Abstract
Functional gastrointestinal disorders—recently renamed into disorders of gut–brain interaction—such as irritable bowel syndrome and functional dyspepsia are highly prevalent conditions with bothersome abdominal symptoms in the absence of structural abnormalities. While traditionally considered as motility disorders or even psychosomatic conditions, our understanding of the pathophysiology has evolved significantly over the last two decades. Initial observations of subtle mucosal infiltration with immune cells, especially mast cells and eosinophils, are since recently being backed up by mechanistic evidence demonstrating increased release of nociceptive mediators by immune cells and the intestinal epithelium. These mediators can activate sensitised neurons leading to visceral hypersensitivity with bothersome symptoms. The interaction between immune activation and an impaired barrier function of the gut is most likely a bidirectional one with alterations in the microbiota, psychological stress and food components as upstream players in the pathophysiology. Only few immune-targeting treatments are currently available, but an improved understanding through a multidisciplinary scientific approach will hopefully identify novel, more precise treatment targets with ultimately better outcomes.
Keywords: FUNCTIONAL BOWEL DISORDER, FUNCTIONAL DYSPEPSIA, IRRITABLE BOWEL SYNDROME, INTESTINAL MAST CELLS, Intestinal eosinophils
Key messages.
Functional gastrointestinal disorders—or disorders of gut–brain interaction—are highly prevalent conditions with limited effective treatment options.
Mucosal sensory neurons in irritable bowel syndrome patients are sensitised through an increased release of nociceptive mediators from immune cells and the epithelium.
Subtle infiltration and activation of mast cells and eosinophils, both a source of nociceptive mediators, have been demonstrated in irritable bowel syndrome and functional dyspepsia.
Psychological stress, food components, microbiota and an impaired barrier function may all contribute to immune activation in functional gastrointestinal disorders.
Novel treatment options, specifically targeting neuroimmune interactions in irritable bowel syndrome and functional dyspepsia, are currently being investigated.
Introduction
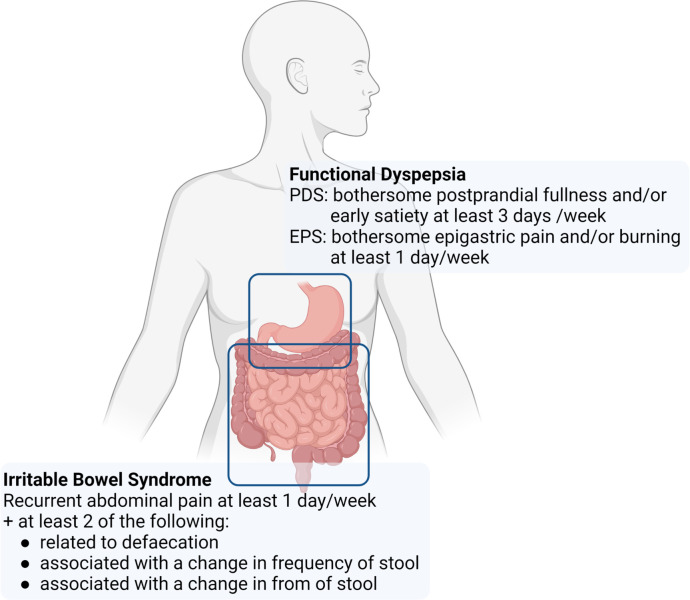
Functional gastrointestinal (GI) disorders are defined by abdominal symptoms in the absence of a structural abnormality explaining the problems. Among the most frequent conditions are irritable bowel syndrome (IBS) characterised by abdominal pain in association with an altered stool pattern and functional dyspepsia (FD) with upper abdominal symptoms (figure 1).1 2 FD symptoms can be triggered by a meal in the postprandial distress syndrome (PDS) or present as epigastric pain or burning not related to food intake in case of the epigastric pain syndrome.2 In the absence of a biomarker, diagnosis relies on symptom criteria of which the Rome IV criteria are the most recent and best validated.3 In a recent large-scale population study by the Rome foundation, the prevalence of IBS and FD was estimated at 4.1 and 7.2%, respectively, using the strict Rome criteria,4 even if for IBS the current criteria have been criticised for being too restrictive in terms of symptom description (only pain, not discomfort) and frequency.5
Figure 1.
Rome IV criteria of functional dyspepsia and irritable bowel syndrome. EPS, epigastric pain syndrome; PDS, postprandial distress syndrome.
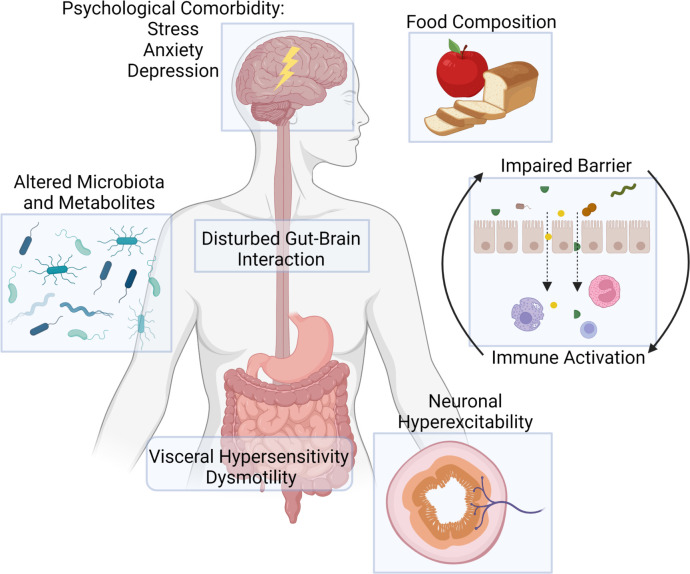
The research focus on the pathophysiology of functional GI disorders has significantly evolved over the last 50 years. Functional GI disorders were initially considered to be purely psychosomatic disorders without a disease substrate in the GI tract. Subsequently, with the development of new tools to examine the function of the GI tract in the 70s and 80s including manometry, GI scintigraphy and breath testing, attention shifted to changes in GI motility including an exaggerated gastrocolonic reflex in IBS and delayed gastric emptying in FD.6–8 Disappointingly, the link between symptoms and disordered motility is limited and has not translated into efficacious treatment options. For example, even if delayed gastric emptying can be found in up to 25% of patients with FD, the correlation to symptoms is inconsistent9–11 and the efficacy of currently available prokinetics is suboptimal and therefore not recommended as first-line therapy in the American, Canadian and European guidelines.12–15 The recognition of the interplay between central (eg, psychological comorbidity including stress and anxiety, altered brain processing) and peripheral, GI alterations (eg, disordered motility, visceral hypersensitivity, immune activation, …) led to the renaming of ‘functional gastrointestinal disorders’ to ‘disorders of gut-brain interaction’ (DGBI) in the Rome IV consensus definition (figure 2).3 In the past 20 years, visceral hypersensitivity, rather than dysmotility, has emerged as a central theme in the pathophysiology of DGBI. Visceral hypersensitivity, that is, abnormal pain signalling to chemical stimuli and/or mechanical distention, has been reported in a variety of patient populations ranging from patients suffering from non-cardiac chest pain,16 gastro-oesophageal reflux disease,17 18 FD19–22 and IBS.23–25 In the current review, we will discuss recent advances in basic and translational research in humans which help us to understand how changes in the GI microenvironment contribute to the development of visceral hypersensitivity in IBS and FD, with a focus on immune activation and neuroimmune interactions. In particular, we will critically discuss the literature on potential mechanisms leading to activation of eosinophils and mast cells, including impaired barrier function, reactions to food, diet–microbiome interactions and psychological comorbidity, and how improved insight in the pathophysiology could be potentially translated into novel treatments.
Figure 2.
Pathophysiological mechanisms in disorders of gut–brain interaction.
Visceral pain signalling and visceral hypersensitivity
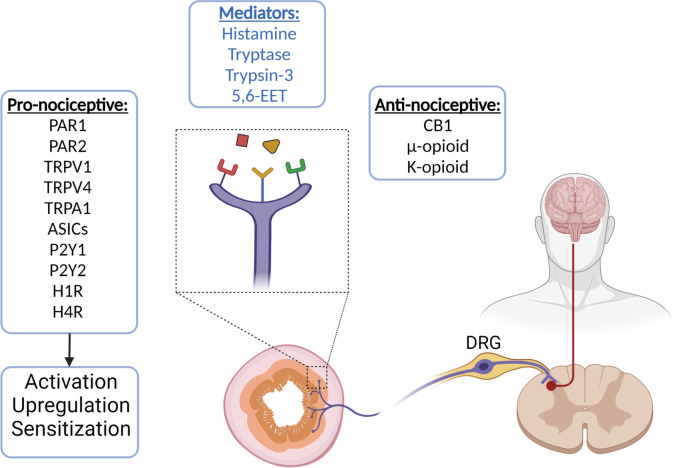
Although sensory neurons located in the enteric nervous system represent key sensors in local neural circuits involved in secretion, absorption, motility and so on, stimuli triggering visceral sensations are mainly conveyed by extrinsic sensory pathways that link the gut to the central nervous system.26 These extrinsic nerve fibres have their cell body in the dorsal root ganglia, synapse in the dorsal horn of the spinal cord, and can be classified into different subtypes of sensory endings depending on the layer they innervate; mucosal, muscular, muscular/mucosal, vascular, serosal and mesenteric afferents.27 28 Visceral pain in particular is detected by pain sensing neurons or nociceptors dedicated to detect harmful thermal, mechanical or chemical stimuli. To this end, their peripheral nerve terminals are equipped with a variety of receptors and ion channels that can be classified into pronociceptive (or excitatory) and antinociceptive (or inhibitory) (figure 3). Transient receptor potential (TRP) channels (TRPV1, TRPV4), acid-sensing ion channels, purinergic and histaminergic receptors, protease activated receptors (PAR1-2) are examples of pronociceptive receptors while typical examples of antinociceptive receptors are opioid and cannabinoid receptors. Depending on the balance between pronociceptive and antinociceptive signals, the nociceptor will signal to the spinal cord transmitting pain to the brain stem, thalamus and cortex. While acute pain is a physiological mechanism of protection informing the organism on a potentially harmful condition or injury, chronic visceral pain is debilitating and can result from either peripheral or central mechanisms affecting pain signalling. Central mechanisms include sensitisation at the level of the spinal cord and abnormal pain processing in the brain. The latter has been elegantly demonstrated mainly by functional MRI revealing abnormal local and global connectivity in the areas related to pain processing.29 In this review, we will focus on peripheral mechanisms affecting the function of visceral nociceptors in patients suffering from functional GI disorders.
Figure 3.
Neuronal mechanisms of visceral hypersensitivity. 5,6-EET, 5,6-epoxyeicosatrienoic acid; ASIC, acid-sensing ion channel; CB1, cannabinoid receptor 1; DRG, dorsal root ganglion; H1R, histamine 1 receptor; P2Y1/2, purinergic receptor P2Y1/2; PAR1/2, protease-activated receptor 1/2; TRPA1, transient receptor potential ankyrin 1; TRPV1/4, transient receptor potential vanilloid 1/4.
Visceral hypersensitivity consists of a painful response to a normally innocuous stimulus (allodynia) and/or an exaggerated response to a painful stimulus (hyperalgesia). This condition can result from increased levels of pronociceptive or decreased levels of antinociceptive mediators in the tissue, upregulation of excitatory receptors or sensitisation of these receptors located on nerve terminals.
Increased release of soluble mediators
Although decreased release by peripheral blood mononuclear cells (PBMCs) of antinociceptive mediators, in particular opioids, has been described in patients with constipation-predominant IBS,30 further evidence supporting this mechanism of aberrant pain signalling is rather limited. In contrast, increased levels of pronociceptive mediators activating sensory neurons has been repeatedly reported in supernatant of biopsies collected from patients with IBS. IBS supernatant indeed contains more histamine and tryptase than supernatant of healthy control biopsies and activates murine visceral afferents or human submucosal neurons, a phenomenon blocked by histamine antagonists and serine protease inhibition.31–33 Intestinal epithelial cells of patients with IBS produce and release increased levels of the active protease trypsin-3, able to signal to enteric neurons and induce visceral hypersensitivity.34 Of interest, increased levels of the TRPV4 agonist 5,6-epoxyeicosatrienoic acid, a metabolite of polyunsaturated fatty acids, have also been reported in IBS biopsies (figure 3).35 The evidence indicating altered synthesis and release of pronociceptive mediators in FD is limited, although increased spontaneous release of histamine and serotonin by gastric biopsies has been reported, in particular in patients suffering from postinfectious FD.36 Besides immune cells, enteroendocrine cells (EEC) represent another source of released mediators such as serotonin. Increased numbers of EEC have been reported in colonic biopsies of patients with diarrhoea-predominant IBS (IBS-D) which was linked to symptom severity, but evidence on EEC activation in DGBI is missing.37 38 Besides production of soluble mediators, subgroups of EECs, labelled as neuropod cells, have been demonstrated to synapse directly with neurons.39 These neuropod cells may function as sensors for luminal signals, but their role in pathophysiology and symptom generation in DGBI remains to be investigated.
Not only in the gut, but also increased release of pronociceptive mediators in peripheral blood of patients has been reported. Medium collected from cultured PBMCs from patients with IBS-D or FD contains more interleukin (IL)−1b, IL-10, tumor-necrosis factor (TNF)-a and IL-6 than that of healthy controls.30 40 Of note, murine visceral afferents respond with direct activation to IL-6 and IL-10, not to TNF-a.30 To what extent peripheral blood cells behave similar to tissue resident immune cells remains however to be confirmed.
Increase excitability of pronociceptive receptors
Increased firing of visceral afferent can also result from increased expression (upregulation) of pronociceptive receptors or ion channels in the plasma membrane or sensitisation of these sensors. Increased expression of TRPV1 and TRPV2 has been reported in gastric biopsies of patients with FD,41 while TRPV142–44 and the purinoreceptors P2Y1 and P2Y242 are increased in rectosigmoid biopsies of patients with IBS (figure 3). A recent study also found elevated expression of TRPV1 and TRPV3 in duodenal biopsies of patients with IBS which correlated with abdominal pain scores in case of TRPV1.45 Inflammatory mediators such as nerve growth factor or inflammatory cytokines can induce increased ion channel expression and transport to the cell membrane of visceral afferents. Of interest, also exposure of DRG neurons to serotonin or histamine results in increased expression of TRPV4 receptors at the plasma membrane surface. This effect was already observed 5 min after treatment and blocked by a specific mitogen-activated protein kinase (MAPK) inhibitor indicating that this effect was not due to new synthesis of TRPV4 but rather to redistribution to the plasma membrane, a phenomenon involving a MAPK pathway.46
Sensitisation of nociceptors involves a reduction in the threshold of activation associated with an increase in the response to noxious stimuli. Using live Ca2+ imaging, we previously demonstrated that submucosal neurons in rectal biopsies from patients with IBS were significantly more excited by TRPA1, TRPV1 and TRPV4 agonists than neurons of healthy controls. Of note, gene expression of these receptors was similar between patients with IBS and controls, indicating that the respective TRP channels are sensitised rather than upregulated,47 48 and may contribute to aberrant pain signalling in IBS. In contrast to IBS, structural abnormalities and decreased responses of duodenal submucosal neurons to general stimuli, including high concentrations of potassium and electrical stimulation, were noted in FD, which correlated with the degree of duodenal eosinophil and mast cell infiltration.49
Besides neurons, enteric glial cells are a second important cell type of the enteric nervous system and are involved in the modulation of neuronal function, but also in epithelial barrier function.50 Activation of enteric glial cells (ie, gliosis) was shown in FD49 and IBS,51 although an earlier study found decreased glial cell responses during Ca2+ imaging on exposure to IBS-D supernatant which was dependent on activation of the histamine receptor 1 (H1R).52
Clearly, as noted above, submucosal enteric neurons are not transmitting nociceptive information to the central nervous system. However, TRP channel sensitisation of submucosal neurons strongly suggests that the microenvironment in which these neurons reside contains mediators with sensitising properties and thus can equally affect neighbouring nerve terminals of extrinsic visceral afferents. Of note, incubation of biopsies from healthy controls with histamine results in sensitisation of TRPV1 on submucosal neurons, suggesting the possible involvement of histamine in the hyperexcitability of pain signalling pathways.47 Obviously, other mediators such as cytokines, serotonin, proteases and metabolites of polyunsaturated fatty acids, present in supernatant of colonic/rectal biopsies can all contribute.
Aberrant visceral pain signalling induced by biopsy supernatant
Finally, preclinical studies involving both in vivo assessment of visceral pain and in vitro recording of visceral afferents have provided convincing evidence that supernatant, in particular collected from IBS biopsies, contains mediators that excite nociceptors and induce visceral hypersensitivity.28 For example, Cenac et al elegantly demonstrated increased trypsin and tryptase expression and release in colonic biopsies of patients with IBS and sensitisation of murine sensory neurons in vitro.53 Interestingly, administration of IBS supernatant into the colon of mice resulted in increased pain responses evoked by colorectal distension. These pronociceptive effects were blocked by serine protease inhibitors and a PAR2 antagonist and absent in PAR2-deficient mice, clearly demonstrating the involvement of proteases. Along the same line, application of IBS supernatant to murine visceral nociceptive afferents32 54 or human submucosal neurons33 results in increased neuronal firing, effects mediated by histamine, serotonin and proteases.
Taken together, the above evidence clearly indicates that the microenvironment in the intestine of IBS and most likely also of functional dyspeptic patients possesses pronociceptive properties increasing pain signalling in these patients.
Immune activation
The most likely source of nociceptive mediators in the intestinal microenvironment described above is the mucosal immune system. Even if obvious macroscopic or microscopic inflammation is not compatible with a diagnosis of a functional GI disorder following the Rome criteria, cumulative evidence from the last two decades suggests an important role for mucosal immune activation in both IBS and FD.
Immune cell infiltration and activation
Immune cell infiltration in DGBI was first demonstrated in patients who developed IBS after an infectious gastroenteritis with a persistently elevated number of mononuclear cells and T lymphocytes in rectal biopsies.55 56 Since then, several groups reported similar findings and a meta-analysis confirmed increased T-cells in the lamina propria of the rectosigmoid.57 In contrast, gastroduodenal lymphocyte infiltration, including intra-epithelial lymphocyte counts, does not come up as a consistent feature of FD,58 59 even if two small studies reported elevated CD8+T cell counts and aggregates in postinfectious FD.60 61 Peripheral blood lymphocytes expressing gut-homing markers were increased in FD40 and IBS.62–64 Whether the circulating gut-homing lymphocytes have a role in the pathogenesis of the disease, including GI immune activation remains to be demonstrated.
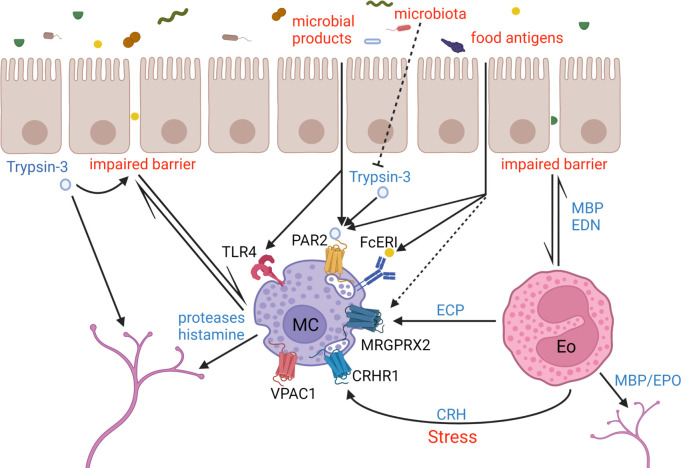
More importantly, the most reproducible finding in the literature relates to the involvement of mast cells and eosinophils in the pathophysiology of IBS and FD, respectively (figure 4). In 2004, the Bologna group was the first to report increased mast cell numbers in rectal biopsies of 44 patients with IBS.31 These findings were corroborated by several other studies, mainly in the left hemicolon and to a similar extent in the different IBS subtypes, with some studies demonstrating an association between mast cell numbers and symptom.57 65 Nevertheless, the mast cell infiltration is not an undisputed finding as some groups have not detected a difference or even lower mast cell counts.57 66 67 These differences may relate to the patient selection, location of the biopsy but also the quantification methodology (eg, slide preparation, staining, counting). Moreover, it is unlikely that the subtle increase in immune cell numbers is relevant for symptom induction and may be an epiphenomenon in the context of immune cell activation. Indeed, mast cell activation—rather than just the numbers—demonstrated by degranulation and release of mast cell mediators such as histamine and tryptase in supernatant emerges as a more consistent finding with more relevance to symptom generation, based on a correlation between symptom severity and the distance of the mast cells to mucosal nerves.31 68 Moreover, as discussed above, IBS supernatant-induced hyperreactivity of neurons which could be blocked by histamine receptor H1 antagonists and protease inhibitors, underscoring the importance of mast cell derived soluble mediators in neuronal hyperreactivity.32 33 47 The role of mast cells in FD is less established, although a few studies have shown increased duodenal mast cell counts.59 69 Mast cell activation in FD is suggested by a study using electron microscopy showing more heterogeneous granules in duodenal mast cells in FD and evidence of increased release of histamine and tryptase from gastric biopsies.36 70 Similar to IBS, mast cells were more likely to be localised near mucosal nerves in the stomach and the duodenum.36 71
Figure 4.
Immune activation in irritable bowel syndrome and functional dyspepsia. Food and microbiota-derived antigens gain access to the subepithelial space through an impaired intestinal barrier function. These antigens and epithelium-derived proteases such as trypsin-3 can activate mast cells and eosinophils through a variety of mechanisms. Mediators from activated mast cells and eosinophils can subsequently activate sensory neurons. Finally, in conditions of psychological stress locally secreted CRH from eosinophils can activate mast cells. CRH, corticotropin-releasing hormone; CRHR1, CRH receptor 1; ECP, eosinophil cationic protein; EDN, eosinophil-derived neurotoxin; Eo, eosinophil; EPO, eosinophil peroxidase; FcERI, high-affinity IgE receptor; MBP, major basic protein; MC, mast cell; MRGPRX2, Mas-related G-protein coupled receptor member X2; NK1/2, neurokinin receptor 1/2; PAR2, Protease-activated receptor 2; SP, Substance P; TLR4, Toll-like receptor 4; VPAC1, vasoactive intestinal peptide receptor type 1. Dashed line indicates a potential link.
In FD, however, a more prominent role has been attributed to duodenal eosinophil infiltration which has been reported by multiple groups (figure 4).69 Duodenal eosinophils as well as their activation and degranulation were often also linked to symptoms, although only based on statistical correlations and mechanistic evidence is still largely lacking.59 69 In the initial reports, it was suggested that duodenal eosinophilia was a feature of PDS,72 but a recent meta-analysis showed similar counts in the FD subgroups.69 Nevertheless—and similar to data on mast cell counts in IBS—elevated duodenal eosinophil counts were not detected in all cohorts: in the largest study to date, Järbrink-Seghal et al found no association between duodenal eosinophil counts and FD in 178 patients and 258 controls, while degranulation, that is, activation, was associated to FD and symptoms of early satiety.73 These discrepancies in the literature may again come down to patient selection, the variability in quantification methodology and stress that research should focus on activation of immune cells rather than quantification of cell numbers, similar to IBS. Another important confounder in the FD literature is also the use of comedication, most notably proton-pump inhibitors (PPIs), which is not appropriately addressed in most studies. In a recent prospective study, we have shown that the use of a PPI, which is still the first-line treatment for FD as recommended by guidelines,14 lowered duodenal eosinophil and mast cell counts in the short term, although prolonged treatment may provoke opposite, proinflammatory effects, potentially through duodenal dysbiosis by overgrowth of oropharyngeal flora.74 75 Indeed, the increased numbers of duodenal eosinophils after longer term PPI in patients without symptomatic benefit of the PPI and in healthy individuals treated with PPI for 4 weeks, were linked to increased abundance of Streptococci in duodenal brushings.75 Increased duodenal abundance of streptococci has recently been linked to symptom severity in FD.76 In IBS, a few studies have found eosinophil infiltration in the colon77 and a recent Swedish study found increased levels of faecal eosinophil-derived neurotoxin in patients with IBS on top of mucosal infiltration and degranulation as shown by histology.78 Surprisingly, few data are available on gastric immune activation in FD with only one study reporting higher mast cell counts in gastric biopsies of patients with FD.36
Unfortunately, the large majority studies described above have only considered histological cell counts rather than immune cell activation which is most likely the critical factor in DGBI. For example, healthy subjects treated with PPI also displayed increased duodenal eosinophil counts in the absence of abdominal symptoms,74 highlighting that the reported limited increases in cell counts cannot be hold accountable for symptoms just by their presence and that future research should focus on activation of immune cells and the produced mediators.
Circulating and tissue cytokines
The data on systemic and mucosal cytokine expression and release are contradictory and interpretation is hampered by small patient numbers and in most cases selective analysis of a limited number of cytokines. In a meta-analysis, a tendency towards increased circulating TNF-α and lower colonic release of IL-10 was the only signal that emerged in IBS, although the magnitude was limited.79 Another possible reason for the lack of a clear immune signal is the heterogeneity of the condition itself which is only defined by subjective symptoms. It is to be expected that only a subgroup of DGBI patients will exhibit a mild immune activation signature. Indeed, in several larger studies, 1/5 to 1/3 of patients with IBS demonstrated an ‘immuno-active’ phenotype in terms of systemic release or local expression of inflammatory mediators.80–82 In a recent study, we demonstrated that 1/3 of patients with IBS exhibited signs of immune activation in biopsies from the descending colon.82 This group showed higher expression of IL1β, prostaglandin synthase PTGS2 and the G-protein coupled receptor Mas-related G-protein coupled receptor member X2. The latter is of interest since it is a receptor involved in mast cell activation and eosinophil-mast cell interaction,83 even if it was only expressed in a minority of the biopsies.82 Nevertheless, in none of the mentioned studies, a link between symptom pattern or severity and the immune phenotype could be established. Similarly, large studies are lacking in FD and even if mildly elevated IL-1 and IL6 expression in the duodenal and gastric mucosa have been reported, the findings are limited to single studies with a small sample size.59 One small study found a higher production of the Th2 related cytokines IL-5 and IL-13 by stimulated PBMCs, which is of interest based on the eosinophil-predominant immune signal in FD, although confirmatory data are needed.84 Finally, discretely increased high-sensitive C-reactive protein levels in patients with FD in our recent study, which normalised after PPI treatment, also suggest a systemic immune signal although the relevance to symptom generation of these minute systemic changes is most likely limited.74
Aetiology of GI immune activation
We selected four potential mechanisms leading to immune activation for which reasonable evidence is available in the current literature demonstrating a mechanism of action, beyond the existence of an associative link with immune activation.
Impaired barrier function
The intestinal barrier represents the largest interface between the environment and the internal milieu of the organism. The barrier has a dual function of protecting the organism from unwanted penetration of luminal, potentially noxious substances including pathogens and their secreted products, while at the same time allowing absorption of fluid and (micro)nutrients. The barrier also plays a critical role in suppressing immune activation towards orally ingested innocuous antigens, mainly food, in a process defined as oral tolerance.85 To achieve oral tolerance, luminal antigens are sampled by microfold (M) cells in Peyer patches, mucosal macrophages or goblet cells, subsequently acquired by tolerogenic dendritic cells which then migrate to mesenteric lymph nodes with induction of immunosuppressive regulatory T-cells.
Disruption of this tightly regulated barrier function has been described in a variety of conditions, including coeliac disease, inflammatory bowel disease, food allergy and functional GI disorders.86 However, measurement of intestinal barrier function is complex and contributes to the variability of the reported data. Direct measurement of permeability of endoscopic biopsies in Ussing chambers is still the gold standard, but less frequently used.86 87
In a recent systematic review, most of the 66 analysed studies in IBS found a reduced small intestinal or colonic barrier function, which was mainly present in postinfectious and diarrhoea-predominant IBS and to a lesser extent in constipation predominant IBS.88 Positive associations with altered stool pattern and pain were identified in the majority of the studies, although an inverse association has been reported as well.89 Similarly, multiple studies have reported increased duodenal permeability in FD using in vivo techniques (urinary sugar excretion tests, mucosal impedance and confocal laser endomicroscopy (CLE)) and biopsies in Ussing chambers, although a link with symptom severity was absent in most studies.87
The expression of several tight junction related proteins, which are the key determinants of epithelial barrier function, in different regions of the GI tract was shown to be altered in IBS and FD, although the reported changes were not consistent between studies.87 88 Colonic infusion of faecal supernatant of patients with IBS in mice or application of biopsy supernatant on cell lines resulted in lower barrier function, indicating the involvement of luminal mediators.90–93 The identity of these mediators is still unclear, but proteases, microbial products, food and bile acids are the main candidates (figure 4). Currently, most of the evidence supports a role for proteases. Faecal supernatant of patients with IBS contains increased levels of serine and cysteine proteases which were associated with impaired barrier function and altered tight junction expression.90 91 94 95 Several studies demonstrated that these proteases are from human origin, including the epithelium-derived trypsin-3.34 93 95 A recent elegant study demonstrated that high proteolytic activity in patients with postinfectious IBS was related to impaired suppression of the host-derived proteases by the microbiota.93
It is often hypothesised that the impaired barrier function allows uncontrolled penetration of antigens in the lamina propria, inciting an immune response. However, whether the increase permeability plays a causal role in these conditions or is rather a consequence of the immune activation remains a topic of ongoing controversy. In support of the last option, tryptase released from mast cells96 97 and major basic protein from eosinophils98 have been shown to impair permeability in cell lines. Moreover, stabilising mast cells by ketotifen or blocking the receptors for vasoactive intestinal polypeptide on mast cells reduced transcellular passage of commensal and pathogenic bacteria in colonic biopsies of patients with IBS.99 We previously demonstrated a correlation between duodenal eosinophil levels and decreased protein expression of phosphorylated occludin and E-cadherin, but mechanistic data in humans with FD are lacking.100
Interpretation of the relevance of intestinal barrier function in the pathophysiology of DGBI is hampered by the lack of treatments which can stabilise the barrier. A possible exception is glutamine which improved symptoms and normalised barrier function in patients with postinfectious IBS,101 possibly through an upregulation of claudin 1.102 However, replication of these data in a larger multicentric study is warranted.
Food
It is well documented that a majority of patients with FD and IBS link their symptoms to food intake (figure 2).103 104 Especially the role of FODMAPs or fermentable oligosaccharides, disaccharides, monosaccharides and polyols in symptom generation is well established in IBS and is thought to mainly result from their osmotic effects and fermentation in the colon with gaseous distension.105 Of interest, recent studies have provided exciting evidence that certain foods can also trigger immune-mediated reactions in the GI tract. Bischoff et al injected food antigen extracts in the caecal mucosa of patients with chronic abdominal symptoms and suspected food allergy, 77% of whom reacted to at least one injection with a weal and flare reaction, which was associated with local eosinophil and mast cell activation.106 This concept was further developed by studies using CLE, using in vivo fluorescence microscopy after intravenous fluorescein administration, with food extract (wheat, yeast, gluten, cow’s milk) application to the duodenal mucosa. Frischer-Ravens et al demonstrated a positive reaction, with increases in intraepithelial lymphocytes, formation of interepithelial gaps and cell shedding, in 2/3 of patients in 2 cohorts of patients with IBS without elevated systemic IgE levels.107 108 Wheat was the trigger in more than half of the patients. Patients with a positive reaction had a favourable response to an exclusion diet, although a sham diet and a diet in the CLE negative group were lacking, hampering interpretation of the relevance of the test-based diet in clinical practice. Of interest, even if the total number of eosinophils and mast cells were not different in patients with IBS with or without a positive reaction versus healthy controls, a positive reaction was associated with release of eosinophil-cationic protein, highlighting again the importance of released mediators rather than cell counts.108 This observation is somewhat surprising as the mucosal immune system is educated not to react against innocuous antigens such as dietary antigens, that is, oral tolerance.28 85 Only failure to develop or loss of oral tolerance to food antigens, for example, following an infection will lead to the development of diseases such as food allergy and coeliac disease.109–112 In case of food allergy, antigen-specific IgE antibodies directed against dietary antigens can be detected in the systemic circulation. These antibodies bind to IgE receptors on mast cells resulting in mast cell sensitisation and ultimately activation when crosslinked by binding of the respective antigen.112 Notably, increased levels of systemic IgE antibodies against food epitopes can however not be detected in patients with IBS or FD.113 114 These findings suggest that either non-IgE mediated eosinophil/mast cell activation or an IgE-mediated reaction confined to the GI mucosa is involved.
Recently, Aguilera-Lizarraga et al elegantly highlighted a role of local but not systemic IgE antibodies against a dietary antigen in an animal model of visceral hypersensitivity.68 Feeding of the dietary antigen ovalbumin during an infectious colitis (Citrobacter rodentium) resulted in loss of oral tolerance resulting in the local production of ovalbumin-specific IgE antibodies in the colon. Feeding of ovalbumin after clearance of the infection resulted in IgE-dependent mast cell activation and sensitisation of visceral afferents leading to abnormal pain signalling. These data are in line with the clinical observation that an infectious gastroenteritis is a major risk factor to develop IBS.115 Moreover, and similar as in the studies described above, injection of food antigens in the rectosigmoid mucosa of patients with IBS elicited a mucosal reaction. Finally, IgE positive mast cells were more prevalent in patients with IBS and located at a shorter distance from nerve endings, correlating to severity of symptoms.68 Taken together, these data indicate a role for local IgE antibodies against dietary antigens in food-induced symptoms in IBS and introduce a new mechanism explaining mast cell activation in IBS. We propose that IBS may be part of a spectrum of food-induced disorders mediated by mast cell activation with systemic food allergy at the extreme end of the spectrum. Similar data in FD are currently still lacking.
Diet–microbiome interactions
Altered microbial composition and metabolomic profiles have been found in patients with IBS compared with healthy controls.116–118 It is unclear, whether these changes are consequence or cause of altered bowel function, diet and symptoms, but accumulating data suggest that at least in some patients, the symptoms of IBS could be attributed to specific microbial neuroactive metabolites, such as serotonin or tryptamine.118 119 This is not surprising as gut bacteria can produce many neurochemical mediators that are found in mammals using their dietary precursors.120
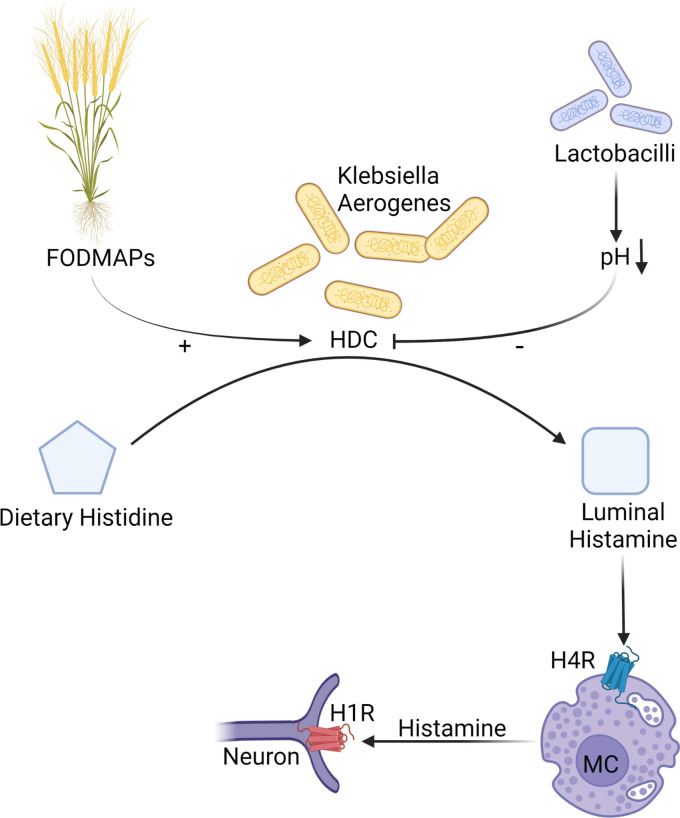
Histamine, previously linked to visceral hypersensitivity, increased permeability and altered motility, can be produced by the microbiome as many bacteria possess the enzyme histidine decarboxylase (HDC) that converts dietary histidine into histamine (figure 5).121 122 There are two main types of bacterial HDC, pyridoxal phosphate-dependent and pyruvoyl-dependent ones.122 While the former is found in Gram-negative bacteria (such as Enterobacter aerogenes, Raoultella ornytolytica or Morganella morganii), the latter is encountered in Gram-positive bacteria, especially in lactic acid producing strains such as Lactobacilli.
Figure 5.
Food–microbiota interaction in visceral hypersensitivity. Bacterial histidine decarboxylase (HDC) in high-histamine producing species such as Klebsiella aerogenes metabolises dietary histidine into histamine which is stimulated by high intake of fermentable carbohydrates. Higher abundance of Lactobacilli reduces histamine production by lowering luminal pH through production of lactic acid. Bacteria-derived histamine can subsequently activate H4R on mast cells contributing to visceral hypersensitivity. FODMAPs, Fermentable Oligo-, Di-, Monosaccharides and Polyols; H1R, histamine 1 receptor; H4R, histamine 4 receptor; HDC, histidine decarboxylase; MC, mast cell; SCFA, short-chain fatty acids.
Bacterial histamine has been identified as a culprit in food poisoning, for example, after consumption of certain fish, which can contain high histamine producing bacteria.121 However, histamine producers can be a permanent part of the human microbiome, as recent studies found increased incidence of histamine producing bacteria in the gut of patients with inflammatory bowel disease,123 as well as those with asthma.124
Several studies identified histamine as one of the key compounds that separates urine metabolomic profiles, which result from the metabolic activity of both the host and the microbiome, between patients with IBS and healthy controls.125 126 Furthermore, levels of urinary histamine seem to correlate with levels of IBS symptoms and abdominal pain.126
In a dietary intervention study, GI symptoms and pain improved in patients with IBS after reducing intake of FODMAPs, which was associated with decrease in urinary histamine and changes in gut microbiota composition.125 A subsequent translational study, which used faecal microbiome from those patients to colonise germ-free mice, found that mice colonised with microbiota from patients with high urinary histamine developed visceral hyperalgesia compared with mice colonised with microbiota from patients with low urinary histamine or healthy controls.127 The increased visceral sensitivity was associated with colonic mast cell hyperplasia, increased density of neural fibres and frequent colocalisation of mast cells with neural cells, findings previously observed in patients with IBS.31 The microbiota from patients with high urinary histamine produced large amount of histamine, and a specific strain of Klebsiella aerogenes was identified as the main histamine producer, with ability to generate 100× more histamine than other isolated bacterial strains. Histamine production was highly pH dependent, suggesting that acidity of the colonic milieu, which is largely determined by bacterial fermentation of dietary fibre, can regulate bacterial histamine production (figure 5). Indeed, placing mice colonised with high histamine producing microbiota on a low fermentable diet, which was associated with changes in luminal lactic acid, decreased histamine production.
Mice colonised with high histamine producing bacteria had increased expression of histamine 4 receptors (H4R), which was detected in multiple cell types, including mast cells. H4R is known to mediate mast cell migration and recruitment,128 as well as regulate visceral sensitivity.129 Administration of H4R antagonists to mice colonised with histamine producing microbiota prevented mast cell hyperplasia and visceral hyperalgesia.127 Thus, while in inflammation induced food hypersensitivity the H1R pathways are key,68 in case of bacterial histamine-to-host signalling the H4R dependent pathways play a major role. Bacterial histamine is therefore a likely trigger in a subset of patients with IBS, and increased urinary histamine or high abundance of bacterial histidine decarboxylase gene may serve as biomarkers to identify those subjects.
Altered microbiome in patients with IBS, who are on high fermentable diet, may also lead to production of other immunomodulatory mediators. Recent studies found that levels of faecal and serum lipopolysaccharide (LPS) are elevated in patients with IBS-D,130 131 and that faecal LPS decreases when the patients are placed on a low FODMAP diet.132 A mouse study then showed that faecal LPS induces impairment of the barrier function and mast cell activation through TLR-4 pathways, thus demonstrating another pathway by which microbiome could trigger symptoms of IBS.132
Also in FD changes in the composition of the gastric and duodenal microbiota have been described, although the findings across studies are quite diverse.75 76 133 134 Increased abundance of Streptococci has been described by several groups, but this finding may be confounded by the effect of PPI treatment.75 76 In a recent study from our group, a lower abundance of duodenal mucosa-associated Porphyromonas correlated with increased symptoms and eosinophils, although causality remains to be demonstrated.75
Psychological comorbidity
Psychological comorbidity, including stress, anxiety and depression, is common in patients with DGBI and affects symptoms and outcome (figure 2).135–138 Two studies have suggested a link between anxiety and depression on the one hand and duodenal eosinophilia139 and mast cell degranulation140 on the other hand, although at this stage it is unclear whether this association was confounded by the presence of FD itself, which is known to be linked to both.69 141
Stress may impact the intestinal barrier through a mast-cell dependent mechanism.142 143 A key mediator of this response is corticotropin-releasing hormone (CRH) which is not only secreted in the hypothalamus as part of the classical hypothalamo–pituitary–adrenal axis activation, but also by eosinophils in the GI tract which can then subsequently activate mast cells.144 145 CRH release by eosinophils is triggered by psychological stress, possibly through activation of NK1/2 purinergic receptors on eosinophils by nerve-derived Substance P.144 Moreover, increased expression of CRH in eosinophils correlated with symptom severity and life stress in patients with IBS-D.146 Mast cells carry CRH receptors and exogenous CRH has been shown to increase intestinal permeability in healthy individuals which was blocked by mast cell stabilisation and vagal nerve stimulation.142 147 148 Additionally, reduced expression of the antiinflammatory, antinociceptive and barrier-stabilising CRH receptor 2 (CRHR2) in duodenal biopsies of patients with FD was recently reported and may also contribute to stress-related mast cell activation in FD.149 150 Polymorphisms in both CRHR1 and 2 have been associated with IBS and anxiety, but how this relates to mucosal immune activation is unexplored.151 152
Treatments targeting the GI immune microenvironment
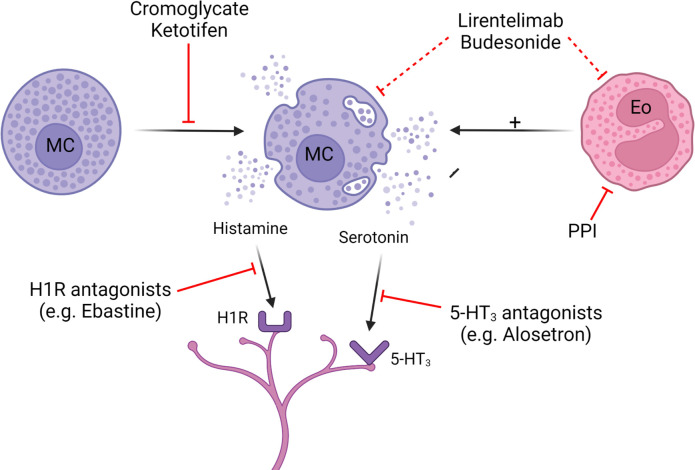
Despite the available evidence for mucosal immune activation in IBS and FD, there is a paucity of therapies targeting this mechanism (figure 6).
Figure 6.
Treatments targeting immune activation. Solid lines: a demonstrated benefit. Dashed lines: potential benefit, but not formally tested. 5-HT3, Serotonin 3 receptor; Eo, eosinophil; H1R, histamine 1 receptor; MC, mast cell; PPI, proton-pump inhibitor.
Previous attempts with broad antiinflammatory agents in IBS have been disappointing. Prednisolone 30 mg for 3 weeks did not improve symptoms in a placebo-controlled study with 29 patients with postinfectious IBS and there was no significant effect on rectal lymphocytes or mast cells.153 Similarly, a liquid formulation of budesonide, a corticosteroid with a high first pass metabolism was not effective in lowering duodenal eosinophil counts or improving symptoms in a small pilot study in FD.154
Based on the observation that 5-aminosalicylic acid was able to reduce release of histamine and prostaglandin D2 from mast cell cultures155 and reduced mast cell counts and histamine release in the rectum of patients with IBS,156 mesalazine was evaluated in two large multicentre trials in patients with IBS, which were both negative.157 158 A post-hoc analysis of the British study also failed to demonstrate increased mast cell counts or a gene expression signature indicative of inflammation in patients with IBS in comparison to healthy controls.159
Because mediator release from specific immune cells is more likely to be involved in symptom generation than a classic inflammatory reaction as seen in, for example, inflammatory bowel disease, more specific mast-cell targeted therapies were trialled in IBS. The mast cell stabiliser disodium cromoglycate reduced abdominal pain and improved stool consistency in a small pilot study in patients with IBS-D, which was also associated with reduced signs of mast cell activation.160 Ketotifen, another mast cell stabiliser, decreased visceral hypersensitivity to rectal distention and improved symptoms, but had no impact on mast cell counts or measured mediator release from rectal biopsies.66 These results were confirmed in a more recent study from China with similar improvement of symptoms and reduced mast cell counts in the terminal ileum but not in the colon.161 More specific eosinophil depleting and mast cell inhibiting therapies have not been evaluated in IBS and FD yet, but a study of lirentelimab, a monoclonal antibody against Siglec 8, in eosinophilic gastritis and duodenitis, showed promising results.162 Notably, case reports describing clinical improvement of IBS symptoms in patients with severe asthma treated with the anti-IgE antibody omalizumab may indirectly support the role of IgE-mediated mast cell activation in IBS.163
An alternative approach to prevent mast cell or eosinophil activation is to block the nociceptive effect of their released mediators. Based on the observations that blocking the histamine receptor H1 prevented the neuronal excitation by supernatant from IBS biopsies, the histamine receptor H1 antagonist ebastine was evaluated in a pilot study showing improved symptom relief and reduced abdominal pain.47 Blockade of serotonin, another mast cell mediator known to activate and sensitise visceral afferents, is another interesting approach to treat abdominal pain. 5-HT3 antagonists such as alosetron and ramosetron have indeed been repeatedly shown to be effective as treatment of diarrhoea-predominant IBS, but possibly also involves an effect on colonic transit.164 Protease inhibitors have proven efficacy in preclinical models of visceral hypersensitivity, but clinical studies are lacking.165
In eosinophilic esophagitis, PPI have been shown to exert direct anti-inflammatory effects, independent of the acid-suppressive effects, based on a reduction of eotaxin-3 expression through inhibition of binding of STAT6 to its promotor, resulting in reduced eosinophil numbers.166 Starting from this observation, we investigated the effect of a 4-week treatment with 40 mg pantoprazole in patients with FD.74 Upper abdominal symptoms and duodenal eosinophils and barrier function improved. Of interest, the effect of pantoprazole on symptoms was mediated by the reduced eosinophil counts and not by changes in luminal pH, suggesting anti-inflammatory effects similar to its mechanism in eosinophilic esophagitis. Nevertheless, the effect of PPI on duodenal eosinophil activation—besides reduction in cell counts—has not been reported to date. However, in the longer term, the beneficial effect of PPI may be overshadowed by induction of dysbiosis through duodenal overgrowth of oropharyngeal flora (cf. supra).75
Conclusion
Recent insights in the mechanisms underlying aberrant pain signalling in patients with FD and IBS indicate a role for immune activation in the GI tract, but clearly we have just started to unravel the complex pathophysiology of functional bowel disorders. Accumulating evidence suggests immune activation of these conditions, but the underlying causes remain a hot topic for further research. Moreover, the therapeutic tools to address this immune activation in DGBI remain scarce. Intense collaboration between immunologists, neurogastroenterologists, neuroscientists, microbiologists, psychologists, bioinformaticians and many more is undoubtedly needed to be successful. Only with this multidisciplinary scientific approach, new targets can be discovered to improve clinical management of these challenging conditions.
Acknowledgments
All figures were created with BioRender.com.
Correction notice: This article has been corrected since it published Online First. The second author affiliation has been amended.
Contributors: All authors contributed to the writing of the manuscript. The figures were designed by TV.
Funding: TV is supported by a senior clinical research fellowship of the Flanders Research Foundation (FWO Vlaanderen; 1830517N). PB is supported by Canadian Institutes of Health Research (CIHR) Foundation grant no. 143253. GB is supported by a KU Leuven internal funding C1 grant (C14/18/086).
Competing interests: TV has received research grants from Danone and MyHealth; has served on the speaker bureau of Abbott, Biocodex, Dr. Falk Pharma, Menarini, MyHealth, Schwabe and Truvion, has provided scientific advice to Biocodex, BMS and Dr. Falk Pharma. PB and GB have no competing interests.
Provenance and peer review: Commissioned; externally peer reviewed.
Please add "Intestinal eosinophils" to Keywords
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016;150:1393–407. 10.1053/j.gastro.2016.02.031 [DOI] [PubMed] [Google Scholar]
- 2. Stanghellini V, Talley NJ, Chan F. Rome IV - Gastroduodenal Disorders. Gastroenterology 2016;150:1380–92. [DOI] [PubMed] [Google Scholar]
- 3. Drossman DA, Hasler WL. Rome IV—Functional Gi disorders: disorders of gut-brain interaction. Gastroenterology 2016;150:1257–61. 10.1053/j.gastro.2016.03.035 [DOI] [PubMed] [Google Scholar]
- 4. Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation global study. Gastroenterology 2021;160:e3:99–114. 10.1053/j.gastro.2020.04.014 [DOI] [PubMed] [Google Scholar]
- 5. Oka P, Parr H, Barberio B, et al. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020;5:908–17. 10.1016/S2468-1253(20)30217-X [DOI] [PubMed] [Google Scholar]
- 6. Rees WD, Miller LJ, Malagelada JR. Dyspepsia, antral motor dysfunction, and gastric stasis of solids. Gastroenterology 1980;78:360–5. 10.1016/0016-5085(80)90589-2 [DOI] [PubMed] [Google Scholar]
- 7. Sarnelli G, Caenepeel P, Geypens B, et al. Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol 2003;98:783–8. 10.1111/j.1572-0241.2003.07389.x [DOI] [PubMed] [Google Scholar]
- 8. Rogers J, Henry MM, Misiewicz JJ. Increased segmental activity and intraluminal pressures in the sigmoid colon of patients with the irritable bowel syndrome. Gut 1989;30:634–41. 10.1136/gut.30.5.634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut 2014;63:1972–8. 10.1136/gutjnl-2013-306084 [DOI] [PubMed] [Google Scholar]
- 10. Vijayvargiya P, Jameie-Oskooei S, Camilleri M, et al. Association between delayed gastric emptying and upper gastrointestinal symptoms: a systematic review and meta-analysis. Gut 2019;68:804–13. 10.1136/gutjnl-2018-316405 [DOI] [PubMed] [Google Scholar]
- 11. Pasricha PJ, Grover M, Yates KP, et al. Functional dyspepsia and gastroparesis in tertiary care are interchangeable syndromes with common clinical and pathologic features. Gastroenterology 2021;160:2006–17. 10.1053/j.gastro.2021.01.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pittayanon R, Yuan Y, Bollegala NP, et al. Prokinetics for functional dyspepsia: a systematic review and meta-analysis of randomized control trials. Am J Gastroenterol 2019;114:233–43. 10.1038/s41395-018-0258-6 [DOI] [PubMed] [Google Scholar]
- 13. Tack J, Van den Houte K, Carbone F. The unfulfilled promise of Prokinetics for functional Dyspepsia/Postprandial distress syndrome. Am J Gastroenterol 2019;114:204–6. 10.14309/ajg.0000000000000072 [DOI] [PubMed] [Google Scholar]
- 14. Wauters L, Dickman R, Drug V, et al. United European gastroenterology (UEG) and European Society for neurogastroenterology and motility (ESNM) consensus on functional dyspepsia. United European Gastroenterol J 2021;9:307–31. 10.1002/ueg2.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moayyedi P, Lacy BE, Andrews CN, et al. ACG and CAG clinical guideline: management of dyspepsia. Am J Gastroenterol 2017;112:988–1013. 10.1038/ajg.2017.154 [DOI] [PubMed] [Google Scholar]
- 16. Sarkar S, Aziz Q, Woolf CJ, et al. Contribution of central sensitisation to the development of non-cardiac chest pain. Lancet 2000;356:1154–9. 10.1016/S0140-6736(00)02758-6 [DOI] [PubMed] [Google Scholar]
- 17. Weijenborg PW, Smout AJPM, Verseijden C, et al. Hypersensitivity to acid is associated with impaired esophageal mucosal integrity in patients with gastroesophageal reflux disease with and without esophagitis. Am J Physiol Gastrointest Liver Physiol 2014;307:G323–9. 10.1152/ajpgi.00345.2013 [DOI] [PubMed] [Google Scholar]
- 18. Rohof WO, Bennink RJ, de Jonge H, et al. Increased proximal reflux in a hypersensitive esophagus might explain symptoms resistant to proton pump inhibitors in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2014;12:1647–55. 10.1016/j.cgh.2013.10.026 [DOI] [PubMed] [Google Scholar]
- 19. Barbera R, Feinle C, Read NW. Abnormal sensitivity to duodenal lipid infusion in patients with functional dyspepsia. Eur J Gastroenterol Hepatol 1995;7:1051–7. 10.1097/00042737-199511000-00007 [DOI] [PubMed] [Google Scholar]
- 20. Boeckxstaens GE, Hirsch DP, van den Elzen BD, et al. Impaired drinking capacity in patients with functional dyspepsia: relationship with proximal stomach function. Gastroenterology 2001;121:1054–63. 10.1053/gast.2001.28656 [DOI] [PubMed] [Google Scholar]
- 21. Tack J, Caenepeel P, Fischler B, et al. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology 2001;121:526–35. 10.1053/gast.2001.27180 [DOI] [PubMed] [Google Scholar]
- 22. Hammer J. Identification of individuals with functional dyspepsia with a simple, minimally invasive test: a single center cohort study of the oral capsaicin test. Am J Gastroenterol 2018;113:584–92. 10.1038/ajg.2018.16 [DOI] [PubMed] [Google Scholar]
- 23. Ritchie J. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut 1973;14:125–32. 10.1136/gut.14.2.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mertz H, Naliboff B, Munakata J, et al. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology 1995;109:40–52. 10.1016/0016-5085(95)90267-8 [DOI] [PubMed] [Google Scholar]
- 25. Kuiken SD, Lindeboom R, Tytgat GN, et al. Relationship between symptoms and hypersensitivity to rectal distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther 2005;22:157–64. 10.1111/j.1365-2036.2005.02524.x [DOI] [PubMed] [Google Scholar]
- 26. Brookes SJH, Spencer NJ, Costa M, et al. Extrinsic primary afferent signalling in the gut. Nat Rev Gastroenterol Hepatol 2013;10:286–96. 10.1038/nrgastro.2013.29 [DOI] [PubMed] [Google Scholar]
- 27. Brierley SM, Linden DR. Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat Rev Gastroenterol Hepatol 2014;11:611–27. 10.1038/nrgastro.2014.103 [DOI] [PubMed] [Google Scholar]
- 28. Aguilera-Lizarraga J, Hussein H, Boeckxstaens GE. Immune activation in irritable bowel syndrome: what is the evidence? Nat Rev Immunol 2022;22:674–86. 10.1038/s41577-022-00700-9 [DOI] [PubMed] [Google Scholar]
- 29. Kano M, Dupont P, Aziz Q, et al. Understanding neurogastroenterology from neuroimaging perspective: a comprehensive review of functional and structural brain imaging in functional gastrointestinal disorders. J Neurogastroenterol Motil 2018;24:512–27. 10.5056/jnm18072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hughes PA, Harrington AM, Castro J, et al. Sensory neuro-immune interactions differ between irritable bowel syndrome subtypes. Gut 2013;62:1456–65. 10.1136/gutjnl-2011-301856 [DOI] [PubMed] [Google Scholar]
- 31. Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 2004;126:693–702. 10.1053/j.gastro.2003.11.055 [DOI] [PubMed] [Google Scholar]
- 32. Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 2007;132:26–37. 10.1053/j.gastro.2006.11.039 [DOI] [PubMed] [Google Scholar]
- 33. Buhner S, Li Q, Vignali S, et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology 2009;137:1425–34. 10.1053/j.gastro.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 34. Rolland-Fourcade C, Denadai-Souza A, Cirillo C, et al. Epithelial expression and function of trypsin-3 in irritable bowel syndrome. Gut 2017;66:1767–78. 10.1136/gutjnl-2016-312094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cenac N, Bautzova T, Le Faouder P, et al. Quantification and potential functions of endogenous agonists of transient receptor potential channels in patients with irritable bowel syndrome. Gastroenterology 2015;149:433–44. 10.1053/j.gastro.2015.04.011 [DOI] [PubMed] [Google Scholar]
- 36. Li X, Chen H, Lu H, et al. The study on the role of inflammatory cells and mediators in post-infectious functional dyspepsia. Scand J Gastroenterol 2010;45:573–81. 10.3109/00365521003632576 [DOI] [PubMed] [Google Scholar]
- 37. El-Salhy M, Hausken T, Gilja OH, et al. The possible role of gastrointestinal endocrine cells in the pathophysiology of irritable bowel syndrome. Expert Rev Gastroenterol Hepatol 2017;11:139–48. 10.1080/17474124.2017.1269601 [DOI] [PubMed] [Google Scholar]
- 38. Park JH, Rhee P-L, Kim G, et al. Enteroendocrine cell counts correlate with visceral hypersensitivity in patients with diarrhoea-predominant irritable bowel syndrome. Neurogastroenterol Motil 2006;18:539–46. 10.1111/j.1365-2982.2006.00771.x [DOI] [PubMed] [Google Scholar]
- 39. Kaelberer MM, Rupprecht LE, Liu WW, et al. Neuropod cells: the emerging biology of gut-brain sensory transduction. Annu Rev Neurosci 2020;43:337–53. 10.1146/annurev-neuro-091619-022657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liebregts T, Adam B, Bredack C, et al. Small bowel homing T cells are associated with symptoms and delayed gastric emptying in functional dyspepsia. Am J Gastroenterol 2011;106:1089–98. 10.1038/ajg.2010.512 [DOI] [PubMed] [Google Scholar]
- 41. Cheung CKY, Lan LL, Kyaw M, et al. Up-regulation of transient receptor potential vanilloid (TRPV) and down-regulation of brain-derived neurotrophic factor (BDNF) expression in patients with functional dyspepsia (FD). Neurogastroenterol Motil 2018;30. 10.1111/nmo.13176. [Epub ahead of print: 07 08 2017]. [DOI] [PubMed] [Google Scholar]
- 42. Luo Y, Feng C, Wu J, et al. P2Y1, P2Y2, and TRPV1 receptors are increased in diarrhea-predominant irritable bowel syndrome and P2Y2 correlates with abdominal pain. Dig Dis Sci 2016;61:2878–86. 10.1007/s10620-016-4211-5 [DOI] [PubMed] [Google Scholar]
- 43. Zhou Q, Yang L, Larson S, et al. Decreased miR-199 augments visceral pain in patients with IBS through translational upregulation of TRPV1. Gut 2016;65:797–805. 10.1136/gutjnl-2013-306464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Akbar A, Yiangou Y, Facer P, et al. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 2008;57:923–9. 10.1136/gut.2007.138982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Grover M, Berumen A, Peters S, et al. Intestinal chemosensitivity in irritable bowel syndrome associates with small intestinal TRPV channel expression. Aliment Pharmacol Ther 2021;54:1179–92. 10.1111/apt.16591 [DOI] [PubMed] [Google Scholar]
- 46. Cenac N, Altier C, Motta J-P, et al. Potentiation of TRPV4 signalling by histamine and serotonin: an important mechanism for visceral hypersensitivity. Gut 2010;59:481–8. 10.1136/gut.2009.192567 [DOI] [PubMed] [Google Scholar]
- 47. Wouters MM, Balemans D, Van Wanrooy S, et al. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology 2016;150:875–87. 10.1053/j.gastro.2015.12.034 [DOI] [PubMed] [Google Scholar]
- 48. Balemans D, Aguilera-Lizarraga J, Florens MV, et al. Histamine-mediated potentiation of transient receptor potential (TRP) ankyrin 1 and TRP vanilloid 4 signaling in submucosal neurons in patients with irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2019;316:G338–49. 10.1152/ajpgi.00116.2018 [DOI] [PubMed] [Google Scholar]
- 49. Cirillo C, Bessissow T, Desmet A-S, et al. Evidence for neuronal and structural changes in submucous ganglia of patients with functional dyspepsia. Am J Gastroenterol 2015;110:1205–15. 10.1038/ajg.2015.158 [DOI] [PubMed] [Google Scholar]
- 50. Neunlist M, Rolli-Derkinderen M, Latorre R, et al. Enteric glial cells: recent developments and future directions. Gastroenterology 2014;147:1230–7. 10.1053/j.gastro.2014.09.040 [DOI] [PubMed] [Google Scholar]
- 51. Meira de-Faria F, Casado-Bedmar M, Mårten Lindqvist C, et al. Altered interaction between enteric glial cells and mast cells in the colon of women with irritable bowel syndrome. Neurogastroenterol Motil 2021;33:e14130. 10.1111/nmo.14130 [DOI] [PubMed] [Google Scholar]
- 52. Lilli NL, Quénéhervé L, Haddara S, et al. Glioplasticity in irritable bowel syndrome. Neurogastroenterol Motil 2018;30:e13232. 10.1111/nmo.13232 [DOI] [PubMed] [Google Scholar]
- 53. Cenac N, Andrews CN, Holzhausen M, et al. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest 2007;117:636–47. 10.1172/JCI29255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Balemans D, Mondelaers SU, Cibert-Goton V, et al. Evidence for long-term sensitization of the bowel in patients with post-infectious-IBS. Sci Rep 2017;7:13606. 10.1038/s41598-017-12618-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gwee KA, Leong YL, Graham C, et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut 1999;44:400–6. 10.1136/gut.44.3.400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 2000;47:804–11. 10.1136/gut.47.6.804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bashashati M, Moossavi S, Cremon C, et al. Colonic immune cells in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil 2018;30:e13192. 10.1111/nmo.13192 [DOI] [PubMed] [Google Scholar]
- 58. Burns G, Carroll G, Mathe A, et al. Evidence for local and systemic immune activation in functional dyspepsia and the irritable bowel syndrome: a systematic review. Am J Gastroenterol 2019;114:429–36. 10.1038/s41395-018-0377-0 [DOI] [PubMed] [Google Scholar]
- 59. Ceulemans M, Jacobs I, Wauters L, et al. Immune activation in functional dyspepsia: bystander becoming the suspect. Front Neurosci 2022;16:831761. 10.3389/fnins.2022.831761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kindt S, Tertychnyy A, de Hertogh G, et al. Intestinal immune activation in presumed post-infectious functional dyspepsia. Neurogastroenterol Motil 2009;21:832–56. 10.1111/j.1365-2982.2009.01299.x [DOI] [PubMed] [Google Scholar]
- 61. Gargala G, Lecleire S, Francois A. Duodenal intraepithelial T lymphocytes in patients with functional dyspepsia. WJG 2007;13:2333–8. 10.3748/wjg.v13.i16.2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ohman L, Isaksson S, Lundgren A, et al. A controlled study of colonic immune activity and beta7+ blood T lymphocytes in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2005;3:980–6. 10.1016/S1542-3565(05)00410-6 [DOI] [PubMed] [Google Scholar]
- 63. Ohman L, Isaksson S, Lindmark A-C, et al. T-Cell activation in patients with irritable bowel syndrome. Am J Gastroenterol 2009;104:1205–12. 10.1038/ajg.2009.116 [DOI] [PubMed] [Google Scholar]
- 64. Nasser Y, Petes C, Simmers C, et al. Activation of peripheral blood CD4+ T-cells in IBS is not associated with gastrointestinal or psychological symptoms. Sci Rep 2019;9:3710. 10.1038/s41598-019-40124-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Krammer L, Sowa AS, Lorentz A. Mast cells in irritable bowel syndrome: a systematic review. J Gastrointestin Liver Dis 2019;28:463–72. 10.15403/jgld-229 [DOI] [PubMed] [Google Scholar]
- 66. Klooker TK, Braak B, Koopman KE, et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut 2010;59:1213–21. 10.1136/gut.2010.213108 [DOI] [PubMed] [Google Scholar]
- 67. Braak B, Klooker TK, Wouters MM, et al. Mucosal immune cell numbers and visceral sensitivity in patients with irritable bowel syndrome: is there any relationship? Am J Gastroenterol 2012;107:715–26. 10.1038/ajg.2012.54 [DOI] [PubMed] [Google Scholar]
- 68. Aguilera-Lizarraga J, Florens MV, Viola MF, et al. Local immune response to food antigens drives meal-induced abdominal pain. Nature 2021;590:151–6. 10.1038/s41586-020-03118-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shah A, Fairlie T, Brown G, et al. Duodenal eosinophils and mast cells in functional dyspepsia: a systematic review and meta-analysis of case-control studies. Clin Gastroenterol Hepatol 2022;20:2229–42. 10.1016/j.cgh.2022.01.014 [DOI] [PubMed] [Google Scholar]
- 70. Vanheel H, Vicario M, Boesmans W, et al. Activation of eosinophils and mast cells in functional dyspepsia: an ultrastructural evaluation. Sci Rep 2018;8:5383. 10.1038/s41598-018-23620-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Giancola F, Volta U, Repossi R, et al. Mast cell-nerve interactions correlate with bloating and abdominal pain severity in patients with non-celiac gluten / wheat sensitivity. Neurogastroenterol Motil 2020;32:e13814. 10.1111/nmo.13814 [DOI] [PubMed] [Google Scholar]
- 72. Talley NJ, Walker MM, Aro P, et al. Non-ulcer dyspepsia and duodenal eosinophilia: an adult endoscopic population-based case-control study. Clin Gastroenterol Hepatol 2007;5:1175–83. 10.1016/j.cgh.2007.05.015 [DOI] [PubMed] [Google Scholar]
- 73. Järbrink-Sehgal ME, Sparkman J, Damron A, et al. Functional dyspepsia and duodenal eosinophil count and degranulation: a multiethnic us veteran cohort study. Dig Dis Sci 2021;66:3482–9. 10.1007/s10620-020-06689-2 [DOI] [PubMed] [Google Scholar]
- 74. Wauters L, Ceulemans M, Frings D, et al. Proton pump inhibitors reduce duodenal eosinophilia, mast cells, and permeability in patients with functional dyspepsia. Gastroenterology 2021;160:1521–31. 10.1053/j.gastro.2020.12.016 [DOI] [PubMed] [Google Scholar]
- 75. Wauters L, Tito RY, Ceulemans M, et al. Duodenal dysbiosis and relation to the efficacy of proton pump inhibitors in functional dyspepsia. Int J Mol Sci 2021;22. 10.3390/ijms222413609. [Epub ahead of print: 19 Dec 2021]. 10.3390/ijms222413609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shanahan ER, Kang S, Staudacher H, et al. Alterations to the duodenal microbiota are linked to gastric emptying and symptoms in functional dyspepsia. Gut 2022;396:gutjnl-2021-326158. 10.1136/gutjnl-2021-326158 [DOI] [PubMed] [Google Scholar]
- 77. Burns GL, Talley NJ, Keely S. Immune responses in the irritable bowel syndromes: time to consider the small intestine. BMC Med 2022;20:115. 10.1186/s12916-022-02301-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Casado‐Bedmar M, de‐Faria FM, Biskou O, et al. Elevated F‐EDN correlates with mucosal eosinophil degranulation in patients with IBS—A possible association with microbiota? J Leukoc Biol 2022;111:655–65. 10.1002/JLB.4A0521-228R [DOI] [PubMed] [Google Scholar]
- 79. Bashashati M, Rezaei N, Shafieyoun A, et al. Cytokine imbalance in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil 2014;26:1036–48. 10.1111/nmo.12358 [DOI] [PubMed] [Google Scholar]
- 80. Bennet SMP, Palsson O, Whitehead WE, et al. Systemic cytokines are elevated in a subset of patients with irritable bowel syndrome but largely unrelated to symptom characteristics. Neurogastroenterol Motil 2018;30:e13378. 10.1111/nmo.13378 [DOI] [PubMed] [Google Scholar]
- 81. Bennet SMP, Polster A, Törnblom H, et al. Global cytokine profiles and association with clinical characteristics in patients with irritable bowel syndrome. Am J Gastroenterol 2016;111:1165–76. 10.1038/ajg.2016.223 [DOI] [PubMed] [Google Scholar]
- 82. Aguilera‐Lizarraga J, Florens MV, Van Brussel T, et al. Expression of immune‐related genes in rectum and colon descendens of Irritable bowel syndrome patients is unrelated to clinical symptoms. Neurogastroenterology Motil 2019;31:e13579. 10.1111/nmo.13579 [DOI] [PubMed] [Google Scholar]
- 83. Ogasawara H, Noguchi M. Therapeutic potential of MRGPRX2 inhibitors on mast cells. Cells 2021;10:2906. 10.3390/cells10112906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kindt S, Van Oudenhove L, Broekaert D, et al. Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol Motil 2009;21:389–98. 10.1111/j.1365-2982.2008.01220.x [DOI] [PubMed] [Google Scholar]
- 85. Liu EG, Yin X, Swaminathan A, et al. Antigen-presenting cells in food tolerance and allergy. Front Immunol 2020;11:616020. 10.3389/fimmu.2020.616020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Vanuytsel T, Tack J, Farre R. The role of intestinal permeability in gastrointestinal disorders and current methods of evaluation. Front Nutr 2021;8:717925. 10.3389/fnut.2021.717925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wauters L, Ceulemans M, Schol J, et al. The role of leaky gut in functional dyspepsia. Front Neurosci 2022;16:851012. 10.3389/fnins.2022.851012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hanning N, Edwinson AL, Ceuleers H, et al. Intestinal barrier dysfunction in irritable bowel syndrome: a systematic review. Therap Adv Gastroenterol 2021;14:1756284821993586. 10.1177/1756284821993586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Witt ST, Bednarska O, Keita AV, et al. Interactions between gut permeability and brain structure and function in health and irritable bowel syndrome. Neuroimage Clin 2019;21:101602. 10.1016/j.nicl.2018.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Annaházi A, Ferrier L, Bézirard V, et al. Luminal cysteine-proteases degrade colonic tight junction structure and are responsible for abdominal pain in constipation-predominant IBS. Am J Gastroenterol 2013;108:1322–31. 10.1038/ajg.2013.152 [DOI] [PubMed] [Google Scholar]
- 91.Gecse K, Roka R, Ferrier L, et al. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut 2008;57:591–9. 10.1136/gut.2007.140210 [DOI] [PubMed] [Google Scholar]
- 92.Piche T, Barbara G, Aubert P, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut 2009;58:196–201. 10.1136/gut.2007.140806 [DOI] [PubMed] [Google Scholar]
- 93.Edwinson AL, Yang L, Peters S, et al. Gut microbial β-glucuronidases regulate host luminal proteases and are depleted in irritable bowel syndrome. Nat Microbiol 2022;7:680–94. 10.1038/s41564-022-01103-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Edogawa S, Edwinson AL, Peters SA, et al. Serine proteases as luminal mediators of intestinal barrier dysfunction and symptom severity in IBS. Gut 2020;69:62–73. 10.1136/gutjnl-2018-317416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tooth D, Garsed K, Singh G, et al. Characterisation of faecal protease activity in irritable bowel syndrome with diarrhoea: origin and effect of gut transit. Gut 2014;63:753–60. 10.1136/gutjnl-2012-304042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jacob C, Yang P-C, Darmoul D, et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J Biol Chem 2005;280:31936–48. 10.1074/jbc.M506338200 [DOI] [PubMed] [Google Scholar]
- 97.Wilcz-Villega EM, McClean S, O'Sullivan MA. Mast cell tryptase reduces junctional adhesion molecule-A (JAM-A) expression in intestinal epithelial cells: implications for the mechanisms of barrier dysfunction in irritable bowel syndrome. Am J Gastroenterol 2013;108:1140–51. 10.1038/ajg.2013.92 [DOI] [PubMed] [Google Scholar]
- 98.Furuta GT, Nieuwenhuis EES, Karhausen J, et al. Eosinophils alter colonic epithelial barrier function: role for major basic protein. Am J Physiol Gastrointest Liver Physiol 2005;289:G890–7. 10.1152/ajpgi.00015.2005 [DOI] [PubMed] [Google Scholar]
- 99.Bednarska O, Walter SA, Casado-Bedmar M, et al. Vasoactive intestinal polypeptide and mast cells regulate increased passage of colonic bacteria in patients with irritable bowel syndrome. Gastroenterology 2017;153:948–60. 10.1053/j.gastro.2017.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vanheel H, Vicario M, Vanuytsel T, et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut 2014;63:262–71. 10.1136/gutjnl-2012-303857 [DOI] [PubMed] [Google Scholar]
- 101.Zhou Q, Verne ML, Fields JZ, et al. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut 2019;68:996–1002. 10.1136/gutjnl-2017-315136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bertrand J, Ghouzali I, Guérin C, et al. Glutamine restores tight junction protein claudin-1 expression in colonic mucosa of patients with diarrhea-predominant irritable bowel syndrome. JPEN J Parenter Enteral Nutr 2016;40:1170–6. 10.1177/0148607115587330 [DOI] [PubMed] [Google Scholar]
- 103.Simrén M, Månsson A, Langkilde AM, et al. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion 2001;63:108–15. 10.1159/000051878 [DOI] [PubMed] [Google Scholar]
- 104.Böhn L, Störsrud S, Törnblom H, et al. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol 2013;108:634–41. 10.1038/ajg.2013.105 [DOI] [PubMed] [Google Scholar]
- 105.Spiller R. Impact of diet on symptoms of the irritable bowel syndrome. Nutrients 2021;13:575. 10.3390/nu13020575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bischoff SC, Mayer J, Wedemeyer J, et al. Colonoscopic allergen provocation (COLAP): a new diagnostic approach for gastrointestinal food allergy. Gut 1997;40:745–53. 10.1136/gut.40.6.745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fritscher-Ravens A, Schuppan D, Ellrichmann M, et al. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2014;147:1012–20. 10.1053/j.gastro.2014.07.046 [DOI] [PubMed] [Google Scholar]
- 108.Fritscher-Ravens A, Pflaum T, Mösinger M, et al. Many patients with irritable bowel syndrome have atypical food allergies not associated with immunoglobulin E. Gastroenterology 2019;157:109–18. 10.1053/j.gastro.2019.03.046 [DOI] [PubMed] [Google Scholar]
- 109.Caminero A, Meisel M, Jabri B, et al. Mechanisms by which gut microorganisms influence food sensitivities. Nat Rev Gastroenterol Hepatol 2019;16:7–18. 10.1038/s41575-018-0064-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bouziat R, Hinterleitner R, Brown JJ, et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 2017;356:44–50. 10.1126/science.aah5298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fonseca DMda, Hand TW, Han S-J, et al. Microbiota-Dependent sequelae of acute infection compromise tissue-specific immunity. Cell 2015;163:354–66. 10.1016/j.cell.2015.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol 2016;16:751–65. 10.1038/nri.2016.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ismail FW, Abid S, Awan S, et al. Frequency of food hypersensitivity in patients with functional gastrointestinal disorders. Acta Gastroenterol Belg 2018;81:253–6. [PubMed] [Google Scholar]
- 114.Nybacka S, Öhman L, Störsrud S, et al. Neither self-reported atopy nor IgE-mediated allergy are linked to gastrointestinal symptoms in patients with irritable bowel syndrome. Neurogastroenterol Motil 2018;30:e13379. 10.1111/nmo.13379 [DOI] [PubMed] [Google Scholar]
- 115.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology 2009;136:1979–88. 10.1053/j.gastro.2009.02.074 [DOI] [PubMed] [Google Scholar]
- 116.Pittayanon R, Lau JT, Yuan Y, et al. Gut microbiota in patients with irritable bowel Syndrome—A systematic review. Gastroenterology 2019;157:97–108. 10.1053/j.gastro.2019.03.049 [DOI] [PubMed] [Google Scholar]
- 117.Jeffery IB, Das A, O'Herlihy E, et al. Differences in fecal Microbiomes and metabolomes of people with vs without irritable bowel syndrome and bile acid malabsorption. Gastroenterology 2020;158:1016–28. 10.1053/j.gastro.2019.11.301 [DOI] [PubMed] [Google Scholar]
- 118.Mars RAT, Yang Y, Ward T, et al. Longitudinal multi-omics reveals subset-specific mechanisms underlying irritable bowel syndrome. Cell 2020;182:e17:1460–73. 10.1016/j.cell.2020.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mujagic Z, Kasapi M, Jonkers DMAE, et al. Integrated fecal microbiome–metabolome signatures reflect stress and serotonin metabolism in irritable bowel syndrome. Gut Microbes 2022;14:2063016. 10.1080/19490976.2022.2063016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lyte M. Microbial endocrinology: Host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes 2014;5:381–9. 10.4161/gmic.28682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim SH, Field KG, Morrissey MT, et al. Source and identification of histamine-producing bacteria from fresh and temperature-abused albacore. J Food Prot 2001;64:1035–44. 10.4315/0362-028x-64.7.1035 [DOI] [PubMed] [Google Scholar]
- 122.Landete JM, De las Rivas B, Marcobal A, et al. Updated molecular knowledge about histamine biosynthesis by bacteria. Crit Rev Food Sci Nutr 2008;48:697–714. 10.1080/10408390701639041 [DOI] [PubMed] [Google Scholar]
- 123.Mou Z, Yang Y, Hall AB, et al. The taxonomic distribution of histamine-secreting bacteria in the human gut microbiome. BMC Genomics 2021;22:695. 10.1186/s12864-021-08004-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Barcik W, Pugin B, Westermann P, et al. Histamine-secreting microbes are increased in the gut of adult asthma patients. J Allergy Clin Immunol 2016;138:1491–4. 10.1016/j.jaci.2016.05.049 [DOI] [PubMed] [Google Scholar]
- 125.McIntosh K, Reed DE, Schneider T, et al. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut 2017;66:1241–51. 10.1136/gutjnl-2015-311339 [DOI] [PubMed] [Google Scholar]
- 126.Keshteli AH, Madsen KL, Mandal R, et al. Comparison of the metabolomic profiles of irritable bowel syndrome patients with ulcerative colitis patients and healthy controls: new insights into pathophysiology and potential biomarkers. Aliment Pharmacol Ther 2019;49:723–32. 10.1111/apt.15141 [DOI] [PubMed] [Google Scholar]
- 127.De Palma G, Shimbori C, Reed DE, et al. Histamine production by the gut microbiota induces visceral hyperalgesia through histamine 4 receptor signaling in mice. Sci Transl Med 2022;14:eabj1895. 10.1126/scitranslmed.abj1895 [DOI] [PubMed] [Google Scholar]
- 128.Godot V, Arock M, Garcia G, et al. H4 histamine receptor mediates optimal migration of mast cell precursors to CXCL12. J Allergy Clin Immunol 2007;120:827–34. 10.1016/j.jaci.2007.05.046 [DOI] [PubMed] [Google Scholar]
- 129.Deiteren A, De Man JG, Ruyssers NE, et al. Histamine H4 and H1 receptors contribute to postinflammatory visceral hypersensitivity. Gut 2014;63:1873–82. 10.1136/gutjnl-2013-305870 [DOI] [PubMed] [Google Scholar]
- 130.Dlugosz A, Nowak P, D'Amato M, et al. Increased serum levels of lipopolysaccharide and antiflagellin antibodies in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil 2015;27:1747–54. 10.1111/nmo.12670 [DOI] [PubMed] [Google Scholar]
- 131.Zhou S-Y, Gillilland M, Wu X, et al. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. J Clin Invest 2018;128:267–80. 10.1172/JCI92390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Singh P, Grabauskas G, Zhou S-Y, et al. High FODMAP diet causes barrier loss via lipopolysaccharide-mediated mast cell activation. In: JCI insight. 6, 2021. 10.1172/jci.insight.146529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhong L, Shanahan ER, Raj A, et al. Dyspepsia and the microbiome: time to focus on the small intestine. Gut 2017;66:1168–9. 10.1136/gutjnl-2016-312574 [DOI] [PubMed] [Google Scholar]
- 134.Zhou L, Zeng Y, Zhang H, et al. The role of gastrointestinal microbiota in functional dyspepsia: a review. Front Physiol 2022;13:910568. 10.3389/fphys.2022.910568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Faresjö A, Grodzinsky E, Johansson S, et al. Psychosocial factors at work and in every day life are associated with irritable bowel syndrome. Eur J Epidemiol 2007;22:473–80. 10.1007/s10654-007-9133-2 [DOI] [PubMed] [Google Scholar]
- 136.Aro P, Talley NJ, Ronkainen J, et al. Anxiety is associated with uninvestigated and functional dyspepsia (Rome III criteria) in a Swedish population-based study. Gastroenterology 2009;137:94–100. 10.1053/j.gastro.2009.03.039 [DOI] [PubMed] [Google Scholar]
- 137.Klaassen T, Vork L, Smeets FGM, et al. The interplay between stress and fullness in patients with functional dyspepsia and healthy controls: an exploratory experience sampling method study. Psychosom Med 2022;84:306–12. 10.1097/PSY.0000000000001012 [DOI] [PubMed] [Google Scholar]
- 138.Schaper SJ, Stengel A. Emotional stress responsivity of patients with IBS - a systematic review. J Psychosom Res 2022;153:110694. 10.1016/j.jpsychores.2021.110694 [DOI] [PubMed] [Google Scholar]
- 139.Ronkainen J, Aro P, Jones M, et al. Duodenal eosinophilia and the link to anxiety: a population‐based endoscopic study. Neurogastroenterology & Motility 2021;33:e14109. 10.1111/nmo.14109 [DOI] [PubMed] [Google Scholar]
- 140.Yuan H-P, Li Z, Zhang Y, et al. Anxiety and depression are associated with increased counts and degranulation of duodenal mast cells in functional dyspepsia. Int J Clin Exp Med 2015;8:8010–4. [PMC free article] [PubMed] [Google Scholar]
- 141.Koloski NA, Jones M, Talley NJ. Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study. Aliment Pharmacol Ther 2016;44:592–600. 10.1111/apt.13738 [DOI] [PubMed] [Google Scholar]
- 142.Vanuytsel T, van Wanrooy S, Vanheel H, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 2014;63:1293–9. 10.1136/gutjnl-2013-305690 [DOI] [PubMed] [Google Scholar]
- 143.Alonso C, Guilarte M, Vicario M, et al. Maladaptive intestinal epithelial responses to life stress may predispose healthy women to gut mucosal inflammation. Gastroenterology 2008;135:163–72. 10.1053/j.gastro.2008.03.036 [DOI] [PubMed] [Google Scholar]
- 144.Zheng P-Y, Feng B-S, Oluwole C, et al. Psychological stress induces eosinophils to produce corticotrophin releasing hormone in the intestine. Gut 2009;58:1473–9. 10.1136/gut.2009.181701 [DOI] [PubMed] [Google Scholar]
- 145.Wallon C, Persborn M, Jönsson M, et al. Eosinophils express muscarinic receptors and corticotropin-releasing factor to disrupt the mucosal barrier in ulcerative colitis. Gastroenterology 2011;140:1597–607. 10.1053/j.gastro.2011.01.042 [DOI] [PubMed] [Google Scholar]
- 146.Salvo-Romero E, Martínez C, Lobo B, et al. Overexpression of corticotropin-releasing factor in intestinal mucosal eosinophils is associated with clinical severity in diarrhea-predominant irritable bowel syndrome. Sci Rep 2020;10:20706. 10.1038/s41598-020-77176-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mogilevski T, Rosella S, Aziz Q, et al. Transcutaneous vagal nerve stimulation protects against stress-induced intestinal barrier dysfunction in healthy adults. Neurogastroenterol Motil 2022;34:e14382. 10.1111/nmo.14382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wallon C, Yang P-C, Keita AV, et al. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut 2008;57:50–8. 10.1136/gut.2006.117549 [DOI] [PubMed] [Google Scholar]
- 149.Bruce JK, Burns GL, Sinn Soh W, et al. Defects in NLRP6, autophagy and goblet cell homeostasis are associated with reduced duodenal CRH receptor 2 expression in patients with functional dyspepsia. Brain Behav Immun 2022;101:335–45. 10.1016/j.bbi.2022.01.019 [DOI] [PubMed] [Google Scholar]
- 150.Fukudo S. Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J Gastroenterol 2007;42 Suppl 17:48–51. 10.1007/s00535-006-1942-7 [DOI] [PubMed] [Google Scholar]
- 151.Orand A, Naliboff B, Gadd M, et al. Corticotropin-Releasing hormone receptor 1 (CRH-R1) polymorphisms are associated with irritable bowel syndrome and acoustic startle response. Psychoneuroendocrinology 2016;73:133–41. 10.1016/j.psyneuen.2016.07.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Komuro H, Sato N, Sasaki A, et al. Corticotropin-releasing hormone receptor 2 gene variants in irritable bowel syndrome. PLoS One 2016;11:e0147817. 10.1371/journal.pone.0147817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dunlop SP, Jenkins D, Neal KR, et al. Randomized, double-blind, placebo-controlled trial of prednisolone in post-infectious irritable bowel syndrome. Aliment Pharmacol Ther 2003;18:77–84. 10.1046/j.1365-2036.2003.01640.x [DOI] [PubMed] [Google Scholar]
- 154.Talley NJ, Walker MM, Jones M, et al. Letter: budesonide for functional dyspepsia with duodenal eosinophilia—randomised, double‐blind, placebo‐controlled parallel‐group trial. Aliment Pharmacol Ther 2021;53:1332–3. 10.1111/apt.16396 [DOI] [PubMed] [Google Scholar]
- 155.Fox CC, Moore WC, Lichtenstein LM. Modulation of mediator release from human intestinal mast cells by sulfasalazine and 5-aminosalicylic acid. Dig Dis Sci 1991;36:179–84. 10.1007/BF01300753 [DOI] [PubMed] [Google Scholar]
- 156.Corinaldesi R, Stanghellini V, Cremon C, et al. Effect of mesalazine on mucosal immune biomarkers in irritable bowel syndrome: a randomized controlled proof-of-concept study. Aliment Pharmacol Ther 2009;30:245–52. 10.1111/j.1365-2036.2009.04041.x [DOI] [PubMed] [Google Scholar]
- 157.Lam C, Tan W, Leighton M, et al. A mechanistic multicentre, parallel group, randomised placebo-controlled trial of mesalazine for the treatment of IBS with diarrhoea (IBS-D). Gut 2016;65:91–9. 10.1136/gutjnl-2015-309122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Barbara G, Cremon C, Annese V, et al. Randomised controlled trial of mesalazine in IBS. Gut 2016;65:82–90. 10.1136/gutjnl-2014-308188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jalanka J, Lam C, Bennett A, et al. Colonic gene expression and fecal microbiota in diarrhea-predominant irritable bowel syndrome: increased Toll-like receptor 4 but minimal inflammation and NO response to mesalazine. J Neurogastroenterol Motil 2021;27:279–91. 10.5056/jnm20205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lobo B, Ramos L, Martínez C, et al. Downregulation of mucosal mast cell activation and immune response in diarrhoea‐irritable bowel syndrome by oral disodium cromoglycate: a pilot study. United European Gastroenterol J 2017;5:887–97. 10.1177/2050640617691690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang J, Wang Y, Zhou H, et al. Clinical efficacy and safety of ketotifen in treating irritable bowel syndrome with diarrhea. Eur J Gastroenterol Hepatol 2020;32:706–12. 10.1097/MEG.0000000000001737 [DOI] [PubMed] [Google Scholar]
- 162.Dellon ES, Peterson KA, Murray JA, et al. Anti–siglec-8 antibody for eosinophilic gastritis and duodenitis. N Engl J Med Overseas Ed 2020;383:1624–34. 10.1056/NEJMoa2012047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pearson JS, Niven RM, Meng J, et al. Immunoglobulin E in irritable bowel syndrome: another target for treatment? A case report and literature review. Therap Adv Gastroenterol 2015;8:270–7. 10.1177/1756283X15588875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Black CJ, Burr NE, Camilleri M, et al. Efficacy of pharmacological therapies in patients with IBS with diarrhoea or mixed stool pattern: systematic review and network meta-analysis. Gut 2020;69:74–82. 10.1136/gutjnl-2018-318160 [DOI] [PubMed] [Google Scholar]
- 165.Ceuleers H, Hanning N, Heirbaut J, et al. Newly developed serine protease inhibitors decrease visceral hypersensitivity in a post-inflammatory rat model for irritable bowel syndrome. Br J Pharmacol 2018;175:3516–33. 10.1111/bph.14396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Odiase E, Zhang X, Chang Y, et al. In esophageal squamous cells from eosinophilic esophagitis patients, Th2 cytokines increase eotaxin-3 secretion through effects on intracellular calcium and a non-gastric proton pump. Gastroenterology 2021;160:2072–88. 10.1053/j.gastro.2021.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]