Abstract
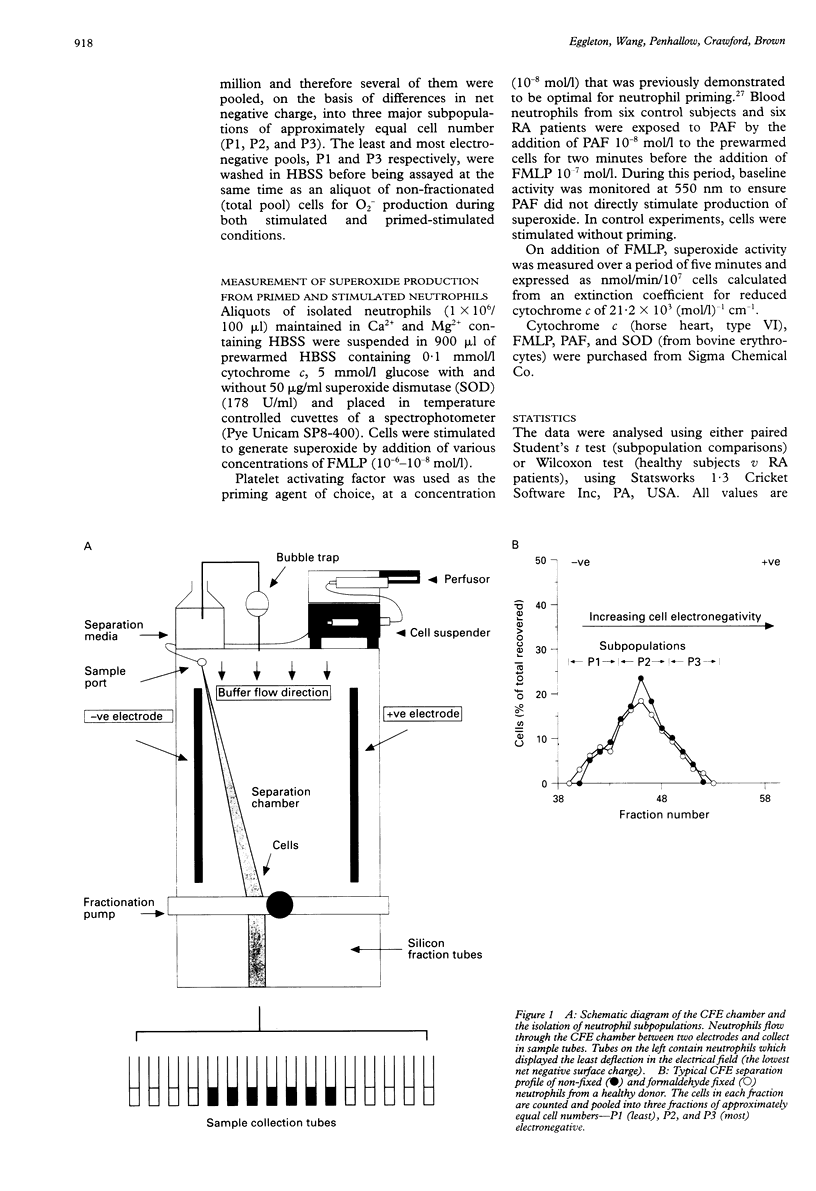
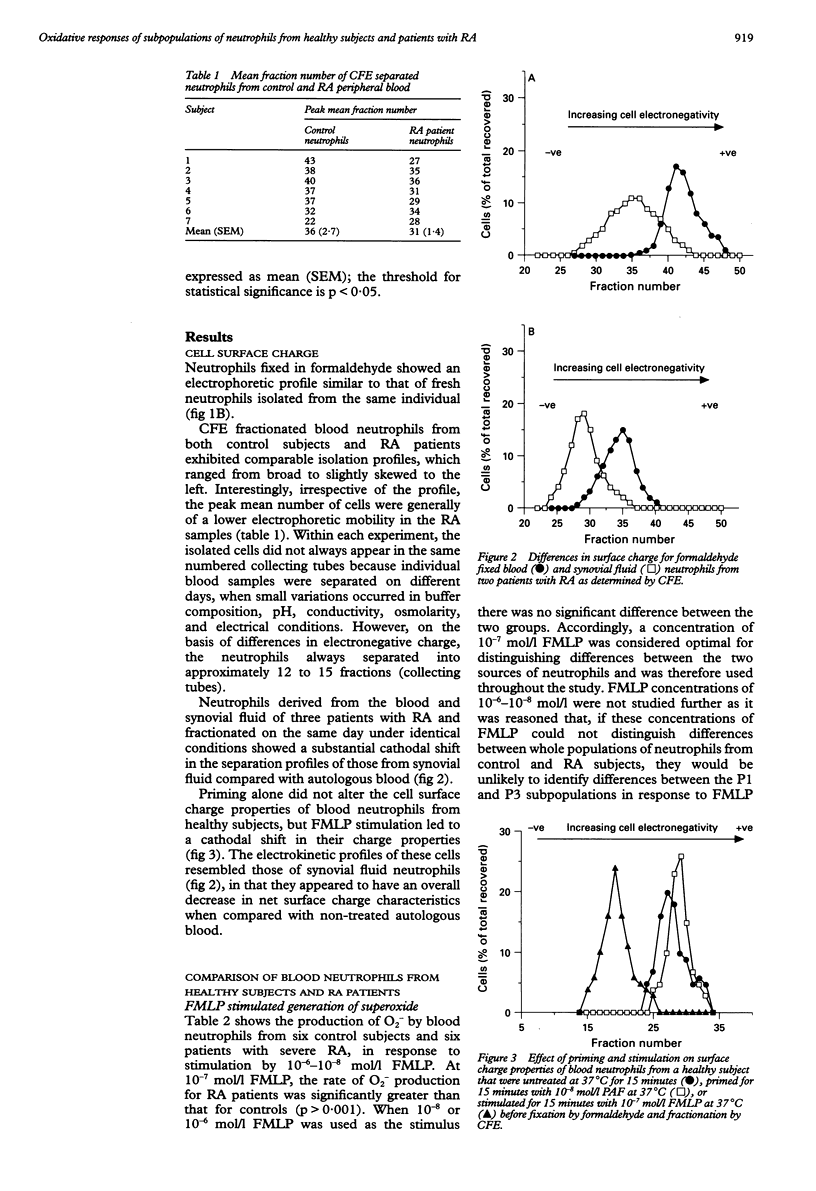
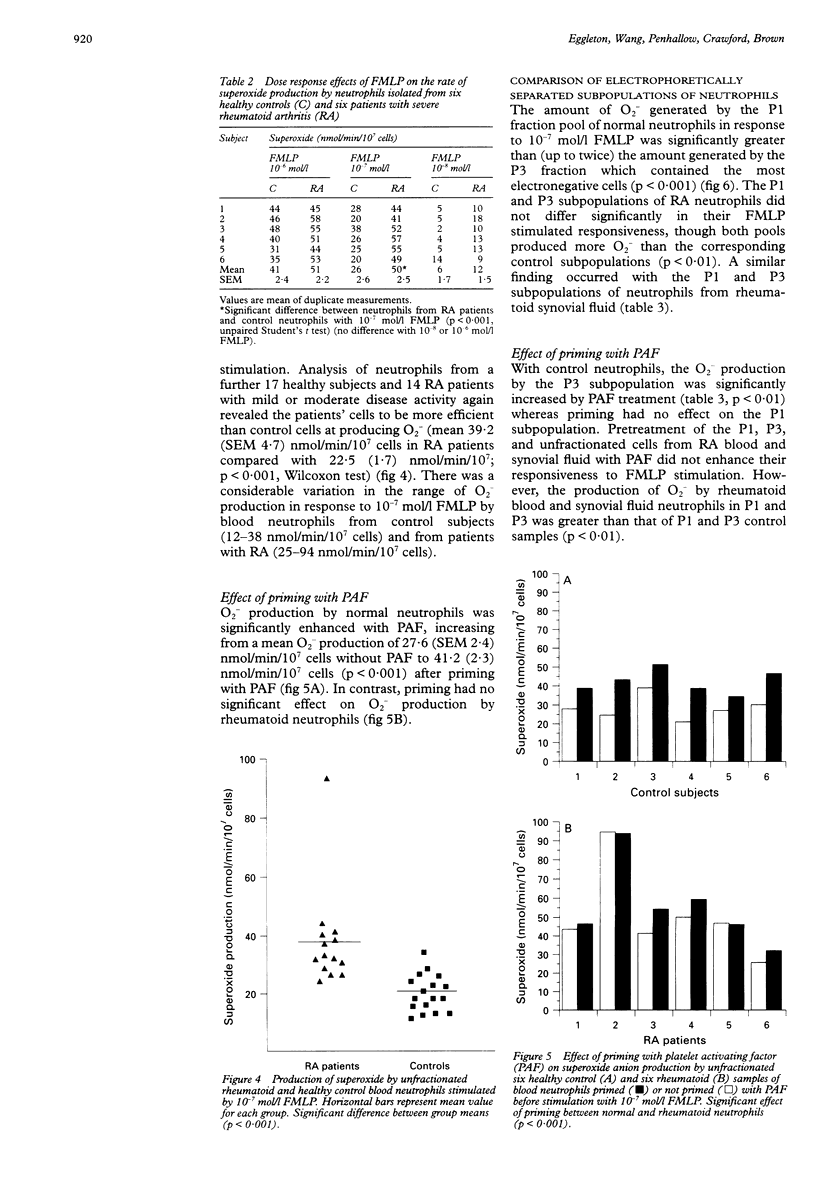
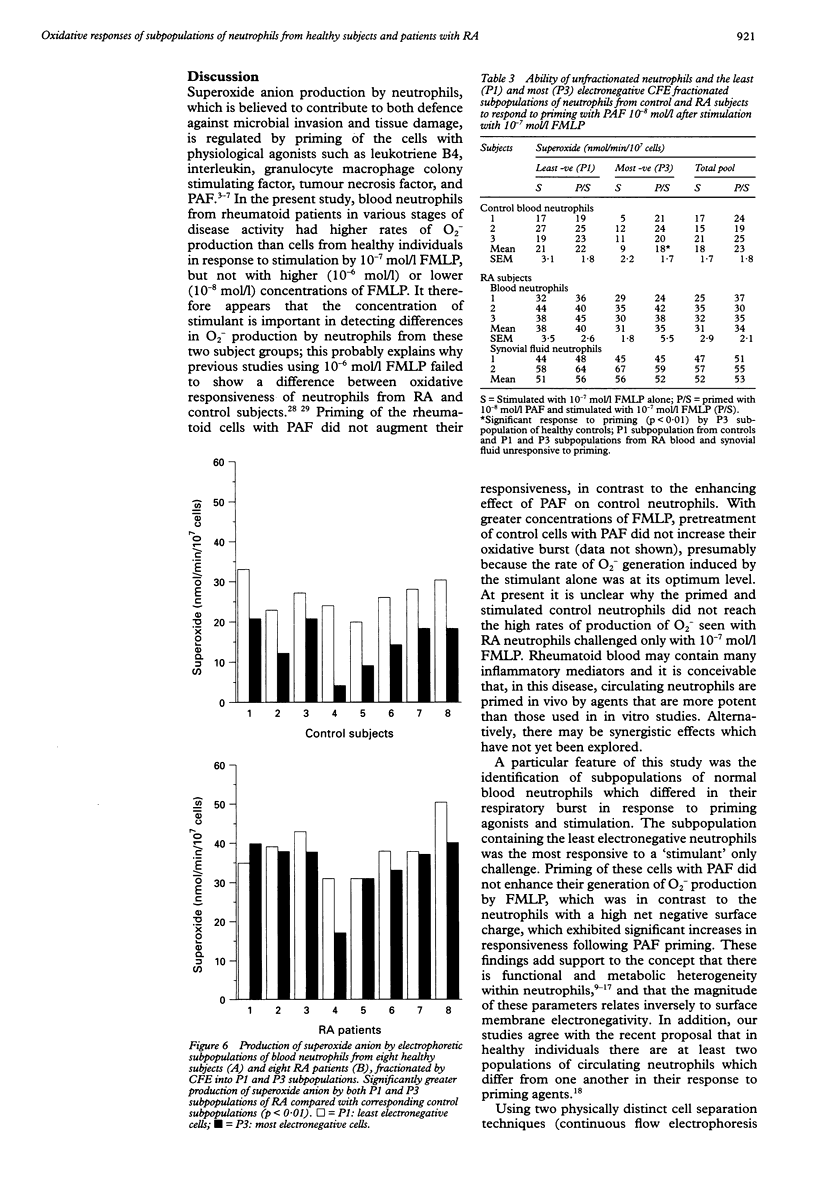
OBJECTIVES--To determine whether blood neutrophils from healthy individuals and blood and synovial fluid neutrophils from patients with rheumatoid arthritis (RA) responded differently to priming agonists and stimuli of the oxidative burst and, if so, whether this was a property of a subpopulation of neutrophils. METHODS--Continuous flow electrophoresis was used to separate neutrophils into subpopulations based upon quantitative differences in net negative surface charge. The generation of superoxide anion (O2-) was used as a measure of oxidative activity using 10(-7) mol/l N-formyl-methionylleucyl-phenylalanine (FMLP) as the stimulating agonist and 10(-8) mol/l platelet activating factor (PAF) as the priming agent. RESULTS--The production of O2- by blood and synovial fluid neutrophils from RA patients in response to FMLP was greater than that observed with control blood neutrophils (p < 0.001). Priming of normal blood neutrophils with PAF increased their FMLP induced oxidative burst (p < 0.001), but PAF treatment had no effect on rheumatoid neutrophils. Neutrophils from synovial fluid of RA patients were less electronegative than paired blood samples and exposure of blood neutrophils to FMLP but not PAF reduced their surface charge. Continuous flow electrophoresis isolated three neutrophil subpopulations: cells of least surface electronegativity were ascribed to pool P1 and cells of greatest surface electro-negativity to P3. Normal blood neutrophils from P3, but not P1, showed increased oxidative activity after PAF priming (twofold increase; p < 0.01), whereas the responsiveness of rheumatoid blood and synovial fluid neutrophils from P1 and P3 was not modified by PAF treatment under the same conditions. CONCLUSION--It is suggested that most of the circulating neutrophils in RA are already in a state of readiness to generate O2- upon activation by an inflammatory stimulus. This is in contrast to normal blood neutrophils, which have both responsive and non-responsive subpopulations with respect to priming agonists.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984 Nov;64(5):959–966. [PubMed] [Google Scholar]
- Bass D. A., Olbrantz P., Szejda P., Seeds M. C., McCall C. E. Subpopulations of neutrophils with increased oxidative product formation in blood of patients with infection. J Immunol. 1986 Feb 1;136(3):860–866. [PubMed] [Google Scholar]
- Brown K. A., Holborow E. J., Collins A. J. Cell electrophoretic analysis of lymphocytes and polymorphonuclear cells from patients with rheumatoid arthritis. Lancet. 1977 Jan 15;1(8003):114–117. doi: 10.1016/s0140-6736(77)91704-4. [DOI] [PubMed] [Google Scholar]
- Brown K. A., McCarthy D., Perry J. D., Dumonde D. C. Reduction of the surface charge of blood polymorphonuclear cells by rheumatoid sera and heat induced aggregated human IgG (HAGG). Ann Rheum Dis. 1988 May;47(5):359–363. doi: 10.1136/ard.47.5.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. A., Perry J. D., Black C., Dumonde D. C. Identification by cell electrophoresis of a subpopulation of polymorphonuclear cells which is increased in patients with rheumatoid arthritis and certain other rheumatological disorders. Ann Rheum Dis. 1988 May;47(5):353–358. doi: 10.1136/ard.47.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. A. The polymorphonuclear cell in rheumatoid arthritis. Br J Rheumatol. 1988 Apr;27(2):150–155. doi: 10.1093/rheumatology/27.2.150. [DOI] [PubMed] [Google Scholar]
- Daniels R. H., Elmore M. A., Hill M. E., Shimizu Y., Lackie J. M., Finnen M. J. Priming of the oxidative burst in human neutrophils by physiological agonists or cytochalasin B results from the recruitment of previously non-responsive cells. Immunology. 1994 Jul;82(3):465–472. [PMC free article] [PubMed] [Google Scholar]
- Dularay B., Elson C. J., Dieppe P. A. Enhanced oxidative response of polymorphonuclear leukocytes from synovial fluids of patients with rheumatoid arthritis. Autoimmunity. 1988;1(3):159–169. doi: 10.3109/08916938808997161. [DOI] [PubMed] [Google Scholar]
- Eggleton P., Crawford N., Fisher D. Fractionation of human neutrophils into subpopulations by countercurrent distribution: surface charge and functional heterogeneity. Eur J Cell Biol. 1992 Apr;57(2):265–272. [PubMed] [Google Scholar]
- Eggleton P., Fisher D., Crawford N. Heterogeneity in the circulating neutrophil pool: studies on subpopulations separated by continuous flow electrophoresis. J Leukoc Biol. 1992 Jun;51(6):617–625. doi: 10.1002/jlb.51.6.617. [DOI] [PubMed] [Google Scholar]
- Eggleton P., Ghebrehiwet B., Coburn J. P., Sastry K. N., Zaner K. S., Tauber A. I. Characterization of the human neutrophil C1q receptor and functional effects of free ligand on activated neutrophils. Blood. 1994 Sep 1;84(5):1640–1649. [PubMed] [Google Scholar]
- Eggleton P., Penhallow J., Crawford N. Priming action of inositol hexakisphosphate (InsP6) on the stimulated respiratory burst in human neutrophils. Biochim Biophys Acta. 1991 Sep 24;1094(3):309–316. doi: 10.1016/0167-4889(91)90091-b. [DOI] [PubMed] [Google Scholar]
- Elbim C., Chollet-Martin S., Bailly S., Hakim J., Gougerot-Pocidalo M. A. Priming of polymorphonuclear neutrophils by tumor necrosis factor alpha in whole blood: identification of two polymorphonuclear neutrophil subpopulations in response to formyl-peptides. Blood. 1993 Jul 15;82(2):633–640. [PubMed] [Google Scholar]
- Gallin J. I. Degranulating stimuli decrease the neagative surface charge and increase the adhesiveness of human neutrophils. J Clin Invest. 1980 Feb;65(2):298–306. doi: 10.1172/JCI109672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I. Human neutrophil heterogeneity exists, but is it meaningful? Blood. 1984 May;63(5):977–983. [PubMed] [Google Scholar]
- Gay J. C., Beckman J. K., Brash A. R., Oates J. A., Lukens J. N. Enhancement of chemotactic factor-stimulated neutrophil oxidative metabolism by leukotriene B4. Blood. 1984 Oct;64(4):780–785. [PubMed] [Google Scholar]
- Ingraham L. M., Coates T. D., Allen J. M., Higgins C. P., Baehner R. L., Boxer L. A. Metabolic, membrane, and functional responses of human polymorphonuclear leukocytes to platelet-activating factor. Blood. 1982 Jun;59(6):1259–1266. [PubMed] [Google Scholar]
- Jaswon M. S., Khwaja A., Roberts P. J., Jones H. M., Linch D. C. The effects of rhGM-CSF on the neutrophil respiratory burst when studied in whole blood. Br J Haematol. 1990 Jun;75(2):181–187. doi: 10.1111/j.1365-2141.1990.tb02646.x. [DOI] [PubMed] [Google Scholar]
- Lichtman M. A., Weed R. I. Alteration of the cell periphery during granulocyte maturation: relationship to cell function. Blood. 1972 Mar;39(3):301–316. [PubMed] [Google Scholar]
- Nurcombe H. L., Bucknall R. C., Edwards S. W. Neutrophils isolated from the synovial fluid of patients with rheumatoid arthritis: priming and activation in vivo. Ann Rheum Dis. 1991 Mar;50(3):147–153. doi: 10.1136/ard.50.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Ritchie D. M., Boyle J. A., McInnes J. M., Jasani M. K., Dalakos T. G., Grieveson P., Buchanan W. W. Clinical studies with an articular index for the assessment of joint tenderness in patients with rheumatoid arthritis. Q J Med. 1968 Jul;37(147):393–406. [PubMed] [Google Scholar]
- Sample A. K., Czuprynski C. J. Priming and stimulation of bovine neutrophils by recombinant human interleukin-1 alpha and tumor necrosis factor alpha. J Leukoc Biol. 1991 Feb;49(2):107–115. doi: 10.1002/jlb.49.2.107. [DOI] [PubMed] [Google Scholar]
- Spangenberg P., Crawford N. Surface membrane electrokinetic properties of polymorphonuclear leucocytes: subpopulation heterogeneity and phagocytic competence. J Cell Biochem. 1987 Aug;34(4):259–268. doi: 10.1002/jcb.240340405. [DOI] [PubMed] [Google Scholar]
- Test S. T. Effect of tumor necrosis factor on the generation of chlorinated oxidants by adherent human neutrophils. J Leukoc Biol. 1991 Aug;50(2):131–139. doi: 10.1002/jlb.50.2.131. [DOI] [PubMed] [Google Scholar]
- Vercellotti G. M., Yin H. Q., Gustafson K. S., Nelson R. D., Jacob H. S. Platelet-activating factor primes neutrophil responses to agonists: role in promoting neutrophil-mediated endothelial damage. Blood. 1988 Apr;71(4):1100–1107. [PubMed] [Google Scholar]
- Watson F., Robinson J. J., Phelan M., Bucknall R. C., Edwards S. W. Receptor expression in synovial fluid neutrophils from patients with rheumatoid arthritis. Ann Rheum Dis. 1993 May;52(5):354–359. doi: 10.1136/ard.52.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L., Mayhew E., Ulrich K. The effect of neuraminidase on the phagocytic process in human monocytes. Lab Invest. 1966 Aug;15(8):1304–1309. [PubMed] [Google Scholar]


