Key words: Human foodborne trematode, intestinal fluke, liver fluke, lung fluke, paratenic host
Abstract
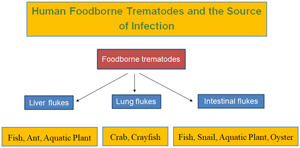
Foodborne trematodes (FBT) of public health significance include liver flukes (Clonorchis sinensis, Opisthorchis viverrini, O. felineus, Fasciola hepatica and F. gigantica), lung flukes (Paragonimus westermani and several other Paragonimus spp.) and intestinal flukes, which include heterophyids (Metagonimus yokogawai, Heterophyes nocens and Haplorchis taichui), echinostomes (Echinostoma revolutum, Isthmiophora hortensis, Echinochasmus japonicus and Artyfechinostomum malayanum) and miscellaneous species, including Fasciolopsis buski and Gymnophalloides seoi. These trematode infections are distributed worldwide but occur most commonly in Asia. The global burden of FBT diseases has been estimated at about 80 million, however, this seems to be a considerable underestimate. Their life cycle involves a molluscan first intermediate host, and a second intermediate host, including freshwater fish, crustaceans, aquatic vegetables and freshwater or brackish water gastropods and bivalves. The mode of human infection is the consumption of the second intermediate host under raw or improperly cooked conditions. The major pathogenesis of C. sinensis and Opisthorchis spp. infection includes inflammation of the bile duct which leads to cholangitis and cholecystitis, and in a substantial number of patients, serious complications, such as liver cirrhosis and cholangiocarcinoma, may develop. In lung fluke infections, cough, bloody sputum and bronchiectasis are the most common clinical manifestations. However, lung flukes often migrate to extrapulmonary sites, including the brain, spinal cord, skin, subcutaneous tissues and abdominal organs. Intestinal flukes can induce inflammation in the intestinal mucosa, and they may at times undergo extraintestinal migration, in particular, in immunocompromised patients. In order to control FBT infections, eating foods after proper cooking is strongly recommended.
Introduction
Foodborne trematodes (FBT) are defined as trematodes infecting humans, which are transmitted by consumption of foods, globally or locally available (including traditional foods) or those taken rarely or accidentally. In 1995, the World Health Organization (WHO) estimated the total global number of people infected with FBT at more than 40 million (WHO, 1995). A decade later (in 2005), about 56.2 million people were estimated to be infected with FBT, with 7.9 million having severe sequelae and 7158 people dying mostly from cholangiocarcinoma and cerebral infections (Fürst et al., 2012a). The most recent estimate extrapolated the total global number of people infected with FBT at 74.7 million as of 2015–2016 with 0.2 million new cases annually and 2 million disability-adjusted life years (Fürst et al., 2019; WHO, 2020). However, there are problems of low sensitivity and specificity of diagnostic tools as well as possible numerous endemic areas so far undetected, and these global estimates of the FBT burden seem to be much underestimated. Thus, the WHO road map for ‘control of FBT diseases 2021–2030’ recommends critical actions, including the development of accurate surveillance and mapping tools and methods, with information on environmental factors involved in infection, and the promotion of application and awareness of preventive chemotherapy (WHO, 2020).
Taxonomically, the FBTs are highly diverse, and at least 99 species (15 liver flukes, 9 lung flukes and 75 intestinal flukes) have been reported from human infections (Chai et al., 2005; Chai, 2007, 2019; Fürst et al., 2012a; Chai and Jung, 2019, 2020; Cho et al., 2020). Among them, those of greatest public health significance are Clonorchis sinensis, Opisthorchis spp., Fasciola hepatica, Paragonimus westermani, and several species of intestinal flukes, such as Metagonimus spp., Haplorchis spp. and echinostomes (WHO, 1995; Chai and Jung, 2019).
The origin of FBTs may date back to almost 8000 BCE when humans started to switch from a nomadic, hunter-gatherer lifestyle to a settled, agricultural way of life (Steverding, 2020). However, from the evolutionary point of view, intestinal flukes seem to be the oldest group, which subsequently evolved to parasitize the bile duct and liver (liver flukes) or lungs (lung flukes) in the human body.
The mode of human infection with FBT is closely linked to human behavioural patterns in endemic localities, specifically the methods of food production, preparation and consumption (WHO, 1995). Thus, the epidemiology of FBT infection is determined by ecological and environmental factors related to food and is strongly influenced by poverty, pollution and population growth (WHO, 1995). The infection source, i.e. food, is diverse, including aquatic or semi-terrestrial snails (gastropods and bivalves), fish, crustaceans, water plants, amphibians and reptiles (WHO, 1995; Chai, 2019). Rarely insects and mammals may serve as the source of human infection for some species (Chai, 2019; Chai and Jung, 2019).
The geographical distribution of FBT is almost worldwide. However, as FBT infections are closely related to food habits, they are predominantly found in East Asian and Western Pacific countries where local people prefer to eat various types of foods raw or under improperly cooked conditions (WHO, 1995). This kind of food habit is in most cases traditional and one of the longstanding customs, so it is very difficult to change within a short period of time. Health education targeting schoolchildren is an important strategy for long-term control of FBT infections. Control of infection sources, including intermediate hosts, is very difficult and practically almost impossible. Mass drug administration (MDA) using an effective anthelmintic, such as praziquantel, may be feasible to lower the infection rate of people in endemic communities. However, if reinfection persists, the prevalence would rise again to the previous level. Thus, control of FBT infections by infrequent MDA may be insufficient. Repeated MDA with sustained health education of young people in endemic communities is an ideal control strategy.
In this review, the authors briefly summarized the current status of FBT occurring around the world. The taxonomy and phylogeny of FBT, their life cycles, mode of transmission, epidemiology and geographical distribution, global disease burden, pathogenicity and clinical manifestations, diagnostic problems, anthelmintics used, and control strategies are briefly reviewed.
Liver flukes
At least 15 species of liver flukes are known to cause human infections. They include C. sinensis, Opisthorchis viverrini, O. felineus, Metorchis conjunctus, M. bilis, M. orientalis, Amphimerus sp., Amphimerus noverca, A. pseudofelineus, Pseudamphistomum truncatum, P. aethiopicum, Dicrocoelium dendriticum, D. hospes, Fasciola hepatica and F. gigantica (Table 1) (Chai and Jung, 2019). They can be divided into small liver flukes (C. sinensis, Opisthorchis spp., Metorchis spp., Amphimerus spp., Pseudamphistomum spp. and Dicrocoelium spp.) and large liver flukes (F. hepatica and F. gigantica) according to the size of the adult worms. The first intermediate host is freshwater or brackish water snails, and the second intermediate host is freshwater fish (C. sinensis, Opisthorchis spp., Metorchis spp., Amphimerus spp. and Pseudamphistomum spp.), ants (Dicrocoelium spp.) or aquatic vegetation (Fasciola spp.) (Table 1). The morphological similarity of opisthorchiid eggs to heterophyid as well as lecithodendriid-like fluke eggs frequently poses diagnostic problems in human fecal examinations. Molecular techniques using internal transcribed spacers (ITS) and mitochondrial cytochrome c oxidase 1 (cox1) have been used to differentiate the eggs as well as larvae and adults of opisthorchiid and heterophyid flukes (Duflot et al., 2021).
Table 1.
Liver flukes infecting humans with biological, clinical characteristics and geographical distribution
| Group/Species of flukes | First intermediate host (snail) | Second intermediate host | Reservoir host | Major clinical characteristics | Geographical distributiona |
|---|---|---|---|---|---|
| Clonorchis sinensis | Parafossarulus sp. | Freshwater fish | Dog, cat, rat, pig, badger, weasel, camel, buffalo | Cholangitis, cirrhosis, cholangiocarcinoma | China, Taiwan, South Korea, Vietnam, Russia |
| Opisthorchis viverrini | Bithynia sp. | Freshwater fish | Dog, cat, rat, pig | Cholangitis, cirrhosis, cholangiocarcinoma | Thailand, Laos, Vietnam, Cambodia, Malaysia, Myanmar |
| Opisthorchis felineus | Bithynia leachi | Freshwater fish | Dog, cat, fox, chipmunk, beaver, Caspian seal, pig | Cholangitis, cirrhosis, cholangiocarcinoma | Germany, Greece, Poland, Romania, Italy, Spain, Belarus, Ukraine, Kazakhstan, Russia |
| Metorchis conjunctus | Amnicola sp. | Freshwater fish | Dog, cat, wolf, fox, coyote, raccoon, muskrat, mink, fisher | Fever, abdominal pain | Canada, Greenland |
| Metorchis bilis | Bithynia sp. | Freshwater fish | Wolf, white-tailed eagle, muskrat, otter, mink | Unknown | Central and Eastern Europe, Russia |
| Metorchis orientalis | Parafossarulus sp. | Cyprinoid fish | Dog, cat, duck, chicken, goose | Unknown | Japan, China, South Korea |
| Amphimerus sp. | Freshwater snail | Freshwater fish | Duck | Liver fibrosis, cirrhosis | Ecuador |
| Amphimerus noverca | Aquatic snail (?) | Freshwater fish | Dog, wolverine, pig | Periductal fibrosis | India |
| Amphimerus pseudofelineus | Aquatic snail (?) | Freshwater fish (?) | Dog, cat, coyote | Unknown | North and South Americas |
| Pseudamphistomum truncatum | Freshwater snail | Cyprinoid fish | Dog, cat, fox, seal, skunk, mink,otter | Liver pathology | Russia, Europe, North America |
| Pseudamphistomum aethiopicum | Freshwater snail | Fish | Unknown | Small tumour at intestinal wall | Ethiopia |
| Dicrocoelium dendriticum | Various snail species | Ant | Cattle, sheep, ox, deer, marmot, rabbit, goat | Mild cholangitis | Russia, Iran, Java, China, Lebanon, Syria, Egypt, Tunisia, France, Italy, Sweden, Germany, Switzerland, Spain, Hungary, Romania, Armenia, Czech Republic, Turkey, Uzbekistan, Iran, Saudi Arabia, Brazil, and USA |
| Dicrocoelium hospes | Limicolaria sp. | Ant | Cattle, sheep, zebus, goat, monkey | Probably mild cholangitis | DR Congo, Ghana, Sierra Leone, Nigeria |
| Fasciola hepatica | Galba sp. | Aquatic plant | Cattle, sheep, goat, European hare | Obstructive cholangitis, ectopic lesions | Ecuador, Bolivia, Chile, Peru, Cuba, Egypt, Portugal, France, Spain, Iran, Turkey, South Korea, Japan, China, Thailand, Vietnam |
| Fasciola gigantica | Radix sp. | Aquatic plant | Cattle, sheep, goat, buffalo, camel, pig, horse, domestic animal | Obstructive cholangitis, ectopic lesions | Japan, South Korea, China, Russia, Vietnam, Thailand, Malaysia, India, Iran, Sudan, Senegal, Chad, Ghana, Niger, Central African Republic, Tanzania, Kenya, USA (Hawaii) |
The geographical distributions of liver flukes are mostly referred from Chai and Jung (2019).
Species involved
Clonorchis sinensis
Clonorchis sinensis (Cobbold, 1875) Looss, 1907 (the Chinese liver fluke) was first found in the bile passages of a Chinese carpenter in Calcutta, India, and an endemic focus was discovered in south China (Beaver et al., 1984). Eggs of this fluke were also found in the coprolites of human mummies (estimated date; 1647) in South Korea (Seo et al., 2007) and China (date back to the 5th century BCE) (Ye and Mitchell, 2016). This fluke infects the bile duct of humans and animals and can cause inflammation of the bile duct and gall bladder which leads to obstruction of the bile duct, cholangitis and cholecystitis (Chai and Jung, 2019). In severe cases, liver cirrhosis and cholangiocarcinoma may develop.
Phylogenetic relationships of small liver flukes have been studied using analysis of nuclear (18S rDNA, ITS region and 28S rDNA) and mitochondrial genes (cox1, cox2, cox3, nd1, ATPase subunit 6 and others) (Thaenkham et al., 2012; Besprozvannykh et al., 2018; Saijuntha et al., 2019, 2021; Duflot et al., 2021). Within the genus Clonorchis, there is only a single valid species, C. sinensis. ITS2 can clearly separate C. sinensis, O. viverrini, O. felineus, Amphimerus sp. and Metorchis spp. from the Heterophyidae and Cryptogonimidae flukes (Thaenkham et al., 2012; Besprozvannykh et al., 2018).
Freshwater snails take the role of the first intermediate host for C. sinensis, which includes Parafossarulus manchouricus, P. anomalospiralis and Bithynia fuchsiana (Chen et al., 1994). At least 113 species of freshwater fish, including Pseudorasbora parva, Carassius spp., Cyprinus spp., Zacco spp. and Puntungia herzi, are known to serve as the second intermediate hosts and the source of human and animal infections (Chen et al., 1994; Chai et al., 2005; Rim, 2005). Shrimps were also reported to be a second intermediate host (Chen et al., 1994).
The mode of infection is related to traditional food habits, in particular, the consumption of raw or improperly cooked freshwater fish (Chai et al., 2005). In South Korea, the major type of fish dish responsible for C. sinensis infection is the sliced raw freshwater fish with red pepper sauce (Chai et al., 2005). In southern China and Hong Kong, the major type of fish dish responsible is the morning congee (rice gruel) with slices of raw freshwater fish (Chen et al., 1994), whilst in the Guangdong Province of China, half-roasted or undercooked fish is commonly linked to infection (Chen et al., 1994).
The endemic areas are located mostly in the Far East and East Asia (Chen et al., 1994; Chai et al., 2005; Rim, 2005). In South Korea, a national survey in 2012 reported an egg positive rate of 1.9%, and the number of infected people was estimated to be about 1 million (Korea Association of Health Promotion, 2013). In China, a total of 24 major endemic localities were reported, with 12.5 million infected people nationwide (Chen et al., 1994; Hong and Fang, 2012; Lai et al., 2016). In Taiwan, the prevalence of clonorchiasis was high in some localities; however, the current status is unknown (Chai and Jung, 2019). In Vietnam, northern parts, especially along the Red River Delta, including Haiphong and Hanoi, are well-known endemic areas, with the number of infected people estimated at about 1 million (Rim, 1982a; Chai et al., 2005; Chai and Jung, 2019). In Russia, human infections were reported in the Amur River territory, although the exact prevalence is unknown; however, at least 1 million people are estimated to be infected (Hong and Fang, 2012). The total global number of infected people is estimated at about 20 million (Hong and Fang, 2012), and the number at risk is about 601 million (Garcia, 2016).
The metacercariae excyst in the duodenum, migrate through the ampulla of Vater to the distal biliary ducts, where they begin to lay eggs 3–4 weeks after infection (Beaver et al., 1984). Cholangitis and cholecystitis are the main pathological features in association with secondary bacterial infections (Rim, 1982a). The major histopathological features of the involved bile ducts include irregular bile duct dilatation, glandular hyperplasia, mucin-secreting cell metaplasia, cystic degeneration and periductal fibrosis (Rim, 1982a; 2005; Hong and Fang, 2012). In some patients, biliary cirrhosis and biliary obstruction with blockage of the common bile duct by adult worms or stones, or both, may occur (Beaver et al., 1984). The pathogenesis and pathology depend on the intensity of infection as well as the frequency of continuous reinfection over a period of years and the total length of the infection which can last for 15–20 years or longer (Beaver et al., 1984). The clinical symptoms include jaundice accompanied by transient urticaria and pain at the site of the liver; in heavy infections, weakness, lassitude, epigastric discomfort, paraesthesia, loss of weight, palpitation, tachycardia, diarrhoea and toxaemic symptoms may occur (Rim, 1982a). In chronic stages, suppurative cholangitis, biliary stone, pancreatitis, liver cirrhosis and cholangiocarcinoma are important complications (Rim, 1982a; Kim, 1984b).
C. sinensis is one of the well-known carcinogenic flukes that can cause cholangiocarcinoma of the bile duct (Rim, 1982a; 2005; Hong and Fang, 2012). The scientific evidence for this was first raised in Hong Kong, an endemic area of clonorchiasis, where it was shown that at least 15% of 200 primary cancers in the liver were induced by infection with C. sinensis (Hou, 1956). In South Korea, close correlations were also found between the cholangiocarcinoma incidence and the prevalence of C. sinensis infection in a southern endemic area (Rim, 1982a; Kim, 1984b).
In known endemic areas, it is relatively easy to diagnose C. sinensis infection through fecal examinations to detect parasite eggs. The daily number of eggs produced per worm was estimated at about 4000 (Rim, 1982a); therefore, the egg detectability in fecal examinations is considerably high. However, in areas previously unknown for FBT infections, the eggs should be differentiated from those of other small liver fluke species (such as O. viverrini, O. felineus, Metorchis spp., or Amphimerus spp.) and also of heterophyid (Metagonimus yokogawai, Heterophyes nocens and various others) and lecithodendriid-like trematode species (Caprimorgorchis molenkampi and others) (Lee et al., 1984; Chai and Lee, 2002; Chai, 2007). Serological tests, such as enzyme-linked immunosorbent assay (ELISA), and radiologic techniques, including ultrasound and CT, are also helpful for diagnosis (Hong and Fang, 2012). Detection of parasite DNA using varied genetic techniques is another method applicable for the diagnosis of clonorchiasis (Hong and Fang, 2012).
Treatment of C. sinensis infection can be done using a potent anthelmintic, praziquantel (Chai, 2013a), or alternatively, albendazole (Table 2). Although such treatment is effective at clearing the infection, fluke-induced bile duct pathology did not recover within 9–12 weeks after praziquantel treatment (Lee et al., 1988; Chai, 2013a). Control strategies include MDA of infected people in endemic areas, environmental sanitation (sterilization of feces and protection of fish ponds from contamination with night-soil), control of snail hosts, and health education to avoid eating raw or improperly cooked freshwater fish (Rim, 1982a).
Table 2.
Drugs used for the treatment of FBT infections
| Anthelmintic drug | Species of FBT | Drug regimen | Remark |
|---|---|---|---|
| Praziquantel | Clonorchis sinensis | 25 mg kg−1 tid for 2 days | Individual treatment |
| 40 mg kg−1 single dose | mass treatment | ||
| Opisthorchis viverrini | 25 mg kg−1 tid for 1–2 days | Individual treatment | |
| 40 mg kg−1 single dose | Mass treatment | ||
| Opisthorchis felineus | 25 mg kg−1 tid for 1 day | Individual treatment | |
| 40 mg kg−1 single dose | Mass treatment | ||
| Dicrocoelium dendriticum | 20–25 mg kg−1 tid for 1–4 days | Satisfactory effects | |
| Paragonimus spp. | 25 mg kg−1 tid for 2 days | Individual and mass treatment | |
| Intestinal flukes | 10–20 mg kg−1 in single dose | Individual and mass treatment | |
| Triclabendazole | Dicrocoelium dendriticum | 10 mg kg−1 single dose | Satisfactory effects |
| Fasciola spp. | 10 mg kg−1 single dose | Repeated after 12–24 h in heavy infections | |
| Paragonimus spp. | 10 mg kg−1 single dose | Repeated after 12–24 h in heavy infections | |
| Niclosamide | Fasciolopsis buski | 40 mg kg−1 daily for 1–2 days | Alcohol should be avoided |
| Heterophyes heterophyes | Total 6 g over 3 alternate days | Repeated if necessary | |
| Metagonimus yokogawai | 100 mg kg−1 daily for 1–2 days | Moderate efficacy | |
| Haplorchis taichui | 40 mg kg−1 single dose | 100% cure rate in experimental mice | |
| Albendazole | Clonorchis sinensis | 1200–1800 mg daily for 3–5 days | Cure rate; 84.6% |
| Opisthorchis viverrini | 800 mg daily for 3–7 days | Cure rare; 60–96% | |
| Dicrooelium dendriticum | 400 mg bid for 7 days | Satisfactory effects | |
| Metagonimus yokogawai | 400 mg daily for 2 days | Cure rate; 61.1% | |
| Fasciola spp. | 1200 mg daily for 7 days | Triclabendazole-resistant cases |
Opisthorchis viverrini and O. felineus
In the genus Opisthorchis, at least 53 nominal species were described (Nawa et al., 2013). Among them, 30 species were recorded from avian hosts, and the remaining 23 were from molluscs, fish, reptiles and mammals (Nawa et al., 2013).
Opisthorchis viverrini (Poirier, 1886) Stiles and Hassall, 1896 (the cat liver fluke) and Opisthorchis felineus (Rivolta, 1884) Blanchard, 1895 (the cat liver flukes) are known to infect humans (Rim, 1982b; Saijuntha et al., 2019). O. viverrini was first discovered in the liver of a civet cat (Felis viverrini) brought from India to France (Miyazaki, 1991). Its first human infection was reported by Leiper in 1911 from the autopsy of two prisoners in Chiang Mai, Thailand (Wykoff et al., 1965). O. felineus was first discovered in the liver of a cat (Miyazaki, 1991), and human infection was first reported by Winogradoff in 1892 in Tomsk, Siberia (Beaver et al., 1984). Eggs of O. felineus were found in the coprolites of humans and dogs in Russia (Slepchenko, 2020). Phylogenetic studies of Opisthorchis spp. have been performed using sequences of nuclear (18S rDNA, ITS region and 28S rDNA) and mitochondrial genes (cox1, cox2, cox3, nd1, ATPase subunit 6 and others) (Besprozvannykh et al., 2018; Saijuntha et al., 2019, 2021; Duflot et al., 2021). Molecular genetic investigations showed that O. viverrini is a species complex ‘O. viverrini sensu lato’, containing two evolutionary lineages with many cryptic species (morphologically similar but genetically distinct species) occurring in Thailand and Laos (Saijuntha et al., 2021).
These flukes can infect the bile duct of humans and animals and can cause hepatobiliary disorders, as in C. sinensis (Chai and Jung, 2019). O. viverrini seems to have a higher potential for inducing cholangiocarcinoma than C. sinensis (Chai et al., 2005; Chai and Jung, 2019). Regarding O. felineus, studies are required for a proper understanding of its carcinogenic potential (Maksimova et al., 2017).
The molluscan intermediate host of O. viverrini is freshwater snails, including Bithynia goniomphalos, B. funiculata, B. siamensis and Melanoides tuberculata (Chai and Jung, 2019). The second intermediate hosts are various species of freshwater fish, i.e. Hampala dispar, Puntius orphoides, P. brevis, P. gonionotus, P. proctozysron and P. viehoever (Rim, 1982b; Chai and Jung, 2019; Chai et al., 2019a). O. viverrini may survive for 10–20 years in the human host (Saijuntha et al., 2019).
With regard to O. felineus, the molluscan host is Bithynia leachi species complex (B. leachi, B. troscheli and B. inflata) distributed in Eastern Europe (Mordvinov et al., 2012). The second intermediate hosts are various species of freshwater fish, including the chub (Idus melanotus), tench (Tinca tinca and T. vulgaris), bream (Abramis brama and A. sapa), barbel (Barbus barbus) and carp (Cyprinus carpio) (Rim, 1982b).
The geographical distribution of O. viverrini, which is determined in close relationship with the distribution of the snail host, is mostly along the Mekong River basin and Indochina peninsula (Chai et al., 2005; Chai and Jung, 2019). In Thailand, O. viverrini is distributed mainly in the north and northeastern regions (Sithithaworn and Haswell-Elkins, 2003), however, the prevalence has been declining since the 1990s (Chai and Jung, 2019). By contrast, in Laos, mainly along the Mekong River, the prevalence has been reported to be high even after the 1990s, with the prevalence being 51–67% among the riparian villagers (Rim et al., 2003; Chai et al., 2007; Nakamura, 2017). In Vietnam, endemic areas are located in southern parts, especially along the Mekong River (Chai and Jung, 2019). In Cambodia, Phnom Penh Municipality, Takeo, Kratie, Stung Treng and Preah Vihear Provinces were reported to be low-grade endemic areas (Yong et al., 2014; Chai and Jung, 2019; Khieu et al., 2019). In Myanmar, a low prevalence of O. viverrini infection has recently been confirmed in a suburban population around Yangon (Sohn et al., 2019). The number of global people infected with O. viverrini is estimated at over 10 million (Sripa et al., 2018), with 80 million at risk for Opisthorchis spp. infections (Garcia, 2016).
O. felineus is known to be distributed from the Iberian Peninsula (Portugal and Spain) to Eastern Europe and West Siberia (Mordvinov et al., 2012). Many riverside areas, including the Ob, Irtysh, Ural and Volga Rivers, are the most important endemic localities; the highest prevalence of 20–60% or above was reported in areas of West Siberia (Fedorova et al., 2018). Human infections were recorded previously in Lithuania (before 1901), Poland (before 1937), Romania (before 1957) and Spain (before 1932) but recently no cases seem to occur in these countries (Mordvinov et al., 2012). However, in the last 50 years, human cases have been reported in some European Union (EU) countries, Eastern Europe, and Kazakhstan (Mordvinov et al., 2012; Pozio et al., 2013). In Italy, from 2003 to 2011, there were 8 small outbreaks of O. felineus infection involving a total of 211 people through consumption of raw fillet of the tench (T. tinca) fished from two lakes named Bolsena and Bracciano (Pozio et al., 2013). Thus, O. felineus infection is regarded as an emerging disease in Italy (Pozio et al., 2013). The worldwide estimated number of people infected with O. felineus is 1.2–1.6 million (Saijuntha et al., 2019).
The pathogenesis and pathology as well as clinical symptoms of O. viverrini and O. felineus infections are basically the same as those of C. sinensis infection. Cholangiocarcinoma due to O. viverrini infection accounts for about 15% of all liver cancers and is the second most frequent primary liver cancer worldwide (Saijuntha et al., 2019). The highest incidence of this disease occurs in northern Thailand, where 5000 cases of cholangiocarcinoma are diagnosed annually (Sripa et al., 2012; Saijuntha et al., 2019). In Laos and Vietnam, the precise incidence of cholangiocarcinoma due to O. viverrini infection has been unknown. However, the global ‘Cholangiocarcinoma Foundation’ recently launched a Vietnam Veterans project regarding developing cholangiocarcinoma among the veterans due to their military service in Vietnam in the 1970s (https://cholangiocarcinoma.org/vietnam-veterans/). In endemic areas of O. felineus infection in eastern Europe and Russia, around 400 cases of cholangiocarcinoma occur every year (Hotez and Alibek, 2011). No precise carcinogenic mechanisms of Opisthorchis-induced cholangiocarcinoma have been completely understood (Sripa et al., 2018). However, a two-stage carcinogenic process was proposed; some kinds of carcinogenic substances, including dimethylnitrosamine (DMN), as an ‘initiator’, and parasites, like O. viverrini and O. felineus, act as a ‘promoter’ (Flavell, 1981; Maksimova et al., 2017). This hypothesis has been further developed into a three-stage (multistage) theory, including initiation, promotion and tumour progression (Sripa et al., 2018).
The diagnosis of O. viverrini or O. felineus infection can be done by fecal examinations to detect eggs. The daily number of eggs produced per worm of O. viverrini was estimated at 160–900, considerably less than the figure of 4000 as reported for C. sinensis (Rim, 1982a, 1982b). Serological tests and fecal antigen detection using ELISA (Sripa et al., 2010; Teimoori et al., 2017) and radiologic techniques, including ultrasound (Pungpak et al., 1997; Kim et al., 2018) and CT (Mairiang and Mairiang, 2003), are helpful for the diagnosis of opisthorchiasis and related cholangiocarcinoma. Detection of parasite DNA is another method applicable for the diagnosis of opisthorchiasis (Lovis et al., 2009; Sripa et al., 2010).
Treatment of O. viverrini and O. felineus infection can be done using a potent anthelmintic, praziquantel (Chai, 2013a), or alternatively, albendazole (Table 2). For prevention and control, see C. sinensis section. It is noteworthy that the metacercariae of O. felineus are tolerant to low temperatures, drying and high salt concentration and can be killed only by a high temperature (Pakharukova and Mordvinov, 2016).
Metorchis spp.
Metorchis conjunctus (Cobbold, 1860) Looss, 1899 (the North American liver fluke) is a common parasite of carnivorous mammals, including sledge dogs (causing fatality), in northern Canada (MacLean et al., 1996). Asymptomatic sporadic human infections were reported in Canada since 1945, particularly in aboriginal populations from Quebec to Saskatchewan and the eastern coast of Greenland (Babbott et al., 1961; MacLean et al., 1996). In 1993, an outbreak of a common-source infection with this fluke, with acute clinical symptoms of fatigue, upper abdominal tenderness and pain, low-grade fever, headache, weight loss and anorexia, occurred among 19 Korean immigrants in a river north of Montreal, Canada; the patients consumed wild-caught fish (Catostomus commersoni) that had been undercooked (MacLean et al., 1996). Laboratory findings included high blood eosinophilia and raised liver enzymes (MacLean et al., 1996). However, serious liver diseases have not been reported in human infections.
Metorchis bilis (Braun, 1790) Odening, 1962 is a liver fluke of carnivorous mammals in Central and Eastern Europe and Western Siberia of Russia (Mordvinov et al., 2012). The geographical range of M. bilis considerably overlaps with that of O. felineus (Mordvinov et al., 2012). Human infections with M. bilis were first suggested by demonstrating serum antibodies to M. bilis in 51.3% of 37 patients (37.8% of them reacted against both O. felineus and M. bilis antigens) residing in the Novosibirsk area of Russia (Kuznetsova et al., 2000). Another serological study also demonstrated positive immunoreactivity of 29 (63.2%) of 46 small liver fluke egg-positive patients to both O. felineus and M. bilis antigens and of 3 (7.9%) patients only to M. bilis antigen (Fedorov et al., 2002). Now it seems that infections of both humans and piscivorous vertebrates by M. bilis are under-diagnosed (Sitko et al., 2016).
Metorchis orientalis Tanabe, 1920 is a small liver fluke of piscivorous birds and mammals in Japan, China and South Korea (Chai and Jung, 2019). The first report of human infection was published in Guangdong Province, China (Lin et al., 2001); 4 (4.2%) of 95 residents examined in Ping Yuan County were egg positive and from 2 purged patients a total of 12 adult flukes were recovered (Lin et al., 2001). However, the epidemiological status and potential impact of human infection are unknown (Chai and Jung, 2019). The geographical range of this fluke considerably overlaps with that of C. sinensis (Chai and Jung, 2019).
Amphimerus spp.
Amphimerus sp. (species undetermined) was found to be a human parasitic liver fluke in Ecuador. In 2009, 4 human fecal samples from indigenous Chachi communities along the Cayapas River on the northern coast of Ecuador were found to be positive for opisthorchiid eggs (Calvopiña et al., 2011). Next year, a follow-up survey was conducted in the same communities, and a high proportion, 71 (24%) of 297 fecal samples, of residents, were positive for the same eggs (Calvopiña et al., 2011). The biliary liquid was collected from 4 patients under duodenoscopy, and eggs identical to those found in the feces were found; they were treated with praziquantel followed by purging, and adult worms recovered were identified as a species of Amphimerus (Calvopiña et al., 2011). The patients had a history of consuming smoked or lightly cooked fish; the number of people living in endemic communities is about 24 000 (Calvopiña et al., 2011). A further study reported a very high prevalence of infection with this fluke in domestic cats and dogs living in Chachi communities, Ecuador (Calvopiña et al., 2015). Several species of freshwater fish were confirmed molecularly to have metacercariae of Amphimerus sp. (Romero-Alvarez et al., 2020). Human Amphimerus sp. infection is in most cases asymptomatic; however, it occasionally can cause non-specific generalized symptoms (Cevallos et al., 2017). Liver cirrhosis and pancreatitis occurred in cats and a cormorant infected with this fluke (Cevallos et al., 2017).
Amphimerus noverca (Braun, 1902) Baker, 1911 is a common parasite in the bile duct or pancreatic duct of dogs, wolverines and pigs in India (Beaver et al., 1984; Roy and Tandon, 1992). Human infection with A. noverca was mentioned two times (in 1876 and 1878) in India (Mas-Coma and Bargues, 1997). No further reports on human infections are available.
Amphimerus pseudofelineus (Rodriguez, Gomez Lince et Montalvan, 1949) Artigas and Perez, 1964 was originally reported under the name Opisthorchis guayaquilensis from 18 of 245 humans (based on eggs in feces) and several dogs (based on eggs in feces and adult worms) in a rural area of Ecuador by Rodriguez and colleagues (Mas-Coma and Bargues, 1997). Then, Thatcher (1970) transferred this fluke to the genus Amphimerus. This fluke is now known to be distributed in North and South America, probably taking aquatic snails shedding cercariae and fish harbouring encysted metacercariae (Mas-Coma and Bargues, 1997).
Pseudamphistomum truncatum and P. aethiopicum
Pseudamphistomum truncatum (Rudolphi, 1819) Lühe, 1908 is a species of liver or gall bladder fluke in mammals in Europe and North America (Yamaguti, 1971; Mas-Coma and Bargues, 1997). Its human infection was briefly mentioned in 1928 in Europe (Mas-Coma and Bargues, 1997). Later, in the Aleksee District of Tartaria, Russia, 31 human cases were diagnosed among 61 patients who had hepatobiliary system damage and had undergone bile analysis (Khamidullin et al., 1991). Human infections were also detected in five areas along the Volga, Kama and Belaya Rivers in Russia (Khamidullin et al., 1995). The eggs and metacercariae of P. truncatum can be morphologically differentiated from those of O. felineus (Mas-Coma and Bargues, 1997). The pathogenicity in humans is unknown but in otters and mink, P. truncatum can cause cholecystitis (Simpson et al., 2005; Sherrad-Smith et al., 2009).
Pseudamphistomum aethiopicum Pierantoni, 1942 was reported, on one occasion, to have caused a human infection with a small tumour in the intestinal wall (Yamaguti, 1971).
Dicrocoelium dendriticum and D. hospes
Within the genus Dicrocoelium, 5 species are currently recognized to be valid; D. dendriticum (Rudolphi, 1819) Looss, 1899, D. chinensis Tang and Tang, 1978, D. hospes Looss, 1907, D. orientale Sudarikov and Ryjikov, 1951 and D. petrowi Kassimov, 1952 (Manga-González and Ferreras, 2014).
Dicrocoelium dendriticum and Dicrocoelium hospes are known to cause human infections in rare instances. D. dendriticum (the lancet fluke) is a small liver fluke infecting domestic animals in the Northern coasts of Africa, Asia and the Americas (Mas-Coma and Bargues, 1997; Traversa et al., 2013; Manga-González and Ferreras, 2014). Using mitochondrial nd1 and cox1 sequences, D. dendriticum could be distinguished from D. chinensis and D. hospes (Hayashi et al., 2017; Khan et al., 2021). In human dicrocoeliasis, there may be true (genuine) and false (spurious) infections. The geographical localities where human infections were reported are very wide, and the incidence is undoubtedly underestimated (Mas-Coma and Bargues, 1997).
The first intermediate host includes more than 90 species of land snails, with Cionella (= Cochlicopa) lubrica as the most important species (Traversa et al., 2013). The cercariae are shed from the snails in clusters of thousands forming a so-called ‘slime ball’ (Traversa et al., 2013). These cercariae are ingested by ants (Formica fusca, F. pratensis and F. rufibarbis) (Traversa et al., 2013). Humans are infected accidentally by swallowing an infected ant together with food (Traversa et al., 2013).
In human infections, the pathogenicity depends on the number of flukes infected and the duration of infection, and in many instances, there may be no notable symptoms. However, a prolonged period of constipation or diarrhoea, nausea, vomiting, abdominal discomfort and epigastric pain may occur (Mas-Coma and Bargues, 1997). The diagnosis is based on the recovery of eggs in feces. However, an egg-positive result does not necessarily indicate a ‘true infection’, and a ‘spurious infection’ should be ruled out.
Serodiagnosis, including immunofluorescence, immunoblot, haemagglutination, complement fixation and ELISA have been introduced (Traversa et al., 2013; Dar et al., 2020). For treatment, albendazole (Magi et al., 2009), praziquantel (Drabick et al., 1988; Khandelwal et al., 2008), triclabendazole (Khandelwal et al., 2008; Ashrafi, 2010) and myrrh (herbal drug in Egypt; Mirazid®) (Abdul-Ghani et al., 2009) showed favourable results (Table 2).
D. hospes is a small liver fluke (more slender and smaller than D. dendriticum) infecting the bile duct and gall bladder of domestic animals in sub-Saharan Africa (Mas-Coma and Bargues, 1997). This liver fluke can also cause genuine and spurious infections in humans (Mas-Coma and Bargues, 1997). Two human patients in Sierra Leone (spurious infection was not ruled out) showed hepatitis-like symptoms; one patient had jaundice, and both had elevated bilirubin and transaminase levels (King, 1971). In animals, D. hospes does not seem to cause much damage even in heavy infections (Mas-Coma and Bargues, 1997). Diagnosis can be made by the recovery of eggs in feces; however, the eggs are morphologically indistinguishable from those of D. dendriticum and Eurytrema pancreaticum (Mas-Coma and Bargues, 1997). Anthelmintic drugs used against D. dendriticum may have similar effects on D. hospes.
Fasciola hepatica and F. gigantica
Fasciola hepatica Linnaeus, 1758 (the sheep liver fluke) infects the bile duct of domestic animals, including cattle and sheep, and occasionally humans (Beaver et al., 1984; Mas-Coma et al., 2005; Liu and Zhu, 2013). The parasite life cycle, biology, pathogenesis, pathology, epidemiology and clinical symptoms are similar between F. hepatica and F. gigantica (Chai and Jung, 2019). Hybrid forms between F. hepatica and F. gigantica have been found in Asia (Tang et al., 2021). Humans are accidental hosts (Liu and Zhu, 2013). They are infected through eating aquatic vegetables or raw liver of infected livestock animals (Liu and Zhu, 2013). The history of human infection with Fasciola spp. seems to be very long, since archaeological studies in ancient mummies in Egypt revealed remains of parasites (possibly part of F. hepatica worm) in liver tissue (David, 1997). Sporadic cases of human Fasciola spp. infections (genuine or spurious infection unclear) were reported in Egypt in the 1950s (Kuntz et al., 1958), and an epidemic occurred in France in 1956 (Mas-Coma et al., 2005). An extensive review of human fascioliasis was performed by Chen and Mott (1990) in which 2595 cases from over 40 countries in Europe, Asia, Western Pacific, the Americas and Africa from 1970 to 1990 were analysed. Now human fascioliasis (F. hepatica and F. gigantica) is recognized by the WHO as an important FBT disease with an estimated 2.4 million people infected annually and 180 million at risk in over 61 countries (Haseeb et al., 2002). Large-scale epidemics occurred in France, Egypt and Iran (Parkinson et al., 2011).
In the genus Fasciola, three species are currently recognized; F. hepatica, F. gigantica Cobbold, 1855, and F. nyanzae Leiper, 1910 (Mas-Coma et al., 2009; Rajapakse et al., 2020). Among them, the most common species worldwide are F. hepatica, F. gigantica and their hybrid forms (Mas-Coma et al., 2009, 2019). In Europe, the Americas and Oceania, only F. hepatica is found, whereas both species and their hybrids are commonly found in Asia and Africa (Mas-Coma et al., 2005, 2019). For phylogenetic analyses of Fasciola spp. worms, sequences of nuclear rDNA, including ITS1-5.8S-ITS2 and 28S rDNA, and mitochondrial genes, including nd1 and cox1, have been found to be useful and popularly used to differentiate F. hepatica, F. gigantica and the intermediate forms (Mas-Coma et al., 2009, 2018, 2019). Molecular analyses of five Korean Fasciola worms from cattle using ITS-2 and 28S rDNA revealed that one possessed the F. hepatica-type sequence, two F. gigantica-type sequences, and two possessed sequences of both types (Agatsuma et al., 2000). An adult specimen recovered from a Korean male patient revealed F. hepatica ITS-1 genotype (Kang et al., 2014). In China, using ITS-2 sequences to perform RFLP analysis, the Fasciola worms from Sichuan Province represented F. hepatica, those from Guangxi Province represented F. gigantica, and the ones (from sheep) from Heilongjiang Province may represent an ‘intermediate genotype’ (Huang et al., 2004). In Japan, both ITS-1 and ITS-2 were useful to discriminate Fsp1 (F. hepatica), Fsp2 (F. gigantica) and Fsp1/2 (intermediate form), but nd1 and cox1 could not discriminate the intermediate form properly (Itagaki et al., 2005). Also in Europe, ITS-2 showed low variability for both fasciolid species and appeared to furnish valuable information (Mas-Coma et al., 2009). It is also notable that in Asian countries there are aspermic Fasciola flukes that may reproduce parthenogenetically (Itagaki et al., 2005).
A wide variety of freshwater snails that belong to the Lymnaeidae play the role of the first intermediate host (Bargues and Mas-Coma, 2005). In Asia, Europe, Africa and the Western Pacific, Galba truncatula (syn. Lymnaea truncatula) and Austropeplea ollula (syn. Austropeplea viridis) snails are most frequently involved (Bargues and Mas-Coma, 2005; Chai and Jung, 2019). Encysted metacercariae of F. hepatica are found on leaves of aquatic vegetables (watercress, alfalfa, water lettuce etc.) or green vegetation, bark or other smooth surfaces above or below the waterline (Beaver et al., 1984; Mas-Coma and Bargues, 1997; Liu and Zhu, 2013). People in Latin America, France and Algeria frequently acquire this disease by eating raw watercress (Nasturtium officinale) (Beaver et al., 1984). In Asia, water bamboo, water caltrop and morning glory were suspected to be important sources of human infections. Recently in South Korea, the water parsley (water dropwort) and its juice have been suspected as the potential source of sporadic human infections (unpublished data). Humans can also be infected by eating raw livers of infected animals containing immature or young worms, and these worms attach to the pharyngeal mucosa and cause pain, bleeding, oedema and dyspnoea, and this condition was once called ‘halzoun’ (Beaver et al., 1984).
F. hepatica is distributed almost worldwide, especially where extensive sheep and cattle raising is popularly done (Liu and Zhu, 2013). Human fascioliasis is one of the major public health problems in northern Africa, western Europe, Andean countries, the Caribbean area and the Caspian areas (Mas-Coma and Bargues, 1997). In South Korea, Japan, China, Thailand and Vietnam, sporadic cases have been reported (Chen and Mott, 1990; Mas-Coma and Bargues, 1997; Chai and Jung, 2019). It is of particular note that the highest prevalence (up to 72% by coprological and 100% by serological tests) and intensity ever have been found in some communities in the Northern Bolivian Altiplano (Mas-Coma and Bargues, 1997).
The pathological changes in the liver and bile duct of infected humans or animals depend primarily on the number of flukes infected (Chen and Mott, 1990), and the pathology in human infections is similar to that reported in animals (Mas-Coma and Bargues, 1997). However, in about 50% of cases, human fascioliasis is asymptomatic probably due to infection with a low number of flukes (Liu and Zhu, 2013). In rare instances (<10% of all patients), ectopic fascioliasis can occur (Beaver et al., 1984; Mas-Coma and Bargues, 1997). The pathogenicity of F. hepatica and F. gigantica in humans is not recognizably different, although in sheep F. gigantica was found to be more pathogenic (Mas-Coma and Bargues, 1997).
After excystation in the duodenum or jejunum of their definitive host, the metacercariae of F. hepatica migrate through the intestinal wall and peritoneal cavity, and then penetrate into the liver parenchyma through Glisson's capsule and finally reside in the bile duct and gall bladder or go astray to other ectopic locations (Beaver et al., 1984; Liu and Zhu, 2013). They can cause mechanical damage with focal haemorrhage, inflammatory reactions and necrotic lesions (Beaver et al., 1984). Adult worms may live between 9 and 13.5 years in humans (Mas-Coma et al., 2005). Stone formation in the bile duct or gall bladder is frequent, but liver cirrhosis is less common (Mas-Coma et al., 2019). The pathogenesis of ectopic fascioliasis is not fully understood. The ectopic locations include the skin, subcutaneous tissue, skeletal muscle, blood vessel, lungs, orbit, ventricles of the brain, stomach, appendix, pancreas, intestinal wall, heart, spleen, epididymis and lymph nodes (Beaver et al., 1984; Chen and Mott, 1990).
The diagnosis of human fascioliasis can be done directly using parasitological techniques and indirectly by immunological tests and radiologic images, including ultrasound, CT, MRI and radioisotope scanning (Mas-Coma et al., 2019).
Triclabendazole is currently the drug of choice for human fascioliasis (Fairweather, 2009; Mas-Coma et al., 2019) (Table 2). A problem related to triclabendazole is the appearance of drug resistance particularly in livestock animals; it was first described in Australia and then in many European and South American countries (Mas-Coma et al., 2019). Praziquantel was also used for human fascioliasis but treatment failure was experienced even at high doses (Mas-Coma et al., 2019). Albendazole, nitazoxanide, Mirazid® and artesunate are alternative drugs for potential use in human and animal fascioliasis (Mas-Coma et al., 2019; Chai et al., 2021a). Infection may be prevented by strict avoidance of consuming watercress and other metacercaria-carrying aquatic plants in endemic areas (Mas-Coma et al., 2019).
Fasciola gigantica Cobbold, 1855 (the giant cattle liver fluke) is a large liver fluke species infecting the bile duct of domestic animals, including cattle and sheep, and occasionally humans (Beaver et al., 1984; Mas-Coma and Bargues, 1997; Liu and Zhu, 2013). In Asia and Africa, F. hepatica, F. gigantica and their hybrid forms are found, but in Europe, the Americas and Oceania, only F. hepatica is found (Mas-Coma et al., 2005, 2019). The parasite's life cycle, biology, pathogenesis, pathology, epidemiology and clinical symptoms are similar to those of F. hepatica (Chai and Jung, 2019). The most important snail host for F. gigantica is Radix auricularia (Liu and Zhu, 2013). The absence of F. gigantica in the New World may be explained by the absence of these snail species (Radix spp.) (Mas-Coma et al., 2009). The metacercariae are encysted on the leaves of aquatic plants (Garcia, 2016).
Lung flukes
Paragonimiasis is a zoonotic disease caused by lung flukes of the genus Paragonimus (Chai and Jung, 2018). As of 1999, more than 50 nominal species had been described in this genus (Blair et al., 1999a; Narain et al., 2010; Doanh et al., 2013a). However, 16–17 of them were synonymized with the others, and the remaining 36 species were regarded as valid or potentially valid (Blair et al., 1999a). Thereafter, five new species were described from Asia and Africa; P. vietnamensis (Doanh et al., 2007), P. pseudoheterotremus (Waikagul, 2007), P. sheni (Shan et al., 2009), P. gondwanensis (Bayssade-Dufour et al., 2014) and P. kerberti (Bayssade-Dufour et al., 2015). Among the 41 nominal species (or subspecies), at least 9 are known to cause human infections, including P. westermani, P. africanus, P. gondawanensis, P. heterotremus, P. kellicotti, P. mexicanus, P. skrjabini, P. skrjabini miyazakii and P. uterobilateralis (Table 3) (Chai, 2013b; Bayssade-Dufour et al., 2014; Chai and Jung, 2018; Blair, 2019). The lung flukes can cause pulmonary as well as extrapulmonary infections in humans. The global number of people infected with Paragonimus spp. was estimated at about 23 million in 48 countries (mostly in China) with 292 million people at risk worldwide (Blair, 2019).
Table 3.
Lung flukes infecting humans with biological, clinical characteristics and geographical distribution
| Species of flukes | First intermediate host (snail) | Second intermediate host | Reservoir host | Major clinical characteristics | Geographical distributiona |
|---|---|---|---|---|---|
| Paragonimus westermani |
Semisulcospira spp. Juga spp. Melanoides tuberculata Brotia spp. Tarebia granifera |
Crab, crayfish | Dog, cat, pig, leopard, tiger, fox, wolf, opossum, mink, monkey | Haemoptysis, bronchiectasis | China, Taiwan, South Korea, Japan, Southeast Siberia (Russia), The Philippines, Malaysia, Thailand, Cambodia, Laos, Vietnam, Myanmar, Sri Lanka, India, Nepal, Pakistan, Papua New Guinea |
| Paragonimus africanus | Potadoma freethii Melania sp. | Crab | Dog, mongoose, civet, drill, monkey | Haemoptysis, bronchiectasis | Guinea, Cameroon, Nigeria, Ivory Coast |
| Paragonimus gondwanensis | Unknown | Crab | Cat, civet | Unknown | Cameroon |
| Paragonimus heterotremus | Various snail species | Crab | Dog, cat, monkey, Mongolian gerbil, rabbit | Haemoptysis, bronchiectasis | China, Vietnam, Laos, Thailand, Myanmar, Sri Lanka, India |
| Paragonimus kellicotti | Pomatiopsis spp. | Crab, crayfish | Dog, cat, pig, skunk, red fox, coyote, mink, bobcat | Haemoptysis, bronchiectasis | Canada, USA |
| Paragonimus mexicanus | Aroapygrus spp. | Crab | Dog, cat, opossum | Haemoptysis, bronchiectasis | Mexico, Peru, Ecuador, Coasta Rica, Panama, Guatemala |
| Paragonimus skrjabini | Various snail species | Crab | Dog, cat, rat, mouse, weasel, monkey | Ectopic lesions | China, Vietnam, northeastern India |
|
Paragonimus skrjabini miyazakii |
Oncomelania sp. | Crab | Dog, cat, marten, weasel, wild boar | Haemoptysis, ectopic lesions | Japan |
| Paragonimus uterobilateralis | Unknown | Crab | Dog, cat, otter, mongoose, swamp, shrew | Haemoptysis, bronchiectasis | Cameroon, Nigeria, Liberia, Guinea, Ivory Coast, Gabon |
The geographical distributions of lung flukes are mostly referred from Chai and Jung (2019).
Species involved
Paragonimus westermani
Paragonimus westermani (Kerbert, 1878) Braun, 1899 (the Oriental lung fluke) was originally reported from the lungs of a Bengal tiger (India) that died in the Zoological Garden in Amsterdam (Beaver et al., 1984). Since then, this species has been reported mainly in Asian countries (Blair et al., 1999a; Narain et al., 2010). Human infections were first found in a Portuguese residing in Taiwan through recovery of adult worm (s) after autopsy in 1880 and also in Japanese patients through detecting eggs from bloody sputum in 1880 and then adult worms in 1883 in Japan (Beaver et al., 1984). Now human infections with this lung fluke continue to occur in Asian countries, including China, Taiwan, Japan, South Korea, Far Southeast Russia, the Philippines and recently India (Chai, 2013b; Singh et al., 2015; Blair, 2019). While human infections may be decreasing in Japan and South Korea, new endemic foci have been detected in several other countries (Yoshida et al., 2019). Morphologically P. westermani differs from other Paragonimus species in the patterns of lobation of the ovary and testes (Miyazaki, 1991). The ovary of P. westermani has 6 simple lobes, whereas that of P. mexicanus or P. ohirai displays many delicate branches (Miyazaki, 1991).
Paragonimus siamensis was described as a new species from experimental cats infected with metacercariae from the freshwater crab Parathelphusa germaini in Thailand (Miyazaki and Wykoff, 1965). Later, this lung fluke was found in animals in Sri Lanka (Kannagara and Karunaratne, 1969), India (Devi et al., 2013) and the Philippines (Yoshida et al., 2019). An adult specimen recovered from a New Guinea native man in 1926 was preserved in School of Public Health and Tropical Medicine, Australia, and it was restudied and assigned as P. siamensis (Wang et al., 2011). Filopaludina martensi martensi snails are the first intermediate host (Yaemput et al., 1994), and freshwater crabs, including Ceylonthelphusa rugosa, play the role of the second intermediate hosts (Blair et al., 1999a). Recent molecular studies reported that P. siamensis is nested within the P. westermani complex (Devi et al., 2013; Blair, 2019).
Phylogenetic studies revealed that P. westermani is a species complex comprising of two groups; the East Asia group (Japan, South Korea, China and Taiwan) and the Southeast Asia group (Malaysia and the Philippines) based on morphological and molecular data (Blair et al., 1997; Doanh et al., 2009). However, recent discoveries of P. westermani in Thailand (Sugiyama et al., 2007), India (Tandon et al., 2007) and Sri Lanka (Iwagami et al., 2008) provided evidence that P. westermani complex was constructed with more than two groups (Blair et al., 2007).
The first intermediate host is variable species of freshwater snails (Table 3). The second intermediate host is crustaceans, including freshwater crayfish Cambaroides spp. (C. similis, C. dauricus and C. schrenki), Eriocheir spp. crabs (E. japonicus and E. sinensis) and a variety of other crab species belonging to the family Potamidae or Parathelphusidae (Blair et al., 1999a). In China, Taiwan and South Korea, freshwater shrimps (Macrobrachium nipponensis and other species) were also reported as the second intermediate hosts (Blair et al., 1999a). There are several kinds of paratenic hosts, for example, wild boars, bears, wild pigs and rats; in these hosts worms do not mature to be adults but remain at a juvenile stage; they can be an important source of human infection (Miyazaki and Habe, 1976; Shibahara et al., 1992). The egg-laying capacity of Paragonimus worms was reported to be 11 000– 104 000 eggs per day per worm (EPD/worm) in experimental dogs (Yokogawa, 1965).
The principal mode of human P. westermani infection is consumption of raw or improperly cooked (pickled) freshwater crabs or crayfish (Chai, 2013b). In Asian countries, famous dishes causing human paragonimiasis have been known, for example, ‘drunken crab’ in China, ‘Kejang (= sauced crab)’ in South Korea, ‘Oboro-kiro (= crab juice soup)’ in Japan, ‘Goong ten (= raw crayfish salad)’ in Thailand, and ‘Kinuolao (= raw crab)’ in the Philippines (Kim, 1984a; Nakamura-Uchiyama et al., 2002). It is of note that freshwater crab or crayfish juice was used in South Korea and Japan for traditional treatment of febrile diseases, such as measles, asthma and urticaria (Kim, 1984a; Nakamura-Uchiyama et al., 2002). This kind of practice was formerly an important mode of contracting paragonimiasis, especially in children. Another important mode of infection in Japan is ingestion of raw or undercooked boar meat containing P. westermani metacercariae or juvenile worms (Miyazaki and Habe, 1976; Shibahara et al., 1992; Nakamura-Uchiyama et al., 2002). In paratenic hosts, worms do not mature and stay in muscles and tissues; when they were eaten by humans, they could develop into adult worms (Nakamura-Uchiyama et al., 2002; Chai, 2013b).
The geographical distribution of P. westermani is wide, including East Asia, Southeast Asia, South Asia (including India) and even the Western Pacific (Blair, 2019). Human cases of P. westermani were reported also in the USA (Fried and Abruzzi, 2010; Boland et al., 2011); however, the existence of the life cycle of P. westermani in North America needs to be verified.
When the definitive host, including humans, consumes crab or crayfish meat containing metacercariae, they excyst in the duodenum and penetrate into the intestinal wall; during this process they mature into juvenile flukes (Chai and Jung, 2018). The juvenile flukes enter the inner wall (abdominal muscle) of the abdominal cavity and reappear in the abdominal cavity; they then penetrate the diaphragm and enter the pleural cavity in about 14 days after infection (Yokogawa, 1965). In the pleural cavity, two worms mate and then move to the lung parenchyma where a fibrous cyst called the ‘worm cyst’ or ‘worm capsule’ develops around them (Narain et al., 2010; Chai, 2013b). The two worms exchange sperm and produce eggs within the worm cyst, which accumulate for some time in acute stages but in chronic stages when tissue necrosis occurs around the cyst, the eggs escape from the cyst into small bronchioles (Yokogawa, 1965; Blair et al., 1999a). There are triploid and tetraploid forms of P. westermani which do not produce sperm and reproduce parthenogenetically (Miyazaki, 1991; Terasaki et al., 1995; Agatsuma et al., 2003). The patients may undergo variable clinical types, including pulmonary, thoracic, abdominal, cerebral, spinal and cutaneous paragonimiasis (Nakamura-Uchiyama et al., 2002; Chai, 2013b).
In pulmonary infections, P. westermani worms lie in worm cysts of host origin, 1–2 cm in diameter, within the lung parenchyma (Blair et al., 1999a). The lung lesions can be classified into infiltrative, nodular and cavitating shadow types, or combination of these types (Nakamura-Uchiyama et al., 2002). In early stages of infection, there is no exit from the worm cyst, and eggs as well as excretions, metabolic products and tissue debris may gather progressively within the cyst, which becomes distended, and the cyst wall becomes thick and fibrotic (Yokogawa, 1965). In chronic stages, eggs can be demonstrated in the blood-tinged portion of the sputum (Chai, 2013b). The earliest possible clinical manifestations by day 15 after infection include abdominal pain, fever, chill, fatigue and diarrhoea (Choi, 1990; Nakamura-Uchiyama et al., 2002; Procop, 2009). Eosinophilia of up to 25% can occur at 2 months after infection (Choi, 1990; Procop, 2009). In chronic stages, the most common and important manifestations include chronic cough, rusty-coloured sputum (= haemoptysis), chest pain, dyspnoea and crepitation (Im et al., 1993; Nakamura-Uchiyama et al., 2002; Procop, 2009). In chest radiographs, cavitating lesions called ‘ring shadows’ or ‘cysts’ are commonly seen (Im et al., 1993). Pleural effusion is frequently seen in Korean and Japanese patients (Im et al., 1993; Nakamura-Uchiyama et al., 2002).
The extrapulmonary migration of Paragonimus worms has been suggested to occur due to several reasons. The most important reason is the complex migration route of the worms (Chai, 2013b). Another reason was suggested to be seeking sexual partners to exchange sperm (Miyazaki, 1991). The brain is the most commonly involved ectopic site, although the mechanisms and route of worm migration to the brain are not well understood (Choi, 1990).
Cerebral paragonimiasis occurs quite commonly in P. westermani infection, about 1% of all paragonimiasis patients (Oh, 1969). Five major symptoms include the Jacksonian type seizure, headache, visual disturbance, motor and sensory disturbances, and 5 major signs are optic atrophy, mental deterioration, hemiplegia, hemi-hypalgesia and homonymous hemianopsia (Oh, 1969). Intracranial haemorrhage can also occur though rare in the incidence (Choo et al., 2003; Koh et al., 2012). In skull radiography, cerebral calcification is the most commonly encountered finding, and temporal, occipital and parietal lobes of the brain are the predilection sites (Oh, 1968b). Compared with cerebral infections, spinal paragonimiasis is relatively rare (Oh, 1968a). The predilection site is extradural areas of the thoracic level (Choi, 1990).
Abdominal paragonimiasis is probably more common than cerebral and spinal infections (Meyers and Neafie, 1976). The affected organs include the abdominal wall (muscle), peritoneal cavity, liver, spleen, pancreas, heart, greater omentum, appendix, ovary, uterus, scrotum, inguinal regions, thigh and urinary tract (Chai, 2013b). Cutaneous and subcutaneous paragonimiasis are very rare compared to pleuropulmonary and other ectopic types (Chai, 2013b). P. westermani can cause abscesses and ulcers in the skin or subcutaneous tissue, but P. skrjabini can cause migrating subcutaneous nodules (Meyers and Neafie, 1976).
Conventional methods to diagnose human Paragonimus infections are microscopic examinations of the sputum or fecal samples for detecting eggs, chest radiography to observe the lung lesions (differential diagnosis needed with tuberculosis and lung cancers), and serological tests, including intradermal test, indirect haemagglutination test, ELISA, and others to detect antibodies (Lee et al., 2003; Sugiyama et al., 2013; Chai, 2013b). Sputum eggs can usually be detected in chronic cases when the worm cyst is ruptured and connected to bronchioles. In children, aged-people or handicapped individuals, sputum is frequently swallowed, and in such cases, eggs could be detected in feces (Chai, 2013b). Among the serological tests, ELISA (such as microplate ELISA and multiple-dot ELISA) is so far the most reliable tool because this assay method shows high sensitivity and high specificity (Lee et al., 2003; Yoshida et al., 2019). Molecular methods, including PCR and DNA sequencing, have become useful for specific identification of Paragonimus eggs and worms (Sugiyama et al., 2013). Other methods, including PCR-RFLP, multiplex PCR, random amplified polymorphic DNA (RAPD), and DNA hybridization, have also been applied for specific identification of Paragonimus (Narain et al., 2010; Sugiyama et al., 2013).
Praziquantel is the drug of choice for treating paragonimiasis (P. westermani and other Paragonimus spp.), including cerebral infections (Chai, 2013b) (Table 2). Rarely, allergic reactions may occur following praziquantel treatment; however, such patients can be successfully treated by desensitization to praziquantel (Kyung et al., 2011). Triclabendazole is a new promising drug for the treatment of pulmonary paragonimiasis (Keiser et al., 2005). However, its efficacy on cerebrospinal paragonimiasis remains to be determined (Chai, 2013b). Prevention of contamination of the crab and crayfish with the cercariae of P. westermani depends on environmental control of surface waters through the elimination of freshwater snails. Long-term health education of young people to avoid eating raw or improperly cooked crayfish or crabs seems to be one of the best ways to prevent P. westermani infection in endemic areas.
Paragonimus africanus
Paragonimus africanus Voelker and Vogel, 1965 was originally described from the mongoose in Cameroon and is now known to be distributed throughout sub-Saharan Africa (Narain et al., 2010). Up to 2008, it was estimated that there had been 2295 confirmed cases of human paragonimiasis in Africa mostly due to P. africanus and P. uterobilateralis with the vast majority occurring in Nigeria and Cameroon (Aka et al., 2008; Cumberlidge et al., 2018). Freshwater crabs Liberonautes spp. and Sudanonautes spp. carry the metacercariae (Aka et al., 2008). Its morphological characters include the distinctly larger oral sucker than the ventral sucker, a delicately branched ovary, and highly branched testes which are significantly larger than the ovary (Narain et al., 2010). Uncooked crab meat is an important source of human infection (Aka et al., 2008). Nucleotide sequences of ITS2 and cox1 have been used for specific diagnosis (as P. africanus) of fecal eggs from monkeys (Friant et al., 2015) and humans (Nkouawa et al., 2009). Clinical manifestations are similar to P. westermani infection. Cerebral infection has been suspected but never proved (Aka et al., 2008). Sputum and stool examinations to detect eggs are the main diagnostic procedures.
Paragonimus gondwanensis
Paragonimus gondwanensis Bayssade-Dufour et al., 2014 was described as a new species from humans (only eggs) and carnivorous mammals (adult worms) in Cameroon (Bayssade-Dufour et al., 2014). This species is morphologically distinct from all other Paragonimus species in having a very short excretory bladder, once used as a character for a different genus, Euparagonimus (Bayssade-Dufour et al., 2014). However, molecular data are lacking, and the validity of this species remains to be determined (Rabone et al., 2021). The second intermediate host is freshwater crabs, Sudanonautes africanus (Bayssade-Dufour et al., 2014).
Paragonimus heterotremus
Paragonimus heterotremus Chen and Hsia, 1964 was first discovered from rats in China and is now known to be distributed in China, Indochina peninsula, Sri Lanka and India (Sing et al., 2009; Narain et al., 2010; Yoshida et al., 2019). In 2007, P. pseudoheterotremus was reported as a new species from a cat experimentally infected with the metacercariae in crabs from a mountainous area of Thailand (Waikagul, 1997). Later, however, this species is considered a geographical variation of the P. heterotremus complex, based on molecular studies using ITS2 and cox1 sequences (Sanpool et al., 2013; Doanh et al., 2015; Tantrawatpan et al., 2021). Human infection with P. heterotremus was first identified by the recovery of an adult worm from a 13-year-old boy in Nakorn-Nayok Province, Thailand in 1965 (Miyazaki and Harinasuta, 1966). In the same year, eggs presumed to be of P. heterotremus were detected in the bloody sputum of a patient in Guangxi, China (Zhou et al., 2021). Since then, a lot of human cases have been reported (Miyazaki, 1991; Singh et al., 2009; Doanh et al., 2013a). Freshwater snails, Assiminea sp., Oncomelania hupensis and Neotricula aperta (syn. Tricula aperta), are known to serve as the first intermediate host, and freshwater crabs, including Larnaudia beusekomae, Siamthelphusa paviei and Potamiscus smithianus (Thailand), Potamon flexum and Sinolapotamon patellifer (China), take the role of the second intermediate hosts (Blair et al., 1999a). In adult specimens, the ventral sucker is characteristically small, about a half the diameter of its oral sucker (Narain et al., 2010). The ovary and testes are both delicately branched (Miyazaki, 1991). The pathology and clinical manifestations in P. heterotremus infection is similar to those seen in P. westermani infection. The diagnosis is based on the recovery of eggs in bloody sputum, serology, chest radiography and molecular genetic analysis.
Paragonimus kellicotti
Paragonimus kellicotti Ward, 1908 was originally discovered in a cat and a dog in the USA (Blair et al., 1999a; Procop, 2009). The lobation of the ovary and testes is more complex than in P. westermani (Procop, 2009). Now this species is known to occur in central and eastern parts of the USA and adjacent areas of Canada (Procop, 2009). Beaver et al. (1984) considered the first human case to be a German labourer who worked in the USA in the 1890s and had eaten crayfish. Another case was a Canadian man aged 51 years who had never been outside of Quebec and complained of systemic and pulmonary symptoms (Béland et al., 1969; Coogle et al., 2021). Thereafter, from 1984 until 2017, a total of 20 human cases have been documented (Lane et al., 2009, 2012; Procop, 2009; Fried and Abruzzi, 2010; Coogle et al., 2021). Molecular studies were done on ITS2, cox1 and other genetic loci (Blair et al., 1999b; Fischer et al., 2011; McNulty et al., 2014). Based on ITS2 and cox1 sequences, P. kellicotti was genetically closest to P. macrorchis and P. mexicanus, and then to P. heterotremus, but distant from P. westermani and P. siamensis (Blair et al., 1999b). The draft genome of P. kellicotti has been established and analysed in comparison with those of P. westermani, P. skrjabini miyazakii and P. heterotremus (Rosa et al., 2020). The second intermediate host is the crayfish of Cambarus bartoni, C. robustus and C. virilis, Orconectes propinquus, O. rusticus and Procambarus blandingi acutus, or crabs Geothelphusa dehaani (Blair et al., 1999a). In the USA, frozen or pickled crabs available at markets are suspected to be the source of P. kellicotti infection (Procop, 2009). Pleuropulmonary infection is dominant in P. kellicotti infection, and the most frequent clinical manifestations among 21 North American patients were cough, pleural effusion, fever, fatigue or malaise, weight loss, chest pain, dyspnoea, haemoptysis and eosinophilia (Coogle et al., 2021). Their diagnosis was based on microscopic examinations of sputum, pleural fluid or bronchoalveolar lavage and serological tests using complement fixation test, immunoblot or western blot (Coogle et al., 2021).
Paragonimus mexicanus
Paragonimus mexicanus Miyazaki and Ishii, 1968 (syn. Paragonimus peruvianus) was first described from opossums in Colima, Mexico (Miyazaki and Ishii, 1968). Its characteristic morphology includes a somewhat larger oral sucker than the ventral sucker and a strongly lobed ovary and two testes (Miyazaki, 1991). Metacercariae have no cyst wall (Sugiyama et al., 2013). P. mexicanus is now known to be distributed in Central and South Americas (Blair et al., 1999a). A human case reported by Báez M. and Galán J. in 1961, a 35-year-old Mexican man from whom eggs were detected in lung tissue, was believed to have been due to P. mexicanus infection (Miyazaki and Ishii, 1968). In Costa Rica, 2 human cases (total 4) were reported in 1968 and 1982 (Beaver et al., 1984), and since then, 28 additional cases were described until 2013 (Hernández-Chea et al., 2017). In Ecuador, as early as in 1922, a paragonimiasis patient was found from a coastal region of Chone-Manabi (Calvopiña et al., 2014); this seems to be the first human case infected with P. mexicanus. Thereafter, until 2007, the total number of human infections in Ecuador was 3822 patients (Calvopiña et al., 2014). Using a combination of ITS2, 28S and cox1 sequences, the phylogenetic position of P. mexicanus was analysed, and it was closest to P. heteretremus and then P. macrorchis; however, far from P. westermani and P. siamensis (Devi et al., 2013). However, in Mexico, Ecuador (López-Caballero et al., 2013) and Guatemala (Landaverde-González et al., 2022), several new genetic groups distinct from P. mexicanus (origin; Colima, Mexico) were found based on cox1 sequences. One from Ecuador could be assigned as P. ecuadoriensis Voelker and Arzube, 1979 (once synonymized with P. mexicanus by Vieira et al. in 1992), and two possible new species (or subspecies) groups included one from Chiapas, Mexico and San José, Guatemala and the other from Veracruz, Mexico (Iwagami et al., 2003; López-Caballero et al., 2013). Freshwater crabs, Hypolobocera aequatorialis, H. chilensis and H. guayaquilensis, Pseudothelphusa dilatata, P. nayaritae, P. propinqua and P. terrestris carry the metacercariae (Blair et al., 1999a; Calvopiña et al., 2018). Raw crabs with vegetables and lemon juice is the main infection source for Peruvians (Nakamura-Uchiyama et al., 2002), and ceviche that contains uncooked crustacean meat is an important source for Mexicans (Procop, 2009). Clinically P. mexicanus infection is mostly of the pulmonary type (99.7% of 3822 cases in Ecuador) (Calvopiña et al., 2014). However, cerebral infection with intracerebral haemorrhage can occur (Brenes Madrigal et al., 1982).
Paragonimus skrjabini
Paragonimus skrjabini Chen, 1959 (syn. P. szechuanensis, P. hueitungensis and P. veocularis) was first described from the lungs of viverrid cats in Guangzhou, China (Blair et al., 1999a). Phylogenetic studies revealed that there exists a P. skrjabini complex, which includes P. skrjabini, P. skrjabini miyazakii and P. proliferus (syn. P. hocuoensis) (Doanh et al., 2013b; Yang et al., 2021; Shu et al., 2021a). P. skrjabini is now known to occur in China, Thailand, Vietnam and northeast India (Singh et al., 2006; Doanh et al., 2013b; Blair, 2019). Human infections were reported for the first time in Sichuan and then in Hunan (Blair et al., 1999a). Numerous provinces in China have been found to be endemic for P. skrjabini infection (Zhou et al., 2021). The first intermediate host is freshwater snails, including Assiminea lutea, Tricula spp. and Neotricula spp., and the second host is freshwater crabs, Aprapotamon grahami, Isolapotamon spp., Sinopotamon spp., Tenuilapotamon spp., Stigmatomma denticulatum and Haberma nanum (Blair et al., 1999a; Shu et al., 2021b). Morphologically this species is characterized by profusely branched ovary and testes and an elongated body (Blair et al., 2005; Narain et al., 2010). P. skrjabini more frequently cause cutaneous or cerebral infections than pulmonary lesions, and the skin lesions usually contain juvenile flukes; thus, humans are considered an abnormal definitive host (Nakamura-Uchiyama et al., 2002). Diagnosis is most commonly done by serological tests, including intradermal test and ELISA (Yu et al., 2017).
Paragonimus skrjabini miyazakii
Paragonimus skrjabini miyazakii (Kamo, Nishida, Hatsushika and Tomimura, 1961) Blair et al., 2005 (syn. P. miyazakii) was first described from dogs experimentally fed the metacercariae from a freshwater crab in Japan (Kamo et al., 1961). Human infections were first identified in the Kanton District of Honshu, Japan (Yokogawa et al., 1974). This lung fluke has been found in Shikoku, Kyushu, and the southern half of Honshu, but never from outside of Japan (Miyazaki, 1991). In the University of Miyazaki, Japan, approximately 800 cases (17–49 cases annually) of human paragonimiasis (predominantly by P. westermani and less frequently by P. skrjabini miyazakii) were diagnosed between 1986 and 2018 (Yoshida et al., 2019). Yatera et al. (2015) reviewed 46 P. skrjabini miyazakii patients reported from 1974 to 2009 having pulmonary involvement. Phylogenetic studies revealed that P. skrjabini miyazakii is clearly included among the P. skrjabini complex (Blair et al., 2005; Doanh et al., 2013b; Yang et al., 2021; Shu et al., 2021a; 2021b). Freshwater crabs, Geothelphusa dehaani carry the metacercariae (Blair et al., 1999a). The adult flukes are somewhat elongated with severely branched ovary and testes (Miyazaki, 1991; Blair et al., 2005). Whereas the main clinical features of P. skrjabini miyazakii infection are pleural manifestations with pneumothorax and pleural effusion (in most cases worms cannot mature to become adults in humans), those of P. westermani infection are pulmonary involvement (Yatera et al., 2015).
Paragonimus uterobilateralis
Paragonimus uterobilateralis Voelker and Vogel, 1965 was originally described from the mongoose in Cameroon (Narain et al., 2010), and is now known to be distributed in mid-western sub-Saharan Africa (Blair et al., 1999a). Human infection was first found in Nigeria (Blair et al., 1999a). Up to 2008, there had been 2295 confirmed cases of human paragonimiasis in Africa, which was mostly due to P. africanus and P. uterobilateralis; the vast majority of these cases occurred in Nigeria and Cameroon (Aka et al., 2008; Cumberlidge et al., 2018). Freshwater crabs, Liberonautes spp. and Sudanonautes spp., are the second intermediate hosts (Blair et al., 1999a). This lung fluke is morphologically similar to P. africanus but differs in having similar-sized oral and ventral suckers (Miyazaki, 1991). It has a delicately branched ovary; however, it has moderately branched testes larger than the ovary (Blair et al., 1999a). Uncooked crab meat is an important source of human infection (Aka et al., 2008). Clinical manifestations are similar to P. westermani infection. Cerebral infection has been suspected but never proved (Aka et al., 2008).
Intestinal flukes
Intestinal flukes are taxonomically diverse, including three large groups, namely, heterophyids (family Heterophyidae), echinostomes (family Echinostomatidae), and other groups, including amphistomes, brachylaimids, cyathocotylids, diplostomes, fasciolids, gymnophallids, isoparorchiids, lecithodendriid-like flukes, microphallids, nanophyetids, plagiorchiids and strigeids (Yamaguti, 1971; Chai, 2019; Chai and Jung, 2020). At least 75 different species have been described which infect an estimated 40–50 million people (Chai et al., 2009; Chai and Jung, 2020). Among them, heterophyids include 28 species (14 species are with more than 10 human cases; Table 4), echinostomes include 24 species (16 species; Table 5), and other groups are comprised of 23 species (10 species; Table 6). In this section, 40 species having more than 10 human cases are briefly introduced in the text, and the other 35 miscellaneous species are briefly mentioned in Supplementary Table. Molecular techniques using ITS and cox1 genes have been applied to differentiate eggs, larvae, as well as adults of various heterophyid species (Duflot et al., 2021). Intestinal flukes are easily treated with 10 mg kg−1 single dose of praziquantel or 2 g single dose of niclosamide (Table 2).
Table 4.
Heterophyid intestinal flukes infecting humans with their life cycle and geographical distributiona
| Species of heterophyids | First intermediate host (snail) | Second intermediate host | Reservoir host | Geographical distribution |
|---|---|---|---|---|
| Metagonimus yokogawai | Semisulcospira sp. | Freshwater fish (sweetfish, dace) | Dog, cat, rat, fox, kite | China, India, Japan, South Korea, Taiwan, Russia |
| Metagonimus miyatai | Semisulcospira sp. | Freshwater fish (chub, minnow) | Dog, red fox, raccoon, kite | Japan, South Korea |
| Metagonimus takahashii | Semisulcospira sp. Koreanomelania sp. | Freshwater fish (carp) | Dog, cat, kite, pelican | Japan, South Korea |
| Heterophyes heterophyes | Pirenella conica | Brackish water fish (mullet, goby, tilapia) | Dog, fox, jackal | Egypt, Greece, India, Iran, Israel, Italy, Kuwait, Sri Lanka, Saudi Arabia, Spain, Sudan, Thailand, Tunisia, Turkey, United Arab Emirate, Yemen |
| Heterophyes nocens | Cerithidea sp. | Brackish water fish (mullet, goby) | Dog, cat | China, Japan, Korea, Thailand (?) |
| Haplorchis taichui | Melania sp. Melanoides sp. | Freshwater fish (carp, minnow) | Dog, cat, bird | Bangladesh, China, Egypt, India, Iraq, Israel, Kuwait, Lao PDR, Malaysia, Myanmar, The Philippines, Sri Lanka, Taiwan, Thailand, USA (Hawaii), Vietnam |
| Haplorchis pumilio | Melania sp. | Cyprinoid fish silurid fish cobitid fish |
Dog, cat | Australia, Cambodia, China, Egypt, India, Iraq, Israel, Kenya, Lao PDR, Malaysia, Mexico, Myanmar, Peru, The Philippines, Sri Lanka, Taiwan, Thailand, USA, Venezuela, Vietnam |
| Haplorchis yokogawai | Melanoides sp. | Freshwater fish (carp, loach) brackish water fish (mullet) |
Dog, cat, cattle | Australia, Cambodia, China, Egypt, India, Indonesia, Kuwait, Lao PDR, Malaysia, Myanmar, The Philippines, Sri Lanka, Taiwan, Thailand, USA (Hawaii), Vietnam |
| Centrocestus formosanus | Stenomelania sp. | Cyprinoid fish | Dog, fox, chicken, duck | Brazil, China, Colombia, Costa Rica, Croatia, Egypt, India, Japan, Lao PDR, Mexico, The Philippines, Taiwan, Thailand, Tunisia, Turkey, USA, Vietnam |
| Heterophyopsis continua | Unknown | Brackish water fish (perch, goby, shad) | Cat, duck, sea-gull | China, Japan, Korea, Saudi Arabia, Thailand, United Arab Emirates, Vietnam |
| Pygidiopsis summa | Cerithidea sp. | Brackish water fish (mullet, goby) | Cat | South Korea, Japan, Vietnam |
| Pygidiopsis genata | Melanoides sp. | Brackish water fish (mullet, tilapia) | Dog, cat, rat, fox, wolf, shrew, kite, pelican, duck | Egypt, Iran, Israel, Kuwait, Romania, The Philippines, Tunisia, Ukraine |
| Stellantchasmus falcatus | Stenomelania sp. | Brackish water fish (mullet) | Dog, cat | Australia, China, India, Iran, Israel, Japan, Korea, Lao PDR, The Philippines, Taiwan, Thailand,USA (Hawaii), Vietnam |
| Stictodora fuscata | Unknown | Freshwater and brackish water fish (carp, goby) | Dog, cat | South Korea, Japan, Kuwait |
Table 5.
Echinostomes infecting humans with their life cycle and geographical distribution
| Species of echinostomes | First intermediate host (snail) | Second intermediate host | Reservoir host | Geographical distributiona |
|---|---|---|---|---|
| Echinostoma revolutum |
Lymnaea sp. Physa sp. Segmentina sp. |
Freshwater snail, clam, tadpole | Dog, cat, rat, muskrat, duck, goose | Asia (Bangladesh, Cambodia, China, India, Indonesia, Japan, Korea, Lao PDR, Malaysia, Taiwan, Thailand, Vietnam), Europe (Austria, Belarus, Bulgaria, Czech Rep., England, Finland, France, Germany, Greece, Hungary, Iceland, The Netherlands, Poland, Russia, Slovak Rep., Yugoslavia), The Middle East (Iran), Oceania (New Zealand), North America (USA), South America (Brazil) |
| Echinostoma cinetorchis |
Hippeutis cantori Segmentina hemisphaerula |
Freshwater snail, fish (loach), tadpole | Rat | South Korea, Japan, China, Taiwan, Vietnam |
| Echinostoma ilocanum |
Gyraulus sp. Hippeutis sp. |
Large snails (Viviparus sp., Pila sp.) | Dog, rat | The Philippines, Cambodia, China, India, Indonesia, Lao PDR, Malaysia, Thailand |
| Echinostoma lindoense | Anisus spp. | Lymnaea, Planorbis, Gyraulus, Viviparus, Biomphalaria spp. | Bird, mammal | Indonesia, Malaysia, Thailand, The Philippines |
| Echinostoma mekongi | Unknown | Freshwater snail (Filopaludina sp.) | Unknown | Cambodia (probably also in Lao PDR, Thailand, Vietnam) |
| Isthmiophora hortensis |
Lymnaea sp. Radix sp. |
Freshwater fish (loach, etc.) | Dog, cat, rat | Japan, South Korea, China |
| Echinochasmus japonicus | Parafossarulus sp. | Cyprinoid fish | Cat, duck, egret | Japan, South Korea, China, Kuwait, Lao PDR, Russia, Taiwan, Thailand, Vietnam |
| Echinochasmus perfoliatus |
Parafossarulus sp. Bithynia sp. Lymnaea sp. |
Freshwater fish (carp, chub) | Dog, cat, rat, fox, wild boar, fowl | Bulgaria, China, Croatia, Denmark, Egypt, England, Hungary, India, Italy, Japan, Korea, Poland, Romania, Russia, Serbia, Taiwan, Thailand, Ukraine, Vietnam |
| Echinochasmus liliputanus | Parafossarulus sp. | Freshwater fish (carp) | Dog, cat, fox, badger, raccoon | Egypt, China, Israel, Sri Lanka, Syria |
| Echinochamus fujianensis | Bellamya sp. | Freshwater fish (carp) | Dog, cat, rat, pig | China |
| Echinochasmus caninus | Unknown | Freshwater fish (Macropodus sp.) | Dog | India, China, Thailand, Vietnam, Lao PDR |
| Artyfechinostomum malayanum |
Indoplanorbis sp. Gyraulus sp. |
Large snails (Pila, Gyraulus, Indoplanorbis spp.) | Dog, cat, rat, pig, mouse, hamster, house-shrew | China, India, Indonesia, Lao PDR, Malaysia, The Philippines, Singapore, Thailand |
| Artyfechinostomum sufrartyfex |
Indoplanorbis sp. Lymnaea sp. |
Freshwater snail, fish, frog | Dog, rat, pig | India, Vietnam (?) |
| Artyfechinostomum oraoni | Lymnaea sp. | Unknown | Pig | India |
| Hypoderaeum conoideum |
Indoplanorbis sp. Planorbis sp. Lymnaea sp. |
Large snail, tadpole | Duck, goose, fowl | Bangladesh, China, Indonesia, Japan, Mexico, North America, Russia, Spain, Taiwan, Thailand |
| Acanthoparyphium tyosenense | Marine megagastropod marine gastropod |
Brackish water bivalve, gastropod | Duck | South Korea, Japan |
Table 6.
Other intestinal fluke species infecting humans with their life cycle and geographical distribution
| Species of intestinal fluke | First intermediate host (snail) | Second intermediate host | Reservoir host | Geographical distributiona |
|---|---|---|---|---|
| Brachylaima cribbi | Land snail (Theba pisana) | Land snail (Cernuella virgata) | Bird, reptile, mammal | Australia |
| Caprimorgorchis molenkampi | Bithynia sp. | Naiad of dragonfly | Rat, bat | Cambodia, Indonesia, Lao PDR, Thailand |
| Fasciolopsis buski |
Segmentina sp. Hippeutis sp. Gyraulus sp. |
Aquatic plant (water chestnut, water bamboo, water caltrop, water hyacinth, root of lotus) | Dog, pig, rabbit | Bangladesh, Cambodia, China, India, Indonesia, Japan, Korea, Lao PDR, Malaysia, Myanmar, Nepal, Pakistan, The Philippines, Singapore, Taiwan, Thailand, Vietnam |
| Gastrodiscoides hominis | Helicorbis coenosus | Aquatic plant, tadpole, frog, crayfish | Pig, mouse deer, rat, monkey | Cambodia, China, England, India, Indonesia, Japan, Kazakhstan, Malaysia, Myanmar, Nigeria, Pakistan, The Philippines, Russia, Thailand, USA, Vietnam, Zambia |
| Gymnophalloides seoi | Unknown | Oyster (Crassostrea gigas) | Palearctic oystercatcher, cat | South Korea |
| Microphallus brevicaeca | Unknown | Brackish water crab (Carcinus maenas), shrimp (Macrobrachium sp.) | Bird, monkey, mammal | Papua New Guinea, The Philippines |
| Nanophyetus salmincola | Oxytrema silicula | Salmonid fish non-salmonid fish |
Dog, cat, raccoon, fox, bird | Canada, USA |
| Nanophyetus schikhobalowi | Semisulcospira sp. | Salmonid fish non-salmonid fish |
Rat, fox | Russia |
| Neodiplostomum seoulense |
Hippeutis cantori Segmentina hemisphaerula |
Tadpole, frog, snake (paratenic host) | Rat, mouse | South Korea, China |
| Phaneropsolus bonnei | Bithynia sp. (?) | Naiad of dragonfly | Monkey | Cambodia, India, Indonesia, Lao PDR, Malaysia, Thailand |
Heterophyid species
Metagonimus spp.
Metagonimus yokogawai (Katsurada, 1912) Katsurada, 1912 was originally described from an experimentally infected dog in Taiwan and then reported mostly from Asian countries (Yu and Chai, 2013). Sequences of nuclear 28S rDNA and mitochondrial cox1 genes were used to discriminate this species from the related ones, M. miyatai and M. takahashii (Chai, 2019). The main clinical symptoms of M. yokogawai infection are mild-to-severe gastrointestinal trouble, including abdominal pain, diarrhoea and indigestion (Chai and Jung, 2020). The histopathology of the small intestine is characterized by villous atrophy and crypt hyperplasia accompanied by inflammatory reactions (Chai, 2019). The first case of human infection was discovered in Japan by Yokogawa S. in 1913, and thereafter numerous succeeding reports were published (Ito, 1964). The sweetfish Plecoglossus altivelis, dace Tribolodon hokonensis or T. taczanowskii, and perch Lateolabrax japonicus are the major fish intermediate host (Yu and Chai, 2013). The principal mode of human infection is ingestion of raw or improperly cooked fish (Chai et al., 2009; Chai and Jung, 2017). M. yokogawai is distributed mainly in the Far East and East Asia (Chai, 2019). In South Korea, endemic areas are scattered along almost all large and small rivers and streams in eastern and southern coastal areas (Chai and Lee, 2002; Chai et al., 2009). In Japan, the prevalence reported in humans has been generally lower than that in South Korea (Chai and Jung, 2020). In China, human infections were reported in Guangdong, Anhui, Hubei and Zhejiang Province (Yu and Mott, 1994). The diagnosis is based on the recovery of eggs in fecal examinations. In Russia, Metagonimus infection has been reported under the name M. yokogawai in the Amur and Ussuri valleys of the Khabarovsk Territory, and the prevalence was 20–70% among the ethnic minority group (Yu and Mott, 1994). Recently, however, the parasite in this area (obtained from experimental rats) was reported as a new species (Metagonimus suifunensis) based on molecular analyses (Shumenko et al., 2017).
Metagonimus miyatai Saito, Chai, Kim, Lee et Rim, 1997 was first found in Japan in 1941 but reported a long time later as a distinct species (Saito et al., 1997). Human infections seem to have existed in Japan but there had been no confirmed reports. In South Korea, human infections were first recognized along the Geum River in 1980 by detecting eggs in feces (Chai, 2019). Adult flukes were recovered from 32 people living along the Namhan River in Umsong and Yongwol County (Chai et al., 1993). Freshwater fish, including Zacco platypus, Z. temminckii, P. altivelis, Tribolodon hakonensis and T. taczanowskii, are the second intermediate hosts (Yu and Chai, 2013). In Japan, fish from small rivers in the Shizuoka Prefecture were found to have M. miyatai infection (Kino et al., 2006).
Metagonimus takahashii (Takahashi, 1929) Suzuki, 1930 was originally described in Japan and now known to exist also in South Korea (Yu and Chai, 2013). The crussian carp C. carassius, carp C. carpio, dace T. taczanowskii and perch L. japonicus carry the metacercariae (Yu and Chai, 2013). In Japan, this fluke was reported in Okayama, Hiroshima and around the Lake Biwa (Ito, 1964; Urabe, 2003). In South Korea, human infections were first identified from riparian people along the Hongcheon River, Gangwon-do (Province) (Ahn and Ryang, 1988). Subsequently, an endemic focus was discovered in 1993 from Umsong County along the upper reaches of the Namhan River (Chai et al., 1993).
Heterophyes heterophyes and H. nocens
Heterophyes heterophyes (v. Siebold, 1852) Stiles and Hassall, 1900 was first described based on specimens obtained at autopsy of an Egyptian, and the Nile Delta of Egypt and Sudan is now known to be important endemic areas (Yu and Mott, 1994; Chai, 2007). The distribution of H. heterophyes is also known in Europe, the Middle East and North Africa (Yu and Mott, 1994; Chai, 2007). Sequences of nuclear ITS2 and 28S rDNA and mitochondrial cox1 have been used to differentiate Heterophyes species (Chai, 2019). Brackish water fish, including the mullet Mugil cephalus and tilapia Tilapia nilotica, are the second intermediate hosts (Chai, 2007). Humans are infected by eating raw or inadequately cooked brackish water fish.
Heterophyes nocens Onji and Nishio, 1916 was first described from experimental dogs and cats fed mullets in Japan (Chai, 2007). This fluke has been reported also in South Korea and Japan (Chai et al., 2009; Chai and Jung, 2017). Brackish water fish, including the mullet and goby Acanthogobius flavimanus, are the second intermediate hosts (Guk et al., 2007). Humans are infected by consuming raw or inadequately cooked brackish water fish. In South Korea, residents of southwestern coastal areas and islands showed 10–70% egg-positive rates (Chai et al., 2004, 2009). In Japan, Kochi, Chiba, Yamaguchi, Chugoku, Hiroshima and Shizuoka Prefectures were the places where human infections were recorded (Kino et al., 2002). Diagnosis is based on the recovery of eggs in fecal examinations, but eggs should be differentiated from those of other heterophyids and small liver flukes.
Haplorchis spp.
Haplorchis taichui (Nishigori, 1924) Chen, 1936 was originally described from birds and mammals in Taiwan (Chai, 2007; Chai and Jung, 2017). The first report of human infections was published from the Philippines (Africa et al., 1940). Now this fluke is known to be distributed widely in Asia and the Middle East (Chai et al., 2007, 2009, 2012a; Sohn et al., 2014). PCR targeting ITS1, ITS2 and cox1 has been proved to be useful for genetic studies of Haplorchis species and opisthorchiids (Chai, 2019). In Laos, hyperendemic areas with average individual worm loads of 12 079 (Champasak Province) and 21 565 specimens (Saravane Province) were reported (Chai et al., 2013a). Freshwater fish, Cyprinus spp., Gambusia affinis, Hampala dispar and Puntius spp., harbour the metacercariae (Chai et al., 2009).
Haplorchis pumilio (Looss, 1896) Looss, 1899 was first recorded from birds and mammals in Egypt (Chai et al., 2009). Later, in Taiwan, a successful experimental infection of a human volunteer was reported in 1924 (Chai et al., 2009). Numerous human infections were found thereafter, and now this fluke is known to be distributed widely from Africa to Asia, Oceania and the Americas (Chai et al., 2009, 2012a, 2014, 2015; Chung et al., 2011). The second intermediate host is freshwater fish of the Cyprinidae, Siluridae and Cobitidae (Chai et al., 2009).
Haplorchis yokogawai (Katsuta, 1932) Chen, 1936 was first reported from dogs and cats experimentally fed mullets in Taiwan (Chai et al., 2009). An experimental human infection was successful in Taiwan but natural human infections were reported for the first time in the Philippines (Africa et al., 1940). This fluke is now known to be distributed in Asia, Australia and Egypt (Chai et al., 2007, 2009, 2014). Freshwater fish Cyclocheilichthys armatus, Hampala dispar, Misgurnus sp., Mugil spp. and Puntius spp. harbour the metacercariae (Chai et al., 2009).
Centrocestus formosanus
Centrocestus formosanus (Nishigori, 1924) Price, 1932 (syn. Centrocestus caninus) was first found in a dog experimentally infected with metacercariae from freshwater fish in Taiwan (Chai et al., 2009; Chai and Jung, 2017). A successful experimental infection of a human volunteer was reported at the same time (Chai et al., 2009; Chai and Jung, 2017). Two human infections reported in Thailand under the name C. caninus (Waikagul et al., 1997) are now considered as C. formosanus infection. In Laos, 7 human infections were detected (Chai et al., 2013b). Now this fluke is distributed almost all over the world, including Asia, Europe and North and South America (Chai et al., 2009, 2012a, 2013b, 2014; Wanlop et al., 2017). Human infections were reported from China, Taiwan, the Philippines, Thailand, Laos and Vietnam (Yu and Mott, 1994; Chai et al., 2009; Chai, 2019). The freshwater fish, including Cyclocheilichthys repasson and Puntius brevis, are the second host (Chai et al., 2009).
Heterophyopsis continua
Heterophyopsis continua (Onji and Nishio, 1916) Price, 1940 was originally described from experimental cats infected with metacercariae in the mullet in Japan. Without proper literature background, the presence of human infections was mentioned in Japan (Yamaguti, 1958). In South Korea, adult flukes were recovered from more than 10 human cases (Chai, 2019). The second intermediate host includes the perch L. japonicus, goby A. flavimanus, shad Clupanodon punctatus, conger eel Conger myriaster, and sweetfish P. altivelis (Chai et al., 2009). This fluke is distributed in Asia and the Middle East (Chai et al., 2012a; Chai, 2019).
Pygidiopsis summa and P. genata
Pygidiopsis summa Onji and Nishio, 1916 was originally described from dogs experimentally infected with metacercariae from brackish water fish in Japan and thereafter this fluke was reported also from South Korea (Chai et al., 2009; Chai and Jung, 2017). In Japan, human infections were suggested by the recovery of eggs in feces in 1929, and adult flukes were subsequently isolated in 1965 (Chai et al., 2009). In South Korea, human infections (8 cases) were first discovered from a salt-farm village in a western costal area of Jeollabuk-do (Province) (Seo et al., 1981). Now this fluke is known to be distributed on many western and southern coastal islands of South Korea (Chai et al., 2004). Its presence was also reported in Vietnam (Vo et al., 2008). Sequences of 28S rDNA and cox1 were used to assess the phylogenetic relationships of P. summa with C. sinensis, M. yokogawai and M. takahashii (Chai, 2019).
Pygidiopsis genata Looss, 1907 was originally described from a pelican in Egypt, and then also found in pelicans in Romania, experimental dogs and cats in the Philippines, a Persian wolf (in Berlin Museum), and dogs and cats in Israel (Palestine) (Witenberg, 1929; Africa et al., 1940). This parasite is distributed in Egypt, the Middle East and the Philippines (Chai and Jung, 2020). Human infections were first found in Egypt (Youssef et al., 1987a). Brackish water fish, including tilapia, mullet and Barbus canis, are the second intermediate hosts (Witenberg, 1929; Youssef et al., 1987b).
Stellantchasmus falcatus
Stellantchasmus falcatus Onji and Nishio, 1916 was first described from cats experimentally fed the mullet harbouring the metacercariae in Japan (Chai et al., 2009). Human infections were first reported in Japan and thereafter in many Asian-Pacific countries, including South Korea, the Philippines, Hawaii, Thailand and Vietnam (Chai et al., 2009, 2012a, 2016).
Stictodora fuscata
Stictodora fuscata (Onji and Nishio, 1916) Yamaguti, 1958 was first discovered from experimental cats fed infected mullets in Japan (Chai and Jung, 2017). Human infection was first found in South Korea in a young man who regularly consumed mullets and gobies raw (Chai et al., 1988). Subsequently, 13 additional cases were detected in a southwestern coastal area (Chai and Lee, 2002). Gobies A. flavimanus and topmouth gudgeons Pseudorasbora parva are the fish hosts (Yamaguti, 1958; Chai et al., 2009).
Miscellaneous heterophyid species
Fourteen species of miscellaneous heterophyid flukes having less than 10 human infection cases are briefly introduced in Supplementary Table. See Chai and Jung (2020) for details.
Echinostome species
Echinostoma spp.
Echinostoma revolutum (Froelich, 1802) Dietz, 1909 is the oldest echinostome species ever recorded in the literature (Chai, 2009; Chai et al., 2020). It was originally described in 1798 from a wild duck Anas boschas fereae naturally infected in Germany (Kanev, 1994). This species is now known to be distributed widely in Asia, Europe, Africa, Oceania and North and South America (Chai et al., 2009). The first report of human infection was published from Taiwan in 1929, where its prevalence among people was 2.8–6.5% (Yu and Mott, 1994). Subsequently, human infections were reported in China, Indonesia, Thailand and Russia (Chai et al., 2009). The flukes can cause gastrointestinal trouble, mucosal ulceration and bleeding (Chai, 2009). Tadpoles, gastropod snails (Physa occidentalis, Lymnaea sp. and Filopaludina sp.) and clams (Corbicula producta) can be the second intermediate hosts (Beaver et al., 1984; Yu and Mott, 1994; Chai et al., 2011; Chantima et al., 2013).
Echinostoma cinetorchis Ando and Ozaki, 1923 was first discovered from naturally infected rats in Japan (Chai et al., 2009). Adult flukes of this echinostome species are morphologically characterized by having a reduced number (none or 1, rarely 2) and abnormal location of testes (Chai, 2009). Human infections were first documented in Japan, and subsequently also detected in South Korea and China (Chai et al., 2009).
Echinostoma ilocanum (Garrison, 1908) Odhner, 1911 was first described from 5 prisoners in Manila, the Philippines (Chai et al., 2009). Subsequently, numerous human infection cases were discovered in Asian countries (Sohn et al., 2011; Chai et al., 2018). For example, in northern Luzon, the Philippines, the prevalence among the people was high, in the range from 7% to 17% (Chai et al., 2009). In Oddar Meanchey Province, Cambodia, the general population and the student group revealed 1.8% and 0.7% egg-positive rates, respectively (Sohn et al., 2011). Large snails, Pila conica (the Philippines) and Viviparus javanicus (Java) are the second intermediate hosts (Beaver et al., 1984; Yu and Mott, 1994).
Echinostoma lindoense Sandground and Bonne, 1940 was described from human population in the Lake Lindoe region of Central Celebes, Indonesia (Chai, 2019). A high prevalence (24–96%) of human infection was reported in Indonesia (Chai et al., 2009). It is now known to be distributed in South Asian countries (Chai, 2009).
Echinostoma mekongi Cho, Jung, Chang, Sohn, Sinuon and Chai, 2020 was described as a new species based on adult flukes recovered from 6 humans residing along the Mekong River in Kratie and Takeo Province, Cambodia (Cho et al., 2020). Further human cases (more than 10) were found in Kandal Province, Cambodia (unpublished data). The freshwater snails, Filopaludina martensi cambodjiensis, from Pursat Province, Cambodia were found to carry the metacercariae (Chai et al., 2021b). This echinostome seems to exist in neighbouring countries, such as Laos, Thailand and Vietnam.
Isthmiophora hortensis
Isthmiophora hortensis (Asada, 1926) Kostadinova and Gibson, 2002 (syn. Echinostoma hortense) was first found from rats in Japan, and then also from rats in South Korea and China (Chai and Jung, 2019). Human infections were first documented in Japan in 1976 and then also found in South Korea and China (Chai and Lee, 2002; Chai et al., 2009). In South Korea, clinical cases with significant abdominal symptoms were diagnosed by extracting living worms by gastroduodenal endoscopy (Chai et al., 2009). Loaches, Misgurnus anguillicaudatus and M. mizolepis, and other freshwater fish, including Odontobutis obscura interrupta and Moroco oxycephalus, are the second intermediate hosts (Chai et al., 2009).
Echinochasmus spp.
Echinochasmus japonicus Tanabe, 1926 was originally reported in Japan from dogs, cats, rats, mice and birds experimentally fed the metacercariae encysted in freshwater fish (Chai and Jung, 2019). This fluke is now known to occur mainly in Far Eastern countries (Chai and Lee, 2002; Chai and Jung, 2019). In Japan, an experimental human infection was reported to be successful, and in China and South Korea natural human infections were discovered (Chai et al., 2009). Freshwater fish, including P. parva, Hypomesus olidus and Gnathopogon strigatus, are the second intermediate hosts (Chai et al., 1985; Choi et al., 2006).
Echinochasmus perfoliatus (Ratz, 1908) Gedoelst, 1911 was originally reported from dogs in Romania, and then found again in dogs and cats in Hungary (Chai et al., 2009). Now its distribution is known to be wide from Asia to Europe (Chai et al., 2009). Human infections were reported in Japan (Chai et al., 2009). In Guangdong, Fujian, Anhui and Hubei Provinces of China, 1.8% prevalence was reported among people (Yu and Mott, 1994). Freshwater fish, including Carassius sp., Zacco platypus, Z. temminckii, and P. parva, are the second intermediate hosts (Rim, 1982c; Yu and Mott, 1994).
Echinochasmus liliputanus (Looss, 1896) Odhner, 1910 was found from dogs, cats, and birds in Egypt, Syria and Israel (Palestine) (Yamaguti, 1958). The first human infections, with a high prevalence of 13.4% among 2426 people, were reported in Anhui Province, China in 1991 (Yu and Mott, 1994). Since that time, more than 2500 human cases had been reported in Anhui Province, China (Xiao et al., 2005). P. parva and goldfish are the second intermediate hosts (Xiao et al., 2005). The mode of human infections is consumption of raw or improperly cooked fish or drinking untreated water containing the cercariae (Chai et al., 2009).
Echinochasmus fujianensis Cheng, Lin, Chen, et al., 1992 was originally reported from humans, dogs, cats, pigs and rats in Fujian Province, China (Yu and Mott, 1994). The prevalence among residents (mostly children) in Fujian Province ranged 1.6–7.8% (Yu and Mott, 1994). P. parva and Cyprinus carpio are the second hosts (Yu and Mott, 1994).
Echinochasmus caninus (Verma, 1935) Chai et al., 2019 (syn. Episthochasmus caninum, Episthmium caninum) was first described from dogs in India. Its metacercariae were found in fish Macropodus opercularis, and adult flukes were detected in dogs in Hainan Island, China (Chai et al., 2019b). Human infections were reported in northeastern Thailand and Lao PDR (Chai et al., 2019b).
Artyfechinostomum spp.
Artyfechinostomum malayanum (Leiper, 1911) Mendheim, 1943 (syn. Echinostoma malayanum) was originally reported from a human in Malaysia (Beaver et al., 1984), and subsequently found in other Asian countries (Chai et al., 2009, 2012b; Sohn et al., 2017). Large snails, i.e. I. exustus, A. convexiusculus and Pila scutate, play the role of the second intermediate hosts (Yu and Mott, 1994; Belizario et al., 2007).
Artyfechinostomum sufrartyfex Lane, 1915 (syn. Artyfechinostomum mehrai) was originally discovered from an Assamese girl in India (Yu and Mott, 1994) and later in Vietnam (Tran et al., 2016). Freshwater snails, fish or frogs harbour the metacercariae (Raghunathan and Srinivasan, 1962). Further human cases were reported in India (Chai and Jung, 2020).
Artyfechinostomum oraoni Bandyopadhyay, Manna et Nandy, 1989 was originally reported from 20 human infection cases in a tribal community near Calcutta, India (Bandyopadhyay et al., 1989, 1995). The life cycle is to be determined. This fluke may provoke fatal diarrhoea in pigs (Bandyopadhyay et al., 1989).
Hypoderaeum conoideum
Hypoderaeum conoideum (Bloch, 1872) Dietz, 1909 was originally reported from various species of birds in Europe (Yamaguti, 1958). This echinostome is now known to be distributed in Europe, Asia and Siberia (Yamaguti, 1958; Rim, 1982c; Beaver et al., 1984). The first human infections were reported in northeastern Thailand; the prevalence among 254 residents was 55% (Yokogawa et al., 1965). Large snails and tadpoles play the role of the second intermediate hosts (Chai et al., 2009).
Acanthoparyphium tyosenense
Acanthoparyphium tyosenense Yamaguti, 1939 was originally reported from the duck Melanitta fusca stejnegeri and M. nigra americana caught in South Korea (Chai, 2009; Chai and Jung, 2019). Its geographical distribution is confined to South Korea and Japan (Kim et al., 2004). Humans infected with this echinostome were first discovered in coastal villages of Jeollabuk-do (Province), South Korea (Chai et al., 2001). Brackish water bivalves, including Mactra veneriformis and Solen spp., and brackish water gastropod Neverita bicolor, were found to harbour the metacercariae (Chai et al., 2001; Kim et al., 2004).
Miscellaneous echinostome species
Eight species of miscellaneous echinostome flukes having less than 10 human infection cases are briefly introduced in Supplementary Table. See Chai and Jung (2020) for details.
Other intestinal fluke species
Brachylaima cribbi
Brachylaima cribbi Butcher and Grove, 2001 was first described by Butcher and Grove (2001) in Australia. Human infections (about 15 cases) were reported in Australia (Butcher et al., 1998, 2003). Helicid land snails, such as, Cernuella virgata, serve as the source of human infection (Butcher and Grove, 2005). Clinical symptoms include diarrhoea, abdominal pain, low-grade fever and fatigue (Butcher et al., 2003).
Caprimolgorchis molenkampi
Caprimolgorchis molenkampi (Lie, 1951) Baugh, 1957 (syn. Prosthodendrium molenkampi) was originally reported from 2 human autopsies in Indonesia (Manning and Lertprasert, 1973). Later, this fluke was found again in 14 human autopsies in northeastern Thailand (Manning et al., 1970; Manning and Lertprasert, 1973). Currently, this fluke shows a considerable prevalence in northeast Thailand and Laos (Chai et al., 2009). Naiads and adults of dragon- and damselflies serve as the second intermediate hosts (Manning and Lertprasert, 1973).
Fasciolopsis buski
Fasciolopsis buski (Lankester, 1857) Odhner, 1902 was originally described in 1843 based on the worms in the duodenum of an Indian sailor (Beaver et al., 1984; Mas-Coma et al., 2005; Tandon et al., 2013). Now this fluke is known to be a common intestinal parasite of humans and pigs in Asia (Chai et al., 2009; Tandon et al., 2013). The estimated number of Asian populations infected with F. buski is about 10 million (Tandon et al., 2013). The prevalence ranged from 0.04% in Cambodia to 8.6–50% in Bangladesh, 25–61% in Taiwan and up to 85% in some areas of China (Tandon et al., 2013). Metacercariae can attach to the surface of aquatic plants, such as, water chestnut, water caltrop, water hyacinth, roots of the lotus, water bamboo and other aquatic vegetations; they may also float on the water (Beaver et al., 1984; Yu and Mott, 1994; Tandon et al., 2013). The main mode of human infection is the consumption of raw or improperly cooked aquatic plants, or peeling off the hull or skin of the plants by mouth before eating the raw nut (Yu and Mott, 1994).
Gastrodiscoides hominis
Gastrodiscoides hominis (Lewis and McConnell, 1876) Leiper, 1913 was first found from an Indian patient (Beaver et al., 1984). This fluke is now known to be a common parasite of humans and pigs in various countries of Asia and Africa (Mas-Coma et al., 2005). Adult worms attach to the caecum and ascending colon of humans and may produce mucous diarrhoea (Beaver et al., 1984). The cercariae encyst on aquatic plants, or in tadpoles, frogs and crayfish (Yu and Mott, 1994).
Gymnophallloides seoi
Gymnophalloides seoi Lee, Chai et Hong, 1993 was originally reported from a woman suffering from acute pancreatitis and gastrointestinal discomfort in South Korea (Lee et al., 1993; Chai et al., 2003). Subsequently, a southwestern coastal island was found to be a highly endemic area with 49% prevalence and heavy worm loads (Chai et al., 2003). This fluke is now known to be distributed in 25 seashore villages of western and southern coastal islands and 3 coastal inlands of South Korea (Chai et al., 2009). Oysters, Crassostrea gigas were verified to be the second intermediate hosts (Chai et al., 2003). Consumption of raw oysters is the main mode of human infection.
Microphallus brevicaeca
Microphallus brevicaeca (Africa and Garcia, 1935) Baer, 1943 (syn. Spelotrema brevicaeca) was first described from birds and subsequently also found from 12 human autopsies in the Philippines (Africa et al., 1940). Eggs were shown to cause acute cardiac dilatation and egg granuloma in the heart, brain and spinal cord, especially in immunocompromised patients (Africa et al., 1940). Brackish water crabs Carcinus maenas and shrimps Macrobrachium sp. serve as the second intermediate hosts (Beaver et al., 1984).
Nanophyetus salmincola and N. schikhobalowi
Nanophyetus salmincola (Chapin, 1926) Chapin in Hall, 1927 (syn. Troglotrema salmincola) was first found in dogs suffering from a fatal disease after the ingestion of uncooked salmon in the Pacific Coast of North America (Witenberg, 1932). Since 1974, at least 20 human infections were found in the USA (Witenberg, 1932; Eastburn et al., 1987). N. salmincola has been proven to be the vector of a rickettsia Neorickettsia helmintheca (Chai and Jung, 2020). This rickettsial infection can cause a serious and often fatal systemic disease known as salmon poisoning in animals such as dogs and foxes (Chai and Jung, 2020). However, salmon poisoning has never been reported in humans (Chai and Jung, 2020).
Nanophyetus schikhobalowi Skrjabin and Podjapolskaja, 1931 was discovered from native people in far eastern Siberia (Skrjabin and Podjapolskaja, 1931). It was described as a new species mainly because of their smaller egg size compared to N. salmincola (Skrjabin and Podjapolskaja, 1931). The far eastern part of Russia including Amur and Ussuri valleys of Khabarovsk Territory and north Sakhalin is an important endemic area, with an average prevalence of 5% (Yu and Mott, 1994).
Neodiplostomum seoulense
Neodiplostomum seoulense (Seo, Rim et Lee, 1964) Hong and Shoop, 1995 was first found in house rats in South Korea (Seo, 1990). Its geographical distribution is confined to mountainous areas of South Korea (Seo, 1990) and a northeastern part of China (Chai et al., 2009). Adult flukes were recovered from a young man who suffered from acute abdominal pain and fever and had a history of consuming improperly cooked snakes (Seo, 1990). Experimental mice and rats showed severe mucosal damage in the intestinal tract with frequent host death and fecundity reduction (Lee et al., 1985; Chai et al., 2009; Shin et al., 2016). Military soldiers who had eaten raw snakes during their survival training were found to be infected with this fluke (Hong et al., 1984; Chai et al., 2009). Tadpoles and frogs of Rana sp. are the second intermediate hosts, and the snake Rhabdophis tigrina is a paratenic host (Seo, 1990).
Phaneropsolus bonnei
Phaneropsolus bonnei Lie, 1951 was originally reported based on adult flukes recovered from a human autopsy in Indonesia, and subsequently this fluke was found in monkeys in Malaysia and India (Manning et al., 1970). Human infections with this fluke were first reported in 15 human autopsies in northeastern Thailand (Manning et al., 1970). Thailand and Laos are currently important countries where this fluke infection is considerably high among the people (Chai et al., 2009). Insects, particularly naiads and adults of dragon- and damselflies, serve as the second intermediate hosts (Manning and Lertprasert, 1973).
Other miscellaneous species
Thirteen species of miscellaneous intestinal flukes having less than 10 human cases are presented in Supplementary Table. See Chai and Jung (2020) for details.
Issues and perspectives
FBT are taxonomically diverse, and a lot of species have been involved in causing zoonotic human and animal infections. Recently, FBT infections have been included among the neglected tropical diseases by the World Health Organization. Because of the increased risk of human exposure to these parasites, the public health significance of each FBT species is expected to increase. The life cycles of many kinds of FBT have been elucidated, and a variety of human infection sources (including types of foods) have been identified. However, problems still remain including difficulties in the diagnosis of infection (fecal examination alone frequently do not provide exact/specific diagnosis) as well as poor understanding of the epidemiological situation, including the geographical distribution, prevalence, intensity of infection and other characteristics. In addition, the clinicopathological significance of each FBT infection requires further investigation.
Acknowledgements
The authors would like to thank the members of the MediCheck Research Institute, Korea Association of Health Promotion, Seoul, South Korea for their help in gathering information and references used in this article.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182022000725.
click here to view supplementary material
Author contributions
J.-Y. C. and B.-K. J. designed this review and wrote the article.
Financial support
This review received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
The authors declare that they have no conflicts of interest related to this review article.
References
- Abdul-Ghani RA, Loutfy N and Hassan A (2009) Myrrh and trematodes in Egypt: an overview of safety, efficacy and effectiveness profiles. Parasitology International 58, 210–214. [DOI] [PubMed] [Google Scholar]
- Africa CM, de Leon W and Garcia EY (1940) Visceral complications in intestinal heterophyidiasis of man. Acta Medica Philippina (Monographic Series), 1–132. [Google Scholar]
- Agatsuma T, Arakawa Y, Iwagami M, Honzako Y, Cahyangsih U, Kang SY and Hong SJ (2000) Molecular evidence of natural hybridization between Fasciola hepatica and F. gigantica. Parasitology International 49, 231–238. [DOI] [PubMed] [Google Scholar]
- Agatsuma T, Iwagami M, Sato Y, Iwashita J, Hong SJ, Kang SY, Ho LY, Su KE, Kawashima K and Abe T (2003) The origin of the triploid in Paragonimus westermani on the basis of variable regions in the mitochondrial DNA. Journal of Helminthology 77, 279–285. [DOI] [PubMed] [Google Scholar]
- Ahn YK and Ryang YS (1988) Epidemiological studies on Metagonimus infection along the Hongcheon River, Kangwon Province. Korean Journal of Parasitology 26, 207–213 (in Korean). [DOI] [PubMed] [Google Scholar]
- Aka NA, Adoubryn K, Rondelaud D and Dreyfuss G (2008) Human paragonimiasis in Africa. Annals of African Medicine 7, 153–162. [DOI] [PubMed] [Google Scholar]
- Ashrafi K (2010) Human dicrocoeliasis in northern Iran: two case reports from Gilan province. Annals of Tropical Medicine and Parasitology 104, 351–353. [DOI] [PubMed] [Google Scholar]
- Babbott FL Jr, Frye WWW and Gordon JE (1961) Intestinal parasites of man in Arctic Greenland. American Journal of Tropical Medicine and Hygiene 10, 185–190. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay AK, Manna B and Nandy A (1989) Human infection of Artyfechinostomum oraoni n. sp. (Paryphostominae: Echinostomatidae) in a tribal community, ‘Oraons’ in West Bengal, India. Indian Journal of Parasitology 13, 191–196. [Google Scholar]
- Bandyopadhyay AK, Maji AK, Manna B, Bera DK, Addy M and Nandy A (1995) Pathogenicity of Artyfechinostomum oraoni in naturally infected pigs. Tropical Medicine and Parasitology 46, 138–139. [PubMed] [Google Scholar]
- Bargues MD and Mas-Coma S (2005) Reviewing lymnaeid vectors of fascioliasis by ribosomal DNA sequence analyses. Journal of Helminthology 79, 257–267. [DOI] [PubMed] [Google Scholar]
- Bayssade-Dufour C, Chermette R, Šundić D and Radujković B (2014) Paragonimus gondwanensis n. sp. (Digenea, Paragonimidae), parasite of mammals (humans and carnivores) in Cameroon. Ecologica Montenegrina 1, 256–267. [Google Scholar]
- Bayssade-Dufour C, Chermette R, Šundić D and Radujković B (2015) Paragonimus kerberti n. sp. (Digenea, Paragonimidae), a parasite of carnivores in Cameroon. Ecologica Montenegrina 2, 271–277. [Google Scholar]
- Beaver PC, Jung RC and Cupp EW (1984) Clinical Parasitology, 9th Edn. Philadelphia, USA: Lea & Febiger. [Google Scholar]
- Béland JE, Boone J, Donevan RE and Mankiewicz E (1969) Paragonimiasis (the lung fluke) report of four cases. American Review of Respiratory Disease 99, 261–271. [DOI] [PubMed] [Google Scholar]
- Belizario VY, Geronilla GG, Anastacio MBM, de Leon WU, Suba-an AP, Sebastian AC and Bangs MJ (2007) Echinostoma malayanum infection, the Philippines. Emerging Infectious Diseases 13, 1130–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besprozvannykh VV, Tatonova YV and Shumenko PG (2018) Life cycle, morphology of developmental stages of Metorchis ussuriensis sp. nov. (Trematoda: Opisthorchiidae), and phylogenetic relationships with other opisthorchiids. Journal of Zoological Systematics and Evolutionary Research 57, 24–40. 10.1111/jzs.12230 [DOI] [Google Scholar]
- Blair D (2019) Paragonimiasis. In Toledo R and Fried B (eds), Digenetic Trematodes. Advances in Experimental Medicine and Biology, vol. 1154. pp. 105–138. Available at 10.1007/978-3-030-18616-6_5. [DOI] [PubMed] [Google Scholar]
- Blair D, Agatsuma T, Watanobe T, Okamoto M and Ito A (1997) Geographical genetic structure within the human lung fluke, Paragonimus westermani, detected from DNA sequences. Parasitology 115, 411–417. [DOI] [PubMed] [Google Scholar]
- Blair D, Xu ZB and Agatsuma T (1999a) Paragonimiasis and the genus Paragonimus. Advances in Parasitology 42, 113–122. [DOI] [PubMed] [Google Scholar]
- Blair D, Wu B, Chang ZS, Gong X, Agatsuma T, Zhang YN, Chen SH, Lin JX, Chen MG, Waikagul J, Guevara AG, Feng Z and Davis GM (1999b) A molecular perspective on the genera Paragonimus Braun, Euparagonimus Chen and Pagumogonimus Chen. Journal of Helminthology 73, 295–299. [PubMed] [Google Scholar]
- Blair D, Chang Z, Chen M, Cui A, Wu B, Agatsuma T, Iwagami M, Corlis D, Fu C and Zhan X (2005) Paragponimus skrjabini Chen, 1959 (Digenea: Paragonimidae) and related species in eastern Asia: a combined molecular and morphological approach to identification and taxonomy. Systematic Parasitology 60, 1–21. [DOI] [PubMed] [Google Scholar]
- Blair D, Agatsuma T and Wang W (2007) Paragonimiasis. In Murrell KD and Fried B (eds), Food-borne Parasitic Zoonoses: Fish and Plant-Borne Parasites. World Class Parasites, vol. 11. New York, USA: Springer, pp. 117–150. [Google Scholar]
- Boland JM, Vaszar LT, Jones JL, Mathison BA, Rovzar MA, Colby TV, Leslie KO and Tazelaar HD (2011) Pleuropulmonary infection by Paragonimus westermani in the United States: a rare cause of eosinophilic pneumonia after ingestion of live crabs. American Journal of Surgical Pathology 35, 707–713. [DOI] [PubMed] [Google Scholar]
- Brenes Madrigal R, Rodriguez-Ortiz B, Vargas Solano G, Ocamp Obando EM and Ruiz Sotela PJ (1982) Cerebral hemmorhagic lesions produced by Paragonimus mexicanus. Report of three cases in Costa Rica. American Journal of Tropical Medicine and Hygiene 31, 522–526. [DOI] [PubMed] [Google Scholar]
- Butcher AR and Grove DI (2001) Description of life-cycle stages of Brachylaima cribbi n. sp. (Digenea: Brachylaimidae) derived from eggs recovered from human feces in Australia. Systematic Parasitology 49, 211–221. [DOI] [PubMed] [Google Scholar]
- Butcher AR and Grove DI (2005) Second intermediate host land snails and definitive host animals of Brachylaima cribbi in southern Australia. Parasite 12, 31–37. [DOI] [PubMed] [Google Scholar]
- Butcher AR, Parasuramar P, Thompson CS and Grove DI (1998) First report of the isolation of an adult worm of the genus Brachylaima (Digenea: Brachylaimidae), from the gastrointestinal tract of a human. International Journal for Parasitology 28, 607–610. [DOI] [PubMed] [Google Scholar]
- Butcher AR, Palethope HM and Grove DI (2003) Response to re-infection with Brachylaima cribbi in immunocompetent and immunodeficient mice. Parasitology International 52, 219–228. [DOI] [PubMed] [Google Scholar]
- Calvopiña M, Cevallos W, Kumazawa H and Eisenberg J (2011) High prevalence of human liver infection by Amphimerus spp. flukes, Ecuador. Emerging Infectious Diseases 17, 2331–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvopiña M, Romero D, Castañeda B, Hashiguchi Y and Sugiyama H (2014) Current status of Paragonimus and paragonimiasis in Ecuador. Memorias Instituto Oswaldo Cruz 109, 849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvopiña M, Cevallos W, Atherton R, Saunders M, Small A, Kumazawa H and Sugiyama H (2015) High prevalence of the liver fluke Amphimerus sp. In domestic cats and dogs in an area for human amphimeriasis in Ecuador. PLoS Neglected Tropical Diseases 9, e000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvopiña M, Romero-Alvarez D, Rendon M, Takagi H and Sugiyama H (2018) Hypolobocera guayaquilensis (Decapoda: Pseudothelphusidae): a new crab intermediate host of Paragonimus mexicanus in Manabí Province, Ecuador. Korean Journal of Parasitology 56, 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevallos W, Calvopiña M, Nipáz V, Vicente-Santiago B, López-Alban J, Fernández-Soto P, Guevara A and Muro A (2017) Enzyme-linked immunosorbent assay for diagnosis of Amphimerus spp. liver fluke infection in humans. Memorias Instituto Oswaldo Cruz (Rio de Janeiro) 112, 364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai JY (2007) Intestinal flukes. In Murrell KD and Fried B (eds), Food-borne Parasitic Zoonoses: Fish and Plant-borne Parasites. World Class Parasites, vol. 11. New York, USA: Springer, pp. 53–115. [Google Scholar]
- Chai JY (2009) Echinostomes in humans. In Fried B and Toledo R (eds), The Biology of Echinostomes. New York, USA: Springer, pp. 147–183. [Google Scholar]
- Chai JY (2013a) Praziquantel treatment in trematode and cestode infections: an update. Infection and Chemotherapy 45, 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai JY (2013b) Paragonimiasis. In Garcia HH, Tanowitz HB and Del Brutto OH (eds), Neuroparasitology and Tropical Neurology. Handbook of Clinical Neurology. Vol. 114 (3rd Series). Amsterdam, The Netherlands: Elsevier, pp. 283–296. [Google Scholar]
- Chai JY (2019) Human Intestinal Flukes. From Discovery to Treatment and Control. Dordrecht, The Netherlands: Springer Nature B.V. [Google Scholar]
- Chai JY and Jung BK (2017) Fishborne zoonotic heterophyid infections: an update. Food and Waterborne Parasitology 8–9, 33–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai JY and Jung BK (2018) Paragonimus spp. In Robertson L (ed.), Global Water Pathogen Project. Part 3. Specific Excreted Pathogens: Environmental and Epidemiological Aspects. Section IV. Helminths. Michigan State University, E. Lansing, MI, USA: UNESCO, pp. 1–18. [Google Scholar]
- Chai JY and Jung BK (2019) Epidemiology of trematode infections: an update. In Toledo R and Fried B (eds), Digenetic Trematodes. Advances in Experimental Medicine and Biology, vol. 1154. pp. 359–409. Available at 10.1007/978-3-030-18616-6_12. [DOI] [PubMed] [Google Scholar]
- Chai JY and Jung BK (2020) Foodborne intestinal flukes: a brief review of epidemiology and geographical distribution. Acta Tropica 201, 105210. [DOI] [PubMed] [Google Scholar]
- Chai JY and Lee SH (2002) Food-borne intestinal trematode infections in the Republic of Korea. Parasitology International 51, 129–154. [DOI] [PubMed] [Google Scholar]
- Chai JY, Hong SJ, Son DW, Lee SH and Seo BS (1985) Metacercariae of the Echinochasmus japonicus encysted in a fresh water fish, Pseudorasbora parva, and their development in experimental mice. Korean Journal of Parasitology 23, 221–229. [DOI] [PubMed] [Google Scholar]
- Chai JY, Hong SJ, Lee SH and Seo BS (1988) Stictodora sp. (Trematoda: Heterophyidae) recovered from a man in Korea. Korean Journal of Parasitology 26, 127–132. [DOI] [PubMed] [Google Scholar]
- Chai JY, Huh S, Yu JR, Kook J, Jung KC, Park EC, Sohn WM, Hong ST and Lee SH (1993) An epidemiological study of metagonimiasis along the upper reaches of the Namhan River. Korean Journal of Parasitology 31, 99–108. [DOI] [PubMed] [Google Scholar]
- Chai JY, Han ET, Park YK, Guk SM and Lee SH (2001) Acanthoparyphium tyosenense: the discovery of human infection and identification of its source. Journal of Parasitology 87, 794–800. [DOI] [PubMed] [Google Scholar]
- Chai JY, Choi MH, Yu JR and Lee SH (2003) Gymnophalloides seoi: a new human intestinal trematode. Trends in Parasitology 19, 109–112. [DOI] [PubMed] [Google Scholar]
- Chai JY, Park JH, Han ET, Shin EH, Kim JL, Guk SM, Hong KS, Lee SH and Rim HJ (2004) Prevalence of Heterophyes nocens and Pygidiopsis summa infections among residents of the western and southern coastal islands of the Republic of Korea. American Journal of Tropical Medicine and Hygiene 71, 617–622. [PubMed] [Google Scholar]
- Chai JY, Murrell KD and Lymbery AJ (2005) Fish-borne parasitic zoonoses: status and issues. International Journal for Parasitology 35, 1233–1254. [DOI] [PubMed] [Google Scholar]
- Chai JY, Han ET, Guk SM, Shin EH, Sohn WM, Yong TS, Eom KS, Lee KH, Jeong HG, Ryang YS, Hoang EH, Phommasack B, Insisiengmay B, Lee SH and Rim HJ (2007) High prevalence of liver and intestinal fluke infections among residents of Savannakhet Province, Laos. Korean Journal of Parasitology 45, 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai JY, Shin EH, Lee SH and Rim HJ (2009) Foodborne intestinal flukes in Southeast Asia. Korean Journal of Parasitology 47, S69–S102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai JY, Sohn WM, Na BK and Nguyen VD (2011) Echinostoma revolutum: metacercariae in Filopaludina snails from Nam Dihn Province, Vietnam, and adults from experimental hamsters. Korean Journal of Parasitology 49, 449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai JY, De NV and Sohn WM (2012a) Foodborne trematode metacercariae in fish from northern Vietnam and their adults recovered from experimental hamsters. Korean Journal of Parasitology 50, 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai JY, Sohn WM, Yong TS, Eom KS, Min DY, Hoang EH, Phammasack B, Insisiengmay B and Rim HJ (2012b) Echinostome flukes recovered from humans in Khammouane Province, Lao PDR. Korean Journal of Parasitology 50, 269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai JY, Yong TS, Eom KS, Min DY, Jeon HK, Kim TY, Jung BK, Sisabath L, Insisiengmay B, Phommasack B and Rim HJ (2013a) Hyperendemicity of Haplorchis taichui infection among riparian people in Saravane and Champasak Province, Lao PDR. Korean Journal of Parasitology 51, 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai JY, Sohn WM, Yong TS, Eom KS, Min DY, Lee MY, Lim H, Insisiengmay B, Phommasack B and Rim HJ (2013b) Centrocestus formosanus (Heterophyidae): human infections and the infection source in Lao PDR. Journal of Parasitology 99, 531–536. [DOI] [PubMed] [Google Scholar]
- Chai JY, Sohn WM, Na BK, Yong TS, Eom KS, Yoon CH, Hoang EH, Jeoung HG and Socheat D (2014) Zoonotic trematode metacercariae in fish from Phnom Penh and Pursat, Cambodia. Korean Journal of Parasitology 52, 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai JY, Sohn WM, Jung BK, Yong TS, Eom KS, Min DY, Insisiengmay B, Insisiengmay S, Phommasack B and Rim HJ (2015) Intestinal helminths recovered from humans in Xieng Khouang Province, Lao PDR with a particular note on Haplorchis pumilio infection. Korean Journal of Parasitology 53, 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai JY, Sohn WM, Na BK, Jeoung HG, Sinuon M and Socheat D (2016) Stellantchasmus falcatus (Digenea: heterophyidae) in Cambodia: discovery of metacercariae in mullets and recovery of adult flukes in an experimental hamster. Korean Journal of Parasitology 54, 537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai JY, Sohn WM, Cho J, Eom KS, Yong TS, Min DY, Hoang EH, Phommasack B, Insisiengmay B and Rim HJ (2018) Echinostoma ilocanum infection in two residents of Savannakhet Province, Lao PDR. Korean Journal of Parasitology 56, 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai JY, Lee SH, Rim HJ, Sohn WM and Phommasack B (2019a) Infection status with zoonotic trematode metacercariae in fish from Lao PDR. Acta Tropica 199, 105100. [DOI] [PubMed] [Google Scholar]
- Chai JY, Chang T, Jung BK, Shin H, Soh WM, Eom KS, Yong TS, Min DY, Phommasack B, Insisiengmay B and Rim HJ (2019b) Echinostoma caninus n. comb. (Trematoda: Echinostomatidae) infection in eleven riparian people in Khammouane Province, Lao PDR. Korean Journal of Parasitology 57, 451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai JY, Cho J, Chang T, Jung BK and Sohn WM (2020) Taxonomy of Echinostoma revolutum and 37-collar-spined Echinostoma spp.: a historical review. Korean Journal of Parasitology 58, 343–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai JY, Jung BK and Hong SJ (2021a) Albendazole and mebendazole as anti-parasitic and anti-cancer agents: an update. Korean Journal of Parasitology 59, 189–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai JY, Sohn WM, Cho J, Jung BK, Chang T, Lee KH, Khieu V and Huy R (2021b) Echinostoma mekongi: discovery of its metacercarial stage in snails, Filopaludina martensi cambodjiensis, in Pursat Province, Cambodia. Korean Journal of Parasitology 59, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantima K, Chai JY and Wongsawad C (2013) Echinostoma revolutum: freshwater snails as the second intermediate hosts in Chiang Mai, Thailand. Korean Journal of Parasitology 51, 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M and Mott KE (1990) Progress in assessment of morbidity due to Fasciola hepatica infection: a review of recent literature. Tropical Diseases Bulletin 87, R1–R38. [Google Scholar]
- Chen M, Lu Y, Hua X and Mott KE (1994) Progress in assessment of morbidity due to Clonorchis sinensis infection: a review of recent literature. Tropical Diseases Bulletin 91, R7–65. [Google Scholar]
- Cho J, Jung BK, Chang T, Sohn WM, Sinuon M and Chai JY (2020) Echinostoma mekongi n. sp. (Digenea: Echinostomatidae) from riparian people along the Mekong River in Cambodia. Korean Journal of Parasitology 58, 431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW (1990) Paragonimus and paragonimiasis in Korea. Korean Journal of Parasitology 28(suppl.), 79–102. [DOI] [PubMed] [Google Scholar]
- Choi MH, Kim SH, Chung JH, Jang HJ, Eom JH, Chung BS, Sohn WM, Chai JY and Hong ST (2006) Morphological observations of Echinochasmus japonicus cercariae and the in vitro maintenance of its life cycle from cercariae to adults. Journal of Parasitology 92, 236–241. [DOI] [PubMed] [Google Scholar]
- Choo JD, Suh BS, Lee HS, Lee JS, Song CJ, Shin DW and Lee YH (2003) Chronic cerebral paragonimiasis combined with aneurysmal subarachnoid hemorrhage. American Journal of Tropical Medicine and Hygiene 69, 466–469. [PubMed] [Google Scholar]
- Chung OS, Lee HJ, Kim YM, Sohn WM, Kwak SJ and Seo M (2011) First report of human infection with Gynaecotyla squatarolae and first Korean record of Haplorchis pumilio in a patient. Parasitology International 60, 227–229. [DOI] [PubMed] [Google Scholar]
- Coogle B, Sosland S and Bahr NC (2021) A clinical review of human disease due to Paragonimus kellicotti in North America. Parasitology 148, 1–7. 10.1017/s0031182021001359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberlidge N, Rollinson D, Vercruysse J, Tchuem Tchuenté LA, Webster B and Clark PF (2018) Paragonimus and paragonimiasis in West and Central Africa: unsolved questions. Parasitology 145, 1748–1757. 10.1017/S0031192018001439 [DOI] [PubMed] [Google Scholar]
- Dar JS, Shabir U, Dar SA and Ganai BA (2020) Molecular characterization and immunodiagnostics of Dicrocoelium dendriticum species isolated from sheep of north-west Himalayan region. Journal of Helminthology 94, e174. [DOI] [PubMed] [Google Scholar]
- David AR (1997) Disease in Egyptian mummies: the contribution of new technologies. Lancet (London, England) 349, 1760–1763. [DOI] [PubMed] [Google Scholar]
- Devi KR, Narain K, Mahanta J, Nirmolia T, Blair D, Saikia SP and Agatsuma T (2013) Presence of three distinct genotypes within the Paragonimus westermani complex in northern India. Parasitology 140, 76–86. [DOI] [PubMed] [Google Scholar]
- Doanh PN, Shinohara A, Horii Y, Habe S, Nawa Y and Le NT (2007) Description of a new lung fluke species, Paragonimus vietnamensis sp. nov. (Trematoda: Paragonimidae), found in northern Vietnam. Parasitology Research 101, 1495–1501. [DOI] [PubMed] [Google Scholar]
- Doanh PN, Shinohara A, Horii Y, Habe S and Nawa Y (2009) Discovery of Paragonimus westermani in Vietnam and its molecular phylogenetic status in P. westermani complex. Parasitology Research 104, 1149–1159. [DOI] [PubMed] [Google Scholar]
- Doanh PN, Horii Y and Nawa Y (2013a) Paragonimus and paragonimiasis in Vietnam: an update. Korean Journal of Parasitology 51, 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doanh PN, Hien HV, Nonaka N, Horii Y and Nawa Y (2013b) Discovery of Paragonimus skrjabini in Vietnam and its phylogenetic status in the Paragonimus skrjabini complex. Journal of Helminthology 87, 450–460. [DOI] [PubMed] [Google Scholar]
- Doanh PN, Thaenkham U, An PT, Hien HV, Horii Y and Nawa Y (2015) Metacercarial polymorphism and genetic variation of Paragonimus heterotremus (Digenea: Paragonimidae), and a re-appraisal of the taxonomic status of Paragonimus pseudoheterotremus. Journal of Helminthology 89, 182–188. [DOI] [PubMed] [Google Scholar]
- Drabick JJ, Egan JE, Brown SL, Vick RG, Sandman BM and Neafie RC (1988) Dicrocoeliasis (lancet fluke disease) in an HIV seropositive man. Journal of the American Medical Association 259, 567–568. [PubMed] [Google Scholar]
- Duflot M, Setbon T, Midelet G, Brauge T and Gay M (2021) A review of molecular identification tools for the Opisthorchioidea. Journal of Molecular Methods 187, 106258. [DOI] [PubMed] [Google Scholar]
- Eastburn RL, Tritsche TR and Terhune CA Jr (1987) Human intestinal infection with Nanophyetus salmincola from salmonid fishes. American Journal of Tropical Medicine and Hygiene 36, 586–591. [DOI] [PubMed] [Google Scholar]
- Fairweather I (2009) Triclabendazole progress report, 2005–2009: an advancement of learning? Journal of Helminthology 83, 139–150. [DOI] [PubMed] [Google Scholar]
- Fedorov KP, Naumov VA, Kuznetsova VG and Belov GF (2002) [Some real problems of human opisthorchiasis]. Meditsinskaia Parazitologiia i Parazitarnye Bolezni 3, 7–9. [PubMed] [Google Scholar]
- Fedorova OS, Fedotova MM, Sokolova TS, Golovach EA, Kovshirina YV, Ageeva TS, Kovshirina AE, Kobyakova OS, Ogorodova LM and Odermatt P (2018) Opisthorchis felineus infection prevalence in Western Siberia: a review of Russian literature. Acta Tropica 178, 196–204. [DOI] [PubMed] [Google Scholar]
- Fischer PU, Curtis KC, Marcos LA and Weil GJ (2011) Molecular characterization of the North American lung fluke Paragonimus kellicotti in Missouri and its development in Mongolian gerbils. American Journal of Tropical Medicine and Hygiene 84, 1005–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell DJ (1981) Liver-fluke infection as an aetiological factor in bile-duct carcinoma of man. Transactions of the Royal Society of Tropical Medicine and Hygiene 75, 814–824. [DOI] [PubMed] [Google Scholar]
- Friant S, Brown K, Saari MT, Segel NH, Slezak J and Goldberg TL (2015) Lung fluke (Paragonimus africanus) infects Nigerian red-capped mangabeys and causes respiratory disease. International Journal for Parasitology: Parasites and Wildlife 4, 329–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried B and Abruzzi A (2010) Food-borne trematode infections of humans in the United States of America. Parasitology Research 106, 1263–1280. [DOI] [PubMed] [Google Scholar]
- Fürst T, Keiser J and Utzinger J (2012a) Global burden of human food-borne trematodiasis: a systematic review and meta-analysis. Lancet Infectious Diseases 12, 210–221. [DOI] [PubMed] [Google Scholar]
- Fürst T, Sayasone S, Odermatt P, Keiser J and Utzinger J (2012b) Manifestation, diagnosis, and management of food-borne trematodiasis. British Medical Journal 344, e4093. [DOI] [PubMed] [Google Scholar]
- Fürst T, Yongvanit P, Khuntikeo N, Lun ZR, Haagsma JA, Torgerson PR, Odermatt P, Bürli C, Chitnis N and Sithithaworn P (2019) Food-borne trematodiases in East Asia: epidemiology and burden. In Utzinger Z, Yap P, Bratschi M and Steinmann P (eds), Neglected Tropical Diseases-East Asia, Neglected Tropical Diseases. Switzerland AG: Springer Cham, pp. 13–38. 10.1007/978-3-030-12008-5_2 [DOI] [Google Scholar]
- Garcia LS (2016) Diagnostic Medical Parasitology, 6th Edn. Washington, DC, USA: ASM Press. [Google Scholar]
- Guk SM, Shin EH, Kim JL, Sohn WM, Hong KS, Yoon CH, Lee SH, Rim HJ and Chai JY (2007) A survey of Heterophyes nocens and Pygidiopsis summa metacercariae in mullets and gobies along the coastal areas of the Republic of Korea. Korean Journal of Parasitology 45, 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseeb AN, El Shazly AM, Arafa MAS and Morsy AT (2002) A review on fascioliasis in Egypt. Journal of the Egyptian Society of Parasitology 32, 317–335. [PubMed] [Google Scholar]
- Hayashi K, Tang WQ, Ohari Y, Ohtori M, Mohanta UK, Matsuo K, Sato H and Itagaki T (2017) Phylogenetic relationship between Dicrocoelium chinensis populations in Japan and China based on mitochondrial nad1 gene sequences. Parasitology Research 116, 2605–2609. [DOI] [PubMed] [Google Scholar]
- Hernández-Chea R, Jiménez-Rocha AE, Castro R, Blair D and Dolz G (2017) Morphological and molecular characterization of the metacercaria of Paragonimus caliensis, as a separate species from P. mexicanus in Costa Rica. Parasitology International 66, 126–133. [DOI] [PubMed] [Google Scholar]
- Hong ST and Fang Y (2012) Clonorchis sinensis and clonorchiasis, an update. Parasitology International 61, 17–24. [DOI] [PubMed] [Google Scholar]
- Hong ST, Cho TK, Hong SJ, Chai JY, Lee SH and Seo BS (1984) Fifteen human cases of Fibricola seoulensis infection in Korea. Korean Journal of Parasitology 22, 61–65. [DOI] [PubMed] [Google Scholar]
- Hotez P and Alibek K (2011) Central Asia's hidden burden of neglected tropical diseases. PLoS Neglected Tropical Diseases 5, e1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou PC (1956) The relationship between primary carcinoma of the liver and infestation with Clonorchis sinensis. Journal of Pathology and Bacteriology 72, 239–246. [DOI] [PubMed] [Google Scholar]
- Huang WY, He B, Wang CR and Zhu XQ (2004) Characterisation of Fasciola species from Mainland China by ITS2 ribosomal DNA sequence. Veterinary Parasitology 120, 75–83. [DOI] [PubMed] [Google Scholar]
- Im JG, Kong Y, Shin YM, Yang SO, Song JG, Han MC, Kim CW, Cho SY and Ham EK (1993) Pulmonary paragonimiasis: clinical and experimental studies. Radiographics 13, 575–586. [DOI] [PubMed] [Google Scholar]
- Itagaki T, Kikawa M, Sakaguchi K, Shimo J, Terasaki K, Shibahara T and Fukuda F (2005) Genetic characterization of parthenogenic Fasciola sp. in Japan on the basis of the sequences of ribosomal and mitochondrial DNA. Parasitology 131, 679–685. [DOI] [PubMed] [Google Scholar]
- Ito J (1964) Metagonimus and other human heterophyid trematodes. Progress of Medical Parasitology in Japan 1, 314–393. [Google Scholar]
- Iwagami M, Monroy C, Rosas MA, Pinto MR, Guevara AG, Vieira JC, Agatsuma Y and Agatsuma T (2003) A molecular phylogeographic study based on DNA sequences from individual metacercariae of Paragonimus mexicanus from Guatemala and Ecuador. Journal of Helminthology 77, 33–38. [DOI] [PubMed] [Google Scholar]
- Iwagami M, Rajapakse RPVJ, Paranagama W, Okada T, Kano S and Agatsuma T (2008) Ancient divergence of Paragonimus westermani in Sri Lanka. Parasitology Research 102, 845–852. [DOI] [PubMed] [Google Scholar]
- Kamo H, Nishida H, Hatsushika R and Tomimura T (1961) On the occurrence of a new lung fluke, Paragonimus miyazakii n. sp. in Japan (Trematoda: Troglotrematidae). Yonago Acta Medica 5, 43–52 (in Japanese). [Google Scholar]
- Kanev I (1994) Life-cycle, delimitation and redescription of Echinostoma revolutum (Froelich, 1982) (Trematoda: Echinostomatidae). Systematic Parasitology 28, 125–144. [Google Scholar]
- Kang BK, Jung BK, Lee YS, Hwang IK, Lim H, Cho J, Hwang JH and Chai JY (2014) A case of Fasciola hepatica infection mimicking cholangiocarcinoma and ITS-1 sequencing of the worm. Korean Journal of Parasitology 52, 193–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagara DWW and Karunaratne GMS (1969) Paragonimus siamensis: the fourth lung fluke reported from Ceylon. Ceylon Journal of Medical Science 18, 61–65. [Google Scholar]
- Keiser J, Engels D, Büscher G and Utzinger J (2005) Triclabendazole for the treatment of fascioliasis and paragonimiasis. Expert Opinion on Investigational Drugs 14, 1513–1526. [DOI] [PubMed] [Google Scholar]
- Khamidullin RI, Liubina VS, Khamidullin IR and Medinskiĭ BL (1991) [Trematodiases in Tartaria]. Meditsinskaia Parazitologiia I Parazitarnye Bolezi 2, 60–61. [PubMed] [Google Scholar]
- Khamidullin RI, Fomina OA, Sultanaeva EG and Khamidullin IR (1995) [Opisthorchiasis and pseudamphistomiasis on the territory of the middle Volga valley]. Meditsinskaia Parazitologiia I Parazitarnye Bolezi 1, 40–42. [PubMed] [Google Scholar]
- Khan MA, Afshan K, Nazar M, Firasat S, Chaudhry U and Sargison ND (2021) Molecular confirmation of Dicrocoelium dendriticum in the Himalayan ranges of Pakistan. Parasitology International 81, 102276. [DOI] [PubMed] [Google Scholar]
- Khandelwal N, Shaw J and Jain MK (2008) Biliary parasites: diagnostic and therapeutic strategies. Current Treatment Opinions in Gastroenterology 11, 85–95. [DOI] [PubMed] [Google Scholar]
- Khieu V, Fürst T, Miyamoto K, Yong TS, Chai JY, Huy R, Muth S and Odermatt P (2019) Is Opisthorchis viverrini emerging in Cambodia? Advances in Parasitology 103, 31–73. [DOI] [PubMed] [Google Scholar]
- Kim DC (1984a) Paragonimus westermani: life cycle, intermediate hosts, transmission to man and geographical distribution in Korea. Arzneimittel-Forschung/Drug Research 34, 1180–1183. [PubMed] [Google Scholar]
- Kim YI (1984b) Liver carcinoma and liver fluke infection. Arzneimittel-Forschung/Drug Research 34, 1121–1126. [PubMed] [Google Scholar]
- Kim YK, Yu JE, Chung EY and Chung PR (2004) Acanthoparyphium tyosenense (Digenea: Echinostomatidae): experimental confirmation of the cercaria and its complete life history in Korea. Journal of Parasitology 90, 97–102. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Kim SH, Lee SO, Choi SH, Kim YS, Woo JH, Yoon YS, Kim KW, Cho J, Chai JY and Chong YP (2017) A case of ectopic peritoneal paragonimiasis mimicking diverticulitis or abdominal abscess. Korean Journal of Parasitology 55, 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Yong TS, Rim HJ, Chai JY, Min DY, Eom KS, m Sohn WM, Lim JH, Choi D, Insisiengmay S, Phommasack B and Insisiengmay B (2018) Ultrasonographic investigation of cholangiocarcinoma in Lao PDR. Acta Tropica 182, 128–134. [DOI] [PubMed] [Google Scholar]
- King EVJ (1971) Human infection with Dicrocoelium hospes in Sierra Leone. Journal of Parasitology 57, 989. [PubMed] [Google Scholar]
- Kino H, Oishi H, Ohno Y and Mitsuru I (2002) An endemic human infection with Heterophyes nocens Onji et Nishio, 1916 at Mikkabi-cho, Shizuoka, Japan. Japanese Journal of Tropical Medicine and Hygiene 30, 301–304. [Google Scholar]
- Kino H, Suzuki T, Oishi H, Suzuki S, Yamagiwa S and Ishiguro M (2006) Geographical distribution of Metagonimus yokogawai and M. miyatai in Shizuoka Prefecture, Japan, and their site preferences in the sweetfish, Plecoglossus altivelis, and hamsters. Parasitology International 55, 201–206. [DOI] [PubMed] [Google Scholar]
- Koh EJ, Kim SK, Wang KC, Chai JY, Chong S, Park SH, Cheon JE and Phi JH (2012) The return of an old worm: cerebral paragonimiasis presenting with intracerebral hemorrhage. Journal of the Korean Medical Science 27, 1428–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korea Association of Health Promotion (2013) Statistics on the Prevalence of Intestinal Parasitic Infections in Korea – The 8th Report (Monographic Series in Korean). Seoul, Korea: Korean CDC and Korea Association of Health Promotion. [Google Scholar]
- Kumchoo K, Wongsawad C, Vanittanakom P, Chai JY and Rojanapaibul A (2007) Effect of niclosamide on the tegumental surface of Haplorchis taichui using scanning electron microscopy. Journal of Helminthology 81, 329–337. [DOI] [PubMed] [Google Scholar]
- Kuntz RE, Lawless DK, Langbehn HR and Malakatis GM (1958) Intestinal protozoa and helminths in the peoples of Egypt living in different type localities. American Journal of Tropical Medicine and Hygiene 7, 630–639. [DOI] [PubMed] [Google Scholar]
- Kusner DR and King CH (1993) Cerebral paragonimiasis. Seminar in Neurology 13, 201–208. [DOI] [PubMed] [Google Scholar]
- Kuznetsova VG, Naumov VA and Belov GF (2000) Metorchiasis in the residents of Novosibirsk area, Russia. Cytobios 102, 33–34. [PubMed] [Google Scholar]
- Kyung SY, Cho YK, Kim YJ, Park JW, Jeong SH, Lee JI, Sung YM and Lee SP (2011) A paragonimiasis patient with allergic reaction to praziquantel and resistant to triclabendazole: successful treatment after desensitization to praziquantel. Korean Journal of Parasitology 49, 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai DH, Hong XK, Su BX, Liang C, Hide G, Zhang X, Yu X and Lun ZR (2016) Current status of Clonorchis sinensis and clonorchiasis in China. Transactions of the Royal Society of Tropical Medicine and Hygiene 110, 21–27. [DOI] [PubMed] [Google Scholar]
- Landaverde-González P, Osgood J, Quinoñez CAM, Monzón V, Rodas A and Monroy C (2022) The effect of landscape and human settlement on the genetic differentiation of Paragonimus species in Mesoamerica. International Journal for Parasitology 52, 13–21. [DOI] [PubMed] [Google Scholar]
- Lane MA, Barsanti MC, Santos CA, Young M, Lubner SJ and Weil GJ (2009) Human paragonimiasis in North America following ingestion of raw crayfish. Clinical Infectious Diseases 49, e55–e61. [DOI] [PubMed] [Google Scholar]
- Lane MA, Marcos LA, Onen NF, Demertzis LM, Hayes EV, Davilla SZ, Nurutdinova DR, Bailey TC and Weil GJ (2012) Paragonimus kellicotti fluke infections in Missouri, USA. Emerging Infectious Diseases 18, 1263–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Hwang SW, Chai JY and Seo BS (1984) Comparative morphology of eggs of heterophyids and Clonorchis sinensis causing human infections in Korea. Korean Journal of Parasitology 22, 171–180. [DOI] [PubMed] [Google Scholar]
- Lee SH, Yoo BH, Hong ST, Chai JY, Seo BS and Chi JG (1985) A histopathological study on the intestine of mice and rats experimentally infected by Fibricola seoulensis. Korean Journal of Parasitology 23, 58–72. [DOI] [PubMed] [Google Scholar]
- Lee SH, Chai JY, Yang EC, Yun CK, Hong ST and Lee JB (1988) Observation of liver pathology after praziquantel treatment in experimental Clonorchis sinensis infection in guinea pigs. Seoul Journal of Medicine 29, 253–262. [Google Scholar]
- Lee SH, Chai JY and Hong ST (1993) Gymnophalloides seoi n. sp. (Digenea: Gymnophallidae), the first report of human infection by a gymnophallid. Journal of Parasitology 79, 677–680. [PubMed] [Google Scholar]
- Lee SH, Kim MN, Back BY, Chai JY, Kim TH and Hwang YS (2003) Analysis of parasite-specific antibody positive patients for Clonorchis sinensis, Paragonimus westermani, cysticercus and sparganum using ELISA. Korean Journal of Laboratory Medicine 23, 126–131 (in Korean). [Google Scholar]
- Lin J, Chen Y and Li Y (2001) The discovery of natural infection of human with Metorchis orientalis and the investigation of its focus. Chinese Journal of Zoonoses 17, 38–53 (in Chinese). [Google Scholar]
- Liu D and Zhu XQ (2013) Fasciola. In Liu D (ed.), Molecular Detection of Human Parasitic Pathogens. Boca Raton, London, New York: CRC Press Taylor & Francis Group, pp. 343–351. [Google Scholar]
- López-Caballero J, Oceguera-Figueroa A and León-Règagnon V (2013) Detection of multiple species of human Paragonimus from Mexico using morphological data and molecular barcodes. Molecular Ecology Resources 13, 1–12. [DOI] [PubMed] [Google Scholar]
- Lovis L, Mak TK, Phongluxa K, Soukhathammavong P, Sayasone S, Akkhavong K, Odermatt P, Keiser J and Felger I (2009) PCR diagnosis of Opisthorchis viverrini and Haplorchis taichui infections in a Lao community in an area of endemicity and comparison of diagnostic methods for parasitological field surveys. Journal of Clinical Microbiology 47, 1517–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean JD, Arthur JR, Ward BJ, Gyorkos TW, Curtis MA and Kokoskin E (1996) Common-source outbreak of acute infection due to the North American liver fluke Metorchis conjunctus. Lancet (London, England) 347, 154–158. [DOI] [PubMed] [Google Scholar]
- Magi B, Frati E, Bernini L, Sansoni A and Zanelli C (2009) Dicrocoelium dendriticum: a true infection? Le Infezioni in Medicina 2, 115–116. [PubMed] [Google Scholar]
- Mairiang E and Mairiang P (2003) Clinical manifestation of opisthorchiasis and treatment. Acta Tropica 88, 221–227. [DOI] [PubMed] [Google Scholar]
- Maksimova GA, Pakharukova MY, Kashina EV, Zhukova NA, Kovner AV, Lvova MN, Katokhin AV, Tolstikova TG, Sripa B and Mordvinov VA (2017) Effect of Opisthorchis felineus infection and dimethylnitrosamine administration on the induction of cholangiocarcinoma in Syrian hamsters. Parasitology International 66, 458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manga-González MY and Ferreras MC (2014) Dicrocoelidae family: major species causing veterinary diseases. In Toledo R and Fried B (eds), Digenetic Trematodes. Advances in Experimental Medicine and Biology, vol. 766. pp. 393–428. Available at 10.1007/978-1-4939-0915-5_12. [DOI] [PubMed] [Google Scholar]
- Manning GS and Lertprasert P (1973) Studies on the life cycle of Phaneropsolus bonnei and Prosthodendrium molenkampi in Thailand. Annals of Tropical Medicine and Parasitology 67, 361–365. [DOI] [PubMed] [Google Scholar]
- Manning GS, Diggs CL, Viyanant V, Lertprasert P and Watanasirmkit K (1970) Preliminary report on Phaneropsolus bonnei Lie Kian Joe, 1951. A newly discovered human intestinal fluke from northeastern Thailand. Journal of the Medical Association of Thailand 53, 173–178. [PubMed] [Google Scholar]
- Mas-Coma S and Bargues MD (1997) Human liver flukes: a review. Research and Reviews in Parasitology (Revista Iberica de Parasitologia) 57, 145–218. [Google Scholar]
- Mas-Coma S, Bargues MD and Valero MA (2005) Fascioliasis and other plant-borne trematode zoonoses. International Journal for Parasitology 35, 1255–1278. [DOI] [PubMed] [Google Scholar]
- Mas-Coma S, Valero MA and Bargues MD (2009) Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Advances in Parasitology 69, 41–146. [DOI] [PubMed] [Google Scholar]
- Mas-Coma S, Bargues MD and Valero MA (2018) Human fascioliasis infection sources, their diversity, incidence factors, analytical methods and prevention measures. Parasitology 145, 1665–1669. [DOI] [PubMed] [Google Scholar]
- Mas-Coma S, Valero MA and Bargues MD (2019) Fascioliasis. In Toledo R and Fried B (eds), Digenetic Trematodes. Advances in Experimental Medicine and Biology, vol. 1154. pp. 71–103. Available at 10.1007/978-3-030-18616-6_4. [DOI] [PubMed] [Google Scholar]
- McNulty SN, Fischer PU, Townsend RR, Curtis KC, Weil GJ and Mitreva M (2014) Systems biology studies of adult Paragonimus lung flukes facilitate the identification of immunodominant parasite antigens. PLoS Neglected Tropical Diseases 8, e3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers WM and Neafie RN (1976) Paragonimiasis. In Binford CH and Conner DH (eds), Pathology of Tropical and Extraordinary Diseases, vol. 2. Washington, DC, USA: Armed Forces Institute of Pathology, pp. 517–523. [Google Scholar]
- Miyazaki I (1991) An Illustrated Book of Helminthic Zoonoses. Tokyo, Japan: International Medical Foundation of Japan, pp. 76–146. [Google Scholar]
- Miyazaki I and Habe S (1976) A newly recognized mode of human infection with lung flukes, Paragonimus westermani (Kerbert, 1878). Journal of Parasitology 48, 23–24. [PubMed] [Google Scholar]
- Miyazaki I and Harinasuta T (1966) The first case of human paragonimiasis caused by Paragonimus heterotremus Chen et Hsia, 1964. Annals of Tropical Medicine and Parasitology 60, 509–514. [Google Scholar]
- Miyazaki I and Ishii Y (1968) Studies on the Mexican lung flukes, with special reference to a description of Paragonimus mexicanus sp. nov. (Trematoda: Troglotrematidae). Japanese Journal of Parasitology 17, 445–453. [Google Scholar]
- Miyazaki I and Wykoff DE (1965) On a new lung fluke Paragonimus siamensis n. sp. found in Thailand (Trematoda: Troglotrematidae). Japanese Journal of Parasitology 14, 251–257. [Google Scholar]
- Mordvinov VA, Yurlova NI, Ogorodova LM and Katokhin AV (2012) Opisthorchis felineus and Metorchis bilis are the main agents of liver fluke infection of humans in Russia. Parasitology International 61, 25–31. [DOI] [PubMed] [Google Scholar]
- Nakamura-Uchiyama F, Mukae H and Nawa Y (2002) Paragonimiasis: a Japanese perspective. Clinics in Chest Medicine 23, 409–420. [DOI] [PubMed] [Google Scholar]
- Nakamura S (2017) Present situation of opisthorchiasis in Vientiane Capital, Lao Peoples’ Democratic Republic. Japanese Journal of Hygiene 72, 101–105 (in Japanese). [DOI] [PubMed] [Google Scholar]
- Narain K, Agatsuma T and Blair D (2010) Paragonimus. In Liu D (ed.), Molecular Detection of Foodborne Pathogens. Boca Raton, USA: CRC Press, Taylor & Francis Group, pp. 827–837. [Google Scholar]
- Nawa Y, Doanh PN and Thaenkham U (2013) Is Opisthorchis viverrini an avian liver fluke? Journal of Helminthology 89, 255–256. [DOI] [PubMed] [Google Scholar]
- Nkouawa A, Okamoto M, Mabou AK, Edinga E, Yamasaki H, Sako Y, Nakao M, Nakaya K, Blair D, Agatsuma T, Enyong P, Shibahara T, Moyou-Somo R and Ito A (2009) Paragonimiasis in Cameroon: molecular identification, serodiagnosis and clinical manifestations. Transactions of the Royal Society of Tropical Medicine and Hygiene 103, 255–261. [DOI] [PubMed] [Google Scholar]
- Oh SJ (1968a) Paragonimus meningitis. Journal of the Neurological Sciences 6, 419–433. [DOI] [PubMed] [Google Scholar]
- Oh SJ (1968b) Spinal paragonimiasis. Journal of the Neurological Sciences 6, 125–140. [DOI] [PubMed] [Google Scholar]
- Oh SJ (1969) Cerebral and spinal paragonimiasis. A histopathological study. Journal of the Neurological Sciences 9, 205–236. [DOI] [PubMed] [Google Scholar]
- Pakharukova MY and Mordvinov VA (2016) The liver fluke Opisthorchis felineus: biology, epidemiology and carcinogenic potential. Transactions of the Royal Society of Tropical Medicine and Hygiene 110, 28–36. [DOI] [PubMed] [Google Scholar]
- Parkinson M, Dalton JP and O'Neill SM (2011) Fasciolosis. In Palmer SR, Soulsby L, Torgerson P and Brown DWG (eds), Oxford Textbook of Zoonoses (2nd ed.). Biology, Clinical Practice, and Public Health Control. Oxford, UK: Oxford University Press, pp. 862–872. [Google Scholar]
- Pozio E, Armigbacco O, Ferri F and Morales MAG (2013) Opisthorchis felineus, an emerging infection in Italy and its implication for the European Union. Acta Tropica 126, 54–62. [DOI] [PubMed] [Google Scholar]
- Procop GW (2009) North American paragonimiasis (caused by Paragonimus kellicoti) in the context of global paragonimiasis. Clinical Microbiology Reviews 22, 415–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pungpak S, Viravan C, Radomyos B, Chalermrut K, Yemput C, Plooksawasdi W, Ho M, Harinasuta C and Bunnag D (1997) Opisthorchis viverrini infection in Thailand: studies on the morbidity of the infection and resolution following praziquantel treatment. American Journal of Tropical Medicine and Hygiene 56, 311–314. [DOI] [PubMed] [Google Scholar]
- Rabone M, Wiethase J, Clark PF, Rollinson D, Cumberlidge N and Emery AM (2021) Endemicity of Paragonimus and paragonimiasis in Sub-Saharan Africa: a systematic review and mapping reveals stability of transmission in endemic foci for a multi-host parasite system. PLoS Neglected Tropical Diseases 15, e0009120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan VS and Srinivasan T (1962) Artyfechinostomum mehrai infestation. A case report. Journal of Indian Medical Association 38, 485–487. [PubMed] [Google Scholar]
- Rajapakse RPVJ, Pham KLT, Karunathilake KJK, Lawton SP and Le TH (2020) Characterization and phylogenetic properties of the complete mitochondrial genome of Fascioloides jacksoni (syn. Fasciola jaksoni) support the suggested intergeneric change from Fasciola to Fascioloides (Platyhelminthes: Trematoda: Plagiorchiida). Infection, Genetics and Evolution 82, 104281. [DOI] [PubMed] [Google Scholar]
- Rim HJ (1982a) Clonorchiasis. In Steele JH, Hillyer GV and Hopla CE (eds), CRC Handbook Series in Zoonoses, Section C: Parasitic Zoonoses, Vol. III. Trematode Zoonoses. Boca Raton, FL, USA: CRC Press, pp. 17–32. [Google Scholar]
- Rim HJ (1982b) Opisthorchiasis. In Steele JH, Hillyer GV and Hopla CE (eds), CRC Handbook Series in Zoonoses, Section C: Parasitic Zoonoses, Vol. III. Trematode Zoonoses. Boca Raton, FL, USA: CRC Press, pp. 109–121. [Google Scholar]
- Rim HJ (1982c) Echinostomiasis. In Steele JH, Hillyer GV and Hopla CE (eds), CRC Handbook Series in Zoonoses, Section C: Parasitic Zoonoses, Vol. III. Trematode Zoonoses. Boca Raton, FL, USA: CRC Press, pp. 53–69. [Google Scholar]
- Rim HJ (2005) Clonorchiasis: an update. Journal of Helminthology 79, 269–281. [DOI] [PubMed] [Google Scholar]
- Rim HJ, Chai JY, Min DY, Cho SY, Eom KS, Hong SJ, Sohn WM, Yong TS, Deodato G, Standgaard H, Phommasack B, Yun CH and Hoang EH (2003) Prevalence of intestinal parasite infections on a national scale among primary schoolchildren in Laos. Parasitology Research 91, 267–272. [DOI] [PubMed] [Google Scholar]
- Romero-Alvarez D, Valverde-Muñoz G, Calvopina M, Rojas M, Cevallos W, Kumazawa H, Takagi H and Sugiyama H (2020) Liver fluke infections by Amphimerus sp. (Digenea: Opisthorchiidae) in definitive and fish intermediate hosts in Manabí province, Ecuador. PLoS Neglected Tropical Diseases 14, e0008286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa BA, Choi YJ, McNulty SN, Jung H, Martin J, Agatsuma T, Sugiyama H, Le TH, Doanh PN, Maleewong W, Blair D, Brindley PJ, Fischer PU and Mitreva M (2020) Comparative genomics and transcriptomes of 4 Paragonimus species provide insights into lung fluke parasitism and pathogenesis. GigaScience 9, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B and Tandon V (1992) Opisthorchis noverca Braun, 1902: first record from a bovine host and a comparative stereoscan study of the surface topography of flukes of swine and cattle origin. Acta Parasitologica 37, 179–181. [Google Scholar]
- Saijuntha W, Sithithaworn P, Kiasopit N, Andrews RH and Petney TN (2019) Liver flukes: Clonorchis and Opisthorchis. In Toledo R and Fried B (eds), Digenetic Trematodes. Advances in Experimental Medicine and Biology, vol. 1154. pp. 139–180. Available at 10.1007/978-3-030-18616-6_6. [DOI] [PubMed] [Google Scholar]
- Saijuntha W, Sithithaworn P, Petney TN and Andrews RH (2021) Foodborne zoonotic parasites of the family Opisthorchiidae. Research in Veterinary Science 135, 404–411. [DOI] [PubMed] [Google Scholar]
- Saito S, Chai JY, Kim KH, Lee SH and Rim HJ (1997) Metagonimus miyatai sp. nov. (Digenea: Heterophyidae), a new intestinal trematode transmitted by freshwater fishes in Japan and Korea. Korean Journal of Parasitology 35, 223–232. [DOI] [PubMed] [Google Scholar]
- Sanpool O, Intapan PM, Thanchomnang T, Janwan P, Nawa Y, Blair D and Maleewong W (2013) Molecular variation in the Paragonimus heterotremus complex in Thailand and Myanmar. Korean Journal of Parasitology 51, 677–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo BS (1990) Fibricola seoulensis Seo, Rim and Lee, 1964 (Trematoda) and fibricoliasis in man. Seoul Journal of Medicine 31, 61–96. [Google Scholar]
- Seo BS, Hong ST and Chai JY (1981) Studies on intestinal trematodes in Korea III. Natural human infection of Pygidiopsis summa and Heterophyes heterophyes nocens. Seoul Journal of Medicine 22, 228–235. [Google Scholar]
- Seo M, Guk SM, Kim J, Chai JY, Bok GD, Park SS, Oh CS, Kim MJ, Yi YS, Shin MH, Kang IU and Shin DH (2007) Paleoparasitological report on the stool from a medieval child mummy in Yangju, Korea. Journal of Parasitology 93, 589–592. [DOI] [PubMed] [Google Scholar]
- Shan X, Lin C, Li Y, Hu Y, Shen X and Lou H (2009) A new species of Paragonimus sheni-with a key to the species of adult worms and metacercariae of the genus Paragonimus in China. Chinese Journal of Zoonoses 25, 1143–1148. [Google Scholar]
- Sherrad-Smith E, Cable J and Chadwick EA (2009) Distribution of Eurasian otter biliary parasites, Pseudamphistomum truncatum and Metorchis albidus (Family Opisthorchiidae), in England and Wales. Parasitology 136, 1015–1022. [DOI] [PubMed] [Google Scholar]
- Shibahara T, Nishida H, Torii M, Gyoten J, Tsuboi T and Sakai M (1992) Experimental infection of wild boars with metacercariae of Paragonimus miyazakii (Trematoda: Troglotrematidae). Japanese Journal of Parasitology 41, 274–278. [Google Scholar]
- Shin EH, Im TK, Park YK, Cho J, Kim JL and Chai JY (2016) Fecundity reduction of BALB/c mice after survival from lethal Neodiplostomum seoulense infection. Parasitology Research 115, 2051–2059. [DOI] [PubMed] [Google Scholar]
- Shoriki T, Ichigawa-Seki M, Suganuma K, Naito I, Hayashi K, Nakao M, Aita J, Mohanta UK, Inoue N, Murakami K and Itagaki T (2016) Novel methods for the molecular discrimination of Fasciola spp. on the basis of nuclear protein-coding genes. Parasitology International 65, 180–183. [DOI] [PubMed] [Google Scholar]
- Shu QH, Li SD, Tian M, Meng Y, He SMQ, Wang MM and Wang WL (2021a) Morphological and molecular characterization of Paragonimus skrjabini complex from Yunnan, China: a brief report. Acta Parasitologica 67, 316–321. 10.1007/s11686-021-00461-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu QH, Yang Y, Wang MM, Li SD, Tian M, Bai WW, Meng Y, He SMQ and Wang WL (2021b) Morphological and molecular characterization of Paragonimus species isolated from freshwater crabs in southern Yunnan, China. Journal of Tropical Medicine 2021, 5646291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumenko PG, Tatanova YV and Besprozvannykh VV (2017) Metagonimus suifunensis sp. n. (Trematoda: Heterophyidae) from the Russian Southern Far East: morphology, life cycle, and molecular data. Parasitology International 66, 982–991. [DOI] [PubMed] [Google Scholar]
- Simpson VR, Gibbons LM, Khalil LF and Williams JLR (2005) Cholecystitis in otters (Lutra lutra) and mink (Mustela vison) caused by the fluke Pseudamphistomum truncatum. Veterinary Record 157, 49–52. [DOI] [PubMed] [Google Scholar]
- Singh ST, Singh DL and Sugiyama H (2006) Possible discovery of Chinese lung fluke, Paragonimus skrjabini in Manipur, India. Southeast Asian Journal of Tropical Medicine and Public Health 37(suppl.), 53–56. [PubMed] [Google Scholar]
- Singh TS, Sugiyama H, Umehara A, Hiese S and Khalo K (2009) Paragonimus heterotremus infection in Nagaland: a new focus of paragonimiasis in India. Indian Journal of Medical Microbiology 27, 123–127. [DOI] [PubMed] [Google Scholar]
- Singh TS, Sugiyama H, Devi KR and Singh WA (2015) First case of Paragonimus westermani infection in a female patient in India. Indian Journal of Medical Microbiology 33(suppl. 1), S156–S159. [DOI] [PubMed] [Google Scholar]
- Sithithaworn P and Haswell-Elkins M (2003) Epidemiology of Opisthorchis viverrini. Acta Tropica 88, 187–194. [DOI] [PubMed] [Google Scholar]
- Sitko J, Bizos J, Sherrad-Smith E, Stanton DWG, Komorova P and Heneberg P (2016) Integrative taxonomy of European parasitic flatworms of the genus Metorchis Looss, 1899 (Trematoda: Opisthorchiidae). Parasitology International 65, 258–267. [DOI] [PubMed] [Google Scholar]
- Skrjabin KJ and Podjapolskaja WP (1931) Nanophyetus schikhobalowi n. sp., ein neuer Trematode aus dem Darm des Menschen. Zentralblatt für Bakteriologie und Parasitenkunde (Orig.) 119, 294–297 (in Germany). [Google Scholar]
- Slepchenko S (2020) Opisthorchis felineus as the basis for the reconstruction of migrations using archaeparasitogical materials. Journal of Archaeological Science: Reports 33, 102548. [Google Scholar]
- Sohn WM, Kim HJ, Yong TS, Eom KS, Jeong HG, Kim JK, Kang AR, Kim MR, Park JM, Ji SH, Sinuon M, Socheat D and Chai JY (2011) Echinostoma ilocanum infection in Oddar Meanchey Province, Cambodia. Korean Journal of Parasitology 49, 187–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn WM, Yong TS, Eom KS, Min DY, Lee D, Jung BK, Banouvong V, Insisiengmay B, Phammasack B, Rim HJ and Chai JY (2014) Prevalence of Haplorchis taichui among humans and fish in Luang Prabang Province, Lao PDR. Acta Tropica 136, 74–80. [DOI] [PubMed] [Google Scholar]
- Sohn WM, Yong TS, Eom KS, Sinuon M, Jeoung WG and Chai JY (2017) Artyfechinostomum malayanum: metacercariae encysted in Pila sp. snails purchased from Phnom Penh, Cambodia. Korean Journal of Parasitology 55, 341–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn WM, Jung BK, Hong SJ, Lee KH, Park HB, Kim HS, Cho S, Htoon TT, Tin HH and Chai JY (2019) Low-grade endemicity of opisthorchiasis, Yangon, Myanmar. Emerging Infectious Diseases 25, 1435–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S, Intapan PM, Maleewong W and Brindley PJ (2010) Food-borne trematodiases in Southeast Asia: epidemiology, pathology, clinical manifestations and control. Advances in Parasitology 72, 305–349. [DOI] [PubMed] [Google Scholar]
- Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E, Bethony JM and Loukas A (2012) The tumorigenic liver fluke Opisthorchis viverrini-multiple pathways to cancer. Trends in Parasitology 28, 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Tangkawattana S and Brindley PJ (2018) Update on pathogenesis of opisthorchiais and cholangiocarcinoma. Advances in Parasitology 102, 97–113. [DOI] [PubMed] [Google Scholar]
- Steverding D (2020) The spreading of parasites by human migratory activities. Virulence 11, 1177–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama H, Morishima Y, Binchai S, Rangsiruji A and Ketudat P (2007) New form of Paragonimus westermani discovered in Thailand: morphological characteristics and host susceptibility. Southeast Asian Journal of Tropical Medicine and Public Health 38, 87–91. [Google Scholar]
- Sugiyama H, Singh TS and Rangsiruji A (2013) Paragonimus. In Liu D (ed.), Molecular Detection of Human Parasitic Pathogens. Boca Raton, USA: CRC Press, Taylor & Francis Group, pp. 423–435. [Google Scholar]
- Tandon V, Prasad PK, Chatterjee A and Bhutia PT (2007) Surface fine topography and PCR-based determination of metacercaria of Paragonimus sp. from edible crabs in Arunachal Pradesh, Northeast India. Parasitology Research 102, 21–28. [DOI] [PubMed] [Google Scholar]
- Tandon V, Roy B and Prasad PK (2013) Fasciolopsis. In Liu D (ed.), Molecular Detection of Human Parasitic Pathogens. Boca Raton, USA: CRC Press, Taylor & Francis Group, pp. 353–364. [Google Scholar]
- Tang M, Zhou Y, Liu Y, Cheng N, Zhang J and Xu X (2021) Molecular identification and genetic-polymorphism analysis of Fasciola flukes in Dali Prefecture, Yunnan Province, China. Parasitology International 85, 102416. [DOI] [PubMed] [Google Scholar]
- Tantrawatpan C, Tapdara S, Agatsuma T, Sanpool O, Intapan PM, Maleewong W and Saijuntha W (2021) Genetic differentiation of Southeast Asian Paragonimus Braun, 1899 (Digenea: Paragonimidae) and genetic variation in the Paragonimus heterotremus complex examined by nuclear DNA sequences. Infection, Genetics and Evolution 90, 104761. [DOI] [PubMed] [Google Scholar]
- Teimoori S, Arimatsu Y, Laha T, Kaewkes S, Sereerak P, Sripa M, Tangkawattana S, Brindley PJ and Sripa B (2017) Chicken IgY-based coproantigen capture ELISA for diagnosis of human opisthorchiasis. Parasitology International 66, 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki K, Habe S, Ho LY, Jian H, Agatsuma T, Shibahara T, Sugiyama H and Kawashima K (1995) Tetraploid of the lung fluke Paragonimus westermani found in China. Parasitology Research 81, 627–630. [DOI] [PubMed] [Google Scholar]
- Thaenkham U, Nuamthanong S, Vonghachck Y, Yoonuan T, Sanguankiat S, Dekumyoy P, Phommasack B, Kobayashi J and Waikagul J (2011) Discovery of Opisthorchis lobatus (Trematoda: Opisthorchiidae): a new record of small liver flukes in the Great Mekong Sub-region. Journal of Parasitology 97, 1152–1158. [DOI] [PubMed] [Google Scholar]
- Thaenkham U, Blair D, Nawa Y and Waikagul J (2012) Families Opisthorchiidae and Heterophyidae: are they distinct? Parasitology International 61, 90–93. [DOI] [PubMed] [Google Scholar]
- Thatcher VE (1970) The genus Amphimerus Barker, 1911 (Trematoda: Opisthorchiidae) in Colombia with the description of a new species. Proceedings of the Helminthological Society of Washington 37, 207–211. [Google Scholar]
- Tran BT, Nguyen ST, Nguyen TT, Luc PV, Mafie E, Rupa FH and Sato H (2016) Endoparasites of Vietnamese lizards recorded in the last 50 years (1966–2015). Japanese Journal of Veterinary Parasitology 15, 34–58. [Google Scholar]
- Traversa D, Lorusso V and Otranto D (2013) Dicrocoelium. In Liu D (ed.), Molecular Detection of Human Parasitic Pathogens. Boca Raton, London, New York: CRC Press Taylor & Francis Group, pp. 323–331. [Google Scholar]
- Urabe M (2003) Trematode fauna of prosobranch snails of the genus Semisulcospira in Lake Biwa and the connected drainage system. Parasitology International 52, 21–34. [DOI] [PubMed] [Google Scholar]
- Vo DT, Murrell D, Dalsgaard A, Bristow G, Nguyen DH, Bui TN and Vo DT (2008) Prevalence of zoonotic metacercariae in two species of groupers, Epinephelus coioides and Epinephelus bleekeri, and flathead mullet, Mugil cephalus, in Vietnam. Korean Journal of Parasitology 46, 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waikagul J (2007) A new species of Paragonimus (Trematoda: Troglotrematidae) from a cat infected with metacercariae from mountain crabs Larnaudia larnaudii. Journal of Parasitology 93, 1496–1500. [DOI] [PubMed] [Google Scholar]
- Waikagul J, Wongsaroj T, Radomyos P, Meesomboon V, Praewanich R and Jongsuksuntikul P (1997) Human infection of Centrocestus caninus in Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 28, 831–835. [PubMed] [Google Scholar]
- Wang W, Blair D, Min T, Li F and Wang D (2011) Paragonimus worm from a New Guinea native in 1926. Asian Pacific Journal of Tropical Medicine 4, 76–78. [DOI] [PubMed] [Google Scholar]
- Wanlop A, Wongsawad C, Prattapong P, Wongsawad P, Chontananarth T and Chai JY (2017) Prevalence of Centrocestus formosanus metacercariae in ornamental fish from Chiang Mai, Thailand, with molecular approach using ITS2. Korean Journal of Parasitology 55, 445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witenberg G (1929) Studies on the trematode-family Heterophyidae. Annals of Tropical Medicine and Parasitology 23, 131–268. [Google Scholar]
- Witenberg G (1932) On the anatomy and systematic position of the causative agent of so-called salmon poisoning. Journal of Parasitology 18, 258–263. [Google Scholar]
- World Health Organization (1995) Control of foodborne trematode infections. Report of a WHO Study Group. WHO Technical Report Series 849, 1–158. [PubMed] [Google Scholar]
- World Health Organization (2020) Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030. Geneva: World Health Organization, pp. 1–196. Available at https://www.who.int/publications/i/item/9789240010352. [Google Scholar]
- Wykoff DE, Harinasuta C, Juttijudata P and Winn MM (1965) Opisthorchis viverrini in Thailand – the life cycle and comparison with O. felineus. Journal of Parasitology 51, 207–214. [PubMed] [Google Scholar]
- Xiao X, Wang T, Zheng X, Shen G and Tian Z (2005) In vivo and in vitro encystment of Echinochasmus liliputanus cercariae and biological activity of the metacercariae. Journal of Parasitology 91, 492–498. [DOI] [PubMed] [Google Scholar]
- Yaemput S, Dekumyoy P and Visiassuk K (1994) The natural first intermediate host of Paragonimus siamensis (Miyazaki and Wykoff, 1965) in Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 25, 284–290. [PubMed] [Google Scholar]
- Yamaguti S (1958) Part I. The digenetic trematodes of vertebrates. In Systema Helminthum, vol. I. New York, USA: Interscience Publishers Inc., pp. 1–1074. [Google Scholar]
- Yamaguti S (1971) Synopsis of Digenetic Trematodes of Vertebrates. Vols. I (pp. 1–1074) & II (349 Figure Plates). Tokyo, Japan: Keigaku Publishing Co. [Google Scholar]
- Yang B, Li J and Zhou B (2021) Analysis of internal transcribed spacer regions II gene and morphology of Paragonimus from Yunnan Province, China. Research Square 2021, 1–19. 10.21203/rs.3.rs-699536/v1 [DOI] [Google Scholar]
- Yatera K, Hanaka M, Hanaka T, Yamasaki K, Nishida C, Kawanami T, Kawanami Y, Ishimoto H, Kanazawa T and Mukae H (2015) A rare case of paragonimiasis miyazakii with lung involvement diagnosed 7 years after infection: a case report and literature review. Parasitology International 64, 274–280. [DOI] [PubMed] [Google Scholar]
- Ye HY and Mitchell PD (2016) Ancient human parasites in ethnic Chinese populations. Korean Journal of Parasitology 54, 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokogawa M (1965) Paragonimus and paragonimiasis. Advances in Parasitology 3, 99–158. [DOI] [PubMed] [Google Scholar]
- Yokogawa M, Harinasuta C and Charoenlarp P (1965) Hypoderaeum conoideum (Block, 1782) Dietz, 1909, a common intestinal fluke of man in the north-east Thailand. Japanese Journal of Parasitology 14, 148–153. [Google Scholar]
- Yokogawa M, Araki K, Saito K, Momose T, Kimura M, Suzuki S, Chiba N, Kutsumi H and Minai M (1974) Paragonimus miyazakii infections in man first found in Kanto district, Japan. Japanese Journal of Parasitology 23, 167–179. [Google Scholar]
- Yong TS, Chai JY, Sohn WM, Eom KS, Jeoung HG, Hoang EH, Yoon CH, Jung BK, Lee SH, Sinuon M and Socheat D (2014) Prevalence of intestinal helminths among inhabitants of Cambodia (2006–2011). Korean Journal of Parasitology 52, 661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Doanh PN and Maruyama H (2019) Paragonimus and paragonimiasis in Asia: an update. Acta Tropica 199, 105074. [DOI] [PubMed] [Google Scholar]
- Youssef MM, Mansour NS, Awadalla HN, Hammouda NA, Khalifa R and Boulos LM (1987a) Heterophyid parasites of man from Idku, Maryut and Manzala Lakes areas in Egypt. Journal of the Egyptian Society of Parasitology 17, 475–479. [PubMed] [Google Scholar]
- Youssef MM, Mansour NS, Hammouda NA, Awadalla HN, Khalifa R and Boulos LM (1987b) Studies on some developmental stages in the life cycle of Pygidiopsis genata Looss, 1907 (Trematoda: Heterophyidae) from Egypt. Journal of the Egyptian Society of Parasitology 17, 463–474. [PubMed] [Google Scholar]
- Yu JR and Chai JY (2013) Metagonimus. In Liu D (ed.), Molecular Detection of Human Parasitic Pathogens. Boca Raton, USA: CRC Press, Taylor, Francis Group, pp. 389–398. [Google Scholar]
- Yu SH and Mott KE (1994) Epidemiology and morbidity of food-borne intestinal trematode infections. Tropical Diseases Bulletin 91, R125–R152. [Google Scholar]
- Yu S, Zhang X, Chen W, Zheng H, Ai G, Ye N and Wang Y (2017) Development of an immunodiagnosis method using recombinant PsCP for detection of Paragonimus skrjabini infection in humans. Parasitology Research 116, 377–385. [DOI] [PubMed] [Google Scholar]
- Zhou XJ, Yang Q, Tan QH, Zhang LY, Shi LB and Zhou JX (2021) Paragonimus and its host in China: an update. Acta Tropica 223, 106094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182022000725.
click here to view supplementary material


