Abstract
Hepatitis B, caused by the hepatitis B virus (HBV), is a global public health issue that affects 290 million people worldwide. Most people with hepatitis B are in low‐ and middle‐income countries (LMIC), where health systems and resources are often constrained. Refugees, asylum seekers and internally displaced persons (IDPs) often face barriers in seeking health care and are a priority population at risk of hepatitis B. No systematic review to date has evaluated the prevalence of hepatitis B amongst refugees in in LMIC. We undertook a systematic review of the literature identifying 28 studies addressing this topic. Though few studies on this topic exist, the available evidence suggests a high prevalence amongst refugees in LMIC, with wide variation between and within countries. Possible risk factors contributing to hepatitis B include unsafe injections, low immunization coverage, low awareness, mother‐to‐child transmission, and limited health services. Further study is needed to better understand the prevalence and risk factors for hepatitis B amongst refugees in LMIC, to inform public health responses. Vulnerable populations such as refugees are an important group to consider in national and global efforts to eliminate hepatitis B.
Keywords: hepatitis B, hepatitis B virus, prevalence, refugees, systematic review
Abbreviations
- Anti‐HBs
hepatitis B surface antibody
- CHB
chronic hepatitis B
- DNA
Deribooxynucleic acid
- ELISA
enzyme‐linked immunoassay
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B Virus
- IDP
internally displaced person
- LMIC
low‐ and middle‐income countries
1. INTRODUCTION
Around 290 million people worldwide are living with chronic hepatitis B (CHB), caused by the hepatitis B virus (HBV). 1 , 2 Untreated, CHB can lead to cirrhosis, liver cancer and causes over 800,000 deaths worldwide each year, but only 10% of people are diagnosed and 22% receiving treatment. 2 Virtually all people with CHB are in low‐ and middle‐income countries (LMIC) 3 with 20 countries accounting for 75% of infections. 4 The highest prevalence occurs at in the WHO Western Pacific Region at 6.2% and the WHO African region at 6.1% and varies on national and subnational levels. 3 Transmission occurs through exposure to infected blood or bodily fluids; vertical transmission from mother to child at birth is an important cause of infection in LMIC. 5 The World Health Assembly in 2016 adopted a goal to eliminate hepatitis B as a public health problem by 2030. 6 This was defined as 90% reduction in incidence and 65% reduction in mortality compared with 2015 baseline, 6 with recently added targets to reduce mother‐to‐child transmission to ≤2% and hepatitis B surface antigen (HBsAg) prevalence amongst under 5 year olds to ≤0.1%. 7 HBV is more common amongst marginalized and socioeconomically disadvantaged groups who face barriers in accessing health care. 3 , 8
Conflict and humanitarian crises have displaced approximately 84 million people worldwide, including refugees, asylum seekers and internally displaced persons (IDPs), 85% of whom are living in LMIC. Refugees are defined by the 1951 Refugee Convention as persons who are ‘unable or unwilling to return to their country of origin owing to a well‐founded fear of being persecuted for reasons of race, religion, nationality, membership of a particular social group, or political opinion’. 9 Asylum seekers are persons seeking international protection but whose application is yet to be processed. 10 IDPs are defined as ‘persons or groups of persons who have been forced or obliged to flee or to leave their homes or places of habitual residence… and who have not crossed an internationally recognized state border’. 11
The health needs of refugees are significant and include communicable diseases, 12 , 13 , 14 , 15 vaccine‐preventable diseases, 15 , 16 mental health conditions, 17 sexual and reproductive health issues 15 , 18 and micronutrient deficiencies. 12 Refugees have often experienced conflict, poverty, discrimination or exclusion from health care and education and may face difficulty accessing health services as well as lower health literacy, cultural and language barriers. 19 , 20
High rates of hepatitis B have been reported amongst refugees and migrants arriving in low prevalence (high‐income countries), with an overall prevalence of 7.2% in a recent systematic review. 21 Reasons for increased risk of HBV may include undiagnosed infection and barriers to treatment 22 ; low knowledge 23 , 24 ; low vaccination coverage, or exposure to other risk factors. No systematic review to date has assessed the prevalence of hepatitis B amongst refugees and displaced populations living in LMIC, where the highest number of refugees reside. We aim to address this gap by undertaking a systematic review of the prevalence of HBV amongst refugees, asylum seekers and IDPs in LMIC.
2. METHODS
We developed a systematic review protocol in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines (Appendix 1). 25 A protocol was not registered.
2.1. Data sources and search strategy
We used the databases MEDLINE, EMBASE, Scopus, Cochrane and Virtual Health Library and performed electronic searches on 5 December 2021. Additional sources were the database WHO Institutional Repository for Information Sharing (IRIS), websites (including Google Scholar) and manual search of the reference list of included studies and review articles. The search strategy is shown in Table 1. The CoCoPop framework was used as this review aims to address a question of prevalence. 26 Due to few existing studies on this topic, the search strategy was intentionally broad with no limits placed on country or year of publication.
TABLE 1.
Search strategy
| Database | Search Strategy |
|---|---|
| Medline |
|
| Embase |
|
| Scopus | (TITLE‐ABS‐KEY(“hepatitis B") OR TITLE‐ABS‐KEY(“hepatitis b virus”) OR TITLE‐ABS‐KEY(“chronic hepatitis B")) AND (TITLE‐ABS‐KEY(refugee$) OR TITLE‐ABS‐KEY(“displaced person$”) OR TITLE‐ABS‐KEY(“asylum seeker$”) OR TITLE‐ABS‐KEY(“displaced people”) OR TITLE‐ABS‐KEY(“displaced population”)) AND (TITLE‐ABS‐KEY(prevalence) OR TITLE‐ABS‐KEY(screening) OR TITLE‐ABS‐KEY(rate$) OR TITLE‐ABS‐KEY(epidemiology)) AND NOT INDEX(medline) |
| Cochrane |
#1 (hepatitis B) OR (hepatitis B virus) OR (chronic hepatitis B):ti,ab,kw #2 refugee* OR (refugee camp) OR (asylum seeker*) OR (displaced person) OR (displaced population) OR (forced migrant*) #3 MeSH descriptor: [Refugees] explode all trees #4 #1 AND (#2 OR #3) |
| Virtual Health Library | ((hepatitis B) OR (hepatitis B virus) OR (chronic hepatitis B)) AND (refugee OR (asylum seeker) OR (forced migrant) OR (displaced person) OR (displaced people) OR (displaced population)) AND (prevalence OR epidemiology OR screening OR rate) |
2.2. Eligibility criteria
Inclusion criteria were articles in English language with full‐text availability reporting the prevalence of HBV amongst refugees, asylum seekers and/or IDPs currently living in LMIC. LMIC were defined according to World Bank classification. 27 We included studies that measured HBV prevalence using hepatitis B surface antigen (HBsAg) and/or hepatitis B virus DNA. Given few existing studies on this topic, inclusion criteria were broad and no limits were applied for age, setting (e.g. camp, clinic, hospital, community), sampling method or number of participants. Refugees, asylum seeker or IDP status was determined based on article terminology, or by described characteristics of the population, noting most articles did not include definitions. The primary outcome was HBV prevalence, but this did not need to be the primary outcome of the study to be eligible. Exclusion criteria were case studies, case series, conference abstracts, modelling studies, economic evaluations or studies from high‐income countries, including amongst people in camps in high‐income countries or those had been resettled. Review articles were excluded but noted for background and additional references.
2.3. Data collection and screening
After retrieval of articles from electronic searches to Endnote, we undertook further article screening using the systematic review tool Covidence. 28 Duplicates were removed, and title and abstracts were screened by two independent reviewers. Conflicts were resolved through discussion between the two reviewers, with escalation to a third independent investigator if unable to be resolved. Full‐text articles were reviewed by a single reviewer. Data were extracted by a single reviewer to a Microsoft Excel Spreadsheet with the following outcomes: lead author, year of publication, total number of participants, mean age, sex (number and proportion of males), population (refugee/asylum seeker/IDP), country of study, country of refugee origin, setting (camp, clinic, hospital, other), HBV prevalence and HBV measure (HBsAg rapid diagnostic test, HBsAg ELISA, HBV DNA).
2.4. Risk of bias assessment
As all included studies were cross‐sectional, the Joanna Briggs Institute critical appraisal tool for cross‐sectional studies was used to assess the risk of bias of included studies. 29 , 30
3. RESULTS
3.1. Study selection and characteristics
We identified 758 articles from the electronic database search and five articles from other sources (websites, citation searching) (Figure 1). Following duplicate removal; screening of titles and abstracts; and review of full texts; we included 28 studies in this review (Appendix 2 and 3).
FIGURE 1.
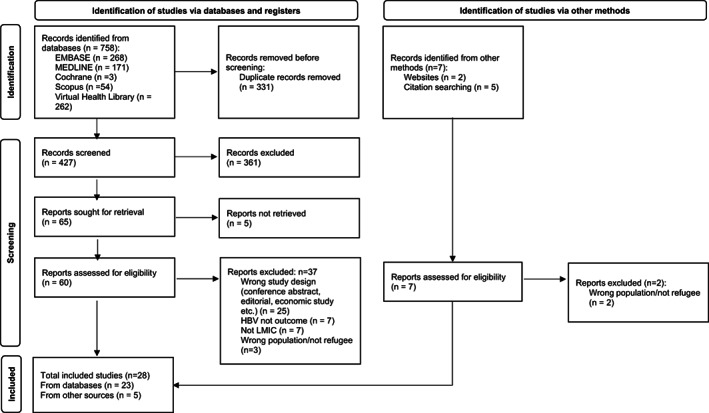
PRISMA Flow diagram. An electronic database search identified 758 articles; 331 duplicates were removed. Following screening by title and abstract, followed by full‐text review, 23 of these articles were included in the final review. An additional five articles were identified through other methods. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
The characteristics of included studies is presented in Table 2. Countries of study included Turkey (n = 7), Pakistan (n = 6), Thailand (n = 3), Bangladesh (n = 2) and others. Countries of refugee origin included Syria (n = 6), Myanmar (n = 5), Afghanistan (n = 4), Pakistan (n = 3) and others; there were no studies from Central or South America. Most studies included refugees from a single country of origin, except for two studies of pre‐arrival screening outcomes for refugees from multiple countries 31 , 32 and one study from Turkey amongst detained asylum seekers from multiple countries. 33
TABLE 2.
Study characteristics
| Author | Year | Study Country | Country of Refugee Origin | Setting | Status | Total | Male | Mean age (years) | Prevalence of HBV | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | N | % | N | % | 95% CI | |||||||
| Ali | 2022 | Bangladesh | Myanmar | Camp | Refugee | 2000 | 1000 | 50 | 82 | 4 | ||
| Ayele | 2020 | Ethiopia | South Sudan | Camp | Refugee | 453 | 83 | 18.3 | 29.6 | 33 | 7.3 | |
| Banks | 2016 | Thailand | Myanmar | Clinic | Refugee & Migrant | 6158 | 0 | 0 | 26 | 511 | 8.3 | |
| Bierhoff | 2019 | Thailand | Myanmar | Clinic | Refugee & Migrant | 11,025 | 0 | 0 | 26 | 687 | 6.2 | 5.7–6.7 |
| Crawshaw | 2018 | International | Various | Clinic | Refugee | 9228 | 130 | 69 | 188 | 2.04 | 1.77–2.35 | |
| Gungor | 2018 | Turkey | Syria | Hospital | Refugee | 704 | 0 | 0 | 23 | 31 | 4.3 | |
| Hussein | 2016 | Iraq | Syria | Camp | Refugee | 880 | 406 | 46.1 | 24 | 34 | 3.86 | |
| Inci (a) | 2017 | Turkey | Syria | Hospital | Refugee | 2158 | 0 | 0 | 24 | 1.1 | ||
| Inci (b) | 2017 | Turkey | Syria | Hospital | Refugee | 300 | 144 | 48 | 9 | 3 | ||
| Kamali | 2021 | Rwanda | Burundi | Camp | Refugee | 26,498 | 12,149 | 46.2 | 1006 | 3.8 | 3.57–4.03 | |
| Karasahin | 2021 | Turkey | Afghanistan | Detention | Asylum seeker | 9197 | 8195 | 89.1 | 23 | 505 | 5.5 | |
| Kazmi | 2021 | Pakistan | India (Kashmir) | Camp | Refugee | 1225 | 585 | 48 | 86 | 7 | ||
| Khan | 2011 | Pakistan | Pakistan | Clinic | IDP | 950 | 550 | 61 | 200 | 21.05 | ||
| Khan | 2018 | Pakistan | Pakistan | Camp | IDP | 1000 | 570 | 57 | 45 | 4.5 | ||
| Khanani | 2010 | Pakistan | Afghanistan | Clinic | Refugee | 556 | 410 a | 73.7 | 51 | 9.17 | ||
| Kose | 2015 | Turkey | Various | Detention | Asylum seeker | 222 | 211 | 95.0 a | 27.8 | 14 | 6.36 | |
| Kose | 2017 | Turkey | Syria | Clinic | Refugee | 140 | 69 a | 49 | 6.5 | 6 | 4.2 | |
| Kowo | 2021 | Cameroon | Central African Republic | Camp | Refugee | 970 | 417 | 43 | 29 | 75 | 7.7 | |
| Mazhar | 2011 | Bangladesh | Myanmar | Camp | Refugee | 275 | 156 | 57 | 36 | 13 | ||
| Mitchell | 2018 | Thailand | Myanmar | Camp | Refugee | 2004 | 1038 | 52 | 191 | 10 | ||
| Mixson‐Hayden | 2014 | International | Various | Camp | Refugee | 4890 | 2409 | 49.3 | 34.8 | 331 | 12.1 | |
| Odimayo | 2020 | Nigeria | Nigeria | Camp | IDP | 346 | 111 | 32.1 | 18.5 | 55 | 15.9 | |
| Pourkarim | 2008 | Iran | Afghanistan | Camp | Refugee | 74 | 37 | 50 | 44.7 | 45 | 60.8 | |
| Quddus | 2006 | Pakistan | Afghanistan | Camp | Refugee | 903 | 75 | 8.3 | 6.6–10.3 | |||
| Rauf | 2011 | Pakistan | Pakistan | Camp | IDP | 590 | 290 | 49.2 a | 2 | 0.34 a | ||
| Shah | 2005 | Nepal | Bhutan | Camp | Refugee | 467 | 204 | 43.7 a | 4 | 0.9 | ||
| Stevens | 2016 | India | Tibet | Camp/Community | Refugee | 2769 | 1780 | 61 | 18 b | 247 | 8.9 | 7.9–9.9 |
| Tumturk | 2019 | Turkey | Syria | Hospital | Refugee | 244 | 154 | 63.1 | 32.6 | 14 | 5.74 | |
Note: Values to the nearest 1dp.
Abbreviations: CI, confidence interval; HBV, hepatitis B virus; IDP, internally displaced persons.
Calculated from available data.
Median.
There were 22 studies reporting outcomes amongst refugees, four for IDPs and two for asylum seekers. Study settings ranged from refugee/IDP camp (n = 16), clinics (n = 6), hospitals (n = 4) and detention centres (n = 2). One study included participants from a refugee camp, a school and a monastery. 34 Half of the included studies mentioned age of participants, of these, the mean age ranged from 6.5 to 44.7 years. One study included children only. 35 The proportion of males varied; one study had only 18% male participants, 36 whilst seven studies had over 60% males. Four articles reported outcomes amongst pregnant women.
Most studies used HBsAg as the screening method for HBV, with the exception of three studies which used HBV DNA only. Four studies did not specify which test was used. Five studies used HBsAg rapid antigen testing, five studies used a rapid antigen test followed by confirmatory ELISA, one study used rapid antigen test followed by confirmatory HBV DNA, 10 studies used HBV ELISA only, and three studies used HBV DNA only.
3.2. Prevalence of hepatitis B virus
The prevalence of HBV ranged significantly from <1% to 60% and significant differences were observed between and within countries. Three studies reported <2% prevalence, 15 studies 2%–8% and 10 studies over 8%.
Prevalence of HBV amongst Syrian refugees was described in five studies from Turkey and one study from Iraq, with rates of 1.1%–5.4%. Two studies amongst pregnant women referred to hospitals in Turkey showed a prevalence of 1.1% and 4.3%, respectively. 37 , 38 One study amongst Syrian children referred to clinics in Turkey found a prevalence of 4.2%. 35 Two other studies from hospitalized Syrian refugees in Turkey described prevalence of 3.86% 39 and 5.74%, the latter included 63% males. 40
Five studies reported outcomes amongst refugees from Myanmar. Two were from clinics on the Thai–Myanmar Border, one was in camps in Thailand, and two studies amongst Rohingya refugees living in Bangladesh. A prevalence of 8.3% was reported amongst 6100 pregnant refugee and migrant women presenting to antenatal clinics 41 ; an expanded study in the same setting then found a prevalence of 6.2% amongst over 11,000 refugees (95% CI 5.7–6.7). 42 Prevalence of 10% was reported amongst 2000 US‐bound refugees living in camps on the Thai–Myanmar border. 43 In this study, multivariate analysis showed significantly higher odds of HBV amongst males and age older than 15 years, but not amongst those with tattoos. Authors reported 47% of HBV‐positive people who attended an ultrasound had abnormal findings of hepatomegaly or parenchymal disease. Only 13% of HBV‐negative individuals had completed HBV immunization, of which 89% were children under 8 years of age. 43 A recent study amongst 2000 Rohingya refugees in Cox's Bazaar in Bangladesh found a prevalence of 4%. 44 Another study of Rohingya refugees in Cox's Bazaar evaluated causes of 272 cases of acute jaundice, reporting 13% were positive for hepatitis B. 45 This is unlikely to representative of the true hepatitis B prevalence in this population as only acute jaundice cases were included, which mostly reflects cases of acute hepatitis A (154 cases, 56%).
Prevalence amongst Afghan refugees and asylum seekers in Pakistan, Turkey and Iran was described in four studies. Prevalence of 8.3% was reported amongst 900 Afghan refugees in Pakistan (95% CI 6.6–10.3). 46 Risk factors for HBV in multivariate analysis included those who received over 10 injections for medical treatment in the past year or were treated by private practitioners; authors highlighted the significance of unsafe infection in this population and the need for further education. High prevalence of 5.9% was reported amongst under 5‐year‐old children, and there was increased odds of HBV‐positive adults having HBV‐positive children, suggesting vertical and possibly horizontal transmission is another significant cause of infection. 46 A small study of 74 Afghan couples in a refugee camp in Balochistan in southwest Iran reported significantly high prevalence of 60.8%. 47 Approximately, 30% of couples were both HBsAg positive, 8% were both negative, and serodiscordance was present in 62% (husbands positive, wives negative). The study methods or reasons for significantly high prevalence were not described. Another study of 500 refugees presenting to clinics in Pakistan reported prevalence of 9.17%, over 70% of participants were male. 48 Another study of over 9100 Afghan asylum seekers detained in Turkey reported a prevalence of 5.5%, almost 90% were male. 49
Three studies reported prevalence amongst IDPs in Pakistan, ranging from <1% to >20%. Prevalence of 4.5% was reported amongst 1000 IDPs in North Wazirstan Agency, Pakistan. 50 Amongst those with HBV, 33% had previous blood transfusion, 60% attended barber shops, 71% had multiple injections, and 80% had general/dental surgery. Another study amongst 950 IDPs in Malakand Division of Pakistan reported prevalence of 21.05%. 51 Authors suggested low immunization rates in rural areas may have contributed to this high prevalence. Both studies of IDPs in Pakistan were conflict‐affected regions and multiple risk factors for HBV were present including household exposure, unsafe medical injections, dental/surgical procedures, barber shops. 50 , 51 High proportions of males may have been an additional contributing factor to one study, where 61% of the total study cohort and 78% of HBV‐positive individuals were male. 51 Authors suggested transmission from males to females may be occurring, given exposure to risk factors such as barber shaving and drug use. 51 Prevalence of 0.35% was reported in one study of 590 IDPs in the north Pakistan; however, this estimate is likely invalid given the method used was screening for hepatitis B surface antibody (positive in five cases), followed by HBV DNA in those samples (positive in two cases). 52
Four studies in Africa reported prevalence of HBV. A study of 2000 South Sudanese refugees living in a camp in Ethiopia found a prevalence of 7.3%. 36 A lack of awareness of HBV was identified: 79% did not know of HBV infection, 91% did not know of its transmission, and 87% did not know a vaccine was available. Additionally, 98% of participants were not vaccinated, and other risk factors including sharp material exchange, tattoos and multiple sex partners were present but not statistically significant. 36 Another study of 26,000 Burundian refugees in Mahama camp in Rwanda reported prevalence of 3.8% (95% CI 3.57–4.03). 53 Of the samples tested for viral load, 85% had a detectable HBV DNA. 53 Household contact of an individual with HBV and family history of hepatitis were associated with infection. The study found that refugees compared with the Rwandan general population had a 1.86 times higher prevalence of HBsAg, after standardizing to the age of the Rwandan population. 53 Another study amongst 970 refugees from the Central African Republic living in Cameroon reported a prevalence of 7.7%; risk factors included age 20–39 years, self‐employment, previous surgery and multiple sex partners. 54 A study of 346 IDPs in Nigeria reported high prevalence of 15.9% 55 ; 68% were female and the mean age was 18.5 years. Over 80% of all participants and 100% of HBV‐positive individuals had not been vaccinated. 55
Two international studies reported outcomes amongst US‐ and UK‐bound refugees attending pre‐arrival screening clinics in LMIC. In a study of 12,000 UK‐bound refugees, of whom 9228 were tested for HBV, the prevalence was 2.04% (95% CI 1.77–2.35). 31 Prevalence varied between refugees from different countries of origin, for example, 0.58% amongst 514 Iraqi refugees compared with 12.5% amongst 40 refugees from South Sudan. Another study of 4890 US‐bound refugees from Bhutan, Myanmar, Laos (Hmong), Thailand (Hmong), Somalia and Iraq found an overall prevalence of 12.1%. 32 In this study, testing was performed by initial screening for anti‐HBs, with negative samples proceeding to testing with HBV DNA; this may under‐estimate prevalence.
3.3. Risk of bias
There was a high risk of bias amongst included studies (Table 3). All except one received a score under 5 out of 8 using the Joanna Briggs Institute critical appraisal tool for cross‐sectional studies. Only one study addressed confounding factors. Most studies had limited description of methods used such as sampling, inclusion and exclusion criteria.
TABLE 3.
Risk of bias assessment, Joanna Briggs Institute Checklist for cross‐sectional studies
| Author (Year) | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Total |
|---|---|---|---|---|---|---|---|---|---|
| Ali (2022) | Y | Y | N/A | Y | N | N | Y | Y | 5 |
| Ayele (2020) | Y | Y | N/A | Y | N | N | Y | Y | 5 |
| Banks (2016) | Y | Y | N/A | Y | N | N | Y | Y | 5 |
| Bierhoff (2019) | Y | Y | N/A | Y | N | N | Y | Y | 5 |
| Gungor (2018) | N | Y | N/A | Y | N | N | U | Y | 3 |
| Crawshaw (2018) | Y | Y | N/A | Y | N | N | U | Y | 4 |
| Hussein (2016) | N | Y | N/A | Y | N | N | Y | Y | 4 |
| Inci (a) 2017 | Y | Y | N/A | Y | N | N | Y | Y | 5 |
| Inci (b) 2017 | Y | Y | N/A | Y | N | N | Y | Y | 5 |
| Kamali (2021) | Y | Y | N/A | Y | Y | Y | Y | Y | 7 |
| Karasahin (2021) | Y | Y | N/A | Y | N | N | U | Y | 4 |
| Kazmi (2022) | Y | Y | N/A | Y | N | N | Y | Y | 5 |
| Khan (2011) | Y | Y | N/A | Y | N | N | Y | Y | 5 |
| Khan (2018) | Y | Y | N/A | Y | N | N | Y | Y | 5 |
| Khanani (2010) | N | Y | N/A | Y | N | N | Y | Y | 4 |
| Kose (2015) | Y | Y | N/A | Y | N | N | Y | Y | 5 |
| Kose (2017) | Y | Y | N/A | Y | N | N | Y | Y | 5 |
| Kowo (2021) | Y | Y | N/A | Y | N | N | Y | Y | 5 |
| Mazhar (2021) | Y | Y | N/A | Y | N | N | Y | Y | 5 |
| Mitchell (2018) | Y | Y | N/A | Y | N | N | Y | Y | 5 |
| Mixson‐Hayden (2014) | Y | Y | N/A | Y | N | N | Y | Y | 5 |
| Odimayo (2020) | Y | Y | N/A | Y | N | N | Y | U | 4 |
| Pourkarim (2008) | N | Y | N/A | Y | N | N | U | Y | 3 |
| Quddus (2006) | Y | Y | N/A | Y | N | N | Y | Y | 5 |
| Rauf (2010) | N | Y | N/A | Y | N | N | N | Y | 3 |
| Shah (2005) | Y | Y | N/A | Y | N | N | Y | Y | 5 |
| Stevens (2016) | Y | Y | N/A | Y | N | N | Y | Y | 5 |
| Tumturk (2019) | N | Y | N/A | Y | N | N | Y | Y | 4 |
Note: N, no; N/A, not applicable; U, unclear; Y, yes.
Q1: Were the criteria for inclusion in the sample clearly defined?
Q2: Were the study subjects and the setting described in detail?
Q3: Was the exposure measured in a valid and reliable way?
Q4: Were objective, standard criteria used for measurement of the condition?
Q5: Were confounding factors identified?
Q6: Were strategies to deal with confounding factors stated?
Q7: Were the outcomes measured in a valid and reliable way?
Q8: Was appropriate statistical analysis used?
4. DISCUSSION
This systematic review is the first to describe the prevalence of hepatitis B amongst refugees, asylum seekers and IDPs in LMIC. This topic is of public health relevance as LMIC have the highest burden of hepatitis B, and host the most refugees, but also face health system challenges and constrained resources. Refugees are a highly diverse, mobile population and have increased risk of poorer health outcomes and barriers in access to health care. Though few studies on this topic exist, the available evidence suggests a high prevalence amongst refugees in LMIC, with wide variation between and within countries. A small number of studies reported very high prevalence rates of over 10%. The wide range of results reflects differences in study design and quality, and that hepatitis B prevalence varies on national and subnational levels. 1 It also highlights the diversity of refugee populations, whose health status and access to health care may be influenced by differences in living conditions, health systems in their country of origin and country of current residence and exposure to varying risk factors.
Previous literature about HBV amongst refugees has mostly reported outcomes amongst those in high‐income countries. A systematic review of HBV by Rossi et al. identified an overall prevalence of 7.2% amongst refugees and migrants arriving in low‐endemic regions, and a higher risk of HBV amongst refugees compared with migrants (OR 1.42, 95% CI 1.03–2.43). 21 Prevalence generally correlated with that of the regions of origins, with highest rates amongst those from East Africa, the Pacific and Sub‐Saharan Africa. Lack of information about age and gender was a confounding factor, given older age and male gender have been previously shown to be associated with higher prevalence of HBV. 21 Similarly, around half of the studies in this review did not include data regarding age distribution. Another systematic review of refugee children arriving in high‐income countries reported hepatitis B prevalence of 3%. 12 A meta‐analysis of infectious diseases amongst refugees and migrants in Africa found overall prevalence of 10.2%, with higher rates amongst refugees from Sub‐Saharan Africa (13.6%) compared with North Africa (3.25%). 13
Hepatitis B prevalence amongst refugees in LMIC may be different compared with those in high‐income countries for several reasons, including the ‘healthy migrant effect’, 56 unstable living conditions, scarce access to health resources, higher baseline prevalence of HBV in their country of origin and exposure to risk factors causing ongoing transmission. Disrupted health services, including in conflict zones and protracted humanitarian crises, may increase the risk of iatrogenic infection through unsafe injection and blood transfusion. 57 Exposure to unsafe injections and procedures was a risk factor identified in several studies 46 , 50 , 51 and may reflect a lack of awareness about safe injection, unsterilized equipment and poor health infrastructure or frequent exposure to violence and conflict requiring hospitalization and treatment such as blood transfusions or surgeries. Some risk factors are unique to certain areas, such as barber shops amongst studies from Central Asia, 50 or scarification in some areas in Africa. Some studies identified risk factors of multiple sex partners and unprotected sexual intercourse; none mentioned the potential of gender‐based violence (GBV) on HBV transmission. GBV is highly prevalent amongst refugees and may be a risk factor for infection, especially in conflict settings and camps. 58 Low awareness of HBV 36 may reflect limited access to education and health care, and low health literacy about HBV amongst refugees and migrants has been described in a previous systematic review. 24 Alarmingly, low rates of HBV immunization, 43 , 55 is a preventable cause of infection. Refugees may have missed out due to displacement, difficulty accessing health care or lack of national immunization programmes in their country of origin.
Due to the paucity of existing literature, we used a broad eligibility criterion and included many low‐quality studies. Most studies had limited description of methods and inclusion criteria, all were cross‐sectional in design, the risk of bias was generally high. We included studies that used either HBsAg or HBV DNA as screening methods. However, some used an invalid screening method (e.g. testing for anti‐HBs followed by HBV DNA as a confirmation marker). Demographics including age and gender, where described, varied greatly. Higher age or higher male proportion may be a confounding factor in some studies given the association of older age and males with HBV infection. Due to all these factors, these studies are not nationally representative nor directly comparable. Some described risk factors for hepatitis B, however, the interpretation of this is limited given they were cross‐sectional studies—this is a potential area for further study.
Hepatitis B has garnered increasing public health attention over the last decade, but the burden is disproportionately high in LMIC and amongst marginalized groups. Refugees and migrants are at higher risk of hepatitis B and are a priority population in the public health response to HBV. 8 The limited high‐quality evidence about prevalence and risk factors for hepatitis B amongst refugees in LMIC highlights the need for further characterization to inform appropriate public health responses, noting that services should adopt a human rights approach and be tailored to the circumstances of local refugee populations—not a ‘one size fits all’ approach. Low awareness and low immunization levels for HBV identified in this review suggests this is an important area to be addressed, especially given the availability of a low cost, highly effective vaccine. Delivering hepatitis care is challenging in resource‐limited settings, particularly in conflict zones and humanitarian crises where health systems are fragile. However, it is feasible to provide treatment for HIV in conflict‐affected regions 59 and prevent mother‐to‐child transmission of HBV with tenofovir in resource‐limited settings. 60
In conclusion, hepatitis B is a significant public health issue with a high burden in LMIC, where service delivery is challenging in the context of constrained resources. Refugees are a highly diverse and mobile group who often face difficulties accessing health care and are a priority population at risk of hepatitis B. No previous studies have assessed the prevalence of HBV amongst refugees in LMIC, where most of the global population of refugees reside. This review highlights an intermediate to high prevalence of hepatitis B amongst refugees, asylum seekers and IDPs in LMIC, with a wide variation between countries and regions. Reasons for higher rates of HBV may include risk factors such as unsafe medical treatments, and exposures to sharps; mother‐to‐child transmission; low immunization coverage; barriers in access to health care; and high baseline prevalence rates in their country of origin. The available literature is scarce and of low quality, and further detailed studies are needed to further characterize the prevalence and risk factors for HBV amongst refugees in LMIC and inform robust and equitable health responses.
FUNDING INFORMATION
No funding was given for this review.
CONFLICT OF INTEREST
There are no conflicts of interest; a separate conflict of interest statement will be supplied at time of manuscript submission.
ACKNOWLEDGEMENT
The authors would like to thank the contribution of Maya Sebesteyen for librarian assistance. Open access publishing facilitated by James Cook University, as part of the Wiley ‐ James Cook University agreement via the Council of Australian University Librarians. Open access publishing facilitated by James Cook University, as part of the Wiley ‐ James Cook University agreement via the Council of Australian University Librarians.
APPENDIX 1. PRISMA 2020 Checklist
| Section and Topic | Item # | Checklist item | Location where item is reported |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review. | P1 |
| Abstract | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | P2 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | P3 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | P3 |
| Methods | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | P4 |
| Information sources | 6 | Specify all databases, registers, websites, organizations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | P4 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | Table 1 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | P5 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | P5 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g. for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | P5 |
| 10b | List and define all other variables for which data were sought (e.g. participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | P5 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | P5 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g. risk ratio, mean difference) used in the synthesis or presentation of results. | N/A |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g. tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | N/A |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | N/A | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | N/A | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta‐analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | N/A | |
| 13 e | Describe any methods used to explore possible causes of heterogeneity amongst study results (e.g. subgroup analysis, meta‐regression). | N/A | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | N.A | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | N/A |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | N/A |
| Results | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | P5, Figure 1 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | P5, Figure 1, Appendix 3 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | Table 2, Appendix 2 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | Table 3 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g. confidence/credible interval), ideally using structured tables or plots. | Table 2 |
| Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and risk of bias amongst contributing studies. | P6 |
| 20b | Present results of all statistical syntheses conducted. If meta‐analysis was done, present for each the summary estimate and its precision (e.g. confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | N/A | |
| 20c | Present results of all investigations of possible causes of heterogeneity amongst study results. | N/A | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | N/A | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | N/A |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | N/A |
| Discussion | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | P9 |
| 23b | Discuss any limitations of the evidence included in the review. | P10 | |
| 23c | Discuss any limitations of the review processes used. | P10 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | P10 | |
| Other information | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | P4 |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | P4 | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | N/A | |
| Support | 25 | Describe sources of financial or non‐financial support for the review, and the role of the funders or sponsors in the review. | P12 |
| Competing interests | 26 | Declare any competing interests of review authors. | P12 |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | N/A |
APPENDIX 2. List of included studies
Ali M, Rahman MA, Njuguna H, Rahman S, Hossain R, Sayeed A, et al. High Prevalence of Hepatitis B and C Virus Infections Amongst Rohingya Refugees in Bangladesh: A Growing Concern for the Refugees and the Host Communities. Clinical Liver Disease. 2022;19(1):1–6.
Ayele A, Abera D, Hailu M, Birhanu M, Desta K. Prevalence and associated risk factors for Hepatitis B and C viruses amongst refugees in Gambella, Ethiopia. BMC Public Health. 2020;20(1):721‐.
Banks T, Kang J, Watts I, Tyrosvoutis MEG, Min AM, Tun NW, et al. High hepatitis B seroprevalence and risk factors for infection in pregnant women on the Thailand‐Myanmar Border. Journal of infection in developing countries. 2016;10(4):384–8.
Bierhoff M, Angkurawaranon C, Myat Min A, Gilder ME, Win Tun N, Keereevijitt A, et al. Maternal Hepatitis B Infection Burden, Comorbidity and Pregnancy Outcome in a Low‐Income Population on the Myanmar‐Thailand Border: A Retrospective Cohort Study. J Pregnancy. 2019;2019:8435019.
Crawshaw AF, Pareek M, Were J, Schillinger S, Gorbacheva O, Wickramage KP, et al. Infectious disease testing of UK‐bound refugees: a population‐based, cross‐sectional study. BMC Med. 2018;16(1):143‐.
Güngör ES, Seval O, İlhan G, Verit FF. Do syrian refugees have increased risk for worser pregnancy outcomes? Results of a tertiary center in İstanbul. Turkish Journal of Obstetrics and Gynaecology. 2018;15(1):23–7.
Hussein N, Muhammed I, Younus O, Taher A, Salim A, Shahab F. Prevalence of HBV, HCV and HIV Infections Amongst Syrian Refugees in Kurdistan Region, Iraq. International Journal of Infection. 2016;4.
Inci A, Sarici IS, Çalişkan G, Kalayci MU. Investigation of frequency of HBSAG, anti HBS, anti HCV and anti HIV in refugee patients from Syria who admit to a training and research hospital department of surgery. Acta Medica Mediterranea. 2017;33(1):59–63.
Inci A, Yildirim D, Seckin KD, Gedikbasi A. Analysis of HbsAg positivity rate before and after vaccination in Turkish and Syrian refugee pregnant women. Journal of Infection in Developing Countries. 2017;11(10):815–8.
Kamali I, Ndahimana JdA, Nyirahabihirwe F, Gakuru JdlP, Musafiri T, Urusaro S, et al. Prevalence and associated risk factors for hepatitis B and C viruses amongst refugee populations living in Mahama, Rwanda: A cross‐sectional study. PLoS ONE. 2021;16(10 October):e0257917.
Karasahin EF, Karasahin O, Kalkan IA. Results of viral hepatitis and human immunodeficiency virus screening in afghan irregular migrants: A cross‐sectional study (2011–2019). Viral Hepatitis Journal. 2021;27(2):98–102.
Kazmi SA, Rauf A, Shafique F, Asim N, Shafi N, Hassan MU. Kashmiri refugees at the verge of hepatitis B and C epidemic in the State of Azad Jammu and Kashmir, Pakistan. Rev Saude Publica. 2022;56:33.
Khan A, Qazi J. Risk factors and molecular epidemiology of HBV and HCV in internally displaced persons (IDPs) of North Waziristan Agency, Pakistan. Journal of the Pakistan Medical Association. 2018;68(2):165–9.
Khan F, Akbar H, Idrees M, Khan H, Shahzad K, Kayani MA. The prevalence of HBV infection in the cohort of IDPs of war against terrorism in Malakand Division of Northern Pakistan. BMC infectious diseases. 2011;11:176.
Khanani MR, Ansari AS, Khan S, Somani M, Kazmi SU, Ali SH. Concentrated epidemics of HIV, HCV, and HBV amongst Afghan refugees. Journal of Infection. 2010;61(5):434–7.
Kose S, Kuzucu L, Gozaydin A, Yilmazer T. Prevalence of hepatitis B and C viruses amongst asylum seekers in Izmir. Journal of immigrant and minority health. 2015;17(1):76–8.
Kose S, Odemis I, Celik D, Gireniz Tatar B, Akbulut I, Ciftdogan DY. Hepatitis A, B, C and HIV seroprevalence amongst Syrian refugee children admitted to outpatient clinics. Le infezioni in medicina. 2017;25(4):339–43.
Kowo MP, Frungwa CN, Andoulo FA, Ndam AWN, Njonnou SRS, Yemeli LD, et al. Epidemiologic patterns of hiv, hepatitis b and c virus infections amongst refugees of the mbile camp in the east region of cameroon (hepatitis and hiv amongst refugees). Journal of Gastroenterology and Hepatology Research. 2021;10(3):3524–30.
Mazhar MKA, Finger F, Evers ES, Kuehne A, Ivey M, Yesurajan F, et al. An outbreak of acute jaundice syndrome (AJS) amongst the Rohingya refugees in Cox's Bazar, Bangladesh: Findings from enhanced epidemiological surveillance. PloS one. 2021;16(4):e0250505.
Mitchell T, Lee D, Weinberg M, Phares C, James N, Amornpaisarnloet K, et al. Impact of Enhanced Health Interventions for United States‐Bound Refugees: Evaluating Best Practices in Migration Health. The American journal of tropical medicine and hygiene. 2018;98(3):920–8.
Mixson‐Hayden T, Lee D, Ganova‐Raeva L, Drobeniuc J, Stauffer WM, Teshale E, et al. Hepatitis B virus and hepatitis C virus infections in United States‐bound refugees from Asia and Africa. The American journal of tropical medicine and hygiene. 2014;90(6):1014–20.
Odimayo MS, Adebimpe WO, Jeff‐Agboola YA, Oyeyemi OT, Okiei BN, Adejumo OA, et al. Screening, Vaccination, and Referrals as Viral Hepatitis Elimination Triad Amongst Internally Displaced Persons in Edo State, Nigeria. Clinical Liver Disease. 2020;16(5):218–22.
Pourkarim MR, Zandi K, Davani NA, Pourkarim HR, Amini‐Bavil‐Olyaee S. An aberrant high prevalence of hepatitis B infection amongst Afghans residing in one of the Bushehr refugee camps (Dalaki camp) in the southwest of Iran. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2008;12(1):101–2.
Quddus A, Luby SP, Jamal Z, Jafar T. Prevalence of hepatitis B amongst Afghan refugees living in Balochistan, Pakistan. International Journal of Infectious Diseases. 2006;10(3):242–7.
Rauf A, Nadeem MS, Ali A, Iqbal M, Mustafa M, Latif MM, et al. Prevalence of hepatitis B and C in internally displaced persons of war against terrorism in Swat, Pakistan. European journal of public health. 2011;21(5):638–42.
Shah BK, Bhattacharya S, Parija SC. Seroprevalence of hepatitis B virus amongst Bhutanese refugees residing in Nepal. Biomedicine. 2005;25(1):36–40.
Stevens K, Palmo T, Wangchuk T, Solomon S, Dierberg K, Hoffmann CJ. Hepatitis B prevalence and treatment needs amongst Tibetan refugees residing in India. Journal of medical virology. 2016;88(8):1357–63.
Tümtürk A, Yeşil B. Hepatitis B, Hepatitis C and HIV seroprevalence amongst Syrian refugees: A cross‐sectional study from a tertiary referral center in Turkey. Journal of Surgery and Medicine. 2019;3.
APPENDIX 3. List of excluded studies
The following 37 studies were excluded following full‐text review.
Alam I, Alam ZT, Saeed S, Shamim N, Rafiqui N, Alam I. LOW COST HEPATITIS C TREATMENT WITH GENERIC SOFOSBUVIR AND RIBAVIRIN IN POOR SOCIOECONOMIC POPULATION IN PAKISTAN BY PRIMARY CARE PROVIDERS. Gastroenterology. 2019;156(6 Supplement 1):S‐1346.
Apte M, Shamala N, Ramakrishnan T. Antigenic relationship between reactivity to hepatitis B e antigen and 19 kDa protein of Mycobacterium tuberculosis amongst the Tibetan settlers in Karnataka. Journal of Biosciences. 1992;17(3):305–12.
Beldjebel I. Infectious diseases amongst refugees in Beirut. International Journal of Infectious Diseases. 2012;16(SUPPL.1):e6.
Beldjebel I, Krcmery V. Infectious diseases amongst Iraqi refugees in Lebanon. International Journal of Infectious Diseases. 2012;16(SUPPL.1):e341‐e2.
Caruana SR, De Silva SL, Kelly HA, Chea L, Nuon S, Saykao P, et al. Knowledge about hepatitis and previous exposure to hepatitis viruses in immigrants and refugees from the Mekong region. Australian and New Zealand journal of public health. 2005;29(1):64–8.
Chaudhary RK, Nicholls ES, Kennedy DA. Prevalence of hepatitis B markers in Indochinese refugees. Can Med Assoc J. 1981;125(11):1243–6.
Chiesa A, Ochola E, Oreni L, Vassalini P, Rizzardini G, Galli M. Hepatitis B and HIV coinfection in Northern Uganda: Is a decline in HBV prevalence on the horizon? PLoS One. 2020;15(11):e0242278.
Chironna M, Germinario C, Lopalco PL, Barbuti S, Quarto M, Carrozzini F. Prevalence rates of viral hepatitis infections in refugee Kurds from Iraq and Turkey. Infection. 2003;31(2):70–4.
Debes JD. Hepatitis B in refugees, guessing the prevalence. Hepatology (Baltimore, Md). 2010;52(2):802–3.
Denburg A, Rashid M, Brophy J, Curtis T, Malloy P, Audley J, et al. Initial health screening results for Karen refugees: a retrospective review. Canada communicable disease report = Releve des maladies transmissibles au Canada. 2007;33(13):16–22.
Devine A, Harvey R, Min AM, Gilder MET, Paw MK, Kang J, et al. Strategies for the prevention of perinatal hepatitis B transmission in a marginalized population on the Thailand‐Myanmar border: a cost‐effectiveness analysis. BMC infectious diseases. 2017;17(1):552.
Djukanovic L, Radovic M, Bakovic J, Budosan I, Bukvic D, Cveticanin A, et al. Epidemiology of end‐stage renal disease and current status of haemodialysis in Yugoslavia. The International journal of artificial organs. 2002;25(9):852–9.
Dulger AC, Colak B, Satcan M, Turkdotan K, Kotan C. Hepatitis B prevalence amongst Syrian immigrants in Turkey. Hepatology International. 2015;9(1 SUPPL. 1):S211‐S2.
Dulger AC, Yakarisik M. Higher prevalences of helicobacter pylori infection, intestinal metaplasia and atrophic gastritis amongst Afghan refugees in coastal blacksea region of Turkey. Turkish Journal of Gastroenterology. 2019;30(Supplement 3):S289‐S90.
Fitzpatrick S, Johnson J, Shragg P, Felice ME. Health care needs of Indochinese refugee teenagers. Paediatrics. 1987;79(1):118–24.
Gargano LM, Cookson ST, Hajjeh R. Pneumonia prevention: Cost‐effectiveness analyses of two vaccines amongst refugee children aged under two years, Haemophilus influenzae type b‐containing and pneumococcal conjugate vaccines, during a humanitarian emergency, Yida camp, South Sudan. Vaccine. 2017;35(3):435–42.
Khan S, Attaullah S. Share of afghanistan populace in hepatitis B and hepatitis C infection's pool: Is it worthwhile? Virology Journal. 2011;8:216.
Kulstrunk M, Evéquoz D, Dubach VC, Bänziger W, Lütschg W, Stalder GA. Prevalence of hepatitis B virus in Kurdish refugees. J Hepatol. 1992;15(3):418–9.
Leblebicioglu H. Managing health and infections in refugees: Turkey's experience. International Journal of Infectious Diseases. 2016;45(SUPPL. 1):56.
Litch JA, Shackleton JR, Bishop RA. Prevalence of hepatitis B infection amongst Tibetan refugees in northern India. Tropical doctor. 1998;28(4):229–30.
McCarthy AE, So H, Weld LH, Greenaway C, Barnett ED, Coyle C, et al. Spectrum of illness in international migrants seen at geosentinel clinics in 1997–2009, part 2: Migrants resettled internationally and evaluated for specific health concerns. Clinical Infectious Diseases. 2013;56(7):925–33.
McCarthy MC, Burans JP, Constantine NT, el‐Hag AA, el‐Tayeb ME, el‐Dabi MA, et al. Hepatitis B and HIV in Sudan: a serosurvey for hepatitis B and human immunodeficiency virus antibodies amongst sexually active heterosexuals. Am J Trop Med Hyg. 1989;41(6):726–31.
Mehmood S, Raza H, Abid F, Saeed N, Rehman HM, Javed S, et al. National prevalence rate of hepatitis B and C in Pakistan and its risk factors. Journal of Public Health (Germany). 2020;28(6):751–64.
Mixson‐Hayden T, Lee D, Ganova‐Raeva L, Drobeniuc J, Stauffer W, Teshale EH, et al. Hepatitis A to E virus infections in selected united states‐bound refugee populations. Hepatology. 2013;58(4 SUPPL. 1):315A.
Mujeeb SA, Khan A, Korejo R. Hepatitis B serum markers amongst pregnant women in Sana'a, Yemen [5]. Annals of Saudi Medicine. 2003;23(3–4):232.
Oztas D, Kurt B, Akbaba M, Akyol M, Mollahaliloglu S, Topac O. Vaccination rates for Syrian population under temporary protection in Turkey. Central European journal of public health. 2020;28(2):130–4.
Pehlivanoǧlu F, Yaşar KK, Şengöz G. Asylum seekers: Do we need new approachs to their health problems? Nobel Medicus. 2011;7(1):102–5.
Rajabali A, Moin O, Ansari AS, Ali SH, Khanani MR. Communicable disease amongst displaced Afghans: Refuge without shelter. Nature Reviews Microbiology. 2009;7(8):609–14.
Sami S, Baloch SN. Vaginitis and sexually transmitted infections in a hospital based study. JPMA The Journal of the Pakistan Medical Association. 2005;55(6):242–4.
Santantonio T, Lo Caputo S, Germinario C, Squarcione S, Greco D, Laddago V, et al. Prevalence of hepatitis virus infections in Albanian refugees. Eur J Epidemiol. 1993;9(5):537–40.
Sekkarie MA, Karah N, Abbara A, Alashawi HS. Viral hepatitis infections in haemodialysis facilities in a non‐government controlled area in Syria. Journal of the American Society of Nephrology. 2019;30:1021.
Skinhøj P, Aldershvile J, Black F, Kjersem H, Kryger P, Mathiesen L. Viral hepatitis in southeast Asian refugees. J Med Virol. 1981;7(2):149–55.
Skinhøj P, Aldershvile J, Kjersem M, Black F. Hepatitis B infection in Vietnamese families. J Med Virol. 1983;11(2):125–9.
Wahid B, Waqar M, Rasool N, Rehman Z, Saeed J, Wasim M, et al. Recent trends in molecular epidemiology of Hepatitis C virus in Mardan, KPK Pakistan. Infect Genet Evol. 2018;66:66–71.
Ward JW, Averhoff F. Refugees, mass casualties, and hepatitis B transmission. Journal of Infectious Diseases. 2011;204(3):338–9.
Yotyingaphiram W, Pimanpanarak M, Bierhoff M, Van Vugt M, Rijken MJ, Angkurawaranon C, et al. Tenofovir for prevention of mother to child transmission of hepatitis B in migrant women in a resource‐limited setting on the Thailand‐Myanmar border: A commentary on challenges of implementation. International Journal for Equity in Health. 2020;19(1):156.
Yousif DE. Seroprevalence of undiagnosed transfusion transmissible infections (HIV, hepatitis B and C and syphilis) in donated blood in National Blood Banks, Khartoum, Sudan. British Journal of Haematology. 2015;169(SUPPL. 1):90.
Lee C, Emeto TI, Walsh N. Prevalence of hepatitis B virus amongst refugees, asylum seekers and internally displaced persons in low‐ and middle‐income countries: A systematic review. J Viral Hepat. 2023;30:4‐18. doi: 10.1111/jvh.13770
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546‐1555. doi: 10.1016/s0140-6736(15)61412-x [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Global progress report on HIV, viral hepatitis and sexually transmitted infections 2021. Accountability for the global health sector strategies 2016–2021: action for impact; 2021.
- 3. World Health Organization . Global Hepatitis Report 2017; 2017.
- 4. Cooke GS, Andrieux‐Meyer I, Applegate TL, et al. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2019;4(2):135‐184. doi: 10.1016/s2468-1253(18)30270-x [DOI] [PubMed] [Google Scholar]
- 5. Liu J‐F, Chen T‐Y, Zhao Y‐R. Vertical transmission of hepatitis B virus: propositions and future directions. Chin Med J (Engl). 2021;134(23):2825‐2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Global health sector strategy on viral hepatitis 2016‐2021. Towards Ending Viral Hepatitis; 2016.
- 7. World Health Organization . Interim Guidance for Country Validation of Viral Hepatitis Elimination; 2021. [DOI] [PubMed]
- 8. World Health Organization . Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022–2030; 2022.
- 9. United Nations High Commissioner for Refugees . Convention and Protocol Relating to the Status of Refugees; 2010. [PubMed]
- 10. United Nations High Commissioner for Refugees . Asylum‐Seekers; 2022. Accessed March 1, 2022 https://www.unhcr.org/en‐au/asylum‐seekers.html
- 11. United Nations . Guiding Principles on Internal Displacement; 2004.
- 12. Baauw A, Kist‐van Holthe J, Slattery B, Heymans M, Chinapaw M, van Goudoever H. Health needs of refugee children identified on arrival in reception countries: a systematic review and meta‐analysis. BMJ Paediatrics Open. 2019;3(1):e000516. doi: 10.1136/bmjpo-2019-000516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chernet A, Utzinger J, Sydow V, et al. Prevalence rates of six selected infectious diseases among African migrants and refugees: a systematic review and meta‐analysis. Eur J Clin Microbiol Infect Dis. 2018;37:605‐619. doi: 10.1007/s10096-017-3126-1 [DOI] [PubMed] [Google Scholar]
- 14. Eiset AH, Wejse C. Review of infectious diseases in refugees and asylum seekers‐current status and going forward. Public Health Rev. 2017;38:22. doi: 10.1186/s40985-017-0065-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cantor D, Swartz J, Roberts B, et al. Understanding the health needs of internally displaced persons: a scoping review. J Migr Health. 2021;4:100071. doi: 10.1016/j.jmh.2021.100071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rahman MR, Islam K. Massive diphtheria outbreak among Rohingya refugees: lessons learnt. J Travel Med. 2019;26(1):tay122. doi: 10.1093/jtm/tay122 [DOI] [PubMed] [Google Scholar]
- 17. Blackmore R, Boyle JA, Fazel M, et al. The prevalence of mental illness in refugees and asylum seekers: a systematic review and meta‐analysis. PLoS Med. 2020;17(9):e1003337. doi: 10.1371/journal.pmed.1003337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ivanova O, Rai M, Kemigisha E. A systematic review of sexual and reproductive health knowledge, experiences and access to services among refugee, migrant and displaced girls and young women in Africa. Int J Environ Res Public Health. 2018;15(8):1583. doi: 10.3390/ijerph15081583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lebano A, Hamed S, Bradby H, et al. Migrants' and refugees' health status and healthcare in Europe: a scoping literature review. BMC Public Health. 2020;20(1):1039. doi: 10.1186/s12889-020-08749-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization . Common health needs of refugees and migrants: literature review; 2021.
- 21. Rossi C, Shrier I, Marshall L, et al. Seroprevalence of chronic hepatitis B virus infection and prior immunity in immigrants and refugees: a systematic review and meta‐analysis. PLoS One. 2012;7(9):e44611. doi: 10.1371/journal.pone.0044611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim JU, Ingiliz P, Shimakawa Y, Lemoine M. Improving care of migrants is key for viral hepatitis elimination in Europe. Bull World Health Organ. 2021;99(4):280‐286. doi: 10.2471/BLT.20.260919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caruana SR, Kelly HA, De Silva SL, et al. Knowledge about hepatitis and previous exposure to hepatitis viruses in immigrants and refugees from the Mekong region. Aust NZ J Public Health. 2005;29(1):64‐68. doi: 10.1111/j.1467-842x.2005.tb00751.x [DOI] [PubMed] [Google Scholar]
- 24. Owiti JA, Greenhalgh T, Sweeney L, Foster GR, Bhui KS. Illness perceptions and explanatory models of viral hepatitis B & C among immigrants and refugees: a narrative systematic review. BMC Public Health. 2015;15(1):151. doi: 10.1186/s12889-015-1476-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Munn Z, Stern C, Aromataris E, Lockwood C, Jordan Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol. 2018;18(1):5. doi: 10.1186/s12874-017-0468-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. The World Bank . World Bank Country and Lending Groups. Accessed March 1, 2021. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519‐world‐bank‐country‐and‐lending‐groups
- 28. Covidence . Covidence. Accessed March 1, 2022. https://www.covidence.org/
- 29. S M, Z M, C T, et al. Chapter 7: systematic reviews of etiology and risk. Joanna Briggs Institute Reviewer's Manuel. The Joanna Briggs Institute; 2017. [Google Scholar]
- 30. Ma L‐L, Wang Y‐Y, Yang Z‐H, Huang D, Weng H, Zeng X‐T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Military Medical Research. 2020;7(1):7. doi: 10.1186/s40779-020-00238-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crawshaw AF, Pareek M, Were J, et al. Infectious disease testing of UK‐bound refugees: a population‐based, cross‐sectional study. BMC Med. 2018;16(1):143. doi: 10.1186/s12916-018-1125-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mixson‐Hayden T, Lee D, Ganova‐Raeva L, et al. Hepatitis B virus and hepatitis C virus infections in United States‐bound refugees from Asia and Africa. Am J Trop Med Hyg. 2014;90(6):1014‐1020. doi: 10.4269/ajtmh.14-0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kose S, Kuzucu L, Gozaydin A, Yilmazer T. Prevalence of hepatitis B and C viruses among asylum seekers in Izmir. J Immigr Minor Health. 2015;17(1):76‐78. doi: 10.1007/s10903-013-9876-7 [DOI] [PubMed] [Google Scholar]
- 34. Stevens K, Palmo T, Wangchuk T, Solomon S, Dierberg K, Hoffmann CJ. Hepatitis B prevalence and treatment needs among Tibetan refugees residing in India. J Med Virol. 2016;88(8):1357‐1363. doi: 10.1002/jmv.24488 [DOI] [PubMed] [Google Scholar]
- 35. Kose S, Odemis I, Celik D, Gireniz Tatar B, Akbulut I, Ciftdogan DY. Hepatitis a, B, C and HIV seroprevalence among Syrian refugee children admitted to outpatient clinics. Infez Med. 2017;25(4):339‐343. [PubMed] [Google Scholar]
- 36. Ayele A, Abera D, Hailu M, Birhanu M, Desta K. Prevalence and associated risk factors for hepatitis B and C viruses among refugees in Gambella, Ethiopia. BMC Public Health. 2020;20(1):721. doi: 10.1186/s12889-020-08893-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Güngör ES, Seval O, İlhan G, Verit FF. Do Syrian refugees have increased risk for worser pregnancy outcomes? Results of a tertiary center in İstanbul. Turk J Obstetrics Gynecol. 2018;15(1):23‐27. doi: 10.4274/tjod.64022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Inci A, Yildirim D, Seckin KD, Gedikbasi A. Analysis of HbsAg positivity rate before and after vaccination in Turkish and Syrian refugee pregnant women. J Infect Dev Ctries. 2017;11(10):815‐818. doi: 10.3855/jidc.8162 [DOI] [PubMed] [Google Scholar]
- 39. Hussein N, Muhammed I, Younus O, Taher A, Salim A, Shahab F. Prevalence of HBV, HCV and HIV infections among Syrian refugees in Kurdistan region, Iraq. International J Infect. 2016;4(2):e39420. doi: 10.5812/iji.39420 [DOI] [Google Scholar]
- 40. Tümtürk A, Yeşil B. Hepatitis B, hepatitis C and HIV seroprevalence among Syrian refugees: a cross‐sectional study from a tertiary referral center in Turkey. J Surg Med. 2019;3(12):845‐847. doi: 10.28982/josam.650891 [DOI] [Google Scholar]
- 41. Banks T, Kang J, Watts I, et al. High hepatitis B seroprevalence and risk factors for infection in pregnant women on the Thailand‐Myanmar border. J Infect Dev Ctries. 2016;10(4):384‐388. doi: 10.3855/jidc.7422 [DOI] [PubMed] [Google Scholar]
- 42. Bierhoff M, Angkurawaranon C, Myat Min A, et al. Maternal hepatitis B infection burden, comorbidity and pregnancy outcome in a low‐income population on the Myanmar‐Thailand border: a retrospective cohort study. J Pregnancy. 2019;2019:8435019. doi: 10.1155/2019/8435019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mitchell T, Lee D, Weinberg M, et al. Impact of enhanced health interventions for United States‐bound refugees: evaluating best practices in migration health. Am J Trop Med Hyg. 2018;98(3):920‐928. doi: 10.4269/ajtmh.17-0725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ali M, Rahman MA, Njuguna H, et al. High prevalence of hepatitis B and C virus infections among Rohingya refugees in Bangladesh: a growing concern for the refugees and the host communities. Clin Liver Dis. 2022;19(1):1‐6. doi: 10.1002/cld.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mazhar MKA, Finger F, Evers ES, et al. An outbreak of acute jaundice syndrome (AJS) among the Rohingya refugees in Cox's Bazar, Bangladesh: findings from enhanced epidemiological surveillance. PLoS One. 2021;16(4):e0250505. doi: 10.1371/journal.pone.0250505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Quddus A, Luby SP, Jamal Z, Jafar T. Prevalence of hepatitis B among afghan refugees living in Balochistan, Pakistan. Int J Infect Dis. 2006;10(3):242‐247. doi: 10.1016/j.ijid.2005.04.007 [DOI] [PubMed] [Google Scholar]
- 47. Pourkarim MR, Zandi K, Davani NA, Pourkarim HR, Amini‐Bavil‐Olyaee S. An aberrant high prevalence of hepatitis B infection among afghans residing in one of the Bushehr refugee camps (Dalaki camp) in the southwest of Iran. Int J Infect Dis. 2008;12(1):101‐102. [DOI] [PubMed] [Google Scholar]
- 48. Khanani MR, Ansari AS, Khan S, Somani M, Kazmi SU, Ali SH. Concentrated epidemics of HIV, HCV, and HBV among afghan refugees. J Infect. 2010;61(5):434‐437. doi: 10.1016/j.jinf.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 49. Karasahin EF, Karasahin O, Kalkan IA. Results of viral hepatitis and human immunodeficiency virus screening in afghan irregular migrants: a cross‐sectional study (2011–2019). Viral Hepatitis J. 2021;27(2):98‐102. doi: 10.4274/VHD.GALENOS.2021.2020-12-8 [DOI] [Google Scholar]
- 50. Khan A, Qazi J. Risk factors and molecular epidemiology of HBV and HCV in internally displaced persons (IDPs) of North Waziristan agency, Pakistan. J Pak Med Assoc. 2018;68(2):165‐169. [PubMed] [Google Scholar]
- 51. Khan F, Akbar H, Idrees M, Khan H, Shahzad K, Kayani MA. The prevalence of HBV infection in the cohort of IDPs of war against terrorism in Malakand division of northern Pakistan. BMC Infect Dis. 2011;11:176. doi: 10.1186/1471-2334-11-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rauf A, Nadeem MS, Ali A, et al. Prevalence of hepatitis B and C in internally displaced persons of war against terrorism in swat, Pakistan. Eur J Public Health. 2011;21(5):638‐642. doi: 10.1093/eurpub/ckq084 [DOI] [PubMed] [Google Scholar]
- 53. Kamali I, Ndahimana JA, Nyirahabihirwe F, et al. Prevalence and associated risk factors for hepatitis B and C viruses among refugee populations living in Mahama, Rwanda: a cross‐sectional study. PLoS One. 2021;16(10 October):e0257917. doi: 10.1371/journal.pone.0257917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kowo MP, Frungwa CN, Andoulo FA, et al. Epidemiologic patterns of HIV, hepatitis b and c virus infections among refugees of the Mbile camp in the east region of Cameroon (hepatitis and HIV among refugees). J Gastroenterol Hepatol Res. 2021;10(3):3524‐3530. doi: 10.17554/j.issn.2224-3992.2021.10.989 [DOI] [Google Scholar]
- 55. Odimayo MS, Adebimpe WO, Jeff‐Agboola YA, et al. Screening, vaccination, and referrals as viral hepatitis elimination triad among internally displaced persons in Edo state, Nigeria. Clin Liver Dis. 2020;16(5):218‐222. doi: 10.1002/cld.1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McDonald JT, Kennedy S. Insights into the ‘healthy immigrant effect’: health status and health service use of immigrants to Canada. Soc Sci Med. 2004;59(8):1613‐1627. doi: 10.1016/j.socscimed.2004.02.004 [DOI] [PubMed] [Google Scholar]
- 57. Goldwater PN. Iatrogenic blood‐borne viral infections in refugee children from war and transition zones. Emerg Infect Dis. 2013;19(6):892‐898. doi: 10.3201/eid1906.120806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Araujo JO, Souza FM, Proença R, Bastos ML, Trajman A, Faerstein E. Prevalence of sexual violence among refugees: a systematic review. Rev Saude Publica. 2019;53:78. doi: 10.11606/s1518-8787.2019053001081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Griffiths K, Ford N. Provision of antiretroviral care to displaced populations in humanitarian settings: a systematic review. Med Confl Surviv. 2013;29(3):198‐215. doi: 10.1080/13623699.2013.813108 [DOI] [PubMed] [Google Scholar]
- 60. Bierhoff M, Rijken MJ, Yotyingaphiram W, et al. Tenofovir for prevention of mother to child transmission of hepatitis B in migrant women in a resource‐limited setting on the Thailand‐Myanmar border: a commentary on challenges of implementation. Int J Equity Health. 2020;19(1):156. doi: 10.1186/s12939-020-01268-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


