Objectives
Developing new high relaxivity gadolinium-based contrast agents (GBCAs) for magnetic resonance imaging (MRI) allowing dose reduction while maintaining similar diagnostic efficacy is needed, especially in the context of gadolinium retention in tissues. This study aimed to demonstrate that contrast-enhanced MRI of the central nervous system (CNS) with gadopiclenol at 0.05 mmol/kg is not inferior to gadobutrol at 0.1 mmol/kg, and superior to unenhanced MRI.
Materials and Methods
PICTURE is an international, randomized, double-blinded, controlled, cross-over, phase III study, conducted between June 2019 and September 2020. Adult patients with CNS lesions were randomized to undergo 2 MRIs (interval, 2–14 days) with gadopiclenol (0.05 mmol/kg) then gadobutrol (0.1 mmol/kg) or vice versa. The primary criterion was lesion visualization based on 3 parameters (border delineation, internal morphology, and contrast enhancement), assessed by 3 off-site blinded readers. Key secondary outcomes included lesion-to-background ratio, enhancement percentage, contrast-to-noise ratio, overall diagnostic preference, and adverse events.
Results
Of the 256 randomized patients, 250 received at least 1 GBCA administration (mean [SD] age, 57.2 [13.8] years; 53.6% women). The statistical noninferiority of gadopiclenol (0.05 mmol/kg) to gadobutrol (0.1 mmol/kg) was achieved for all parameters and all readers (n = 236, lower limit 95% confidence interval of the difference ≥−0.06, above the noninferiority margin [−0.35], P < 0.0001), as well as its statistical superiority over unenhanced images (n = 239, lower limit 95% confidence interval of the difference ≥1.29, P < 0.0001).
Enhancement percentage and lesion-to-background ratio were higher with gadopiclenol for all readers (P < 0.0001), and contrast-to-noise ratio was higher for 2 readers (P = 0.02 and P < 0.0001). Three blinded readers preferred images with gadopiclenol for 44.8%, 54.4%, and 57.3% of evaluations, reported no preference for 40.7%, 21.6%, and 23.2%, and preferred images with gadobutrol for 14.5%, 24.1%, and 19.5% (P < 0.001).
Adverse events reported after MRI were similar for gadopiclenol (14.6% of patients) and gadobutrol (17.6%). Adverse events considered related to gadopiclenol (4.9%) and gadobutrol (6.9%) were mainly injection site reactions, and none was serious.
Conclusions
Gadopiclenol at 0.05 mmol/kg is not inferior to gadobutrol at 0.1 mmol/kg for MRI of the CNS, confirming that gadopiclenol can be used at half the gadolinium dose used for other GBCAs to achieve similar clinical efficacy.
Key Words: gadopiclenol, GBCA, contrast agent, relaxivity, MRI, CNS, phase III, efficacy, safety
Contrast-enhanced magnetic resonance imaging (MRI), using gadolinium-based contrast agents (GBCAs), significantly improves the detection and characterization of diseases compared with unenhanced MRI. These agents are administered intravenously and enhance the detail of pathology allowing more accurate diagnoses. The overall use of GBCAs is generally considered to be safe. However, there is mounting evidence that, after the administration of GBCAs, traces of gadolinium may be retained within various tissues such as the brain, bone, and other organs.1–3 Gadolinium-based contrast agents can be categorized based on their molecular structure as linear or macrocyclic. It is acknowledged that linear GBCAs show higher propensity to induce gadolinium retention in the body compared with macrocyclic GBCAs.4–6
After reports of nephrogenic systemic fibrosis and gadolinium retention associated with the use of some GBCAs, the major health authorities recommend reducing the dose of gadolinium wherever possible, but without affecting diagnostic quality.7–9 Similarly, although the retention of gadolinium in the brain has not been directly associated with any clinically significant consequences, reducing the use and/or dose of gadolinium when possible without compromising care is desired.
Increasing the relaxivity of the GBCA is one way to accentuate contrast enhancement. Higher relaxivity can be achieved by increasing the hydration number (number of bound water nuclei per Gd3+ ion). Gadopiclenol (Elucirem, Guerbet, France) is a GBCA with a hydration number of 2, allowing a 2- to 3-fold greater relaxivity than currently approved GBCAs.10 Thus, it could be hypothesized that using a lower gadolinium dose of a GBCA, whose relaxivity is twice higher than that of currently available GBCAs, may result in fine in similar contrast enhancement obtained with other GBCAs used at the usual dose of 0.1 mmol/kg.
In a previous phase IIb study on patients with central nervous system (CNS) diseases, gadopiclenol at 0.05 mmol/kg provided similar contrast-to-noise ratio (CNR) as gadobenate dimeglumine at 0.1 mmol/kg, whereas the statistical superiority of gadopiclenol over gadobenate dimeglumine, both at 0.1 mmol/kg, was demonstrated with an increase in CNR >30%.11
Given the aforementioned relaxivity data, and the results from the phase IIb study, the objective of this study was to demonstrate that gadopiclenol at 0.05 mmol/kg is statistically not inferior to gadobutrol at 0.1 mmol/kg for contrast-enhanced MRI of the CNS, and that it is superior to unenhanced MRI, in terms of lesion visualization.
MATERIALS AND METHODS
Study Design and Population
PICTURE (gadopiclenol for CNS magnetic resonance) is a multicenter, prospective, randomized, double-blinded, controlled, and cross-over phase III study, conducted in 33 medical centers from 11 countries (in Europe, America, and Asia), between June 2019 and September 2020. The study was approved by independent ethics committees and authorized by national regulatory authorities. All patients provided informed written consent. The study was registered on ClinicalTrials.gov (NCT03996447).
Adult female or male patients aged 18 years or older, with known or highly suspicious brain or spinal lesions based on previous imaging examination, were eligible for inclusion. Patients with multiple sclerosis and acute or chronic renal insufficiency (estimated glomerular filtration rate <30 mL/min/1.73 m2) were excluded.
MRI Examination
Patients received both gadopiclenol and gadobutrol (as an active comparator), in a random order (using an interactive Web response system, in a 1:1 ratio), separated by 2 to 14 days. Magnetic resonance imaging was performed before and after administration of each contrast agent. A safety visit was scheduled 1 day after each MRI examination.
Magnetic resonance imaging was performed using 1.5 T or 3 T systems, and the same system had to be used for a single patient. The following unenhanced sequences in the axial plane of the brain were applied: 2D T1-weighted spin echo (SE)/turbo spin echo (TSE), 3D T1-weighted gradient echo (GRE), 2D T2-weighted fluid attenuated inversion recovery (FLAIR), and T2-weighted TSE. After contrast administration, the following sequences were applied: 2D T1-weighted SE/TSE and 3D T1-weighted GRE. For the spine, unenhanced sagittal T2-weighted TSE and T1-weighted SE/TSE sequences, as well as contrast-enhanced axial T1-weighted SE/TSE and sagittal T1-weighted SE/TSE sequences, were applied.
Both gadopiclenol at 0.05 mmol/kg (0.5 M solution, 0.1 mL/kg) and gadobutrol at 0.1 mmol/kg (1.0 M solution, 0.1 mL/kg) were administered, manually or with a power injector, as a single intravenous bolus injection at a recommended rate of 2 mL/second followed by a saline flush.
Efficacy Evaluation
Images evaluations were performed both off-site and on-site. For off-site reading, the evaluations were performed in a centralized manner by 3 independent blinded readers. Three other readers were involved for overall diagnostic preference evaluation, as well as one additional reader for lesion tracking to allow an unambiguous matching of lesions between pre and paired images of the same MR examination, and between the 2 MR examinations for the same patient. All off-site readers were neuroradiologists with more than 5 years of experience. For on-site reading, at least 1 radiologist with more than 5 years of experience was appointed at each site to read images of patients.
Primary Criteria
Primary criteria were based on off-site evaluations. Lesion visualization was based on 3 parameters: border delineation, internal morphology, and contrast enhancement. Up to 3 most representative lesions (based on lesion size and contrast enhancement) were scored using a 4-point scale (1 = poor [internal morphology] or none [border delineation, contrast enhancement], 2 = moderate, 3 = good, 4 = excellent), and a mean of scores was calculated. Lesion visualization parameters were compared between unenhanced (pre) and combined unenhanced and contrast-enhanced (paired) images with gadopiclenol, and between gadopiclenol and gadobutrol paired images.
Secondary Criteria
The off-site readers evaluated the number of detected lesions, quantitative parameters, and the overall diagnostic preference. The quantitative parameters (ie, lesion-to-background ratio [LBR], CNR, and enhancement percentage [E%]) were calculated for each patient (a mean was calculated for up to 3 most representative lesions) as follow:
wherein SIlesion indicates lesion signal intensity (SI); SIht, healthy tissue SI; SDnoise, background noise standard deviation; SIpost, lesion SI on postinjection images; and SIpre, lesion SI on preinjection images.
Overall diagnostic preference was evaluated in a global matched pairs fashion (a reader evaluates paired images with gadopiclenol and gadobutrol from the same patient side by side).
The on-site investigators evaluated lesion visualization parameters and assessed, overall and according to tumor malignancy (assessed before contrast administration), if treatment plan could have been changed for each contrast-enhanced MRI, and if yes, to specify the proposed therapeutic management.
Safety Evaluation
Adverse events (AEs) were recorded throughout the entire study period and evaluated by intensity (mild, moderate, severe) and seriousness.12 Blood samples were collected before and 1 day after each MRI to measure hematology and biochemistry parameters. Vital signs and tolerance at injection site were assessed before, 1 hour, and 1 day after each contrast agent administration.
Statistical Methods
Based on the phase IIb study results,11 and taking into account a possible greater heterogeneity of patient population to be included and a patient dropout rate of 20%, an enrollment of 250 patients was deemed necessary for the 95% confidence interval (CI) lower limit to exceed the noninferiority margin set to −0.35 (10% of the expected mean score).
Differences in terms of lesions visualization were analyzed using a general linear model. Lesion visualization parameter was the dependent variable; contrast agent, period (ie, first or second MRI; for noninferiority analysis), and MRI modality (for superiority analysis) were the fixed factors; and patient was the random factor. A paired t test was used for statistical analyses of the primary criteria. To conclude on the superiority of gadopiclenol paired over pre images, the difference in mean scores had to be significantly greater than 0 for at least 2 of 3 readers and for all 3 parameters (type 1 error set at 0.025). The analysis was performed on patients who had both pre and paired images with gadopiclenol assessable for at least 1 matching lesion for at least 1 off-site reader (full analysis set). The noninferiority of gadopiclenol toward gadobutrol could be concluded if the lower limit of the 95% CI of the difference in mean scores was above the noninferiority margin (−0.35) for at least 2 of 3 readers and for all 3 parameters. The analysis was performed on patients, without major protocol deviation, who have both paired images for gadopiclenol and gadobutrol assessable for at least 1 matching lesion for at least 1 off-site reader (per-protocol set).
All secondary and subgroup analyses are considered as exploratory; therefore, no adjustment for type 1 error was performed.
Differences between contrast agents in quantitative parameters were analyzed using a general linear model. The quantitative parameter was the dependent variable; contrast agent, period, and unenhanced value (only for LBR and CNR) were the fixed factors; and patient was the random factor. Differences were tested using a t test. Diagnostic preference results were evaluated using a Wilcoxon signed rank test. Safety results were compared descriptively. Statistical analyses were performed using SAS (Version 9.4; SAS Institute Inc, Cary, NC).
RESULTS
Overall, 260 patients were screened, and 256 were randomized to a sequence of 2 MRIs (128 patients in each sequence). Among randomized patients, 250 underwent the first MRI with gadopiclenol (n = 125) or gadobutrol (n = 125) and the first safety follow-up, and 242 underwent the second MRI with gadobutrol (n = 120) or gadopiclenol (n = 122) and the second safety follow-up, and completed the study (Fig. 1). Fourteen patients prematurely discontinued the study: due to the COVID-19 pandemic preventing patients from following the protocol schedule (n = 3), inclusion criteria not met/noninclusion criteria met (n = 1), withdrawal of patient's consent (n = 4), AE (n = 4), patient unavailable the day of visit (n = 1), and due to planned surgery (n = 1).
FIGURE 1.
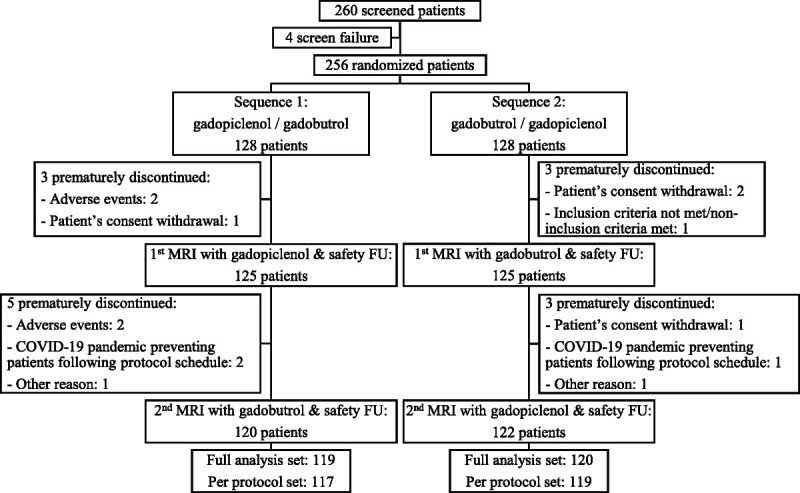
Flowchart of patients from PICTURE study. FU indicates follow-up; full analysis set (superiority analysis), patients who had both unenhanced (pre) and combined unenhanced and contrast-enhanced (paired) images with gadopiclenol assessable for at least 1 matching lesion for at least 1 off-site reader; per-protocol set (noninferiority analysis), patients without major protocol deviation, who had both paired images for gadopiclenol and gadobutrol assessable for at least 1 matching lesion for at least 1 off-site reader.
Patients were 18 to 84 years old, with a mean (SD) age of 57.2 (13.8) years and a mean (SD) weight of 78.3 (20.4) kg. Overall, 53.6% were women (see Table, Supplemental Digital Content 1, http://links.lww.com/RLI/A781). Magnetic field strength was 1.5 T for 45.6% of patients and 3 T for 54.4% of patients. The most frequent diagnoses after MRI were meningiomas (32.1%), brain metastases (22.9%), and glial tumors (21.7%) (see Table, Supplemental Digital Content 2, http://links.lww.com/RLI/A782).
Efficacy Results
The per-protocol set included 236 patients, and full analysis set included 239 patients. When comparing gadopiclenol to gadobutrol paired images, the differences in mean of scores for border delineation, internal morphology, and contrast enhancement were close to 0 in all cases, with a lower limit of the 95% CI of the difference (≥−0.06) above the noninferiority margin for all 3 readers (P < 0.0001 in all cases) (see Table, Supplemental Digital Content 3, http://links.lww.com/RLI/A783; Fig. 2). Therefore, the statistical noninferiority of gadopiclenol (0.05 mmol/kg) versus gadobutrol (0.1 mmol/kg) paired images was achieved. The differences in mean of scores were significantly in favor of gadopiclenol paired compared with pre images for all 3 readers and all 3 parameters (P < 0.0001 in all cases) (see Table, Supplemental Digital Content 4, http://links.lww.com/RLI/A784; Fig. 2). Therefore, the statistical superiority of paired gadopiclenol (0.05 mmol/kg) over pre images was achieved.
FIGURE 2.
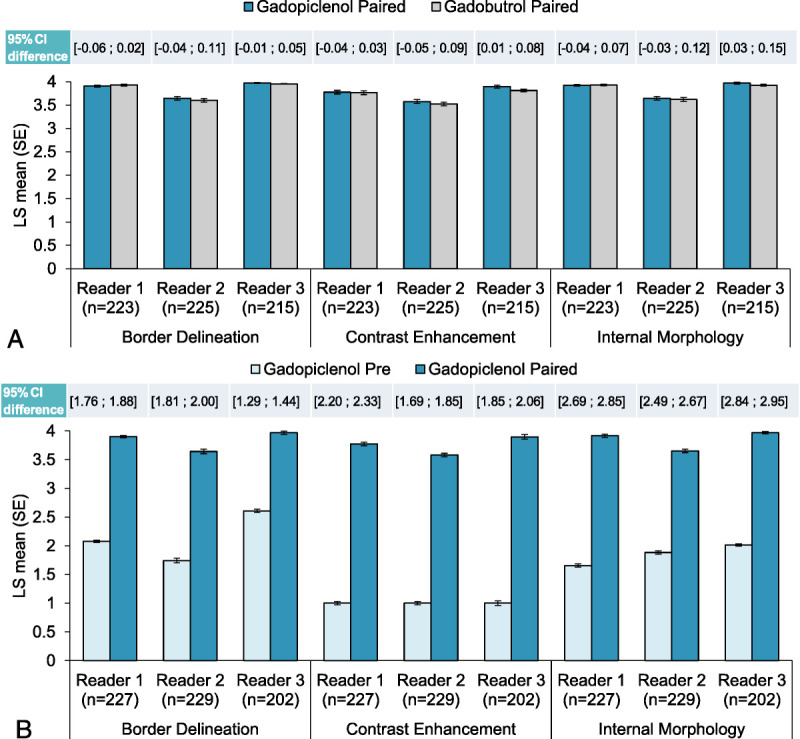
Noninferiority and superiority analyses for lesion visualization. Up to 3 most representative lesions in each patient were qualitatively scored (from 1 to 4) for each parameter by 3 offsite independent blinded readers, and a mean of scores was calculated. A noninferiority analysis between gadopiclenol (0.05 mmol/kg) and gadobutrol (0.1 mmol/kg) combined unenhanced (pre) and contrast-enhanced (paired) images (A), and a superiority analysis between pre and gadopiclenol (0.05 mmol/kg) paired images (B) were performed. Data are presented as bar graphs showing the least squares (LS) mean (standard error [SE]) of the scores, and the confidence interval (CI) of the difference between the scores are shown above each bar graph, with a P value >0.0001 for all readers and all visualization criteria.
With on-site reading, the noninferiority of gadopiclenol versus gadobutrol was also achieved (lower limit of the 95% CI of the difference, ≥−0.05). Subgroup analyses showed homogeneous results within each demographic parameter (age, sex, race, ethnicity, geographic region) and for 1.5 T and 3 T systems.
Most patients presented with only 1 lesion, and the number of identified lesions was similar for gadopiclenol and gadobutrol paired images (95% CI of the difference: [−0.21; 0.45], [−0.60; 0.61], [−0.72; 0.16] for the 3 readers, respectively [P ≥ 0.22]) (see Table, Supplemental Digital Content 5, http://links.lww.com/RLI/A785).
LBR and E% were significantly higher with gadopiclenol for all 3 readers (95% CI of the difference not including 0, P < 0.0001). For CNR, the difference was statistically significant for 2 readers (95% CI of the difference: [3.14; 33.52], P = 0.02; [8.70; 18.22], P < 0.0001; Fig. 3).
FIGURE 3.
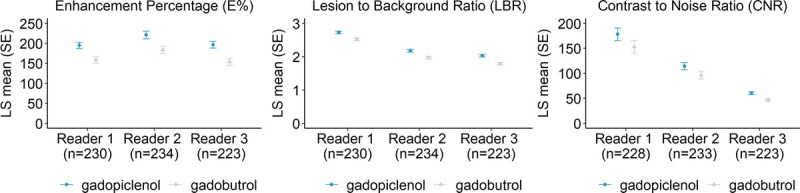
Quantitative parameters for MRI with gadopiclenol (0.05 mmol/kg) versus MRI with gadobutrol (0.1 mmol/kg). CNR, E%, and LBR were calculated for each patient (mean for up to 3 most representative lesions) by 3 offsite independent blinded readers. Data are presented as bar graphs showing the least squares (LS) mean (standard error [SE]) of the quantitative parameters. Differences between gadopiclenol (0.05 mmol/kg) and gadobutrol (0.1 mmol/kg) were statistically significant for LBR and E% for all 3 readers (P < 0.0001), and with CNR for readers 2 and 3 (P = 0.02 and P < 0.0001, respectively).
Images with gadopiclenol were in majority preferred to images with gadobutrol (44.8%, 54.4%, and 57.3% of cases, depending on the reader, P < 0.001). The readers reported no preference for 40.7%, 21.6%, and 23.2% of cases, and images with gadobutrol were preferred in 14.5%, 24.1%, and 19.5% of cases (Fig. 4).
FIGURE 4.
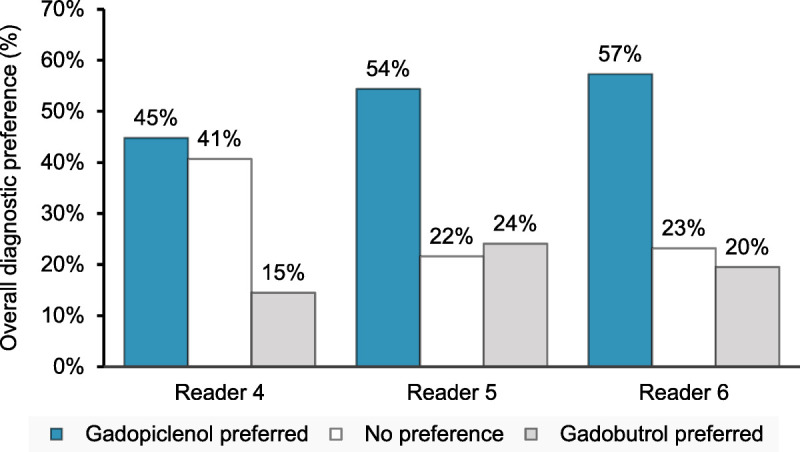
Overall diagnostic preference. Side by side comparison of paired images of 241 patients, from MRI with gadopiclenol (0.05 mmol/kg) and gadobutrol (0.1 mmol/kg). Data are presented as bar graphs. The preference for images with gadopiclenol was statistically significant for all 3 readers (P < 0.001).
Examples of contrast-enhanced MRI scans obtained with gadopiclenol (0.05 mmol/kg) and gadobutrol (0.1 mmol/kg) are shown in Figure 5.
FIGURE 5.
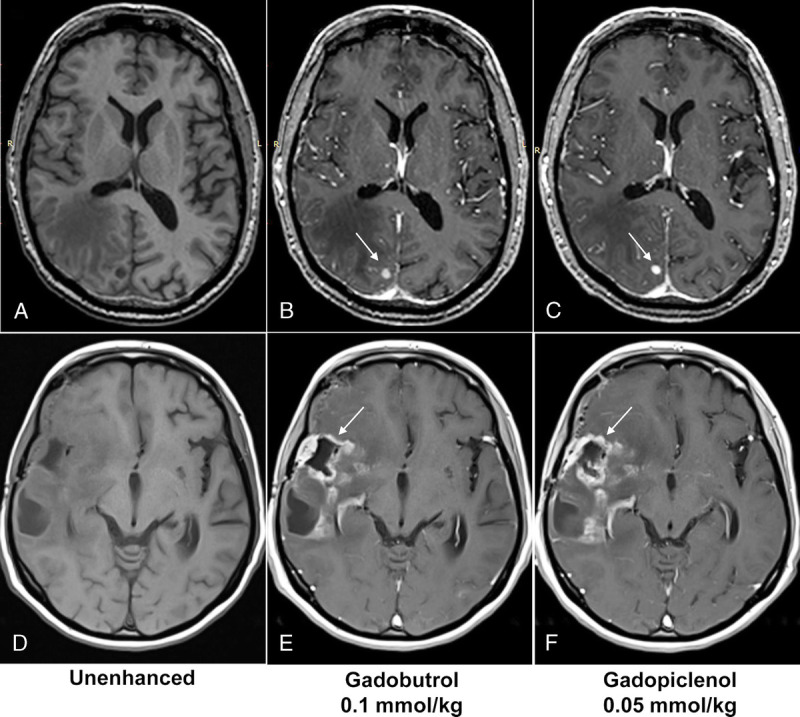
Examples of contrast-enhanced MRI scans after gadopiclenol (0.05 mmol/kg) or gadobutrol (0.1 mmol/kg) administration. Axial 3D T1-weighted GRE MRI scans of a 65-year-old male patient with brain metastases from lung cancer (A–C) and a 48-year-old female patient with glioblastoma (D–F) obtained before (A and D) and after gadopiclenol administration at 0.05 mmol/kg (C and F) or gadobutrol administration at 0.1 mmol/kg (B and E). Arrows point toward the corresponding lesion of each MRI scan.
The change in treatment plan was similar between gadopiclenol (23.3% of patients) and gadobutrol (23.7%). There was no difference in the proposed therapeutic management based on paired images with gadopiclenol or gadobutrol, with chemotherapy, radiotherapy, or surgery being the most frequent. The treatment plan could be changed for 28% of patients with malignant lesions and approximately 12% patients with nonmalignant lesions, similarly for both contrast agents.
Safety Results
Overall, 49 AEs were reported for 36 patients (14.6%) after MRI with gadopiclenol and 55 AEs for 43 patients (17.6%) after MRI with gadobutrol, most of which were of mild intensity (48/49 [98%] with gadopiclenol and 50/55 [91%] with gadobutrol). Adverse events considered related to gadopiclenol (12 patients [4.9%]) and gadobutrol (17 patients [6.9%]) were mostly similar in nature and mainly injection site reactions (Table 1).
TABLE 1.
Postinjection Adverse Events Related to Contrast Agent
| Gadopiclenol (n = 247) | Gadobutrol (n = 245) | |
|---|---|---|
| At least 1 adverse event related to contrast agent | 12 (4.9%) | 17 (6.9%) |
| Injection site pain | 2 (0.8%) | 4 (1.6%) |
| Injection site coldness | 2 (0.8%) | 1 (0.4%) |
| Injection site warmth | 1 (0.4%) | 2 (0.8%) |
| Dysgeusia | 1 (0.4%) | 2 (0.8%) |
| Blood creatinine increased | 1 (0.4%) | 2 (0.8%) |
| Injection site paraesthesia | 1 (0.4%) | 1 (0.4%) |
| Headache | 1 (0.4%) | 1 (0.4%) |
| Nausea | 1 (0.4%) | 1 (0.4%) |
| Drug ineffective* | 1 (0.4%) | 0 |
| Feeling hot | 1 (0.4%) | 0 |
| Injection site hypoaesthesia | 0 | 1 (0.4%) |
| Dizziness | 1 (0.4%) | 0 |
| Paraesthesia oral | 1 (0.4%) | 0 |
| Vomiting | 0 | 1 (0.4%) |
| Ecchymosis | 0 | 1 (0.4%) |
| Erythema | 1 (0.4%) | 0 |
| Rash macular | 0 | 1 (0.4%) |
| Groin pain | 0 | 1 (0.4%) |
*Extravasation was suspected in this patient.
Four serious AEs were reported during the study: 3 occurred before any contrast agent administration, and 1 (fatal respiratory failure) occurred 5 days after the first MRI performed with gadobutrol and was due to patient's underlying disease.
No safety concerns were raised from laboratory results and vital signs.
DISCUSSION
Within the context of nephrogenic systemic fibrosis and gadolinium retention in the brain and other organs, using the lowest dose of GBCA and/or limiting repeated examinations while not compromising diagnostic quality and efficacy in routine practice is recommended.13 Deep learning MRI solutions were recently developed for this purpose and showed that they could potentially help in reducing the injected gadolinium dose for brain MRI while maintaining a good image quality and contrast enhancement.11,14 Nevertheless, these approaches are still exploratory and have not been validated in larger cohorts. Another means to maintain image quality and diagnostic accuracy while reducing contrast dose is by increasing GBCA relaxivity.7 Gadopiclenol has the advantage of having the highest relaxivity compared with currently available GBCAs, with a stable r1 relaxivity value with increasing clinical magnetic field (r1 = 12.8 mM−1·s−1 at 1.41 T and 11.6 mM−1·s−1 at 3 T measured in human serum at 37°C).10 Bendszus and colleagues11 previously showed that, for CNS, gadopiclenol at 0.1 mmol/kg was statistically superior to gadobenate dimeglumine at 0.1 mmol/kg in terms of CNR (at least 32% higher), and gadopiclenol at 0.05 mmol/kg had a similar CNR as gadobenate dimeglumine at 0.1 mmol/kg.
The linear GBCA gadobenate dimeglumine has the highest relaxivity among marketed GBCAs with an r1 value in biological medium of 5.5 mM−1·s−1 at 3 T.10 However, since 2017, its use was restricted in Europe to liver scans.15
In this study, gadobutrol was chosen as comparator, as it has the highest relaxivity among currently marketed macrocyclic GBCAs with an r1 value in biological medium of 5 mM−1·s−1 at 3 T.10 The interval between the 2 MRI examinations (2 to 14 days) was strictly controlled, in line with previous investigations,16 to allow contrast agent washout between the 2 examinations and avoid potential changes due to tumor growth or disease progression. This study demonstrated that gadopiclenol at 0.05 mmol/kg is statistically not inferior to gadobutrol at 0.1 mmol/kg in terms of lesion visualization and superior to unenhanced MRI. This reduction in the injected gadolinium dose could be beneficial for patients needing repeated contrast-enhanced MRI for screening, follow-up, or disease management,17–19 but also in pediatric20 and renally impaired patients.21 The increased use of GBCAs, especially in neuroimaging, has raised concerns about their potential environmental impact.22–25 After being eliminated in urine, and released into wastewater systems, gadolinium ends up in rivers and seawater where its concentrations are regularly increasing, with potential consequences on aquatic organisms.26–28 Therefore, reducing the gadolinium dose, as with gadopiclenol, could lower the environmental impact of GBCAs.
At equivalent dose (0.1 mmol/kg), gadobenate dimeglumine achieved greater morphologic information and lesion enhancement compared with gadobutrol, in patients who underwent brain contrast-enhanced MRI.16 Given these results, and those from the phase IIb study,11 better CNR was expected when comparing gadopiclenol at 0.05 mmol/kg with gadobutrol at 0.1 mmol/kg. In this study, E%, CNR, and LBR were 20%, 16%, and 11% higher with gadopiclenol than gadobutrol and may explain the difference in global paired reads. Images with gadopiclenol were in majority preferred to images with gadobutrol, mainly for superior contrast enhancement and better delineation of normal structures and lesions, but also better visualization of lesions internal structure. Although the intraindividual side by side comparison used to assess the overall diagnostic preference is not usually performed in the clinical setting, the higher lesion contrast enhancement obtained with gadopiclenol could facilitate the diagnostic process for the radiologist in the usual clinical setting.
A precise identification of lesions, especially cerebral metastases, is important for patient management. Furthermore, a change in the number of identified lesions could have major clinical implications and impact patient management, especially regarding stereotactic radiosurgery, or when invasive surgery was the treatment option.29 In this study, no difference in the number of detected lesions was observed between gadopiclenol and gadobutrol. Furthermore, the change in treatment plan and the proposed therapeutic management were similar between gadopiclenol and gadobutrol.
Because both gadoterate meglumine and gadoteridol have lower relaxivity than gadobutrol, similar results, if not more favorable for gadopiclenol at 0.05 mmol/kg, would be expected in comparison with these GBCAs.
The good safety profile of gadopiclenol has been previously highlighted in studies including healthy volunteers,30,31 patients with CNS lesions,11,30 pediatric patients with CNS and other body lesions,32 and patients with renal impairment.33 Results from this study showed a slightly lower rate of AEs related to gadopiclenol (4.9%) as compared with gadobutrol (6.9%), and the most frequent AEs were various types of reactions at the injection site for both GBCAs. The safety profile was consistent with previous studies with gadopiclenol and other approved GBCAs.34
This study comes with some limitations. Patients without previous imaging undergoing contrast-enhanced MRI for a first diagnosis were not included. However, this would have further increased the under representation of some contrast-enhancing CNS diseases. Patients with multiple sclerosis were not included, as it is very challenging in such cross-over study. Indeed, enhancement in inflammatory demyelinating lesions is a short-lived feature, and as patients were undergoing 2 MRIs, it could potentially produce bias for the direct comparison between the 2 GBCAs. There was no long-term safety follow-up performed, as the study was not designed for this purpose. The diagnostic performance (sensitivity and specificity) of gadopiclenol and gadobutrol was not evaluated in this study, as no histopathology data were collected.
In summary, PICTURE study demonstrated that gadopiclenol at 0.05 mmol/kg, which is half the dose of gadolinium injected as compared with other approved GBCAs, was as good as gadobutrol at 0.1 mmol/kg, and superior to unenhanced MRI, in terms of CNS lesion visualization. Furthermore, images with gadopiclenol were in majority preferred, in correlation with the higher values for lesion quantitative parameters observed with gadopiclenol. Gadopiclenol showed a good safety profile.
Supplementary Material
ACKNOWLEDGMENT
The authors thank all the investigators of the PICTURE study, Benoît Piednoir (Guerbet) for statistical input, and Ibrahim Bedioune (Guerbet) for providing editorial support. This study was funded by Guerbet, which was involved in study design and conduct and data analysis.
Footnotes
Conflicts of interest and sources of funding: All authors reported no relevant conflicts of interest. This study was funded by Guerbet.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.investigativeradiology.com).
Contributor Information
Balint Kolumban, Email: kolumban.balint@pte.hu.
Gábor Hutóczki, Email: hutoczkigabor@gmail.com.
Katarzyna Dziadziuszko, Email: kado@gumed.edu.pl.
Daniel Bereczki, Email: bereczki.daniel@med.semmelweis-univ.hu.
Attila Bago, Email: bagoatt@hotmail.com.
Anna Pichiecchio, Email: anna.pichiecchio@unipv.it.
REFERENCES
- 1.Kanda T Ishii K Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270:834–841. [DOI] [PubMed] [Google Scholar]
- 2.White GW, Gibby WA, Tweedle MF. Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-DO3A) (ProHance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Invest Radiol. 2006;41:272–278. [DOI] [PubMed] [Google Scholar]
- 3.Maximova N Gregori M Zennaro F, et al. Hepatic gadolinium deposition and reversibility after contrast agent-enhanced MR imaging of pediatric hematopoietic stem cell transplant recipients. Radiology. 2016;281:418–426. [DOI] [PubMed] [Google Scholar]
- 4.Radbruch A Weberling LD Kieslich PJ, et al. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology. 2015;275:783–791. [DOI] [PubMed] [Google Scholar]
- 5.Robert P Lehericy S Grand S, et al. T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Invest Radiol. 2015;50:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanescu AL Shaw DW Murata N, et al. Brain tissue gadolinium retention in pediatric patients after contrast-enhanced magnetic resonance exams: pathological confirmation. Pediatr Radiol. 2020;50:388–396. [DOI] [PubMed] [Google Scholar]
- 7.Lancelot E, Raynaud J-S, Desché P. Current and future MR contrast agents: seeking a better chemical stability and relaxivity for optimal safety and efficacy. Invest Radiol. 2020;55:578–588. [DOI] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration . FDA Drug Safety Communication: FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warnings. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-gadolinium-based-contrast-agents-gbcas-are-retained-body. Accessed August 8, 2022.
- 9.European Medicines Agency . EMA's final opinion confirms restrictions on use of linear gadolinium agents in body scans. Available at: https://www.ema.europa.eu/en/documents/press-release/emas-final-opinion-confirms-restrictions-use-linear-gadolinium-agents-body-scans_en.pdf. Accessed August 8, 2022.
- 10.Robic C Port M Rousseaux O, et al. Physicochemical and pharmacokinetic profiles of gadopiclenol: a new macrocyclic gadolinium chelate with high T1 relaxivity. Invest Radiol. 2019;54:475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bendszus M Roberts D Kolumban B, et al. Dose finding study of gadopiclenol, a new macrocyclic contrast agent, in MRI of central nervous system. Invest Radiol. 2020;55:129–137. [DOI] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration . What is a Serious Adverse Event? Available at: https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event. Accessed July 19, 2022.
- 13.Lancelot E, Desche P. Gadolinium retention as a safety signal: experience of a manufacturer. Invest Radiol. 2020;55:20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong E Pauly JM Wintermark M, et al. Deep learning enables reduced gadolinium dose for contrast-enhanced brain MRI. J Magn Reson Imaging. 2018;48:330–340. [DOI] [PubMed] [Google Scholar]
- 15.European Medicines Agency . Gadolinium-containing contrast agents. European Commission Decision, July 1, 2010. Available at: https://www.ema.europa.eu/en/medicines/human/referrals/gadolinium-containing-contrast-agents-0. Accessed June 16, 2022.
- 16.Seidl Z Vymazal J Mechl M, et al. Does higher gadolinium concentration play a role in the morphologic assessment of brain tumors? Results of a multicenter intraindividual crossover comparison of gadobutrol versus gadobenate dimeglumine (the MERIT study). AJNR Am J Neuroradiol. 2012;33:1050–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wattjes MP Rovira A Miller D, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis—establishing disease prognosis and monitoring patients. Nat Rev Neurol. 2015;11:597–606. [DOI] [PubMed] [Google Scholar]
- 18.Bougias H, Stogiannos N. Breast MRI: where are we currently standing? J Med Imaging Radiat Sci. 2022;53:203–211. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann TJ Smits M Boxerman J, et al. Consensus recommendations for a standardized brain tumor imaging protocol for clinical trials in brain metastases. Neuro Oncol. 2020;22:757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah R D'Arco F Soares B, et al. Use of gadolinium contrast agents in paediatric population: Donald Rumsfeld meets Hippocrates! Br J Radiol. 2019;92:20180746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudnick MR Wahba IM Leonberg-Yoo AK, et al. Risks and options with gadolinium-based contrast agents in patients with CKD: a review. Am J Kidney Dis. 2021;77:517–528. [DOI] [PubMed] [Google Scholar]
- 22.Hatje V, Bruland KW, Flegal AR. Increases in anthropogenic gadolinium anomalies and rare earth element concentrations in San Francisco Bay over a 20 year record. Environ Sci Technol. 2016;50:4159–4168. [DOI] [PubMed] [Google Scholar]
- 23.Verplanck PL Furlong ET Gray JL, et al. Evaluating the behavior of gadolinium and other rare earth elements through large metropolitan sewage treatment plants. Environ Sci Technol. 2010;44:3876–3882. [DOI] [PubMed] [Google Scholar]
- 24.Elbaz-Poulichet F, Seidel JL, Othoniel C. Occurrence of an anthropogenic gadolinium anomaly in river and coastal waters of Southern France. Water Res. 2002;36:1102–1105. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt K Bau M Merschel G, et al. Anthropogenic gadolinium in tap water and in tap water-based beverages from fast-food franchises in six major cities in Germany. Sci Total Environ. 2019;687:1401–1408. [DOI] [PubMed] [Google Scholar]
- 26.Martino C Costa C Roccheri MC, et al. Gadolinium perturbs expression of skeletogenic genes, calcium uptake and larval development in phylogenetically distant sea urchin species. Aquat Toxicol. 2018;194:57–66. [DOI] [PubMed] [Google Scholar]
- 27.Hanana H Turcotte P Andre C, et al. Comparative study of the effects of gadolinium chloride and gadolinium-based magnetic resonance imaging contrast agent on freshwater mussel, Dreissena polymorpha. Chemosphere. 2017;181:197–207. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y Cao XD Lu Y, et al. Effects of rare earth metal ions and their EDTA complexes on antioxidant enzymes of fish liver. Bull Environ Contam Toxicol. 2000;65:357–365. [DOI] [PubMed] [Google Scholar]
- 29.Paek SH Audu PB Sperling MR, et al. Reevaluation of surgery for the treatment of brain metastases: review of 208 patients with single or multiple brain metastases treated at one institution with modern neurosurgical techniques. Neurosurgery. 2005;56:1021–1034. [PubMed] [Google Scholar]
- 30.Hao J, Bourrinet P, Desché P. Assessment of pharmacokinetic, pharmacodynamic profile, and tolerance of gadopiclenol, a new high relaxivity GBCA, in healthy subjects and patients with brain lesions (phase I/IIa study). Invest Radiol. 2019;54:396–402. [DOI] [PubMed] [Google Scholar]
- 31.Funck-Brentano C Felices M Le Fur N, et al. Randomized study of the effect of gadopiclenol, a new gadolinium-based contrast agent, on the QTc interval in healthy subjects. Br J Clin Pharmacol. 2020;86:2174–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jurkiewicz E Tsvetkova S Grinberg A, et al. Pharmacokinetics, safety, and efficacy of gadopiclenol in pediatric patients aged 2 to 17 years. Invest Radiol. 2022;57:510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradu A Penescu M Pitrou C, et al. Pharmacokinetics, dialysability, and safety of gadopiclenol, a new gadolinium-based contrast agent, in patients with impaired renal function. Invest Radiol. 2021;56:486–493. [DOI] [PubMed] [Google Scholar]
- 34.Gutierrez JE Rosenberg M Seemann J, et al. Safety and efficacy of gadobutrol for contrast-enhanced magnetic resonance imaging of the central nervous system: results from a multicenter, double-blind, randomized, comparator study. Magn Reson Insights. 2015;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


