Abstract
The triglyceride glucose‐body mass index (TyG‐BMI) has been considered an alternative marker of insulin resistance (IR). This cross‐sectional study was designed to mainly investigate the association between TyG‐BMI, triglyceride glucose combined with body mass index, and hypertension in Chinese adults. The relationship between TyG‐BMI and hypertension was examined by multivariate logistic regression and restricted cubic spline model. Multiple logistic regression models were also performed to examine the associations between the individual components of TyG‐BMI (BMI, TyG index, TG and FBG) and hypertension. The incremental ability of TyG‐BMI versus its individual components for hypertension discrimination was evaluated by C‐statistic and net reclassification index. Subgroup analysis was performed to examine potential interactions. A total of 92,545 participants (38.9% men, mean age 53.7 years) were included for final analysis. Logistic regression models showed TyG‐BMI and its individual components were all significantly associated with the odds of hypertension (p for trend < .001). The restricted cubic spline regression manifested a linear association between TyG‐BMI and hypertension (p for non‐linear = .062). The addition of TyG‐BMI, in comparison with each individual component, exhibited the maximum incremental value for the discrimination of hypertension on the basis of base model (C‐statistic: 0.679, 95% CI: 0.675‐0.683 for base model vs. 0.695, 95% CI: 0.691‐0.699 for base model + TyG‐BMI; net reclassification index: 0.226, 95% CI: 0.215‐0.234). TyG‐BMI was significantly associated with the odds of hypertension and can be a better discriminator of hypertension.
Keywords: hypertension, insulin resistance, triglyceride glucose‐body mass index (TyG‐BMI)
1. INTRODUCTION
Hypertension represents the primary cause of cardiovascular disease (CVD) and premature mortality throughout the world. 1 Despite the widespread use of antihypertensive drugs, the prevalence of hypertension continues to increase. It was expected an estimated 60% increase in adults with hypertension in 2025 compared to 2000, reaching 1.56 billion, especially in economically developing countries. 2 In China, the prevalence of hypertension was approximately 27.9% among adults and 60% among the elderly population in 2012−2015, while the awareness, treatment, and control of hypertension were low. 3 , 4
Insulin resistance (IR) is generally recognized as decreased insulin sensitivity, resulting in reduced cellular glucose uptake and utilization. 5 As part of metabolic syndrome, IR affects insulin‐regulated pathways and contributes significantly to the progression of numerous cardiovascular risk factors. 6 , 7 Body mass index (BMI) and insulin levels have been widely reported to be associated with hypertension, and BMI affects blood pressure through multiple mechanisms including IR. 8 , 9 The triglyceride glucose (TyG) index has been proven to be a surrogate marker of IR. 10 , 11 Since the strong correlation between obesity and IR was well established, the integration of TyG index and obesity parameters theoretically has an advantage of reflecting IR. 12 , 13 In addition, previous studies have demonstrated that both lipotoxicity and glucotoxicity are contributors to the development of IR. 14 , 15 Recently, the triglyceride glucose‐body mass index (TyG‐BMI), the logarithmic product of fasting blood glucose (FBG) and fasting triglyceride (TG) multiplied by BMI, has been suggested as an alternative indicator of IR. 16 Unlike traditional methods of assessing IR, such as the hyperinsulinemic‐euglycemic clamp (HEC) and homeostasis model assessment of IR (HOMA‐IR), TyG‐BMI does not require measuring insulin but only FBG, TG and BMI (the three most frequently used clinical indicators), which is not only more stable for the IR evaluation but also relatively inexpensive. 10 , 12
Several studies have explored the association of TyG‐BMI with hypertension. 12 , 17 , 18 , 19 , 20 However, the results were inconclusive due to limited sample size or lack of representative population. Moreover, few studies have specialized in the only one indicator TyG‐BMI, rather than a mixture with other indicators. Therefore, we aimed to investigate the association between TyG‐BMI and hypertension among Chinese adults.
2. METHODS
2.1. Participants
The China‐PEACE MPP (Patient‐centered Evaluative Assessment of Cardiac Events Million Persons Project) was a government‐funded public health project and a large‐scale screening initiative to detect population at high risk of CVD all over China. 21 The China‐PEACE MPP design and methods have previously been described. 21 , 22 , 23 We analyzed data from participants of the Early Screening and Comprehensive Intervention Program for High Risk Population of CVD between January 1, 2016 and December 31, 2020 in Guangdong province, China. This program was a vital branch of the China‐PEACE MPP. Participants who were aged 35–75 years old and dwelled locally more than 6 months before screening met the inclusion criteria. Totally, 102,358 subjects were enrolled. Considering the calculation of TyG‐BMI needs FBG and TG, subjects who had taken hypoglycemic agents or lipid‐lowering drugs during the past two weeks that may affect FBG or TG were excluded. Finally, 92,545 participants were included for analysis (Figure 1). The current research was approved by the Institutional Ethics Review Committee of Guangdong Provincial People's Hospital. We have obtained signed informed consent from each participant.
FIGURE 1.
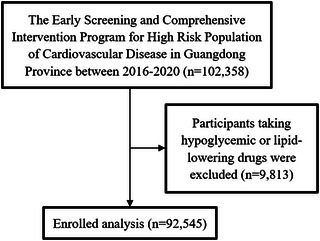
The research flow chart of study participants.
2.2. Data collection and definitions
Standardized questionnaires were administered by well‐trained staff to gather socio‐demographic information, lifestyle behaviors, medication in the past 2 weeks and personal history of disease. Socio‐demographic information included age, gender, nationality, education level, marital status, current job, household income and medical insurance. Lifestyle behaviors included smoking status and alcohol drinking status. Medication included use of antidiabetic, lipid‐lowering, antiplatelet and antihypertensive agents. Personal history of disease included self‐reported diabetes, cancer, and CVD. Physical measurements were performed to exam height, weight, waist circumference (WC), systolic blood pressure (SBP), as well as diastolic blood pressure (DBP). Sitting in a comfortable position, the right upper arm was measured twice for blood pressure using the HEM‐7430 electronic monitor (Omron, Kyoto, Japan). Height and weight were measured without shoes and hats, accurate to 0.1 cm and 0.1 kg, respectively. Fingertip blood samples were collected from all subjects on an empty stomach to determine FBG, TG, total cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐C), and high‐density lipoprotein cholesterol (HDL‐C). Diabetes was defined as FBG ≥ 7 mmol/L, current taking hypoglycemic agents or self‐report of physician diagnosis. Dyslipidemia was defined as TC ≥ 6.2 mmol/L, LDL‐C ≥ 4.1 mmol/L, or self‐reported receiving hypolipidemic medication. Hypertension was defined as SBP/DBP ≥ 140/90 mmHg or taking antihypertensive drugs. 24
2.3. TyG‐BMI calculation
BMI was calculated as weight in kilograms divided by height in meters squared. TyG index was calculated by the formula Ln [FBG (mg/dl) × fasting TG (mg/dl)/2]. 11 TyG‐BMI was calculated as BMI multiplied by TyG index. 16
2.4. Statistical analysis
Baseline characteristics of analyzed population were grouped by quartiles of TyG‐BMI (Q1, Q2, Q3, Q4) and the presence or absence of hypertension. Continuous variables were presented as medians (inter quartile range) due to their non‐normal distribution, which was examined by the Kolmogorov‐Smirnov test. Categorical variables were reported as number (percentage) and compared using the Wilcoxon Mann‐Whitney test, Kruskal‐Wallis H‐test, or chi‐square tests as appropriate. Three sets of multivariate logistic regression models were constructed to estimate odds ratio (OR) with 95% confident interval of hypertension for TyG‐BMI versus its individual components (BMI, TyG index, TG and FBG). In Model 1, no adjustment was made. In Model 2, only age and gender were adjusted. Model 3 was additionally adjusted for marriage, farmer, annual household income, smoking, drinking, having medical insurance, diabetes, dyslipidemia, history of CVD, WC, TC, LDL‐C, and HDL‐C. Next, a restricted cubic spine was applied to assess the correlation between TyG‐BMI and hypertension. Moreover, C‐statistic and net reclassification index were determined to evaluate the incremental value of TyG‐BMI versus its individual components for the discrimination of hypertension. Finally, we conducted subgroup analysis and calculated the p‐value for interactions, including gender (male or female), age (<65 or ≥65 years), and diabetes (no or yes). Receiver operating characteristics (ROC) curve analysis was also employed to assess the specificity and sensitivity of TyG‐BMI in identifying hypertension, compared with each individual component. p < .05 was considered statistically significant.
3. RESULTS
3.1. Baseline characteristics
In the current study, a total of 92,545 participants (men: 38.9% and median age: 53.7 years) were included. Baseline participant characteristics by quartiles of TyG‐BMI and hypertension status were listed in Tables 1 and 2, respectively. We observed all baseline characteristics variables differed significantly in groups except the variable of having medical insurance (p < .05). Participants with hypertension were older, having higher levels of WC, BMI, TC, TG, FBG, TyG‐BMI, more likely to be farmers, smokers, alcohol drinkers, and more likely to have diabetes, dyslipidemia and CVD when compared with subjects without hypertension (Table 2).
TABLE 1.
Baseline characteristics of study participants according to quartiles of triglyceride glucose‐body mass index.
| Q1 | Q2 | Q3 | Q4 | p‐Value | |
|---|---|---|---|---|---|
| Number | 23137 | 23134 | 23138 | 23136 | |
| Age, years | 52.5 (44.3‐62.4) | 53.6 (45.9‐62.5) | 54.2 (47.0‐62.6) | 54.2 (47.0‐62.3) | <.001 |
| SBP, mmHg | 120.5 (110.0‐133.0) | 125.5 (114.5‐137.5) | 129.0 (118.0‐142.0) | 134.0 (123.0‐147.5) | <.001 |
| DBP, mmHg | 74.0 (68.0‐81.5) | 77.0 (70.5‐84.5) | 79.5 (72.5‐87.0) | 82.5 (75.5‐90.5) | <.001 |
| BMI, kg/m2 | 20.4 (19.3‐21.4) | 22.9 (22.1‐23.8) | 24.8 (23.9‐25.8) | 27.6 (26.3‐29.3) | <.001 |
| WC, cm | 74.0 (70.0‐78.0) | 80.1 (76.0‐85.0) | 85.0 (81.0‐90.0) | 92.0 (87.0‐97.0) | <.001 |
| TC, mg/dl | 179.0 (151.9‐209.9) | 185.2 (157.7‐215.3) | 188.7 (160.1‐220.0) | 193.3 (163.9‐226.5) | <.001 |
| TG, mg/dl | 84.2 (68.2‐106.3) | 106.3 (83.3‐137.3) | 132.0 (100.1‐177.2) | 179.9 (131.1‐254.3) | <.001 |
| LDL‐C, mg/dl | 99.0 (75.8‐117.5) | 105.5 (82.0‐126.8) | 105.6 (82.7‐130.7) | 105.6 (82.7‐129.5) | <.001 |
| HDL‐C, mg/dl | 64.2 (53.4‐77.3) | 56.8 (47.2‐68.8) | 52.2 (43.3‐63.4) | 48.3 (39.8‐58.8) | <.001 |
| FBG, mg/dl | 93.6 (84.6‐102.6) | 97.2 (88.2‐106.2) | 100.8 (91.8‐111.6) | 106.2 (95.4‐120.6) | <.001 |
| TyG index | 8.3 (8.1‐8.5) | 8.5 (8.3‐8.8) | 8.8 (8.5‐9.1) | 9.2 (8.8‐9.6) | <.001 |
| TyG‐BMI | 171.0 (160.6‐178.5) | 196.6 (191.0‐201.9) | 219.0 (213.1‐225.4) | 251.5 (240.9‐268.0) | <.001 |
| Gender, n% | <.001 | ||||
| Male | 7695 (33.3) | 8437 (36.5) | 9587 (41.4) | 10312 (44.6) | |
| Female | 15442 (66.7) | 14697 (63.5) | 13551 (58.6) | 12824 (55.4) | |
| Education level, n% | <.001 | ||||
| Less than high school | 15749 (68.1) | 15937 (68.9) | 16365 (70.7) | 16920 (73.1) | |
| High school or above | 7388 (31.9) | 7197 (31.1) | 6773 (29.3) | 6216 (26.9) | |
| Marital status, n% | .015 | ||||
| Married | 20849 (90.1) | 21002 (90.8) | 21028 (90.9) | 21006 (90.8) | |
| Other | 2288 (9.9) | 2132 (9.2) | 2110 (9.1) | 2130 (9.2) | |
| Annual household income, n% | <.001 | ||||
| <50,000 yuan | 12987 (56.1) | 12734 (55.0) | 12593 (54.4) | 12260 (53.0) | |
| >50,000 yuan | 10150 (43.9) | 10400 (45.0) | 10545 (45.6) | 10876 (47.0) | |
| Farmer, n% | <.001 | ||||
| No | 19981 (86.4) | 20397 (88.2) | 20494 (88.6) | 20644 (89.2) | |
| Yes | 3156 (13.6) | 2737 (11.8) | 2644 (11.4) | 2492 (10.8) | |
| Having medical insurance, n% | .042 | ||||
| No | 1556 (6.7) | 1637 (7.1) | 1559 (6.7) | 1483 (6.4) | |
| Yes | 21581 (93.3) | 21497 (92.9) | 21579 (93.3) | 21653 (93.6) | |
| Smoking, n% | <.001 | ||||
| No | 19393 (83.8) | 19573 (84.6) | 19171 (82.9) | 18620 (80.5) | |
| Yes | 3744 (16.2) | 3561 (15.4) | 3967 (17.1) | 4516 (19.5) | |
| Alcohol drinking, n% | <.001 | ||||
| No | 22079 (95.4) | 22073 (95.4) | 21856 (94.5) | 21657 (93.6) | |
| Yes | 1058 (4.6) | 1061 (4.6) | 1282 (5.5) | 1479 (6.4) | |
| Diabetes, n% | <.001 | ||||
| No | 22436 (97.0) | 21767 (94.1) | 20818 (90.0) | 18491 (79.9) | |
| Yes | 701 (3.0) | 1367 (5.9) | 2320 (10.0) | 4645 (20.1) | |
| Dyslipidemia, n% | <.001 | ||||
| No | 20456 (88.4) | 19855 (85.8) | 19350 (83.6) | 18675 (80.7) | |
| Yes | 2681 (11.6) | 3279 (14.2) | 3788 (16.4) | 4461 (19.3) | |
| History of CVD, n% | <.001 | ||||
| No | 22968 (99.3) | 22923 (99.1) | 22893 (98.9) | 22859 (98.8) | |
| Yes | 169 (0.7) | 211 (0.9) | 245 (1.1) | 277 (1.2) | |
| Hypertension, n% | <.001 | ||||
| No | 17974 (77.7) | 15764 (68.1) | 13567 (58.6) | 10843 (46.9) | |
| Yes | 5163 (22.3) | 7370 (31.9) | 9571 (41.4) | 12293 (53.1) |
Note: Data are presented as medians (inter quartile range) or number (%).
Abbreviations: BMI, body mass index; CVD, cardiovascular disease.; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol;Q, quartiles; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; TyG, triglyceride glucose; TyG‐BMI, triglyceride glucose‐body mass index; WC, waist circumference.
TABLE 2.
Baseline characteristics between study participants with and without hypertension.
| Overall | Non‐hypertension | Hypertension | p‐Value | |
|---|---|---|---|---|
| Number | 92545 | 59096 | 33449 | |
| Age, years | 53.7 (46.1‐62.4) | 50.8 (44.0‐59.7) | 59.1 (51.0‐65.6) | <.001 |
| SBP, mmHg | 127.0 (116.0‐140.5) | 120.0 (111.0‐128.0) | 146.0 (138.0‐158.0) | <.001 |
| DBP, mmHg | 78.5 (71.0‐86.0) | 74.5 (68.5‐80.0) | 88.5 (81.0‐94.5) | <.001 |
| BMI, kg/m2 | 23.8 (21.8‐26.0) | 23.3 (21.3‐25.4) | 24.7 (22.7‐27.0) | <.001 |
| WC, cm | 83.0 (76.7‐89.2) | 81.0 (75.0‐87.1) | 86.0 (80.0‐92.0) | <.001 |
| TC, mg/dl | 186.7 (158.1‐218.0) | 184.4 (156.6‐215.3) | 190.6 (161.2‐222.3) | <.001 |
| TG, mg/dl | 117.8 (86.8‐168.3) | 110.8 (82.4‐156.8) | 131.1 (95.7‐188.7) | <.001 |
| LDL‐C, mg/dl | 105.5 (80.8‐126.4) | 104.0 (79.6‐124.5) | 105.6 (82.7‐129.9) | <.001 |
| HDL‐C, mg/dl | 55.3 (44.8‐67.7) | 56.1 (45.6‐68.8) | 53.4 (43.7‐65.3) | <.001 |
| FBG, mg/dl | 99.0 (88.9‐109.8) | 97.2 (88.2‐108.0) | 102.6 (91.8‐115.2) | <.001 |
| TyG index | 8.7 (8.3‐9.1) | 8.6 (8.3‐9.0) | 8.8 (8.5‐9.2) | <.001 |
| TyG‐BMI | 207.5 (185.2‐232.5) | 200.9 (180.0‐224.6) | 219.5 (196.6‐244.2) | <.001 |
| Gender, n% | <.001 | |||
| Male | 36031 (38.9) | 21609 (36.6) | 14422 (43.1) | |
| Female | 56514 (61.1) | 37487 (63.4) | 19027 (56.9) | |
| Education level, n% | <.001 | |||
| Less than high school | 64971 (70.2) | 39374 (66.6) | 25597 (76.5) | |
| High school or above | 27574 (29.8) | 19722 (33.4) | 7852 (23.5) | |
| Marital status, n% | <.001 | |||
| Married | 83885 (90.6) | 5172 (8.8) | 3488 (10.4) | |
| Other | 8660 (9.4) | 53924 (91.2) | 29961 (89.6) | |
| Annual household income, n% | <.001 | |||
| <50,000 yuan | 50574 (54.6) | 31407 (53.1) | 19167 (57.3) | |
| >50,000 yuan | 41971 (45.4) | 27689 (46.9) | 14282 (42.7) | |
| Farmer, n% | <.001 | |||
| No | 81516 (88.1) | 52863 (89.5) | 28653 (85.7) | |
| Yes | 11029 (11.9) | 6233 (10.5) | 4796 (14.3) | |
| Having medical insurance, n% | .071 | |||
| No | 6235 (6.7) | 4048 (6.8) | 2187 (6.5) | |
| Yes | 86310 (93.3) | 55048 (93.2) | 31262 (93.5) | |
| Smoking, n% | <.001 | |||
| No | 76757 (82.9) | 49300 (83.4) | 27457 (82.1) | |
| Yes | 15788 (17.1) | 9796 (16.6) | 5992 (17.9) | |
| Alcohol drinking, n% | <.001 | |||
| No | 87665 (94.7) | 56393 (95.4) | 31272 (93.5) | |
| Yes | 4880 (5.3) | 2703 (4.6) | 2177 (6.5) | |
| Diabetes, n% | <.001 | |||
| No | 83512 (90.2) | 54830 (92.8) | 28682 (85.7) | |
| Yes | 9033 (9.8) | 4266 (7.2) | 4767 (14.3) | |
| Dyslipidemia, n% | <.001 | |||
| No | 78336 (84.6) | 50755 (85.9) | 27581 (82.5) | |
| Yes | 14209 (15.4) | 8341 (14.1) | 5868 (17.5) | |
| History of CVD, n% | <.001 | |||
| No | 91643 (99.0) | 58728 (99.4) | 32915 (98.4) | |
| Yes | 902 (1.0) | 368 (0.6) | 534 (1.6) |
Note: Data are presented as medians (inter quartile range) or number (%).
Abbreviations: BMI, body mass index; CVD, cardiovascular disease.; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol;Q, quartiles; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; TyG, triglyceride glucose; TyG‐BMI, triglyceride glucose‐body mass index; WC, waist circumference.
3.2. Odds ratios of TyG‐BMI for hypertension
Covariables including age, gender, marriage, farmer, annual household income, smoking, drinking, having medical insurance, diabetes, dyslipidemia, history of CVD, WC, TC, LDL‐C, and HDL‐C were controlled in Model 3. When treating TyG‐BMI as a continuous (per SD increment) variable, odds ratio in the multiple logistic regression model was 1.51 (1.48, 1.54). When treating TyG‐BMI as a categorical variable, the multivariate ORs for hypertension were 1.44 (1.38, 1.51) for Q2, 1.89 (1.80, 1.98) for Q3, and 2.76 (2.61, 2.91) for Q4 (p for trend < .001), compared with Q1 of TyG‐BMI (Table 3). Multiple logistic regression models were also performed to examine the associations between each individual component of TyG‐BMI and hypertension. As seen in Table 3, BMI, TyG index, TG and FBG were all significantly associated with hypertension and P for trend of each individual component was < .001. TyG‐BMI was linearly related to hypertension in the multivariate‐adjusted restricted cubic spine model (p for non‐linear = .062) (Figure 2).
TABLE 3.
The associations between TyG‐BMI versus its individual components and hypertension by multivariate logistic regression analysis.
| Case/total | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| TyG‐BMI | ||||
| Per SD increment | 1.68 (1.66, 1.71) | 1.72 (1.69, 1.74) | 1.51 (1.48, 1.54) | |
| Quartiles | ||||
| Q1 | 5015/23137 | 1.0 | 1.0 | 1.0 |
| Q2 | 7162/23134 | 1.62 (1.55, 1.69) | 1.65 (1.58, 1.72) | 1.44 (1.38, 1.51) |
| Q3 | 9267/23138 | 2.41 (2.32, 2.51) | 2.48 (2.37, 2.58) | 1.89 (1.80, 1.98) |
| Q4 | 12005/23136 | 3.90 (3.74, 4.06) | 4.09 (3.92, 4.26) | 2.76 (2.61, 2.91) |
| p for trend | <.001 | <.001 | <.001 | |
| BMI | ||||
| Per SD increment | 1.57 (1.55, 1.59) | 1.61 (1.59, 1.64) | 1.40 (1.37, 1.42) | |
| Quartiles | ||||
| Q1 | 5494/23160 | 1.0 | 1.0 | 1.0 |
| Q2 | 7243/23120 | 1.47 (1.41, 1.53) | 1.52 (1.46, 1.58) | 1.29 (1.23, 1.34) |
| Q3 | 9135/23138 | 2.10 (2.02, 2.18) | 2.19 (2.10, 2.28) | 1.60 (1.52, 1.68) |
| Q4 | 11577/23127 | 3.22 (3.10, 3.35) | 3.46 (3.32, 3.60) | 2.26 (2.15, 2.38) |
| p for trend | <.001 | <.001 | <.001 | |
| TyG index | ||||
| Per SD increment | 1.47 (1.45, 1.49) | 1.45 (1.43, 1.47) | 1.28 (1.25, 1.30) | |
| Quartiles | ||||
| Q1 | 5787/23159 | 1.0 | 1.0 | 1.0 |
| Q2 | 7504/23164 | 1.44 (1.38, 1.50) | 1.41 (1.35, 1.47) | 1.25 (1.20, 1.30) |
| Q3 | 9087/23108 | 1.95 (1.87, 2.02) | 1.87 (1.80, 1.95) | 1.48 (1.42, 1.55) |
| Q4 | 11071/23114 | 2.76 (2.65, 2.87) | 2.67 (2.57, 2.78) | 1.79 (1.70, 1.88) |
| p for trend | <.001 | <.001 | <.001 | |
| TG | ||||
| Per SD increment | 1.32 (1.30, 1.34) | 1.32 (1.30, 1.33) | 1.16 (1.14, 1.18) | |
| Quartiles | ||||
| Q1 | 6294/23694 | 1.0 | 1.0 | 1.0 |
| Q2 | 7638/22910 | 1.38 (1.33, 1.44) | 1.35 (1.30, 1.41) | 1.20 (1.15, 1.25) |
| Q3 | 8917/22817 | 1.77 (1.71, 1.84) | 1.72 (1.65, 1.79) | 1.37 (1.31, 1.43) |
| Q4 | 10600/23124 | 2.34 (2.25, 2.43) | 2.29 (2.20, 2.38) | 1.58 (1.50, 1.66) |
| p for trend | <.001 | <.001 | <.001 | |
| FBG | ||||
| Per SD increment | 1.31 (1.29, 1.33) | 1.29 (1.28, 1.31) | 1.14 (1.12, 1.16) | |
| Quartiles | ||||
| Q1 | 6610/23149 | 1.0 | 1.0 | 1.0 |
| Q2 | 7886/24671 | 1.18 (1.13, 1.22) | 1.18 (1.13, 1.22) | 1.14 (1.09, 1.18) |
| Q3 | 8157/21941 | 1.48 (1.42, 1.54) | 1.47 (1.42, 1.53) | 1.35 (1.29, 1.41) |
| Q4 | 10796/22784 | 2.25 (2.17, 2.34) | 2.19 (2.10, 2.27) | 1.65 (1.58, 1.73) |
| p for trend | <.001 | <.001 | <.001 |
Note: Data are represented as odds ratio (95% confident interval).
Model 1 adjust for none.
Model 2 adjust for age and gender.
Model 3 adjust for age, gender, marriage, farmer, annual household income, smoking, drinking, having medical insurance, diabetes, dyslipidemia, history of cardiovascular disease, waist circumference, total cholesterol, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol.
Abbreviations: BMI, body mass index; FBG, fasting blood glucose; SD, standard deviation; TG, triglyceride; TyG, triglyceride glucose; TyG‐BMI, triglyceride glucose‐body mass index; Q, quartiles.
FIGURE 2.
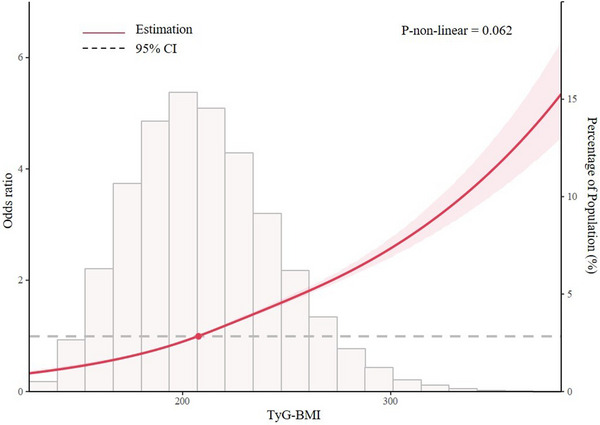
Multivariate‐adjusted restricted cubic spine model of the association between triglyceride glucose‐body mass index and hypertension.
3.3. Subgroup analysis
As stratified by age, gender and diabetes, we performed subgroup analysis shown by forest plots to explore the association between TyG‐BMI and hypertension (Figure 3). No interaction was observed between the three subgroups and the odds of hypertension.
FIGURE 3.
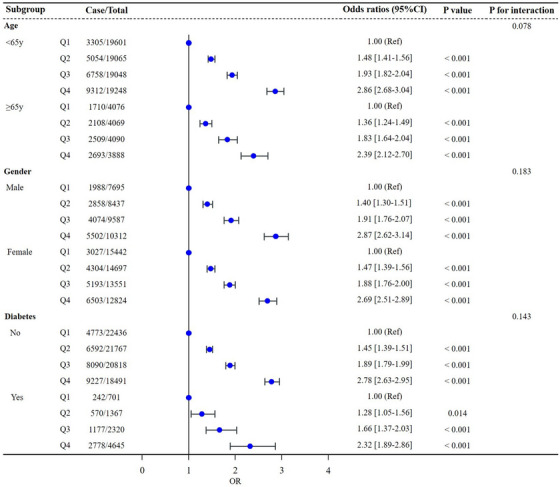
Forest plots of subgroups analysis.
3.4. Incremental value of TyG‐BMI for the discrimination of hypertension
In comparison with its individual components, the addition of TyG‐BMI exhibited the maximum incremental value for the discrimination of hypertension on the basis of base model, in terms of increased C‐statistic (0.679, 95% CI: 0.675‐0.683 for base model vs. 0.695, 95% CI: 0.691‐0.699 for base model + TyG‐BMI), and net reclassification index (0.226, 95% CI: 0.215‐0.234). Base model for hypertension includes age, gender, marriage, farmer, annual household income, smoking, drinking, having medical insurance, diabetes, dyslipidemia, history of CVD, WC, TC, LDL‐C, HDL‐C (Table 4).
TABLE 4.
Incremental value of triglyceride glucose‐body mass index versus its individual components for the discrimination of hypertension.
| C‐statistic (95% CI) | Net reclassification index (95% CI) | |
|---|---|---|
| Base model | 0.679 (0.675‐0.683) | / |
| + BMI | 0.692 (0.688‐0.696) | 0.190 (0.173‐0.198) |
| + TyG index | 0.685 (0.681‐0.689) | 0.174 (0.167‐0.178) |
| + TG | 0.682 (0.678‐0.686) | 0.111 (0.095‐0.118) |
| + FBG | 0.682 (0.678‐0.686) | 0.108 (0.102‐0.123) |
| + TyG‐BMI | 0.695 (0.691‐0.699) | 0.226 (0.215‐0.234) |
Note: Base model: age, gender, marriage, farmer, annual household income, smoking, drinking, having medical insurance, diabetes, dyslipidemia, history of cardiovascular disease, waist circumference, total cholesterol, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol.
Abbreviations: BMI, body mass index; CI, confidence interval; FBG, fasting blood glucose; TG, triglyceride; TyG, triglyceride glucose; TyG‐BMI, triglyceride glucose‐body mass index.
3.5. ROC curve analysis for hypertension
The results of ROC analysis of TyG‐BMI and its individual components for identifying hypertension were summarized in Supplementary Table S1 and Supplementary Figure S1. TyG‐BMI had a maximum area under the curve (AUC) for detection of hypertension (0.645, 95% CI: 0.642‐0.649). FBG presented an optimal cut‐off value of 0.359 and the highest specificity of 0.619. BMI had the highest sensitivity of 0.627.
4. DISCUSSION
We have examined the association between TyG‐BMI and hypertension among Chinese adults aged from 35 to 75 years old. We found TyG‐BMI was positively associated with hypertension and its individual components BMI, TyG index, TG and FBG were all significantly associated with the odds of hypertension. In the incremental value for the discrimination of hypertension, TyG‐BMI was superior to each individual component.
The current study mainly revealed that TyG‐BMI was positively related to hypertension, which was similar to previous studies. A cross‐sectional study, including a total of 105,070 Chinese lean individuals without hypertension, showed that TyG‐BMI was an effective assessment indicator positively correlated with prehypertension. 12 Bala and colleagues found that TyG‐BMI was independently connected to the presence of hypertension, but no superiority could be demonstrated over BMI alone. 18 In another cross‐sectional study with 117,056 subjects, Cheng and colleagues pointed out that TyG‐BMI was positively linked to the prevalence of hypertension (p for trend < .001), which was parallel to our findings. 19 Yuan and colleagues held that TyG‐BMI and TyG‐waist circumference (WC) had better abilities in the prediction of hypertension compared with HOMA‐IR based on an ongoing open cohort study, however, TyG‐WC presented the highest AUC value (0.618, 95% CI: 0.597‐0.639) for identifying hypertension. 20 The results of above studies consistently presented a strong association of TyG‐BMI with hypertension but the predictive value of TyG‐BMI for hypertension remains to be further explored. As an IR surrogate, TyG‐BMI was linked to hypertension possibly through IR‐related hyperinsulinemia, which may influence the activity of sympathetic nervous system or enhance activation of the renin‐angiotensin‐aldosterone system. 25 , 26 To date, only a few studies reported the relationship between TyG‐BMI and hypertension. In our study, we put the focus on Chinese population aged 35−75 and investigated the association of TyG‐BMI with hypertension, compared with its individual components.
Our study indicated that BMI, TyG index, TG and FBG were all independently associated with hypertension, but the discriminative power of them was inferior to TyG‐BMI. Findings from Zhu and colleagues demonstrated that discriminative ability of TyG index for hypertension was superior to lipid and glycemic parameters such as TG and PBG (2 h post‐load blood glucose) in the Chinese elderly population. 27 Linderman and colleagues pointed out that the association between BMI and blood pressure was positive, indicating that increased BMI was associated with the prevalence of hypertension across nearly children, adolescence or adults. 28 In addition, Cheng and colleagues found that the predictive value of TyG‐BMI for the prevalence of hypertension was higher than that of TyG index, which was consistent to our study. 19 However, Bala and colleagues found no superiority of TyG‐BMI over the use of BMI as predictor of hypertension. 18 IR and obesity both may lead to hypertension through a serious of pathophysiological mechanisms. Nevertheless, not all individuals with obesity or excessive weight will develop hypertension. Furthermore, different from previous studies, our study population was Chinese population aged 35−75 and therefore may lead to differences in results. TyG‐BMI combined the effects of TyG index and BMI, which may account for its better discriminative performance for hypertension.
Several speculations are being made regarding the mechanisms linking TyG‐BMI and hypertension. First, elevated TyG‐BMI represents some degree of glucose intolerance, an atherogenic lipoprotein phenotype characterized by low HDL‐C and high TG levels, smaller and more intensive LDL particles, and endothelial dysfunction. 29 Second, hypertension can cause increased TyG‐BMI by altering insulin and glucose delivery to skeletal muscle cells, leading to impaired glucose uptake. 30 Third, the common pathogenesis of IR and hypertension may be sympathetic nervous system activation, which contributes to vasoconstriction and, possibly, changes in vascular structure. 9 , 30
Some limitations with regard to this study should be noted. First, this study did not use HEC, the gold standard for assessing IR, and consequently could not evaluate true insulin levels. 12 Second, the current study did not suggest causality due to its cross‐sectional nature, which requires a well‐designed prospective cohort study to elucidate. Finally, the findings applied mainly to Chinese adults and may not be directly extrapolated to people in other populations.
In conclusion, TyG‐BMI was linearly associated with hypertension in Chinese adults and superior to its individual components BMI, TyG index, TG and FBG in discriminative ability for hypertension. Therefore, we indicated that TyG‐BMI could be a better and more efficient index for the screening of hypertension.
AUTHOR CONTRIBUTIONS
Danying Deng participated in the design of this study and drafted the manuscript. Chaolei Chen performed statistical analysis and data interpretation. Jiabin Wang collected the data. Chaolei Chen and Songyuan Luo helped to draft the manuscript. Yingqing Feng conceived of the study and participated in its design. All authors reviewed and approved this manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Supporting information
Supplementary Figure 1 The receiver operating characteristics curve analysis
Supporting Information
ACKNOWLEDGMENTS
We thank all the participants included in this project. This work was supported by the Key Area R&D Program of Guangdong Province (No.2019B020227005), the Climbing Plan of Guangdong Provincial People's Hospital (DFJH2020022), and Guangdong Provincial Clinical Research Center for Cardiovascular disease (2020B1111170011).
Deng D, Chen C, Wang J, Luo S, Feng Y. Association between triglyceride glucose‐body mass index and hypertension in Chinese adults: A cross‐sectional study. J Clin Hypertens. 2023;25:370–379. 10.1111/jch.14652
Danying Deng and Chaolei Chen contributed equally for this work.
DATA AVAILABILITY STATEMENT
The original contributions are presented in the article/supplementary material, further inquiries can be directed to the corresponding authors.
REFERENCES
- 1. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217‐223. [DOI] [PubMed] [Google Scholar]
- 3. Wang Z, Chen Z, Zhang L, et al. Status of hypertension in China: results from the China hypertension survey, 2012–2015. Circulation. 2018;137(22):2344‐2356. [DOI] [PubMed] [Google Scholar]
- 4. Lu J, Lu Y, Wang X, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from 1.7 million adults in a population‐based screening study (China PEACE Million Persons Project). Lancet. 2017;390(10112):2549‐2558. [DOI] [PubMed] [Google Scholar]
- 5. Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. 2008;31(2):S262‐268. Suppl. [DOI] [PubMed] [Google Scholar]
- 6. DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173‐194. [DOI] [PubMed] [Google Scholar]
- 7. Jonk AM, Houben AJ, de Jongh RT, Serne EH, Schaper NC, Stehouwer CD. Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity‐associated insulin resistance and hypertension. Physiology (Bethesda). 2007;22:252‐260. [DOI] [PubMed] [Google Scholar]
- 8. Nurdiantami Y, Watanabe K, Tanaka E, Pradono J, Anme T. Association of general and central obesity with hypertension. Clin Nutr. 2018;37(4):1259‐1263. [DOI] [PubMed] [Google Scholar]
- 9. Xie Y, Guo R, Li Z, et al. Temporal relationship between body mass index and triglyceride‐glucose index and its impact on the incident of hypertension. Nutr Metab Cardiovasc Dis. 2019;29(11):1220‐1229. [DOI] [PubMed] [Google Scholar]
- 10. Guerrero‐Romero F, Simental‐Mendia LE, Gonzalez‐Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic‐hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347‐3351. [DOI] [PubMed] [Google Scholar]
- 11. Simental‐Mendia LE, Rodriguez‐Moran M, Guerrero‐Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299‐304. [DOI] [PubMed] [Google Scholar]
- 12. Zeng ZY, Liu SX, Xu H, Xu X, Liu XZ, Zhao XX. Association of triglyceride glucose index and its combination of obesity indices with prehypertension in lean individuals: a cross‐sectional study of Chinese adults. J Clin Hypertens (Greenwich). 2020;22(6):1025‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Virdis A, Neves MF, Duranti E, Bernini G, Taddei S. Microvascular endothelial dysfunction in obesity and hypertension. Curr Pharm Des. 2013;19(13):2382‐2389. [DOI] [PubMed] [Google Scholar]
- 14. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98(4):2133‐2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parikh H, Carlsson E, Chutkow WA, et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med. 2007;4(5):e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Er LK, Wu S, Chou HH, et al. Triglyceride glucose‐body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS One. 2016;11(3):e0149731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Y, You A, Tomlinson B, et al. Insulin resistance surrogates predict hypertension plus hyperuricemia. J Diabetes Investig. 2021;12(11):2046‐2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bala C, Gheorghe‐Fronea O, Pop D, et al. The association between six surrogate insulin resistance indexes and hypertension: a population‐based study. Metab Syndr Relat Disord. 2019;17(6):328‐333. [DOI] [PubMed] [Google Scholar]
- 19. Cheng W, Kong F, Chen S. Comparison of the predictive value of four insulin resistance surrogates for the prevalence of hypertension: a population‐based study. Diabetol Metab Syndr. 2022;14(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yuan Y, Sun W, Kong X. Comparison between distinct insulin resistance indices in measuring the development of hypertension: the China Health and Nutrition Survey. Front Cardiovasc Med. 2022;9:912197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu J, Xuan S, Downing NS, et al. Protocol for the China PEACE (patient‐centered evaluative assessment of cardiac events) million persons project pilot. BMJ Open. 2016;6(1):e010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li X, Wu C, Lu J, et al. Cardiovascular risk factors in China: a nationwide population‐based cohort study. Lancet Public Health. 2020;5(12):e672‐e681. [DOI] [PubMed] [Google Scholar]
- 23. Lu J, Lu Y, Yang H, et al. Characteristics of high cardiovascular risk in 1.7 million Chinese adults. Ann Intern Med. 2019;170(5):298‐308. [DOI] [PubMed] [Google Scholar]
- 24. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021‐3104. [DOI] [PubMed] [Google Scholar]
- 25. Tack CJ, Smits P, Willemsen JJ, Lenders JW, Thien T, Lutterman JA. Effects of insulin on vascular tone and sympathetic nervous system in NIDDM. Diabetes. 1996;45(1):15‐22. [DOI] [PubMed] [Google Scholar]
- 26. Fonseca VA. Insulin resistance, diabetes, hypertension, and renin‐angiotensin system inhibition: reducing risk for cardiovascular disease. J Clin Hypertens (Greenwich). 2006;8(10):713‐720. quiz 721‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu B, Wang J, Chen K, et al. A high triglyceride glucose index is more closely associated with hypertension than lipid or glycemic parameters in elderly individuals: a cross‐sectional survey from the Reaction Study. Cardiovasc Diabetol. 2020;19(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Linderman GC, Lu J, Lu Y, et al. Association of body mass index with blood pressure among 1.7 million Chinese adults. JAMA Netw Open. 2018;1(4):e181271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reaven GM. Relationships among insulin resistance, type 2 diabetes, essential hypertension, and cardiovascular disease: similarities and differences. J Clin Hypertens (Greenwich). 2011;13(4):238‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salvetti A, Brogi G, Di Legge V, Bernini GP. The inter‐relationship between insulin resistance and hypertension. Drugs. 1993;46(2):149‐159. Suppl. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 The receiver operating characteristics curve analysis
Supporting Information
Data Availability Statement
The original contributions are presented in the article/supplementary material, further inquiries can be directed to the corresponding authors.


