Key Points
Question
Among adults hospitalized with severe COVID-19, does treatment with synthetic angiotensin (1-7) (TXA-127) or an angiotensin II type 1 receptor–biased ligand (TRV-027) improve clinical outcomes?
Findings
In 2 placebo-controlled, randomized clinical trials, the number of days alive and free from supplemental oxygen during the 28 days after trial enrollment (oxygen-free days) was not significantly different from placebo for TXA-127 (adjusted odds ratio, 0.88) or TRV-027 (adjusted odds ratio, 0.74).
Meaning
These findings do not support the hypothesis that pharmacological modulation of the renin-angiotensin system with exogenous administration of synthetic angiotensin (1-7) or blockade of the angiotensin II type 1 receptor results in clinical benefit for patients with severe COVID-19.
Abstract
Importance
Preclinical models suggest dysregulation of the renin-angiotensin system (RAS) caused by SARS-CoV-2 infection may increase the relative activity of angiotensin II compared with angiotensin (1-7) and may be an important contributor to COVID-19 pathophysiology.
Objective
To evaluate the efficacy and safety of RAS modulation using 2 investigational RAS agents, TXA-127 (synthetic angiotensin [1-7]) and TRV-027 (an angiotensin II type 1 receptor–biased ligand), that are hypothesized to potentiate the action of angiotensin (1-7) and mitigate the action of the angiotensin II.
Design, Setting, and Participants
Two randomized clinical trials including adults hospitalized with acute COVID-19 and new-onset hypoxemia were conducted at 35 sites in the US between July 22, 2021, and April 20, 2022; last follow-up visit: July 26, 2022.
Interventions
A 0.5-mg/kg intravenous infusion of TXA-127 once daily for 5 days or placebo. A 12-mg/h continuous intravenous infusion of TRV-027 for 5 days or placebo.
Main Outcomes and Measures
The primary outcome was oxygen-free days, an ordinal outcome that classifies a patient’s status at day 28 based on mortality and duration of supplemental oxygen use; an adjusted odds ratio (OR) greater than 1.0 indicated superiority of the RAS agent vs placebo. A key secondary outcome was 28-day all-cause mortality. Safety outcomes included allergic reaction, new kidney replacement therapy, and hypotension.
Results
Both trials met prespecified early stopping criteria for a low probability of efficacy. Of 343 patients in the TXA-127 trial (226 [65.9%] aged 31-64 years, 200 [58.3%] men, 225 [65.6%] White, and 274 [79.9%] not Hispanic), 170 received TXA-127 and 173 received placebo. Of 290 patients in the TRV-027 trial (199 [68.6%] aged 31-64 years, 168 [57.9%] men, 195 [67.2%] White, and 225 [77.6%] not Hispanic), 145 received TRV-027 and 145 received placebo. Compared with placebo, both TXA-127 (unadjusted mean difference, −2.3 [95% CrI, −4.8 to 0.2]; adjusted OR, 0.88 [95% CrI, 0.59 to 1.30]) and TRV-027 (unadjusted mean difference, −2.4 [95% CrI, −5.1 to 0.3]; adjusted OR, 0.74 [95% CrI, 0.48 to 1.13]) resulted in no difference in oxygen-free days. In the TXA-127 trial, 28-day all-cause mortality occurred in 22 of 163 patients (13.5%) in the TXA-127 group vs 22 of 166 patients (13.3%) in the placebo group (adjusted OR, 0.83 [95% CrI, 0.41 to 1.66]). In the TRV-027 trial, 28-day all-cause mortality occurred in 29 of 141 patients (20.6%) in the TRV-027 group vs 18 of 140 patients (12.9%) in the placebo group (adjusted OR, 1.52 [95% CrI, 0.75 to 3.08]). The frequency of the safety outcomes was similar with either TXA-127 or TRV-027 vs placebo.
Conclusions and Relevance
In adults with severe COVID-19, RAS modulation (TXA-127 or TRV-027) did not improve oxygen-free days vs placebo. These results do not support the hypotheses that pharmacological interventions that selectively block the angiotensin II type 1 receptor or increase angiotensin (1-7) improve outcomes for patients with severe COVID-19.
Trial Registration
ClinicalTrials.gov Identifier: NCT04924660
These 2 randomized clinical trials compare the efficacy and safety of renin-angiotensin system (RAS) modulation using 2 investigational RAS agents, TXA-127 vs placebo and TRV-027 vs placebo, in adults hospitalized with severe COVID-19.
Introduction
Despite the success of vaccines for preventing COVID-19 and the success of immunomodulatory and antiviral drugs for treating severe COVID-19,1,2,3,4,5,6 the outcomes for patients admitted to the hospital with COVID-19 remain suboptimal.3,7 Thus, identifying new therapies for severe COVID-19 remains an important unmet need.
SARS-CoV-2 enters pulmonary and myocardial cells through binding of the viral spike protein to the human angiotensin-converting enzyme 2 (ACE2), which is a key component of the renin-angiotensin system (RAS).8 Preclinical models suggest viral binding to ACE2 inhibits the conversion of angiotensin II to angiotensin (1-7), thereby causing a pathological imbalance in the RAS, favoring the angiotensin II pathway (which promotes inflammation, vasoconstriction, and thrombosis) at the expense of the angiotensin (1-7) alternative pathway (which promotes anti-inflammatory, vasodilatory, and antithrombotic activities).8,9,10,11
A prevailing hypothesis since early in the COVID-19 pandemic has been that these changes in the RAS are central to the development of severe clinical disease in the lungs and other organs.11,12,13,14 Thus, the RAS is an appealing target for COVID-19 drug development (eFigure 1 in Supplement 1). However, early trials evaluating investigational and repurposed RAS drugs for the treatment of COVID-19 have not clearly demonstrated efficacy,15,16,17,18,19 and whether pharmacological modulation of the RAS can provide benefit for patients with severe COVID-19 remains unclear.
To test the hypothesis that pharmacological RAS modulation to potentiate the angiotensin (1-7) pathway and mitigate the angiotensin II pathway will improve clinical outcomes among adults hospitalized with COVID-19–associated hypoxemia, 2 placebo-controlled, randomized clinical trials were conducted evaluating 2 potent, investigational RAS agents that increase the relative activity of angiotensin (1-7) compared with angiotensin II via different mechanisms. In the TXA-127 trial, synthetic angiotensin (1-7)20 (TXA-127) was compared with placebo. In the TRV-027 trial, an angiotensin II type 1 receptor–biased ligand19,21 (TRV-027) was compared with placebo.
Methods
Trial Oversight
The National Institutes of Health established the fourth Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-4) program to rapidly evaluate host response therapies for the treatment of COVID-19.22 The multicenter ACTIV-4 Host Tissue platform is the component of the ACTIV-4 program focused on blinded, placebo-controlled, randomized clinical trials of investigational agents for hospitalized patients with COVID-19 and is funded by the National Heart, Lung, and Blood Institute, is coordinated by Vanderbilt University Medical Center, and is overseen by a data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute and a single institutional review board at Vanderbilt University.
The agents were investigated under US Food and Drug Administration investigational new drug application No. 154000. Written informed consent was obtained for all participants prior to initiation of the trial procedures. A master trial protocol governed multiple trials and appears in Supplement 2.
Trial Design
This report describes the first 2 ACTIV-4 Host Tissue trials (TXA-127 vs placebo and TRV-027 vs placebo). The 2 trials ran concurrently between July 22, 2021, and April 20, 2022, at 35 hospitals in the US (eTable 1 in Supplement 1). Each was a blinded, placebo-controlled, randomized clinical trial comparing an active RAS agent (TXA-127 or TRV-027) vs placebo. The active RAS agents (TXA-127 and TRV-027) were not compared with one another.
The trials shared patients for the placebo group such that each trial included the patients randomized to the relevant active agent and the patients eligible for that active agent who were randomized to placebo, regardless of which type of placebo they received. During the conduct of the 2 trials, a third active agent, fostamatinib, was added to the platform on November 17, 2021 (Figure 1). Some patients who received a placebo mimic of fostamatinib were included in the placebo groups in the TXA-127 and TRV-027 trials. The fostamatinib trial continued enrolling patients beyond the 2 concurrent trials of the RAS agents.
Figure 1. Patient Screening, Randomization, and Participation in the TXA-127 Trial and in the TRV-027 Trial.
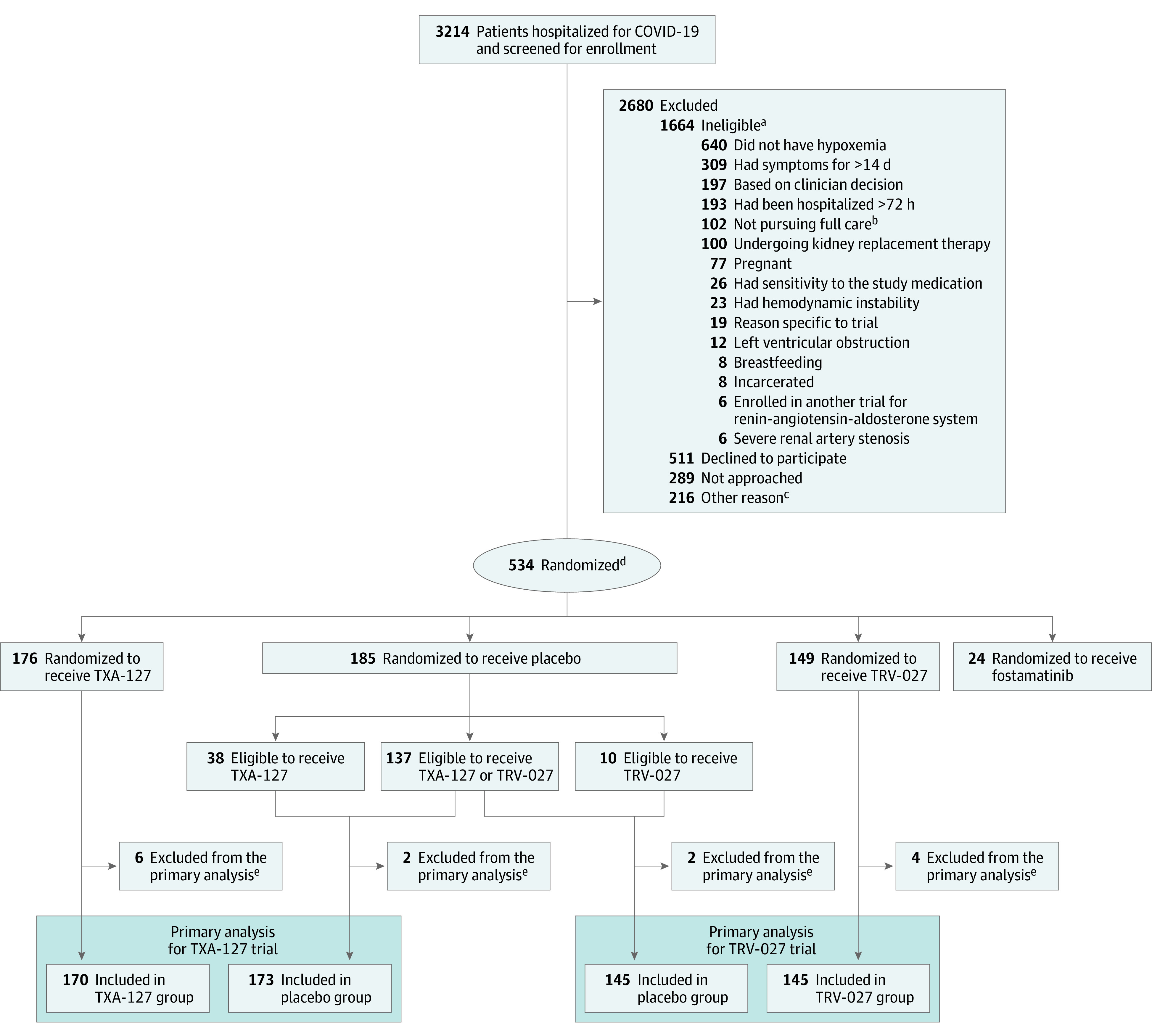
aThe criteria were not mutually exclusive; some potential participants met multiple criteria for ineligibility.
bThe patient, clinical team, or both, was not pursuing full medical management (eg, a do not intubate order).
cIncluded (1) the patient was to be discharged from the hospital before the study procedures could be initiated, (2) the patient was enrolled in another trial, and (3) the patient’s situation presented logistical challenges for trial enrollment.
dPatients eligible for more than 1 trial were randomized with equal probability to a specific trial. Patients were randomized to active vs placebo in an m:1 ratio, with m representing the number of trials for which the patient was eligible. Patients randomized to placebo were included in the placebo groups of each trial for which they were eligible. Patients randomized to receive an active drug contributed only to the trial they were randomized. Thus, patients randomized to receive active fostamatinib did not contribute to either the TXA-127 trial or the TRV-027 trial, whereas patients assigned to placebo fostamatinib were included in the placebo groups for the TXA-127 and TRV-027 trials if they were eligible for those trials. This design with a shared placebo group reduces the total number of patients who received placebo while retaining a statistically efficient 1:1 active vs placebo allocation for each trial.
eDid not receive the randomized study drug or were randomized but did not meet the enrollment criteria.
Study treatment (TXA-127, TRV-027, or placebo) was administered in addition to usual care COVID-19 therapies selected by treating clinicians independent of the trial protocol. The trial protocol did not influence treatment choices regarding antiviral medications, immunomodulatory therapies, or anticoagulation. There was no limitation on the clinical use of ACE inhibitor medications in either trial. Because TRV-027 binds at the same receptor (angiotensin II type 1 receptor) as angiotensin receptor blockers (ARBs), patients in the TRV-027 trial were restricted from using ARB medications during the 5-day study drug treatment course.
Patient Population
The inclusion criteria included being aged 18 years or older; being hospitalized for COVID-19; having a positive SARS-CoV-2 molecular or antigen test; and being diagnosed with new-onset hypoxemia, which was defined as an oxygen saturation as measured by pulse oximetry (Spo2) less than 92% on room air, use of supplemental oxygen to maintain an Spo2 greater than 92%, or an increase in oxygen for patients who were receiving supplemental oxygen before COVID-19 to maintain their baseline Spo2. A complete list of eligibility criteria appears in eTable 2 in Supplement 1.
The exclusion criteria included having COVID-19 symptoms for longer than 14 days; being hospitalized for longer than 3 days; undergoing kidney replacement therapy; having hemodynamic instability (mean arterial pressure <65 mm Hg or vasopressor use at a rate equivalent to ≥0.1 μg/kg/min of norepinephrine); and ARB use for the TRV-027 trial only.
Race and ethnicity were collected by self-report using mutually exclusive categories provided by the trial’s case report form. Race and ethnicity were collected to report the demographic characteristics of the trial population.
Randomization
Patients were assessed for eligibility for each of the trials currently running on the platform at the site where they were hospitalized. Study personnel entered which trial or multiple trials that the patient was eligible for into a centralized electronic randomization system (REDCap23). Patient-level randomization was conducted using permuted blocks, stratified by site and the trials for which the patient was eligible. To accomplish this, each randomization block contained a multiple of m(m + 1) assignments, with m active and 1 placebo assignment for each trial. The block size multiple was either 1 or 2, selected randomly. Patients eligible for multiple trials were randomized in equal ratios to those trials.
Patients were randomized to an active agent or placebo in an m:1 ratio, with m representing the number of trials for which the patient was eligible. Thus, a patient eligible for both the TXA-127 and TRV-027 trials would have been randomized in a 1:1 ratio to TXA-127 or TRV-027 and then in a 2:1 ratio to active vs placebo. If randomized to placebo, this patient would have been equally likely to receive a placebo mimic of TXA-127 or a placebo mimic of TRV-027 and would have been included in the analytical placebo group for both the TXA-127 and TRV-027 trials (additional details appear in §6.7 of the trial protocol; Supplement 2). This design with a shared placebo group was selected to reduce the total number of patients who received placebo while retaining a statistically efficient 1:1 active vs placebo allocation.24
Blinding
Patients, treating clinicians, and trial personnel were blinded to active vs placebo assignment but were not blinded to the specific trial. Thus, whether the patient was receiving study drug on a dosing regimen consistent with TXA-127 or TRV-027 was unblinded, but whether the study drug was active or placebo was blinded. This design enabled blinded comparisons of active vs placebo for multiple trials on the same platform using agents administered by different routes and dosing schedules.
Trial Interventions
TXA-127 (Constant Therapeutics, LLC) activates the Mas receptor, thereby promoting anti-inflammatory, vasodilatory, and antithrombotic activity (eFigure 1 in Supplement 1).12 TXA-127 was administered as a 0.5-mg/kg intravenous infusion over 3 hours once daily for 5 days or until hospital discharge, whichever occurred first. This dose was selected based on pharmacokinetic data suggesting successful activation of the Mas receptor and clinical safety in prior trials.20
TRV-027 (Trevena, Inc) produces a unique receptor conformation that results in antagonism of angiotensin II activity at the angiotensin II type 1 receptor and also triggers a β-arrestin pathway that activates the Mas receptor.25 Thus, TRV-027 both blocks angiotensin II signaling and activates angiotensin (1-7) signaling. TRV-027 was administered as a 12-mg/h continuous intravenous infusion for 5 days or until hospital discharge, whichever occurred first. This dosing regimen was selected based on prior data demonstrating successful angiotensin II type 1 receptor antagonism and clinical safety.21,25
The matching placebo for TXA-127 and TRV-027 was 0.9% sodium chloride (saline) administered in a regimen to mimic dosing of the active RAS agents. Patients eligible for TXA-127, TRV-027, or both, and who were randomly assigned to receive the placebo mimic of fostamatinib were included in the TXA-127 trial, the TRV-027 trial, or both trials. The fostamatinib placebo was an oral tablet administered twice daily for 14 days.
Outcomes
The primary outcome was oxygen-free days, an ordinal outcome that classifies a patient’s status at day 28 based on mortality and duration of supplemental oxygen use. The rationale and design of this outcome was published.26 It was calculated as 28 days minus the number of days between initiation and final liberation from new supplemental oxygen use during the 28 days after randomization. Patients receiving long-term supplemental oxygen before COVID-19 were classified as liberated from new supplemental oxygen when their oxygen flow rate returned to their baseline level prior to COVID-19. Patients who died before day 28 were coded as having −1 oxygen-free days. Patients who started the trial receiving new supplemental oxygen and continued with it through day 28 were coded as having 0 oxygen-free days. Hence, oxygen-free days was an ordinal outcome with 30 levels, ranging from −1 to 28 days.
The 3 key secondary efficacy outcomes included 28-day all-cause mortality; alive and free from respiratory failure (defined as alive and not receiving high-flow nasal oxygen, noninvasive ventilation, or invasive ventilation) at day 28; and status on the 8-level World Health Organization COVID-19 clinical progression ordinal scale at day 28.27 The safety outcomes included allergic reaction (including angioedema) through day 7; undergoing new kidney replacement therapy through day 28; and hypotension through day 7, which was defined as a low blood pressure leading to an initiation or increase in vasopressor therapy, administration of a 500-mL fluid bolus or greater, or a pause of the study drug.
A complete list of the trial outcomes appears in eTable 3 in Supplement 1 and a list of protocol-specified exempt serious events appears in eTable 4 in Supplement 1.
Statistical Analysis
Details of the statistical approach appear in the statistical analysis plan (Supplement 3). The 2 trials (TXA-127 vs placebo and TRV-027 vs placebo) were analyzed independently. The population for analysis included eligible, randomized patients who received any amount of the assigned study drug, hereafter referred to as the primary analysis population.
The primary outcome, oxygen-free days, was analyzed using a bayesian multivariable proportional odds regression model with the assigned treatment (active study drug vs placebo) as the primary independent variable, the 30-level oxygen-free days ordinal scale (range, −1 to 28 days) as the dependent variable, and the following co-variables: age group (18-30, 31-64, and ≥65 years), sex assigned at birth, and baseline level of oxygen support (level 4 on World Health Organization COVID-19 ordinal scale: standard nasal cannula or mask; level 5: high-flow nasal cannula or noninvasive ventilation; or level 6 or 7: invasive mechanical ventilation).
A noninformative prior was used for all model parameters. The treatment effect on oxygen-free days was quantified using an adjusted odds ratio (OR) and a bayesian 95% credible interval (CrI). An adjusted OR greater than 1.0 indicated superiority (more oxygen-free days in the active group than in the placebo group) and an adjusted OR less than 1.0 indicated inferiority. The efficacy probability was the posterior probability that the adjusted OR exceeded 1.0. Regression diagnostics (including assessment of the proportional odds assumption) and the sensitivity analyses were implemented as described in the statistical analysis plan in Supplement 3. No evidence was found that the proportional odds assumption was violated.
Planned interim analyses were scheduled after one-third (200 patients) and two-thirds (400 patients) of the total planned sample size had reached ascertainment of the primary outcome. Prespecified protocol guidelines recommended halting enrollment if the efficacy probability at an interim analysis was less than 5%, indicating greater than 95% posterior probability that the active drug was inferior to placebo for oxygen-free days. At the final analysis, a conclusion of superiority was indicated if the efficacy probability exceeded 0.976, which was a threshold selected to ensure a type I error rate of less than 2.5%.
The trials were powered based on the primary outcome of oxygen-free days and its distribution in a recently published COVID-19 trial.28 Prior to enrollment, a sample size of 600 patients (300 in an active group and 300 in a placebo group) was calculated using statistical simulation to provide 85% power to detect an OR of 1.65. An OR of 1.65 corresponds to an increase of 3.1 oxygen-free days and an absolute mortality reduction of 7.8% in the active group compared with the placebo group. Although the minimum clinically important difference in oxygen-free days is not definitively known, a difference of at least 2 oxygen-free days is frequently considered clinically important.26,29,30,31
Heterogeneity of treatment effect by prespecified baseline characteristics for oxygen-free days was evaluated by adding an interaction term to the primary model.32 The baseline characteristics, including sex, age, COVID-19 vaccination status, and respiratory support, which was classified with the World Health Organization COVID-19 ordinal scale, and concomitant use of a usual care RAS medication were used to evaluate for heterogeneity of treatment effect.
The secondary and safety outcomes were analyzed with regression models using the same covariables as the primary model. A gatekeeping method was used to ensure a type I error rate of less than 2.5% across the primary outcome and 3 key secondary outcomes. The key secondary outcomes were tested in a prespecified order only if the active drug was superior to placebo for the preceding outcome. Systematically collected safety events (protocol-specified exempt serious events) and adverse events were reported with frequency counts and proportions.
The statistical analyses were conducted using R version 4.2.0 (R Foundation for Statistical Computing). Notwithstanding the described formal superiority and inferiority assessments, estimates with a 95% CrI that excluded the null were considered statistically significant. When the 95% CrI excludes the null value, the posterior probability of values more extreme than the null is less than 0.025. The widths of the 95% CrIs were not adjusted for multiplicity. For the primary outcome of oxygen-free days, missing and partially observed data were analyzed using likelihood-based methods without imputation (details appear in the statistical analysis plan in Supplement 3). For the mortality outcomes, the number of patients who died among those with known vital status was reported.
Halting Enrollment
Both the TXA-127 and TRV-027 trials were halted at the first interim analysis due to meeting the prespecified stopping criterion of less than 0.05 for probability of efficacy. At the first interim analysis, the efficacy probability was 0.040 for TXA-127 and was 0.031 for TRV-027 (eTable 5 in Supplement 1).
Results
Participants
Between July 22, 2021, and April 20, 2022, a total of 3214 patients were screened and 534 were randomized (Figure 1). The last follow-up visit occurred on July 26, 2022. Twenty-four patients were randomized to the active fostamatinib group and were not included in either the TXA-127 trial or the TRV-027 trial. Of the 510 patients randomized to either the TXA-127 trial or the TRV-27 trial, 13 were not included in the primary analyses due to not receiving any study drug (n = 10 where 1 patient assigned to placebo would have otherwise contributed to both trials) or being identified as ineligible after randomization (n = 3). This resulted in a primary analysis population of 343 patients (170 in TXA-127 group and 173 in placebo group) for the TXA-127 trial and 290 patients (145 in TRV-027 group and 145 in placebo group) for the TRV-027 trial (Figure 1 and eTable 6 in Supplement 1).
In the TXA-127 trial, 18 patients (5.2%) were between the ages of 18 and 30 years, 226 (65.9%) were between the ages of 31 and 64 years, and 99 (28.9%) were aged 65 years or older. At baseline, 218 patients (63.6%) were receiving standard-flow oxygen therapy, 100 (29.2%) were receiving high-flow nasal oxygen or noninvasive mechanical ventilation, and 25 (7.3%) were receiving invasive mechanical ventilation. Patients in the TXA-127 group were older compared with patients in the placebo group (31.2% vs 26.6% were aged ≥65 years) and more were receiving invasive mechanical ventilation (9.4% vs 5.2%, respectively) (Table 1 and eTable 7 in Supplement 1).
Table 1. Patient Characteristics at Baseline in the TXA-127 Trial and in the TRV-027 Trial.
| TXA-127 trial | TRV-027 trial | |||
|---|---|---|---|---|
| TXA-127 (n = 170) |
Placebo (n = 173) |
TRV-027 (n = 145) |
Placebo (n = 145) |
|
| Demographics, No. (%) | ||||
| Age group, y | ||||
| 18-30 | 11 (6.5) | 7 (4.1) | 5 (3.5) | 7 (4.8) |
| 31-64 | 106 (62.4) | 120 (69.4) | 100 (69.0) | 99 (68.3) |
| ≥65 | 53 (31.2) | 46 (26.6) | 40 (27.6) | 39 (26.9) |
| Sex assigned at birth | ||||
| Female | 67 (39.4) | 76 (43.9) | 53 (36.6) | 69 (47.6) |
| Male | 103 (60.6) | 97 (56.1) | 92 (63.4) | 76 (52.4) |
| Racea | ||||
| Asian | 3 (1.8) | 4 (2.3) | 2 (1.4) | 3 (2.1) |
| Black | 29 (17.1) | 29 (16.8) | 25 (17.2) | 21 (14.5) |
| Native American or Alaska Native | 5 (2.9) | 1 (0.6) | 2 (1.4) | 1 (0.7) |
| Native Hawaiian or Other Pacific Islander | 0 | 1 (0.6) | 0 | 0 |
| White | 112 (65.9) | 113 (65.3) | 99 (68.3) | 96 (66.2) |
| Other or prefer not to answer | 21 (12.4) | 25 (14.5) | 17 (11.7) | 24 (16.6) |
| Ethnicitya | ||||
| Hispanic | 26 (15.3) | 26 (15.0) | 24 (16.6) | 23 (15.9) |
| Not Hispanic | 136 (80.0) | 138 (79.8) | 111 (76.6) | 114 (78.6) |
| Other or prefer not to answer | 8 (4.7) | 9 (5.2) | 10 (6.9) | 8 (5.5) |
| Medical history, No. (%) b | ||||
| Obesity (BMI ≥30c) | 107 (62.9) | 109 (63.0) | 91 (62.8) | 90 (62.1) |
| Hypertension | 85 (50.0) | 91 (52.6) | 68 (46.9) | 69 (47.6) |
| Diabetes (with or without end-organ damage) | 47 (27.6) | 56 (32.4) | 39 (26.9) | 40 (27.6) |
| Chronic kidney disease (not undergoing kidney replacement therapy) | 22 (12.9) | 11 (6.4) | 13 (9.0) | 12 (8.3) |
| Chronic heart failure | 15 (8.8) | 10 (5.8) | 13 (9.0) | 7 (4.8) |
| Active cancer | 13 (7.6) | 14 (8.1) | 10 (6.9) | 9 (6.2) |
| Chronic obstructive pulmonary disease | 13 (7.6) | 32 (18.5) | 25 (17.2) | 25 (17.2) |
| Supplemental oxygen use prior to COVID-19 | 6 (3.5) | 7 (4.1) | 6 (4.1) | 5 (3.5) |
| Dementia | 5 (2.9) | 5 (2.9) | 1 (0.7) | 5 (3.5) |
| Cirrhosis | 3 (1.8) | 4 (2.3) | 5 (3.4) | 2 (1.4) |
| Medication use before COVID-19 | ||||
| Angiotensin-converting enzyme inhibitor | 7 (4.1) | 9 (5.2) | 7 (4.8) | 9 (6.2) |
| Angiotensin receptor blocker | 11 (6.5) | 11 (6.4) | 0 | 0 |
| COVID-19 characteristics | ||||
| Predominant SARS-CoV-2 variant in US, No. (%)b | ||||
| Delta (prior to and including Dec 26, 2021) | 108 (63.5) | 98 (56.6) | 103 (71.0) | 89 (61.4) |
| Omicron (after Dec 26, 2021) | 62 (36.5) | 75 (43.4) | 42 (29.0) | 56 (38.6) |
| Receipt of ≥1 COVID-19 vaccine dose, No. (%) | 53 (31.2) | 57 (32.9) | 41 (28.3) | 49 (33.8) |
| WHO COVID-19 clinical progression scale, No. (%)d | ||||
| Level 4: hospitalized and receiving supplemental oxygen by nasal prongs or mask | 100 (58.8) | 118 (68.2) | 84 (57.9) | 98 (67.6) |
| Level 5: hospitalized and receiving high-flow nasal oxygen or noninvasive ventilation | 54 (31.8) | 46 (26.6) | 48 (33.1) | 40 (27.6) |
| Level 6 or 7: hospitalized and receiving invasive mechanical ventilation alone or with other organ support | 16 (9.4) | 9 (5.2) | 13 (9.0) | 7 (4.8) |
| Time from hospital admission to randomization, median (IQR), d | 1 (1-2) | 1 (1-2) | 1 (1-2) | 1 (1-2) |
| Vasopressor use, No. (%)e | 13 (7.7) | 10 (5.8) | 12 (8.3) | 8 (5.5) |
| Acute in-hospital treatments for COVID-19 prior to randomization, No. (%)f | ||||
| Corticosteroids | 149 (87.6) | 136 (78.6) | 113 (77.9) | 112 (77.2) |
| Anticoagulantsg | 129 (75.9) | 129 (74.6) | 102 (70.3) | 104 (71.7) |
| Remdesivir | 120 (70.6) | 121 (69.9) | 95 (65.5) | 95 (65.5) |
| Baricitinib | 25 (14.7) | 22 (12.7) | 22 (15.2) | 18 (12.4) |
| Tocilizumab | 0 | 1 (0.6) | 0 | 1 (0.7) |
Abbreviations: BMI, body mass index; WHO, World Health Organization.
Self-reported using mutually exclusive categories.
Data were obtained from the medical record for each patient.
Calculated as weight in kilograms divided by height in meters squared.
Assessed at randomization. Eight-level ordinal scale representing the worst clinical status on a given day. Level 1 was defined as ambulatory and not hospitalized, no limitation of activities; level 2, ambulatory and not hospitalized with limitation of activities or receiving home oxygen therapy; level 3, hospitalized with mild disease, not receiving oxygen therapy; level 4, hospitalized with mild disease and receiving oxygen by mask or nasal prongs; level 5, hospitalized with severe disease and receiving noninvasive ventilation or high-flow oxygen; level 6, hospitalized with severe disease and receiving invasive mechanical ventilation; level 7, hospitalized with severe disease and receiving invasive mechanical ventilation plus additional organ support with vasopressors, kidney replacement therapy, or extracorporeal membrane oxygenation; and level 8, dead.
Prior to or on the day of randomization and included use of any vasopressors or inotropes (eg, dobutamine, dopamine, epinephrine, milrinone, norepinephrine, phenylephrine, and vasopressin).
Additional baseline patient characteristics and usual care in-hospital medications before randomization appear in eTables 7-8 in Supplement 1.
Collected as part of the medical history questionnaire and did not distinguish specific medications or doses.
In the TRV-027 trial, 12 patients (4.1%) were between the ages of 18 and 30 years, 199 (68.6%) were between the ages of 31 and 64 years, and 79 (27.2%) patients were aged 65 years or older. At baseline, 182 patients (62.8%) were receiving standard-flow oxygen therapy, 88 (30.3%) were receiving high-flow nasal oxygen or noninvasive mechanical ventilation, and 20 (6.9%) were receiving invasive mechanical ventilation. Patients in the TRV-027 group were similar in age compared with patients in the placebo group, but more were receiving invasive mechanical ventilation (9.0% vs 4.8%, respectively) (Table 1 and eTable 7 in Supplement 1).
Receipt of in-hospital COVID-19 treatments before randomization was similar between the active group and placebo group in both trials (Table 1 and eTable 8 in Supplement 1).
Study Drug Delivery
To quantify the amount of study drug received, drug dosing was evaluated for patients who received TXA-127 active drug or TXA-127 placebo in the TXA-127 trial and those who received TRV-027 active drug or TRV-027 placebo in the TRV-027 trial. This included 262 of 343 patients in the TXA-127 trial and 224 of 290 patients in the TRV-027 trial. Among the 262 patients in the primary analysis who received TXA-127 or its matching placebo, 164 (62.6%) received the maximum 5 daily doses. Among 224 patients in the primary analysis who received TRV-027 or its matching placebo, 144 (64.3%) received the maximum 5-day infusion. In both trials, the most common reason for not receiving the full 5 days of treatment was because of death or hospital discharge prior to day 5 (eTables 9-10 in Supplement 1).
Primary Outcome
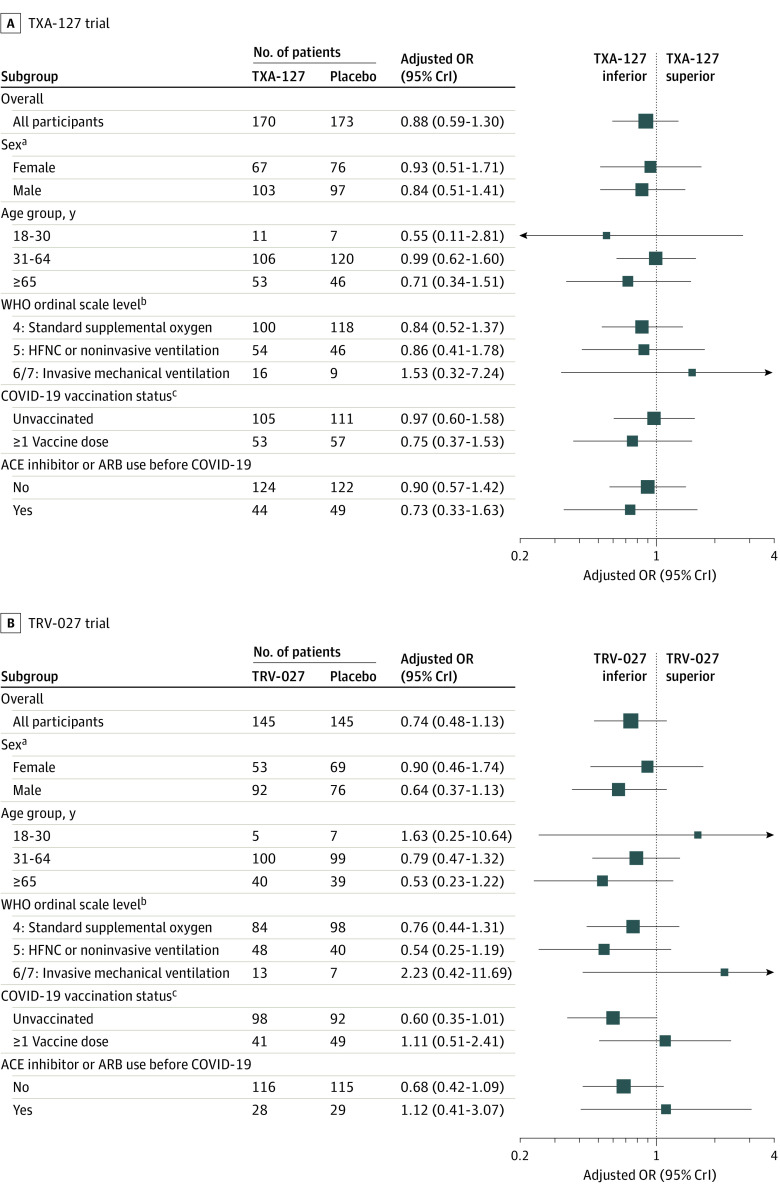
In the TXA-127 trial, the primary outcome of oxygen-free days through day 28 was not statistically different in the TXA-127 group compared with the placebo group (unadjusted mean difference, −2.3 [95% CrI, −4.8 to 0.2]; adjusted OR, 0.88 [95% CrI, 0.59 to 1.30]; posterior probability of superiority, 0.259) (Table 2, Figure 2, and eFigure 2 in Supplement 1). In the analyses to assess heterogeneity of treatment effect, the point estimates were similar across subgroups and none of the treatment estimates were statistically significant (Figure 3A and eTable 11 in Supplement 1).
Table 2. Primary Outcome, Key Secondary Outcomes, and Safety Outcomes in the TXA-127 Trial and in the TRV-027 Trial.
| TXA-127 trial | TRV-027 trial | |||||||
|---|---|---|---|---|---|---|---|---|
| TXA-127 (n = 170) | Placebo (n = 173) | Unadjusted absolute difference (95% CrI)a | Adjusted OR (95% CrI)b |
TRV-027 (n = 145) | Placebo (n = 145) | Unadjusted absolute difference (95% CrI)a | Adjusted OR (95% CrI)b |
|
| Primary outcome | ||||||||
| Oxygen-free days at 28 d, mean (SD)c | 9.0 (10.9) | 11.3 (11.5) | −2.3 (−4.8 to 0.2) | 0.88 (0.59 to 1.30) | 8.1 (10.8) | 10.5 (11.5) | −2.4 (−5.1 to 0.3) | 0.74 (0.48 to 1.13) |
| Key secondary outcomes d | ||||||||
| Mortality at 28 d, No./total (%) | 22/163 (13.5) | 22/166 (13.3) | 0.2 (−7.1 to 7.6) | 0.83 (0.41 to 1.66) | 29/141 (20.6) | 18/140 (12.9) | 7.7 (−0.9 to 16.4) | 1.52 (0.75 to 3.08) |
| Alive and free from respiratory failure at 28 d, No./total (%)e | 123/155 (79.4) | 125/160 (78.1) | 1.2 (−7.8 to 10.2) | 1.43 (0.78 to 2.63) | 96/136 (70.6) | 106/136 (77.9) | −7.4 (−17.7 to 3.0) | 0.88 (0.47 to 1.66) |
| WHO COVID-19 clinical progression level at 28 d, No. (%)f | (n = 154) | (n = 158) | (n = 135) | (n = 134) | ||||
| 1 | 58 (37.7) | 62 (39.2) | −1.6 (−12.3 to 9.2) | 0.88 (0.58 to 1.35) | 48 (35.6) | 47 (35.1) | 0.5 (−10.9 to 11.9) | 0.97 (0.62 to 1.53) |
| 2 | 53 (34.4) | 58 (36.7) | −2.3 (−12.9 to 8.3) | 44 (32.6) | 53 (39.6) | −7.0 (−18.3 to 4.5) | ||
| 3 | 4 (2.6) | 2 (1.3) | 1.3 (−1.6 to 4.6) | 0 | 2 (1.5) | NE | ||
| 4 | 7 (4.6) | 1 (0.6) | 3.9 (0.8 to 7.8) | 3 (2.2) | 2 (1.5) | 0.7 (−2.5 to 4.2) | ||
| 5 | 1 (0.7) | 3 (1.9) | −1.2 (−4.0 to 1.1) | 3 (2.2) | 3 (2.2) | 0 (−3.7 to 3.6) | ||
| 6 | 4 (2.6) | 5 (3.2) | −0.6 (−4.4 to 3.2) | 3 (2.2) | 4 (3.0) | −0.8 (−4.7 to 3.0) | ||
| 7 | 5 (3.3) | 5 (3.2) | 0.1 (−3.9 to 4.1) | 5 (3.7) | 5 (3.7) | 0 (−4.6 to 4.6) | ||
| 8 | 22 (14.3) | 22 (13.9) | 0.4 (−7.3 to 8.1) | 29 (21.5) | 18 (13.4) | 8.0 (−0.9 to 17.1) | ||
| Safety outcomes, No. (%) g | ||||||||
| ≥1 Hypotensive event through 7 d | 19 (11.2) | 21 (12.1) | −1.0 (−7.7 to 5.8) | 0.66 (0.31 to 1.38) | 21 (14.5) | 16 (11.0) | 3.4 (−4.2 to 11.2) | 1.04 (0.48 to 2.25) |
| New kidney replacement therapy through 28 d | 11 (6.5) | 12 (6.9) | −0.5 (−5.8 to 4.9) | 0.75 (0.31 to 1.84) | 9 (6.2) | 11 (7.6) | −1.4 (−7.3 to 4.4) | 0.59 (0.22 to 1.56) |
| Allergic reaction through 7 d | 3 (<1) | 0 | NE | NE | 0 | 0 | NE | NE |
Abbreviations: OR, odds ratio; CrI, credible interval; NE, not estimable; WHO, World Health Organization.
Calculated using normal distribution with a flat prior for both the mean and variance for oxygen-free days, excluding partially observed values. A binomial distribution with an improper β prior was used for each binary outcome.
The adjusted ORs were calculated using regression techniques with covariable adjustment for age group, sex, and baseline level on the WHO COVID-19 clinical progression ordinal scale. For oxygen-free days at 28 days, multivariable proportional odds was used (adjusted OR <1.0 is the direction of inferiority for the active agent); for mortality at 28 days, multivariable logistic regression was used (OR <1.0 is the direction of superiority for the active agent); for alive and free from respiratory failure at 28 days, multivariable logistic regression was used (OR <1.0 is the direction of inferiority for the active agent); for WHO COVID-19 clinical progression level at 28 days, multivariable proportional odds was used (OR <1.0 is the direction of superiority for the active agent); for all safety outcomes, multivariable logistic regression was used (OR <1.0 is the direction of fewer safety events for the active group).
Calculated as 28 days minus the number of days between initiation and final liberation from new supplemental oxygen use during the 28 days after randomization. Patients who died before day 28 were coded as having −1 oxygen-free days (worst possible outcome). The primary analysis of oxygen-free days included all patients, including those with partially observed data (ie, patients for whom the number of oxygen-free days was not known precisely but was known to be within a certain range). In the TXA-127 trial, there were 17 patients with partially observed data in the TXA-127 group and 18 patients in the placebo group. In the TRV-027 trial, there were 10 patients with partially observed data in the TRV-027 group and 14 patients in the placebo group. The mean (SD) estimates exclude patients with partially observed oxygen-free days.
Additional secondary and safety outcomes appear in eTables 13-18 in Supplement 1. The outcomes presented in this Table plus those in eTables 13-18 in Supplement 1 are an exhaustive list of prespecified outcomes for the trials.
Defined as alive and not requiring high-flow nasal oxygen, noninvasive ventilation, or invasive ventilation.
Eight-level ordinal scale representing the worst patient clinical status on a given day. Level 1 was defined as ambulatory and not hospitalized, no limitation of activities; level 2, ambulatory and not hospitalized with limitation of activities or home oxygen therapy; level 3, hospitalized with mild disease, not receiving oxygen therapy; level 4, hospitalized with mild disease and receiving oxygen by mask or nasal prongs; level 5, hospitalized with severe disease and receiving noninvasive ventilation or high-flow oxygen; level 6, hospitalized with severe disease and receiving invasive mechanical ventilation; level 7, hospitalized with severe disease and receiving invasive mechanical ventilation plus additional organ support with vasopressors, kidney replacement therapy or extracorporeal membrane oxygenation; and level 8, dead.
Prespecified and data were collected by clinicians blinded to treatment assignment in real time.
Figure 2. Primary Outcome of Oxygen-Free Days Between Randomization and Day 28 in the TXA-127 Trial and in the TRV-027 Trial.
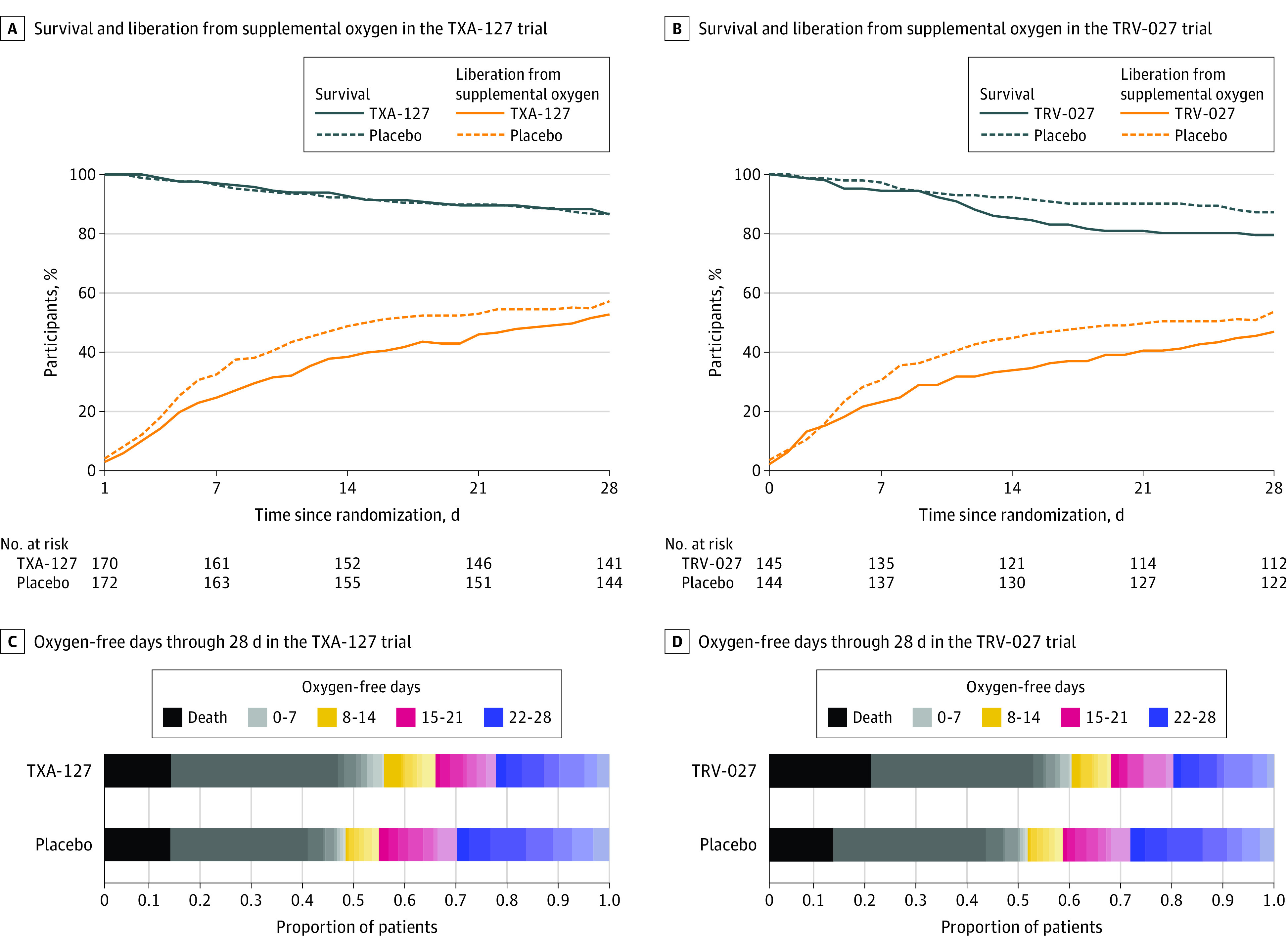
The day of randomization was study day 0. The total sample size was 343 patients in the TXA-127 trial. The total sample size was 290 patients in the TRV-027 trial. Patients were followed up until the earlier of death or day 28. The numbers at risk shown in panels A and B are the numbers of patients who were not deceased, withdrawn, or lost to follow-up. The plots in panels C and D display the proportion of patients in each of the 30 levels (range, −1 to 28 days) of the oxygen-free days ordinal scale at day 28. The oxygen-free days outcome demonstrated null results for TXA-127 vs placebo and TRV-027 vs placebo with point estimates in the direction of inferiority for the TXA-127 trial (adjusted OR, 0.88 [95% credible interval, 0.59 to 1.30]) and for the TRV-027 trial (adjusted OR, 0.74 [95% credible interval, 0.48 to 1.13]).
Figure 3. Primary Outcome of Oxygen-Free Days by Baseline Characteristics in the TXA-127 Trial and in the TRV-027 Trial.
ACE indicates angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CrI, credible interval; HFNC, high-flow nasal cannula; OR, odds ratio; and WHO, World Health Organization. The ORs were adjusted for sex, age group, and baseline WHO ordinal scale level. An oxygen-free day was calculated as 28 minus the number of days between randomization (day 0) and liberation from new supplemental oxygen use during the 28 days. Patients who died before day 28 were coded as having −1 oxygen-free days (worst possible outcome). The subgroup analyses of oxygen-free days included patients with partially observed data (only known to be within a certain range). Additional information appears in eTables 11 and 12 in Supplement 1, including the number of patients with partially observed oxygen-free days.
aNot prespecified as a subgrouping variable; thus, heterogeneity of treatment effect by sex is a post hoc analysis.
bThe WHO COVID-19 clinical progression scale is an 8-level ordinal scale representing the worst patient clinical status on a given day. The descriptions for levels 1-8 appear in footnote d in Table 1.
cPatients with unknown vaccination status were excluded (17 patients in the TXA-127 trial and 10 patients in the TRV-027 trial).
In the TRV-027 trial, the primary outcome of oxygen-free days through day 28 was not statistically different in the TRV-027 group compared with the placebo group (unadjusted mean difference, −2.4 [95% CrI, −5.1 to 0.3]; adjusted OR, 0.74 [95% CrI, 0.48 to 1.13]; posterior probability of superiority, 0.083) (Figure 2 and eFigure 3 in Supplement 1). In the analyses to assess heterogeneity of treatment effect, the point estimates were similar across subgroups and none of the treatment effects were statistically significant (Figure 3B and eTable 12 in Supplement 1).
Secondary Outcomes
In the TXA-127 trial, 28-day all-cause mortality occurred in 22 of 163 patients (13.5%) in the TXA-127 group compared with 22 of 166 patients (13.3%) in the placebo group (adjusted OR, 0.83 [95% CrI, 0.41-1.66]). In the TRV-027 trial, 28-day all-cause mortality occurred in 29 of 141 patients (20.6%) in the TRV-027 group compared with 18 of 140 patients (12.9%) in the placebo group (adjusted OR, 1.52 [95% CrI, 0.75-3.08]). Other secondary efficacy outcomes did not differ between the active drug and placebo groups in either trial (eTables 13-14 and eFigures 4 and 5 in Supplement 1).
Safety Outcomes and Adverse Events
The percentage of patients who experienced at least 1 hypotensive event through day 7 was similar for patients in the TXA-127 group (11.2%) compared with its placebo group (12.1%) and was also similar in the TRV-027 group (14.5%) compared with its placebo group (11.0%). The frequency of other safety outcomes, protocol-specified exempt serious events (eTables 15-16 in Supplement 1), and adverse events (eTables 17-18 in Supplement 1) was similar between the active group and placebo group in both trials.
Discussion
In these multicenter, blinded, placebo-controlled randomized clinical trials including 510 adults hospitalized with COVID-19–associated hypoxemia, pharmacological interventions aimed at blocking angiotensin II and increasing angiotensin (1-7) activity did not improve clinical outcomes, including days alive and free from supplemental oxygen therapy (oxygen-free days) or mortality. In these trials, RAS modulation with either synthetic angiotensin (1-7) (TXA-127) or an angiotensin II type 1 receptor–biased ligand (TRV-027) did not improve the number of oxygen-free days and trended toward inferiority (worse outcomes compared with placebo). These results suggest attempting to reverse RAS imbalances anticipated from SARS-CoV-2 infection through exogenous angiotensin (1-7) administration or blockade of the primary receptor for angiotensin II does not provide clinical benefit.
SARS-CoV-2 binds to ACE2, which leads to cellular entry of the virus and also a reduction in the conversion of host angiotensin II to angiotensin (1-7).8,9,10,11 An elevated ratio of angiotensin II to angiotensin (1-7) has been hypothesized as a key mechanism driving lung injury in COVID-19, and potentially in acute respiratory distress syndrome (ARDS) from other etiologies as well.11,12,13 Thus, there has been significant interest in RAS modulation as a potential therapeutic approach for severe COVID-19 and for ARDS generally.11,33,34,35 The COVID-19 pandemic presented an opportunity to evaluate RAS modulation in a population of severely ill patients with lung injury caused by a single etiology known to directly result in RAS dysfunction (SARS-CoV-2 infection).36
The current trial results add to the growing literature suggesting RAS modulation with on-market agents, such as ARBs, and investigational agents intended to reverse the effects of an elevated ratio of angiotensin II to angiotensin (1-7) do not provide benefit for patients with COVID-19.15,16,17,18,37 Why this therapeutic approach has failed to demonstrate benefit is not definitively known. One possibility is that SARS-CoV-2 infection may not result in unopposed angiotensin II activity in the lung as hypothesized. The physiology of the RAS is complex, with multiple feedback mechanisms that could prevent SARS-CoV-2 infection from resulting in end-organ exposure to elevated angiotensin II. For example, in a recent postmortem study, Gerard et al38 found that patients who died of COVID-19 ARDS had increased expression of ACE2 in the lung and serum and increased concentration of serum angiotensin (1-7) compared with control patients without COVID-19. This suggests that endogenous host feedback mechanisms may respond to SARS-CoV-2–induced destruction of ACE2 with increased expression of additional ACE2, thereby rendering pharmacological RAS interventions (eg, ARBs, TXA-127, and TRV-027) as not beneficial and potentially harmful. Mechanistic changes in components of the RAS, including angiotensin II, ACE2, and angiotensin (1-7), in response to SARS-CoV-2 infection and RAS modulation therapies will be important to evaluate to gain further understanding of COVID-19 pathophysiology and the host response to these therapies.
The 2 trials had several strengths, including their multicenter, blinded, placebo-controlled design, high adherence to treatment assignment, robust collection of outcome and safety data, use of oxygen-free days as an outcome to capture death and lung-related morbidity, use of a shared placebo group, bayesian stopping rules to efficiently conduct the trials, and the evaluation of 2 agents active in the RAS pathway with distinct mechanisms.
Limitations
The 2 trials also had limitations. First, study drug administration stopped at hospital discharge, using the rationale that once a patient had clinically improved to the point of discharge, further RAS modulation would be unlikely to mitigate lung injury. Longer treatment courses were not evaluated; however, with outcomes trending toward inferiority, increased drug exposure is unlikely to have improved outcomes. Second, the RAS agents studied in these trials were added to usual care COVID-19 treatments, which included corticosteroids for most patients; thus, the effect of these drugs independent of other treatments could not be evaluated.
Third, the trials were stopped at the first interim analysis because of a probability of inferiority greater than 95% for each active agent compared with placebo based on the primary outcome. In the final analysis, which included more patients than the interim analysis, point estimates for the primary outcome were in the direction of inferiority for both agents but the probabilities for inferiority were less than 95%. Thus, while lack of benefit could be concluded with high confidence, inferiority of the active agents compared with placebo could not be definitively concluded.
Fourth, halting enrollment at the first interim analysis resulted in low power for the secondary outcomes. Fifth, by chance, the active groups had more severe COVID-19 at baseline than the placebo groups. Many of the same patients in the shared placebo group likely contributed to both trials having similar imbalances in the baseline characteristics. Prespecified statistical adjustment was used to mitigate the effects of baseline imbalances and all results were adjusted for patient age and baseline COVID-19 severity. Sixth, additional mechanistic studies are indicated to understand the effect of TXA-127 and TRV-027 on the RAS pathway; these analyses are ongoing using biospecimens collected during the 2 trials.
Conclusions
Among adults hospitalized with severe COVID-19, RAS modulation with either TXA-127 or TRV-027 did not improve oxygen-free days compared with placebo. These results do not support the hypotheses that pharmacological interventions that selectively block the angiotensin II type 1 receptor or increase angiotensin (1-7) improve outcomes for patients with severe COVID-19.
eAppendix. ACTIV-4 Host Tissue Platform Collaborators
eFigure 1. Schematic of the renin-angiotensin system (RAS) and trial agent targets
eFigure 2. Distribution of oxygen-free days in TXA-127 trial
eFigure 3. Distribution of oxygen-free days in TRV-027 trial
eFigure 4. TXA-127 trial WHO COVID-19 ordinal scale results over time
eFigure 5. TRV-027 trial WHO COVID-19 ordinal scale results over time
eTable 1. Enrolling sites
eTable 2. Eligibility criteria
eTable 3. Trial outcomes
eTable 4. Protocol-specified exempt serious events (PSESEs)
eTable 5. Results at the first interim analyses
eTable 6. Details of placebo groups
eTable 7. Additional baseline patient characteristics
eTable 8. Usual care in-hospital medications before randomization
eTable 9. Study drug delivery in the TXA-127 trial
eTable 10. Study drug delivery in the TRV-027 trial
eTable 11. Summaries of oxygen-free days for subgroup analyses in TXA-127 trial
eTable 12. Summaries of oxygen-free days for subgroup analyses in TRV-027 trial
eTable 13. Additional outcome results in TXA-127 trial
eTable 14. Additional outcome results in TRV-027 trial
eTable 15. PSESEs results in TXA-127 trial
eTable 16. PSESEs results in TRV-027 trial
eTable 17. Adverse events (AEs) in TXA-127 trial
eTable 18. Adverse events (AEs) in TRV-027 trial
Trial protocol
Statistical analysis plan
Nonauthor collaborators
Data sharing statement
References
- 1.Tenforde MW, Self WH, Adams K, et al. ; Influenza and Other Viruses in the Acutely Ill (IVY) Network . Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA. 2021;326(20):2043-2054. doi: 10.1001/jama.2021.19499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauring AS, Tenforde MW, Chappell JD, et al. ; Influenza and Other Viruses in the Acutely Ill (IVY) Network . Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376:e069761. doi: 10.1136/bmj-2021-069761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams K, Rhoads JP, Surie D, et al. ; Influenza and Other Viruses in the Acutely Ill (IVY) Network . Vaccine effectiveness of primary series and booster doses against covid-19 associated hospital admissions in the United States: living test negative design study. BMJ. 2022;379:e072065. doi: 10.1136/bmj-2022-072065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beigel JH, Tomashek KM, Dodd LE, et al. ; ACTT-1 Study Group Members . Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383(19):1813-1826. doi: 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sterne JAC, Murthy S, Diaz JV, et al. ; WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group . Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330-1341. doi: 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalil AC, Patterson TF, Mehta AK, et al. ; ACTT-2 Study Group Members . Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795-807. doi: 10.1056/NEJMoa2031994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raman B, Bluemke DA, Lüscher TF, Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J. 2022;43(11):1157-1172. doi: 10.1093/eurheartj/ehac031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318(5):H1084-H1090. doi: 10.1152/ajpheart.00217.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112-116. doi: 10.1038/nature03712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wösten-van Asperen RM, Lutter R, Specht PA, et al. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin (1-7) or an angiotensin II receptor antagonist. J Pathol. 2011;225(4):618-627. doi: 10.1002/path.2987 [DOI] [PubMed] [Google Scholar]
- 11.Zoufaly A, Poglitsch M, Aberle JH, et al. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir Med. 2020;8(11):1154-1158. doi: 10.1016/S2213-2600(20)30418-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu M, Wang T, Zhou Y, Zhao Y, Zhang Y, Li J. Potential role of ACE2 in coronavirus disease 2019 (COVID-19) prevention and management. J Transl Int Med. 2020;8(1):9-19. doi: 10.2478/jtim-2020-0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Files DC, Gibbs KW, Schaich CL, et al. A pilot study to assess the circulating renin-angiotensin system in COVID-19 acute respiratory failure. Am J Physiol Lung Cell Mol Physiol. 2021;321(1):L213-L218. doi: 10.1152/ajplung.00129.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monteil V, Kwon H, Prado P, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905-913.e7. doi: 10.1016/j.cell.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puskarich MA, Ingraham NE, Merck LH, et al. ; Angiotensin Receptor Blocker Based Lung Protective Strategies for Inpatients With COVID-19 (ALPS-IP) Investigators . Efficacy of losartan in hospitalized patients with COVID-19–induced lung injury: a randomized clinical trial. JAMA Netw Open. 2022;5(3):e222735. doi: 10.1001/jamanetworkopen.2022.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopes RD, Macedo AVS, de Barros E Silva PGM, et al. ; BRACE CORONA Investigators . Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325(3):254-264. doi: 10.1001/jama.2020.25864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen JB, Hanff TC, William P, et al. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med. 2021;9(3):275-284. doi: 10.1016/S2213-2600(20)30558-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tornling G, Batta R, Porter JC, et al. Seven days treatment with the angiotensin II type 2 receptor agonist C21 in hospitalized COVID-19 patients; a placebo-controlled randomised multi-centre double-blind phase 2 trial. EClinicalMedicine. 2021;41:101152. doi: 10.1016/j.eclinm.2021.101152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robbins AJ, Che Bakri NA, Toke-Bjolgerud E, et al. The effect of TRV027 on coagulation in COVID-19: a pilot randomized, placebo-controlled trial. Br J Clin Pharmacol. 2023;89(4):1495-1501. doi: 10.1111/bcp.15618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagener G, Goldklang MP, Gerber A, et al. A randomized, placebo-controlled, double-blinded pilot study of angiotensin 1-7 (TXA-127) for the treatment of severe COVID-19. Crit Care. 2022;26(1):229. doi: 10.1186/s13054-022-04096-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pang PS, Butler J, Collins SP, et al. Biased ligand of the angiotensin II type 1 receptor in patients with acute heart failure: a randomized, double-blind, placebo-controlled, phase IIB, dose ranging trial (BLAST-AHF). Eur Heart J. 2017;38(30):2364-2373. doi: 10.1093/eurheartj/ehx196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins FS, Stoffels P. Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV): an unprecedented partnership for unprecedented times. JAMA. 2020;323(24):2455-2457. doi: 10.1001/jama.2020.8920 [DOI] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium . The REDCap Consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saville BR, Berry SM. Efficiencies of platform clinical trials: a vision of the future. Clin Trials. 2016;13(3):358-366. doi: 10.1177/1740774515626362 [DOI] [PubMed] [Google Scholar]
- 25.Violin JD, Soergel DG, Boerrigter G, Burnett JC Jr, Lark MW. GPCR biased ligands as novel heart failure therapeutics. Trends Cardiovasc Med. 2013;23(7):242-249. doi: 10.1016/j.tcm.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 26.Moskowitz A, Shotwell MS, Gibbs KW, et al. ; Fourth Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-4) Host Tissue Investigators . Oxygen-free days as an outcome measure in clinical trials of therapies for COVID-19 and other causes of new-onset hypoxemia. Chest. 2022;162(4):804-814. doi: 10.1016/j.chest.2022.04.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO Working Group on the Clinical Characterisation and Management of COVID-19 Infection . A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192-e197. doi: 10.1016/S1473-3099(20)30483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Self WH, Wheeler AP, Stewart TG, Bernard GR, Rice TW; Passive Immunity Trial for Our Nation (PassITON) Investigators . Neutralizing COVID-19 convalescent plasma in adults hospitalized with COVID-19: a blinded, randomized, placebo-controlled trial. Chest. 2022;162(5):982-994. doi: 10.1016/j.chest.2022.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network . Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564-2575. [DOI] [PubMed] [Google Scholar]
- 30.Laterre P-F, Berry SM, Blemings A, et al. ; SEPSIS-ACT Investigators . Effect of selepressin vs placebo on ventilator- and vasopressor-free days in patients with septic shock: the SEPSIS-ACT randomized clinical trial. JAMA. 2019;322(15):1476-1485. doi: 10.1001/jama.2019.14607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Self WH, Semler MW, Leither LM, et al. ; National Heart, Lung, and Blood Institute PETAL Clinical Trials Network . Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(21):2165-2176. doi: 10.1001/jama.2020.22240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine–reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189-2194. doi: 10.1056/NEJMsr077003 [DOI] [PubMed] [Google Scholar]
- 33.Imai Y, Kuba K, Penninger JM. The renin-angiotensin system in acute respiratory distress syndrome. Drug Discov Today Dis Mech. 2006;3(2):225-229. doi: 10.1016/j.ddmec.2006.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Li S, Niu S. ACE2 and COVID-19 and the resulting ARDS. Postgrad Med J. 2020;96(1137):403-407. doi: 10.1136/postgradmedj-2020-137935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—lessons from available evidence and insights into COVID-19. Hypertens Res. 2020;43(7):648-654. doi: 10.1038/s41440-020-0455-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collins SP, Chappell MC, Files DC. The renin-angiotensin-aldosterone system in COVID-19-related and non-COVID-19-related acute respiratory distress syndrome: not so different after all? Am J Respir Crit Care Med. 2021;204(9):1007-1008. doi: 10.1164/rccm.202108-1904ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauer A, Schreinlechner M, Sappler N, et al. ; ACEI-COVID investigators . Discontinuation versus continuation of renin-angiotensin-system inhibitors in COVID-19 (ACEI-COVID): a prospective, parallel group, randomised, controlled, open-label trial. Lancet Respir Med. 2021;9(8):863-872. doi: 10.1016/S2213-2600(21)00214-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerard L, Lecocq M, Bouzin C, et al. Increased angiotensin-converting enzyme 2 and loss of alveolar type II cells in COVID-19-related acute respiratory distress syndrome. Am J Respir Crit Care Med. 2021;204(9):1024-1034. doi: 10.1164/rccm.202012-4461OC [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. ACTIV-4 Host Tissue Platform Collaborators
eFigure 1. Schematic of the renin-angiotensin system (RAS) and trial agent targets
eFigure 2. Distribution of oxygen-free days in TXA-127 trial
eFigure 3. Distribution of oxygen-free days in TRV-027 trial
eFigure 4. TXA-127 trial WHO COVID-19 ordinal scale results over time
eFigure 5. TRV-027 trial WHO COVID-19 ordinal scale results over time
eTable 1. Enrolling sites
eTable 2. Eligibility criteria
eTable 3. Trial outcomes
eTable 4. Protocol-specified exempt serious events (PSESEs)
eTable 5. Results at the first interim analyses
eTable 6. Details of placebo groups
eTable 7. Additional baseline patient characteristics
eTable 8. Usual care in-hospital medications before randomization
eTable 9. Study drug delivery in the TXA-127 trial
eTable 10. Study drug delivery in the TRV-027 trial
eTable 11. Summaries of oxygen-free days for subgroup analyses in TXA-127 trial
eTable 12. Summaries of oxygen-free days for subgroup analyses in TRV-027 trial
eTable 13. Additional outcome results in TXA-127 trial
eTable 14. Additional outcome results in TRV-027 trial
eTable 15. PSESEs results in TXA-127 trial
eTable 16. PSESEs results in TRV-027 trial
eTable 17. Adverse events (AEs) in TXA-127 trial
eTable 18. Adverse events (AEs) in TRV-027 trial
Trial protocol
Statistical analysis plan
Nonauthor collaborators
Data sharing statement