Abstract
“Obesity paradox” describes the counterintuitive finding that aged overweight and obese people with a particular disease may have better outcomes than their normal weight or underweight counterparts. This systematic review was performed to summarize the publications related to the obesity paradox in older adults, to gain an in-depth understanding of this phenomenon. PubMed©, Embase©, and Scopus© were used to perform literature search for all publications up to 20 March 2022. Studies were included if they reported data from older adults on the relation between BMI and mortality. The following article types were excluded from the study: reviews, editorials, correspondence, and case reports and case series. Publication year, study setting, medical condition, study design, sample size, age, and outcome(s) were extracted. This review has been registered with PROSPERO (no. CRD42021289015). Overall, 2226 studies were identified, of which 58 were included in this systematic review. In all, 20 of the 58 studies included in this review did not find any evidence of an obesity paradox. Of these 20 studies, 16 involved patients with no specific medical condition, 1 involved patients with chronic diseases, and 2 involved patients with type 2 diabetes mellitus. Seven out of the nine studies that looked at short-term mortality found evidence of the obesity paradox. Of the 28 studies that examined longer-term mortality, 15 found evidence of the obesity paradox. In the studies that were conducted in people with a particular medical condition (n = 24), the obesity paradox appeared in 18 cases. Our work supports the existence of an obesity paradox, especially when comorbidities or acute medical problems are present. These findings should help guide strategies for nutritional counselling in older populations.
Keywords: obesity paradox, aged adults, body mass index, mortality
1. Introduction
Obesity, usually defined by the body mass index (BMI), is considered a public health problem, and is associated with many diseases [1,2,3]. The prevalence of obesity is high in younger adults but also in older people [4], and evidence suggests that prevalence of obesity will continue to increase [5]. The term “obesity paradox” is used to describe the counterintuitive finding that aged overweight and obese people with a particular disease may have better outcomes than their normal weight or underweight counterparts. However, there is wide heterogeneity between studies regarding the association between obesity and mortality in older adults, depending on the diseases concerned, the presence or absence of a particular disease, or the BMI level considered [6,7,8]. In aged people, body composition tends to change, and body weight tends to decrease, and some authors have suggested that fatness could be healthy [9]. Thus, it is important to confirm whether an “obesity paradox” truly exists, with a view to adapting management policies for overweight or obese old people.
In this context, the objective of the study was to summarize the publications in the literature relating to the obesity paradox in older adults, to enhance our understanding of this phenomenon.
2. Methods
2.1. Literature Search
A preliminary check was made in PubMed©, Scopus©, Embase©, Prospero©, and the Cochrane Library© to ensure that no systematic reviews had previously been conducted on this specific topic.
A literature search was performed using PubMed©, Embase©, and Scopus© to cover all publications up to March 20, 2022. The search terms defined by the two researchers (LG, MD) included the following keywords in the title and/or the abstract: (“obesity paradox” OR “reverse epidemiology” OR “body mass index” OR BMI OR overweight OR obesity) AND (mortality OR death OR survival)). The search included studies in the French or English language and studies on human subjects, and excluded the following publication types: reviews, editorials, correspondence, and case reports and case series. A manual check was performed for potential additional studies. This systematic review was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. This study was registered with PROSPERO (an International prospective register of systematic reviews) (number CRD42021289015), available at https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021289015, accessed on 20 March 2023.
2.2. Study Selection
Study eligibility criteria were defined a priori by the two researchers (LG, MD) within the PICOS framework. Studies were eligible if they reported data on “obesity paradox” (using body mass index as a nutritional indicator). The population was restricted to studies that included persons 65 years or older, whatever their sex, ethnicity, or living place. The intervention (exposure) was a presence of overweight or obesity as defined by the baseline BMI value. The control was those who were underweight or a normal weight. The outcome was death, whatever the timepoint. When the study was not specifically conducted in older adults, only data concerning those aged 65 years or over were taken into account (provided that the information was available). Correspondence, editorials, reviews, basic science articles, and case reports and case series were excluded.
2.3. Data Extraction
The Covidence systematic review software© (Veritas Health Innovation, Melbourne, Australia), available at www.covidence.org, was used to perform data analysis. After elimination of duplicates, the two researchers (LG, MD) made a blind review of titles and abstracts of all articles. When there was disagreement about whether or not to include an article, they discussed the case until consensus was reached. Overlap between studies was verified. Data extraction was realised independently by the two researchers (LG, MD), using the same extraction form. The following data were extracted: publication year, study setting, medical condition, study design, sample size, age (mean or median and their statistical dispersion parameters, when available), and outcome(s). To check whether the obesity paradox was present or not, the following information was collected: outcome(s), BMI classes, type of analysis (whether multivariable or not), statistical estimates (Hazard ratio, Odds ratio, Rate ratio, Rates) and their respective 95% confidence intervals (95% CI), and the level of significance (p-values).
2.4. Quality Assessment
The Newcastle–Ottawa Scale (NOS) [10] was used to assess the quality of included studies. This scale is composed of three quality criteria: selection (4 points), comparability (2 points), and outcome assessment (3 points). This gives a total of between 0 and 9 points. Scores of 7 or more are considered high quality studies, scores of 5–6 as moderate quality, and scores below 5 as low quality. Disagreements in scoring were resolved by a joint review of the manuscript to reach consensus.
Where possible and appropriate, some parameters were calculated from available data (e.g., mean age and/or standard deviation, rate ratio, odds ratio, etc.).
3. Results
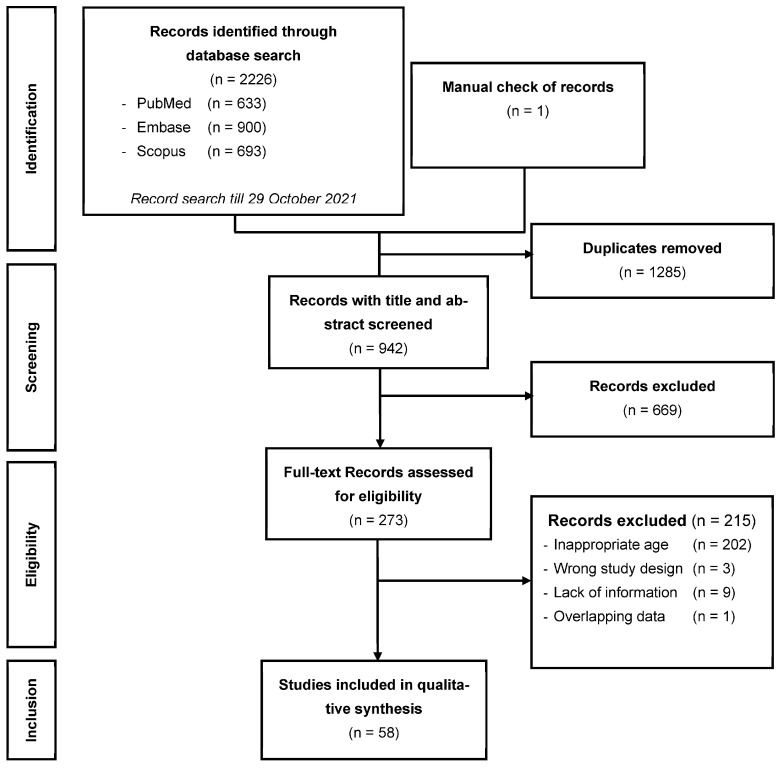
As shown in Figure 1, 2226 studies were identified by the literature search. Among these, 1285 duplicates were found and excluded. After checking titles and abstracts of the remaining 942 studies, 273 articles were included for full-text assessment. After full-text examination of these 273 studies, 215 were excluded for at least one of the following reasons: lack of relevant information, overlapping data, or inappropriate age of the study population. Thus, 58 studies were retained in this review.
Figure 1.
PRISMA flow diagram of the records included in the systematic review.
Table 1 summarizes the characteristics of the studies included in the review. All studies were observational cohorts; 41 were prospective [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51] and 17 were retrospective [52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68].
Table 1.
Description of the studies included in the present systematic review.
| Author, Year | Country | Study Design | Study Setting | Medical Condition | Sample Size | Age (Years) Mean ± SD |
|---|---|---|---|---|---|---|
| Kananen, 2022 [68] | Sweden | Retrospective cohort | Hospital, Geriatrics | COVID-19 | 1409 | 77 [65–104] ♣ |
| Amin, 2021 [11] | USA | Prospective cohort | Hospital, Surgery | Hip fracture | 52,729 | x ± x |
| Danninger, 2021 [52] | USA | Retrospective cohort | Hospital, ICU | Sepsis | 8707 | x ± x |
| El Moheb, 2021 [12] | USA | Prospective cohort | Hospital, Surgery | Emergent surgery | 78,704 | 75 ± x |
| Lin, 2021 [13] | Taiwan | Prospective cohort | Community | None specific | 81,221 | 74 ± 6 |
| Martinez-Tapia, 2021 [14] | France | Prospective cohort | Hospital, Geriatrics | Cancer | 2071 | 81 ± 6 |
| Lai, 2020 [15] | Taiwan | Prospective cohort | LTCF | None specific | 182 | 79 ± 8 |
| Schneider, 2020 [16] | Germany | Prospective cohort | Hospital, Neurosurgery | Glioblastoma | 110 | 72 [65–86] ♣ |
| Seino, 2020 [53] | Japan | Retrospective cohort | Community | None specific | 1977 | 72 ± 6 * |
| Nishida, 2019 [17] | Japan | Prospective cohort | Community | None specific | 1229 | 74 ± 5 |
| Om, 2019 [18] | Korea | Prospective cohort | Hospital, Cardiology | Aortic stenosis | 379 | 79 ± x * |
| Tokarek, 2019 [54] | Poland | Retrospective cohort | Hospital, Cardiology | TAVI patients | 147 | 82 [x–x] ♣ |
| Yoshihisa, 2019 [19] | Japan | Prospective cohort | Hospital, Cardiology | Acute heart failure | 2410 | x ± x |
| Crotti, 2018 [20] | Italy | Prospective cohort | Community | None specific | 4970 | 72 ± 5 |
| De Palma, 2018 [21] | Sweden | Prospective cohort | Hospital, Cardiology | TAVI patients | 492 | 83 ± 6 |
| Keller, 2018 [55] | Germany | Retrospective cohort | Hospital, Cardiology | AMI | 122,607 | 80 ± x |
| Kim, 2018 [22] | Korea | Prospective cohort | Community | None specific | 170,639 | 72 ± 5 |
| Lee, 2018 [56] | Korea | Retrospective cohort | Community | None specific | 11,844 | 72 ± 5 |
| Lv, 2018 [23] | China | Prospective cohort | Community | None specific | 4361 | 92 ± 8 |
| de Souto Barreto, 2017 [24] | France | Prospective cohort | Nursing home | Dementia | 3741 | 86 ± 8 |
| Wu, 2017 [25] | China | Prospective cohort | Hospital, ED | Atrial fibrillation | 1321 | x ± x |
| Cheng, 2016 [57] | USA | Retrospective cohort | Community | None specific | 4565 | 74 ± 5 |
| Flodin, 2016 [26] | Sweden | Prospective cohort | Hospital | Hip fracture | 843 | 82 ± 7 |
| Calabia, 2015 [58] | Spain | Retrospective cohort | Hospital, Nephrology | Haemodialysis | 3978 | 75 ± 6 |
| Kim, 2015 [59] | Korea | Retrospective cohort | Community | Chronic diseases | x | x ± x |
| Kubota, 2015 [60] | Japan | Retrospective cohort | Community | T2DM | 16,304 # | x ± x |
| Kuo, 2015 [27] | Taiwan | Prospective cohort | Outpatients | T2DM | x | x ± x |
| Shil Hong, 2015 [61] | Korea | Retrospective cohort | Community | None specific | 1000 | 76 ± 9 |
| Buys, 2014 [28] | USA | Prospective cohort | Community | None specific | 1257 | 75 ± 7 |
| Clark, 2014 [62] | USA/Nigeria | Retrospective cohort | Community | None specific | 2466 | 77 ± 5 * |
| Ford, 2014 [29] | USA | Prospective Cohort | Community | None specific | 2995 | 81 ± 4 |
| Lang, 2014 [30] | France | Prospective cohort | Hospital, ED | None specific | 1306 | 85 ± 6 |
| Lee, 2014 [31] | Korea | Prospective cohort | Community | None specific | 11,844 | 73 ± 7 |
| Murphy, 2014 [63] | Iceland | Retrospective cohort | Community | T2DM | 637 | 77 [66–96] ♠ |
| Wu, 2014 [32] | Taiwan | Prospective cohort | Community | None specific | 77,541 | 73 ± 7 |
| Yamauchi, 2014 [64] | Japan | Retrospective cohort | Hospital, Pulmonology | COPD | 263,940 | 78 ± 7 |
| Chen, 2013 [33] | Taiwan | Prospective cohort | Veterans | None specific | 1257 | 83 ± 5 |
| Dahl, 2013 [34] | Sweden | Prospective cohort | Community | None specific | 882 | 80 ± 6 |
| Nakazawa, 2013 [35] | Japan | Prospective cohort | Nursing home | None specific | 8510 | 84 ± 8 |
| Takata, 2013 [36] | Japan | Prospective cohort | Community | None specific | 675 | 80 ± 0 |
| Tseng, 2013 [37] | Taiwan | Prospective cohort | Community | T2DM | 34,825 | x ± x |
| Veronese, 2013 [38] | Italy | Prospective cohort | Nursing home | None specific | 181 | 81 ± 8 |
| Woo, 2013 [39] | China | Prospective cohort | Community | None specific | 4000 | 73 ± 5 |
| Yamamoto, 2013 [40] | France | Prospective cohort | Hospital, Cardiology | TAVI patients | 3072 | 83 ± 7 |
| Zekry, 2013 [41] | Switzerland | Prospective cohort | Hospital, Geriatric | None specific | 444 | 85 ± 7 |
| de Hollander, 2012 [42] | Netherlands | Prospective cohort | Community | None specific | 1980 | 73 ± 2 |
| Kvamme, 2012 [43] | Norway | Prospective cohort | Community | None specific | 16,711 | 73 ± 5 |
| Mihel, 2012 [44] | Croatia | Prospective cohort | Community | Hypertension | 2507 | x ± x |
| Tsai, 2012 [65] | Taiwan | Retrospective cohort | Community | None specific | 2892 | x ± x |
| Cereda, 2011 [45] | Italy | Prospective cohort | LTCF | None specific | 533 | 84 ± 8 |
| Berraho, 2010 [46] | France | Prospective cohort | Community | None specific | 3646 | 75 ± 7 |
| Han, 2010 [47] | Korea | Prospective cohort | Community | None specific | 877 | 75 ± 8 |
| Kitamura, 2010 [48] | Japan | Prospective cohort | Home care | None specific | 205 | 84 ± 8 |
| Lea, 2009 [66] | USA | Retrospective cohort | Hospital, Cardiology | AMI | 74,167 | 77 ± x * |
| Luchsinger, 2008 [49] | USA | Prospective cohort | Community | None specific | 1372 | 78 ± 6 |
| Locher, 2007 [50] | USA | Prospective cohort | Community | None specific | 983 | 75 ± 7 |
| Takata, 2007 [51] | Japan | Prospective cohort | Community | None specific | 697 | 80 ± 0 |
| Grabowski, 2001 [67] | USA | Retrospective cohort | Community | None specific | 7527 | 77 ± 6 |
SD: Standard deviation; ICU: Intensive care unit; ED: Emergency department; TAVI: Transcatheter Aortic Valve Implementation; COPD: Chronic Obstructive Pulmonary Disease; AMI: Acute Myocardial Infarction; T2DM: Type 2 Diabetes Mellitus; LTCF: Long-term care facility. x: Missing information; #: Person-years; *: Pooled mean and/or standard deviation have been calculated with the information available in these articles; ♣: Median [range]; ♠: Mean [range].
As shown in Table 2, 20 of the 58 studies included in this review did not find any evidence of an obesity paradox [17,27,28,29,36,39,42,43,46,47,49,50,53,56,59,62,63,65,68,69]. Of these 20 studies, 16 involved patients with no specific medical condition [17,28,29,36,39,42,43,46,47,49,50,53,56,62,65,69]. One involved patients with chronic diseases [59], and two involved patients with type 2 diabetes mellitus [27,63]. Of the 58 studies, 34 used the threshold of BMI ≥ 25.0 kg/m2 [11,12,14,16,19,20,21,22,24,26,30,31,32,34,38,40,41,44,45,51,52,54,55,57,58,60,66,67,68]. A further 10 studies used a threshold different from 25 kg/m2 and found evidence of the obesity paradox [13,18,23,25,33,35,37,48,61,64]. Regarding the time points, 9 studies looked at short-term mortality (less than 12-month mortality, ICU mortality, hospital mortality) [11,12,19,30,40,52,55,64,68]. All of these, except Yamamoto et al. [40] and Kananen et al. [68], found evidence of the obesity paradox. Of the 28 studies that examined longer-term mortality (time point ≥ 5 years) [13,14,15,20,22,27,28,32,34,36,37,38,39,42,44,45,46,49,53,56,57,58,59,60,61,62,63,66,67], 15 (54%) found evidence of the obesity paradox [13,14,20,22,32,34,37,38,44,45,57,58,60,61,66,67]. In the studies that were conducted in people with a particular medical condition (n = 24) [11,12,14,16,18,19,21,24,25,26,27,37,40,44,52,54,55,58,59,60,63,64,66,68], the obesity paradox appeared in 18 (75%) cases [11,12,14,16,18,19,21,24,25,26,37,40,44,52,54,55,58,60,64,66]. In the studies that were carried out among people with no specific medical condition (n = 34) [13,15,17,20,22,23,28,29,30,31,32,33,34,35,36,38,39,41,42,43,45,46,47,48,49,50,51,53,56,57,61,62,65,67], the obesity paradox appeared in 17 (50%) cases [22,23,30,31,32,33,34,35,38,41,45,48,51,57,61,67].
Table 2.
Outcomes and association between body mass index group and mortality in aged adults.
| Author(s), Year | Age (Mean ± SD) |
Medical Condition | Outcome | Obesity Paradox | BMI Thresholds # (kg/m2) |
|---|---|---|---|---|---|
| Kananen, 2022 [68] | x ± x | COVID-19 | In-hospital mortality | No | 18.5 < BMI < 25.0 |
| Amin, 2021 [11] | x ± x | Hip fracture | 30-day mortality | Yes | BMI ≥ 25.0 (No, if BMI > 40.0) |
| Danninger, 2021 [52] | x ± x | Sepsis | ICU mortality | Yes | BMI ≥ 30.0 |
| El Moheb, 2021 [12] | 75 ± x | Emergent Surgery | 30-day mortality | Yes | BMI ≥ 25.0 |
| Lin, 2021 [13] | 74 ± 6 | None specific | 84-month mortality | Yes | BMI ≥ 24.0 |
| Martinez-Tapia, 2021 [14] | 81 ± 6 | Cancer | 12-month mortality (men) | Yes | BMI ≥ 30.0 |
| 12-month mortality (women) | No | ||||
| 60-month mortality (men) | Yes | BMI ≥ 30.0 | |||
| 60-month mortality (women) | Yes | BMI ≥ 30.0 | |||
| Lai, 2020 [15] | 79 ± 8 | None specific | 72-month mortality | No | |
| Schneider, 2020 [16] | 72 ± x | Glioblastoma | 12-month mortality | Yes | BMI ≥ 30.0 |
| Seino, 2020 [53] | 72 ± 6 | None specific | All-cause mortality (men) | No | |
| All-cause mortality (women) | No | ||||
| Nishida, 2019 [17] | 74 ± 5 | None specific | 36-month mortality | No | |
| Om, 2019 [18] | 79 ± x | Aortic stenosis | 12-month mortality | Yes | BMI ≥ 24.9 |
| Tokarek, 2019 [54] | 82 ± x | TAVI patients | 12-month survival | Yes | BMI ≥ 30.0 |
| Yoshihisa, 2019 [19] | x ± x | AHF | In-hospital mortality | Yes | BMI ≥ 25.0 |
| Crotti, 2018 [20] | 72 ± 5 | None specific | 68-month mortality | Yes | BMI ≥ 25.0 (No, if BMI > 30.0) |
| 68-month CVD mortality | No | ||||
| 68-month cancer mortality | No | ||||
| De Palma, 2018 [21] | 83 ± 6 | TAVI patients | 12-month mortality | Yes | BMI ≥ 25.0 |
| 50-month mortality | Yes | BMI ≥ 25.0 | |||
| Keller, 2018 [55] | 80 ± x | AMI | In-hospital mortality | Yes | BMI ≥ 30.0 |
| Kim, 2018 [22] | 72 ± 5 | None specific | 60-month mortality | Yes | BMI ≥ 25.0 (No, if BMI > 27.5) |
| Lee, 2018 [56] | 72 ± 5 | None specific | 60-month mortality | No | |
| Lv, 2018 [23] | 92 ± 8 | None specific | 36-month mortality | Yes | BMI ≥ 18.5 |
| De Souto Barreto, 2017 [24] | 86 ± 8 | Dementia | 18-month mortality (dementia) | Yes | BMI ≥ 25.0 |
| 18-month mortality (without dementia) | Yes | BMI ≥ 25.0 | |||
| Wu, 2017 [25] | x ± x | Atrial fibrillation | 12-month mortality (65–74 years) | No | |
| 12-month mortality (≥75 years) | Yes | BMI ≥ 24.0 | |||
| Cheng, 2016 [57] | 74 ± 5 | None specific | 132-month mortality | Yes | BMI ≥ 25.0 (No, if BMI ≥ 35.0) |
| Diabetes | Yes | BMI ≥ 25.0 (No, if BMI ≥ 35.0) |
|||
| Hypertension | Yes | BMI ≥ 25.0 (No, if BMI ≥ 35.0) |
|||
| Dyslipidaemia | Yes | BMI ≥ 25.0 (No, if BMI ≥ 35.0) |
|||
| Flodin, 2016 [26] | 82 ± 7 | Hip fracture | 12-month survival | Yes | BMI > 26.0 |
| Calabia, 2015 [58] | 75 ± 6 | Haemodialysis | 120-month mortality | Yes | BMI = 30.0–34.9 (No, if BMI = 27.5–29.9 or BMI ≥ 35.0) |
| Kim, 2015 [59] | x ± x | Chronic diseases | 108-month mortality | No | |
| Kubota, 2015 [60] | x ± x | T2DM | 132-month ID mortality | Yes | BMI ≥ 25.0 |
| Kuo, 2015 [27] | x ± x | T2DM | 66-month mortality | No | |
| Shil hong, 2015 [61] | 76 ± 9 | None specific | 72-month mortality | Yes | BMI ≥ 23.8 |
| Buys, 2014 [28] | 75 ± 7 | None specific | 102-month mortality | No | |
| Clark, 2014 [62] | 77 ± 5 | None specific | 120-month mortality (Africans) | No | |
| 120-month mortality (African Americans) | No | ||||
| Ford, 2014 [29] | 81 ± 4 | None specific | 40-month mortality | No | |
| Lang, 2014 [30] | 85 ± 6 | None specific | 6-week mortality | Yes | BMI ≥ 30.0 |
| 12-month mortality | Yes | BMI ≥ 25.0 | |||
| 24-month mortality | Yes | BMI ≥ 25.0 | |||
| Lee, 2014 [31] | 73 ± 7 | None specific | 36-month mortality | Yes | BMI ≥ 25.0 (No, if BMI ≥ 30.0) |
| Murphy, 2014 [63] | 77 ± x | T2DM | 84-month mortality | No | |
| Wu, 2014 [32] | 73 ± 7 | None specific | 60-month mortality | Yes | BMI ≥ 25.0 (No, if BMI ≥ 35.0) |
| 60-month CVD mortality | BMI ≥ 25.0 (No, if BMI ≥ 30.0) |
||||
| Yamauchi, 2014 [64] | 78 ± 7 | COPD | In-hospital mortality | Yes | BMI ≥ 23.0 |
| Chen, 2013 [33] | 83 ± 5 | None specific | 18-month mortality | Yes | BMI ≥ 23.0 |
| Dahl, 2013 [34] | 80 ± 6 | None specific | 216-month mortality | Yes | BMI ≥ 25.0 (No, if BMI ≥ 30.0) |
| Nakazawa, 2013 [35] | 84 ± 8 | None specific | 12-month mortality | Yes | BMI ≥ 23.6 |
| Takata, 2013 [36] | 80 ± 0 | None specific | 144-month mortality | No | |
| 144-month CVD mortality | No | ||||
| 144-month cancer mortality | No | ||||
| Tseng, 2013 [37] | x ± x | T2DM | 144-month mortality | Yes | BMI ≥ 23.0 |
| Veronese, 2013 [38] | 81 ± 8 | None specific | 60-month | Yes | BMI ≥ 30.0 |
| Woo, 2013 [39] | 73 ± 5 | None specific | 84-month mortality | No | |
| Yamamoto, 2013 [40] | 83 ± 7 | TAVI patients | 30-day mortality | No | |
| 12-month mortality | Yes | BMI ≥ 25.0 | |||
| Zekry, 2013 [41] | 85 ± 7 | None specific | 48-month mortality | Yes | BMI ≥ 30.0 |
| de Hollander, 2012 [42] | 73 ± 2 | None specific | 120-month mortality | No | |
| Kvamme, 2012 [43] | 73 ± 5 | None specific | 12-month mortality (men) | No | |
| 12-month mortality (women) | No | ||||
| Respiratory diseases | 12-month mortality (men) | No | |||
| 12-month mortality (women) | No | ||||
| CVD | 12-month mortality (men) | No | |||
| 12-month mortality (women) | No | ||||
| Cancer | 12-month mortality (men) | No | |||
| 12-month mortality (women) | No | ||||
| Mihel, 2012 [44] | x ± x | Hypertension | 60-month mortality (men) | Yes | BMI ≥ 30.0 |
| 60-month mortality (women) | No | ||||
| Tsai, 2012 [65] | x ± x | None specific | 48-month mortality (65–74 y; men) | No | |
| 48-month mortality (≥75 y; men) | No | ||||
| 48-month mortality (65–74 y; women) | No | ||||
| 48-month mortality (≥75 y; women) | No | ||||
| Cereda, 2011 [45] | 84 ± 8 | None specific | 72-month mortality | Yes | BMI ≥ 25.0 |
| Berraho, 2010 [46] | 75 ± 7 | None specific | 156-month mortality | No | |
| Han, 2010 [47] | 75 ± 8 | None specific | 42-month mortality | No | |
| Kitamura, 2010 [48] | 84 ± 8 | None specific | 24-month mortality | Yes | BMI ≥ 17.1 |
| Lea, 2009 [66] | 77 ± x | AMI | 125-month mortality | Yes | BMI ≥ 25.0 (No, if BMI > 40.0) |
| Luchsinger, 2008 [49] | 78 ± 6 | None specific | 144-month mortality | No | |
| Locher, 2007 [50] | 75 ± 7 | None specific | 36-month mortality | No | |
| Takata, 2007 [51] | 80 ± 0 | None specific | 48-month mortality | Yes | BMI ≥ 25.0 |
| 48-month CVD mortality | No | ||||
| 48-month cancer mortality | No | ||||
| Grabowski, 2001 [67] | 77 ± 6 | None specific | 96-month mortality | Yes | BMI ≥ 28.5 |
# BMI thresholds at which an obesity paradox was demonstrated. SD: Standard deviation; ICU: Intensive Care Unit; TAVI: Transcatheter Aortic Valve Implementation; COPD: Chronic Obstructive Pulmonary Disease; AHF: Acute Heart Failure; AMI: Acute Myocardial Infarction; T2DM: Type 2 Diabetes Mellitus; CVD: Cardiovascular disease; y, years. x: Missing information.
An appendix provides detailed information of the analyses and results of the relationship between BMI and mortality in aged adults. Of the analyses tested for the existence of an obesity paradox, 48 were adjusted for confounders, and 10 were unadjusted analyses (see Supplementary Materials).
The quality of the included studies, as assessed using the NOS, was considered high for all 58 studies (Table 3).
Table 3.
Quality assessment of the different studies included in this systematic review, using the Newcastle–Ottawa scale (NOS).
| Author, Year | Study Design | Selection | Comparability | Outcome | Total Score | Quality Rating |
|---|---|---|---|---|---|---|
| Kananen, 2022 [68] | Retrospective cohort | **** | ** | *** | 9 | High |
| Amin, 2021 [11] | Prospective cohort | **** | ** | *** | 9 | High |
| Danninger, 2021 [52] | Retrospective cohort | **** | ** | *** | 9 | High |
| El Moheb, 2021 [12] | Prospective cohort | **** | ** | *** | 9 | High |
| Lin, 2021 [13] | Prospective cohort | *** | ** | *** | 8 | High |
| Martinez-Tapia, 2021 [14] | Prospective cohort | **** | ** | *** | 9 | High |
| Lai, 2020 [15] | Prospective cohort | **** | ** | *** | 9 | High |
| Schneider, 2020 [16] | Prospective cohort | **** | ** | *** | 9 | High |
| Seino, 2020 [53] | Retrospective cohort | **** | ** | *** | 9 | High |
| Nishida, 2019 [17] | Prospective cohort | **** | ** | *** | 9 | High |
| Om, 2019 [18] | Prospective cohort | **** | * | *** | 8 | High |
| Tokarek, 2019 [54] | Retrospective cohort | **** | * | *** | 8 | High |
| Yoshihisa, 2019 [19] | Prospective cohort | **** | * | *** | 8 | High |
| Crotti, 2018 [20] | Prospective cohort | **** | ** | *** | 9 | High |
| De Palma, 2018 [21] | Prospective cohort | **** | * | *** | 8 | High |
| Keller, 2018 [55] | Retrospective cohort | **** | * | *** | 8 | High |
| Kim, 2018 [22] | Prospective cohort | **** | ** | *** | 9 | High |
| Lee, 2018 [56] | Retrospective cohort | **** | ** | *** | 9 | High |
| Lv, 2018 [23] | Prospective cohort | **** | ** | *** | 9 | High |
| de Souto Barreto, 2017 [24] | Prospective cohort | **** | ** | *** | 9 | High |
| Wu, 2017 [25] | Prospective cohort | **** | ** | *** | 9 | High |
| Cheng, 2016 [57] | Retrospective cohort | **** | ** | *** | 9 | High |
| Flodin, 2016 [26] | Prospective cohort | **** | ** | *** | 9 | High |
| Calabia, 2015 [58] | Retrospective cohort | **** | ** | *** | 9 | High |
| Kim, 2015 [59] | Retrospective cohort | **** | ** | *** | 9 | High |
| Kubota, 2015 [60] | Retrospective study | **** | ** | *** | 9 | High |
| Kuo, 2015 [27] | Prospective cohort | **** | * | *** | 8 | High |
| Shil Hong, 2015 [61] | Retrospective cohort | **** | ** | *** | 9 | High |
| Buys, 2014 [28] | Prospective cohort | *** | ** | *** | 8 | High |
| Clark, 2014 [62] | Retrospective cohort | **** | ** | *** | 9 | High |
| Ford, 2014 [29] | Prospective cohort | *** | ** | *** | 8 | High |
| Lang, 2014 [30] | Prospective cohort | **** | ** | *** | 9 | High |
| Lee, 2014 [31] | Prospective cohort | **** | ** | *** | 9 | High |
| Murphy, 2014 [63] | Retrospective cohort | **** | ** | *** | 9 | High |
| Wu, 2014 [32] | Prospective cohort | **** | ** | *** | 9 | High |
| Yamauchi, 2014 [64] | Retrospective cohort | **** | ** | *** | 9 | High |
| Chen, 2013 [33] | Prospective cohort | *** | ** | *** | 8 | High |
| Dahl, 2013 [34] | Prospective cohort | *** | ** | *** | 8 | High |
| Nakazawa, 2013 [35] | Prospective cohort | **** | ** | *** | 9 | High |
| Takata, 2013 [36] | Prospective cohort | *** | ** | *** | 8 | High |
| Tseng, 2013 [37] | Prospective cohort | **** | ** | *** | 9 | High |
| Veronese, 2013 [38] | Prospective cohort | *** | ** | *** | 8 | High |
| Woo, 2013 [39] | Prospective cohort | **** | ** | *** | 9 | High |
| Yamamoto, 2013 [40] | Prospective cohort | **** | ** | *** | 9 | High |
| Zekry, 2013 [41] | Prospective cohort | **** | ** | *** | 9 | High |
| de Hollander, 2012 [42] | Prospective cohort | *** | ** | *** | 8 | High |
| Kvamme, 2012 [43] | Prospective cohort | **** | ** | *** | 9 | High |
| Mihel, 2012 [44] | Prospective cohort | *** | * | *** | 7 | High |
| Tsai, 2012 [65] | Retrospective cohort | **** | ** | *** | 9 | High |
| Cereda, 2011 [45] | Prospective cohort | *** | ** | *** | 8 | High |
| Berraho, 2010 [46] | Prospective cohort | **** | ** | *** | 9 | High |
| Han, 2010 [47] | Prospective cohort | **** | ** | *** | 9 | High |
| Kitamura, 2010 [48] | Prospective cohort | **** | ** | *** | 9 | High |
| Lea, 2009 [66] | Retrospective cohort | **** | ** | *** | 9 | High |
| Luchsinger, 2008 [49] | Prospective cohort | **** | ** | *** | 9 | High |
| Locher, 2007 [50] | Prospective cohort | **** | ** | *** | 9 | High |
| Takata, 2007 [51] | Prospective cohort | **** | ** | *** | 9 | High |
| Grabowski, 2001 [67] | Retrospective cohort | **** | ** | *** | 9 | High |
Each star is equal to one point. The sum of the stars gives the total score of the NOS. NOS score of ≥7 were considered as high quality studies, NOS score of 5–6 as moderate quality, and NOS Scores less than 5 as low quality.
4. Discussion
In this systematic review of studies exploring the relationship between BMI and mortality in patients aged 65 years or older, 28 out of the 58 studies included observed longer survival in patients with a BMI ≥ 25 kg/m2 (the so-called obesity paradox) [11,12,14,16,19,20,21,22,24,26,30,31,32,34,38,40,41,44,45,51,52,54,55,57,58,60,66,67]. Among these 28 studies, 16 involved patients with a specific or acute medical condition [11,12,14,16,19,21,24,26,40,44,52,54,55,58,60,66]. Seven studies found improved survival in overweight and obese older people when focussing on short-term mortality [11,12,19,30,52,55,64,70]. One showed increased survival only in the oldest patients [25]. Two showed increased survival only in men [14,44]. Of the 23 studies that did not observe an obesity paradox [14,15,17,25,27,28,29,36,39,40,42,43,46,47,49,50,53,56,59,62,63,65,68], 7 involved populations selected according to the presence of a particular medical condition [14,25,27,40,59,63,68].
Nearly two-thirds of the studies included in this work report better survival in overweight or obese older people. Several factors may influence the relationship between obesity and survival in the older population, including age, degree of obesity, presence or absence of comorbidities, and occurrence of an acute event.
Regarding age, the studies in this review that failed to show better survival in overweight or obese individuals included populations that were, on average, younger than those demonstrating an obesity paradox. Wu et al. [25], in their study of the impact of age on the association between BMI and all-cause mortality in patients with atrial fibrillation, found better survival in overweight or obese patients aged 75 years or older but not in patients aged between 65 and 74 years. Observations made in older populations must therefore take into account the intrinsic characteristics of the survivors. For the same BMI, patient profiles can be different, and this profile can influence survival. For instance, body composition may differ due to ethnicity, sex, or advancing age [71,72]. BMI does not provide information on body composition, and is less correlated with percentage of body mass or fat mass index, especially in younger people [72]. Abdominal obesity has direct metabolic consequences (adipose tissue inflammation, dysglycaemia, alteration of blood pressure regulation, etc.). Conversely, subcutaneous fat accumulation in the hips, for example, appears to have benign effects on cardiovascular risk. Other indicators, such as waist circumference or waist-to-hip ratio, are strongly associated with higher mortality risk [73,74]. Taking only BMI into account does not make it possible to differentiate between these situations [9]. In all studies included in this work, BMI was defined as an obesity index. If obesity is defined by “body adiposity”, BMI level is probably not the best criterion [75]. The term “BMI paradox” may be more appropriate than “obesity paradox”, as suggested by Antonopoulos et al. [9].
Obesity is a factor associated with higher mortality in younger populations [76,77,78], but it is also associated with an increased risk of developing and dying from a number of diseases [3], such as cancer [79,80], Some authors point to the obesity-related cellular and immune changes that make obese people more vulnerable, including an increased risk of infections [1]. Older obese people could be considered constitutionally more robust as they have survived the risk factor of obesity into adulthood. The degree of obesity could also be a factor. In this review, not all authors differentiated between different classes of obesity. However, the positive effect on survival in cases of overweight and obesity was not found for morbid obesity (BMI ≥ 35.0 kg/m²) in 5 studies [11,32,57,58,66]. Furthermore, weight is not a reflection of body composition, in particular the muscle mass/fat mass ratio. Loss of muscle mass and strength (sarcopenia) is a factor associated with an increased risk of death. Tian et al. reported that obese people with sarcopenia have a higher risk of death than obese people without sarcopenia [81]. Obese people may be less frequently sarcopenic than non-obese people. In 1493 subjects aged 65 years or more (median age 74 ± 11 years), Sousa-Santos et al. [82] found a prevalence of 0.8% of obese sarcopenic individuals versus 11.6% of sarcopenic individuals of all BMI status.
The presence of a chronic pathology or an acute event may also influence survival. In this review, 20 studies [11,12,14,16,18,19,21,24,25,26,37,40,44,52,54,55,58,60,64,66] of the 38 which found a favourable effect of overweight or obesity on survival involved patients with a particular chronic condition or facing a specific medical event. This finding suggests that even moderately overweight older individuals with chronic disease or acute medical events have better survival. Obesity in older people with a chronic disease could be a sign of greater robustness or higher reserves (better appetite, less risk of undernutrition). Overweight or obese older subjects would be less undernourished than the general older population. Cereda et al. [83], in their meta-analysis of the prevalence of undernutrition in an older population, found a prevalence of undernutrition ranging from 3.1 to 29.4%, depending on the setting. Sousa-Santos et al. [84] showed that 6% of obese elderly subjects (BMI ≥ 30 kg/m2) were also undernourished or at risk of undernutrition. In the event of an acute event, obese elderly people may have a better chance of survival, particularly because of their greater functional reserves. This observation is also made in younger obese or overweight subjects. Akinnusi et al. [85] show in their meta-analysis of patients admitted to intensive care that obese subjects have a similar mortality to non-obese subjects. In 2013, the meta-analysis by Flegal et al. [76] confirmed in a population without any particular pathology that overweight people (BMI > 25 kg/m²) (all types of obesity and all ages) had a higher overall mortality rate, whatever the cause. However, mildly overweight people (BMI ≥ 25 and <30 kg/m²) had lower all-cause mortality than normal weight people (BMI < 25 kg/m²). Thus, this advantage was found regardless of age.
Several mechanisms could explain “obesity paradox”. Probably, there are “good adipose tissues” in elderly subjects. In the literature, overweight or obesity, defined by high level of BMI, is shown to have positive influence on prothrombotic factors, production of certain cytokines, or NT-proBNP levels. Adipokine produced by adipose tissue seems to be cardioprotective [86]. Obesity could have a protective effect against progression or consequences of some chronic diseases. High BMI could also reflect better nutritional status and adequate muscle reserves. Casas-Vara et al. [87] showed better nutritional status in overweight or obese elderly people with heart failure.
Our systematic review has limitations. Although the WHO has proposed thresholds for BMI, the authors used different thresholds in their respective studies. In addition, the outcomes were also different between the studies. This made it difficult to compare the studies, and precluded meta-analysis. The age variable was missing in 14.0% of cases (8/57).
However, this work covers a large number of studies, totalling more than 1,120,000 people aged 65 years or over, with varying medical conditions and in different settings. The follow-up time of the studies ranged from 30 days to 156 months (even though the majority of studies have a long-term follow-up). These differences in follow-up time may make comparison difficult. In addition, there is no information on BMI variation over time, especially for studies with long-term follow-up. Weight loss or gain between baseline measurement and death could have a significant impact. The fact that only studies conducted in subjects aged 65 years or older were selected gives a certain homogeneity to this systematic review in terms of population. Finally, all studies were evaluated for methodological quality using the NOS, and were found to be of high quality.
5. Conclusions
The findings of this systematic review are in favour of the existence of an obesity paradox, which could more specifically concern older subjects with a comorbidity and/or experiencing an acute event. Nevertheless, because BMI does not reflect body composition, the term “BMI paradox” would be more appropriate. The influence of the level of BMI remains unclear. These findings should help guide strategies for nutritional counselling in the older population.
Acknowledgments
Thanks to Fiona Ecarnot for editorial assistance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15071780/s1, Table S1: Outcome and results of association between body mass index groups and mortality in aged adults (detailed information).
Author Contributions
L.G. and M.D. conceived and designed the study, prepared the material, collected the data, and performed the analysis. They wrote the first draft of the manuscript, and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data could be made available on reasonable request at moustapha.drame@chu-martinique.fr.
Conflicts of Interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding Statement
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. The APC was funded by tht University Hospitals of Martinique.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Frydrych L.M., Bian G., O’Lone D.E., Ward P.A., Delano M.J. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J. Leukoc. Biol. 2018;104:525–534. doi: 10.1002/JLB.5VMR0118-021RR. [DOI] [PubMed] [Google Scholar]
- 2.Liu Z., Sanossian N., Starkman S., Avila-Rinek G., Eckstein M., Sharma L.K., Liebeskind D., Conwit R., Hamilton S. Adiposity and Outcome After Ischemic Stroke: Obesity Paradox for Mortality and Obesity Parabola for Favorable Functional Outcomes. Stroke. 2021;52:144–151. doi: 10.1161/STROKEAHA.119.027900. [DOI] [PubMed] [Google Scholar]
- 3.Powell-Wiley T.M., Poirier P., Burke L.E., Després J.-P., Gordon-Larsen P., Lavie C.J., Lear S.A., Ndumele C.E., Neeland I.J., Sanders P., et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2021;143:e984–e1010. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hales C.M., Carroll M.D., Fryar C.D., Ogden C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States. 2017–2018. NCHS Data Brief. 2020;360:1–8. [PubMed] [Google Scholar]
- 5.Ward Z.J., Bleich S.N., Cradock A.L., Barrett J.L., Giles C.M., Flax C., Gortmaker S.L. Projected, U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019;381:2440–2450. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
- 6.Kwon Y., Kim H.J., Park S., Park Y.G., Cho K.H. Body mass index-related mortality in patients with type 2 diabetes and heterogeneity in obesity paradox studies: A dose-response meta-analysis. PLoS ONE. 2017;12:e0168247. doi: 10.1371/journal.pone.0168247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skinner J.S., Abel W.M., McCoy K., Wilkins C.H. Exploring the “Obesity Paradox” as a Correlate of Cognitive and Physical Function in Community-dwelling Black and White Older Adults. Ethn. Dis. 2017;27:387–394. doi: 10.18865/ed.27.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamazaki K., Suzuki E., Yorifuji T., Tsuda T., Ohta T., Ishikawa-Takata K., Doi H. Is there an obesity paradox in the Japanese elderly population? A community-based cohort study of 13,280 men and women. Geriatr. Gerontol. Int. 2017;17:1257–1264. doi: 10.1111/ggi.12851. [DOI] [PubMed] [Google Scholar]
- 9.Antonopoulos A.S., Tousoulis D. The molecular mechanisms of obesity paradox. Cardiovasc. Res. 2017;113:1074–1086. doi: 10.1093/cvr/cvx106. [DOI] [PubMed] [Google Scholar]
- 10.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomised Studies in Meta-Analyses: The Ottawa Hospital. 2013. [(accessed on 1 November 2022)]. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 11.Amin R.M., Raad M., Rao S.S., Musharbash F., Best M.J., Amanatullah D.F. Survival bias may explain the appearance of the obesity paradox in hip fracture patients. Osteoporos. Int. 2021;32:2555–2562. doi: 10.1007/s00198-021-06046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Moheb M., Jia Z., Qin H., El Hechi M.W., Nordestgaard A.T., Lee J.M., Kaafarani H.M. The Obesity Paradox in Elderly Patients Undergoing Emergency Surgery: A Nationwide Analysis. J. Surg. Res. 2021;265:195–203. doi: 10.1016/j.jss.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Lin Y.-K., Wang C.-C., Yen Y.-F., Chen L.-J., Ku P.-W., Chen C.-C., Lai Y.-J. Association of body mass index with all-cause mortality in the elderly population of Taiwan: A prospective cohort study. Nutr. Metab. Cardiovasc. Dis. NMCD. 2021;31:110–118. doi: 10.1016/j.numecd.2020.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Tapia C., Diot T., Oubaya N., Paillaud E., Poisson J., Gisselbrecht M., Morisset L., Caillet P., Baudin A., Pamoukdjian F., et al. The obesity paradox for mid- and long-term mortality in older cancer patients: A prospective multicenter cohort study. Am. J. Clin. Nutr. 2020;113:129–141. doi: 10.1093/ajcn/nqaa238. [DOI] [PubMed] [Google Scholar]
- 15.Lai K.-Y., Wu T.-H., Liu C.-S., Lin C.-H., Lin C.-C., Lai M.-M., Lin W.-Y. Body mass index and albumin levels are prognostic factors for long-term survival in elders with limited performance status. Aging. 2020;12:1104–1113. doi: 10.18632/aging.102642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider M., Potthoff A.-L., Scharnböck E., Heimann M., Schäfer N., Weller J., Schaub C., Jacobs A.H., Güresir E., Herrlinger U., et al. Newly diagnosed glioblastoma in geriatric (65+) patients: Impact of patients frailty, comorbidity burden and obesity on overall survival. J. Neurooncol. 2020;149:421–427. doi: 10.1007/s11060-020-03625-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishida M.M., Okura M., Ogita M., Aoyama T., Tsuboyama T., Arai H. Two-Year Weight Loss but Not Body Mass Index Predicts Mortality and Disability in an Older Japanese Community-Dwelling Population. J. Am. Med. Dir. Assoc. 2019;20:1654.e11–1654.e18. doi: 10.1016/j.jamda.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 18.Om S.Y., Ko E., Ahn J.-M., Kang D.-Y., Lee K., Kwon O., Lee P.H., Lee S.-W., Kim H.J., Kim J.B., et al. Relation of Body Mass Index to Risk of Death or Stroke in Patients Who Underwent Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2019;123:638–643. doi: 10.1016/j.amjcard.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Yoshihisa A., Sato T., Kajimoto K., Sato N., Takeishi Y. Acute Decompensated Heart Failure Syndromes i. Heterogeneous impact of body mass index on in-hospital mortality in acute heart failure syndromes: An analysis from the ATTEND Registry. Eur. Heart J. Acute Cardiovasc. Care. 2019;8:589–598. doi: 10.1177/2048872617703061. [DOI] [PubMed] [Google Scholar]
- 20.Crotti G., Gianfagna F., Bonaccio M., Di Castelnuovo A., Costanzo S., Persichillo M., Iacoviello L. Body Mass Index and Mortality in Elderly Subjects from the Moli-Sani Study: A Possible Mediation by Low-Grade Inflammation? Immunol. Investig. 2018;47:774–789. doi: 10.1080/08820139.2018.1538237. [DOI] [PubMed] [Google Scholar]
- 21.De Palma R., Ivarsson J., Feldt K., Saleh N., Ruck A., Linder R., Settergren M. The obesity paradox: An analysis of pre-procedure weight trajectory on survival outcomes in patients undergoing transcatheter aortic valve implantation. Obes. Res. Clin. Pract. 2018;12:51–60. doi: 10.1016/j.orcp.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Kim H., Yoon J.L., Lee A., Jung Y., Kim M.Y., Cho J.J., Ju Y.S. Prognostic effect of body mass index to mortality in Korean older persons. Geriatr. Gerontol. Int. 2018;18:538–546. doi: 10.1111/ggi.13213. [DOI] [PubMed] [Google Scholar]
- 23.Lv Y.-B., Liu S., Yin Z.-X., Gao X., Kraus V.B., Mao C., Yuan J.-Q., Zhang J., Luo J.-S., Chen H.-S., et al. Associations of Body Mass Index and Waist Circumference with 3-Year All-Cause Mortality Among the Oldest Old: Evidence from a Chinese Community-Based Prospective Cohort Study. J. Am. Med. Dir. Assoc. 2018;19:672–678.e4. doi: 10.1016/j.jamda.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Souto Barreto P., Cadroy Y., Kelaiditi E., Vellas B., Rolland Y. The prognostic value of body-mass index on mortality in older adults with dementia living in nursing homes. Clin. Nutr. 2017;36:423–428. doi: 10.1016/j.clnu.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Wu S., Yang Y.-M., Zhu J., Wan H.-B., Wang J., Zhang H., Shao X.-H. Impact of Age on the Association Between Body Mass Index and All-Cause Mortality in Patients with Atrial Fibrillation. J. Nutr. Heal. Aging. 2017;21:1125–1132. doi: 10.1007/s12603-016-0863-2. [DOI] [PubMed] [Google Scholar]
- 26.Flodin L., Laurin A., Lokk J., Cederholm T., Hedstrom M. Increased 1-year survival and discharge to independent living in overweight hip fracture patients: A prospective study of 843 patients. Acta Orthop. 2016;87:146–151. doi: 10.3109/17453674.2015.1125282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuo J.F., Hsieh Y.T., Mao I.C., Lin S.D., Tu S.T., Hsieh M.C. The Association Between Body Mass Index and All-Cause Mortality in Patients With Type 2 Diabetes Mellitus: A 5.5-Year Prospective Analysis. Medicine. 2015;94:e1398. doi: 10.1097/MD.0000000000001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buys D.R., Roth D.L., Ritchie C.S., Sawyer P., Allman R.M., Funkhouser E.M., Locher J.L. Nutritional risk and body mass index predict hospitalization, nursing home admissions, and mortality in community-dwelling older adults: Results from the UAB Study of Aging with 8.5 years of follow-up. J. Gerontol. Biol. Sci. Med. Sci. 2014;69:1146–1153. doi: 10.1093/gerona/glu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford D.W., Hartman T.J., Do C.S., Wood C., Mitchell D.C., Erickson P., Bailey R., Smiciklas-Wright H., Coffman D.L., Jensen G.L. Body mass index, poor diet quality, and health-related quality of life are associated with mortality in rural older adults. J. Nutr. Gerontol. Geriatr. 2014;33:23–34. doi: 10.1080/21551197.2014.875819. [DOI] [PubMed] [Google Scholar]
- 30.Lang P.O., Mahmoudi R., Novella J.-L., Tardieu E., Bertholon L.-A., Nazeyrollas P., Blanchard F., Jolly D., Dramé M. Is obesity a marker of robustness in vulnerable hospitalized aged populations? Prospective, multicenter cohort study of 1306 acutely ill patients. J. Nutr. Health Aging. 2014;18:66–74. doi: 10.1007/s12603-013-0352-9. [DOI] [PubMed] [Google Scholar]
- 31.Lee Y., Kim J., Han E.S., Ryu M., Cho Y., Chae S. Frailty and body mass index as predictors of 3-year mortality in older adults living in the community. Gerontology. 2014;60:475–482. doi: 10.1159/000362330. [DOI] [PubMed] [Google Scholar]
- 32.Wu C.Y., Chou Y.C., Huang N., Chou Y.J., Hu H.Y., Li C.P. Association of body mass index with all-cause and cardiovascular disease mortality in the elderly. PLoS ONE. 2014;9:e102589. doi: 10.1371/journal.pone.0102589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L., Peng L., Liu L., Lin M., Lan C., Chang P. Body mass index, health status, and mortality of older Taiwanese men: Overweight good, underweight bad, obesity neutral. J. Am. Geriatr. Soc. 2013;61:2233–2234. doi: 10.1111/jgs.12587. [DOI] [PubMed] [Google Scholar]
- 34.Dahl A.K., Fauth E.B., Ernsth-Bravell M., Hassing L.B., Ram N., Gerstof D. Body mass index, change in body mass index, and survival in old and very old persons. J. Am. Geriatr. Soc. 2013;61:512–518. doi: 10.1111/jgs.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakazawa A., Nakamura K., Kitamura K., Yoshizawa Y. Association between body mass index and mortality among institutionalized elderly adults in Japan. Environ. Health Prev. Med. 2013;18:502–506. doi: 10.1007/s12199-013-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takata Y., Ansai T., Soh I., Awano S., Nakamichi I., Akifusa S., Goto K., Yoshida A., Fujii H., Fujisawa R., et al. Body mass index and disease-specific mortality in an 80-year-old population at the 12-year follow-up. Arch. Gerontol. Geriatr. 2013;57:46–53. doi: 10.1016/j.archger.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Tseng C.H. Obesity paradox: Differential effects on cancer and noncancer mortality in patients with type 2 diabetes mellitus. Atherosclerosis. 2013;226:186–192. doi: 10.1016/j.atherosclerosis.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Veronese N., De Rui M., Toffanello E.D., De Ronch I., Perissinotto E., Bolzetta F., D’Avanzo B., Cardin F., Coin A., Manzato E., et al. Body mass index as a predictor of all-cause mortality in nursing home residents during a 5-year follow-up. J. Am. Med. Dir. Assoc. 2013;14:53–57. doi: 10.1016/j.jamda.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Woo J., Yu R., Yau F. Fitness, fatness and survival in elderly populations. Age. 2013;35:973–984. doi: 10.1007/s11357-012-9398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto M., Mouillet G., Oguri A., Gilard M., Laskar M., Eltchaninoff H., Fajadet J., Iung B., Donzeau-Gouge P., Leprince P., et al. Effect of body mass index on 30- and 365-day complication and survival rates of transcatheter aortic valve implantation (from the FRench Aortic National CoreValve and Edwards 2 [FRANCE 2] registry) Am. J. Cardiol. 2013;112:1932–1937. doi: 10.1016/j.amjcard.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 41.Zekry D., Herrmann F.R., Vischer U.M. The association between the body mass index and 4-year all-cause mortality in older hospitalized patients. J. Gerontol. Biol. Sci. Med. Sci. 2013;68:705–711. doi: 10.1093/gerona/gls207. [DOI] [PubMed] [Google Scholar]
- 42.de Hollander E.L., Van Zutphen M., Bogers R.P., Bemelmans W.J., De Groot L.C. The impact of body mass index in old age on cause-specific mortality. J. Nutr. Health Aging. 2012;16:100–106. doi: 10.1007/s12603-011-0077-6. [DOI] [PubMed] [Google Scholar]
- 43.Kvamme J.M., Holmen J., Wilsgaard T., Florholmen J., Midthjell K., Jacobsen B.K. Body mass index and mortality in elderly men and women: The Tromso and HUNT studies. J. Epidemiol. Community Health. 2012;66:611–617. doi: 10.1136/jech.2010.123232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mihel S., Milanovic S.M. Association of elevated body mass index and hypertension with mortality: The CroHort study. Coll. Antropol. 2012;36:183–188. doi: 10.5671/ca.2012361s.183. [DOI] [PubMed] [Google Scholar]
- 45.Cereda E., Pedrolli C., Zagami A., Vanotti A., Piffer S., Opizzi A., Rondanelli M., Caccialanza R. Body mass index and mortality in institutionalized elderly. J. Am. Med. Dir Assoc. 2011;12:174–178. doi: 10.1016/j.jamda.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 46.Berraho M., Nejjari C., Raherison C., El Achhab Y., Tachfouti N., Serhier Z., Dartigues J.F., Barberger-Gateau P. Body mass index, disability, and 13-year mortality in older French adults. J. Aging Health. 2010;22:68–83. doi: 10.1177/0898264309349422. [DOI] [PubMed] [Google Scholar]
- 47.Han S.S., Kim K.W., Na K.Y., Chae D.-W., Kim S., Chin H.J. Lean mass index: A better predictor of mortality than body mass index in elderly Asians. J. Am. Geriatr. Soc. 2010;58:312–317. doi: 10.1111/j.1532-5415.2009.02672.x. [DOI] [PubMed] [Google Scholar]
- 48.Kitamura K., Nakamura K., Nishiwaki T., Ueno K., Hasegawa M. Low body mass index and low serum albumin are predictive factors for short-term mortality in elderly Japanese requiring home care. Tohoku J. Exp. Med. 2010;221:29–34. doi: 10.1620/tjem.221.29. [DOI] [PubMed] [Google Scholar]
- 49.Luchsinger J.A., Patel B., Tang M.X., Schupf N., Mayeux R. Body mass index, dementia, and mortality in the elderly. J. Nutr. Health Aging. 2008;12:127–131. doi: 10.1007/BF02982565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Locher J.L., Roth D.L., Ritchie C.S., Cox K., Sawyer P., Bodner E.V., Allman R.M. Body mass index, weight loss, and mortality in community-dwelling older adults. J. Gerontol. Biol. Sci. Med. Sci. 2007;62:1389–1392. doi: 10.1093/gerona/62.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takata Y., Ansai T., Soh I., Akifusa S., Sonoki K., Fujisawa K., Awano S., Kagiyama S., Hamasaki T., Nakamichi I., et al. Association between body mass index and mortality in an 80-year-old population. J. Am. Geriatr. Soc. 2007;55:913–917. doi: 10.1111/j.1532-5415.2007.01170.x. [DOI] [PubMed] [Google Scholar]
- 52.Danninger T., Rezar R., Mamandipoor B., Dankl D., Koköfer A., Jung C., Wernly B., Osmani V. Underweight but not overweight is associated with excess mortality in septic ICU patients. Wien. Klin. Wochenschr. 2021;134:139–147. doi: 10.1007/s00508-021-01912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seino S., Kitamura A., Abe T., Taniguchi Y., Yokoyama Y., Amano H., Nishi M., Nofuji Y., Narita M., Ikeuchi T., et al. Dose-Response Relationships Between Body Composition Indices and All-Cause Mortality in Older Japanese Adults. J. Am. Med. Dir. Assoc. 2020;21:726–733.e4. doi: 10.1016/j.jamda.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 54.Tokarek T.A., Dziewierz A., Sorysz D., Bagienski M., Rzeszutko Ł., Krawczyk-Ożóg A., Kleczyński P. The obesity paradox in patients undergoing transcatheter aortic valve implantation: Is there any effect of body mass index on survival? Kardiol. Pol. 2019;77:190–197. doi: 10.5603/KP.a2018.0243. [DOI] [PubMed] [Google Scholar]
- 55.Keller K., Munzel T., Ostad M.A. Sex-specific differences in mortality and the obesity paradox of patients with myocardial infarction ages > 70 y. Nutrition. 2018;46:124–130. doi: 10.1016/j.nut.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Lee S.H., Kim D.H., Park J.H., Kim S., Choi M., Kim H., Park Y.G. Association between body mass index and mortality in the Korean elderly: A nationwide cohort study. PLoS ONE. 2018;13:e0207508. doi: 10.1371/journal.pone.0207508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng F.W., Gao X., Mitchell D.C., Wood C., Still C.D., Rolston D., Jensen G.L. Body mass index and all-cause mortality among older adults. Obesity. 2016;24:2232–2239. doi: 10.1002/oby.21612. [DOI] [PubMed] [Google Scholar]
- 58.Calabia J., Arcos E., Carrero J.J., Comas J., Valles M. Does the obesity survival paradox of dialysis patients differ with age? Blood Purif. 2015;39:193–199. doi: 10.1159/000374102. [DOI] [PubMed] [Google Scholar]
- 59.Kim N.H., Lee J., Kim T.J., Kim N.H., Choi K.M., Baik S.H., Choi D.S., Pop-Busui R., Park Y., Kim S.G. Body Mass Index and Mortality in the General Population and in Subjects with Chronic Disease in Korea: A Nationwide Cohort Study (2002–2010) PLoS ONE. 2015;10:e0139924. doi: 10.1371/journal.pone.0139924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kubota Y., Iso H., Tamakoshi A., Group J.S. Association of Body Mass Index and Mortality in Japanese Diabetic Men and Women Based on Self-Reports: The Japan Collaborative Cohort (JACC) Study. J. Epidemiol. 2015;25:553–558. doi: 10.2188/jea.JE20150011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shil Hong E., Khang A.R., Roh E., Jeong Ku E.U., An Kim Y.E., Min Kim K., Lim S. Counterintuitive relationship between visceral fat and all-cause mortality in an elderly Asian population. Obesity. 2015;23:220–227. doi: 10.1002/oby.20914. [DOI] [PubMed] [Google Scholar]
- 62.Clark D.O., Gao S., Lane K.A., Callahan C.M., Baiyewu O., Ogunniyi A., Hendrie H.C. Obesity and 10-year mortality in very old African Americans and Yoruba-Nigerians: Exploring the obesity paradox. J. Gerontol. Biol. Sci. Med. Sci. 2014;69:1162–1169. doi: 10.1093/gerona/glu035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murphy R.A., Reinders I., Garcia M.E., Eiriksdottir G., Launer L.J., Benediktsson R., Gudnason V., Jonsson P.V., Harris T.B. Adipose tissue, muscle, and function: Potential mediators of associations between body weight and mortality in older adults with type 2 diabetes. Diabetes Care. 2014;37:3213–3219. doi: 10.2337/dc14-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamauchi Y., Hasegawa W., Yasunaga H., Sunohara M., Jo T., Matsui H., Fushimi K., Takami K., Nagase T. Paradoxical association between body mass index and in-hospital mortality in elderly patients with chronic obstructive pulmonary disease in Japan. Int. J. Chronic Obstr. Pulm. Dis. 2014;9:1337–1346. doi: 10.2147/COPD.S75175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsai A.C., Hsiao M.L. The association of body mass index (BMI) with all-cause mortality in older Taiwanese: Results of a national cohort study. Arch Gerontol. Geriatr. 2012;55:217–220. doi: 10.1016/j.archger.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 66.Lea J.P., Crenshaw D.O., Onufrak S.J., Newsome B.B., McClellan W.M. Obesity, end-stage renal disease, and survival in an elderly cohort with cardiovascular disease. Obesity. 2009;17:2216–2222. doi: 10.1038/oby.2009.70. [DOI] [PubMed] [Google Scholar]
- 67.Grabowski D.C., Ellis J.E. High body mass index does not predict mortality in older people: Analysis of the Longitudinal Study of Aging. J. Am. Geriatr. Soc. 2001;49:968–979. doi: 10.1046/j.1532-5415.2001.49189.x. [DOI] [PubMed] [Google Scholar]
- 68.Kananen L., Eriksdotter M., Boström A., Kivipelto M., Annetorp M., Metzner C., Jerlardtz V.B., Engström M., Johnson P., Lundberg L., et al. Body mass index and Mini Nutritional Assessment-Short Form as predictors of in-geriatric hospital mortality in older adults with COVID-19. Clin. Nutr. 2022;41:2973–2979. doi: 10.1016/j.clnu.2021.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lai C.C., Wang C.Y., Wang Y.H., Hsueh S.C., Ko W.C., Hsueh P.R. Global epidemiology of coronavirus disease 2019: Disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. Int. J. Antimicrob. Agents. 2020;55:105946. doi: 10.1016/j.ijantimicag.2020.105946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yaffe K., Fox P., Newcomer R., Sands L., Lindquist K., Dane K., Covinsky K.E. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287:2090–2097. doi: 10.1001/jama.287.16.2090. [DOI] [PubMed] [Google Scholar]
- 71.Heymsfield S.B., Peterson C.M., Thomas D.M., Heo M., Schuna J.M., Jr. Why are there race/ethnic differences in adult body mass index-adiposity relationships? A quantitative critical review. Obes. Rev. 2016;17:262–275. doi: 10.1111/obr.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jeong S.M., Lee D.H., Rezende L.F.M., Giovannucci E.L. Different correlation of body mass index with body fatness and obesity-related biomarker according to age, sex and race-ethnicity. Sci Rep. 2023;13:3472. doi: 10.1038/s41598-023-30527-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coutinho T., Goel K., de Sá D.C., Kragelund C., Kanaya A.M., Zeller M., Park J.-S., Kober L., Torp-Pedersen C., Cottin Y., et al. Central obesity and survival in subjects with coronary artery disease: A systematic review of the literature and collaborative analysis with individual subject data. J. Am. Coll. Cardiol. 2011;57:1877–1886. doi: 10.1016/j.jacc.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 74.de Koning L., Merchant A.T., Pogue J., Anand S.S. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: Meta-regression analysis of prospective studies. Eur. Heart J. 2007;28:850–856. doi: 10.1093/eurheartj/ehm026. [DOI] [PubMed] [Google Scholar]
- 75.Okorodudu D.O., Jumean M.F., Montori V.M., Romero-Corral A., Somers V.K., Erwin P.J., Lopez-Jimenez F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: A systematic review and meta-analysis. Int. J. Obes. 2010;34:791–799. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 76.Flegal K.M., Kit B.K., Orpana H., Graubard B.I. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gao F., Wang Z.J., Shen H., Yang S.W., Nie B., Zhou Y.J. Impact of obesity on mortality in patients with diabetes: Meta-analysis of 20 studies including 250,016 patients. J. Diabetes Investig. 2018;9:44–54. doi: 10.1111/jdi.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao X., Gang X., He G., Li Z., Lv Y., Han Q., Wang G. Obesity Increases the Severity and Mortality of Influenza and COVID-19: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2020;11:595109. doi: 10.3389/fendo.2020.595109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Golabek T., Bukowczan J., Szopinski T., Chlosta P., Lipczynski W., Dobruch J., Borowka A. Obesity and renal cancer incidence and mortality--a systematic review of prospective cohort studies. Ann. Agric. Environ. Med. 2016;23:37–43. doi: 10.5604/12321966.1196850. [DOI] [PubMed] [Google Scholar]
- 80.Liu X., Ju W., Huo C., Zhang S., Wang X., Huang K. Overweight and Obesity as Independent Factors for Increased Risk of Hepatocellular Cancer-Related Mortality: A Meta-Analysis. J. Am. Coll. Nutr. 2021;40:287–293. doi: 10.1080/07315724.2020.1751007. [DOI] [PubMed] [Google Scholar]
- 81.Tian S., Xu Y. Association of sarcopenic obesity with the risk of all-cause mortality: A meta-analysis of prospective cohort studies. Geriatr. Gerontol. Int. 2016;16:155–166. doi: 10.1111/ggi.12579. [DOI] [PubMed] [Google Scholar]
- 82.Sousa-Santos A.R., Afonso C., Borges N., Santos A., Padrão P., Moreira P., Amaral T.F. Sarcopenia and Undernutrition Among Portuguese Older Adults: Results from Nutrition UP 65 Study. Food Nutr. Bull. 2018;39:487–492. doi: 10.1177/0379572118765801. [DOI] [PubMed] [Google Scholar]
- 83.Cereda E., Pedrolli C., Klersy C., Bonardi C., Quarleri L., Cappello S., Caccialanza R. Nutritional status in older persons according to healthcare setting: A systematic review and meta-analysis of prevalence data using MNA((R)) Clin. Nutr. 2016;35:1282–1290. doi: 10.1016/j.clnu.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 84.Sousa-Santos A.R., Afonso C., Borges N., Santos A., Padrão P., Moreira P., Amaral T.F. Sarcopenia, physical frailty, undernutrition and obesity cooccurrence among Portuguese community-dwelling older adults: Results from Nutrition UP 65 cross-sectional study. BMJ Open. 2020;10:e033661. doi: 10.1136/bmjopen-2019-033661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Akinnusi M.E., Pineda L.A., El Solh A.A. Effect of obesity on intensive care morbidity and mortality: A meta-analysis. Crit. Care Med. 2008;36:151–158. doi: 10.1097/01.CCM.0000297885.60037.6E. [DOI] [PubMed] [Google Scholar]
- 86.Donini L.M., Pinto A., Giusti A.M., Lenzi A., Poggiogalle E. Obesity or BMI Paradox? Beneath the Tip of the Iceberg. Front. Nutr. 2020;7:53. doi: 10.3389/fnut.2020.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Casas-Vara A., Santolaria F., Fernandez-Bereciartua A., Gonzalez-Reimers E., Garcia-Ochoa A., Martinez-Riera A. The obesity paradox in elderly patients with heart failure: Analysis of nutritional status. Nutrition. 2012;28:616–622. doi: 10.1016/j.nut.2011.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data could be made available on reasonable request at moustapha.drame@chu-martinique.fr.