Summary
In some patients, the inflammatory‐immune response to surgical injury progresses to a harmful, dysregulated state. We posit that postoperative systemic inflammatory dysregulation forms part of a pathophysiological response to surgical injury that places patients at increased risk of complications and subsequently prolongs hospital stay. In this narrative review, we have outlined the evolution, measurement and prediction of postoperative systemic inflammatory dysregulation, distinguishing it from a healthy and self‐limiting host response. We reviewed the actions of glucocorticoids and the potential for heterogeneous responses to peri‐operative corticosteroid supplementation. We have then appraised the evidence highlighting the safety of corticosteroid supplementation, and the potential benefits of high/repeated doses to reduce the risks of major complications and death. Finally, we addressed how clinical trials in the future should target patients at higher risk of peri‐operative inflammatory complications, whereby corticosteroid regimes should be tailored to modify not only the a priori risk, but also further adjusted in response to markers of an evolving pathophysiological response.
Keywords: corticosteroids, dysregulation, inflammation, postoperative complications, surgical injury
Introduction
Over 300 million major surgical procedures are performed every year and these are associated with an estimated 8 million peri‐operative deaths [1]. Despite this relatively high mortality rate, the value of surgery in managing symptoms of disease and prolonging life is not in question. However, it highlights the potential harm caused by the stress response to surgery [2]. This stress response is made up of neurohumoral and inflammatory‐immune elements and is largely determined by the magnitude of surgical injury. It is modified by age [3, 4, 5], comorbidities (immune modulation) and anaesthesia [6]. Inflammation is essential to limit exposure to harmful cellular debris and pathogens, and to promote healing. It is fundamentally balanced by pro‐ and anti‐inflammatory processes within the innate and adaptive immune systems [7, 8]. Imbalances in this response, resulting in hyperinflammation and altered immune competence, may increase the risk of postoperative complications and organ dysfunction (so called secondary injury) resulting in increased risks of death or persistent disability [2, 9].
The importance of post‐traumatic hyperinflammation and systemic inflammatory response syndrome (SIRS) has long been recognised [10, 11]; however, the biphasic model in which SIRS is followed by a compensatory anti‐inflammatory response has now changed, recognising that pro‐inflammatory and balancing anti‐inflammatory (immunosuppressive) processes commence simultaneously [12]. This was initially demonstrated in an analysis of genome‐wide immune cell gene expression following major trauma (Fig. 1) [13]. Xiao et al. also proposed that the dramatically altered immune cell gene expression in patients experiencing complications represented a state of immune dysregulation characterised by prolonged hyperinflammation and immunosuppression [13]. Dysregulation of the host response is now integral in the definition of sepsis [14] and trauma [15], highlighting a pathophysiological state of both pro‐inflammatory and immunosuppressive responses rather than hyperinflammation or SIRS alone.
Figure 1.
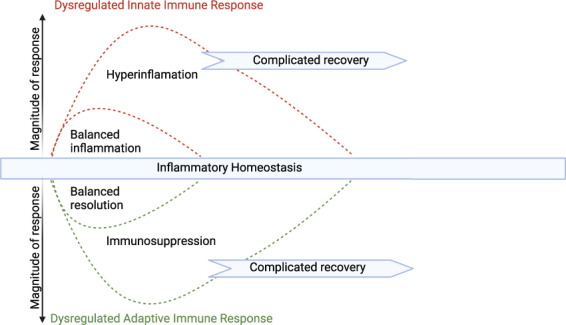
Post‐traumatic dysregulated inflammatory‐immune state. Paradigm shift in the nature of the systemic inflammatory‐immune response to trauma based on analysis of differential gene expression. Initial rapid upregulation of proinflammatory genes involved in the innate immune response is accompanied by simultaneous downregulation of genes involved in adaptive immunity. Dysregulation of the host response is reflected by excessively altered gene expression for a prolonged period associated with complicated outcomes. Adapted with permission from reference [13] Creativecommons license by‐nc‐sa/3.0/.
Surgery is unique in that it creates a predictable surgical injury‐induced inflammatory stimulus, which leads to a measurable immune response that can subsequently contribute to postoperative morbidity and mortality. Over the last 30 years, there have been many attempts to understand the impact of supplemental administration of synthetic glucocorticoids (collectively now referred to as corticosteroids, e.g. methylprednisolone, dexamethasone, hydrocortisone) on potentially harmful postoperative inflammatory processes. Despite numerous clinical trials in the setting of surgery and sepsis, it remains unclear whether there are benefits of corticosteroids in modifying inflammation. In this review, we aim to better define peri‐operative systemic inflammatory dysregulation, assess how it is measured and explore the underlying mechanisms that place patients at higher risk. Furthermore, we will address how the peri‐operative administration of corticosteroids can modify the inflammatory response to major surgery, the likelihood of inflammatory dysregulation and subsequent complications. Finally, the next challenge is to explore why, to date, clinical trials of peri‐operative anti‐inflammatory therapy have not been able to provide definitive answers regarding effectiveness in preventing inflammation‐related complications.
Methods
We conducted an electronic literature search of PubMed and Google Scholar for peer reviewed English language articles published mainly after 2000 to synthesise into an expert narrative review. The search focused on systemic inflammation, immune responses to surgical trauma, clinical outcomes and corticosteroid administration in adults undergoing surgery. There was no pre‐defined strategy, and the scope of the search was broad to explore both translational science and related historical and emerging clinical outcomes research. We did not exclude commonly referenced or highly regarded older, definitive publications. Examples of literature search terms included: major surgery; cardiac surgery; trauma; postoperative; stress response; inflammatory dysregulation; glucocorticoid; corticosteroids; and dexamethasone. A wide range of publications, including basic science, translational science, clinical trials, systematic reviews, narrative reviews, consensus guidelines and the most recently published clinical trials were reviewed. This provided a framework for discussions and numerous meetings between the first and last authors to select the articles that best describe a state of postoperative systemic inflammatory dysregulation, its potential impact on surgical outcomes and relate this to landmark clinical trial evidence for corticosteroid supplementation.
The inflammatory‐immune response to surgical injury
Systemic inflammation following major surgery is initially the result of the highly conserved innate immune response. The magnitude varies widely depending on the surgical environment and is proportional to the degree of the surgical injury [16]. Sensing of cellular injury occurs at a molecular level by pattern recognition receptors within the cells of the innate immune system (e.g. monocytes/macrophages; dendritic cells; neutrophils; natural killer cells; mast cells; and eosinophils). Pattern recognition receptors are a diverse group, consisting of toll‐like receptors (TLR), nucleotide‐binding and oligomerisation domain‐like receptors, C‐type lectin, purinergic receptors and complement receptors that recognise molecules released from damaged and necrotic cells known as damage‐associated molecular patterns (DAMPs) or alarmins. Pattern recognition receptors also recognise highly conserved molecules derived from exposed micro‐organisms (pathogens), known as pathogen‐associated molecular patterns (PAMPs) [7, 17, 18, 19, 20, 21].
Damage‐associated molecular patterns are the key molecular ligands responsible for triggering the inflammatory‐immune response to surgical injury. At the site of injury, DAMPs such as heat shock proteins, S100 proteins, high‐mobility group protein B, nucleic acids, DNA and adenosine triphosphate, bind to pattern recognition receptors to signal the cells of the innate immune system. Pattern recognition receptor activation induces multiple downstream signalling pathways, resulting in activation of transcription factors such as nuclear factor‐κB (NF‐κB), activator protein 1 and interferon regulatory factors [17]. This drives the production and release of proinflammatory cytokines and chemokines (e.g. interleukin (IL)‐6; tumour necrosis factor‐α (TNF‐ɑ); IL‐1β; IL‐8; IL‐12; type 1 interferons); leukotrienes (e.g. leukotriene B4); and DAMPs (e.g. high‐mobility group box protein 1) promoting inflammation. This results in increased production of neutrophils and monocytes and their recruitment to the site of injury. It also leads to increased activation of natural killer cells, release of reactive oxygen species, increased phagocytosis and modified endothelial permeability. Dendritic cells and monocytes/macrophages present antigen to naïve T cells, thereby activating the different T‐cell subsets (e.g. cluster of differentiation (CD)8+ cytotoxic T cells (Tcyto) and CD4+ T helper type 1 (Th1) cells) of the adaptive immune system to further bolster cell‐mediated immunity and cytotoxicity.
The inflammatory‐immune response is balanced, with immune‐suppressing processes commencing at the same time as activation. Principally released from monocytes, IL‐6 is the dominant postoperative inflammatory cytokine in this response. IL‐6 levels strongly correlate with injury severity and postoperative synthesis and secretion of the acute phase reactants C‐reactive protein (CRP) and procalcitonin. However, in combination with IL‐1β and TNF‐ɑ it also directly stimulates the hypothalamic–pituitary–adrenal (HPA) axis, thereby increasing cortisol secretion and influencing glucocorticoid‐mediated immunoregulation. Furthermore, along with glucocorticoids and anti‐inflammatory cytokines (e.g. IL‐4), IL‐6 promotes polarisation of naïve T cells to the immunosuppressive type 2 (Th2) phenotype that produce anti‐inflammatory cytokines (IL‐10, IL‐4, IL‐13) depressing cell‐mediated immunity [22, 23]. IL‐6 also induces the release of prostaglandin E2, a powerful immunosuppressant, from macrophages, further negatively regulating monocyte, macrophage and T‐cell function. Collectively, these actions demonstrate the double‐sided effects of IL‐6, acting both as a pro‐inflammatory cytokine driving the initial host response and simultaneously contributing to immune regulation and suppression.
The ratio of the type 1/type 2 T helper cell response (Th1/Th2) denotes the status of immune balance, and suppression of Th1‐mediated immunity has been linked to increased risk of infectious complications [24, 25, 26, 27]. IL‐10 plays a significant role in regulating the Th1/Th2 balance [28], limiting the level of Th1‐mediated immune activation and hyperinflammation. However, IL‐10 may induce profound immunosuppression by deactivating monocytes and cytotoxic T cells [29, 30, 31]. It also induces TNF‐α secretion and monocyte human leukocyte antigen‐DR isotope expression, thereby impairing antigen presentation. In addition, IL‐10 supports differentiation of naïve T cells into regulatory T cells [23, 32], which further downregulates Th1 responses. Major surgery is associated with the appearance of an immature myeloid cell type known as myeloid‐derived suppressor cells. Catecholamines, prostaglandin E2 and Th2 cytokines induce arginase 1 in myeloid‐derived suppressor cells, resulting in the consumption of arginine. Arginine is an essential protein for T‐cell proliferation and responses and the resultant low levels further exacerbate post‐traumatic immune suppression [33].
Postoperative systemic inflammatory dysregulation
A point of transition exists when postoperative systemic inflammation either begins to resolve or persists and progresses to a state of dysregulation and imbalance. For most patients, the response is self‐limiting. Neutrophils (polymorphonucleocytes) and monocyte‐derived macrophages are key in triggering the process of resolution and clearance. Prostoglandin E2 plays a critical role stimulating a switch in neutrophil lipid mediator biosynthesis away from the synthesis of the strongly chemo‐attractant and pro‐inflammatory leukotriene (e.g. leukotriene B4), towards lipoxin (lipoxin A) synthesis, indicating the beginning of the end of acute inflammation. Lipoxin A production is an initial stop signal; however, other metabolites are generated – known as resolvins, protectins and maresins – that function as specialised pro‐resolving lipid mediators [34]. Collectively these resolving substances downregulate neutrophil recruitment, reprogramme macrophages to a resolving phenotype and promote tissue repair. Resolving macrophages clear apoptotic neutrophils and debris in a process known as efferocytosis [34, 35, 36]. Together with monocytes and neutrophils, resolving macrophages also release cytokine scavengers such as interleukin‐1 receptor antagonists that reduce the activity of pro‐inflammatory cytokines.
In some patients, however, postoperative systemic inflammation does not resolve and persists in a much less regulated, pathophysiological state. We posit that pre‐existing patient and surgical factors combine with intra‐operative events to escalate the response to a pathophysiological inflammatory‐immune state we have termed ‘postoperative systemic inflammatory dysregulation’. This is part of a dysregulated host response to surgical injury, in which progression to SIRS and infectious complications is more likely. Postoperative systemic inflammatory dysregulation is a state of immune imbalance due to DAMP‐driven excessive immune activation and suppression. Similar processes have been extensively described in the setting of severe trauma in which excessive immune responses, reactive oxygen species release and coagulopathy can lead to increasing endotheliopathy and barrier dysfunction facilitating further DAMP production and PAMP release. Rather than being protective, the response becomes a self‐amplifying cycle of tissue injury in which DAMPs and PAMPs combine to further drive uncontrolled, harmful hyperinflammation [20, 37]. Hyperinflammation, however, also triggers significant immunosuppression and collectively the net effect is an imbalanced immune response that increases susceptibility to infection (Fig. 2) [15].
Figure 2.
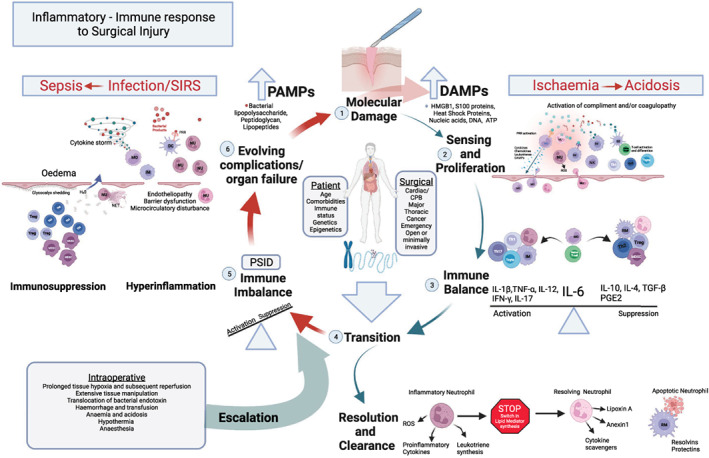
The inflammatory‐immune response to surgical injury. (1) The host response is primarily driven by damage that is apparent at a molecular level; (2) danger‐associated molecular patterns (DAMPs), sensed by cells of the innate immune system, proliferate the response and activate the adaptive immune system further bolstering cell‐mediated immunity; (3) the response is balanced with the actions of interleukin 6 (IL‐6) driving the initial host response and simultaneously contributing to immune regulation and suppression; (4) Transition towards resolution and clearance is marked by a switch within inflammatory neutrophil lipid mediator (LM) biosynthesis pathways away from strongly chemoattractant and proinflammatory leukotrienes to lipoxins (Lipoxin A), proresolving LMs (resolvins and protectins) and resolving proteins (annexin 1). Monocyte‐derived macrophages reprogramme to a resolving macrophage (RM) promoting neutrophil apoptosis and tissue repair; (5) patient and surgical factors combine with intra‐operative events to escalate the response resulting in a pathophysiological state of immune imbalance and postoperative systemic inflammatory dysregulation (PSID); (6) this response may become a cycle of increasing tissue injury, with endotheliopathy, barrier dysfunction, further DAMP production and the release pathogen‐associated molecular patterns (PAMPs). Uncontrolled hyperinflammation simultaneously triggers significant immunosuppression, the net effect being increased risk of organ dysfunction, infection and complications.
CPB, cardiopulmonary bypass; DC, dendritic cells; ROS, reactive oxygen species; HMGB1, high‐mobility group box protein B; IFN‐γ, interferon gamma; IL‐1β, interleukin 1 beta; IL‐6, interleukin 6; IL‐10, interleukin 10; IL‐12, interleukin 12; IM, inflammatory macrophage; MDSC, myeloid‐derived suppressor cells; MO, monocytes; NET, neutrophil extracellular traps; NK, natural killer; NU, neutrophil; PGE2, prostaglandin E2; PRR, pattern recognition receptor; SIRS, systemic inflammatory response syndrome; TGF‐β, transforming growth factor β; TNF‐ɑ, tumour necrosis factor alpha; T reg, regulatory T cell; T cyto, cytotoxic T cell; Th1, T helper type 1; Th2, T helper type 2; Th17, T helper type 17. Adapted with permission from reference [20].
Distinguishing postoperative systemic inflammatory dysregulation from a normal, beneficial inflammatory response is critical to understanding the impact of inflammation on clinical outcomes. Also, this understanding is of key importance for measuring the effects of interventions aimed at modulating or preventing the transition to undesirable inflammation. Recognising postoperative systemic inflammatory dysregulation within the spectrum of normal and pathophysiological immune responses is valuable in distinguishing the different immune effects of surgical injury and other forms of trauma. Of course, within the context of surgery, this will be different in largely variable surgical environments: for example, emergency vs. elective; cardiac vs. non‐cardiac; major vs. minor; and infected vs. ‘non‐infected’. Furthermore, it will likely also be affected by underlying patient factors such as medical and surgical comorbidity, immune and HPA axis function, and genetic and epigenetic factors.
A systemic inflammatory response according to the ‘classic’ SIRS criteria following major surgery remains an important indicator of harmful hyperinflammation during the postoperative period [38], as it is strongly associated with wound infections, pulmonary and cardiac complications, and a 13‐fold increase in mortality [39]. However, postoperative systemic inflammatory dysregulation may be a better reflection of the much more heterogeneous imbalance between hyperinflammation and immunosuppression. A retrospective analysis of ICU patients presenting with sepsis and organ failure found that up to 12.5% of patients were SIRS negative [40]. Importantly, surgical patients were a very significant proportion (39%) of this cohort, suggesting that immunosuppression rather than hyperinflammation alone was underlying the progression to sepsis in the postoperative period. Furthermore, the risk associated with postoperative SIRS appears to be dependent on the surgical environment. For example, following cardiac surgery up to 96% of patients may briefly demonstrate two SIRS criteria with little impact on outcomes [41]. Notwithstanding the existing evidence indicating the significance of SIRS, it is likely that the detection of early postoperative changes within the immune system, occurring at a molecular level well in advance of clinically evident of SIRS and complications, may provide important insights into the hyperinflammatory and immunosuppressive mechanisms contributing to postoperative systemic inflammatory dysregulation.
Measuring and predicting postoperative systemic inflammatory dysregulation
Significant progress in understanding the relationship between excessive or persistent systemic inflammation and surgical outcomes has come from studies on postoperative plasma CRP concentrations. Baseline CRP levels are normally < 10 mg.l−1, and are unaffected by age, sex, diurnal rhythm, diet and organ function. Following surgery, due to increased hepatic synthesis, CRP levels can undergo up to a 1000‐fold rise [42]. Kinetic analyses have demonstrated that CRP concentrations that persisted at a level > 100 mg.l−1 by postoperative day 4 are indicative of increased risk of postoperative infection [43]. Subsequent meta‐analyses confirmed this pattern following major abdominal and colorectal surgery [44, 45, 46], and also suggested that higher CRP levels (>150 mg.l−1) on postoperative days 3–5 appear to reflect a degree of exaggerated inflammation associated with increased risk of complications and worse outcomes [47, 48, 49]. A recent prospective study in 350 patients undergoing major abdominal surgery has provided further insight into the utility of postoperative CRP concentrations for detecting inflammation‐associated complications [50]. The median (IQR) postoperative day‐3 CRP level associated with major infectious complications was 265 (178–324) mg.l−1, while there was an approximately 75% probability of such complications at 400 mg.l−1. Importantly the safe discharge cut off was 105 mg.l−1 with a negative predictive value of 97% and a probability of major infection < 10%.
Collectively, these data indicate the potential value of CRP levels to identify patients likely to have deleterious postoperative systemic inflammation in advance of the development of significant complications. Furthermore, it supports the notion that this pathophysiology forms part of a dysregulated host response to surgery, which in principle is very similar to the dysregulated inflammatory responses to infection in the settings of sepsis [14, 51] and trauma [20, 52], where pathophysiological immune imbalance is contributing to increased risk of a complicated recovery [2].
The peri‐operative period, especially in the setting of clinical trials, has proven to be an ideal environment to utilise powerful emerging investigative methods for exploring novel biomarkers and pathways to detect (and predict) evolving undesirable levels of inflammation. This approach continues to be an important, albeit complex, future research direction to enhance our understanding of the nuances of postoperative systemic inflammatory dysregulation in different surgical environments. Flow cytometric analysis of peripheral blood mononuclear cells demonstrated the TLR/NF‐κB/IL‐6 pathway in monocytes to be significantly upregulated in patients who later develop SIRS following major abdominal surgery. Increased TLR4/5 protein and TLR5 gene (TLR5) expression was predictive of SIRS (AUC 0.89–1) [53]. Single‐cell mass cytometry demarcated a surgical ‘immune signature’ in a specific monocyte subset following orthopaedic surgery that strongly correlated with clinical recovery. Signal transducer and activation of transcription (STAT3), adenosine 3′5′ monophosphate response element binding protein (CREB) and NF‐κB activity as early as 1 h after surgery were strong markers of delayed functional recovery and pain following total hip replacement [54]. This approach also identified patient‐specific pre‐surgical immune states in monocytes that correlate with recovery [55].
Measuring the changing levels of expression of genes (e.g. IL‐10, TNF‐a, RORɣT) has highlighted the role of specific innate and adaptive immune cell subsets in postoperative immunosuppression and complications following major abdominal [56] and cardiac [57] surgery. Genome‐wide profiling (transcriptomic) approaches identified that inflammatory gene expression was dramatically altered following colorectal [58] and cardiac surgery [59]. Epigenetic profiling of peripheral blood mononuclear cell DNA methylation (methylomic analysis), a key regulator of gene expression, detected altered methylation states in genes involved with immunity following major orthopaedic surgery [60]. We recently conducted an integrated methylomic and transcriptomic analysis of postoperative systemic inflammatory dysregulation. This analysis focused on patients demonstrating extremes of the postoperative inflammation phenotype, based on CRP levels on postoperative day 3 (postoperative systemic inflammatory dysregulation: CRP > 250 mg.l−1; low CRP < 75 mg.l−1) after major abdominal surgery [61]. We identified important changes in both DNA methylation and gene expression in patients experiencing postoperative systemic inflammatory dysregulation.
In summary, while much is yet to be discovered, it is increasingly apparent that the impact of surgical injury on the immune system is widespread, affecting cellular function and activity at multiple levels. Changes appear to occur at the level of the epigenome (e.g. DNA methylation), transcriptome (gene expression), cellular differentiation (signalling cell subsets and surface markers) and function (effector cell subsets), in both the innate and adaptive arms of the immune system. These changes are not universal, but specific to an individual's response and to different surgical environments. It is not surprising that it has proven difficult to clearly determine the impact of the peri‐operative use of non‐selective immune modifying drugs such as corticosteroids, on inflammatory dysregulation and surgical outcomes. In the next part of this manuscript, we will therefore review the evidence for the potential interactions between corticosteroids and postoperative systemic inflammatory dysregulation. We will also propose an approach to maximise the opportunity to discover the utility of corticosteroids to prevent or beneficially suppress postoperative systemic inflammatory dysregulation.
Glucocorticoid actions
The inflammatory response is regulated by increased levels of glucocorticoid (cortisol) at the site of injury [62]. Surgical stress, pain and inflammation (IL‐6, TNF‐ɑ and IL‐1) directly stimulate the HPA axis disrupting basal circadian and ultradian patterns of cortisol secretion, resulting in cortisol levels rising up to four‐fold and remaining elevated for up to 7 days [63]. Cortisol is transported to the site of injury and inflammation by cortisol binding globulin, and is enzymatically released by activated neutrophils [62]. Following minor surgery, cortisol levels rise transiently and return to normal within hours. Following major surgery, however, cortisol levels remain elevated [64, 65], while adrenocorticotropic hormone levels, the key HPA axis regulator of adrenal cortisol secretion, initially rise and return to baseline within 24 h. This uncoupling of the HPA axis with persistently increased cortisol levels is a hallmark of evolving critical illness [62, 63, 64, 65, 66].
The genomic and non‐genomic actions of glucocorticoids have been extensively reviewed [67, 68, 69, 70, 71, 72], and the complexity of the response is increasingly appreciated. Glucocorticoids primarily act by binding and activating glucocorticoid receptors. The anti‐inflammatory actions of glucocorticoids result from activated glucocorticoid receptors binding nuclear transcription factors, such as activator protein 1 and NF‐κB, repressing pro‐inflammatory genes and inducing lymphocyte apoptosis [73]. However, activated glucocorticoid receptors are able to act as transcription factors in their own right, interacting directly with DNA (via glucocorticoid response elements) or competing, sequestering and blocking other transcription factors and their cofactors to modulate gene expression in processes known as transactivation and transrepression [74]. The net glucocorticoid effect is, therefore, dependent on the cell type and the level of activity of target genes. In addition, structural variation in glucocorticoid receptors due to altered translation initiation (glucocorticoid receptor isoforms) and post‐translational modification, add another layer for diversity in the cellular response [73, 75, 76]. To further add to this complexity, cell type‐specific differences in chromatin accessibility (access to DNA) mediated by epigenetic factors such as histone modifications further predetermine the actions of the glucocorticoid receptors [77].
There is, therefore, enormous potential for variability in glucocorticoid receptor‐mediated responses to corticosteroid administration during the peri‐operative period. Rather than being strictly immunosuppressive, the effects may depend on the level of systemic inflammation, the driving molecular signals and the status of the HPA axis. This is well summarised by the five ‘Rs’ of glucocorticoid actions: the primary phase of pro‐inflammatory or permissive actions to ‘ready’ and ‘reinforce’ the innate immune system before and during the acute inflammatory response; followed by the secondary phase of anti‐inflammatory actions to ‘repress’ and ‘resolve’ inflammation, which also involve the adaptive immune system; and ultimately the contribution to the ‘restoration’ of homeostasis [69]. These biphasic actions are considered to be dose responsive [78], effectively recognising glucocorticoids and glucocorticoid receptors as key regulators of immune balance. In the absence of inflammation, permissive actions in the homeostatic state sensitise cells by enhancing the expression of pattern recognition receptors, cytokines and complement receptors, thereby maximising the rate of the response to DAMPs/PAMPs. During the state of inflammatory stress, they act to suppress and resolve inflammation. As a result, when there is a relative lack of glucocorticoids, the immune response is slower to develop and resolution more prolonged (Fig. 3) [79, 80]. This simple unified model, proposed by Cain and Cidlowski, provides a framework to consider how supplementary corticosteroid administration, especially with synthetic medicines that act almost exclusively on the glucocorticoid receptors (e.g. dexamethasone and methylprednisolone), in the peri‐operative period may interact with the immune system to optimise the response to surgical injury.
Figure 3.
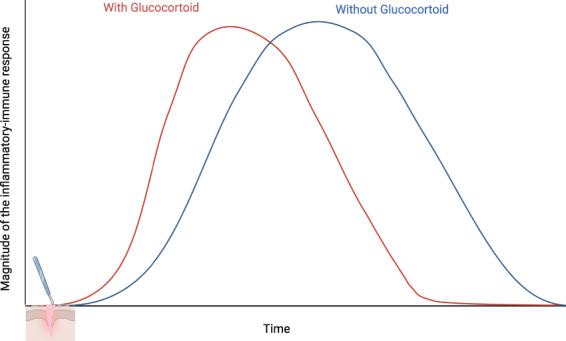
Unified model of glucocorticoid mediation of the immune response. Hypothetical timeline of the immune response to surgery in the presence (red) and absence (blue) of glucocorticoids as proposed by Cain and Cidowoski, based on the biphasic action of glucocorticoids. At low levels, glucocorticoids promote expression of innate immune genes and a rapid initial response to surgical injury. Postoperatively, the stress response and/or corticosteroid supplementation suppresses signalling and inflammation promoting resolution and restoration of homeostasis. When there is an absence or relative lack of glucocorticoid (adrenalectomy or antagonism), the initial response is delayed and the duration of the response is prolonged. Adapted with permission from reference [80].
Postoperative systemic inflammatory dysregulation and peri‐operative corticosteroids
The peri‐operative period is a relatively ‘controlled model’ to further define the utility of prophylactic corticosteroids to modify major surgical inflammatory stress, postoperative systemic inflammatory dysregulation and subsequent outcomes. This is in distinct contrast to the dysregulated systemic inflammation that follows severe sepsis and non‐surgical trauma, where the exposure to infection and injury is not predictable a priori, which may involve multiple regions simultaneously (brains, bones, viscera, soft tissues), and which generally presents with shock and established organ injury before corticosteroids can be administered.
In the peri‐operative period, early administration of low‐dose corticosteroid (primarily for prevention of postoperative nausea and vomiting (e.g. dexamethasone 4–8 mg)) is widespread [81, 82]. However, the value of higher or repeated doses of steroids to prevent excessive systemic inflammation in major surgery is of increasing interest. Typically, one or more doses of either dexamethasone (0.2–1 mg.kg−1) or methylprednisolone (15–30 mg.kg−1) are used for such indications [83, 84].
When it comes to peri‐operative corticosteroid administration for the latter indication, while this makes sense from a mechanistic point of view, it is still largely unknown to whom they should be given and what the optimal administration regimen is. The first question refers to distinguishing those patients who are more likely to develop the phenotype of significant inflammatory dysregulation, who would therefore be most likely to benefit and who, based on safety and efficacy evidence, are at low risk of harm from corticosteroid supplementation. The second question recognises the complexity of assessing the impact of corticosteroids, given the potential heterogeneity of their actions and effects in different surgical populations. Therefore, the challenges to design clinical trials that can more definitively detect the impact of corticosteroids in patients at high risk of postoperative systemic inflammatory dysregulation, major complications and death, are significant.
Targeting patients
Identifying patients who may benefit most from peri‐operative corticosteroid therapy recognises the need to determine both surgical and patient factors that interact to adversely alter systemic inflammation. Surgical factors reflect the likelihood of extensive tissue manipulation and injury, exposure of exogenous ligands (e.g. translocation of bacterial endotoxin) and periods of significant tissue hypoxia/ischaemia and subsequent reperfusion (e.g. vascular compromise) [16, 85]. Different surgical environments (Fig. 2) likely play an important role here as well. Furthermore, intra‐operative factors such as haemorrhage, transfusion, anaemia and hypothermia will also contribute to this risk [86]. In contrast, patient factors are less well defined. Advanced age (>72 y) has been independently associated with reduced postoperative SIRS following cardiac surgery [4], suggesting that younger age groups may be more likely to develop postoperative systemic inflammatory dysregulation. This effect may be due to age‐related impaired immune function, especially involving T cells, known as immunosenescence [87]. However, age‐related changes in endocrine systems and the HPA axis that alter hormonal negative feedback mechanisms, cortisol and glucocorticoid receptor levels, have the potential to modify systemic inflammation, and by extension the utility of supplemental corticosteroids [68, 88]. Chronic comorbid diseases such as diabetes, obesity and cancer also potentially affect the pre‐operative immune status. Increased background levels of pre‐operative inflammation have been extensively investigated in the setting of cancer surgery with both the neutrophil lymphocyte ratio and the modified Glasgow prognostic score (CRP > 10 mg.l−1, albumin < 35 g.l−1) being strongly (p < 0.0001) associated with overall and cancer‐specific survival [89, 90, 91].
Underlying these clinical patient characteristics will be genetic (static) and epigenetic (dynamic) factors that modify inflammation. For example, specific genetic variation within the TLR4 gene has been associated with postoperative adrenocorticotropic hormone and cytokine levels [92]. Also, a meta‐analysis of epigenome‐wide association studies of over 8000 patients was able to identify specific patterns of DNA methylation at genetic loci underlying low‐grade inflammation associated with chronic disease [93]. Given both the phenotypic complexity of postoperative systemic inflammatory dysregulation and the potential heterogeneity of peri‐operative supplemental corticosteroid actions, integrated multi‐omic analyses examining the genome, metabolome, proteome and cellular networks are required to further define predictive variants that are interacting to significantly impact on clinical outcomes [94, 95, 96].
Safety
The evidence supporting the effectiveness of peri‐operative administration of corticosteroids to prevent postoperative nausea and vomiting (PONV) is extensive [97, 98, 99], and the use of dexamethasone 4 mg for the prevention of PONV has become common practice worldwide as a result. Initial safety concerns around the peri‐operative use of steroids for this indication, which were mainly related to hyperglycaemia, diabetes control, infection risk and wound healing [100, 101] have now largely been addressed. A Cochrane systematic review and meta‐analysis of 37 trials (4603 patients) demonstrated no impact on wound healing, infection and only mildly increased blood glucose in the first 12 h postoperatively [102]. The trial by Corcoran et al. (peri‐operative administration of dexamethasone and infection – PADDI) [98], a pragmatic randomised trial that enrolled 8725 patients undergoing non‐cardiac surgery, has provided the most compelling evidence of safety. The non‐inferiority design of this study specifically addressed the effects on surgical site infection risk, with a significant number of randomised patients being diabetic (13%). Dexamethasone 8 mg was clearly non‐inferior for the primary outcome at 30 days (8.1% vs. 9.1%, risk difference (95%CI) adjusted for diabetes −0.9% (−2.1–0.3)), while within the secondary outcomes there was a trend towards a reduced risk of both infection (RR (95%CI) 0.92 (−0.82–1.03)) and sepsis at discharge (RR (95%CI) 0.58 (−0.39–0.87)).
Two meta‐analyses of corticosteroid use in non‐cardiac surgery have demonstrated slight increases in blood glucose levels [103, 104]. In the PADDI trial, dexamethasone was associated with increases in blood glucose levels (median peak rise pre‐operative to postoperative day 2 of 3.6 vs. 2.4 mmol.l−1); however, only a small proportion of non‐diabetic patients required insulin (0.5% vs. 0.1%, respectively). In cardiac surgical trials, larger corticosteroid doses resulted in increased blood glucose levels, requiring significantly more insulin to control it [105, 106]. Nonetheless, no association of increases in blood glucose levels with adverse outcomes has been demonstrated in any of these studies. Recent data support a recommendation to preferentially administer dexamethasone 8 mg in adult patients to maximise potential benefit [99]. Higher intra‐operative dosing is, therefore, now increasingly acceptable and further supported by evidence that dexamethasone is an effective analgesic adjunct [107, 108, 109, 110] that can further facilitate enhanced recovery [110, 111, 112, 113].
Major complications and death
Higher (or multiple) doses of corticosteroids are often administered to modify inflammation‐associated outcomes, such as death or composite major complications. The best evidence for effectiveness of these higher doses is mainly derived from two large trials in cardiac surgery [105, 106] and one in major non‐cardiac surgery [114]. The trials by Dieleman et al. and Whitlock et al. (dexamethasone in cardiac surgery (DECS) and steroids in cardiac surgery (SIRS)), respectively, administered intra‐operative dexamethasone (1 mg.kg−1) and methylprednisolone (total 500 mg) or placebo to nearly 12,000 patients undergoing cardiac surgery with cardiopulmonary bypass [105, 115]. The trial by Asehnoune et al. (peri‐operative administration of corticotherapy on morbidity and mortality after non‐cardiac surgery – PACMAN) administered dexamethasone 0.2 mg.kg−1 or placebo immediately after surgery and repeated the dose on the first postoperative day in 1222 patients having major non‐cardiac surgery [114]. None of these trials were able to demonstrate a statistically significant benefit on their respective primary composite endpoints (Table 1) [114, 115]. However, there was a consistent trend towards reduced mortality and morbidity, with point estimates (OR (95% CI)) for all‐cause mortality at 28–30 days consistently < 1 (0.84 (0.52–1.38) and 0.87 (0.72–1.07)) for PACMAN and DECS/SIRS, respectively [114, 115].
Table 1.
Summary of primary and secondary endpoints of landmark randomised controlled trials on peri‐operative corticosteroid supplementation on major complications and death. Values are OR (95%CI).
| Endpoint | PACMAN [114] | DECS/SIRS [105, 115] |
|---|---|---|
| n = 1222 | n = 11,989 | |
| Death* | 0.81 (0.60–1.08) | 0.87 (0.72–1.07) |
| Respiratory failure** | 0.70 (0.53–0.93) | 0.86 (0.75–0.99) |
| AKI*** | 0.52 (0.30–0.91) | 0.83 (0.67–1.02) |
| Infection**** | 0.82 (0.60–1.11) | 0.80 (0.72–0.89) |
PACMAN: Peri‐operative administration of corticotherapy on morbidity and mortality after non‐cardiac surgery [114]; DECS/SIRS: Meta‐analysis of two randomised trials of the effect of steroids in patients undergoing cardiopulmonary bypass [115].
Primary endpoint: PACMAN, complications or death at 14 days; DECS/SIRS, all cause mortality at 30 days.
Secondary endpoint: PACMAN, need for mechanical ventilation for respiratory failure; DECS/SIRS, uninterrupted mechanical ventilation for > 48 h.
PACMAN, KDIGO ≥ 2; DECS/SIRS, increase in postoperative serum creatinine of at least three times the pre‐operative value or serum creatinine level > 4 mg.dl−1 associated with an acute increase in serum creatinine of at least 0.5 mg.dl−1.
SIRS/PACMAN; as per International Sepsis Consensus definitions; DECS, requirement for antibiotic treatment beyond routine peri‐operative prophylaxis.
Pre‐planned analyses of secondary endpoints and relevant subgroups also consistently demonstrated very similar treatment effects (Table 1) [114, 115], with a reduced risk of respiratory failure, acute kidney injury and infection reaching statistical significance. The “moderate repeated dose” approach in the PACMAN trial resulted in significantly reduced risk of complications or death at 14 days in the non‐thoracic surgical subgroup (OR (95%CI) 0.70 (0.50–0.99)). We believe this may be evidence of potential benefit, as the directions of the point estimates are consistent across multiple studies. The only outcome not consistent with such benefit was an increase in postoperative myocardial injury (defined by peak CK‐MB levels > 6 or > 15 times the upper limit of normal (depending on the type of surgery)) in the patients receiving corticosteroids in both cardiac surgical trials. However, the clinical significance of this unexpected finding remains unclear, as it did not translate into increased mortality or associate with any other adverse outcomes [115].
Furthermore, a more recent study [116] demonstrated that when using the much more sensitive troponin‐I, increases of 218 and 499 times the upper limit of normal, respectively, were required to represent a clinically relevant rise, indicating that the cut‐off that was used for myocardial injury in the SIRS trial may have been much too low. The PACMAN trial demonstrated a trend towards increased complications or all‐cause mortality in the thoracic surgical subgroup (OR (95%CI) 1.46 (0.74–2.85)) indicating that the beneficial effects may not be apparent when the lungs are manipulated directly.
Again, the clinical significance of this finding is not clear or consistent with perceived benefits in transthoracic oesophageal resection or major abdominal surgery. Results of a meta‐analysis of 381 patients enrolled in seven randomised controlled trials of transthoracic oesophagectomy [117] did not find an association with adverse effects from the use of corticosteroids or added risk of postoperative pulmonary complications (OR (95%CI) 0.69 (0.23–1.61)). A meta‐analysis of 439 patients in 11 randomised controlled trials of major abdominal surgery showed a reduced risk of major complications (OR (95%CI) 0.37 (0.21–0.64)), infections (OR (95%CI) 0.35 (0.18–0.67)) and adverse events [118]. The absence of safety issues, especially with respect to wound healing and infection, has resulted in higher or repeated doses now being incorporated into several enhanced recovery programmes targeting reduction in major complications, such as pancreatic fistula formation following pancreaticoduodenectomy [119, 120], and post‐implantation syndrome after endovascular aortic aneurysm repair [121].
High‐dose corticosteroids to enhance recovery following total joint arthroplasty are also now tentatively recommended [122]. However, this is with caution as it is recognised that optimal dose‐finding studies are still required, and a need to specifically identify those patients who are likely to benefit the most (e.g. higher risk of excessive inflammation, infection, severe pain and immobility) [122]. Numerous meta‐analyses have indicated that higher doses, in addition to effects on postoperative nausea and vomiting, are associated with reduced postoperative pain [123, 124] and early fatigue [125], without evidence of significant harm. Safety with respect to the risk of infection in this population has been supported by a recent meta‐analysis, which included 29 studies evaluating single and repeated low‐dose as well as high‐dose regimens [126]. This evidence was limited to low‐risk patients and is consistent with the findings of the PADDI trial [98]. However further data are required in patients at higher risk of infections, such as patients with poorly controlled diabetes, raised BMI and infected revisions.
Future clinical studies
Given the apparent safety of peri‐operative corticosteroids and appreciating the consistent signals of potential benefit, future research designs need to be further refined such that meaningful outcomes can be achieved. For this several key elements, mainly revolving around administration regimens and patient selection, need to be further addressed.
Good quality evidence around optimal dosing of corticosteroids in the peri‐operative setting is still sparse. Doses in most trials have been based on contemporary practice of either a single 4–8 mg dose of dexamethasone for PONV prophylaxis, or much higher doses for inflammatory prophylaxis in major surgery. Although there is some evidence indicating a dose–response effect of intra‐operative dexamethasone (0.05–0.1 mg.kg−1) on quality of recovery and analgesia‐related outcomes [111], the relationships between corticosteroid dosing regimens and inflammation‐related outcomes are not well‐defined. In recent large cardiac surgery trials, the high dosing regimen was pragmatically chosen based on long‐standing practices where dexamethasone doses of up to 1 mg.kg−1 (or methylprednisolone 10–30 mg.kg−1) were routinely used. The dose of methylprednisolone used in the SIRS trial (2 x 250 mg intra‐operatively) was chosen more conservatively based on meta‐analysis evidence suggesting that higher doses might be associated with prolonged postoperative ventilation [127]. Interestingly, more recent data suggest that high doses could in fact have a mode of action that has a different balance of genomic and non‐genomic effects [71] when compared with lower doses, making it harder to directly compare anti‐inflammatory effects between different doses. In the recent PACMAN trial in major non‐cardiac surgery [114], an even more conservative regimen was used, consisting of a postoperative first dose of dexamethasone 0.2 mg.kg−1 (max 20 mg) with a repeat dose on the first postoperative day. The rationale for this was to target the biphasic actions of glucocorticoids. While the first immediate postoperative dose was targeted to reinforce the inflammatory response, the second dose aims to promote restoration of homeostasis [114]. This “moderate repeated dose” approach may prove to be more effective than traditional single large doses and is consistent with current evidence for the treatment of sepsis [128, 129], where lower doses (hydrocortisone ≤ 400 mg.day−1) reduce ICU length of stay and mortality. The absence of major safety outcomes in the PACMAN trial further supports the potential of the peri‐operative “moderate repeated dose” regimen to be incorporated in future study designs. In fact, such a delayed dosing regimen may even be preferable when the decision to administer steroids beyond routine PONV prophylaxis cannot be made solely based on pre‐operative information.
Since the extent of the inflammatory response to surgery is determined by a combination of a priori individual susceptibility and peri‐operative factors, the resulting response phenotype can vary significantly between patients, ranging from very mild in some to a severely dysregulated response (postoperative systemic inflammatory dysregulation) in others. Given the heterogeneity of this phenotype, combined with the lack of adequate tools to predict – in an early phase – how it will develop and who the most appropriate patients for treatment are, the clinical effects of anti‐inflammatory therapy will vary significantly between patients as a result. It is therefore unlikely that clinical studies will demonstrate very strong clinical effects of any immune‐modulating treatment in a largely unselected patient population [130]. Furthermore, within the multidimensional peri‐operative context – where the (single) intervention can be multiple causal steps away from the clinical outcome of interest – the strength of an overall association will be further reduced [131, 132]. With this in mind, the consistent signal of potential benefit of peri‐operative corticosteroids across multiple studies is actually encouraging.
The inter‐individual variability of the peri‐operative inflammatory response phenotype is a major challenge that researchers will have to face in future clinical studies of peri‐operative anti‐inflammatory therapy. An improved understanding of this variability should ultimately lead to better targeting of therapy to only those patients who are at an increased risk of developing postoperative systemic inflammatory dysregulation, ideally by developing prediction models for this phenotype and using these in an adaptive manner in clinical effectiveness studies.
Future clinical studies into peri‐operative anti‐inflammatory therapy will have to increasingly focus on improving the understanding of the ‘high postoperative systemic inflammatory dysregulation – risk phenotype’, rather than on effectiveness alone. Embedding this focus in comparative effectiveness trials can increase our ability to better define pre‐operative risk factors, and to recognise patterns of peri‐operative inflammatory dysregulation at an early stage. However, the process to get to that point is not one of ‘definitive’ large, randomised trials per se. A rather more stepwise approach, such as a series of clinical trials that combine the collection of adequate inflammatory phenotype data with relevant clinical outcomes to inform the intervention and patient selection for the next step, will more likely lead to useful results in the longer term.
Good examples of such approaches in the context of critical care are the trials by Angus et al. (randomised embedded multifactorial adaptive platform for community‐acquired pneumonia – REMAP‐CAP) [133] and the RECOVERY collaborative group [134]. Both are phenotype‐focused adaptive platform trials in the context of biologically heterogeneous syndromes such as sepsis and adult respiratory distress syndrome. These trials are partly embedded in clinical practice in a large number of intensive care units around the world, and the adaptive design allows for assessment of multiple interventions at the same time, with the option to preferentially randomly allocate participants to interventions with the strongest signal of clinical benefit.
A study design similar to these trials in the context of postoperative systemic inflammatory dysregulation would be an attractive option. This would simultaneously and efficiently allow for studying phenotype characteristics, optimal dosing of anti‐inflammatory therapy, as well as clinical effectiveness. Emerging evidence around phenotypic detail (appreciating phenotypes of both inflammation and pharmacodynamics) in such a design can be used to further increase precision to target ‘at risk’ individuals. Also, it will allow for further improvements in the definition of relevant, patient‐centred endpoints [135]. The design and setup of such a research environment would ideally be a collaborative effort, in which previously available evidence from clinical trials such as DECS, SIRS, PADDI, PACMAN and studies by Kehlet et al. should be optimally utilised for the derivation and validation of initial prediction models for postoperative systemic inflammatory dysregulation.
In summary, in the future, clinical trials investigating anti‐inflammatory therapies to modify postoperative systemic inflammatory dysregulation and associated outcomes should specifically target patients at higher peri‐operative risk. Corticosteroid dosing regimens could be tailored to modify not only the a priori risk but supplemented postoperatively in response to early markers of an evolving pathological inflammatory‐immune response. The integration of basic science research and technologies will enhance the clinical findings, improve prediction and detect underlying modifiable factors contributing to postoperative systemic inflammatory dysregulation.
Acknowledgements
No external funding or competing interests declared. Figures were created in BioRender.com. Open access publishing facilitated by Monash University, as part of the Wiley ‐ Monash University agreement via the Council of Australian University Librarians.
References
- 1. Weiser TG, Haynes AB, Molina G, et al. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet 2015; 385: S11. [DOI] [PubMed] [Google Scholar]
- 2. Dobson GP. Trauma of major surgery: a global problem that is not going away. International Journal of Surgery 2020; 81: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hearps AC, Martin GE, Angelovich TA, et al. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 2012; 11: 867–75. [DOI] [PubMed] [Google Scholar]
- 4. Dieleman JM, Peelen LM, Coulson TG, et al. Age and other perioperative risk factors for postoperative systemic inflammatory response syndrome after cardiac surgery. British Journal of Anaesthesia 2017; 119: 637–44. [DOI] [PubMed] [Google Scholar]
- 5. Vester H, Huber‐Lang MS, Kida Q, et al. The immune response after fracture trauma is different in old compared to young patients. Immunity and Ageing 2014; 11: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sanders RD III. Perioperative immunity: is there an anaesthetic hangover? British Journal of Anaesthesia 2014; 112: 210–2. [DOI] [PubMed] [Google Scholar]
- 7. Alazawi W, Pirmadjid N, Lahiri R, Bhattacharya S. Inflammatory and immune responses to surgery and their clinical impact. Annals of Surgery 2016; 264: 73–80. [DOI] [PubMed] [Google Scholar]
- 8. Watanabe S, Alexander M, Misharin AV, Budinger GRS. The role of macrophages in the resolution of inflammation. Journal of Clinical Investigation 2019; 129: 2619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kehlet H. Enhanced postoperative recovery: good from afar, but far from good? Anaesthesia 2020; 75: e54–e61. [DOI] [PubMed] [Google Scholar]
- 10. Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 1992; 101: 1644–55. [DOI] [PubMed] [Google Scholar]
- 11. Bone RC, Grodzin CJ, Balk RA. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest 1997; 112: 235–43. [DOI] [PubMed] [Google Scholar]
- 12. Binkowska AM, Michalak G, Słotwiński R. Current views on the mechanisms of immune responses to trauma and infection. Central‐European Journal of Immunology 2015; 40: 206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. Journal of Experimental Medicine 2011; 208: 2581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis‐3). Journal of the American Medical Association 2016; 315: 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Relja B, Land WG. Damage‐associated molecular patterns in trauma. European Journal of Trauma and Emergency Surgery 2020; 46: 751–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watt DG, Horgan PG, McMillan DC. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: a systematic review. Surgery 2015; 157: 362–80. [DOI] [PubMed] [Google Scholar]
- 17. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010; 140: 805–20. [DOI] [PubMed] [Google Scholar]
- 18. O'Dwyer MJ, Owen HC, Torrance HDT. The perioperative immune response. Current Opinion in Critical Care 2015; 21: 342–2. [DOI] [PubMed] [Google Scholar]
- 19. Lord JM, Midwinter MJ, Chen Y‐F, et al. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet 2014; 384: 1455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huber‐Lang M, Lambris JD, Ward PA. Innate immune responses to trauma. Nature Immunology 2018; 19: 327–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gong T, Liu L, Jiang W, Zhou R. DAMP‐sensing receptors in sterile inflammation and inflammatory diseases. Nature Reviews Immunology 2020; 20: 95–112. [DOI] [PubMed] [Google Scholar]
- 22. Diehl S, Rincón M. The two faces of IL‐6 on Th1/Th2 differentiation. Molecular Immunology 2002; 39: 531–6. [DOI] [PubMed] [Google Scholar]
- 23. Marik PE, Flemmer M. The immune response to surgery and trauma: implications for treatment. Journal of Trauma and Acute Care Surgery 2012; 73: 801–8. [DOI] [PubMed] [Google Scholar]
- 24. O'Sullivan ST, Lederer JA, Horgan AF, Chin DHL, Mannick JA, Rodrick ML. Major injury leads to predominance of the T helper‐2 lymphocyte phenotype and diminished interleukin‐12 production associated with decreased resistance to infection. Annals of Surgery 1995; 222: 482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brune IB, Wilke W, Hensler T, Holzmann B, Siewert J‐R. Downregulation of T helper type 1 immune response and altered pro‐inflammatory and anti‐inflammatory T cell cytokine balance following conventional but not laparoscopic surgery. American Journal of Surgery 1999; 177: 55–60. [DOI] [PubMed] [Google Scholar]
- 26. Decker D, Schöndorf M, Bidlingmaier F, Hirner A, von Ruecker AA. Surgical stress induces a shift in the type‐1/type‐2 T‐helper cell balance, suggesting down‐regulation of cell‐mediated and up‐regulation of antibody‐mediated immunity commensurate to the trauma. Surgery 1996; 119: 316–25. [DOI] [PubMed] [Google Scholar]
- 27. Matsuda A, Furukawa K, Suzuki H, et al. Does impaired Th1/Th2 balance cause postoperative infectious complications in colorectal cancer surgery? Journal of Surgical Research 2007; 139: 15–21. [DOI] [PubMed] [Google Scholar]
- 28. Mingomataj EÇ, Bakiri AH. Regulator versus effector paradigm: interleukin‐10 as indicator of the switching response. Clinical Reviews in Allergy and Immunology 2016; 50: 97–113. [DOI] [PubMed] [Google Scholar]
- 29. Döcke W‐D, Randow F, Syrbe U, et al. Monocyte deactivation in septic patients: restoration by IFN‐γ treatment. Nature Medicine 1997; 3: 678–81. [DOI] [PubMed] [Google Scholar]
- 30. Williams LM, Ricchetti G, Sarma U, Smallie T, Foxwell BMJ. Interleukin‐10 suppression of myeloid cell activation—a continuing puzzle. Immunology 2004; 113: 281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith LK, Boukhaled GM, Condotta SA, et al. Interleukin‐10 directly inhibits CD8+ T cell function by enhancing N‐glycan branching to decrease antigen sensitivity. Immunity 2018; 48: 299–312.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang J, Xia S, Ren H, Shi X. The role and function of CD4+ T cells in hepatic ischemia‐reperfusion injury. Expert Review of Gastroenterology and Hepatology 2022; 16: 5–11. [DOI] [PubMed] [Google Scholar]
- 33. Zhu X, Herrera G, Ochoa JB. Immunosupression and infection after major surgery: a nutritional deficiency. Critical Care Clinics 2010; 26: 491–500. [DOI] [PubMed] [Google Scholar]
- 34. Serhan CN. Pro‐resolving lipid mediators are leads for resolution physiology. Nature 2014; 510: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro‐resolving superfamily of mediators. Journal of Clinical Investigation 2018; 128: 2657–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jones HR, Robb CT, Perretti M, Rossi AG. The role of neutrophils in inflammation resolution. Seminars in Immunology 2016; 28: 137–45. [DOI] [PubMed] [Google Scholar]
- 37. Netea MG, Balkwill F, Chonchol M, et al. A guiding map for inflammation. Nature Immunology 2017; 18: 826–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nunnally ME, Patel A. Sepsis – what's new in 2019? Current Opinion in Anaesthesiology 2019; 32: 163–8. [DOI] [PubMed] [Google Scholar]
- 39. Ferraris VA, Ballert EQ, Mahan A. The relationship between intraoperative blood transfusion and postoperative systemic inflammatory response syndrome. American Journal of Surgery 2013; 205: 457–65. [DOI] [PubMed] [Google Scholar]
- 40. Kaukonen K, Bailey M, Pilcher D, Cooper D, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. New England Journal of Medicine 2015; 372: 1629–38. [DOI] [PubMed] [Google Scholar]
- 41. MacCallum NS, Finney SJ, Gordon SE, Quinlan GJ, Evans TW. Modified criteria for the systemic inflammatory response syndrome improves their utility following cardiac surgery. Chest 2014; 145: 1197–203. [DOI] [PubMed] [Google Scholar]
- 42. Epstein FH, Gabay C, Kushner I. Acute‐phase proteins and other systemic responses to inflammation. New England Journal of Medicine 1999; 340: 448–54. [DOI] [PubMed] [Google Scholar]
- 43. Santonocito C, Loecker ID, Donadello K, et al. C‐reactive protein kinetics after major surgery. Anesthesia and Analgesia 2014; 119: 624–9. [DOI] [PubMed] [Google Scholar]
- 44. Adamina M, Steffen T, Tarantino I, Beutner U, Schmied BM, Warschkow R. Meta‐analysis of the predictive value of C‐reactive protein for infectious complications in abdominal surgery. British Journal of Surgery 2015; 102: 590–8. [DOI] [PubMed] [Google Scholar]
- 45. Singh PP, Zeng ISL, Srinivasa S, Lemanu DP, Connolly AB, Hill AG. Systematic review and meta‐analysis of use of serum C‐reactive protein levels to predict anastomotic leak after colorectal surgery. British Journal of Surgery 2014; 101: 339–46. [DOI] [PubMed] [Google Scholar]
- 46. McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. British Journal of Surgery 2015; 102: 462–79. [DOI] [PubMed] [Google Scholar]
- 47. McSorley ST, Watt DG, Horgan PG, McMillan DC. Postoperative systemic inflammatory response, complication severity, and survival following surgery for colorectal cancer. Annals of Surgical Oncology 2016; 23: 2832–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Watt DG, McSorley ST, Park JH, Horgan PG, McMillan DC. A postoperative systemic inflammation score predicts Short‐ and long‐term outcomes in patients undergoing surgery for colorectal cancer. Annals of Surgical Oncology 2017; 24: 1100–9. [DOI] [PubMed] [Google Scholar]
- 49. McSorley ST, Roxburgh CSD, Horgan PG, McMillan DC. The impact of preoperative dexamethasone on the magnitude of the postoperative systemic inflammatory response and complications following surgery for colorectal cancer. Annals of Surgical Oncology 2017; 24: 2104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Plat VD, Voeten DM, Daams F, van der Peet DL, Straatman J. C‐reactive protein after major abdominal surgery in daily practice. Surgery 2021; 170: 1131–9. [DOI] [PubMed] [Google Scholar]
- 51. Ding R, Meng Y, Ma X. The central role of the inflammatory response in understanding the heterogeneity of sepsis‐3. BioMed Research International 2018; 2018: 5086516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cole E, Gillespie S, Vulliamy P, et al. Multiple organ dysfunction after trauma. British Journal of Surgery 2020; 107: 402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lahiri R, Derwa Y, Bashir Z, et al. Systemic inflammatory response syndrome after major abdominal surgery predicted by early upregulation of TLR4 and TLR5. Annals of Surgery 2016; 263: 1028–37. [DOI] [PubMed] [Google Scholar]
- 54. Gaudillière B, Fragiadakis GK, Bruggner RV, et al. Clinical recovery from surgery correlates with single‐cell immune signatures. Science Translational Medicine 2014; 6: 255ra131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fragiadakis GK, Gaudillière B, Ganio EA, Aghaeepour N, Tingle M, Nolan GP, Angst MS. Patient‐specific immune states before surgery are strong correlates of surgical recovery. Anesthesiology 2015; 123: 1241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fragkou P, Torrance H, Pearse R, et al. Perioperative blood transfusion is associated with a gene transcription profile characteristic of immunosuppression: a prospective cohort study. Critical Care 2014; 18: 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Duggan E, Caraher E, Gately K, et al. Tumor necrosis factor‐alpha and interleukin‐10 gene expression in peripheral blood mononuclear cells after cardiac surgery. Critical Care Medicine 2006; 34: 2134–9. [DOI] [PubMed] [Google Scholar]
- 58. Watt SK, Hasselbalch HC, Skov V, et al. Whole blood gene expression profiling in patients undergoing colon cancer surgery identifies differential expression of genes involved in immune surveillance, inflammation and carcinogenesis. Surgical Oncology 2018; 27: 208–15. [DOI] [PubMed] [Google Scholar]
- 59. Liangos O, Domhan S, Schwager C, et al. Whole blood transcriptomics in cardiac surgery identifies a gene regulatory network connecting ischemia reperfusion with systemic inflammation. PLoS One 2010; 5: e13658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sadahiro R, Knight B, James F, et al. Major surgery induces acute changes in measured DNA methylation associated with immune response pathways. Scientific Reports 2020; 10: 5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bain CR, Myles PS, Taylor R, et al. Methylomic and transcriptomic characterisation of postoperative systemic inflammatory dysregulation. Translational Research 2022; 247: 79–98. [DOI] [PubMed] [Google Scholar]
- 62. Miller T, Gibbison B, Russell GM. Hypothalamic–pituitary–adrenal function during health, major surgery, and critical illness. British Journal of Anaesthesia Education 2017; 17: 16–21. [Google Scholar]
- 63. Prete A, Yan Q, Al‐Tarrah K, et al. The cortisol stress response induced by surgery: a systematic review and meta‐analysis. Clinical Endocrinology 2018; 89: 554–67. [DOI] [PubMed] [Google Scholar]
- 64. Gibbison B, Spiga F, Walker JJ, et al. Dynamic pituitary‐adrenal interactions in response to cardiac surgery. Critical Care Medicine 2015; 43: 800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Peeters B, Langouche L, Van den Berghe G. Adrenocortical stress response during the course of critical illness. Comprehensive Physiology 2017; 8: 283–98. [DOI] [PubMed] [Google Scholar]
- 66. Bornstein SR, Engeland WC, Ehrhart‐Bornstein M, Herman JP. Dissociation of ACTH and glucocorticoids. Trends in Endocrinology and Metabolism 2008; 19: 175–80. [DOI] [PubMed] [Google Scholar]
- 67. Vandevyver S, Dejager L, Libert C. Comprehensive overview of the structure and regulation of the glucocorticoid receptor. Endocrine Reviews 2014; 35: 671–93. [DOI] [PubMed] [Google Scholar]
- 68. Manou‐Stathopoulou V, Korbonits M, Ackland GL. Redefining the perioperative stress response: a narrative review. British Journal of Anaesthesia 2019; 123: 570–83. [DOI] [PubMed] [Google Scholar]
- 69. Busillo JM, Cidlowski JA. The five Rs of glucocorticoid action during inflammation: ready, reinforce, repress, resolve, and restore. Trends in Endocrinology and Metabolism 2013; 24: 109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nature Clinical Practice Rheumatology 2008; 4: 525–33. [DOI] [PubMed] [Google Scholar]
- 71. Timmermans S, Souffriau J, Libert C. A general introduction to glucocorticoid biology. Frontiers in Immunology 2019; 10: 1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Baschant U, Tuckermann J. The role of the glucocorticoid receptor in inflammation and immunity. Journal of Steroid Biochemistry and Molecular Biology 2010; 120: 69–75. [DOI] [PubMed] [Google Scholar]
- 73. Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in health and disease. Trends in Pharmacological Sciences 2013; 34: 518–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gerber AN, Newton R, Sasse SK. Repression of transcription by the glucocorticoid receptor: a parsimonious model for the genomics era. Journal of Biological Chemistry 2021; 296: 100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Vandevyver S, Dejager L, Tuckermann J, Libert C. New insights into the anti‐inflammatory mechanisms of glucocorticoids: an emerging role for glucocorticoid‐receptor‐mediated transactivation. Endocrinology 2013; 154: 1007–7. [DOI] [PubMed] [Google Scholar]
- 76. Quatrini L, Ugolini S. New insights into the cell‐ and tissue‐specificity of glucocorticoid actions. Cellular and molecular immunology 2021; 18: 269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. John S, Sabo PJ, Thurman RE, et al. Chromatin accessibility pre‐determines glucocorticoid receptor binding patterns. Nature Genetics 2011; 43: 264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Munck A, Náray‐Fejes‐Tóth A. The ups and downs of glucocorticoid physiology permissive and suppressive effects revisited. Molecular and Cellular Endocrinology 1992; 90: C1–C4. [DOI] [PubMed] [Google Scholar]
- 79. Wiegers GJ, Reul JMHM. Induction of cytokine receptors by glucocorticoids: functional and pathological significance. Trends in Pharmacological Sciences 1998; 19: 317–21. [DOI] [PubMed] [Google Scholar]
- 80. Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nature Reviews Immunology 2017; 17: 233–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Corcoran TB, Edwards T. A survey of antiemetic dexamethasone administration—frequency of use and perceptions of benefits and risks. Anaesthesia and Intensive Care 2015; 43: 167–74. [DOI] [PubMed] [Google Scholar]
- 82. Golder AM, McSorley ST, Kearns RJ, McMillan DC, Horgan PG, Roxburgh CS. Attitudes towards the use of perioperative steroids in resectional colorectal cancer surgery in the UK: a qualitative study. Annals of Medicine and Surgery 2019; 48: 23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ng KT, Paassen JV, Langan C, et al. The efficacy and safety of prophylactic corticosteroids for the prevention of adverse outcomes in patients undergoing heart surgery using cardiopulmonary bypass: a systematic review and meta‐analysis of randomized controlled trials. European Journal of Cardio‐Thoracic Surgery 2020; 57: 620–7. [DOI] [PubMed] [Google Scholar]
- 84. Dvirnik N, Belley‐Cote EP, Hanif H, et al. Steroids in cardiac surgery: a systematic review and meta‐analysis. British Journal of Anaesthesia 2018; 120: 657–67. [DOI] [PubMed] [Google Scholar]
- 85. Schwartz RS, Eltzschig HK, Carmeliet P. Hypoxia and inflammation. New England Journal of Medicine 2011; 364: 656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. McSorley ST, Tham A, Dolan RD, et al. Perioperative blood transfusion is associated with postoperative systemic inflammatory response and poorer outcomes following surgery for colorectal cancer. Annals of Surgical Oncology 2020; 27: 833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Aiello A, Farzaneh F, Candore G, et al. Immunosenescence and its hallmarks: how to oppose aging strategically? A review of potential options for therapeutic intervention. Frontiers in Immunology 2019; 10: 2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. van den Beld AW, Kaufman J‐M, Zillikens MC, Lamberts SWJ, Egan JM, van der Lely AJ. The physiology of endocrine systems with ageing. Lancet Diabetes and Endocrinology 2018; 6: 647–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncology 2010; 6: 149–63. [DOI] [PubMed] [Google Scholar]
- 90. McMillan DC. The systemic inflammation‐based Glasgow prognostic score: a decade of experience in patients with cancer. Cancer Treatment Reviews 2013; 39: 534–40. [DOI] [PubMed] [Google Scholar]
- 91. Dolan RD, Lim J, McSorley ST, Horgan PG, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: systematic review and meta‐analysis. Scientific Reports 2017; 7: 16717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Koch A, Hamann L, Schott M, et al. Genetic variation of TLR4 influences immunoendocrine stress response: an observational study in cardiac surgical patients. Critical Care 2011; 15: R109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ligthart S, Marzi C, Aslibekyan S, et al. DNA methylation signatures of chronic low‐grade inflammation are associated with complex diseases. Genome Biology 2016; 17: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Stelzer IA, Ghaemi MS, Han X, et al. Integrated trajectories of the maternal metabolome, proteome, and immunome predict labor onset. Science Translational Medicine 2021; 13: eabd9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Culos A, Tsai AS, Stanley N, et al. Integration of mechanistic immunological knowledge into a machine learning pipeline improves predictions. Nature Machine Intelligence 2020; 2: 619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nirvik P, Kertai MD. Future of perioperative precision medicine: integration of molecular science, dynamic health care informatics, and implementation of predictive pathways in real time. Anesthesia and Analgesia 2022; 134: 900–8. [DOI] [PubMed] [Google Scholar]
- 97. Weibel S, Pace NL, Schaefer MS, et al. Drugs for preventing postoperative nausea and vomiting in adults after general anesthesia: an abridged Cochrane network meta‐analysis. Journal of Evidence‐Based Medicine 2021; 14: 188–97. [DOI] [PubMed] [Google Scholar]
- 98. Corcoran TB, Myles PS, Forbes AB, et al. Dexamethasone and surgical‐site infection. New England Journal of Medicine 2021; 384: 1731–41. [DOI] [PubMed] [Google Scholar]
- 99. Myles PS, Corcoran T. Benefits and risks of dexamethasone in noncardiac surgery. Anesthesiology 2021; 135: 895–903. [DOI] [PubMed] [Google Scholar]
- 100. Bartlett R, Hartle AJ. Routine use of dexamethasone for postoperative nausea and vomiting: the case against. Anaesthesia 2013; 68: 892–6. [DOI] [PubMed] [Google Scholar]
- 101. Short TG, Leslie K. Dexamethasone—an effective antiemetic, but is it safe? Anaesthesia and Intensive Care 2015; 43: 155–6. [DOI] [PubMed] [Google Scholar]
- 102. Polderman JAW, Farhang‐Razi V, Dieren S, et al. Adverse side‐effects of dexamethasone in surgical patients – an abridged Cochrane systematic review. Anaesthesia 2019; 74: 929–39. [DOI] [PubMed] [Google Scholar]
- 103. Waldron NH, Jones CA, Gan TJ, Allen TK, Habib AS. Impact of perioperative dexamethasone on postoperative analgesia and side‐effects: systematic review and meta‐analysis. British Journal of Anaesthesia 2013; 110: 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Toner AJ, Ganeshanathan V, Chan MT, Ho KM, Corcoran TB. Safety of perioperative glucocorticoids in elective noncardiac surgery: a systematic review and meta‐analysis. Anesthesiology 2017; 126: 234–48. [DOI] [PubMed] [Google Scholar]
- 105. Dieleman JM, Nierich AP, Rosseel PM, et al. Intraoperative high‐dose dexamethasone for cardiac surgery: a randomized controlled trial. Journal of the American Medical Association 2012; 308: 1761–7. [DOI] [PubMed] [Google Scholar]
- 106. Whitlock RP, Devereaux PJ, Teoh KH, et al. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double‐blind, placebo‐controlled trial. Lancet 2015; 386: 1243–53. [DOI] [PubMed] [Google Scholar]
- 107. Doleman B, Sutton AJ, Sherwin M, Lund JN, Williams JP. Baseline morphine consumption may explain between‐study heterogeneity in meta‐analyses of adjuvant analgesics and improve precision and accuracy of effect estimates. Anesthesia and Analgesia 2018; 126: 648–60. [DOI] [PubMed] [Google Scholar]
- 108. Lex JR, Edwards TC, Packer TW, Jones GG, Ravi B. Perioperative systemic dexamethasone reduces length of stay in total joint arthroplasty: a systematic review and meta‐analysis of randomized controlled trials. Journal of Arthroplasty 2021; 36: 1168–86. [DOI] [PubMed] [Google Scholar]
- 109. Peng C, Li C, Yuan B, Jiao J. The efficacy of dexamethasone on pain management for knee arthroscopy. Medicine 2020; 99: e19417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tolska HK, Hamunen K, Takala A, Kontinen VK. Systematic review on analgesics and dexamethasone for post‐tonsillectomy pain in adults. British Journal of Anaesthesia 2019; 123: e397–e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Oliveira GSD, Ahmad S, Fitzgerald PC, et al. Dose ranging study on the effect of preoperative dexamethasone on postoperative quality of recovery and opioid consumption after ambulatory gynaecological surgery. British Journal of Anaesthesia 2011; 107: 362–71. [DOI] [PubMed] [Google Scholar]
- 112. Surender AP, Khurana G, Sachan PK. Comparison of postoperative quality of recovery and pain relief with preoperative single‐dose dexamethasone and lignocaine after laparoscopic cholecystectomy. Anesthesia, Essays and Researches 2018; 12: 630–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mihara T, Ishii T, Ka K, Goto T. Effects of steroids on quality of recovery and adverse events after general anesthesia: meta‐analysis and trial sequential analysis of randomized clinical trials. PLoS One 2016; 11: e0162961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Asehnoune K, Moal CL, Lebuffe G, et al. Effect of dexamethasone on complications or all cause mortality after major non‐cardiac surgery: multicentre, double blind, randomised controlled trial. British Medical Journal 2021; 373: n1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Whitlock RP, Dieleman JM, Belley‐Cote E, et al. The effect of steroids in patients undergoing cardiopulmonary bypass: an individual patient meta‐analysis of two randomized trials. Journal of Cardiothoracic and Vascular Anesthesia 2020; 34: 99–105. [DOI] [PubMed] [Google Scholar]
- 116. Devereaux PJ, Lamy A, Chan MTV, et al. High‐sensitivity troponin I after cardiac surgery and 30‐day mortality. New England Journal of Medicine 2022; 386: 827–36. [DOI] [PubMed] [Google Scholar]
- 117. Weijs TJ, Dieleman JM, Ruurda JP, Kroese AC, Knape HJTA, van Hillegersberg R. The effect of perioperative administration of glucocorticoids on pulmonary complications after transthoracic oesophagectomy: a systematic review and meta‐analysis. European Journal of Anaesthesiology 2014; 31: 685–94. [DOI] [PubMed] [Google Scholar]
- 118. Srinivasa S, Kahokehr AA, Yu T‐C, Hill AG. Preoperative glucocorticoid use in major abdominal surgery: systematic review and meta‐analysis of randomized trials. Annals of Surgery 2011; 254: 183–91. [DOI] [PubMed] [Google Scholar]
- 119. Laaninen M, Sand J, Nordback I, Vasama K, Laukkarinen J. Perioperative hydrocortisone reduces major complications after pancreaticoduodenectomy. Annals of Surgery 2016; 264: 696–702. [DOI] [PubMed] [Google Scholar]
- 120. Antila A, Siiki A, Sand J, Laukkarinen J. Perioperative hydrocortisone treatment reduces postoperative pancreatic fistula rate after open distal pancreatectomy. A randomized placebo‐controlled trial. Pancreatology 2019; 19: 786–92. [DOI] [PubMed] [Google Scholar]
- 121. de la Motte L, Kehlet H, Vogt K, et al. Preoperative methylprednisolone enhances recovery after endovascular aortic repair: a randomized, double‐blind, placebo‐controlled clinical trial. Annals of Surgery 2014; 260: 540–8; discussion 548–9. [DOI] [PubMed] [Google Scholar]
- 122. Kehlet H, Lindberg‐Larsen V. High‐dose glucocorticoid before hip and knee arthroplasty: to use or not to use—that's the question. Acta Orthopaedica 2018; 89: 477–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Yue C, Wei R, Liu Y. Perioperative systemic steroid for rapid recovery in total knee and hip arthroplasty: a systematic review and meta‐analysis of randomized trials. Journal of Orthopaedic Surgery and Research 2017; 12: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Li D, Wang C, Yang Z, Kang P. Effect of intravenous corticosteroids on pain management and early rehabilitation in patients undergoing total knee or hip arthroplasty: a meta‐analysis of randomized controlled trials. Pain Practice 2018; 18: 487–99. [DOI] [PubMed] [Google Scholar]
- 125. Lunn TH, Kristensen BB, Andersen LØ, Husted H, Otte KS, Gaarn‐Larsen L, Kehlet H. Effect of high‐dose preoperative methylprednisolone on pain and recovery after total knee arthroplasty: a randomized, placebo‐controlled trial. British Journal of Anaesthesia 2011; 106: 230–8. [DOI] [PubMed] [Google Scholar]
- 126. Feeley AA, Feeley TB, Feeley IH, Sheehan E. Postoperative infection risk in total joint arthroplasty after perioperative IV corticosteroid administration: a systematic review and meta‐analysis of comparative studies. Journal of Arthroplasty 2021; 36: 3042–53. [DOI] [PubMed] [Google Scholar]
- 127. Ho KM, Tan JA. Benefits and risks of corticosteroid prophylaxis in adult cardiac surgery. Circulation 2009; 119: 1853–66. [DOI] [PubMed] [Google Scholar]
- 128. Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids for treating sepsis in children and adults. Cochrane Database of Systematic Reviews 2019; 12: CD002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Pastores SM, Annane D, Rochwerg B, et al. Guidelines for the diagnosis and management of critical illness‐related corticosteroid insufficiency (CIRCI) in critically ill patients (part II): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Medicine 2018; 44: 474–7. [DOI] [PubMed] [Google Scholar]
- 130. Marshall JC. Why have clinical trials in sepsis failed? Trends in Molecular Medicine 2014; 20: 195–203. [DOI] [PubMed] [Google Scholar]
- 131. Gaskell A, Sleigh J. The quagmire of postoperative delirium: does dose matter? British Journal of Anaesthesia 2021; 127: 664–6. [DOI] [PubMed] [Google Scholar]
- 132. Wijeysundera DN, Mistry N, Mazer CD. The future of clinical trials methodology: accomplishments and challenges ahead. Anesthesia and Analgesia 2022; 134: 664–7. [DOI] [PubMed] [Google Scholar]
- 133. Angus DC, Berry S, Lewis RJ, et al. The REMAP‐CAP (randomized embedded multifactorial adaptive platform for community‐acquired pneumonia) study. Rationale and design. Annals of the American Thoracic Society 2020; 17: 879–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Recovery Collaborative Group . Dexamethasone in hospitalized patients with Covid‐19. New England Journal of Medicine 2020; 384: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Barnes J, Hunter J, Harris S, et al. Systematic review and consensus definitions for the standardised endpoints in perioperative medicine (StEP) initiative: infection and sepsis. British Journal of Anaesthesia 2019; 122: 500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


