Abstract
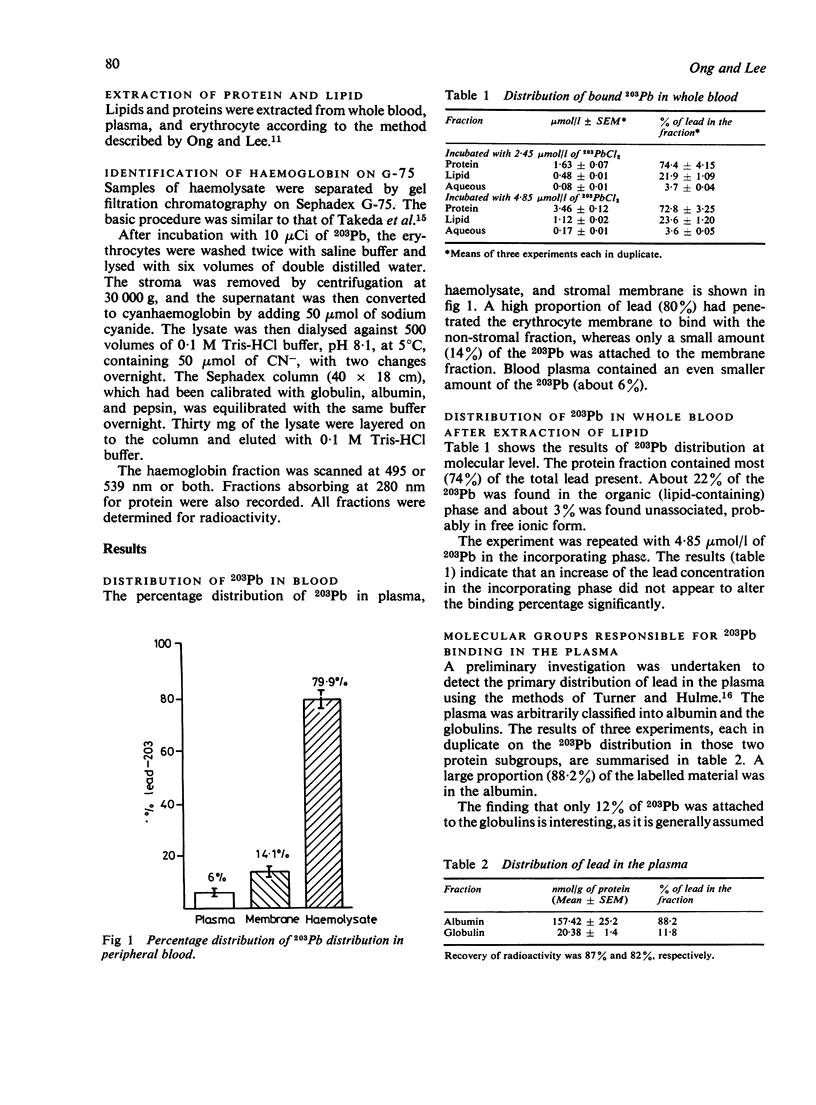
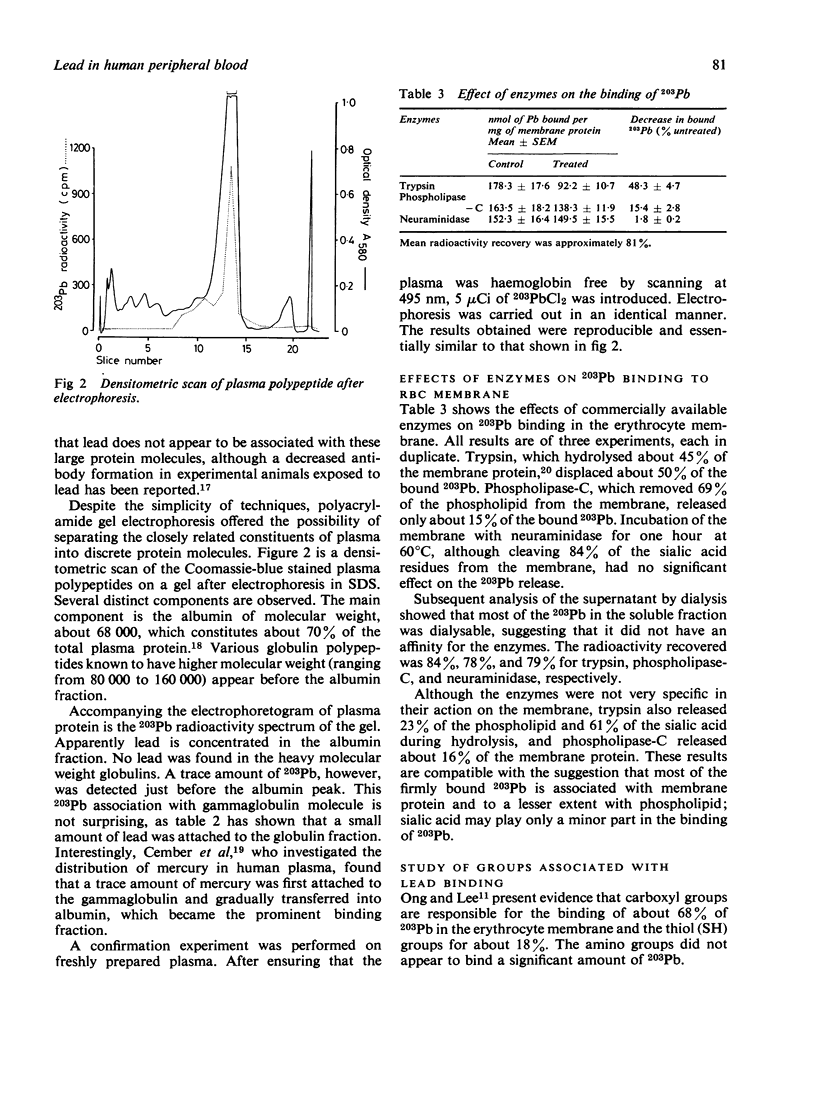
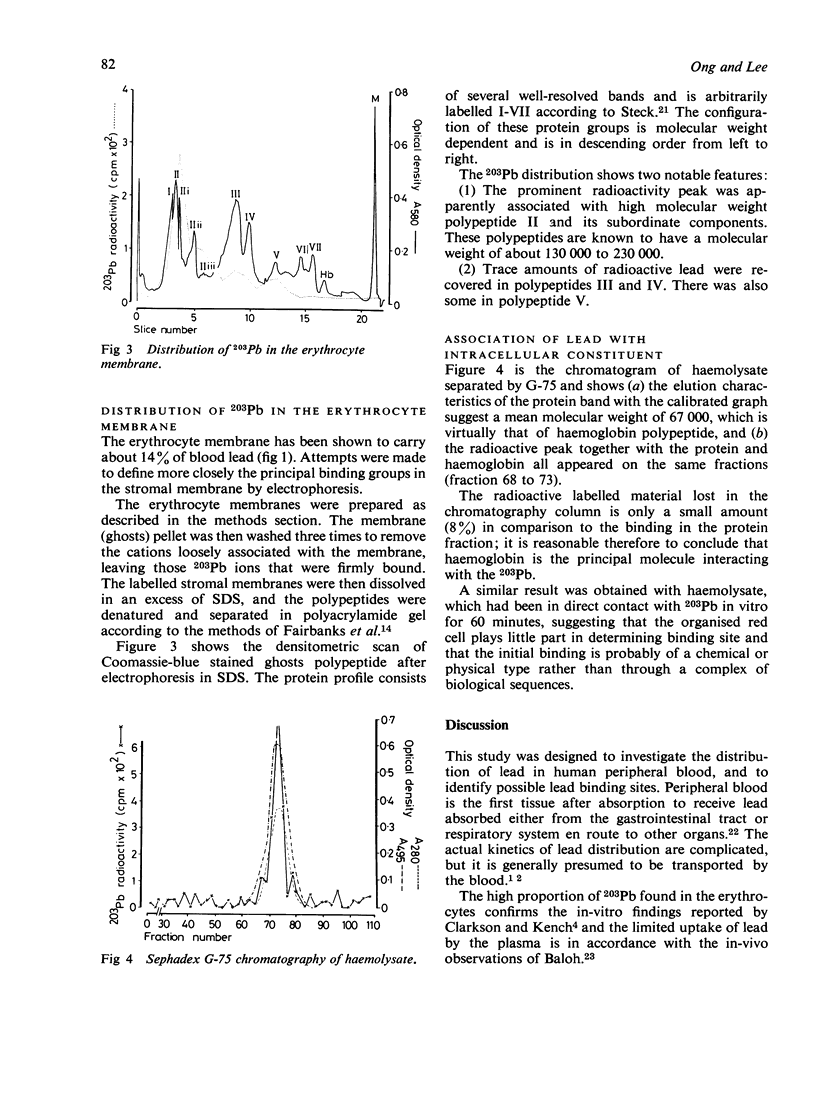
In-vitro experiments using 203Pb were performed to identify the lead binding components in human peripheral blood. The distribution of lead in plasma, in the red cell membrane, and within the red cell was also investigated. Studies of the distribution of 203Pb in whole blood showed that at a lead concentration of 2.45 mumol/l (50 micrograms/100 ml) about 94% of lead had been incorporated by the erythrocytes and 6% remained in the plasma. After extraction of lipid by a methanol/chloroform mixture, about 75% of the lead was found to be associated with the protein fraction. The lipid contained about 21% of the 203Pb, the remainder being in the aqueous plasma. SDS polyacrylamide gel electrophoresis of blood plasma showed that almost 90% of the 203Pb was present in the albumin fraction; the remainder was likely to be associated with high molecular weight globulins. Several binding sites were identified on the erythrocyte membrane. The high molecular weight component, about 130 000-230 000, was the most important 203Pb binding site. Chemical modification of membrane proteins suggested that the carboxyl groups are the major ligand responsible for most of the lead binding. SH groups of the membrane may have a minor role, but amino groups did not appear to affect the lead binding. The binding of lead to erythrocytes was not confined to membranes, over 80% of lead in blood penetrates into erythrocytes and binds to intracellular components. Gel chromatography of the haemolysate showed that over 90% of the 203Pb was attached to the haemoglobin molecule.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Fairbanks G. Demonstration of a phosphopeptide intermediate in the Mg ++ -dependent, Na + - and K + -stimulated adenosine triphosphatase reaction of the erythrocyte membrane. Proc Natl Acad Sci U S A. 1972 May;69(5):1216–1220. doi: 10.1073/pnas.69.5.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloh R. W. Laboratory diagnosis of increased lead absorption. Arch Environ Health. 1974 Apr;28(4):198–208. doi: 10.1080/00039896.1974.10666469. [DOI] [PubMed] [Google Scholar]
- Barltrop D. Environmental lead and its paediatric significance. Postgrad Med J. 1969 Feb;45(520):129–134. doi: 10.1136/pgmj.45.520.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barltrop D., Smith A. Interaction of lead with erythrocytes. Experientia. 1971 Jan 15;27(1):92–93. doi: 10.1007/BF02137760. [DOI] [PubMed] [Google Scholar]
- Barltrop D., Smith A. Lead binding to human haemoglobin. Experientia. 1972 Jan 15;28(1):76–77. doi: 10.1007/BF01928273. [DOI] [PubMed] [Google Scholar]
- Beattie A. D., Moore M. R., Goldberg A. Tetraethyl-lead poisoning. Lancet. 1972 Jul 1;2(7766):12–15. doi: 10.1016/s0140-6736(72)91276-7. [DOI] [PubMed] [Google Scholar]
- Booker D. V., Chamberlain A. C., Newton D., Stott A. N. Uptae of radioactive lead following inhalation and injection. Br J Radiol. 1969 Jun;42(498):457–466. doi: 10.1259/0007-1285-42-498-457. [DOI] [PubMed] [Google Scholar]
- Brown P. A., Feinstein M. B., Sha'afi R. I. Membrane proteins related to water transport in human erythrocytes. Nature. 1975 Apr 10;254(5500):523–525. doi: 10.1038/254523a0. [DOI] [PubMed] [Google Scholar]
- Bruenger F. W., Stevens W., Stover B. J. The association of 210Pb with constituents of erythrocytes. Health Phys. 1973 Jul;25(1):37–42. doi: 10.1097/00004032-197307000-00004. [DOI] [PubMed] [Google Scholar]
- CLARKSON T. W., KENCH J. E. Uptake of lead by human erythrocytes in vitro. Biochem J. 1958 Jul;69(3):432–439. doi: 10.1042/bj0690432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cember H., Gallagher P., Faulkner A. Distribution of mercury among blood fractions and serum proteins. Am Ind Hyg Assoc J. 1968 May-Jun;29(3):233–237. doi: 10.1080/00028896809342994. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hasan J., Vihko V., Hernberg S. Deficient red cell membrane /Na++K+/-ATPase in lead poisoning. Arch Environ Health. 1967 Feb;14(2):313–318. doi: 10.1080/00039896.1967.10664738. [DOI] [PubMed] [Google Scholar]
- KILLANDER J. SEPARATION OF HUMAN HEME- AND HEMOGLOBIN-BINDING PLASMA PROTEINS, CERULOPLASMIN AND ALBUMIN BY GEL FILTRATION. Biochim Biophys Acta. 1964 Oct 9;93:1–14. doi: 10.1016/0304-4165(64)90254-5. [DOI] [PubMed] [Google Scholar]
- Koller L. D., Kovacic S. Decreased antibody formation in mice exposed to lead. Nature. 1974 Jul 12;250(462):148–150. doi: 10.1038/250148a0. [DOI] [PubMed] [Google Scholar]
- Ong C. N., Lee W. R. Interaction of calcium and lead in human erythrocytes. Br J Ind Med. 1980 Feb;37(1):70–77. doi: 10.1136/oem.37.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASSOW H., ROTHSTEIN A., CLARKSON T. W. The general pharmacology of the heavy metals. Pharmacol Rev. 1961 Jun;13:185–224. [PubMed] [Google Scholar]
- REDDI K. K. Isolation of thorium B (lead)-binding substance from the erythrocytes of rabbit blood. Nature. 1953 Aug 1;172(4370):202–202. doi: 10.1038/172202a0. [DOI] [PubMed] [Google Scholar]
- Rabinowitz M., Wetherill G. W., Kopple J. D. Studies of human lead metabolism by use of stable isotope tracers. Environ Health Perspect. 1974 May;7:145–153. doi: 10.1289/ehp.747145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secchi G. C., Alessio L., Cambiaghi G. Na plus-K plus-ATPase activity of erythrocyte membranes: in urban populations not occupationally exposed to lead. Arch Environ Health. 1973 Dec;27(6):399–400. doi: 10.1080/00039896.1973.10666412. [DOI] [PubMed] [Google Scholar]
- Selhi H. S., White J. M. The effect of lead on the red cell membrane. Postgrad Med J. 1975 Nov;51(601):765–769. doi: 10.1136/pgmj.51.601.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Kunugi T., Hoshino O., Ukita T. Distribution of inorganic, aryl, and alkyl mercury compounds in rats. Toxicol Appl Pharmacol. 1968 Sep;13(2):156–164. doi: 10.1016/0041-008x(68)90089-6. [DOI] [PubMed] [Google Scholar]
- White J. M., Selhi H. S. Lead and the red cell. Br J Haematol. 1975 Jun;30(2):133–138. doi: 10.1111/j.1365-2141.1975.tb00526.x. [DOI] [PubMed] [Google Scholar]


