Abstract
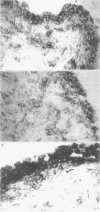
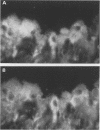
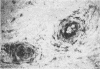
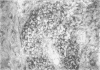
OBJECTIVES--To examine the distribution of four annexins in non-inflamed rheumatoid arthritic and osteoarthritic synovial tissue. METHODS--Frozen sections were stained with monoclonal antibodies (MAb) specific for annexins-I, -II, -IV, and -VI, and for cell lineage related markers including CD68 and CD14 (macrophages), prolyl hydroxylase (fibroblasts), and CD3 (T cells). RESULTS--Each of the annexins was present in synovial tissues in significant amounts in the three groups studied. Annexin-I was predominantly found within the synovial lining layer and double labelling showed it to be present predominantly in cells of the macrophage lineage. In rheumatoid specimens there was increased staining within the lining layer, perivascularly and on macrophages within the tissue stroma. Annexin-II was present in a distribution similar to that of annexin-I, but with more prominent perivascular staining. Annexins-IV and -VI were seen chiefly in association with areas of lymphocyte infiltration in rheumatoid tissue, whereas annexins-I and -II were absent from these areas. Endothelial cells stained weakly positive for annexins-I and -II, and more strongly for -IV and -VI. CONCLUSIONS--This study demonstrates that annexins (particularly annexin-I, a putative mediator of the anti-inflammatory activities of glucocorticoids) are abundant in rheumatoid and non-rheumatoid synovial tissue, annexins-IV and -VI having a distribution distinct from that of -I and -II.
Full text
PDF


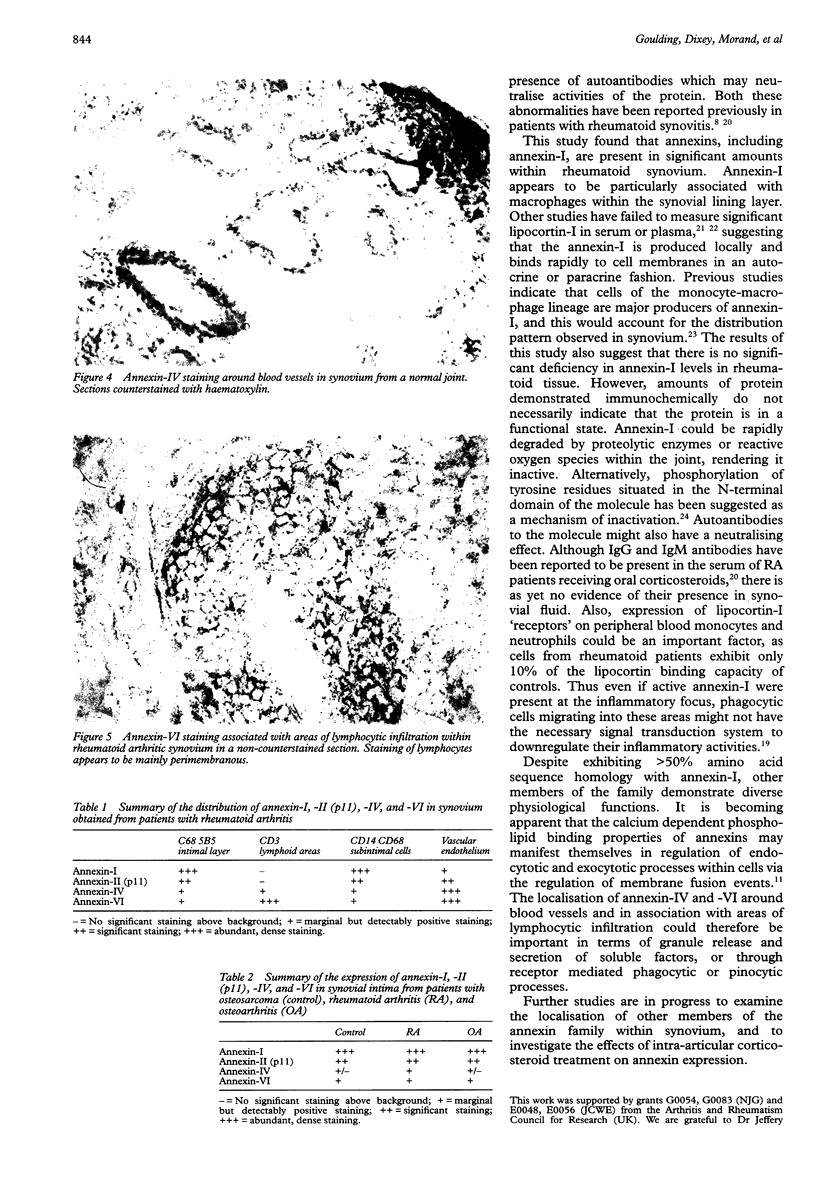

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. M., Geisow M. J., Burgoyne R. D. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature. 1989 Jul 27;340(6231):313–315. doi: 10.1038/340313a0. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Boumpas D. T., Paliogianni F., Anastassiou E. D., Balow J. E. Glucocorticosteroid action on the immune system: molecular and cellular aspects. Clin Exp Rheumatol. 1991 Jul-Aug;9(4):413–423. [PubMed] [Google Scholar]
- Fava R. A., McKanna J., Cohen S. Lipocortin I (p35) is abundant in a restricted number of differentiated cell types in adult organs. J Cell Physiol. 1989 Nov;141(2):284–293. doi: 10.1002/jcp.1041410209. [DOI] [PubMed] [Google Scholar]
- Flower R. J. Lipocortin. Prog Clin Biol Res. 1990;349:11–25. [PubMed] [Google Scholar]
- Franklin W. A., Mason D. Y., Pulford K., Falini B., Bliss E., Gatter K. C., Stein H., Clarke L. C., McGee J. O. Immunohistological analysis of human mononuclear phagocytes and dendritic cells by using monoclonal antibodies. Lab Invest. 1986 Mar;54(3):322–335. [PubMed] [Google Scholar]
- Goulding N. J., Godolphin J. L., Sharland P. R., Peers S. H., Sampson M., Maddison P. J., Flower R. J. Anti-inflammatory lipocortin 1 production by peripheral blood leucocytes in response to hydrocortisone. Lancet. 1990 Jun 16;335(8703):1416–1418. doi: 10.1016/0140-6736(90)91445-g. [DOI] [PubMed] [Google Scholar]
- Goulding N. J., Guyre P. M. Regulation of inflammation by lipocortin 1. Immunol Today. 1992 Aug;13(8):295–297. doi: 10.1016/0167-5699(92)90040-E. [DOI] [PubMed] [Google Scholar]
- Goulding N. J., Jefferiss C. M., Pan L., Rigby W. F., Guyre P. M. Specific binding of lipocortin-1 (annexin I) to monocytes and neutrophils is decreased in rheumatoid arthritis. Arthritis Rheum. 1992 Nov;35(11):1395–1397. doi: 10.1002/art.1780351126. [DOI] [PubMed] [Google Scholar]
- Morand E. F., Jefferiss C. M., Dixey J., Mitra D., Goulding N. J. Impaired glucocorticoid induction of mononuclear leukocyte lipocortin-1 in rheumatoid arthritis. Arthritis Rheum. 1994 Feb;37(2):207–211. doi: 10.1002/art.1780370209. [DOI] [PubMed] [Google Scholar]
- Munck A., Guyre P. M., Holbrook N. J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984 Winter;5(1):25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B. Phosphorylation of lipocortin-1 by the epidermal growth factor receptor. Methods Enzymol. 1991;198:260–272. doi: 10.1016/0076-6879(91)98027-4. [DOI] [PubMed] [Google Scholar]
- Podgorski M. R., Goulding N. J., Hall N. D., Flower R. J., Maddison P. J. Autoantibodies to lipocortin-1 are associated with impaired glucocorticoid responsiveness in rheumatoid arthritis. J Rheumatol. 1992 Nov;19(11):1668–1671. [PubMed] [Google Scholar]
- Raynal P., Pollard H. B. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994 Apr 5;1197(1):63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Rocha V., Lozano J. J., Haindl A. H. Evidence for differential localization of annexin VI during mammary secretory differentiation. Biochem Soc Trans. 1990 Dec;18(6):1110–1113. doi: 10.1042/bst0181110. [DOI] [PubMed] [Google Scholar]
- Rothwell N. J., Flower R. Lipocortin-1 exhibits novel actions, providing clinical opportunities. Trends Pharmacol Sci. 1992 Feb;13(2):45–46. doi: 10.1016/0165-6147(92)90019-3. [DOI] [PubMed] [Google Scholar]
- Tait J. F., Gibson D., Fujikawa K. Phospholipid binding properties of human placental anticoagulant protein-I, a member of the lipocortin family. J Biol Chem. 1989 May 15;264(14):7944–7949. [PubMed] [Google Scholar]
- Vishwanath B. S., Frey F. J., Bradbury M., Dallman M. F., Frey B. M. Adrenalectomy decreases lipocortin-I messenger ribonucleic acid and tissue protein content in rats. Endocrinology. 1992 Feb;130(2):585–591. doi: 10.1210/endo.130.2.1531128. [DOI] [PubMed] [Google Scholar]
- Zaks W. J., Creutz C. E. Ca(2+)-dependent annexin self-association on membrane surfaces. Biochemistry. 1991 Oct 8;30(40):9607–9615. doi: 10.1021/bi00104a007. [DOI] [PubMed] [Google Scholar]
- Zaks W. J., Creutz C. E. Evaluation of the annexins as potential mediators of membrane fusion in exocytosis. J Bioenerg Biomembr. 1990 Apr;22(2):97–120. doi: 10.1007/BF00762942. [DOI] [PubMed] [Google Scholar]