Abstract
ABSTRACT Twenty lead-exposed men were selected on the basis of a maximum level of lead in the blood of 70-140 μg/100 ml within the past year. There was no clinical evidence of neuropathy attributable to lead and haemoglobin levels were normal. In individuals, maximum motor and sensory conduction and the amplitude of the evoked potentials were normal or borderline in the median, peroneal and sural nerves, except in the distal portion of the deep peroneal nerve. In this nerve, motor conduction was slowed because of compression by metal-lined safety shoes; changes in this segment are not included in the findings. When the average conduction velocity in lead-exposed men was compared with the average in nerves of controls matched for age, distal motor latency was slightly prolonged in the median nerve. The average latency for proximal muscle supplied by the peroneal nerve was prolonged, and the maximum motor conduction velocity was slowed in the median nerve from elbow to wrist (0·01 > p <0·001). In addition, the average maximum sensory conduction was slightly slowed along the distal and intermediate portion of the superficial peroneal and sural nerves (p <0·001). The average minimum sensory conduction velocities were normal, as were the average amplitudes of the evoked muscle action potentials and the average ratio of amplitude of the muscle action potential evoked by stimuli at a proximal and a distal nerve site. The average amplitude of the sensory potentials recorded in the median and the superficial peroneal nerves tended to be increased. Electromyography of the abductor pollicis brevis and anterior tibial muscles showed that the only abnormality was an increased incidence of polyphasic potentials in the anterior tibial muscle of seven men. Neither the slowing in conduction nor the histological findings in the sural nerves of eight men were related to the level of lead in the blood. The slight slowing in conduction suggests a minor defect in the excitable membrane of the nerve fibre: it was not attributable to histological abnormalities in the sural nerve, in which the number of myelinated and unmyelinated nerve fibres was normal and demyelination was absent. In teased fibres, those with paranodal remyelination were slightly increased, and few fibres had segments with diminished diameter. The mechanism of the defect causing the slight slowing in conduction in lead-exposed men seems to differ from the lesion in patients with clinical evidence of lead neuropathy, which is axonal in type. It is, therefore, doubtful whether the slight slowing in the nerves of the group of lead-exposed men should be classified as a subclinical neuropathy.
Full text
PDF

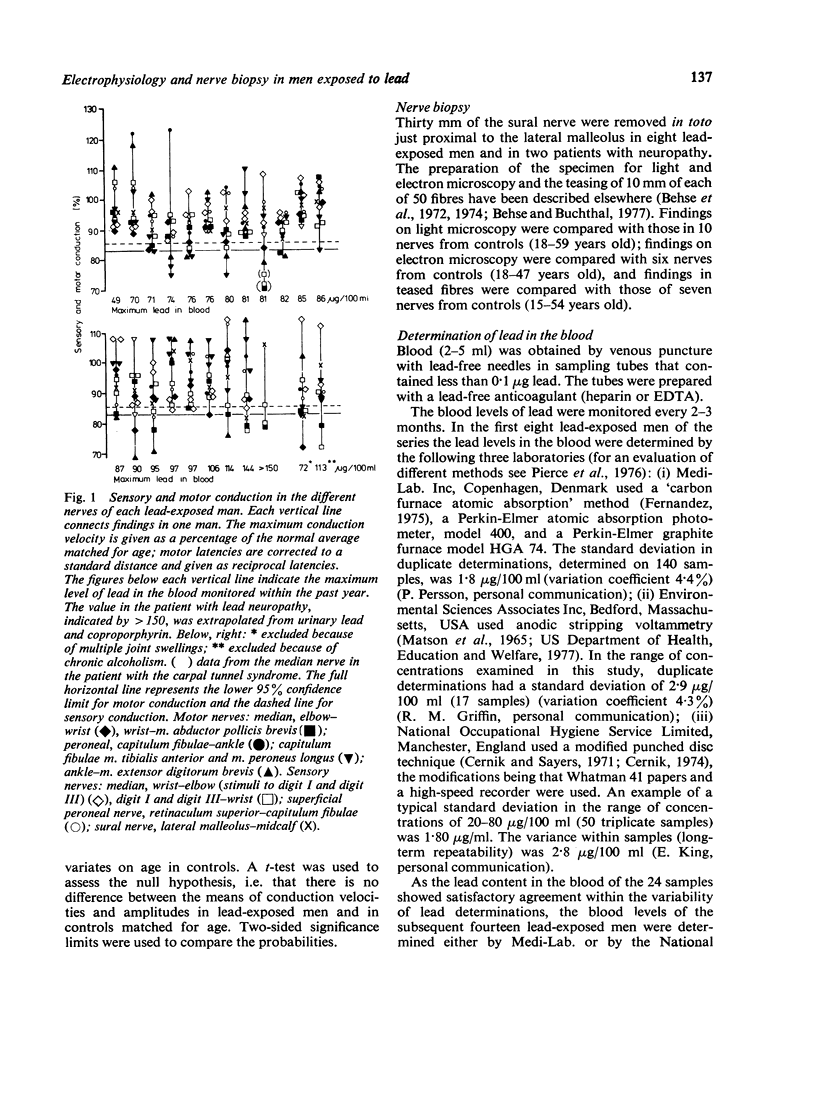
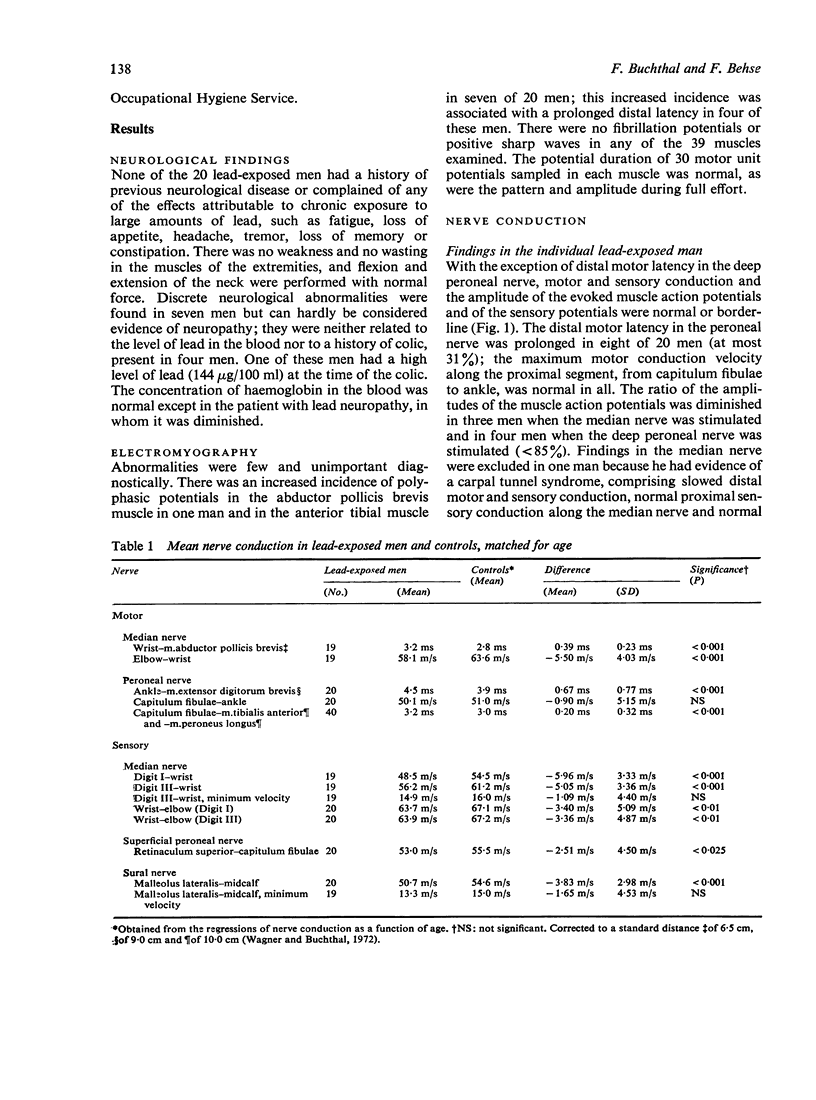
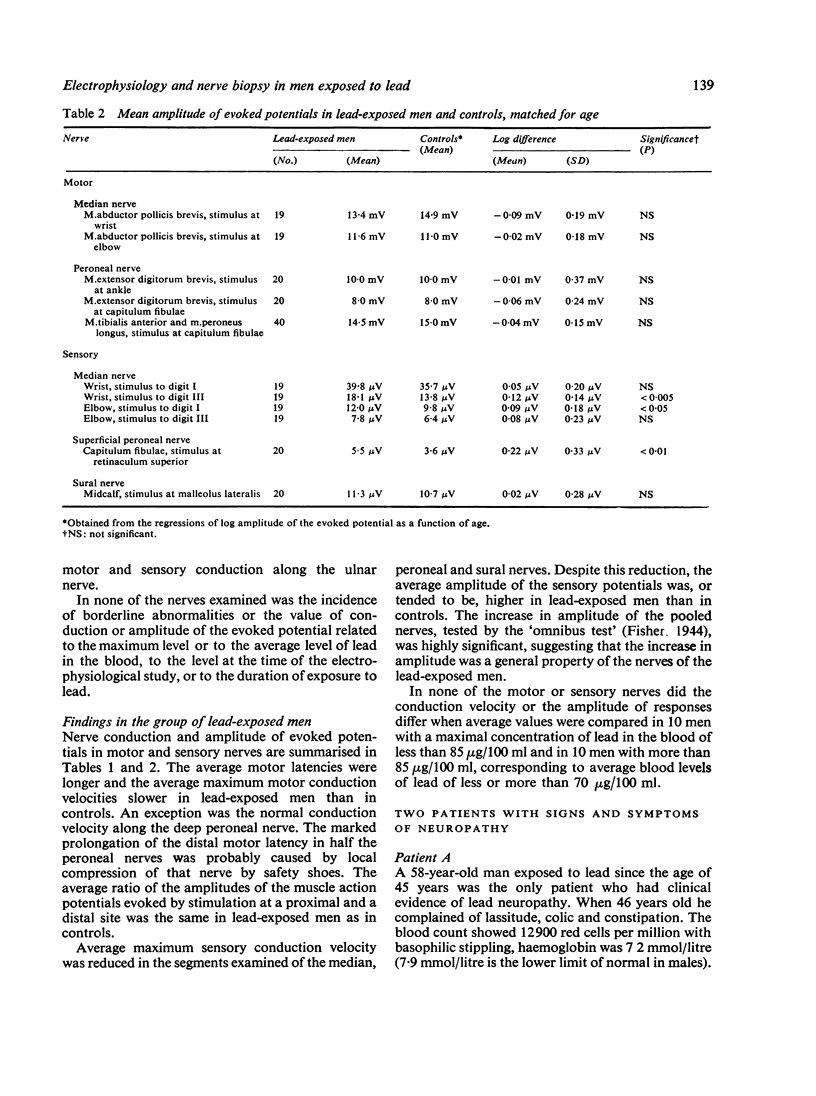



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki S., Honma T. Relationships between lead absorption and peripheral nerve conduction velocities in lead workers. Scand J Work Environ Health. 1976 Dec;2(4):225–231. doi: 10.5271/sjweh.2800. [DOI] [PubMed] [Google Scholar]
- Behse F., Buchthal F., Carlsen F., Knappeis G. G. Endoneurial space and its constituents in the sural nerve of patients with neuropathy. Brain. 1974 Dec;97(4):773–784. doi: 10.1093/brain/97.1.773. [DOI] [PubMed] [Google Scholar]
- Behse F., Buchthal F., Carlsen F., Knappeis G. G. Hereditary neuropathy with liability to pressure palsies. Electrophysiological and histopathological aspects. Brain. 1972;95(4):777–794. doi: 10.1093/brain/95.4.777. [DOI] [PubMed] [Google Scholar]
- Behse F., Buchthal F., Carlsen F., Knappeis G. G. Unmyelinated fibres and Schwann cells of sural nerve in neuropathy. Brain. 1975 Sep;98(3):493–510. doi: 10.1093/brain/98.3.493. [DOI] [PubMed] [Google Scholar]
- Behse F., Buchthal F. Peroneal muscular atrophy (PMA) and related disorders. II. Histological findings in sural nerves. Brain. 1977 Mar;100(Pt 1):67–85. doi: 10.1093/brain/100.1.67. [DOI] [PubMed] [Google Scholar]
- Behse F., Buchthal Sensory action potentials and biopsy of the sural nerve in neuropathy. Brain. 1978 Sep;101(3):473–493. doi: 10.1093/brain/101.3.473. [DOI] [PubMed] [Google Scholar]
- Buchthal F., Rosenfalck A. Sensory potentials in polyneuropathy. Brain. 1971;94(2):241–262. doi: 10.1093/brain/94.2.241. [DOI] [PubMed] [Google Scholar]
- Catton M. J., Harrison M. J., Fullerton P. M., Kazantzis G. Subclinical neuropathy in lead workers. Br Med J. 1970 Apr 11;2(5701):80–82. doi: 10.1136/bmj.2.5701.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernik A. A. Determination of blood lead using a 4-0 mm paper punched disc carbon sampling cup technique. Br J Ind Med. 1974 Jul;31(3):239–244. doi: 10.1136/oem.31.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernik A. A., Sayers M. H. Determination of lead in capillary blood using a paper punched disc atomic absorption technique. Application to the supervision of lead workers. Br J Ind Med. 1971 Oct;28(4):392–398. doi: 10.1136/oem.28.4.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl K., Buchthal F. Digital memory recorder in electromyography and nerve conduction studies. Electroencephalogr Clin Neurophysiol. 1978 Oct;45(4):538–544. doi: 10.1016/0013-4694(78)90298-5. [DOI] [PubMed] [Google Scholar]
- Diagnosis of inorganic lead poisoning: a statement. Br Med J. 1968 Nov 23;4(5629):501–501. doi: 10.1136/bmj.4.5629.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck P. J., Hopkins A. P. Electron microscopic observations on degeneration and regeneration of unmyelinated fibres. Brain. 1972;95(2):233–234. doi: 10.1093/brain/95.2.223. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Johnson W. J., Lambert E. H., O'Brien P. C. Segmental demyelination secondary to axonal degeneration in uremic neuropathy. Mayo Clin Proc. 1971 Jun;46(6):400–431. [PubMed] [Google Scholar]
- Fernandez F. J. Micromethod for lead determination in whole blood by atomic absorption, with use of the graphite furnace. Clin Chem. 1975 Apr;21(4):558–561. [PubMed] [Google Scholar]
- Fullerton P. M. Chronic peripheral neuropathy produced by lead poisoning in guinea-pigs. J Neuropathol Exp Neurol. 1966 Apr;25(2):214–236. doi: 10.1097/00005072-196604000-00003. [DOI] [PubMed] [Google Scholar]
- Gilliatt R. W., Hopf H. C., Rudge P., Baraitser M. Axonal velocities of motor units in the hand and foot muscles of the baboon. J Neurol Sci. 1976 Oct;29(2-4):249–258. doi: 10.1016/0022-510x(76)90174-x. [DOI] [PubMed] [Google Scholar]
- HAUSMANOWA-PETRUSEWICZ I., EMERYK B., SOBKOWICZ H., WASOWICZ B., TUR J. [Electromyographic studies in lead poisoning]. Pol Tyg Lek. 1962 Sep 3;17:1405–1408. [PubMed] [Google Scholar]
- Hausmanowa-Petrusewicz I., Emeryk B., Wasowicz B., Kopeć A. Electromyography in neuro-muscular diagnostics. Electromyography. 1967 Aug-Oct;7(3):203–225. [PubMed] [Google Scholar]
- Hopkins A. Experimental lead poisoning in the baboon. Br J Ind Med. 1970 Apr;27(2):130–140. doi: 10.1136/oem.27.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary D., Ochoa J., Gilliatt R. W. Sub-clinical entrapment neuropathy in man. J Neurol Sci. 1975 Mar;24(3):283–298. doi: 10.1016/0022-510x(75)90248-8. [DOI] [PubMed] [Google Scholar]
- Payan J. Electrophysiological localization of ulnar nerve lesions. J Neurol Neurosurg Psychiatry. 1969 Jun;32(3):208–220. doi: 10.1136/jnnp.32.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prineas J. W., McLeod J. G. Chronic relapsing polyneuritis. J Neurol Sci. 1976 Apr;27(4):427–458. doi: 10.1016/0022-510x(76)90213-6. [DOI] [PubMed] [Google Scholar]
- SIMPSON J. A., SEATON D. A., ADAMS J. F. RESPONSE TO TREATMENT WITH CHELATING AGENTS OF ANAEMIA, CHRONIC ENCEPHALOPATHY, AND MYELOPATHY DUE TO LEAD POISONING. J Neurol Neurosurg Psychiatry. 1964 Dec;27:536–541. doi: 10.1136/jnnp.27.6.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppäläinen A. M., Hernberg S. Sensitive technique for detecting subclinical lead neuropathy. Br J Ind Med. 1972 Oct;29(4):443–449. doi: 10.1136/oem.29.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS P. K., SEARS T. A., GILLIATT R. W. The range of conduction velocity in normal motor nerve fibers to the small muscles of the hand and foot. J Neurol Neurosurg Psychiatry. 1959 Aug;22:175–181. doi: 10.1136/jnnp.22.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A. L., Buchthal F. Motor and sensory conduction in infancy and childhood: reappraisal. Dev Med Child Neurol. 1972 Apr;14(2):189–216. doi: 10.1111/j.1469-8749.1972.tb02576.x. [DOI] [PubMed] [Google Scholar]
- Wiśniewski H., Prineas J., Raine C. S. An ultrastructural study of experimental demyelination and remyelination. I. Acute experimental allergic encephalomyelitis in the peripheral nervous system. Lab Invest. 1969 Aug;21(2):105–118. [PubMed] [Google Scholar]
- Zielhuis R. L. Second international workshop permissible levels for occupational exposure to inorganic lead. Int Arch Occup Environ Health. 1977 Jun 30;39(2):59–72. doi: 10.1007/BF00380886. [DOI] [PubMed] [Google Scholar]