Abstract
Objective
Cancer remains one of the most enduring health crises of the modern world. Prehabilitation is a relatively new intervention aimed at preparing individuals for the stresses associated with treatment from diagnosis. Prehabilitation can include exercise, psychological and nutrition‐based interventions. The present systematic review aimed to assess the efficacy of prehabilitation on affective and functional outcomes for young to midlife adult cancer patients (18–55 years). Outcomes of interest included prehabilitation programme composition, duration, mode of delivery and measures used to determine impact on affective and functional outcomes.
Methods
The following databases were searched with controlled and free text vocabulary; Psychological Information database (PsychINFO), Culmunated Index to Nursing and Allied Health Literature (CINAHL), Medical Literature Analysis and Retrieval System Online (MEDLINE), Excerpta Medica Database (EMBASE) and Public MEDLINE (PubMed). Abstract and full‐text screening was conducted with a secondary reviewer and final texts were subject to risk of bias analysis.
Results
Thirteen texts were included at full‐text. These included data of 797 prehabilitation participants (mean age 53 years) and a large representation of female participants (71% average). Evidence was found for the efficacy of psychological prehabilitation for anxiety reduction. Prehabilitation did not significantly affect health related quality of life. Findings moderately supported the therapeutic validity of exercise prehabilitation for functional outcomes, both in terms of clinical and experimental improvement with respect to the quality of evidence. Variation between all prehabilitation types was observed. There was insufficient evidence to support the efficacy of psychological prehabilitation on stress, distress or depression.
Conclusion
Implications for future research are highlighted and then discussed with respect to this young to midlife age group.
Keywords: anxiety, cancer, exercise, functional‐capacity, intervention, nutrition, oncology, pre‐surgery, pre‐treatment, prehabilitation, psycho‐oncology, young to mid‐life
Cancer is one of the most prevalent global health crises, with 19 million new cases projected in 2022. 1 Improvements to preventative treatment are at the forefront of policy and research on national 2 and international 3 , 4 levels. The burden of cancer also results in loss of productivity, income and family time for millions of adults every year as they recover. 5 Prehabilitation is a relatively new approach based on the principle of pre‐emptive preparation of patients to reduce treatment or surgery related risks and enhance recovery after a stressful event by optimizing physical and psychological status. This can be through exercise, nutrition and psychological interventions implemented before surgery or treatment occurs. 6 Interventions can be unimodal, whereby one intervention is employed or bi or multimodal which uses combinations of interventions. 6 For the purpose of this review, we have categorised lifestyle prehabilitation as proposed by the Macmillan Cancer Support model 7 including exercise, nutrition and psychological interventions as key pillars of treatment. 7 Exercise prehabilitation is the use of light aerobic or resistance‐based exercises with an aim to increase cardiorespiratory fitness and strength. 8 , 9 , 10 , 11 , 12 , 13 , 14 Patients who exercised frequently post‐diagnosis have lower mortality rates, fewer rates of recurrence and less severe side effects. 15 Qualitative evidence suggests that patients support aerobic exercise prehabilitation as a means of preparation both physically and emotionally for surgery. 16 Nutritional prehabilitation, the optimization of nutrient and metabolic stores to compensate for malnutrition and deficiencies is common during invasive cancer treatment or surgery. 17 , 18 Malnutrition for cancer patients can be as a result of reduced food intake and metabolic derangements that can be induced by the cancer or tumour itself. 19 The use of nutritional support improves nutritional status before cancer surgery, 7 decreases mortality and increases health related quality of life (HrQoL) and functional outcomes across cancer types. 20 The final category of prehabilitation is psychological interventions used to counter the adverse affective impacts of treatment, 21 including depression, anxiety, stress and can also increase self efficacy 22 and HrQoL. 23 Evidence suggests that even short forms of psychological interventions are effective at reducing depression and fatigue post‐surgery, compared to care as usual. 24 Psychological benefits can include increased resilience against anxiety, depression and cancer‐related cognitive impairment, 25 , 26 , 27 , 28 as well as an increase in HrQoL. 29 Evidence suggests that the combination of two or more interventions, either bimodal or multimodal prehabilitation, increases efficacy. 30
The aim of this review is to assess the impact of prehabilitation interventions on patient outcomes. To date, reviews tend to focus on specific cancer subtypes, 31 and by default, include older 32 , 33 or younger age groups. 34 , 35 , 36 The review will specifically address age‐related gaps by focussing on the young to midlife adult age range of 18–55 years. Young adult cancer patients are defined as those between the ages of 18–39. 37 However, research indicates that midlife is a period where the prevalence of multiple risk factors is high, and it is at this point when the prevalence of cancer increases for many cancer types. 38 The present review also builds on the existing literature by focussing on both functional and affective outcomes. Functional outcomes are those which measure a patient's functional capacity or ability to take part in daily activity. Affective outcomes, defined in the present review those that include a measure of anxiety, depression, stress or HrQoL, have been explored within prehabilitation literature when referring to psychological interventions, 27 but for other types of prehabilitation they are rarely the focus in favour of post‐operative and functional outcomes such as walk tests. 13 , 14 This currently leads to a lack of evidence currently to support psychological based interventions due to mixed efficacy. 17 , 26 , 39 , 40 , 41 This review will address this gap providing an overview of both psychological and physical outcomes which is important for the development of standardized prehabilitation programs. 7 , 8 , 42
1. METHODS
The present systematic review was reported and conducted in line with the preferred reporting for systematic reviews and meta‐analyses (PRISMA) statements.
1.1. Search strategy
A systematic review of literature relating to prehabilitation for cancer treatment was conducted between 31 January 2021 and October 2021. Five relevant databases were identified for inclusion: Psychological Information database (PsychINFO), Medical Literature Analysis and Retrieval System Online (MEDLINE), Culmunated Index to Nursing and Allied Health Literature (CINAHL), Excerpta Medica Database (EMBASE) and Public MEDLINE (PubMed). Search terms were chosen based on relevance and previous literature: 1. Cancer related terms 2. Prehabilitation related terms 3. Intervention related terms and 4. Lifestyle related terms. Searches were limited to peer reviewed articles, published in English and conducted on human participants. Figure 1 below represents the PRISMA screening strategy.
FIGURE 1.
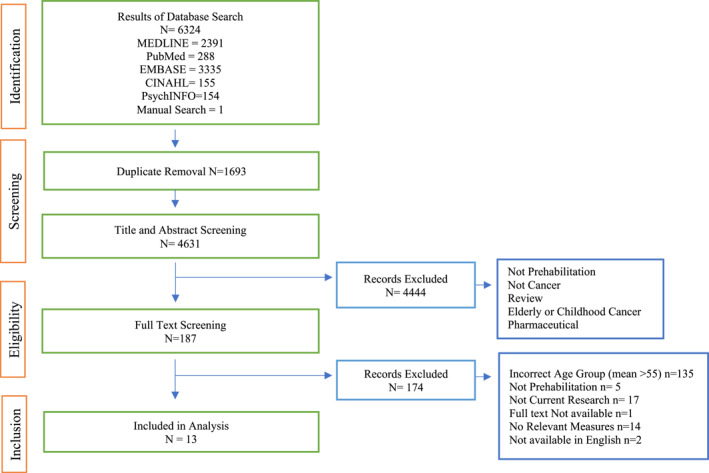
PRISMA Flowchart of screening and review process for included studies
1.2. Inclusion criteria
Studies were included if they involved a prehabilitation intervention that began any time before the patient's treatment occurred and the mean age of participants was 55. Full inclusion and exclusion criteria are displayed in Table 1 below:
TABLE 1.
Inclusion and Exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Cancer of any type or stage | Wrong disease type |
| The mean age of total participants within a study of between 18 and 55 years | Child, adolescent or geriatric focus |
| Peer reviewed journal articles | Not current research ‐ study protocol or review article |
| Prehabilitation | Not prehabilitation – no pre‐treatment or surgery intervention |
| Artificial nutritional support | |
| Affective and/or functional outcome measures | No relevant outcome measures |
| Published in English | Not available in English |
1.3. Screening process
A two‐stage screening process was employed using Covidence™. 43 Abstract and full text screening included a secondary reviewer. All conflicts were addressed in partnership with the second reviewer.
1.4. Quality assessment
Articles were assessed for quality using a checklist based on previous literature. 44 , 45 The checklist contained 12 items relating to the quality of methodological, statistical and clarity of design and reporting of results. The Checklist scores articles in a range of 0–2. Scores of two indicated a “yes” response, scores of one indicated a “partial” response and scores of 0 indicated a “no” or “don't know” response. A higher score indicates a higher quality article, with 17–24 representing good quality research, a score of 9–16 is acceptable quality and a score of 0–8 represents low quality research (See Table 2) (See Supplementary Information S1 for full quality assessment). Figure 1 below outlines the screening and review process for included studies.
TABLE 2.
Characteristics of included studies
| Author | Design | Nature of prehabilitation | Country | Gender breakdown | Patients (n) | Age (mean) | Primary outcome | Type of treatment | Follow up | Quality appraisal |
|---|---|---|---|---|---|---|---|---|---|---|
| Ambler et al., 1999 | Controlled trial | Intervention began at 2nd diagnostic visit | England | 100% female | 110 | 49.5 | Anxiety | Breast cancer surgery | 2 weeks, 6 Months | Acceptable |
| Belleau et al., 2001 | Randomised control trial | Intervention approx.2 weeks pre surgery | Canada | 100% female | 60 | 51.82 | Anxiety | Mastectomy, segmentectomy, modified radical mastectomy | N/A | Good |
| Brahmbhatt et al., 2020 | Feasibility trial a | Duration of surgical wait time. Average 31 days | Canada | 100% female | 22 | 54.18 | Feasibility | Mastectomy or lumpectomy | 6 weeks, 12 Weeks | Good |
| Can et al., 2009 | Randomised control trial | Began at chemotherapy cycle start | Turkey | 65% male, 35% female | 40 | 54.32 | Quality of life (QoL) and reduction in gastrointestinal complaints | Chemotherapy | 6 Weeks in treatment | Good |
| de Moor et al., 2008 | Randomised control trial | 3 weeks before surgery | United States | 100% female | 49 | 53.60 | Distress and perceived stress management | Breast cancer surgery | 2 weeks | Good |
| de Paleville et al., 2007 | Case study | 1 week before chemotherapy | United States | 100% female | 1 | 42 | Functional tasks | Chemotherapy | 9 weeks | Acceptable |
| Foley et al., 2010 | Randomised control trial | Varied due to multiple cancer stages and types | Australia | 77% female, 23% male | 115 | 55.17 | Depression, anxiety, health‐related QoL | Varied | 3 Month | Good |
| Galantino et al., 2012 | Case study series | After diagnosis and before chemotherapy | United States | 100% female | 4 | 54.75 | Cognition, perceived cognition, QoL, mood | Breast cancer | 1 month, 3 Months post | Acceptable |
| Garssen et al., 2013 | Randomised control trial | 5 days before surgery | Netherlands | 100% female | 70 | 53 | Psychological effects | Mastectomy or lumpectomy | Day 2, 5, 1 month, 3 Months | Good |
| Li et al., 2017 | Randomised control trial | 1 week before surgery | China | 57.6% male, 42.4% female | 210 | 55.3 | QoL | Non‐specific therapy | N/A | Good |
| Millstine et al., 2019 | Randomised control trial | Minimum 1 week before surgery | United States | 100% female | 28 | 55.6 | Fatigue, stress, QoL | Breast cancer surgery | 3 Months | Good |
| Singh et al., 2017 | Feasibility trial 1 | Intervention approx. 16 weeks before surgery | Australia | 50% male, 50% female | 10 | 54.4 | Muscle performance | Curative resection | 2 Months | Good |
| Zaman et al., 2021 | Randomised control trial | Workshop session 1 before treatment | Netherlands | 64% male, 36% female | 88 | 55 | Number of days sick leave | Gastrointestinal | 3,6,9,12 Months | Good |
aFeasibility Trials: Research that takes place prior to main research with the aim of assessing parameters required for the main study inclusing satsfaction, adherence, recruitment path and eligibility (Arain et al., 2010). 58
1.5. Data extraction
Eligible articles were screened for inclusion based on the criteria outlined in Table 1 above. Extracted data included: (1) Reference, (2) Study design, (3) Prehabilitation type, (4) Participant demographics, (5) Intervention design and description, (6) Points of data collection and follow up, (7) Outcomes of interest, (8) Relevant measures, (9) Description of results, (10) Effect sizes where applicable. Prehabilitation was coded as either psychological, nutritional or exercise based and whether it was unimodal or multimodal. Study design was also coded. If available, the pooled effect size of interventions on relevant outcome measures were calculated. Depending on study design these may be between or within‐group. Due to the heterogeneity of outcome measures resulting from full‐text data extraction, and to increase clarity, a narrative synthesis approach was employed. 46 As studies varied in design, significance was coded based on the guidelines outlined within each study and included both statistical significance and clinical significance as defined within each study where applicable.
2. RESULTS
2.1. Search results
Figure 1 below represents data screening and inclusion. 6324 studies were included for abstract screening and after duplicate removal, 4631 studies remained. Of those, 4444 studies were deemed ineligible for full text inclusion and 187 studies were included for full text screening. One study within this was added by manual search. 47 In total, 13 were eligible for inclusion based on the criteria (See Table 1). Study characteristics are outlined below in Table 2. For search strategy see Supplementary Information 2.
2.2. Study characteristics
Eight of the 13 studies were randomised control trials RCTs. 24 , 28 , 48 , 49 , 50 , 51 , 52 , 53 Year of publication ranged from 1999 to 2021. All studies used unimodal interventions. Eight studies employed various psychological interventions. These included psychological counselling 24 , 28 , 54 psychoeducation, 48 mindfulness‐based interventions 50 , 51 , 52 and psychosocial return to work support. 53 Only one study employed a nutrition‐based intervention 49 and four studies employed exercise‐based interventions. 47 , 55 , 56 , 57
The mean age of participants was 53 years. A total of 797 participants were included. Participants were largely female. Eight studies included all female participants. 24 , 47 , 48 , 50 , 52 , 54 , 56 , 57 Across the other studies, male participation accounted for 22.6%, 51 50%, 55 57.6%, 28 65% 49 and 64% 53 of the recruited participants. Cancer type predominantly consisted of breast cancer (n = 8), 24 , 47 , 48 , 50 , 52 , 54 , 56 , 57 other cancer types represented included laryngeal, 28 rectal 49 , 55 and multiple cancer types. 51 , 53 A breakdown of study characteristics is presented in Table 2 below:
2.3. Measures
Large heterogeneity existed between measures. For measures such as HrQoL, affective subscales of interest were extracted and assessed where available. Functional measures included variations of the 6 min walk test, the sit to stand test, and other tasks such as ascending and descending stairs. Affective outcome measures are summarised in Table 3 below:
TABLE 3.
Summary of affective outcome assessment measures of final studies
| Construct | Measures | Description | Studies |
|---|---|---|---|
| Health related quality of life | European organisation for research and treatment of cancer quality‐of‐life questionnaire core 30 (EORTC‐QLQ‐C30) | Subscales: Physical, role, cognitive, *emotional, *social & *total global health score | Garssen et al., 2013, Li et al., 2017, Singh et al., 2017, Zaman et al., 2021 |
| Short form health survey (SF‐36 (V2)) | Subscales: Eight subdomains, two composites; physical component summary & *mental component summary | Can et al., 2009, Singh et al.,2017, 58.Brahmbhatt et al., 2020, Zaman et al., 2021 | |
| Functional assessment of cancer therapy‐general (FACT‐G) | Subscales: Physical wellbeing, *social/family wellbeing, *emotional wellbeing, functional wellbeing | Can et al., 2009, Foley et al., 2010, Millstein et al., 2019 | |
| Functional assessment of cancer therapy‐ breast (FACT‐B) | Subscales: Physical wellbeing, social/family wellbeing, emotional wellbeing, functional wellbeing | Galantino et al., 2012 | |
| Depression | Hospital anxiety and depression scale (HADS) | Subscales: Anxiety, *depression | Ambler et al., 1999 |
| Hamilton rating scale for depression HAM‐D | Subscales: One 17 item scale, higher score indicating symptom severity | Foley et al., 2010, Li et al., 2017 | |
| Profile of mood states | Subscales: Depression subscale | Garssen et al., 2013 | |
| Profile of mood states | Subscales: Tension‐anxiety, anger‐hostility, vigour‐activity, fatigue‐inertia, depression‐dejection, confusion‐bewilderment | Galantino et al., 2012 | |
| Anxiety | Hospital anxiety and depression scale (HADS) | Subscales: *Anxiety, depression | Ambler et al., 1999 |
| Hamilton rating scale for anxiety (HAM‐A) | Subscales: One 14 item scale, higher score indicating symptom severity | Foley et al., 2010, Li et al., 2017 | |
| The situational anxiety inventory | Subscales: Adapted from the state‐trait anxiety inventory, 20 items, 10 to decent absence of and 10 to detect presence of unpleasant emotional states | Belleau et al., 2001 | |
| The state trait anxiety inventory | Subscales: Dutch edition, state anxiety 20 item scale | Garssen et al., 2013 | |
| Stress | Rotterdam symptom checklist (RCSL) | Subscales: *Psychological scale, physical scale | Ambler et al., 1999 |
| Perceived stress scale (PSS) | Subscales: One scale 14 items apprising stress within one's life | de Moor et al., 2008, Millstein et al., 2019 | |
| Depression anxiety stress scale (DASS) | Subscales: Depression, anxiety and *stress | Foley et al., 2010 |
Note: * Indicates subscale of relevance for measures with multiple scales.
2.4. Prehabilitation exercise interventions
Four studies involved exercise interventions. 47 , 55 , 56 , 57 Variation existed between type and mode of delivery. Two studies employed both aerobic exercise and resistance training. 55 , 57 Intervention length varied from one to 3 months. One study used aerobic exercise only. 56 One study employed Iyengar Yoga exercises, 47 and duration and frequency of interventions varied from 2 to 5 times weekly and 15–70 min per session. In some cases qualitative components and feasibility analysis were also introduced at follow‐up assessments. 57 Two studies increased exercise intensity as participants progressed based on a gradually increasing duration by 5 min 56 and using rates of perceived exertion. 57 A breakdown of exercise study characteristics is presented below in Table 4.
TABLE 4.
Characteristics of exercise intervention studies
| Author | Exercise type | Duration (max) | Frequency | Mode of delivery | Exercises | Equipment |
|---|---|---|---|---|---|---|
| Brahmbhatt et al., 2020 | Aerobic & resistance | 40 min | 3–5 times weekly | Remote supervision | Aerobic: Brisk walking | Resistance bands and exercise manual |
| Resistance: Standing rows, shoulder external rotation, front raise, lateral raise, bicep curls, tricep extensions, wall push ups and chest press | ||||||
| Mobility and stretching | ||||||
| de Paleville et al., 2007 | Aerobic only | 35 min | 5 time weekly | Supervised and home based | Aerobic: Walking | Pedometer, training log |
| Warm up and stretching | ||||||
| Galantino et al., 2012 | Iyengar‐inspired yoga program | 70 min | 2 times weekly | Small group sessions and home‐based | Aerobic: Iyengar yoga class with 14 poses | A mat, two bolsters, two chairs, two blankets, one eye bandage, belt, two blocks and a 1‐lb. Weight. |
| Singh et al., 2017 | Aerobic & resistance | 60 min | 2 times weekly (supervised) | Supervised | Aerobic: Walking or jogging, cycling, rowing | Treadmill, exercise bike, rowing machine, standard resistance training gym machinery |
| Resistance: Chest press, seated row, latissimus pull down, leg extension, leg curl, leg press |
2.4.1. Outcomes
Affective Measures: Measures of HrQoL were employed across three studies. These included European organisation for research and treatment of cancer quality‐of‐life questionnaire core 30 (EORTC‐QLQ‐C30) 55 and the Short form health survey (SF‐36), 55 , 57 the Functional Assessment of Cancer Therapy‐ Breast (FACT‐B) 47 (Table 3). Results of the EORTC‐QLQ‐C30 indicated that exercise interventions had a modest non‐significant effect on global health (d baseline‐pre = 0.55), but returned to baseline levels post‐surgery. Scores on the social and emotional subscales of the EORTC‐QLQ‐C30 also showed a minimal effect of exercise (d = 0.09–0.10). 55 Results of the short form health survey indicated a small non‐significant decline post‐surgery in mental composite scores (d = baseline to pre = 0.08, baseline to post = 0.07). For Brahmbhatt and colleagues 57 single arm feasibility study, the SF‐36 mental composite score worsened from baseline to pre‐op but increased by 4.36 points post‐op representing a clinically significant improvement. In terms of effect, the baseline to preoperative effect for the mental composite score was low from baseline to 3 month follow up. Galantino et al., 2012 47 reported improvements in quality of life (QoL) across participants in their case study series, but did not provide sufficient data for further interpretation. Qualitatively, improvements were reported at 3 months follow‐up across all participants. This study also reported decreases in mood disturbances across participants during the intervention, but that these decreases were not maintained at follow‐up.
Functional Measures: Primary outcomes included muscle strength, 55 functional ability, 47 , 56 feasibility. 57 Walk tests measured functional capacity across three studies. 55 , 56 , 57 Other measures of functional capacity included The Disabilities of Arm Shoulder and Hand measure (DASH), 57 stair climbs, chair raises, arm curls, sit and reach and related reach tasks. 47 , 55 , 56 Results indicated promising improvements in functional capacity as a result of exercise prehabilitation. The 6‐min walk backward test (d baseline‐pre = 0.55) showed statistically significant improvements (p < 0.035) 55 and results of the 6‐min walk test distance were of clinical significance in another study, 37 m over the clinically significant distance. 57
Results of the 400 m walk test were statistically significant (d baseline‐pre = 0.68). 55 Case study data also indicated an over 40% improvement in 12‐min walk distance from baseline to post. 56 Results of the other measures varied. Ability to ascend stairs improved. 56 Overall, results of other measures were non‐significant but moderate in effect (d = 0.39–0.68) 55 and represented case study improvements of 5%–41%. 56 The DASH was employed in one study 57 and found a non‐statistically significant increase in DASH scores but reported a clinically meaningful increase in DASH scores pre‐post‐op of over 15 points. Qualitative case series data from the yoga intervention also indicated increases in flexibility and balance across all participants from measures of functional arm reach. 47
2.5. Prehabilitation nutrition interventions
Only one study used a nutrition based intervention. 49 This involved the use of probiotic kefir to supplement patients as they underwent chemotherapy for colorectal cancer. Kefir is a natural probiotic that mimics the beneficial bacteria found in dairy products. Forty participants were randomised to either the control or intervention condition. Intervention participants ingested 500 ml of weekly kefir before and during chemotherapy in two 250 ml rounds. Data was collected at baseline, week three and week six of treatment cycles.
2.5.1. Outcomes
Affective Measures: HrQoL was assessed at the three time points using the Functional assessment of cancer therapy‐general (FACT‐G) questionnaire. The effect of kefir on HrQoL was non‐significant. With regards to the affective subscales, all between group differences were non‐significant at all time points.
2.6. Prehabilitation psychological interventions
Eight studies employed psychological interventions. 24 , 28 , 48 , 50 , 51 , 52 , 53 , 54 Intervention duration varied from days to weeks pre‐surgery. 24 , 52 Follow‐ups ranged from 2 weeks to 12 months. 53 Follow‐up assessments generally included repeated administration of study outcome measures. One study did not assess patients post intervention. 48 Treatments focused on the pre‐surgical/treatment and surgical consultation phases. One study continued the intervention into the perioperative phase, 51 and another study continued support up to 9 months post‐surgery. 53 Anxiety reduction was the primary outcome in five studies. 24 , 28 , 48 , 51 , 54 As with the physical interventions, modes of intervention varied across studies. Two studies employed the use of tailored nurse advocacy ‐ These included counselling from diagnosis to surgery and offering psychoeducational support. 28 , 54 Mindfulness‐based practice and meditation were commonly used, including mindfulness‐based training with cognitive elements, 51 and mindfulness‐based home neurofeedback. 52 Many interventions used multidisciplinary approaches combining education, counselling and cognition. 28 , 48 Other interventions included expressive writing 50 and stress management. 24 One study employed a tailored psychosocial return‐to‐work support intervention. 53 Three interventions took place onsite, 48 , 54 two were offsite 50 , 52 and three used a combination. 28 , 51 , 53
2.6.1. Outcomes
Affective Measures: Anxiety was assessed across five studies. 24 , 28 , 48 , 51 , 54 Anxiety measures varied widely and included the Hospital anxiety and depression Scale (HADS) Scale, The Situational Anxiety Inventory, The Hamilton rating Scale for anxiety and The State Trait Anxiety Inventory (See Table 3). Overall, psychological interventions were moderately successful for anxiety. Four of seven interventions showed significant anxiety improvements. 28 , 48 , 51 , 54 Another study produced a clinically significant result pre‐surgery, but with no intervention effects. 24 Foley and colleagues 51 reported large effects of their mindfulness‐based intervention from baseline to 3 months follow‐up (p < 0.002, d = 0.59). Ambler and colleagues 54 reported a significant reduction in anxiety pre‐treatment to 2‐week follow‐up (P < 0.003) (d = 0.45). Other studies had small to moderate effects of interventions on anxiety scores ranging from d = 0.26‐0.4. 28 , 48 However these effects were not always maintained at 6‐month follow‐up. 54 Less successful interventions included expressive writing 50 and stress management training. 24
Depression was assessed in five studies 24 , 28 , 50 , 51 , 54 and assessed as primary in one study. 51 Measures included The Brief Symptom Inventory, The HAM‐D, The HADS and The Profile of Mood States: Depression (See Table 3). Three studies found significant moderate‐large effects on depression. 24 , 28 , 51 Foley and colleagues 51 reported clinically significant decreases in symptoms over time (p < 0.001, d = 0.83). Li and colleagues in 2017 reported a significant (p < 0.05) effect on depression (d = 0.33). Significant effects were also found between groups day‐5 post treatment as a result of the stress management intervention but these were not significant after day 5. 24 The nurse advocacy counselling intervention 54 and the expressive writing intervention 48 reported no effects.
Health related quality of life was assessed in five studies. 24 , 28 , 51 , 52 , 53 HrQoL was included as a primary outcome within two studies. 24 Measures employed included The FACT‐G and The EORTC‐QLQ‐C30 and the Short form health survey (See Table 3). Only Li and colleagues 28 reported significant effects of treatment on QoL as a result of their psycho‐cognitive and psychoeducational intervention. They found a significant pre‐post effect on global health (d = 1.3), mental health (d = 0.53) and social function (d = 0.31). Foley and colleagues 51 reported clinically significant effects of mindfulness based cognitive behavioural therapy (CBT) on overall HrQoL (d = 0.30) and these improvements were above clinical significance. Stress management training 24 and electroencephalogram (EEG) mindfulness meditation 52 reported no effect.
Finally, stress and distress were assessed within four studies. 48 , 51 , 52 , 54 Measures employed included The Perceived Stress Scale, The Depression Anxiety Stress Scale and The Rotterdam Symptom Checklist (See Table 3). Only one study 51 found a significant reduction in symptoms as a result of mindfulness based CBT (p < 0.001, d = 0.53). Other studies reported non‐significant trends relating to stress and distress. Millstine and colleagues 52 reported a small decrease in perceived stress as a result of EEG mindfulness‐based meditation. Ambler and colleagues 54 found a decrease in psychological distress pre‐post treatment for the nurse advocacy counselling intervention.
2.7. Risk of bias
All included studies were of acceptable quality based criteria outlined. 44 There was no evidence of reporting bias. Risk of selection bias was low as many studies employed a RCT design. However some studies did not include a control group and did not adequately outline recruitment strategy and eligibility. For full quality assessment scoring see Supplementary Information 1.
3. DISCUSSION
3.1. Prehabilitation interventions
To our knowledge, this is the first review of its kind assessing the effects of prehabilitation interventions on affective and functional outcomes for young to midlife adult (18–55) participants. Evidence was found to support the alleviation of anxiety as a result of prehabilitation interventions, with support for health‐related QoL, stress and depression being less‐robust and underrepresented. Findings moderately supported the therapeutic validity of exercise prehabilitation to improve functional capacity both in terms of clinical and experimental improvements. All studies included in this review were unimodal, involving only one prehabilitation intervention as opposed to multimodal, where multiple interventions are employed in combination. 59 , 60 This is not in line with previous systematic evidence, in which multimodal interventions are usually represented. 58 , 60 , 61 This could be due to the age‐range of interest, as previous multimodal pre‐surgery interventions report an average age of diagnosis ranging from 63 to 72 years. 62 The majority of studies retained were psychological interventions, indicating that exercise prehabilitation is not the primary focus of prehabilitation interventions within the 18–55 age range, despite younger patients being reported to be in favour of exercise prehabilitation. 16 Only one study employed a nutritional intervention. This could relate to the age of diagnosis for cancers that required the most nutritional support such as colon or gastrointestinal. Regardless of cancer type, general nutritional support is highlighted as a key pillar of prehabilitation treatment. 7
Studies were largely heterogeneous in design. The majority of studies applied a randomised control trial design. Feasibility as a construct appeared to be assessed in exercise studies that occurred more recently, within the past 5 years. 55 , 57 Despite large variance in psychological techniques and the development of novel interventions, no psychological intervention identified feasibility as a primary outcome. These data could provide a context for interpreting non‐significant results observed, as some of the psychological interventions, particularly mindfulness and meditation‐focused, require high levels of commitment. 51 , 52 Assessments included a high battery of measures, with the majority of studies reporting over five outcome assessments. Both the time commitment of interventions and assessments could be contributing to the mixed efficacy of results, particularly for psychological interventions as patient distress could be affecting adherence. 63 For exercise, levels of adherence can also relate to baseline level of fitness, which could also impact upon the results of smaller exercise intervention studies. 64
3.2. Participants
The majority of studies focused on breast cancer. This could be due to a number of factors including the age range of interest, the relatively quick timeline from diagnosis to treatment or the distressing nature of breast cancer surgery for women requiring increased psychological support. 65 The studies represented in this review focus on psychological intervention, however these results are heavily confounded by the treatment pathways related to cancer subtypes.
There was an over‐representation of female participants. This could have had implications for the prevalence of anxiety‐related research, as women report increased levels of cancer treatment‐related anxiety compared to men. 66 , 67
Variation existed across treatment and surgery types and heterogeneity between follow‐up assessments (See Table 2) also made it difficult to ascertain any distinct differences between treatment or surgery with regards to outcomes. Two studies 47 , 56 also employed a case study and case series design, such that results for relevant outcomes were largely descriptive in nature and difficult to generalise to functional and affective outcomes for prehabilitation interventions at large.
3.3. Affective outcomes
Assessment of affective outcomes resulted in thought‐provoking findings. Study measures varied with some studies employing a combined measure to assess affective outcomes (See Table 3). Interventions produced a moderate effect on anxiety. This is in line with research trends, which indicate a positive effect of prehabilitation on anxiety. 25 Similar to functional capacity and exercise, anxiety measures were only represented within the unimodal psychological interventions. It is interesting to note that only one exercise intervention 47 employed a measure of anxiety, as that previous literature indicates that exercise can aid in alleviating preoperative anxiety 68 and therefore would been expected as an included outcome. This could be due to the nature of the yoga‐based intervention, which is known to aid in anxiety reduction. 69
The evidence for depression was less definitive and not statistically supported, as only seven studies included assessments. Although the evidence was limited, the effect size of the psychological interventions on depression did exceed that of anxiety (d = 0.50–1.8). The positive reported effects on depression are surprising, as it was not the primary outcome for many interventions. Measures of depression were included mainly within the psychological interventions, and not alongside measures of functional capacity within exercise interventions, apart from Galantino and colleagues 2012 47 yoga‐based intervention. This was unanticipated, due to literature supporting the link between exercise and treatment for even moderate depression. 70 The lack of affective measures assessed within the nutritional intervention was unanticipated, due to trends exploring the positive impact of nutrition on affective outcomes. 71
Stress and distress were under‐represented, appearing in four psychological interventions. Of those, two shared a common measure. Low numbers and large heterogeneity made it difficult to ascertain the effect of the interventions on stress and distress, with clinical significance only found in one study.
Health related quality of life was the only affective measure to be assessed across the three modes of prehabilitation. Results did not support a statistically significant increase in HrQoL as a result of any intervention. However, clinically significant increases were found for both exercise and psychological intervention types within studies. The effect of psychological interventions on HrQoL was broad. These mixed results for the mental components of HrQoL across studies was surprising due to the relatively consistent effects of the interventions on outcomes, like anxiety.
3.4. Functional outcomes
Overall, the functional outcomes assessed indicated moderate to large effects of exercise prehabilitation for improving functional capacity. Only studies employing exercise prehabilitation assessed functional outcomes. The overall effect of exercise prehabilitation was large, indicating particular improvements across functional walking tasks. Some measures focused on time taken to travel certain distances, whilst others focused on distance travelled within certain timeframes as well as other markers of function such as flexibility and balance. This variation amongst measures made it difficult to directly compare efficacy. Three studies did not have sufficient power to report significance. 47 , 56 , 57 The results of the present review provides moderate support for the clinical efficacy of exercise prehabilitation. The present review also found a low number of eligible studies to provide evidence for the efficacy of nutritional or psychological support on functional outcomes.
3.5. Limitations
Large heterogeneity across all prehabilitation outcome measures prevented assessment via a meta‐analysis, limiting generalisability. The age range of 18–55 years possibly confounded study findings by over representing certain cancer types and female gender. This age range was also selected using mean values as it was not possible to obtain the range for all studies, this may have skewed the representation of ages. This was interesting as this unintentionally resulted in a narrow focus of cancer type which further highlights the need for increased focus of young to midlife adults across cancer types. The large representation across female cancer types would be generalisable to the population at large, as research from cancer research UK suggests that for adults in the 24–59 age bracket, rates of female cancer nearly doubles that of male indicine rates, 72 the large representation of breast cancer was also representative, as breast cancer was the most common cancer type reported by the National Cancer Institute. 37 Due to the relatively new development of prehabilitation within cancer treatment, no restrictions in publication date were placed on studies. As a result of study design and participant count, some studies lacked sufficient power to report statistical significance, 47 , 56 , 57 but did find clinically significant results, which may have resulted in an underestimation of the true effectiveness of interventions. Some studies failed to report the relevant affective scores for each subscale, which also created data extraction issues for some HrQoL subscales. Heterogeneity of study design also meant that follow up assessments were not consistent (See Table 2).
3.6. Clinical implications
In terms of functional capacity, studies also reported large improvements within their populations despite lacking power to report statistical significance. 47 , 56 , 57 This was also the case for anxiety, where clinical improvements were also observed. 24 It would appear that the results of the present review moderately supports the clinical efficacy of exercise prehabilitation. The use of clinical change metrics and change scores is common in health related intervention research, and allows for an increasingly patient‐centred approach. 73 From a clinical perspective, the results of the present review provide increasing evidence for the use of prehabilitation among cancer patients between the ages of 18–55 to improve anxiety and functional capacity.
4. CONCLUSION
The present study adds to prehabilitation literature by assessing the impact of prehabilitation on affective outcomes, and by focussing on the young to midlife age range of cancer patients. There exists large heterogeneity in both study design and assessment within cancer prehabilitation. There is also a lack of young to midlife cancer research across cancer types, which should be addressed in future. The area would benefit from the development of a standardised functional and affective battery in order to allow for increased generalisability of findings. Future research could aim to combine feasibility measures into prehabilitation interventions of all kinds, in order to understand the psychological load of the prehabilitation commitment on patients, whilst also expanding participant count to provide sufficient power.
CONFLICT OF INTEREST
The authors have no conflicts of interest statements to disclose.
Supporting information
Supplementary Information 1
Supplementary Information 2
ACKNOWLEDGEMENTS
This work was undertaken as part of an MSc. In Psychology and Wellbeing at Dublin City University. This work was not funded.
Open access funding provided by IReL.
Scriney A, Russell A, Loughney L, Gallagher P, Boran L. The impact of prehabilitation interventions on affective and functional outcomes for young to midlife adult cancer patients: a systematic review. Psychooncology. 2022;31(12):2050‐2062. 10.1002/pon.6029
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Globocan . All Cancers Fact Sheet. World Health Organisation; 2020. https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf [Google Scholar]
- 2. Department of Health. National Cancer Strategy 2017 ‐ 2026. [Internet]. [cited 2021 Jun 16]. https://www.gov.ie/en/publication/a89819-national-cancer-strategy-2017-2026/
- 3. Cormie P, Atkinson M, Bucci L, et al. Clinical Oncology Society of Australia position statement on exercise in cancer care. Med J Aust. 2018;209(4):184‐187. 10.5694/mja18.00199 [DOI] [PubMed] [Google Scholar]
- 4. European Commission , Directorate‐General for Research and Innovation , Pita Barros P, Beets‐Tan R, Chomienne C, et al. Conquering Cancer: Mission Possible. Publications Office; 2020. 10.2777/045403 [DOI] [Google Scholar]
- 5. Association of European Cancer Leagues . European Code against Cancer Policy Framework; 2020.
- 6. Bausys A, Mazeikaite M, Bickaite K, Bausys B, Bausys R, Strupas K. The role of prehabilitation in modern esophagogastric cancer surgery: a comprehensive review. Cancers. 2022;14(9):2096. 10.3390/cancers14092096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Macmillan Cancer Support . Prehabilitation Guidance for Healthcare Professionals. [Internet]. [cited 2022 Mar 21]. https://www.macmillan.org.uk/healthcare-professionals/news-and-resources/guides/principles-and-guidance-for-prehabilitation
- 8. van Rooijen S, Carli F, Dalton S, et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation. BMC Cancer. 2019;19(1):98. 10.1186/s12885-018-5232-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loughney L, Cahill R, O’Malley K, McCaffrey N, Furlong B. Compliance, adherence and effectiveness of a community‐based pre‐operative exercise programme: a pilot study. Perioperat Med. 2019;8(1):17. 10.1186/s13741-019-0126-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kehoe B, Skelly F, Moyna N, et al. The effect of participating in MedEx Wellness, a community‐based chronic disease exercise rehabilitation programme, on physical, clinical and psychological health: a study protocol for a cohort trial. Contemporary Clinical Trials Communications. 2020;19:100591. 10.1016/j.conctc.2020.100591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loughney LA, West MA, Kemp GJ, Grocott MP, Jack S. Exercise interventions for people undergoing multimodal cancer treatment that includes surgery. Cochrane Database Syst Rev. 2018. [Internet][cited 2022 Mar 20];(12). http://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012280.pub2/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heger P, Probst P, Wiskemann J, Steindorf K, Diener MK, Mihaljevic AL. A systematic review and meta‐analysis of physical exercise prehabilitation in major abdominal surgery (PROSPERO 2017 CRD42017080366). J Gastrointest Surg. 2020;24(6):1375‐1385. 10.1007/s11605-019-04287-w [DOI] [PubMed] [Google Scholar]
- 13. Waterland JL, McCourt O, Edbrooke L, et al. Efficacy of prehabilitation including exercise on postoperative outcomes following abdominal cancer surgery: a systematic review and meta‐analysis. Front Surg. 2021;8:628848. 10.3389/fsurg.2021.628848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Campbell KL, Winters‐Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375‐2390. 10.1249/mss.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cormie P, Zopf EM, Zhang X, Schmitz KH. The impact of exercise on cancer mortality, recurrence, and treatment‐related adverse effects. Epidemiol Rev. 2017;39(1):71‐92. 10.1093/epirev/mxx007 [DOI] [PubMed] [Google Scholar]
- 16. Banerjee S, Semper K, Skarparis K, et al. Patient perspectives of vigorous intensity aerobic interval exercise prehabilitation prior to radical cystectomy: a qualitative focus group study. Disabil Rehabil. 2021;43(8):1084‐1091. 10.1080/09638288.2019.1651907 [DOI] [PubMed] [Google Scholar]
- 17. West MA, Wischmeyer PE, Grocott MPW. Prehabilitation and nutritional support to improve perioperative outcomes. Curr Anesthesiol Rep. 2017;7(4):340‐349. 10.1007/s40140-017-0245-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McMillan DC. An inflammation‐based prognostic score and its role in the nutrition‐based management of patients with cancer: nutrition Society and BAPEN Medical Symposium on ‘Nutrition support in cancer therapy. Proc Nutr Soc. 2008;67(3):257‐262. 10.1017/s0029665108007131 [DOI] [PubMed] [Google Scholar]
- 19. Arends J, Baracos V, Bertz H, et al. ESPEN expert group recommendations for action against cancer‐related malnutrition. Clin Nutr. 2017;36(5):1187‐1196. 10.1016/j.clnu.2017.06.017 [DOI] [PubMed] [Google Scholar]
- 20. Bargetzi L, Brack C, Herrmann J, et al. Nutritional support during the hospital stay reduces mortality in patients with different types of cancers: secondary analysis of a prospective randomized trial. Ann Oncol. 2021;32(8):1025‐1033. 10.1016/j.annonc.2021.05.793 [DOI] [PubMed] [Google Scholar]
- 21. Fradelos EC, Papathanasiou IV, Veneti A, et al. Psychological distress and resilience in women diagnosed with breast cancer in Greece. Asian Pac J Cancer Prev APJCP. 2017. [Internet][cited 2022 Mar 22];18(9). 10.22034/APJCP.2017.18.9.2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marinelli V, Danzi OP, Mazzi MA, et al. PREPARE: PreoPerative anxiety REduction. One‐year feasibility RCT on a brief psychological intervention for pancreatic cancer patients prior to major surgery. Front Psychol. 2020. [Internet][cited 2021 Apr 20];11. https://www.frontiersin.org/articles/10.3389/fpsyg.2020.00362/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fortmann J, Fisher A, Hough R, Gregory A, Pugh G. Sleep quality, fatigue, and quality of life among teenage and young adult cancer survivors. J Adolesc Young Adult Oncol. 2018;7(4):465‐471. 10.1089/jayao.2018.0004 [DOI] [PubMed] [Google Scholar]
- 24. Garssen B, Boomsma MF, Meezenbroek EdeJ, et al. Stress management training for breast cancer surgery patients. Psycho‐Oncology. 2013;22(3):572‐580. 10.1002/pon.3034 [DOI] [PubMed] [Google Scholar]
- 25. Zhu H, Moffa ZJ, Gui X, Carroll JM. Prehabilitation: care challenges and technological opportunities. In: Proceedings of the 2020 CHI Conference on Human Factors in Computing Systems [Internet]. Association for Computing Machinery; 2020. [cited 2021 Jul 5]. p. 1–13. 10.1145/3313831.3376594 [DOI] [Google Scholar]
- 26. Silver JK, Baima J. Cancer prehabilitation: an opportunity to decrease treatment‐related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil. 2013;92(8):715‐727. 10.1097/phm.0b013e31829b4afe [DOI] [PubMed] [Google Scholar]
- 27. Tsimopoulou I, Pasquali S, Howard R, et al. Psychological prehabilitation before cancer surgery: a systematic review. Ann Surg Oncol. 2015;22(13):4117‐4123. 10.1245/s10434-015-4550-z [DOI] [PubMed] [Google Scholar]
- 28. Li X, Li J, Shi Y, et al. Psychological intervention improves life quality of patients with laryngeal cancer. Patient Prefer Adherence. 2017;11:1723‐1727. 10.2147/ppa.s147205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ngo‐Huang A, Parker NH, Bruera E, et al. Home‐Based exercise prehabilitation during preoperative treatment for pancreatic cancer is associated with improvement in physical function and quality of life. Integr Cancer Ther. 2019;18:1534735419894061. 10.1177/1534735419894061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luther A, Gabriel J, Watson RP, Francis NK. The impact of total body prehabilitation on post‐operative outcomes after major abdominal surgery: a systematic review. World J Surg. 2018;42(9):2781‐2791. 10.1007/s00268-018-4569-y [DOI] [PubMed] [Google Scholar]
- 31. Bundred JR, Kamarajah SK, Hammond JS, Wilson CH, Prentis J, Pandanaboyana S. Prehabilitation prior to surgery for pancreatic cancer: a systematic review. Pancreatology. 2020;20(6):1243‐1250. 10.1016/j.pan.2020.07.411 [DOI] [PubMed] [Google Scholar]
- 32. Bruns ERJ, van den Heuvel B, Buskens CJ, et al. The effects of physical prehabilitation in elderly patients undergoing colorectal surgery: a systematic review. Colorectal Dis. 2016;18(8):O267‐O277. 10.1111/codi.13429 [DOI] [PubMed] [Google Scholar]
- 33. Looijaard SMLM, Slee‐Valentijn MS, Otten RHJ, Maier AB. Physical and nutritional prehabilitation in older patients with colorectal carcinoma: a systematic review. J Geriatr Phys Ther. 2018;41(4):236‐244. 10.1519/jpt.0000000000000125 [DOI] [PubMed] [Google Scholar]
- 34. Zhi X, Xie M, Zeng Y, Liu J, Cheng ASK. Effects of exercise intervention on quality of life in adolescent and young adult cancer patients and survivors: a meta‐analysis. Integr Cancer Ther. 2019;18:1534735419895590. 10.1177/1534735419895590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Daniels SL, Burton M, Lee MJ, et al. Healthcare professional preferences in the health and fitness assessment and optimization of older patients facing colorectal cancer surgery. Colorectal Dis. 2021;23(9):2331‐2340. 10.1111/codi.15758 [DOI] [PubMed] [Google Scholar]
- 36. Ospina PA, McComb A, Wiart LE, Eisenstat DD, McNeely ML. Physical therapy interventions, other than general physical exercise interventions. In: Children and adolescents before, during and following treatment for cancer. Cochrane Database of Systematic Reviews; 2018. [Internet][cited 2022 Mar 22];(1). http://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012924/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. National Cancer Institute . Adolescents and Young Adults (AYAs) with Cancer. National Cancer Institute; [Internet]. 2015 [cited 2022 Mar 22]. https://www.cancer.gov/types/aya [Google Scholar]
- 38. White MC, Holman DM, Boehm JE, Peipins LA, Grossman M, Henley SJ. Age and cancer risk: a potentially modifiable relationship. Am J Prev Med. 2014;46(3):S7‐S15. 10.1016/j.amepre.2013.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Levett DZH, Grimmett C. Psychological factors, prehabilitation and surgical outcomes: evidence and future directions. Anaesthesia. 2019;74(S1):36‐42. 10.1111/anae.14507 [DOI] [PubMed] [Google Scholar]
- 40. EuroQol. EuroQol ‐ a new facility for the measurement of health‐related quality of life. Health Pol. 1990;16(3):199‐208. [DOI] [PubMed] [Google Scholar]
- 41. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365‐376. 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 42. Campbell KL, Zadravec K, Bland KA, Chesley E, Wolf F, Janelsins MC. The effect of exercise on cancer‐related cognitive impairment and applications for physical therapy: systematic review of randomized controlled trials. Phys Ther. 2020;100(3):523‐542. 10.1093/ptj/pzz090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Veritas Health Innovation. Covidence Systematic Review Software. VH Innovation; 2017. [Google Scholar]
- 44. Dunne S, Mooney O, Coffey L, et al. Psychological variables associated with quality of life following primary treatment for head and neck cancer: a systematic review of the literature from 2004 to 2015. Psycho Oncol. 2017;26(2):149‐160. 10.1002/pon.4109 [DOI] [PubMed] [Google Scholar]
- 45. Jefferies P, Gallagher P, Dunne S. The Paralympic athlete: a systematic review of the psychosocial literature. Prosthetics Orthot Int. 2012;36(3):278‐289. 10.1177/0309364612450184 [DOI] [PubMed] [Google Scholar]
- 46. Popay J, Roberts H, Sowden A, et al. Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme version. 2006;1(1):b92. [Google Scholar]
- 47. Galantino ML, Greene L, Daniels L, Dooley B, Muscatello L, O’Donnell L. Longitudinal impact of yoga on chemotherapy‐related cognitive impairment and quality of life in women with early stage breast cancer: a case series. Explore. 2012;8(2):127‐135. 10.1016/j.explore.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 48. Belleau FP, Hagan L, Mâsse B. Effects of an educational intervention on the anxiety of women awaiting mastectomies. Can Oncol Nurs J. 2001;11(4):172‐180. 10.5737/1181912x114177180 [DOI] [PubMed] [Google Scholar]
- 49. Can G, Topuz E, Derin D, Durna Z, Aydiner A. Effect of kefir on the quality of life of patients being treated for colorectal cancer. Oncol Nurs Forum. 2009;36(6):E335‐E342. 10.1188/09.onf.e335-e342 [DOI] [PubMed] [Google Scholar]
- 50. de Moor JS, Moyé L, Low D, et al. Expressive writing as a presurgical stress management intervention for breast cancer patients. J Soc Integr Oncol. 2008;6(2):59‐66. [PubMed] [Google Scholar]
- 51. Foley E, Baillie A, Huxter M, Price M, Sinclair E. Mindfulness‐based cognitive therapy for individuals whose lives have been affected by cancer: a randomized controlled trial. J Consult Clin Psychol. 2010;78(1):72‐79. 10.1037/a0017566 [DOI] [PubMed] [Google Scholar]
- 52. Millstine DM, Bhagra A, Jenkins SM, et al. Use of a wearable EEG headband as a meditation device for women with newly diagnosed breast cancer: a randomized controlled trial. Integr Cancer Ther. 2019;18:1534735419878770. 10.1177/1534735419878770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zaman ACGNM, Tytgat KMAJ, Klinkenbijl JHG, et al. Effectiveness of a tailored work‐related support intervention for patients diagnosed with gastrointestinal cancer: a multicenter randomized controlled trial. J Occup Rehabil. 2021;31(2):323‐338. 10.1007/s10926-020-09920-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ambler N, Rumsey N, Harcourt D, Khan F, Cawthorn S, Barker J. Specialist nurse counsellor interventions at the time of diagnosis of breast cancer: comparing ‘advocacy’ with a conventional approach. J Adv Nurs. 1999;29(2):445‐453. 10.1046/j.1365-2648.1999.00902.x [DOI] [PubMed] [Google Scholar]
- 55. Singh F, Newton RU, Baker MK, Spry NA, Taaffe DR, Galvão DA. Feasibility and efficacy of presurgical exercise in survivors of rectal cancer scheduled to receive curative resection. Clin Colorectal Cancer. 2017;16(4):358‐365. 10.1016/j.clcc.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 56. de Paleville DT, Topp RV, Swank AM. Effects of aerobic training prior to and during chemotherapy in a breast cancer patient: a case study. J Strength Condit Res. 2007;21(2):635‐637. 10.1519/r-19735.1 [DOI] [PubMed] [Google Scholar]
- 57. Brahmbhatt P, Sabiston CM, Lopez C, et al. Feasibility of prehabilitation prior to breast cancer surgery: a mixed‐methods study. Front Oncol. 2020. [Internet][cited 2021 Mar 26];10. https://www.frontiersin.org/articles/10.3389/fonc.2020.571091/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Arain M, Campbell MJ, Cooper CL, Lancaster GA. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol. 2010;10(1):67. 10.1186/1471-2288-10-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gillis C, Buhler K, Bresee L, et al. Effects of nutritional prehabilitation, with and without exercise, on outcomes of patients who undergo colorectal surgery: a systematic review and meta‐analysis. Gastroenterology. 2018;155(2):391‐410. e4. 10.1053/j.gastro.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 60. Bolshinsky V, Li MH.‐G, Ismail H, Burbury K, Riedel B, Heriot A. Multimodal prehabilitation programs as a bundle of care in gastrointestinal cancer surgery: a systematic review. Dis Colon Rectum. 2018;61(1):124‐138. 10.1097/dcr.0000000000000987 [DOI] [PubMed] [Google Scholar]
- 61. Hijazi Y, Gondal U, Aziz O. A systematic review of prehabilitation programs in abdominal cancer surgery. Int J Surg. 2017;39:156‐162. 10.1016/j.ijsu.2017.01.111 [DOI] [PubMed] [Google Scholar]
- 62. Cancer.net . Colorectal Cancer ‐ Risk Factors and Prevention. [Internet]. Cancer.Net. 2012 [cited 2022 Mar 22]. https://www.cancer.net/cancer-types/colorectal-cancer/risk-factors-and-prevention
- 63. Brebach R, Sharpe L, Costa DSJ, Rhodes P, Butow P. Psychological intervention targeting distress for cancer patients: a meta‐analytic study investigating uptake and adherence. Psycho‐Oncology. 2016;25(8):882‐890. 10.1002/pon.4099 [DOI] [PubMed] [Google Scholar]
- 64. Halliday LJ, Doganay E, Wynter‐Blyth V, Osborn H, Buckley J, Moorthy K. Adherence to pre‐operative exercise and the response to prehabilitation in oesophageal cancer patients. J Gastrointest Surg. 2021;25(4):890‐899. 10.1007/s11605-020-04561-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Perez‐Tejada J, Labaka A, Pascual‐Sagastizabal E, Garmendia L, Iruretagoyena A, Arregi A. Predictors of psychological distress in breast cancer survivors: a biopsychosocial approach. Eur J Cancer Care. 2019;28(6):e13166. 10.1111/ecc.13166 [DOI] [PubMed] [Google Scholar]
- 66. Jimenez‐Fonseca P, Calderón C, Hernández R, et al. Factors associated with anxiety and depression in cancer patients prior to initiating adjuvant therapy. Clin Transl Oncol. 2018;20(11):1408‐1415. 10.1007/s12094-018-1873-9 [DOI] [PubMed] [Google Scholar]
- 67. Pitman A, Suleman S, Hyde N, Hodgkiss A. Depression and anxiety in patients with cancer. BMJ. 2018;361:k1415. 10.1136/bmj.k1415 [DOI] [PubMed] [Google Scholar]
- 68. Quist M, Adamsen L, Rørth M, Laursen JH, Christensen KB, Langer SW. The impact of a multidimensional exercise intervention on physical and functional capacity, anxiety, and depression in patients with advanced‐stage lung cancer undergoing chemotherapy. Integr Cancer Ther. 2015;14(4):341‐349. 10.1177/1534735415572887 [DOI] [PubMed] [Google Scholar]
- 69. Saeed SA, Cunningham K, Bloch RM. Depression and anxiety disorders: benefits of exercise, yoga, and meditation. AFP. 2019;99(10):620‐627. [PubMed] [Google Scholar]
- 70. Netz Y. Is the comparison between exercise and pharmacologic treatment of depression in the clinical practice guideline of the American college of physicians evidence‐based? Front Pharmacol [Internet]. 2017 [cited 2022 Mar 22];8. https://www.frontiersin.org/article/10.3389/fphar.2017.00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Duarte‐Silva E, Clarke G, Dinan TG, Peixoto CA. Personalized nutrition for depression: impact on the unholy trinity. Nucl Instrum Methods. 2021;28(2):47‐51. 10.1159/000514094 [DOI] [PubMed] [Google Scholar]
- 72. Cancer Incidence by Age [Internet]. Cancer Research UK. 2015. [cited 2022 Mar 3]. https://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/age [Google Scholar]
- 73. Berger S, Saut AM, Berssaneti FT. Using patient feedback to drive quality improvement in hospitals: a qualitative study. BMJ Open. 2020;10(10):e037641. 10.1136/bmjopen-2020-037641 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information 1
Supplementary Information 2
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


