Migraine reduces the ability to work and to function optimally at work. Suboptimal acute treatment for migraine attacks is linked to greater lost productive time. Optimizing an individual’s acute treatment for migraine may reduce migraine-related losses in work productivity and reduce indirect costs.
Keywords: absenteeism, presenteeism, headache, migraine, employment, disability, productivity
Objective
This study aimed to ascertain whether level of optimization of acute treatment of migraine is related to work productivity across the spectrum of migraine.
Methods
Data were from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study, an internet-based longitudinal survey. Respondents with migraine who reported full-time employment and use of ≥1 acute prescription medication for migraine were included. We determined relationships among lost productive time (LPT; measured with the Migraine Disability Assessment Scale), acute treatment optimization (Migraine Treatment Optimization Questionnaire- ), and monthly headache days (MHDs).
Results
There was a direct relationship between LPT and MHD category. Greater acute treatment optimization was associated with lower total LPT, less absenteeism, and less presenteeism within each MHD category.
Conclusions
Optimizing acute treatment for migraine may reduce LPT in people with migraine and reduce indirect costs.
LEARNING OUTCOMES
Identify the relationship between optimization of acute treatment of migraine and work productivity.
Critically analyze how the level of acute treatment optimization affects the relationship between MHDs and LPT.
Migraine, a chronic disease with episodic attacks defined by various combinations of symptoms including moderate to severe headache pain, pulsatile pain, photophobia, phonophobia, and nausea, is a leading cause of global disease burden.1–3 Migraine attacks are often incapacitating, with an adverse impact on physical and emotional functioning, engagement in activities of daily living, and health-related quality of life, both during and between attacks.4–7
Migraine-associated disability results in absence from work (absenteeism) and reduced productivity while at work (presenteeism), both of which contribute to substantial economic burden on individuals, employers, and society.4,8–13 Lost productivity increases in relation to headache frequency and headache pain intensity in people with migraine.8 Higher frequency of headache days is associated with greater lost productive time (LPT) and increased likelihood of unemployment.14
Acute medications for migraine are used at the time of an attack to relieve pain and restore function, whereas preventive treatments are intended to reduce the frequency, severity, and duration of future attacks.15–17 Prescription medications used for the acute treatment of migraine include triptans, nonsteroidal anti-inflammatory drugs (prescription or over-the-counter [OTC]), ergot derivatives, opioids, and barbiturates,18,19 and, more recently, gepants and lasmiditan.20 An acute treatment regimen is considered optimized when it consistently relieves pain, restores function with minimal need for repeat dosing or rescue medication, and is well tolerated with minimal adverse events and the patient feels that it is reliable.21 Some medications for the acute treatment of migraine may be ineffective or poorly tolerated in some people with migraine.21,22 Suboptimal treatment may lead to uncontrolled migraine and acute medication overuse, as well as medication overuse headache, potentially further compounding the disability and burden of migraine on all aspects of an individual's life, including work productivity.1,23,24
To our knowledge, the relationship between acute treatment optimization and LPT has not been previously reported. To test the hypothesis that optimization of acute treatment is associated with reduced adverse impact on work productivity across the spectrum of monthly headache day (MHD) frequency in persons with migraine, we analyzed data from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study.25 The potential impact of education level achieved, annual household income, race, and ethnicity was also evaluated.
METHODS
Study Design
The CaMEO Study (ClinicalTrials.gov identifier: NCT01648530) was a longitudinal internet-based survey designed to characterize the course of migraine for 1 year in a broad cohort of respondents representative of the US population. Details of the study design have been published.25 Between September and October 2012, participants were screened and recruited from an internet research panel (Research Now, renamed Dynata [https://www.dynata.com/], Plano, TX) with 2.4 million active US members. A web survey captured headache information and demographics and was used to identify eligible participants with migraine as detailed hereinafter. Those who met the criteria for migraine received a series of cross-sectional surveys that assessed health care use, family burden, and comorbidities or endophenotypes. The current analysis evaluated data from the screening and core modules (administered at baseline) and the endophenotype module (administered to the same individuals approximately 1–2 months after baseline). The core module included assessments of migraine treatments, treatment optimization (5-item Migraine Treatment Optimization Questionnaire [mTOQ-5]), migraine-related disability (Migraine Disability Assessment [MIDAS]), depression (9-item Patient Health Questionnaire [PHQ-9]), and anxiety (7-item Generalized Anxiety Disorder [GAD-7]). The endophenotype module assessed allodynia using the 12-item Allodynia Symptom Checklist. The CaMEO Study was approved by the Albert Einstein College of Medicine Institutional Review Board (12-04-177E); the institutional review board waived written informed consent for study volunteers, who had the right to accept or refuse participation in the survey.
Study Population
The CaMEO Study used quota sampling to recruit a demographically representative sample of the US population based on age, sex, and income.25 Eligible respondents were adults (18 years or older) who met the criteria for migraine consistent with the International Classification of Headache Disorders, third edition (ICHD-3),1 based on the validated American Migraine Study/American Migraine Prevalence and Prevention study migraine diagnostic module,26 and provided data on headache frequency (Note: The American Migraine Study/American Migraine Prevalence and Prevention diagnostic module was based on ICHD-2 migraine criteria25,27; however, no significant changes occurred between ICHD-2,27 ICHD-3-β,28 and the final ICHD-31 criteria related to classification of migraine in this study). Analysis was confined to eligible respondents who, at the time of the survey, were using a prescription medication for the acute treatment of migraine attacks, were employed full time (>35 h/wk), and had valid data for the MIDAS and mTOQ-5. Participant-reported race and annual household income were determined based on responses to questions in the screening Module. Prescription acute medications for migraine or headache were identified by participants with migraine from a prespecified list of available acute prescription medications.
Estimation of Absenteeism, Presenteeism, and Total LPT
Absenteeism, presenteeism, and total LPT were estimated from responses to the MIDAS questionnaire.29 Two of the five MIDAS questions ask about the number of days of missed work or school (absenteeism) in the past 3 months because of headache and number of days while at work or school where productivity was reduced by half or more (presenteeism) because of headache. We defined total LPT as missed workdays (absenteeism) plus workdays where productivity was reduced by half or more (presenteeism).8,30 Based on prior validation research, a workday reduced by half or more is defined as being equivalent to a full day of missed work.31 Diary data showed that, on days where work productivity was reduced by half or more per MIDAS, the average diary-reported reduction in productivity was 72.7%.31 Furthermore, days where productivity was reduced by less than half were not included in the MIDAS estimate; thus, LPT is unlikely to be overestimated using this method.
Variables
Level of acute treatment optimization was assessed from the mTOQ-5,32 which asks about all acute treatments that an individual may be using (OTC and prescription) with the general statement about “times when you take treatment for your headaches” with dichotomous (yes/no) responses to the following five questions:
Were you able to quickly return to your normal activities (ie, work, family, leisure, social activities) after taking your headache medication?
Could you count on your headache medication to relieve your pain within 2 hours for most attacks?
Did one dose of your headache medication usually relieve your headache and keep it away for at least 24 hours?
Was your headache medication well tolerated?
Were you comfortable enough with your headache medication to be able to plan your daily activities?
The total mTOQ-5 score ranged from 0 to 5 with 1 point allocated for each “yes” response. A score of 0 represents the worst possible treatment optimization, and a score of 5 represents the best treatment optimization.
Monthly average headache day frequency was categorized as ≤3 MHDs, 4 to 7 MHDs, 8 to 14 MHDs, and ≥15 MHDs, consistent with recent convention.33–35 Other characteristics evaluated included participant-reported age (10-year categories), sex (male/female), race (White only, Black or African American only, ≥2 races, and other), ethnicity (Hispanic/Latinx vs not Hispanic/Latinx), education level (high school or less; some college, technical school, or technical school degree; 4-year college degree; and postcollege degree), and annual household income (<$30,0000, $30,000–$49,999, $50,000–$74,999, and ≥ $75,000). We calculated body mass index from self-reported height and weight. The Migraine Symptom Severity Score (MSSS) is a composite index based on the frequency of seven ICHD-3 diagnostic migraine symptoms (unilateral pain, pulsatile pain, moderate or severe pain intensity, routine activities worsening pain, nausea, photophobia, and phonophobia)36; item responses range from 0 to 3, with the overall sum score ranging from 0 to 21. The presence of cutaneous allodynia was determined using the 12-item Allodynia Symptom Checklist; Allodynia Symptom Checklist scores of ≥3 indicate the presence of allodynia.37,38 Depression was assessed using the 9-item depression module of the PHQ-9; sum scores for the PHQ-9 range from 0 to 27, with scores ≥10 categorized as moderate to severe depression.39 Anxiety was assessed using the GAD-7, with scores ranging from 0 to 21 and scores of ≥10 categorized as moderate to severe anxiety.40
Statistical Analysis
A cross-sectional analysis compared sociodemographic, clinical, and headache characteristics across levels of treatment optimization. Scores for mTOQ-5 ranged from 0 (worst possible treatment optimization) to 5 (best possible treatment optimization). With the exception of age and body mass index, sociodemographic covariates were coded as categorical variables. For clinical characteristics, the mean MHD frequency and MSSSs were coded as continuous variables; allodynia, depression, and anxiety were coded as categorical variables, as defined previously. Mean absenteeism, presenteeism, and total LPT were plotted by mTOQ-5 score for each MHD category. The linearity of the relationship between each productivity measure and mTOQ-5 score was assessed within each MHD category using a test for linearity, which is part of the analysis of variance procedure in SPSS (IBM SPSS Statistics for Macintosh, version 27.0; IBM, Armonk, NY). The CaMEO study was conducted in 2012, and respondents reported their sex (male or female) in response to the question: “Please indicate your gender.” The study did not include a separate question to distinguish respondents' sex at birth and gender. Given this limitation, we have chosen to use the exact survey terms for all reported results.
A series of nested multivariable negative binomial regression models, in which absenteeism, presenteeism, or total LPT was the dependent variable, assessed factors associated with each productivity measure. Negative binomial regression is used when modeling count outcome variables (eg, number of days of absenteeism or presenteeism or total LPT), particularly when the distribution is overdispersed (ie, the variance is greater than the mean).41 An inspection of the distribution of the mean LPT confirmed the presence of these qualities and guided our decision that negative binomial regression was an appropriate methodology. Covariates were entered in sequential blocks, starting with MHD group and mTOQ-5 score, followed by sociodemographic variables, allodynia and MSSS, and depression and anxiety. Rate ratios (95% confidence intervals) were calculated for each variable.
RESULTS
Participants
Of the 16,789 CaMEO respondents with migraine, 4902 endorsed current use of preselected prescription acute headache medications, and 2455 (50.1%) of these respondents were employed full time, had valid MIDAS and mTOQ-5 scores, and were included in the analysis population. Within this analysis population of respondents who were employed full time, 142 (5.8%) reported that they were also currently students. The most commonly reported acute prescription migraine medications in this analysis population were triptans (40.7%), prescription nonsteroidal anti-inflammatory drugs (37.7%), and opioids (36.3%) (Table 1); 88.8% of respondents also reported current use of at least one OTC medication for their headaches.
TABLE 1.
Baseline Characteristics by mTOQ-5 Acute Treatment Optimization Responses
| No. Positive mTOQ Responses | |||||||
|---|---|---|---|---|---|---|---|
| Total (n = 2455) | 0 (Not at All Optimized) (n = 87) |
1 (n = 186) |
2 (n = 310) |
3 (n = 463) |
4 (n = 596) |
5 (Fully Optimized) (n = 813) |
|
| Age, mean (SD), y | 42.0 (11.2) | 39.3 (9.2) | 39.1 (10.7) | 41.2 (11.0) | 40.9 (10.7) | 42.0 (11.1) | 43.9 (11.7) |
| Sex | |||||||
| Female | 1908 | 72 (3.8) | 143 (7.5) | 249 (13.1) | 369 (19.3) | 460 (24.1) | 615 (32.2) |
| Male | 547 | 15 (2.7) | 43 (7.9) | 61 (11.2) | 94 (17.2) | 136 (24.9) | 198 (36.2) |
| Race | |||||||
| White only | 1820 | 61 (3.4) | 148 (8.1) | 246 (13.5) | 340 (18.7) | 425 (23.4) | 600 (33.0) |
| Black only | 383 | 17 (4.4) | 23 (6.0) | 37 (9.7) | 69 (18.0) | 101 (26.4) | 136 (35.5) |
| Other single race | 162 | 5 (3.1) | 8 (4.9) | 20 (12.3) | 32 (19.8) | 41 (25.3) | 56 (34.6) |
| ≥2 Races | 83 | 3 (3.6) | 5 (6.0) | 7 (8.4) | 21 (25.3) | 28 (33.7) | 19 (22.9) |
| Ethnicity | |||||||
| Hispanic/Latinx | 421 | 20 (4.8) | 34 (8.1) | 55 (13.1) | 79 (18.8) | 112 (26.6) | 121 (28.7) |
| Not Hispanic/Latinx | 2033 | 67 (3.3) | 152 (7.5) | 255 (12.5) | 383 (18.8) | 484 (23.8) | 692 (34.0) |
| College degree (≥4 y) | 1249 | 43 (3.4) | 85 (6.8) | 160 (12.8) | 242 (19.4) | 303 (24.3) | 416 (33.3) |
| Obese (BMI ≥30 kg/m2) | 958 | 40 (4.2) | 67 (7.0) | 118 (12.3) | 169 (17.6) | 237 (24.7) | 327 (34.1) |
| Income (yearly) | |||||||
| <$30,000 | 257 | 13 (5.1) | 22 (8.6) | 41 (16.0) | 56 (21.8) | 60 (23.3) | 65 (25.3) |
| $30,000–49,999 | 452 | 19 (4.2) | 30 (6.6) | 54 (11.9) | 88 (19.5) | 111 (24.6) | 150 (33.2) |
| $50,000–74,999 | 624 | 14 (2.2) | 57 (9.1) | 86 (13.8) | 129 (20.7) | 135 (21.6) | 203 (32.5) |
| ≥$75,000 | 1116 | 41 (3.7) | 75 (6.7) | 129 (11.6) | 190 (17.0) | 288 (25.8) | 393 (35.2) |
| Has health insurance | 2282 | 81 (3.5) | 164 (7.2) | 288 (12.6) | 426 (18.7) | 563 (24.7) | 760 (33.3) |
| MHD frequency | |||||||
| ≤3 d/mo | 1182 | 33 (2.8) | 71 (6.0) | 129 (10.9) | 205 (17.3) | 266 (22.5) | 478 (40.4) |
| 4–7 d/mo | 602 | 18 (3.0) | 52 (8.6) | 82 (13.6) | 124 (20.6) | 138 (22.9) | 188 (31.2) |
| 8–14 d/mo | 379 | 19 (5.0) | 31 (8.2) | 55 (14.5) | 71 (18.7) | 112 (29.6) | 91 (24.0) |
| ≥15 d/mo | 292 | 17 (5.8) | 32 (11.0) | 44 (15.1) | 63 (21.6) | 80 (27.4) | 56 (19.2) |
| Allodynia (ASC-12 ≥3) | 1037 | 39 (3.8) | 100 (9.6) | 145 (14.0) | 213 (20.5) | 240 (23.1) | 300 (28.9) |
| MSSS, median (IQR) | – | 19.0 (4.0) | 19.0 (4.0) | 18.0 (4.0) | 17.0 (5.0) | 16.0 (5.0) | 16.0 (5.5) |
| PHQ-9, moderate-severe depression (score, ≥10) | 906 | 44 (4.9) | 91 (10.0) | 144 (15.9) | 213 (23.5) | 217 (24.0) | 197 (21.7) |
| GAD-7, moderate-severe anxiety (score, ≥10) | 844 | 42 (5.0) | 76 (9.0) | 139 (16.5) | 183 (21.7) | 199 (23.6) | 205 (24.3) |
| Current medication for acute treatment of headaches | |||||||
| OTC medication use* | |||||||
| Any | 2179 | 74 (3.4) | 166 (7.6) | 271 (12.4) | 434 (19.9) | 533 (24.5) | 701 (32.2) |
| Ibuprofen | 1031 | 41 (4.0) | 84 (8.1) | 138 (13.4) | 207 (20.1) | 273 (26.5) | 288 (27.9) |
| Acetaminophen | 1009 | 33 (3.3) | 78 (7.7) | 137 (13.6) | 204 (20.2) | 250 (24.8) | 307 (30.4) |
| Acetaminophen-aspirin-caffeine combo | 930 | 36 (3.9) | 82 (8.8) | 119 (12.8) | 188 (20.2) | 216 (23.2) | 289 (31.1) |
| Naproxen sodium | 850 | 29 (3.4) | 70 (8.2) | 110 (12.9) | 169 (19.9) | 213 (25.1) | 259 (30.5) |
| Aspirin | 468 | 16 (3.4) | 36 (7.7) | 62 (13.2) | 95 (20.3) | 117 (25.0) | 142 (30.3) |
| Prescription medication use | |||||||
| Triptan | 998 | 42 (4.2) | 87 (8.7) | 123 (12.3) | 165 (16.5) | 243 (24.3) | 338 (33.9) |
| Prescription NSAID | 926 | 28 (3.0) | 67 (7.2) | 126 (13.6) | 185 (20.0) | 235 (25.4) | 285 (30.8) |
| Opioid | 891 | 27 (3.0) | 73 (8.2) | 114 (12.8) | 199 (22.3) | 214 (24.0) | 264 (29.6) |
| Barbiturate | 233 | 6 (2.6) | 14 (6.0) | 35 (15.0) | 46 (19.7) | 63 (27.0) | 69 (29.6) |
| Isometheptene | 83 | 6 (7.2) | 6 (7.2) | 13 (15.7) | 17 (20.5) | 20 (24.1) | 21 (25.3) |
| Ergotamine | 61 | 0 | 3 (4.9) | 7 (11.5) | 18 (29.5) | 16 (26.2) | 17 (27.9) |
Data are presented as n (%) unless indicated otherwise. Percentages were calculated as n/N, with N being the value reported in the total column of each row. Higher mTOQ scores indicate better treatment optimization.
ASC-12, 12-item Allodynia Symptom Checklist; BMI, body mass index; GAD-7, 7-item Generalized Anxiety Disorder; IQR, interquartile range; OTC, over-the-counter; PHQ-9, 9-item Patient Health Questionnaire; MSSS, Migraine Symptom Severity Score; NSAID, nonsteroidal anti-inflammatory drug.
*Respondents may endorse use of more than one type of medication at a time.
Monthly headache day frequency was ≤3 MHDs in 48.1% (1182 of 2455) of respondents, 4 to 7 MHDs in 24.5% (602 of 2455), 8 to 14 MHDs in 15.4% (379 of 2455), and ≥15 MHDs in 11.9% (292 of 2455). Approximately one third (813 of 2455 [33.1%]) of respondents had a total mTOQ-5 score of 5 positive responses, the highest possible score for optimization of acute treatment. The remaining respondents (66.9%) had mTOQ-5 scores as follows: 24.3% (596 of 2455) scored 4, 18.9% (463 of 2455) scored 3, 12.6% (310 of 2455) scored 2, 7.6% (186 of 2455) scored 1, and 3.5% (87 of 2455) scored 0 (no positive responses; worst possible score) ( see Supplemental Digital Content, Supplementary Table S1, http://links.lww.com/JOM/B287, for a summary of responses to each individual mTOQ-5 question).
LPT by Level of Acute Treatment Optimization
In the analysis population, greater total LPT, absenteeism, and presenteeism days were associated with worse treatment optimization scores (P < 0.0001 for all three outcomes) (Fig. 1). In those with poorly optimized acute treatment (mTOQ-5 score, ≤1) 3-month LPT was 12.5 days compared with 4.3 days in those with well-optimized acute treatment (mTOQ-5 score, 5). Mean 3-month absenteeism was 3.8 days in those with poorly optimized acute treatment (mTOQ-5 score, ≤1) and 1.3 days in those with well-optimized acute treatment (mTOQ-5 score, 5). Similarly, the mean 3-month presenteeism was 8.6 days in those with poorly optimized acute treatment and 3.0 days in the well-optimized group.
FIGURE 1.
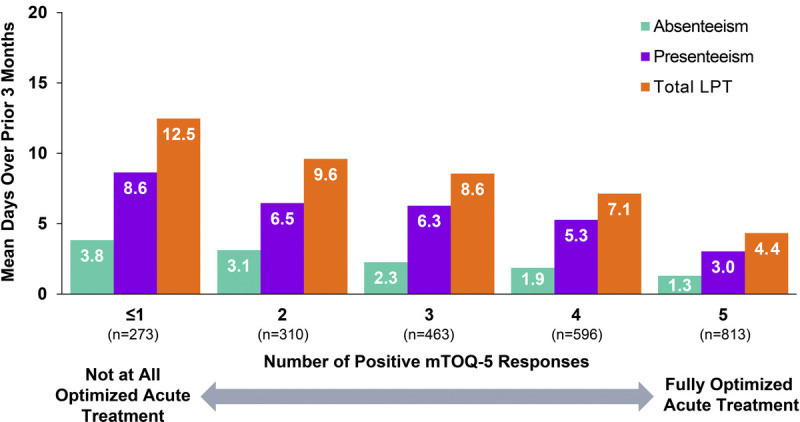
Mean absenteeism, presenteeism, and total LPT for 3 months by acute treatment optimization (mTOQ-5) score. LPT, lost productive time; mTOQ-5, 5-Item Migraine Treatment Optimization Questionnaire.
When evaluating LPT within each MHD category, total LPT was inversely related with mTOQ-5 scores in three of four MHD groups (Fig. 2A; test for linearity, P ≤ 0.01). The relationship between treatment optimization and LPT was most pronounced in the ≥15 MHD group where the mean 3-month LPT was 30.4 days among those with poorly optimized acute treatment (mTOQ-5 score, ≤1) and 7.1 days among those with well-optimized acute treatment (mTOQ-5 score, 5). Mean 3-month absenteeism showed an inverse relationship with mTOQ-5 score in all MHD groups (test for linearity, P ≤ 0.01), with the exception of the 8 to 14 MHD group (test for linearity, P = 0.197) (Fig. 2B). In the ≥15 MHD group, the mean 3-month absenteeism ranged from 7.6 days (mTOQ-5 score, ≤1) to 1.0 days (mTOQ-5 score, 5). Similarly, the mean 3-month presenteeism was significantly lower in association with higher mTOQ-5 scores in all MHD groups (test for linearity, P ≤ 0.01), with the exception of the 8 to 14 MHD group (test for linearity, P = 0.140) (Fig. 2C).
FIGURE 2.
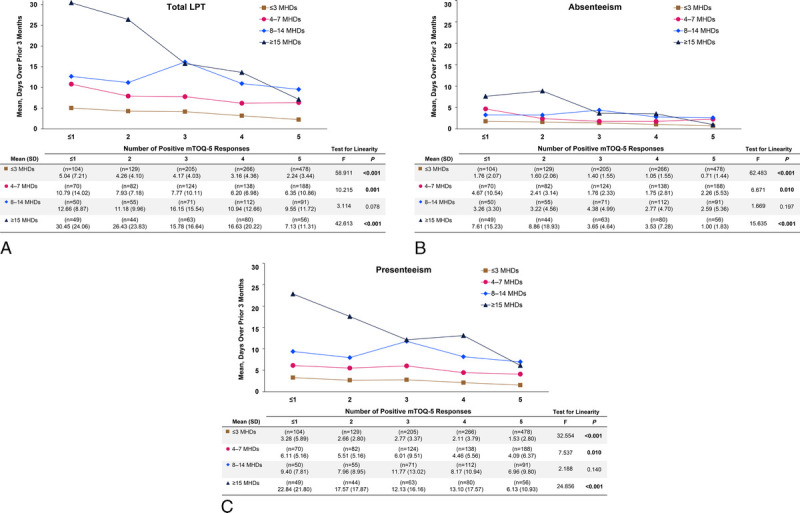
Mean 3-month (A) total LPT, (B) absenteeism, and (C) presenteeism by acute treatment optimization (mTOQ-5) score and MHD group. LPT, lost productive time; MHD, monthly headache day; mTOQ-5, 5-Item Migraine Treatment Optimization Questionnaire.
Negative Binomial Regression Modeling
To identify factors associated with total LPT, absenteeism, and presenteeism, we used a series of negative binomial regression models. Results of the final models are shown in Table 2 (full models are shown in Supplemental Digital Content, Supplementary Table S2, http://links.lww.com/JOM/B288; Supplementary Table S3, http://links.lww.com/JOM/B289; and Supplementary Table S4, http://links.lww.com/JOM/B290). Increasing MHDs and decreasing mTOQ-5 score were significantly associated with increased total LPT, absenteeism, and presenteeism in the fully adjusted models (Table 2). Compared with the ≤3 MHD group, those with ≥15 MHD would be expected to have a 4.35 times greater rate for total LPT while holding the other variables in the model constant. The 8 to 14 MHD group was associated with an LPT rate 3.01 times higher, and the 4 to 7 MHD group was associated with an LPT rate 1.98 times higher. Additional factors associated with increased total LPT in the fully adjusted model were Black/African American race (rate ratio, 1.32; P < 0.0001), presence of allodynia (rate ratio, 1.27; P < 0.0001), elevated MSSS (rate ratio, 1.04; P < 0.0001), moderate to severe depression (rate ratio, 1.36; P < 0.0001), and anxiety (rate ratio, 1.21; P = 0.006; Table 2). Self-reported female sex (P = 0.008) and increasing age (10-year intervals; P < 0.0001) were associated with less LPT. Lost productive time rates for women would be expected to decrease by a factor of 0.84, compared with the LPT rates for men (with other variables held constant). Factors associated with greater absenteeism were Black/African American race (P < 0.0001), Hispanic/Latinx ethnicity (P = 0.039), presence of allodynia (P = 0.003), elevated MSSS (P = 0.009), and depression (P < 0.0001). Female sex (P < 0.0001), having a postcollege degree (P = 0.025), and increasing age (P = 0.004) were associated with lower absenteeism. Greater presenteeism was associated with presence of allodynia (P < 0.0001), elevated MSSS (P < 0.0001), depression (P < 0.0001), and anxiety (P < 0.0001). Increasing age (P = 0.001) was significantly associated with lower presenteeism.
TABLE 2.
Factors Associated With LPT, Absenteeism, and Presenteeism: Final Multivariable Negative Binomial Regression Models
| Rate Ratio (95% CI) | |||
|---|---|---|---|
| Total LPT | Absenteeism | Presenteeism | |
| MHDs (reference: ≤3 MHDs) | |||
| 4–7 | 1.98 (1.74–2.25) | 1.82 (1.53–2.16) | 2.01 (1.73–2.32) |
| 8–14 | 3.01 (2.60–3.50) | 2.31 (1.89–2.81) | 3.31 (2.79–3.92) |
| ≥15 | 4.35 (3.67–5.15) | 3.42 (2.75–4.25) | 4.72 (3.90–5.72) |
| mTOQ-5 score (reference: 5) | |||
| 4 | 1.23 (1.07–1.42) | 1.10 (0.91–1.33) | 1.28 (1.09–1.50) |
| 3 | 1.48 (1.28–1.72) | 1.34 (1.10–1.64) | 1.54 (1.30–1.83) |
| 2 | 1.46 (1.23–1.74) | 1.61 (1.28–2.02) | 1.43 (1.17–1.74) |
| 0/1 | 1.60 (1.33–1.93) | 1.89 (1.49–2.41) | 1.51 (1.22–1.87) |
| Female (reference: male) | 0.84 (0.74–0.96) | 0.66 (0.56–0.78) | 0.91 (0.79–1.06) |
| Annual household income (reference: <$30,000) | |||
| $30,000–$49,999 | 1.00 (0.81–1.22) | 1.10 (0.84–1.44) | 0.95 (0.76–1.20) |
| $50,000–$74,999 | 0.99 (0.82–1.21) | 1.18 (0.91–1.52) | 0.94 (0.75–1.17) |
| ≥$75,000 | 0.95 (0.78–1.16) | 1.07 (0.83–1.38) | 0.91 (0.73–1.14) |
| Race (reference: White only) | |||
| Black/African American only | 1.32 (1.14–1.53) | 1.71 (1.41–2.09) | 1.18 (1.00–1.40) |
| Other | 0.90 (0.71–1.14) | 1.16 (0.86–1.58) | 0.80 (0.61–1.05) |
| ≥2 Races | 1.12 (0.83–1.50) | 1.35 (0.92–1.97) | 1.09 (0.78–1.52) |
| Hispanic/Latinx (reference: Not Hispanic/Latinx) | 1.12 (0.97–1.29) | 1.22 (1.01–1.46) | 1.08 (0.92–1.27) |
| Education (reference: high school or less) | |||
| Some college, technical school, or technical school degree | 1.04 (0.88–1.24) | 0.99 (0.79–1.24) | 1.06 (0.87–1.29) |
| 4-y College degree | 1.01 (0.84–1.21) | 0.89 (0.70–1.13) | 1.06 (0.86–1.30) |
| Postcollege degree | 1.00 (0.81–1.22) | 0.73 (0.56–0.96) | 1.11 (0.88–1.40) |
| Health insurance, yes (reference: no) | 1.00 (0.81–1.25) | 1.25 (0.93–1.68) | 0.95 (0.74–1.21) |
| Age (10-y intervals) | 0.91 (0.87–0.96) | 0.91 (0.85–0.97) | 0.91 (0.87–0.97) |
| BMI | 1.00 (1.00–1.01) | 0.99 (0.99–1.00) | 1.00 (1.00–1.01) |
| Allodynia, yes (ASC ≥3; reference: ASC <3) | 1.27 (1.14–1.42) | 1.25 (1.08–1.45) | 1.28 (1.13–1.46) |
| Migraine symptom severity (MSSS) | 1.04 (1.02–1.06) | 1.03 (1.01–1.06) | 1.04 (1.02–1.06) |
| Depression, yes (PHQ-9 ≥10; reference: PHQ-9 <10) | 1.36 (1.19–1.56) | 1.50 (1.26–1.80) | 1.32 (1.13–1.54) |
| Anxiety, yes (GAD-7 ≥10; reference: GAD-7 <10) | 1.21 (1.06–1.38) | 0.99 (0.83–1.18) | 1.28 (1.10–1.50) |
Boldfaced values indicate that the P value for the odds ratio was statistically significant (P < 0.05).
ASC, Allodynia Symptom Checklist; BMI, body mass index; CI, confidence interval; GAD-7, 7-item Generalized Anxiety Disorder; LPT, lost productive time; MHD, monthly headache day; MIDAS, Migraine Disability Assessment; MSSS, Migraine Symptom Severity Score; mTOQ-5, 5-Item Migraine Treatment Optimization Questionnaire; PHQ-9, 9-item Patient Health Questionnaire.
Higher frequencies of MHDs were associated with greater total LPT, absenteeism, and presenteeism in separate models of Black/African American respondents, White respondents (Supplemental Digital Content, Supplementary Table S5, http://links.lww.com/JOM/B291), and Hispanic/Latinx and non-Hispanic/Latinx respondents (Supplemental Digital Content, Supplementary Table S6, http://links.lww.com/JOM/B292). Significance was more consistent across mTOQ-5 scores with White and non-Hispanic/Latinx respondents, possibly because of the larger sample sizes.
DISCUSSION
This analysis of the overall population from the CaMEO Study showed that well-optimized acute migraine treatment was associated with less total LPT, absenteeism, and presenteeism across MHD categories. People with higher MHDs consistently reported greater losses in work productivity. Among respondents experiencing ≥15 MHDs, average total LPT was more than fourfold greater in people reporting poor/very poor acute treatment optimization compared with those reporting well-optimized acute treatment, with a mean total LPT of 30.4 days for 3 months (or approximately 10 d/mo) versus 7.1 days for 3 months (or approximately 2 d/mo). In respondents with ≤3 MHDs, the average total LPT was 5.0 days for 3 months (approximately 1.67 d/mo) in those with poor/very poor acute treatment optimization compared with 2.2 days for 3 months (0.73 d/mo) in those with well-optimized acute treatment. The effect of acute treatment optimization on average total LPT was statistically significant after adjusting for MHDs, as well as sociodemographic and disease characteristics. We observed that both mTOQ-5 score and MHDs are related to LPT; greater MHDs and poorer acute treatment optimization predicted greater losses in productivity. By extension, one could potentially reduce LPT either by reducing MHDs, improving acute treatment optimization, or both.
Migraine-related lost productivity is substantial and contributes to considerable economic burden on individuals, employers, and society.4,8–12 Migraine is most common between the ages of 18 and 55 years, the peak productive years in a person's life, and thus a time period when the disease can substantially disrupt educational and occupational outcomes. The economic burden of migraine comprises direct costs (eg, cost of medical care) and indirect costs (eg, work productivity loss). A recent analysis of the Medical Expenditure Panel Survey database found that migraine was associated with substantially higher annualized societal costs compared with a propensity-matched nonmigraine cohort.42 An analysis of US commercial insurance claims data between 2007 and 2013 reported that the mean annual total indirect cost due to workdays lost because of absence, short-term disability, and long-term disability was $2350 higher per individual in patients with migraine compared with matched patients without migraine.43 Addressing the indirect costs of migraine requires providing individuals with optimized acute treatment regimens that restore function compromised by migraine attacks.
Undertreatment and suboptimal acute treatment of migraine are common. A separate analysis of CaMEO data from 13,624 people with migraine revealed that 64.5% of the respondents had never used an acute prescription medication for migraine attacks, 22.9% reported current use of acute prescription medication for migraine attacks, and 12.6% had previously used an acute prescription medication but were no longer doing so.44 Despite current use of an acute prescription medication, 50.7% of the current users reported ≥5 MHDs, 59.8% had moderate-to-severe headache-related disability (MIDAS grade III/IV), 37.4% had comorbid moderate to severe depression (PHQ-9 score, ≥10), and 33.0% had comorbid moderate to severe anxiety (GAD-7 score, >10).44 Among respondents who had discontinued acute prescription medication, only 21.3% discontinued because their disease had improved; 28.2% discontinued because of lack of efficacy, and 24.9% discontinued because of tolerability or safety concerns.22 In addition, the use of opioids for the acute treatment of migraine attacks is not ideal because of the association of opioids and dependency; individuals using opioids for migraine were also found to have additional comorbidities and higher utilization of healthcare resources.45
Our results suggest that optimizing an individual's acute treatment regimen may translate into reductions in LPT. We recommend caution, as this is a cross-sectional study, and longitudinal within-person acute treatment optimization and its relationship to LPT were not assessed. Results of randomized controlled trials of triptans suggest that use of effective treatments can reduce LPT.46–48 In addition, patients who do not respond to triptans have greater losses in work productivity than triptan responders.49 Preventive treatment of migraine has also been associated with reductions in lost work productivity.50 This may be attributed to preventive treatments reducing the frequency or severity of migraine attacks. In addition, preventive treatment may improve the efficacy of acute medication taken for breakthrough migraine attacks.21
It is important for clinicians to periodically assess the efficacy and tolerability of a patient's acute medications, as well as preventive treatments, if indicated, to work toward optimization of the patient's treatment regimen. There are several validated patient-reported outcome questionnaires that may be used clinically to assess level of acute treatment optimization (eg, mTOQ, Migraine—Assessment of Current Therapy, Patient Perception of Migraine Questionnaire—Revised, Functional Impairment Scale, Patient Global Impression of Change).21,51,52 There are several factors to consider when assessing acute treatment.21 Patients should be encouraged to treat early after the onset of a migraine attack, adopt appropriate lifestyle modifications, and avoid overexposure to acute medications.
Our modeling analyses identified several sociodemographic and disease characteristics that were associated with LPT. Employed women had lower levels of total LPT and absenteeism compared with men. Hispanic/Latinx ethnicity was associated with increased time lost to absenteeism, but not total LPT or presenteeism. Higher education (≥4-year degree) was associated with reduced time lost due to absenteeism, but not presenteeism or total LPT. One factor contributing to this finding may be the ability of better educated individuals to modify their work demands in the workplace when they are ill.53 Increased age was associated with lower levels of total LPT, absenteeism, and presenteeism. Allodynia, greater severity of migraine symptoms, and moderate/severe depression all were associated with greater absenteeism, presenteeism, and LPT, perhaps because allodynia is a marker of more severe headache.37,54 One previous study reported that LPT decreased with increasing age and was significantly higher for Black/African American individuals compared with White individuals.8 The CaMEO Study did not collect data on type of work or job category. Evaluating differences in job type (low control/high-demand vs high control/low-demand)55,56 may provide some insight to the relationship observed in our study. It is possible that differences in type of work among demographic groups in our analysis population may explain the impact on LPT observed. For example, careers associated with a high level of education or increased age may have greater autonomy that allows the employee to control their schedule and modify their work environment around migraine attacks.
Employer-sponsored programs with the goal of improving optimization of migraine management have shown increased work productivity and quality of life for employees. A systematic review of prospective cohort studies found that employee education programs focused on migraine management in the workplace were associated with a 29% to 36% increase in productivity.57 One study found that implementation of an employer-sponsored disease management program that included coaching lessons and action plans focused on healthy lifestyle changes (sleep, hydration, nutrition, etc) resulted in a reduction in absenteeism and presenteeism and increased migraine-free days for employees in their private/social life.58 Our results further support the use of these employer-sponsored programs and suggest that improving treatment optimization can result in increased productivity and reduced absenteeism.
This study has limitations. The results rely on self-reported data, and errors in self-report could lead to biased estimates. The mTOQ-5 has dichotomous response options, restricting the range of response possibilities. In addition, the mTOQ-5 asks respondents about “times when you take treatment for your headaches.” Therefore, we cannot know what treatment(s) the respondent had in mind when answering the questions, and the majority of respondents were using acute polypharmacy. We assume that it is a compilation of all acute treatments that they were using, including OTC and prescription treatments. Estimates of LPT were based on responses to the MIDAS questionnaire using a recall period of 3 months. The MIDAS scores and questions on work impact have been shown to be highly reliable in test-retest studies59 and highly valid when compared with measures based on a 3-month diary study.31 However, the MIDAS asks about lost productivity at both work and school, and since all participants were required to be employed full-time, we assumed that all lost productivity was related to work; however, they could be responding related to lost productivity at school as well. In addition, the small effect of the Migraine Symptom Severity Scale observed may be a consequence of the large number of points in the scale relative to categorical variables. The CaMEO study population is a volunteer cohort, intended to be representative of the US population with regard to selected demographics; however, bias from selective participation is possible (completion required a computer and English literacy). Last, we did not investigate effect of treatment optimization and MHDs on employment status, which may have resulted in an underestimation of work impact of migraine, especially in people with chronic migraine (≥15 MHDs) and high-frequency episodic migraine.30
In conclusion, in people with migraine, more optimal acute treatment is associated with less productivity loss. Optimizing acute treatment for people with migraine may lead to reduced LPT and may result in indirect cost savings.
Supplementary Material
ACKNOWLEDGMENTS
Medical writing support was provided by Cory Hussar, PhD, of Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and was funded by AbbVie.
Footnotes
Ethical considerations and disclosures: The CaMEO Study was approved by the Albert Einstein College of Medicine Institutional Review Board (12-04-177E); the institutional review board waived written informed consent for study volunteers, who had the right to accept or refuse participation in the survey.
Funding sources: Allergan (before its acquisition by AbbVie) funded this study and contributed in the interpretation of data and in writing, reviewing, and approval of the final version. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
Conflict of interest: D.C.B. has received research support from the US Food and Drug Administration and the National Headache Foundation. She serves as consultant or advisory board member, or has received honoraria or research support from AbbVie/Allergan, Amgen, Biohaven, Colleguium, Eli Lilly and Company, Lundbeck, and Teva and for work on the editorial board of Current Pain and Headache Reports in the past year. S.J.N. has received honoraria for consulting from Alder/Lundbeck, Allergan/AbbVie, Amgen/Novartis (ended April 2021), Axsome (ended January 2022), BioDelivery Sciences (ended February 2022), Biohaven (ended November 2021), Eli Lilly, Fenix Group International (ended July 2021), Teva (ended October 2021), and Theranica (ended September 2022). She has received honoraria for speaking from Allergan/AbbVie (ended July 2021), Amgen/Novartis (ended June 2021), Eli Lilly (ended September 2021), and Teva (ended June 2021); research support from Teva (Ended March 2021); and honoraria for work in education or publishing from American Academy of Neurology, American Headache Society, Evolve Med Ed, Massachusetts Medical Society, MedLink Neurology, MJH Life Sciences, NACCME, Neurology Learning Network, Pennsylvania Neurologic Society, Springer, WebMD/Medscape, and Wolters-Kluwer. Furthermore, she has received legal fees for serving as a medical expert to Jackson & Campbell.
W.F.S. has served as a consultant to Amgen, Grifols, and Promius/Dr. Reddy's. C.E.A. has served as an advisory board member and has received honoraria from Biohaven and Impel Regional and from AbbVie and Teva. She is web editor of JAMA Neurology. M.L.R. is managing director of Vedanta Research, which has received research funding from AbbVie, Allay Lamp, Dr. Reddy's Laboratories, Eli Lilly, GlaxoSmithKline, and Promius via grants to the National Headache Foundation. Vedanta Research has received funding directly from AbbVie for work on the CaMEO and CaMEO-I Studies. K.M.F. is managing director of MIST Research, which, in collaboration with Vedanta Research, has received research funding from AbbVie, Allay Lamp, Dr. Reddy's Laboratories, Eli Lilly, GlaxoSmithKline, and NYU Langone Health via grants to the National Headache Foundation. MIST Research has received funding directly from AbbVie for work on the CaMEO and CaMEO-I Studies. A.M.A. is an employee of AbbVie and may hold AbbVie stock. R.B.L. is the Edwin S. Lowe Professor of Neurology at the Albert Einstein College of Medicine in New York. He receives research support from the National Institutes of Health: 2PO1 AG003949 (mPI), 1RF1 AG057531 (site PI), RF1 AG054548 (investigator), 1RO1 AG048642 (investigator), R56 AG057548 (investigator), RO1 AG060933 (investigator), RO1 AG062622 (investigator), 1UG3FD006795 (mPI), 1U24NS113847 (investigator), U01 AT011005 (investigator), 1R01 AG075758 (pending — investigator), 1R01 AG077639 (pending — investigator), and K23 NS107643 (mentor). He also receives support from the Migraine Research Foundation and the National Headache Foundation and research grants from TEVA, Satsuma, and Amgen. He serves on the editorial board of Neurology and as senior advisor to Headache and associate editor to Cephalalgia. He has reviewed for the NIA and NINDS and holds stock and stock options in Biohaven Holdings and stock options in Manistee; serves as consultant or advisory board member, or has received honoraria from AbbVie (Allergan), American Academy of Neurology, American Headache Society, Amgen, Avanir, Axsome, Biohaven, Biovision, Boston Scientific, Dr. Reddy's (Promius), Electrocore, Eli Lilly, eNeura Therapeutics, Equinox, GlaxoSmithKline, Grifols, Lundbeck (Alder), Merck, Pernix, Pfizer, Satsuma, Supernus, Teva, Trigemina, Vector, and Vedanta. He receives royalties from Wolff's Headache 7th and 8th Edition, Oxford Press University, 2009, Wiley and Informa.
Supplemental digital contents are available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.joem.org).
Contributor Information
Dawn C. Buse, Email: dawnbuse@gmail.com.
Stephanie J. Nahas, Email: Stephanie.Nahas@jefferson.edu.
Walter (Buzz) F. Stewart, Email: wfs502000@yahoo.com.
Cynthia E. Armand, Email: CARMAND@montefiore.org.
Michael L. Reed, Email: mlreed@vedantaresearch.com.
Kristina M. Fanning, Email: Kfanning@mistresearch.com.
Richard B. Lipton, Email: richard.lipton@einsteinmed.edu.
REFERENCES
- 1.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- 2.Lipton RB Bigal ME Diamond M Freitag F Reed ML Stewart WF, AMPP Advisory Group . Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. [DOI] [PubMed] [Google Scholar]
- 3.Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1859–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanteri-Minet M, Duru G, Mudge M, Cottrell S. Quality of life impairment, disability and economic burden associated with chronic daily headache, focusing on chronic migraine with or without medication overuse: a systematic review. Cephalalgia. 2011;31:837–850. [DOI] [PubMed] [Google Scholar]
- 5.Blumenfeld AM Varon SF Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS). Cephalalgia. 2011;31:301–315. [DOI] [PubMed] [Google Scholar]
- 6.Leonardi M, Raggi A, Bussone G, D'Amico D. Health-related quality of life, disability and severity of disease in patients with migraine attending to a specialty headache center. Headache. 2010;50:1576–1586. [DOI] [PubMed] [Google Scholar]
- 7.Freitag FG. The cycle of migraine: patients' quality of life during and between migraine attacks. Clin Ther. 2007;29:939–949. [DOI] [PubMed] [Google Scholar]
- 8.Stewart WF, Wood GC, Razzaghi H, Reed ML, Lipton RB. Work impact of migraine headaches. J Occup Environ Med. 2008;50:736–745. [DOI] [PubMed] [Google Scholar]
- 9.Serrano D, Manack AN, Reed ML, Buse DC, Varon SF, Lipton RB. Cost and predictors of lost productive time in chronic migraine and episodic migraine: results from the American Migraine Prevalence and Prevention (AMPP) Study. Value Health. 2013;16:31–38. [DOI] [PubMed] [Google Scholar]
- 10.D'Amico D Grazzi L Curone M, et al. Difficulties in work activities and the pervasive effect over disability in patients with episodic and chronic migraine. Neurol Sci. 2015;36(Suppl 1):9–11. [DOI] [PubMed] [Google Scholar]
- 11.Raggi A Giovannetti AM Quintas R, et al. A systematic review of the psychosocial difficulties relevant to patients with migraine. J Headache Pain. 2012;13:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart WF, Bruce C, Manack A, Buse DC, Varon SF, Lipton RB. A case study for calculating employer costs for lost productive time in episodic migraine and chronic migraine: results of the American Migraine Prevalence and Prevention Study. J Occup Environ Med. 2011;53:1161–1171. [DOI] [PubMed] [Google Scholar]
- 13.Buse DC, Yugrakh MS, Lee LK, Bell J, Cohen JM, Lipton RB. Burden of illness among people with migraine and ≥4 monthly headache days while using acute and/or preventive prescription medications for migraine. J Manag Care Spec Pharm. 2020;26:1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart WF, Wood GC, Bruce C, Buse DC, Runken MC, Lipton RB. Longitudinal change in migraine headache-days and indirect cost consequences. J Occup Environ Med. 2011;53:478–487. [DOI] [PubMed] [Google Scholar]
- 15.Silberstein SD. Migraine. Lancet. 2004;363:381–391. [DOI] [PubMed] [Google Scholar]
- 16.Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754–762. [DOI] [PubMed] [Google Scholar]
- 17.Silberstein SD. Preventive migraine treatment. Continuum (Minneap Minn). 2015;21:973–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker WJ. Acute migraine treatment in adults. Headache. 2015;55:778–793. [DOI] [PubMed] [Google Scholar]
- 19.Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3–20. [DOI] [PubMed] [Google Scholar]
- 20.Drugs for migraine. Med Lett Drugs Ther. 2020;62:153–160. [PubMed] [Google Scholar]
- 21.Ailani J Burch RC Robbins MS, Board of Directors of the American Headache Society . The American Headache Society Consensus Statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61:1021–1039. [DOI] [PubMed] [Google Scholar]
- 22.Lipton RB Hutchinson S Ailani J, et al. Discontinuation of acute prescription medication for migraine: results from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache. 2019;59:1762–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48:1157–1168. [DOI] [PubMed] [Google Scholar]
- 24.Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37:631–649. [DOI] [PubMed] [Google Scholar]
- 25.Manack Adams A Serrano D Buse DC, et al. The impact of chronic migraine: the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study methods and baseline results. Cephalalgia. 2015;35:563–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–657. [DOI] [PubMed] [Google Scholar]
- 27.Headache Classification Subcommittee of the International Headache Society . The International Classification of Headache Disorders, 2nd ed. Cephalalgia. 2004;24(Suppl 1):9–160. [DOI] [PubMed] [Google Scholar]
- 28.Headache Classification Committee of the International Headache Society . The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629–808. [DOI] [PubMed] [Google Scholar]
- 29.Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology. 2001;56:S20–S28. [DOI] [PubMed] [Google Scholar]
- 30.Stewart WF, Wood GC, Manack A, Varon SF, Buse DC, Lipton RB. Employment and work impact of chronic migraine and episodic migraine. J Occup Environ Med. 2010;52:8–14. [DOI] [PubMed] [Google Scholar]
- 31.Stewart WF, Lipton RB, Kolodner KB, Sawyer J, Lee C, Liberman JN. Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain. 2000;88:41–52. [DOI] [PubMed] [Google Scholar]
- 32.Lipton RB Kolodner K Bigal ME, et al. Validity and reliability of the Migraine-Treatment Optimization Questionnaire. Cephalalgia. 2009;29:751–759. [DOI] [PubMed] [Google Scholar]
- 33.Buse DC, Reed ML, Fanning KM, Bostic RC, Lipton RB. Demographics, headache features, and comorbidity profiles in relation to headache frequency in people with migraine: results of the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2020;60:2340–2356. [DOI] [PubMed] [Google Scholar]
- 34.Buse DC Scher AI Dodick DW, et al. Impact of migraine on the family: perspectives of people with migraine and their spouse/domestic partner in the CaMEO study. Mayo Clin Proc. 2016;91:596–611. [DOI] [PubMed] [Google Scholar]
- 35.Buse DC Fanning KM Reed ML, et al. Life with migraine: effects on relationships, career, and finances from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache. 2019;59:1286–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipton RB Buse DC Friedman BW, et al. Characterizing opioid use in a US population with migraine: results from the CaMEO study. Neurology. 2020;95:e457–e468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipton RB Bigal ME Ashina S, et al. Cutaneous allodynia in the migraine population. Ann Neurol. 2008;63:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bigal ME Ashina S Burstein R, et al. Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology. 2008;70:1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. [DOI] [PubMed] [Google Scholar]
- 41.Hilbe JM. Negative Binomial Regression. New York, NY: Cambridge University Press; 2011. [Google Scholar]
- 42.Joshi S Ford JH Ye W, et al. Economic impact of migraine in employed adult patients with migraine from the United States: results from the Medical Expenditure Panel Survey (MEPS) [poster]. In: Migraine Trust Virtual Symposium; October 3–9, 2020.
- 43.Bonafede M, Sapra S, Shah N, Tepper S, Cappell K, Desai P. Direct and indirect healthcare resource utilization and costs among migraine patients in the United States. Headache. 2018;58:700–714. [DOI] [PubMed] [Google Scholar]
- 44.Hutchinson S Lipton RB Ailani J, et al. Characterization of acute prescription migraine medication use: results from the CaMEO study. Mayo Clin Proc. 2020;95:709–718. [DOI] [PubMed] [Google Scholar]
- 45.Buse DC, Pearlman SH, Reed ML, Serrano D, Ng-Mak DS, Lipton RB. Opioid use and dependence among persons with migraine: results of the AMPP study. Headache. 2012;52:18–36. [DOI] [PubMed] [Google Scholar]
- 46.Silberstein SD, Cady RK, Sheftell FD, Almas M, Parsons B, Albert KS. Efficacy of eletriptan in migraine-related functional impairment: functional and work productivity outcomes. Headache. 2007;47:673–682. [DOI] [PubMed] [Google Scholar]
- 47.Lisotto C, Guidotti M, Zava D, Savi L. Frovatriptan and rizatriptan economic EVAluation: the FREEVA study. J Headache Pain. 2013;14:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schulman EA Cady RK Henry D, et al. Effectiveness of sumatriptan in reducing productivity loss due to migraine: results of a randomized, double-blind, placebo-controlled clinical trial. Mayo Clin Proc. 2000;75:782–789. [DOI] [PubMed] [Google Scholar]
- 49.Lombard L Farrar M Ye W, et al. A global real-world assessment of the impact on health-related quality of life and work productivity of migraine in patients with insufficient versus good response to triptan medication. J Headache Pain. 2020;21:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blumenfeld AM, Patel AT, Turner IM, Mullin KB, Manack Adams A, Rothrock JF. Patient-reported outcomes from a 1-year, real-world, head-to-head comparison of onabotulinumtoxina and topiramate for headache prevention in adults with chronic migraine. J Prim Care Community Health. 2020;11:2150132720959936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buse DC, Sollars CM, Steiner TJ, Jensen RH, Al Jumah MA, Lipton RB. Why HURT? A review of clinical instruments for headache management. Curr Pain Headache Rep. 2012;16:237–254. [DOI] [PubMed] [Google Scholar]
- 52.Haywood KL, Mars TS, Potter R, Patel S, Matharu M, Underwood M. Assessing the impact of headaches and the outcomes of treatment: a systematic review of patient-reported outcome measures (PROMs). Cephalalgia. 2018;38:1374–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zavala SK, French MT, Zarkin GA, Omachonu VK. Decision latitude and workload demand: implications for full and partial absenteeism. J Public Health Policy. 2002;23:344–361. [PubMed] [Google Scholar]
- 54.Dodick DW Reed ML Fanning KM, et al. Predictors of allodynia in persons with migraine: results from the Migraine in America Symptoms and Treatment (MAST) study. Cephalalgia. 2019;39:873–882. [DOI] [PubMed] [Google Scholar]
- 55.de Jonge J, van Vegchel N, Shimazu A, Schaufeli W, Dormann C. A longitudinal test of the demand-control model using specific job demands and specific job control. Int J Behav Med. 2010;17:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karasek RA, Theorell T. Healthy Work: Stress, Productivity and the Reconstruction of Working Life. New York, NY: Basic Books; 1990. [Google Scholar]
- 57.Begasse de Dhaem O, Gharedaghi MH, Bain P, Hettie G, Loder E, Burch R. Identification of work accommodations and interventions associated with work productivity in adults with migraine: a scoping review. Cephalalgia. 2021;41:760–773. [DOI] [PubMed] [Google Scholar]
- 58.Schaetz L Rimner T Pathak P, et al. Employee and employer benefits from a migraine management program: disease outcomes and cost analysis. Headache. 2020;60:1947–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stewart WF Lipton RB Whyte J, et al. An international study to assess reliability of the Migraine Disability Assessment (MIDAS) score. Neurology. 1999;53:988–994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


