Abstract
Background
Despite the advancement in our understanding of cholera and its etiological agent, Vibrio cholerae, the prevention and treatment of the disease are often hindered due to rapid changes in drug response pattern, serotype, and the major genomic islands namely, the CTX-prophage, and related genetic characteristics. In the present study, V. cholerae (n = 172) associated with endemic cholera in Dhaka during the years 2015–2021 were analyzed for major phenotypic and genetic characteristics, including drug resistance patterns.
Results
Results revealed that the V. cholerae strains belonged to serogroup O1 biotype El Tor carrying El Tor -specific genes rtxC, tcpA El Tor, and hlyA El Tor, but possessed classical-biotype cholera toxin. Serotypes of V. cholerae strains differed temporally in predominance with Inaba during 2015–2017, and again in 2020–2021, while Ogawa was the predominant serotype in 2018–2019. Also, ctxB1 was predominant in V. cholerae associated with cholera during 2015–2017, while ctxB7 was predominant in 2018, and in the subsequent years, as observed until 2021. V. cholerae strains differed in their antibiotic resistance pattern with a majority (97%) being multi-drug resistant (MDR) and belonging to six sub-groups. Notably, one of these MDR strains was resistant to eleven of the eighteen antibiotics tested, with resistance to fourth-generation cephalosporin (cefepime), and aztreonam. This extreme drug resistant (XDR) strain carried resistance-related genes namely, extended-spectrum β-lactamases (ESBL), blaOXA-1 and blaPER-3.
Conclusion
The observed temporal switching of serotypes, as well as the ctxB genotype, and the emergence of MDR/XDR V. cholerae and their association with endemic cholera in Dhaka underscore the need for routine monitoring of the pathogen for proper patient management.
Keywords: Vibrio cholerae, Temporal transition, Multi-drug resistant
Background
Cholera is an infectious diarrheal disease, which if not treated timely, can be fatal [1]. The disease has been endemic in the Ganges Delta of the Bay of Bengal, Bangladesh and India for centuries [2]. The etiological agent of the disease is a Gram negative Gammaproteobacteria, Vibrio cholerae. Based on the variation of ‘O’ antigenic lipopolysaccharide (LPS), more than 200 serogroups of V. cholerae have been identified, of which serogroups O1 and O139 are primarily associated with epidemic cholera [3]. V. cholerae O1 has two biotypes, classical and El Tor. The two biotypes are universally recognized, differing in major phenotypic and genetic characteristics [4], including genes associated with important virulence factors, for example, toxin coregulated pilus (tcpA) and cholera toxin B-subunit (ctxB) [5]. Seven pandemics of cholera have been documented, with the seventh pandemic being the longest that started in the 1960s and is still present. Of the seven recognized cholera pandemics, the first six are believed to have been caused by the classical biotype of V. cholerae O1, and the ongoing seventh pandemic is being caused by the El Tor biotype.
The seventh cholera pandemic is significantly different from the others partly because of its rapid global spread and remarkable evolution of V. cholerae. The emergence of atypical El Tor variants with a few classical biotype attributes and its global spread, the predominance of Haitian variants in endemic regions of Asia and Africa, and the acquisition of virulence-related gene islands are examples of a few documented evolutionary events that potentially changed the course of the disease scenario [2, 4, 6–9]. Recent evolutionary genetic changes in V. cholerae O1 El Tor include several non-synonymous mutations in the cholera toxin B (ctxB) gene, resulting in alleles such as ctxB1 and ctxB7 [9, 10]. With documentation of the emerging new alleles, there are records of notable events such as periodic switching of serotype and ctxB genotype. For instance, following the spread of the Haitian variant ctxB allele, there was evidence of switching of the classical and Haitian ctxB in endemic regions [11]. As far we know, there is no evidence of an association between these two genotypes and the periodic switching with cholera outbreak in Bangladesh. Similarly, serotype conversion from Inaba to Ogawa, and back to Inaba again was also documented for many regions [11, 12]. There have been a few speculations to describe this periodic serotype switching, which presumably is attributable to the selection pressures prevailing in the environments [11]. Although the epidemiological implications of these switching are yet to be understood, these events are presumed to have a definite genetic basis and could therefore contribute to the infectious propensity of V. cholerae O1 [12].
Cholera intervention includes treatment of the affected individuals with an effective antibiotic alongside the oral rehydration therapy, although V. cholerae has already developed resistance towards a number of antimicrobials posing new challenges in the clinical management of cholera. Such antibiotic resistance is associated with the acquisition of mobile genetic elements such as the SXT integrative and conjugative element (ICE) by V. cholerae. The SXT-ICE contributes not only to the spread of antimicrobial resistance genes, but also drives V. cholerae diversity [13]. In the present study, V. cholerae O1 associated with endemic cholera in Dhaka during 2015–2021, were analyzed for major phenotypic (culture, serology, and antimicrobial susceptibility assay) and genetic characteristics, including the drug response pattern. The results presented in this study highlight a few notable temporal changes in the genotypic and phenotypic characteristics, including the emergence of an extreme drug-resistant V. cholerae O1 strain, providing new insights that could aid the prevention of cholera in Bangladesh.
Methods
Bacterial strains
In this study, a set of 172 Vibrio cholerae O1 strains were isolated from clinical samples collected in Dhaka, Bangladesh, between 2015 and 2021, as a part of an epidemiological surveillance conducted by the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b). The strains were isolated from the stool of suspected cholera patients seeking treatment at icddr,b Dhaka Hospital. Informed consent was obtained from the patients or legal guardians for minor patients prior to the collection of stool samples. For the years 2015, 2016, and 2017, we tried to select strains covering each month of the year, given that V. cholerae O1 was isolated in those months. For the years 2018–2020, the rate of isolation of V. cholerae O1 decreased drastically. Therefore, we selected almost all the strains that were isolated in those years. V. cholerae O1 isolation rate increased again in 2021 and we selected strains representing each month until the studied period. Isolation and identification of V. cholerae were performed according to standard cultural and molecular methods as described previously [14]. Genomic DNA was extracted from the strains following boiling lysis method, described elsewhere [5]. Additionally, genomic DNA of the XDR strain was extracted using Qiagen Blood and Tissue Kit as per the manufacturer’s instructions.
Serogrouping
The serogroup of the V. cholerae isolates was confirmed by slide agglutination test, using V. cholerae O1 and O139 specific polyvalent antisera and were tested further using serotype specific monoclonal antibodies, Inaba and Ogawa as described previously [5]. For molecular confirmation of serogroups, and detection of cholera toxin, multiplex PCR targeting O1-(wbe), O139-(wbf), and ctxA genes were performed [10].
Antibiotic susceptibility assay
Bacterial susceptibility to antimicrobials was determined by standard disc diffusion test on Muller-Hinton agar (BD, USA) according to Clinical and Laboratory Standards Institute guidelines as described previously [5]. All strains of V. cholerae were tested for susceptibility to ampicillin (AMP, 10 μg), ceftriaxone (CRO, 30 μg), ciprofloxacin (CIP, 5 μg), mecillinam (MEL, 25 μg), erythromycin (E, 15 μg), nalidixic acid (NA, 30 μg), imipenem (IMP, 10 μg), sulfamethoxazole/trimethoprim (SXT, 25 μg), streptomycin (S, 10 μg), levofloxacin (LEV, 5 μg), cephalothin (KF, 30 μg), cefixime (CFM, 5 μg), cefepime (FEP, 30 μg), tetracycline (TE, 30 μg), aztreonam (ATM, 30 μg), azithromycin (AZM, 15 μg), chloramphenicol (C, 30 μg), and gentamicin (CN, 10 μg) using commercially available discs (BD BBL SensiDisc). The minimum inhibitory concentration (MIC) was determined by E-test (Biomeuriex). Suspected Extended Spectrum Beta Lactamase (ESBL) producing V. cholerae isolates were screened for the production of ESBLs by using a double disk synergy test (DDST) [15].
Polymerase chain reaction (PCR) assay
PCR assays were performed to detect genes encoding zonula occludens toxin (zot), accessory cholera enterotoxin (ace), hemolysin (hlyA), SXT-related integrase (intSXT) and biotype-specific (El Tor and Classical) phage encoded repressor (rstR), toxin coregulated pilus (tcpA), and repeat in toxin (rtxC) using primers and conditions described previously [5]. All antibiotic-resistant V. cholerae O1 strains were tested for gene encoding the class 1 and class 2 integron using primers and procedures described elsewhere [16]. Double mismatch amplification mutation assay (DMAMA)-PCR was performed to determine three genotypes of the cholera toxin gene, Classical (ctxB genotype 1, ctxB1), El Tor (ctxB genotype 3, ctxB3), and Haitian type (ctxB genotype 7, ctxB7) based on nucleotide substitution at position 58, 115, and 203 [17]. V. cholerae O1 strains O395 (Classical), N16961 (El Tor), and EL-1786 (Haitian variant, ctxB7) were used as control for the PCR.
PCR was also performed to detect genes for ESBL, AmpC, carbapenemase, and quinolone resistance [18–22]. Specifically, multiplex PCR (6 sets) was performed to detect ESBL genes blaTEM/blaSHV/blaOXA-1-like genes, blaCTX-M including phylogenetic groups 1, 2 and 9, carbapenemase genes blaVEB/blaGES/blaPER, blaVIM/blaIMP/blaKPC, blaGES/blaOXA-48- like genes, and the AmpC group of genes blaMOX/blaFOX/blaEBC/blaCIT/blaDHA/blaACC. Simplex PCR was used to detect the following genes: ESBL (blaCTX-M-8/-25) [18]; carbapenemase (blaAIM, blaGIM, blaSIM, and blaDIM) [19]; blaNDM [22], quinolone resistance related qnrVC [20], efflux pump encoding qepA, acetylase encoding aac(6’)-Ib-cr [21].
Sequencing of antibiotic resistant genes
PCR amplified antibiotic resistant genes were sequenced using an ABI Big-Dye Terminator v.3.1 Cycle Sequencing Reaction kit (Applied Biosystems) on an ABI PRISM 3500 XL genetic analyzer (AppliedBiosystems, Foster City, USA). The BLASTN program (www.ncbi.nlm.nih.gov/BLAST) was used for homology searching. The partial sequences of the genes were submitted to GenBank (Accession Numbers: MK992813, MK992814, MK992815).
MLST and MLVA typing
We examined the draft genome assemblies of the 30 recently sequenced strains from 2018 to 2019 [23] and determined their multi-locus sequence type (MLST) based on seven housekeeping genes, adk, gyrB, mdh, metE, pntA, purM, and pyrC, using PubMLST scheme [24]. Additionally, multiple-locus variable-number tandem repeat (MLVA) analysis of selected isolates were performed based on five MLVA loci (VC01747, VC0436, VC1650, VCA0171 & VCA0283) following methods as described previously [25].
Results
Phenotypic and genotypic characteristics
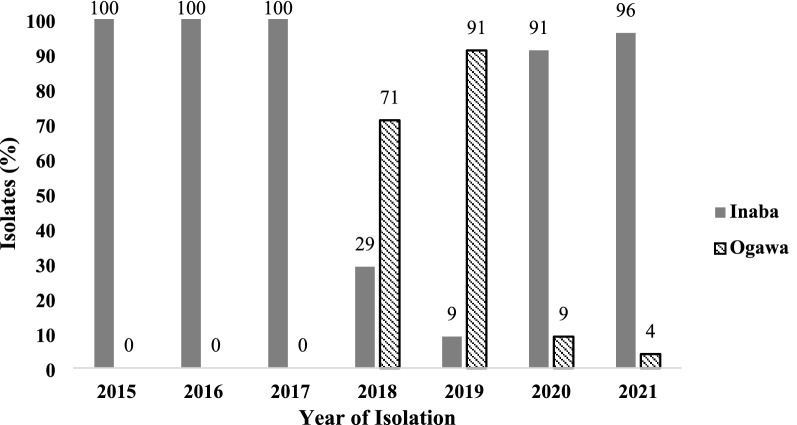
All of the total 172 isolates tested produced characteristic colonies typical of V. cholerae on both thiosulfate citrate bile-salts sucrose (TCBS) agar and taurocholate tellurite gelatin agar (TTGA). Positive agglutination with a polyvalent antibody for V. cholerae serogroup O1 followed by positive agglutination with monovalent Inaba (143/172) and Ogawa antisera (29/172) confirmed both Inaba and Ogawa serotypes were associated with endemic cholera in Dhaka, Bangladesh (Table 1). Although, the serotypes of V. cholerae strains differed temporally in predominance, one or the other of the two serotypes dominated in clinical cholera cases in a given year. For instance, Inaba was exclusively found to be associated with cholera in the first 3 years (2015–2017) while Ogawa was not found among the strains tested during this period. Thereafter, Ogawa was found to be the predominant serotype (71–91%) in 2018–2019, which then dipped to a low (9–4%) in 2020–2021 with the sharp rise of the Inaba serotype strains (91–96%) (Fig. 1). Overall, Inaba was the predominant serotype among the V. cholerae O1 strains associated with cholera for most of the study period.
Table 1.
Genetic characteristics and drug resistance profile of Vibrio cholerae O1 strains isolated from Dhaka, 2015–2021
| Year of isolation | No. of strains | Serotype | wbeO1 | ace | zot | tcpA | rtxC | ctxA | ctxB type | rstR | hlyA | intSXT | intl1 | Drug resistance profile | Deduced genetic characteristics and AMR profile | MLST type | MLVA type |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 16 | Inaba | + | + | + | ET | + | + | B1 | ET | ET | + | – | NA, S, SXT | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Inaba, ctxB1, intSXT + , intl1–, NA, S, SXT | ND | ND |
| 1 | Inaba | + | + | + | ET | + | + | B7 | ET | ET | + | – | NA, S, SXT | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Inaba, ctxB7, intSXT + , intl1–, NA, S, SXT | ND | ND | |
| 3 | Inaba | + | + | + | ET | + | + | B1 | ET | ET | – | – | NA | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Inaba, ctxB1, intSXT–, intl1–,NA | ND | ND | |
| 2016 | 29 | Inaba | + | + | + | ET | + | + | B1 | ET | ET | + | – | NA, S, SXT | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Inaba, ctxB1, intSXT + , intl1–, NA, S, SXT | ND | ND |
| 1 | Inaba | + | + | + | ET | + | + | B7 | ET | ET | + | – | NA, S, SXT | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Inaba, ctxB7, intSXT + , intl1–, NA, S, SXT | ND | ND | |
| 1 | Inaba | + | + | + | ET | + | + | B1 | ET | ET | – | – | NA | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Inaba, ctxB1, intSXT–, intl1–, NA | ND | ND | |
| 2017 | 32 | Inaba | + | + | + | ET | + | + | B1 | ET | ET | + | – | NA, S, SXT | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Inaba, ctxB1, intSXT + , intl1–, NA, S, SXT | ND | ND |
| 2018 | 2 | Inaba | + | + | + | ET | + | + | B1 | ET | ET | + | – | NA, SXT | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Inaba, ctxB1, intSXT + , intl1–, NA, SXT | ST69 | 1 |
| 1 | Inaba | + | + | + | ET | + | + | B1 | ET | ET | + | – | NA, SXT | ST69 | 4 | ||
| 1 | Inaba | + | + | + | ET | + | + | B1 | ET | ET | + | – | NA, S, SXT | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Inaba, ctxB1, intSXT + , intl1–, NA, S, SXT | ST69 | 1 | |
| 1 | Inaba | + | + | + | ET | + | + | B1 | ET | ET | + | – | NA, S, SXT | ST69 | 2 | ||
| 1 | Ogawa | + | + | + | ET | + | + | B7 | ET | ET | + | – | NA | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Ogawa, ctxB7, intSXT + , intl1–, NA | ST69 | 5 | |
| 3 | Ogawa | + | + | + | ET | + | + | B7 | ET | ET | + | – | NA, S, SXT | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Ogawa, ctxB7, intSXT + , intl1–, NA, S, SXT | ST69 | 3 | |
| 4 | Ogawa | + | + | + | ET | + | + | B7 | ET | ET | + | – | NA, S, SXT | ST69 | 5 | ||
| 1 | Inaba | + | + | + | ET | + | + | B7 | ET | ET | + | + | NA, S, SXT | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Inaba, ctxB7, intSXT + , intl 1 + , NA, S, SXT | ND | ND | |
| 3 | Ogawa | + | + | + | ET | + | + | B7 | ET | ET | + | – | NA, S, SXT, MEL | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Ogawa, ctxB7, intSXT + , intl1–, NA, S, SXT, MEL | ST69 | 3 | |
| 2 | Ogawa | + | + | + | ET | + | + | B7 | ET | ET | + | – | NA, S, SXT, MEL | ST69 | 5 | ||
| 1 | Ogawa | + | + | + | ET | + | + | B7 | ET | ET | + | + | NA, S, SXT, MEL | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Ogawa, ctxB7, intSXT + , intl1 + , NA, S, SXT, MEL | ST69 | 5 | |
| 1 | Ogawa | + | + | + | ET | + | + | B7 | ET | ET | + | + | NA, S, SXT | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Ogawa, ctxB7, intSXT + , intl1 + , NA, S, SXT | ST69 | 6 | |
| 2019 | 5 | Ogawa | + | + | + | ET | + | + | B7 | ET | ET | + | – | NA, S, SXT | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Ogawa, ctxB7, intSXT + , intl1–, NA, S, SXT | ST69 | 5 |
| 3 | Ogawa | + | + | + | ET | + | + | B7 | ET | ET | + | – | NA, S, SXT | ST69 | 3 | ||
| 1 | Ogawa | + | + | + | ET | + | + | B7 | ET | ET | + | + | NA, S, SXT | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Ogawa, ctxB7, intSXT + , intl1 + , NA, S, SXT | ST69 | 5 | |
| 1 | Ogawa | + | + | + | ET | + | + | B7 | ET | ET | + | + | NA, S, SXT | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Ogawa, ctxB7, intSXT + , intl1 + , NA, S, SXT | ND | ND | |
| 1 | Inaba | + | + | + | ET | + | + | B1 | ET | ET | + | – | KF, S, CFM, CRO, NA, SXT, FEP, MEL, CIP, AMP, ATM | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Inaba, ctxB1, KF, S, CFM, CRO, NA, SXT, FEP, MEL, CIP, AMP, ATM | ST69 | 2 | |
| 2020 | 31 | Inaba | + | + | + | ET | + | + | B7 | ET | ET | + | – | NA, S, SXT | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Inaba, ctxB7, intSXT + , intl1–, NA, S, SXT | ND | ND |
| 3 | Ogawa | + | + | + | ET | + | + | B7 | ET | ET | + | – | NA, S, SXT | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Ogawa, ctxB7, intSXT + , intl1–, NA, S, SXT | ND | ND | |
| 2021 | 22 | Inaba | + | + | + | ET | + | + | B7 | ET | ET | + | – | AMP, NA, S, SXT | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Inaba, ctxB7, intSXT + , intl1–, AMP, NA, S, SXT | ND | ND |
| 1 | Ogawa | + | + | + | ET | + | + | B7 | ET | ET | + | – | AMP, NA, S, SXT | wbeO1 + , ace + , zot + , tcpA(ET), rtxC + , ctxA + , rstR(ET), hlyA(ET), Ogawa, ctxB7, intSXT + , intl1-, AMP, NA, S, SXT | ND | ND |
*ET El Tor, ND Not Done, NA nalidixic acid, S streptomycin, SXT sulfamethoxazole/trimethoprim, KF cephalothin, CFM cefixime, CRO ceftriaxone, FEP cefepime, MEL mecillinam, CIP ciprofloxacin, AMP ampicillin, ATM aztreonam
Fig. 1.
Temporal distribution of serotypes among Vibrio cholerae O1 strains isolated from Dhaka, 2015–2021
Genomic DNA of all of the isolates (n = 172) supported the amplification of V. cholerae species-specific gene, ompW, and O1-antigen biosynthetic gene, wbe (O1), further confirming that the bacteria were V. cholerae O1. All V. cholerae isolates amplified the CTX-prophage-mediated genes ctxA, ace, and zot, confirming that all of the isolates were toxigenic and harbored CTX-prophage in their genome (Table 1).
All of the V. cholerae isolates tested carried the El Tor biotype-specific genes for tcpA, rstR and rtxC. V. cholerae strains, classified as hybrid El Tor may carry both rstREl Tor and rstRClassical in the same genome. However, none of the isolates in this study were found to carry either rstRClassical or both rstREl Tor and rstRClassical together, only rstREl Tor was found (Table 1). Gene for cholera toxin B-subunit of classical biotype (ctxB1) and Haitian variant type (ctxB7) was detected in 87 and 85 V. cholerae O1 isolates, respectively (Table 1). PCR results suggested that all the V. cholerae O1 strains in this study were atypical El Tor, carrying either ctxB1 or ctxB7 and rstREl Tor.
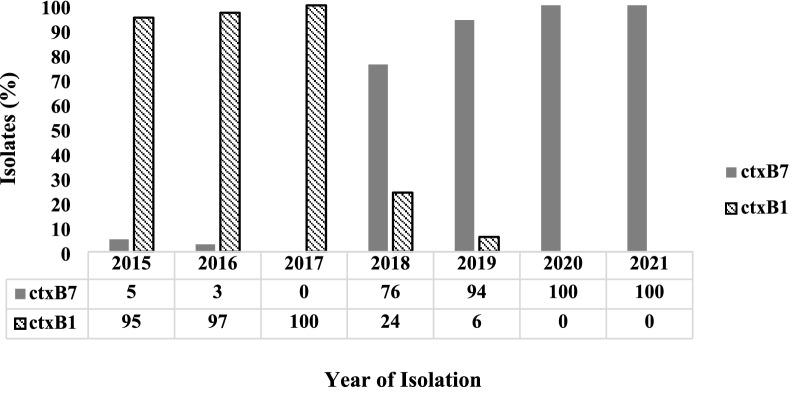
The ctxB genotype also showed a temporal shift in predominance during the study period. The classical biotype cholera toxin, ctxB1, was predominant having been detected in 95% (n = 19/20), 97% (n = 30/31), and 100% (n = 32/32) of the strains isolated from cholera patients in 2015, 2016 and 2017, respectively (Fig. 2). The Haitian variant cholera toxin, ctxB7, was found only in 5% (n = 1/20) and 3% (n = 1/31) V. cholerae O1 clinical strains in 2015 and 2016, respectively, while it was not detected in the strains associated with cholera in 2017. Subsequently, V. cholerae strains associated with cholera in 2018 and 2019 carried ctxB7 amongst 76% (n = 16/21) and 94% (n = 10/11) of the strains, but ctxB1 was detected only in 24% (n = 5/21) and 6% (n = 1/11) of the strains, respectively. All of the V. cholerae strains tested in 2020–2021 carried ctxB7 (n = 23/23, 100%) as the predominant type in Dhaka (Fig. 2).
Fig. 2.
Temporal distribution of ctxB alleles among Vibrio cholerae O1 strains isolated from Dhaka, 2015–2021
Antimicrobial susceptibility patterns
Overall, antimicrobial susceptibility pattern of the studied strains fluctuated slightly across the years. The majority of the strains were multi drug resistant (MDR), showing resistance to at least three different antibiotics (Table 1). Eighteen different antibiotics were tested and 97% (n = 167/172) of the strains circulating among cholera patients in the last 7 years were MDR. All of the isolates were resistant to nalidixic acid (NA), and all, except one strain, sensitive to azithromycin (AZM), erythromycin (E), cephalothin (KF), cefixime (CFM), ceftriaxone (CRO), cefepime (FEP), imipenem (IMP), tetracycline (TE), ciprofloxacin (CIP), levofloxacin (LEV), chloramphenicol (C), gentamicin (CN) and aztreonam (ATM). A unique resistance profile was observed for one of the tested isolates, which was resistant to eleven of the eighteen antibiotics tested, namely ampicillin (AMP), CRO, CIP, mecillinam (MEL), NA, sulfamethoxazole/trimethoprim (SXT), streptomycin (S), KF, CFM, FEP, and ATM. It is important to note that the MIC of the fourth-generation cephalosporin (FEP) for this strain was determined to be ≥ 256 μg/mL. The double disc synergy test (DDST) for ESBL detection was also positive for this strain indicating the production of ESBL.
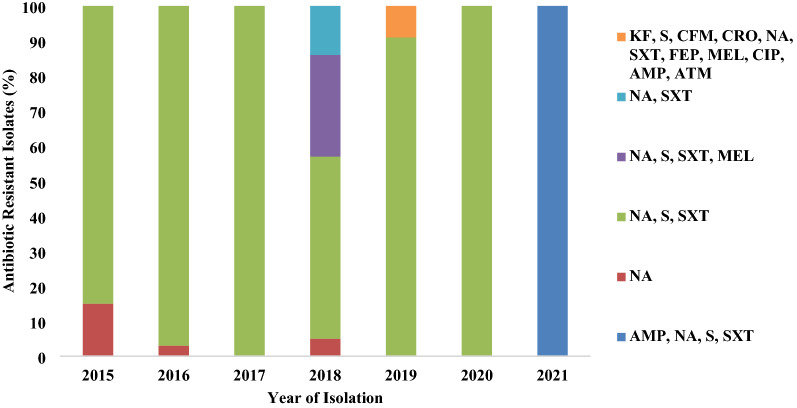
Six distinct resistance profiles were found among the 172 isolates, with a combination of resistance against one to eleven antibiotics during 2015–2021, as shown in Fig. 3. Resistance against S, NA, and SXT was most frequently detected in individual V. cholerae isolated from 2015 to 2021 (Fig. 3). Co-occurrence of resistance against S, NA, and SXT has been observed in 84%, 97%, 97%, 54%, 89%, and 97% of the strains isolated from cholera cases during 2015, 2016, 2017, 2018, 2019, and 2020, respectively (Fig. 3). Fluctuation in susceptibility profile was found higher in 2018 when a proportion (29%) of the strains acquired resistance against MEL, which was not recorded in any other year. Contrary to what we observed in 2015–2020, all of the strains associated with cholera were found to have acquired resistance against ampicillin (AMP) in 2021. All of the tested V. cholerae strains in 2021 were resistant against AMP, NA, S, and SXT (Fig. 3).
Fig. 3.
Antibiotic resistance profile of Vibrio cholerae O1 strains isolated from Dhaka, 2015–2021. *NA, nalidixic acid; S, streptomycin; SXT, sulfamethoxazole/trimethoprim; KF, cephalothin; CFM, cefixime; CRO, ceftriaxone; FEP, cefepime; MEL, mecillinam; CIP, ciprofloxacin; AMP, ampicillin; ATM, aztreonam
Most of the strains carried intSXT (168/172) gene encoding integrase enzyme of the SXT, a mobile genetic element carrying multidrug resistance gene cassettes [10]. Only 3% of the tested strains harbored intl1 in their genomes, confirming the presence of class 1 integron. None of the tested strains in the present study was found to carry the intl2 gene encoding class 2 integron (Table 1).
PCR assays and sequencing of genes responsible for antibiotic resistance
PCR assays for the detection of all of the targeted ESBL genes in the ESBL producing V. cholerae O1 isolate (DP19) were negative except blaPER-3 (520 bp) and blaOXA-1 (564 bp). The strain did not carry any of the genes responsible for quinolone and fluoroquinolone resistance, except for aac(6’)-Ib-cr (482 bp) encoding the enzyme acetylase. None of the genes for AmpC group and carbapenemase were detected by PCR assays.
Next, PCR amplicons of the blaPER-3, blaOXA-1, and aac(6´)-Ib-cr genes were sequenced. BLAST homology search revealed that the deduced sequence of 458 bp blaPER-3 (Accession No.MK992813) gene had 100% identity with blaPER-3 gene of Aeromonas caviae reported from China [26]. The sequence of a 496 bp blaOXA-1 (Accession No.MK992814) detected in this study had 99.80% identity with blaOXA-1 gene found in Proteus spp. isolated in China [27]. The DNA sequence of a 415 bp fragment of aac(6´)-Ib-cr (Accession No. MK992815) found in V. cholerae was identical to that of the aac(6´)-Ib-cr existing in Aeromonas spp. and Shigella spp. isolated from water samples [28].
MLST and MLVA profiles
To determine the genetic relatedness among the isolates we performed molecular fingerprinting analysis on selected strains using Multi Locus Sequence Typing (MLST). We leveraged the genome sequencing data of 30 isolates (20 isolated in 2018, and 10 in 2019) published elsewhere by Monir et al. [23] to deduce their MLST and multi-locus variable number tandem repeat analysis (MLVA) types. MLST typing results based on seven housekeeping genes, adk, gyrB, mdh, metE, pntA, purM, and pyrC showed that all of the tested strains belong to sequence type (ST)—69 (Table 1). On the other hand, MLVA results of the tested strains revealed 6 different profiles indicating better discriminatory power of the tool in comparison to MLST for this setting.
Discussion
Vibrio cholerae O1 biotype El Tor, the causative agent of the ongoing 7th cholera pandemic, continues to evolve and cause more severe disease acquiring the classical biotype cholera toxin (ctxB1 and ctxB7), and related classical biotype attributes under the El Tor biotype background [4, 9, 29]. The present research provides insights into the current scenario of cholera in Dhaka, Bangladesh showing the temporal changes in serotype and molecular profiles, including the antimicrobial resistance (AMR) patterns of V. cholerae O1 biotype El Tor strains associated with endemic cholera from 2015 to 2021. Our results reaffirm that endemic cholera occurs each year in Bangladesh with V. cholerae O1 biotype El Tor strains showing temporal shifts in serotype, ctxB genotype, and AMR profile, and call for routine monitoring to aid intervention and preventive measures against the persistent disease resulting in significant morbidity and mortality each year in Bangladesh.
In V. cholerae, serotype switching from Inaba to Ogawa, and back to Inaba, occurs in every 2–3 years [11, 12], although the epidemiological significance of the sero-switching is not fully understood for cholera. In the present study, a temporal shift in the predominance of V. cholerae serotype was observed twice during 2015–2021, once from Inaba to Ogawa in 2018, and Ogawa to Inaba in 2020. V. cholerae associated with endemic cholera in Dhaka had also shown transient switching of ctxB1 to ctxB7 during 2018–2019, as reported earlier from India, and then in Bangladesh in the subsequent years [9, 10]. It is therefore, evident from the results presented in this study that V. cholerae O1 strains carrying ctxB7, which is found in association with cholera in Haiti (Haitian variant), was responsible for the recent cholera outbreaks in Dhaka. Our data appear in agreement with reports from other regions of Asia and Africa [30]. Several previous reports and analysis on the origin of Haitian cholera outbreak suggested that ctxB7 genotype existed in South Asia before 2010. In fact, it was shown that the strains carrying ctxB7 allele originated in South Asia. For example, there are a few studies which reported that ctxB7 allele was circulating among V. cholerae O1 outbreak causing strains isolated from Kolkata in 2006 and Orissa in 2007 [17]. Epidemiological and phylogenetic analysis particularly supported the hypothesis that strains introduced from Nepal were responsible for the Haitian outbreak in 2010 [7]. Although the biological significance of these mutations in ctxB allele is not fully defined, phenotypic analysis revealed that Haitian stains have increased production of cholera toxin and other virulence factors thus contributing to the infectious propensity of V. cholerae O1 [31].
When the relatedness of the strains from the transition period (2018–2019) was compared using multi-locus sequence typing (MLST), all isolates belonged to the same MLST type—ST69 (Table 1). This is in agreement with previous report on clinical V. cholerae O1 isolated from an urban community in Dhaka, which also belonged to ST69 [32]. Recent studies in Asia and Africa also reported that the majority of the seventh pandemic El Tor (7PET) V. cholerae O1 strains belongs to ST69 [33, 34]. It corroborates the observation that the recent epidemic cholera outbreak in Dhaka is predominantly caused by the 7PET lineage, although further study is needed to confirm the clonal relatedness of V. cholerae strains responsible for endemic cholera in Bangladesh. Our multi-locus variable-number-tandem-repeat analysis (MLVA) results however differentiated the ST69 strains in the present study into 6 different MLVA types. Although whole genome analysis would be needed to better understand the relatedness, a recent study conducted on V. cholerae El Tor isolated from clinical and water sources in Dhaka, Bangladesh, showed similar results with 124 distinct MLVA types from among 621 strains [35].
Temporal switching of serotype and ctxB allele is common among V. cholerae O1 strains associated with endemic cholera. These strains can usually be divided into two clades at a genomic level—one clade represents strains predominantly having Inaba serotype and carrying ctxB1 allele while the other clade includes strains of the Ogawa serotype carrying ctxB7 allele [12, 36]. As a result, switching of serotype and ctxB allele usually occurs simultaneously. Similarly, here we noted that during the transition period of serotype conversion and ctxB allele switching, the strains had a change in the predominance of serotypes from Inaba to Ogawa in 2018, which continued until 2019, while ctxB genotype switching also occurred from ctxB1 to ctxB7 during the same period. However, in 2020, Inaba serotype became the predominant serotype again, but ctxB allele switching did not occur. As a result, ctxB7, instead of ctxB1, were found to be the predominant ctxB genotype among Inaba strains. These results suggest that the serotype switching is most likely to be an independent event, not causally related to ctxB allele switching.
Antibiotic use is a convention for the clinical management of cholera. For this, it is essential to have an up-to-date surveillance data of antibiotic susceptibility patterns of the pathogen, which tend to change over time [5]. V. cholerae is a resident flora of wide ranging natural aquatic environments [37, 38], which usually lack antibiotics and related selection pressure essential for the bacterium to steadily carry antibiotic resistance-related plasmids and other mobile genetic elements. As a result, the organism tends to have a fluctuating antibiotic susceptibility pattern. However, owing to the overuse of antibiotics, recent environmental and clinical V. cholerae strains appear to be multi-drug resistant acquiring different resistance genes [39]. Such acquisition of resistance and related genes can be both temporary and persisting, as demonstrated by the results presented in this study. For example, tetracycline (TE) was the priority drug widely used for the treatment of cholera, but its clinical use was reduced drastically because of the emergence of TE resistant V. cholerae strains in Asia and Africa [5]. The recently isolated V. cholerae O1 strains in South Asia, including Bangladesh, were found to be susceptible against TE [40–42]. In the present study all of the V. cholerae isolates tested were susceptible towards TE. This could be due to decrease in the use of TE and lack of antibiotic selection pressure [43, 44]. Nonetheless, the increasing susceptibility of V. cholerae to TE indicates the effectiveness of this highly cost-effective drug to be in use again for the treatment of cholera. The transient resistance observed against other antibiotics such as mecillinam and ampicillin indicates exposure of V. cholerae O1 to these drugs. Consistent with the results from other studies [5, 42], the observed high susceptibility of V. cholerae O1 to azithromycin, chloramphenicol, ciprofloxacin, cephalosporins and carbapenems in the present study shows promise for these drugs to be used for treating cholera.
Recent studies have reported compromised drug susceptibility pattern of V. cholerae O1 against newer drugs, including those which are not commonly used for the treatment of cholera [41, 42, 45]. For example, recently isolated V. cholerae O1 strains were reported to be resistant against extended spectrum beta-lactam antibiotics, such as the fourth generation cephalosporins. Although the underlying molecular mechanism for the drug resistance is yet to be elucidated, it is presumed to be attributed to the activity of either extended spectrum of β-lactamase (ESBL) or AmpC β-lactamase [41, 45]. Beta-Lactams are broad-spectrum drugs used to treat a wide range of infectious diseases and in post-operative infection management [46]. Thus, increasing resistance against beta-lactams and carriage of multiple beta-lactamase genes could not only hinder the clinical management of cholera but also pose a major threat for other infection control measures. In the present study, we report cholera caused by the lone V. cholerae O1 strain that was resistant towards eleven different antibiotics, including the fourth generation cephalosporin (FEP), and carrying ESBL encoding genes blaPER-3 and blaOXA-1 together with fluoroquinolone resistance gene aac(6´)-Ib-cr. To our knowledge, this is the first report of the association of an extensively drug resistant (XDR) V. cholerae O1 with cholera in Bangladesh. Previously, chromosomal integration of blaNDM-1 was reported from the genome of an XDR V. cholerae non-O1/O139 strain associated with diarrhea in India [46]. Although V. cholerae can develop AMR via single or multiple point mutations in the chromosome, the bacterium can gain resistance genes via horizontal gene transfer (HGT) from other populations sharing niches with them [46]. Owing to the high genetic plasticity of V. cholerae, MDR/XDR strains can emanate through the acquisition of extrachromosomal mobile genetic elements such as self-replicating plasmids or integrative mobile genetic elements (MGEs) including SXT integrating conjugative elements (SXT ICEs), class 1 integrons, and transposable genetic elements [43, 46].
In Silico resistome analysis revealed that the XDR isolate (DP19) possessed a bunch of genes related to antimicrobial resistance phenotypes: aac(6')-Ib-cr5, aph(3'')-Ib, aph(6)-Id, blaOXA-1, blaPER-3, catB3, catB9, dfrA1, dfrA15, mph(A), sul1, sul2, tetA(D), and varG. blaOXA-1 found in the strain showed 99.88% nucleotide sequence identity with the plasmid-borne gene found in several enteric bacteria such as Escherichia coli, Kleibsiella pneumoniae, Shigella, and Citrobacter. blaPER-3 showed 100% nucleotide sequence similarity with the plasmid borne gene found in a Vibiro parahaemolyticus isolate (GenBank: KY014464.1). In addition to the blaOXA-1 and blaPER-3 beta-lactamase genes, for several other antibiotic resistance genes (catB3, aac(6')-Ib-cr5, dfrA1, dfrA15, mph(A), tetA(D), aph(6)-Id, aph(3'')-Ib, sul2, and sul1), identical homologues found in the database are all listed as plasmid borne sourcing in marine and enteric bacteria. These indicates that the resistance related gene cluster in the XDR strain of this study might also be plasmid mediated. However, we could not isolate any plasmid from the strain using plasmid extraction kit as well as following the standard alkaline lysis method. There is a possibility of the presence of a large plasmid and perhaps modification of the protocol would enable isolation of plasmid from the strains, if there is any. In the present study, we also detected SXT-related integrase (intSXT) in all of the V. cholerae isolates that were resistant to S and SXT, indicating the possible role of SXT/R391 ICE in carrying multiple drug resistance genes for the bacterium.
Conclusion
The treatment regimen and prevention of cholera relies greatly on deciphering the dynamics of genotypic and phenotypic characteristics, of V. cholerae, the causative agent of cholera. In this study, we present data on the genotypic and phenotypic characteristics, including the serotype profiles and drug resistance of V. cholerae O1 associated with endemic cholera in Dhaka, Bangladesh, between 2015 and 2021. This study provides new insights on the temporal genetic changes in serotype, genotypes of major virulence genes, and drug resistance profiles of V. cholerae, including the emergence of MDR/XDR V. cholerae resistant to eleven of eighteen tested drugs including resistance to fourth-generation cephalosporin (cefepime) and aztreonam, which would be important in designing therapeutic intervention and preventive measures for the deadly disease in the global hotspot of transmission. The observed serotype switching occurred among the strains with the Inaba serotype predominant over Ogawa for most of the 7 year study period from 2015 to 2021. Also, we have recorded the transition of the ctxB genotype in 2018 from ctxB1 to ctxB7, which became predominant in 2019, and continued as the predominant ctxB genotype until 2021. Though it is difficult to foresee how the changing genotype and phenotypic characteristics of V. cholerae O1 will affect cholera epidemiology in this region, our results underscore the need for routine monitoring to decide on effective intervention and preventive measures against cholera.
Acknowledgements
icddr,b gratefully acknowledges the following donors who provide unrestricted support: Governments of Bangladesh, Canada, Sweden and the UK.
Author contributions
FTJ and KSN: conceptualization, data curation, formal analysis, investigation, methodology, writing—original draft; IB: conceptualization, data curation, formal analysis, investigation, methodology, writing—review and editing; FTJ: investigation, methodology; MTI: investigation, writing—review and editing; MS: investigation; WU, JT, SRB and MMM: methodology, writing—review and editing; CMG, AC, NA, AGR and JDC: writing—review and editing; MA: conceptualization, supervision, writing—review and editing. All authors read and approved the final manuscript.
Funding
This research was partially supported by the Foreign, Commonwealth and Development Office (FCDO/Wellcome) (215704/Z/19/Z), icddr,b, Tufts University, USA and University of California, Berkeley, USA.
Availability of data and materials
The PCR amplicon sequence data of blaPER-3, blaOXA-1, and aac(6´)-Ib-cr genes were deposited in NCBI Gene Bank under Accession No. MK992813, MK992814 and MK992815, respectively. All the data are available on request from the corresponding author.
Declarations
Ethics approval and consent to participate
Ethical approval and consent to participate was obtained from the ethical committee of International Center for Diarrheal Disease Research, Bangladesh (icddr, b).
Consent for publication
Yes.
Competing interests
NA is the Editor in Chief of Gut Pathogens but he did not oversee peer review and/or decision-making process for this article. All other authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fatema Tuz Jubyda, Kazi Sumaita Nahar and Indrajeet Barman contributed equally to this article
References
- 1.Kaper JB, Morris JG, Levine MM. Cholera. Clin Microbiol Rev. 1995;8(1):48–86. doi: 10.1128/CMR.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mutreja A, Kim DW, Thomson NR, Connor TR, Lee JH, Kariuki S, et al. Evidence for several waves of global transmission in the seventh cholera pandemic. Nature. 2011;477(7365):462–465. doi: 10.1038/nature10392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sack DA, Sack RB, Nair GB, Siddique AK. Cholera. Lancet. 2004;363(9404):223–233. doi: 10.1016/S0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- 4.Safa A, Nair GB, Kong RYC. Evolution of new variants of Vibrio cholerae O1. Trends Microbiol. 2010;18(1):46–54. doi: 10.1016/j.tim.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Rashed SM, Hasan NA, Alam M, Sadique A, Sultana M, Hoq MM, et al. Vibrio cholerae O1 with reduced susceptibility to ciprofloxacin and azithromycin isolated from a rural coastal area of Bangladesh. Front Microbiol. 2017 doi: 10.3389/fmicb.2017.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim EJ, Lee CH, Nair GB, Kim DW. Whole-genome sequence comparisons reveal the evolution of Vibrio cholerae O1. Trends Microbiol. 2015;23(8):479–89. doi: 10.1016/j.tim.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh P, Naha A, Pazhani GP, Ramamurthy T, Mukhopadhyay AK. Genetic traits of Vibrio cholerae O1 haitian isolates that are absent in contemporary strains from Kolkata India. PLoS ONE. 2014;9(11):112973. doi: 10.1371/journal.pone.0112973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar P, Mishra DK, Deshmukh DG, Jain M, Zade AM, Ingole KV, et al. Vibrio cholerae O1 Ogawa El Tor strains with the ctxB7 allele driving cholera outbreaks in south-western India in 2012. Infect Genet Evol. 2014;1(25):93–96. doi: 10.1016/j.meegid.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Rashid MU, Rashed SM, Islam T, Johura FT, Watanabe H, Ohnishi M, et al. CtxB1 outcompetes CtxB7 in Vibrio cholerae O1 Bangladesh. J Med Microbiol. 2016;65(1):101–103. doi: 10.1099/jmm.0.000190. [DOI] [PubMed] [Google Scholar]
- 10.Rashed SM, Mannan SB, Johura tuz F, Tarequl Islam M, Sadique A, Watanabe H, et al. Genetic characteristics of drug-resistant Vibrio cholerae O1 causing endemic cholera in Dhaka, 2006–2011. J Med Microbiol. 2012;61(PART12):1736–45. doi: 10.1099/jmm.0.049635-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlsson SL, Thomson N, Mutreja A, Connor T, Sur D, Ali M, et al. Retrospective analysis of serotype switching of Vibrio cholerae O1 in a cholera endemic region shows it is a non-random process. PLoS Negl Trop Dis. 2016;10:10. doi: 10.1371/journal.pntd.0005044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baddam R, Sarker N, Ahmed D, Mazumder R, Abdullah A, Morshed R, et al. Genome dynamics of Vibrio cholerae isolates linked to seasonal outbreaks of cholera in Dhaka Bangladesh. MBio. 2020;11:1. doi: 10.1128/mBio.03339-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeGault KN, Hays SG, Angermeyer A, McKitterick AC, Johura FT, Sultana M, et al. Temporal shifts in antibiotic resistance elements govern phage-pathogen conflicts. Science. 2021;373(6554):1–29. doi: 10.1126/science.abg2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alam M, Sultana M, Nair GB, Siddique AK, Hasan NA, Sack RB, et al. Viable but nonculturable Vibrio cholerae O1 in biofilms in the aquatic environment and their role in cholera transmission. Proc Natl Acad Sci USA. 2007 doi: 10.1073/pnas.0705599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereckaite L, Tatarunas V, Giedraitiene A. Current antimicrobial susceptibility testing for beta-lactamase-producing enterobacteriaceae in clinical settings. J Microbiol Methods. 2018;1(152):154–164. doi: 10.1016/j.mimet.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Thungapathra M, Sinha KK, Chaudhuri SR, Garg P, Ramamurthy T, Nair GB, et al. Occurrence of antibiotic resistance gene cassettes. Society. 2002;46(9):2948–2955. doi: 10.1128/AAC.46.9.2948-2955.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naha A, Pazhani GP, Ganguly M, Ghosh S, Ramamurthy T, Nandy RK, et al. Development and evaluation of a PCR assay for tracking the emergence and dissemination of haitian variant ctxB in Vibrio cholerae O1 strains isolated from Kolkata India. J Clin Microbiol. 2012;50(5):1733–1736. doi: 10.1128/JCM.00387-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dallenne C, Da Costa A, Decré D, Favier C, Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important b-lactamases in enterobacteriaceae. J Antimicrob Chemother. 2010;65(3):490–5. doi: 10.1093/jac/dkp498. [DOI] [PubMed] [Google Scholar]
- 19.Daoud Z, Sokhn SE, Salem Sokhn E, Masri K, Cheaito K, Haidar-Ahmad N, et al. Escherichia coli isolated from urinary tract infections of lebanese patients between 2005 and 2012: epidemiology and profiles of resistance. Front Med. 2015 doi: 10.3389/fmed.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bin Kim H, Wang M, Ahmed S, Park CH, LaRocque RC, Faruque ASG, et al. Transferable quinolone resistance in Vibrio cholerae. Antimicrob Agents Chemother. 2010;54(2):799–803. doi: 10.1128/AAC.01045-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bin Kim H, Park CH, Kim CJ, Kim E, Jacoby GA, Hooper DC. Prevalence of plasmid-mediated quinolone resistance determinants over a 9-year period. Antimicrob Agents Chemother. 2009;53(2):639–45. doi: 10.1128/AAC.01051-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulvey MR, Grant JM, Plewes K, Roscoe D, Boyd DA. New Delhi metallo-β-lactamase in klebsiella pneumoniae and Escherichia coli Canada. Emerg Infect Dis. 2011;17(1):103–6. doi: 10.3201/eid1701.101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monir MM, Islam MT, Mazumder R, Mondal D, Nahar KSSM, et al. Genomic attributes of Vibrio cholerae O1 responsible for 2022 massive cholera outbreak in Bangladesh Res Sq. Basel: Preprint; 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page AJ, Taylor B, Keane JA. Multilocus sequence typing by blast from de novo assemblies against PubMLST. J Open Source Softw. 2016;1(8):118. doi: 10.21105/joss.00118. [DOI] [Google Scholar]
- 25.Ambroise J, Irenge LM, Durant JF, Bearzatto B, Bwire G, Colin Stine O, et al. Backward compatibility of whole genome sequencing data with MLVA typing using a new MLVAtype shiny application for Vibrio cholerae. PLoS ONE. 2019;14(12):1–12. doi: 10.1371/journal.pone.0225848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu CJ, Chuang YC, Lee MF, Lee CC, Lee HC, Lee NY, et al. Bacteremia due to extended-spectrum-β-lactamase-producing Aeromonas spp. at a medical center in Southern Taiwan. Antimicrob Agents Chemother. 2011;55(12):5813–8. doi: 10.1128/AAC.00634-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo X, Xia R, Han N, Xu H. Genetic diversity analyses of class 1 integrons and their associated antimicrobial resistance genes in enterobacteriaceae strains recovered from aquatic habitats in China. Lett Appl Microbiol. 2011 doi: 10.1111/j.1472-765X.2011.03059.x. [DOI] [PubMed] [Google Scholar]
- 28.Wen Y, Pu X, Zheng W, Hu G. High prevalence of plasmid-mediated quinolone resistance and incq plasmids carrying qnrs2 gene in bacteria from rivers near hospitals and aquaculture in China. PLoS ONE. 2016 doi: 10.1371/journal.pone.0159418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nair GB, Qadri F, Holmgren J, Svennerholm AM, Safa A, Bhuiyan NA, et al. Cholera due to altered El Tor strains of Vibrio cholerae O1 in Bangladesh. J Clin Microbiol. 2006;44(11):4211–4213. doi: 10.1128/JCM.01304-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pal BB, Nayak AK, Nayak SR. Emergence and spread of different ctxB alleles of Vibrio cholerae O1 in Odisha. India Int J Infect Dis. 2021;1(105):730–732. doi: 10.1016/j.ijid.2021.03.042. [DOI] [PubMed] [Google Scholar]
- 31.Satchell KJF, Jones CJ, Wong J, Queen J, Agarwal S, Yildiz FH. Phenotypic analysis reveals that the 2010 haiti cholera epidemic is linked to a hypervirulent strain. Infect Immun. 2016;84(9):2473–2481. doi: 10.1128/IAI.00189-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hossain ZZ, Leekitcharoenphon P, Dalsgaard A, Sultana R, Begum A, Jensen PKM, et al. Comparative genomics of Vibrio cholerae O1 isolated from cholera patients in Bangladesh. Lett Appl Microbiol. 2018;67(4):329–336. doi: 10.1111/lam.13046. [DOI] [PubMed] [Google Scholar]
- 33.Ramamurthy T, Mutreja A, Weill FX, Das B, Ghosh A, Nair GB. Revisiting the global epidemiology of cholera in conjuction with the genomics of Vibrio cholerae. Front Public Heal. 2019;7(July):1–10. doi: 10.3389/fpubh.2019.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith AM, Weill FX, Njamkepo E, Ngomane HM, Ramalwa N, Sekwadi P, et al. Emergence of Vibrio cholerae o1 sequence type 75, South Africa, 2018–2020. Emerg Infect Dis. 2021;27(11):2927–2931. doi: 10.3201/eid2711.211144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.George CM, Rashid M, Almeida M, Monira S, Bhuyian SI, Hasan K, et al. Genetic relatedness of Vibrio cholerae isolates within and between households during outbreaks in Dhaka, Bangladesh. BMC Genomics. 2017 doi: 10.1186/s12864-017-4254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monir MM, Hossain T, Morita M, Ohnishi M, Johura F-T, Sultana M, et al. Genomic characteristics of recently recognized Vibrio cholerae El Tor lineages associated with cholera in Bangladesh, 1991 to 2017. Microbiol Spectr. 2022 doi: 10.1128/spectrum.00391-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alam M, Kasan NA, Sadique A, Bhuiyan NA, Ahmed KU, Nusrin S, et al. Seasonal cholera caused by Vibrio cholerae serogroups O1 and O139 in the coastal aquatic environment of Bangladesh. Appl Environ Microbiol. 2006;72(6):4096–4104. doi: 10.1128/AEM.00066-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alam M, Sultana M, Nair GB, Sack RB, Sack DA, Siddique AK, et al. Toxigenic Vibrio cholerae in the aquatic environment of mathbaria Bangladesh. Appl Environ Microbiol. 2006;72(4):2849–2855. doi: 10.1128/AEM.72.4.2849-2855.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan X, Li Y, Vaziri AZ, Kaviar VH, Jin Y, Jin Y, et al. Global status of antimicrobial resistance among environmental isolates of Vibrio cholerae O1/O139: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2022;11(1):62. doi: 10.1186/s13756-022-01100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garbern SC, Chu TC, Yang P, Gainey M, Nasrin S, Kanekar S, et al. Clinical and socio-environmental determinants of multidrug-resistant Vibrio cholerae 01 in older children and adults in Bangladesh. Int J Infect Dis. 2021;105:436–441. doi: 10.1016/j.ijid.2021.02.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishna K, Anas A, Kuttan SP, Vijayakumar S, Chekidhenkuzhiyil J, Philomina B, et al. Multiple drug-resistant Vibrio Cholerae responsible for cholera outbreak among migrant domestic workers in Kerala, South India. Multidiscip Digit Publish Inst Proc. 2021;66(1):26. [Google Scholar]
- 42.Parvin I, Shahunja KM, Khan SH, Alam T, Shahrin L, Mahmuda Ackhter M, et al. Changing susceptibility pattern of Vibrio cholerae o1 isolates to commonly used antibiotics in the largest diarrheal disease hospital in Bangladesh during 2000–2018. Am J Trop Med Hyg. 2020;103(2):652–8. doi: 10.4269/ajtmh.20-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitaoka M, Miyata ST, Unterweger D, Pukatzki S. Antibiotic resistance mechanisms of Vibrio cholerae. London: Microbiology Society; 2011. [DOI] [PubMed] [Google Scholar]
- 44.Chokshi A, Sifri Z, Cennimo D, Horng H. Global contributors to antibiotic resistance. J Glob Infect Dis. 2019;11(1):36–42. doi: 10.4103/jgid.jgid_110_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mandal J, Sangeetha V, Ganesan V, Parveen M, Preethi V, Harish BN, et al. Third-generation cephalosporin-resistant Vibrio cholerae. India Emerg Infect Dis. 2012;18(8):1326–1328. doi: 10.3201/eid1808.111686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verma J, Bag S, Saha B, Kumar P, Ghosh TS, Dayal M, et al. Genomic plasticity associated with antimicrobial resistance in Vibrio cholerae. Proc Natl Acad Sci USA. 2019;116(13):6226–6231. doi: 10.1073/pnas.1900141116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The PCR amplicon sequence data of blaPER-3, blaOXA-1, and aac(6´)-Ib-cr genes were deposited in NCBI Gene Bank under Accession No. MK992813, MK992814 and MK992815, respectively. All the data are available on request from the corresponding author.