Abstract
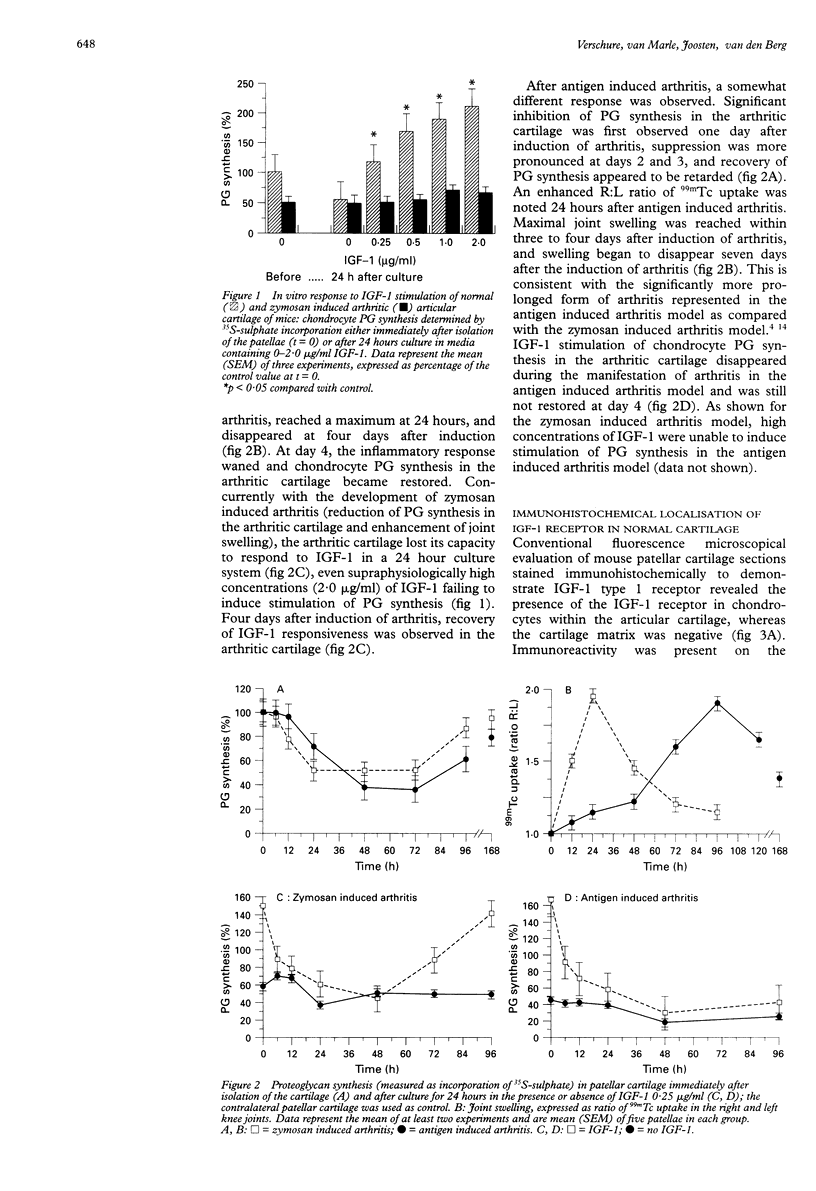
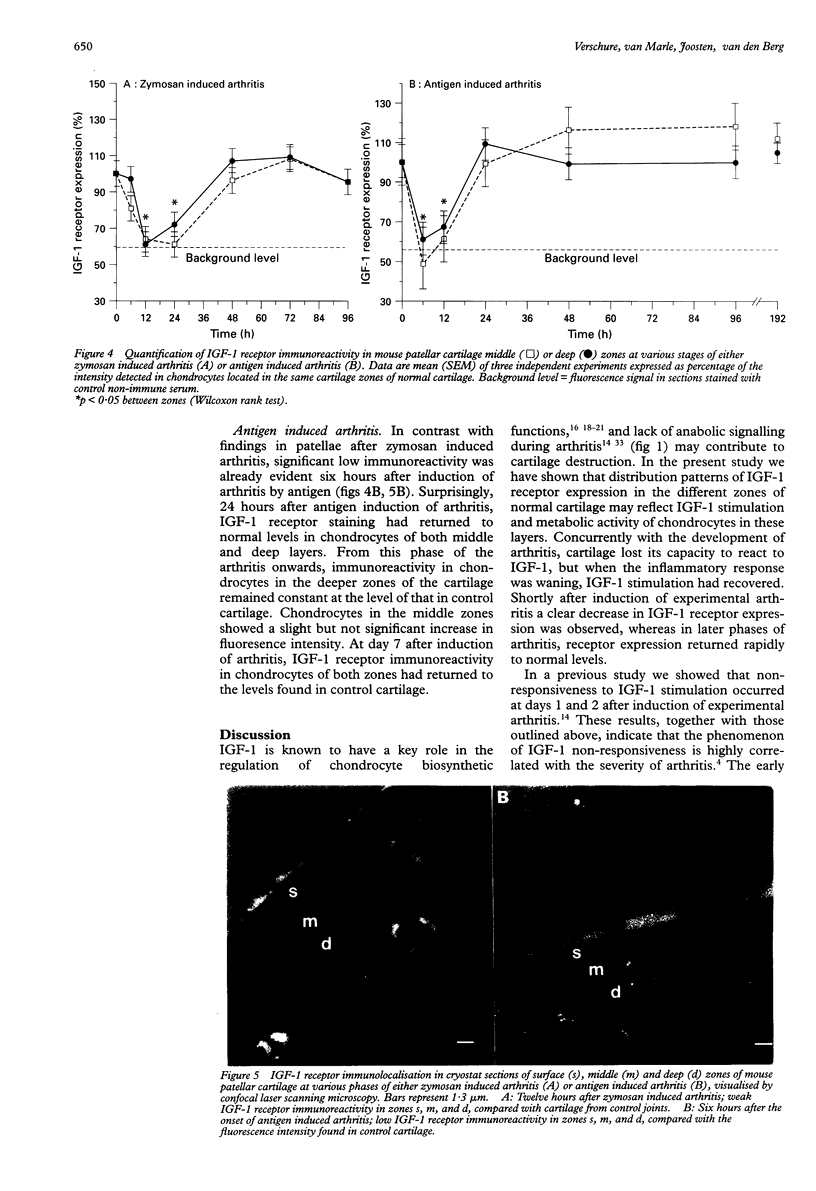
OBJECTIVE--To examine the distribution of insulin like growth factor-1 (IGF-1) receptors and the biological response to IGF-1 stimulation in articular cartilage of normal mouse knee joints and arthritic joints taken at various stages of experimentally induced arthritis. METHODS--In situ IGF-1 receptor expression and responsiveness to IGF-1 stimulation were examined in murine articular cartilage at different phases in two models of experimentally induced arthritis. IGF-1 receptor expression was visualised in joint sections with the use of anti-IGF-1 receptor antibodies and quantified by confocal laser scanning microscopy. Chondrocyte proteoglycan (PG) synthesis was measured by incorporation of 35S-sulphate. RESULTS--In control cartilage, the majority of IGF-1 receptors were found on chondrocytes localised in the middle and deeper zones of the cartilage, whereas receptor expression in surface zone chondrocytes was very low. During culture of normal articular cartilage, IGF-1 was able to maintain chondrocyte PG synthesis at the in vivo level. Concurrently with the development of arthritis, cartilage lost its capacity to react to IGF-1, but IGF-1 stimulation recovered when the inflammatory response waned. Shortly after induction of arthritis, IGF-1 receptor expression initially declined, but it had returned to normal levels by day 1 and remained increased thereafter. CONCLUSION--The distribution of IGF-1 receptor expression in the different zones of normal articular cartilage reflects IGF-1 stimulation and metabolic activity of chondrocytes in these layers. This correlation is disturbed in arthritic cartilage, suggesting inadequate or overruled signalling.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman F. P. A gelatin embedding technique as an aid in the preparation of unfixed cryostat sections. Histochem J. 1978 Sep;10(5):617–620. doi: 10.1007/BF01003143. [DOI] [PubMed] [Google Scholar]
- Altman F. P. A metabolic dysfunction in early murine osteoarthritis. Ann Rheum Dis. 1981 Jun;40(3):303–306. doi: 10.1136/ard.40.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baici A. Die Arthrose aus biochemischer Sicht. Ther Umsch. 1991 Jan;48(1):13–17. [PubMed] [Google Scholar]
- Benton H. P., Tyler J. A. Inhibition of cartilage proteoglycan synthesis by interleukin I. Biochem Biophys Res Commun. 1988 Jul 15;154(1):421–428. doi: 10.1016/0006-291x(88)90703-6. [DOI] [PubMed] [Google Scholar]
- Bhaumick B., Bala R. M. Differential effects of insulin-like growth factors I and II on growth, differentiation and glucoregulation in differentiating chondrocyte cells in culture. Acta Endocrinol (Copenh) 1991 Aug;125(2):201–211. doi: 10.1530/acta.0.1250201. [DOI] [PubMed] [Google Scholar]
- Brackertz D., Mitchell G. F., Mackay I. R. Antigen-induced arthritis in mice. I. Induction of arthritis in various strains of mice. Arthritis Rheum. 1977 Apr;20(3):841–850. doi: 10.1002/art.1780200314. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H., Rotwein P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev. 1989 Feb;10(1):68–91. doi: 10.1210/edrv-10-1-68. [DOI] [PubMed] [Google Scholar]
- Demarquay D., Dumontier M. F., Tsagris L., Bourguignon J., Nataf V., Corvol M. T. In vitro insulin-like growth factor I interaction with cartilage cells derived from postnatal animals. Horm Res. 1990;33(2-4):111–115. doi: 10.1159/000181493. [DOI] [PubMed] [Google Scholar]
- Doré S., Pelletier J. P., DiBattista J. A., Tardif G., Brazeau P., Martel-Pelletier J. Human osteoarthritic chondrocytes possess an increased number of insulin-like growth factor 1 binding sites but are unresponsive to its stimulation. Possible role of IGF-1-binding proteins. Arthritis Rheum. 1994 Feb;37(2):253–263. doi: 10.1002/art.1780370215. [DOI] [PubMed] [Google Scholar]
- Dunham J., Shackleton D. R., Nahir A. M., Billingham M. E., Bitensky L., Chayen J., Muir I. H. Altered orientation of glycosaminoglycans and cellular changes in the tibial cartilage in the first two weeks of experimental canine osteoarthritis. J Orthop Res. 1985;3(3):258–268. doi: 10.1002/jor.1100030302. [DOI] [PubMed] [Google Scholar]
- Froesch E. R., Schmid C., Schwander J., Zapf J. Actions of insulin-like growth factors. Annu Rev Physiol. 1985;47:443–467. doi: 10.1146/annurev.ph.47.030185.002303. [DOI] [PubMed] [Google Scholar]
- Good M. J., Hage W. J., Mummery C. L., De Laat S. W., Boonstra J. Localization and quantification of epidermal growth factor receptors on single cells by confocal laser scanning microscopy. J Histochem Cytochem. 1992 Sep;40(9):1353–1361. doi: 10.1177/40.9.1506672. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Handley C. J., McQuillan D. J., Hascall G. K., Robinson H. C., Lowther D. A. The effect of serum on biosynthesis of proteoglycans by bovine articular cartilage in culture. Arch Biochem Biophys. 1983 Jul 1;224(1):206–223. doi: 10.1016/0003-9861(83)90205-9. [DOI] [PubMed] [Google Scholar]
- Johnstone J., Bitensky L., Chayen J. Cryostat sections of undemineralized bone. J Clin Pathol. 1972 Aug;25(8):742–742. doi: 10.1136/jcp.25.8.742-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten L. A., Helsen M. M., van den Berg W. B. Transient chondrocyte nonresponsiveness to insulin-like growth factor-1 upon H2O2 exposure is not related to IGF receptor damage. J Rheumatol. 1991 Apr;18(4):585–590. [PubMed] [Google Scholar]
- Kato H., Faria T. N., Stannard B., Levy-Toledano R., Taylor S. I., Roberts C. T., Jr, LeRoith D. Paradoxical biological effects of overexpressed insulin-like growth factor-1 receptors in Chinese hamster ovary cells. J Cell Physiol. 1993 Jul;156(1):145–152. doi: 10.1002/jcp.1041560120. [DOI] [PubMed] [Google Scholar]
- Kruijsen M. W., van den Berg W. B., van de Putte L. B., van den Broek W. J. Detection and quantification of experimental joint inflammation in mice by measurement of 99mTc-pertechnetate uptake. Agents Actions. 1981 Dec;11(6-7):640–642. doi: 10.1007/BF01978775. [DOI] [PubMed] [Google Scholar]
- Luyten F. P., Hascall V. C., Nissley S. P., Morales T. I., Reddi A. H. Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys. 1988 Dec;267(2):416–425. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- MEACHIM G., GHADIALLY F. N., COLLINS D. H. REGRESSIVE CHANGES IN THE SUPERFICIAL LAYER OF HUMAN ARTICULAR CARTILAGE. Ann Rheum Dis. 1965 Jan;24:23–30. doi: 10.1136/ard.24.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton J. F., Tyler J. A. Upregulation of insulin-like growth factor I gene expression in the lesions of osteoarthritic human articular cartilage. Ann Rheum Dis. 1992 Apr;51(4):440–447. doi: 10.1136/ard.51.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely E. K., Beukers M. W., Oh Y., Cohen P., Rosenfeld R. G. Insulin-like growth factor receptors. Acta Paediatr Scand Suppl. 1991;372:116–124. doi: 10.1111/j.1651-2227.1991.tb17985.x. [DOI] [PubMed] [Google Scholar]
- Oemar B. S., Foellmer H. G., Hodgdon-Anandant L., Rosenzweig S. A. Regulation of insulin-like growth factor I receptors in diabetic mesangial cells. J Biol Chem. 1991 Feb 5;266(4):2369–2373. [PubMed] [Google Scholar]
- Pearse A. D., Gardner D. L. Preparation of unfixed undemineralized bone sections: the Bright bone cryostat. J Clin Pathol. 1972 Jan;25(1):26–29. doi: 10.1136/jcp.25.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteu F., Nathan C. Shedding of tumor necrosis factor receptors by activated human neutrophils. J Exp Med. 1990 Aug 1;172(2):599–607. doi: 10.1084/jem.172.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijntjes N. V., Van de Putte L. B., Van der Pol M., Guelen P. J. Cryosectioning of undecalcified tissues for immunofluorescence. J Immunol Methods. 1979;30(3):263–268. doi: 10.1016/0022-1759(79)90100-5. [DOI] [PubMed] [Google Scholar]
- Rosenfeld R. G., Dollar L. A. Characterization of the somatomedin-C/insulin-like growth factor I (SM-C/IGF-I) receptor on cultured human fibroblast monolayers: regulation of receptor concentrations by SM-C/IGF-I and insulin. J Clin Endocrinol Metab. 1982 Sep;55(3):434–440. doi: 10.1210/jcem-55-3-434. [DOI] [PubMed] [Google Scholar]
- Rosenfeld R. G., Hintz R. L. Characterization of a specific receptor for somatomedin C (SM-C) on cultured human lymphocytes: evidence that SM-C modulates homologous receptor concentration. Endocrinology. 1980 Dec;107(6):1841–1848. doi: 10.1210/endo-107-6-1841. [DOI] [PubMed] [Google Scholar]
- Sachs D. H., Kiszkiss P., Kim K. J. Release of Ia antigens by a cultured B cell line. J Immunol. 1980 May;124(5):2130–2136. [PubMed] [Google Scholar]
- Sampson H. W., Cannon M. S. Zonal analysis of metabolic profiles of articular-epiphyseal cartilage chondrocytes: a histochemical study. Histochem J. 1986 May;18(5):233–238. doi: 10.1007/BF01676232. [DOI] [PubMed] [Google Scholar]
- Sandy J. D., Lowther D. A., Brown H. L. Antigen-induced arthritis. Studies on the inhibition of proteoglycan synthesis observed in articular cartilage during short-term joint inflammation. Arthritis Rheum. 1980 Apr;23(4):433–447. doi: 10.1002/art.1780230406. [DOI] [PubMed] [Google Scholar]
- Saxne T., Palladino M. A., Jr, Heinegård D., Talal N., Wollheim F. A. Detection of tumor necrosis factor alpha but not tumor necrosis factor beta in rheumatoid arthritis synovial fluid and serum. Arthritis Rheum. 1988 Aug;31(8):1041–1045. doi: 10.1002/art.1780310816. [DOI] [PubMed] [Google Scholar]
- Schalkwijk J., Joosten L. A., van den Berg W. B., van Wyk J. J., van de Putte L. B. Insulin-like growth factor stimulation of chondrocyte proteoglycan synthesis by human synovial fluid. Arthritis Rheum. 1989 Jan;32(1):66–71. doi: 10.1002/anr.1780320111. [DOI] [PubMed] [Google Scholar]
- Schalkwijk J., Joosten L. A., van den Berg W. B., van de Putte L. B. Chondrocyte nonresponsiveness to insulin-like growth factor 1 in experimental arthritis. Arthritis Rheum. 1989 Jul;32(7):894–900. [PubMed] [Google Scholar]
- Siczkowski M., Watt F. M. Subpopulations of chondrocytes from different zones of pig articular cartilage. Isolation, growth and proteoglycan synthesis in culture. J Cell Sci. 1990 Oct;97(Pt 2):349–360. doi: 10.1242/jcs.97.2.349. [DOI] [PubMed] [Google Scholar]
- Tesch G. H., Handley C. J., Cornell H. J., Herington A. C. Effects of free and bound insulin-like growth factors on proteoglycan metabolism in articular cartilage explants. J Orthop Res. 1992 Jan;10(1):14–22. doi: 10.1002/jor.1100100103. [DOI] [PubMed] [Google Scholar]
- Trippel S. B., Chernausek S. D., Van Wyk J. J., Moses A. C., Mankin H. J. Demonstration of type I and type II somatomedin receptors on bovine growth plate chondrocytes. J Orthop Res. 1988;6(6):817–826. doi: 10.1002/jor.1100060605. [DOI] [PubMed] [Google Scholar]
- Tyler J. A. Insulin-like growth factor 1 can decrease degradation and promote synthesis of proteoglycan in cartilage exposed to cytokines. Biochem J. 1989 Jun 1;260(2):543–548. doi: 10.1042/bj2600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986 Oct;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Noorden C. J., Smith R. E., Rasnick D. Cysteine proteinase activity in arthritic rat knee joints and the effects of a selective systemic inhibitor, Z-Phe-AlaCH2F. J Rheumatol. 1988 Oct;15(10):1525–1535. [PubMed] [Google Scholar]
- Van Noorden C. J., Vogels I. M. Enzyme histochemical reactions in unfixed and undecalcified cryostat sections of mouse knee joints with special reference to arthritic lesions. Histochemistry. 1986;86(2):127–133. doi: 10.1007/BF00493377. [DOI] [PubMed] [Google Scholar]
- Van Noorden C. J., Vogels I. M., Smith R. E. Localization and cytophotometric analysis of cathepsin B activity in unfixed and undecalcified cryostat sections of whole rat knee joints. J Histochem Cytochem. 1989 May;37(5):617–624. doi: 10.1177/37.5.2703699. [DOI] [PubMed] [Google Scholar]
- Verschure P. J., Joosten L. A., van der Kraan P. M., Van den Berg W. B. Responsiveness of articular cartilage from normal and inflamed mouse knee joints to various growth factors. Ann Rheum Dis. 1994 Jul;53(7):455–460. doi: 10.1136/ard.53.7.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschure P. J., Van Noorden C. J. The effects of interleukin-1 on articular cartilage destruction as observed in arthritic diseases, and its therapeutic control. Clin Exp Rheumatol. 1990 May-Jun;8(3):303–313. [PubMed] [Google Scholar]
- Verschure P. J., van Marle J., Joosten L. A., Van den Berg W. B. Localization and quantification of the insulin-like growth factor-1 receptor in mouse articular cartilage by confocal laser scanning microscopy. J Histochem Cytochem. 1994 Jun;42(6):765–773. doi: 10.1177/42.6.8189038. [DOI] [PubMed] [Google Scholar]
- Verschure P. J., van der Kraan P. M., Vitters E. L., van den Berg W. B. Stimulation of proteoglycan synthesis by triamcinolone acetonide and insulin-like growth factor 1 in normal and arthritic murine articular cartilage. J Rheumatol. 1994 May;21(5):920–926. [PubMed] [Google Scholar]
- White J. G., Amos W. B., Fordham M. An evaluation of confocal versus conventional imaging of biological structures by fluorescence light microscopy. J Cell Biol. 1987 Jul;105(1):41–48. doi: 10.1083/jcb.105.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries B. J., van den Berg W. B., Vitters E., van de Putte L. B. Quantitation of glycosaminoglycan metabolism in anatomically intact articular cartilage of the mouse patella: in vitro and in vivo studies with 35S-sulfate, 3H-glucosamine, and 3H-acetate. Rheumatol Int. 1986;6(6):273–281. doi: 10.1007/BF00541319. [DOI] [PubMed] [Google Scholar]
- van Beuningen H. M., Arntz O. J., van den Berg W. B. In vivo effects of interleukin-1 on articular cartilage. Prolongation of proteoglycan metabolic disturbances in old mice. Arthritis Rheum. 1991 May;34(5):606–615. doi: 10.1002/art.1780340513. [DOI] [PubMed] [Google Scholar]
- van de Loo A. A., van Beuningen H. M., van Lent P. L., van den Berg W. B. Direct effect of murine rIL-1 on cartilage metabolism in vivo. Agents Actions. 1989 Jan;26(1-2):153–155. doi: 10.1007/BF02126590. [DOI] [PubMed] [Google Scholar]
- van de Loo A. A., van den Berg W. B. Effects of murine recombinant interleukin 1 on synovial joints in mice: measurement of patellar cartilage metabolism and joint inflammation. Ann Rheum Dis. 1990 Apr;49(4):238–245. doi: 10.1136/ard.49.4.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Loo F. A., Arntz O. J., Otterness I. G., van den Berg W. B. Protection against cartilage proteoglycan synthesis inhibition by antiinterleukin 1 antibodies in experimental arthritis. J Rheumatol. 1992 Mar;19(3):348–356. [PubMed] [Google Scholar]
- van den Berg W. B., Kruijsen M. W., van de Putte L. B., van Beusekom H. J., van der Sluis-van der Pol M., Zwarts W. A. Antigen-induced and zymosan-induced arthritis in mice: studies on in vivo cartilage proteoglycan synthesis and chondrocyte death. Br J Exp Pathol. 1981 Jun;62(3):308–316. [PMC free article] [PubMed] [Google Scholar]
- van der Kraan P. M., Vitters E. L., Postma N. S., Verbunt J., van den Berg W. B. Maintenance of the synthesis of large proteoglycans in anatomically intact murine articular cartilage by steroids and insulin-like growth factor I. Ann Rheum Dis. 1993 Oct;52(10):734–741. doi: 10.1136/ard.52.10.734. [DOI] [PMC free article] [PubMed] [Google Scholar]