Key words: Acanthocephalans, generalist, Mexico, Polymorphidae, phylogeography, specialist
Abstract
Polymorphidae is a monophyletic group of acanthocephalans distributed worldwide. Within this family, Hexaglandula corynosoma is a specialist species that uses a single bird species as a definitive host. Southwellina hispida is a generalist species that uses a broad spectrum of definitive hosts to complete its life cycle. In the current research, sequences of cytochrome c oxidase subunit 1 (cox1) from mitochondrial DNA were generated from 44 specimens of H. corynosoma and 76 of S. hispida distributed sympatrically in 6 biogeographic provinces of Mexico with the objective of characterizing and comparing the population genetic structure of 2 acanthocephalan species with opposing life strategies. The phylogeographic studies indicated that the populations of both species lacked a phylogeographic structure and exhibited high haplotype diversity, low nucleotide diversity and low Fst values among the biogeographic provinces; in combination with negative values on the neutrality test, this suggests that the populations of acanthocephalans are expanding. Paratenic hosts are key for the transmission from intermediate to definitive hosts in the generalist species. However, the inclusion of paratenic hosts does not play a principal role in the population genetic structure of S. hispida within its distribution along the coasts of Mexico.
Introduction
Parasitism is a highly successful lifestyle and has evolved independently at least 60 times in different groups of metazoans worldwide (Price, 1980; Poulin and Morand, 2004). Parasites have been traditionally divided into 2 major groups depending on their life cycle: generalists and specialists (Thompson, 1994, 2005). The generalist parasites use a wide range of definitive hosts, whereas the specialist parasites use a single definitive host to complete their life cycle. Under these 2 opposing strategies, generalist parasites infect a broad spectrum of hosts resulting in an optimal or suboptimal level of fitness, whereas specialist parasites prioritize a single optimal host in which fitness is maximized (Rigaud et al., 2010; Lievens et al., 2018). Some studies have suggested that parasite life cycle complexity (generalists vs specialists) could influence population genetic structure (Nadler, 1995; Criscione and Blouin, 2004; Barrett et al., 2008; Archie and Ezenwa, 2011). According to Li et al. (2014), a specialist parasite shows significantly less genetic flow; therefore, populations are less connected and are subdivided into smaller populations, leading to strong genetic differentiation. In some cases, the populations might experience bottlenecks, decreasing the effective population size. Moreover, specialist species are more sensitive to stochastic fluctuations that can cause local extinction. In contrast, a generalist parasite shows a high effective population size, high genetic flow and a population that is structured or panmictic. Moreover, a generalist parasite may show greater persistence of populations over the long term because the generalist may be less sensitive to stochastic fluctuations in any given resource as it is able to replace a scarce resource with another (see Sehgal et al., 2001; Brant and Ortí, 2003; Archie and Ezenwa, 2011; Li et al., 2014).
The recent application of molecular markers has helped establishing a more robust classification scheme in acanthocephalans (Near et al., 1998; García-Varela et al., 2000). In particular, cytochrome c oxidase subunit 1 (cox1) from mitochondrial DNA is among the most useful molecular markers for defining, recognizing and delineating species and better understanding the population genetic structure in acanthocephalans (Steinauer et al., 2007; Rosas-Valdez et al., 2012, 2020; Alcántar-Escalera et al., 2013; Goulding and Cohen, 2014; Perrot-Minnot et al., 2018; Pinacho-Pinacho et al., 2018; García-Varela et al., 2021; Sereno-Uribe et al., 2022). Polymorphidae (Meyer, 1931) is an emblematic group of obligate endoparasites with complex life cycles that use vertebrates (marine mammals, fish-eating birds and waterfowl) as definitive hosts and invertebrates (amphipods, decapods and euphausiids) as intermediate hosts to complete their life cycle (Schmidt, 1985; Hoberg, 1986; Pichelin et al., 1998; Nickol et al., 1999, 2002; Kennedy, 2006). Currently, the family is classified into 14 genera with approximately 129 species (Schmidt, 1973; Dimitrova and Georgiev, 1994; Nickol et al., 1999, 2002; Aznar et al., 2006; Amin, 2013; García-Varela et al., 2013b; Presswell et al., 2020). Phylogenetic analyses based on multiple molecular markers have suggested that the family is monophyletic as are the genera Hexaglandula (Petrochenko, 1950) and Southwellina (Witenberg, 1932) (see García-Varela et al., 2011, 2013b).
Members of the Polymorphidae form a monophyletic group that contains generalist and specialist species. Therefore, this family represents an interesting system to explore and compare the population genetic structure of species with 2 opposing life history strategies. Hexaglandula corynosoma (Travassos, 1915) is a specialist species that has been recorded as adult only in the intestine of the yellow-crowned night-heron (Nyctanassa violacea) (Linnaeus, 1758), and cystacanths have been recorded in 2 decapod species [the fiddler crabs Leptuca spinicarpa (Rathbun) and Minuca rapax (Smith)], which serve as intermediate hosts in the Americas (Nickol et al., 2002; Guillén-Hernández et al., 2008; García-Prieto et al., 2010). In contrast, the acanthocephalan Southwellina hispida (Van Cleave, 1925) Witenberg, 1932 is a generalist species and is considered one of the most abundant species of polymorphids associated with piscivorous birds throughout the world (see Amin et al., 2022). In Mexico, S. hispida has been documented in 13 piscivorous bird species (García-Prieto et al., 2010; García-Varela et al., 2012), and the cystacanth has been recorded in the red swamp crayfish Procambarus clarkii (Girard) in the USA (Font, 2007), which serves as an intermediate host that is ingested by several vertebrates that act as paratenic hosts such as frogs and freshwater and brackish fishes (Schmidt, 1985). The paratenic hosts must be ingested by piscivorous birds to complete their life cycle (Schmidt, 1985).
In the current study, we examined the sequences of cox1 from mitochondrial DNA of 2 closely related polymorphid species, a specialist species (H. corynosoma) and a generalist species (S. hispida), distributed sympatrically in 6 biogeographic provinces (California, Baja California, Pacific Lowlands, Tamaulipas, Veracruzan and the Yucatán Peninsula) of Mexico with the objective of characterizing and comparing the population genetic structure of 2 acanthocephalan species with opposing life history strategies.
Materials and methods
Specimen collection
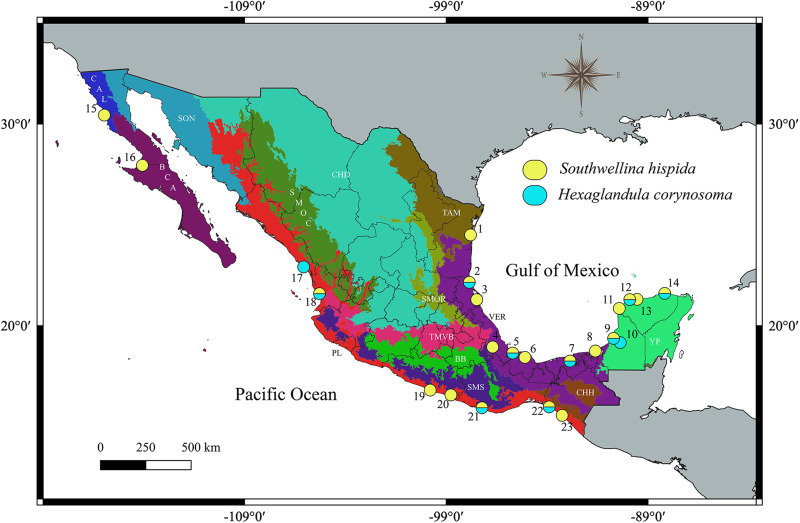
A total of 75 birds, and 36 fishes, were collected between October 2006 and December 2021 in 23 localities across 6 biogeographic provinces (California, Baja California, Pacific Lowlands, Tamaulipas, Veracruzan and the Yucatán Peninsula) of Mexico (Table 1; Fig. 1). Birds and fishes were dissected within the following 4 h, and their viscera were placed in separate Petri dishes containing a 0.75% saline solution and examined under a dissecting microscope. The acanthocephalans recovered were washed in 0.75% saline solution and placed overnight in distilled water at 4°C and subsequently preserved in 100% ethanol. Birds and fishes were identified using the field guide of Howell and Webb (1995) and Miller et al. (2005), respectively.
Table 1.
Specimens’ information, collection sites (CS), host name; number of host examined/infected (prevalence of infection); number of specimens analysed (n); locality, geographical coordinates, GenBank accession number for specimens studied in this study
| Coordinates | GenBank | |||||||
|---|---|---|---|---|---|---|---|---|
| CS | State | Species | Host | n | Locality | North | West | Cox1 |
| Tamaulipas | ||||||||
| 1 | Southwellina hispida | Pelecanus occidentalis (5/1) | 1 | Laguna Madre, Tamaulipas | 24°29′00″ | 97°45′00″ | FJ824179 | |
| Veracruz | ||||||||
| 2 | Hexaglandula corynosoma | Nyctanassa violacea (2/2) | 5 | Rivera, Veracruz | 22°06′54.4″ | 97°46′37.6″ | OQ152151–155 | |
| S. hispida | Ardea alba (1/1) | 1 | Rivera, Veracruz | OQ152190 | ||||
| 3 | S. hispida | Thalasseus maximus (5/3) | 5 | Tamiahua, Veracruz | 21°15′49″ | 97°27′41″ | OQ152203-207 | |
| 4 | S. hispida | Nycticorax nycticorax (4/3) | 1 | Los Chivos, Veracruz | 18°56′13″ | 95°58′08″ | FJ824173 | |
| 5 | H. corynosoma | N. violacea (8/6) | 6 | Tlacotalpan, Veracruz | 18°36′00″ | 95°39′00″ |
EF467869 OQ152124-128 |
|
| S. hispida | Tigrisoma mexicanum (1/1) | 1 | Tlacotalpan, Veracruz |
EF467867 OQ152164-166 |
||||
| S. hispida | A. alba (1/1) | 1 | Tlacotalpan, Veracruz | HM636468 | ||||
| S. hispida | Nannopterum brasilianus (7/5) | 4 | Tlacotalpan, Veracruz |
HM636469 OQ152167-169 |
||||
| S. hispida | Rupornis magnirostris (2/2) | 2 | Tlacotalpan, Veracruz | OQ152218-219 | ||||
| S. hispida | Cichlasoma urophthalmums (10/5) | 1 | Tlacotalpan, Veracruz | FJ824172 | ||||
| 6 | H. corynosoma | N. violacea (1/1) | 5 | Catemaco, Veracruz | 18°24′21.6″ | 95°6′7.2″ | OQ152139-143 | |
| S. hispida | N. nycticorax (4/3) | 1 | FJ824173 | |||||
| Tabasco | ||||||||
| 7 | H. corynosoma | N. violacea (1/1) | 1 | Espino, Tabasco | 18°14′47″ | 92°49′57″ | EU189486 | |
| S. hispida | Egretta garzetta (1/1) | 1 | Espino, Tabasco | EF467868 | ||||
| Campeche | ||||||||
| 8 | S. hispida | Egretta tricolor (1/1) | 1 | Laguna de Términos, Campeche | 18°44′01″ | 91°34′50″ | FJ824174 | |
| 9 | H. corynosoma | N. violacea (1/1) | 4 | Champoton, Campeche | 19°16′50″ | 90°36′51″ | OQ152156-159 | |
| S. hispida | Pandion haliaetus (1/1) | 1 | Champoton, Campeche | OQ152208 | ||||
| 10 | H. corynosoma | N. violacea (1/1) | 3 | Ulumal, Campeche | 19°17′50″ | 90°37′15″ | OQ152144-146 | |
| Yucatán | ||||||||
| 11 | S. hispida | A. alba (1/1) | 5 | Celestún, Yucatán | 20° 50′ 53.5″ | 90° 24′ 22″ |
FJ824175 OQ152170-173 |
|
| S. hispida | C. urophthalmum (6/4) | 1 | Celestún, Yucatán | FJ824176 | ||||
| 12 | H. corynosoma | Leptuca spinicarpa | 1 | Chuburna, Yucatán | 21°13′18″ | 89°49′44″ | EU189485 | |
| S. hispida | Egretta caerulea (1/1) | 6 | Chuburna, Yucatán | OQ152191-196 | ||||
| 13 | S. hispida | C. urophthalmum (10/5) | 5 | Progreso, Yucatán | 21°16′24.2″ | 89°39′34.9″ |
FJ824178 OQ152186-189 |
|
| 14 | H. corynosoma | N. violacea (1/1) | 6 | Ría Lagartos, Yucatán | 21°35′32″ | 88°09′48″ |
EU189487 OQ152129-133 |
|
| S. hispida | C. urophthalmum (5/4) | 1 | Ría Lagartos, Yucatán | FJ824177 | ||||
| Baja California Norte | ||||||||
| 15 | S. hispida | Botaurus lentiginosus (1/1) | 1 | Bahía de San Quintin, Baja California Norte | 30°24′57″ | 115°54′72″ | FJ824189 | |
| Baja California Sur | ||||||||
| 16 | S. hispida | Nannopterum auritus (3/1) | 3 | Guerrero, Negro, Baja California Sur | 27°57′32″ | 114°03′22″ |
OQ152180-181 FJ824187 |
|
| S. hispida | N. nycticorax (1/1) | 3 | Guerrero, Negro, Baja California Sur |
FJ824188 OQ152182-183 |
||||
| Sinaloa | ||||||||
| 17 | H. corynosoma | N. violacea (1/1) | 4 | Caimanero, Sinaloa | 22°53′4.65″ | 106°03′39.15″ | OQ152147-150 | |
| Nayarit | ||||||||
| 18 | H. corynosoma | N. violacea (1/1) | 6 | La Tovara, Nayarit | 21°32′43.66″ | 105°16′24.12″ |
EU189488 OQ152134-138 |
|
| S. hispida | N. brasilianus (2/1) | 1 | La Tovara, Nayarit | FJ824185 | ||||
| S. hispida | Anhinga anhinga (3/1) | 2 | La Tovara, Nayarit |
OQ152174 FJ824186 |
||||
| S. hispida | Butorides virescens (2/1) | 3 | La Tovara, Nayarit |
OQ152175-176 FJ824184 |
||||
| S. hispida | Ardea herodias (2/1) | 3 | La Tovara, Nayarit |
OQ152177-178 FJ824183 |
||||
| S. hispida | T. mexicanum (1/1) | 2 | La Tovara, Nayarit |
OQ152179 FJ824182 |
||||
| Guerrero | ||||||||
| 19 | S. hispida | Cichlasoma trimaculatum (5/3) | 3 | Tres Palos, Guerrero | 16°48′00″ | 99°47′00″ |
FJ824180 OQ152184-185 |
|
| S. hispida | N. brasilianus (1/1) | 1 | Tres Palos, Guerrero |
FJ824181 |
||||
| 20 | S. hispida | N. nycticorax (1/1) | 1 | Marquelia, Guerrero | 16°33′19.75″ | 98°48′38.89″ | OQ152217 | |
| Oaxaca | ||||||||
| 21 | H. corynosoma | N. violacea (1/1) | 1 | Laguna Manialtepec, Oaxaca | 15°56′25″ | 97°11′40″ | OQ152123 | |
| S. hispida | A. alba (1/1) | 4 | Laguna Manialtepec, Oaxaca | OQ152197-200 | ||||
| S. hispida | N. brasilianus (2/1) | 2 | Laguna Manialtepec, Oaxaca | OQ152201-202 | ||||
| Chiapas | ||||||||
| 22 | H. corynosoma | N. violacea (1/1) | 4 | Tonala, Chiapas | 15°58′20″ | 93°51′23″ | OQ152160-163 | |
| S. hispida | A. alba (2/2) | 5 | Tonala, Chiapas | OQ152212-216 | ||||
| 23 | S. hispida | N. nycticorax (1/1) | 3 | Pijijiapan, Chiapas | 15°33′02″ | 93°15′53″ | OQ152209-211 | |
The sample number for each locality corresponds with the same number in Fig. 1. Sequences in bold were generated in the current study.
Fig. 1.
Map of Mexico showing the sampled sites for the birds. Localities with a circle of yellow and turquoise colour were positive for the infection with Southwellina hispida and Hexaglandula corynosoma, respectively; localities correspond to those in Table 1.
Morphological analyses
Selected adult acanthocephalans were gently punctured with a fine needle in the trunk, stained with Mayer's paracarmine, destained in 70% acid ethanol, dehydrated in a graded ethanol series, cleared in methyl salicylate and mounted in Canada balsam. Specimens were examined using a compound microscope Leica DM 1000 LED equipped with bright field (Leica, Wetzlar, Germany). The acanthocephalans were identified by conventional morphological criteria following Petrochenko (1958). In addition, descriptions of H. corynosoma and S. hispida were consulted as needed (Schmidt, 1973; Nickol et al., 2002; Amin et al., 2022). For scanning electron microscopy, 2 adult specimens of each species were dehydrated with an ethanol series, critical point dried, sputter coated with gold and examined with a Hitachi Stereoscan Model S-2469N scanning electron microscope operating at 15 kV from the Instituto de Biología, Universidad Nacional Autónoma de México (UNAM). Adult specimens were deposited in the Colección Nacional de Helmintos, under numbers CNHE: 11823 and 11824, Instituto de Biología, Universidad Nacional Autónoma de México, Mexico City
DNA isolation, amplification and sequencing
A total of 97 specimens, 41 identified as H. corynosoma and 56 as S. hispida were placed individually in tubes and digested overnight at 56°C in a solution containing 10 mm Tris–HCl (pH 7.6), 20 mm NaCl, 100 mm Na2-EDTA (pH 8.0), 1% Sarkosyl and 0.1 mg mL−1 proteinase K. Following digestion, DNA was extracted from the supernatant using the DNAzol reagent (Molecular Research Center, Cincinnati, Ohio, USA) according to the manufacturer's instructions. The cox1 of the mitochondrial DNA was amplified using the forward primer 5′-AGTTCTAATCATAA(R)GATAT(Y)GG-3′ and reverse primer 5′-TAAACTTCAGGGTGACCAAAAAATCA-3′ (Folmer et al., 1994). Polymerase chain reactions (PCRs) (25 μL) consisted of 10 μL of each primer, 2.5 μL of 10× buffer, 2 mm MgCl2 and 1 U of Taq DNA polymerase (Platinum Taq, Invitrogen Corporation, São Paulo, Brazil). PCR cycling parameters for the molecular marker consisted of denaturation at 94°C for 1 min, 35 cycles of 94°C for 1 min, 40°C for 1 min and 72°C for 1 min, followed by a post-amplification incubation at 72°C for 10 min. Sequencing reactions were performed using ABI Big Dye (Applied Biosystems, Boston, Massachusetts, USA) terminator sequencing chemistry, and reaction products were separated and detected using an ABI 3730 capillary DNA sequencer. Contigs were assembled and base-calling differences resolved using Codoncode Aligner version 9.0.1 (Codoncode Corporation, Dedham, Massachusetts, USA) and submitted to the GenBank dataset (Table 1).
Alignments, population genetic structure and historical demographic
Newly obtained sequences in the current research of S. hispida were aligned with 22 other sequences of S. hispida (EF467867–868, HM636468–469, FJ824172–189) downloaded from GenBank (Table 1), forming a dataset of 78 sequences with 646 characters, and the new sequences of H. corynosoma were aligned with other sequences of H. corynosoma (EU189485–486, EU189488 and EF467869), downloaded from GenBank (Table 1), forming a dataset of 46 sequences with 644 characters. Sequences of each dataset were aligned separately using software ClustalW with default parameters implemented in MEGA version 7.0 (Kumar et al., 2016).
To analyse the molecular information in the framework of population genetics, we grouped individuals of H. corynosoma and S. hispida into populations considering the biogeographic provinces (California, Baja California, Pacific Lowlands, Tamaulipas, Veracruzan and the Yucatán Peninsula). Intrapopulation variation was summarized using standard statistics: number of haplotypes (H), number of segregating sites (S), haplotype diversity (Hd), nucleotide diversity (Pi) and average number of nucleotide differences (K), were all calculated using the program DnaSP v.5.10 (Rozas et al., 2003). To examine haplotype frequency among the populations of H. corynosoma and S. hispida a statistical network was constructed independently, using the program PopART with the median joining algorithm (Bandelt et al., 1999). The degree of genetic differentiation among the populations was estimated using the fixation indices Fst (Hudson et al., 1992), with the program Arlequin v.3.5 (Excoffier and Lischer, 2010). To investigate the genetic variation among populations or within populations, the analysis of molecular variance was performed, considering genetic distance among the haplotypes using Arlequin v.3.5. To investigate the population history and demography, Tajima's D (Tajima, 1989) and Fu's Fs (Fu, 1997) were calculated using DnaSP v.5.10 (Rozas et al., 2003). The values were considered significant when the P values were less than 0.05.
Results
Morphological identification
The acanthocephalans recovered from diverse definitive hosts such as herons, gulls, cormorants, pelicans and hawks on both coasts of Mexico show similar morphological characteristics compared with those assigned to S. hispida by García-Varela et al. (2012) and Amin et al. (2022), including (i) an elongated cylindrical trunk with 2 fields of somatic spines on the anterior region of the trunk, (ii) a cylindrical proboscis with a swollen region, (iii) proboscis hooks arranged in 16–17 longitudinal rows of 12–15 hooks per row, (iv) a double-walled proboscis receptacle and (v) 4 tubular cement glands in males (Fig. 2A–E). The acanthocephalans recovered from the intestine of the yellow-crowned night-heron (N. violacea) in coastal Mexico show morphological characteristics that match those assigned to H. corynosoma by Nickol et al. (2002), Guillén-Hernández et al. (2008) and Amin et al. (2022), including (i) an elongate cylindrical trunk, swollen anteriorly with a single field of somatic spines in the anterior region of the trunk; (ii) a cylindrical proboscis; (iii) proboscis hooks arranged in 16 longitudinal rows of 11–12 hooks per row; (iv) a double-walled proboscis receptacle and (v) 6 tubular cement glands in males (Fig. 3A–E).
Fig. 2.
Scanning electron micrographs and photomicrographs of S. hispida from Ardea alba from Tonala, Chiapas, Mexico (locality 22 in Fig. 1 and Table 1): adult male, whole worm (A); male anterior region (B); proboscis (C); adult male, whole worm (D) and adult female, whole worm (E).
Fig. 3.
Scanning electron micrographs and photomicrographs of H. corynosoma from Nyctanassa violacea from La Tovara Nayarit, Mexico (locality 18 in Fig. 1 and Table 1): adult male, whole worm (A); proboscis (B); male anterior region (C); adult male, whole worm (D) and adult female, whole worm (E).
Population genetic structure and demographic analysis
The mitochondrial marker was successfully amplified for 56 S. hispida individuals and 41 H. corynosoma individuals. The complete alignment of the cox1 dataset contained 78 S. hispida individuals with a total length of 646 bp, whereas the cox1 dataset of H. corynosoma contained 46 individuals with a total length of 644 bp. No insertions or deletions were detected in any of the sequences, and when the sequences were translated into proteins, no stop codons were found.
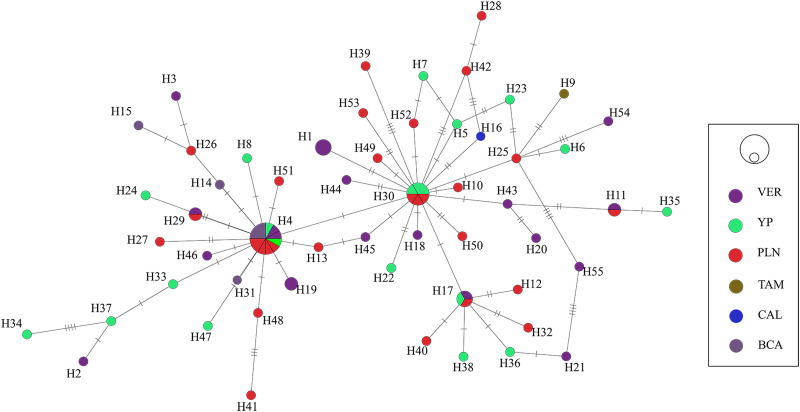
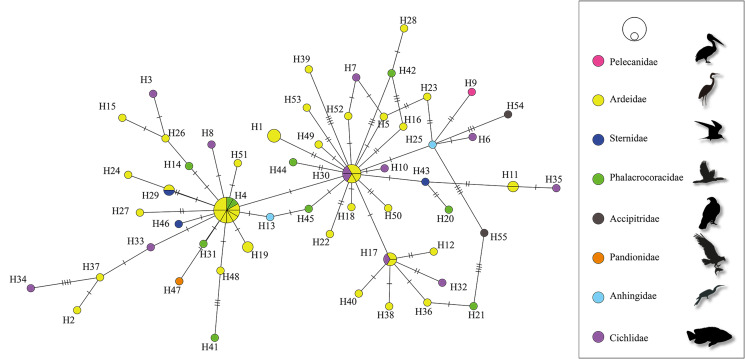
The haplotype network built for the generalist species S. hispida did not show a phylogeographic structure; in fact, of the 55 mtDNA haplotypes detected, 50 were unique (singlets), and 5 others were shared haplotypes (H4, H11, H17, H29 and H30). The most frequent haplotype (H4, n = 12) corresponded to specimens from 4 biogeographic provinces (Pacific Lowlands, Baja California, Veracruzan and the Yucatán Peninsula). Most of the identified haplotypes were separated from one another by 1, 2, 3 or up to 5 substitutions (see Fig. 4). The haplotype diversity was very high (Hd = 0.970) and nucleotide diversity was low (pi = 0.00555) among the populations from the 4 biogeographic provinces sampled (Pacific Lowlands, Baja California, Veracruzan and the Yucatán Peninsula) of Mexico. Neutrality tests (Tajima's D, −2.473 and Fu's Fs, −34.252) were negative for all regions (see Table 2), indicating an excess of rare alleles greater than what would be expected under neutrality and suggesting a recent population expansion of S. hispida. The Fst values were estimated to assess genetic differentiation among populations from the 4 biogeographic provinces analysed (Pacific Lowlands, Baja California, Veracruzan and the Yucatán Peninsula). Despite the large geographic distances, the Fst values were low, ranging from −0.007 to 0.058 (Table 3), which indicates that the populations were poorly genetically differentiated from one another. The sampled definitive and paratenic host species of S. hispida were grouped into 8 families (Fig. 5). The host haplotype network did not show a pattern; 30 haplotypes were found in herons scattered throughout the network. In the paratenic hosts sampled (cichlid fishes), 11 haplotypes were detected (H3, H6, H7, H8, H10, H17, H30, H32, H33, H34 and H35), suggesting that the paratenic hosts are able to harbour and transmit diverse haplotypes to definitive hosts (Fig. 5).
Fig. 4.
Haplotype network of samples of S. hispida, built with the gene cox1 from mitochondrial DNA. Each circle represents a haplotype, with size proportional to the haplotype's frequency in the populations. Mutational steps are symbolized by dashes. Biogeographic provinces: Veracruzan (VER); Yucatán Peninsula (YUC); Pacific Lowlands (PLN); Tamaulipas (TAM); California (CAL) and Baja California (BCA).
Table 2.
Molecular diversity indices and neutrality tests calculated for cox1 datasets among the populations of S. hispida used in this study
| Biogeographic provinces | n | S | H | Hd ± s.d. | Pi ± s.d. | K | Tajima's D | Fu's Fs |
|---|---|---|---|---|---|---|---|---|
| Veracruzan | 21 | 29 | 18 | 0.981 ± 0.023 | 0.00663 ± 0.00064 | 4.285 | −1.811 | −13.228 |
| Yucatán Peninsula | 19 | 26 | 17 | 0.988 ± 0.021 | 0.00607 ± 0.00083 | 3.918 | −1.859 | −13.746 |
| Baja California | 7 | 7 | 5 | 0.857 ± 0.137 | 0.00310 ± 0.00095 | 2.000 | −1.553 | −1.548 |
| Pacific Lowlands | 31 | 33 | 23 | 0.955 ± 0.026 | 0.00488 ± 0.00064 | 3.152 | −2.282 | −21.079 |
| All | 78 | 67 | 55 | 0.970 ± 0.012 | 0.00555 ± 0.00041 | 3.587 | −2.473 | −34.252 |
n, number of sequences; H, number of haplotypes; S, number of segregating sites; Hd, haplotype diversity; Pi, nucleotide diversity; K, average number of nucleotide differences.
Table 3.
Pairwise Fst values estimated for cox1
| VER | YP | BCAL | PL | |
|---|---|---|---|---|
| VER | – | |||
| YP | −0.007 | – | ||
| BCAL | 0.088 | 0.082 | – | |
| PL | 0.010 | 0.005 | 0.058 | – |
VER, Veracruzan; YP, Yucatan Peninsula; BCAL, Baja California; PL, Pacific Lowlands.
Fst average among 3 biogeographic provinces = 0.03672.
Significance level = 0.05.
Fig. 5.
Host haplotype network of samples of S. hispida, built with the gene cox1 from mitochondrial DNA. The paratenic and definitive hosts were grouped by families. Each circle represents a haplotype, with size proportional to the haplotype's frequency in the populations. Mutational steps are symbolized by dashes.
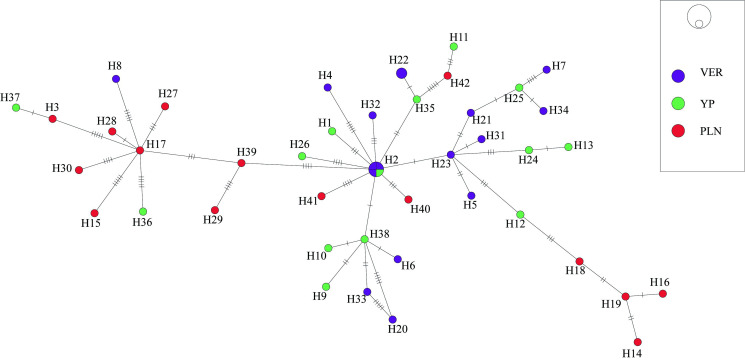
The haplotype network of the specialist species H. corynosoma did not show a phylogeographic structure for the 42 mtDNA haplotypes detected; 41 were unique (singlets), and only 1 was shared (H2, n = 4) between 2 biogeographic provinces (Veracruzan and the Yucatán Peninsula). Most of the identified haplotypes were separated by several substitutions (see Fig. 6). The haplotype diversity (Hd = 0.993) was very high and nucleotide diversity was low (pi = 0.01166) among the populations from the 3 biogeographic provinces sampled (Pacific Lowlands, Veracruzan and the Yucatán Peninsula). Neutrality tests (Tajima's D, −1.875 and Fu's Fs, −33.964) were negative for all regions (see Table 4), which indicates an excess of rare alleles greater than what would be expected under neutrality, suggesting a recent population expansion of H. corynosoma. The Fst values were estimated to assess genetic differentiation among the populations from the 3 biogeographic provinces analysed (Pacific Lowlands, Veracruzan and the Yucatán Peninsula). Despite the large geographic distances, the Fst values were low, ranging from −0.019 to 0.059 (Table 5), which indicates that the populations were poorly genetically differentiated from one another.
Fig. 6.
Haplotype network of samples of H. corynosoma, built with the gene cox1 from mitochondrial DNA. Each circle represents a haplotype, with size proportional to the haplotype's frequency in the populations. Mutational steps are symbolized by dashes. Biogeographic provinces: Veracruzan (VER); Yucatán Peninsula (YUC) and Pacific Lowlands (PLN).
Table 4.
Molecular diversity indices and neutrality tests calculated for cox1 datasets among the populations of H. corynosoma used in this study
| Biogeographic provinces | n | S | H | Hd ± s.d. | Pi ± s.d. | K | Tajima's D | Fu's Fs |
|---|---|---|---|---|---|---|---|---|
| Veracruzan | 17 | 32 | 14 | 0.971 ± 0.032 | 0.00884 ± 0.00132 | 5.691 | −1.707 | −6.056 |
| Yucatán Peninsula | 14 | 38 | 14 | 1.0 ± 0.027 | 0.01165 ± 0.00177 | 7.505 | −1.613 | −8.104 |
| Pacific Lowlands | 15 | 35 | 15 | 1.0 ± 0.024 | 0.01344 ± 0.00114 | 8.657 | −0.926 | −8.273 |
| All | 46 | 66 | 42 | 0.993 ± 0.007 | 0.01166 ± 0.00083 | 7.510 | −1.875 | −33.964 |
n, number of sequences; H, number of haplotypes; S, number of segregating sites; Hd, haplotype diversity; Pi, nucleotide diversity; K, average number of nucleotide differences.
Table 5.
Pairwise Fst values estimated for cox
| VER | YP | PL | |
|---|---|---|---|
| VER | – | ||
| YP | −0.019 | – | |
| PL | 0.112 | 0.059 | – |
VER, Veracruzan; YP, Yucatan Peninsula; PL, Pacific Lowlands
Fst average among 3 biogeographic provinces = 0.05609.
Significance level = 0.05.
Discussion
To the best of our knowledge, S. hispida and H. corynosoma are 2 species that use piscivorous birds as definitive hosts and decapods as intermediate hosts, share a common ancestor within Polymorphidae and are distributed sympatrically along the coastline of Mexico (Guillén-Hernández et al., 2008; García-Prieto et al., 2010; García-Varela et al., 2013b). The generalist species S. hispida was described in Japan by Van Cleave in 1925, and since then it has been recorded in the body cavities of fishes and reptiles as a cystacanth (larval stage) and as an adult form in the intestines of diverse piscivorous birds in Asia, Europe and the Americas (see Amin et al., 2022). In Mexico, adults of S. hispida have been recorded in 15 piscivorous bird species (García-Prieto et al., 2010; García-Varela et al., 2012). It is well known that helminths with a broad spectrum of definitive hosts show phenotypic plasticity in their morphological traits. Schmidt (1973), García-Varela et al. (2012) and Amin et al. (2022) documented the phenotypic plasticity of S. hispida recovered from diverse definitive hosts including in traits such as body size, leminisci, proboscis receptacle, testes, cement glands and the reproductive system in females. However, diagnostic characters such as proboscis shape, number of proboscis hooks and the presence of 2 fields of spines on the anterior trunk region in both sexes did not vary among specimens, including our specimens collected from the coasts of the Gulf of Mexico and the Pacific Ocean slopes (see Fig. 2A–E). In Brazil, the specialist species H. corynosoma was described as parasite of the yellow-crowned night heron (N. violacea) by Travassos in 1915. Since then, H. corynosoma has been recorded in Puerto Rico, the USA and Mexico in the same definitive host species (Cable and Quick, 1954; Nickol et al., 2002; Guillén-Hernández et al., 2008). Our specimens identified as H. corynosoma agree morphologically with those previously described by Nickol et al. (2002) and Guillén-Hernández et al. (2008). For example, an elongated trunk, swollen anteriorly; covered with spines on the anterior region of the trunk; a cylindrical proboscis with 16 longitudinal rows of 11–12 hooks each; a conical neck; ovoid testes located in the swollen portion of the trunk and long, tubular cement glands (see Fig. 3A–E).
The intraspecific genetic divergence estimated in the current study among the 78 isolates of S. hispida and the 46 isolates of H. corynosoma ranged from 0.00 to 1.5% and from 0.00 to 2.6%, respectively. These values of intraspecific genetic divergence are similar to those previously reported for isolates of polymorphid species such as Andracantha sigma (Presswell et al., 2017) recovered from 3 definitive hosts, the Otago shag, Leucocarbo chalconotus (Gray), spotted shag Phalacrocorax punctatus (Sparrman) and Otago little blue penguin, Eudyptula novaehollandiae (Forster) from New Zealand, which ranged from 0.00 to 0.32% (Presswell et al., 2017); similar to those among 14 adults, 3 acanthella and 4 cystacanths of Pseudocorynosoma constrictum (Van Cleave, 1918) Aznar et al. (2006) recovered from 7 wild duck species and the freshwater amphipod Hyalella azteca (Saussure) in central Mexico, which ranged from 0.0 to 3.0% (García-Varela et al., 2013a) and similar to those among 19 adults recovered from 3 fish-eating bird species and 33 cystacanths recovered from 19 freshwater fish species identified as Polymorphus brevis (Van Cleave, 1916) Travassos, 1926, which ranged from 0.00 to 1.6% (Alcántar-Escalera et al., 2013). Furthermore, the values from this study were also similar to those among adults and cystacanths of Corynosoma hannae Zdzitowiecki, 1984 recovered from the New Zealand sea lion (Phocarctos hookeri Grey), Stewart Island shag (L. chalconotus Gray), spotted shags (P. punctatus Sparrman), yellow-eyed penguins (Megadyptes antipodes Hombron and Jacquinot), New Zealand brill (Colistium guntheri Hutton) and New Zealand sole (Peltorhamphus novaezeelandiae Gunther), which ranged from 0.00 to 2.8% (Hernández-Orts et al., 2017) and those among Corynosoma australe (Johnston, 1937) recovered from the California sea lion (Zalophus californianus Lesson), South American sea lions (Otaria flavescens Shaw), South American fur seals (Arctocephalus australis Zimmermann), Magellanic penguins (Spheniscus magellanicus Forster) and cystacanths recovered from marine fishes in Argentina, which ranged from 1.0 to 1.7% (García-Varela et al., 2021).
The haplotype network genealogy generated in this study based on cox1 sequences from S. hispida (generalist) and H. corynosoma (specialist) did not show a phylogeographic structure; therefore, the haplotypes could not be grouped into their own geographic clusters. In fact, the specimens of S. hispida were not correlated with their definitive host family (see Figs 4 and 5). The populations analysed for both species of acanthocephalans were classified into biogeographical provinces separated by geographical barriers as follows: mountains, the dry lowlands of the Isthmus of Tehuantepec, the Balsas Depression and the central Trans-Mexicana Volcanic Belt (Barrier et al., 1998; Ferrari et al., 2012; Morrone et al., 2017). Despite the large geographic distances, the Fst values estimated among the populations of both species were very low (Tables 3 and 5), indicating that the populations were poorly genetically differentiated from each other; this can be explained by the migration patterns of birds along the coasts of Mexico. Historical events leave signatures in the DNA, and neutrality tests can infer the demographic history of populations. In both species analysed (generalist and specialist), the estimated values of Fu's Fs and Tajima's D among the populations were negative (see Tables 2 and 4). In addition, high haplotype diversity and an excess of low-frequency haplotypes were detected in both networks (Figs 4 and 6), although the haplotypes differed from one another by fewer than 5 nucleotide substitutions. These findings confirmed that both populations of acanthocephalans had experienced rapid population growth in the past.
In this study, we found that 2 species of acanthocephalans with opposing life history strategies (generalist and specialist) showed similar population genetics patterns. This pattern was not consistent with the specialist–generalist variation hypothesis (SGVH), which predicts that: (i) populations of specialists may be less connected and more subdivided into smaller populations than generalists; (ii) specialists are expected to have lower effective population sizes than generalists and to be composed of populations with less gene flow and (iii) generalists will show high effective population sizes, high genetic flow and highly structured populations (see Dennis et al., 2011; Li et al., 2014). According to the SGVH, the populations of S. hispida (generalist species) resemble the specialist model. To date, only a few phylogeographic studies of generalist acanthocephalans have been conducted using cox1 as a molecular marker. For example, Profilicollis altmani (Perry, 1942), which has a broad distribution across North and South America and parasitizes multiple species of intermediate hosts and diverse species of marine birds that act as definitive hosts (gulls, ducks, sanderling and common tern), showed a lack of population genetic structure with high haplotype diversity and low nucleotide diversity, suggesting that P. altmani have experienced a period of rapid population growth in the past (see Goulding and Cohen, 2014). Similarly, Profilicollis novaezelandensis Brockerhoff and Smales (2002) is a parasite that has been recorded as an adult in gulls (Larus spp.) and oystercatchers (Haematopus spp.) and as cystacanths in the shore crab Hemigrapsus crenulatus (Milne-Edwards) distributed along the east coast of New Zealand's South Island (Brockerhoff and Smales, 2002); population genetic analyses from 50 P. novaezelandensis individuals from 8 localities showed a lack of population genetic structure with high haplotype diversity and low nucleotide diversity (Hay et al., 2018).
The 2 species of acanthocephalans analysed here are considered to be typical components of the helminth fauna of piscivorous birds in the Americas. Of the 19 piscivorous birds recorded as definitive hosts, 11 belong to the family Ardeidae, which represent 58% of the host diversity, suggesting that the ardeids could be the ancestral hosts of S. hispida and H. corynosoma with secondary and independent colonization events to other piscivorous birds. In particular, S. hispida was recorded in diverse piscivorous birds such as pelicans, cormorants, eagles, hawks, anhingas and royal terns with a lower level of infection (prevalence values <20%) than those of ardeid hosts (prevalence values from 80 to 100%), suggesting that these piscivorous birds may act as suboptimal definitive hosts.
The population genetic structures of S. hispida and H. corynosoma showed similar patterns. However, these species use different strategies to complete their life cycles. The main ecological difference between S. hispida and H. corynosoma is the inclusion of paratenic hosts in S. hispida (fishes from the families Cichlidae, Eleotridae, Characidae, Lutjanidae, Centropomidae, Sparidae, Scianidae and Paralichthyidae) (García-Prieto et al., 2010). The paratenic hosts act as a trophic bridge and facilitate the transmission between the intermediate host and definitive host, but apparently do not play a central role in the population genetic structure of S. hispida within its distribution along the coasts of Mexico.
Acknowledgements
We thank Laura Márquez and Nelly López Ortiz from LaNabio for their help during the sequencing of the DNA fragments. We also thank Berenit Mendoza Garfias for her help in obtaining the scanning electron microphotographs. A. L.-J. and M. T. G.-G. acknowledge the support of the Programa de Posgrado en Ciencias Biológicas, UNAM and CONACYT (A. L.-J. CVU No. 706119; M. T. G.-G. CVU No. 956064), for granting a scholarship to complete her PhD and his Master program, respectively. L. A.-G. acknowledges the Coordinación de la Investigación Científica and Dirección General de Asuntos de Personal Académico (DGAPA-UNAM), Mexico, for granting the Postdoctoral Fellowship.
Author's contributions
M. G.-V. and A. L. S.-U. conceived and designed the study. M. G.-V., A. L.-J. and M. T. G.-G. conducted data gathering. A. L.-J. and M. T. G.-G. performed statistical analyses. A. L. S.-U., L. A.-G. and M. G.-V. wrote and edited the article.
Financial support
This research was supported by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT-UNAM) IN201122.
Conflict of interest
No conflict of interest exists among the authors.
Ethical standards
The sampling in this work complies with the current laws and animal ethics regulations of México. Specimens were collected under the Cartilla Nacional de Colector Científico (FAUT 0202) issued by the Secretaría del Medio Ambiente y Recursos Naturales (SEMARNAT), to M. G.-V.
References
- Alcántar-Escalera FJ, García-Varela M, Vázquez-Domínguez E and Pérez Ponce de León G (2013) Using DNA barcoding to link cystacanths and adults of the acanthocephalan Polymorphus brevis in Central Mexico. Molecular Ecology Resources 13, 1116–1124. [DOI] [PubMed] [Google Scholar]
- Amin OM (2013) Classification of the Acanthocephala. Folia Parasitologica 60, 273–305. [DOI] [PubMed] [Google Scholar]
- Amin OM, Chaudhary A and Singh HS (2022) Morphological and molecular description of immature Southwellina hispida (Van Cleave, 1925) Witenberg, 1932 (Acanthocephala: Polymorphidae) from the body cavity of the paratenic host Gillichthys mirabilis Cooper (Gobiidae) in California, with analyses of the chemical composition of hooks and spines. Acta Parasitologica 67, 1107–1125. [DOI] [PubMed] [Google Scholar]
- Archie AE and Ezenwa VO (2011) Population genetic structure and history of a generalist parasite infecting multiple sympatrict host species. International Journal for Parasitology 41, 89–98. [DOI] [PubMed] [Google Scholar]
- Aznar FJ, Pérez Ponce de León G and Raga JA (2006) Status of Corynosoma (Acanthocephala: Polymorphidae) based on anatomical, ecological and phylogenetic evidence, with the erection of Pseudocorynosoma n. gen. Journal of Parasitology 92, 548–564. [DOI] [PubMed] [Google Scholar]
- Bandelt H, Forster P and Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution 16, 37–48. [DOI] [PubMed] [Google Scholar]
- Barrett LG, Thrall PH, Burdon JJ and Linde CC (2008) Life history determines genetic structure and evolutionary potential of host–parasite interactions. Trends in Ecology & Evolution 23, 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrier E, Velasquillo L, Chavez M and Gaulon R (1998) Neotectonic evolution of the Isthmus of Tehuantepec (southeastern Mexico). Tectonophysics 287, 77–96. [Google Scholar]
- Brant SV and Ortí G (2003) Evidence for gene flow in parasitic nematodes between two host species of shrews. Molecular Ecology 12, 2853–2859. [DOI] [PubMed] [Google Scholar]
- Brockerhoff AM and Smales LR (2002) Profilicollis novaezelandensis n. sp. (Polymorphidae) and two other acanthocephalan parasites from shore birds (Haematopodidae and Scolopacidae) in New Zealand, with records of two species in intertidal crabs (Decapoda: Grapsidae and Ocypodidae). Systematic Parasitology 52, 55–65. [DOI] [PubMed] [Google Scholar]
- Cable RM and Quick LA (1954) Some acanthocephalan from Puerto Rico with the description of a new genus and three new species. Transactions of the American Microscopical Society 73, 393–400. [Google Scholar]
- Criscione CD and Blouin MS (2004) Life cycles shape parasite evolution: comparative population genetics of salmon trematodes. Evolution 58, 198–202. [DOI] [PubMed] [Google Scholar]
- Dennis RLH, Dapporto L, Fattorini S and Cook LM (2011) The generalism specialism debate: the role of generalists in the life and death of species. Biological Journal of the Linnean Society 104, 725–737. [Google Scholar]
- Dimitrova ZM and Georgiev BB (1994) Ardeirhynchus n. gen. (Palaeacanthocephala: Polymorphida: Polymorphidae), with a redescription of A. spiralis (Rudolphi, 1809) n. comb. Systematic Parasitology 29, 149–158. [Google Scholar]
- Excoffier L and Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources 10, 564–567. [DOI] [PubMed] [Google Scholar]
- Ferrari L, Orozco-Esquivel T, Manea V and Manea M (2012) The dynamic history of the Trans-Mexican Volcanic Belt and the Mexico subduction zone. Tectonophysics 522, 122–149. [Google Scholar]
- Folmer O, Black M, Hoeh W, Lutz R and Vrijenhoek R (1994) DNA primers for the amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3, 294–299. [PubMed] [Google Scholar]
- Font WF (2007) Parasites of Hawaiian stream fishes: sources and impacts. Bishop Museum Bulletin in Cultural and Environmental Studies 3, 157–169. [Google Scholar]
- Fu YX (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147, 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Prieto L, García-Varela M, Mendoza-Garfias B and Pérez-Ponce de León G (2010) Checklist of the Acanthocephala in wildlife vertebrates of Mexico. Zootaxa 2419, 1–50. [Google Scholar]
- García-Varela M, Pérez-Ponce de León G, de la Torre P, Cummings MP, Sarma SS and Laclette JP (2000) Phylogenetic relationships of Acanthocephala based on analysis of 18S ribosomal RNA gene sequences. Journal of Molecular Evolution 50, 532–540. [DOI] [PubMed] [Google Scholar]
- García-Varela M, Pérez-Ponce de León G, Aznar FJ and Nadler SA (2011) Ibirhynchus dimorpha n. gen. (Acanthocephala: Polymorphidae), inferred through morphological, ecological and molecular data. Journal of Parasitology 97, 97–105. [DOI] [PubMed] [Google Scholar]
- García-Varela M, Aznar FJ, Rodríguez RP and Pérez-Ponce de León G (2012) Genetic and morphological characterization of Southwellina hispida Van Cleave, 1925 (Acanthocephala: Polymorphidae), a parasite of fish-eating birds. Comparative Parasitology 79, 192–201. [Google Scholar]
- García-Varela M, Pinacho-Pinacho CD, Sereno-Uribe AL and Mendoza-Garfías B (2013a) First record of the intermediate host of Pseudocorynosoma constrictum Van Cleave, 1918 (Acanthocephala: Polymorphidae) in Central Mexico. Comparative Parasitology 80, 171–178. [Google Scholar]
- García-Varela M, Pérez-Ponce de León G, Aznar FJ and Nadler SA (2013b) Phylogenetic relationship among genera of Polymorphidae (Acanthocephala), inferred from nuclear and mitochondrial gene sequences. Molecular Phylogenetics and Evolution 68, 176–184. [DOI] [PubMed] [Google Scholar]
- García-Varela M, Masper A, Crespo EA and Hérnandez-Orts JS (2021) Genetic diversity and phylogeography of Corynosoma australe Johnston, 1937 (Acanthocephala: Polymorphidae), an endoparasite of otariids from the Americas in the Northern and Southern Hemispheres. Parasitology International 80, 102205. [DOI] [PubMed] [Google Scholar]
- Goulding CT and Cohen CS (2014) Phylogeography of a marine acanthocephalan: lack of cryptic diversity in a cosmopolitan parasite of mole crab. Journal of Biogeography 41, 965–976. [Google Scholar]
- Guillén-Hernández S, García-Varela M and Pérez-Ponce de León G (2008) First record of Hexaglandula corynosoma (Travassos, 1915) Petrochenko, 1958 (Acanthocephala: Polymorphidae) in intermediate and definitive hosts in Mexico. Zootaxa 1873, 61–68. [Google Scholar]
- Hay E, Jorge F and Poulin R (2018) The comparative phylogeography of shore crabs and their acanthocephalan parasites. Marine Biology 165, 69. [Google Scholar]
- Hernández-Orts JS, Brandão M, Georgieva S, Raga JA, Crespo EA, Luque JL and Aznar FJ (2017) From mammals back to birds: host-switch of the acanthocephalan Corynosoma australe from pinnipeds to the Magellanic penguin Spheniscus magellanicus. PLoS ONE 12, e0183809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoberg EP (1986) Aspects of ecology and biogeography of Acanthocephala in Antarctic seabirds. Annales de Parasitologie Humaine et Comparée 61, 199–214. [Google Scholar]
- Howell SNG and Webb S (1995) A Guide to the Birds of Mexico and Northern Central America. New York: Oxford University. [Google Scholar]
- Hudson RR, Boos DD and Kaplan NL (1992) A statistical test for detecting population subdivision. Molecular Biology and Evolution 9, 138–151. [DOI] [PubMed] [Google Scholar]
- Kennedy CR (2006) Ecology of the Acanthocephala. New York: Cambridge University Press. [Google Scholar]
- Kumar S, Stecher G and Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33, 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jovelin R, Yoshiga T, Tanaka R and Cutter AD (2014) Specialist versus generalist life histories and nucleotide diversity in Caenorhabditis nematodes. Proceedings of the Royal Society B 281, 20132858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievens EJP, Perreau J, Agnew P, Michalakis Y and Lenormand T (2018) Decomposing parasite fitness reveals the basis of specialization in a two-host, two-parasite system. Evolution Letters 2, 390–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RR, Minckley WL and Norris SM (2005) Freshwater Fishes of Mexico. Chicago, IL: The University of Chicago Press. [Google Scholar]
- Morrone JJ, Escalante T and Rodríguez-Tapia G (2017) Mexican biogeographic provinces: map and shapefiles. Zootaxa 4277, 277–279. [DOI] [PubMed] [Google Scholar]
- Nadler SA (1995) Microevolution and the genetic structure of parasite populations. Journal of Parasitology 81, 395–403. [PubMed] [Google Scholar]
- Near TJ, Garey JR and Nadler SA (1998) Phylogenetic relationships of the Acanthocephala inferred from 18S ribosomal DNA sequences. Molecular Phylogenetics and Evolution 10, 287–298. [DOI] [PubMed] [Google Scholar]
- Nickol BB, Crompton DWT and Searle DW (1999) Reintroduction of Profilicollis Meyer, 1931, as a genus in Acanthocephala: significance of the intermediate host. Journal of Parasitology 85, 716–718. [PubMed] [Google Scholar]
- Nickol BB, Heard RW and Smith NF (2002) Acanthocephalans from crabs in the southeastern US, with the first intermediate hosts known for Arhythmorhynchus frassoni and Hexaglandula corynosoma. Journal of Parasitology 88, 79–83. [DOI] [PubMed] [Google Scholar]
- Perrot-Minnot MJ, Špakulová M, Wattier R, Kotlík P, Düsen S, Ayogdu A and Tougard C (2018) Contrasting phylogeography of two Western Palaeartic fish parasites despite similar life cycle. Journal of Biogeography 45, 101–115. [Google Scholar]
- Petrochenko VI (1958) Acanthocephala of Domestic and Wild Animals, Vol. II. Moscow, Russia: Izdatel'stvo Akademii Nauk SSSR, Vsesoyuznoe Obshchestvo Gel'mintologov; (in Russian). [Google Scholar]
- Pichelin S, Kuris AM and Gurney R (1998) Morphological and biological notes on Polymorphus (Profilicollis) sphaerocephalus and Corynosoma stanleyi (Polymorphidae: Acanthocephala). Journal of Parasitology 84, 798–801. [PubMed] [Google Scholar]
- Pinacho-Pinacho CD, García-Varela M, Sereno-Uribe AL and Pérez Ponce de León G (2018) A hyper-diverse genus of acanthocephalans revealed by tree-base and non-tree-base species delimitation methods: ten cryptic species of Neoechinorhynchus in Middle American freshwater fishes. Molecular Phylogenetics and Evolution 127, 30–45. [DOI] [PubMed] [Google Scholar]
- Poulin R and Morand S (2004) Parasite Biodiversity. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- Presswell B, García-Varela M and Smales LR (2017) Morphological and molecular characterization of two new species of Andracantha (Acanthocephala: Polymorphidae) from New Zealand shags (Phalacrocoracidae) and penguins (Spheniscidae) within a key to the species. Journal of Helminthology 16, 1–12. [DOI] [PubMed] [Google Scholar]
- Presswell B, Bennett JDL and Smales LR (2020) Morphological and molecular characterisation of a new genus and species of acanthocephalan, Tenuisoma tarapungi n. g., n. sp. (Acanthocephala: Polymorphidae) infecting red-billed gulls in New Zealand, with a key to the genera of the Polymorphidae Meyer, 1931. Systematic Parasitology 97, 25–39. [DOI] [PubMed] [Google Scholar]
- Price PW (1980) Evolutionary Biology of Parasites. Princeton, NJ: Princeton University Press. [Google Scholar]
- Rigaud T, Perrot-Minnot MJ and Brown MJF (2010) Parasites and host assemblages: embracing the reality will improve our knowledge of parasites transmission and virulence. Proceedings of the Royal Society B 277, 3693–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Valdez R, Morrone JJ and García-Varela M (2012) Molecular phylogenetics of Floridosentis Ward, 1953 (Acanthocephala: Neoechinorhynchidae) parasites of mullets (Osteichthyes) from Mexico, using 28S rDNA sequences. Journal of Parasitology 98, 855–862. [DOI] [PubMed] [Google Scholar]
- Rosas-Valdez R, Morrone JJ, Pinacho-Pinacho CD, Domínguez-Domínguez O and García-Varela M (2020) Genetic diversification of acanthocephalans of the genus Floridosentis Ward, 1953 (Acanthocephala: Neoechinorhynchidae), parasites of mullets from the Americas. Infection, Genetics and Evolution 85. doi: 10.1016/j.meegid.2020.104535. [DOI] [PubMed] [Google Scholar]
- Rozas J, Sánchez-DelBarrio JC, Messeguer X and Rozas R (2003) DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics (Oxford, England) 19, 2496–2497. [DOI] [PubMed] [Google Scholar]
- Schmidt GD (1973) Resurrection of Southwellina Witenberg, 1932, with a description of Southwellina dimorpha sp. n., a key to genera in Polymorphida (Acanthocephala). Journal of Parasitology 59, 299–305. [PubMed] [Google Scholar]
- Schmidt, GD (1985) Development and life cycles. In Nickol BB and Crompton DWT (eds), Biology of the Acanthocephala. Cambridge, UK: Cambridge University Press, pp. 273–286. [Google Scholar]
- Sehgal RRM, Jones H and Smith TB (2001) Host specificity and incident of Trypanosoma in some African rainforest birds: a molecular approach. Molecular Ecology 10, 2319–2327. [DOI] [PubMed] [Google Scholar]
- Sereno-Uribe AL, López-Jiménez A, González-García MT, Pinacho-Pinacho CD, Macip Ríos R and García-Varela M (2022) Phenotypic plasticity, genetic structure, and systematic position of Neoechinorhynchus emyditoides Fisher, 1960 (Acanthocephala: Neoechinorhynchidae) a parasite of emydid turtles from the Nearctic and Neotropical regions. Parasitology 149, 991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinauer ML, Nickol BB and Ortí G (2007) Cryptic speciation and patterns of phenotypic variation of variable acanthocephalan parasite. Molecular Ecology 16, 4097–4109. [DOI] [PubMed] [Google Scholar]
- Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123, 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JN (1994) The Coevolutionary Process. Chicago: University of Chicago Press. [Google Scholar]
- Thompson JN (2005) The Geographic Mosaic of Coevolution. Chicago: University of Chicago Press. [Google Scholar]
- Travassos L (1915) Revisão dos acanthocephalos brazileiros. II. Familia Echinorhynchidae Hamann, 1892 (2 nota previa). Brazil Medico 48, 377. [Google Scholar]
- Van Cleave HJ (1925) Acanthocephala from Japan. Parasitology 17, 149–156. [Google Scholar]