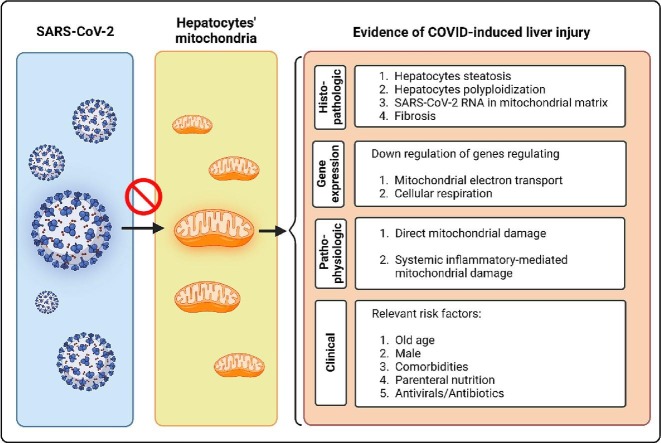
Graphical abstract
Histopathologic, transcriptomic, pathophysiologic, and clinical characteristics of COVID-induced liver injury from the mitochondria’ eye view.
Keywords: ATP, COVID-19, Cytokine storm, Hepatic impairment, Mitochondria, SARS-CoV-2
Abstract
Liver damage is a common sequela of COVID-19 (coronavirus disease 2019), worsening the clinical outcomes. However, the underlying mechanism of COVID-induced liver injury (CiLI) is still not determined. Given the crucial role of mitochondria in hepatocyte metabolism and the emerging evidence denoting SARS-CoV-2 can damage human cell mitochondria, in this mini-review, we hypothesized that CiLI happens following hepatocytes’ mitochondrial dysfunction. To this end, we evaluated the histologic, pathophysiologic, transcriptomic, and clinical features of CiLI from the mitochondria’ eye view. Severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2), the causative agent of COVID-19, can damage hepatocytes through direct cytopathic effects or indirectly after the profound inflammatory response. Upon entering the hepatocytes, the RNA and RNA transcripts of SARS-CoV-2 engages the mitochondria. This interaction can disrupt the mitochondrial electron transport chain. In other words, SARS-CoV-2 hijacks the hepatocytes’ mitochondria to support its replication. In addition, this process can lead to an improper immune response against SARS-CoV-2. Besides, this review outlines how mitochondrial dysfunction can serve as a prelude to the COVID-associated cytokine storm. Thereafter, we indicate how the nexus between COVID-19 and mitochondria can fill the gap linking CiLI and its risk factors, including old age, male sex, and comorbidities. In conclusion, this concept stresses the importance of mitochondrial metabolism in hepatocyte damage in the context of COVID-19. It notes that boosting mitochondria biogenesis can possibly serve as a prophylactic and therapeutic approach for CiLI. Further studies can reveal this notion.
Nomenclature
Abbreviations
- ACE2
Angiotensin-converting enzyme 2
- AMPK
Adenosine monophosphate-activated protein kinase
- AST
Aspartate aminotransferase
- AT1R
Angiotensin II type 1 receptor
- ATP
Adenosine triphosphate
- CiLI
COVID-induced liver injury
- COVID-19
Coronavirus disease 2019
- DaG
Diammonium glycyrrhizinate
- DAMPs
Damage-associated molecular patterns
- dsRNA
Double-stranded RNA
- GPX4
Glutathione peroxidase-4
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HIF-1
Hypoxia-inducible factor 1
- IFN
Interferon-1
- IL
Interleukin
- MAVS
Mitochondrial antiviral signaling
- MgIG
Magnesium isoglycyrrhizinate
- MRSA
Methicillin-resistant staphylococcus aureus
- MSSA
Methicillin-sensitive staphylococcus aureus
- mTOR
Mammalian target of rapamycin
- NADH
Nicotinamide adenine dinucleotide
- NASH
Non-alcoholic steatohepatitis
- NSP
Non-structural protein
- ORF
Open reading frame
- pDC
Plasmacytoid dendritic cells
- PGC-1α
Proliferator-activated receptor gamma coactivator 1-alpha
- RAS
Renin-angiotensin system
- ROS
Reactive oxygen species
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- TCA
Tricarboxylic acid
- TLR
Toll-like receptor
- TMPRSS2
Transmembrane serine protease 2
- TNF-α
Tumor necrosis factor-α
- USP30
Ubiquitin-specific peptidase 30
1. Introduction
The ongoing coronavirus disease 2019 (COVID-19) is a major health threat worldwide. This viral disease happens upon colonization and proliferation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the respiratory tract. Thereafter, COVID-19 enters the inflammatory phase, in which dysregulated systemic inflammatory response (termed as cytokine storm) can lead to multiorgan failure (Griffin et al., 2021). After the lungs, the liver is the second most affected organ in the context of COVID-19 (Wanner et al., 2022). Abnormally high levels of liver enzymes, a marker of hepatocyte damage and death, are commonly observed in patients with COVID-19, resulting in hospitalization, mechanical ventilation, and death (Lei et al., 2020). COVID-induced liver injury (CiLI) is defined as any liver damage occurring during the disease course regardless of pre-existing liver disease (Gracia-Ramos et al., 2021). In different studies, the incidence of CiLI varied from 14 to 53 % of infected cases (Chen et al., 2021). SARS-CoV-2 can damage hepatic tissue through direct effects on hepatocytes or indirectly through systemic inflammation (see Section 2). The histologic manifestations of CiLI are widely distinct, including hepatocyte steatosis, polyploidization, and portal and lobular inflammation, which can lead to hepatic sinus congestion and microthrombosis (Wang et al., 2020). It has been demonstrated that CiLI can significantly increase the risk of COVID severity and mortality (Lei et al., 2020). This is because liver is a major organ in the human body and regulates the metabolic flexibility of the whole body in response to fluctuations in energy demands and supplies (Morio et al., 2021). Despite significant findings, it is still not clarified how SARS-CoV-2 injures hepatic cells. Considering mitochondria are the master regulators of cellular metabolism (Spinelli and Haigis, 2018), and given that SARS-CoV-2 can damage human cell mitochondria (De la Cruz-Enríquez et al., 2021), we hypothesized that COVID-induced liver damage manifests mitochondrial dysfunction.
Mitochondria are the major organelles responsible for energy production for cell survival and function, so named the human cells’ powerhouses. Besides, mitochondria regulate numerous signaling pathways, including apoptosis and calcium homeostasis (Murphy et al., 2016). In addition, functional mitochondria rapidly sense and respond to intrinsic (e.g., DNA mutation, nutrient deprivation) and extrinsic stressors (e.g., toxins and infectious agents) in different ways—including fusion/fission, intracellular movements, changing in their mass and metabolism, and remodeling their cristae—to improve the cellular tolerance and reaction to the imposed stressor (Eisner et al., 2018). In hepatocytes, mitochondria have specific roles in regulating metabolism (e.g., gluconeogenesis, lipogenesis, ureagenesis, and ketogenesis) and coordinating innate immune response (Morio et al., 2021). A large body of evidence noted that mitochondria are the targets for the common risk factors of liver diseases, including alcohol (Abdallah and Singal, 2020), hepatitis B virus (HBV) (Hossain et al., 2020), hepatitis C virus (HCV) (Kim et al., 2014), and antibiotics (e.g., ciprofloxacin and amoxicillin/clavulanate) (Oyebode et al., 2019). In addition, it has been demonstrated that mitochondrial dysfunction is associated with chronic liver diseases in the context of obesity, diabetes mellitus, and non-alcoholic steatohepatitis (NASH) (Morio et al., 2021).
With these understandings in mind, we sought to find if hepatic cells’ mitochondria are the main targets of SARS-CoV-2. This review summarizes the current knowledge about the CiLI from the mitochondria’ eye view. The following sections describe the mechanisms underlying the CiLI (2, 3) and its risk factors (Section 4). This concept can open an avenue to reduce liver damage incidence and severity in the context of COVID-19 by applying tactics to improve mitochondria biogenesis, as outlined in Section 5.
2. Mechanisms underlying COVID-induced liver injury: Current understandings
Patients with COVID-19 have different degrees of liver damage, but the underlying mechanism remains unclear. Liver damage in the context of COVID-19 may occur through direct cytopathic effects of SARS-CoV-2, or secondary to excessive systemic inflammatory response (Zhang et al., 2022). This section outlines these two mechanisms. Section 3 presents how mitochondria might involve in these processes.
2.1. Direct effects
SARS-CoV-2 may access liver tissue through blood circulation or retrograde migration via the portal venous system (Zhang et al., 2022). The latter is more probable because the infectious virus is generally at low viral loads in the systemic circulation of patients with COVID-19 (Andersson et al., 2020). Upon access to liver tissue, the viruses bind the angiotensin-converting enzyme 2 (ACE2) receptor on hepatocytes by their surface spike proteins (so-called S proteins). The SARS-CoV-2 attachment to the host cells is 10–20 times more potent than previous coronavirus variants (Wrapp et al., 2020). After binding, the host transmembrane serine protease 2 (TMPRSS2) primes ACE2 and facilitates virus entry into the cell. Once entering the hepatocyte, the virus becomes uncoated and releases its RNA into the cytosol to run intracellular proliferation by applying the host cells’ facilities (Nardo et al., 2021). In this process, the virus's RNA is translated into structural (e.g., M protein) and nonstructural proteins (NSP1 to 16). In parallel, the viral genome is replicated to generate more RNA molecules. The newly synthesized viral genome is enveloped by the proteins, and the new viruses are released from host cells via exocytosis (Nardo et al., 2021). In this context, SARS-Cov-2 can damage hepatocytes through different mechanisms: (1) hepatocytes are metabolically active cells, a feature making them sensitive to hypoxia. COVID-19 lead to different degrees of hypoxemia, secondary to pulmonary involvement. In addition, SARS-CoV-2 can directly damage hepatic sinusoidal endothelial cells, leading more liver tissue hypoxia. Under hypoxic conditions, reactive oxygen species (ROSs) accumulate in hepatocytes, propelling them toward cell death (Bhogal et al., 2011). Furthermore, with a decrease in oxygen pressure, the expression of ACE2 receptor on hepatocytes gradually increases, which can facilitate the entry of viruses into hepatocytes (Paizis et al., 2005); (2) claudin proteins on cholangiocytes maintain the barrier function of bile ductal epithelium and keep the bile acid flow in the intercellular space. It has been demonstrated that SARS-CoV-2 can downregulate the claudin expression in cholangiocytes, leading bile acid reflux and hepatocytes damages (Zhao et al., 2020). However, further evaluation of COVID patients’ liver tissues has opened new dimensions of CiLI, the mitochondria. Section 3 describes how mitochondria can be involved in the CiLI process.
2.2. Systemic inflammation
Besides direct effects on tissue, SARS-CoV-2 can induce a profound systemic inflammation (a.k.a cytokine storm) that damages vital organs, including the liver (Taneva et al., 2021). In this process, SARS-CoV-2 dysregulates the renin-angiotensin system (RAS) signaling pathway and promotes angiotensin toward the angiotensin II type 1 receptor (AT1R) axis, leading to a robust release of pro-inflammatory cytokines, such as interferon-1 (IFN-1), interleukine-6 (IL-6), prostaglandins, and tumor necrosis factor-α (TNF-α), from immune cells (Ali et al., 2021). The RAS imbalance into the pro-inflammatory state has pro-oxidative, pro-fibrotic, and pro-apoptotic consequences on human tissues (El-Arif et al., 2022). In a study on patients with COVID-19, Effenberger et al. found that aspartate aminotransferase (AST), as a marker of hepatocytes damage and death, was significantly elevated in patients with a higher IL-6, ferritin, and c-reactive protein (CRP) (Effenberger et al., 2021). This finding demonstrates that although pro-inflammatory cytokines are required for a proper anti-viral immune activation, their release must be carefully controlled due to the detrimental effects of profound cytokine production on normal tissues. In addition, acute and exaggerated immune activation against SARS-CoV-2 can deplete the energy repositories of immune cells, which reduce their sustained immunosurveillance against the pathogen (Taghizadeh-Hesary and Akbari, 2020). Section 3.2 discusses how mitochondria can be involved in the COVID 19-induced massive cytokine release.
3. Mechanisms underlying COVID-induced liver injury: How mitochondria are involved
3.1. Direct effects
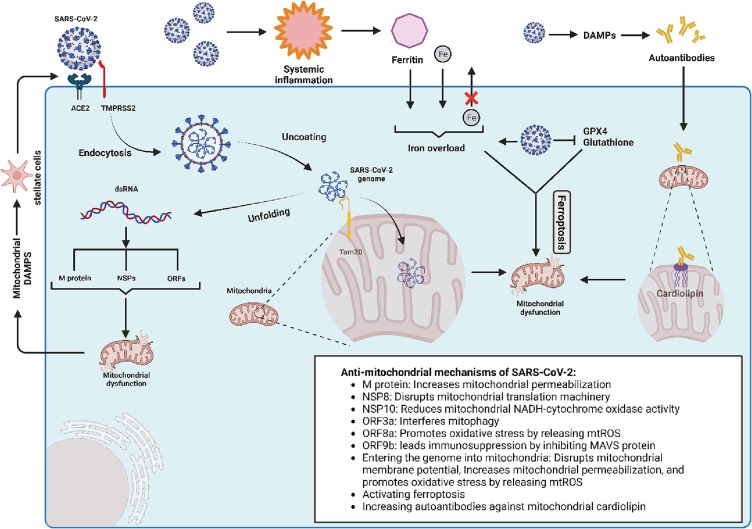
Liver autopsy specimens of individuals with COVID-19 have revealed three crucial findings: binuclear hepatocytes (a.k.a polyploidization), steatosis, and SARS-CoV-2 particles besides the swollen mitochondria (Wang et al., 2020). The latter finding might address that hepatocytes’ mitochondria are the targets of SARS-CoV-2. Further studies endorsed this notion. In a study on Huh7 cells, Shang et al. found the double-stranded RNA (dsRNA) of SARS-CoV-2 in the mitochondria matrix. The investigators pointed out that the viral RNA can enter the mitochondria via the Tom20 receptor to facilitate replication. This interaction can disrupt mitochondrial membrane depolarization and increase mitochondrial membrane permeability resulting in mitochondrial ROS release to the cytosol and host cell apoptosis (Shang et al., 2021). This process has also been demonstrated in immune cells (Ganji and Reddy, 2020). In a normal state, mitochondria need to constantly run fission and fusion to maintain cellular homeostasis. However, in the case of COVID-19, the fission process is utterly inhibited, causing mitochondrial elongation and providing a receptive intracellular environment for viral replication (Holder and Reddy, 2021). The release of SARS-CoV-2′s RNA to the cytosol can disrupt mitochondrial function in several other ways (Fig. 1 ):
Fig. 1.
Schematic view of COVID-induced liver injury from the perspective of mitochondria. ACE2 indicates angiotensin-converting enzyme 2; DAMPs, damage-associated molecular patterns; dsRNA, double-stranded RNA; Fe, iron; GPX4, glutathione peroxidase-4; MAVS, mitochondrial antiviral signaling; mtROS, mitochondrial reactive oxygen species; NADH, nicotinamide adenine dinucleotide; NSP, non-structural protein; ORF, open reading frame; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMPRSS2, transmembrane serine protease 2.
(1) the structural M protein of SARS-CoV-2 can increase mitochondrial outer membrane permeabilization, leading to host cell apoptosis through the release of caspases (Srinivasan et al., (2021)); (2) the NSP8 interferes with mitochondrial translation machinery, leading to mitochondrial dysfunction (Gordon et al., 2020); (3) the NSP10 reduces the NADH-cytochrome oxidase II activity, leading mitochondrial dysfunction through mitochondrial membrane depolarization (Srinivasan et al., 2021); (4) the open reading frame 3a (ORF3a) encoded protein interacts with ubiquitin-specific peptidase 30 (USP30) and disrupt the mitochondrial homeostasis by inhibiting the mitophagy of infected mitochondria (Shang et al., 2021); (5) the ORF9b impedes the IFN-mediated antiviral response by inhibiting mitochondrial antiviral signaling (MAVS) protein (Shi et al., 2014); (6) the ORF8a can lead to oxidative stress through an increase in ROS production (Srinivasan et al., 2021).
Recent evidence has pointed out another mechanism of CiLI, ferroptosis. It is an iron-dependent programmed cell death characterized by iron overload and lipid peroxidation through the Fenton reaction. During ferroptosis, mitochondria are mainly targeted by iron-caused oxidative stress, representing mitochondrial shrinkage, cristae deformation, and increased mitochondrial membrane potential (Dixon et al., 2012). It has been demonstrated that SARS-CoV-2 can lead to ferroptosis through several mechanisms, including (1) intracellular iron overload through transferrin receptor-1 (Chen et al., 2022, Suriawinata and Mehta, 2022); (2) suppression of GPX4 (glutathione peroxidase-4) expression that is a negative regulator of ferroptosis (Wang et al., 2021); (3) intracellular iron overload by increasing the serum ferritin (as an acute phase reactant) and running ferritniophagy (Mancias et al., 2014); (4) decrease in cellular glutathione level that inhibits lipid peroxidation (Bartolini et al., 2021); and (5) inhibiting the cellular efflux of iron through ferroportin by activating hepcidin (Banchini et al., 2020). Ferroptosis is an immunogenic process (Sun et al., 2020). Therefore, COVID-induced ferroptosis can aggravate CiLI. In a histopathological examination of patients with COVID-19, Del Nonno et al. demonstrated that iron overload is the possible mechanism of CiLI (Del Nonno et al., 2021). In a clinical study, Szabo et al. demonstrated that higher baseline hepcidin and ferritin levels were associated with a higher multi-organ dysfunction rate in patients with COVID-19 (Szabo et al., 2022). The negative impacts of SARS-CoV-2 on mitochondria and the resultant reduced cellular glutathione levels can impair redox homeostasis and further propose the mitochondria to systemic inflammation and ferroptosis-induced oxidative damage (Bartolini et al., 2021, Grossini et al., 2021).
COVID-19 can instantly increase liver fibrogenesis (Kolesova et al., 2021). This event can be justified by the negative impacts of SARS-CoV-2 on hepatocyte mitochondria. It has been demonstrated that releasing mitochondria-derived damage-associated molecular patterns (DAMPs) from damaged mitochondria can drive liver fibrosis by activating hepatic stellate cells (An et al., 2020). Under the condition of liver fibrosis, the expression of ACE2 increases (Li et al., 2022). This effect can further aggravate the CiLI. COVID-induced oxidative stress can lead to polyploidization of hepatocytes (Wang et al., 2020), which has also been reported in the context of oxidative stress due to mitochondrial dysfunction in patients with NASH (Gentric et al., 2015). Another histopathological manifestation of CiLI is micro/macrovesicular steatosis, which is a hallmark of mitochondria dysfunction in patients with NASH (Xu et al., 2021). Therefore, mitochondria dysfunction can justify the histopathological appearance of damaged hepatocytes in the context of COVID-19.
Besides the histopathological findings, the gene expression signature of SARS-CoV 2-infected hepatocytes addresses the mitochondrial dysfunction. In a bioinformatic analysis of forty-two liver autopsy specimens, Wanner et al. found that SARS-CoV-2 can lead to a downregulation of several genes in hepatocytes, most of which are responsible for regulating mitochondrial electron transport chain and cellular respiration (Wanner et al., 2022). These findings reflect that mitochondrial engagement by SARS-CoV-2 can dysregulate mitochondrial function, leading to hepatocyte dysfunction and death.
3.2. Indirect effects
3.2.1. Systemic inflammation
As alluded to above, SARS-CoV-2 dysregulates the immune cells to produce massive pro-inflammatory cytokines. Among the immune cells, plasmacytoid dendritic cells (pDCs) are of special interest as they are the sentinels in the innate immune response to SARS-CoV-2. In addition, pDCs are the main source of IFN-1 as a pro-inflammatory cytokine in the COVID-induced cytokine storm (Van der Sluis et al., 2022). The pDCs detect the viruses by their superficial toll-like receptor 7 (TLR7). Upon binding to SARS-CoV-2, TLR7 mediates the production of IFN-1 (Van der Sluis et al., 2022). The regulated release of IFN-1 is dependent on the endo-lysosomal PH; in order that an increase in the endo-lysosomal PH would enhance the IFN production (Rebbapragada et al., 2016). It has been demonstrated that regulating the endo-lysosomal PH is adenosine triphosphate (ATP)-dependent process; in order that a decrease in cellular ATP can increase the endo-lysosomal PH (Rebbapragada et al., 2016). Therefore, COVID-induced cytokine storm might happen upon mitochondrial damage in the following two ways: (a) SARS-CoV-2 damages the pDCs’ mitochondria directly (see Section 3.1 or through systemic inflammation (see below); mitochondrial dysfunction results in a reduction of the pDCs’ ATP content; reduce in ATP would increase the endo-lysosomal PH of pDCs; IFN-1 production disproportionately increases in a high endo-lysosomal PH, which would result in COVID’s cytokine storm. (b) SARS-CoV-2 can trigger systemic inflammation by damaging the mitochondria and releasing the mitochondrial DNA (Singh et al., 2020). This effect is mediated through several signaling pathways, including TLR and cGAS-cGAMP-STING pathways (Mahmoodpoor et al., 2022). It has been demonstrated that human alveolar epithelial cells with dysfunctional mitochondria release large amounts of cytokines following SARS-CoV-2 infection. This event aggravates systemic inflammation-induced oxidative stress, which impairs the coagulation cascade and contributes to microbiota dysbiosis. The resultant vicious cycle aggravates clotting events and systemic inflammation (Saleh et al., 2020).
In patients with COVID-19, angiotensin II leads to intracellular oxidative stress through AT1R-dependent induction of NADPH oxidase, which can damage intracellular organelles-including mitochondria (El-Arif et al., 2022). Subsequent to the impaired mitochondrial membrane potential, mitochondrial K-ATP channels will open that further aggravating the oxidative stress by releasing the mitochondrial ROS (El-Arif et al., 2022). This process can propel the status to a vicious cycle; in order that the oxidative stress can damage the pDCs’ mitochondria, which in turn enhances the TLR7-dependent IFN-1 production. This section illustrated how mitochondrion can serve as either victim or murderer of COVID-associated cytokine storm.
3.2.2. Autoimmunity
Section 3.1 noted that COVID-19 can damage hepatocytes’ mitochondria through ferroptosis. In this process, iron-activated ROSs oxidize cellular lipidic structures, including mitochondrial membrane phospholipids. Notably, Wiernicki et al. demonstrated that cardiolipin (a phospholipid in the mitochondrial inner membrane) is resistant to ferroptosis (Wiernicki et al., 2020). It is a key phospholipid playing crucial roles in mitochondrial processes, including respiration and energy production (Dudek, 2017). It has been shown that COVID-19 can damage mitochondrial cardiolipin by damaging human cells and releasing DAMPs, such as extracellular DNA. Bertin et al. demonstrated that in response to extracellular DNA, the IgG autoantibodies directly attack mitochondrial cardiolipins (Bertin et al., 2022).
4. Risk factors of COVID-induced liver injury and how mitochondria play a role
The available studies have determined different risk factors of liver damage in patients with COVID-19. However, the underlying mechanism(s) still needs to be determined. This section outlines how mitochondrial dysfunction can link the proposed risk factors and CiLI.
4.1. Old age
It has been evidenced that old age is a significant risk factor for CiLI in terms of incidence (Zhang et al., (2021)) and severity (Chen et al., 2021). However, the underlying reasons are still unknown. The association between aging and mitochondrial dysfunction is well established. Aging is typified by a progressive decline in mitochondrial activity. This can be manifested as impaired redox homeostasis, fission/fusion, mitophagy, and apoptosis (Lima et al., 2022). Therefore, one may link the increased risk of CiLI in older ages because of impaired mitochondrial function.
4.2. Gender
The literature regarding gender as a risk factor for CiLI is inconsistent. Shen et al. demonstrated that the male sex is an independent RF of CiLI. (Shen et al., (2021)) This finding was confirmed in a large cohort by Chen et al. from Huazhong University (Chen et al., 2021). On the other hand, Zhang et al. reported more CiLI in female patients (Zhang et al., 2021). The latter study’s findings might enface bias due to not excluding patients with a history of chronic liver diseases (e.g., viral hepatitis, alcoholic liver disease, liver cancer, and autoimmune liver disease), a crucial issue which was considered by Shen et al. With this preface, we considered male sex, as a risk factor of CiLI in this paper. Emerging evidence has noted that both mitochondria content and function are higher in females, Silaidos et al. (2018) which can justify why male patients are more susceptible to developing CiLI.
4.3. Comorbidities
It has been demonstrated that patients with diabetes mellitus, hypertension, cardiovascular disease, or cancer are at a greater risk of developing CiLI (Shen et al., 2021). However, the underlying mechanisms are remained to be defined. Available studies have demonstrated that mitochondrial biogenesis is impaired in patients with diabetes mellitus in terms of redox homeostasis, fission/fusion, and respiratory chain activity (Rovira-Llopis et al., 2017). Also, it has been demonstrated that patients with essential hypertension and cardiovascular disorders have some extent of mitochondrial dysfunction (Ding et al., 2013, Yang et al., 2022). Therefore, patients’ comorbidities, as risk factors of CiLI, are justifiable through mitochondrial dysfunction.
4.4. Parenteral nutrition
A large cohort study on hospitalized patients with COVID-19 indicated that patients receiving intravenous nutrition are at greater risk for developing CiLI (Zhang et al., 2021). However, the underlying mechanism was not outlined. In a rat model receiving parenteral nutrition —containing dextrose, amino acids, fat emulsion, minerals, and vitamins— the investigators found degrees of hepatic mitochondrial dysfunction (Katayama et al., 1990). In this study, the hepatocytes’ mitochondria of rats receiving parenteral nutrition had a lower capacity for oxidative respiration and ATP production. The detrimental effects of parenteral nutrition on mitochondria activity may justify how it increases the risk of liver injury in patients with COVID-19.
4.5. Medications
Hepatotoxicity is an adverse effect of a subset of drugs that COVID patients receive during hospitalization. This issue complicates the clinical judgment on the reason for CiLI. This section outlines how the common medications administered for patients with COVID-19 can damage liver tissue and how mitochondria can be involved in this process.
4.5.1. Antivirals
In a retrospective study on 192 hospitalized COVID-19 patients, Zhan et al. found that the administration of ritonavir (a protease inhibitor) is a significant risk factor for CiLI (Zhan et al., 2021). It has been demonstrated that ritonavir can dysregulate the mitochondrial membrane potential, which results in hepatocytes apoptosis following the release of mitochondrial cytochrome c into the cytosol (Ganta and Chaubey, 2019). Studies on the clinical safety of other antiviral agents used in patients with COVID-19 have demonstrated their potential hepatotoxicity. For example, remdesivir (an RNA-dependent RNA polymerase inhibitor) can damage liver tissue, usually in the form of mild-to-moderate elevation in transaminases (Aleem et al., 2021). Besides viral RNA, remdesivir can also inhibit the human mitochondrial RNA polymerase. This interference leaves open the possibility of mitochondrial toxicity. An experimental study on marine renal and cardiac cells demonstrated that remdesivir disrupted the metabolism and proliferation of kidney cells and reduced the heartbeat and contractility. These adverse effects were due to interference with mitochondrial membrane potential and respiratory chain (Merches et al., 2022). However, in another study on hepatocytes, remdesivir’s inhibitory effect on mitochondrial RNA polymerase was weak and did not translate to the defect in mitochondrial respiration (Bjork and Wallace, 2021). These findings demonstrated that different tissues have different sensitivity to the mitochondrial toxicities of remdesivir.
4.5.2. Antibiotics
In a large cohort study, Zhang et el. noted that antibiotic administration significantly increased the risk of liver damage in hospitalized patients with COVID-19 (Zhang et al., 2021). However, the study did not outline the name/group of antibiotics. For hospitalized patients with COVID-19, antibiotics are commonly applied for its complications, such as bacterial pneumonia. To this end, the common antibiotics are vancomycin, oxacillin, and amoxicillin/clavulanate (for methicillin-susceptible staphylococcus aureus [MSSA]), linezolid (for methicillin-resistant staphylococcus aureus [MRSA]), and colistin (for acinetobacter baumannii) (Posteraro et al., 2021). Among these, the hepatocytes’ mitotoxicity of vancomycin (Wu and Zhou, 2022), amoxicillin/clavulanate (Oyebode et al., 2019), and linezolid (De Vriese et al., 2006) has been evidenced. Therefore, commonly used antibiotics during the COVID-19 course can lead to CiLI by dysregulating the mitochondrial function of hepatocytes.
5. How boosting mitochondria can protect liver from COVID-induced injury
In Zhang et al.’s cohort, three hepatoprotective agents (diammonium glycyrrhizinate, magnesium isoglycyrrhizinate, and polyene phosphatidylcholine) significantly reduced the risk of CiLI (Zhang et al., 2021). However, the underlying mechanisms were not noted. This section describes the effects of the aforementioned agents on mitochondria biogenesis and how this action can reduce the incidence/severity of CiLI.
Diammonium glycyrrhizinate (DaG) is a glycyrrhizic acid derived from the root of Radix Glycyrrhizae. It has been shown that DaG improves the cellular peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) content (Zhu et al., 2012), which in turn upregulates mitochondria biogenesis (Onishi et al., 2014). Magnesium isoglycyrrhizinate (MgIG) is a novel preparation of glycyrrhizic acid. MgIG can improve mitochondria biogenesis through upregulating 5′-adenosine monophosphate-activated protein kinase (AMPK) expression (Herzig and Shaw, 2018, Jiang et al., 2020). Polyene phosphatidylcholine, a phospholipid enriched in polyunsaturated fatty acids, has a similar mechanism of action to boost mitochondrial metabolism via AMPK upregulation (Herzig and Shaw, 2018, Lu et al., 2022). But how can mitochondrial activation reduce the risk of CiLI?
(1) As noted in Section 1, SARS-CoV-2 can modulate the hepatocytes’ mitochondrial function to impede a proper innate immune response. Hence, patients with mitochondrial dysfunction are more susceptible to SARS-CoV-2 invasion due to an imperfect frontline immune reaction (Morio et al., 2021). Therefore, more potent mitochondria can reduce the severity of developing COVID-19, which further reduces the risk of CiLI. Another possible mechanism is by impeding/reducing the COVID-associated cytokine storm. (2) As noted in Section 3.2, cytokine storm can lead to mitochondrial dysfunction, which in turn aggravates the release of IFN-1 from pDCs (Rebbapragada et al., 2016). Therefore, boosting mitochondrial activity can break this vicious cycle and alleviate/prevent the massive release of cytokines. (3) Another mechanism by which boosting mitochondrial metabolism can inhibit hepatocyte damage is by inhibiting COVID-induced ferroptosis. Functional mitochondria enhance the cellular glutathione level (Taghizadeh-Hesary et al., 2022), directly inhibiting cellular lipid peroxidation through glutathione-GPX4 pathway (Maiorino et al., 2018). (4) Section 3.1 denoted how COVID-induced mitochondrial-DAMPs release can increase SARS-CoV-2 entry into hepatocytes. Improving mitochondrial function can disrupt this vicious cycle by safely recycling the damaged mitochondria by mitophagy. Functional mitochondria can serve as a regulator of mitophagy given the following two assumptions: (a) hypoxia-inducible factor 1 (HIF-1) is one of the primary regulators of autophagy in cancer cells (Nazio et al., 2019), (b) HIF-1 requires mitochondrial support to function (Van Gisbergen et al., 2020). This approach has been applied in recent studies using hydroxytyrosol. Dong et al. examined hydroxytyrosol, derived from olive oil, in the setting of NASH. This study showed hydroxytyrosol can alleviate NASH by inducing mitophagy upon mitochondrial activation through the AMPK/PINK1 pathway (Dong et al., 2022).
Enhancing the mitochondria metabolism can impede/reduce the devastating effects of SARS-CoV-2 on hepatocytes’ mitochondria, preventing/alleviating the CiLI. To this end, it is recommended to apply strategies to enhance mitochondria content and activity. The mitochondria quality can increase by two strategies; (1) improving the lifestyle by regular exercise (Memme et al., 2021), specific diets (low-specific dynamic action diet (Luoma et al., 2016), branched-chain amino acid-rich diet (D'Antona et al., 2010), and Mediterranean diet (Khalil et al., 2022); good sleep (Rodrigues et al., 2018), healthy weight (de Mello et al., 2018), alcohol abstinence (Abdallah and Singal, 2020), and smoking cessation (Malińska et al., 2019); and (2) mitochondria boosting agents (e.g., Coenzyme Q10, activators of adenosine AMPK, acetyl-l-Carnitine; mammalian target of rapamycin [mTOR], PGC-1α, etc.) (Chamoto et al., 2017, Pizzorno, 2014), as denoted in the previous paragraph. In addition, recently, liver-targeted nanomedicines have been applied to improve the mitochondrial function of hepatocytes (Wu et al., 2019). Exercise is an established non-pharmacological method to maintain mitochondrial integrity. It has been demonstrated that exercise can improve mitochondrial quality control, which further improves the quality of mitochondrial biogenesis, trafficking, and repair (Sorriento et al., 2021). In addition, the human gut microbiota is another modulator of mitochondrial fitness. It has been demonstrated that microbiota-derived metabolites are necessary for the proper action of mitochondrial metabolisms, including glycolysis, tricarboxylic acid (TCA) cycle, oxidative phosphorylation, as well as amino acid and fatty acid metabolism. In addition, gut microbiota-derived short-chain fatty acids can enhance mitochondrial function by activating the AMPK pathway (Imdad et al., 2022). The mitochondrial boosting strategies are diverse. Detailed information is presented in the following sources (Pizzorno, 2014, Burtscher et al., 2022).
6. Conclusions
This review noted how mitochondrial dysfunction leads to deranged liver function in the context of COVID-19. This hypothesis is originated from the crucial role of mitochondria in the metabolism of hepatocytes and further justified by the histopathologic and transcriptomic appearances of CiLI. Recent evidence demonstrated that SARS-CoV-2 enters its genome into the hepatocytes’ mitochondria, leading to mitochondrial malfunction. In other words, SARS-CoV-2 exploits host cells’ mitochondria for its replication. This article indicated how the interaction between SARS-CoV-2 and mitochondria can justify the risk factors of CiLI. It also illustrated how SARS-CoV-2 can directly damage the hepatocytes’ mitochondria and how mitochondrial dysfunction can aggravate the COVID-associated cytokine storm. Preventing the devastating impacts of SARS-CoV-2 on hepatocytes’ mitochondria is a potential area of research to prevent and treat liver damage in the context of COVID-19. Detailed and comprehensive studies are required to clarify the interaction between SARS-CoV-2 and hepatocytes’ mitochondria.
Authors' contributions
F.T-H and H.A. designed the research; F.T-H acquired data. F.T.H. performed the research; F.T.H. wrote the paper; H.A. provided funding; supervised the research. All authors gave final approval of the version to be submitted.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We dedicate this article to the loving memory of our beloved mentor Moslem Bahadori, who was a pioneer in structuring the “National Council for Medical Education” and “National Organization for Educational Evaluation”, and a founder of the “Iranian National Tuberculosis and Lung Diseases Research Center” in Iran.
References
- Abdallah M.A., Singal A.K. Mitochondrial dysfunction and alcohol-associated liver disease: a novel pathway and therapeutic target. Signal Transduct. Target. Ther. 2020;5(1):26. doi: 10.1038/s41392-020-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleem A., Mahadevaiah G., Shariff N., Kothadia J.P. Hepatic manifestations of COVID-19 and effect of remdesivir on liver function in patients with COVID-19 illness. Baylor Univ. Med. Center Proc. 2021;34:473–477. doi: 10.1080/08998280.2021.1885289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali F.E.M., Mohammedsaleh Z.M., Ali M.M., Ghogar O.M. Impact of cytokine storm and systemic inflammation on liver impairment patients infected by SARS-CoV-2: prospective therapeutic challenges. World J. Gastroenterol. 2021;27(15):1531–1552. doi: 10.3748/wjg.v27.i15.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An P., Wei L.-L., Zhao S., et al. Hepatocyte mitochondria-derived danger signals directly activate hepatic stellate cells and drive progression of liver fibrosis. Nat. Commun. 2020;11(1):2362. doi: 10.1038/s41467-020-16092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M.I., Arancibia-Carcamo C.V., Auckland K., et al. SARS-CoV-2 RNA detected in blood products from patients with COVID-19 is not associated with infectious virus. Wellcome Open Res. 2020;5 doi: 10.12688/wellcomeopenres.16002.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchini F., Vallisa D., Maniscalco P., Capelli P. Iron overload and Hepcidin overexpression could play a key role in COVID infection, and may explain vulnerability in elderly, diabetics, and obese patients. Acta Biomed. 2020;91(3) doi: 10.23750/abm.v91i3.9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini D., Stabile A.M., Bastianelli S., et al. SARS-CoV2 infection impairs the metabolism and redox function of cellular glutathione. Redox Biol. 2021;45 doi: 10.1016/j.redox.2021.102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin D., Brodovitch A., Lopez A., et al. Anti-cardiolipin IgG autoantibodies associate with circulating extracellular DNA in severe COVID-19. Sci. Rep. 2022;12(1):12523. doi: 10.1038/s41598-022-15969-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogal R.H., Weston C.J., Curbishley S.M., Bhatt A.N., Adams D.H., Afford S.C. Variable responses of small and large human hepatocytes to hypoxia and hypoxia/reoxygenation (H-R) FEBS Lett. 2011;585(6):935–941. doi: 10.1016/j.febslet.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.A., Wallace K.B. Remdesivir; molecular and functional measures of mitochondrial safety. Toxicol. Appl. Pharmacol. 2021;433 doi: 10.1016/j.taap.2021.115783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtscher J., Romani M., Bernardo G., et al. Boosting mitochondrial health to counteract neurodegeneration. Prog. Neurobiol. 2022;215 doi: 10.1016/j.pneurobio.2022.102289. [DOI] [PubMed] [Google Scholar]
- Chamoto K., Chowdhury P.S., Kumar A., et al. Mitochondrial activation chemicals synergize with surface receptor PD-1 blockade for T cell-dependent antitumor activity. PNAS. 2017;114(5):E761–E770. doi: 10.1073/pnas.1620433114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Chen W., Chen J., et al. Clinical features and risk factors of COVID-19-associated liver injury and function: a retrospective analysis of 830 cases. Ann. Hepatol. 2021;21 doi: 10.1016/j.aohep.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Xu Y., Zhang K., Shen L., Deng M. Ferroptosis in COVID-19-related liver injury: a potential mechanism and therapeutic target. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.922511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Antona G., Ragni M., Cardile A., et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010;12(4):362–372. doi: 10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- De la Cruz-Enríquez J., Rojas-Morales E., Ruíz-García M.G., Tobón-Velasco J.C., Jiménez-Ortega J.C. SARS-CoV-2 induces mitochondrial dysfunction and cell death by oxidative stress/inflammation in leukocytes of COVID-19 patients. Free Radic. Res. 2021;55(9–10):982–995. doi: 10.1080/10715762.2021.2005247. [DOI] [PubMed] [Google Scholar]
- de Mello A.H., Costa A.B., Engel J.D.G., Rezin G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018;192:26–32. doi: 10.1016/j.lfs.2017.11.019. [DOI] [PubMed] [Google Scholar]
- De Vriese A.S., Coster R.V., Smet J., et al. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin. Infect. Dis. 2006;42(8):1111–1117. doi: 10.1086/501356. [DOI] [PubMed] [Google Scholar]
- Del Nonno F., Nardacci R., Colombo D., et al. Hepatic failure in COVID-19: is iron overload the dangerous trigger? Cells. 2021;10(5):1103. doi: 10.3390/cells10051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Xia B., Yu J., Leng J., Huang J. Mitochondrial DNA mutations and essential hypertension (review) Int. J. Mol. Med. 2013;32(4):768–774. doi: 10.3892/ijmm.2013.1459. [DOI] [PubMed] [Google Scholar]
- Dixon S.J., Lemberg K.M., Lamprecht M.R., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Yu M., Wu Y., et al. Hydroxytyrosol promotes the mitochondrial function through activating mitophagy. Antioxidants. 2022;11(5):893. doi: 10.3390/antiox11050893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek J. Role of cardiolipin in mitochondrial signaling pathways. Review. Front. Cell Dev. Biol. 2017;5 doi: 10.3389/fcell.2017.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effenberger M., Grander C., Grabherr F., et al. Systemic inflammation as fuel for acute liver injury in COVID-19. Dig. Liver Dis. 2021;53(2):158–165. doi: 10.1016/j.dld.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner V., Picard M., Hajnóczky G. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat. Cell Biol. 2018;20(7):755–765. doi: 10.1038/s41556-018-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Arif G., Khazaal S., Farhat A., et al. Angiotensin II type I receptor (AT1R): the gate towards COVID-19-associated diseases. Molecules. 2022;27(7) doi: 10.3390/molecules27072048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganji R., Reddy P.H. Impact of COVID-19 on mitochondrial-based immunity in aging and age-related diseases. Front. Aging Neurosci. 2020;12 doi: 10.3389/fnagi.2020.614650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganta K.K., Chaubey B. Endoplasmic reticulum stress leads to mitochondria-mediated apoptosis in cells treated with anti-HIV protease inhibitor ritonavir. Cell Biol. Toxicol. 2019;35(3):189–204. doi: 10.1007/s10565-018-09451-7. [DOI] [PubMed] [Google Scholar]
- Gentric G., Maillet V., Paradis V., et al. Oxidative stress promotes pathologic polyploidization in nonalcoholic fatty liver disease. J. Clin. Invest. 2015;125(3):981–992. doi: 10.1172/jci73957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D.E., Jang G.M., Bouhaddou M., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583(7816):459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia-Ramos A.E., Jaquez-Quintana J.O., Contreras-Omaña R., Auron M. Liver dysfunction and SARS-CoV-2 infection. World J. Gastroenterol. 2021;27(26):3951–3970. doi: 10.3748/wjg.v27.i26.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D.O., Brennan-Rieder D., Ngo B., et al. The importance of understanding the stages of COVID-19 in treatment and trials. AIDS Rev. 2021;23(1):40–47. doi: 10.24875/AIDSRev.200001261. [DOI] [PubMed] [Google Scholar]
- Grossini E., Concina D., Rinaldi C., et al. Association between plasma redox state/mitochondria function and a flu-like syndrome/COVID-19 in the elderly admitted to a long-term care unit. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.707587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S., Shaw R.J. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018;19(2):121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder K., Reddy P.H. The COVID-19 effect on the immune system and mitochondrial dynamics in diabetes, obesity, and dementia. Neuroscientist. 2021;27(4):331–339. doi: 10.1177/1073858420960443. [DOI] [PubMed] [Google Scholar]
- Hossain M.G., Akter S., Ohsaki E., Ueda K. Impact of the interaction of hepatitis B virus with mitochondria and associated proteins. Viruses. 2020;12(2) doi: 10.3390/v12020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imdad S., Lim W., Kim J.H., Kang C. Intertwined relationship of mitochondrial metabolism, gut microbiome and exercise potential. Int. J. Mol. Sci. 2022;23(5) doi: 10.3390/ijms23052679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Xu S., Guo H., et al. Magnesium isoglycyrrhizinate prevents the nonalcoholic hepatic steatosis via regulating energy homeostasis. J. Cell Mol. Med. 2020;24(13):7201–7213. doi: 10.1111/jcmm.15230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama T., Tanaka M., Tanaka K., et al. Alterations in hepatic mitochondrial function during total parenteral nutrition in immature rats. JPEN J. Parenter. Enteral Nutr. 1990;14(6):640–645. doi: 10.1177/0148607190014006640. [DOI] [PubMed] [Google Scholar]
- Khalil M., Shanmugam H., Abdallah H., et al. The potential of the Mediterranean diet to improve mitochondrial function in experimental models of obesity and metabolic syndrome. Nutrients. 2022;14(15) doi: 10.3390/nu14153112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-J., Syed G.H., Khan M., et al. Hepatitis C virus triggers mitochondrial fission and attenuates apoptosis to promote viral persistence. Proc. Natl. Acad. Sci. 2014;111(17):6413–6418. doi: 10.1073/pnas.1321114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesova O., Vanaga I., Laivacuma S., et al. Intriguing findings of liver fibrosis following COVID-19. BMC Gastroenterol. 2021;21(1):370. doi: 10.1186/s12876-021-01939-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei F., Liu Y.M., Zhou F., et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology. 2020;72(2):389–398. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Liu Y., Cheng Z., Yu X., Li Y. COVID-19-associated liver injury: clinical characteristics, pathophysiological mechanisms and treatment management. Biomed. Pharmacother. 2022;154 doi: 10.1016/j.biopha.2022.113568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima T., Li T.Y., Mottis A., Auwerx J. Pleiotropic effects of mitochondria in aging. Nat. Aging. 2022;2(3):199–213. doi: 10.1038/s43587-022-00191-2. [DOI] [PubMed] [Google Scholar]
- Lu Y., Feng T., Zhao J., et al. Polyene phosphatidylcholine ameliorates high fat diet-induced non-alcoholic fatty liver disease via remodeling metabolism and inflammation. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.810143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoma R.L., Butler M.W., Stahlschmidt Z.R. Plasticity of immunity in response to eating. J. Exp. Biol. 2016;219(Pt 13):1965–1968. doi: 10.1242/jeb.138123. [DOI] [PubMed] [Google Scholar]
- Mahmoodpoor A., Sanaie S., Ostadi Z., et al. Roles of mitochondrial DNA in dynamics of the immune response to COVID-19. Gene. 2022;836 doi: 10.1016/j.gene.2022.146681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorino M., Conrad M., Ursini F. GPx4, lipid peroxidation, and cell death: discoveries, rediscoveries, and open issues. Antioxid. Redox Signal. 2018;29(1):61–74. doi: 10.1089/ars.2017.7115. [DOI] [PubMed] [Google Scholar]
- Malińska D., Więckowski M.R., Michalska B., et al. Mitochondria as a possible target for nicotine action. J. Bioenerg. Biomembr. 2019;51(4):259–276. doi: 10.1007/s10863-019-09800-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancias J.D., Wang X., Gygi S.P., Harper J.W., Kimmelman A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509(7498):105–109. doi: 10.1038/nature13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memme J.M., Erlich A.T., Phukan G., Hood D.A. Exercise and mitochondrial health. J. Physiol. 2021;599(3):803–817. doi: 10.1113/jp278853. [DOI] [PubMed] [Google Scholar]
- Merches K., Breunig L., Fender J., et al. The potential of remdesivir to affect function, metabolism and proliferation of cardiac and kidney cells in vitro. Arch. Toxicol. 2022;96(8):2341–2360. doi: 10.1007/s00204-022-03306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morio B., Panthu B., Bassot A., Rieusset J. Role of mitochondria in liver metabolic health and diseases. Cell Calcium. 2021;94 doi: 10.1016/j.ceca.2020.102336. [DOI] [PubMed] [Google Scholar]
- Murphy E., Ardehali H., Balaban R.S., et al. Mitochondrial function, biology, and role in disease: a scientific statement from the American Heart Association. Circ. Res. 2016;118(12):1960–1991. doi: 10.1161/res.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardo A.D., Schneeweiss-Gleixner M., Bakail M., Dixon E.D., Lax S.F., Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41(1):20–32. doi: 10.1111/liv.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazio F., Bordi M., Cianfanelli V., Locatelli F., Cecconi F. Autophagy and cancer stem cells: molecular mechanisms and therapeutic applications. Cell Death Differ. 2019;26(4):690–702. doi: 10.1038/s41418-019-0292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi Y., Ueha T., Kawamoto T., et al. Regulation of mitochondrial proliferation by PGC-1α induces cellular apoptosis in musculoskeletal malignancies. Sci. Rep. 2014;4(1) doi: 10.1038/srep03916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyebode O.T., Adebiyi O.R., Olorunsogo O.O. Toxicity of some broad-spectrum antibacterials in normal rat liver: the role of mitochondrial membrane permeability transition pore. Toxicol. Mech. Methods. 2019;29(2):128–137. doi: 10.1080/15376516.2018.1528651. [DOI] [PubMed] [Google Scholar]
- Paizis G., Tikellis C., Cooper M.E., et al. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54(12):1790–1796. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorno J. Mitochondria-fundamental to life and health. Integr. Med. (Encinitas) 2014;13(2):8–15. [PMC free article] [PubMed] [Google Scholar]
- Posteraro B., Cortazzo V., Liotti F.M., et al. Diagnosis and treatment of bacterial pneumonia in critically Ill patients with COVID-19 using a multiplex PCR assay: a large Italian hospital’s five-month experience. Microbiology Spectrum. 2021;9(3) doi: 10.1128/Spectrum.00695-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbapragada I., Birkus G., Perry J., Xing W., Kwon H., Pflanz S. Molecular determinants of GS-9620-dependent TLR7 activation. PLoS ONE. 2016;11(1) doi: 10.1371/journal.pone.0146835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues N.R., Macedo G.E., Martins I.K., et al. Short-term sleep deprivation with exposure to nocturnal light alters mitochondrial bioenergetics in Drosophila. Free Radic. Biol. Med. 2018;120:395–406. doi: 10.1016/j.freeradbiomed.2018.04.549. [DOI] [PubMed] [Google Scholar]
- Rovira-Llopis S., Bañuls C., Diaz-Morales N., Hernandez-Mijares A., Rocha M., Victor V.M. Mitochondrial dynamics in type 2 diabetes: pathophysiological implications. Redox Biol. 2017;11:637–645. doi: 10.1016/j.redox.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh J., Peyssonnaux C., Singh K.K., Edeas M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion. 2020;54:1–7. doi: 10.1016/j.mito.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang C., Liu Z., Zhu Y., et al. SARS-CoV-2 causes mitochondrial dysfunction and mitophagy impairment. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.780768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J.X., Zhuang Z.H., Zhang Q.X., et al. Risk factors and prognosis in patients with COVID-19 and liver injury: a retrospective analysis. J. Multidiscip. Healthc. 2021;14:629–637. doi: 10.2147/jmdh.S293378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C.S., Qi H.Y., Boularan C., et al. SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J. Immunol. 2014;193(6):3080–3089. doi: 10.4049/jimmunol.1303196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silaidos C., Pilatus U., Grewal R., et al. Sex-associated differences in mitochondrial function in human peripheral blood mononuclear cells (PBMCs) and brain. Biol. Sex Differ. 2018;9(1):34. doi: 10.1186/s13293-018-0193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K.K., Chaubey G., Chen J.Y., Suravajhala P. Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am. J. Physiol. Cell Physiol. 2020;319(2):C258–C267. doi: 10.1152/ajpcell.00224.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorriento D., Di Vaia E., Iaccarino G. Physical exercise: a novel tool to protect mitochondrial health. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.660068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli J.B., Haigis M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018;20(7):745–754. doi: 10.1038/s41556-018-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan K., Pandey A.K., Livingston A., Venkatesh S. Roles of host mitochondria in the development of COVID-19 pathology: could mitochondria be a potential therapeutic target? Mol. Biomed. 2021;2:38. doi: 10.1186/s43556-021-00060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Chen P., Zhai B., et al. The emerging role of ferroptosis in inflammation. Biomed. Pharmacother. 2020;127 doi: 10.1016/j.biopha.2020.110108. [DOI] [PubMed] [Google Scholar]
- Suriawinata, E., Mehta, K.J., 2022. Iron and iron-related proteins in COVID-19. Clin. Exp. Med. 1–23. doi: 10.1007/s10238-022-00851-y. [DOI] [PMC free article] [PubMed]
- Szabo R., Petrisor C., Bodolea C., et al. Hyperferritinemia, low circulating iron and elevated hepcidin may negatively impact outcome in COVID-19 patients: a pilot study. Antioxidants. 2022;11(7):1364. doi: 10.3390/antiox11071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghizadeh-Hesary F., Akbari H. The powerful immune system against powerful COVID-19: a hypothesis. Med. Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghizadeh-Hesary F., Akbari H., Bahadori M., Behnam B. Targeted anti-mitochondrial therapy: the future of oncology. Genes (Basel) 2022;13(10) doi: 10.3390/genes13101728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneva G., Dimitrov D., Velikova T. Liver dysfunction as a cytokine storm manifestation and prognostic factor for severe COVID-19. World J. Hepatol. 2021;13(12):2005–2012. doi: 10.4254/wjh.v13.i12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Sluis R.M., Holm C.K., Jakobsen M.R. Plasmacytoid dendritic cells during COVID-19: ally or adversary? Cell Rep. 2022;40(4) doi: 10.1016/j.celrep.2022.111148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gisbergen M.W., Offermans K., Voets A.M., et al. Mitochondrial dysfunction inhibits hypoxia-induced HIF-1α stabilization and expression of its downstream targets. Front. Oncol. 2020;10:770. doi: 10.3389/fonc.2020.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Liu S., Liu H., et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J. Hepatol. 2020;73(4):807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Huang J., Sun Y., et al. SARS-CoV-2 suppresses mRNA expression of selenoproteins associated with ferroptosis, endoplasmic reticulum stress and DNA synthesis. Food Chem. Toxicol. 2021;153 doi: 10.1016/j.fct.2021.112286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner N., Andrieux G., Badia-i-Mompel P., et al. Molecular consequences of SARS-CoV-2 liver tropism. Nat. Metab. 2022;4(3):310–319. doi: 10.1038/s42255-022-00552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiernicki B., Dubois H., Tyurina Y.Y., et al. Excessive phospholipid peroxidation distinguishes ferroptosis from other cell death modes including pyroptosis. Cell Death Dis. 2020;11(10):922. doi: 10.1038/s41419-020-03118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J., Zhou, Y., 2022. Case analysis of hepatotoxicity caused by vancomycin. Research Square. http://europepmc.org/abstract/PPR/PPR526427. doi: 10.21203/rs.3.rs-1873168/v1. [DOI] [PMC free article] [PubMed]
- Wu M., Liao L., Jiang L., et al. Liver-targeted Nano-MitoPBN normalizes glucose metabolism by improving mitochondrial redox balance. Biomaterials. 2019;222 doi: 10.1016/j.biomaterials.2019.119457. [DOI] [PubMed] [Google Scholar]
- Xu J., Shen J., Yuan R., et al. Mitochondrial targeting therapeutics: promising role of natural products in non-alcoholic fatty liver disease. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.796207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Guo Q., Feng X., Liu Y., Zhou Y. Mitochondrial dysfunction in cardiovascular diseases: potential targets for treatment. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.841523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan K., Liao S., Li J., et al. Risk factors in patients with COVID-19 developing severe liver injury during hospitalisation. Gut. 2021;70(3):628–629. doi: 10.1136/gutjnl-2020-321913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.S., Dong L., Wang G.M., et al. Progressive liver injury and increased mortality risk in COVID-19 patients: a retrospective cohort study in China. World J. Gastroenterol. 2021;27(9):835–853. doi: 10.3748/wjg.v27.i9.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Yu Y., Zhang C., et al. Mechanism of SARS-CoV-2 invasion into the liver and hepatic injury in patients with COVID-19. Mediterr. J. Hematol. Infect. Dis. 2022;14(1) doi: 10.4084/mjhid.2022.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Ni C., Gao R., et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11(10):771–775. doi: 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Chen C., Ye D., et al. Diammonium glycyrrhizinate upregulates PGC-1α and protects against Aβ1-42-induced neurotoxicity. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0035823. [DOI] [PMC free article] [PubMed] [Google Scholar]