ABSTRACT
Background
Kidney disease registries typically report populations incident to kidney replacement therapy (KRT) after excluding reversible disease. Registry-based audit and quality assurance is thus based on populations depleted of those with the highest early mortality. It is now mandatory for UK kidney units to report all recipients of dialysis, both acute and chronic. This work presents 90-day survival and recovery outcomes for all reported adults.
Methods
Seventy adult centres reporting to the UK Renal Registry were included. Those assessed as underreporting death and recovery were excluded. Survival was evaluated using a Kaplan–Meier estimator. Cox regression was used to describe hazard ratios (HRs) for age, sex and acute/chronic dialysis coding on day 1. Analysis of all-cause 90-day mortality with recovery as a competing risk is presented.
Results
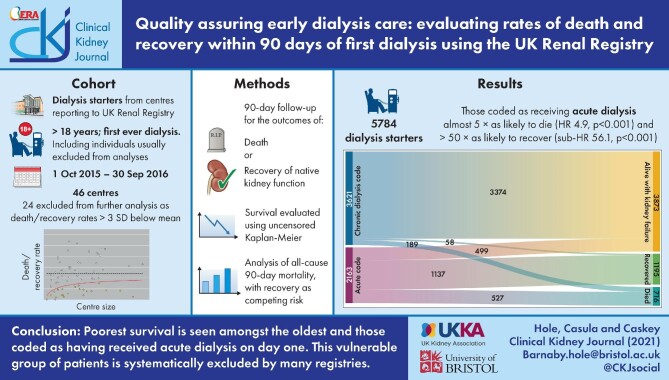
Twenty-four centres were assessed as underreporting, with rates of death/recovery below the 99.7th centile. Of 5784 dialysis starters in the remaining 46 centres, 2163 (37.4%) were coded as receiving acute dialysis on day 1. Ninety days after starting, 3860 (66.7%) of all starters were receiving KRT, 1157 (20.0%) were alive having stopped, 716 (12.4%) were dead and 51 (0.9%) were lost to follow-up. Mortality was higher among those coded as receiving acute dialysis on day 1 (HR 4.88, P < 0.001). The sub-HR for recovery among those coded as receiving acute compared with chronic dialysis was 56.14 (P < 0.001).
Conclusions
Death and recovery rates are substantially higher than reported in conventional incident populations. This work highlights a vulnerable subgroup of patients largely overlooked by most national quality assurance systems.
Keywords: AKD, dialysis, recovery, registry, survival analysis
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Data on kidney disease and treatment rates can be used to advocate for changes aimed at reducing the burden of kidney disease in individuals and populations [1–3]. Most high-income countries have some form of kidney health surveillance system, usually in the form of a kidney replacement therapy (KRT) registry [4]. Many low- and middle-income countries are yet to implement the capture of data, while the human and economic impacts of kidney failure are highest in these settings [2, 5]. Kidney registries report outcomes for an ‘incident population’ of individuals who started KRT, usually applying criteria to identify those with kidney failure believed to be chronic [6–8]. Inclusion of all individuals still receiving KRT 90 days after initiation is widely accepted. Some registries also include individuals who died before day 90 if a clinical code indicates that their kidney failure was believed to be chronic. Individuals with acute kidney disease (AKD) represent an important and vulnerable subgroup whose care only features in registry data if their kidney failure is considered irreversible and they are reported to the registry.
Those who start KRT and subsequently recover native kidney function (NKF) can be identified in real time or retroactively. Predicting the reversibility of an individual's kidney failure on day 1 is an individual clinical judgement, and some whose kidney failure is irreversible are likely to die without a code indicating chronicity. Exclusion of individuals who die without a code indicating chronicity limits the options for quality assurance for an important group of patients. This is a problem faced by all chronic dialysis surveillance systems and the proportion of such individuals is likely to vary between countries and centres.
Furthermore, where KRT initiation is manually captured, entry may be made contemporaneously or long after initiation—by which time an individual may have recovered, died or been diagnosed with irreversible kidney failure [9]. Centres where timeline entries are made retrospectively may label dialysis starters differently from centres that code in ‘real time’.
As such, the population of individuals conventionally reported to have started dialysis in each kidney centre is sensitive to the availability and practice patterns of nephrology and critical care units, coding practices and completeness of reporting. Centre-level variation in case mix and reporting practices is likely to drive systematic differences in reporting of KRT initiation in the contexts of AKD, late presentation and critical illness [10, 11].
Survival statistics derived from conventional ‘incident populations’ of individuals deemed to require chronic dialysis dominate the kidney literature. While these may generalize to a subgroup of individuals making planned starts on dialysis, they cannot be applied to other subgroups that are only partially reported to registries. The primary aim of this work is to describe 90-day survival and NKF recovery for all UK adults reported as having had a first dialysis to the UK Renal Registry (UKRR), where reporting of all starters has been mandatory since 2009. It is hoped that this will stimulate discussion about how kidney health surveillance systems can be enhanced to quality assure the complete dialysis experience.
MATERIALS AND METHODS
The UKRR is part of the UK Kidney Association and provides independent audit of kidney care in the UK, where KRT can be initiated in any of 71 National Health Service (NHS) adult kidney centres or 263 adult critical care units. The UKRR acquires data on KRT initiation by direct extraction from electronic health records held by kidney centres in England and Wales and via the Scottish Renal Registry. The UKRR does not extract data from critical care units, where KRT for AKD is usually overseen by critical care specialists [12]. The UKRR is funded directly by participating kidney units through an annual fee per patient registered. The information technology (IT) infrastructure and data provision are not incentivized by the NHS, although reporting is mandated. The data reported to the UKRR are for audit and quality assurance and are not linked with reimbursement.
Since 2009, clinicians working in UK kidney centres have been required to record the date of initiation of first dialysis or haemofiltration for all individuals receiving KRT under their care using the ‘timeline’ field of their renal IT system [13]. Separate codes are used to indicate whether an individual started ‘acute’ dialysis or dialysis ‘deemed to be chronic’ and subsequent codes are used to indicate when an individual transitioned to needing ‘chronic’ dialysis or recovered NKF [Box 1]. The UKRR derives an annual incident population deemed to require chronic dialysis of ∼8000, but this includes only three quarters of those reported to the registry as having started KRT [14]. The UKRR timeline codes provide a unique dataset to describe early survival and recovery outcomes. Initiation data include those who made planned starts in the context of chronic kidney disease (CKD), those who made unplanned starts under nephrology care and those who started in critical care and still required KRT on transfer. No data are available regarding the reason for initiation or to indicate whether the first session was on a critical care unit.
Box 1.
Timeline codes used by centres reporting to the UKRR to record the date of first dialysis or haemofiltration (acute and chronic dialysis codes) and indicate whether an individual recovered NKF or had enduring kidney failure. *This is not an exhaustive list—for further details and a full list of treatment codes, visit www.renalreg.org/datasets. [13].
Acute dialysis codes
81 Acute haemodialysis
82 Acute haemofiltration
83 Acute peritoneal dialysis
Example codes indicating kidney failure ‘deemed to be chronic’*
1 Haemodialysis
3 Haemodiafiltration
11 Continuous ambulatory peritoneal dialysis
12 Automated peritoneal dialysis
For individuals who start with an acute code, a separate code must subsequently indicate
84 recovered NKF
85 stopped dialysis without recovery of NKF
Kidney failure ‘deemed to be chronic’ (codes above, plus transplantation)
Routinely collected data were included from 70 of the 71 adult kidney centres providing individual patient records to the UKRR. (One centre did not provide individual patient-level data for the period of study.) Individuals were included if they were >18 years of age and were reported by an adult kidney unit as having received their first-ever haemodialysis/haemodiafiltration/haemofiltration or peritoneal dialysis session between 1 October 2015 and 30 September 2016. Thereafter, analysis was based on KRT receipt, without consideration for modality. Those who received a kidney transplant within 90 days of dialysis initiation were included, but individuals who were pre-emptively transplanted were excluded. Date of death was identified from linkage with the NHS Digital Spine (IT partner to the UK Health and Social Care system [15]). Since KRT may be discontinued without recovery of NKF, recovery was assigned in the following three settings: where a treating centre provided a timeline code indicating recovery of NKF and KRT was not restarted within 90 days (irrespective of date of death); where an individual discontinued dialysis and was alive without restarting KRT 90 days later; and where an individual lost to follow-up from kidney centre extracts had no linked death certification data within 90 days. All other individuals who discontinued KRT were classified as not having recovered NKF. A 90-day period was chosen to reflect the disease duration definition for AKD/CKD [16]. A sensitivity analysis was also conducted using 21 days in place of the 90-day period in all three rules, chosen to approximate survival following withdrawal of dialysis in the absence of NKF recovery [17–20].
Routinely collected clinical and demographic characteristics for all individuals reported to have started dialysis are described. Individuals’ statuses on the 90th day after dialysis initiation (receiving KRT, not receiving KRT, dead, lost to follow-up) and by recovery of NKF are also shown. Individuals were stratified by age and sex and by whether they were coded as receiving acute or chronic dialysis on day 1. Individuals coded as receiving acute dialysis on day 1 were further stratified by whether a later code indicated their kidney failure was subsequently deemed to be chronic.
To identify sites likely to be systematically underreporting KRT initiation, a centre ‘filter’ was applied. The combined rate of ‘death and NKF recovery’ in the 90 days after dialysis initiation was used as an index of dialysis initiation among individuals experiencing AKD, late presentation or critical illness. Kidney centres that fell below the 99.7th centile for death/NKF recovery were excluded from the filtered dataset.
The filtered dataset was used for the following survival and recovery analyses, all based on KRT continuation/discontinuation, irrespective of modality. Transplantation was neither censored for nor treated as a competing event. Survival was evaluated using a Kaplan–Meier estimator, with and without censoring at recovery of NKF. Cox regression was used to describe hazard ratios (HRs) for age, sex and acute/chronic dialysis coding on day 1. Recovery of NKF cannot be assumed to be a random, independent event when analysing death as the event of interest (i.e. those who recovered NKF cannot be assumed to have had the same risk of death as those still receiving KRT). As such, a competing risk analysis of all-cause 90-day mortality, with recovery of NKF as a competing risk, was conducted. The cumulative incidence of death by day 90 was calculated, stratifying by age and acute/chronic dialysis coding on day 1.
All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA) and Stata/MP version 12 (StataCorp, College Station, TX, USA) within the UKRR. Approval to conduct this work using the UKRR's audit and research permissions was granted by the UKRR's Research Methods Study Group. The UKRR has Human Research Authority (HRA) Confidentiality Advisory Group section 251 approvals to perform both audit and research analyses, as well as HRA Research Ethics Committee approval of its research database.
RESULTS
Full dataset
A total of 8671 dialysis starters were identified between 1 October 2015 and 30 September 2016, with centres contributing between 11 and 374 individuals. The median age was 66.4 years [interquartile range (IQR) 53.5–75.9] and 63.3% were male (Table 1). Of the 8671 starters, 2436 (28.1%) were coded as receiving acute dialysis on day 1. The acute dialysis proportion ranged between 0% and 66% [median 27 (IQR 9–39)] by centre. Ninety days after starting dialysis, 6506 individuals (75.0%) were alive and receiving KRT, 1257 (14.5%) were alive and having stopped KRT, 848 (9.8%) were dead, 60 (0.7%) had been lost to follow-up and 1429 (16.5%) were classified as having recovered NKF, of whom 134 (9.4%) were dead by day 90 (see Supplementary data). Using a 21-day period in place of the 90-day period had a marginal influence on the number of individuals classified as having recovered NKF, so the 90-day period was maintained for all analyses (see Supplementary data).
Table 1.
Clinical, demographic and outcome data for the 8671 individuals reported by the 70 centres to have received their first-ever dialysis session between 1 October 2015 and 30 September 2016
| Coded as receiving acute dialysis on day 1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Coded as receiving chronic dialysis on day 1 | All | Coded as chronic by day 90 | Not coded as chronic by day 90 | ||||||
| Characteristics | N | % | n | % | N | % | n | % | n | % |
| Total | 8671 | 100.0 | 6235 | 71.9 | 2436 | 28.1 | 684 | 7.9 | 1752 | 20.2 |
| Age (years), median (IQR) | 66.4 (53.5–75.9) | 65.1 (52.4–74.9) | 69.0 (57–77.5) | 69.6 (57.9–78.1) | 67.5 (54.9–75.9) | |||||
| 18–59 | 3156 | 36.4 | 2430 | 39.0 | 726 | 29.8 | 237 | 34.6 | 489 | 27.9 |
| 60–74 | 3167 | 36.5 | 2247 | 36.0 | 920 | 37.8 | 267 | 39.0 | 653 | 37.3 |
| ≥75 | 2348 | 27.1 | 1558 | 25.0 | 790 | 32.4 | 180 | 26.3 | 610 | 34.8 |
| Male | 5491 | 63.3 | 3938 | 63.2 | 1553 | 63.8 | 450 | 65.8 | 1103 | 63.0 |
| White* | 5984 | 78.7 | 4207 | 75.4 | 1777 | 87.8 | 520 | 79.5 | 1257 | 91.8 |
| % missing data | 1067 | 12.3 | 655 | 10.5 | 412 | 16.9 | 30 | 4.4 | 382 | 21.8 |
| Status 90 days after initiating dialysis | ||||||||||
| Alive on KRT | 6506 | 75.0 | 5824 | 93.4 | 682 | 28.0 | 609 | 89.0 | 73 | 4.2 |
| Alive off KRT | 1257 | 14.5 | 99 | 1.6 | 1158 | 47.5 | 33 | 4.8 | 1125 | 64.2 |
| Dead | 848 | 9.8 | 297 | 4.8 | 551 | 22.6 | 42 | 6.1 | 509 | 29.1 |
| Lost to follow-up | 60 | 0.7 | 15 | 0.2 | 45 | 1.8 | 0 | 0.0 | 45 | 2.6 |
| Recovery of NKF | ||||||||||
| Alive without recovery of NKF | 6528 | 75.3 | 5836 | 93.6 | 692 | 28.4 | 611 | 89.3 | 81 | 4.6 |
| Alive following recovery of NKF** | 1295 | 14.9 | 102 | 1.6 | 1193 | 49.0 | 31 | 4.5 | 1162 | 66.3 |
| Dead following recovery of NKF | 134 | 1.5 | 1 | 0.0 | 133 | 5.5 | 0 | 0.0 | 133 | 7.6 |
| Dead without recovery of NKF | 714 | 8.2 | 296 | 4.7 | 418 | 17.2 | 42 | 6.1 | 376 | 21.5 |
Age is presented both as median age and categorized as <60, 60–74 and ≥75. Ethnicity is presented as % White. NKF recovery was assigned in the following three settings: where a treating centre provided a timeline code indicating recovery of NKF and KRT was not restarted within 90 days (irrespective of date of death), where an individual discontinued dialysis and was alive without restarting KRT 90 days later and where an individual was lost to follow-up but was shown to be alive 90 days after dialysis initiation. All other individuals were assumed to not have recovered NKF. *Percentages in those with ethnicity data. **Individuals lost to follow-up, but alive at day 90 were presumed to be alive following recovery of NKF.
Filtered dataset
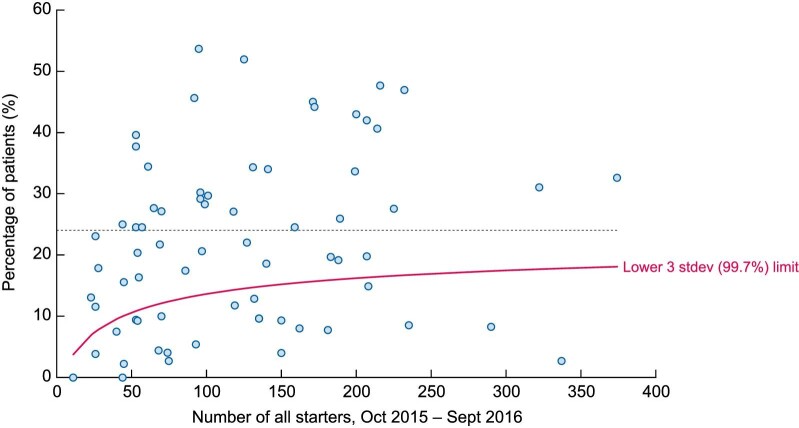
Of the 70 centres, 24 (34%) were identified as reporting rates of death/NKF recovery below the 99.7th centile (Figure 1). A total of 5784 dialysis starters (66.7% of the original cohort) were reported to have started dialysis in the 46 centres remaining in the filtered dataset. Centres reported initiation of 23 to 374 individuals. The median age was 67.6 years (IQR 54.4–76.6) and 63.9% were male (Table 2). Higher rates of white ethnicity (85.1% vs 78.7%) were seen in the filtered dataset, likely reflective of differences in the populations served by the included centres. Of the 5784 starters in the filtered dataset, 2163 (37.4%) were coded as receiving acute dialysis on day 1. The acute dialysis proportion ranged between 0% and 66% [median 32 (IQR 26–42)] by centre. Ninety days after starting dialysis, 3860 individuals (66.7%) were alive and receiving KRT, 1157 (20.0%) were alive having stopped KRT, 716 (12.4%) were dead, 51 (0.9%) had been lost to follow-up and 1326 (22.9%) were classified as having recovered NKF, of whom 131 (9.9%) were dead by day 90. By day 90, 73 individuals had received a transplant, of whom one died.
FIGURE 1:
Funnel plot displaying the percentages of individuals who died or recovered NKF within 90 days of starting dialysis. Data are included for all individuals starting dialysis between 1 October 2015 and 30 September 2016 in 70 UK kidney centres. Recovery of NKF classified using the 90-day window. All 24 centres that fell below the 99.7th limit were deemed ‘underreporters’ and excluded from further analysis. Separate funnel plots for death and NKF recovery are presented in the Supplementary material. No adjustment was made for the case mix reported at the centre level.
Table 2.
Clinical, demographic and outcome data for the 5784 individuals in the 46 centres in the filtered dataset reported to have received their first-ever dialysis session between 1 October 2015 and 30 September 2016
| Coded as receiving acute dialysis on day 1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Coded as receiving chronic dialysis on day 1 | All | Coded as chronic by day 90 | Not coded as chronic by day 90 | ||||||
| Characteristics | N | % | n | % | N | % | n | % | n | % |
| Total | 5784 | 100.0 | 3621 | 62.6 | 2163 | 37.4 | 483 | 8.4 | 1680 | 29.1 |
| Age (years), median (IQR) | 67.6 (54.4–76.6) | 66.1 (53.0–75.8) | 69.2 (57.4–77.8) | 68.5 (55.1–76.5) | 69.5 (57.9–78.1) | |||||
| 18–59 | 1994 | 34.5 | 1372 | 37.9 | 622 | 28.8 | 157 | 32.5 | 465 | 27.7 |
| 60–74 | 2089 | 36.1 | 1273 | 35.2 | 816 | 37.7 | 189 | 39.1 | 627 | 37.3 |
| ≥75 | 1701 | 29.4 | 976 | 27.0 | 725 | 33.5 | 137 | 28.4 | 588 | 35.0 |
| Male | 3695 | 63.9 | 2310 | 63.8 | 1385 | 64.0 | 322 | 66.7 | 1063 | 63.3 |
| White* | 4431 | 85.1 | 2848 | 82.5 | 1583 | 90.1 | 381 | 83.9 | 1202 | 92.3 |
| % missing data | 577 | 10.0 | 170 | 4.7 | 407 | 18.8 | 29 | 6.0 | 378 | 22.5 |
| Status 90 days after initiating dialysis | ||||||||||
| Alive on KRT | 3860 | 66.7 | 3370 | 93.1 | 490 | 22.7 | 428 | 88.6 | 62 | 3.7 |
| Alive off KRT | 1157 | 20.0 | 55 | 1.5 | 1102 | 50.9 | 22 | 4.6 | 1080 | 64.3 |
| Dead | 716 | 12.4 | 189 | 5.2 | 527 | 24.4 | 33 | 6.8 | 494 | 29.4 |
| Lost to follow up | 51 | 0.9 | 7 | 0.2 | 44 | 2.0 | 0 | 0.0 | 44 | 2.6 |
| Recovery of NKF | ||||||||||
| Alive without recovery of NKF | 3873 | 67.0 | 3374 | 93.2 | 499 | 23.1 | 430 | 89.0 | 69 | 4.1 |
| Alive following recovery of NKF** | 1195 | 20.7 | 58 | 1.6 | 1137 | 52.6 | 20 | 4.1 | 1117 | 66.5 |
| Dead following recovery of NKF | 131 | 2.3 | 0 | 0.0 | 131 | 6.1 | 0 | 0.0 | 131 | 7.8 |
| Dead without recovery of NKF | 585 | 10.1 | 189 | 5.2 | 396 | 18.3 | 33 | 6.8 | 363 | 21.6 |
Age is presented both as median age and categorized as <60, 60–74 and ≥75. Ethnicity is presented as % White. NKF recovery was assigned in the following three settings: where a treating centre provided a timeline code indicating recovery of NKF and KRT was not restarted within 90 days (irrespective of date of death), where an individual discontinued dialysis and was alive without restarting KRT 90 days later and where an individual was lost to follow-up but was shown to be alive 90 days after dialysis initiation. All other individuals were assumed to not have recovered NKF. *Percentages in those with ethnicity data. **Individuals lost to follow-up but alive at day 90 were presumed to be alive following recovery of NKF.
Of those coded as receiving chronic dialysis on day 1, 3370 (93.1%) were alive and receiving KRT at day 90, 55 (1.5%) were alive having stopped KRT, 189 (5.2%) were dead and 7 (0.2%) had been lost to follow-up. Of those coded as receiving acute dialysis, 490 (22.7%) were alive receiving KRT, 1102 (50.9%) were alive having stopped KRT, 527 (22.4%) were dead and 44 (2.0%) had been lost to follow-up.
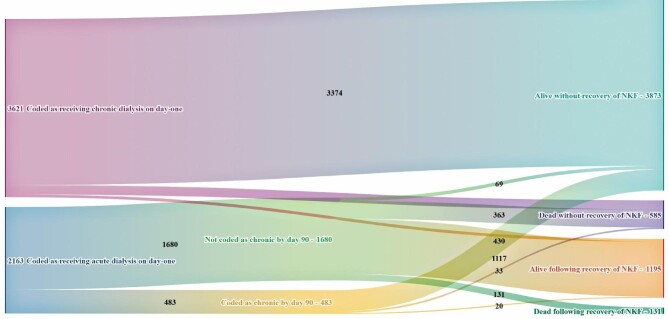
Of the 3873 individuals who were alive and had not recovered NKF by day 90, 499 (12.9%) were coded as having received acute dialysis on day 1, of whom 430 (86.2%) were relabelled as receiving chronic dialysis before day 90 (Figure 2). Of the 585 individuals who started dialysis and died without recovery of NKF, 396 (67.7%) were coded as receiving acute dialysis on day 1, of whom 33 (5.6%) were relabelled as receiving chronic dialysis before they died. Of the 1195 individuals who started dialysis and were alive having recovered NKF by day 90, 58 (4.9%) were coded as receiving chronic dialysis on day 1 and an additional 20 (1.7%) subsequently received such a code—meaning 1117 (93.5%) were coded as receiving acute dialysis on day 1 and were never relabelled. All 131 individuals who started dialysis and died following recovery of NKF (2.3% of all starters) came from the group of individuals who were coded as receiving acute dialysis on day 1 and were never relabelled.
FIGURE 2:
Outcomes 90 days after starting dialysis for the 5784 individuals in the 46 centres in the filtered dataset who were reported to have received their first-ever dialysis session between 1 October 2015 and 30 September 2016. NKF recovery was assigned in the following three settings: where a treating centre provided a timeline code indicating recovery of NKF and KRT was not restarted within 90 days (irrespective of date of death), where an individual discontinued dialysis and was alive without restarting KRT 90 days later and where an individual was lost to follow-up but was shown to be alive 90 days after dialysis initiation. All other individuals were assumed to not have recovered NKF. Individuals lost to follow-up but alive at day 90 were presumed to be alive following recovery of NKF. A Sankey diagram for the full 8671 individuals who started dialysis is in the Supplementary material.
The 24 excluded centres reported initiation of 11 to 337 individuals and coded between 0% and 37% as receiving acute dialysis on day 1 [median 1 (IQR 0–13)]. An association between the percentage of individuals coded as receiving acute dialysis on day 1 and the proportion who were dead or had recovered kidney function by day 90 was evident. Of the 20 centres reporting <10% of their dialysis starters as receiving acute dialysis on day 1, 17 were excluded as under reporters (see Supplementary data).
Survival and recovery analyses
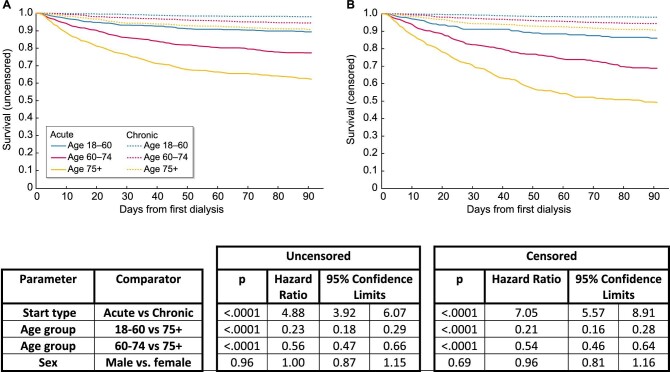
The following analyses were conducted using the filtered dataset. Uncensored Kaplan–Meier survival analysis showed higher mortality among individuals who were coded as receiving acute dialysis on day 1, with a HR for death of 4.99 [confidence interval (CI) 3.92–6.07; P < 0.001] (Figure 3). Older age groups had higher mortality than younger. Compared with those ≥75 years of age, Cox regression indicated a HR for death of 0.23 (CI 0.18–0.29; P < 0.001) for those ages 18–60 years and 0.56 (CI 0.47–0.66; P < 0.001) for those ages 60–75 years. There was no association between sex and hazard of death. After censoring for recovery, the HR for death associated with being coded as receiving acute dialysis on day 1 rose to 7.05 (CI 5.57–8.91; P < 0.001). No evidence of non-proportionality was identified using visual observation of log-log estimated survivor function graphs. Testing for interaction with log(time) and calculation of Schoenfeld residuals provided no strong indication of non-proportionality (P = 0.44, including all variables).
FIGURE 3:
Kaplan–Meier and Cox regression analysis of survival in the first 90 days for the 5784 individuals in the 46 centres included in the filtered dataset reported to have received their first-ever dialysis session between 1 October 2015 and 30 September 2016, stratified by age and whether they were coded as receiving acute or chronic dialysis on day 1: (A) uncensored; (B) censored for recovery.
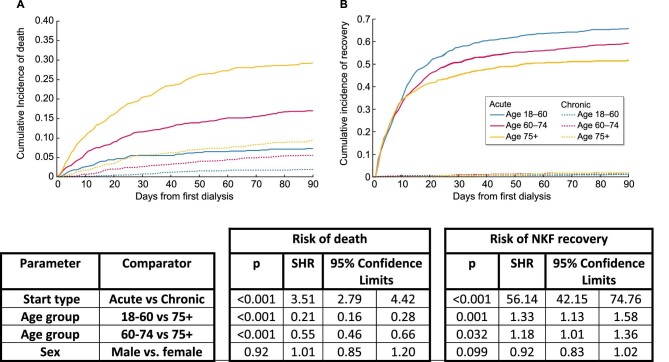
Using a competing risks analysis, the sub-HR for recovery of individuals coded as receiving acute dialysis compared with chronic dialysis on day 1 was 56.14 (CI 42.15–74.76). The sub-HR for recovery was higher among younger age groups at 1.18 (CI 1.01–1.36) for ages 60–75 years and 1.33 (CI 1.13–1.58) for ages 18–60 years, when compared with >75 years (Figure 4).
FIGURE 4:
Cumulative incidence curves and sub-HR of death and NKF recovery in the first 90 days for the 5784 individuals in the 46 centres included in the filtered dataset reported to have received their first-ever dialysis session between 1 October 2015 and 30 September 2016, stratified by age and coding at dialysis initiation, estimated using competing risk regression analysis. NKF recovery was assigned in the following three settings: where a treating centre provided a timeline code indicating recovery of NKF and KRT was not restarted within 90 days (irrespective of date of death), where an individual discontinued dialysis and was alive without restarting KRT 90 days later and where an individual was lost to follow-up but was shown to be alive 90 days after dialysis initiation. All other individuals were assumed to not have recovered NKF.
DISCUSSION
The UKRR has reported outcomes for an incident KRT population since 1996 [21]. Like other kidney health surveillance systems, analyses have focussed on individuals deemed to have reached irreversible kidney failure. Indeed, the medical literature relating to KRT initiation is largely founded upon populations of individuals defined as having ‘end-stage kidney disease’, reflecting American healthcare funding [7, 22, 23]. As such, even seminal work on early outcomes after dialysis initiation often neglects to highlight the inherent overestimation of survival and under-estimation of recovery inherent in the derivation of conventionally defined incident populations [24]. There is an obligation to take account of those who are routinely excluded—especially older people who start in unanticipated settings, for whom mortality and recovery rates differ markedly from conventional statistics [25]. The presented analyses reinforce this message. After exclusion of centres that appear to report implausibly low rates of death and recovery, within 90 days of starting, 2 in 3 dialysis recipients are still receiving KRT, 1 in 5 has recovered and 1 in 10 is dead.
That mortality is highest early after dialysis initiation is recognized as a universal phenomenon [26–28]. Mortality continues to be elevated until at least 120 days, even among cohorts with very low levels of NKF recovery [24, 28–30]. When efforts are made to account for survivorship bias, the earlier peak in mortality becomes increasingly evident [23]. Low levels of mortality in the very early period after initiation have been interpreted as suggestive of non-registration of individuals who die in the early weeks after starting KRT [9, 11, 23]. Few previous attempts have been made to integrate anticipated and unanticipated dialysis starts [23, 29]. The studies that have looked at early mortality among conventionally defined incident dialysis recipients have reported rates between 2% and 8%, compared with the 12.4% here [26, 29, 31, 32]. The very much lower total and relative risk of mortality among those classified as having AKD in these cohorts reflects exclusion or non-reporting of individuals with the worst outcomes.
Individuals labelled as starting acute dialysis were almost five times as likely to have died and 56 times as likely to have recovered NKF within 90 days than those who started with a chronic code. While these codes present a simple means by which to segregate starters in registry data, to what degree the strong association with mortality and recovery reflects retroactive coding versus clinicians’ ability to forecast recovery is unclear. Predicting whether an individual will recover NKF is open to clinical interpretation, and individuals are less likely to be identified and recoded as having irreversible disease the earlier they die. As such, biased, subjective decisions decide which individuals feature in conventional outcome data, undermining the value of analyses. These issues are not unique to the UKRR—they are relevant to any registry that relies on manual registration of individuals starting KRT.
Marked variation in coding patterns and clinical outcomes between UK centres was identified. Possible explanations include local IT system features, true variation in case mix, the proportion of individuals treated in critical care units and the limitations of manual coding described above. No amount of statistical adjustment can satisfactorily correct for biases in reporting, nor should it ‘adjust’ for true differences in practice. While the UKRR can collect data for all dialysis recipients, dependable AKD coding is unavailable and no data are routinely captured to describe the indication for or clinical setting of initiation. The close association between centre rates of starts coded as chronic and rates of death/recovery suggest that patients are missing rather than misclassified. Removing under reporting centres adjusts for this, but does not facilitate quality assurance for the care of individuals in removed centres. The greatest weakness of this work is its inability to shed light upon unaccounted for individuals or to explain between-centre variation. Bespoke studies or linkage to other routinely collected statistics—such as critical care registries—are needed to fully understand the patterns depicted. The greatest strength of this work is inclusivity, which mitigates against the biases inherent in deriving conventional incident populations, with profound influence upon both mortality and recovery patterns. We propose that in addition to conventionally derived incident populations, kidney health surveillance systems should analyse outcomes for all individuals reported to have started KRT. The challenge they face is to report these data in a way that supports both clinical utility, and quality assurance of care for all individuals receiving KRT under a nephrologist. Presentation of results by accepted subgroups—such as by age, planned/unplanned status and acute/chronic start type—enables, for the first time, generalizability of results to these groups and ensures that individuals with the worst outcomes are not forgotten.
CONCLUSION
The analyses presented use registry data to generate early outcome statistics for a comprehensive population of people starting dialysis. The poorest 90-day survival is seen among the oldest and those who are coded as having received acute dialysis on day 1, yet this most vulnerable group of patients is systematically excluded by many registries. A fresh perspective for defining the scope and rigor required of registries and to specify data sets and target populations will support the global community's ambition of kidney health for everyone, everywhere. Fledgling and established registries must identify local and
national solutions to ensure quality assurance for people entering a particularly vulnerable time in their kidney care—the initiation of dialysis for ACD or CKD.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to express thanks for technical assistance and manuscript preparation to Prof Dorothea Nitsch, Prof James Medcalf, Dr Shalini Santhakumaran and Dr Lucy Plumb. The authors would like to acknowledge the work done by all UKRR staff and reporting centres to capture the data upon which this article relies.
Contributor Information
Barnaby D Hole, Population Health, University of Bristol, Bristol, UK; UK Renal Registry, UK Renal Association, Bristol, UK.
Anna Casula, UK Renal Registry, UK Renal Association, Bristol, UK.
Fergus J Caskey, Population Health, University of Bristol, Bristol, UK.
AUTHORS’ CONTRIBUTIONS
B.H. led on concept and manuscript preparation. A.C. led on data analysis. B.H., A.C. and F.C. contributed equally to all other aspects of the project, supervised by F.C.
FUNDING
This work was completed without external funding. B.H. and A.C. are employees of the UKRR.
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the UKRR. Information on how to apply are available at https://renal.org/audit-research/how-access-data/ukrr-data.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Crews D, Bello A, Saadi G. Burden, access, and disparities in kidney disease. Kidney Int 2019; 95: 242–248 [DOI] [PubMed] [Google Scholar]
- 2.Bello A, Levin A, Lunney M.. Status of care for end stage kidney disease in countries and regions worldwide: international cross sectional survey. BMJ 2019; 367: l5873. [DOI] [PubMed] [Google Scholar]
- 3.Jha V, Garcia-Garcia G, Iseki K.. Chronic kidney disease: global dimension and perspectives. Lancet 2013; 382: 260–272 [DOI] [PubMed] [Google Scholar]
- 4.Hole B, Evans K, Pyart Ret al. . International collaborative efforts to establish kidney health surveillance systems. Kidney Int 2020; 98: 812–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris D, Davies S, Finkelstein F. Increasing access to integrated ESKD care as part of universal health coverage. Kidney Int 2019; 95(4 Suppl): S1–S33 [DOI] [PubMed] [Google Scholar]
- 6.ERA-EDTA Registry Annual Report 2018 . Amsterdam: Department of Medical Informatics, Amsterdam UMC. 2020 [Google Scholar]
- 7.USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2019 [Google Scholar]
- 8.ANZDATA 43rd Annual Report 2020 (data to 2019). https://www.anzdata.org.au/report/anzdata-43rd-annual-report-2020-data-to-2019/ (17 March 2021, date last accessed) [Google Scholar]
- 9.Caskey F, Schober-Halstenberg HJ, Roderick PJet al. . Exploring the differences in epidemiology of treated ESRD between Germany and England and Wales. Am J Kidney Dis 2006; 47: 445–454 [DOI] [PubMed] [Google Scholar]
- 10.Foley RN. Epidemiology and risk factors for early mortality after dialysis initiation. Semin Nephrol 2017; 37: 114–119 [DOI] [PubMed] [Google Scholar]
- 11.Foley RN, Chen SC, Solid CAet al. . Early mortality in patients starting dialysis appears to go unregistered. Kidney Int 2014; 86: 392–398 [DOI] [PubMed] [Google Scholar]
- 12.Jones S, Devonald M. How acute kidney injury is investigated and managed in UK intensive care units—a survey of current practice. Nephrol Dial Transplant 2013; 28: 1186–1190 [DOI] [PubMed] [Google Scholar]
- 13.UK Renal Registry . UK Renal Registry 19th annual report: appendix B definitions and analysis criteria. Nephron 2017; 137: 333–338 [DOI] [PubMed] [Google Scholar]
- 14.Hole B, Gilg J, Casula Aet al. . Chapter 1 UK renal replacement therapy adult incidence in 2016: national and centre-specific analyses. Nephron 2018; 139(Suppl 1): 13–46 [DOI] [PubMed] [Google Scholar]
- 15.National Health Service . https://digital.nhs.uk/ (4 October 2021, date last accessed) [Google Scholar]
- 16.Levey AS, Eckardt KU, Dorman NMet al. . Nomenclature for kidney function and disease: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int 2020; 97: 1117–1129 [DOI] [PubMed] [Google Scholar]
- 17.Birmele B, Francois M, Pengloan J. et al. Death after withdrawal from dialysis: the most common cause of death in a French dialysis population. Nephrol Dial Transplant 2004; 19: 686–691 [DOI] [PubMed] [Google Scholar]
- 18.Sekkarie MA, Moss AH.. Withholding and withdrawing dialysis: the role of physician specialty and education and patient functional status. Am J Kidney Dis 1998; 31: 464–472 [DOI] [PubMed] [Google Scholar]
- 19.Cohen LM, Germain MJ, Poppel DMet al. . Dying well after discontinuing the life-support treatment of dialysis. Arch Intern Med 2000; 160: 2513–2518 [DOI] [PubMed] [Google Scholar]
- 20.O'Connor NR, Dougherty M, Harris PSet al. . Survival after dialysis discontinuation and hospice enrollment for ESRD. Clin J Am Soc Nephrol 2013; 8: 2117–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Will EJ. A short cultural history of the UK Renal Registry 1995–2020. BMC Nephrol 2020; 21: 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins AJ, Foley RN, Gilbertson DTet al. . The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol 2009; 4(Suppl 1): S5–11 [DOI] [PubMed] [Google Scholar]
- 23.Chan KE, Maddux FW, Tolkoff-Rubinet al. . Early outcomes among those initiating chronic dialysis in the United States. Clin J Am Soc Nephrol 2011; 6: 2642–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson BM, Zhang J, Morgenstern Het al. . Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int 2014; 85: 158–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wachterman MW, O'Hare AM, Rahman OK. et al. One-year mortality after dialysis initiation among older adults. JAMA Intern Med 2019; 179: 987–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradbury BD, Fissell RB, Albert JMet al. . Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 2007; 2: 89–99 [DOI] [PubMed] [Google Scholar]
- 27.Noordzij M, Jager KJ.. Increased mortality early after dialysis initiation: a universal phenomenon. Kidney Int 2014; 85: 12–14 [DOI] [PubMed] [Google Scholar]
- 28.Lukowsky LR, Kheifets L, Arah OA. et al. Patterns and predictors of early mortality in incident hemodialysis patients: new insights. Am J Nephrol 2012; 35: 548–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah S, Leonard AC, Harrison K. et al. Mortality and recovery associated with kidney failure due to acute kidney injury. Clin J Am Soc Nephrol 2020; 15: 995–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovesdy CP, Naseer A, Sumida K. et al. Abrupt decline in kidney function precipitating initiation of chronic renal replacement therapy. Kidney Int Rep 2018; 3: 602–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wingard RL, Pupim LB, Krishnan M. et al. Early intervention improves mortality and hospitalization rates in incident hemodialysis patients: RightStart program. Clin J Am Soc Nephrol 2007; 2: 1170–1175 [DOI] [PubMed] [Google Scholar]
- 32.Soucie JM, McClellan WM.. Early death in dialysis patients: risk factors and impact on incidence and mortality rates. J Am Soc Nephrol 1996; 7: 2169–2175 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the UKRR. Information on how to apply are available at https://renal.org/audit-research/how-access-data/ukrr-data.