Abstract
Coronavirus disease 2019 (COVID-19) has caused tremendous morbidity and mortality worldwide. The large number of post-COVID survivors has drawn attention to the management of post-COVID condition, known as long COVID. This review examines current knowledge of long COVID, regarding its epidemiology, mechanism, and clinical presentations in both adults and children. We also review the rehabilitation principles, modules, and effects, and share Taiwan's efforts to provide a top-down, nationwide care framework for long COVID patients. Dyspnea, chronic cough, and fatigue are the most commonly reported symptoms in the first 6 months after infection, but cognitive impairment and psychological symptoms may persist beyond this time. Several possible mechanisms behind these symptoms were proposed, but remained unconfirmed. These symptoms negatively impact individuals' function, activities, participation and quality of life. Rehabilitation is a key element of management to achieve functional improvement. Early management should start with comprehensive evaluation and identification of red flags. Exercise-based therapy, an essential part of management of long COVID, can be conducted with different modules, including telerehabilitation. Post-exertional symptom exacerbation and orthostatic hypotension should be carefully monitored during exercise. Randomized control trials with a large sample size are needed to determine the optimal timing, dosage, and modules.
Keywords: Activities of daily living, COVID-19, Long COVID, Rehabilitation
Introduction
The global pandemic of Coronavirus disease 2019 (COVID-19) has resulted in more than 635 million confirmed infections and more than 6.5 million deaths in the past 3 years.1 COVID-19 infection is characterized by a broad spectrum of symptoms and disease severity, from asymptomatic to severe acute respiratory distress syndrome and death.2 Many COVID-19 survivors are at risk of long-term impairment and disability, especially critical cases.2 A relatively high proportion of individuals experience variable and persistent symptoms after infection, which are collectively called long COVID or post-COVID condition. Taiwan experienced its first significant COVID-19 outbreak a year after the pandemic began. A second surge of case numbers occurred in May 2022, with an accumulated number of 7.75 million cases reported as of October 2022. As the number of post-infection cases increases, concerns about long COVID have grown. The persistent symptoms affect not only physical and psychological function, but also the individuals’ health-related quality of life, activities, and participation.3 , 4
Rehabilitation is a key element for improving functional outcomes in patients with long COVID. We review studies regarding the current knowledge of long COVID, including its epidemiology, clinical features, and possible mechanisms. We also focus on the modules and effects of rehabilitation, which are important for evidence-based clinical practice. Additionally, we share Taiwan's experience of providing a nationwide, comprehensive care framework for post-COVID-19 patients.
Definition and epidemiology
Long COVID, or chronic/post-COVID-19 syndrome, refers to persistent symptoms and dysfunctions that last more than 2–3 months after the resolution of acute COVID-19 infection and cannot be attributed to alternative diagnoses.5 , 6 According to the Centers for Disease Control and Prevention in the United States (US), it is described as symptoms and signs that persist for more than 4 weeks after acute COVID-19 infection.7 Long COVID encompasses a variety of manifestations involving different organ systems, including but not limited to pulmonary, cardiovascular, gastrointestinal, endocrine, and neuropsychiatric systems.5, 6, 7, 8, 9 One study based on a retrospective matched cohort study using a United Kingdom (UK)-based primary care database identified up to 62 symptoms significantly associated with COVID-19 infection after 12 weeks.10
The prevalence of long COVID varies depending on the definition, and is affected by the particular stages of the pandemic, among which the dominant viral strain has differed. The majority of long COVID patients had more than one persistent symptom at 2–6 months follow-up (50.9%–87.4%).2 , 10, 11, 12, 13, 14, 15 A Scottish cohort followed up 33,281 COVID-19 patients with questionnaires at 6, 12, and 18 months post-infection.16 Fatigue occurred in 44%, dyspnea in 20%, sleep disturbance in 24%, and muscle pain/weakness in 24% of cases with symptomatic infection. Four European studies reported a prevalence rate of fatigue of 35%–53% and of dyspnea of 34%–43% 2–3 months after infection.11, 12, 13 A Chinese cohort prospectively followed up 1733 COVID-19 patients in person at 6 months from symptom onset; overall, 76% of these patients reported persistent symptoms.2 Fatigue and weakness were noted in 63%, while dyspnea was reported in 23%. Moreover, 26% of post-COVID patients had sleep disturbance and 23% had anxiety or depression. A German prospective cohort (n = 667) reported that fatigue (57%) and sleep disturbance (57%) were the most common symptoms at 6–12 months post-infection.17 Furthermore, in a survey from the US, dyspnea (23%) and cough (15%) were the most common findings at 3 months.18
Overall, fatigue, dyspnea, sleep disturbance, and cognitive impairment are the most commonly reported symptoms (Table 1 ). Other common symptoms include loss of taste/smell (7%–23%), cough (12%–21%), joint pain (5%–27%), chest pain (5%–22%), and headache (2%–18%).11, 12, 13, 14, 15, 16 , 18 19 Hair loss and diarrhea have also been reported.11 , 13 , 14 , 16 19
Table 1.
Most common symptoms of long COVID.
Clinical course and risk factors
The clinical course of long COVID is still unclear and probably varies depending on the symptoms. Overall, the prevalence of different symptoms was found to gradually decrease over 6–12 months.8 , 9 , 12 Cough and dyspnea are prevalent within 2–3 months after infection, the rates of which decrease at 6 months, while fatigue and neuropsychiatric symptoms tend to persist for a longer period of time.8 , 9 Perez et al. reported that the proportion of COVID patients with dyspnea decreased from 34% to 11%, and those with cough from 21% to 2%, from 2–3 months–4 months after infection.13 In contrast, a meta-analysis by Kim et al. indicated that cognitive impairment and psychological symptoms, including the so-called “brain fog”, became the most prevalent symptoms after 6–12 months.8 In the Scottish cohort, no significant decrease was found in the rate of patients reporting incomplete recovery in the serial questionnaire survey, with 6% reporting no recovery and 42% reporting partial recovery at 6–18 months.16 However, these results may have been biased by the low follow-up rate at 18 months.
The risk factors for long COVID vary depending on the symptoms and across studies. The most consistently reported risk factor is disease severity at the time of initial infection.2 , 15, 16, 17 Older age was also found to be associated with persistent symptoms in two studies.15 , 16 However, one study found that age above 30 years was associated with a higher risk of reporting long COVID symptoms in the univariate analysis, but a lower risk in the multivariate analysis.10 Other risk factors associated with long COVID included female, smoking, co-morbidities (including respiratory disease and depression), socioeconomic deprivation and the level of personal resilience.10 , 16 , 17 In contrast, a Spanish study identified no clinical characteristics as predictors of the occurrence of long COVID, but this study had the limitation of a relatively small size (N = 277).13 Ethnicity has been associated with persistent symptoms inconsistently in studies from the UK and Scotland.10 , 16
Mechanisms of long COVID
Possible mechanisms include ongoing sequelae of acutely damaged tissues, persistence of virus in the organs, dysregulation of the immune system with the triggering of autoimmunity and chronic inflammation, chronic tissue hypoxia caused by coagulopathy, and endothelial damage.20, 21, 22, 23, 24, 25, 26 Several mechanisms are proposed for different symptoms.
Fatigue, probably the most common symptom, was attributed to dysfunction of the brain glymphatic system causes cerebrospinal fluid congestion and subsequent toxic build-up within the central nervous system (CNS).27 Virus infecting the skeletal muscle, causing inflammation of myofibers and neuromuscular junctions, as well as mitochondrial dysfunction, may also contribute to weakness and fatigue.28 , 29 Hypothetically, mitochondrial dysfunction may alter metabolic sate and O2 diffusion capacity in muscle, leading to fatigue and exercise intolerancc.30
The dyspnea suffered in long COVID may result from substantial injury to the pulmonary system via viral replication, endothelial cell dysfunction, triggering of inflammatory and immune responses, and micro-vessel damage.26 , 31 , 32 Patients with premorbid lung disease are prone to lung fibrosis, possibly provoked by cytokines such as IL-6.31 , 33
Chest pain and arrhythmia following SARS-CoV-2 virus infection have been reported.2 Mechanisms postulated to be behind these symptoms include direct viral infection of cardiomyocytes, sarcomere disruption and fragmentation, transcriptional change, local immune response, and the sustained immune response activating the fibrotic pathway with subsequent structural remodeling.34, 35, 36
Impaired cognitive and mental function in long COVID are challenging to explain. Possible mechanisms include SARS-CoV-2 virus entering the CNS via a hematogenous or retrograde neuro-invasive route, affecting the permeability of the blood–brain barrier, enabling peripheral cytokine influx into the CNS, and driving persistent neuro-inflammation.37 Imaging studies of long COVID patients revealed regional brain alterations, including of gray matter volume, cerebral blood flow, and hypometabolism.38, 39, 40 The abnormal taste and smell in long COVID patients may result from the virus entering the olfactory support cells, stem cells, and perivascular cells, causing subsequent inflammation, and an altered gustatory threshold.41, 42, 43
Research addressing the underlying pathophysiological mechanism in long COVID is scarce and heterogeneous, and there is a need for further studies correlating the symptoms to immunological markers, and structural and functional imaging.
Rehabilitation of patients with long COVID
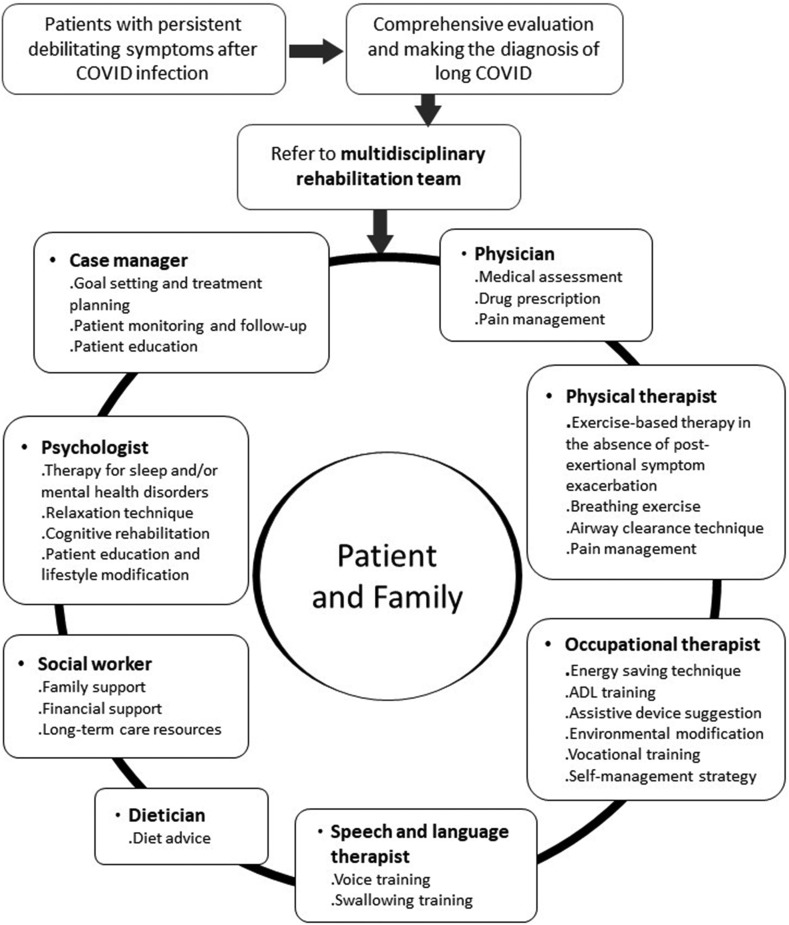
The management of long COVID starts with a comprehensive evaluation to differentially diagnose patients with persistent symptoms.5 , 6 , 44 Diagnoses such as direct organ damage, post-ICU syndrome, and metabolic or endocrine disorders should be identified and managed accordingly, since various factors might lead to persistent symptoms after COVID infection. Once other diseases are ruled out and the diagnosis of post-COVID condition is made, patients with debilitating symptoms should be referred to a multidisciplinary rehabilitation team at an early stage for a comprehensive assessment of rehabilitation needs and planning of further management.6 , 44 The multidisciplinary rehabilitation team should include healthcare professionals to meet the individual needs and symptoms of each patient. The team may include case managers, physicians, physical therapists, occupational therapists, speech and language therapists, psychologists, dietitians, and social workers (Fig. 1 ).45, 46, 47
Figure 1.
A multidisciplinary team approach to the rehabilitation of long COVID patients.
The most common symptoms or impairments requiring rehabilitation in patients with long COVID include fatigue, post-exertional symptom exacerbation (PESE), dyspnea, exercise intolerance, orthostatic intolerance, cognitive impairment, anxiety and depression, sleep problems and arthralgia.48 No evidence of the effectiveness of rehabilitation on long COVID has yet been provided by randomized controlled trials and most recommendations from international guidance are based on expert opinion or evidence from other diseases.7 , 48 Patient-centered management with continuous follow-up to lessen the severity of presented symptoms is suggested.7 Emphasis should be placed on establishing therapeutic alliances and setting reasonable expectations and goals via thorough explanation and discussion with patients and caregivers.
Evaluation should be performed first to rule out red flags such as exertional desaturation or cardiac impairment before starting rehabilitation with increasing oxygen demand for long COVID patients.48 Chest X-ray, spirometry, 1-min sit-to-stand test or 6-min walking test, or cardiopulmonary exercise testing (CPET) can be used to assess pulmonary function, exercise capacity, and oxygen saturation change as needed. When patients present with cardiopulmonary symptoms such as chest pain, dyspnea, palpitation, or syncope, electrocardiography (EKG), cardiac troponin, N-terminal pro-BNP, echocardiography, or further cardiac testing help to evaluate the cardiac impairment.49 The underlying pathology of red flags should be managed first to assuage any safety concerns.48
Once the presence of red flags has been ruled out, screening for PESE and orthostatic intolerance should also be performed prior to rehabilitation.48 PESE is defined as the worsening of symptoms that could previously be tolerated, such as fatigue, pain, dyspnea, cognitive impairment, and other symptoms after physical, mental, or emotional exertion. The worsening of symptoms may occur immediately or 12–72 h after exertion and last for hours to weeks.48 , 50 It is recommended that patients with PESE monitor the onset, duration, and intensity of symptom exacerbation and explore the potential triggering factors to recognize their individual limitations. Self-management training of an energy conservation technique is suggested to reduce exacerbation,51 while elevating the intensity of rehabilitation without considering PESE should be avoided.48 , 51
In patients presenting with dizziness, breathlessness, presyncope, or syncope after a prolonged period in an upright position, orthostatic intolerance should be identified by an active standing test to measure the change of blood pressure and heart rate.52 Postural orthostatic tachycardia syndrome is defined as an increase of heart rate greater than 30 beats per min after standing for more than 30 s. Postural hypotension is defined as a drop of systolic blood pressure of more than 20 mmHg after standing for more than 3 min.52 Patients with orthostatic intolerance should be given adequate fluid and salt, and avoid exacerbating factors such as prolonged standing, large meals, and warm environments. Compression stockings and an abdominal binder or pharmacological intervention can be considered. Regular aerobic exercise in a non-upright position, such as swimming and use of a recumbent ergometer, and resistance exercise are preferred to avoid orthostatic intolerance.48 , 52
When patients have a mental health problem or sleep disorder, psychological therapy and pharmacological therapy can be considered. Mindfulness-based stress reduction may be beneficial to improve stress, anxiety, depression, and sleep-related problems.53 , 54 Exercise training has also been shown to alleviate mental distress.55 , 56 With regard to cognitive impairment, compensatory strategies including pacing, environmental modification, and assistive tools as well as restorative cognitive exercise could be used.48
Exercise-based therapy for long COVID conditions
Exercise-based rehabilitation, an essential part of long COVID rehabilitation, is also suggested for patients with long COVID to manage fatigue, exercise intolerance, dyspnea, mental health and sleep-related problems, and musculoskeletal pain. Deconditioning and peripheral oxygen extraction abnormality may be a main limiting factor of exercise capacity.57 A meta-analysis on CPET showed that the mean peak oxygen uptake decreased by 4.9 ml/kg/min (95% confidence interval: −3.4 to 6.4) in patients with persistent symptoms 3 months after COVID infection, compared with the level in those without symptoms.57 One study evaluating patients after COVID infection using invasive CPET demonstrated lower peak oxygen uptake and an arteriovenous oxygen difference compared with controls; meanwhile, there were no significant between-group differences in cardiac output, decrease in dead space ventilation, and total pulmonary resistance at peak exercise.58 In addition, abnormal breathing pattern and chronotropic incompetence were also reported.59, 60, 61
Only a few small studies have examined the effectiveness of exercise-based rehabilitation programs in patients after COVID infection.45 , 46 , 62, 63, 64, 65 The exercise training usually comprises aerobic exercise, resistance training, and/or respiratory muscle training lasting from 6 to 8 weeks.45 , 46 , 62 Exercise-based rehabilitation has been shown to improve dyspnea, fatigue, functional capacity, strength, quality of life, and mental health disorders.45 , 46 , 62 A randomized controlled trial recruited 39 long COVID patients with mild COVID infection in the acute stage.62 The intervention group underwent an 8-week supervised aerobic and resistance exercise program at low to moderate intensity, whereas the control group undertook general physical activity in line with WHO guidelines, without supervised sessions. There were significant between-group differences in changes of exercise capacity, strength, quality of life, dyspnea, fatigue, and depression in favor of the intervention group. The mean VO2max improved by 2.1 ml/kg/min−1 (5.7%, standard deviation 9.2%) in the intervention group, while it remained stationary in the control group. There was a tendency for a greater proportion of participants in the intervention group to report a symptom-free status at a post-intervention test (42.1% vs. 16.7%, p = 0.091).62 Another observational study in 58 patients with persistent symptoms after COVID infection exhibited an increase of 6-min walking distance by 62.9 ± 48.2 m after a 6-week pulmonary rehabilitation program.45 About 70% of the participants had an increase greater than the minimal clinically important difference.
Owing to the risk of COVID infection and temporary cessation of in-person rehabilitation, telerehabilitation has attracted increasing attention during the COVID-19 pandemic. A limited number of clinical trials with small sample sizes showed that telerehabilitation might decrease dyspnea and improve functional capacity in patients with COVID infection in the acute phase and after hospital discharge, although the rates of adverse events were similar between patients undergoing telerehabilitation and their controls.63 While telerehabilitation has the advantage of greater accessibility, it also faces some challenges, such as low technological literacy among some patients, technical issues, and the lack of physical contact.66
Pediatric long COVID
Children with acute COVID infection are often asymptomatic or have mild symptoms, with a hospitalization rate of only 0.1%–1.9%.67 Very few of these cases develop severe illness, such as multisystem inflammatory syndrome in children (MIS-C). However, a recent survey found that 4%–66% of pediatric patients experienced at least one persistent symptom beyond 2 months after infection.68, 69, 70 The definition of long COVID in children and adolescents resembles that in adults, which has been described as involving (1) a history of confirmed SARS-CoV-2 infection, and (2) at least one symptom persisting beyond 12 weeks after initial testing, that cannot be explained by an alternative diagnosis, and that affects daily functioning.71 Long COVID in children is regarded as a persistent and chronic condition occurring after the resolution of acute COVID-19 infection, which includes over 200 less serious symptoms.72 In contrast, MIS-C occurs 2–6 weeks after COVID infection and is often severe enough to require intensive care support in 80% of cases.73 , 74 The symptoms of long COVID in children and adolescents have some similarities to those symptoms seen in adults, including fatigue, dyspnea, headache, difficulty concentrating, memory issues, myalgia/arthralgia, abdominal pain, irritability, mood changes, sleep disturbance, rhinorrhea, coughing, anosmia/dysgeusia, and sensory problems.69 , 75, 76, 77, 78 These symptoms may have a significant impact on children's daily routine and affect school attendance.79 Notably, most of the symptoms are from patient- or parent-reported questionnaires, while few psycho-physiological and functional assessments have been performed.80
There has been a lack of research regarding the prevalence, symptoms, and etiology of long COVID in children and adolescents. Conflicting results were obtained due to small sample sizes, recruiting both COVID and non-COVID participants, and different health policies, such as lockdown, school closures, and social distancing.81 Recently, a two-year cohort study disclosed that, compared to adults, children and adolescents after COVID infection have different trajectories in mental health. Children/adolescents do not have an increased risk of mood or anxiety disorders and the risk of cognitive deficit is transient.82 The prevalence of long COVID symptoms in children/adolescents steadily declines over time upon 6–9 months of follow-up.75 Risk factors associated with long COVID in children and adolescents are older age (>6 years) and a history of allergic disease.75 The mechanisms underlying long COVID in children and adolescents remain unclear, but dysregulation of the immune system following COVID-19 infection may play a predominant role.83, 84, 85, 86
Treatment of long COVID in children is challenging due to the large variety of symptoms and unclear etiology. Because of the multi-organ involvement of long COVID in children, a cross-specialty team is mandatory for regular follow-up.87 Initial evaluation includes symptom surveys and comprehensive physical examination.47 Blood exams including biochemistry and immunological status, lung function tests, chest X-ray, EKG, cardiac echography, muscle strength test, exercise tolerance test, sleep evaluation, psychological-cognitive assessment, abdominal ultrasound, and skin evaluations could be arranged depending on the patient's symptoms.88 , 89
Pediatric rehabilitation, including behavioral intervention and an exercise program, plays important roles in managing long COVID in children.47 The multidisciplinary rehabilitation program encompasses paced aerobic exercise, resistance training, relaxing exercise, pulmonary exercise, management of postural orthostatic tachycardia syndrome, brain cognitive training, and linking to school- and community-based resources.47 Future studies are needed to investigate the underlying mechanisms, management, and long-term physiological/psychological development in children/adolescents with long COVID.68
A framework for long COVID management in Taiwan
The coverage of the National Health Insurance exceeds 99.9% in Taiwan, and more than 90% of the clinics in Taiwan participate in the service. Given the rising numbers of post-COVID patients in the country towards the end of 2021, the healthcare authorities in Taiwan became concerned about the management of such patients. The Ministry of Health and Welfare in Taiwan initiated a nationwide Integrated Healthcare Plan to provide comprehensive care for COVID-19 survivors from December 2021. For inclusion in this care plan, patients had to be within 6 months after de-isolation for COVID-19 infection for outpatients, and 3 months for inpatients. Pulmonologists and infection specialists were designated as the first-line care providers. They are responsible for screening patients and can consult with other specialists as needed. This top-down care infrastructure is unique in several ways. First, the cost is fully covered by the National Health Insurance, which eliminates the financial barriers to accessing the care. Second, the participating hospitals get reimbursement to establish the case management system. Third, it incorporates a multi-disciplinary approach to address the wide range of symptoms. The last, it establishes a case registration system with standardized outcome measures, including the Barthel Index, EuroQol Instrument, modified Medical Research Council Dyspnea Scale, Numerical Rating Scale for pain, Brief Symptom Rating Scale, and mini Nutrition Assessment. This program aims to reduce the physical and psychological consequences of COVID-19 infection. The Center of Disease Control in Taiwan also issued guidelines for clinical care of the post-COVID condition.90 This program is different from the approach suggested by the National Health System, UK, which established 90 post COVID services to provide access to specialist diagnosis, treatment and rehabilitation. The care pathway is based on three core principles: personalized care, multidisciplinary and rehabilitation and supporting and enabling self-care, and general practitioners play important roles.91
As of the end of September 2022, around 130 hospitals are participating in this program. According to a press release in early September 2022, a total of 4489 patients have used this program, the majority of them on an outpatient basis. Around 70% of them are aged between 20 and 60 years, with the largest proportion observed in the age group of 30–40 years. Meanwhile, accumulated infections in Taiwan at that time have reached more than 5.6 million cases, implying that a relatively low proportion of those infected have used the program. To the best of our knowledge, no data have yet been published regarding the epidemiology of long COVID in Taiwan or the outcomes of the participants of the program yet. The significance and clinical efficacy should be explored in future work.
Conclusion
Post-COVID condition includes multiple symptoms and appears to be particularly prevalent in the first 6 months after infection. Although the symptoms can be self-limiting, the impact on individuals’ daily function and quality of life cannot be overlooked. With a large variety of clinical presentations, a multidisciplinary approach for clinical care is essential. Rehabilitation plays an important role in the management of both adults and children with post-COVID symptoms. However, the optimal timing, dosage, and modules of rehabilitation are yet to be studied via well-designed randomized control studies.
Funding/support statement
This work was partly supported by the funding from the National Taiwan University Hospital, Taiwan, ROC (grant number: 111-N0086).
Declaration of competing interest
None.
Acknowledgments
The authors thank the Integrated Healthcare Team for post-COVID patients of the National Taiwan University Hospital, Taiwan, ROC, led by Professor Shur-Fen Gau, for their generous help providing the information regarding the program.
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabacof L., Tosto-Mancuso J., Wood J., Cortes M., Kontorovich A., McCarthy D., et al. Post-acute COVID-19 syndrome negatively impacts physical function, cognitive function, health-related quality of life, and participation. Am J Phys Med Rehabil. 2022;101(1):48–52. doi: 10.1097/PHM.0000000000001910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandmann F.G., Tessier E., Lacy J., Kall M., Van Leeuwen E., Charlett A., et al. Long-term health-related quality of life in non-hospitalized coronavirus disease 2019 (COVID-19) cases with confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in England: longitudinal analysis and cross-sectional comparison with controls. Clin Infect Dis. 2022;75(1):e962–e973. doi: 10.1093/cid/ciac151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V. Condition WHOCCDWGoP-C-. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22(4):e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.COVID-19 rapid guideline: managing the long-term effects of COVID-19. 2021. https://www.nice.org.uk/guidance/ng188 Accessed 16th, November, 2022. [PubMed]
- 7.CDC. Post-COVID conditions: information for healthcare providers. 2022. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html Accessed.
- 8.Kim Y., Kim S.E., Kim T., Yun K.W., Lee S.H., Lee E., et al. Preliminary guidelines for the clinical evaluation and management of long COVID. Infect Chemother. 2022;54(3):566–597. doi: 10.3947/ic.2022.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V, McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramanian A., Nirantharakumar K., Hughes S., Myles P., Williams T., Gokhale K.M., et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706–1714. doi: 10.1038/s41591-022-01909-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnold D.T., Hamilton F.W., Milne A., Morley A.J., Viner J., Attwood M., et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2021;76(4):399–401. doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carfi A., Bernabei R., Landi F., Gemelli Against C-P-ACSG Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno-Perez O., Merino E., Leon-Ramirez J.M., Andres M., Ramos J.M., Arenas-Jimenez J., et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;82(3):378–383. doi: 10.1016/j.jinf.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrigues E., Janvier P., Kherabi Y., Le Bot A., Hamon A., Gouze H., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81(6):e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvalho-Schneider C., Laurent E., Lemaignen A., Bourbao-Tournois E., Lemaignen C., Laribi S., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27(2):258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hastie C.E., Lowe D.J., McAuley A., Winter A.J., Mills N.L., Black C., et al. Outcomes among confirmed cases and a matched comparison group in the Long-COVID in Scotland study. Nat Commun. 2022;13(1):5663. doi: 10.1038/s41467-022-33415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahmer T., Borzikowsky C., Lieb W., Horn A., Krist L, Fricke J., et al. Severity, predictors and clinical correlates of post-COVID syndrome (PCS) in Germany: a prospective, multi-centre, population-based cohort study. EClinicalMedicine. 2022;51:101549. doi: 10.1016/j.eclinm.2022.101549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chopra V., Flanders S.A., O'Malley M., Malani A.N., Prescott H.C. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576–578. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Meijenfeldt F.A., Havervall S., Adelmeijer J., Lundstrom A., Magnusson M., Mackman N., et al. Sustained prothrombotic changes in COVID-19 patients 4 months after hospital discharge. Blood Adv. 2021;5(3):756–759. doi: 10.1182/bloodadvances.2020003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afrin L.B., Weinstock L.B., Molderings G.J. Covid-19 hyperinflammation and post-Covid-19 illness may be rooted in mast cell activation syndrome. Int J Infect Dis. 2020;100:327–332. doi: 10.1016/j.ijid.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Q., Zheng X.S., Shen X.R., Si H.R., Wang X, Wang Q., et al. Prolonged shedding of severe acute respiratory syndrome coronavirus 2 in patients with COVID-19. Emerg Microb Infect. 2020;9(1):2571–2577. doi: 10.1080/22221751.2020.1852058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan F.J., Hope C.M., Masavuli M.G., Lynn M.A., Mekonnen Z.A., Yeow A.E.L., et al. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med. 2022;20(1):26. doi: 10.1186/s12916-021-02228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karlsson A.C., Humbert M., Buggert M. The known unknowns of T cell immunity to COVID-19. Sci Immunol. 2020;5(53) doi: 10.1126/sciimmunol.abe8063. [DOI] [PubMed] [Google Scholar]
- 26.Evans P.C., Rainger G.E., Mason J.C., Guzik T.J., Osto E., Stamataki Z., et al. Endothelial dysfunction in COVID-19: a position paper of the ESC working group for atherosclerosis and vascular biology, and the ESC Council of basic cardiovascular science. Cardiovasc Res. 2020;116(14):2177–2184. doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wostyn P. COVID-19 and chronic fatigue syndrome: is the worst yet to come? Med Hypotheses. 2021;146 doi: 10.1016/j.mehy.2020.110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrandi P.J., Alway S.E., Mohamed J.S. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol. 2020;129(4):864–867. doi: 10.1152/japplphysiol.00321.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wood E., Hall K.H., Tate W. Role of mitochondria, oxidative stress and the response to antioxidants in myalgic encephalomyelitis/chronic fatigue syndrome: a possible approach to SARS-CoV-2 'long-haulers. Chronic Dis Transl Med. 2021;7(1):14–26. doi: 10.1016/j.cdtm.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serviente C., Decker S.T., Layec G. From heart to muscle: pathophysiological mechanisms underlying long-term physical sequelae from SARS-CoV-2 infection. J Appl Physiol. 2022;132(3):581–592. doi: 10.1152/japplphysiol.00734.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J., Xu X., Jiang L., Dua K., Hansbro P.M., Liu G. SARS-CoV-2 induces transcriptional signatures in human lung epithelial cells that promote lung fibrosis. Respir Res. 2020;21(1):182. doi: 10.1186/s12931-020-01445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhawan R.T., Gopalan D., Howard L., Vicente A., Park M., Manalan K., et al. Beyond the clot: perfusion imaging of the pulmonary vasculature after COVID-19. Lancet Respir Med. 2021;9(1):107–116. doi: 10.1016/S2213-2600(20)30407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao B., Liu Z., Tang L., Li L., Gan Q., Shi H., et al. Longitudinal clinical and radiographic evaluation reveals interleukin-6 as an indicator of persistent pulmonary injury in COVID-19. Int J Med Sci. 2021;18(1):29–41. doi: 10.7150/ijms.49728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez-Bermejo J.A., Kang S., Rockwood S.J., Simoneau C.R., Joy D.A., Ramadoss G.N., et al. SARS-CoV-2 infection of human iPSC-derived cardiac cells predicts novel cytopathic features in hearts of COVID-19 patients. bioRxiv. 2020 doi: 10.1126/scitranslmed.abf7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moody W.E., Liu B., Mahmoud-Elsayed H.M., Senior J., Lalla S.S., Khan-Kheil A.M., et al. Persisting adverse ventricular remodeling in COVID-19 survivors: a longitudinal echocardiographic study. J Am Soc Echocardiogr. 2021;34(5):562–566. doi: 10.1016/j.echo.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korompoki E., Gavriatopoulou M., Hicklen R.S., Ntanasis-Stathopoulos I., Kastritis E., Fotiou D., et al. Epidemiology and organ specific sequelae of post-acute COVID19: a narrative review. J Infect. 2021;83(1):1–16. doi: 10.1016/j.jinf.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero-Sanchez C.M., Diaz-Maroto I., Fernandez-Diaz E., Sanchez-Larsen A., Layos-Romero A., Garcia-Garcia J., et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95(8):e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y., Li X., Geng D., Mei N., Wu P.Y., Huang C.C., et al. Cerebral micro-structural changes in COVID-19 patients - an MRI-based 3-month follow-up study. EClinicalMedicine. 2020;25:100484. doi: 10.1016/j.eclinm.2020.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin Y., Wu J., Chen T., Li J, Zhang G., Wu D., et al. Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological manifestations. J Clin Invest. 2021;131(8) doi: 10.1172/JCI147329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guedj E., Campion J.Y., Dudouet P., Kaphan E., Bregeon F., Tissot-Dupont H., et al. (18)F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. 2021;48(9):2823–2833. doi: 10.1007/s00259-021-05215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kandemirli S.G., Altundag A., Yildirim D., Tekcan Sanli D.E., Saatci O. Olfactory bulb MRI and paranasal sinus CT findings in persistent COVID-19 anosmia. Acad Radiol. 2021;28(1):28–35. doi: 10.1016/j.acra.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Melo G.D., Lazarini F., Levallois S., Hautefort C., Michel V. F.Larrous ,et al., COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. 2021;13(596) doi: 10.1126/scitranslmed.abf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asadi M.M., Shankayi Z., Bahrami F., Mohammadzadeh T., Amini H., Naderi M. Quantitative analysis of taste disorder in COVID-19 patients, the hypersensitivity to salty quality. New Microbes New Infect. 2021;43:100919. doi: 10.1016/j.nmni.2021.100919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clinical management of patients with COVID-19: rehabilitation of patients with COVID-19. World Health Organization; 2020. https://openwho.org/courses/clinical-management-COVID-19-rehabilitation Accessed 16th November, 2022. [Google Scholar]
- 45.Nopp S., Moik F., Klok F.A., Gattinger D., Petrovic M., Vonbank K., et al. Outpatient pulmonary rehabilitation in patients with long COVID improves exercise capacity, functional status, dyspnea, fatigue, and quality of life. Respiration. 2022;101(6):593–601. doi: 10.1159/000522118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Compagno S., Palermi S., Pescatore V., Brugin E., Sarto M., Marin R., et al. Physical and psychological reconditioning in long COVID syndrome: results of an out-of-hospital exercise and psychological - based rehabilitation program. IJC Heart Vasculat. 2022;41:101080. doi: 10.1016/j.ijcha.2022.101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrow A.K., Ng R., Vargas G., Jashar D.T., Henning E., Stinson N., et al. Postacute/long COVID in pediatrics: development of a multidisciplinary rehabilitation clinic and preliminary case series. Am J Phys Med Rehabil. 2021;100(12):1140–1147. doi: 10.1097/PHM.0000000000001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clinical management of COVID-19: living guideline, 15 September 2022. World Health Organization; 2022. [PubMed] [Google Scholar]
- 49.Writing Committee, Gluckman T.J., Bhave N.M., Larry A.A., Eugene H.C., Erica S.S., et al. 2022 ACC expert consensus decision pathway on cardiovascular sequelae of COVID-19 in adults: myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection, and return to play. J Am Coll Cardiol. 2022;79(17):1717–1756. doi: 10.1016/j.jacc.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stussman B., Williams A., Snow J., Gavin A., Scott R., Nath A., et al. Characterization of post–exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. 2020:1025. doi: 10.3389/fneur.2020.01025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown D., Oller D., Hassell H., DeChane T., Appel C., Hagey S., et al. Physical therapists living with long COVID, part 1: defining the Indefinable. 2021. [Google Scholar]
- 52.Dani M., Dirksen A., Taraborrelli P., Torocastro M., Panagopoulos D., Sutton R., et al. Autonomic dysfunction in ‘long COVID’: rationale, physiology and management strategies. Clin Med. 2021;21(1):e63–e67. doi: 10.7861/clinmed.2020-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rusch H.L., Rosario M., Levison L.M., Olivera A., Livingston W.S., Wu T., et al. The effect of mindfulness meditation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. Ann N Y Acad Sci. 2019;1445(1):5–16. doi: 10.1111/nyas.13996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kriakous S.A., Elliott K.A., Lamers C., Owen R. The effectiveness of mindfulness-based stress reduction on the psychological functioning of healthcare professionals: a systematic review. Mindfulness. 2021;12(1):1–28. doi: 10.1007/s12671-020-01500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayden M.C., Limbach M., Schuler M., Merkl S., Schwarzl G., Jakab K., et al. 2021. Effectiveness of a three-week inpatient pulmonary rehabilitation program for patients after COVID-19: a prospective observational study. 18(17):9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmed I., Inam A.B., Belli S., Ahmad J., Khalil W., Jafar MJoP. 2021. Effectiveness of aerobic exercise training program on cardio-respiratory fitness and quality of life in patients, recovered from COVID-19; pp. 1–6. [Google Scholar]
- 57.Durstenfeld M.S., Sun K., Tahir P., Peluso M.J., Deeks S.G., Aras M.A., et al. Use of cardiopulmonary exercise testing to evaluate long COVID-19 symptoms in adults: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(10) doi: 10.1001/jamanetworkopen.2022.36057. e2236057–e2236057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh I., Joseph P., Heerdt P.M., Cullinan M., Lutchmansingh D.D., Gulati M., et al. Persistent exertional intolerance after COVID-19: insights from invasive cardiopulmonary exercise testing. Chest. 2022;161(1):54–63. doi: 10.1016/j.chest.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mancini D.M., Brunjes D.L., Lala A., Trivieri M.G., Contreras J.P., Natelson B.H. Use of cardiopulmonary stress testing for patients with unexplained dyspnea post-coronavirus disease. JACC Heart Fail. 2021;9(12):927–937. doi: 10.1016/j.jchf.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abdallah S.J., Voduc N., Corrales-Medina V.F., McGuinty M., Pratt A., Chopra A., et al. Symptoms, pulmonary function, and functional capacity four months after COVID-19. Ann Am Thorac Soc. 2021;18(11):1912–1917. doi: 10.1513/AnnalsATS.202012-1489RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szekely Y., Lichter Y., Sadon S., Lupu L., Taieb P., Banai A., et al. Cardiorespiratory abnormalities in patients recovering from coronavirus disease 2019. J Am Soc Echocardiogr. 2021;34(12):1273–1284.e1279. doi: 10.1016/j.echo.2021.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jimeno-Almazán A., Franco-López F., Buendía-Romero Á, et al. Rehabilitation for post-COVID-19 condition through a supervised exercise intervention: a randomized controlled trial. Scand J Med Sci Sports. 2022;32(12):1791–1801. doi: 10.1111/sms.14240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vieira A., Pinto A., Garcia B., Eid R.A.C., Mól C.G., Nawa R.K. Telerehabilitation improves physical function and reduces dyspnoea in people with COVID-19 and post-COVID-19 conditions: a systematic review. J Physiother. 2022;68(2):90–98. doi: 10.1016/j.jphys.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen H., Shi H., Liu X., Sun T., Wu J., Liu Z. Effect of pulmonary rehabilitation for patients with post-COVID-19: a systematic review and meta-analysis. Front Med. 2022;9:837420. doi: 10.3389/fmed.2022.837420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahmadi Hekmatikar A.H., Ferreira Júnior J.B., Shahrbanian S., Suzuki K. Functional and psychological changes after exercise training in post-COVID-19 patients discharged from the hospital: a PRISMA-compliant systematic review. Int J Environ Res Publ Health. 2022;19(4) doi: 10.3390/ijerph19042290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saaei F., Klappa S.G. Rethinking telerehabilitation: attitudes of physical therapists and patients. J Patient Exp. 2021;8 doi: 10.1177/23743735211034335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6) doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 68.Stephenson T., Shafran R., De Stavola B., Rojas N., Aiano F., Amin-Chowdhury Z., et al. Long COVID and the mental and physical health of children and young people: national matched cohort study protocol (the CLoCk study) BMJ Open. 2021;11(8):e052838. doi: 10.1136/bmjopen-2021-052838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parisi G.F., Diaferio L., Brindisi G., Indolfi C., Umano G.R., Klain A., et al. Cross-sectional survey on long term sequelae of pediatric COVID-19 among Italian pediatricians. Children. 2021;8(9) doi: 10.3390/children8090769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Radtke T., Ulyte A., Puhan M.A., Kriemler S. Long-term symptoms after SARS-CoV-2 infection in children and adolescents. JAMA. 2021;326(9):869–871. doi: 10.1001/jama.2021.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stephenson T., Allin B., Nugawela M.D., Rojas N., Dalrymple E., Pinto Pereira S., et al. Long COVID (post-COVID-19 condition) in children: a modified Delphi process. Arch Dis Child. 2022;107(7):674–680. doi: 10.1136/archdischild-2021-323624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zimmermann P., Pittet L.F., Curtis N. Long covid in children and adolescents. BMJ. 2022;376:o143. doi: 10.1136/bmj.o143. [DOI] [PubMed] [Google Scholar]
- 73.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F., et al. Multisystem inflammatory syndrome in U.S. Children and adolescents. N Engl J Med. 2020;383(4):334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Multisystem inflammatory syndrome in children and adolescents with COVID-19: scientific brief, 15 May 2020. World Health Organization; 2020. [Google Scholar]
- 75.Osmanov I.M., Spiridonova E., Bobkova P., Gamirova A., Shikhaleva A., Andreeva M., et al. Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC Global follow-up protocol: a prospective cohort study. Eur Respir J. 2022;59(2) doi: 10.1183/13993003.01341-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borch L., Holm M., Knudsen M., Ellermann-Eriksen S., Hagstroem S. Long COVID symptoms and duration in SARS-CoV-2 positive children - a nationwide cohort study. Eur J Pediatr. 2022;181(4):1597–1607. doi: 10.1007/s00431-021-04345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Behnood S.A., Shafran R., Bennett S.D., Zhang A.X.D., O'Mahoney L.L., Stephenson T.J., et al. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: a meta-analysis of controlled and uncontrolled studies. J Infect. 2022;84(2):158–170. doi: 10.1016/j.jinf.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Funk A.L., Kuppermann N., Florin T.A., Tancredi D.J., Xie J., Kim K., et al. Post-COVID-19 conditions among children 90 Days after SARS-CoV-2 infection. JAMA Netw Open. 2022;5(7) doi: 10.1001/jamanetworkopen.2022.23253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brackel C.L.H., Lap C.R., Buddingh E.P., van Houten M.A., van der Sande L., Langereis E.J., et al. Pediatric long-COVID: an overlooked phenomenon? Pediatr Pulmonol. 2021;56(8):2495–2502. doi: 10.1002/ppul.25521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buonsenso D., Pujol F.E., Munblit D., Pata D., McFarland S., Simpson F.K. Clinical characteristics, activity levels and mental health problems in children with long coronavirus disease: a survey of 510 children. Future Microbiol. 2022;17:577–588. doi: 10.2217/fmb-2021-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zimmermann P., Pittet L.F., Curtis N. How common is long COVID in children and adolescents? Pediatr Infect Dis J. 2021;40(12):e482–e487. doi: 10.1097/INF.0000000000003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taquet M., Sillett R., Zhu L., Mendel J., Camplisson I., Dercon Q., et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatr. 2022;9(10):815–827. doi: 10.1016/S2215-0366(22)00260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Piazza M., Di Cicco M., Pecoraro L., Ghezzi M., Peroni D., Comberiati P. Long COVID-19 in children: from the pathogenesis to the biologically plausible roots of the syndrome. Biomolecules. 2022;12(4) doi: 10.3390/biom12040556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Crook H., Raza S., Nowell J., Young M., Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 85.Buonsenso D., Di Gennaro L., De Rose C., Morello R., D'Ilario F., Zampino G., et al. Long-term outcomes of pediatric infections: from traditional infectious diseases to long Covid. Future Microbiol. 2022;17:551–571. doi: 10.2217/fmb-2022-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Howard-Jones A.R., Burgner D.P., Crawford N.W., Goeman E., Gray P.E., Hsu P., et al. COVID-19 in children. II: pathogenesis, disease spectrum and management. J Paediatr Child Health. 2022;58(1):46–53. doi: 10.1111/jpc.15811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Izquierdo-Pujol J., Moron-Lopez S., Dalmau J., Gonzalez-Aumatell A., Carreras-Abad C., Mendez M., et al. Post COVID-19 condition in children and adolescents: an Emerging problem. Front Pediatr. 2022;10:894204. doi: 10.3389/fped.2022.894204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ashkenazi-Hoffnung L., Shmueli E., Ehrlich S., Ziv A., Bar-On O., Birk E., et al. Long COVID in children: observations from a designated pediatric clinic. Pediatr Infect Dis J. 2021;40(12):e509–e511. doi: 10.1097/INF.0000000000003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fainardi V., Meoli A., Chiopris G., Motta M., Skenderaj K., Grandinetti R., et al. Long COVID in children and adolescents. Life. 2022;12(2) doi: 10.3390/life12020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.新冠肺炎. ( COVID 19 ) 染疫康復者 指引. Center of Disease Control, Ministry of Health and Welfare, Taiwan, ROC; 2021. [Google Scholar]
- 91.National Health Service The NHS plan for improving long COVID services. 2022. https://www.england.nhs.uk/wp-content/uploads/2022/07/C1607_The-NHS-plan-for-improving-long-COVID-services_July-2022.pdf Published June, 2022. Accessed 18 th Feburary, 2023.