Keywords: glomerular and tubulointerstitial diseases, ANCA, ANCA-associated vasculitis, ANCA-negative vasculitis, cohort studies, end-stage kidney disease, glomerulonephritis, outcomes, pauci-immune glomerulonephritis, renal biopsy, vasculitis
Abstract
Key Points
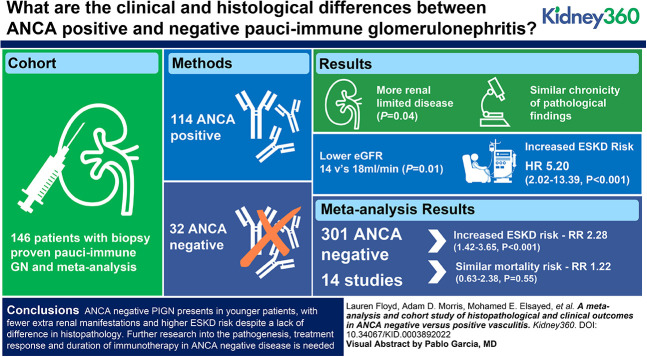
ANCA-negative pauci-immune glomerulonephritis presents in younger patients, with fewer extrarenal manifestations and higher risk of ESKD.
The absence of positive ANCA serology should not discourage immunosuppressive treatment.
Further research into the pathogenesis, treatment response, and duration of immunotherapy in ANCA-negative disease is needed.
Background
ANCA-negative pauci-immune glomerulonephritis (PIGN) represents a rare and often under-studied subgroup of the vasculitides. This study aims to investigate differences in the clinical phenotype, renal histological features, and clinical outcomes of patients with PIGN, with and without serum ANCA positivity.
Methods
A cohort of biopsy-proven PIGN with and without detectable circulating ANCA was constructed from a single center between 2006 and 2016. Primary outcomes compared clinical presentation and histopathological features according to ANCA status, with multivariate Cox regression to compare mortality and ESKD. A systematic review and meta-analysis of the published literature was undertaken.
Results
In our cohort of 146 patients, 22% (n=32) had ANCA-negative disease, with a comparatively younger mean age at diagnosis; 51.4 versus 65.6 years (P<0.001). In total, 14 studies, inclusive of our cohort, were eligible for meta-analysis, totaling 301 patients who were ANCA negative. Those with ANCA-negative disease tended to have fewer extrarenal symptoms and a higher frequency of renal-limited disease, but both failed to reach statistical significance (P=0.92 and P=0.07). The risk of ESKD was significantly higher in seronegative disease (RR, 2.28; 95% confidence interval, 1.42 to 3.65; P<0.001), reflecting our experience, with a fivefold increased risk of ESKD in ANCA-negative disease (P<0.001). No significant difference in the chronicity of histopathological findings was seen and the meta-analysis showed no difference in morality (RR, 1.22; 95% confidence interval, 0.63 to 2.38; P=0.55).
Conclusion
Our findings demonstrate that ANCA-negative PIGN presents in younger patients, with fewer extrarenal manifestations and higher ESKD risk, despite a lack of difference in histopathology. This study provides the impetus for further research into the pathogenesis, treatment response, and duration of immunotherapy in ANCA-negative disease. We suggest that the absence of positive ANCA serology should not discourage treatment and for clinical trials to include patients who are ANCA negative.
Introduction
Pauci-immune small vessel vasculitis is characterized by inflammation and necrosis of small vessels with potential end-organ damage and life-threatening consequences. The role of ANCA is central to the understanding of pathogenesis.1,2 Its detection is closely associated with disease, but despite this, its clinical utility as a reliable biomarker remains imperfect.3 As such, a proportion of patients develop new and relapsing disease in the absence of detectable circulating ANCA.
The finding of seronegative pauci-immune glomerulonephritis (PIGN) has previously been reported to occur in up to 30% of patients.4–6 This was the case in both European and South-East Asian cohorts, with data spanning several decades. More recent cohort studies from Asia and Mexico estimate a more modest disease incidence of 7%–12%.7,8 These studies solely utilize data from the last two decades, and it could be considered the lower rate of seronegative disease may reflect improvements in assay sensitivity over the years. However, it is worth noting these studies reflect different health care systems, ethnicities, and latitudinal gradients, meaning these findings may not be transferable to all population groups.9
It has been suggested that pauci-immune small-vessel vasculitis exhibits a different spectrum of disease, in the absence of detectable circulating ANCA. Reports vary on the clinical presentation and phenotype, with disparity in the degree of renal and extrarenal disease occurrence among patients who are ANCA positive and negative.4,5 Due to the rarity of the disease, there are limited studies evaluating the histopathological differences between the two groups. This study aims to investigate the differences in clinical presentation, renal histopathological features, and clinical outcomes among patients with PIGN, with and without detectable circulating ANCA.
Methods
Participants and Study Design
A retrospective cohort study with biopsy-proven PIGN was constructed from a single center between 2006 and 2016, and followed-up until November 2021. Patients were stratified by ANCA status from the time of diagnosis. Patients with anti-glomerular basement membrane antibody positivity, a non-diagnostic biopsy or where the electron microscopy report could not ensure a lack of significant deposits were excluded. Patients who were ANCA positive were categorized to either myeloperoxidase (MPO) or proteinase 3 (PR3). An ANCA-negative diagnosis was defined as disease activity without detectable circulating ANCA in the context of biopsy-proven PIGN. All diagnosed patients were in keeping with the Chapel Hill Consensus Conference.10 Patients with secondary ANCA-negative disease as a result of infection, drugs, or malignancies were excluded. Ethical approval for this study was obtained from the UK Health Research Authority and Confidentiality Advisory Group. The research was performed in accordance with the Declaration of Helsinki.
Data Acquisition
Patient demographics, presentation, immunotherapy, and clinical outcomes were collected and examined alongside the histopathology outcomes from renal biopsy findings. Data were collected retrospectively from electronic patient records. Birmingham vasculitis activity scores were not included, due to the presence of mainly renal-limited disease and the limitations of retrospective scoring.
Laboratory Data
Renal function was measured using the standardized CKD Epidemiology Collaboration formula (ml/min per 1.73 m2).11 ANCA serology was tested by indirect immunofluorescence microscopy assay with subsequent testing by Phadia ELiA fluorescence enzyme immunoassay in patients with a positive result. Anti-myeloperoxidase titers of >5.0 IU/ml and anti-proteinase 3 titers of >3.0 IU/ml were defined as positive.
Histologic Data
All included biopsy specimens underwent standard processing for light microscopy, immunohistochemistry, and electron microscopy. All biopsies were independently reviewed by a pathologist at the time of initial diagnosis and pathology reports were used to collect the histologic variables retrospectively. Alongside characteristic features on light microscopy, PIGN was defined as few or no electron-dense deposits on electron microscopy.12 Arteries and arterioles were also evaluated for the presence of vasculitis and the degree of interstitial fibrosis and tubular atrophy (IFTA). Biopsies were taken before the initiation of immunosuppressive treatment, and were categorized according to the Berden et al.13 histopathological classification.
Treatment
The dosing regimen of intravenous cyclophosphamide was adjusted for renal function and patient age in accordance with recommendations by the European vasculitis study group.14 Rituximab was administered at a dose of 1 g every 2 weeks (total of 2 g). Pulsed intravenous methylprednisolone and plasma exchange were administered according to local physician discretion. Considerations for plasma exchange at the time included dialysis dependence, serum creatinine >500 µmol/L, and/or pulmonary hemorrhage.
Outcomes
Primary outcomes compared the histopathological classification, clinical presentation, and outcomes including mortality and ESKD of those with ANCA-negative and positive disease. ESKD was defined by the use of continued RRT or transplantation at follow-up. Secondary outcomes included remission and relapse rates among the two groups. Disease relapse was defined as a change in signs and symptoms that were attributed to vasculitis disease and required the reintroduction or increase in immunosuppressive treatment.
Systematic Review and Meta-Analysis
A systematic review and meta-analysis of the available published studies, inclusive of our presented cohort data, was undertaken. Literature searches were performed using PubMed, Embase, and Cochrane databases until March 1, 2022. Search strategies included the terms “ANCA,” “anti-neutrophil cytoplasmic antibodies,” “vasculitis,” and “pauci-immune.”
All outcome studies on biopsy-proven ANCA-negative disease, inclusive of those presenting data as a subgroup analysis, were included. Eligible studies were screened and reviewed by two independent reviewers (L.F. and A.M.) who were blinded to the other selections, reducing the risk of bias. In cases of disagreement, a subsequent discussion took place with the resolution of discrepancies agreed by joint consensus.
The studies had to meet at least one of the following criteria for inclusion in meta-analysis: (1) comparison of histopathologic findings between patients who were ANCA negative and positive, (2) Clinical presentation and phenotype of ANCA-negative and positive disease, and (3) clinical outcomes including, mortality, relapse, and/or ESKD between the two groups. Studies that were unable to exclude the presence of glomerular deposits or where secondary vasculitides were included, along with case reports, editorials, letters to the editor, review articles, conference abstracts and studies not published in English were excluded from review.
The protocol for this review was registered and published in PROSPERO. The systematic review study selection was conducted in accordance with the PRISMA guidelines (Figure 1). The methodological quality and risk of bias of eligible studies were assessed using the Newcastle–Ottawa scale for observational studies (Supplemental Table 1). Supplemental Table 2 describes the characteristics of included studies.
Figure 1.
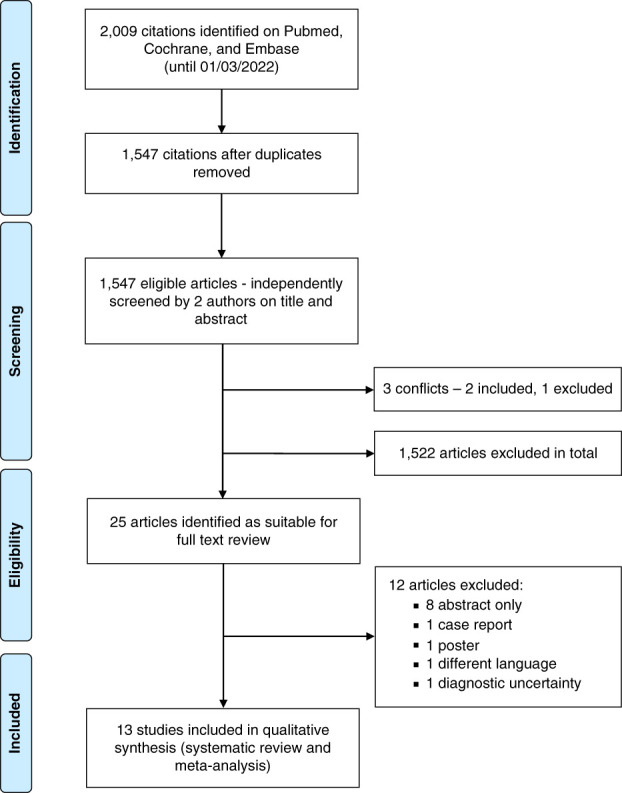
PRISMA flow diagram.
Statistical Analysis
Cohort data analyses were conducted using R statistical software v4.1.2 (R Foundation, Vienna, Austria). Patient characteristics by ANCA status were presented as mean±SD or median for continuous variables and proportions for categorical variables. Comparisons between the two groups were examined utilizing t tests, Mann–Whitney, chi-squared, or Fisher exact tests, as appropriate. Patient survival times were calculated from the point of diagnosis until death, loss to follow-up or end of study. Renal survival was calculated similarly with the addition of a censor for death. Risk of ESKD and death were estimated using Cox regression models and presented as hazard ratios (HR) with 95% confidence intervals (95% CIs). The proportional hazards assumption was checked using Schoenfeld residuals.
The meta-analysis was conducted using R meta package (v5.1–1). We measured outcomes (death, ESKD, relapse), presentation (renal-limited, extrarenal manifestations) and histology (Berden classification).13 For outcomes and presentation, we used “metabin” for the dichotomous outcomes with the Mantel–Haenszel method. For Berden classification we used “metabin” to consider the proportion of patients who were ANCA-negative or positive in a Berden subgroup, for example, crescentic compared with all of the other subgroups, such as focal, mixed, and sclerosed. This was done for each subgroup in turn. We calculated the random-effect forms of these analyses and reported the unadjusted relative risk ratio, P value, I2 (measure of heterogeneity), and τ2 (measure of variance of true effect size). We applied a Bonferroni correction for multiple hypothesis testing and used this to filter nominally significant results.
Results
Demographics and Clinical Features
In total, 146 patients with biopsy-proven PIGN were included in our cohort. Baseline characteristics are shown in Table 1. ANCA-negative disease accounted for 22% (n=32) of the cohort and was associated with more renal-limited disease (62% versus 39%, P=0.04). Of the four patients who were ANCA negative with pulmonary involvement, three had features of bilateral pulmonary infiltrates identified on radiologic imaging, and one presented with acute pulmonary hemorrhage. At the time of diagnosis, patients who were ANCA negative reported no constitutional symptoms, including weight loss, night sweats or fever, and less ear, nose, and throat involvement (0% versus 27%, P <0.001).
Table 1.
Comparison of the demographics, clinical features, and histopathologic findings between patients who are ANCA positive and negative with pauci-immune glomerulonephritis
| Characteristics of Study Population | ANCA Positive n=114 (%) | ANCA Negative n=32 (%) | P Value |
|---|---|---|---|
| Patient demographics | |||
| Age, yr (mean±SD) | 65.6±12.10 | 51.44±19.57 | <0.001 |
| Sex, M:F | 59:55 | 21:11 | 0.23 |
| eGFR at presentation, median, IQR | 18.0 (11.0–39.3) | 14.0 (7.0–26.0) | 0.10 |
| Clinical features and systems involvement, % | |||
| Constitutional symptoms | 32 (28) | 0 | <0.001 |
| Renal | 113 (99) | 31 (97) | 0.39 |
| Pulmonary | 28 (25) | 4 (13) | 0.22 |
| ENT | 27 (24) | 0 | <0.001 |
| CNS | 14 (12) | 1 (3) | 0.19 |
| Cutaneous | 16 (14) | 8 (25) | 0.18 |
| Ophthalmic | 6 (5) | 0 | 0.34 |
| Gastrointestinal | 4 (4) | 1 (3) | 1.00 |
| Histopathologic findings, % | |||
| Normal glomeruli (N0 >25%) | 73 (64) | 16 (50) | 0.01 |
| IFTA 0 | 21 (18) | 8 (25) | 0.42 |
| Extraglomeruli arteritis | 15 (13) | 7 (22) | 0.26 |
| Berden Classification, % | |||
| Mixed | 30 (26) | 11 (34) | 0.38 |
| Focal | 51 (45) | 12 (38) | 0.55 |
| Sclerotic | 6 (5) | 4 (13) | 0.23 |
| Crescentic | 28 (25) | 5 (16) | 0.35 |
M, male; F, female; IQR, interquartile range; ENT, ears, nose, and throat; CNS, central nervous system; IFTA, interstitial fibrosis and tubular atrophy.
Histological Outcomes
There was a comparable spread across the Berden classification, with no significant difference seen between the positive and negative groups (Table 1). The odds ratio (OR) associated with ANCA serology and Berden’s classification are shown in Table 2. Patients who were ANCA negative had a higher degree of abnormal glomeruli than patients who were ANCA positive. In accordance with the ANCA renal risk score (15), the number of patients with >25% normal glomeruli was lower in the ANCA negative group, compared with ANCA positive (50% versus 64%, P=0.01). Although, of note, only 13% (n=4) of patients who were ANCA negative had features diagnostic of sclerotic classification. IFTA was present in 80% of all patients with vasculitis, with no significant difference between the two cohorts.
Table 2.
Demonstrates the odds ratio of the four Berden classifications associated with ANCA-negative versus ANCA-positive disease
| Berden Classification | Odds Ratio | 95% Confidence Interval |
|---|---|---|
| Mixed | 1.47 | 0.62 to 3.36 |
| Focal | 0.74 | 0.32 to 1.64 |
| Sclerotic | 2.57 | 0.62 to 9.63 |
| Crescentic | 0.57 | 0.18 to 1.51 |
Clinical Outcomes
At the time of diagnosis, patients who were ANCA negative had a lower eGFR than those with positive ANCA serology (14.0 versus 18.0 ml/min, P=0.10). A higher percentage of patients who were ANCA negative were dialysis dependent at the time of diagnosis (28%, n=9, versus 11%, n=13) and at 30 days from presentation (59%, n=19 versus 20%, n=23). At the end of follow-up, a higher proportion of ANCA negative progressed to ESKD (53% versus 12%). The hazard ratio (HR) for ESKD in patients who were ANCA negative compared with ANCA positive was 6.23 (95% CI, 2.98 to 13.05, P<0.001). When adjusting for age, sex, and induction therapy, the HR for ESKD was five times higher in patients who were ANCA negative (HR, 5.20; 95% CI, 2.02 to 13.39, P<0.001) and this remained largely unchanged on sensitivity analyses with restricted follow-up to 1 and 2 years from the time of diagnosis (Supplemental Table 3). Figure 2A shows the risk of ESKD associated with positive and negative serology.
Figure 2.
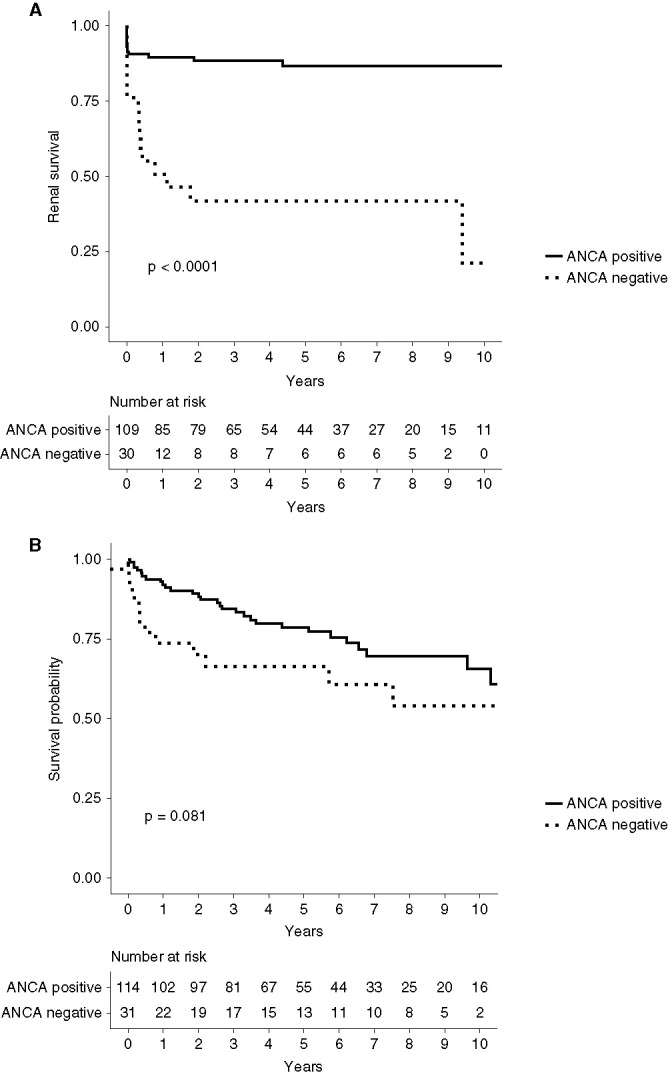
Outcomes according to ANCA-positive and -negative serology. (A) Kaplan-Meier curve demonstrating renal survival of patients with ANCA-positive and -negative serology. (B) Kaplan-Meier curve demonstrating patient survival according to ANCA-positive and -negative serology.
Overall patient survival was 87%, 82%, and 71% at 12 months, 24 months, and end of study, respectively. Where the cause of death was known 13 patients died from infection-related illnesses, three from malignancy, one from pulmonary fibrosis, one from a myocardial infarction, and two patients died as a result of active vasculitis. On univariate analysis, mortality rates were higher in the ANCA negative cohort (HR, 1.81; 95% CI, 0.92 to 3.55, P=0.09). Multivariable analysis adjusting for age, sex, and induction therapy, identified an HR for death that was three times higher in the ANCA negative cohort (HR 3.0; 95% CI, 1.36 to 6.63, P=0.007) and when adjusting further for the addition of ESKD, the HR for death was 2.31 (95% CI, 0.97 to 5.49, P=0.06). Figure 2B shows the mortality associated with positive and negative serology. The mean time from diagnosis to death was 177 days (interquartile range, 100–709) in the ANCA-negative cohort. Among the ANCA-negative cohort, one death was attributed to vasculitis. Seven patients (58%) were dialysis dependent at the time of death, which in part may account for the increase mortality rate. Approximately 77% of patients who were ANCA-negative and 95% who were ANCA-positive achieved remission at 6 months. Relapsing disease was significantly higher in the patients who were ANCA positive; 35% compared with 16% in patients who were ANCA negative. There was no significant difference in follow-up duration between the two groups (P=0.09).
Treatment
Most patients (n=130, 89%) received remission induction treatment, as shown in Table 3. Fewer patients who were ANCA negative received induction treatment than those with ANCA-positive disease; 72% and 94%, respectively. Of those patients who were ANCA negative that did not receive remission induction treatment, five presented with renal-limited disease and dialysis dependence. Two patients were considered clinically frail with a Rockwood Clinical Frailty Score16 of 4 and 5.
Table 3.
Shows the remission induction treatment given for those with ANCA-positive versus ANCA-negative disease
| Remission Induction Treatment | ANCA Positive (n=114) | ANCA Negative (n=32) |
|---|---|---|
| IV methylprednisolone, n (%) | 33 (29) | 5 (15.6) |
| IV methylprednisolone mean dose, g (±SD) | 1.5±0.63 | 1.5±0.76 |
| Cyclophosphamide use, n (%) | 104 (91) | 22 (68.8) |
| Cyclophosphamide mean dose, g (±SD) | 5.54±3.58 | 4.47±3.34 |
| Rituximab use, n (%) | 6 (5) | 0 |
| Rituximab mean dose, g (±SD) | 1.9±0.2 | 0 |
| Plasmapheresis, n (%) | 23 (20) | 3 (9) |
IV, intravenous.
Maintenance treatment was given to 87% of patients. The majority of ANCA-negative patients (n=18, 78%) received azathioprine and/or oral glucocorticoids as maintenance treatment, which was similar to the 70% of patients who were ANCA positive.
Systematic Review and Meta-Analysis
In total, 14 studies were eligible for inclusion, with 301 patients who were ANCA negative available for analysis, inclusive of our cohort (Supplemental Table 2). The pooled age was lower in the patients who were ANCA negative (50.9 years, 95% CI, 44.6 to 58.2) in comparison to patients who were ANCA positive (57.6 years, 95% CI, 53.1 to 62.5). There was a slightly higher male proportion in the ANCA negative cohort (53.3% versus 50.9%). Supplemental Table 4 shows the meta-analysis findings, which were limited due to the moderate to large heterogeneity of results. Ten studies describe the clinical features associated with patients who were ANCA positive and negative. Patients with ANCA-negative disease tended to have fewer extrarenal manifestations and present with more renal-limited disease in our cohort, compared with those with ANCA positivity (22% versus 6%), although both failed to reach statistical significance, with respective P values of 0.92 and 0.07. On review of the histopathologic classification between the two cohorts, five studies reported Berden’s classification (Supplemental Table 2). The most common classification was focal (38%) in those with ANCA-positive serology, and crescentic (35%) in those with ANCA-negative disease.
When analyzing clinical outcomes, the relative risk (RR) of death was similar across the studies (n=8) and there was no significant difference in mortality between patients who were ANCA positive and negative (RR, 1.22; 95% CI, 0.63 to 2.38; P=0.55). There was, however, a significantly increased risk of ESKD in patients who were ANCA negative (RR, 2.28; 95% CI, 1.42 to 3.65; P<0.001) (Figure 3). Meta-analysis of relapse rates was not possible as only two studies reported on this. Shah et al.17 reported a shorter time to remission in ANCA-negative disease (51 versus 78 days, P=0.01) and relapse rates were lower in patients who were ANCA negative, which is in keeping with our findings.
Figure 3.
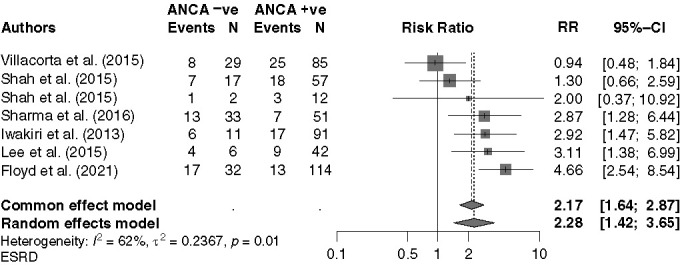
Forest plot for meta-analysis, comparing ESKD associated with ANCA-negative versus ANCA-positive disease.
Discussion
To date, patients with ANCA-negative vasculitis have been largely excluded from trials with limited data guiding their care. Our study and meta-analysis help address this, demonstrating that patients with ANCA-negative PIGN tend to be younger, exhibit fewer extrarenal symptoms, and have a higher risk of ESKD compared with those with ANCA-positive disease. The latter occurred despite no significant difference in the severity of scarring or chronicity on histology between the two groups.
Increased physician awareness, and widely available and highly sensitive ANCA assays with a short turnaround time, have contributed to improved diagnostics and a reduction in therapeutic delay in patients who are seropositive. It has previously been hypothesized that because patients who are ANCA negative experience fewer constitutional and extrarenal symptoms, there is a delay in self-presentation and investigation.17,18 As such, it has often been presumed patients with ANCA-negative PIGN were more likely have a higher degree of sclerosed glomeruli and IFTA at presentation, accounting for their worse outcomes.
Nevertheless, there is some discrepancy in the histopathologic findings among previously reported studies. Cohorts from the United States and South America have tended to display a higher degree of interstitial fibrosis and glomerular sclerosis,8,17,19 compared with larger cohorts form Europe, South-East Asia, and India, which typically observed a predominance for crescents with sclerosis only occurring in 10%–20% of lesions.4,18,20 Within our cohort and subsequent meta-analysis, there was no significant difference in sclerotic lesions among ANCA-positive and ANCA-negative disease, and we observed no significant difference in IFTA severity. In light of these findings, and a twofold increased risk of ESKD in ANCA-negative PIGN, an important question arises about treatment practice, therapeutic response, and the underlying pathogenesis. Treatment data was not available from the pooled studies to help shed light on this, but there was a slightly lower tendency for patients who were ANCA negative to receive induction therapy within our center.
The exact pathophysiology of seronegative PIGN and the reason for its clinical phenotype as a predominately single organ disease is beyond the scope of the data presented in this paper. Previous studies have suggested that patients who are ANCA negative have circulating autoantibodies with the potential for neutrophil and monocyte activation that are below the detection limit of modern assays, or that there are epitopes present, masking their detection.21,22 Furthermore, the role of more novel autoantibodies including anti–LAMP-2 antibodies and anti-tissue plasminogen activator antibodies23,24 are other possible factors contributing to disease activity and endothelial damage in the absence of circulating ANCA, although many of these have not been validated or require further research. In addition, most commercial assays are designed to detected IgG ANCA; however, other Ig subclasses, such as IgA ANCA, may be present.25 Although these theories attempt to explain the presence of disease in the absence of ANCA serology, it fails to account for the observed difference in presentation and outcomes. Much remains to be elucidated in ANCA-negative disease. Although seropositive and seronegative disease share the same histopathological endpoints, it is possible they remain two different disease processes, which could account for the heterogeneity in clinical features, outcomes, and pathologic findings observed in our study.
Just over half of the studies (n=8) included in the systematic review and meta-analysis presented mortality data that showed no significant difference in death between patients who were ANCA positive and negative. Although our cohort showed an increased risk of mortality associated with ANCA-negative disease, despite being comparatively younger, when adjusting for ESKD on multivariate analysis, this was not found to be statistically significant (P=0.06). This highlights the known implications of ESKD on mortality, with both advanced CKD and ANCA associated vasculitis representing independent risk factors for cardiovascular disease.26–28
The risk of relapse reported in some studies ranges from 20% to 60%.29,30 The meta-analysis findings showed the relapse rates were lower in patients who were ANCA negative (RR, 0.49; 95% CI, 0.25 to 0.97; P=0.04), which was similar to our findings. This may be in part explained by the higher rates of renal-limited disease and ESKD, which makes relapse less likely in itself. However, given the lower rates of relapse in ANCA-negative disease, it raises the question about duration of maintenance treatment in this subcategory. Both the REMAIN (prolonged REmission-MAINtenance therapy in systemic vasculitis) and MAINRITSAN (Maintenance of Remission using Rituximab in Systemic ANCA-Associated Vasculitis) trials looked at duration of maintenance immunosuppression in ANCA-associated vasculitis.31,32 Although these trials did not look specifically at those with initial ANCA-negative serology, they do raise the risk benefit balance of long-term maintenance immunosuppression.
Limitations
Our study was limited by its single-center, retrospective character. Reduced reporting of constitutional symptoms at presentation may be limited by the accuracy of capturing subtle nonrenal symptoms from retrospective data collection. Classification and biopsy reporting are potential limitations due to human variability and interpretation and the histological findings reported are from clinical records; however, this is the case across all comparable studies. We applied robust inclusion criteria to our meta-analysis, although not all studies reported the same outcomes and many were unadjusted, creating the potential for under-reporting of clinical features in the ANCA-negative cohorts. Treatment data on the included studies were not available, but we appreciate variation in treatment practice compared with seropositive disease is an important factor and requires future evaluation.
ANCA-negative vasculitis has been largely excluded from mainstream trials and previous studies looking at the differences in ANCA-negative disease have been limited, partly due to the small sample sizes, degree of heterogeneity, and lack of multivariable analysis.
With an incident rate approaching 30% of all patients with biopsy-proven PIGN, a better understanding this patient group is needed. Our findings demonstrate that PIGN exhibits a different clinical phenotype in the absence of detectable circulating ANCA, occurring in younger patients, with fewer extrarenal manifestations and higher rates of ESKD. Further research is needed to understand its pathogenesis, current treatment practice, and therapeutic response, so as to improve patient outcomes.
Supplementary Material
Acknowledgments
We acknowledge the support of the Department of Renal Medicine, Manchester Royal Infirmary, Manchester University NHS Foundation Trust, the Division of Cardiovascular Sciences, Manchester Academy of Health Sciences Centre, University of Manchester, and the Department of Renal Medicine at Royal Preston Hospital, Lancashire NHS Foundation Trust. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Disclosures
A. Dhaygude reports receiving honoraria from AstraZeneca; and reports having an advisory or leadership role with Vifor Pharma. D. Geetha reports having consultancy agreements with ChemoCentryx and as an adjudicator for Birmingham vasculitis activity scores (BVAS)/vasculitis damage index (VDI); and reports being a consultant for Aurinia and GlaxoSmithKline (GSK). S. Mitra is supported by The National Institute for Health and Care Research (NIHR) Devices for Dignity MedTech Co-operative and reports receiving honoraria from GSK and Vifor Pharma; reports having an advisory or leadership role with Baxter and Quanta; and reports speakers bureau from AstraZeneca. All remaining authors have nothing to disclose.
Funding
None.
Author Contributions
A. Dhaygude and A.D. Morris conceptualized the study; A. Baksi, L. Floyd, and D. Geetha were responsible for the data curation; M.E. Elsayed, L. Floyd, A.D. Morris, and A. Shetty were responsible for the formal analysis; A. Dhaygude, M.E. Elsayed, L. Floyd, A.D. Morris, and A. Shetty were responsible for the investigation; A. Dhaygude, M.E. Elsayed, L. Floyd, A.D. Morris, and A. Shetty were responsible for the methodology; A. Dhaygude, L. Floyd, and A.D. Morris were responsible for the project administration; A. Shetty was responsible for the resources and software; A. Dhaygude, D. Geetha, and S. Mitra provided supervision; M.E. Elsayed, L. Floyd, and A. Shetty were responsible for the validation; A. Dhaygude, L. Floyd, and A.D. Morris were responsible for the visualization; A. Dhaygude, M.E. Elsayed, L. Floyd, D. Geetha, A.D. Morris, and A. Shetty wrote the original draft; A. Baksi, A. Dhaygude, M.E. Elsayed, L. Floyd, D. Geetha, S. Mitra, A.D. Morris, and A. Shetty reviewed and edited the manuscript; and all authors approved the final version of the manuscript.
Data Sharing Statement
All data are included in the manuscript and/or supporting information.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/KN9/A313.
Supplemental Table 1. Risk of bias of included studies on the Newcastle–Ottawa scale.
Supplemental Table 2. Systematic review of publications comparing clinical presentation, histopathologic findings, and outcomes in patients who were ANCA negative versus positive.
Supplemental Table 3. When applying sensitivity analyses with restricted follow-up to 1 and 2 years from the time of diagnosis, multivariate models show comparable hazard ratios when adjusting for ANCA status, sex, eGFR at diagnosis, age, and induction therapy.
Supplemental Table 4. Meta-analysis results reporting the unadjusted relative risk ratio and moderate to large degree of heterogeneity between studies.
References
- 1.Jennette JC, Falk RJ, Gasim AH: Pathogenesis of antineutrophil cytoplasmic autoantibody vasculitis. Curr Opin Nephrol Hypertens 20: 263–270, 2011. 10.1097/MNH.0b013e3283456731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falk RJ, Terrell RS, Charles LA, Jennette JC: Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci U S A 87: 4115–4119, 1990. 10.1073/pnas.87.11.4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris AD, Rowbottom AW, Martin FL, Woywodt A, Dhaygude AP: Biomarkers in ANCA-associated vasculitis: Potential pitfalls and future prospects. Kidney360 2: 586–597, 2021. 10.34067/KID.0006432020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedger N, Stevens J, Drey N, Walker S, Roderick P: Incidence and outcome of pauci-immune rapidly progressive glomerulonephritis in Wessex, UK: A 10-year retrospective study. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc 15: 1593–1599, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Chen M, Yu F, Wang S-X, Zou W-Z, Zhao M-H, Wang H-Y: Antineutrophil cytoplasmic autoantibody-negative Pauci-immune crescentic glomerulonephritis. J Am Soc Nephrol 18: 599–605, 2007. 10.1681/ASN.2006091021 [DOI] [PubMed] [Google Scholar]
- 6.Hung P-H, Chiu Y-L, Lin W-C, Chiang W-C, Chen Y-M, Lin S-L, Wu K-D, Tsai T-J: Poor renal outcome of antineutrophil cytoplasmic antibody negative Pauci-immune glomerulonephritis in Taiwanese. J Formos Med Assoc 105: 804–812, 2006. 10.1016/S0929-6646(09)60267-9 [DOI] [PubMed] [Google Scholar]
- 7.Lee SW, Yu M-Y, Baek SH, Ahn S-Y, Kim S, Na KY, Chae D-W, Chin HJ: Long-term prognosis of anti-neutrophil cytoplasmic antibody-negative renal vasculitis: Cohort study in Korea. J Korean Med Sci 31: 542–546, 2016. 10.3346/jkms.2016.31.4.542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Córdova-Sánchez BM, Mejía-Vilet JM, Morales-Buenrostro LE, Loyola-Rodríguez G, Uribe-Uribe NO, Correa-Rotter R: Clinical presentation and outcome prediction of clinical, serological, and histopathological classification schemes in ANCA-associated vasculitis with renal involvement. Clin Rheumatol 35: 1805–1816, 2016. 10.1007/s10067-016-3195-z [DOI] [PubMed] [Google Scholar]
- 9.Weiner M, Bjørneklett R, Hrušková Z, Mackinnon B, Poulton CJ, Sindelar L, Mohammad AJ, Eriksson P, Gesualdo L, Geetha D, Crnogorac M, Jayne D, Hogan SL, Geddes C, Tesar V, Aasarød K, Segelmark M: Proteinase-3 and myeloperoxidase serotype in relation to demographic factors and geographic distribution in anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. Nephrol Dial Transplant 34: 301–308, 2019. 10.1093/ndt/gfy106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jennette JC: Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol 17: 603–606, 2013. 10.1007/s10157-013-0869-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS Stevens LA Schmid CH Zhang YL Castro AF 3rd Feldman HI Kusek JW Eggers P Van Lente F Greene T Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas M, Eustace JA: Immune complex deposits in ANCA-associated crescentic glomerulonephritis: a study of 126 cases. Kidney Int 65: 2145–2152, 2004. 10.1111/j.1523-1755.2004.00632.x [DOI] [PubMed] [Google Scholar]
- 13.Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, Neumann I, Noël L-H, Pusey CD, Waldherr R, Bruijn JA, Bajema IM: Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 21: 1628–1636, 2010. 10.1681/ASN.2010050477 [DOI] [PubMed] [Google Scholar]
- 14.Jayne D: Update on the European Vasculitis Study Group trials. Curr Opin Rheumatol 13: 48–55, 2001. 10.1097/00002281-200101000-00008 [DOI] [PubMed] [Google Scholar]
- 15.Brix SR, Noriega M, Tennstedt P, Vettorazzi E, Busch M, Nitschke M, Jabs WJ, Özcan F, Wendt R, Hausberg M, Sellin L, Panzer U, Huber TB, Waldherr R, Hopfer H, Stahl RAK, Wiech T: Development and validation of a renal risk score in ANCA-associated glomerulonephritis. Kidney Int 94: 1177–1188, 2018. 10.1016/j.kint.2018.07.020 [DOI] [PubMed] [Google Scholar]
- 16.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A: A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J 173: 489–495, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah S, Havill J, Rahman MH, Geetha D: A historical study of American patients with anti-neutrophil cytoplasmic antibody negative pauci-immune glomerulonephritis. Clin Rheumatol 35: 953–960, 2016. 10.1007/s10067-015-3086-8 [DOI] [PubMed] [Google Scholar]
- 18.Eisenberger U, Fakhouri F, Vanhille P, Beaufils H, Mahr A, Guillevin L, Lesavre P, Noël L-H: ANCA-negative pauci-immune renal vasculitis: Histology and outcome. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc–Eur Ren Assoc 20: 1392–1399, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Shah S, Hruskova Z, Segelmark M, Morgan MD, Hogan J, Lee SK, Dale J, Harper L, Tesar V, Jayne DRW, Geetha D: Treatment of severe renal disease in ANCA positive and negative small vessel vasculitis with rituximab. Am J Nephrol 41: 296–301, 2015. 10.1159/000431336 [DOI] [PubMed] [Google Scholar]
- 20.Villacorta J, Diaz-Crespo F, Acevedo M, Guerrero C, Mollejo M, Fernandez-Juarez G: Antineutrophil cytoplasmic antibody negative pauci-immune extracapillary glomerulonephritis. Nephrology (Carlton) 21: 301–307, 2016. 10.1111/nep.12608 [DOI] [PubMed] [Google Scholar]
- 21.Rowaiye OO, Kusztal M, Klinger M: The kidneys and ANCA-associated vasculitis: from pathogenesis to diagnosis. Clin Kidney J 8: 343–350, 2015. 10.1093/ckj/sfv020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tateyama K, Kodama S, Kishibe K, Harabuchi Y, Suzuki M: A novel strategy with combined assays for detection of anti-neutrophil cytoplasmic antibody (ANCA) in clinically ANCA-negative granulomatosis with polyangiitis patients. Auris Nasus Larynx 44: 735–741, 2017. 10.1016/j.anl.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 23.Kain R, Exner M, Brandes R, Ziebermayr R, Cunningham D, Alderson CA, Davidovits A, Raab I, Jahn R, Ashour O, Spitzauer S, Sunder-Plassmann G, Fukuda M, Klemm P, Rees AJ, Kerjaschki D: Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med 14: 1088–1096, 2008. 10.1038/nm.1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berden AE, Nolan SL, Morris HL, Bertina RM, Erasmus DD, Hagen EC, Hayes DP, van Tilburg NH, Bruijn JA, Savage COS, Bajema IM, Hewins P: Anti-plasminogen antibodies compromise fibrinolysis and associate with renal histology in ANCA-associated vasculitis. J Am Soc Nephrol 21: 2169–2179, 2010. 10.1681/ASN.2010030274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelley JM, Monach PA, Ji C, Zhou Y, Wu J, Tanaka S, Mahr AD, Johnson S, McAlear C, Cuthbertson D, Carette S, Davis JCJ, Jr, Dellaripa PF, Hoffman GS, Khalidi N, Langford CA, Seo P, St Clair EW, Specks U, Stone JH, Spiera RF, Ytterberg SR, Merkel PA, Edberg JC, Kimberly RP: IgA and IgG antineutrophil cytoplasmic antibody engagement of Fc receptor genetic variants influences granulomatosis with polyangiitis. Proc Natl Acad Sci U S A 108: 20736–20741, 2011. 10.1073/pnas.1109227109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jankowski J, Floege J, Fliser D, Böhm M, Marx N: Cardiovascular disease in chronic kidney disease: Pathophysiological insights and therapeutic options. Circulation 143: 1157–1172, 2021. 10.1161/CIRCULATIONAHA.120.050686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Floyd L, Morris AD, Woywodt A, Dhaygude A: Cardiovascular disease and ANCA-associated vasculitis: Are we missing a beat? Clin Kidney J [Internet], 2022. Available at: 10.1093/ckj/sfac009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas R, Kanso A, Sedor JR: Chronic kidney disease and its complications. Prim Care 35: 329–344, 2008. 10.1016/j.pop.2008.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gapud EJ, Manno R, Seo P, Hanouneh M, Geetha D: Long-term clinical course of antineutrophil cytoplasmic antibody-associated vasculitis patients off maintenance therapy. Cureus 10: e2372, 2018. 10.7759/cureus.2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronsin C, Georges M, Chapelet-Debout A, Augusto J-F, Audard V, Lebourg L, Rubin S, Quemeneur T, Bataille P, Karras A, Daugas E, Titeca-Beauport D, Boffa J-J, Vigneau C, Halimi J-M, Isnard-Bagnis C, Durault S, Renaudineau E, Bridoux F, Testa A, Le Quintrec M, Renaudin K, Fakhouri F: ANCA-negative pauci-immune necrotizing glomerulonephritis: A case series and a new clinical classification. Am J Kidney Dis 79: 56–68.e1, 2022. 10.1053/j.ajkd.2021.03.027 [DOI] [PubMed] [Google Scholar]
- 31.Karras A Pagnoux C Haubitz M Groot K Puechal X Tervaert JWC Segelmark M Guillevin L Jayne D; European Vasculitis Society : Randomised controlled trial of prolonged treatment in the remission phase of ANCA-associated vasculitis. Ann Rheum Dis 76: 1662–1668, 2017. 10.1136/annrheumdis-2017-211123 [DOI] [PubMed] [Google Scholar]
- 32.Terrier B, Pagnoux C, Karras A, Khouatra C, Aumaitre O, Cohen P, Maurier F, Decaux O, Demurs-Clavel H, Gobert P, Quemeneur T, Godmer P, Puechal X, Mouthon L, Guillevin L: OP0213 rituximab versus azathioprine for maintenance in antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis (MAINRITSAN): Follow up at 34 months. Ann Rheum Dis 72: 778–779, 2013 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript and/or supporting information.