Abstract
Nodulisporic acids (NAs) are structurally complex potent antiinsectan indole diterpenes. We previously reported the biosynthetic gene cluster for these metabolites in Hypoxylon pulicicidum and functionally characterised the first five steps of the biosynthetic pathway. Here we reveal a highly complex biosynthetic array, furnishing multiple end products through expression of cluster components in Penicillium paxilli. We show that seven additional cluster‐encoded gene products comprise the biosynthetic machinery that elaborate precursor NAF in this highly branched pathway. The combined action of these enzymes delivers 37 NA congeners including four major end products, NAA, NAA1, NAA2 and NAA4. The plethora of intermediates arises due to modification of the carboxylated prenyl tail by a single promiscuous P450 monooxygenase, NodJ, a pivotal branchpoint enzyme which produces four distinct biosynthetic products giving rise to the complex metabolic grid that characterises NA biosynthesis.
Keywords: Alkaloids, Biosynthesis, Fungal Natural Products, Heterologous Expression, Indole Diterpenes
Nodulisporic acids are structurally complex potent antiinsectan natural products produced by the filamentous fungus Hypoxylon pulicicidum. A gene cluster, consisting of 13 genes, has been identified and biosynthetic machinery functionally characterised. This work reveals a highly complex biosynthetic array, furnishing multiple nodulisporic acid congeners. The variation arises due to the action of a single promiscuous P450 monooxygenase, NodJ.
The generation of structural diversity through divergent natural product biosynthetic pathways furnishes organisms with an arsenal of bioactive compounds that may confer fitness. The mechanisms that deliver pathway divergence vary widely and offer intriguing insights towards understanding the principles of biochemical evolution. [1]
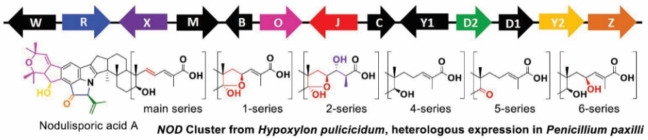
A diverse set of highly decorated bioactive indole diterpenes (IDTs) known as the nodulisporic acids have been isolated from the endophytic fungus Hypoxylon pulicicidum (prev. Nodulisporium sp.) found in the tropical shrub Bontia daphnoides. [2] Three assumed biosynthetic end products, nodulisporic acids A, A1 and A2 (NAA, NAA1, and NAA2) were initially identified, but chemical mutagenesis of H. pulicicidum uncovered a complex mixture of congeners.[ 2 , 3 ] This extensive array has been rationalised by placing the metabolites into series based on the modification to the right‐hand prenyl tail (main‐, 1‐, 2‐, and 4‐ series, Figure 1, Scheme 1). Singh et al. proposed that each series was comprised of four analogous biosynthetic intermediates (D, C, B, and A), representing stepwise construction of the western hemisphere and D ring common to all nodulisporic acid end products (Figure 1, Scheme 1). [3a] However, the biosynthetic machinery responsible for generating this plethora of metabolites remained unknown.
Figure 1.
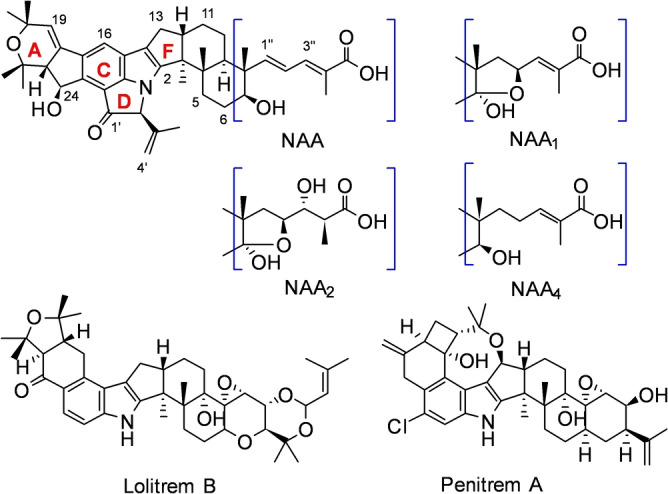
Nodulisporic acid end products and examples of other highly decorated indole diterpenes.
Scheme 1.
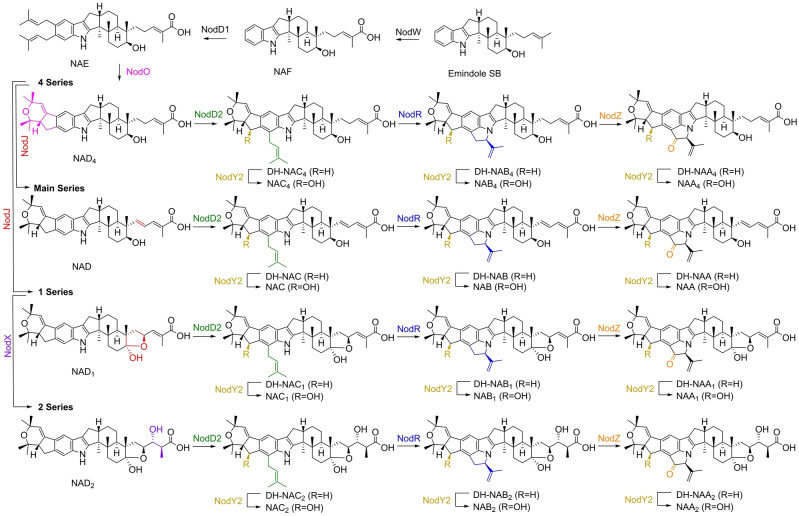
Biosynthetic array of the nodulisporic acids. Compounds lacking the 24‐OH installed by NodY2 have been given the prefix DH‐ (dehydroxy), except for the Ds which do not have 24‐OH equivalents. Stereochemistry has been assigned in line with the previously established configurations.[ 3a , 3c , 3d ]
The potent activity of NAA against blood feeding insects and absence of mammalian toxicity spurred a significant medicinal chemistry programme led by Merck Research Laboratories. Modification of the dienoic acid side chain enhanced the activity of NAA and cemented its potential as a viable flea and tick treatment for companion animals.[ 3a , 3b , 3c , 4 ] Unfortunately, production of NAA in H. pulicicidum has proved both challenging and low‐yielding.[ 2 , 5 ] Recent advances in synthetic approaches have led to the total synthesis of the biosynthetic precursors NAF, NAD, NAC and NAB (Scheme 1), including construction of the highly strained D ring, but access to NAA itself remains elusive. [6]
Heterologous biosynthesis presents an alternative approach to produce NAA in useful yields and elucidate its complex and intriguing biosynthesis. Partial pathway reconstruction, feeding studies, and in vitro experiments have been used to shed light on the biosynthesis of other structurally complex IDTs. Notable examples include the highly elaborated lolitrems and penitrems, both derived from the IDT paspaline, which require 10 and 15 biosynthetic genes to produce their respective end products via apparently linear pathways (Figure 1). [7]
We have previously reported a gene cluster in H. pulicicidum containing 13 genes, six of which have close homologues in other IDT biosynthetic gene clusters (Figure 2). [8] Through heterologous expression in Penicillium paxilli the functions of NodC (geranylgeranyl transferase), NodM (FAD‐dependent monoepoxidase) and NodB (IDT cyclase) were confirmed, and led to the production of the IDT emindole SB. The P450 monooxygenase NodW was subsequently found to install the carboxylic acid functionality characteristic of the nodulisporic acids, while the prenyl transferase NodD1 diprenylated the indole giving rise to nodulisporic acid E (NAE), laying the foundation for the complex ring systems observed in NAA (Scheme 1).[ 8a , 9 ]
Figure 2.
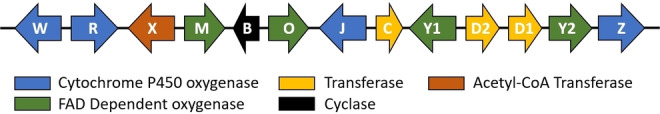
Nodulisporic acid biosynthetic gene cluster from Hypoxylon pulicicidum.
Herein we report the complete elucidation of nodulisporic acid biosynthesis, uncovering the genetic and biosynthetic relationships between congeners to reveal a highly complex biosynthetic array encompassing a plethora of new nodulisporic acid intermediates. The biosynthetic machinery responsible for this metabolic grid comprised seven further NOD cluster genes, which we functionally characterise by heterologous expression in P. paxilli. We demonstrate that this vast array of intermediates and the corresponding complexity of this highly branched biosynthetic pathway arises primarily due to the multifunctional P450 monooxygenase NodJ.
Besides the five genes responsible for the production of NAE there were eight genes in the NOD cluster with functions yet to be elucidated. These genes were predicted to encode three P450 monooxygenases (NodR, Z, and J), two paralogous FAD‐dependent monooxygenases (NodY1 and Y2), an FAD‐dependent cyclo‐oxidase (NodO), a second aromatic prenyl transferase (NodD2), and an acetyl‐CoA transferase (NodX) (Figure 2).[ 8a , 9 ]
To investigate the functions of these gene products and directly establish their respective roles in nodulisporic acid biosynthesis, we used the previously described Modular Idempotent DNA Assembly System (MIDAS) to construct multigene plasmids that were used to transform appropriate P. paxilli host strains for heterologous expression. [10] Chemotypes of the resulting transformants were characterised by reversed‐phase liquid chromatography‐mass spectrometry (LC‐MS) and high‐resolution tandem mass spectrometry (HR‐ESI‐MS2). Key biosynthetic intermediates were purified using flash chromatography and semi‐preparative HPLC, and their structures were confirmed by NMR spectroscopy.
The A/B ring system of the nodulisporic acids most closely resembles that of shearinine A. [11] In shearinine biosynthesis the oxidative cyclisation of two adjacent prenyl groups was shown to be catalysed by JanO. NodO shares 69 % amino acid sequence identity with JanO and its addition to an NAE‐producing strain yielded a new compound consistent with the expected cyclised product which we named NAD4 in line with the naming system used by Singh et al. (Figure 3, Ia, Scheme 1). [3a] NAD4 is a key intermediate as it represents the entry point into the metabolic grid in which multiple decorations of this core structure can take place. Thus, we used an NAD4‐producing strain to determine the function of the remaining NOD genes.
Figure 3.
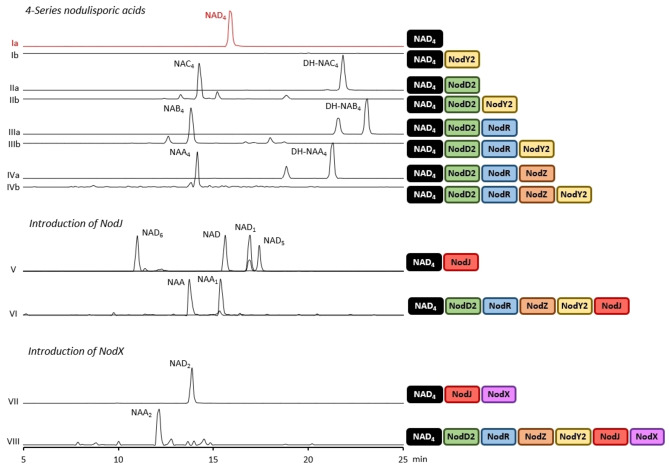
LC‐MS analysis of transformants producing nodulisporic acids leading to the assignment of all clustered genes except nodY1 (genetic information for transformants is listed in the Supporting Information, Table S1). Traces are combined EICs of key fragments associated with each metabolite from HR‐ESI‐MS2 analysis (Supp. Info., Table S3). Note that the appearance of two peaks in traces IIIa, IIIb and IVa was attributed to the presence of 2′‐epimers (Supp. Info., Pg 79), and the smaller peak under NAD1 in trace V is the NAD6 EIC‐MS2 trace which also captures NAD1 due to the close parent mass and identical fragments (Supp. Info. Table S3).
Elaboration of NAD4 to produce advanced nodulisporic acids requires further decoration of the indole functionality through additional prenylation and oxidation steps. To perform these modifications, we screened the remaining unassigned NOD genes by sequential addition to the NAD4 producing strain with subsequent chemical analysis to characterise nodulisporic acid products. In this manner, it was found that NodD2, an aromatic prenyl transferase closely related to other enzymes of the same class in IDT biosynthesis, installed the final prenyl group at C‐24 to give the full carbon complement of the advanced nodulisporic acids (Figure 3, IIa). [9] The unique oxidative annulation functionality required to construct the highly strained D ring was assigned to NodR (Figure 3, IIIa), while oxidation to furnish a ketone at C‐1′ was mediated by NodZ (Figure 3, IVa). Lastly, NodY2 was found to install the 24‐OH which in combination with NodD2, R and Z produced NAA4, and thus completed the left‐hand side of the molecule (Figure 3, IVb). Interestingly NAD4 could not be oxidised by NodY2 suggesting that prior prenylation by NodD2 is required for hydroxylation at C‐24 (Figure 3, Ib).
Access to the fully decorated nodulisporic acids requires further oxidation of the prenyl tail on the right‐hand side of the structure. The remaining three unassigned NOD genes were first screened for biosynthetic function in an NAD4 producing strain. While introduction of nodY1 or nodX did not result in the production of new IDTs, incorporation of nodJ led to the identification of four new nodulisporic acids (Figure 3, V). Isolation and characterisation of the two major products by NMR spectroscopy confirmed these metabolites were NAD, with the 1′′‐2′′‐alkene, and NAD1, bearing the hemiketal through further oxidation of the secondary hydroxyl at C‐7. The remaining two compounds were also characterised and found to be alternative NAD4 oxidation products. The first, 7‐oxo‐NAD4, was designated NAD5 and the second, 2′′‐hydroxy‐NAD4, termed NAD6 (Scheme 2).
Scheme 2.
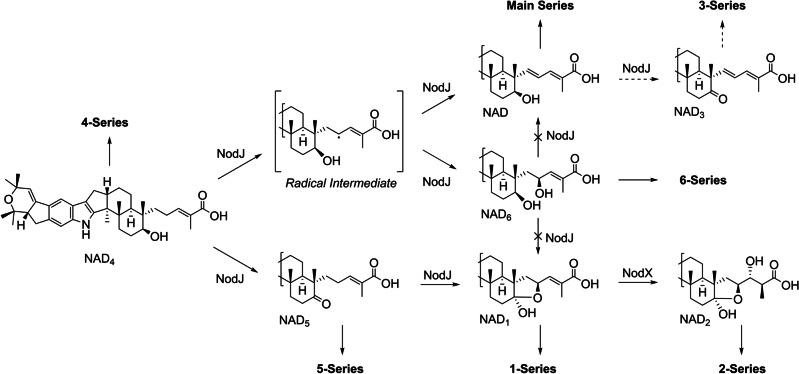
Introduction of NodJ leads to five possible parallel series of nodulisporic acids and NodX further functionalises 1‐series compounds to produce 2‐series nodulisporic acids. NAD3 or downstream products in this series were not identified in this study. Proposed mechanistic details can be found in the Supp. Info. (Figure S60). The configuration of C2′′ of NAD6 was assigned based on the previously established configuration of this position for NAA1. [3d]
The promiscuous activity of NodJ can be rationalised as oxidation at two separate sites, occurring independently or in combination. From NAD4, oxidation at C‐7 leads to NAD5 whereas oxidation at C‐2′′ gives NAD6, oxidation at both sites results in hemiketal formation to give NAD1 (Scheme 2). Singh et al. proposed that advanced nodulisporic acids with the fully unsaturated prenyl tail were formed through desaturation of the allylic alcohol of NAD6 to produce the diene functionality of the main series nodulisporic acids. [3a]
To test this hypothesis and establish the biosynthetic relationship between the NADs, we conducted in vivo feeding studies to determine whether NodJ is able to modify NAD5 and NAD6 (Supp. Info. Pg. 143). A P. paxilli strain expressing only nodJ was cultured in media spiked with NAD6 and did not yield NAD. Similarly, treating NAD6 with acid did not facilitate the hypothesised elimination (Supp. Info. Pg 145). An alternative explanation to that of Singh et al. is that hydrogen abstraction at C‐2′′ gives an allylic radical intermediate which partitions either to NAD through the loss of hydrogen or to NAD6 through reaction with an iron‐bound hydroxyl radical (Scheme 2, Figure S60). [12] Such bifurcation of a radical intermediate has been reported for other P450 oxidases.[ 12 , 13 ] These experiments demonstrated that NAD6 is a shunt metabolite that cannot be further utilised by NodJ. In contrast, NAD5 was successfully converted to NAD1 when added to a culture of the nodJ‐expressing strain, thus confirming that it is a precursor to the advanced 1‐series nodulisporic acids. Surprisingly, NAD3, a metabolite identified by Singh et al., was not identified in these experiments despite its obvious biosynthetic relationship to the other metabolites produced by NodJ (Scheme 2). [3a]
While NAD, NAD1 and their downstream products have been previously identified in H. pulicicidum cultures, NAD5 and NAD6 have not. [3a] It is conceivable that these new metabolites could be modified by the previously described NOD enzymes, expanding the metabolic grid and giving rise to two additional series of metabolites leading to the theoretical NAA5 and NAA6 (in analogy to Scheme 2). To determine the impact of NodJ on downstream nodulisporic acids, NodJ was introduced into an NAD4 producing strain along with all previously assigned NOD genes (nodD2, R, Z, Y2) (Figure 3, VI). NAA and NAA1 were both identified in this strain, demonstrating successful heterologous production of two key biosynthetic end products via complete pathway reconstruction. NAC5, NAC6 and NAB5, as well as their dehydroxy equivalents, were tentatively identified by HR‐ESI‐MS2, but no evidence for the proposed NAA5 and NAA6 was observed. This result suggested that while the 5‐ and 6‐series nodulisporic acids are possible, these are not preferred pathways.
The multitude of products generated downstream of NAD4 as a result of the promiscuous action of NodJ confirms that the action of this enzyme represents a key branch point in nodulisporic acid biosynthesis and expands an already complex array of known congeners to include 5‐ and 6‐series nodulisporic acids. While IDT biosynthesis is rich with oxidative chemistry, unlike many other NOD enzymes, NodJ lacks a homologue among other IDT P450 monooxygenases. Perhaps the most apt comparisons are to AtmQ, AceQ, and JanQ, which are responsible for conversion of 13‐desoxypaxilline to paspalinine via oxidation at two separate sites.[ 11 , 14 ] However, these steps appear to occur in a linear fashion and do not generate parallel series of metabolites. NodJ is therefore unique in its ability to perform multiple oxidations that are independent of one another, and the ability of downstream enzymes to accept these as substrates leads to a degree of branching unprecedented in IDT biosynthesis. [7]
The functional characterisation of NodJ and subsequent heterologous production of NAA and NAA1 left only the 2‐series nodulisporic acids unaccounted for. To access these metabolites the 3′′‐4′′‐alkene, derived from GGPP and present in all other nodulisporic acid series, is hydrated in an anti‐Markovnikov manner to give the 3′′‐OH (Scheme 2). The two remaining NOD genes, nodX (acetyl‐CoA transferase) and nodY1 (FAD‐dependent oxygenase), had shown no activity in an NAD4 producing strain; therefore, it was predicted that this modification must occur after the C‐7 hemiketal was constructed by NodJ. When nodJ and nodX were simultaneously introduced into an NAD4 producing strain a new metabolite of mass consistent with NAD2 was identified (Figure 3, VII). This metabolite was isolated and structurally confirmed by NMR spectroscopy as the 18–23‐alkene of NAD2, a non‐enzymatic isomerisation of the 18–19‐alkene which has been reported in previous isolations of nodulisporic acids. [3a] Finally, to produce the most elaborated nodulisporic acid, NAA2, all NOD genes with assigned functions were incorporated into P. paxilli and heterologous production of NAA2 was confirmed by HR‐ESI‐MS2 (Figure 3, VIII).
The conversion of NAD1 to NAD2 by NodX is interesting as while online databases identify this protein as belonging to the acyl‐CoA transferase superfamily, it appears here to act as a hydratase catalysing syn‐addition of H2O across the 3′′‐4′′‐alkene to deliver the previously reported 3′′R, 4′′S stereochemistry. [15] No analogous modifications have been reported in IDT biosynthesis, and we have found no precedent in the literature for this unusual chemistry. However, the current iterations of the abovementioned online databases are known to have erroneously identified several non‐CoA transferase proteins as members of the CoA transferase superfamily. [16] Phylogenetic analysis of the NodX protein sequence showed that NodX is similarly not closely related to any characterised CoA transferase protein (Figure S61).
This work represents a complete pathway reconstruction of nodulisporic acid biosynthesis and describes the genetic basis for all known biosynthetic modifications via the functional characterisation of 12 NOD genes. The plethora of congeners identified arise from a single precursor, NAD4, and comprise significant structural diversity leading to a broad range of biological activities.[ 2 , 3 , 4d , 4f , 17 ] Many natural product biosynthesis pathways generate similar chemical diversity through branching, for example a multiplicity of xanthanones are derived from chrysophanol via extensive pathway divergence.[ 1b , 18 ] The mechanisms that deliver such divergence vary widely, with either a pathway intermediate being accepted by different biosynthetic enzymes or through promiscuous enzymic action.[ 1 , 18 ] In nodulisporic acid biosynthesis we have revealed that the catalytic promiscuity of NodJ, and resulting mechanistic bifurcation of the pathway, combined with the substrate tolerance of all downstream biosynthetic enzymes delivers diverse chemical output at a very low cost. Such interconnected pathways are extremely efficient and have been termed metabolic matrices. [1b] A similarly efficient matrix, if much smaller, is illustrated by the oxidation of β‐carotene to astaxanthin by two gene products, CrtW and CrtZ, each of which can perform two distinct oxidative modifications, and may act in any order to produce 9 different products. [19] The full description of this biosynthetic diversity exposes the catalytic versality of natural product biosynthetic enzymes and aids our understanding of the principles of biochemical evolution.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
The authors thank Dr Jan Tkacz for insightful discussions regarding nodulisporic acid biosynthesis. We acknowledge funding from the New Zealand Ministry of Business, Innovation and Employment (RTVU1909) and the New Zealand Marsden Fund (Grant # VUW1819). Open Access publishing facilitated by Victoria University of Wellington, as part of the Wiley ‐ Victoria University of Wellington agreement via the Council of Australian University Librarians.
A. T. Richardson, R. C. Cameron, L. J. Stevenson, A. J. Singh, Y. Lukito, D. Berry, M. J. Nicholson, E. J. Parker, Angew. Chem. Int. Ed. 2022, 61, e202213364; Angew. Chem. 2022, 134, e202213364.
Contributor Information
Dr. Matthew J. Nicholson, Email: matthew.nicholson@wellingtonuniventures.nz.
Prof. Emily J. Parker, Email: emily.parker@vuw.ac.nz.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.
- 1a. Noda-Garcia L., Tawfik D. S., Curr. Opin. Chem. Biol. 2020, 59, 147–154; [DOI] [PubMed] [Google Scholar]
- 1b. Firn R. D., Jones C. G., Nat. Prod. Rep. 2003, 20, 382–391. [DOI] [PubMed] [Google Scholar]
- 2. Ondeyka J. G., Helms G. L., Hensens O. D., Goetz M. A., Zink D. L., Tsipouras A., Shoop W. L., Slayton L., Dombrowski A. W., Polishook J. D., Ostlind D. A., Tsou N. N., Ball R. G., Singh S. B., J. Am. Chem. Soc. 1997, 119, 8809–8816. [Google Scholar]
- 3.
- 3a. Singh S. B., Ondeyka J. G., Jayasuriya H., Zink D. L., Ha S. N., Dahl-Roshak A., Greene J., Kim J. A., Smith M. M., Shoop W., Tkacz J. S., J. Nat. Prod. 2004, 67, 1496–1506; [DOI] [PubMed] [Google Scholar]
- 3b. Ondeyka J. G., Byrne K., Vesey D., Zink D. L., Shoop W. L., Goetz M. A., Singh S. B., J. Nat. Prod. 2003, 66, 121–124; [DOI] [PubMed] [Google Scholar]
- 3c. Ondeyka J. G., Dahl-Roshak A. M., Tkacz J. S., Zink D. L., Zakson-Aiken M., Shoop W. L., Goetz M. A., Singh S. B., Bioorg. Med. Chem. Lett. 2002, 12, 2941–2944; [DOI] [PubMed] [Google Scholar]
- 3d. Hensens O. D., Ondeyka J. G., Dombrowski A. W., Ostlind D. A., Zink D. L., Tetrahedron Lett. 1999, 40, 5455–5458. [Google Scholar]
- 4.
- 4a. Chakravarty P. K., Shih T. L., Colletti S. L., Ayer M. B., Snedden C., Kuo H., Tyagarajan S., Gregory L., Zakson-Aiken M., Shoop W. L., Schmatz D. M., Wyvratt M., Fisher M. H., Meinke P. T., Bioorg. Med. Chem. Lett. 2003, 13, 147–150; [DOI] [PubMed] [Google Scholar]
- 4b. Ok D., Li C., Shih T. L., Salva S., Ayer M. B., Colletti S. L., Chakravarty P. K., Wyvratt M. J., Fisher M. H., Gregory L., Zakson-Aiken M., Shoop W. L., Schmatz D. M., Meinke P. T., Bioorg. Med. Chem. Lett. 2002, 12, 1751–1754; [DOI] [PubMed] [Google Scholar]
- 4c. Chakravarty P. K., Tyagarajan S., Shih T. L., Salva S., Snedden C., Wyvratt M. J., Fisher M. H., Meinke P. T., Org. Lett. 2002, 4, 1291–1294; [DOI] [PubMed] [Google Scholar]
- 4d. Shoop W. L., Zakson-Aiken M., Gregory L. M., Michael B. F., Pivnichny J., Meinke P. T., Fisher M. H., Wyvratt M. J., Pikounis B., Schmatz D. M., J. Parasitol. 2001, 87, 1150–1154; [DOI] [PubMed] [Google Scholar]
- 4e. Berger R., Shoop W. L., Pivnichny J. V., Warmke L. M., Zakson-Aiken M., Owens K. A., deMontigny P., Schmatz D. M., Wyvratt M. J., Fisher M. H., Meinke P. T., Colletti S. L., Org. Lett. 2001, 3, 3715–3718; [DOI] [PubMed] [Google Scholar]
- 4f. Smith M. M., Warren V. A., Thomas B. S., Brochu R. M., Ertel E. A., Rohrer S., Schaeffer J., Schmatz D., Petuch B. R., Tang Y. S., Meinke P. T., Kaczorowski G. J., Cohen C. J., Biochemistry 2000, 39, 5543–5554; [DOI] [PubMed] [Google Scholar]
- 4g. Meinke P. T., Ayer M. B., Colletti S. L., Li C., Lim J., Ok D., Salva S., Schmatz D. M., Shih T. L., Shoop W. L., Warmke L. M., Wyvratt M. J., Zakson-Aiken M., Fisher M. H., Bioorg. Med. Chem. Lett. 2000, 10, 2371–2374; [DOI] [PubMed] [Google Scholar]
- 4h. Kane N. S., Hirschberg B., Qian S., Hunt D., Thomas B., Brochu R., Ludmerer S. W., Zheng Y., Smith M., Arena J. P., Cohen C. J., Schmatz D., Warmke J., Cully D. F., Proc. Natl. Acad. Sci. USA 2000, 97, 13949–13954; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4i. Ostlind D. A., Felcetto T., Misura A., Ondeyka J., Smith S., Goetz M., Shoop W., Mickle W., Med. Vet. Entomol. 1997, 11, 407–408. [DOI] [PubMed] [Google Scholar]
- 5. Polishook J. D., Ondeyka J. G., Dombrowski A. W., Peláez F., Platas G., Teran A. M., Mycologia 2001, 93, 1125–1137. [Google Scholar]
- 6.
- 6a. Zou Y., Li X., Yang Y., Berritt S., Melvin J., Gonzales S., Spafford M., Smith A. B., J. Am. Chem. Soc. 2018, 140, 9502–9511; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6b. Godfrey N. A., Schatz D. J., Pronin S. V., J. Am. Chem. Soc. 2018, 140, 12770–12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.
- 7a. Jiang Y., Ozaki T., Harada M., Miyasaka T., Sato H., Miyamoto K., Kanazawa J., Liu C., Maruyama J.-i., Adachi M., Nakazaki A., Nishikawa T., Uchiyama M., Minami A., Oikawa H., Angew. Chem. Int. Ed. 2020, 59, 17996–18002; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 18152–18158; [Google Scholar]
- 7b. Liu C., Tagami K., Minami A., Matsumoto T., Frisvad J. C., Suzuki H., Ishikawa J., Gomi K., Oikawa H., Angew. Chem. Int. Ed. 2015, 54, 5748–5752; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 5840–5844. [Google Scholar]
- 8.
- 8a. Van de Bittner K. C., Nicholson M. J., Bustamante L. Y., Kessans S. A., Ram A., van Dolleweerd C. J., Scott B., Parker E. J., J. Am. Chem. Soc. 2018, 140, 582–585; [DOI] [PubMed] [Google Scholar]
- 8b. Nicholson M. J., Bittner K. C. V. d., Ram A., Bustamante L. Y., Scott B., Parker E. J., Genome 2018, 6, e01380-01317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van de Bittner K. C., Cameron R. C., Bustamante L. Y., Bundela R., Kessans S. A., Vorster J., Nicholson M. J., Parker E. J., MedChemComm 2019, 10, 1160–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Dolleweerd C. J., Kessans S. A., Van de Bittner K. C., Bustamante L. Y., Bundela R., Scott B., Nicholson M. J., Parker E. J., ACS Synth. Biol. 2018, 7, 1018–1029. [DOI] [PubMed] [Google Scholar]
- 11. Liu C., Minami A., Dairi T., Gomi K., Scott B., Oikawa H., Org. Lett. 2016, 18, 5026–5029. [DOI] [PubMed] [Google Scholar]
- 12.
- 12a. Guengerich F. P., Chem. Res. Toxicol. 2001, 14, 611–650; [DOI] [PubMed] [Google Scholar]
- 12b. Guengerich F. P., ACS Catal. 2018, 8, 10964–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.
- 13a. Rettie A. E., Rettenmeier A. W., Howald W. N., Baillie T. A., Science 1987, 235, 890–893; [DOI] [PubMed] [Google Scholar]
- 13b. Kramlinger V. M., Nagy L. D., Fujiwara R., Johnson K. M., Phan T. T. N., Xiao Y., Enright J. M., Toomey M. B., Corbo J. C., Guengerich F. P., FEBS Lett. 2016, 590, 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.
- 14a. McLellan R. M., Cameron R. C., Nicholson M. J., Parker E. J., Org. Lett. 2022, 24, 2332–2337; [DOI] [PubMed] [Google Scholar]
- 14b. Nicholson M. J., Koulman A., Monahan B. J., Pritchard B. L., Payne G. A., Scott B., Appl. Environ. Microbiol. 2009, 75, 7469–7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.
- 15a. Mistry J., Chuguransky S., Williams L., Nucleic Acids Res. 2021, 49, D412–D419; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15b. Andreeva A., Kulesha E., Gough J., Murzin A., Nucleic Acids Res. 2020, 48, D376–D382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hackmann T., Protein Sci. 2022, 31, 864–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.
- 17a. Choudhary S., Abongwa M., Kashyap S. S., Verma S., Mair G. R., Kulke D., Martin R. J., Robertson A. P., Proc. Natl. Acad. Sci. USA 2022, 119, e2111932119; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17b. Meinke P. T., Colletti S. L., Fisher M. H., Wyvratt M. J., Shih T. L., Ayer M. B., Li C., Lim J., Ok D., Salva S., Warmke L. M., Zakson M., Michael B. F., deMontigny P., Ostlind D. A., Fink D., Drag M., Schmatz D. M., Shoop W. L., J. Med. Chem. 2009, 52, 3505–3515. [DOI] [PubMed] [Google Scholar]
- 18. Wei X., Wang W.-G., Matsuda Y., Fungal Biol. Biotechnol. 2022, 9, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Misawa N., Satomi Y., Kondo K., Yokoyama A., Kajiwara S., Saito T., Ohtani T., Miki W., J. Bacteriol. 1995, 177, 6575–6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.