Abstract
Background and Objective
Phantom limb pain (PLP) concerns >50% of amputees and has a negative impact on their rehabilitation, mental health and quality of life. Mirror therapy (MT) is a promising strategy, but its effectiveness remains controversial. We performed a systematic review to: (i) evaluate the effectiveness of MT versus placebo in reducing PLP, and (ii) determine MT effect on disability and quality of life.
Databases and data treatment
We selected randomized‐controlled trials in five databases (Medline, Cochrane Library, CINAHL, PEDro and Embase) that included patients with unilateral lower or upper limb amputation and PLP and that compared the effects on PLP of MT versus a placebo technique. The primary outcome was PLP intensity changes and the secondary outcomes were PLP duration, frequency, patients' disability and quality of life.
Results
Among the five studies included, only one reported a significant difference between the MT group and control group, with a positive MT effect at week 4. Only one study assessed MT effect on disability and found a significant improvement in the MT group at week 10 and month 6.
Conclusions
Our systematic review did not allow concluding that MT reduces PLP and disability in amputees. This lack of strong evidence is probably due to (i) the low methodological quality of the included studies, and (ii) the lack of statistical power. Future trials should include a higher number of patients, increase the number and frequency of MT sessions, have a long‐term follow‐up and improve the methodological quality.
Significance
Recent meta‐analyses concluded that MT is effective for reducing phantom limb pain. Conversely, the present systematic review that included only studies with the best level of evidence did not find any evidence about its effectiveness for this condition. We identified many ways to improve future randomized‐controlled trials on this topic: increasing the number of participants, reducing the intra‐group heterogeneity, using a suitable placebo and intensifying the MT sessions and frequency.
1. INTRODUCTION
Phantom limb pain (PLP), which affects between 49% and 88% of amputees (Nikolajsen & Jensen, 2001), is a neuropathic pain defined as a painful or unpleasant sensation perceived in the territory of the amputated or deafferented limb (Niraj & Niraj, 2014). Patients describe PLP as a strong pain like electric currents, or as a burning, stinging, cramping or stabbing pain (Hsu & Cohen, 2013). PLP often occurs suddenly and lasts from few seconds to several hours, and is mostly felt in the distal part of the phantom limb (fingers or toes). PLP affects negatively rehabilitation, mental health and quality of life (Sinha et al., 2011; van der Schans et al., 2002).
The mechanisms underlying PLP have not been clearly identified and are probably multiple (Flor et al., 2006; Makin et al., 2013). According to the maladaptive cortical plasticity theory, PLP emerges following the progressive occupation of the cortical areas that correspond to the missing limb by adjacent areas. This is due to the absence of sensory afferent signals from the missing limb and spinal dysregulation that increases the transmission of the pain signal (Flor, 2002). Spinal dysregulation contributes to the pro‐nociceptive effect due to a cascade mechanism that implicates increased activity of nociceptors in the dorsal horn, degeneration of C‐fibres in the lamina II, sprouting of A‐fibres, downregulation of GABAergic activity and opioid receptors, expansion of adjacent receptive fields in deafferented zones, and spinal cord hyperexcitability linked to the altered substance P expression by Aß fibres (Flor, 2002; Woolf et al., 1992; Woolf & Mannion, 1999). According to another theory, PLP extent is related to the preserved representation of the phantom limb in the cortex that can be activated and to a reduction of inter‐regional functional connectivity in the primary sensorimotor cortex (Makin et al., 2013).
Several pharmacological, surgical or non‐pharmacological approaches have been proposed for PLP management. The effectiveness of pharmacological treatments remains low (Alviar et al., 2016). Surgical strategies are effective, but invasive and with a long healing time and loss of function (Dumanian et al., 2019; Herr et al., 2020; Knotkova et al., 2012). Therefore, they are not used as first‐line treatment. Among non‐pharmacological interventions, mirror therapy (MT), first proposed by Ramachandran (1996), seems to be a promising strategy. MT consists in placing a mirror in the parasagittal plane between the healthy limb and the amputated limb. The image of the healthy limb is reflected in the mirror and the patient perceives the reflection of the healthy limb instead of the amputated limb. By creating a visual representation of the missing limb, MT might restore the cortical (motor and sensory) areas that correspond to the absent limb. By restoring the body image and body schema disturbed by the amputation, MT might reduce PLP (Flor, 2002; Foell et al., 2014; Ramachandran & Altschuler, 2009).
MT effectiveness remains controversial (Barbin et al., 2016; Batsford et al., 2017; Herrador Colmenero et al., 2018; Plumbe et al., 2013; Rothgangel et al., 2011; Thieme et al., 2016, 2018; Xie et al., 2021) because previous reviews and meta‐analyses found inconclusive evidence. This could be due to the fact that the studies included in these reviews assessed MT in various pathologies (Rothgangel et al., 2011; Thieme et al., 2016) or in combination with other techniques (Batsford et al., 2017; Herrador Colmenero et al., 2018). Barbin et al. (2016) analysed MT use specifically in amputees with PLP (Barbin et al., 2016). They found only three randomized‐controlled trials and concluded that the level of evidence was not adequate, due to the low methodological quality of the included studies and the small sample sizes. Two recent meta‐analyses on this question concluded that MT is effective for PLP management (Wang et al., 2021; Xie et al., 2021). One found a positive effect of MT at month 1 of treatment (I 2 = 0%, standardized mean difference = −0.46, 95% CI: −0.79 to 0.13, p = 0.007), but not at month 3 or 6 of follow‐up (Xie et al., 2021). The other meta‐analysis showed that MT reduces pain with a larger effect size (I 2 = 82%; standardized mean difference = −0.81; 95% CI = −1.36 to −0.25; p = 0.005) compared with other methods (Wang et al., 2021). However, the conclusion of these meta‐analyses are subject of debate due to questionable methodological and statistical choices: the use of fixed‐effect analyses (Borenstein et al., 2010), the inclusion of low‐powered and poor‐quality studies, the strong heterogeneity and the interpretation of non‐clinically relevant effects (Moore et al., 2022). Furthermore, no systematic review has evaluated MT impact on the patients' quality of life and disability. Pain reduction is a good indicator of PLP improvement, but changes in disability and well‐being also are important (World Health Organization, World Bank, 2011).
Therefore, we performed a systematic review: (i) to evaluate the effectiveness of MT versus placebo in reducing PLP; and (ii) to determine MT effect on disability and quality of life. To overcome the previously stated limitations, we included only randomized‐controlled trials that compared MT versus a placebo in unilateral amputees.
2. LITERATURE SEARCH METHODS
This systematic review of the literature followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines (Liberati et al., 2009).
2.1. Eligibility criteria
We defined the eligibility criteria to include studies according to the Population, Intervention, Comparison, Outcomes and Study (PICOS) model:
Population: adult patients with unilateral, lower or upper limb amputation due to any cause and with PLP.
Intervention: MT to reduce PLP. It has been hypothesized that MT restores the cortical (motor and sensory) areas corresponding to the missing limb through the co‐occurrence of visual, proprioceptive information from the intact limb, and motor inputs from both limbs. This input combination is considered to be the active component of MT (Schone et al., 2022). To avoid heterogeneity and to specifically assess the association of these components, we chose MT only as the intervention. We considered that other techniques, such as mental visualization, tactile discrimination and virtual reality, target a similar process (i.e. maladaptive plasticity), but have different active components. Mental visualization does not induce motor commands or sensory inputs. Tactile discrimination enhances sensory stimulation on the stump; however, it does not implicate movements, motor commands and proprioceptive inputs. Virtual reality can act as MT, but virtual reality approaches are heterogenous (e.g. using the residual or the intact limb, moving one or two arms, interacting with exergames or avatars).
Comparison: any other technique considered as placebo (covered mirror or mirrorless visualization). The covered mirror is classically considered as the best controls for MT (Chan et al., 2007; Finn et al., 2017; Ramadugu et al., 2017; Schone et al., 2022). To test the effect of MT active components, an adequate control is a technique as similar as possible. When using the covered mirror and the mirrorless approaches as placebo, the only difference, compared with MT, is the suppression of the visual feedback, while the other inputs remain the same.
Outcomes:
Primary: PLP intensity (measured with a scale).
Secondary:
PLP duration and frequency
Disability and quality of life (measured with questionnaires)
Study types: randomized‐controlled trials published between 1996 (first description of MT) and 01 July 2021.
Non‐inclusion criteria were:
Articles without available full text.
Articles published in a language other than English or French.
Articles comparing the effects of MT with another technique (TENS, motor imaging, phantom exercise, tactile discrimination).
2.2. Information sources and search strategy
We used Medline, Cochrane Library, CINAHL, PEDro and Embase as information sources and searched them using the index terms and syntax listed in Table 1.
TABLE 1.
Index terms and syntax for each database
| Database | Index terms and syntax |
|---|---|
| PubMed | (“Amputation”[Mesh] OR “Amputees”[Mesh]) AND (“Phantom Limb/therapy”[Mesh]) AND (mirror* OR mirror therap* OR mirror box). |
| Cochrane Library | amput* AND (phantom limb OR phantom pain) AND (mirror therapy* OR mirror* OR mirror box) |
| CINHAL | ((MH “Phantom Limb”) OR (MH “Phantom Pain”)) AND ((MH “Amputation”) OR (MH “Amputees”)) AND ([MH “Mirror Therapy”] OR mirror* OR mirror therapy* OR mirror box) |
| PEDro | amput* AND “phantom limb pain” AND mirror* The “clinical trial” filter was added to limit the search to clinical trials |
| Embase | (‘amputation’/exp OR ‘amputee’/exp) AND ‘phantom pain’/exp AND (‘mirror therapy’/de OR ‘mirror’/de) The filter “Embase” was used to eliminate articles fromPubMed and keep only articles that are indexed only in Embase |
2.3. Study selection
This step was carried out by two independent reviewers (L.R., N.R.) following the established inclusion and non‐inclusion criteria. Duplicates were removed from the list of results. A first selection was made by reading the titles and abstracts to remove articles not meeting the inclusion criteria. The final selection was made by reading the full text of the selected articles. When no agreement could be reached, a third reviewer (M.G.) intervened to reach an agreement.
2.4. Data extraction
The data extracted were:
Participants: sample size, age, sex, location and cause of amputation, PLP intensity and time of onset since amputation.
Intervention: data were extracted based on the French translation of the Template for Intervention Description and Replication (TIDieR) item list.
Measurements: tools used to measure PLP and the number of measurements performed.
Intervention effect: we extracted results of intra‐ and inter‐group assessments. We extracted only results that compared the effect of MT versus placebo. We did not consider comparisons with other techniques (mental visualization, virtual reality).
2.5. Methodological quality of the included studies
We assessed the methodological quality of the retained randomized‐controlled trials using the PEDro scale (Brosseau et al., 2015). The PEDro scale includes 11 criteria in order to help the PEDro database users to rapidly identify which clinical studies (i.e. randomized clinical trials and controlled clinical trials) are likely to be internally valid (criteria 2–9) and to have sufficient statistical information to make their results interpretable (criteria 10–11). Criterion 1, which relates to external validity, is not used to calculate the PEDro score. Two reviewers (L.R., N.R.) independently scored the articles, and discussed areas of disagreement. When no agreement could be reached, a third reviewer (M.G.) intervened to reach an agreement.
3. RESULTS
3.1. Study selection
The literature search identified 155 articles (flowchart in Figure 1). We excluded 43 articles after the removal of duplicates and 94 after reading the titles and abstracts. The reasons for exclusions are detailed in Figure 1. We did not find one study (text unavailable; Perry, 2013). Following this selection, we examined the full text of 17 articles and retained five articles (Brodie et al., 2007; Chan et al., 2007; Finn et al., 2017; Ramadugu et al., 2017; Rothgangel et al., 2018).
FIGURE 1.
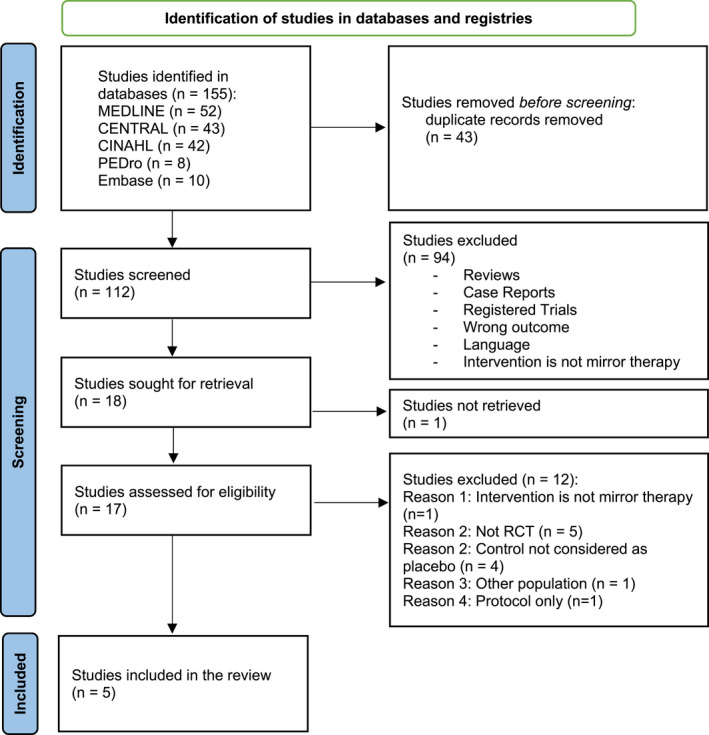
Study selection flowchart according to the PRISMA guidelines. RCT, randomized‐controlled trial
3.2. Study methodological quality
We assessed the methodological quality of the five studies with the PEDro scale (Table 2). Their scores ranged from 4 to 8. Three studies had a score >6 and could be considered as having a limited risk of bias (Brodie et al., 2007; Ramadugu et al., 2017; Rothgangel et al., 2018). The other two studies [27,28] had a score <5 and were at higher risk of bias.
TABLE 2.
PEDro score of the selected studies
| First author | Items | Total score/10 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Brodie et al. | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Chan et al. | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 4 |
| Finn et al. | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 4 |
| Ramadugu et al. | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Rothgangel et al. | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
Note: Criterion 1: eligibility criteria were specified; criterion 2: subjects were randomly allocated to groups; criterion 3: allocation was concealed; criterion 4: at baseline, the most important prognostic indicators were similar between groups; criterion 5: blinding of all subjects; criterion 6: blinding of all therapists who administered the therapy; criterion 7: blinding of all assessors who measured at least one key outcome; criterion 8: measures of at least one key outcome were obtained from more than 85% of the subjects initially allocated to groups; criterion 9: all subjects for whom outcome measures were available received the treatment or control condition, as allocated; criterion 10: the results of between‐group statistical comparisons are reported for at least one key outcome; criterion 11: the study provides both point measures and variability measures for at least one key outcome.
3.3. Study characteristics
Three studies used a parallel group design (Brodie et al., 2007; Finn et al., 2017; Rothgangel et al., 2018) and two a cross‐over design (Chan et al., 2007; Ramadugu et al., 2017). In total, 256 patients were included, with sample sizes varying from 15 to 80 patients. Participant characteristics (age, location and cause of amputation, time of PLP onset since amputation, and PLP intensity at inclusion) varied among the studies (Table 3). Conversely, the type of exercise (i.e., the study intervention) and the control intervention (opaque sheet or mirrorless) were similar among studies. However, the duration, number and frequency of MT sessions varied (Table 4). The intervention was performed under the supervision of an examiner in four studies (Brodie et al., 2007; Chan et al., 2007; Finn et al., 2017; Ramadugu et al., 2017). PLP intensity was assessed with different tools: visual analogue scale (VAS; Brodie et al., 2007; Finn et al., 2017; Ramadugu et al., 2017), numeric rating scale (Rothgangel et al., 2018) and McGill Pain Questionnaire, original (Brodie et al., 2007) or shorter version (Ramadugu et al., 2017). Only one study assessed MT impact on the patients' disability and quality of life (Rothgangel et al., 2018). Disability was assessed with the Patient Specific Functional Scale (PSFS) and the Pain Disability Index (PDI). Quality of life was assessed with the EuroQol 5‐Dimensional Questionnaire (EQ‐5D‐5L) and two numeric rating scales that measure the impact of pain on sleep and mood.
TABLE 3.
Characteristics of the patients included in the selected studies
| Author, years | Pathology | Age (years), sex | PLP intensity and/or number of PLP episodes at recruitment | Interval between amputation and inclusion in the study |
|---|---|---|---|---|
| Brodie et al., 2007 |
Unilateral lower limb amputees Reason for amputation: congenital (1%), cancer (5%), accident (33%), medical (61%) |
N = 80; ♂ (79%) Mean age: 55 (20–83) |
— |
<2 weeks: 65% <1 year: 20% >1 year: 6% |
| Chan et al. (2007) | Unilateral lower limb amputees Reason for amputation: not specified | N = 22 | — | — |
| Finn et al. (2017) | Unilateral upper extremity amputees Reason for amputation: accident (100%) | N = 15; ♂ (100%) Mean age: 28.7 (19–68) | Episodes per week: 3 | Mean: 4.51 months (0.55–24) |
| Ramadugu et al. (2017) |
Unilateral lower limb (84%) and unilateral upper extremity amputees (16%) Reason for amputation: not specified Non‐inclusion criteria: traumatic brain injury or major psychiatric illness |
N = 64; ♂ (100%) Mean age: NA (17–62) | Intensity (VAS): 30 (10.7) | — |
| Rothgangel et al. (2018) | Unilateral lower limb amputees Reason for amputation: accident (33%), diabetes (11%), vascular problems (29%), tumour (13%), other (e.g., infection; 13%) | N = 75; ♂ (69%) Mean age: 61.1 (NP) | Episodes per week: at least one Intensity (VAS): 5.7 | Mean: 3 years |
Abbreviations: NA, not available; VAS, visual analogue scale.
TABLE 4.
Summary of the characteristics, intervention and outcomes of the selected studies
| Author, year, study type | Intervention group | Control intervention | Measurements: Primary outcome secondary outcome(s) | Procedure | Supervised intervention (yes/no) | Number of sessions and session duration | Fidelity to the programmed intervention |
|---|---|---|---|---|---|---|---|
|
Brodie et al. (2007) RCT—parallel group |
Mirror therapy N = 41 | Covered mirror N = 39 |
|
Ten movements with both limbs, each repeated 10 times, with a break between each movement type | Yes | Only one session | — |
| Chan et al. (2007) RCT‐crossover for the control group | Mirror therapy N = 6 (number of participants who completed the study) | Covered mirror N = 6 (number of participants who completed the study) |
|
Movements with both limbs | Yes |
15 minutes per day, for 8 weeks (for the control group, covered mirror the first 4 weeks then change to mirror therapy) |
At 4 weeks, all patients completed the study |
| Finn et al. (2017) RCT–parallel group | Mirror therapy N = 9 | Covered mirror N = 3 |
|
Both limbs perform slow movements with progressive increase in amplitude | Yes | 15 minutes per day, 5 days per week for 4 weeks | Due to lack of efficacy of the control treatment, all participants in the control group received the MT intervention after 11 sessions (planned change at 20 sessions initially) |
| Ramadugu et al. (2017) RCT‐crossover for the control group | Mirror therapy N = 32 | Covered mirror N = 28 |
|
Movements with both limbs | Yes | 15 minutes per day, for 4 weeks | All patients in the mirror group completed the study and 86% of participants in the control group completed the study |
| Rothgangel et al. (2018) RCT—parallel group | Mirror therapy N = 25 | Mirrorless N = 24 |
|
Intervention group: exercise program adapted to the patient's preferences with both limbs. ‐Observation of different positions ‐Motor exercises ‐Exercises using sensory stimuli (e.g. vibration, heat) ‐ Functional motor exercises with objects |
Yes, during the first 4 weeks. Then unsupervised for the next 6 weeks (home) | 10 sessions of at least 30 min/each for the first 4 sessions As many sessions as the patient wishes during the following 6 weeks | 84% of participants in the intervention group completed the study and 80% of participants in the control group completed the study |
Abbreviations: MPQ, McGill pain questionnaire; NPSI, Neuropathic Pain symptom inventory; NRS, numerical rating scale; PDI, pain disability index.; PSFS, patient‐specific functional scale; RCT, randomized‐controlled trial; SF‐MPQ, Short McGill pain questionnaire; VAS, visual analogue scale.
3.4. Synthesis of the results
The results obtained for each group and the conclusion on the primary outcome of each study are presented in Table 4.
3.4.1. Primary outcome: PLP intensity
Among the four studies with an intergroup comparison (Brodie et al., 2007; Chan et al., 2007;Ramadugu et al., 2017; Rothgangel et al., 2018), only one found a significant difference between MT group and control (placebo) group with a positive effect of MT at week 4 of follow‐up (Chan et al., 2007). Furthermore, in the study by Rothgangel et al. (2018) a subgroup analysis suggested clinically significant effects of MT on the mean PLP intensity in women and in patients with a motor component (cramps or unnatural phantom limb position) of PLP.
For intra‐group comparisons, three studies showed a significant decrease in pain in the MT group after the intervention (Chan et al., 2007; Finn et al., 2017; Ramadugu et al., 2017).
3.4.2. Secondary outcomes
PLP duration and frequency
The included studies did not find any inter‐group difference in PLP duration and frequency at 4 and 6 weeks of MT (Ramadugu et al., 2017; Rothgangel et al., 2018), but PLP duration was significantly reduced at 6 months in the MT group compared with placebo (Rothgangel et al., 2018). For intra‐group comparisons, three studies found that PLP duration and frequency were decreased in both (MT and control) groups (Finn et al., 2017; Ramadugu et al., 2017; Rothgangel et al., 2018).
Effect on disability and quality of life
Only the study by Rothgangel et al. assessed MT effect on the patients' disability level and quality of life. According to some PSFS items (daily living activities), disability was significantly reduced in the MT group at week 10 and 6 months (Rothgangel et al., 2018). Conversely, quality of life (EQ‐5D–5 L questionnaire) was comparable between groups.
4. DISCUSSION
Among the five studies included in this systematic review, three found that MT decreased PLP intensity, but mainly in the intra‐group comparison. Only Chan et al. (2007) found a specific positive effect of MT on PLP at week 4 compared with the control group, but this study has a low methodological quality score. One study reported an improvement of disability by MT through the reduction of PLP impact on daily living activities (Rothgangel et al., 2018). Thus, our systematic review did not allow concluding that MT reduces PLP intensity, frequency and duration, disability and quality of life.
The lack of strong evidence of MT efficiency in PLP is probably due to (i) the low methodological quality (Table 2), and (ii) the lack of statistical power of the included studies. Several studies included fewer than 10 patients per group (Chan et al., 2007; Finn et al., 2017) although Rothgangel et al. calculated that at least 30 patients per group were needed to detect a 2‐point difference, with high effect size (Cohen's d close to 0.8). As they did not detect any effect, the effect size for MT might be smaller (Cohen's d close to 0.5). This would imply that more than 50 patients per group should be recruited to detect an effect with 80% of power. Furthermore, intra‐group heterogeneity was high and several factors should have been considered, such as sex, type of prosthesis, ability to move the phantom limb, and presence of a PLP motor component (cramps or unnatural position of the phantom limb; Rothgangel et al., 2018). Future studies should take into account these factors when deciding the inclusion and exclusion criteria.
Our conclusion is similar to that of previous reviews (Aternali & Katz, 2019; Barbin et al., 2016), but differs from that of the two most recent meta‐analyses that reported a significant positive effect of MT on PLP (Wang et al., 2021; Xie et al., 2021). However, the methodological quality of these meta‐analyses raises questions. In the meta‐analysis by Xie et al. (2021), the risk of bias of the included studies (GRADE method) was not evaluated. Moreover, a fixed effect model was used instead of a random effect model that considers trials as different parameters. The observed statistical effect is questionable because the study that contributed to 54% of the conclusion had a low methodological quality (PEDro score = 2). A sensitivity analysis should have been carried out to assess the effect of removing low‐quality studies (Borenstein et al., 2010). In the meta‐analysis by Wang et al. (Wang et al., 2021) the conclusion displayed a large heterogeneity (I 2 > 80%), suggesting the need of a subgroup analysis. When this subgroup analysis was carried out, MT effect, compared with a covered mirror, disappeared. However, the authors concluded that MT is an effective technique to reduce PLP in amputees. On the basis of these different points, we disagree with the interpretation and conclusions of these two meta‐analyses.
Although evidence is lacking concerning MT effectiveness for PLP, the beneficial effects of MT in other conditions suggest that it may be useful also for PLP. The latest Cochrane review shows a significant effect of MT on motor function (effect size: 0.47 [0.27, 0.67]) following stroke (Thieme et al., 2018), and a meta‐analysis found an effect of MT on pain associated with post‐stroke complex regional pain syndrome (Duong et al., 2018). In these reviews, the MT intervention modalities were more intense (30‐minute sessions, 5 times per week) than those used in the five studies included in this review (Table 4; Thieme et al., 2018). Therefore, increasing the MT session frequency and duration (per week and/or for a longer period) might influence brain plasticity and consequently PLP (Andoh et al., 2017; Flor, 2008; Flor et al., 2006; Kuner & Flor, 2016). It has also been shown that MT effects last for several months after treatment, particularly on motor functions, daily living activities and pain (Sütbeyaz et al., 2007). In agreement, Rothgangel et al. found that MT still modulated pain episode frequency at 6 months of follow‐up (Rothgangel et al., 2018). This could be due to its stabilizing effect on brain plasticity.
4.1. Limitations and strengths
This review presents some limitations. First, we limited the search to five databases, and the selected articles had to be in French or English. Second, we did not register our study in the PROSPERO database, although we followed a pre‐established protocol (wrote in French) before the start of our study that was approved by the ethics committee. Third, during the literature search, we identified many study protocols to address the research question, but the results of these trials have not been published yet, or they might have been negative, and the authors did not try to publish them (publication bias).
The strengths of this review are: (i) we followed the PRISMA reporting recommendations, (ii) we only included studies with a placebo control group, (iii) we assessed the methodology quality of the studies with the PEDro scale, and (iv) we interpreted their results in function of the PEDro scores. We also assessed secondary outcomes: pain frequency and duration, disability and quality of life.
5. CONCLUSION
The results obtained in this systematic review do not allow us to conclude that MT reduces PLP intensity, frequency, duration or disability. Future clinical studies should enrol a larger number of patients to increase the statistical power, use a covered mirror or mirrorless exercises as placebo, intensify MT sessions and frequency (Duong et al., 2018; Thieme et al., 2018), include a long‐term follow‐up (Rothgangel et al., 2018), reduce intra‐group heterogeneity (sex, prosthesis type, ability to move the phantom limb, presence of a PLP motor component), improve their methodological quality (PEDro scale), and evaluate also other criteria (e.g. duration and frequency of painful episodes, disability and quality of life). Other study designs could be considered, for instance, single‐case experimental design (SCED) studies that are adapted to rare diseases with a variety of specific and clinical situations (Ganz & Ayres, 2018; Krasny‐Pacini & Evans, 2018; Smith, 2012; Tate et al., 2008).
AUTHOR CONTRIBUTIONS
All authors contributed to the study conception and design. NR and LR extracted data from the trials and evaluated the inclusion and exclusion criteria. NR, LR and MG assessed the methodological quality of the trials. All authors contributed to the interpretation of the data and drafted the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interests related to this manuscript.
ACKNOWLEDGEMENTS
We thank Elisabetta ANDERMARCHER and Kathlyne DUPUIS MAURIN for their careful proofreading.
Guémann, M. , Olié, E. , Raquin, L. , Courtet, P. , & Risch, N. (2023). Effect of mirror therapy in the treatment of phantom limb pain in amputees: A systematic review of randomized placebo‐controlled trials does not find any evidence of efficacy. European Journal of Pain, 27, 3–13. 10.1002/ejp.2035
REFERENCES
- Alviar, M. J. M. , Hale, T. , & Dungca, M. (2016). Pharmacologic interventions for treating phantom limb pain. Cochrane Database of Systematic Reviews, 10, CD006380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh, J. , Diers, M. , Milde, C. , Frobel, C. , Kleinböhl, D. , & Flor, H. (2017). Neural correlates of evoked phantom limb sensations. Biological Psychology, 126, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aternali, A. , Katz, J. (2019). Recent advances in understanding and managing phantom limb pain [version 1; peer review: 2 approved]. F1000Research 8. [DOI] [PMC free article] [PubMed]
- Barbin, J. , Seetha, V. , Casillas, J. M. , Paysant, J. , & Pérennou, D. (2016). The effects of mirror therapy on pain and motor control of phantom limb in amputees: A systematic review. Annals of Physical and Rehabilitation Medicine, 59, 270–275. [DOI] [PubMed] [Google Scholar]
- Batsford, S. , Ryan, C. G. , & Martin, D. J. (2017). Non‐pharmacological conservative therapy for phantom limb pain: A systematic review of randomized controlled trials. Physiotherapy Theory and Practice, 33, 173–183. [DOI] [PubMed] [Google Scholar]
- Borenstein, M. , Hedges, L. V. , Higgins, J. P. T. , & Rothstein, H. R. (2010). A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Research Synthesis Methods, 1, 97–111. [DOI] [PubMed] [Google Scholar]
- Brodie, E. E. , Whyte, A. , & Niven, C. A. (2007). Analgesia through the looking‐glass? A randomized controlled trial investigating the effect of viewing a ‘virtual’ limb upon phantom limb pain, sensation and movement. European Journal of Pain, 11, 428–436. [DOI] [PubMed] [Google Scholar]
- Brosseau, L. , Laroche, C. , Sutton, A. , Guitard, P. , King, J. , Poitras, S. , Casimiro, L. , Tremblay, M. , Cardinal, D. , Cavallo, S. , Laferrière, L. , Grisé, I. , Marshall, L. , Smith, J. R. , Lagacé, J. , Pharand, D. , Galipeau, R. , Toupin‐April, K. , Loew, L. , … Vaillancourt, V. (2015). Une version franco‐canadienne de la physiotherapy evidence database (PEDro) scale: L'Échelle PEDro. Physiotherapy Canada, 67, 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, B. L. , Da, B. , Jb, W. , Jj, C. , & Jt, H. (2007). Mirror therapy for phantom limb pain. The New England Journal of Medicine, 2, 2206–2207. [DOI] [PubMed] [Google Scholar]
- Hsu, E. , & Cohen, S. P. (2013). Postamputation pain: Epidemiology, mechanisms, and treatment. Journal of Pain Research, 6, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumanian, G. A. , Potter, B. K. , Mioton, L. M. , Ko, J. H. , Cheesborough, J. E. , Souza, J. M. , Ertl, W. J. , Tintle, S. M. , Nanos, G. P. , Valerio, I. L. , Kuiken, T. A. , Apkarian, A. V. , Porter, K. , & Jordan, S. W. (2019). Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: A randomized clinical trial. Annals of Surgery, 270, 238–246. [DOI] [PubMed] [Google Scholar]
- Duong, S. , Bravo, D. , Todd, K. J. , Finlayson, R. J. , & Tran, D. Q. (2018). Treatment of complex regional pain syndrome: An updated systematic review and narrative synthesis. Canadian Journal of Anesthesia, 65, 658–684. [DOI] [PubMed] [Google Scholar]
- Finn, S. B. , Perry, B. N. , Clasing, J. E. , Walters, L. S. , Jarzombek, S. L. , Curran, S. , Rouhanian, M. , Keszler, M. S. , Hussey‐Andersen, L. K. , Weeks, S. R. , Pasquina, P. F. , & Tsao, J. W. (2017). A randomized, controlled trial of Mirror therapy for upper extremity phantom limb pain in male amputees. Frontiers in Neurology, 8, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor, H. (2002). Phantom‐limb pain: Characteristics, causes, and treatment. Lancet Neurology, 1, 182–189. [DOI] [PubMed] [Google Scholar]
- Flor, H. (2008). Maladaptive plasticity, memory for pain and phantom limb pain: Review and suggestions for new therapies. Expert Review of Neurotherapeutics, 8, 809–818. [DOI] [PubMed] [Google Scholar]
- Flor, H. , Nikolajsen, L. , & Jensen, T. S. (2006). Phantom limb pain: A case of maladaptive CNS plasticity? Nature Reviews. Neuroscience, 7, 873–881. [DOI] [PubMed] [Google Scholar]
- Foell, J. , Bekrater‐Bodmann, R. , Diers, M. , & Flor, H. (2014). Mirror therapy for phantom limb pain: Brain changes and the role of body representation: Mirror therapy for phantom limb pain. European Journal of Pain, 18, 729–739. [DOI] [PubMed] [Google Scholar]
- Ganz, J. B. , & Ayres, K. M. (2018). Methodological standards in single‐case experimental design: Raising the bar. Research in Developmental Disabilities, 79, 3–9. [DOI] [PubMed] [Google Scholar]
- Herr, H. M. , Clites, T. R. , Srinivasan, S. , Talbot, S. G. , Dumanian, G. A. , Cederna, P. S. , & Carty, M. J. (2020). Reinventing extremity amputation in the era of functional limb restoration. Ann Surg Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- Herrador Colmenero, L. , Perez Marmol, J. M. , Martí‐García, C. , de Querol Zaldivar, M. , Los, Á. , Tapia Haro, R. M. , Castro Sánchez, A. M. , & Aguilar‐Ferrándiz, M. E. (2018). Effectiveness of mirror therapy, motor imagery, and virtual feedback on phantom limb pain following amputation: A systematic review. Prosthetics and Orthotics International, 42, 288–298. [DOI] [PubMed] [Google Scholar]
- Knotkova, H. , Cruciani, R. A. , Tronnier, V. M. , & Rasche, D. (2012). Current and future options for the management of phantom‐limb pain. Journal of Pain Research, 5, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasny‐Pacini, A. , & Evans, J. (2018). Single‐case experimental designs to assess intervention effectiveness in rehabilitation: A practical guide. Annals of Physical and Rehabilitation Medicine, 61, 164–179. [DOI] [PubMed] [Google Scholar]
- Kuner, R. , & Flor, H. (2016). Structural plasticity and reorganisation in chronic pain. Nature Reviews. Neuroscience, 18, 20–30. [DOI] [PubMed] [Google Scholar]
- Liberati, A. , Altman, D. G. , Tetzlaff, J. , Mulrow, C. , Gøtzsche, P. C. , Ioannidis, J. P. A. , Clarke, M. , Devereaux, P. J. , Kleijnen, J. , & Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: Explanation and elaboration. Journal of Clinical Epidemiology, 62, e1–e34. [DOI] [PubMed] [Google Scholar]
- Makin, T. R. , Scholz, J. , Filippini, N. , Henderson Slater, D. , Tracey, I. , & Johansen‐Berg, H. (2013). Phantom pain is associated with preserved structure and function in the former hand area. Nature Communications, 4, 1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, R. A. , Fisher, E. , & Eccleston, C. (2022). Systematic reviews do not (yet) represent the ‘gold standard’ of evidence: A position paper. European Journal of Pain, 26, 557–566. [DOI] [PubMed] [Google Scholar]
- Nikolajsen, L. , & Jensen, T. S. (2001). Phantom limb pain. British Journal of Anaesthesia, 87, 107–116. [DOI] [PubMed] [Google Scholar]
- Niraj, S. , & Niraj, G. (2014). Phantom limb pain and its psychologic management: A critical review. Pain Management Nursing, 15, 349–364. [DOI] [PubMed] [Google Scholar]
- Perry, B. N. (2013). Twenty‐third meeting of the European neurological society, 8–11 June, 2013 Barcelona, Spain. Journal of Neurology, 260, 1–280. [DOI] [PubMed] [Google Scholar]
- Plumbe, L. , Peters, S. , Bennett, S. , Vicenzino, B. , Coppieters, M.W. (2013). Mirror therapy, graded motor imagery and virtual illusion for the management of chronic pain. In The Cochrane collaboration (Ed.), Cochrane database of systematic reviews. John Wiley & Sons, Ltd. CD010329. [Google Scholar]
- Ramachandran, V. S. (1996). Synaesthesia in phantom limbs induced with mirrors. Proceedings of the Biological Sciences, 263(1369), 377–386. [DOI] [PubMed] [Google Scholar]
- Ramachandran, V. S. , & Altschuler, E. L. (2009). The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain, 132, 1693–1710. [DOI] [PubMed] [Google Scholar]
- Ramadugu, S. , Nagabushnam, S. C. , Katuwal, N. , & Chatterjee, K. (2017). Intervention for phantom limb pain: A randomized single crossover study of mirror therapy. Indian Journal of Psychiatry, 59, 457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothgangel, A. , Braun, S. , Winkens, B. , Beurskens, A. , & Smeets, R. (2018). Traditional and augm ented reality mirror therapy for patients with chronic phantom limb pain (PACT study): Results of a three‐group, multicentre single‐blind randomized controlled trial. Clinical Rehabilitation, 32, 1591–1608. [DOI] [PubMed] [Google Scholar]
- Rothgangel, A. S. , Braun, S. M. , Beurskens, A. J. , Seitz, R. J. , & Wade, D. T. (2011). The clinical aspects of mirror therapy in rehabilitation: A systematic review of the literature. International Journal of Rehabilitation Research, 34, 1–13. [DOI] [PubMed] [Google Scholar]
- van der Schans, C. P. , Geertzen, J. H. B. , Schoppen, T. , & Dijkstra, P. U. (2002). Phantom pain and health‐related quality of life in lower limb amputees. Journal of Pain and Symptom Management, 24, 429–436. [DOI] [PubMed] [Google Scholar]
- Schone, H. R. , Baker, C. I. , Katz, J. , Nikolajsen, L. , Limakatso, K. , Flor, H. , & Makin, T. R. (2022). Making sense of phantom limb pain. Journal of Neurology, Neurosurgery, and Psychiatry, 93, 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha, R. , van den Heuvel, W. J. , & Arokiasamy, P. (2011). Factors affecting quality of life in lower limb amputees. Prosthetics and Orthotics International, 35, 90–96. [DOI] [PubMed] [Google Scholar]
- Smith, J. D. (2012). Single‐case experimental designs: A systematic review of published research and current standards. Psychological Methods, 17, 510–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sütbeyaz, S. , Yavuzer, G. , Sezer, N. , & Koseoglu, B. F. (2007). Mirror therapy enhances lower‐extremity motor recovery and motor functioning after stroke: A randomized controlled trial. Archives of Physical Medicine and Rehabilitation, 88, 555–559. [DOI] [PubMed] [Google Scholar]
- Tate, R. L. , Mcdonald, S. , Perdices, M. , Togher, L. , Schultz, R. , & Savage, S. (2008). Rating the methodological quality of single‐subject designs and n‐of‐1 trials: Introducing the single‐case experimental design (SCED) scale. Neuropsychological Rehabilitation, 18, 385–401. [DOI] [PubMed] [Google Scholar]
- Thieme, H. , Morkisch, N. , Mehrholz, J. , Pohl, M. , Behrens, J. , Borgetto, B. , & Dohle, C. (2018). Mirror therapy for improving motor function after stroke. In 2018. Mirror therapy for improving motor function after stroke. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieme, H. , Morkisch, N. , Rietz, C. , Dohle, C. , & Borgetto, B. (2016). The efficacy of movement representation techniques for treatment of limb pain—A systematic review and meta‐analysis. The Journal of Pain, 17, 167–180. [DOI] [PubMed] [Google Scholar]
- Wang, F. , Zhang, R. , Zhang, J. , Li, D. , Wang, Y. , Yang, Y.‐H. , & Wei, Q. (2021). Effects of mirror therapy on phantom limb sensation and phantom limb pain in amputees: A systematic review and meta‐analysis of randomized controlled trials. Clinical Rehabilitation, 35, 1710–1721. [DOI] [PubMed] [Google Scholar]
- Woolf, C. J. , & Mannion, R. J. (1999). Neuropathic pain: Aetiology, symptoms, mechanisms, and management. The Lancet, 353, 1959–1964. [DOI] [PubMed] [Google Scholar]
- Woolf, C. J. , Shortland, P. , & Coggeshall, R. E. (1992). Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature, 355, 75–78. [DOI] [PubMed] [Google Scholar]
- World Health Organization, World Bank (2011). World report on disability 2011. https://apps.who.int/iris/handle/10665/44575 [PubMed] [Google Scholar]
- Xie, H.‐M. , Zhang, K.‐X. , Wang, S. , Wang, N. , Wang, N. , Li, X. , & Huang, L.‐P. (2021). Effectiveness of Mirror therapy for phantom limb pain: A systematic review and meta‐analysis. Archives of Physical Medicine and Rehabilitation S0003999321013794, 102, 1775–1787. [DOI] [PubMed] [Google Scholar]


