syndrome, and infertility: a new avenue for GLP‐1 receptor agonists. J Clin Endocrinol Metab. 2020;105(8):e2695‐e2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Paoli A, Mancin L, Giacona MC, Bianco A, Caprio M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mavropoulos JC, Yancy WS, Hepburn J, Westman EC. The effects of a low‐carbohydrate, ketogenic diet on the polycystic ovary syndrome: a pilot study. Nutr Metab (Lond). 2005;2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Watanabe M, Tuccinardi D, Ernesti I, et al. Scientific evidence underlying contraindications to the ketogenic diet: an update. Obes Rev. 2020;21:e13053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Watanabe M, Risi R, Masi D, et al. Current evidence to propose different food supplements for weight loss: a comprehensive review. Nutrients. 2020;12(9):2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Akbari Sene A, Tabatabaie A, Nikniaz H, et al. The myo‐inositol effect on the oocyte quality and fertilization rate among women with polycystic ovary syndrome undergoing assisted reproductive technology cycles: a randomized clinical trial. Arch Gynecol Obstet. 2019;299(6):1701‐1707. [DOI] [PubMed] [Google Scholar]
- 114. Balen AH, Morley LC, Misso M, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. 2016;22(6):687‐708. [DOI] [PubMed] [Google Scholar]
- 115. Zeng L, Yang K. Effectiveness of myoinositol for polycystic ovary syndrome: a systematic review and meta‐analysis. Endocrine. 2018;59(1):30‐38. [DOI] [PubMed] [Google Scholar]
- 116. Maurizi AR, Menduni M, Del Toro R, et al. A pilot study of D‐chiro‐inositol plus folic acid in overweight patients with type 1 diabetes. Acta Diabetol. 2017;54(4):361‐365. [DOI] [PubMed] [Google Scholar]
- 117. Clarke SF, Murphy EF, O'Sullivan O, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63(12):1913‐1920. [DOI] [PubMed] [Google Scholar]
- 118. Ahmadi S, Jamilian M, Karamali M, et al. Probiotic supplementation and the effects on weight loss, glycaemia and lipid profiles in women with polycystic ovary syndrome: a randomized, double‐blind, placebo‐controlled trial. Hum Fertil (Camb). 2017;20(4):254‐261. [DOI] [PubMed] [Google Scholar]
- 119. Guo Y, Qi Y, Yang X, et al. Association between Polycystic Ovary Syndrome and Gut Microbiota. PLoS One. 2016;11(4):e0153196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yang K, Zeng L, Bao T, Ge J. Effectiveness of Omega‐3 fatty acid for polycystic ovary syndrome: a systematic review and meta‐analysis. Reprod Biol Endocrinol. 2018;16(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bjekić‐Macut J, Vukašin T, Velija‐Ašimi Z, et al. Polycystic ovary syndrome: a contemporary clinical approach. Curr Pharm Des. 2021;27(36):3812‐3820. [DOI] [PubMed] [Google Scholar]
- 123. Defeudis G, Khazrai YM, Di Rosa C, et al. Conversation maps, an effective tool for the management of males and females with type 2 diabetes and mildly impaired glycemic control. Hormones (Athens). 2018;17(1):113‐117. [DOI] [PubMed] [Google Scholar]
- 124. Casper RF, Mitwally MF. Review: aromatase inhibitors for ovulation induction. J Clin Endocrinol Metab. 2006;91(3):760‐771. [DOI] [PubMed] [Google Scholar]
- 125. Yun BH, Chon SJ, Park JH, et al. Minimal stimulation using gonadotropin combined with clomiphene citrate or letrozole for intrauterine insemination. Yonsei Med J. 2015;56(2):490‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bansal S, Goyal M, Sharma C, Shekhar S. Letrozole versus clomiphene citrate for ovulation induction in anovulatory women with polycystic ovarian syndrome: a randomized controlled trial. Int J Gynaecol Obstet. 2021;152(3):345‐350. [DOI] [PubMed] [Google Scholar]
- 127. Tsiami AP, Goulis DG, Sotiriadis AI, Kolibianakis EM. Higher ovulation rate with letrozole as compared with clomiphene citrate in infertile women with polycystic ovary syndrome: a systematic review and meta‐analysis. Hormones (Athens). 2021;20(3):449‐461. [DOI] [PubMed] [Google Scholar]
- 128. Mejia RB, Summers KM, Kresowik JD, Van Voorhis BJ. A randomized controlled trial of combination letrozole and clomiphene citrate or letrozole alone for ovulation induction in women with polycystic ovary syndrome. Fertil Steril. 2019;111(3):571‐578. [DOI] [PubMed] [Google Scholar]
- 129. Shi S, Hong T, Jiang F, Zhuang Y, Chen L, Huang X. Letrozole and human menopausal gonadotropin for ovulation induction in clomiphene resistance polycystic ovary syndrome patients: a randomized controlled study. Medicine (Baltimore). 2020;99(4):e18383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kar S. Clomiphene citrate or letrozole as first‐line ovulation induction drug in infertile PCOS women: a prospective randomized trial. J Hum Reprod Sci. 2012;5(3):262‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Costello MF, Misso ML, Balen A, et al. A brief update on the evidence supporting the treatment of infertility in polycystic ovary syndrome. Aust N Z J Obstet Gynaecol. 2019;59(6):867‐873. [DOI] [PubMed] [Google Scholar]
- 132. Thessaloniki EA‐SPCWG . Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril. 2008;89(3):505‐522. [DOI] [PubMed] [Google Scholar]
- 133. Bevilacqua A, Bizzarri M. Inositols in insulin signaling and glucose metabolism. Int J Endocrinol. 2018;2018:1968450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Doldi N, Persico P, Di Sebastiano F, Marsiglio E, Ferrari A. Gonadotropin‐releasing hormone antagonist and metformin for treatment of polycystic ovary syndrome patients undergoing in vitro fertilization‐embryo transfer. Gynecol Endocrinol. 2006;22(5):235‐238. [DOI] [PubMed] [Google Scholar]
- 135. Tanbo T, Mellembakken J, Bjercke S, Ring E, Abyholm T, Fedorcsak P. Ovulation induction in polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2018;97(10):1162‐1167. [DOI] [PubMed] [Google Scholar]
- 136. Glueck CJ, Wang P, Goldenberg N, Sieve L. Pregnancy loss, polycystic ovary syndrome, thrombophilia, hypofibrinolysis, enoxaparin, metformin. Clin Appl Thromb Hemost. 2004;10(4):323‐334. [DOI] [PubMed] [Google Scholar]
- 137. Bjelica A, Trninic‐Pjevic A, Mladenovic‐Segedi L, Cetkovic N, Petrovic D. Comparison of the efficiency of clomiphene citrate and letrozole in combination with metformin in moderately obese clomiphene citrate‐resistant polycystic ovarian syndrome patients. Srp Arh Celok Lek. 2016;144(3‐4):146‐150. [PubMed] [Google Scholar]
- 138. Daneshjou D, Zadeh Modarres S, Soleimani Mehranjani M, Shariat Zadeh SMA. Comparing the effect of sitagliptin and metformin on the oocyte and embryo quality in classic PCOS patients undergoing ICSI. Ir J Med Sci. 2021;190(2):685‐692. [DOI] [PubMed] [Google Scholar]
- 139. Venturella R, Vaiarelli A, Cimadomo D, et al. State of the art and emerging drug therapies for female infertility. Gynecol Endocrinol. 2019;35(10):835‐841. [DOI] [PubMed] [Google Scholar]
- 140. Ovarian Stimulation T, Bosch E, Broer S, et al. ESHRE guideline: ovarian stimulation for IVF/ICSI(dagger). Hum Reprod Open. 2020;2020(2):hoaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Mostinckx L, Segers I, Belva F, et al. Obstetric and neonatal outcome of ART in patients with polycystic ovary syndrome: IVM of oocytes versus controlled ovarian stimulation. Hum Reprod. 2019;34(8):1595‐1607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


