Abstract
Background
Bronchoscopy is generally a safe and efficient procedure. However, the risk of cross-contamination with reusable flexible bronchoscopes (RFB) has been detected in several outbreaks worldwide.
Aim
To estimate the average cross-contamination rate of patient-ready RFBs based on available published data.
Methods
We performed a systematic literature review in PubMed and Embase to investigate the cross-contamination rate of RFB. Included studies identified indicator organisms or colony forming units (CFU) levels, and total number of samples >10. The Contamination threshold was defined according to the European Society of Gastrointestinal Endoscopy and European Society of Gastrointestinal Endoscopy Nurse and Associates (ESGE-ESGENA) guidelines. To calculate the total contamination rate, a random effects model was applied. Heterogeneity was analysed via a Q-test and illustrated in a forest plot. Publication bias was analysed via the Egger’s regression test and illustrated in a funnel plot.
Results
Eight studies fulfilled our inclusion criteria. The random effects model included 2169 samples and 149 events (positive tests). The total RFB cross-contamination rate was 8.69% ± 1.86 (standard division [SD]) (95% confidence interval [CI]: 5.06–12.33%). The result showed significant heterogeneity of 90% and publication bias.
Discussion
Significant heterogeneity and publication bias is likely associated with varying methodology and aversion towards publishing negative findings, respectively. Based on the cross-contamination rate an infection control paradigm shift is needed to ensure patient safety. We recommend to follow the Spaulding classification and classify RFBs as critical items. Accordingly, infection control measures such as obligatory surveillance, and implementing single-use alternatives must be considered where feasible.
Keywords: Equipment contamination, bronchoscopes, cross-infection, bronchoscopy, disinfection, sterilisation
Background
Bronchoscopy is frequently performed in more than 1,100,000 procedures annually in Europe (Eurostat - Your key to European statistics, 2020). Contaminated reusable flexible bronchoscopes (RFB) are frequently associated with outbreaks of healthcare-associated infections (Kovaleva et al., 2013). Bronchoscopy most often results in endogenous infections caused by the microbial flora from the upper respiratory tract, including viridans group streptococci, staphylococci, Moraxella spp., Neisseria spp., and anaerobic bacteria (Spach et al., 1993). Endogenous infections are associated with endoscopy but do not appear to be prevented by well-controlled disinfection procedures (Srinivasan, 2003). The exogenous microorganisms most frequently associated with transmission of infection during bronchoscopy are Pseudomonas aeruginosa, Mycobacterium tuberculosis and nontuberculous mycobacteria (Kovaleva et al., 2013; Srinivasan, 2003). These microorganisms can be transmitted from previous patients or contaminated reprocessing equipment such as contaminated RFBs or its accessory equipment. Exogenous infection should be prevented by strict endoscope disinfection procedures (Botana-Rial et al., 2016; Rutala and Weber, 2013). Accurate reprocessing of flexible endoscopes is a multistep procedure involving manual cleaning followed by high-level disinfection (HLD) via automated endoscope reprocessors (AERs) or ethylene oxide (EtO) or hydrogen peroxide plasma sterilisation with further rinsing and drying before storage (Beilenhoff et al., 2007; Botana-Rial et al., 2016; Kovaleva et al., 2013). Most RFBs belong to semi critical devices which come into contact with mucous membranes during use and have a moderate degree of infection risk if contaminated at the time of use (Spaulding and Gröschel, 1974). They should receive at least HLD resulting in elimination of all vegetative bacteria, mycobacteria, fungi, and viruses, except for small numbers of bacterial spores (Garner and Favero, 1986; Simmons BP, 1983). RFB used for therapeutic purposes in sterile body cavities belong to critical devices and should be sterilised after each procedure to eliminate all forms of microbial life, including bacterial spores (Garner and Favero, 1986; Spaulding and Gröschel, 1974). Given their material composition, most RFBs cannot be steam sterilised (Srinivasan, 2003).
Flexible bronchoscopes are complex instruments with multiple and narrow internal channels which may become heavily contaminated with microorganisms during use (Beilenhoff et al., 2007; Botana-Rial et al., 2016). Because of their complex structure, endoscopes are difficult to clean and disinfect (American National Standard, 2015; Beilenhoff et al., 2007). Infectious outbreaks after flexible bronchoscopy have been linked to inadequate reprocessing (cleaning, disinfection, and drying); contaminated AERs; or malfunctioning RFBs (Cosgrove et al., 2012; Peaper et al., 2015). According to a recent study, visible irregularities and residual contamination after manual cleaning have been observed in up to 100% of RFBs (Ofstead et al., 2018). Microbial growth was found in 58% (14/24) fully reprocessed bronchoscopes (American National Standard, 2015; Beilenhoff et al., 2007; Botana-Rial et al., 2016). The narrow lumens of the RFB are cleaned without visualisation of the lumens, thereby making it difficult to disinfect small scratches and enabling bacterial adhesions and potential biofilm formation (Kovaleva et al., 2009; Pajkos et al., 2004). A biofilm is an assemblage of microbial cells, potentially originating from patient materials, attached to a surface and enclosed in a matrix of exopolymeric substances (Donlan and Costerton, 2002). Biofilms are extremely difficult to remove, show increased resistance to disinfectants and antibiotics, and can result in failure in reprocessing of endoscopes and outbreaks of endoscopy-related infections (Einav and Wiener-Well, 2017; Kenters et al., 2015; Kovaleva et al., 2009; Pajkos et al., 2004). Microbiological surveillance of flexible endoscopes is appropriate to trace contaminations and to prevent contaminations and infections in patients after endoscopic procedures (Kovaleva et al., 2009). Owing to the continuous reprocessing irregularities and endoscope-associated outbreaks, microbiological surveillance is recommended along with a paradigm shift from endoscope HLD to sterilisation (Center for Disease Control and Prevention, 2016; Kenters et al., 2015; Kovaleva et al., 2009; Kovaleva et al., 2013; Mehta and Muscarella, 2020; Ofstead et al., 2018; Rutala and Weber, 2013).
The aim of this study was to estimate the cross-contamination rate of patient-ready RFB based on published literature.
Methods
Study selection
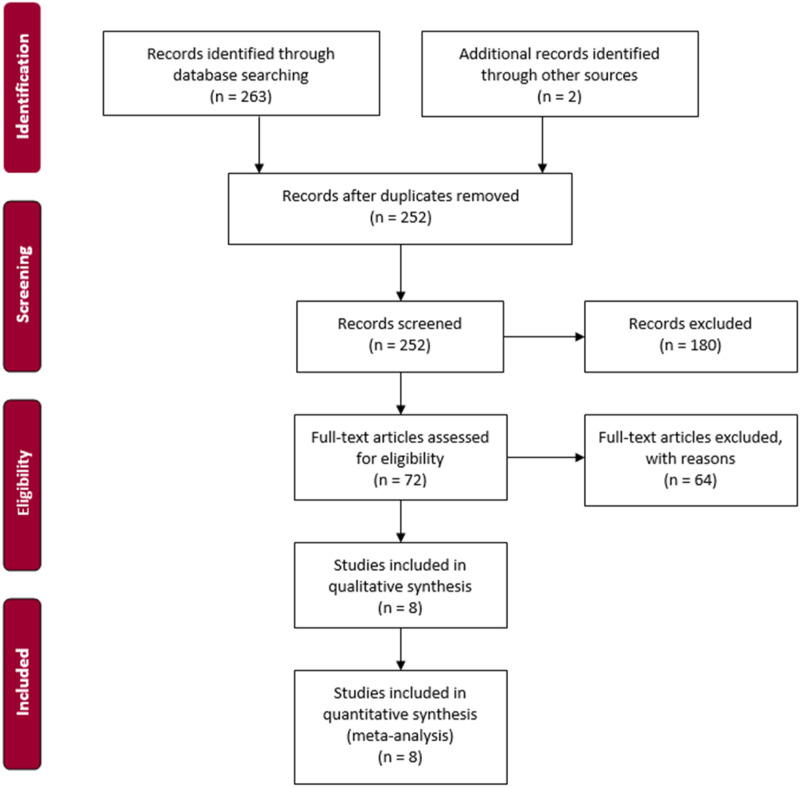
This study is based on a systematic literature search to detect full-text studies on RFBs. All studies should have investigated the potential cross-contamination on RFBs. The methods used to sort the studies was conducted per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses’ (PRISMA) guidelines [Figure 1] (PRISMA, 2015).
Figure 1.
PRISMA.
All included studies must have been published from January 1, 2010, until June 12, 2020, to account for the continuous optimisation of reprocessing guidelines (Larsen et al., 2020). To guarantee a systematic approach, the search terms were inserted in a block chart addressing the problem, intervention, comparator, and outcome. The block searches were reviewed by two authors (HST and RVR). To ensure searches within a broad spectrum, all studies were identified using the following keywords in Embase: bronchoscopy’/exp OR ‘bronchoscope’/exp AND cross contamination’/exp OR ‘contamination’/exp OR ‘outbreak’/exp OR ‘device contamination’. In addition, the following search string was used in PubMed: (((“bronchoscop*”[All Fields] OR (((“bronchial”[All Fields] OR “bronchiale”[All Fields]) OR “bronchials”[All Fields]) AND “endoscop*”[All Fields])) OR “tracheobronchoscop*”[All Fields]) AND (((((((((“cross”[All Fields] OR “crosse”[All Fields]) OR “crossed”[All Fields]) OR “crosses”[All Fields]) OR “crossing”[All Fields]) OR “crossings”[All Fields]) AND “contamina*”[All Fields]) OR “contamina*”[All Fields]) OR “outbreak*”[All Fields])). Truncation was applied for some keywords to allow variations of the words. In addition, Google Scholar was screened along with chain searches for relevant studies. Duplicates were removed with the help of Mendeley Desktop v1.19.2 (Copyright © 2020 Mendeley Ltd).
The study inclusion criteria were total number of samples from bronchoscopes (N), total number of positive samples (contaminated RFB) (n), and samples collected from bronchoscopes and not from patients. The exclusion criteria were in vitro models or studies performed in animals, conference abstracts, editorials, letters, non-original research, and sample size ≤ 10 (to account for bias) (Higgins et al., 2003). Further, studies were excluded if they did not identify microorganisms or if the colony forming unit (CFU) was not declared.
Title and abstract review were conducted independently by two authors (HST and RVR). In case of doubt, particularly with respect to abstract relevance, the full-text was assessed. All publications assessed for eligibility were independently evaluated by two authors (HST and RVR). If any disagreements occurred, they were discussed in plenum by the study authors.
Data extraction, analysis, and statistical methods
Positive samples were defined in accordance with the European Society of Gastrointestinal Endoscopy and European Society of Gastrointestinal Endoscopy Nurse and Associates (ESGE-ESGENA) guidelines for quality assurance in reprocessing: Microbiology surveillance testing in endoscopy is stated below (Beilenhoff et al., 2007).
• Minimum total count<20 CFU/channel (liquid samples from endoscope channels)
• Growth of indicator organisms in swab cultures (Enterobacteriaceae, enterococci, P. aeruginosa, and other gram-negative nonfermenters, Staphylococcus aureus, Staphylococcus epidermidis, atypical mycobacteria, and Legionella spp.)
The primary data extracted from the studies that were assessed for eligibility were authors; country; setting; total number of samples (N); total number of positive samples (n); and whether they fulfilled CFU thresholds or stated indicator organisms, microorganisms, reprocessing method, and sampling method. The data extraction was performed manually and extrapolated into a commercially available spreadsheet (Microsoft® Excel® for Office 365 MSO) Table 1. The statistical analyses were conducted via the metafor package in R V.3.6.2. To overcome the challenges of anticipated heterogeneity, a random effects model was applied (Higgins et al., 2003; Von Hippel, 2015).
Table 1.
Included studies.
| Author | Country | Setting | Bronchoscope samples (N) | Positive events (n) | Outbreak (yes/no) | ESGE indicator organism (A)/CFU threshold (B) | Microorganisms | Reprocessing method | Sample method |
|---|---|---|---|---|---|---|---|---|---|
| (Cosgrove et al., 2012) | USA | Endoscopy suite, operating room, and the intensive care unit | 18 | 2 | Yes | A | Pseudomonas aeruginosa, Pseudomonas putida Stenotrophomonas maltophilia | HLD | Flush |
| (Marino et al., 2012) | Italy | Respiratory care unit | 528 | 24 | No | A | Serratia marcescens, Acinetobacter lowffii, Staphylococcus epidermidis, Staphylococcus spp., haemolytic and nonhemolytic streptococci, Lactobacillus spp., M. luteus, Aerococcus viridans | HLD and EtO | Flush |
| (Vincenti et al., 2014) | Netherlands | Bronchoscopy unit | 658 | 40 | No | A | S. marcescens, A. lowffii, S. epidermidis, Staphylococcus spp., haemolytic and nonhemolytic streptococci, Lactobacillus spp., M. luteus, molds, spore-forming bacilli, A. viridans | N/A | Flush |
| (Gavaldà et al., 2015) | Spain | Bronchoscopy suit and operating room | 620 | 59 | No | A | P. aeruginosa, S. marcescens, S. aureus, Enterobacter agglomerans, Streptococcus viridans, Enterococcus faecium, Candida albicans, Aspergillus fumigatus, Aspergillus niger. | HLD or manual | Flush |
| (Batailler et al., 2015) | France | N/A | 62 | 2 | No | A/B | Enterobacteriaecae, P. aeruginosa, S. maltophilia, Acinetobacter spp., S. aureus a | HLD | Flush |
| (Ofstead et al., 2018) | USA | N/A | 24 | 13 | No | A/B | P. aeruginosa S. maltophilia, E. coli, Shigella spp., Paenibacillus provencensis, Sphingomonas phyllosphaerae, gram-positive cocci; Lecanicilliumlecanii/Verticillium dahliae | HLD | Flush-brush-flush and swab |
| (Troiano et al., 2019) | Italy | N/A | 218 | 45 | No | A | P. aeruginosa, Pseudomonas spp., Enterobacteriaceae, Staphylococcus spp., Clostridium spp,, Enterococcus spp. | HLD | Flush and swab |
| (Cottarelli et al., 2020) | Italy | Endoscopy operating unit | 41 | 4 | No | A | P. aeruginosa, S. aureus, other gram-negative nonfermenters | HLD or manual | Flush |
aUnknown if originating from bronchoscopes or other endoscopes tested in the same study ESGE: European Society of Gastrointestinal Endoscopy, CFU: Colony forming unit, HLD: High-level disinfection, EtO: Ethylene oxide, N/A: Not applicable.
The results of the random effect models were presented as average cross-contamination rate ± standard deviation (SD) and 95% confidence interval (CI). Heterogeneity was presented as an inconsistency index (I2), analysed via a Q-test, and illustrated in a forest plot. Publication bias was analysed via the Egger’s regression test and illustrated in a funnel plot.
Results
Characteristics of included studies
We assessed 265 study abstracts: 130 originated from Embase, 133 from PubMed, and two were identified through chain searches. After duplicates were removed, 252 studies were screened based on title and abstracts. After eliminating 180 studies based on the inclusion and exclusion criteria, 72 studies were assessed for eligibility upon screening of the title and abstracts. After full-text review, an additional 64 studies were excluded. Finally, eight studies were included upon full-text review and included in the analysis. The included studies generated a sample size of 2,169, of which 149 were contaminated. The included studies were differentiated according to countries, including USA (n = 2, 25.0%), Italy (n = 3, 37.5%), The Netherlands (n = 1, 12.5%), France (n = 1, 12.5%), and Spain (n = 1, 12.5%).
Analysis
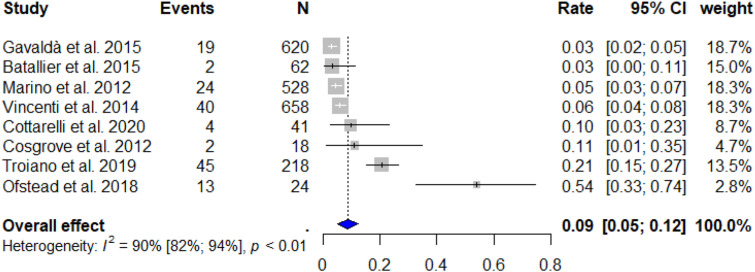
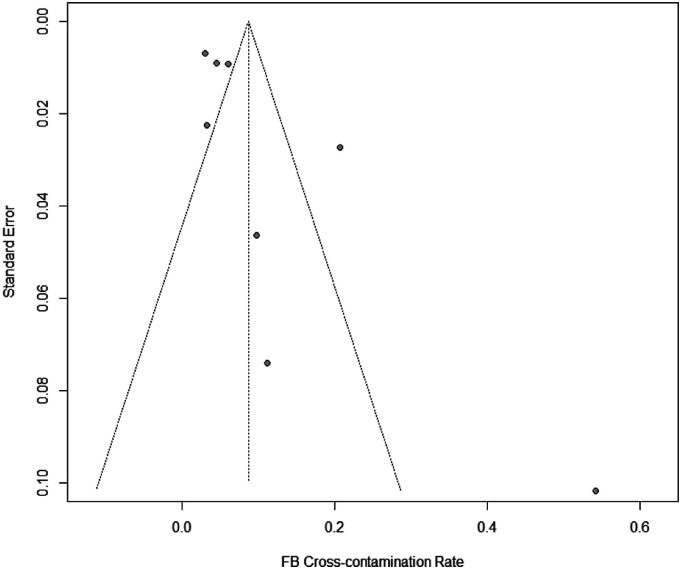
The random effects model resulted in a total RFB cross-contamination rate of 8.69% ± SD 1.86 (95% CI: 5.06–12.33%). Subsequently, this represents that 95,590 (95% CI: 55,660-135,630) bronchoscopic procedures are conducted with contaminated RFBs annually in Europe alone. The forest plot [Figure 2] indicated a significant heterogeneity between the studies (I2 = 90%, Q-test: p < 0.01). Furthermore, the Egger’s regression test showed significant publication bias between the included studies with a significant funnel-plot asymmetry (z = 2.1403, p = 0.0323) [Figure 3].
Figure 2.
Forest plot of included studies. CI: Confidence interval, I2: Inconsistency index.
Figure 3.
Funnel plot. RFB: Reusable flexible bronchoscope.
Discussion
Recently, numerous outbreaks have been linked to cross-contaminated, reusable, patient-ready RFBs, entailing significant cost and potentially health-related quality of life decrement (Mangen et al., 2017; Mouritsen et al., 2020). Therefore, it is important to elucidate the actual cross-contamination rate of RFBs. This study estimated the RFB cross-contamination rate based on the best available evidence via a systematic literature review. In all, eight studies were identified utilising PRISMA, and the estimated total cross-contamination rate was 8.69% via a random effect model. To our best knowledge, this is the first meta-analysis to estimate the total cross-contamination rate of RFBs, including a standardised contamination threshold based on a well-established endoscopy surveillance-testing guideline (Beilenhoff et al., 2007).
This result clearly shows that despite continuous improvement in reprocessing and reprocessing guidelines, the RFB cross-contamination rate is still high. By proving this, it is evident that an infection control paradigm shift is needed to ensure patient safety.
The total cross-contamination rate of 8.69% differs from that reported in other studies. To the authors’ knowledge, two other studies have investigated the total RFB cross-contamination rate. Terjesen et al. (2017) conducted a cost-effectiveness analysis on reusable RFB versus single-use flexible bronchoscopes. The model input included the total RFB cross-contamination rate, the subsequent risk of cross-infection, the flexible bronchoscopy procedure cost, and cost of ventilator-associated pneumonia. In this study, the cost of cross-infection per bronchoscopy was £151 ($199). They estimated the overall cross-contamination rate to be 3.38% using the Delphi method, and subsequently found that single-use FB provided a cost-effective alternative (Terjesen et al., 2017). Our estimate of RFB cross-contamination rate was remarkably higher, and Terjesen et al. (2017) reported an even lower 95% CI than ours (Terjesen et al., 2017). The lower cross-contamination rate was likely caused by an overall perception of low RFB cross-contamination rate amongst the participants in the Delphi survey, rather than an actual decreased rate.
Mouritsen et al. (2019) also conducted a cost-effectiveness analysis on single-use versus RFB. They used a similar model as that in used by Terjesen et al. (2017). However, the direct procedure cost was based on a micro-costing approach, the cost of treating cross-infections was extrapolated from published literature, and the cross-contamination rate and subsequent risk of cross-infection were based on published evidence. The cost of treating cross-infections was £262 ($346) per procedure (Mouritsen et al., 2020). They calculated the cross-contamination rate to be 15.26%, which is notably higher than that reported by us and above our 95% CI value. This difference in the total cross-contamination rate can, in turn, be explained by the mismatch in methodology between our studies. Mouritsen et al. (2019) included studies dating back to 1982, wherein the cross-contamination threshold was not underpinned by a guideline, missing datapoints in the total sample size was inserted via linear regression, and the estimated cross-contamination rate of 15.26% was calculated via a fixed effects rather than a random effects model (Mouritsen et al., 2020).
The majority of included studies in our meta-analysis sampled RFBs by flushing the RFB channels, whereas only Ofstead et al. (2018) sampled the RFBs by the flush-brush-flush method and swabbing the external surfaces (Ofstead et al., 2018). Troiano et al. (2019) flushed the RFB channels and swabbed the external surfaces (Troiano et al., 2019). It has previously been indicated that the flush-brush-flush method is superior to conventional sampling methods, where saline or reverse osmosis water is flushed through the channels of the endoscope. Moreover, the sample recovery improves by adding friction. Hence, the diverse sampling methods entail a potential cofounding factor that should be considered when interpreting the results (Alfa, Ribeiro, et al., 2017a; Alfa, Singh, et al., 2017b).
Our study has some limitations. The heterogeneity in our meta-analysis was high (I2 = 90%) and the subsequent Q-test was significant (p < 0.01), indicating that the variance in the results were rather caused by study heterogeneity than sample variance. The non-randomised design of the included studies, low number of datapoints, varying sampling methods, geography, and clinical settings all potentiate the heterogeneity of results (Glasziou and Sanders, 2002). Furthermore, there is significant publication bias in our analysis (z = 2.1403, p = 0.0323). This bias could potentially be diminished if routine surveillance testing of RFBs were conducted and published (Kovaleva et al., 2009). Accordingly, the aversion towards publishing negative findings likely represents a large proportion of the publication bias (Matosin et al., 2014).
The evidently high RFB cross-contamination rate, high heterogeneity, and bias indicated in our meta-analysis highlights the need for a large multicentre prospective study to assess the RFB cross-contamination rate utilising the flush-brush-flush sampling method. In parallel, it would be valuable to initiate a regional or ideally global endoscope cross-contamination register, to enable statistical analysis of register data. Further, given our findings and considering the persistent risk of cross-infection because RFBs are frequently in contact with non-intact mucous membranes and sterile tissue, we recommend to follow the Spaulding classification and classify RFBs as critical items (Kovaleva et al., 2013; Rutala et al., 2019; Spaulding and Gröschel, 1974).
In conclusion, we showed that RFB cross-contamination is still a relevant and persistent healthcare issue. The current reprocessing methods and surveillance strategies are flawed, and new approaches must be considered. To eliminate the risk of cross-contamination, innovative single-use technologies should replace RFB where feasible. For the remaining bronchoscopy procedures, systematic surveillance using flush-brush-flush and microbiological culturing methods must be made mandatory along with a sterilisation step and strict adherence to periodic servicing (Alfa, Singh, et al., 2017b; Mehta and Muscarella, 2020). This study is uploaded to the Research Registry and is accessible via reviewregistry953.
Appendix.
Abbreviations
- AER
automated endoscope reprocessor
- CFU
colony forming unit
- CI
confidence interval
- ESGE
European Society of Gastrointestinal Endoscopy
- ESGENA
European Society of Gastrointestinal Endoscopy Nurse and Associates
- EtO
ethylene oxide
- HLD
high-level disinfection
- I2
inconsistency index
- RFB
reusable flexible bronchoscope
- SD
standard deviation.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: HST and RVR are employed by Ambu A/S, Ballerup Denmark. No external funding or competing interests are declared for JK.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Helena S Travis https://orcid.org/0000-0002-9231-1863
References
- Alfa MJ, Ribeiro MM, da Costa Luciano C, et al. (2017. a) A novel polytetrafluoroethylene-channel model, which simulates low levels of culturable bacteria in buildup biofilm after repeated endoscope reprocessing. American Society for Gastrointestinal Endoscopy 86(3): 442–451. DOI: 10.1016/j.gie.2017.05.014. [DOI] [PubMed] [Google Scholar]
- Alfa MJ, Singh H, Nugent Z, et al. (2017. b) Sterile reverse osmosis water combined with friction are optimal for channel and lever cavity sample collection of flexible duodenoscopes. Frontiers in Medicine 4(NOV): 1–9. Available at:https://www.frontiersin.org/articles/10.3389/fmed.2017.00191/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- American National Standard (2015) Flexible and Semi-rigid Endoscope Processing in Health Care Facilities. Available at:https://webstore.ansi.org/standards/aami/ansiaamist912015 [Google Scholar]
- Batailler P, Saviuc P, Picot-Gueraud R, Bosson JL, Mallaret MR. (2015) Usefulness of adenosinetriphosphate bioluminescence assay (ATPmetry) for monitoring the reprocessing of endoscopes. Infect. Control Hosp. Epidemiol 36(12): 1437–1443. doi: 10.1017/ice.2015.212. [DOI] [PubMed] [Google Scholar]
- Beilenhoff U, Neumann CS, Rey JF, et al. (2007) ESGE-ESGENA guideline for quality assurance in reprocessing: Microbiological surveillance testing in endoscopy. Endoscopy 39(2): 175–181. Available at:https://pubmed.ncbi.nlm.nih.gov/17327980/ [DOI] [PubMed] [Google Scholar]
- Botana-Rial M, Leiro-Fernández V, Núñez-Delgado M, et al. (2016) A pseudo-outbreak of Pseudomonas putida and Stenotrophomonas maltophilia in a bronchoscopy unit. Respiration 92(4): 274–278. Available at:http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L612291961 [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention (2016) Essential Elements of a Reprocessing Program for Flexible Endoscopes – Recommendations of the Healthcare Infection Control Practices Advisory Committee. Atlanta, GA: Center for Disease Control and Prevention. Available at:https://www.cdc.gov/hicpac/recommendations/flexible-endoscope-reprocessing.html [Google Scholar]
- Cosgrove SE, Ristaino P, Caston-Gaa A, et al. (2012) Caveat emptor: the role of suboptimal bronchoscope repair practices by a third-party vendor in a pseudo-outbreak of pseudomonas in bronchoalveolar lavage specimens. Infection Control & Hospital Epidemiology 33(3): 224–229. United; States. DOI: 10.1086/664051. Available at:https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=25523512 [DOI] [PubMed] [Google Scholar]
- Cottarelli A, De Giusti M, Solimini AG, Venuto G, Palazzo C, Del Cimmuto A, Osborn J, Marinelli L. (2020) Microbiological surveillance of endoscopes and implications for current reprocessing procedures adopted by an Italian teaching hospital. Ann. Ig 32(2): 166–177. doi: 10.7416/ai.2020.2340. [DOI] [PubMed] [Google Scholar]
- Donlan RM, Costerton JW. (2002) Biofilms: Survival Mechanisms of clinically relevant microorganisms. Clinical Microbiology Reviews 15(2): 167–193. Available at:http://arxiv.org/abs/1208.0281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einav S, Wiener-Well Y. (2017) Anesthesia in patients with infectious disease caused by multi-drug resistant bacteria. Current Opinion in Anaesthesiology 30(3): 426–434. United States. Available at:https://pubmed.ncbi.nlm.nih.gov/28319476/ [DOI] [PubMed] [Google Scholar]
- Eurostat - Your key to European statistics (2020) Surgical Operations and Procedures Performed in Hospitals by ICD-9-CM. Available at:https://ec.europa.eu/eurostat/web/products-datasets/-/hlth_co_proc2 [Google Scholar]
- Garner JS, Favero MS. (1986) CDC guidelines for the prevention and control of nosocomial infections. Guideline for handwashing and hospital environmental control, 1985. Supersedes guideline for hospital environmental control published in 1981. American Journal of Infection Control 14(3): 110–129. Available at:https://pubmed.ncbi.nlm.nih.gov/3014924/ [DOI] [PubMed] [Google Scholar]
- Gavaldà L, Olmo AR, Hernández R, Domínguez MA, Robert Salamonsen M, Ayats J, Alcaide F, Soriano A. (2015) Microbiological monitoring of flexible bronchoscopes after high-level disinfection and flushing channels with alcohol: Results and costs. Respir. Med 109(8): 1079–1085. doi: 10.1016/j.rmed.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Glasziou PP, Sanders SL. (2002) Investigating causes of heterogeneity in systematic reviews. Statistics in Medicine 21(11): 1503–1511. Available at:https://pubmed.ncbi.nlm.nih.gov/12111916/ [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Jonathan JD, et al. (2003) Measuring inconsistency in meta-analyses. British Medical Journal 327: 557–560. Available at:https://pubmed.ncbi.nlm.nih.gov/12958120/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenters N, Huijskens E, Meier C, et al. (2015) Infectious diseases linked to cross-contamination of flexible endoscopes. Antimicrobial Resistance and Infection Control 4(S1): 259–265. Available at:https://pubmed.ncbi.nlm.nih.gov/26355428/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovaleva J., Meessen N, Peters F, et al. (2009) Is bacteriologic surveillance in endoscope reprocessing stringent enough? Endoscopy 41(10): 913–916. Available at:https://pubmed.ncbi.nlm.nih.gov/19750453/ [DOI] [PubMed] [Google Scholar]
- Kovaleva J, Peters FTM, van der Mei HC, et al. (2013) Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clinical Microbiology Reviews 26(2): 231–254. Available at:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3623380/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S, Vinther R, Klinten L, et al. (2020) Rate and impact of duodenoscope contamination: A systematic review and meta-analysis. EClinicalMedicine - the LANCET 25: 100451. Available at:https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(20)30195-4/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangen MJJ, Huijts SM, Bonten MJM, et al. (2017) The impact of community-acquired pneumonia on the health-related quality-of-life in elderly. BMC Infectious Diseases 17(1): 1–9. Available at:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5351062/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino M, Grieco G, Moscato U, Bruno S, Orecchio F, Ficarra MG, Turnaturi C. (2012) Is reprocessing after disuse a safety procedure for bronchoscopy?: A cross-sectional study in a teaching hospital in Rome. Gastroenterol Nurs 35(5): 324–330. doi: 10.1097/SGA.0b013e3182608fd5. [DOI] [PubMed] [Google Scholar]
- Matosin N, Frank E, Engel M, et al. (2014) Negativity towards negative results: A discussion of the disconnect between scientific worth and scientific culture. DMM Disease Models and Mechanisms 7(2): 171–173. Available at:https://pubmed.ncbi.nlm.nih.gov/24713271/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AC, Muscarella LF. (2020) Bronchoscope-Related “Superbug” Infections. Chest 157(2): 454–469. Available at:http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L2004306084 [DOI] [PubMed] [Google Scholar]
- Mouritsen JM, Ehlers L, Kovaleva J, et al. (2020) A systematic review and cost effectiveness analysis of reusable vs. single-use flexible bronchoscopes. Anaesthesia 75(4): 529–540. Available at:http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L2003561197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofstead CL, Quick MR, Wetzler HP, et al. (2018) Effectiveness of reprocessing for flexible bronchoscopes and endobronchial ultrasound bronchoscopes. Chest 154(5): 1024–1034. DOI: 10.1016/j.chest.2018.04.045. Available at:http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L2001071163 [DOI] [PubMed] [Google Scholar]
- Pajkos A, Vickery K, Cossart Y. (2004) Is biofilm accumulation on endoscope tubing a contributor to the failure of cleaning and decontamination? Journal of Hospital Infection 58: 224–229. Available at:https://pubmed.ncbi.nlm.nih.gov/15501338/ [DOI] [PubMed] [Google Scholar]
- Peaper DR, Havill NL, Aniskiewicz M, et al. (2015) Pseudo-outbreak of Actinomyces graevenitzii associated with bronchoscopy. Journal of Clinical Microbiology 53(1): 113–117. Available at:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4290908/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRISMA (2015) PRISMA - Transparent Reporting of Systematic Reviews and Meta-Analyses. PRISMA. Available at:http://prisma-statement.org/PRISMAStatement/FlowDiagram.aspx [Google Scholar]
- Rutala WA, Kanamori H, Sickbert-Bennett EE, et al. (2019) What’s new in reprocessing endoscopes: Are we going to ensure “the needs of the patient come first” by shifting from disinfection to sterilization? American Journal of Infection Control 47: A62–A66. DOI: 10.1016/j.ajic.2019.01.017. [DOI] [PubMed] [Google Scholar]
- Rutala WA, Weber DJ. (2013) Disinfection and sterilization in healthcare facilities. Infectious Disease Clinics of North America 30(3): 609–637. Bennett & Brachman’s Hospital Infections: Sixth Edition (May). Available at:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7099662/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons BP (1983) CDC guidelines for the prevention and control of nosocomial infections guideline for hospital environmental control. The American Journal of Infection Control 11: 97–120. doi: 10.1016/0196-6553(83)90122-0. https://pubmed.ncbi.nlm.nih.gov/6309037/. [DOI] [PubMed] [Google Scholar]
- Spach DH, Silverstein FE, Stamm WE. (1993) Transmission of infection by gastrointestinal endoscopy and bronchoscopy. Annals of Internal Medicine 118(2): 117–118. Available at:https://www.acpjournals.org/doi/pdf/10.7326/0003-4819-118-2-199301150-00008 [DOI] [PubMed] [Google Scholar]
- Spaulding EH, Gröschel DH. (1974) Hospital disinfectants and antiseptics. In: Lennette EH, Spaulding EH TJ. (eds), Manual of clinical microbiology. Washington, DC: American Society for Microbiology (ASM), pp. 852–857. Available at:https://onlinelibrary.wiley.com/doi/abs/10.1002/jobm.19750150418 [Google Scholar]
- Srinivasan A. (2003) Epidemiology and prevention of infections related to endoscopy. Current Infectious Diseases Report. 5: 467–472. Available at:https://pubmed.ncbi.nlm.nih.gov/14642186/ [DOI] [PubMed] [Google Scholar]
- Terjesen CL, Kovaleva J, Ehlers L. (2017) Early assessment of the likely cost effectiveness of single-use flexible video bronchoscopes. PharmacoEconomics - Open 1(2): 133–141. Available at:http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L625870764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiano G, Lo Nostro A, Calonico C, et al. (2019) Microbiological surveillance of flexible bronchoscopes after a high-level disinfection with peracetic acid: preliminary results from an Italian teaching hospital. Annali di igiene : medicina preventiva e di comunita 31(1): 13–20. Available at:http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L625560735 [DOI] [PubMed] [Google Scholar]
- Vincenti S, Quaranta G, De Meo C, Bruno S, Giovanna Ficarra M, Carovillano S, Ricciardi W, Laurenti P. (2014) Non-fermentative gram-negative bacteria in hospital tap water and water used for haemodialysis and bronchoscope flushing: Prevalence and distribution of antibiotic resistant strains. Sci. Total Environ 499: 47–54. doi: 10.1016/j.scitotenv.2014.08.041. [DOI] [PubMed] [Google Scholar]
- Von Hippel PT. (2015) The heterogeneity statistic I2 can be biased in small meta-analyses BMC Medical Research Methodology. BioMed Central Ltd 1515(1): 35. Available at:https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/s12874-015-0024-z [DOI] [PMC free article] [PubMed] [Google Scholar]