Abstract
Introduction
Financial toxicity (FT) is a growing concern among cancer survivors that adversely affects the quality of life and survival. Individuals diagnosed with aggressive cancers are often at a greater risk of experiencing FT. The objectives of this study were to estimate FT among prostate cancer (PCa) survivors after 10−15 years of diagnosis, assess the relationship between PCa aggressiveness at diagnosis and FT, and examine whether current cancer treatment status mediates the relationship between PCa aggressiveness and FT.
Methods
PCa patients enrolled in the North Carolina‐Louisiana Prostate Cancer Project (PCaP) were recontacted for long‐term follow‐up. The prevalence of FT in the PCaP cohort was estimated. FT was estimated using the COmprehensive Score for Financial Toxicity, a validated measure of FT. The direct effect of PCa aggressiveness and an indirect effect through current cancer treatment on FT was examined using causal mediation analysis.
Results
More than one‐third of PCa patients reported experiencing FT. PCa aggressiveness was significantly independently associated with high FT; high aggressive PCa at diagnosis had more than twice the risk of experiencing FT than those with low or intermediate aggressive PCa (adjusted odds ratio [aOR] = 2.13, 95% CI = 1.14−3.96). The proportion of the effect of PCa aggressiveness on FT, mediated by treatment status, was 10%, however, the adjusted odds ratio did not indicate significant evidence of mediation by treatment status (aOR = 1.05, 95% CI = 0.95−1.20).
Conclusions
Aggressive PCa was associated with high FT. Future studies should collect more information about the characteristics of men with high FT and identify additional risk factors of FT.
Keywords: Cancer aggressiveness, Cancer treatment, financial toxicity, Mediation analysis, NC‐LA PCaP, Prostate cancer
1. INTRODUCTION
Cancer is one of the most expensive medical conditions in the United States. 1 Cancer‐related expenditures continue to increase as cancer treatment and survival improve. US national cancer costs are projected to increase by 34% to a total cost of $246 billion in 2030 from $183 billion in 2015. 2 New drugs include expensive targeted therapies and immunotherapies. Patients' insurance coverage often may be inadequate to cover the costs associated with these new therapeutics. Consequently, patients may incur substantial out‐of‐pocket costs for their care often leading to financial hardship, stress, and anxiety, which could adversely impact their quality of life (QoL) and survival. 3
Financial toxicity (FT) describes the adverse effects of these medical care costs, usually out‐of‐pocket expenses, such as copayments, deductibles, and coinsurance. 4 , 5 Individuals with cancer are more likely to experience FT than those without cancer. A recent meta‐analysis estimated that the prevalence of FT is between 28% and 48% among cancer survivors, 6 and the risk factors for high FT include younger age, African American (AA) race, longer distance from treatment centers, and unemployment. 7 , 8 , 9 Similarly, the cancer‐related factors such as stage or aggressiveness, comorbidities, treatment choices, and health insurance also increased the risk of high FT. 7 , 8 , 9 , 10 , 11 In particular, the literature suggests that people with aggressive cancer are at a greater risk of FT and may be unable to pay for cancer care and experience cancer‐associated job loss. 12 , 13
Aggressive cancers usually require more resource‐intensive treatment therapies. Therefore, FT is likely to be greater among patients with aggressive cancers than those with less aggressive cancers. Access to health care, higher socioeconomic status, employment, and better education play significant role in detecting cancer early, and those without access to health care are likely to be diagnosed with high aggressive or late‐stage cancer. 14 , 15 , 16 Thus, the risk of experiencing FT among those with low socioeconomic status and lack of health insurance may be further exacerbated if diagnosed with aggressive or higher stage cancer. While the majority of individuals with a cancer diagnosis receive treatment, uninsured individuals may be less likely to receive timely screening and treatment than those with insurance. 17 , 18 , 19
Patients diagnosed with more aggressive cancers often have underlying comorbidities including hypertension, depression, hyperlipidemia, diabetes mellitus, coronary artery disease, and are concurrently receiving multiple treatments, 20 , 21 , 22 , 23 further worsening their financial condition. Sustained FT due to cancer treatment and underlying comorbidities adversely impact an individual's physical, psychological, cognitive functioning, QoL, and overall survival. 4 , 24 , 25 , 26 A study that included 254 cancer patients receiving chemotherapy or hormonal therapy reported that 68% of study participants cut back on leisure activities, 46% reduced spending on food and clothing, and 46% used savings to cover out‐of‐pocket expenses, leading to poor QoL. 7 Using propensity scores that included the sociodemographic characteristics and stage at diagnosis and treatment modality, Ramsey et al. 27 reported that financially distressed cancer patients had an 80% higher risk of mortality than those who did not have financial distress. Furthermore, some cancer survivors reported that FT was more severe than physical, emotional, social, or family distress. 28
Prostate cancer (PCa) is the second most common cancer in American men. 29 In 2019, there were more than 3.6 million US men with a history of PCa. 30 , 31 The 5‐year national relative survival rate of all PCa stages combined was 98%, and approximately 20% of all PCa cases were diagnosed either as regional or distant disease. 32 , 33 Treatment modalities for PCa include active surveillance (AS), radical prostatectomy (RP), androgen deprivation therapy (ADT), and radiation therapy (RT), either external beam radiation therapy or brachytherapy. Low aggressive PCa is often treated with less expensive approaches such as AS or RP, or both, but aggressive PCa or PCa that progresses may require highly expensive ADT, salvage RT, immunotherapy, or chemotherapy. Furthermore, recurrence of PCa during ADT may require additional treatments that are more expensive including immunotherapy, androgen metabolism inhibitors, small molecule anti‐androgens, radiopharmaceuticals, and docetaxel, cabazitaxel, or cisplatin. Men with PCa who receive these treatments are more likely to have higher FT than those who are not receiving such treatments. 34 , 35 Not only are many PCa treatments costly, but cancer also impacts employment. PCa survivors may take more extended time off from work, work fewer hours, and retire earlier due to cancer, which further increases the long‐term impact of FT. 36 Aggressive cancers are prevalent among low income populations who cannot afford the cost of routine cancer screening and health care which may lead to high FT. 37 Factors including income, education, and access to health care influence cancer screening and diagnosis. Delayed diagnosis may increase the frequency of a diagnosis of aggressive PCa, requiring resource intensive cancer treatment, which may increase financial burden.
To date, most studies have examined the association between PCa treatment and FT; however, very little research has been done to explore the relationship between PCa aggressiveness and FT. Therefore, the objectives of this study are to: (1) estimate FT among PCa survivors 10−15 years after diagnosis of PCa, (2) assess the relationship between PCa aggressiveness at diagnosis and FT, and (3) examine whether current cancer treatment status mediates the relationship between PCa aggressiveness and FT.
2. MATERIALS AND METHODS
2.1. Study design and participants
The North Carolina‐Louisiana Prostate Cancer Project (PCaP) was initiated in 2004. A total of 2258 men with PCa were enrolled from North Carolina (NC) and Louisiana (LA) study areas between 2004 and 2009. PCaP is a multidisciplinary population‐based case‐only study designed to address racial differences in PCa survival through a comprehensive evaluation of social, individual, and tumor level influences on PCa aggressiveness. 38 AA and European American (EA) men were enrolled in equal proportions. Eligibility criteria at the time of enrollment included: (1) resident of NC or LA study areas; (2) histologically diagnosed PCa; (3) 40−79 years of age; (4) ability to complete the study interview in English; (5) not institutionalized; and (6) not cognitively impaired.
Potential PCaP participants were identified at time of their initial diagnosis through their respective state tumor registry (North Carolina Central Cancer Registry and Louisiana Tumor Registry [LTR]). Trained registered nurses visited participants in their homes, administered structured questionnaires, and collected biological samples and anthropometric measures. Additional information about the PCaP study design and population can be found in a prior publication. 38
2.1.1. 10−15‐year follow‐up study
The aim of the follow‐up study was to examine FT among a cohort of men 10−15 years after their PCa diagnosis. The latest follow‐up of PCaP participants began in 2018 and was completed in 2020. All living PCaP participants were invited to participate in follow‐up survey. The vital status of PCaP participants is annually ascertained through a linkage with the NC Department of Health and Human Services Vital Records for NC participants and the LTR for LA participants. Of 2258 participants initially enrolled in the PCaP study, a total of 642 died before recruitment started for the 2018 follow‐up study. A total of 1616 eligible participants were invited to complete the follow‐up survey. The study was reviewed and approved by the Institutional Review Board at Louisiana State University Health Sciences Center (LSUHSC) and Department of Defense Human Subjects Research Review Board.
2.1.2. Participant recruitment and data collection
Introductory letters introducing the follow‐up survey were sent to all living PCaP participants. Two weeks after the introductory letters were sent, participants were contacted via telephone by trained interviewers to invite them to complete the survey. A mixed mode approach including telephone, paper, and online survey methods were used for data collection. Online and telephone versions of survey data were collected using Research Electronic Data Capture (REDCap), a secure web application hosted at the Epidemiology Data Center at the LSUHSC School of Public Health. 39 Optically scannable survey forms were developed using TeleForm for the paper survey. The completed paper surveys were scanned and directly entered into the TeleForm database and then transferred to REDCap. Participants who did not respond to calls, voicemails, and mail surveys were subsequently searched using LexisNexis Accurint to obtain their most recent phone number and residential address. LexisNexis Accurint is a database of approximately 84 billion public records that includes information on an individual's contact information and vital status. 40 In total, 6 call attempts at different times on different days of the week were made to recruit a participant to the follow‐up study.
2.1.3. Computer‐assisted telephone interviews (CATI)
The primary method of survey data collection was CATI. Trained interviewers read the survey questions to participants and responses were directly entered into the REDCap database. Participants who preferred not to complete a telephone interview were offered the option to complete either a paper or online survey.
2.1.4. Paper survey
A paper survey with a prepaid return envelope was sent to PCaP participants who opted for a paper survey. Participants were followed up via telephone after 3 weeks to confirm if they received, completed, and returned the survey. Up to 3 follow‐up calls were made to those who received a paper version of the survey to maximize completion.
2.1.5. Online survey
An online survey link was created using REDCap and emailed to participants who requested this option. Up to 3 weekly reminders emails were sent to those who had not completed the survey.
2.2. Surveys
2.2.1. Baseline survey
Trained registered nurses conducted structured in‐person interviews that collected information on demographics, screening history, comorbidities, family history, and behavioral factors. The nurses also collected biological specimens and took anthropometric measurements. Data on clinical stage, Gleason grade, prostate‐specific antigen (PSA), and treatment related information were abstracted from the medical records.
2.2.2. Follow‐up
Information on current household income, employment status, and current treatment were collected. Comorbidities, QoL, and FT were ascertained using validated instruments. Charlson comorbidity index (CCI) for comorbidities, SF‐12 version 2 for QoL, and COmprehensive Score for Financial Toxicity (COST) for FT were used. Participants were also asked to provide contact information for their current health care providers.
2.3. Measures
2.3.1. Outcome assessment: FT
The primary outcome of interest was FT measured at follow‐up. Participants' self‐reported estimate of FT was measured using the COST. 41 COST is a validated screening tool for FT that consists of 11 questions, each with a five‐point Likert scale. 41 , 42 COST captures information about psychological burden and material hardship. The psychological burden‐related questions assess current financial situation, financial stress, and satisfaction with present financial situation. The material hardship questions assess the inability to meet monthly and out‐of‐pocket medical expenses. The COST score ranges 0−44; a higher COST score indicates higher FT. Terciles of COST scores were created; the top tercile was defined as high FT, and the bottom two terciles were classified as low FT. 43
2.3.2. Exposure assessment: PCa aggressiveness
Baseline PCa aggressiveness at the time of diagnosis was the primary exposure of interest. A combination of prognostic factors that included clinical tumor stage, biopsy Gleason grade, and diagnostic serum PSA level were abstracted from medical records and used to define PCa aggressiveness. 44 PCa was dichotomized as high aggressive (Gleason sum ≥8, or PSA >20 ng/ml, or Gleason sum ≥7 and clinical tumor stage cT3−cT4) or low aggressive (Gleason sum <7 and clinical stage cT1−cT2, and PSA <10 ng/ml)/intermediate aggressive (all other cases).
2.3.3. Potential mediator: Current cancer treatment measured at follow‐up
The current cancer treatment measured at follow‐up was assessed as a potential mediator in the relationship between PCa aggressiveness and FT among PCa survivors. The most recent cancer treatment at follow‐up was ascertained by asking participants “Are you currently being treated for cancer?” The response was recorded as “Yes” or “No.”
2.3.4. Covariates
Patients' age at diagnosis, race, marital status, income, education, health insurance status before PCa diagnosis, family history of PCa, PCa screening history, comorbidity status, PCa recurrence, treatment status at follow‐up, self‐reported health status, and employment at follow‐up were included in the analysis.
Age at diagnosis was classified as: (1) ≤50; (2) 51−60; (3) 61−70; or (4) more than 70 years. Education level was dichotomized as more than high school degree or high school degree or less. Income was classified into five categories: (1) less than $10,000; (2) $10,001−$40,000; (3) $40,001−$60,000; (4) $60,001−$80,000; or (5) more than $80,000 per year. The CCI was categorized into no (CCI = 0), mild (CCI < 2), moderate (CCI = 2−4), or severe (CCI > 4) morbidity. Of 562 participants who completed the follow‐up survey, 54 had missing values for COST questionnaires, thus excluded in the final analysis.
2.4. Statistical analysis
Descriptive statistics, frequencies (percentages) for categorical variables, and means (SD) for continuous variables, were calculated. Unadjusted relationships between exposure (PCa aggressiveness), the potential mediator (current cancer treatment status), and covariates with FT were examined using χ 2 tests or t‐tests.
A formal causal mediation analysis was conducted to decompose the total effect of PCa aggressiveness on FT into a direct effect, and indirect effect mediated by current cancer treatment status. The direct effect, the effect of PCa aggressiveness unexplained by the mediator, estimated how FT would change while controlling for treatment status (the mediator). The indirect effect, the effect of PCa aggressiveness on FT that acts through the mediator, estimated how FT would change if the PCa aggressiveness were fixed. The counterfactual framework approach by VanderWeele, extending Baron and Kenny's approach was used to estimate the effects. 45 , 46 The SAS macro developed by Valeri and VanderWeele 45 was used to perform the mediation analysis. The following assumptions were made for the causal mediation analysis: (1) there are no unmeasured exposure‐outcome confounders; (2) there are no unmeasured mediator‐outcome confounders; (3) there are no unmeasured exposure‐mediator confounders; (4) none of the mediator‐outcome confounders are affected by exposure. 45
Crude and adjusted mediation models were created. The adjusted model controlled for race, state, age, education, and baseline income. The exposure‐outcome confounding variables were selected based on a review of the literature and directed acyclic graphs (DAGs) developed using DAGitty. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc.) at the statistical significance level of 0.05.
3. RESULTS
Of 1616 participants, 562 completed the follow‐up survey, 161 refused, and 791 were lost to follow‐up (Figure 1). A total of 106 participants died during the follow‐up survey period (2018−2020).
Figure 1.
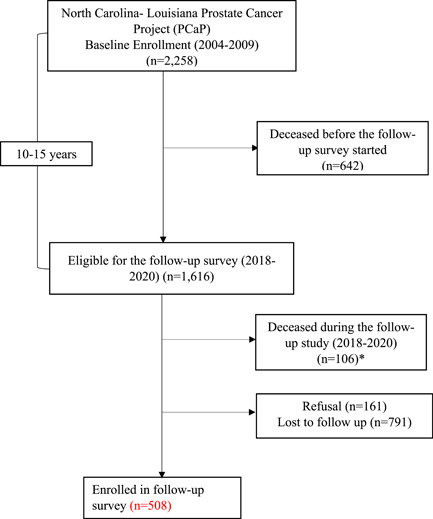
North Carolina‐Louisiana Prostate Cancer Project (PCaP) study flowchart. *Four of 106 deceased participants had completed the follow‐up survey. [Color figure can be viewed at wileyonlinelibrary.com]
Table 1 shows the baseline characteristics of the men enrolled in the initial and follow‐up survey. A total of 562 men completed the follow‐up survey. The response rate was 37% (562/1514). Approximately equal number of AA and EA men were enrolled at baseline, but the majority of respondents in the follow‐up survey were EA (65%). The distribution of age and marital status of men enrolled in the baseline was similar to men who completed the follow‐up survey. Most men who completed the follow‐up survey had more than a high school degree (74%), and 95% of those who completed the follow‐up survey had health insurance at baseline. The majority of participants who completed the follow‐up survey had baseline incomes of more than $80,000 per year. A higher proportion of participants who completed the follow‐up survey did not have any underlying comorbidities (61%) and had low/intermediate aggressive PCa (89%) at the baseline. Likewise, of those participating in follow‐up, the majority had received treatment for their PCa at baseline (39%). The distribution of baseline characteristics among deceased, lost to follow‐up, and those who refused are presented in the supplementary table (Supporting Information: Table S1). Of 2258 men in the original cohort, 748 (33%) died, 791 (35%) were lost to follow‐up, and 161 (7%) refused to participate in the follow‐up study. Men who were lost to follow‐up were more likely to be AAs (60%) than EA (40%). Furthermore, those who were lost to follow‐up were less likely to have health insurance and had low household income than those who participated in the following study.
Table 1.
Baseline characteristics of participants by enrollment
| Characteristics | Baseline survey (2004−2009) | Follow‐up survey (2018−2020) | ||||
|---|---|---|---|---|---|---|
| Overall (N = 2258) | AA (N = 1130) | EA (N = 1128) | Overall (N = 562) | AA (N = 194) | EA (N = 367) | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Age group (years) | ||||||
| ≤50 | 127 (5.62) | 73 (6.46) | 54 (4.79) | 31 (5.52) | 14 (7.18) | 17 (4.63) |
| 51−60 | 738 (32.68) | 428 (37.88) | 310 (27.48) | 245 (43.59) | 99 (50.77) | 146 (39.78) |
| 61−70 | 952 (42.16) | 455 (40.27) | 497 (44.06) | 238 (42.35) | 68 (34.87) | 170 (46.32) |
| More than 70 | 441 (19.53) | 174 (15.40) | 267 (23.67) | 48 (8.54) | 14 (7.18) | 34 (9.26) |
| Marital status | ||||||
| Married | 1689 (74.80) | 755 (66.81) | 934 (82.80) | 446 (79.36) | 137 (70.26) | 309 (84.20) |
| Separated | 86 (3.81) | 73 (6.46) | 13 (1.15) | 14 (2.49) | 9 (4.62) | 5 (1.36) |
| Divorced | 236 (10.45) | 147 (13.01) | 89 (7.89) | 53 (9.43) | 25 (12.82) | 28 (7.63) |
| Single/never married | 102 (4.52) | 68 (6.02) | 34 (3.01) | 24 (4.27) | 12 (6.15) | 12 (3.27) |
| Widowed | 143 (6.33) | 85 (7.52) | 58 (5.14) | 25 (4.45) | 12 (6.15) | 13 (3.54) |
| Unknown/missing | 2 (0.09) | 2 (0.18) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Education | ||||||
| High school or less | 1039 (46.01) | 681 (60.27) | 358 (31.74) | 146 (25.98) | 78 (40.00) | 68 (18.53) |
| More than high school | 1219 (53.99) | 449 (39.73) | 770 (68.26) | 416 (74.02) | 117 (60.00) | 299 (81.47) |
| Insurance status | ||||||
| No | 218 (9.65) | 169 (14.96) | 49 (4.34) | 27 (4.80) | 20 (10.26) | 7 (1.91) |
| Yes | 2025 (89.68) | 951 (84.16) | 1074 (95.21) | 533 (94.84) | 174 (89.23) | 359 (97.82) |
| Unknown/missing | 15 (0.66) | 10 (0.88) | 5 (0.44) | 2 (0.36) | 1 (0.51) | 1 (0.27) |
| Income | ||||||
| ≤$10,000 | 198 (8.77) | 165 (14.60) | 33 (2.93) | 16 (2.85)) | 13 (6.67) | 3 (0.82) |
| $10,001−$40,000 | 786 (34.81) | 480 (42.48) | 306 (27.13) | 127 (22.06) | 69 (35.38) | 58 (15.80) |
| $40,001−$60,000 | 349 (15.46) | 165 (14.60) | 184 (16.31) | 94 (16.73) | 35 (17.95) | 59 (16.08) |
| $60,001−$80,000 | 237 (10.50) | 86 (7.61) | 151 (13.39) | 87 (15.48) | 24 (12.31) | 63 (17.17) |
| $80,000+ | 473 (20.95) | 119 (10.53) | 354 (31.38) | 206 (36.65) | 43 (22.05) | 163 (44.41) |
| Unknown/missing | 215 (9.52) | 115 (10.18) | 100 (8.87) | 32 (5.69) | 11 (5.64) | 21 (5.72) |
| Employment | ||||||
| Full time | 770 (34.10) | 336 (29.73) | 434 (38.48) | 54 (9.61) | 16 (8.21) | 38 (10.35) |
| Part time | 226 (10.01) | 106 (9.38) | 120 (10.64) | 40 (7.12) | 15 (7.69) | 25 (6.81) |
| Retired of PCa/other problem | 365 (16.16) | 266 (23.54) | 99 (8.78) | 56 (9.96) | 35 (17.95) | 21 (5.72) |
| Retired by choice/age | 744 (32.95) | 311 (27.52) | 433 (38.39) | 345 (61.39) | 96 (49.23) | 249 (67.85) |
| Other | 153 (6.78) | 111 (9.82) | 41 (3.63) | 37 (6.58) | 16 (8.21) | 21 (5.72) |
| Unknown/missing | 1 (0.04) | 0 (0.00) | 1 (0.09) | 30 (5.34) | 17 (8.72) | 13 (3.54) |
| CCI | ||||||
| None | 1121 (49.65) | 526 (46.55) | 595 (52.75) | 341 (60.68) | 110 (56.41) | 231 (62.94) |
| Mild | 547 (24.22) | 285 (25.22) | 262 (23.23) | 114 (20.28) | 40 (20.51) | 74 (20.16) |
| Moderate | 508 (22.50) | 274 (24.25) | 234 (20.74) | 96 (17.08) | 41 (21.03) | 55 (14.99) |
| Severe | 73 (3.23) | 37 (3.27) | 36 (3.19) | 10 (1.78) | 3 (1.54) | 7 (1.91) |
| Unknown/missing | 9 (0.40) | 8 (0.71) | 1 (0.09) | 1 (0.18) | 1 (0.51) | 0 (0.00) |
| Family history of PCa | ||||||
| No | 1617 (71.61) | 790 (69.91) | 827 (73.32) | 379 (67.44) | 127 (65.13) | 252 (68.66) |
| Yes | 555 (24.58) | 291 (25.75) | 264 (23.40) | 158 (2811) | 59 (30.26) | 99 (26.98) |
| Unknown/missing | 86 (3.81) | 49 (4.34) | 37 (3.28) | 25 (4.45) | 9 (4.62) | 16 (4.36) |
| PSA screening history | ||||||
| No | 474 (20.99) | 323 (28.58) | 151 (13.39) | 60 (10.68) | 32 (16.41) | 28 (7.63) |
| Yes | 1571 (69.57) | 650 (57.52) | 921 (81.65) | 476 (75.38)) | 147 (75.38) | 329 (89.65)) |
| Unknown/missing | 213 (9.43) | 157 (13.89) | 56 (4.96) | 26 (4.63) | 16 (8.21) | 10 (2.72) |
| PCa aggressiveness | ||||||
| Low/intermediate | 1777 (78.70) | 850 (75.22) | 927(82.18) | 485 (86.30) | 167 (85.64) | 318 (86.65) |
| High | 396 (17.54) | 228 (20.18) | 168 (14.89) | 61 (1085) | 24 (12.31) | 37 (10.08) |
| Unknown/missing | 85 (3.76) | 52 (4.60) | 33 (2.93) | 16 (2.85) | 4 (2.05) | 12 (3.27) |
| Treatment status | ||||||
| Yes | 1501 (66.47) | 740 (65.49) | 761 (67.46) | 408 (72.60) | 138 (70.77) | 270 (73.57) |
| No | 757 (33.53) | 390 (34.51) | 367 (32.54) | 154 (27.40) | 57 (29.23) | 97 (26.43) |
Abbreviations: AA, African American; CCI, Charlson comorbidity index; EA, European American; PCa, prostate cancer; PSA, prostate‐specific antigen.
Table 2 shows the mean FT score by aggressiveness, treatment status, and race. The overall mean (SD) FT score was 11.10 (SD 8.46) and ranged from 0 to 43. The mean (SD) FT score was significantly higher among participants with high aggressive PCa compared to those with low aggressive PCa (14.70 [10.26] vs. 10.70 [8.08], p < 0.0067). Similarly, AA men had almost twice the mean (SD) FT score compared to EA men (15.60 [9.13] vs. 8.81 [7.11], p < 0.0001). The log‐binomial regression adjusted for income, employment, and insurance status indicated a positive association between race and FT. AA men had 1.14 times the risk of experiencing FT compared to EA men 1.14 (95% CI = 1.02−1.27).
Table 2.
Mean (SD) financial toxicity score by PCa aggressiveness and current treatment status (N = 508)
| Mean (SD) FT score | p Value | |
|---|---|---|
| Overall mean (SD) | 11.10 (8.46) | |
| Aggressiveness | ||
| Low/intermediate aggressive | 10.70 (8.08) | 0.0067* |
| High aggressive | 14.70 (10.26) | |
| Current treatment status | ||
| Not receiving treatment | 11.00 (8.30) | 0.3246 |
| Receiving treatment | 12.41 (9.57) | |
| Race | ||
| AA | 15.60 (9.13) | <0.0001* |
| EA | 8.81 (7.11) | |
Abbreviations: AA, African American; FT, financial toxicity; PCa, prostate cancer.
*p Values indicate differences in mean FT score by aggressiveness, current treatment status, and race at type I error = 0.05.
*p Values were calculated using t‐testing.
The mean FT score among those receiving treatment for PCa at the time of follow‐up was slightly higher than those not receiving treatment. However, the mean FT scores were not statistically different.
Table 3 shows the distribution of demographic characteristics of participants who were enrolled at follow‐up and completed the COST questionnaires (n = 508).
Table 3.
Demographic characteristics of participants enrolled at follow‐up (2018−2020) by financial toxicity (N = 508)
| Characteristics | Financial toxicity | ||
|---|---|---|---|
| Low (N = 333) | High (N = 175) | p Value | |
| N (%) | N (%) | ||
| Race | |||
| African American | 79 (23.72) | 92 (52.57) | <0.001* |
| European American | 254 (76.28) | 83 (47.43) | |
| Age (years) | |||
| ≤50 | 16 (4.80) | 13 (7.43) | 0.2600 |
| 51−60 | 142 (42.64) | 85 (48.57) | |
| 61−70 | 146 (43.84) | 65 (37.14) | |
| More than 70 | 29 (8.71) | 12 (6.86) | |
| Marital status | |||
| Married | 270 (81.08) | 129 (73.71) | 0.1005 |
| Separated | 6 (1.80) | 8 (4.57) | |
| Divorced | 32 (9.61) | 16 (9.14) | |
| Single/never married | 11 (3.30) | 12 (6.86) | |
| Widowed | 14 (4.20) | 10 (5.71) | |
| Income | |||
| ≤$10,000 | 3 (0.90) | 11 (6.29) | <0.0001* |
| $10,001−$40,000 | 54 (16.22) | 59 (33.71) | |
| $40,001−$60,000 | 54 (16.22) | 35 (20.00) | |
| $60,001−$80,000 | 52 (1562) | 24 (13.71) | |
| $80,000+ | 156 (46.85) | 37 (21.14) | |
| Unknown/missing | 14 (4,20) | 9 (5.14) | |
| Education | |||
| High school or less | 57 (17.12) | 69 (39.43) | <0.0001* |
| More than high school | 276 (82.88) | 106 (60.57) | |
| Insurance status | |||
| No | 7 (2.10) | 15 (8.57) | 0.0004* |
| Yes | 326 (97.90) | 158 (90.29) | |
| Unknown/missing | 0 (0.00) | 2 (1.14) | |
| Employment at follow‐up | |||
| Full time | 34 (10.21) | 18 (10.29) | <0.0001* |
| Part time | 20 (6.01) | 18 (10.29) | |
| Retired of PCa/other problem | 21 (6.31) | 32 (18.29) | |
| Retired by choice/age | 235 (72.57) | 81 (46.29) | |
| Other | 16 (4.80) | 20 (11.43) | |
| Unknown/missing | 7 (2.10) | 6 (3.43) | |
Note: p Values were calculated from Pearson's χ 2 or Fisher's exact test.
Abbreviations: FT, financial toxicity; PCa, prostate cancer.
p Values indicate differences by participant‐reported FT at type I error = 0.05.
Of 508 participants, 34% (n = 175) reported high FT (Table 3). The majority of participants who had high FT were AA (53%). Of those men with high FT, the majority had an annual household income less than $60,000 compared to those with low FT; a majority had an annual household income of more than $60,000. Of participants with low FT, most (83%) had more than a high school degree than 61% among those in the low FT group. When comparing health insurance coverage at baseline, almost 98% of participants who did not report high FT had health insurance compared to 91% among those with high FT (p = 0.0006). Similarly, FT also differed by participant's employment status as measured at follow‐up. Among participants who reported high FT, almost 48% indicated they were retired by choice compared to those with low FT, where 72% had retired by choice. Of those men with high FT, 19% reported they retired because of PCa, or other health problems compared to only 6% among those in the low FT group (Table 3).
Table 4 shows the clinical and treatment‐related characteristics by FT. Specific clinical characteristics measured at baseline, such as PSA screening history and self‐reported health status were significantly different by FT status. Of those men who reported high FT, 79% were screened for PCa compared to those with low FT, 92% were screened for PCa. Among high FT participants, 16% had high aggressive cancer at diagnosis compared to only 9%. None of the treatments received at baseline was associated with high FT.
Table 4.
Clinical and treatment characteristics of participants enrolled at follow‐up survey (2018−2020) by financial toxicity (N = 508)
| Characteristics | Financial toxicity | ||
|---|---|---|---|
| Low (N = 333) | High (N = 175) | ||
| N (%) | N (%) | p Value | |
| Family history of Pca | |||
| No | 220 (66.07) | 121 (69.14) | 0.7607 |
| Yes | 99 (29.73) | 48 (27.43) | |
| Unknown/missing | 14 (4.20) | 48 (27.43) | |
| PSA screening history | |||
| No | 25 (7.51) | 33 (18.86) | <0.0001* |
| Yes | 300 (90.09) | 127 (72.57) | |
| Unknown/missing | 8 (2.40) | 15 (8.57) | |
| Treatment received at baseline | |||
| No | 239 (71.77) | 131 (74.86) | 0.4575 |
| Yes | 94 (28.23) | 44 (25.14) | |
| Active surveillance | |||
| No | 323 (97.00) | 173 (98.86) | 0.2334 |
| Yes | 10 (3.00) | 2 (1.14) | |
| Radical prostatectomy | |||
| No | 86 (25.83) | 50 (28.57) | 0.5066 |
| Yes | 247 (74.17) | 125 (71.43) | |
| Androgen deprivation | |||
| No | 315 (94.59) | 161 (92.00) | 0.2527 |
| Yes | 18 (5.41) | 14 (8.00) | |
| External beam radiation therapy | |||
| No | 307 (92.19) | 154 (88.00) | 0.1212 |
| Yes | 26 (7.81) | 21 (12.00) | |
| Brachytherapy | |||
| No | 300 (90.09) | 161 (92.00) | 0.4802 |
| Yes | 33 (9.91) | 14 (8.00) | |
| PCa aggressiveness | |||
| Low/intermediate | 296 (88.89) | 143 (81.71) | 0.0519 |
| High | 28 (8.41) | 27 (15.43) | |
| Unknown/missing | 9 (2.70) | 5 (2.86) | |
| CCI | |||
| None | 208 (62.46) | 98 (56.32) | 0.4086 |
| Mild | 64 (19.22) | 40 (22.99) | |
| Moderate | 56 (16.82) | 32 (18.39) | |
| High | 5 (1.50) | 4 (2.30) | |
| Unknown/missing | 0 (0.00) | 1 (0.57) | |
| Recurrence at follow‐up | |||
| No | 251 (75.38) | 128 (73.14) | 0.4230 |
| Yes | 20 (6.01) | 16 (9.14) | |
| Unknown/missing | 62 (18.62) | 31 (17.71) | |
| Getting treated at follow‐up | |||
| No | 296 (88.89) | 150 (85.71) | 0.5393 |
| Yes | 31 (9.31) | 20 (11.43) | |
| Unknown/missing | 6 (1.80) | 5 (2.86) | |
| Self‐reported health status | |||
| Poor/fair | 225 (67.57) | 85 (48.57) | 0.0002 |
| Good | 81 (24.32) | 60 (34.29) | |
| Very good/excellent | 27 (8.11) | 29 (16.57) | |
| Unknown/missing | 0 (0.00) | 1 (0.57) | |
Abbreviations: CCI, Charlson comorbidity index; FT, financial toxicity; PCa, prostate cancer; PSA, prostate‐specific antigen.
p Values indicate differences by participant reported FT at type I error = 0.05. p Values were calculated from Pearson's χ 2 or Fisher's exact test.
The results of the mediation analysis are presented in Table 5. The adjusted estimates (adjusted odds ratio [aOR], 95% CI) are provided for: (1) direct effect of PCa aggressiveness on FT; (2) indirect effect of PCa aggressiveness on FT mediated by the current treatment status; and (3) total effect of PCa aggressiveness on FT that includes both direct and indirect effects. The multivariable model was adjusted for race, state, age, education, and baseline health insurance.
Table 5.
Assessment of the relationship between PCa aggressiveness and FT, mediated by treatment status
| Mediator treatment status | Crude modela | Adjusted modelb | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | aOR (95% CI) | p Value | |
| Controlled direct effect** | 2.08 (1.16−3.72) | 0.01350* | 2.02 (1.07−3.80) | 0.03006* |
| Indirect effect | 1.01 (0.91−1.13) | 0.81630 | 1.05 (0.95−1.20) | 0.44306 |
| Total effect | 2.10 (1.19−3.73) | 0.01047* | 2.13 (1.14−3.96) | 0.01814* |
| Proportion mediated | 0.02 | 0.10 | ||
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; FT, financial toxicity; OR, odds ratio; PCa, prostate cancer.
Statistically significant at 0.05.
Controlled direct effect = natural direct effect.
Crude model assessed the relationship between PCa aggressiveness and FT mediated by current treatment status.
Adjusted for race, state, age, education, and baseline health insurance.
3.1. Direct effect
The adjusted model assessing the direct effect suggested significant effect of PCa aggressiveness on FT. The direct effect slightly attenuated upon adjustment for confounding variables. The adjusted direct effect odds ratios of high aggressive PCa on high FT was 2.02 (95% CI = 1.07−3.80) (Table 5); the PCa aggressiveness was significantly independently associated with high FT. Men with high aggressive PCa had more than twofold the odds of exhibiting FT than men with low/intermediate aggressive PCa.
3.2. Indirect effect
The relationship between PCa aggressiveness and FT was not mediated by current cancer treatment at follow‐up. The adjusted odds of the indirect effect of PCa on FT via treatment status was 1.05 (95% CI = 0.95−1.20) (Table 5).
3.3. Total effect
In the adjusted mediation analysis, the total effect of PCa aggressiveness was statistically significant (OR = 2.13, 95% CI = 1.14−3.96). The adjusted total effect suggests that men with high aggressive PCa had 2.13 times the odds to exhibit FT than those with low/intermediate aggressive PCa.
4. DISCUSSION
This study found more than one‐third of PCaP survivors reported high FT, and men diagnosed with high aggressive PCa had more than twice the risk of FT compared to men with low/intermediate aggressive PCa, after adjusting for potential confounders. The causal mediation analysis suggested that current cancer treatment measured at follow‐up did not mediate the effect of PCa aggressiveness on FT.
In our study, the estimated prevalence of FT among PCaP survivors was higher than those reported in other studies of cancer survivors. Yabroff et al. 47 reported a 22% prevalence of FT in a population‐based study that included 1202 adults with any cancer. Another study reported that approximately 18% of breast, colorectal, head, and neck, lung, PCa survivors had a significant financial burden. 48 Unlike this study, both studies included multiple cancer sites and the cost of cancer treatments might be substantially different that may explain the contrasting results. Additionally, the demographic make‐up of study participants might have contributed to differences in the proportion of FT. Similar to our study, a population‐based study in the United States. that included only PCa survivors reported a FT prevalence of 15%. 10 However, FT was measured using only four questions related to the direct and indirect cost of treatment. To our knowledge, this is the first study that uses COST, a validated measure to assess FT among PCa survivors.
The financial burden of cancer care is not limited to the US. 49 , 50 Studies conducted across the world have reported higher FT among cancer survivors. For example, a study among new cancer patients in 10 Southeast Asian countries reported almost 50% of cancer patients had a financial catastrophe. 50 The study defined financial catastrophe as out‐of‐pocket costs after 12 months equal to or exceeding 30% of annual household income. The same study reported that cancer patients diagnosed with stage IV cancer had a 52% increased odds of financial catastrophe than those diagnosed with stage I cancer. 50 Honda et al. 49 noted higher FT among Japanese cancer patients; however, FT was not associated with disease and treatment‐related factors. Japan's health insurance system that provides comprehensive coverage to all Japanese citizens as a part of universal health coverage may have made a difference in the findings.
To date, studies conducted on FT among PCa survivors have focused their investigation on the role of PCa treatment on FT and reported higher FT among PCa survivors that is largely due to cancer treatments. Our study found that men diagnosed with an aggressive form of PCa are at greater risk of FT regardless of their cancer treatment. In the baseline PCaP cohort, almost 32% of men diagnosed with PCa had not received any treatment. Similarly, a higher proportion of men who completed the follow‐up survey had received treatment at baseline than overall participants. Furthermore, in the follow‐up survey, most men with high aggressive PCa reported that they were not receiving any cancer treatment at follow‐up, suggesting that people may not receive secondary cancer treatment even if there were diagnosed with an aggressive form of PCa. Literature suggests that the primary reasons behind not receiving PCa treatment include lack of health care access, low household income, and expensive cancer treatment. 51 , 52 Therefore, cancer patients who chose not to get any cancer treatment are also at higher risk of financial burden. Rotter et al. 52 highlighted the burden of financial hardship among patients with an aggressive form of cancer. Authors emphasized that low‐income AA men are at a higher risk of both poor cancer outcomes and greater financial burden as aggressive cancers are disproportionally higher among AA due to their poor underlying socioeconomic status and lack of health care access.
The impact of current/secondary treatment 10−15 years after diagnosis on FT was assessed as a mediator in this study. No indirect effect of PCa aggressive on FT through treatment status was observed. The information on specific PCa drugs and treatments that participants received at follow‐up was not available, which may have influenced the results in the mediation analysis.
4.1. Limitations
There are some limitations in this study. First, the attrition rate was relatively high because this was a long‐term (10−15 years) population‐based follow‐up survey. However, there was no difference among men who participated in the follow‐up study compared to those who were lost to follow‐up by cancer aggressiveness and baseline treatment.
Second, current/secondary PCa treatments at follow‐up were not specified. Thus, they were not analyzed as potential mediators in the causal mediation analysis. Since cancer costs differ based on the type of treatment, specific cancer treatment would better clarify the mediating role of PCa treatment in the relationship between PCa aggressiveness and FT.
4.2. Strengths
This study has several strengths. First, the study analyzed the long‐term (10−15 years) FT among PCa survivors in one of the largest population‐based studies of newly diagnosed PCa in AA and EA. The majority of FT studies were hospital‐based consisting of patients from a single hospital. This population‐based study included cancer survivors from a larger geographical area than a single hospital, with diverse sociodemographic and clinical characteristics. Lastly, this is one of the first studies that used a validated measure (COST) to assess FT among PCa patients.
5. CONCLUSIONS
There is a substantial risk of FT among PCa survivors. Men diagnosed with high aggressive PCa were more likely to have higher FT than those with low aggressive PCa. Previous FT studies among cancer survivors were focused on investigating the impact of treatment on FT. In contrast, while this study examined the relationship between PCa aggressiveness and FT and found that PCa aggressiveness, regardless of treatment status at follow‐up, has a significant impact on FT.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supplementary information.
ACKNOWLEDGMENTS
The authors thank the staff, advisory committees, and research subjects participating in the PCaP study for their important contributions. The North Carolina—Louisiana Prostate Cancer Project (PCaP) is carried out as a collaborative study supported by the Department of Defense contract DAMD 17‐03‐2‐0052 and W81XWH‐17‐1‐0119. PCaP is a collaborative study supported by the Department of Defense contract DAMD 17‐03‐2‐0052 and W81XWH‐17‐1‐0119.
KC M, Oral E, Rung AL, et al. Prostate cancer aggressiveness and financial toxicity among prostate cancer patients. The Prostate. 2023;83:44‐55. 10.1002/pros.24434
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from PCaP management team. Restrictions apply to the availability of these data.
REFERENCES
- 1. National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP). Health and economic costs of chronic diseases. 2021. https://www.cdc.gov/chronicdisease/about/costs/index.htm
- 2. Mariotto AB, Enewold L, Zhao J, Zeruto CA, Yabroff KR. Medical care costs associated with cancer survivorship in the United States. Cancer Epidemiol Biomarkers Prev. 2020;29(7):1304‐1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martin MY, Fouad MN, Oster RA, et al. What do cancer patients worry about when making decisions about treatment? Variation across racial/ethnic groups. Supp Care Cancer. 2014;22(1):233‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Cancer Institute . Financial toxicity (financial distress) and cancer treatment (PDQ(R)). In: PDQ cancer information summaries. Bethesda (MD). 2019.
- 5. Zafar SY, Abernethy AP. Financial toxicity, part I: a new name for a growing problem. Oncology. 2013;27(2):80‐81. [PMC free article] [PubMed] [Google Scholar]
- 6. Gordon LG, Merollini KMD, Lowe A, Chan RJ. A systematic review of financial toxicity among cancer survivors: we can't pay the co‐pay. Patient. 2017;10(3):295‐309. [DOI] [PubMed] [Google Scholar]
- 7. Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: a pilot study assessing out‐of‐pocket expenses and the insured cancer patient's experience. Oncologist. 2013;18(4):381‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meropol NJ, Schulman KA. Cost of cancer care: issues and implications. J Clin Oncol. 2007;25(2):180‐186. [DOI] [PubMed] [Google Scholar]
- 9. Lentz R, Benson AB, 3rd , Kircher S. Financial toxicity in cancer care: prevalence, causes, consequences, and reduction strategies. J Surg Oncol. 2019;120(1):85‐92. [DOI] [PubMed] [Google Scholar]
- 10. Stone BV, Laviana AA, Luckenbaugh AN, et al. Patient‐reported financial toxicity associated with contemporary treatment for localized prostate cancer. J Urol. 2021;205(3):761‐768. [DOI] [PubMed] [Google Scholar]
- 11. Bhanvadia SK, Psutka SP, Burg ML, et al. Financial toxicity among patients with prostate, bladder, and kidney cancer: a systematic review and call to action. Eur Urol Oncol. 2021;4(3):396‐404. [DOI] [PubMed] [Google Scholar]
- 12. Blinder VS, Gany FM. Impact of cancer on employment. J Clin Oncol. 2020;38(4):302‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dumas A, Vaz Luis I, Bovagnet T, et al. Impact of breast cancer treatment on employment: results of a multicenter prospective cohort study (CANTO). J Clin Oncol. 2020;38(7):734‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roetzheim RG, Pal N, Tennant C, et al. Effects of health insurance and race on early detection of cancer. J Natl Cancer Inst. 1999;91(16):1409‐1415. [DOI] [PubMed] [Google Scholar]
- 15. Roetzheim RG, Pal N, Tennant C, et al. Effects of health insurance and race on early detection of cancer. JNCI: J Nat Cancer Inst. 1999;91(16):1409‐1415. [DOI] [PubMed] [Google Scholar]
- 16. Calo WA, Vernon SW, Lairson DR, Linder SH. Associations between contextual factors and colorectal cancer screening in a racially and ethnically diverse population in Texas. Cancer Epidemiol. 2015;39(6):798‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yabroff KR, Reeder‐Hayes K, Zhao J, et al. Health insurance coverage disruptions and cancer care and outcomes: systematic review of published research. J Natl Cancer Inst. 2020;112(7):671‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nabi J, Tully KH, Cole AP, et al. Access denied: the relationship between patient insurance status and access to high‐volume hospitals. Cancer. 2021;127(4):577‐585. [DOI] [PubMed] [Google Scholar]
- 19. Fossati N, Nguyen DP, Trinh Q‐D, et al. The impact of insurance status on tumor characteristics and treatment selection in contemporary patients with prostate cancer. J Nat Comp Cancer Net. 2015;13(11):1351‐1358. [DOI] [PubMed] [Google Scholar]
- 20. Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer‐related financial problems more likely to forgo or delay medical care? Cancer. 2013;119(20):3710‐3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dowling EC, Chawla N, Forsythe LP, et al. Lost productivity and burden of illness in cancer survivors with and without other chronic conditions. Cancer. 2013;119(18):3393‐3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hendriksen E, Williams E, Sporn N, Greer J, DeGrange A, Koopman C. Worried together: a qualitative study of shared anxiety in patients with metastatic non‐small cell lung cancer and their family caregivers. Supp Care Cancer. 2015;23(4):1035‐1041. [DOI] [PubMed] [Google Scholar]
- 23. Regenbogen SE, Veenstra CM, Hawley ST, et al. The personal financial burden of complications after colorectal cancer surgery. Cancer. 2014;120(19):3074‐3081. [DOI] [PubMed] [Google Scholar]
- 24. Lynch JW, Kaplan GA, Shema SJ. Cumulative impact of sustained economic hardship on physical, cognitive, psychological, and social functioning. N Engl J Med. 1997;337(26):1889‐1895. [DOI] [PubMed] [Google Scholar]
- 25. Ver Hoeve ES, Ali‐Akbarian L, Price SN, Lothfi NM, Hamann HA. Patient‐reported financial toxicity, quality of life, and health behaviors in insured US cancer survivors. Supp Care Cancer. 2021;29(1):349‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zafar SY, McNeil RB, Thomas CM, Lathan CS, Ayanian JZ, Provenzale D. Population‐based assessment of cancer survivors' financial burden and quality of life: a prospective cohort study. J Oncol Pract. 2015;11(2):145‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramsey SD, Bansal A, Fedorenko CR, et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol. 2016;34(9):980‐986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. PDQ® Adult Treatment Editorial Board. PDQ financial toxicity (financial distress) and cancer treatment. 2021. Accessed May 14, 2021. https://www.cancer.gov/about-cancer/managing-care/track-care-costs/financial-toxicity-pdq
- 29. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7‐33. [DOI] [PubMed] [Google Scholar]
- 30. National Cancer Institute: Surveillance Epidemiology and End Results (SEER) Program . Cancer stat facts. Prostate Cancer . 2021. https://seer.cancer.gov/statfacts/html/prost.html
- 31. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2019−2021. American Cancer Society; 2019. [Google Scholar]
- 32. The American Cancer Society (ACS). Survival rates for prostate cancer. 2021. https://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/survival-rates.html.
- 33. National Cancer Institute: Surveillance Epidemiology and End Results (SEER) Program. Prostate: SEER 5‐year age‐adjusted incidence rates. 2021. 2014‐2018.
- 34. Raldow A, Hamstra DA, Kim S, Yu JB. Salvage external beam radiotherapy for prostate cancer after radical prostatectomy: current status and controversy. Oncology. 2010;24(8):692‐700. [PubMed] [Google Scholar]
- 35. Gustavsen G, Gullet L, Cole D, Lewine N, Bishoff JT. Economic burden of illness associated with localized prostate cancer in the United States. Future Oncol. 2020;16(1):4265‐4277. [DOI] [PubMed] [Google Scholar]
- 36. Nekhlyudov L, Walker R, Ziebell R, Rabin B, Nutt S, Chubak J. Cancer survivors' experiences with insurance, finances, and employment: results from a multisite study. J Cancer Sur. 2016;10(6):1104‐1111. [DOI] [PubMed] [Google Scholar]
- 37. Miller DC, Litwin MS, Bergman J, et al. Prostate cancer severity among low income, uninsured men. J Urol. 2009;181(2):579‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schroeder JC, Bensen JT, Su LJ, et al. The North Carolina‐Louisiana Prostate Cancer Project (PCaP): methods and design of a multidisciplinary population‐based cohort study of racial differences in prostate cancer outcomes. Prostate. 2006;66(11):1162‐1176. [DOI] [PubMed] [Google Scholar]
- 39. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Accurint. LexisNexis commercial location and research online service. 2005. http://www.accurint.com/
- 41. de Souza JA, Yap BJ, Hlubocky FJ, et al. The development of a financial toxicity patient‐reported outcome in cancer: the COST measure. Cancer. 2014;120(20):3245‐3253. [DOI] [PubMed] [Google Scholar]
- 42. de Souza JA, Yap BJ, Wroblewski K, et al. Measuring financial toxicity as a clinically relevant patient‐reported outcome: the validation of the COmprehensive score for financial toxicity (COST). Cancer. 2017;123(3):476‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bouberhan S, Shea M, Kennedy A, et al. Financial toxicity in gynecologic oncology. Gynecol Oncol. 2019;154(1):8‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. DeVita VT, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg's Cancer: principles & Practice of Oncology. 11th ed. Wolters Kluwer; 2019. [Google Scholar]
- 45. Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure‐mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. VanderWeele TJ. Mediation analysis: a practitioner's guide. Annu Rev Public Health. 2016;37:17‐32. [DOI] [PubMed] [Google Scholar]
- 47. Yabroff KR, Dowling EC, Guy GP Jr., et al. Financial hardship associated with cancer in the United States: findings from a population‐based sample of adult cancer survivors. J Clin Oncol. 2016;34(3):259‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ver Hoeve ES, Ali‐Akbarian L, Price SN, Lothfi NM, Hamann HA. Patient‐reported financial toxicity, quality of life, and health behaviors in insured US cancer survivors. Supp Care Cancer. 2021;29(1):349‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Honda K, Gyawali B, Ando M, et al. Prospective survey of financial toxicity measured by the comprehensive score for financial toxicity in Japanese patients with cancer. J Glob Oncol. 2019;5:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Group AS, Kimman M, Jan S, et al. Catastrophic health expenditure and 12‐month mortality associated with cancer in Southeast Asia: results from a longitudinal study in eight countries. BMC Med. 2015;13:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ward MM, Ullrich F, Matthews K, et al. Who does not receive treatment for cancer? J Oncol Pract. 2013;9(1):20‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rotter J, Spencer JC, Wheeler SB. Financial toxicity in advanced and metastatic cancer: overburdened and underprepared. J Oncol Pract. 2019;15(4):e300‐e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Data Availability Statement
The data that support the findings of this study are available from PCaP management team. Restrictions apply to the availability of these data.


