Abstract
Aim
To report prespecified and post hoc analyses of the SoliMix dataset exploring the impact of baseline participant characteristics on the original SoliMix study outcomes, to enable informed treatment choices for people with different biomedical characteristics.
Methods
SoliMix (EudraCT 2017‐003370‐13) compared once‐daily iGlarLixi (a fixed‐ratio combination of insulin glargine 100 U/mL and the glucagon‐like peptide‐1 receptor agonist lixisenatide) with twice‐daily BIAsp 30 (30% insulin aspart and 70% insulin aspart protamine). In this analysis, the original primary outcomes of noninferiority of iGlarLixi versus BIAsp 30 in terms of glycated haemoglobin (HbA1c) change and superiority in terms of body weight change, together with change in basal insulin dose and hypoglycaemia outcomes, were investigated by baseline age, duration of diabetes, insulin dose, HbA1c level, body mass index (BMI), and renal function.
Results
No evidence of difference in comparative treatment effect was detected across baseline age, duration of diabetes, insulin dose, HbA1c level, BMI and renal function subgroups for any endpoint (all heterogeneity P > 0.05), except American Diabetes Association Level 2 hypoglycaemia event rate when stratified by insulin dose (P = 0.011), which may be a chance difference given multiple testing and the small numbers of Level 2 events.
Conclusions
Treatment effects of iGlarLixi were consistent irrespective of baseline HbA1c, insulin dose, BMI, age, duration of diabetes and renal function, supporting the use of iGlarLixi as an efficacious and well‐tolerated treatment option in people with type 2 diabetes with a wide range of biomedical characteristics.
Keywords: basal insulin, GLP‐1 analogue, glycaemic control, hypoglycaemia, iGlarLixi, insulin therapy, randomized trial, type 2 diabetes
1. INTRODUCTION
People with type 2 diabetes (T2D) exhibit diverse clinical characteristics, which in turn can influence therapy outcomes including glucose control, body weight change, and experience of hypoglycaemia. 1 , 2 , 3 , 4 , 5 , 6 Both clinical and demographic factors have been identified as potential predictors of response to therapy, including age at diagnosis, duration of diabetes, baseline insulin dose, baseline glycated haemoglobin (HbA1c), body mass index (BMI), lipid levels, renal function, sex, geographical region, and ethnicity. 5 , 6 , 7 , 8 , 9 Particular concerns with regard to response to glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) have been duration of diabetes, glucose control and insulin dose, all of which, when greater, might suggest less islet β‐cell reserve or reduced function. 10 , 11 Furthermore, there is evidence to suggest a lower risk of hypoglycaemia in people with higher BMI, 12 , 13 so any advantage of GLP‐1RAs over insulin might then be ameliorated.
Recent management guidelines recommend that clinical characteristics and therapeutic priorities be used to inform choice of diabetes medications and targets. 14 , 15 Subgroup analyses of randomized controlled trials may inform improved decision‐making by elucidating differences in efficacy, tolerability and safety among different groups of people with T2D based on data collected at baseline and routinely available to prescribers. 16 , 17
Clinical outcomes with iGlarLixi, a fixed‐ratio combination of insulin glargine 100 U/mL and the GLP‐1RA lixisenatide, in various treatment scenarios are well described. 18 , 19 , 20 The SoliMix trial was a randomized head‐to‐head study comparing the efficacy, tolerability and safety of iGlarLixi with premixed insulin (biphasic insulin aspart 30 [BIAsp 30]; 30% insulin aspart and 70% insulin aspart protamine) in adults with suboptimally controlled T2D on basal insulin plus oral glucose‐lowering drugs (OGLDs). 21 , 22 Analysis of the SoliMix trial demonstrated that iGlarLixi was associated with greater HbA1c reduction, body weight benefit, and lower incidence and event rate of hypoglycaemia compared with BIAsp 30. 21 Here, we explore the SoliMix data in subgroups stratified by baseline age, duration of diabetes, insulin dose, HbA1c, BMI, and renal function (estimated glomerular filtration rate [eGFR]), with the aim of identifying contributing factors to the outcomes studied, which could then be used to inform treatment decisions with regard to choice of medication. 21
2. MATERIALS AND METHODS
2.1. Study design
Detailed methods for the open‐label SoliMix trial and the dose adjustment algorithms for iGlarLixi and BIAsp 30 have been previously described. 21 , 22 In brief, adults with suboptimally controlled T2D (HbA1c 58–86 mmol/mol [7.5%–10.0%]) diagnosed at least 1 year prior to screening, who were receiving basal insulin and one to two OGLDs, were included. Doses of basal insulin and OGLDs had to have been stable (±20% for insulin) for 3 months, and OGLDs could include metformin with or without a sodium‐glucose cotransporter‐2 inhibitor. Following a 2‐week screening period, eligible participants were randomized 1:1 to switch from their prior basal insulins to either iGlarLixi once daily or BIAsp 30 twice daily for 26 weeks. Background treatment with OGLDs was to be continued throughout the study.
The SoliMix study is registered in the European Union Drug Regulating Authorities Clinical Trials Database (EudraCT 2017‐003370‐13) and was conducted in accordance with all usual ethical standards. 21
2.2. Subgroup analyses and endpoints
The primary objectives were, as in the base study, noninferiority of iGlarLixi versus BIAsp 30 in HbA1c change or superiority in body weight change from baseline to Week 26. In the present analysis, participants were split into subgroups according to age (<65 and ≥65 years), duration of diabetes (<10 and ≥10 years), basal insulin dose (<30 and ≥30 U/d), baseline HbA1c (58–64, ≥64–75, and >75–86 mmol/mol [7.5%–8.0%, >8.0%–9.0% and >9.0%–10.0%]), BMI (<25.0, 25.0 to <30.0, 30.0 to <35.0, and ≥35.0 kg/m2) and renal function (eGFR ≥90, 60 to <90, and <60 mL/min/1.73 m2). The cut‐offs for duration of T2D, insulin dose and BMI were prespecified in the original study protocol. The cut‐offs for the HbA1c and eGFR subgroups were chosen prior to any subgroup analysis to be clinically meaningful and provide enough participants per subgroup. Age subgroups were predefined as <50, 50 to <65, 65 to <75, and ≥75 years, but combined in two main subgroups (<65 and ≥65 years) for the purpose of this analysis, aligning with the cut‐offs used in the American Diabetes Association (ADA) guidelines for the management of diabetes in older people. 23
As in the main analysis, the primary endpoints were change in HbA1c and body weight change from baseline to Week 26. Secondary endpoints included confirmed hypoglycaemia (according to ADA Level 2: <3.0 mmol/L [<54 mg/dL] and Level 1: 3.0 to <3.9 mmol/L [54 to <70 mg/dL] criteria), change in insulin dose, and reaching a composite endpoint of HbA1c <53 mmol/mol (<7.0%) with no weight gain and no hypoglycaemia. Severe hypoglycaemia was recorded only three times in the SoliMix study, 21 and thus could not be assessed by subgroup analysis.
2.3. Statistical analysis
Efficacy analyses were based on the intention‐to‐treat (ITT) population, defined as all randomized participants. The safety population was defined as participants who received at least one dose or part of a dose of the study medication.
Primary analyses of between‐treatment differences in HbA1c and body weight changes were protocol‐defined as part of the original study for T2D duration, basal insulin dose, and BMI subgroups. All other assessments were planned after finalization of the main study protocol but used original study data and outcomes. The HbA1c noninferiority margin was set to 3 mmol/mol (0.3%). Heterogeneity testing was conducted post hoc, with a cut‐off of P < 0.05 for statistical significance, with no adjustment for multiple testing. The primary endpoints were assessed using a multiple imputation strategy and an analysis of covariance (ANCOVA) model. The ANCOVA models included fixed categorical effects of randomization strata, treatment group, subgroup, subgroup‐by‐treatment group interaction, country, as well as fixed continuous variables of baseline values.
Odds ratios for categorical key secondary endpoints were analysed using a logistic regression model adjusting for fixed categorical effects of randomization strata, treatment group, subgroup, and subgroup‐by‐treatment group interaction, as well as fixed continuous covariates of baseline values. Hypoglycaemia event rates were estimated using a negative binomial regression model with a log‐link function and the log of the time period in which a hypoglycaemia episode was considered treatment‐emergent as offset. The P values of the heterogeneity test were generated using a subgroup‐by‐treatment interaction test.
3. RESULTS
3.1. Baseline characteristics
In total, 887 participants were randomized, 443 to iGlarLixi and 444 to BIAsp 30; baseline characteristics did not differ between the two treatment groups. 21 , 22 Baseline characteristics were also broadly similar across each of the subgroups (except where determined by the discriminant metric), but age and duration of diabetes did appear to trend upwards with reduced renal function in the eGFR subgroups (Table S1).
3.2. Blood glucose control and body weight
No differences in treatment effect were detected among the baseline subgroups for changes in the primary outcomes of HbA1c or body weight, with all heterogeneity P values > 0.05 (Figure 1 and Tables S2 and S3). Despite reduced power, both primary objectives were met in all 16 subgroups: iGlarLixi was noninferior to BIAsp 30 in HbA1c reductions and superior in body weight change in all subgroups.
FIGURE 1.
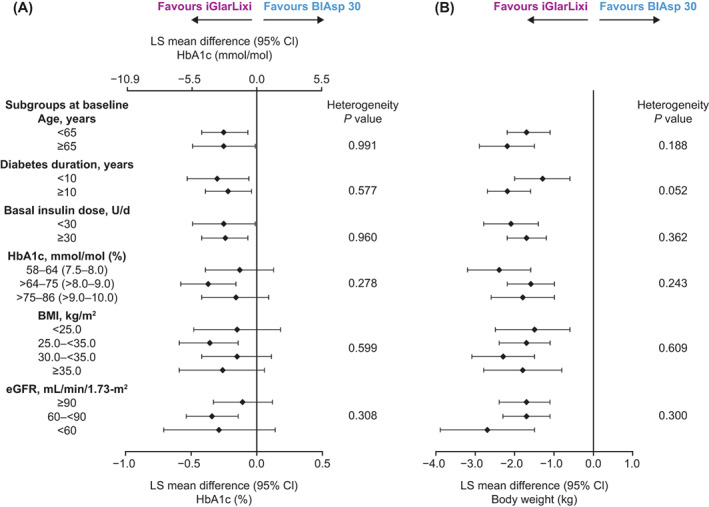
Difference in change in primary endpoints from baseline to Week 26 by baseline characteristics' subgroups (intention‐to‐treat population). (A) Change in glycated haemoglobin (HbA1c; mmol/mol and %). (B) Change in body weight (kg). Least squares (LS) mean difference values are for iGlarLixi versus BIAsp 30. Numerical data for the confidence intervals (CIs) are given in Tables S2 and S3. BIAsp, biphasic insulin aspart; BMI, body mass index; eGFR, estimated glomerular filtration rate; FRC, fixed‐ratio combination; iGlarLixi, FRC of insulin glargine 100 U/mL and the glucagon‐like peptide‐1 receptor agonist, lixisenatide
Subgroups had no detectable effect on the difference in insulin dose increment seen in SoliMix with iGlarLixi versus BIAsp 30 (all heterogeneity P values > 0.05; Figure 2, Table S4).
FIGURE 2.
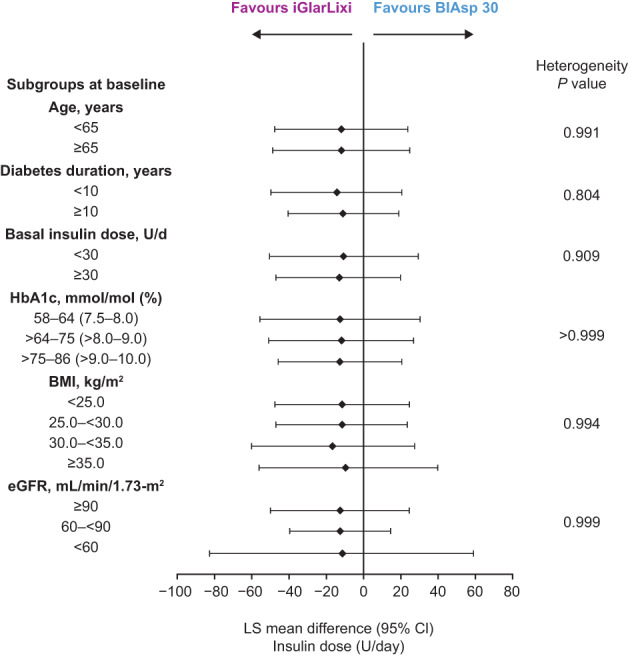
Difference in change in insulin dose (U/d) from baseline to Week 26 by baseline characteristics' subgroups (intention‐to‐treat population). Least squares (LS) mean difference values are for iGlarLixi versus BIAsp 30. Numerical data for the confidence intervals (CIs) are given in Table S4. BIAsp, biphasic insulin aspart; BMI, body mass index; eGFR, estimated glomerular filtration rate; FRC, fixed‐ratio combination; iGlarLixi, FRC of insulin glargine 100 U/mL and the glucagon‐like peptide‐1 receptor agonist, lixisenatide
The incidence of ADA Level 2 hypoglycaemia with iGlarLixi versus BIAsp 30 was not detectably affected by baseline subgroup (all heterogeneity P values > 0.05). This was also true for event rates except for baseline insulin dose subgroups (<30 vs. ≥30 U/d) where the heterogeneity P value was 0.011 (Figure 3, Table S5). However, both the incidence and event rates of ADA Level 1 hypoglycaemia seen with iGlarLixi versus BIAsp 30 in the main analysis were unaffected by any baseline subgroup (all heterogeneity P values > 0.05; Table S6).
FIGURE 3.
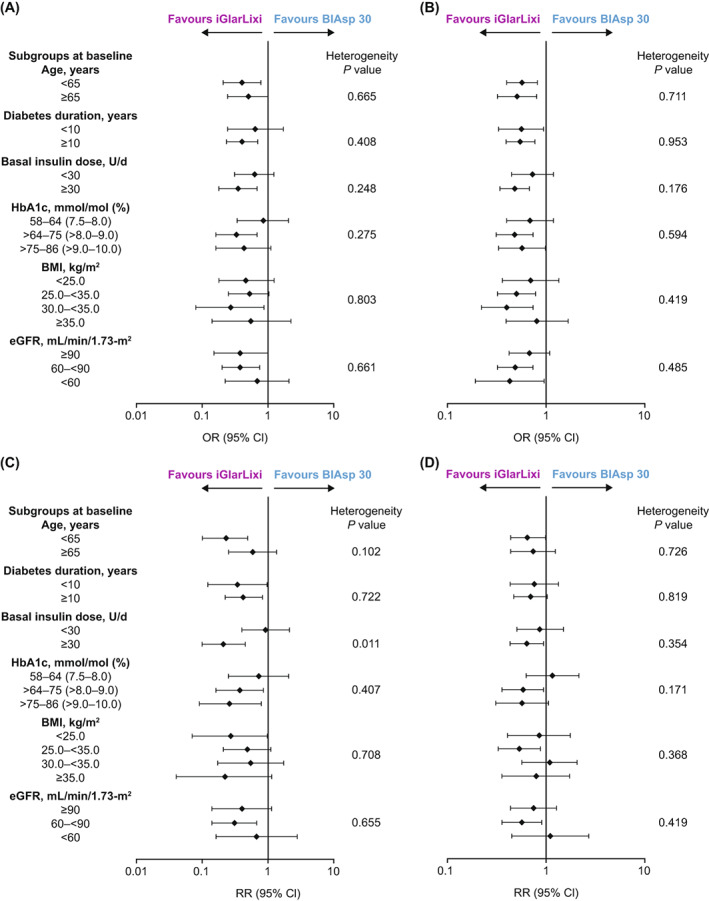
Incidence and event rates of hypoglycaemia comparing iGlarLixi with BIAsp‐30 over the 26‐week treatment period (safety population). (A, C) American Diabetes Association (ADA) Level 2 hypoglycaemia (all confirmed <3.0 mmol/L [<54 mg/dL]). (B, D) ADA Level 1 hypoglycaemia (all confirmed <3.9 to ≥3.0 mmol/L [<70 to ≥54 mg/dL]). Top panels: incidence (odds ratio for iGlarLixi versus BIAsp 30); bottom panels: event rates (rate ratio for iGlarLixi versus BIAsp 30). Numerical data are given in Tables S5 and S6. BIAsp, biphasic insulin aspart; BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; FRC, fixed‐ratio combination; iGlarLixi, FRC of insulin glargine 100 U/mL and the glucagon‐like peptide‐1 receptor agonist, lixisenatide; OR, odds ratio; RR, rate ratio
Similarly, the achievement of HbA1c <53 mmol/mol [7.0%] and the composite targets (HbA1c <53 mmol/mol [<7.0%] with no weight gain and HbA1c <53 mmol/mol [<7.0%] with no weight gain and no hypoglycaemia) seen with iGlarLixi versus BIAsp 30 were consistent across all baseline subgroups (Table S7), having been greater for iGlarLixi than BIAsp 30 in the main study analysis.
4. DISCUSSION
The present subgroup analysis of SoliMix data suggests that individuals with different baseline characteristics of age, diabetes duration, daily insulin dose, HbA1c, BMI, and renal function were indistinguishable in terms of benefit from iGlarLixi compared to BIAsp 30 in regard to HbA1c, body weight change, and incidence of hypoglycaemia. This is inclusive of participants with characteristics typical of more challenging management profiles such as a higher baseline HbA1c (>75 mmol/mol [>9.0 %]; however, >86 mmol/mol [>10.0 %] was an exclusion criterion), longer duration of T2D (≥10 years), higher baseline insulin dose requirement (≥30 U/d), and obesity (≥30.0 to <35.0 and ≥35.0 kg/m2).86 Thus, the conclusion of the main SoliMix study that switching to iGlarLixi rather than BIAsp 30 is likely to be beneficial for people with suboptimally controlled T2D on basal insulin and OGLDs 21 is corroborated by this analysis in people with broadly different biomedical characteristics. Accordingly, a broad T2D population on basal insulin may benefit from the reduced disease burden and treatment simplification that once‐daily GLP‐1RA fixed‐ratio combination therapy offers, as is the case with iGlarLixi and IDegLira.
Assessments for event rates of ADA Level 2 hypoglycaemia according to insulin dose subgroups (<30 vs. ≥30 U/d) gave a heterogeneity P value of 0.011 (Figure 3), but no such effect was evident either for incidence of hypoglycaemia or for Level 1 event rate or incidence. Furthermore, this is a single “significant” P value (unadjusted for multiple testing) out of 42 performed comparisons and is likely to represent a chance difference, given the relatively small numbers of Level 2 events. Therefore, there is insufficient evidence to conclude whether a real difference exists in Level 2 event rates between insulin dose subgroups, although this remains a possibility. A similar statistical argument pertains to the P value of 0.052 for heterogeneity of body weight change by diabetes duration (Figure 1).
The consistency of benefit with iGlarLixi across subgroups mirrors that observed in the LixiLan‐L study, also performed in participants inadequately controlled on prior basal insulin with up to two OGLDs, with the comparator insulin glargine 100 U/mL. 24 Thus, in LixiLan‐L, glycaemic control improved with iGlarLixi and weight gain was mitigated, while changes in hypoglycaemia were negligible irrespective of baseline HbA1c, T2D duration or BMI. 24 Similar findings were reported in the LixiLan‐O study in insulin‐naïve participants (iGlarLixi vs. insulin glargine 100 U/mL) across subgroups, except for the possibility of higher incidence of hypoglycaemia in the HbA1c ≥64 mmol/mol (≥8.0%) versus <64 mmol/mol subgroup, attributed to differences in dosing. 25 Benefits of IDegLira have also been preserved across subgroups in the DUAL VII study with similar HbA1c reductions, less severe or plasma glucose‐confirmed hypoglycaemia, lower end‐of‐trial total daily insulin dose, together with weight loss relative to meal‐time plus basal insulin therapy. 26
The main limitations of the present analysis are, firstly, the exploratory nature of the assessments except for the between‐treatment differences in HbA1c and body weight changes in the basal insulin dose and BMI subgroups. However, the data used were entirely those of the original database, and the endpoints were those specified for the main study analysis. Secondly, many subgroups had small numbers of participants which reduced statistical power for within‐subgroup confidence intervals, which are consequently often wide, but also for heterogeneity comparisons. Correction for multiple testing was not done in order to preserve power for detection of differences, but is only important for interpretation of the single statistically significant finding (P < 0.05), as discussed above.
Accordingly, these data suggest that age, diabetes duration, daily basal insulin dose, HbA1c, BMI and renal function did not significantly impact treatment outcomes in the SoliMix trial, and are not necessary to identify people with T2D who may respond to treatment with iGlarLixi in clinical scenarios where the alternative is an analogue premixed insulin. The improved glycaemic control and body weight management, with no increased risk of hypoglycaemic events, were observed in all baseline characteristics subgroups. These findings, taken together with results from other studies, validate iGlarLixi as an effective and well‐tolerated treatment option for a wide range of people with suboptimally controlled T2D requiring treatment advancement beyond basal insulin.
AUTHOR CONTRIBUTIONS
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, had full access to all the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All authors participated in the interpretation of the data, the writing, reviewing, and editing of the manuscript, and had final responsibility for approving the published version.
CONFLICT OF INTEREST
Philip D. Home, or institutions with which he is associated, have received funding for his research, advisory and lecturing activities from Sanofi and Novo Nordisk, and from other GLP‐1RA and insulin manufacturers including AstraZeneca, Boehringer Ingelheim, Gan & Lee, GlaxoSmithKline, Janssen, and Merck (MSD). Rory J. McCrimmon has acted as an advisor and speaker for Sanofi and Novo Nordisk. Julio Rosenstock has served on advisory panels for Applied Therapeutics, Boehringer Ingelheim, Eli Lilly, Intarcia, Janssen, Novo Nordisk, Oramed, Sanofi and Zealand, and has received research support from Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Genentech, GlaxoSmithKline, Intarcia, Janssen, Lexicon, Merck, Novo Nordisk, Oramed, Pfizer and Sanofi. Matthias Blüher received honoraria as a consultant and speaker from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Lilly, Novo Nordisk, Novartis and Sanofi. Katrin Pegelow and Khier Djaballah are employees of Sanofi and may hold shares and/or stock options in the company. Lydie Melas‐Melt is an employee of IVIDATA Life Sciences, Levallois‐Perret, France, contracted by Sanofi. Francesco Giorgino has served as an advisor for AstraZeneca, a research investigator for Eli Lilly, a speaker for AstraZeneca and Eli Lilly and a consultant for Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novo Nordisk, Roche Diabetes and Sanofi, and has received grants from Eli Lilly, Lifescan and Roche Diabetes Care.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14907.
PRIOR PUBLICATION
This analysis was presented in part within two posters at the 82nd Scientific Sessions of the American Diabetes Association, New Orleans, LA, June 3–7, 2022.
Supporting information
Table S1. Participant characteristics in subgroups determined by baseline characteristics (ITT population).
Table S2. Change in HbA1c from baseline to Week 26 by baseline characteristics subgroups (ITT population).
Table S3. Change in body weight from baseline to Week 26 by baseline characteristics subgroups (ITT population).
Table S4. Change in insulin dose from baseline to Week 26 by baseline characteristics subgroups (ITT population).
Table S5. Incidence and rate of hypoglycaemia ADA Level 2a by baseline characteristics subgroups (safety population).
Table S6. Incidence and rate of hypoglycaemia ADA Level 1a by baseline characteristics subgroups (safety population).
Table S7. Proportion of participants reaching target HbA1c and composite endpoints by subgroups (ITT population).
Data S1. Plain Language Summary
ACKNOWLEDGMENTS
The authors thank the study participants, trial staff, and investigators for their participation. We thank Ana Merino‐Trigo, PhD (Sanofi) for coordinating the development, facilitating author discussions, and providing a courtesy review of this manuscript. We thank Rose Dall, PhD, Emiliana Jelezarova, PhD, CMPP™ and Hannah Brown, PhD, CMPP™ of Fishawack Communications Ltd, part of Fishawack Health, for providing editorial assistance. Sponsorship for this study was funded by Sanofi, Paris, France. Editorial assistance was provided by Fishawack Communications Ltd, part of Fishawack Health, and was funded by Sanofi.
Home PD, McCrimmon RJ, Rosenstock J, et al. Findings for iGlarLixi versus BIAsp 30 confirmed in groups of people with type 2 diabetes with different biomedical characteristics. Diabetes Obes Metab. 2023;25(3):656‐663. doi: 10.1111/dom.14907
This article has an accompanied Plain Language Summary in the Supporting Information Data S1.
Funding information Sanofi
DATA AVAILABILITY STATEMENT
Qualified researchers may request access to participant‐level data and related study documents. Participant‐level data will be anonymised, and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at https://www.vivli.org/.
REFERENCES
- 1. Pearson ER. Type 2 diabetes: a multifaceted disease. Diabetologia. 2019;62:1107‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Diabetes Association . Standards of medical Care in Diabetes—2022. 2. Classification and diagnosis of diabetes. Diabetes Care. 2022;45:S17‐S38. [DOI] [PubMed] [Google Scholar]
- 3. Karalliedde J, Gnudi L. Diabetes mellitus, a complex and heterogeneous disease, and the role of insulin resistance as a determinant of diabetic kidney disease. Nephrol Dial Transplant. 2016;31:206‐213. [DOI] [PubMed] [Google Scholar]
- 4. Porcellati F, Lin J, Lucidi P, Bolli GB, Fanelli CG. Impact of patient and treatment characteristics on glycemic control and hypoglycemia in patients with type 2 diabetes initiated to insulin glargine or NPH: a post hoc, pooled, patient‐level analysis of 6 randomized controlled trials. Medicine (Baltimore). 2017;96:e6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miller ME, Bonds DE, Gerstein HC, et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Home PD, Shen C, Hasan MI, Latif ZA, Chen JW, González GG. Predictive and explanatory factors of change in HbA1c in a 24‐week observational study of 66,726 people with type 2 diabetes starting insulin analogs. Diabetes Care. 2014;37:1237‐1245. [DOI] [PubMed] [Google Scholar]
- 7. Hanefeld M, Fleischmann H, Schiffhorst G, Bramlage P. Predictors of response to early basal insulin treatment in patients with type 2 diabetes‐‐the EARLY experience. Diabetes Technol Ther. 2014;16:241‐246. [DOI] [PubMed] [Google Scholar]
- 8. Mannucci E, Monami M, Dicembrini I, Piselli A, Porta M. Achieving HbA1c targets in clinical trials and in the real world: a systematic review and meta‐analysis. J Endocrinol Invest. 2014;37:477‐495. [DOI] [PubMed] [Google Scholar]
- 9. Hsieh A, Ong PX, Molyneaux L, et al. Age of diabetes diagnosis and diabetes duration associate with glycated haemoglobin. Diabetes Res Clin Pract. 2014;104:e1‐e4. [DOI] [PubMed] [Google Scholar]
- 10. Jones AG, McDonald TJ, Shields BM, et al. Markers of β‐cell failure predict poor glycemic response to GLP‐1 receptor agonist therapy in type 2 diabetes. Diabetes Care. 2016;39:250‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thong KY, Mcgowan BM, Htay T, et al. Insulin treatment and longer diabetes duration both predict poorer glycaemic response to liraglutide treatment in type 2 diabetes: the Association of British Clinical Diabetologists Nationwide Liraglutide Audit. Br J Diabetes Vasc Dis. 2015;15:169‐172. [Google Scholar]
- 12. Plečko D, Bennett N, Mårtensson J, Bellomo R. The obesity paradox and hypoglycemia in critically ill patients. Crit Care. 2021;25:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yun JS, Park YM, Han K, Cha SA, Ahn YB, Ko SH. Association between BMI and risk of severe hypoglycaemia in type 2 diabetes. Diabetes Metab. 2019;45:19‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American Diabetes Association . Standards of medical Care in Diabetes—2022. 6. Glycemic targets. Diabetes Care. 2022;45:S83‐S96. [DOI] [PubMed] [Google Scholar]
- 15. Davies MJ, D'Alessio DA, Fradkin J, et al. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetologia. 2018;2018(61):2461‐2498. [DOI] [PubMed] [Google Scholar]
- 16. Dijkman B, Kooistra B, Bhandari M, Evidence‐Based Surgery Working G . How to work with a subgroup analysis. Can J Surg. 2009;52:515‐522. [PMC free article] [PubMed] [Google Scholar]
- 17. Burke JF, Sussman JB, Kent DM, Hayward RA. Three simple rules to ensure reasonably credible subgroup analyses. BMJ. 2015;351:h5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blonde L, Rosenstock J, Del Prato S, et al. Switching to iGlarLixi versus continuing daily or weekly GLP‐1 RA in type 2 diabetes inadequately controlled by GLP‐1 RA and oral antihyperglycemic therapy: the LixiLan‐G randomized clinical trial. Diabetes Care. 2019;42:2108‐2116. [DOI] [PubMed] [Google Scholar]
- 19. Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed‐ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan‐L randomized trial. Diabetes Care. 2016;39:1972‐1980. [DOI] [PubMed] [Google Scholar]
- 20. Rosenstock J, Aronson R, Grunberger G, et al. Benefits of LixiLan, a titratable fixed‐ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan‐O randomized trial. Diabetes Care. 2016;39:2026‐2035. [DOI] [PubMed] [Google Scholar]
- 21. Rosenstock J, Emral R, Sauque‐Reyna L, et al. Advancing therapy in suboptimally controlled basal insulin‐treated type 2 diabetes: clinical outcomes with iGlarLixi versus premix BIAsp 30 in the SoliMix randomized controlled trial. Diabetes Care. 2021;44:2361‐2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCrimmon R, Al Sifri S, Emral R, et al. Advancing therapy with iGlarLixi versus premix BIAsp 30 in basal insulin‐treated type 2 diabetes: design and baseline characteristics of the SoliMix randomized controlled trial. Diabetes Obes Metab. 2021;23:1221‐1231. [DOI] [PubMed] [Google Scholar]
- 23. American Diabetes Association . Standards of medical Care in Diabetes—2022. 13. Older adults. Diabetes Care. 2022;45:S195‐S207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wysham C, Bonadonna RC, Aroda VR, et al. Consistent findings in glycaemic control, body weight and hypoglycaemia with iGlarLixi (insulin glargine/lixisenatide titratable fixed‐ratio combination) vs insulin glargine across baseline HbA1c, BMI and diabetes duration categories in the LixiLan‐L trial. Diabetes Obes Metab. 2017;19:1408‐1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davies MJ, Leiter LA, Guerci B, et al. Impact of baseline glycated haemoglobin, diabetes duration and body mass index on clinical outcomes in the LixiLan‐O trial testing a titratable fixed‐ratio combination of insulin glargine/lixisenatide (iGlarLixi) vs insulin glargine and lixisenatide monocomponents. Diabetes Obes Metab. 2017;19:1798‐1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Billings LK, Agner BFR, Altuntas Y, et al. The benefit of insulin degludec/liraglutide (IDegLira) compared with basal‐bolus insulin therapy is consistent across participant subgroups with type 2 diabetes in the DUAL VII randomized trial. J Diabetes Sci Technol. 2021;15:636‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Participant characteristics in subgroups determined by baseline characteristics (ITT population).
Table S2. Change in HbA1c from baseline to Week 26 by baseline characteristics subgroups (ITT population).
Table S3. Change in body weight from baseline to Week 26 by baseline characteristics subgroups (ITT population).
Table S4. Change in insulin dose from baseline to Week 26 by baseline characteristics subgroups (ITT population).
Table S5. Incidence and rate of hypoglycaemia ADA Level 2a by baseline characteristics subgroups (safety population).
Table S6. Incidence and rate of hypoglycaemia ADA Level 1a by baseline characteristics subgroups (safety population).
Table S7. Proportion of participants reaching target HbA1c and composite endpoints by subgroups (ITT population).
Data S1. Plain Language Summary
Data Availability Statement
Qualified researchers may request access to participant‐level data and related study documents. Participant‐level data will be anonymised, and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at https://www.vivli.org/.


