Abstract
Little is known about how diet might influence breast cancer prognosis. The current systematic reviews and meta‐analyses summarise the evidence on postdiagnosis dietary factors and breast cancer outcomes from randomised controlled trials and longitudinal observational studies. PubMed and Embase were searched through 31st October 2021. Random‐effects linear dose‐response meta‐analysis was conducted when at least three studies with sufficient information were available. The quality of the evidence was evaluated by an independent Expert Panel. We identified 108 publications. No meta‐analysis was conducted for dietary patterns, vegetables, wholegrains, fish, meat, and supplements due to few studies, often with insufficient data. Meta‐analysis was only possible for all‐cause mortality with dairy, isoflavone, carbohydrate, dietary fibre, alcohol intake and serum 25‐hydroxyvitamin D (25(OH)D), and for breast cancer‐specific mortality with fruit, dairy, carbohydrate, protein, dietary fat, fibre, alcohol intake and serum 25(OH)D. The results, with few exceptions, were generally null. There was limited‐suggestive evidence that predefined dietary patterns may reduce the risk of all‐cause and other causes of death; that isoflavone intake reduces the risk of all‐cause mortality (relative risk (RR) per 2 mg/day: 0.96, 95% confidence interval (CI): 0.92‐1.02), breast cancer‐specific mortality (RR for high vs low: 0.83, 95% CI: 0.64‐1.07), and recurrence (RR for high vs low: 0.75, 95% CI: 0.61‐0.92); that dietary fibre intake decreases all‐cause mortality (RR per 10 g/day: 0.87, 95% CI: 0.80‐0.94); and that serum 25(OH)D is inversely associated with all‐cause and breast cancer‐specific mortality (RR per 10 nmol/L: 0.93, 95% CI: 0.89‐0.97 and 0.94, 95% CI: 0.90‐0.99, respectively). The remaining associations were graded as limited‐no conclusion.
Keywords: breast cancer survival, diet, evidence grading, food, systematic review
What's new?
To date, there are no evidence‐based nutritional guidelines specifically developed for breast cancer survivors due to a lack of knowledge. In this systematic review and meta‐analysis, the Global Cancer Update Programme evaluated the associations between postdiagnosis dietary patterns, dietary intakes, and supplements use and breast cancer outcomes among breast cancer survivors. The independent expert panel concluded that the evidence about potential associations remains limited (likelihood of causality: suggestive or no conclusion). Stronger evidence, contributed by intervention trials and/or well‐conducted observational studies, is needed before specific dietary recommendations for improving breast cancer prognosis can be made.
Abbreviations
- 25(OH)D
25‐hydroxy‐vitamin D
- ABCPP
After Breast Cancer Pooling Project
- AICR
American Institute for Cancer Research
- BMI
body mass index
- CIs
confidence intervals
- CUP Global
Global Cancer Update Programme
- CUP
Continuous Update Project
- CVD
cardiovascular diseases
- HR
hazard ratio
- RCT
randomised control trials
- RR
relative risk
- WCRF
World Cancer Research Fund
- WHEL
Women's Healthy Eating and Living
- WHI
Women's Health Initiative
- WINS
Women's Intervention Nutrition
1. INTRODUCTION
Breast cancer was the most commonly diagnosed cancer (2.3 million incident cases, 24.5% of all cancers) and the leading cause of cancer death (684 996 deaths, 15.5% of all cancer deaths) in women worldwide in 2020. 1 Despite its high public health burden, the 5‐year relative survival in economically developed countries is approximately 91.2%, in part due to tailored adjuvant treatments, improved surgery and the detection of cases at an earlier stage and detection of more cases. 2 , 3 , 4 With the long survival duration, breast cancer survivors are at risk of disease recurrence, second primary cancer, and other comorbidities, such as cardiovascular diseases (CVD) and diabetes. 5 , 6 , 7
Despite the breadth of knowledge on the relationship between modifiable lifestyle factors and breast cancer incidence, 8 , 9 , 10 little is known about how these factors might influence breast cancer prognosis. A growing body of evidence suggests that being overweight or obese or physically inactive are associated with a lower overall survival after breast cancer diagnosis, 11 , 12 but there remains limited data on the role of diet on breast cancer survival. The Third Expert Report from the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR), 4 which included studies up to 30th June, 2012, suggested that having a healthy body weight, being physically active and following a diet rich in dietary fibre and soy after diagnosis was linked to better overall survival. However, the evidence was graded as limited‐suggestive because of the limitations in the design, the few randomised controlled trials (RCTs) available, and the lack of mechanistic evidence. Recommendations specifically for women living with and beyond breast cancer were not developed. 4
In the previous WCRF/AICR systematic review, 4 breast cancer recurrence was not evaluated as an outcome and the analyses were performed separately by exposure measurement timeframe relative to cancer diagnosis. Since its publication, the number of new studies on dietary factors has almost doubled. Importantly, several of these have focused on postdiagnosis exposures such as wholegrains, dairy products, meat, and serum 25‐hydroxyvitamin D (25(OH)D) which could not be reviewed previously due to the lack of publications.
This work aimed to systematically review and meta‐analyse the accumulated evidence on postdiagnosis diet (foods, food groups, dietary patterns, food components, nutrients, and dietary supplements) and breast cancer outcomes (survival, disease recurrence and secondary primary cancers), and update the findings and the Expert Panel's conclusions of the previously published systematic review and meta‐analysis by WCRF/AICR. 4
This article presents the evidence on dietary factors and supplement use and breast cancer outcomes, whereas evidence on body fatness, physical activity, and the overall summary is presented in the accompanied papers. 13 , 14 , 15
2. METHODS
The present systematic review was conducted as part of the ongoing Global Cancer Update Programme (CUP Global), formally known as WCRF/AICR Continuous Update Project (CUP). 16 The protocol is available online. 17 Details on the complete search strategy, data extraction, outcome definition, statistical analysis, and the PRISMA checklist are available in Supplementary Material (Tables S1 and S2 and Appendix S2).
2.1. Search strategy, selection criteria and data extraction
PubMed and Embase were searched from inception to 31 October 2021. The reference lists of relevant articles were hand searched.
Inclusion criteria were: (1) RCTs with study period of at least 6 months; longitudinal observational studies, or pooled analyses thereof; (2) With at least 100 participants; (3) Investigated postdiagnosis dietary factors (dietary patterns, foods, beverages, macro‐ and micronutrients intakes and supplements) and breast cancer outcomes (all‐cause mortality, breast cancer‐specific mortality, breast cancer recurrence [as defined in studies], any second primary cancers, CVD mortality; Table S2).
Among publications with overlapping samples, the publication with the greater number of outcome events was selected.
Relevant data, including participants' characteristics and results of analyses, were extracted in the CUP Global database. Study selection and data extraction was checked by a second reviewer. Any disagreements were resolved by consensus. The quality of individual studies was not graded using a specific tool. Instead, relevant study characteristics that could be used to explore potential sources of bias were included into the CUP Global database. For all the included studies, information on potential for selection bias, information bias of exposure and outcome assessment, and residual confounding by cancer stage and treatment was retrieved after identifying the most likely influential sources of bias in cancer survival studies 18 , 19 (Appendix S2 and Table S3). Details on how the study authors addressed the potential biases were also included. In the Expert Panel meeting, whether the studies had serious quality issues were discussed when judging the evidence for each exposure‐outcome association.
2.2. Statistical methods for meta‐analysis
Summary relative risks (RRs) and 95% confidence intervals (CIs) were calculated using the random‐effects model by DerSimonian‐Laird. 20 When at least three (additional) studies were identified in the updated search, a linear dose‐response meta‐analysis 21 , 22 was conducted (or updated if reviewed previously in WCRF/AICR Third Expert Report with evidence up to 30 June, 2012 4 ) if the studies reported sufficient information for analysis. For evidence that was judged as limited‐suggestive or above in the previous systematic review or was related to the WCRF/AICR Cancer Prevention Recommendations, the accumulated evidence was summarised in an updated meta‐analysis regardless of the number of studies identified during the CUP Global update.
Multivariable adjusted estimates were used in the meta‐analyses. Between‐study heterogeneity was assessed by the Cochran's Q test and I 2 statistic. 23
The Egger's test and visual inspection of funnel plots were used to assess presence of small study effects when there were 10 or more studies in analyses. 24
Nonlinear dose‐response meta‐analysis was conducted using restricted cubic spline regression with three knots at 10%, 50%, and 90% percentiles of the exposure distribution, which were combined using multivariate meta‐analysis when there were more than five studies with at least three exposure categories. 25 , 26 Likelihood ratio test was used to compare between the linear and nonlinear models. 27
When linear and nonlinear dose‐response meta‐analyses were not possible, we performed a descriptive synthesis, where the findings of the individual studies were systematically gathered, tabulated, and descriptively summarised by type of dietary exposure and outcome analysed. A forest plot for the RR comparing extreme exposure categories was presented to aid results interpretation.
Statistical analyses were conducted using Stata 13.1 (StataCorp, College Station, TX).
2.3. Evidence grading criteria
An independent WCRF/AICR Expert Panel (ELG, MJG, AAJ, EK, VL, SKC, AMT) graded the quality of the evidence for all dietary exposures as strong (subgrades evaluating likelihood of causality: convincing or probable or substantial effect on risk unlikely) or limited (subgrades evaluating likelihood of causality: limited‐suggestive or limited‐no conclusion) according to the predefined criteria listed in Table S4, which cover the quantity, consistency, magnitude and precision of the summary estimates, existence of a dose‐response, risk of bias, study design and limitations, generalisability and mechanistic plausibility of the results.
3. RESULTS
3.1. Screening and study characteristics
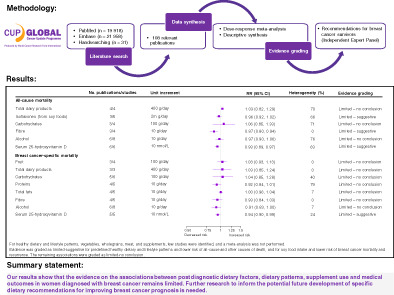
Figure 1 shows the study selection process. One hundred and eight publications (four from RCTs, 104 from observational studies) comprising more than 14 900 all‐cause deaths, 5900 breast cancer deaths and 6000 breast cancer recurrence events among more than 151 000 breast cancer survivors were included.
FIGURE 1.
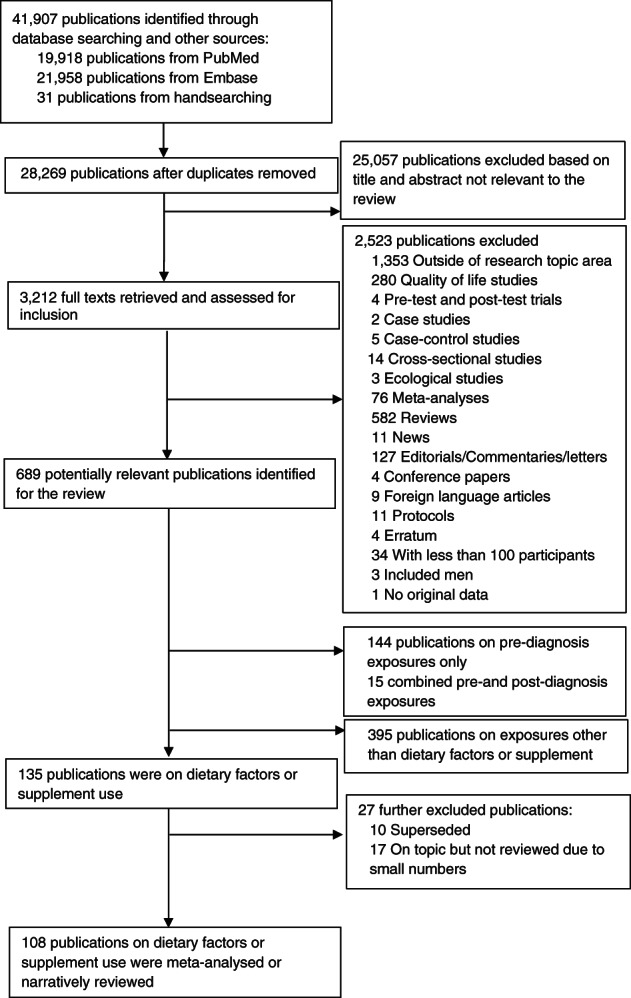
Flowchart of study selection process
Studies reporting results on dietary patterns, fruits and vegetables, wholegrains, meat, fish and eggs, milk and dairy products, soy foods (including isoflavones and soy protein), fibre, alcohol, dietary supplements and 25(OH)D met the review criteria, among which meta‐analysis was only possible for intakes of fruits, dairy products, isoflavones, carbohydrates, proteins, fat, dietary fibre, alcohol, and serum 25(OH)D. Characteristics of the reviewed studies are presented in Tables S5‐S23.
The summary findings and the Expert Panel judgement are shown in Table 1 and explained below for each dietary factor.
TABLE 1.
Evidence grades and main findings from the meta‐analyses and narrative reviews of postdiagnosis dietary patterns, food intake, and supplements use
| 2020 | Diet and survival in women with breast cancer | ||||
|---|---|---|---|---|---|
| Decreases risk | |||||
| Exposure | Outcome | Summary of findings RR (95% CI) | Conclusions | ||
| Strong evidence | Convincing | – | – | – | – |
| Probable | – | – | – | – | |
| Limited evidence | Limited suggestive | Predefined healthy dietary and lifestyle patterns | All‐cause mortality |
Sixteen publications (12 studies; 18 different scores), no meta‐analysis RR ranged from 0.32 to 1.03, and in eight out of 17 patterns showing inverse associations, the 95% CI did not include 1 |
The evidence is substantial, and generally consistent in the direction of an inverse association |
| Other causes of death |
Seven publications (4 studies, 10 patterns), no meta‐analysis RR ranged from 0.44 to 0.95, and in 7 out of 10 patterns the 95% CIs did not include 1 |
||||
| Soy foods | All‐cause mortality |
RR = 0.96 (0.92‐1.02) I 2 = 66%, 5 studies |
The evidence is sparse, but it is suggesting that high isoflavone intake after diagnosis may reduce the risk of all‐cause mortality, breast cancer mortality and recurrence | ||
| Breast cancer‐specific mortality |
Pooled analysis (3 prospective studies), no meta‐analysis RR = 0.83 (0.64‐1.07) Test for heterogeneity not statistically significant |
||||
| Breast cancer recurrence |
Pooled analysis (3 prospective studies), no meta‐analysis RR = 0.75 (0.61‐0.92) Test for heterogeneity not statistically significant |
||||
| Dietary fibre | All‐cause mortality | RR per 10 g/day = 0.87 (0.80‐0.94), I 2 = 0%, 4 studies | The evidence is sparse but is suggestive of inverse association | ||
| Vitamin D status (blood levels) | All‐cause mortality |
RR per 10 nmol/L = 0.93 (0.89‐0.97) I 2 = 62.8%, 6 studies |
The evidence is sparse but is suggestive of inverse association | ||
| Breast cancer‐specific mortality | RR per 10 nmol/L = 0.94 (0.90‐0.99) I 2 = 24%, 5 studies | ||||
| Limited—no conclusion | Low‐fat diet, predefined healthy dietary and lifestyle patterns (for breast cancer‐specific mortality and cardiovascular disease death), data‐driven dietary patterns, high‐fat dietary pattern, alcoholic drinks, fruit and vegetables, cruciferous vegetables, dietary fibre (for breast cancer‐specific mortality and recurrence), wholegrains, red and processes meats, fish, eggs, milk and dairy products, nutrients (fats, carbohydrate, animal protein, plant protein), supplements (multivitamins, antioxidants, vitamins, carotenoids), vitamin D (blood levels on recurrence) | The evidence is sparse and inconsistent | |||
3.2. Postdiagnosis dietary and lifestyle patterns
3.2.1. Randomised controlled trials
Two RCTs (six publications) 28 , 29 , 30 , 31 , 32 , 33 investigated low‐fat dietary pattern (15%‐20% of total energy). Two publications 32 , 33 were superseded by other publications from the same studies. 28 , 31 The results were inconsistent (Table S5). The low‐fat diet intervention did not reduce the all‐cause mortality risk in the Women's Intervention Nutrition Study (WINS) or the Women's Healthy Eating and Living (WHEL) study 31 (trials of breast cancer survivors). Breast cancer recurrence risk was reduced by 24% (RR: 0.76, 95% CI: 0.60‐0.98) in WINS, 28 but not in WHEL. 31
3.2.2. Observational studies
Tables S6 and S7 show the characteristics and main results of the studies identified investigating data driven or predefined dietary and lifestyle patterns. 6 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56
Three studies 51 , 52 , 53 investigated the Prudent (healthy) and Western (unhealthy) data‐driven dietary patterns. The Prudent diet in the Life After Cancer Epidemiology study, and the Western diet in the Nurses' Health Study were negatively and positively associated, respectively, with all‐cause mortality, but not with breast cancer mortality 51 , 52 or recurrence. 52 Neither of these dietary patterns were associated with breast cancer prognosis in the Hong Kong NTEC‐KWC Breast Cancer Survival Study. 53
Twelve studies (16 publications 6 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 ) investigated high vs low categories of 18 predefined healthy dietary and lifestyle patterns (Figure 2). Results were generally consistent in the direction of an inverse association with all‐cause mortality (only the Recommended Foods Score showed a RR > 1) and in eight (Healthy Eating Index‐2005, 35 , 37 , 47 WCRF/AICR Cancer Prevention Recommendations, 41 Dietary Inflammatory Index, 39 , 44 low‐fat, high‐vegetables, fruit, and fibre diet, 42 Chinese Food Pagoda diet‐2007, 43 Dietary Approaches to Stop Hypertension, 43 diabetes risk reduction diet 45 and endogenous acid production diet 48 ) out of 17 patterns showing inverse associations, the 95% CIs did not include 1. There was not a clear pattern for breast cancer‐specific mortality (RRs ranged from 0.12 to 1.54) and breast cancer recurrence (RRs ranged from 0.42 to 1.45). There were some inverse associations for CVD mortality (three patterns 6 , 34 , 41 ; RRs: 0.44 to 0.95, in one 6 of which the 95% CI did not include 1) and other causes of death (10 patterns 34 , 36 , 37 , 40 ; RRs: 0.44 to 0.95, in seven of which the 95% CIs did not include 1). Only two studies 38 , 45 assessed changes in pre‐ to postdiagnosis dietary patterns. In the Women's Health Initiative, a lower risk of mortality from other causes with increased diet quality, but not for all‐cause or breast cancer‐specific mortality was observed. 38 In the Nurses' Health Study, 45 participants who improved their adherence to the diabetes risk reduction diet showed a lower risk of all‐cause and breast‐cancer specific mortality compared to those with consistently low adherence. A lower risk of all‐cause mortality was also reported among those who maintained a higher adherence after diagnosis.
FIGURE 2.
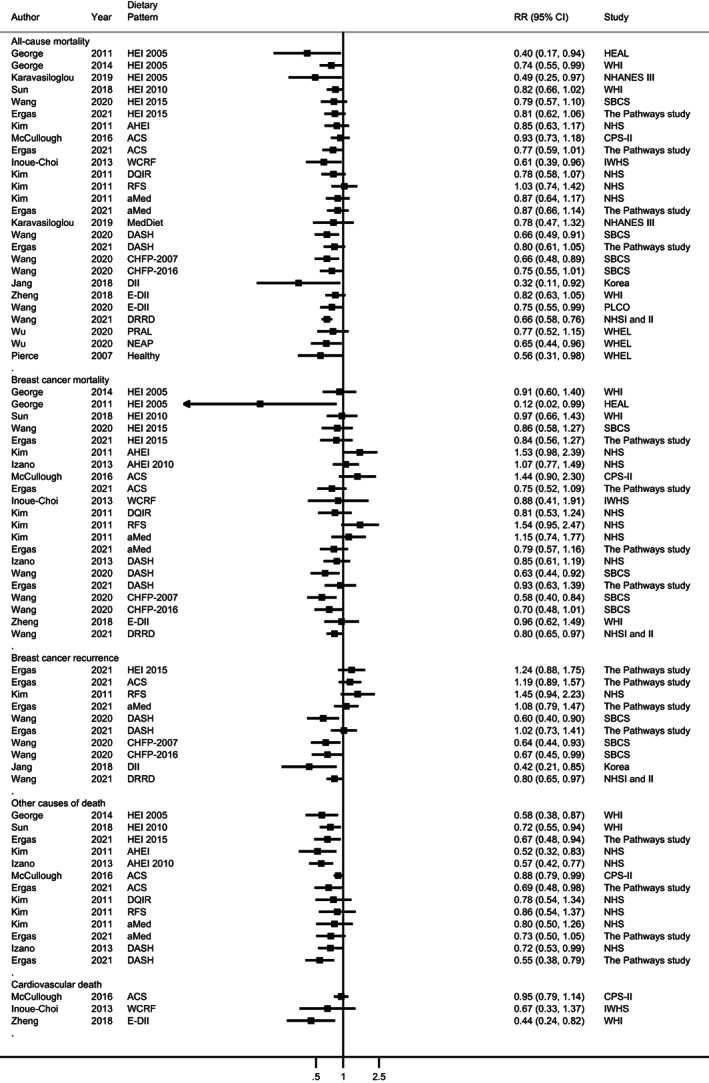
Forest plot of prognostic outcomes for the highest compared to the lowest level of predefined dietary or lifestyle patterns after breast cancer diagnosis. Different patterns are represented in the same forest plot to facilitate the visualisation of the data. Also, the same study may be represented more than once if different dietary patterns were investigated. Each square represents the relative risk (RR) estimate for the highest compared to the lowest level of the predefined dietary or lifestyle pattern and the horizontal line across each square represents the 95% confidence interval (CI) of the RR estimate. The figure should not be interpreted as a quantitative summary. The Pathways study which reported the point estimate per each 10‐point increase of plant‐based dietary index (total, healthy and unhealthy; Anyene 2021) 49 was not included in the figure. Results from Wang 2020 44 and Wu 2020 48 for breast cancer‐specific mortality, and from Wu 2020 56 for recurrence were not included because competing risk regression models were employed. Inoue‐Choi 2013 41 investigated a score of diet plus lifestyle factors; Pierce 2007 42 investigated the combination of fruit and vegetable intake and physical activity. For comparative purposes, data from Jang 2018 39 and Wang 2020 44 (proinflammatory diet), and Wu 2020 48 (dietary acid load) were recalculated to have higher scores as the reference category. ACS, American Cancer Society; AHEI, Alternate Healthy Eating Index; aMed, alternate Mediterranean Diet Score; CHFP, Chinese Food Pagoda; CPS‐II, Cancer Prevention Study II Nutrition Cohort; DASH, Dietary Approaches to Stop Hypertension; DII, Dietary Inflammatory Index; DRRD, Diabetes risk reduction diet; HEAL, Health, Eating, Activity, and Lifestyle Study; E‐DII, energy‐adjusted Dietary Inflammatory Index; HEI, Healthy Eating Index; IWHS, Iowa Women's Health Study; NEAP, Net endogenous acid production; NHANES; National Health and Nutrition Examination Survey; NHS, Nurses' Health Study; MedDiet; Mediterranean Diet; PLCO, Prostate, Lung, Colorectal and Ovarian cancer screening trial; PRAL, Potential renal acid load; RFS, Recommended Food Score; SBCS, Shanghai Breast Cancer Study; WCRF, World Cancer Research Fund; WHEL, Women's Healthy Eating and Living Study; WHI, Women's Health Initiative
Two studies 54 , 55 investigated a high‐fat diet in relation to all‐cause 55 and breast cancer‐specific mortality, 54 showing a higher risk.
3.3. Postdiagnosis fruit, and vegetable intakes
Nine observational studies 31 , 34 , 57 , 58 , 59 , 60 , 61 , 62 , 63 were identified (Table S8). One publication 61 was superseded by another from the same study. 63 Linear dose‐response meta‐analysis was only possible for fruit intake and breast cancer‐specific mortality. No association was observed per each 100 g/day increase (RR: 1.03; 95% CI: 0.93‐1.13, I 2 = 0%, P heterogeneity = .68; three studies 60 , 62 , 63 ; Figure 4A). Studies investigating fruit intake and breast cancer‐specific mortality 60 , 63 , 64 and cardiovascular death 63 did not show an association. There were inconclusive results for all the other exposures (ie, fruit and vegetables combined, vegetables and cruciferous vegetables) and breast cancer outcomes, with the RRs ranging from 0.69 to 1.44 (Figure S1).
FIGURE 4.
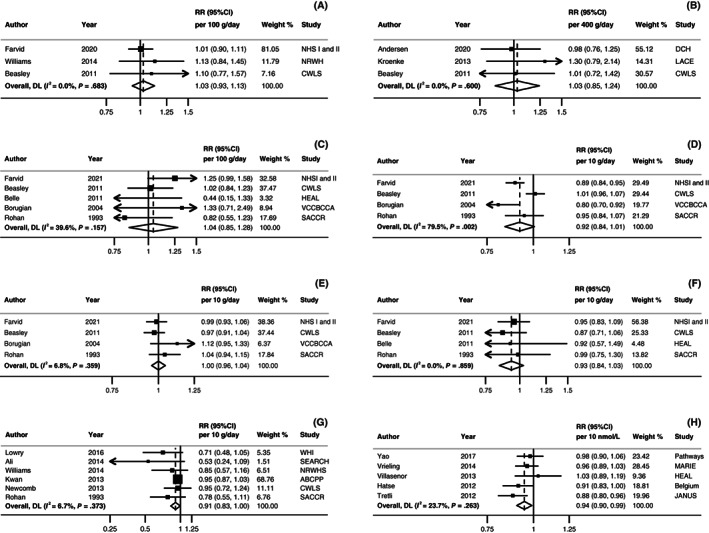
Linear dose‐response meta‐analyses on (A) fruit intake, (B) dairy product intake, (C) carbohydrate intake, (D) protein intake, (E) dietary fat intake, (F) fibre intake, (G) alcohol intake, and (H) serum 25(OH)D and breast cancer‐specific mortality. Forest plots show the linear dose‐response results from the inverse variance DerSimonian‐Laird random‐effects models. Diamonds represent the summary relative risk (RR) estimates. Each square represents the RR estimate of each study and the horizontal line across each square represents the 95% confidence interval (CI) of the RR estimate. The increment units were (A) 100 g/day, (B) 400 g/day, (C) 100 g/day, (D) 10 g/day, (E) 10 g/day, (F) 10 g/day, (G) 10 g/day, and (H) 10 nmol/L. ABCPP, After Breast Cancer Pooling Project; BCFR, Breast Cancer Family Registry; CI, confidence interval; CWLS, Collaborative Women's Longevity Study; DCH, Danish Diet, Cancer, and Health Study; HEAL, Health, Eating, Activity, and Lifestyle Study; MARIE, Mammary carcinoma risk factor Investigation; NHS, Nurses' Health Study; RR, Relative risk; NRWHS, National Runner's and Walker's Health study; SACCR, South Australian Central Cancer Registry; SEARCH, Studies of Epidemiology and Risk Factors in Cancer Heredity Breast Cancer Study; WHI, Women's Health Initiative. [Correction added after first online publication on 29 November, 2022: In Figure 4 legend, there were repeated text and have been removed.]
Only one study investigated individual fruit and vegetable intake and breast cancer outcomes, showing mostly null associations. 63
3.4. Postdiagnosis wholegrains
Three studies 34 , 60 , 65 were identified (Table S9 and Figure S2). The observed associations were generally null. There was not clear pattern for all‐cause (RRs ranged from 0.79 to 1.09) and breast cancer‐specific mortality (RRs ranged from 0.83 to 1.24). No association was observed in the only study analysing recurrence (RR per 200 g/day increase: 0.98, 95% CI: 0.83‐1.13). 65
3.5. Postdiagnosis meat, fish and eggs intake
Six observational studies (seven publications) 34 , 58 , 60 , 61 , 62 , 66 , 67 were identified (Tables S10 and S11 and Figures S3 and S4). One publication 61 was superseded by a new publication of the same study. 66 Only one 34 out of the three 34 , 60 , 66 studies investigating different types of meats and all‐cause mortality reported a 36% (HR for high vs low: 0.64, 95% CI: 0.49‐0.84) lower risk with lower red and processed meat intake. No associations were observed for breast cancer mortality 34 , 58 , 60 , 62 , 66 , 67 or recurrence. 58 , 66
Pre‐ to postdiagnosis changes in different types of meat and fish intake and breast cancer outcomes were assessed in one study, showing mostly null associations. 67
3.6. Postdiagnosis milk and dairy product intake
Four observational studies (five publications) 60 , 61 , 65 , 66 , 68 were included (Table S12). One publication 61 was superseded by another from the same study 66 only for the high vs low forest plots (Figures S5‐S7). The linear dose‐response meta‐analysis showed no association between total dairy product intake and all‐cause mortality (RR per 400 g/day: 1.03, 95% CI: 0.82‐1.29; I 2 = 70%, P heterogeneity = .02; Four publications 60 , 61 , 65 , 68 ; Figure 3A). A pattern of positive associations was observed across the two studies on high‐fat dairy 66 , 68 (RRs: 1.12‐1.64, in one 68 of which the 95% CIs did not include 1) and all‐cause mortality. No associations were observed on low‐fat dairy and all‐cause mortality. 66 , 68 Dairy consumption was not associated with breast cancer‐specific mortality (RR per 400 g/day: 1.03, 95% CI: 0.85‐1.24; I 2 = 0%, P heterogeneity = .6; Three publications 60 , 65 , 68 ; Figure 4B). For high‐fat, risk estimates from both studies 66 , 68 suggested the potential for a higher risk (RRs >1.0), in one 68 of which the 95% CIs did not include the null value (RR for high vs low intake: 1.49, 95% CI: 1.00‐2.24).
FIGURE 3.
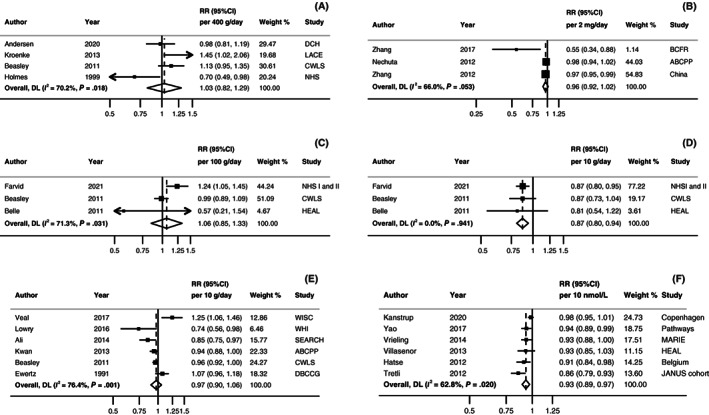
Linear dose‐response meta‐analyses on (A) dairy product intake, (B) isoflavone intake, (C) carbohydrate intake, (D) fibre intake, (E) alcohol intake and (F) serum 25(OH)D and all‐cause mortality. Forest plots show the linear dose‐response results from the inverse variance DerSimonian‐Laird random‐effects models. Diamonds represent the summary relative risk (RR) estimates. Each square represents the RR estimate of each study and the horizontal line across each square represents the 95% confidence interval (CI) of the RR estimate. The increment units were (A) 400g/day, (B) 2 mg/day, (C) 100g/day, (D) 10 g/day, (E) 10 g/day, and (F) 10 nmol/L. ABCPP, After Breast Cancer Pooling Project; BCFR, Breast Cancer Family Registry; CI, confidence interval; CWLS, Collaborative Women's Longevity Study; DBCCG, Danish Breast Cancer Cooperative Group; DCH, Danish Diet, Cancer, and Health Study; HEAL, Health, Eating, Activity, and Lifestyle Study; MARIE, Mammary carcinoma risk factor Investigation; NHS, Nurses' Health Study; RR, Relative risk; SEARCH, Studies of Epidemiology and Risk Factors in Cancer Heredity Breast Cancer Study; WHI, Women's Health Initiative; WISC, Wisconsin In Situ Cohort Study. [Correction added after first online publication on 29 November, 2022: In Figure 3 legend, there were repeated text and have been removed.]
Null associations were reported for breast cancer recurrence. 65 , 66 , 68
3.7. Postdiagnosis soy foods (isoflavones and soy protein) intake
Five observational studies (six publications) 69 , 70 , 71 , 72 , 73 , 74 on isoflavones and proteins from soy foods were reviewed (Table S13). Three publications 70 , 71 , 72 were superseded by a pooled analysis of three prospective studies. 69
Five studies (three publications) 69 , 73 , 74 were included in the dose‐response meta‐analysis showing that a 2 mg/day higher isoflavone intake yielded a 4% lower all‐cause mortality risk but with CIs crossing the null value (RR: 0.96, 95% CI: 0.92‐1.02; I 2 = 66%, P heterogeneity = .05; Figure 3B; isoflavone was assessed on average 69 days to 5 years after diagnosis in the studies). The results from the pooled analysis showed no association between isoflavone intake and breast cancer‐specific mortality (HR for high vs low: 0.83, 95% CI: 0.64‐1.07), whereas a lower cancer recurrence risk was associated with highest intakes (HR for high vs low: 0.75, 95% CI: 0.61‐0.92). 69
Soy protein intake was inversely associated with all‐cause mortality in two 72 , 74 studies, and a reduced risk was also observed for breast cancer‐specific mortality and recurrence combined 72 (Table S13).
3.8. Postdiagnosis carbohydrate, protein and fat intake
Eight observational studies 60 , 61 , 64 , 75 , 76 , 77 , 78 , 79 on carbohydrates, five on protein 60 , 64 , 66 , 76 , 78 and 10 on lipids 42 , 60 , 61 , 64 , 76 , 78 , 80 , 81 , 82 , 83 intake were reviewed (Tables S14‐S16 and Figures S8‐S14). Linear dose‐response meta‐analysis showed no association per each 100 g/day increase in carbohydrate intake and all‐cause mortality (RR: 1.06, 95% CI: 0.85‐1.33; I 2 = 71%, P heterogeneity = .03; three studies 60 , 64 , 75 ; Figure 3C). We did not find evidence of linear (RR per 100 g/day: 1.04, 95% CI: 0.85‐1.28, I 2 = 40%, P heterogeneity = .16; five studies 60 , 64 , 75 , 76 , 78 ; Figure 4C) neither nonlinear association (P nonlinearity = .33; Figure S9) with breast‐cancer specific mortality. One 77 out of the two studies 75 , 77 on total carbohydrate and breast cancer recurrence reported a higher risk (RR for stable/increased vs decreased: 2.00, 95% CI: 1.30‐5.00). One study analysed carbohydrates from different food sources and all‐cause and breast cancer‐specific mortality showing mostly null associations. 79
For total protein, a meta‐analysis was only possible for breast cancer‐specific mortality, showing limited evidence for an association (RR per 10 g/day: 0.92, 95% CI: 0.84‐1.01; I 2 = 79%, P heterogeneity = .002; four studies 60 , 64 , 76 , 78 ; Figure 4D). Higher animal protein intake was associated with lower risk of breast cancer recurrence in one study (RR for high vs low: 0.78, 95% CI: 0.63‐0.95; Figure S12). 66
The results are limited and inconsistent for total and specific types of fats (Table S15 and Figures S13 and S14). Overall, studies reported no association between dietary fat intake, and all‐cause mortality. Only one out 82 of the three 60 , 64 , 81 studies on total fat, and one 60 out of the two 60 , 61 studies on saturated fat and trans fatty acids reported a higher all‐cause mortality risk. Marine fatty acids (eicosapentaenoic acid and docosahexaenoic acid combined or alone) were associated with lower risk of all‐cause mortality in two studies. 61 , 80 For breast cancer‐specific mortality there was no association per each 10 g/day increase in total fat (RR: 1.00, 95% CI: 0.96‐1.04; I 2 = 7%, P heterogeneity = .36; five studies 60 , 64 , 76 , 78 ; Figure 4E). An increased risk was reported in one 78 out of the three 60 , 76 , 78 studies on saturated fat.
3.9. Postdiagnosis dietary fibre intake
Six observational studies (eight publications) 42 , 60 , 61 , 64 , 75 , 76 , 78 , 84 on dietary fibre intake were reviewed (Table S17). One publication 61 was superseded by a new publication 64 from the same study. Three studies 60 , 64 , 75 could be included in the dose‐response meta‐analysis, where a lower all‐cause mortality risk was observed for every 10 g/day increase in fibre (RR: 0.87, 95% CI: 0.80‐0.94; I 2 = 0%, P heterogeneity = .94; Figure 3D). On average, fibre was assessed 2 years after diagnosis. No association was observed in the dose‐response meta‐analysis for fibre and breast cancer‐specific mortality (RR: 0.93, 95% CI: 0.84‐1.03; I 2 = 0%, P heterogeneity = .86; Figure 4F) or for the high vs low comparisons (Figures S15 and S16). Only one study investigated breast cancer recurrence, showing no association. 75
3.10. Postdiagnosis alcohol intake
Twenty‐nine publications from 22 observational studies were reviewed 60 , 61 , 62 , 76 , 78 , 81 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 (Table S18 and Figures S17‐S20). The meta‐analyses showed little evidence for a linear dose‐response association with all‐cause mortality (RR per 10 g/day: 0.97, 95% CI: 0.90‐1.06; I 2 = 76%, P heterogeneity = .001; Figure 3E; 8 studies 60 , 81 , 87 , 89 , 92 , 93 ; alcohol was assessed >1 year to 5 years after diagnosis); the point estimate was inverse with CIs crossing the null value for breast cancer‐specific mortality (RR per 10 g/day: 0.91, 95% CI: 0.83‐1.00; I 2 = 7%, P heterogeneity = .37; Figure 4G; 8 studies 62 , 76 , 89 , 92 , 93 , 94 ; alcohol was assessed on average 4.8 months to 5.8 years after diagnosis). We did not find evidence of nonlinearity for any of the two outcomes (P nonlinearity > .05; Figures S21 and S22).
Alcohol intake after diagnosis was not associated with breast cancer recurrence in any of the three 86 , 91 , 93 studies that conducted high vs low analyses (RR ranged from 0.68 to 1.04; Figure S19). One 105 out of the three 103 , 105 , 107 studies on alcohol intake and second primary cancer showed an increased risk (RR for ≥7 vs 0 drinks/week: 1.90, 95% CI: 1.10‐3.20; Figure S20).
In the only study investigating pre‐ to postdiagnosis changes in alcohol intake and all‐cause and breast cancer‐specific mortality, no associations were observed. 89
3.11. Postdiagnosis dietary supplement use
Fourteen publications 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 were reviewed, showing in general null associations of supplements use with breast cancer outcomes (Tables S19‐S22).
No associations were observed in the two studies on beta‐carotene or lycopene supplement use and breast cancer outcomes. 111 , 113 Frequent use of combination of carotenoid supplements was positively associated with all‐cause and breast cancer‐specific mortality but not recurrence. 111
Evidence regarding other multivitamins and minerals on breast cancer outcomes was reviewed in three studies, 111 , 113 , 115 showing an increased risk of all‐cause mortality with iron supplement use in one study (HR for user vs never user: 1.60, 95% CI: 1.11‐2.31). 113
A significant inverse association was observed for antioxidant use and all‐cause mortality in one study (HR for yes vs no: 0.84, 95% CI: 0.72‐0.99). 112
No association was observed with breast cancer outcomes in the only study investigating pre‐ and postdiagnosis combined use of multivitamin/mineral supplements and antioxidants. 121
Limited studies have analysed the influence of dietary supplements on breast cancer outcomes according to timing of cancer treatment. One publication 120 investigated the association between dietary supplement use before and after chemotherapy and breast cancer prognosis. Results showed that antioxidant supplements, iron, and vitamin B12 use before and during chemotherapy might be associated with an increased risk of all‐cause mortality and recurrence (HRs ranging from 1.41 to 2.04). Multivitamin use was not associated with breast cancer prognosis. In another publication, concurrent use of antioxidants with chemotherapy or radiotherapy was associated with an increased risk of all‐cause mortality (HR: 1.64, 95% CI: 1.01‐2.66) and recurrence (HR: 1.84, 95% CI: 1.26‐2.68). 119
3.12. Postdiagnosis vitamin D from diet and/or supplements, and serum 25‐hydroxyvitamin D (25(OH)D)
No association was observed for vitamin D from diet and supplements for any of the outcomes across the four identified studies. 60 , 61 , 109 , 122 Dietary vitamin D was also not associated with all‐cause mortality (Figure S23) and recurrence. 61 , 122 Vitamin D supplementation was inversely associated with all‐cause mortality in one 117 study out of the four identified 112 , 113 , 117 , 118 (HR for >400 I.U./day vs 1‐400 I.U./day: 0.82, 95% CI: 0.69‐0.99). Vitamin D supplementation was associated with improved disease‐free survival (HR for users vs no users: 0.36, 95% CI: 0.15‐0.88) in another study. 118 A decreased risk of recurrence among women diagnosed with oestrogen receptor‐positive (HR for use vs no use: 0.64, 95% CI: 0.47‐0.87) but not oestrogen receptor‐negative tumours was observed in the After Breast Cancer Pooling Project. 112
Twenty‐three publications 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 on serum 25(OH)D were reviewed (Table S24). Linear dose‐response meta‐analyses were conducted for all‐cause and breast cancer‐specific mortality. Results showed an inverse association between serum 25(OH)D and all‐cause (RR per 10 nmol/L: 0.93, 95% CI: 0.89‐0.97; I 2 = 63% P heterogeneity = .020; 6 studies 125 , 128 , 129 , 130 , 131 , 135 ; 25(OH)D assessed on average 58 days to 36 months after diagnosis; Figure 3F) and breast cancer‐specific mortality (RR per 10 nmol/L: 0.94, 95% CI: 0.90‐0.99; I 2 = 24% P heterogeneity = .26; 5 studies 125 , 128 , 129 , 130 , 131 ; 25(OH)D was assessed on average 69 days to 36 months after diagnosis; Figure 4H). The relationship with all‐cause mortality was somewhat nonlinear (P nonlinearity = .02; Figure S24). The risk of death increased sharply below 50 nmol/L and was null with wide CIs above this. Results remained essentially the same when only studies collecting 25(OH)D before treatment initiation were included (Figures S25‐S27).
Most studies yielded inverse point estimates for breast cancer recurrence, and in seven of them the CIs did not include the null value (Figure S28).
3.13. Evidence grading
Table 1 reports the evidence grading for all dietary factors. Evidence was graded as “limited‐ suggestive” for dietary patterns and lower risk of all‐cause and other causes of death and for soy food intake and lower risk of all‐cause mortality, breast cancer mortality and recurrence. Additionally, there was limited‐suggestive evidence that dietary fibre intake was associated with lower risk of all‐cause mortality and that serum 25(OH)D was associated with a lower risk of all‐cause and breast cancer‐specific mortality.
The evidence on the remaining associations was limited and sparse, thus they were graded as “limited‐no conclusion.”
4. DISCUSSION
The improved survival after breast cancer diagnosis has created an urgent need to understand the relationship between dietary intake, dietary patterns and supplements use and subsequent outcomes. Such insights would direct the development of evidence‐based nutritional guidelines for breast cancer survivors. In the current updated systematic reviews and meta‐analyses, the number of studies for many of the exposures ranged from one to three, often with insufficient information to be included in a dose‐response meta‐analysis. Therefore, dose‐response meta‐analyses were only possible for dairy products, carbohydrates, dietary fibre, alcohol intake and serum 25(OH)D with all‐cause mortality and breast cancer‐specific mortality; for isoflavone intake with all‐cause mortality; and for protein, dietary fat, and fruits with breast cancer‐specific mortality. In general, data on dietary factors and breast cancer outcomes were limited and inconsistent, and no conclusions could be reached. Evidence was judged by the Expert Panel as limited‐suggestive for dietary patterns in association with lower risk of all‐cause and other causes of death, intake of soy in association with lower risk of all‐cause mortality, breast cancer mortality and recurrence, dietary fibre in association with lower risk of all‐cause mortality, and serum 25(OH)D in association with lower risk of all‐cause and breast cancer mortality. The remaining exposures reviewed were judged as limited‐no conclusion.
In our previous WCRF/AICR review, 4 we presented the results separately according to exposure assessment timepoint relative to the cancer diagnosis. For postdiagnosis dietary exposures, dose‐response meta‐analysis was only possible for dietary fibre and alcohol intake (all‐cause and breast cancer‐specific mortality), and for isoflavones (all‐cause mortality), assessed 12 months or more after breast cancer diagnosis. In the present work, all postdiagnosis timepoints were pooled, and subgroup analysis was performed, when possible, by timeframe relative to cancer treatment, which is a more accurate measurement of the relevant periods in the natural history of cancer survivors.
Despite the differences in the synthesis approach, the current analysis confirmed our previous findings on postdiagnosis dietary fibre, alcohol and isoflavone intake and their corresponding conclusions by the independent Expert Panel (ie, limited‐suggestive evidence for a lower risk of outcomes for fibre and isoflavones and limited‐no conclusion for alcohol).
For most of the exposures (ie, low‐fat dietary patterns (RCTs), data‐driven dietary patterns, vegetables, wholegrains, fish, meat, and supplements use), few studies were identified, and their results were not meta‐analysed. We descriptively synthetised these studies and found that associations with breast cancer outcomes were mostly null. These results are in line with other recently published meta‐analyses in breast cancer survivors. 145 , 146 , 147
A considerable amount of research has examined the association between postdiagnosis predefined healthy dietary and lifestyle patterns and breast cancer outcomes. Due to the diversity in the methods and cut‐off points used to derive the patterns, the identified studies were descriptively synthesised instead of being meta‐analysed. Considering the consistency in the direction of an inverse association, the evidence was graded as limited‐suggestive reduced risk of all‐cause and other causes of death. This beneficial association could be partially explained by the individual and synergistic favourable effect of fruits, vegetables, and wholegrains on overall health. 148 , 149 The standardisation of the operationalization of the patterns is crucial to strengthen the evidence in this field.
Despite the small number of studies on isoflavone intake from soy foods, we were able to conduct a dose response meta‐analysis of three publications 69 , 73 , 74 with all‐cause mortality as outcome. Soy isoflavones may have a protective association on breast cancer survival through the modulation of the oestrogen receptor β, which has anticarcinogenic and antiproliferative effects. 150 Besides, isoflavones also exert an antioxidative and anti‐inflammatory function. 151 In our meta‐analysis, there was little evidence to support an association between isoflavone intake from soy foods and all‐cause mortality risk (narrow CI crossing the null value, and with substantial heterogeneity). Three out of the five studies comprised women from Western countries, which may limit the ability to detect an association due to their low soy intake compared to Asian countries. 152 In fact, the two 69 , 74 Chinese studies investigating soy protein intake and all‐cause mortality reported inverse associations. The country‐specific results may at least partly explain the substantial heterogeneity. A recent published categorical (high vs low intakes) meta‐analysis reported a suggestive association between postdiagnosis isoflavone and soy protein intake and overall survival (HR: 0.80, 95% CI: 0.62‐1.04), 153 which is in agreement with our findings. Taken together the consistent direction and magnitude of association for soy foods (including isoflavones and soy protein) intake and all outcomes, the evidence was graded as limited‐suggestive.
We conducted a linear dose‐response meta‐analysis of four observational studies (three publications 60 , 64 , 75 ) on postdiagnosis dietary fibre intake and breast cancer outcomes. Our results demonstrated a 13% lower risk of all‐cause mortality for each 10 g/day increase in fibre intake with no evidence of between‐study heterogeneity. There was no association with breast cancer‐specific mortality. Our findings are in line with a recent categorical meta‐analysis that included three studies 60 , 61 , 75 and showed a 30% lower risk of all‐cause mortality when comparing extreme categories of postdiagnosis fibre intake, but not with breast cancer‐specific mortality. 154 The authors rated the quality of the evidence as moderate for all‐cause mortality and low for breast cancer‐specific mortality based on the NutriGrade scoring system, 155 whereas the evidence of causality using the predefined grading in the present study was graded as limited‐suggestive. Dietary fibre has shown beneficially effects on diabetes, CVD and its associated risk factors, 156 which could partially explain the reduced risk of all‐cause mortality observed in our meta‐analysis. Further studies considering the type of dietary fibre consumed and information on the tumour oestrogen receptor status are needed to thoroughly elucidate the potential association between postdiagnosis fibre intake and breast cancer survival.
Despite alcohol being a risk factor for breast cancer incidence in pre‐ and postmenopausal women, 4 the present analyses did not detect an association between postdiagnosis alcohol intake and all‐cause mortality but the point estimate was inverse with CIs crossing the null value for breast cancer‐specific mortality, as reported in another published meta‐analysis. 92 The included observational studies may be subject to methodological issues as discussed in the limitations paragraph below, and collider‐stratification and heterogeneity disease bias might be present. It has been suggested that alcohol intake could differentially impact breast cancer risk depending on the patient's genotype. 157 Whether this is true for breast cancer prognosis warrants investigation.
The current evidence on dietary supplementation use after breast cancer diagnosis was scarce and did not show any overall benefit on breast cancer outcomes. More detailed investigations are needed, as cancer survivors tend to use dietary supplements to aid with treatment side effects. 158 However, there are still concerns about the use of dietary supplements in patients undergoing certain types of cancer treatment due to the potential compromise of the effectiveness of therapy. 2 Future studies should aim to collect comprehensive information that is currently lacking on the types, dosages and duration of use for the supplements before and after cancer diagnosis, the type of cancer treatments, and account for the dietary sources of the nutrients. Given the limitations of the few studies identified and the inconsistent associations, the evidence on dietary supplements was graded as limited‐no conclusion.
There were limited studies examining the association of postdiagnosis dietary and/or supplemental vitamin D intake and breast cancer outcomes, and the associations observed were generally null. The linear dose‐response inverse association for serum 25(OH)D concentrations and all‐cause and breast cancer‐specific mortality is in agreement with a previous dose‐response meta‐analysis. 159
Experimental evidence suggests a plausible anticancer role of vitamin D mediated by its interaction with the vitamin D receptor. 160 Calcitriol, a metabolite of vitamin D3, is involved in oestrogen receptor signalling pathways that could also have a role in reducing the risk of breast cancer mortality. 159 Factors such as BMI, 161 physical activity, 162 or chemotherapy 163 could modify 25(OH)D levels. However, only three of the included studies in our meta‐analyses adjusted for BMI 125 , 129 , 131 and one for physical activity 129 which could bias the results of the individual studies. When we repeated our meta‐analysis including only studies assessing serum 25(OH)D in participants before or without receiving cancer treatment, results remained essentially the same.
The current systematic reviews and meta‐analyses have limitations that should be considered in the interpretation of the findings. These were also taken into account by the independent Expert Panel when making the decisions on the evidence grading. There are few intervention studies on diet and outcomes in breast cancer survivors and have substantially different follow‐up periods (ranging from 60 months up to 11.5 years), which sometimes might not be large enough before mortality effects are apparent. Moreover, although considered useful in determining causation, intervention studies may be opened to issues such as small sample size, and low adherence to intervention. 164 Most of the data come from observational studies, which are susceptible to several biases, such as reverse causation, survival bias and exposure measurement error. Most of the studies adjusted for breast cancer prognostic factors but had inadequate control for cancer treatment type or completion. In addition, we were unable to conduct subgroup meta‐analyses due to the small number of available studies stratified by cancer treatment, hormone receptor status of the tumour, time frame (before, during and after treatment), socioeconomic status and country, among other factors; and examined small‐study effects, because of the small number of studies, and with insufficient information for analysis.
Observational studies differed in the dietary assessment method used (food frequency questionnaires, 24 h recalls, diet records, medical records, or other instruments), and the average time of assessment (ranging from 90 days up to 6 years postdiagnosis). Moreover, although in general the dietary assessment tools were validated, with few exceptions, most of the studies measured diet after diagnosis only at one point in time. Results from updated diet assessment during the follow‐up, could reduce measurement error due to intraindividual variation. 165 Likewise, only a limited number of studies assessed dietary change from pre‐ to postdiagnosis, 38 , 67 , 77 , 89 and there were no data on dietary changes over time after breast cancer diagnosis which could bias the observed diet‐cancer survival associations.
We also observed variations in the definition of the breast cancer recurrence outcome, which may undermine the quality of the evidence. The clinical trials may use different endpoints as surrogate measures of overall survival. Some studies referred to recurrence as “disease‐free survival,” “progression‐free survival,” “additional breast cancer events” and other studies included different events or combination of events under the term recurrence. Despite these heterogenous definitions, all were reviewed under the general term of recurrence, as the number of studies was small to allow subgroup analyses.
Breast cancer survivors involved in research studies are healthy enough to participate. These women are likely more health‐conscious and may come from a higher socioeconomic background compared to nonparticipants. Therefore, selection bias, when not accounted for, may have an unpredictable impact on study results.
Despite these limitations, the present updated systematic reviews and meta‐analyses are the most comprehensive scientific investigation of postdiagnosis dietary factors and breast cancer outcomes. Each diet‐related exposure was evaluated for all‐cause mortality, breast cancer‐specific mortality and breast cancer recurrence and the evidence was examined and judged by the independent Expert Panel following the standardised evidence grading criteria, as part of the work for the on‐going CUP Global that aims to systematically collect and synthesise the evidence for making lifestyle recommendations and research recommendations. 16
5. CONCLUSIONS
In conclusion, the current assessment of the evidence indicates that the associations between postdiagnostic dietary factors, dietary patterns, and supplement use and breast cancer outcomes in women with breast cancer remains inconclusive, and further research is needed before specific dietary recommendations for improving breast cancer prognosis can be made. Breast cancer survivors are still advised to follow the guidelines developed for the public on cancer prevention once their treatment is completed, 166 which is in line with the general recommendations to cancer survivors recently released by the American Cancer Society. 167 In some specific situations survivors may be advised otherwise by their health care professional. 166 More large, well‐designed RCTs of dietary interventions and observational studies with long follow‐up and repeated measures of dietary exposures and confounders, in diverse populations, and studies exploring the underlying biological mechanisms may strengthen the evidence for specific dietary recommendations for breast cancer survivors.
AUTHOR CONTRIBUTIONS
Konstantinos K. Tsilidis and Doris S. M. Chan are coprincipal investigator of CUP Global at Imperial College London. Konstantinos K. Tsilidis was part of the Expert Panel but was not involved with judging the evidence after becoming a coprincipal investigator of CUP Global. Teresa Norat and Doris S. M. Chan wrote the protocol based on the advice from the Protocol Expertise Group and implemented the study with Konstantinos K. Tsilidis. Doris S. M. Chan and Neesha Nanu did the literature search. Leila Abar, Katia Balducci, Margarita Cariolou, Neesha Nanu, Rita Vieira and Nerea Becerra‐Tomas did the study selections and data extraction. Leila Abar, Katia Balducci, Margarita Cariolou and Nerea Becerra‐Tomas checked, analysed, and interpreted the data. Dagfinn Aune and Georgios Markozannes were CUP Global team members who revised the manuscript. Darren C. Greenwood was statistical adviser. Anne McTiernan, Steven K. Clinton, Edward L. Giovannucci, Ellen Kampman, Alan A. Jackson, Konstantinos K. Tsilidis, Marc J. Gunter, and Vivien Lund (lay member) were the Expert Panel members who provided judgements on the evidence and advised on the interpretation of the review. Elio Riboli and Amanda J. Cross were Expert Panel observers. Kate Allen, Nigel T. Brockton, Helen Croker, Daphne Katsikioti, Deirdre McGinley‐Gieser, Panagiota Mitrou, and Martin Wiseman were the CUP Global Secretariat members who provided overall coordination for the work and convened and facilitated discussions with the Expert Panel. Katia Balducci and Nerea Becerra‐Tomás drafted the original manuscript. All authors reviewed and provided comments on the manuscript. Doris S. M. Chan is the guarantor and has full access to all the data and takes responsibility for the integrity of the data and the accuracy of the data analysis. The work reported in the study has been performed by the authors, unless clearly specified in the text.
FUNDING INFORMATION
This work was funded by the World Cancer Research Fund network of charities (American Institute for Cancer Research (AICR); World Cancer Research Fund (WCRF); Wereld Kanker Onderzoek Fonds (WKOF)) (CUP GLOBAL Special Grant 2018). Konstantinos K. Tsilidis, Doris S. M. Chan, Rita Vieira, Dagfinn Aune, Katia Balducci, Margarita Cariolou, Georgios Markozannes, and Nerea Becerra‐Tomás are supported by the World Cancer Research Fund network of charities. Leila Abar and Neesha Nanu were previously supported by the World Cancer Research Fund network of charities. Teresa Norat was supported by the World Cancer Research Fund network of charities as principal investigator of the WCRF/AICR Continuous Update Project (CUP) and by WCRF International as the CUP Global scientific advisor. Dr McTiernan was supported by grants from the Breast Cancer Research Foundation (BCRF‐19‐107/BCRF‐20‐107/BCRF‐21‐107). The funders of our study had no role in the decisions about the design and conduct of the study; collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript. The process used was based on the method developed by WCRF International's Methodology Task Force for the WCRF/AICR Second Expert Report. The CUP Global Secretariat, led by WCRF International, provided overall coordination for the work and convened and facilitated discussions with a WCRF/AICR Expert Panel who provided judgements on the evidence. The views expressed in this review are the opinions of the authors. They may differ from those in future updates of the evidence related to food, nutrition, physical activity, and cancer incidence and survival.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
Supporting information
Data S1. Supporting information
ACKNOWLEDGEMENTS
We thank Teresa Norat for leading the WCRF/AICR Continuous Update Project (CUP) as principal investigator from 2007 to 2020. We thank the Protocol Expertise Group: Annie Anderson (University of Dundee), Steven Clinton (The Ohio State University), Ellen Copson (Southampton University), Wendy Demark‐Wahnefried (UAB Comprehensive Cancer Center, Birminham, AL), John Mathers (Newcastle University), Anne McTiernan (Fred Hutchinson Cancer Research Center), Andrew Renehan (University of Manchester), Lesley Turner (patient representative), Franzel van Duijnhoven (Wageningen University), and Galina Velikova (University of Leeds), for their expert opinion on the review protocol. We thank the CUP Global team members: Sonia Chemlal, Jakub Sobiecki, Britta Talumaa and Victoria White, for their contribution to the literature search and data extraction; and database managers: Rui Vieira, Christophe Stevens, Yusuf O. Anifowoshe, and Lam Teng for implementing and updating the CUP Global database. We also acknowledge the input of Isobel Bandurek and Susannah Brown as past CUP Global Secretariat members.
Becerra‐Tomás N, Balducci K, Abar L, et al. Postdiagnosis dietary factors, supplement use and breast cancer prognosis: Global Cancer Update Programme (CUP Global) systematic literature review and meta‐analysis. Int J Cancer. 2023;152(4):616‐634. doi: 10.1002/ijc.34321
Nerea Becerra‐Tomás and Katia Balducci contributed equally to this work.
Funding information American Institute for Cancer Research, Grant/Award Number: CUPGLOBAL‐SpecialGrant‐2018; Breast Cancer Research Foundation, Grant/Award Number: BCRF‐19‐107/BCRF‐20‐107/BCRF‐21‐107; Wereld Kanker Onderzoek Fonds, Grant/Award Number: CUPGLOBAL‐SpecialGrant‐2018; World Cancer Research Fund, Grant/Award Number: CUPGLOBAL‐SpecialGrant‐2018
DATA AVAILABILITY STATEMENT
Only publicly available data were used in our study. Data sources and handling of these data are described in the Materials and Methods section. Further details are available from the corresponding author upon request.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. [DOI] [PubMed] [Google Scholar]
- 2. de Cicco P, Catani MV, Gasperi V, Sibilano M, Quaglietta M, Savini I. Nutrition and breast cancer: a literature review on prevention, treatment and recurrence. Nutrients. 2019;11:1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harbeck NP‐L, Penault‐Llorca F, Cortes J, et al. Breast cancer. Nat Rev Dis Primers. 2019;5:66. [DOI] [PubMed] [Google Scholar]
- 4. World Cancer Research Fund International/American Institute for Cancer Research . Diet, nutrition, physical activity and cancer: a global perspective. Continuous Update Project Report . 2018. http://dietandcancerreport.org. Accessed May 18, 2021.
- 5. Ng HSV, Vitry A, Koczwara B, Roder D, McBride ML. Patterns of comorbidities in women with breast cancer: a Canadian population‐based study. Cancer Causes Control. 2019;30:931‐941. [DOI] [PubMed] [Google Scholar]
- 6. Zheng JT, Tabung FK, Zhang J, et al. Association between post‐cancer diagnosis dietary inflammatory potential and mortality among invasive breast cancer survivors in the Women's Health Initiative. Cancer Epidemiol Biomark Prev. 2018;27:454‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rock CLD‐W, Demark‐Wahnefried W. Can lifestyle modification increase survival in women diagnosed with breast cancer? J Nutr. 2002;132:3504S‐3509S. [DOI] [PubMed] [Google Scholar]
- 8. Papadimitriou NM, Markozannes G, Kanellopoulou A, et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat Commun. 2021;12:4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rezende LFMS, Sá TH, Markozannes G, et al. Physical activity and cancer: an umbrella review of the literature including 22 major anatomical sites and 770 000 cancer cases. Br J Sports Med. 2018;52:826‐833. [DOI] [PubMed] [Google Scholar]
- 10. Kyrgiou MK, Kalliala I, Markozannes I, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chan DSMV, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer‐systematic literature review and meta‐analysis of 82 follow‐up studies. Ann Oncol. 2014;25:1901‐1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mctiernan A, Friedenreich CM, Katzmarzyk PT, et al. Physical activity in cancer prevention and survival: a systematic review. Med Sci Sports Exerc. 2019;51:1252‐1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan DSM, Vieira R, Abar L, et al. Post‐diagnosis body fatness, weight change and breast cancer prognosis: Global Cancer Update Programme (CUP Global) systematic literature review and meta‐analysis. Int J Cancer. 2023;152(4):572‐599. 10.1002/ijc.34322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsilidis KK, Cariolou M, Becerra‐Tomás N, et al. Post‐diagnosis body fatness, recreational physical activity, dietary factors and breast cancer prognosis: Global Cancer Update Programme (CUP Global) summary of evidence grading. Int J Cancer. 2023;152(4):635‐644. 10.1002/ijc.34320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cariolou M, Abar L, Aune D, et al. Post‐diagnosis recreational physical activity and breast cancer prognosis: Global Cancer Update Programme (CUP Global) systematic literature review and meta‐analysis. Int J Cancer. 2023;152(4):600‐615. 10.1002/ijc.34324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Global Cancer Update Programme (CUP Global), 2022 . https://www.wcrf.org/diet‐activity‐and‐cancer/global‐cancer‐update‐programme/about‐the‐global‐cancer‐update‐programme/. Accessed September 15, 2022.
- 17. Imperial College London CUP Global Team . Cancer Update Programme on diet and cancer: protocol for the data collection and systematic literature reviews on the role of diet, nutrition and physical activity on outcomes after diagnosis of breast cancer. Version 3. 2019. https://www.imperial.ac.uk/school-public-health/epidemiology-and-biostatistics/research/cancer-and-nutritional-epidemiology/global-cancer-update-programme/. Accessed July 2022.
- 18. Chubak JB, Boudreau DM, Wirtz HS, McKnight B, Weiss NS. Threats to validity of nonrandomized studies of postdiagnosis exposures on cancer recurrence and survival. J Natl Cancer Inst. 2013;105:1456‐1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Savitz DAW, Wellenius GA, Trikalinos TA. The problem with mechanistic risk of bias assessments in evidence synthesis of observational studies and a practical alternative: assessing the impact of specific sources of potential bias. Am J Epidemiol. 2019;188:1581‐1585. [DOI] [PubMed] [Google Scholar]
- 20. DerSimonian RL, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177‐188. [DOI] [PubMed] [Google Scholar]
- 21. Orsini NB, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose‐response data. Stata J. 2006;6:40‐57. [Google Scholar]
- 22. Bekkering GEH, Harris RJ, Thomas S, et al. How much of the data published in observational studies of the association between diet and prostate or bladder cancer is usable for meta‐analysis? Am J Epidemiol. 2008;167:1017‐1026. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539‐1558. [DOI] [PubMed] [Google Scholar]
- 24. Egger MDS, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Orsini NL, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta‐analysis for linear and nonlinear dose‐response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jackson DW, White IR, Thompson SG. Extending DerSimonian and Laird's methodology to perform multivariate random effects meta‐analyses. Stat Med. 2010;29:1282‐1297. [DOI] [PubMed] [Google Scholar]
- 27. Royston P. A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Stat Med. 2000;19:1831‐1847. [DOI] [PubMed] [Google Scholar]
- 28. Chlebowski RTB, Thomson GL, Nixon CA, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition study. J Natl Cancer Inst. 2006;98:1767‐1776. [DOI] [PubMed] [Google Scholar]
- 29. Pierce JPN, Caan L, Flatt BJ, et al. Dietary change and reduced breast cancer events among women without hot flashes after treatment of early‐stage breast cancer: subgroup analysis of the Women's Healthy Eating and Living study. Am J Clin Nutr. 2009;89:1565s‐1571s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gold EBP, Natarajan JP, Stefanick L, et al. Dietary pattern influences breast cancer prognosis in women without hot flashes: the Women's Healthy Eating and Living trial. J Clin Oncol. 2009;27:352‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pierce JPN, Caan L, Parker BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women's Healthy Eating and Living (WHEL) randomized trial. Jama. 2007;298:289‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reddy G, Tripathy D. Dietary fat reduction improves relapse‐free survival in postmenopausal women previously treated for early‐stage breast cancer: results from a phase III Women's Intervention Nutrition study. Clin Breast Cancer. 2005;6:112‐114. [Google Scholar]
- 33. Rock C, Natarajan L, Pu M, et al. Longitudinal biological exposure to carotenoids is associated with breast cancer‐free survival in the Women's Healthy Eating and Living study. Cancer Epidemiol Biomark Prev. 2009;18:486‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCullough MLG, Gapstur SM, Shah R, et al. Pre‐ and postdiagnostic diet in relation to mortality among breast cancer survivors in the CPS‐II nutrition cohort. Cancer Causes Control. 2016;27:1303‐1314. [DOI] [PubMed] [Google Scholar]
- 35. George SMI, Smith ML, Neuhouser AW, et al. Postdiagnosis diet quality, the combination of diet quality and recreational physical activity, and prognosis after early‐stage breast cancer. Cancer Causes Control. 2011;22:589‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Izano MAF, Fung TT, Chiuve SS, Hu FB, Holmes MD. Are diet quality scores after breast cancer diagnosis associated with improved breast cancer survival? Nutr Cancer. 2013;65:820‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. George SMB‐B, Shikany R, Caan JM, et al. Better postdiagnosis diet quality is associated with reduced risk of death among postmenopausal women with invasive breast cancer in the Women's Health Initiative. Cancer Epidemiol Biomark Prev. 2014;23:575‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun YB, Liu W, Caan B, et al. Changes in overall diet quality in relation to survival in postmenopausal women with breast cancer: results from the Women's Health Initiative. J Acad Nutr Diet. 2018;118:1855‐63.e6. [DOI] [PubMed] [Google Scholar]
- 39. Jang HC, Chung M, Kang S, Park Y. Association between the dietary inflammatory index and risk for cancer recurrence and mortality among patients with breast cancer. Nutrients. 2018;10:1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim EHW, Willett W, Fung T, Rosner B, Holmes M. Diet quality indices and postmenopausal breast cancer survival. Nutr Cancer. 2011;63:381‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Inoue‐Choi MRK, Lazovich D. Adherence to the WCRF/AICR guidelines for cancer prevention is associated with lower mortality among older female cancer survivors. Cancer Epidemiol Biomarkers Prev. 2013;22:792‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pierce JPS, Flatt ML, Natarajan SW, et al. Greater survival after breast cancer in physically active women with high vegetable‐fruit intake regardless of obesity. J Clin Oncol. 2007;25:2345‐2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang F, Cai H, Gu K, et al. Adherence to dietary recommendations among long‐term breast cancer survivors and cancer outcome associations. Cancer Epidemiol Biomarkers Prev. 2020;29:386‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang K, Sun JZ, Wu QX, et al. Long‐term anti‐inflammatory diet in relation to improved breast cancer prognosis: a prospective cohort study. NPJ Breast Cancer. 2020;6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang T, Farvid MS, Kang JH, et al. Diabetes risk reduction diet and survival after breast cancer diagnosis. Cancer Res. 2021;81:4155‐4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ergas IJ, Cespedes Feliciano EM, Bradshaw PT, et al. Diet quality and breast cancer recurrence and survival: the pathways study. JNCI Cancer Spectr. 2021;5:pkab019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Karavasiloglou N, Pestoni G, Faeh D, Rohrmann S. Post‐diagnostic diet quality and mortality in females with self‐reported history of breast or gynecological cancers: results from the third National Health and Nutrition Examination Survey (NHANES III). Nutrients. 2019;11:2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu T, Hsu FC, Pierce JP. Increased acid‐producing diet and past smoking intensity are associated with worse prognoses among breast cancer survivors: a prospective cohort study. J Clin Med. 2020;9:1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Anyene IC, Ergas IJ, Kwan ML, et al. Plant‐based dietary patterns and breast cancer recurrence and survival in the pathways study. Nutrients. 2021;13:3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Marinac CRN, Breen SH, Hartman CI, et al. Prolonged nightly fasting and breast cancer prognosis. JAMA Oncol. 2016;2:1049‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kroenke CHF, Fung TT, Hu FB, Holmes MD. Dietary patterns and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:9295‐9303. [DOI] [PubMed] [Google Scholar]
- 52. Kwan MLW, Weltzien E, Kushi LH, Castillo A, Slattery ML, Caan BJ. Dietary patterns and breast cancer recurrence and survival among women with early‐stage breast cancer. J Clin Oncol. 2009;27:919‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lei Y, Ho SC, Kwok C, et al. Dietary pattern at 18‐month post‐diagnosis and outcomes of breast cancer among Chinese women with early‐stage breast cancer. Cancer Manag Res. 2021;13:4553‐4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baghestani AR, Shahmirzalou P, Zayeri F, Akbari ME, Hadizadeh M. Prognostic factors for survival in patients with breast cancer referred to cancer research center in Iran. Asian Pac J Cancer Prev. 2015;16:5081‐5084. [DOI] [PubMed] [Google Scholar]
- 55. Mohseny M, Shekarriz‐Foumani R, Amiri P, et al. Assessment of the fitness of cox and parametric regression models of survival distribution for Iranian breast cancer patients' data. J Adv Pharm Technol Res. 2019;10:39‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wu T, Hsu FC, Wang S, Luong D, Pierce JP. Hemoglobin A1c levels modify associations between dietary acid load and breast cancer recurrence. Nutrients. 2020;12:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nechuta SC, Chen BJ, Kwan WY, et al. Postdiagnosis cruciferous vegetable consumption and breast cancer outcomes: a report from the after breast cancer pooling project. Cancer Epidemiol Biomarkers Prev. 2013;22:1451‐1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hebert JRH, Hurley TG, Ma Y. The effect of dietary exposures on recurrence and mortality in early stage breast cancer. Breast Cancer Res Treat. 1998;51:17‐28. [DOI] [PubMed] [Google Scholar]
- 59. Thomson CAR, Rock CL, Thompson PA, et al. Vegetable intake is associated with reduced breast cancer recurrence in tamoxifen users: a secondary analysis from the Women's Healthy Eating and Living study. Breast Cancer Res Treat. 2011;125:519‐527. [DOI] [PubMed] [Google Scholar]
- 60. Beasley JMN, Trentham‐Dietz PA, Hampton A, et al. Post‐diagnosis dietary factors and survival after invasive breast cancer. Breast Cancer Res Treat. 2011;128:229‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Holmes MDS, Stampfer MJ, Colditz GA, Rosner B, Hunter DJ, Willett WC. Dietary factors and the survival of women with breast carcinoma. Cancer. 1999;86:826‐835. [DOI] [PubMed] [Google Scholar]
- 62. Williams PT. Significantly greater reduction in breast cancer mortality from post‐diagnosis running than walking. Int J Cancer. 2014;135:1195‐1202. [DOI] [PubMed] [Google Scholar]
- 63. Farvid MS, Holmes MD, Chen WY, et al. Postdiagnostic fruit and vegetable consumption and breast cancer survival: prospective analyses in the nurses' health studies. Cancer Res. 2020;80:5134‐5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Farvid MS, Tamimi RM, Poole EM, et al. Postdiagnostic dietary glycemic index, glycemic load, dietary insulin index, and insulin load and breast cancer survival. Cancer Epidemiol Biomarkers Prev. 2021;30:335‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Andersen JLM, Hansen L, Thomsen BLR, Christiansen LR, Dragsted LO, Olsen A. Pre‐ and post‐diagnostic intake of whole grain and dairy products and breast cancer prognosis: the Danish Diet, Cancer and Health cohort. Breast Cancer Res Treat. 2020;179:743‐753. [DOI] [PubMed] [Google Scholar]
- 66. Holmes MDW, Wang J, Hankinson SE, Tamimi RM, Chen WY. Protein intake and breast cancer survival in the nurses' health study. J Clin Oncol. 2017;35:325‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Parada H Jr, Steck SE, Bradshaw PT, et al. Barbecued, and smoked meat intake and survival following breast cancer. J Natl Cancer Inst. 2017;109:djw299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kroenke CHK, Kwan ML, Sweeney C, Castillo A, Caan BJ. High‐ and low‐fat dairy intake, recurrence, and mortality after breast cancer diagnosis. J Natl Cancer Inst. 2013;105:616‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nechuta SJC, Chen BJ, Lu WY, et al. Soy food intake after diagnosis of breast cancer and survival: an in‐depth analysis of combined evidence from cohort studies of US and Chinese women. Am J Clin Nutr. 2012;96:123‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Caan BJN, Parker L, Gold B, et al. Soy food consumption and breast cancer prognosis. Cancer Epidemiol Biomarkers Prev. 2011;20:854‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Guha NK, Kwan ML, Quesenberry CP Jr, Weltzien EK, Castillo AL, Caan BJ. Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: the life after cancer epidemiology study. Breast Cancer Res Treat. 2009;118:395‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shu XO, Zheng Y, Cai Y, et al. Soy food intake and breast cancer survival. Jama. 2009;302:2437‐2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang FFH, Terry DE, Knight MB, et al. Dietary isoflavone intake and all‐cause mortality in breast cancer survivors: the breast cancer family registry. Cancer. 2017;123:2070‐2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang YFK, Kang HB, Li BL, Zhang RM. Positive effects of soy isoflavone food on survival of breast cancer patients in China. Asian Pac J Cancer Prev. 2012;13:479‐482. [DOI] [PubMed] [Google Scholar]
- 75. Belle FNK, McTiernan E, Bernstein A, et al. Dietary fiber, carbohydrates, glycemic index, and glycemic load in relation to breast cancer prognosis in the HEAL cohort. Cancer Epidemiol Biomarkers Prev. 2011;20:890‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rohan TEH, Hiller JE, McMichael AJ. Dietary factors and survival from breast cancer. Nutr Cancer. 1993;20:167‐177. [DOI] [PubMed] [Google Scholar]
- 77. Emond JAP, Natarajan JP, Gapuz L, et al. Risk of breast cancer recurrence associated with carbohydrate intake and tissue expression of IGFI receptor. Cancer Epidemiol Biomarkers Prev. 2014;23:1273‐1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Borugian MJS, Kim‐Sing SB, Van Patten C, et al. Insulin, macronutrient intake, and physical activity: are potential indicators of insulin resistance associated with mortality from breast cancer? Cancer Epidemiol Biomark Prev. 2004;13:1163‐1172. [PubMed] [Google Scholar]
- 79. Farvid MS, Barnett JB, Spence ND, Rosner BA, Holmes MD. Types of carbohydrate intake and breast cancer survival. Eur J Nutr. 2021;60:4565‐4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Patterson REF, Newman SW, Natarajan VA, et al. Marine fatty acid intake is associated with breast cancer prognosis. J Nutr. 2011;141:201‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ewertz MG, Gillanders S, Meyer L, Zedeler K. Survival of breast cancer patients in relation to factors which affect the risk of developing breast cancer. Int J Cancer. 1991;49:526‐530. [DOI] [PubMed] [Google Scholar]
- 82. Nomura AMM, le Marchand L, Kolonel LN, Hankin JH. The effect of dietary fat on breast cancer survival among Caucasian and japanese women in Hawaii. Breast Cancer Res Treat. 1991;18(Suppl 1):S135‐S141. [DOI] [PubMed] [Google Scholar]
- 83. Newman SC, Miller AB, Howe GR. A study of the effect of weight and dietary fat on breast cancer survival time. Am J Epidemiol. 1986;123:767‐774. [DOI] [PubMed] [Google Scholar]
- 84. Holmes MDC, Chen WY, Hankinson SE, Willett WC. Physical activity's impact on the association of fat and fiber intake with survival after breast cancer. Am J Epidemiol. 2009;170:1250‐1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nakamura KO, Ukawa E, Hirata S, et al. Characteristics and prognosis of Japanese female breast cancer patients: the BioBank Japan project. J Epidemiol. 2017;27:S58‐s64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wu XY, Barcenas Y, Chow CH, et al. Personalized prognostic prediction models for breast cancer recurrence and survival incorporating multidimensional data. J Natl Cancer Inst. 2017;109:djw314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Veal CTH, Lakoski V, Hampton SG, et al. Health‐related behaviors and mortality outcomes in women diagnosed with ductal carcinoma in situ. J Cancer Surviv Res Pract. 2017;11:320‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nechuta SC, Cai WY, Poole H, et al. A pooled analysis of post‐diagnosis lifestyle factors in association with late estrogen‐receptor‐positive breast cancer prognosis. Int J Cancer. 2016;138:2088‐2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lowry SJK, Kapphahn K, Chlebowski R, Li CI. Alcohol use and breast cancer survival among participants in the Women's Health Initiative. Cancer Epidemiol Biomarkers Prev. 2016;25:1268‐1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Larsen SBK, Kroman N, Ibfelt EH, Christensen J, Tjønneland A, Dalton SO. Influence of metabolic indicators, smoking, alcohol and socioeconomic position on mortality after breast cancer. Acta Oncol. 2015;54:780‐788. [DOI] [PubMed] [Google Scholar]
- 91. Simonsson MM, Markkula A, Bendahl PO, Rose C, Ingvar C, Jernström H. Pre‐ and postoperative alcohol consumption in breast cancer patients: impact on early events. SpringerPlus. 2014;3:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ali AMS, Bolla MK, Wang MK, et al. Alcohol consumption and survival after a breast cancer diagnosis: a literature‐based meta‐analysis and collaborative analysis of data for 29,239 cases. Cancer Epidemiol Biomarkers Prev. 2014;23:934‐945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kwan MLC, Flatt WY, Weltzien SW, et al. Postdiagnosis alcohol consumption and breast cancer prognosis in the after breast cancer pooling project. Cancer Epidemiol Biomarkers Prev. 2013;22:32‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Newcomb PAK, Trentham‐Dietz E, Egan A, et al. Alcohol consumption before and after breast cancer diagnosis: associations with survival from breast cancer, cardiovascular disease, and other causes. J Clin Oncol. 2013;31:1939‐1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Allin KHN, Nordestgaard BG, Flyger H, Bojesen SE. Elevated pre‐treatment levels of plasma C‐reactive protein are associated with poor prognosis after breast cancer: a cohort study. Breast Cancer Res. 2011;13:R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kwan MLK, Kushi LH, Weltzien E, et al. Alcohol consumption and breast cancer recurrence and survival among women with early‐stage breast cancer: the life after cancer epidemiology study. J Clin Oncol. 2010;28:4410‐4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Flatt SWT, Gold CA, Natarajan EB, et al. Low to moderate alcohol intake is not associated with increased mortality after breast cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:681‐688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Barnett GCS, Shah M, Redman K, Easton DF, Ponder BAJ, Pharoah PDP. Risk factors for the incidence of breast cancer: do they affect survival from the disease? J Clin Oncol. 2008;26:3310‐3316. [DOI] [PubMed] [Google Scholar]
- 99. Brewster AMD, Thompson KA, Hahn PA, et al. Relationship between epidemiologic risk factors and breast cancer recurrence. J Clin Oncol. 2007;25:4438‐4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tominaga KA, Andow J, Koyama Y, et al. Family environment, hobbies and habits as psychosocial predictors of survival for surgically treated patients with breast cancer. Jpn J Clin Oncol. 1998;28:36‐41. [DOI] [PubMed] [Google Scholar]
- 101. Schmidt G, Schneider C, Gerlinger C, et al. Impact of body mass index, smoking habit, alcohol consumption, physical activity and parity on disease course of women with triple‐negative breast cancer. Arch Gynecol Obstet. 2020;301:603‐609. [DOI] [PubMed] [Google Scholar]
- 102. Furrer D, Jacob S, Michaud A, Provencher L, Lemieux J, Diorio C. Association of tobacco use, alcohol consumption and HER2 polymorphisms with response to trastuzumab in HER2‐positive breast cancer patients. Clin Breast Cancer J Transl Name Clin Breast Cancer. 2018;18:E687‐E694. [DOI] [PubMed] [Google Scholar]
- 103. Knight JA, Fan J, Malone KE, et al. Alcohol consumption and cigarette smoking in combination: a predictor of contralateral breast cancer risk in the WECARE study. Int J Cancer. 2017;141:916‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Knight JA, Bernstein L, Largent J, et al. Alcohol intake and cigarette smoking and risk of a contralateral breast cancer. Am J Epidemiol. 2009;169:962‐968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Li CI, Daling JR, Porter PL, Tang MT, Malone KE. Relationship between potentially modifiable lifestyle factors and risk of second primary contralateral breast cancer among women diagnosed with estrogen receptor‐positive invasive breast cancer. J Clin Oncol. 2009;27:5312‐5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ewertz M. Breast cancer in Denmark. Incidence, risk factors, and characteristics of survival. Acta Oncol (Stockholm, Sweden). 1993;32:595‐615. [DOI] [PubMed] [Google Scholar]
- 107. Trentham‐Dietz A, Newcomb PA, Nichols HB, Hampton JM. Breast cancer risk factors and second primary malignancies among women with breast cancer. Breast Cancer Res Treat. 2007;105:195‐207. [DOI] [PubMed] [Google Scholar]
- 108. Fleischauer ATS, Simonsen N, Arab L. Antioxidant supplements and risk of breast cancer recurrence and breast cancer‐related mortality among postmenopausal women. Nutr Cancer. 2003;46:15‐22. [DOI] [PubMed] [Google Scholar]
- 109. Saquib JR, Natarajan CL, Saquib L, et al. Dietary intake, supplement use, and survival among women diagnosed with early‐stage breast cancer. Nutr Cancer. 2011;63:327‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Nechuta SL, Chen W, Zheng Z, et al. Vitamin supplement use during breast cancer treatment and survival: a prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2011;20:262‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Greenlee HK, Kwan ML, Kushi LH, et al. Antioxidant supplement use after breast cancer diagnosis and mortality in the life after cancer epidemiology (LACE) cohort. Cancer. 2012;118:2048‐2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Poole EMS, Caan X, Flatt BJ, et al. Postdiagnosis supplement use and breast cancer prognosis in the after breast cancer pooling project. Breast Cancer Res Treat. 2013;139:529‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Inoue‐Choi MG, Greenlee H, Oppeneer SJ, Robien K. The association between postdiagnosis dietary supplement use and total mortality differs by diet quality among older female cancer survivors. Cancer Epidemiol Biomarkers Prev. 2014;23:865‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bruemmer BP, Patterson RE, Cheney C, Aker SN, Witherspoon RP. The association between vitamin C and vitamin E supplement use before hematopoietic stem cell transplant and outcomes to two years. J Am Diet Assoc. 2003;103:982‐990. [DOI] [PubMed] [Google Scholar]
- 115. Hietala MH, Henningson M, Ingvar C, Jönsson PE, Rose C, Jernström H. Natural remedy use in a prospective cohort of breast cancer patients in southern Sweden. Acta Oncol. 2011;50:134‐143. [DOI] [PubMed] [Google Scholar]
- 116. Harris HRB, Bergkvist L, Wolk A. Vitamin C intake and breast cancer mortality in a cohort of Swedish women. Br J Cancer. 2013;109:257‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Madden JMM, Murphy L, Zgaga L, Bennett K. De novo vitamin D supplement use post‐diagnosis is associated with breast cancer survival. Breast Cancer Res Treat. 2018;172:179‐190. [DOI] [PubMed] [Google Scholar]
- 118. Zeichner SB, Koru‐Sengul T, Shah N, et al. Improved clinical outcomes associated with vitamin D supplementation during adjuvant chemotherapy in patients with HER2+ nonmetastatic breast cancer. Clin Breast Cancer. 2015;15:e1‐e11. [DOI] [PubMed] [Google Scholar]
- 119. Jung AY, Cai X, Thoene K, et al. Antioxidant supplementation and breast cancer prognosis in postmenopausal women undergoing chemotherapy and radiation therapy. Am J Clin Nutr. 2019;109:69‐78. [DOI] [PubMed] [Google Scholar]
- 120. Ambrosone CB, Zirpoli GR, Hutson AD, et al. Dietary supplement use during chemotherapy and survival outcomes of patients with breast cancer enrolled in a cooperative group clinical trial (SWOG S0221). J Clin Oncol. 2020;38:804‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kwan ML, Greenlee H, Lee VS, et al. Multivitamin use and breast cancer outcomes in women with early‐stage breast cancer: the life after cancer epidemiology study. Breast Cancer Res Treat. 2011;130:195‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jacobs ETT, Flatt CA, Al‐Delaimy SW, et al. Vitamin D and breast cancer recurrence in the Women's Healthy Eating and Living (WHEL) study. Am J Clin Nutr. 2011;93:108‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Mizrak Kaya DO, Kubilay B, Onur P, et al. Diagnostic serum vitamin D level is not a reliable prognostic factor for resectable breast cancer. Future Oncol. 2018;14:1461‐1467. [DOI] [PubMed] [Google Scholar]
- 124. Kim JSH, Kim CC, Lim JH, et al. Association between changes in serum 25‐hydroxyvitamin D levels and survival in patients with breast cancer receiving neoadjuvant chemotherapy. J Breast Cancer. 2018;21:134‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Yao SK, Ergas ML, Roh IJ, et al. Association of serum level of vitamin D at diagnosis with breast cancer survival: a case‐cohort analysis in the pathways study. JAMA Oncol. 2017;3:351‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wu YS, Sarkissyan M, Clayton S, Chlebowski R, Vadgama JV. Association of vitamin D3 level with breast cancer risk and prognosis in African‐American and Hispanic women. Cancers. 2017;9:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lohmann AEC, Burnell JA, Levine MJ, et al. Prognostic associations of 25 hydroxy vitamin D in NCIC CTG MA.21, a phase III adjuvant randomized clinical trial of three chemotherapy regimens in high‐risk breast cancer. Breast Cancer Res Treat. 2015;150:605‐611. [DOI] [PubMed] [Google Scholar]
- 128. Vrieling AS, Johnson P, Heinz TS, et al. Circulating 25‐hydroxyvitamin D and postmenopausal breast cancer survival: influence of tumor characteristics and lifestyle factors? Int J Cancer. 2014;134:2972‐2983. [DOI] [PubMed] [Google Scholar]
- 129. Villaseñor AB‐B, Ambs R, Bernstein A, et al. Associations of serum 25‐hydroxyvitamin D with overall and breast cancer‐specific mortality in a multiethnic cohort of breast cancer survivors. Cancer Causes Control. 2013;24:759‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Tretli SS, Schwartz GG, Torjesen PA, Robsahm TE. Serum levels of 25‐hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma: a population‐based study. Cancer Causes Control. 2012;23:363‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hatse SL, Verstuyf D, Smeets A, et al. Vitamin D status at breast cancer diagnosis: correlation with tumor characteristics, disease outcome, and genetic determinants of vitamin D insufficiency. Carcinogenesis. 2012;33:1319‐1326. [DOI] [PubMed] [Google Scholar]
- 132. Pritchard KIS, Chapman LE, Norris JA, et al. Randomized trial of tamoxifen versus combined tamoxifen and octreotide LAR therapy in the adjuvant treatment of early‐stage breast cancer in postmenopausal women: NCIC CTG MA.14. J Clin Oncol. 2011;29:3869‐3876. [DOI] [PubMed] [Google Scholar]
- 133. Vrieling AH, Hein R, Abbas S, Schneeweiss A, Flesch‐Janys D, Chang‐Claude J. Serum 25‐hydroxyvitamin D and postmenopausal breast cancer survival: a prospective patient cohort study. Breast Cancer Res. 2011;13:R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Goodwin PJE, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25‐hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757‐3763. [DOI] [PubMed] [Google Scholar]
- 135. Kanstrup C, Teilum D, Rejnmark L, et al. 25‐Hydroxyvitamin D at time of breast cancer diagnosis and breast cancer survival. Breast Cancer Res Treat. 2020;179:699‐708. [DOI] [PubMed] [Google Scholar]
- 136. Thanasitthichai S, Prasitthipayong A, Boonmark K, Purisa W, Guayraksa K. Negative impact of 25‐hydroxyvitamin D deficiency on breast cancer survival. Asian Pac J Cancer Prev. 2019;20:3101‐3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Robsahm TE, Tretli S, Torjesen PA, Babigumira R, Schwartz GG. Serum 25‐hydroxyvitamin D levels predict cancer survival: a prospective cohort with measurements prior to and at the time of cancer diagnosis. Clin Epidemiol. 2019;11:695‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Viala M, Chiba A, Thezenas S, et al. Impact of vitamin D on pathological complete response and survival following neoadjuvant chemotherapy for breast cancer: a retrospective study. BMC Cancer. 2018;18:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Bouvard B, Chatelais J, Soulie P, et al. Osteoporosis treatment and 10 years' oestrogen receptor+ breast cancer outcome in postmenopausal women treated with aromatase inhibitors. Eur J Cancer. 2018;101:87‐94. [DOI] [PubMed] [Google Scholar]
- 140. Huang YQ, Zhou C, Zhao R, Cui YP, Wu XT. The relationship between vitamin D, ratio of neutrophil to lymphocyte, and ratio of lymphocyte to monocyte in preoperative serum and prognosis of patients with breast conserving surgery in breast cancer. Int J Clin Exp Med. 2019;12:10537‐10548. [Google Scholar]
- 141. Kim HJ, Lee YM, Ko BS, et al. Vitamin D deficiency is correlated with poor outcomes in patients with luminal‐type breast cancer. Ann Surg Oncol. 2011;18:1830‐1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lim ST, Jeon YW, Suh YJ. Association between alterations in the serum 25‐hydroxyvitamin d status during follow‐up and breast cancer patient prognosis. Asian Pac J Cancer Prev. 2015;16:2507‐2513. [DOI] [PubMed] [Google Scholar]
- 143. Lim ST, Jeon YW, Gwak H, Suh YJ. Clinical implications of serum 25‐hydroxyvitamin D status after 5‐year adjuvant endocrine therapy for late recurrence of hormone receptor‐positive breast cancer. J Breast Cancer. 2020;23:498‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Tokunaga E, Masuda T, Ijichi H, et al. Impact of serum vitamin D on the response and prognosis in breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Tokyo. 2022;29:156‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Schwedhelm CB, Boeing H, Hoffmann G, Aleksandrova K, Schwingshackl L. Effect of diet on mortality and cancer recurrence among cancer survivors: a systematic review and meta‐analysis of cohort studies. Nutr Rev. 2016;74:737‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. He JG, Gu Y, Zhang S. Consumption of vegetables and fruits and breast cancer survival: a systematic review and meta‐analysis. Sci Rep. 2017;7:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Terranova COP, Protani MM, Reeves MM. Overall dietary intake and prognosis after breast cancer: a systematic review. Nutr Cancer. 2018;70:153‐163. [DOI] [PubMed] [Google Scholar]
- 148. Wallace TC, Bailey RL, Blumberg JB, et al. Fruits, vegetables, and health: a comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit Rev Food Sci. 2020;60:2174‐2211. [DOI] [PubMed] [Google Scholar]
- 149. Aune D, Keum N, Giovannucci E, et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose‐response meta‐analysis of prospective studies. BMJ Br Med J. 2016;353:i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Poschner SM‐S, Zehl A, Wackerlig M, et al. The impacts of genistein and daidzein on estrogen conjugations in human breast cancer cells: a targeted metabolomics approach. Front Pharmacol. 2017;8:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Uifălean A, Schneider S, Ionescu C, Lalk M, Iuga CA. Soy isoflavones and breast cancer cell lines: molecular mechanisms and future perspectives. Molecules. 2015;21:E13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Messina M. Impact of soy foods on the development of breast cancer and the prognosis of breast cancer patients. Forsch Komplement (2006). 2016;23:75‐80. [DOI] [PubMed] [Google Scholar]
- 153. Qiu SJ, Jiang C. Soy and isoflavones consumption and breast cancer survival and recurrence: a systematic review and meta‐analysis. Eur J Nutr. 2019;58:3079‐3090. [DOI] [PubMed] [Google Scholar]
- 154. Jayedi A, Emadi A, Khan TA, Abdolshahi A, Shab‐Bidar S. Dietary fiber and survival in women with breast cancer: a dose‐response meta‐analysis of prospective cohort studies. Nutr Cancer. 2020;73:1‐11. [DOI] [PubMed] [Google Scholar]
- 155. Schwingshackl LK, Schwedhelm S, Hoffmann C, et al. Perspective: NutriGrade: a scoring system to assess and judge the meta‐evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr. 2016;7:994‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Reynolds AM, Mann J, Cummings J, Winter N, Mete E, te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta‐analyses. Lancet. 2019;393:434‐445. [DOI] [PubMed] [Google Scholar]
- 157. McCarty CAR, Reding DJ, Commins J, et al. Alcohol, genetics and risk of breast cancer in the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Breast Cancer Res Treat. 2012;133:785‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Greenlee HH, Hershman DL, Jacobson JS. Use of antioxidant supplements during breast cancer treatment: a comprehensive review. Breast Cancer Res Treat. 2009;115:437‐452. [DOI] [PubMed] [Google Scholar]
- 159. Hu KC, Callen DF, Li J, Zheng H. Circulating vitamin D and overall survival in breast cancer patients: a dose‐response meta‐analysis of cohort studies. Integr Cancer Ther. 2018;17:217‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Feldman DK, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342‐357. [DOI] [PubMed] [Google Scholar]
- 161. Vimaleswaran KSB, Lu DJ, Tikkanen C, et al. Causal relationship between obesity and vitamin D status: bi‐directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10:e1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wanner MR, Richard A, Martin B, Linseisen J, Rohrmann S. Associations between objective and self‐reported physical activity and vitamin D serum levels in the US population. Cancer Causes Control. 2015;26:881‐891. [DOI] [PubMed] [Google Scholar]
- 163. Kim HJK, Koh BS, Yu JH, et al. Changes in serum hydroxyvitamin D levels of breast cancer patients during tamoxifen treatment or chemotherapy in premenopausal breast cancer patients. Eur J Cancer. 2014;50:1403‐1411. [DOI] [PubMed] [Google Scholar]
- 164. Stull VBS, Snyder DC, Demark‐Wahnefried W. Lifestyle interventions in cancer survivors: designing programs that meet the needs of this vulnerable and growing population. J Nutr. 2007;137:243S‐248S. [DOI] [PubMed] [Google Scholar]
- 165. Hu FBS, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531‐540. [DOI] [PubMed] [Google Scholar]
- 166. World Cancer Research Fund International . https://www.wcrf.org/dietandcancer/after-a-cancer-diagnosis-follow-our-recommendations-if-you-can/. Accessed May 18, 2021.
- 167. Rock CL, Thomson CA, Sullivan KR, et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin. 2022;72:230‐262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information
Data Availability Statement
Only publicly available data were used in our study. Data sources and handling of these data are described in the Materials and Methods section. Further details are available from the corresponding author upon request.


