Abstract
ABSTRACT Changes in nerve conduction velocity were found in 94 workers exposed to lead in a battery factory compared with 94 age-matched controls. There was no clinical evidence of nerve damage in the lead workers. The mean blood lead concentration in the 94 lead workers was 2·9 μmol/l (60 μg/100 ml) and their length of exposure to lead ranged from 6 months to 33 years.
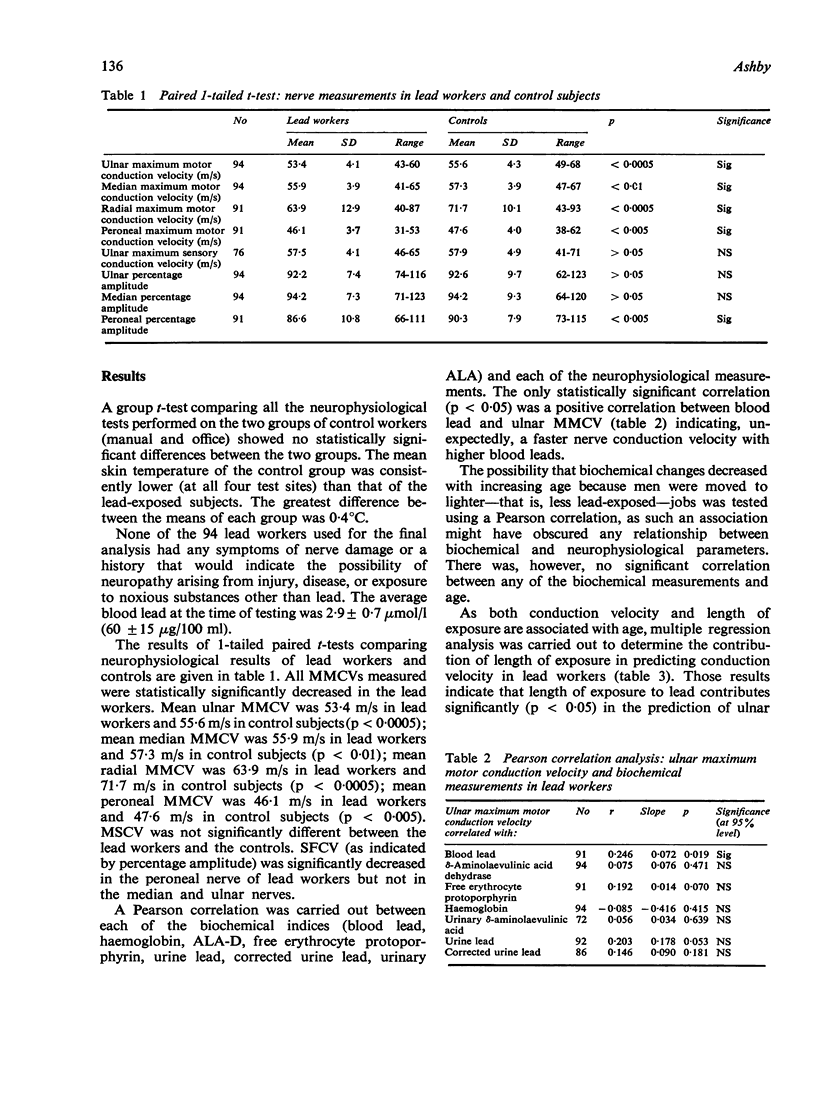
All mean maximum motor nerve conduction velocities (MMCV) measured were highly statistically significantly lower in the lead-exposed group when compared with their age-matched controls. Thus mean ulnar MMCV was 53·4 m/s in lead workers and 55·6 m/s in control subjects (p < 0·0005); mean median MMCV was 55·9 m/s in lead workers and 57·3 m/s in control subjects (p < 0·01); mean radial MMCV was 63·9 m/s in lead workers and 71·1 m/s in control subjects (p < 0·0005); mean peroneal MMCV was 46·1 m/s in lead workers and 47·6 m/s in control subjects (p < 0·005).
The amplitude of the muscle action potential produced by proximal stimulation of a nerve was expressed as a percentage of the amplitude of the muscle action potential produced by distal stimulation and the percentage amplitude thus obtained used as an indicator of the conduction velocity of slower fibres (SFCV). Peroneal nerve percentage amplitude of lead workers was statistically significantly lower (p < 0·005) than in the control group (means 86·6% and 90·3% respectively). There were, however, no significant differences in the percentage amplitude in the ulnar and median nerves. It is suggested that percentage amplitude is an inappropriate indicator of SFCV in ulnar and median nerves.
There was no statistically significant correlation to indicate that progressive slowing of nerve conduction (MMCV and SFCV) was associated with increasing exposure to lead (as indicated by blood and urine lead concentrations) or with the commonly measured biochemical changes associated with disturbed haemopoiesis in lead exposure (δ-aminolaevulinic acid dehydrase; free erythrocytc protoporphyrin; haemoglobin and urinary δ-aminolaevulinic acid). MMCV of the ulnar nerve was the only conduction velocity statistically significantly correlated with length of exposure to lead. Increased length of exposure to lead was associated with a decrease in the ulnar MMCV.
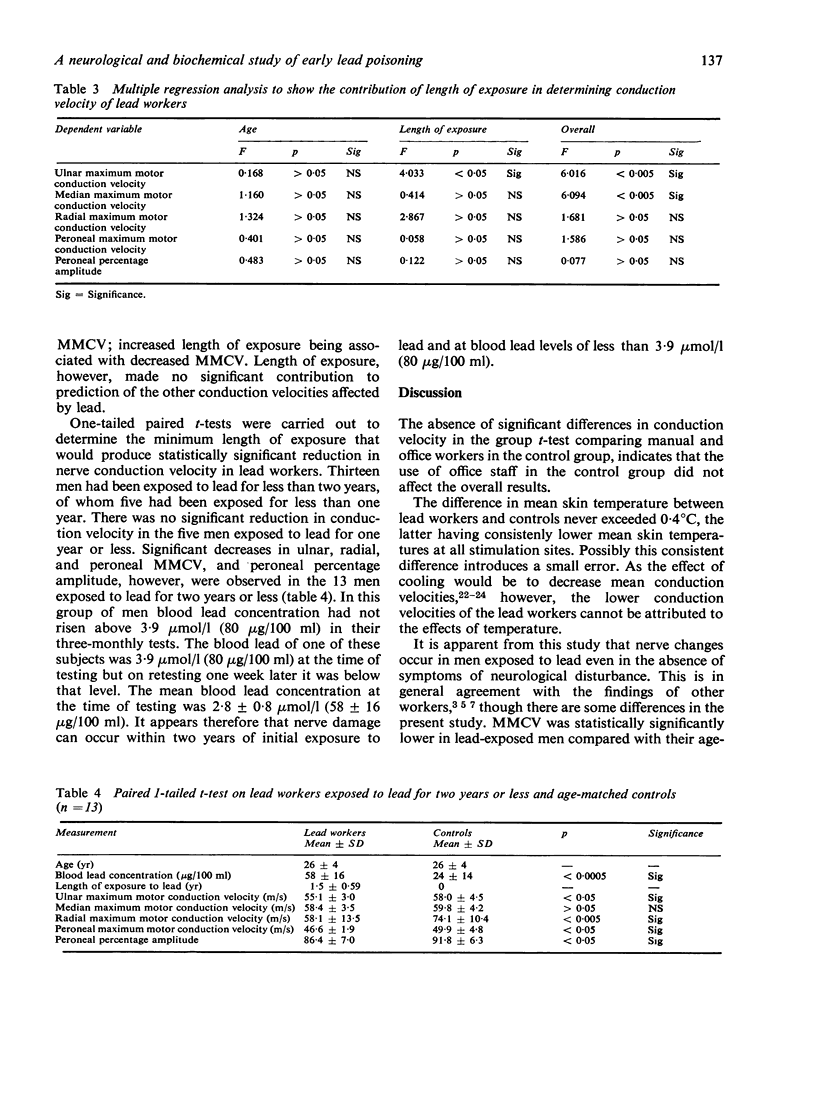
Only 13 of the subjects had been exposed to lead for two years or less and in none of them had the blood lead ever risen above 3·9 μmol/l (80 μg/100 ml) in three-monthly tests (mean blood lead concentration at time of testing: 2·8 μmol/l). In these subjects the MMCV of ulnar, radial, and peroneal nerves and the peroneal percentage amplitude were statistically significantly reduced. The results from this group suggest that the onset of nerve conduction changes occurs within two years and at concentrations of lead in blood of less than 3·9 μmol/l (80 μg/100 ml).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behse F., Pach J., Dorndorf W. Bleipolyneuropathie Klinische, elektrophysiologische und bioptische Befunde. Z Neurol. 1972;202(3):209–216. [PubMed] [Google Scholar]
- Boothby J. A., DeJesus P. V., Rowland L. P. Reversible forms of motor neuron disease. Lead "neuritis". Arch Neurol. 1974 Jul;31(1):18–23. doi: 10.1001/archneur.1974.00490370044005. [DOI] [PubMed] [Google Scholar]
- Catton M. J., Harrison M. J., Fullerton P. M., Kazantzis G. Subclinical neuropathy in lead workers. Br Med J. 1970 Apr 11;2(5701):80–82. doi: 10.1136/bmj.2.5701.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. R., Andelman S. L. Urinary delta-aminolevulinic acid (ALA) levels in lead poisoning. I. A modified method for the rapid determination of urinary delta-aminolevulinic acid using disposable ion-exchange chromatography columns. Arch Environ Health. 1967 Jul;15(1):53–59. doi: 10.1080/00039896.1967.10664873. [DOI] [PubMed] [Google Scholar]
- FAZLULLAH S., RAMAMURTHI B. LEAD INTOXICATION AND THE NERVOUS SYSTEM. J Indian Med Assoc. 1965 Jun 16;44:644–648. [PubMed] [Google Scholar]
- Fullerton P. M. Chronic peripheral neuropathy produced by lead poisoning in guinea-pigs. J Neuropathol Exp Neurol. 1966 Apr;25(2):214–236. doi: 10.1097/00005072-196604000-00003. [DOI] [PubMed] [Google Scholar]
- GASSEL M. M., TROJABORG W. CLINICAL AND ELECTROPHYSIOLOGICAL STUDY OF THE PATTERN OF CONDUCTION TIMES IN THE DISTRIBUTION OF THE SCIATIC NERVE. J Neurol Neurosurg Psychiatry. 1964 Aug;27:351–357. doi: 10.1136/jnnp.27.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoschek R., Schittke H. J. Vergleichsbestimmungen für Blei im Blut sowie für Blei und Kadmium im Urin. Zentralbl Arbeitsmed. 1973 Aug;23(8):243–248. [PubMed] [Google Scholar]
- KATO M. The conduction velocity of the ulnar nerve and the spinal reflex time measured by means of the H wave in average adults and athletes. Tohoku J Exp Med. 1960 Dec 25;73:74–85. doi: 10.1620/tjem.73.74. [DOI] [PubMed] [Google Scholar]
- LAFRATTA C. W., SMITH O. H. A STUDY OF THE RELATIONSHIP OF MOTOR NERVE CONDUCTION VELOCITY IN THE ADULT TO AGE, SEX, AND HANDEDNESS. Arch Phys Med Rehabil. 1964 Aug;45:407–412. [PubMed] [Google Scholar]
- Mostafa M., el-Sewefy A. Z., Hamid T. A. Clinical, biochemical and electromyographic study in Egyptian lead workers. Med Lav. 1972 Mar-Apr;63(3):109–116. [PubMed] [Google Scholar]
- NORRIS A. H., SHOCK N. W., WAGMAN I. H. Age changes in the maximum conduction velocity of motor fibers of human ulnar nerves. J Appl Physiol. 1953 Apr;5(10):589–593. doi: 10.1152/jappl.1953.5.10.589. [DOI] [PubMed] [Google Scholar]
- Nielsen V. K. Sensory and motor nerve conduction in the median nerve in normal subjects. Acta Med Scand. 1973 Nov;194(5):435–443. doi: 10.1111/j.0954-6820.1973.tb19469.x. [DOI] [PubMed] [Google Scholar]
- PREISKEL D. Chronic lead poisoning: myopathy or neuritis. Ann Phys Med. 1958 Nov;4(8):293–296. doi: 10.1093/rheumatology/iv.8.293. [DOI] [PubMed] [Google Scholar]
- SIMPSON J. A., SEATON D. A., ADAMS J. F. RESPONSE TO TREATMENT WITH CHELATING AGENTS OF ANAEMIA, CHRONIC ENCEPHALOPATHY, AND MYELOPATHY DUE TO LEAD POISONING. J Neurol Neurosurg Psychiatry. 1964 Dec;27:536–541. doi: 10.1136/jnnp.27.6.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K. Nerve conduction studies in man. Electromyography. 1967 Aug-Oct;7(3):175–176. [PubMed] [Google Scholar]
- Seppäläinen A. M., Hernberg S. Sensitive technique for detecting subclinical lead neuropathy. Br J Ind Med. 1972 Oct;29(4):443–449. doi: 10.1136/oem.29.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppäläinen A. M., Tola S., Hernberg S., Kock B. Subclinical neuropathy at "safe" levels of lead exposure. Arch Environ Health. 1975 Apr;30(4):180–183. doi: 10.1080/00039896.1975.10666672. [DOI] [PubMed] [Google Scholar]
- Vasilescu C. Motor nerve conduction velocity and electromyogram in chronic lead poisoning. Rev Roum Neurol. 1973;10(3):221–226. [PubMed] [Google Scholar]
- WAGMAN I. H., LESSE H. Maximum conduction velocities of motor fibers of ulnar nerve in human subjects of various ages and sizes. J Neurophysiol. 1952 May;15(3):235–244. doi: 10.1152/jn.1952.15.3.235. [DOI] [PubMed] [Google Scholar]
- Weissberg J. B., Lipschutz F., Oski F. A. Delta-aminolevulinic acid dehydratase activity in circulating blood cells. A sensitive laboratory test for the detection of childhood lead poisoning. N Engl J Med. 1971 Mar 18;284(11):565–569. doi: 10.1056/NEJM197103182841101. [DOI] [PubMed] [Google Scholar]


