Abstract
Magnesium (Mg) has a vital role in the human body, and the kidney is a key organ in the metabolism and excretion of this cation. The objective of this work is to compile the available evidence regarding the role that Mg plays in health and disease, with a special focus on the elderly population with chronic kidney disease (CKD) and the eventual sex differences. A narrative review was carried out by executing an exhaustive search in the PubMed, Scopus, and Cochrane databases. Ten studies were found in which the role of Mg and sex was evaluated in elderly patients with CKD in the last 10 years (2012–2022). The progression of CKD leads to alterations in mineral metabolism, which worsen as the disease progresses. Mg can be used as a coadjuvant in the treatment of CKD patients to improve glomerular filtration, but its use in clinical applications needs to be further characterized. In conclusion, there’s a need for well-designed prospective clinical trials to advise and standardize Mg supplementation in daily clinical practice, taking age and sex into consideration.
Keywords: magnesium, elderly, kidney function, chronic kidney disease, sex
1. Introduction
Magnesium (Mg) is one of the most important cations in the human body, but it has not been fully considered in preclinical and clinical fields. Indeed, the pathophysiological and clinical importance of Mg has only recently been acknowledged. Mg is an essential element in energy storage and utilization. This essential cation is also involved in the synthesis, degradation, and polymerization of deoxyribonucleic acid, ribosome binding to ribonucleic acid, and protein synthesis [1].
The circulating concentrations of magnesium are regulated under normal physiological conditions and are maintained in a narrow range (0.75–0.95 mmol/L). This is managed mainly by its intestinal absorption and renal excretion [1,2].
Mg has special clinical relevance in states of decompensated cirrhosis, renal failure, intestinal malabsorption syndrome, prolonged high-dose diuretic therapy, acute pancreatitis, and extensive burns [3], among others.
1.1. Magnesium Metabolism
Mg is the second most frequent cation within the cell [4]. Further, it can be categorized into three fractions: ionized (55–70%), protein-bound (20–30%), and complexed with anions such as phosphate, bicarbonate, citrate, or sulfate (5–15%). Both the ionized and complexed Mg are the ultrafilterable fraction of Mg, which represents the portion of total serum Mg that can be excreted by the kidney [5].
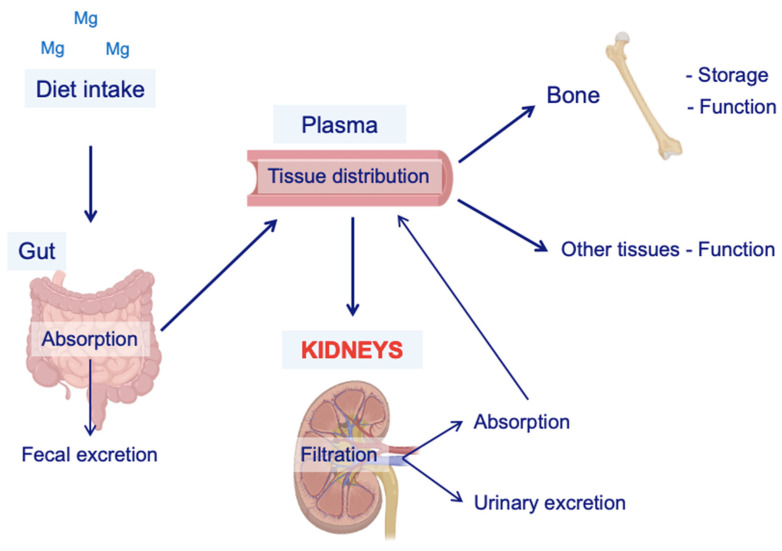
Mg can be stored in different forms (Figure 1). The bone storage of Mg is intimately associated with carboxyl apatite crystals, while the intracellular Mg reservoir is bound to lipoproteins, nucleoproteins, ribonucleic acids, and adenosine diphosphate [5,6]. 20% of the total Mg is in the plasma, bound to the plasma proteins. Thus, Mg concentrations are minimally affected by the variations in proteinemia, contrary to what happens with calcium [7]. Furthermore, the low amount of Mg in the extracellular fluid vs. intracellular and bone deposits explains why serum Mg is not a parameter of absolute reliability for assessing the state of Mg depletion or repletion [8].
Figure 1.
Schematic representation of Mg metabolism. The main source of Mg is diet intake. Once in the body, a fraction of the Mg contained in the food is absorbed by the gut, and the rest is eliminated via fecal excretion. Then, Mg is incorporated into the plasma to be distributed to the other organs for self-consumption and storage (e.g., the bones). Kidneys are the key regulators of Mg balance because of their ability to retake or excrete it, depending on the demands of the body. Mg = magnesium.
Mg balance is highly dependent on dietary absorption. The adult daily Mg requirement is estimated to be 200–400 mg, and the dietary recommendation is 320 mg/day for women and 420 mg/day for men [9]. This cation is present in almost all foods and is especially abundant in green leafy vegetables because it is part of the composition of chlorophyll [10,11]. Intestinal Mg absorption mainly occurs in the distal small intestine, specifically from the distal duodenum (D3) to the ileum, by two reverse flows: a facilitated flow of transcellular absorption and another of passive paracellular secretion (Figure 1). On the other hand, the colon has a limited capacity to absorb Mg [11,12].
In healthy adults, Mg balance depends primarily on adjusting the renal Mg excretion to the intestinal absorption of the cation. This balance should be adjusted to maintain or restore the body’s Mg content within the physiological ranges [13].
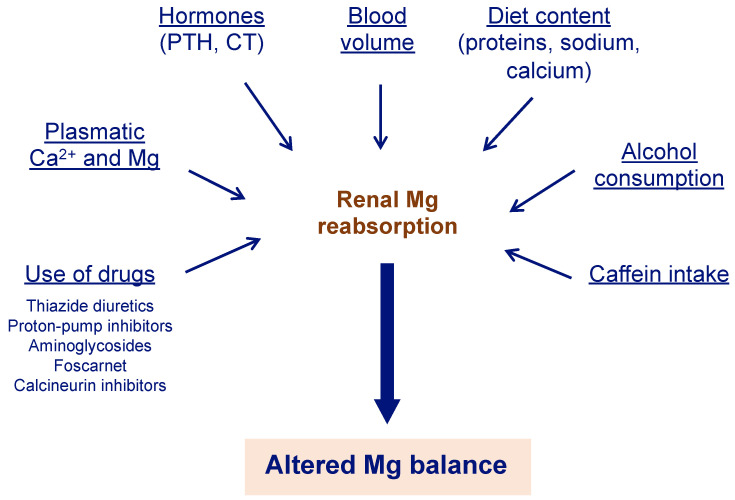
Several factors can alter Mg balance, leading to hypermagnesemia or hypomagnesemia (Figure 2) [14]. Hypomagnesemia is much more common and does not present specific symptoms until the deficiency is severe [15,16].
Figure 2.
Different factors can alter renal Mg reabsorption. Mg absorption in the kidneys is a crucial step to ensure Mg balance. Therefore, different causes that alter this step can cause impaired Mg metabolism, with hypomagnesemia being the most common alteration. Mg can be obtained from legumes, dark and green leafy vegetables, nuts, seeds, whole grains, and fortified cereals, among others [9]. Ca2+ = calcium; CT = calcitonin; Mg = magnesium; PTH = parathyroid hormone.
Moderate hypomagnesemia is defined in a range of 0.50–0.65 mmol/L Mg and is usually asymptomatic, whereas severe and symptomatic hypomagnesemia appears when serum Mg levels are below 0.5 mmol/L [17]. The magnesiuria measurement helps in determining whether the origin of hypomagnesemia is renal (magnesiuria greater than 2 mmol/24 h) or extra-renal (magnesiuria less than or equal to 1 mmol/24 h) [16].
1.2. Kidney Function in Relation to Magnesium and Other Implicated Electrolytes
The kidney has a vital function in the regulation of Mg balance. Mg is filtered in the glomerulus and reabsorbed in the proximal tubule via a paracellular pathway in the loop of Henle [18]. The loop of Henle is a segment with reduced permeability to water, so its reabsorption is carried out through the paracellular route, which has a selective permeability to calcium and Mg. Furthermore, this process is facilitated by the binding proteins, claudin 16 and claudin 19 [19].
The distal convoluted tubule is also an essential segment for the regulation of renal Mg reabsorption. In this segment, 10% of the filtered Mg load is reabsorbed, which allows for adjustment of the final excretion of this cation according to the needs of the organism [20]. In this location, the reabsorption of Mg is independent of sodium reabsorption (transcellular) and involves molecular pathways of transport different from those of calcium [19].
The main determinants of renal reabsorption of Mg include variations in the normal levels of calcium and blood volume, which modify the reabsorption in the kidney of water and solutes, and hence of Mg. In other words, hypervolemia inhibits its reabsorption, while hypovolemia stimulates it [19,21]. In addition, hypermagnesemia and hypercalcemia inhibit the reabsorption of Mg in the loop of Henle by stimulating the calcium-sensing receptor (CaSR) [21].
Parathyroid hormone (PTH) and other hormones, capable of activating the cAMP pathway, stimulate the reabsorption of calcium in the kidney. There are no known hormones that particularly regulate Mg but PTH and calcitonin (CT) are known to have a positive effect on Mg balance (Figure 1) [22]. This effect may be related to the hypermagnesemic tendency of patients with hyperparathyroidism [23].
1.3. Kidney Disease and Magnesium
As mentioned, the kidney has a vital role in maintaining a normal concentration of Mg. Furthermore, when the glomerular filtration rate falls, the kidney’s ability to excrete Mg decreases accordingly [24].
In chronic kidney disease (CKD), there is a tendency towards hypermagnesemia, but it depends on the severity of the disease. For example, in CKD stages 1–3, an increase in fractional Mg excretion compensates for the loss of renal function, and, as a consequence, Mg levels remain within normal ranges. However, in advanced CKD (stages 4–5), compensatory systems are not sufficient, and the fraction of filtered Mg excreted increases as a result of impaired tubular reabsorption [25]. This becomes more evident when the glomerular filtration rate drops below 10 mL/min. In other words, the compensatory increase in fractional excretion of Mg is inadequate to prevent and increase serum Mg concentrations [26].
CKD patients in treatment with dialysis have both ionized and total Mg concentrations that tend to be higher than normal but always depend on the degree of residual kidney function [13]. However, patients with end-stage renal disease who are on treatment with dialysis usually have normal levels or can sometimes present hypomagnesemia. This could be a consequence of the diet, drug side effects (Figure 2) [14], or dialysate Mg concentration [27].
Additionally, it is common for CKD patients to have impaired intestinal Mg absorption compared to healthy individuals [28].
It has been previously shown that CKD patients with hypomagnesemia have a higher risk of increased vascular calcification and consequently increased cardiovascular risk, leading to a higher risk of mortality in dialysis patients [29,30,31]. On the other hand, hypomagnesemia is commonly related to patients that endure renal transplantation, as a consequence of the immunosuppressors that are used in the treatment to avoid graft failure [32]. Other authors have found a relationship with new-onset diabetes after renal transplantation, making a connection with patients that have post-transplant hypomagnesemia [15,33,34,35].
1.4. The Aging Kidney
In world demographics, life expectancy continues to increase; therefore, it is essential to consider the aging process as a variable in clinical and preclinical research. In particular, aging has a pivotal role in the structural and functional changes that occur in the kidney during our lives [36]. For example, the kidney experiences a progressive functional decline along with aging, as well as macroscopic and microscopic histological alterations, which can be accentuated by systemic comorbidities or by pre-existing kidney disease [37].
At the macroscopic scale, there is the formation of simple renal cysts and roughness of the kidney surface [38]. Even if cysts are formed in one or both kidneys, they usually do not cause enlargement of the organ [39,40]. These renal cysts have been correlated with hypertension, decreased renal size, and functional changes [41,42].
Kidney volume is an important indicator of renal dysfunction, and it is demonstrated to progressively decline with age. For example, the study conducted by Roseman and collaborators confirmed that kidney volume decreased by approximately 16 cm3 per decade over the age of 60 years [43]. Likewise, Wang and colleagues reported that kidney volume reduces by 22 cm3 per decade over the age of 50 years, in addition to a reduction in parallel of the renal cortex [44].
Microscopic changes associated with aging include nephrosclerosis, thickening of the glomerular basement membrane, mesangial widening, and increased extracellular matrix accumulation in aging kidneys [45].
Some reports showed that by the age of 30, the kidney losses approximately 6000–6500 nephrons each year, which has been correlated with the annual reduction of the glomerular filtration associated with the aging process [11,37]. Although it has been less studied, there is evidence suggesting that renal tubular function also declines progressively with aging [46].
Kidney function is a vital predictor of longevity, and the age-related decline in renal function shows several consequences for the quality of life [47]. Due to a gradual deterioration of the renal function reserve, kidney aging increases the risk of acute kidney injury and CKD [48,49]. Furthermore, 65-year-old patients (or older) are at higher risk of end-stage renal disease and drug-related nephrotoxicity [50].
1.5. Magnesium Alterations in Older Population
A relationship between decreasing Mg serum levels and aging has been reported. However, it is not clear whether this association is with the aging process or with the presence of diseases or pathological alterations in kidney function [50].
Several studies have shown that, worldwide, the average dietary intake is often inadequate or significantly lower, while the Mg requirements for body functioning do not change with age [51,52,53].
Aging seems to be a risk factor for inadequate Mg levels due to reduced intestinal absorption, and this could be related to the decrease in vitamin D levels [54]. Another reason that supports this statement is the increased urinary excretion of Mg [45]. As mentioned earlier, with advanced age, renal function and tubular reabsorption decline [46]. In addition, there are other factors that influence the decrease in Mg in elderly patients related to comorbidities and polypharmacotherapy [55]. For example, diuretic therapy may cause excessive urinary loss of Mg, and diuretic-induced hypomagnesemia is often accompanied by hypokalemia [14].
Hypomagnesemia may be present in about 40% of patients with hypokalemia, and correction of the Mg deficit is required to achieve correction of the potassium deficit [56]. As a result, it is recommended to assess Mg levels in patients with hypokalemia [11]. Some therapies that are commonly used in older adults can also contribute to Mg deficit (Figure 2) [14]. For example, proton-pump inhibitors are widely used by patients and prescribed by physicians and are highly toxic to the kidneys, causing hypomagnesemia, which, in turn, can lead to acute kidney injury that can progress to chronic kidney disease and worsen if the disease is already established [57].
2. Materials and Methods
The first searches were carried out in June 2022, combining the terms ‘magnesium’ and ‘ageing’ in the PubMed and Scopus databases.
Later, it was expanded with a combination, using the Boolean operators “AND” and “OR” as appropriate, of the terms ‘kidney function’, ‘hypermagnesemia’, ‘hypomagnesemia’, ‘gender’, ‘sex’, ‘ageing’, ‘elderly’, ‘geriatric patient’, and ‘elderly’. These searches yielded a considerable number of results, quite a few of which repeated or were not very useful for review, but they gave us an overview of the breadth of the theme.
During the search process, different databases were consulted (PubMed, Scopus, and Cochrane), of which the articles corresponding to the PICO search and through the MeSH (Medical Subject Headings) were taken into account.
The combination of terms that yielded the best results in all search engines was as follows: (Magnesium) AND (kidney disease): (“magnesium”[MeSH Terms] OR “magnesium”[All Fields] OR “magnesium s”[All Fields] OR “magnesiums”[All Fields]) AND (“kidney diseases”[MeSH Terms] OR (“kidney”[All Fields] AND “diseases”[All Fields]) OR “kidney diseases”[All Fields] OR (“kidney”[All Fields] AND “disease”[All Fields]) OR “kidney disease”[All Fields]). Magnesium: “magnesium”[MeSH Terms] OR “magnesium”[All Fields] OR “magnesium’s”[All Fields] OR “magnesiums”[All Fields] kidney disease: “kidney diseases”[MeSH Terms] OR (“kidney”[All Fields] AND “diseases”[All Fields]) OR “kidney diseases”[All Fields] OR (“kidney”[All Fields] AND “disease”[All Fields]) OR “kidney disease”[All Fields].
PICO question: P: Elderly with chronic kidney disease, I: role of Mg, C: sex O: improvement in kidney function.
The aim of this study was to point out the importance of a better understanding of the role of Mg in the elderly population with CKD and the possible differences by sex.
The inclusion criteria were: (a) observational studies or clinical trials that evaluated older patients with kidney disease, taking the role of magnesium into consideration, and (b) studies published in the period 2012–2022.
The exclusion criteria were: (a) articles outside the study period; (b) articles that included variables outside the scope of this study; (c) articles that did not include elderly patients; (d) systematic reviews or articles under review; and (e) articles in a language other than Spanish or English.
The search process involved the consultation of 1482 articles, of which 10 were finally selected.
3. Results
After the exhaustive search, 10 articles were found according to the conditions applied. As shown in Table 1, the 10 articles selected for this review found a positive relationship between high Mg levels in serum and a decreased risk of CKD-related mortality or cardiological complications. Furthermore, Sakaguchi and collaborators found an association between lower Mg levels and (i) increasing age, (ii) lower levels of albumin, calcium, phosphate, and hemoglobin, (iii) higher levels of C-reactive protein and alkaline phosphate, and (iv) a higher prevalence of diabetes mellitus, a history of cardiovascular disease, and hip fracture [58].
Moreover, telomere attrition has been demonstrated to be more prevalent in patients enduring dialysis and has a strong relationship with chronic systemic inflammation [59,60], which is considered the cause and consequence of the senescence in CKD [61], as well as with oxidative stress [62].
In addition, the protective effect of Mg intake on renal function in CKD patients is well established in all articles except one [63]. However, since the effect was not statistically significant, the low Mg group had a slightly more negative or detrimental monthly eGFR slope than the control group.
The relationship between treatment and Mg concentration was just shown in one study [64], which found an association with decreased Mg for proton-pump inhibitors and calcium supplementation. In particular, a higher concentration of Mg was found in the case of calcitriol. Furthermore, loop diuretics had a positive association (statistically significant) with serum Mg, whereas thiazide diuretics had an inverse association and potassium-sparing diuretics had no significant association.
Table 1.
Characteristics of the studies included in this review are divided into sections relevant to the purpose of this study. Abbreviations: Magnesium (Mg), chronic kidney disease (CKD), Estimated Glomerular Filtration Rate (eGFR), Modification of Diet in Renal Disease Equation (MDRD), Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI).
| Authors | Year | Type of Study | Mean Age or Range and Sex | Population | Date | Measures | Results |
|---|---|---|---|---|---|---|---|
| Kanbay et. al. [65] | 2012 | Observational cohort study. |
|
|
|
|
|
| Wyskida et. al. [66] | 2012 | Prospective, open-label, cross-sectional clinical study. |
|
|
|
|
|
| Van Laecke et. al. [67] | 2013 | Retrospective cohort study. |
|
|
|
|
|
| Sakaguchi et. al. [58] | 2013 | Observational cohort study |
|
|
|
|
|
| Lacson et. al. [68] | 2015 | Observational retrospective cohort study. |
|
|
|
|
|
| Rebholz et. al. [69] | 2016 | Prospective cohort study. |
|
|
|
|
|
| Ferrè et. al. [70] | 2017 | A multiethnic, population-based, cohort study. |
|
|
|
|
|
| Farhadnejad et. al. [71] | 2016 | Prospective population-based cohort study. |
|
|
|
|
|
| Azem et. al. [63] | 2020 | Observational cohort study. |
|
|
|
|
|
| Galán Carrillo et. al. [64] | 2021 | Retrospective observational cohort study. |
|
|
|
|
|
4. Discussion
The role of Mg in renal function is supported by all the articles included and analyzed in this review. Furthermore, this relationship is also observed in patients with kidney disease as well as in healthy elderly patients [72,73].
For patients without kidney disease, maintaining a healthy intake of a micronutrient-rich diet reduces the risk of renal failure [71]. For example, it has been proven that hypomagnesemia can lead to an inflammatory, atherogenic, and thrombotic response in the vasculature [74]. Also, it may affect the healing process after vascular injury by regulating endothelial cell migration and proliferation [75]. Additionally, higher Mg intake through oral supplementation can lower blood pressure levels, which is an established risk factor for kidney disease [69,76].
On the other hand, the progression of CKD leads to alterations in mineral metabolism, and as such, its severity increases as the disease advances. Older age has been associated with the occurrence and higher progression of CKD [77]. Moreover, it has also been proven that among CKD patients, hypomagnesemia can increase the risk of progression to end-stage renal disease (ESRD) and cardiovascular disease [67,68,78].
This might be explained by the protective role the Mg exerts in the prevention of arrhythmia and atherosclerosis in ESRD patients [79,80]. Therefore, as Kanbay et. al. proposed, Mg can be used as an important adjuvant for the management of endothelial dysfunction and cardiovascular disease associated with CKD [57].
Also, there are several studies [81,82,83] that show that Mg could provide better clinical outcomes by preventing the progression of vascular calcification in CKD.
Another important association proposed in this review is the one of Mg concentration with risk of mortality in CKD patients [58,66]. We found that higher Mg levels are beneficial in terms of survival. In support of that, Van Laecke et al. confirm an association between hypomagnesemia with an increased risk for death and accelerated kidney function deterioration [67].
Furthermore, a significant association has been shown between a higher Mg intake and a lower incidence of type 2 diabetes mellitus [84] due to the role that Mg has in the improvement of insulin resistance and beta-cell function [85,86].
Nevertheless, there are still two important questions that need to be addressed to establish an optimal clinical practice: What is the correct target range of serum Mg levels and could it be standardized for every patient? What is the best approach to increase serum Mg levels? The answers to these questions could help to define an optimal strategy to manage Mg-related complications and improve the prognosis of CKD patients [87].
Quality of life is another important aspect to take into consideration. Mobility and pain discomfort are the two aspects in patients with moderate renal dysfunction (eGFR of 30.0–59.9 mL/min/1.73 m2) [88]. This not only affects the quality of daily life by making them more dependent patients but also increases the risk of mortality and leads to more comorbidities.
Regarding sex differences, it has been shown that the parenchymal kidney volume in males increased up to middle age and then progressively declined, more sharply after 70 years of age, whereas females trended towards a gradual decrease in kidney volume through life [44,47]. This can be explained by the kidney sex hormone receptors. In a study conducted in an elderly cohort [89], it was shown that Mg levels are strongly and independently associated with the anabolic hormones, testosterone and IGD-1. Therefore, in terms of eGFR, chronic testosterone deficiency may lead to reduced kidney function [90].
Finally, there was only one article [67] in this review that found an increased incidence of hypermagnesemia in men. Making an association with higher dietary intake of Mg in contrast with women, which can be related to socioeconomic factors [91].
5. Conclusions
Magnesium is a relevant cation that needs further research to expand the knowledge of its implications and have a better understanding of its preventing role.
Altogether, we conclude that well-designed prospective clinical trials are needed to determine in which cases there would be an indication to recommend Mg supplementation, either dietary or pharmacological, taking age and sex into account.
Acknowledgments
To Miguel Ángel Gallegos Mompeán, José Vicente Silla Aliaga, and Jesus Damian Blasco for their administrative and technical support as part of the group of Clinical and Experimental Neuroscience (NiCE), Institute for Aging Research of the University of Murcia.
Author Contributions
L.D. and M.T.H.: Conceptualization, data curation, formal analysis, methodology, funding acquisition, project administration, resources, supervision, writing, review and editing. M.d.C.M.R.: Investigation, translation, writing, review and editing. L.C.B., E.F.V. and A.M.G.C.: Investigation and review. M.B., N.V., V.R., C.M.N., A.K.-W., K.K. and L.P.: Supervision, review and editing. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The GOING-FWD Consortium was funded by the GENDER-NET Plus ERA-NET Initiative [Project Ref. no.: GNP-78]: “La Caixa” Foundation [ID 100010434] with code LCF/PR/DE18/52010001, Canadian Institutes of Health Research [GNP-161904], Swedish Research Council [2018-00932], Austrian Science Fund [FWF, I 4209]; MTH is funded by UM and in addition funded by UM/R714/2021. Open Access Funding by the Austrian Science Fund (FWF). All authors have read and agreed to the published version of the manuscript.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Blanchard A. Metabolismo Normal y Patológico del Magnesio. Elsevier; Amsterdam, The Netherlands: 2007. pp. 1–8. [Google Scholar]
- 2.Glasdam S.M., Glasdam S., Peters G.H. Advances in Clinical Chemistry. Elsevier; Amsterdam, The Netherlands: 2016. [(accessed on 1 June 2022)]. The Importance of Magnesium in the Human Body; pp. 169–193. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0065242315000943. [DOI] [PubMed] [Google Scholar]
- 3.Touyz R.M. Magnesium in clinical medicine. Front. Biosci. 2004;9:1278. doi: 10.2741/1316. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed F., Mohammed A. Magnesium: The Forgotten Electrolyte—A Review on Hypomagnesemia. Med. Sci. 2019;7:56. doi: 10.3390/medsci7040056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van de Wal-Visscher E.R., Kooman J.P., van der Sande F.M. Magnesium in Chronic Kidney Disease: Should We Care? Blood Purif. 2018;45:173–178. doi: 10.1159/000485212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Mier M.P.-R., Rodelo-Haad C., Díaz-Tocados J., Muñoz-Castañeda J., Rodríguez M. Magnesium: An old player revisited in the context of CKD-MBD. Clin. Chim. Acta. 2019;501:53–59. doi: 10.1016/j.cca.2019.11.037. [DOI] [PubMed] [Google Scholar]
- 7.Houillier P. Mechanisms and Regulation of Renal Magnesium Transport. Annu. Rev. Physiol. 2014;76:411–430. doi: 10.1146/annurev-physiol-021113-170336. [DOI] [PubMed] [Google Scholar]
- 8.Leehey D.J. Magnesium Homeostasis in CKD. Adv. Chronic Kidney Dis. 2018;25:222–223. doi: 10.1053/j.ackd.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Cases A., Cigarrán-Guldrís S., Mas S., Gonzalez-Parra E. Vegetable-Based Diets for Chronic Kidney Disease? It Is Time to Reconsider. Nutrients. 2019;11:1263. doi: 10.3390/nu11061263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang R.J., Luan S. Rhythms of magnesium. Nat. Plants. 2020;6:742–743. doi: 10.1038/s41477-020-0706-3. [DOI] [PubMed] [Google Scholar]
- 11.Barbagallo M., Veronese N., Dominguez L.J. Magnesium in Aging, Health and Diseases. Nutrients. 2021;13:463. doi: 10.3390/nu13020463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veronese N., Demurtas J., Pesolillo G., Celotto S., Barnini T., Calusi G., Caruso M.G., Notarnicola M., Reddavide R., Stubbs B., et al. Magnesium and health outcomes: An umbrella review of systematic reviews and meta-analyses of observational and intervention studies. Eur. J. Nutr. 2019;59:263–272. doi: 10.1007/s00394-019-01905-w. [DOI] [PubMed] [Google Scholar]
- 13.Felsenfeld A.J., Levine B.S., Rodriguez M. Pathophysiology of Calcium, Phosphorus, and Magnesium Dysregulation in Chronic Kidney Disease. Semin. Dial. 2015;28:564–577. doi: 10.1111/sdi.12411. [DOI] [PubMed] [Google Scholar]
- 14.William J.H., Richards K., Danziger J. Magnesium and Drugs Commonly Used in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2018;25:267–273. doi: 10.1053/j.ackd.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Cheungpasitporn W., Thongprayoon C., Harindhanavudhi T., Edmonds P.J., Erickson S.B. Hypomagnesemia linked to new-onset diabetes mellitus after kidney transplantation: A systematic review and meta-analysis. Endocr. Res. 2016;41:142–147. doi: 10.3109/07435800.2015.1094088. [DOI] [PubMed] [Google Scholar]
- 16.Van Laecke S. Hypomagnesemia and hypermagnesemia. Rev. Endocr. Metab. Disord. 2019;74:41–47. doi: 10.1080/17843286.2018.1516173. [DOI] [PubMed] [Google Scholar]
- 17.Agus Z.S. Mechanisms and causes of hypomagnesemia. Curr. Opin. Nephrol. Hypertens. 2016;25:301–307. doi: 10.1097/MNH.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 18.Wolf F.I., Trapani V. Cell (patho)physiology of magnesium. Clin. Sci. 2008;114:27–35. doi: 10.1042/CS20070129. [DOI] [PubMed] [Google Scholar]
- 19.Blaine J., Chonchol M., Levi M. Renal Control of Calcium, Phosphate, and Magnesium Homeostasis. Clin. J. Am. Soc. Nephrol. 2015;10:1257–1272. doi: 10.2215/CJN.09750913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliveira B., Cunningham J., Walsh S.B. Magnesium Balance in Chronic and End-Stage Kidney Disease. Adv. Chronic Kidney Dis. 2018;25:291–295. doi: 10.1053/j.ackd.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Dominguez L., Veronese N., Barbagallo M. Magnesium and Hypertension in Old Age. Nutrients. 2020;13:139. doi: 10.3390/nu13010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allgrove J. Physiology of calcium, phosphate, magnesium and vitamin D. In: Allgrove J., Shaw N.J., editors. Endocrine Development. S. Karger AG; Basel, Switzerland: 2015. [(accessed on 1 June 2022)]. pp. 7–32. Available online: https://www.karger.com/Article/FullText/380990. [DOI] [PubMed] [Google Scholar]
- 23.Vetter T., Lohse M.J. Magnesium and the parathyroid. Curr. Opin. Nephrol. Hypertens. 2002;11:403–410. doi: 10.1097/00041552-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Vormann J. Magnesium and Kidney Health-More on the ‘Forgotten Electrolyte’. Am. J. Nephrol. 2016;44:379–380. doi: 10.1159/000450863. [DOI] [PubMed] [Google Scholar]
- 25.Cunningham J., Rodríguez M., Messa P. Magnesium in chronic kidney disease Stages 3 and 4 and in dialysis patients. Clin. Kidney J. 2012;5((Suppl. S1)):i39–i51. doi: 10.1093/ndtplus/sfr166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanbay M., Goldsmith D., Uyar M.E., Turgut F., Covic A. Magnesium in Chronic Kidney Disease: Challenges and Opportunities. Blood Purif. 2010;29:280–292. doi: 10.1159/000276665. [DOI] [PubMed] [Google Scholar]
- 27.Spiegel D.M. Magnesium in Chronic Kidney Disease: Unanswered Questions. Blood Purif. 2011;31:172–176. doi: 10.1159/000321837. [DOI] [PubMed] [Google Scholar]
- 28.Mountokalakis T.D. Magnesium metabolism in chronic renal failure. Magnes. Res. 1990;3:121–127. [PubMed] [Google Scholar]
- 29.Rodríguez-Ortiz M.E., Canalejo A., Herencia C., Martínez-Moreno J.M., Peralta-Ramírez A., Perez-Martinez P., Navarro-González J.F., Rodríguez M., Peter M., Gundlach K., et al. Magnesium modulates parathyroid hormone secretion and upregulates parathyroid receptor expression at moderately low calcium concentration. Nephrol. Dial. Transplant. 2013;29:282–289. doi: 10.1093/ndt/gft400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H., Wang R. Associations between the serum magnesium and all-cause or cardiovascular mortality in chronic kidney disease and end-stage renal disease patients: A meta-analysis. Medicine. 2021;100:e27486. doi: 10.1097/MD.0000000000027486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Francisco A.L.M., Rodríguez M. Magnesium—Its role in CKD. Nefrología. 2013;33:389–399. doi: 10.3265/Nefrologia.pre2013.Feb.11840. [DOI] [PubMed] [Google Scholar]
- 32.Xiong J., He T., Wang M., Nie L., Zhang Y., Wang Y., Huang Y., Feng B., Zhang J., Zhao J. Serum magnesium, mortality, and cardiovascular disease in chronic kidney disease and end-stage renal disease patients: A systematic review and meta-analysis. J. Nephrol. 2019;32:791–802. doi: 10.1007/s40620-019-00601-6. [DOI] [PubMed] [Google Scholar]
- 33.Huang J.W., Famure O., Li Y., Kim S.J. Hypomagnesemia and the Risk of New-Onset Diabetes Mellitus after Kidney Transplantation. J. Am. Soc. Nephrol. 2015;27:1793–1800. doi: 10.1681/ASN.2015040391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Augusto J.-F., Subra J.-F., Duveau A., Rakotonjanahary J., Dussaussoy C., Picquet J., Croue A., Villemain F., Onno C., Sayegh J. Relation Between Pretransplant Magnesemia and the Risk of New Onset Diabetes After Transplantation Within the First Year of Kidney Transplantation. Transplantation. 2014;97:1155–1160. doi: 10.1097/01.TP.0000440950.22133.a1. [DOI] [PubMed] [Google Scholar]
- 35.Garg N., Weinberg J., Ghai S., Bradauskaite G., Nuhn M., Gautam A., Kumar N., Francis J., Chen J.L.T. Lower magnesium level associated with new-onset diabetes and pre-diabetes after kidney transplantation. J. Nephrol. 2014;27:339–344. doi: 10.1007/s40620-014-0072-1. [DOI] [PubMed] [Google Scholar]
- 36.Csiszar A., Toth J., Peti-Peterdi J., Ungvari Z. The aging kidney: Role of endothelial oxidative stress and inflammation. Acta Physiol. Hung. 2007;94:107–115. doi: 10.1556/APhysiol.94.2007.1-2.10. [DOI] [PubMed] [Google Scholar]
- 37.Fang Y., Gong A.Y., Haller S.T., Dworkin L.D., Liu Z., Gong R. The ageing kidney: Molecular mechanisms and clinical implications. Ageing Res. Rev. 2020;63:101151. doi: 10.1016/j.arr.2020.101151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grantham J.J. Solitary Renal Cysts: Worth a Second Look? Am. J. Kidney Dis. 2012;59:593–594. doi: 10.1053/j.ajkd.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Al-Said J., Brumback M.A., Moghazi S., Baumgarten D.A., O’Neill W.C. Reduced renal function in patients with simple renal cysts. Kidney Int. 2004;65:2303–2308. doi: 10.1111/j.1523-1755.2004.00651.x. [DOI] [PubMed] [Google Scholar]
- 40.Al-Said J., Charles O’Neill W. Reduced kidney size in patients with simple renal cysts. Kidney Int. 2003;64:1059–1064. doi: 10.1046/j.1523-1755.2003.00193.x. [DOI] [PubMed] [Google Scholar]
- 41.Chin H.J., Ro H., Lee H.J., Na K.Y., Chae D.W. The clinical significances of simple renal cyst: Is it related to hypertension or renal dysfunction? Kidney Int. 2006;70:1468–1473. doi: 10.1038/sj.ki.5001784. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Y., Jia L., Lu B., Bai L., Cui W. Simple renal cyst as an independent risk factor for hypertension. J. Clin. Hypertens. 2022;24:898–907. doi: 10.1111/jch.14519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roseman D.A., Hwang S.-J., Oyama-Manabe N., Chuang M.L., O’Donnell C.J., Manning W.J., Fox C.S. Clinical associations of total kidney volume: The Framingham Heart Study. Nephrol. Dial. Transplant. 2016;32:1344–1350. doi: 10.1093/ndt/gfw237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X., Vrtiska T.J., Avula R.T., Walters L.R., Chakkera H.A., Kremers W.K., Lerman L.O., Rule A.D. Age, kidney function, and risk factors associate differently with cortical and medullary volumes of the kidney. Kidney Int. 2014;85:677–685. doi: 10.1038/ki.2013.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denic A., Glassock R.J., Rule A.D. Structural and Functional Changes With the Aging Kidney. Adv. Chronic Kidney Dis. 2016;23:19–28. doi: 10.1053/j.ackd.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van der Burgh A.C., Rizopoulos D., Ikram M.A., Hoorn E.J., Chaker L. Determinants of the Evolution of Kidney Function With Age. Kidney Int. Rep. 2021;6:3054–3063. doi: 10.1016/j.ekir.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piras D., Masala M., Delitala A., Urru S.A.M., Curreli N., Balaci L., Ferreli L.P., Loi F., Atzeni A., Cabiddu G., et al. Kidney size in relation to ageing, gender, renal function, birthweight and chronic kidney disease risk factors in a general population. Nephrol. Dial. Transplant. 2018;35:640–647. doi: 10.1093/ndt/gfy270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.James M.T., Hemmelgarn B.R., Wiebe N., Pannu N., Manns B.J., Klarenbach S.W., Tonelli M. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: A cohort study. Lancet. 2010;376:2096–2103. doi: 10.1016/S0140-6736(10)61271-8. [DOI] [PubMed] [Google Scholar]
- 49.Nitta K., Okada K., Yanai M., Takahashi S. Aging and Chronic Kidney Disease. Kidney Blood Press. Res. 2013;38:109–120. doi: 10.1159/000355760. [DOI] [PubMed] [Google Scholar]
- 50.Curry J.N., Yu A.S.L. Magnesium Handling in the Kidney. Adv. Chronic Kidney Dis. 2018;25:236–243. doi: 10.1053/j.ackd.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mazzaferro S., de Martini N., Cannata-Andía J., Cozzolino M., Messa P., Rotondi S., Tartaglione L., Pasquali M., on behalf of the ERA-EDTA CKD-MBD Working Group Focus on the Possible Role of Dietary Sodium, Potassium, Phosphate, Magnesium, and Calcium on CKD Progression. J. Clin. Med. 2021;10:958. doi: 10.3390/jcm10050958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DiNicolantonio J.J., O’Keefe J.H., Wilson W. Subclinical magnesium deficiency: A principal driver of cardiovascular disease and a public health crisis. Open Heart. 2018;5:e000668. doi: 10.1136/openhrt-2017-000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pickering G., Mazur A., Trousselard M., Bienkowski P., Yaltsewa N., Amessou M., Noah L., Pouteau E. Magnesium Status and Stress: The Vicious Circle Concept Revisited. Nutrients. 2020;12:3672. doi: 10.3390/nu12123672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uwitonze A.M., Razzaque M.S. Role of Magnesium in Vitamin D Activation and Function. J. Am. Osteopat. Assoc. 2018;118:181–189. doi: 10.7556/jaoa.2018.037. [DOI] [PubMed] [Google Scholar]
- 55.Van Orten-Luiten A., Janse A., Verspoor E., Brouwer-Brolsma E., Witkamp R. Drug use is associated with lower plasma magnesium levels in geriatric outpatients; possible clinical relevance. Clin. Nutr. 2018;38:2668–2676. doi: 10.1016/j.clnu.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 56.Sakaguchi Y., Hamano T., Isaka Y. Magnesium and Progression of Chronic Kidney Disease: Benefits Beyond Cardiovascular Protection? Adv. Chronic Kidney Dis. 2018;25:274–280. doi: 10.1053/j.ackd.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Al-Aly Z., Maddukuri G., Xie Y. Proton Pump Inhibitors and the Kidney: Implications of Current Evidence for Clinical Practice and When and How to Deprescribe. Am. J. Kidney Dis. 2020;75:497–507. doi: 10.1053/j.ajkd.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 58.Sakaguchi Y., Fujii N., Shoji T., Hayashi T., Rakugi H., Isaka Y. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 2014;85:174–181. doi: 10.1038/ki.2013.327. [DOI] [PubMed] [Google Scholar]
- 59.Chung H.Y., Cesari M., Anton S., Marzetti E., Giovannini S., Seo A.Y., Carter C., Yu B.P., Leeuwenburgh C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sonya V., Giuseppina C., Carmela Rita B., Marco C., Giuseppina C.-R., Maria Paola G., Florinda L., Domenico N., Domenico L., Calogero C. Inflammatory networks in ageing, age-related diseases and longevity. Mech. Ageing Dev. 2007;128:83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 61.Carrero J.J., Stenvinkel P., Fellström B., Qureshi A.R., Lamb K., Heimbürger O., Bárány P., Radhakrishnan K., Lindholm B., Soveri I., et al. Telomere attrition is associated with inflammation, low fetuin-A levels and high mortality in prevalent haemodialysis patients. J. Intern. Med. 2008;263:302–312. doi: 10.1111/j.1365-2796.2007.01890.x. [DOI] [PubMed] [Google Scholar]
- 62.Childs B.G., Durik M., Baker D.J., Van Deursen J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015;21:1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Azem R., Daou R., Bassil E., Anvari E.M., Taliercio J.J., Arrigain S., Schold J.D., Vachharajani T., Nally J., Na khoul G.N. Serum magnesium, mortality and disease progression in chronic kidney disease. BMC Nephrol. 2020;21:49. doi: 10.1186/s12882-020-1713-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carrillo I.G., Vega A., Goicoechea M., Shabaka A., Gatius S., Abad S., López-Gómez J.M. Impact of Serum Magnesium Levels on Kidney and Cardiovascular Prognosis and Mortality in CKD Patients. J. Ren. Nutr. 2021;31:494–502. doi: 10.1053/j.jrn.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 65.Kanbay M., Yilmaz M.I., Apetrii M., Saglam M., Yaman H., Unal H.U., Gok M., Caglar K., Oguz Y., Yenicesu M., et al. Relationship between Serum Magnesium Levels and Cardiovascular Events in Chronic Kidney Disease Patients. Am. J. Nephrol. 2012;36:228–237. doi: 10.1159/000341868. [DOI] [PubMed] [Google Scholar]
- 66.Wyskida K., Witkowicz J., Chudek J., Więcek A. Daily Magnesium Intake and Hypermagnesemia in Hemodialysis Patients With Chronic Kidney Disease. J. Ren. Nutr. 2012;22:19–26. doi: 10.1053/j.jrn.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 67.Van Laecke S., Nagler E.V., Verbeke F., Van Biesen W., Vanholder R. Hypomagnesemia and the Risk of Death and GFR Decline in Chronic Kidney Disease. Am. J. Med. 2013;126:825–831. doi: 10.1016/j.amjmed.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 68.Lacson E., Wang W., Ma L., Passlick-Deetjen J. Serum Magnesium and Mortality in Hemodialysis Patients in the United States: A Cohort Study. Am. J. Kidney Dis. 2015;66:1056–1066. doi: 10.1053/j.ajkd.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 69.Rebholz C.M., Tin A., Liu Y., Kuczmarski M.F., Evans M.K., Zonderman A.B., Crews D.C. Dietary Magnesium and Kidney Function Decline: The Healthy Aging in Neighborhoods of Diversity across the Life Span Study. Am. J. Nephrol. 2016;44:381–387. doi: 10.1159/000450861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferre S., Li X., Adams-Huet B., Maalouf N.M., Sakhaee K., Toto R.D., Moe O.W., Neyra J.A. Association of serum magnesium with all-cause mortality in patients with and without chronic kidney disease in the Dallas Heart Study. Nephrol. Dial. Transplant. 2018;33:1389–1396. doi: 10.1093/ndt/gfx275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Farhadnejad H., Asghari G., Mirmiran P., Yuzbashian E., Azizi F. Micronutrient Intakes and Incidence of Chronic Kidney Disease in Adults: Tehran Lipid and Glucose Study. Nutrients. 2016;8:217. doi: 10.3390/nu8040217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joosten M.M., Gansevoort R.T., Bakker S.J. Low plasma magnesium and risk of developing chronic kidney disease: Results from the PREVEND Study. Kidney Int. 2015;87:1262–1263. doi: 10.1038/ki.2015.33. [DOI] [PubMed] [Google Scholar]
- 73.Tin A., Grams M.E., Maruthur N.M., Astor B.C., Couper D., Mosley T.H., Selvin E., Coresh J., Kao W.H.L. Results from the Atherosclerosis Risk in Communities study suggest that low serum magnesium is associated with incident kidney disease. Kidney Int. 2015;87:820–827. doi: 10.1038/ki.2014.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maier J.A.M., Malpuech-Brugère C., Zimowska W., Rayssiguier Y., Mazur A. Low magnesium promotes endothelial cell dysfunction: Implications for atherosclerosis, inflammation and thrombosis. Biochimica et Biophysica Acta (BBA)-Mol. Basis Dis. 2004;1689:13–21. doi: 10.1016/j.bbadis.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 75.Ferrè S., Baldoli E., Leidi M., Maier J.A. Magnesium deficiency promotes a pro-atherogenic phenotype in cultured human endothelial cells via activation of NFkB. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2010;1802:952–958. doi: 10.1016/j.bbadis.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 76.Zhang X., Li Y., Del Gobbo L.C., Rosanoff A., Wang J., Zhang W., Song Y. Effects of Magnesium Supplementation on Blood Pressure: A Meta-Analysis of Randomized Double-Blind Placebo-Controlled Trials. Hypertension. 2016;68:324–333. doi: 10.1161/HYPERTENSIONAHA.116.07664. [DOI] [PubMed] [Google Scholar]
- 77.Tótoli C., Carvalho A.B., Ammirati A.L., Draibe S.A., Canziani M.E.F. Associated factors related to chronic kidney disease progression in elderly patients. PLoS ONE. 2019;14:e0219956. doi: 10.1371/journal.pone.0219956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sakaguchi Y., Shoji T., Hayashi T., Suzuki A., Shimizu M., Mitsumoto K., Kawabata H., Niihata K., Okada N., Isaka Y., et al. Hypomagnesemia in Type 2 Diabetic Nephropathy. Diabetes Care. 2012;35:1591–1597. doi: 10.2337/dc12-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kyriazis J., Kalogeropoulou K., Bilirakis L., Smirnioudis N., Pikounis V., Stamatiadis D., Liolia E. Dialysate magnesium level and blood pressure. Kidney Int. 2004;66:1221–1231. doi: 10.1111/j.1523-1755.2004.00875.x. [DOI] [PubMed] [Google Scholar]
- 80.Ishimura E., Okuno S., Yamakawa T., Inaba M., Nishizawa Y. Serum magnesium concentration is a significant predictor of mortality in maintenance hemodialysis patients. Magnes. Res. 2007;20:237–244. [PubMed] [Google Scholar]
- 81.Salem S., Bruck H., Bahlmann F.H., Peter M., Passlick-Deetjen J., Kretschmer A., Steppan S., Volsek M., Kribben A., Nierhaus M., et al. Relationship between Magnesium and Clinical Biomarkers on Inhibition of Vascular Calcification. Am. J. Nephrol. 2011;35:31–39. doi: 10.1159/000334742. [DOI] [PubMed] [Google Scholar]
- 82.Turgut F., Kanbay M., Metin M.R., Uz E., Akcay A., Covic A. Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. Int. Urol. Nephrol. 2008;40:1075–1082. doi: 10.1007/s11255-008-9410-3. [DOI] [PubMed] [Google Scholar]
- 83.Kircelli F., Peter M.E., Ok E.S., Celenk F.G., Yilmaz M., Steppan S., Asci G., Passlick-Deetjen J. Magnesium reduces calcification in bovine vascular smooth muscle cells in a dose-dependent manner. Nephrol. Dial. Transplant. 2011;27:514–521. doi: 10.1093/ndt/gfr321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schulze M.B. Fiber and Magnesium Intake and Incidence of Type 2 Diabetes: A Prospective Study and Meta-analysis. Arch. Intern. Med. 2007;167:956. doi: 10.1001/archinte.167.9.956. [DOI] [PubMed] [Google Scholar]
- 85.Rodríguez-Morán M., Guerrero-Romero F. Oral Magnesium Supplementation Improves Insulin Sensitivity and Metabolic Control in Type 2 Diabetic Subjects. Diabetes Care. 2003;26:1147–1152. doi: 10.2337/diacare.26.4.1147. [DOI] [PubMed] [Google Scholar]
- 86.Guerrero-Romero F., Rodríguez-Morán M. Magnesium improves the beta-cell function to compensate variation of insulin sensitivity: Double-blind, randomized clinical trial. Eur. J. Clin. Investig. 2011;41:405–410. doi: 10.1111/j.1365-2362.2010.02422.x. [DOI] [PubMed] [Google Scholar]
- 87.Sakaguchi Y. The emerging role of magnesium in CKD. Clin. Exp. Nephrol. 2022;26:379–384. doi: 10.1007/s10157-022-02182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee H., Oh Y.J., Kim M., Kim H., Lee J.P., Kim S., Oh K.-H., Chin H.J., Joo K.W., Lim C.S., et al. The association of moderate renal dysfunction with impaired preference-based health-related quality of life: 3rdKorean national health and nutritional examination survey. BMC Nephrol. 2012;13:19. doi: 10.1186/1471-2369-13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maggio M., Ceda G.P., Lauretani F., Cattabiani C., Avantaggiato E., Morganti S., Ablondi F., Bandinelli S., Dominguez L.J., Barbagallo M., et al. Magnesium and anabolic hormones in older men: Magnesium and anabolic status in older men. Int. J. Androl. 2011;34:e594–e600. doi: 10.1111/j.1365-2605.2011.01193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kurita N., Horie S., Yamazaki S., Otani K., Sekiguchi M., Onishi Y., Takegami M., Ono R., Konno S.-I., Kikuchi S.-I., et al. Low Testosterone Levels and Reduced Kidney Function in Japanese Adult Men: The Locomotive Syndrome and Health Outcome in Aizu Cohort Study. J. Am. Med. Dir. Assoc. 2016;17:371.e1–371.e6. doi: 10.1016/j.jamda.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 91.Ford E.S., Mokdad A.H. Dietary Magnesium Intake in a National Sample of U.S. Adults. J. Nutr. 2003;133:2879–2882. doi: 10.1093/jn/133.9.2879. [DOI] [PubMed] [Google Scholar]