Abstract
Objectives
Periodontal disease and diabetes have an extensively investigated bidirectional correlation. Non-surgical periodontal treatment (NSPT) was proven to contribute to glycemic control. Moreover, it may benefit from the association of adjunctive therapies. The aim of the present systematic review is to assess the clinical efficacy of NSPT in association with laser (LT) or photodynamic therapy (PDT) in controlled or uncontrolled diabetic patients, and to grade the level of evidence.
Materials and methods
Randomized controlled clinical trials with at least 3-month follow-up were searched in MEDLINE via OVID, EMBASE, and Cochrane Central, screened for inclusion, and grouped based on the performed treatments, follow-up time, type of diabetes, and level of glycemic control.
Results
Eleven RCTs with 504 total subjects were included. The adjunct of PDT showed a statistically significant 6-month difference in PD changes (with low certainty of evidence), but not in CAL changes, while a significant difference in 3-month PD and CAL changes was found with the adjunct of LT (low certainty of evidence). Patients treated with PDT registered a higher decrease in HbA1c levels at 3 months, but no significant difference was noted at 6 months; LT also led to better HbA1c changes at 3 months with a moderate certainty of evidence.
Conclusion
Despite the promising short-term HbA1c decrease, the results should be interpreted with caution due to the small effect sizes and the statistical heterogeneity, and further evidence from well-designed RCTs is needed to support the routine use of PDT or LT in adjunct to NSPT.
Keywords: Antimicrobial photodynamic therapy, Diabetes mellitus, Laser, Non-surgical periodontal treatment, Periodontitis, Systematic review
Introduction
The bidirectional relationship between hyperglycemia (all types of diabetes) and periodontitis is well-known and widely documented in the scientific literature [1]. Several recent studies confirmed that diabetes represents a significant independent risk factor, it influences oral health in general, and it is a known cause of increased tooth loss rate [2–4]. Indeed, diabetes is considered one of the major risk factors for periodontal diseases, being the risk of having periodontitis in subjects with diabetes approximately three-fold higher than in healthy subjects [5].
Several mechanisms were pointed out to explain the linkage between diabetes mellitus and periodontitis. In general, diabetes can trigger an increase of the inflammatory response towards the oral microbiota (e.g., augmenting IL-1, IL-6, TNF-α) and can impair the immune host response, thus creating favorable conditions for the development and worsening of periodontal diseases in predisposed subjects [6, 7].
At the same time, periodontitis is responsible of increasing insulin resistance and may enhance the risk for diabetes or promote an impairment of glucose tolerance mechanisms. Based on the existing literature, there is evidence that periodontitis could be associated with an increased incidence of diabetes in specific cohorts of systemically compromised patients [8], as well as in the general population, since people with normal glycemic control and periodontitis are more prone to develop diabetes than periodontally healthy subjects [9]. Moreover, periodontitis represents an independent risk factor for microvascular complications in diabetic subjects, such as nephropathy, neuropathy, and retinopathy [10]. The biological plausibility of a correlation between periodontitis and diabetes finds a substantial support considering the low-grade inflammatory systemic status that is induced by periodontitis itself, which could be the basis of an increased susceptibility to diabetes in particularly predisposed subjects [11, 12]. Furthermore, periodontitis-induced systemic inflammation could also contribute to hematopoiesis by increasing the production of myeloid cells that are more responsive to inflammation, and this process might potentially be at the basis of different comorbidities [13].
Given the bidirectional correlation between diabetes and periodontitis, it was demonstrated that non-surgical periodontal treatment (NSPT) in subjects with periodontitis and diabetes could influence glycemic control [14–16]. A recent Cochrane systematic review, including 35 studies and accounting for a total of 3249 participants, found a reduction of HbA1c of 0.43% at 3–4 months after non-surgical treatment (any type of subgingival instrumentation), thus suggesting that periodontal therapy contributes to glycemic control [15].
Despite NSPT is considered to be generally effective in the treatment of periodontitis, we expect that a certain number of pockets (about 26% at 6/8 months) will not close because of local factors (e.g., depth of initial pocket, anatomy of the tooth and of the defect) and factors related to the patient (e.g., smoking, systemic diseases, compliance with oral hygiene) or operator (ability to successfully remove the deposits and to motivate the patient) [17]. Therefore, adjunctive measures that could enhance the outcomes of NSPT have been proposed [18–22]. Among these adjunctive therapies, the systematic review published by Salvi and coworkers, considered in the recently published S3-level treatment guideline of the European Federation of Periodontology, examined the efficacy of laser (LT) and photodynamic therapy (PDT) [20]. While the authors did not find differences when focusing on systemically healthy periodontitis patients, a specific analysis of the effects of laser or PDT in a particular susceptible group of subjects, such as diabetic patients, considering both periodontal and glycemic outcomes, is still missing. It might be hypothesized that LT and PDT, due to their anti-inflammatory effect and the ability of modulating the inflammatory response in other systemic clinical conditions [23], can be a valuable adjunctive therapy for the treatment of diabetic periodontitis patients. Moreover, the differences in the subgingival population that exist between diabetic and non-diabetic periodontal patients could be a further reason for the need of different/additional approaches for treating the periodontal disease in diabetic patients [24]. Despite some systematic reviews with heterogeneous methodology are available in this field [25, 26], no meta-analysis and critical appraisal of certainty of evidence have been published comparing PDT/LT as an adjunct to NSPT to NSPT alone. Moreover, the previously published studies reported inconclusive results.
There is therefore the need of systemically addressing the evidence about adjunctive periodontal treatments such as PDT and LT in subjects with diabetes, mainly because of the high prevalence of the disease and the need of considering the effect of this systemic disease on treatment outcome in studies designed for this specific purpose.
The present systematic review of the literature aimed to fill this knowledge gap and to assess the efficacy of NSPT performed with the adjunct of LT or PDT in patients with type II diabetes mellitus and to grade the level of available evidence.
Materials and methods
The protocol of the study was registered in PROSPERO database (number CRD42021237742) before study initiation. The protocol followed the instructions provided by the Cochrane Handbook for Systematic Review of Interventions – Second Edition [27].
The aim of this review was to answer the following focused question: in periodontitis patients affected by type II diabetes mellitus, what is the efficacy of PDT and LT as an adjunct to non-surgical periodontal therapy in terms of pocket closure, probing pocket depth (PPD) reduction, and clinical attachment level (CAL) gain?
Eligibility criteria
The criteria for considering studies for this review based on the PICOS are:
Population (P): ≥ 18 years old, previously untreated periodontitis patients (defined following the current and past classifications [28, 29] as stage II, stage III, or stage IV periodontitis (any grade) or moderate to severe periodontitis) affected by controlled or uncontrolled type II diabetes (T2DM) (code 5A11 following the International Classification of Diseases of the World Health Organization [30]), defined as presence of insulin resistance [31].
Intervention (I): (a) Physical treatment (e.g., LT, PDT) as an adjunct to non-surgical treatment (sub-gingival instrumentation) of periodontitis.
Control (C): The same non-surgical treatment of periodontitis associated with placebo or without adjunctive therapy, or performed according to a different protocol.
-
Outcomes (O):
Primary outcomes:- Proportion or number of pockets closed (defined as PPD < 5 mm and no bleeding on probing (BOP)); reduction in PPD, which is defined as the distance from the gingival margin to the base of the pocket as assessed with a standardized (UNC-15) periodontal probe with a force of 0.2/0.25N; changes in CAL, which is the measurement of the position of the soft tissue in relation to cemento-enamel junction (CEJ).
Secondary outcomes:- Site-specific response to subgingival instrumentation (in horizontal defects, intrabony defects and furcations)
- Changes in HbA1c levels
- Changes in BOP or gingival inflammation and in plaque levels
- Number of teeth lost or extracted during the examination period
- Patient-reported outcome measures (PROMs), including adverse events
Studies (S): Randomized controlled clinical trials with at least 3-month follow-up. Split-mouth studies were excluded due to the risk of carry-over effects
Search and study selection
The electronic search for pertinent articles was performed searching the following databases: MEDLINE via OVID, EMBASE, and Cochrane Central and by using the search strategy presented in Appendix 1. Grey literature was searched for pertinent articles interrogating Greylit and OpenGrey. Trials registers (ClinicalTrials.gov and EU Clinical Trials Register) were also searched through keywords. A manual search was performed for all the issues published since 1990 of the following journals: Journal of Clinical Periodontology, Journal of Periodontology, Journal of Periodontal Research, Journal of Dentistry, and Journal of Dental Research. Besides checking the reference list of all included papers, Scopus was consulted to check the articles citing the papers included. No language limitations were posed. Conference papers and abstracts were excluded.
The last electronic search was performed in all databases on 10 February 2022.
Two reviewers (SC, EC) independently screened titles and abstract for preliminary check of inclusion criteria (1st stage). The second stage of articles selection was performed by the same reviewers, by carefully screening the full texts of the papers retrieved after preliminary check. In case of disagreement, a third reviewer (ND) was interrogated to solve the dispute. Reasons for exclusion in the second step were recorded, and the level of concordance in each step of the selection process was assessed through Cohen’s kappa.
Data extraction
The process of data extraction was performed independently by two authors (AA, PE) who retrieved the following information from the included studies: authors’ names, year of publication, country, characteristics of the sample (age distribution, sex distribution, ethnicity, educational status, smoking status), characteristics of diabetes (definition and type, level of control of the disease, HbA1c levels, drugs), definition/assessment of periodontitis, characteristics of the periodontal treatment and of the adjunctive physical therapy, clinical data before and after the treatment (number of teeth lost, proportion of closed periodontal pockets, mean periodontal probing depth (PD), mean CAL, gingival bleeding indexes (gingival bleeding index, gingival index (GI), percentage of bleeding sites (BOP), plaque indexes (plaque index (PI), Turesky-modified plaque index, proportion of sites with visible plaque) or difference between baseline and follow-up values, occurrence of adverse events or complications, and patients’ reported outcomes (PROMs).
In case of missing/unclear information, an attempt was made to contact the authors by email.
Risk of bias evaluation and quality of evidence assessment
The risk of bias evaluation and the quality of evidence assessment were performed independently by two reviewers (SC, LF) and any disagreement resolved by discussion.
The criteria for evaluating the risk of bias in the included studies were the ones of the Cochrane risk-of-bias tool for randomized trials 2.0 [27]:
Bias arising from the randomization process
Bias due to deviations from intended interventions
Bias due to missing outcome data
Bias in measurement of the outcome
Bias in selection of the reported result
The overall risk-of-bias judgment was considered as high risk if the level of risk of bias was high for at least one domain or if the trial was judged to have some concerns for multiple domains (three). If the trial was judged to have some concerns for less than three domains, the overall risk of bias was “some concerns,” while the study had low risk of bias if all domains were judged to have low risk.
The funding bias was estimated by evaluating if authors disclosed their potential sources of competing conflict of interest and the source of funding for the studies they carried on (if any).
The quality of the available evidence was assessed for each comparison and for each outcome in the meta-analysis by using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach [32]. GRADE provides a system for rating quality of evidence and strength of recommendations that is explicit, comprehensive, transparent, and pragmatic.
Summary measures and synthesis of the results
In order to perform the meta-analysis, studies were grouped based on the treatments that were carried out, follow-up time, and, whenever possible, based on the type of diabetes and on level of control. In particular, we distinguished between photodynamic therapy (PDT) and direct laser application (LT). Meta-analysis was performed by using the software RevMan (Review Manager Version 5.3, 2014; The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark) if at least three papers were available for each comparison.
For each continuous outcome, the difference between baseline and follow-up values was extracted with its specific error measure (standard deviation, standard error, or variance). When difference values were not reported, they were calculated as the difference between baseline and follow-up values and error (namely, standard deviation) was computed following the procedure described in Appendix 2. In the meta-analysis, the effect size was computed through the weighted mean method, and results were combined using the DerSimonian and Laird’s random-effect model [33], assuming heterogeneity among studies. Cochran’s test served to measure the consistency of the results, considering it significant if P < 0.1. I2 statistics was applied to measure heterogeneity (total variation across studies that was due to heterogeneity rather than to chance) [27].
Regression meta-analysis was performed to evaluate the effect of baseline HbA1c% on the primary outcome measures.
Small study effects, as proxy for publication bias, were assessed by testing for funnel plot asymmetry and by calculating Egger’s bias, as described in the Cochrane Handbook [27].
Results
The results of this systematic review are herein presented following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [34].
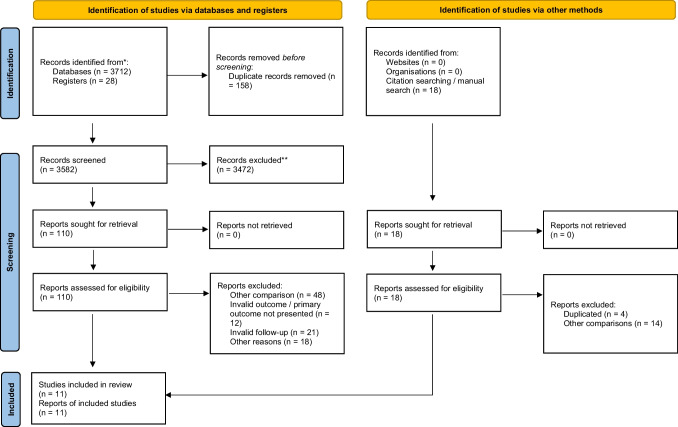
The summary of the article selection process is summarized in Fig. 1. Eleven RCTs were included in the analysis [35–45], which accounted for a total of 504 subjects, examined with a follow-up ranging from 1 to 6 months.
Fig. 1.
PRISMA diagram of article selection process
In particular, seven papers compared NSPT to NSPT and adjunctive PDT in subjects with diabetes [35–37, 40, 42, 43, 45]. In all the studies, in the test groups, non-thermal diode laser was used to irradiate a photosensitizer agent. In one study, NSPT was performed following a “Full-mouth disinfection” protocol in both groups [43].
Four studies compared NSPT to NSPT and adjunctive DL use (with settings varying between 0.8 and 1.8 W) in subjects with diabetes [38, 39, 41, 44]. In all studies, the control groups were treated according to a quadrant-based NSPT protocol. In four studies, the periodontal disease was classified following the 2017 classification [28], including stage II, stage III, and stage IV periodontitis and grade B or C [36, 40, 44, 45]. The other included studies used older classifications and diagnostic parameters [46].
Considering the characteristics of the population, three studies were performed in Saudi Arabia [35, 36, 40], three in Brazil [37, 42, 45], two in India [38, 44], two in Turkey [39, 41], and one in Pakistan [43]. In all studies, only T2DM was considered, with different level of controls defined on the basis of HbA1c: three studies included patients with HbA1c > 7% [39, 42, 45]; one included subjects with HbA1c > 6% [44]; one considered HbA1c ≥ 6.5% [43]; one < 7% [37]; and in one study, subjects with HbA1c between 5.7 and 8.5% were included [41], while other studies adopted different definitions [35, 36, 38, 40]. One study clearly stated that only subjects with decompensated T2DM were included [45], while in four studies, patients with major diabetic complications were excluded [35, 39, 42, 43]. Smokers were excluded in all studies.
Additional details about the characteristics of the studies are shown in Table 1.
Table 1.
Main characteristics of the included studies
| Authors | Study type | Number of subjects; sex (m/f) | Age (mean ± SD (range)) | Systemic conditions/health status | Ethnicity | Periodontal disease | Diabetes | Outcomes | Follow up | Type of probe and n sites/tooth evaluated | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Al-Zahrani et al. 2009 Saudi Arabia |
RCT, single-blind |
43; 17/26 G1: 7/8 G2: 4/10 G3: 6/8 |
52.21 ± 8.35 G1: 53.14 ± 10.91 G2: 51.42 ± 6.24 G3: 51.92 ± 7.28 |
Excluded: atb in the previous 6 mo, pregnancy | NS | CAL loss ≥ 3 mm at ≥ 30% of sites | T2DM, no major diabetic complications | PD, CAL, REC, plaque and bleeding scores, HbA1c | 12 wks | 6 sites per tooth | SRP | SRP + doxycycline 2× 100 mg for day 1 and then 100 mg once a day for 13 days *not considered in the present review | SRP + PDT (670-nm non-thermal diode laser) | - |
|
Koçak et al. 2016 Turkey |
Parallel RCT, single-blind |
60; 30/30 G1 15/15 G2 15/15 |
35–60 G1 53.1 ± 5.1 G2 51.7 ± 5.2 |
Excluded: other systemic diseases, smoking, alcoholism, atb in the previous 6 mo, immunosuppressive medications, pregnancy/lactation | NS | CP, 8 ≤ sites with PD ≥ 5 mm, ≥ 17 remaining teeth | T2DM, no changes in diabetes therapy in the previous 12 mo HbA1c 5.7–8.5% | PD, CAL, GI, PI, HbA1c, GCF levels of IL-1β, IL-6, IL-8, ICAM, VCAM | 1 and 3 mo | PCP-UNC 15 PD/CAL: 6 sites per tooth for, GI/PI: 4 sites per tooth | SRP | SRP + diode laser (940-nm, indium–gallium–aluminum–phosphate diode laser) | - | - |
|
Barbosa et al. 2018 Brazil |
RCT, Pilot study | 12; 4/8 | 52.2 | Excluded: other systemic disease influencing periodontal status, smoking, atb in the previous 3 mo, pregnancy | NS | Moderate to severe periodontitis | T2DM using oral hypoglycemic agents and/or insulin and who had glycated hemoglobin (HbA1c) values below 7% measured no more than 90 days prior to selection were included in the study | PD, CAL, PI, GBP, GSP, HbA1c | 30, 90, 180 d | Williams color-coded probe | SRP+ aPDT (660-nm diode laser) | SRP | - | - |
|
Chandra et al. 2019 India |
RCT, single-blind |
36; 18/18 9/9; 9/9 |
48/50.6 | Excluded: smoking, alcoholism, pregnancy/lactation | NS | Generalized CP, PPD 4–7 mm with CAL ≥ 2 mm, or greater and each quadrant having at least 3 teeth (≥ 3 in each quadrant) | T2DM, non insulin dependent | PD, CAL, PI, GI, microbiological Aa, Pg, HbA1c | 3 mo | UNC-15 | SRP + diode laser (808 nm and a power setting of 1.5–1.8W were used in continuous, contact mode with a thin flexible fiber optic cable (320 nm)) + irrigation with saline | SRP + irrigation with saline | - | - |
|
Dengizek Eltas et al. 2019 Turkey |
RCT, single-blind | 37; 17/20 | 49.7/51.85 | Excluded: other systemic diseases affecting periodontal status, smoking, atb or anti-inflammatory drugs in the previous 6 mo, pregnancy/lactation | NS | Generalized CP, PD 4-7 mm in ≥4 teeth in the upper jaw, ≥ 20 remaining teeth |
T2DM for ≥ 2 yrs, no major diabetes complications HbA1c ≥ 7% |
PD, CAL, PI, GI, HbA1c, CRP | 3 and 6 mo | PCP-12 | SRP + diode laser (810 nm wavelength, 1 W power, contact mode using a 400-μm fiber optic tip) | SRP | - | - |
|
Mirza et al. 2019 Pakistan |
RCT |
30; 20/10 11/4; 9/6 |
51.45/52.93 | Excluded: current/former smokers, atb in the previous 3 mo, pregnancy/lactation | NS | Mild to moderate periodontits, no periodontal treatment in previous 6 mo |
T2DM for ≥ 2 yrs, no major diabetic complications HbA1c ≥ 6.5% |
PD, BOP, PI, AL, HbA1c, Advanced glycation end-products in GCF | 3 and 6 mo |
UNC-15 6 sites per tooth |
FMD+ PDT (670 nm 150 nW fluency of 22 J/cm2 and density of 1.1 W/cm2) | FMD | - | - |
|
Macedo et al. 2013 Brazil |
RCT |
30; 16/29 G2 6/9 G1 5/10 |
48.73 ± 7.11 G2 49.4 ± 6.8 G1 48.1 ± 9 |
excluded: smoking in the previous 5 years, atb in the previous 6 months, pregnancy/lactation | NS | ≥ 1 site with PPD ≥ 5 mm on each quadrant, and ≥ 2 teeth with CAL loss ≥ 6 mm |
T2DM > 5 yrs, no major diabetic complications HbA1c > 7% |
PPD, CAL, PI, BOP, HbA1c, suppuration | 3 mo |
Computerized periodontal probe 6 sites per tooth |
SRP | SRP + aPDT (660-nm diode laser, phenothiazine chloride photosensitizer-induced aPDT) | - | - |
|
Al-Zawawi et al. 2020 Saudi Arabia |
RCT | 128; diabetic subjects 27/6 | diabetic subjects 55.5 | Excluded: other systemic diseases, smoking, tobacco chewing, alcoholism, pregnancy/lactation | NS | Stage II grade C periodontitis according to Consensus report 2017 World Workshop | T2DM | PD, CAL, GI, PI, MBL, cortisol in GCF, HbA1c | 3 and 6 mo |
Click-probe 6 sites per tooth |
(Diabetic patients) SRP + aPDT (diode laser at 660 nm and 150 mW, irradiation was performed for 60 s with a fiber optic tip of 300 μm diameter) | (Diabetic patients) SRP | (Non-diabetic patients) SRP + aPDT (diode laser at 660 nm and 150 mW, irradiation was performed for 60 s with a fiber-optic tip of 300 μm diameter) [not included] | (Non-diabetic patients) SRP [not included] |
|
Elsadek et al. 2020 Saudi Arabia |
RCT |
60; 34/26 11/9; 10/10; 13/7 |
52.16/51.87/52.88 | Excluded: other systemic disease influencing periodontal disease course, current/former smokers, patients on anti-inflammatory/antimicrobials/statin therapy, pregnancy/lactation | NS | Stage III and grade C generalized periodontitis, CAL ≥ 5 mm and radiographic bone loss extending to middle or apical third of root, no previous periodontal therapy | T2DM (ADA 2018) | PD, CAL, REC, BOP, PS, HbA1c | 3 mo |
UNC probe 6 sites per tooth |
SRP + PDT (diode laser670 nm wavelength, 150 mW maximum power, 60 s per site, 20 J/cm2 per site) | SRP + probiotic L. reuteri (2 × 108 CFU/tablet, 2 lozenges/day for 3 wks) | Debridement | - |
|
Soi et al. 2021 India |
RCT | 37; 21/16 | 51.58/51.67 | Excluded: other systemic diseases, smoking, alcoholism, medication other than hypoglycemics, pregnancy/lactation | NS | Stage II or III/grade B or C periodontitis, ≥ 8 sites with CAL loss ≥ 3 mm and PPD ≥ 3 mm, ≥ 20 teeth |
T2DM (FPG ≥ 126 mg/dl, RBS ≥ 200 mg/dl, PP ≥ 200 mg/dl) HbA1c > 6% |
PD, CAL, PI, GI, RBS, FBS, HbA1c | 1, 3, 6 mo |
UNC-15 CAL, PD: 6 sites per tooth, PI, GI: 4 sites per tooth |
SRP + diode laser (0.8 W, pulse interval 1.0 ms, pulse length 1.0 ms, 24 J) | SRP | - | - |
| Claudio et al. 2021 | RCT, double-blind (surgeon and examiner) | 34, 31 examined (22/9) |
G1: 53.13 ± 7.58 G2: 54 ± 8.56 |
Age 30–70; excluded: medical disorders that required antibiotic prophylaxis, antibiotics, anti-inflammatories, anticonvulsants, immunosuppressants or calcium channel blockers in the last 6 mo, smokers in the last 12 mo, pregnancy | NS | Periodontitis stages III and IV, grade C with at least 6 sites with PD and CAL ≥ 5 mm and BOP in at least 15 teeth, excluding third molars; no SRP in the last 6 mo | decompensated DM2: HbA1c ≥ 7.0% | PD, CAL, REC, PI, BOP, number of PD ≥ 5 mm, P. gingivalis and P. intermedia quantification | 3 mo, 6 mo | PCPUNC-15, Hu-Friedy, six sites of each tooth | SRP | SRP + aPDT (immediately after SRP, 48 and 96 h after in pockets with PD ≥ 5 mm) | - | - |
Abbreviations:ADA American Diabetes Association, atb antibiotics, BOP bleeding on probing, CAL clinical attachment level, CFU colony-forming units, CP chronic periodontitis, CRP C-reactive protein, d days, FPG fasting plasma glucose, FBS fasting blood sugar, GCF gingival crevicular fluid, GSP glycated serum proteins, GBP glycated blood proteins, Hb1Ac glycated hemoglobin 1Ac, mo months, NS not specified, PP 2-h post-prandial glucose, PPD probing pocket depth, RBS random blood sugar, REC recession, wks weeks, yrs years, SRP scaling and root 22planing, OHI oral hygiene instructions, PDT p22hotodynamic therapy, FMD full-mouth disinfection, UNC University of North Carolina, T2DM type 2 diabetes mellitus, Aa Aggregatibacter actynomycetemcomitans, Pg Porphyromonas gingivalis
Risk of bias evaluation
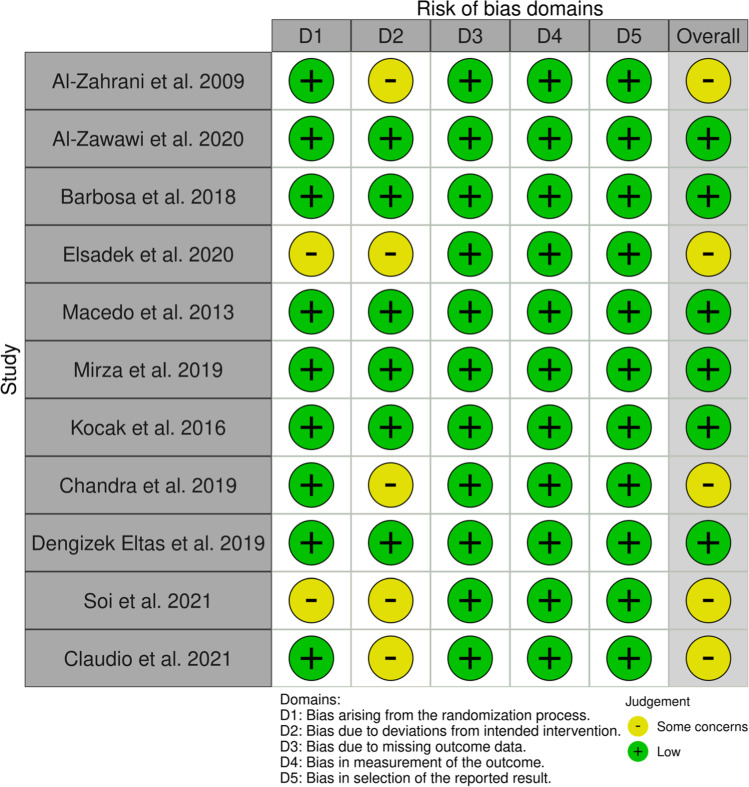
The results of risk of bias evaluation are reported in Table 2. Five studies raised some concerns about the risk of bias due to the methods of randomization and to the blinding of subjects [35, 38, 40, 44, 45], while six studies were at low risk [36, 37, 39, 41–43] (Fig. 2).
Table 2.
Results of risk of bias evaluation
| Study | Randomization process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported result | Overall bias |
|---|---|---|---|---|---|---|
| Al-Zahrani et al. 2009 | Low | Some concerns | Low | Low | Low | Some concerns |
| Al-Zawawi et al. | Low | Low | Low | Low | Low | Low |
| Barbosa et al. 2018 | Low | Low | Low | Low | Low | Low |
| Elsadek et al. 2020 | Some concerns | Some concerns | Low | Low | Low | Some concerns |
| Macedo et al. 2013 | Low | Low | Low | Low | Low | Low |
| Mirza et al. 2019 | Low | Low | Low | Low | Low | Low |
| Kocak et al. 2016 | Low | Low | Low | Low | Low | Low |
| Chandra et al. 2019 | Low | Some concerns | Low | Low | Low | Some concerns |
| Dengizek Eltas et al. 2019 | Low | Low | Low | Low | Low | Low |
| Soi et al. 2021 | Some concerns | Some concerns | Low | Low | Low | Some concerns |
| Claudio et al. 2021 | Low | Some concerns | Low | Low | Low | Some concerns |
Fig. 2.
Diagram showing the results of risk of bias evaluation
Synthesis of the results
Pocket closure, PD changes, CAL changes
NSPT versus NSPT and photodynamic therapy (PDT)
Meta-analysis based on 4 studies indicated a statistically significant difference in PD changes (favoring the test group) and CAL changes favoring control group 6 months after treatment with a low effect size (PD change: 0.26 mm, CI95%: 0.01, 0.50, I2: 57%, 137 subjects; CAL change: − 0.2 mm, CI95%: − 0.23, − 0.17, I2: 0%, 137 subjects) (Table 3).
Table 3.
Results of the meta-analysis (a positive effect size value means an advantage in test group)
| 3 months | 6 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean [95% CI] (n° of studies) | P | I2 | Certainty of evidence (GRADE) | Mean [95% CI] (n° of studies) | P | I2 | Certainty of evidence (GRADE) | ||
| NSPT (with or without placebo) vs NSPT + Photodynamic therapy | PD red | 0.04 [− 0.13, 0.22] (6) | 0.63 | 93% | Very low | 0.26 [0.01, 0.50] (4) | 0.04 | 57% | Low |
| CAL gain | 0.03 [0.00, 0.06] (7) | 0.08 | 0% | Low | − 0.20 [− 0.23, − 0.17] (4) | < 0.001 | 0% | Moderate | |
| BOP% red | − 5.95 [− 9.92, − 1.98] (5) | 0.003 | 0% | Moderate | 0.36 [− 9.53, 10.25] (3) | 0.94 | 0% | Low | |
| PI red | 0.09 [− 0.08, 0.26] (3) | 0.32 | 79% | Very low | |||||
| PI% red | 0.23 [− 5.48, 5.95] (5) | 0.94 | 63% | Very low | 1.64 [− 3.78, 7.06] (3) | 0.55 | 0% | Very low | |
| HbA1c red | 0.24 [0.17, 0.32] (6) | < 0.001 | 14% | Moderate | − 0.04 [− 0.17, 0.10] (4) | 0.62 | 8% | Low | |
| NSPT (with or without placebo) vs NSPT + diode laser | PD red | 0.59 [0.41, 0.76] (4) | < 0.001 | 80% | Low | ||||
| CAL gain | 0.84 [0.09, 1.59] (3) | 0.03 | 86% | Low | |||||
| GI red | 0.34 [0.21, 0.47] (3) | < 0.001 | 0% | Moderate | |||||
| PI red | 0.21 [− 0.01, 0.43] (3) | 0.06 | 40% | Very low | |||||
| HbA1c red | 0.18 [0.07, 0.28] (4) | < 0.001 | 0% | Moderate | |||||
In bold the effect sizes that resulted statistically significant
Three studies reported data about the changes in pockets ≥ 5mm, but they could not be pooled in a meta-analysis because one study reported the mean number of pockets per patient [45] and the others presented the proportions [35, 40]. More specifically, at 3 months, Al-Zahrani and colleagues found a non-significant decrease in the proportion of sites with PD ≥ 5 mm from 11% ± 8% to 6% ± 7% in the test group and from 14% ± 14% to 8% ± 13% in the control group [35]. Likewise, Elsadek et al. [40] indicated a non-significant significant decrease after 3 months in the proportion of sites with PD ≥ 5 mm in both groups (from 12% ± 7% to 4% ± 6% in the test group and from 15% ± 15% to 9% ± 12% in control group). A more recent study reported a decrease in the number of pockets that was significant in both groups after 3 and 6 months from the treatment without a significant intergroup difference [45].
NSPT versus NSPT + diode laser (DL)
Meta-analysis was performed for PD and CAL change at 3 months post-treatment, and it involved 4 and 3 studies, respectively. As reported in Table 3, a significant difference in 3-month PD and CAL change is found when DL was applied as an adjunctive therapy (PD change: 0.59 mm, CI95%: 0.41 mm, 0.76 mm, I2: 80%, 170 subjects; CAL change: 0.84 mm, CI95%: 0.09 mm; 1.59 mm, I2: 86%, 112 subjects).
None of the studies reported data on pocket closure. Regression meta-analysis did not reveal any significant effect of baseline HbA1c% on the examined outcomes.
Secondary outcomes
NSPT versus NSPT and photodynamic therapy (PDT)
Based on 5 studies, meta-analysis indicated a statistically significant difference in 3-month BoP change between test and control groups (− 5.95% [− 9.92%, − 1.98%]), favoring the latter. However, at 6 months, this difference was no longer significant.
Remarkably, HbA1c decreased significantly more in the test groups than in control groups 3 months after the treatment (0.24, CI95%: 0.17, 0.32), but this outcome was not confirmed at 6 months.
No significant differences were suggested for PI changes (Table 3).
No patient-reported outcomes were reported.
NSPT versus NSPT + diode laser (DL)
The quantitative synthesis based on the data from 3 studies indicated a significant difference, in 3-month GI changes (0.34, CI95%: 0.21, 0.47) favoring the test groups (Table 3). Likewise, the adjunctive use of DL led to better HbA1c changes (0.18, CI95%: 0.07, 0.28) at 3 months (Table 3).
No patient-reported outcomes were reported.
Certainty of evidence
The results of the evaluation of the certainty of evidence are summarized in Table 3. Regarding the primary outcomes, in the comparison between NSPT and NSPT + PDT, the certainty was moderate for CAL reduction (6 months) and low for PD changes, while for the comparison between NSPT and NSPT + DL, the certainty of evidence was low. Regarding the secondary outcomes, the adjunctive use of PDT was associated with a moderate certainty of evidence in terms of BoP% reduction (3 months) and HbA1c reduction (3 months), while the adjunctive use of DL was associated with a moderate certainty of evidence both for GI reduction (3 months) and for HbA1c reduction (3 months).
Discussion
The results of the present systematic review and meta-analysis demonstrated a small but significant positive effect of the application of PDT as an adjunct to NSPT in type II diabetic patients regarding PD changes (6 months) and HbA1c changes (3 months) compared to control groups (NSPT only), the latter reporting more favorable CAL changes (6 months). Moreover, LT with DL as an adjunct to NSPT resulted in an enhanced effect for PD, CAL, GI, and HbA1c reductions at 3 months. However, these results need to be interpreted with caution due to the small effect sizes and the relatively high statistical heterogeneity.
Different from several other published systematic reviews that addressed mainly glycemic control [15, 16, 47, 48], the main focus of this systematic review was on post-treatment periodontal. As a matter of fact, our primary outcomes included the percentage of closed pockets, PD reduction, and CAL gain. Therefore, the results of the present research should be interpreted in the light of the existing literature that examined the same outcomes.
PDT was described as an effective antimicrobial strategy towards periodontal pathogens, and its activity depends on the creation of components that are noxious for the microorganisms (such as free radicals) following the activation, by the laser light, of the photosensitive component [49, 50]. Several laser types and applications were described as an adjunct for the treatment of periodontal diseases [51]. The rational of using laser for the treatment of periodontal pockets relates to the decontamination ability of the affected sites, particularly in situation of difficult access [52]. Moreover, laser application could result in accelerated healing and homeostasis, thus potentially improving the treatment outcomes [52].
Regarding the available evidence on the use of LT or PDT as an adjunct to NSPT, the systematic review published by Salvi and coworkers in 2020, using strict inclusion criteria, evaluated a total of 18 papers, of which only 2 could be included in the quantitative synthesis [20]. Their meta-analysis revealed a non-significant beneficial effect of PDT as an adjunct to NSPT in terms of PD changes [20]. Another systematic review about the application of LT for the management of untreated periodontitis and that performed meta-analysis on five papers did not suggest any significant effect on CAL or PD changes as well as PROMS over time [53]. Other recently published papers have provided further data on the topic without solving the controversy, as both favorable results [54, 55] and clinically insignificant benefits [56] were suggested. The results of our meta-analysis, although showing a significant effect in some comparisons of PDT/LT + NSPT, failed to clearly demonstrate a clinically relevant beneficial effect, being coherent with the previously cited studies.
While all the aforementioned systematic reviews focused on systemically healthy subjects, Abduljabbar and coworkers aimed at exploring the role of lasers as adjunct to NSPT in subjects with diabetes [25]. The authors adopted different inclusion criteria than those considered in the present study, and included six articles in the final qualitative synthesis, three about LT and three about PDT, without presenting conclusive results [25]. Another review of the same group on PDT included four RCTs and concluded that no difference between test and control group could be observed in terms of clinical parameters [26]. Compared to the works by Abduljabbar et al., our research included a higher number of recent papers by using different inclusion criteria, thus presenting updated data on the topic. Moreover, we performed a risk of bias evaluation with standardized methods, and we included in the meta-analysis more outcome variables. Additionally, the present research included the evaluation of the quality of evidence, which should be considered a crucial aspect for weighting the validity of the results.
Another important aspect to consider when dealing with diabetic patients is the effect that periodontal treatment might have on glycemic control. A recent Cochrane systematic review on the improvements in glycemic control (measured by the HbA1c changes) in subjects treated with NSPT compared to controls indicated a decrease of 0.43% (CI95%: 0.28–0.59) of HbA1c in test group at 3–4 months, with positive results also in longer follow-ups [15]. Although our main aim was not to assess changes in diabetes control, our meta-analysis suggested an adjunctive effect of PDT on HbA1c after 3 months of 0.24% (CI95%: 0.17–0.32), which was not confirmed after 6 months. Remarkably, studies on the efficacy of other adjunctive treatments to NSPT in subjects with diabetes, such as systemic antibiotics, found no significant additional effects in terms of glycaemic control [57, 58]. The regression meta-analysis performed in the present review failed to reveal a significant effect of baseline HbA1c% on PD changes and CAL changes. However, it should be noted that the relatively low number of papers available for each outcome and for each comparison may have limited the reliability of such analysis. Nevertheless, the risk of bias evaluation revealed a substantially moderate quality of the included studies, being six studies at low risk of bias. We can therefore reasonably assume that the results of the meta-analysis and the quality appraisal of the evidence were not affected by bias.
It is worth to acknowledge that the present systematic review had few shortcomings, as this might help to better consider the validity of the results and to interpret its findings. First, we should highlight that a substantial heterogeneity existed among the included study protocols regarding the characteristics of diabetes and the level of glycaemic control, the ethnicity of the population, the settings of the laser device, and the characteristic of periodontitis (namely severity), and this was probably the main cause of the statistical heterogeneity in the meta-analysis. Moreover, very limited data were available about the proportion of pocket closure, which is considered the most reliable outcome when evaluating the results of NSPT [59]. The lack of data about this outcome is a limiting factor, although PD and CAL changes are surrogate outcomes widely accepted and reported in the literature [60].
On the other hand, one strength of the present review is that to the best of our knowledge, this is the first systematic review on periodontitis and diabetes that also assessed the certainty of evidence for all the comparisons and outcomes included in the meta-analysis based on GRADE. The GRADE is a well-recognized tool for weighting the level of evidence of assumptions derived from a study, ideally a systematic review, in order to provide also clinical recommendations [32]. The GRADE is now fully integrated in Cochrane systematic reviews [27]; however, it is not frequently adopted in systematic reviews in the field of dentistry. In the authors’ opinion, considering the level of evidence and combining it with the statistical significance and the effect size can better inform on a clinically relevant topic such as the efficacy of PDT/LT. This comprehensive approach should be implemented whenever recommendations or clinically oriented guidelines are produced.
Finally, while it was not within the remit of this review to assess the cost-effectiveness of LT and PDT, the extra costs associated with the purchase and use of these physical therapies should be taken into account when considering whether or not to implement them in clinical practice and future studies are warranted to investigate the cost-effectiveness of these therapies.
In conclusion, taking all the aforementioned limitations into consideration, our review suggested that there is currently insufficient scientific evidence (and limited clinical relevance) to suggest the routine use of PDT or LT as an adjunct to NSPT in subjects with type II diabetes, although the promising results in terms of HbA1c decrease in the short term should be further explored in well-designed RCTs with > 6-month follow-up. It is recommended that future studies should consider the percentage of pocket closure as a primary outcome and explore the role of patient-reported outcome measures. It is also important that future studies will apply standard definitions of diabetes.
Appendix 1. Search strategy
Table 4.
MEDLINE via OVID
| MeSH term | Free-Text search | |
| Population |
Exp Periodontal Diseases OR alveolar bone loss/ AND Exp diabetes mellitus, Type 2 OR exp hyperglycemia |
Periodontit* OR Parodontos$s OR periodontal disease OR pyorrhea OR Pericementit* OR gum disease OR (Periodont* ADJ2 pocket*) OR (Periodont* ADJ2 defect*) OR (Periodont* ADJ2 atroph*) OR (periodontal attachment ADJ2 loss) OR (periodontal bone ADJ2 loss*) OR (periodontal ADJ2 resorption*) OR furcation OR (alveolar bone ADJ2 loss) AND diabet* or DM2 OR NIDDM or hyperglyc* |
The limitation to human studies was performed following the double negation strategy suggested by the Cochrane handbook, i.e. combining the results with NOT (exp animals/ not humans.sh.).
In addition, the following filters were applied:
• Filter to exclude systematic reviews:
NOT (((systematic OR state-of-the-art OR scoping OR literature OR umbrella) ADJ (review* OR overview* OR assessment*)) OR "review* of reviews" OR meta-analy* OR metaanaly* OR ((systematic OR evidence) ADJ1 assess*) OR "research evidence" OR metasynthe* OR meta-synthe*).tw. OR systematic review/ OR "systematic review (topic)"/ OR meta analysis/ OR "meta analysis (topic)"/
• Filter to exclude case reports and other non-relevant publications:
NOT (letter/ OR editorial/ OR news/ OR exp historical article/ OR anecdotes as topic/) OR (letter OR comment*.ti) ORcase reports/
• Filter to exclude guidelines:
(clinical adj3 pathway).ti,ab,kw. or (clinical adj3 pathways).ti,ab,kw. or (practice adj3 parameter).ti,ab,kw. or (practice adj3 parameters).ti,ab,kw. or algorithms/ or care pathway.ti,ab,kw. or care pathways.ti,ab,kw. or clinical protocols/ or Consensus/ or Consensus Development Conference.pt. or Consensus Development Conference, NIH.pt. or Consensus Development Conferences as Topic/ or Consensus Development Conferences, NIH as Topic/or critical pathway/ or guidance.ti,ab. or guideline*.ti. or guidelines as topic/ or practice guidelines as topic/ or Health Planning Guidelines/ or practice guideline/
Table 5.
Cochrane library via CENTER
| MeSH term | Free-Text search | |
| Population |
Exp Periodontal Diseases OR exp alveolar bone loss AND Exp diabetes mellitus, Type 2 OR exp hyperglycemia |
Periodontit* OR Parodontos$s OR periodontal disease OR pyorrhea OR Pericementit* OR gum disease OR (Periodont* NEAR/2 pocket*) OR (Periodont* NEAR/2 defect*) OR (Periodont* NEAR/2 atroph*) OR (periodontal attachment NEAR/2 loss) OR (periodontal bone NEAR/2 loss*) OR (periodontal NEAR/2 resorption*) OR furcation OR (alveolar bone NEAR/2 loss) AND diabet* or DM2 OR NIDDM or hyperglyc* |
Table 6.
EMBASE
| EMTREE terms | Free-Text search | |
| Population |
Exp Periodontal Disease OR alveolar bone loss AND Exp non insulin dependent diabetes mellitus OR exp hyperglycemia |
Periodontit* OR Parodontos$s OR periodontal disease OR pyorrhea OR Pericementit* OR gum disease OR (Periodont* ADJ2 pocket*) OR (Periodont* NEAR/2 defect*) OR (Periodont* NEAR/2 atroph*) OR (periodontal attachment NEAR/2 loss) OR (periodontal bone NEAR/2 loss*) OR (periodontal NEAR/2 resorption*) OR furcation OR (alveolar bone NEAR/2 loss) AND diabet* or DM2 OR NIDDM or hyperglyc* |
The results were limited to human studies, clinical studies, articles and articles in press with the dedicated EMBASE limits.
Appendix 2. Procedure for calculating SD
For each presented outcome, the difference between baseline and follow-up values were extracted (with specific error measure such as standard deviation (SD) or standard error (SE) or variance). When such parameter was not presented, it was computed as the difference between baseline and follow-up values. In these cases, following the instructions of the Cochrane Handbook for Systematic Reviews when SDs of changes values were not presented and they were not provided by authors after contacting them by email, they were computed as follows: i) if similar studies were present (similar treatment, similar population, similar sample size), SD was imputed taking the value of the other study; ii) when P value is presented SD was computed by using T tables for retrieving SEs; iii) when P value is presented as a limit (e.g. < 0.05) a conservative value of P (e.g. 0.05 in case of < 0.05) was considered for computing SE as described before; iv) if P value was not present SDs of change values was imputed by using the following formula [27, 61, 62]:
being CORR the correlation coefficient, that could be imputed from similar studies if present, or it was assumed conservatively to be 0.2. For each measure, pooled estimate of 95% CI was calculated.
Author contributions
SC: conceptualization, methodology, formal analysis, investigation, writing–original draft preparation; EC: conceptualization, methodology, investigation, writing–original draft preparation; ND: conceptualization, methodology, investigation, writing–review and editing, supervision; AA: conceptualization, investigation, data curation, writing–review and editing; PE: conceptualization, investigation, data curation, writing–review and editing; LF: conceptualization, methodology, investigation, writing–review and editing, supervision.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement.
Data Availability
The data supporting the findings of this study are available on request from the authors.
Declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55(1):21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmadinia AR, Rahebi D, Mohammadi M, Ghelichi-Ghojogh M, Jafari A, Esmaielzadeh F, et al. Association between type 2 diabetes (T2D) and tooth loss: a systematic review and meta-analysis. BMC Endocr Disord. 2022;22(1):100. doi: 10.1186/s12902-022-01012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldosari M, Aldosari M, Aldosari MA, Agrawal P Diabetes mellitus and its association with dental caries, missing teeth and dental services utilization in the US adult population: results from the 2015-2018 National Health and Nutrition Examination Survey. Diabet Med 2022:e14826. 10.1111/dme.14826 [DOI] [PubMed]
- 4.Steigmann L, Miller R, Trapani VR, Giannobile WV, Braffett BH, Pop-Busui R, et al. Type 1 diabetes and oral health: findings from the epidemiology of diabetes interventions and complications (EDIC) study. J Diabetes Complications. 2022;36(4):108120. doi: 10.1016/j.jdiacomp.2021.108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mealey BL, Ocampo GL. Diabetes mellitus and periodontal disease. Periodontol 2000. 2007;44:127–153. doi: 10.1111/j.1600-0757.2006.00193.x. [DOI] [PubMed] [Google Scholar]
- 6.Barutta F, Bellini S, Durazzo M, Gruden G (2022) Novel insight into the mechanisms of the bidirectional relationship between diabetes and periodontitis. Biomedicines 10(1). 10.3390/biomedicines10010178 [DOI] [PMC free article] [PubMed]
- 7.Kocher T, Konig J, Borgnakke WS, Pink C, Meisel P. Periodontal complications of hyperglycemia/diabetes mellitus: epidemiologic complexity and clinical challenge. Periodontol 2000. 2018;78(1):59–97. doi: 10.1111/prd.12235. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S, Philips KH, Moss K, Wu D, Adam HS, Selvin E, et al. Periodontitis and risk of diabetes in the atherosclerosis risk in communities (ARIC) study: a BMI-modified association. J Clin Endocrinol Metab. 2021;106(9):e3546–e3e58. doi: 10.1210/clinem/dgab337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Iida M, et al. The severity of periodontal disease is associated with the development of glucose intolerance in non-diabetics: the Hisayama study. J Dent Res. 2004;83(6):485–490. doi: 10.1177/154405910408300610. [DOI] [PubMed] [Google Scholar]
- 10.Park MS, Jeon J, Song TJ, Kim J. Association of periodontitis with microvascular complications of diabetes mellitus: a nationwide cohort study. J Diabetes Complications. 2022;36(2):108107. doi: 10.1016/j.jdiacomp.2021.108107. [DOI] [PubMed] [Google Scholar]
- 11.Hajishengallis G. Interconnection of periodontal disease and comorbidities: evidence, mechanisms, and implications. Periodontol 2000. 2022;89(1):9–18. doi: 10.1111/prd.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira KKY, Jara CM, Antunes GL, Gomes MS, Rosing CK, Cavagni J et al (2022) Effects of periodontitis and periodontal treatment on systemic inflammatory markers and metabolic profile in obese and non-obese rats. J Periodontol. 10.1002/JPER.21-0575 [DOI] [PubMed]
- 13.Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021;21(7):426–440. doi: 10.1038/s41577-020-00488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Aiuto F, Gkranias N, Bhowruth D, Khan T, Orlandi M, Suvan J, et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. 2018;6(12):954–965. doi: 10.1016/S2213-8587(18)30038-X. [DOI] [PubMed] [Google Scholar]
- 15.Simpson TC, Clarkson JE, Worthington HV, MacDonald L, Weldon JC, Needleman I, et al. Treatment of periodontitis for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev. 2022;4:CD004714. doi: 10.1002/14651858.CD004714.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbella S, Francetti L, Taschieri S, De Siena F, Fabbro MD. Effect of periodontal treatment on glycemic control of patients with diabetes: a systematic review and meta-analysis. J Diabetes Investig. 2013;4(5):502–509. doi: 10.1111/jdi.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suvan J, Leira Y, Moreno Sancho FM, Graziani F, Derks J, Tomasi C. Subgingival instrumentation for treatment of periodontitis. A systematic review. J Clin Periodontol. 2020;47(Suppl 22):155–175. doi: 10.1111/jcpe.13245. [DOI] [PubMed] [Google Scholar]
- 18.Corbella S, Calciolari E, Alberti A, Donos N, Francetti L. Systematic review and meta-analysis on the adjunctive use of host immune modulators in non-surgical periodontal treatment in healthy and systemically compromised patients. Sci Rep. 2021;11(1):12125. doi: 10.1038/s41598-021-91506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donos N, Calciolari E, Brusselaers N, Goldoni M, Bostanci N, Belibasakis GN. The adjunctive use of host modulators in non-surgical periodontal therapy. A systematic review of randomized, placebo-controlled clinical studies. J Clin Periodontol. 2020;47(Suppl 22):199–238. doi: 10.1111/jcpe.13232. [DOI] [PubMed] [Google Scholar]
- 20.Salvi GE, Stahli A, Schmidt JC, Ramseier CA, Sculean A, Walter C. Adjunctive laser or antimicrobial photodynamic therapy to non-surgical mechanical instrumentation in patients with untreated periodontitis: a systematic review and meta-analysis. J Clin Periodontol. 2020;47(Suppl 22):176–198. doi: 10.1111/jcpe.13236. [DOI] [PubMed] [Google Scholar]
- 21.Herrera D, Matesanz P, Martin C, Oud V, Feres M, Teughels W. Adjunctive effect of locally delivered antimicrobials in periodontitis therapy: a systematic review and meta-analysis. J Clin Periodontol. 2020;47(Suppl 22):239–256. doi: 10.1111/jcpe.13230. [DOI] [PubMed] [Google Scholar]
- 22.Teughels W, Feres M, Oud V, Martin C, Matesanz P, Herrera D. Adjunctive effect of systemic antimicrobials in periodontitis therapy: a systematic review and meta-analysis. J Clin Periodontol. 2020;47(Suppl 22):257–281. doi: 10.1111/jcpe.13264. [DOI] [PubMed] [Google Scholar]
- 23.Wickenheisser VA, Zywot EM, Rabjohns EM, Lee HH, Lawrence DS, Tarrant TK. Laser light therapy in inflammatory, musculoskeletal, and autoimmune disease. Curr Allergy Asthma Rep. 2019;19(8):37. doi: 10.1007/s11882-019-0869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu LS, Gkranias N, Farias B, Spratt D, Donos N. Differences in the subgingival microbial population of chronic periodontitis in subjects with and without type 2 diabetes mellitus-a systematic review. Clin Oral Investig. 2018;22(8):2743–2762. doi: 10.1007/s00784-018-2660-2. [DOI] [PubMed] [Google Scholar]
- 25.Abduljabbar T, Javed F, Shah A, Samer MS, Vohra F, Akram Z. Role of lasers as an adjunct to scaling and root planing in patients with type 2 diabetes mellitus: a systematic review. Lasers Med Sci. 2017;32(2):449–459. doi: 10.1007/s10103-016-2086-5. [DOI] [PubMed] [Google Scholar]
- 26.Abduljabbar T, Vohra F, Javed F, Akram Z. Antimicrobial photodynamic therapy adjuvant to non-surgical periodontal therapy in patients with diabetes mellitus: a meta-analysis. Photodiagnosis Photodyn Ther. 2017;17:138–146. doi: 10.1016/j.pdpdt.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JPT. Cochrane book series. 2. Hoboken, NJ: Wiley-Blackwell; 2020. Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 28.Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. 2018;89(Suppl 1):S173–SS82. doi: 10.1002/JPER.17-0721. [DOI] [PubMed] [Google Scholar]
- 29.Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004;34:9–21. doi: 10.1046/j.0906-6713.2002.003421.x. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization: International Classification of Diseases of the World Health Organization. https://icd.who.int/en (2022). Accessed 2021.
- 31.CDC Centers for Disease Control and Prevention: Diabetes. https://www.cdc.gov/diabetes/index.html (2022). Accessed 2022.
- 32.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 34.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Zahrani MS, Bamshmous SO, Alhassani AA, Al-Sherbini MM. Short-term effects of photodynamic therapy on periodontal status and glycemic control of patients with diabetes. J Periodontol. 2009;80(10):1568–1573. doi: 10.1902/jop.2009.090206. [DOI] [PubMed] [Google Scholar]
- 36.Al-Zawawi AS, Bukhari IA, Bello-Correa FO, Sheikh SA, Albaijan R, Vohra F (2020) Influence of root debridement with adjunct photodynamic therapy on periodontal parameters and gingival crevicular fluid cortisol levels among patients with and without type-2 diabetes mellitus. Photodiagnosis Photodyn Ther 32 [DOI] [PubMed]
- 37.Barbosa FI, Araújo PV, Machado LJC, Magalhães CS, Guimarães MMM, Moreira AN. Effect of photodynamic therapy as an adjuvant to non-surgical periodontal therapy: periodontal and metabolic evaluation in patients with type 2 diabetes mellitus. Photodiagnosis Photodyn Ther. 2018;22:245–250. doi: 10.1016/j.pdpdt.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 38.Chandra S, Shashikumar P. Diode laser - a novel therapeutic approach in the treatment of chronic periodontitis in type 2 diabetes mellitus patients: a prospective randomized controlled clinical trial. J Lasers Med Sci. 2019;10(1):56–63. doi: 10.15171/jlms.2019.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dengizek Eltas S, Gursel M, Eltas A, Alptekin NO, Ataoglu T. Evaluation of long-term effects of diode laser application in periodontal treatment of poorly controlled type 2 diabetic patients with chronic periodontitis. Int J Dent Hyg. 2019;17(4):292–299. doi: 10.1111/idh.12384. [DOI] [PubMed] [Google Scholar]
- 40.Elsadek MF, Ahmed BM, Alkhawtani DM, Zia SA (2020) A comparative clinical, microbiological and glycemic analysis of photodynamic therapy and Lactobacillus reuteri in the treatment of chronic periodontitis in type-2 diabetes mellitus patients. Photodiagnosis Photodyn Ther 29 [DOI] [PubMed]
- 41.Koçak E, Sağlam M, Kayış SA, Dündar N, Kebapçılar L, Loos BG, et al. Nonsurgical periodontal therapy with/without diode laser modulates metabolic control of type 2 diabetics with periodontitis: a randomized clinical trial. Lasers Med Sci. 2016;31(2):343–353. doi: 10.1007/s10103-016-1868-0. [DOI] [PubMed] [Google Scholar]
- 42.Macedo Gde O, Novaes AB, Souza SL, Taba M, Palioto DB, Grisi MF. Additional effects of aPDT on nonsurgical periodontal treatment with doxycycline in type II diabetes: a randomized, controlled clinical trial. Lasers Med Sci. 2014;29(3):881–886. doi: 10.1007/s10103-013-1285-6. [DOI] [PubMed] [Google Scholar]
- 43.Mirza S, Khan AA, Al-Kheraif AA, Khan SZ, Shafqat SS. Efficacy of adjunctive photodynamic therapy on the clinical periodontal, HbA1c and advanced glycation end product levels among mild to moderate chronic periodontal disease patients with type 2 diabetes mellitus: a randomized controlled clinical trial. Photodiagnosis Photodyn Ther. 2019;28:177–182. doi: 10.1016/j.pdpdt.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Soi S, Bains VK, Srivastava R, Madan R (2021) Comparative evaluation of improvement in periodontal and glycemic health status of type 2 diabetes mellitus patients after scaling and root planing with or without adjunctive use of diode laser. Lasers Med Sci [DOI] [PubMed]
- 45.Claudio MM, Nuernberg MAA, Rodrigues JVS, Belizario LCG, Batista JA, Duque C, et al. Effects of multiple sessions of antimicrobial photodynamic therapy (aPDT) in the treatment of periodontitis in patients with uncompensated type 2 diabetes: a randomized controlled clinical study. Photodiagnosis Photodyn Ther. 2021;35:102451. doi: 10.1016/j.pdpdt.2021.102451. [DOI] [PubMed] [Google Scholar]
- 46.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4(1):1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Cao R, Li Q, Wu Q, Yao M, Chen Y, Zhou H. Effect of non-surgical periodontal therapy on glycemic control of type 2 diabetes mellitus: a systematic review and Bayesian network meta-analysis. BMC Oral Health. 2019;19(1):176. doi: 10.1186/s12903-019-0829-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasuike A, Iguchi S, Suzuki D, Kawano E, Sato S. Systematic review and assessment of systematic reviews examining the effect of periodontal treatment on glycemic control in patients with diabetes. Med Oral Patol Oral Cir Bucal. 2017;22(2):e167–ee76. doi: 10.4317/medoral.21555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sales LS, Miranda ML, de Oliveira AB, Ferrisse TM, Fontana CR, Milward M, et al. Effect of the technique of photodynamic therapy against the main microorganisms responsible for periodontitis: a systematic review of in-vitro studies. Arch Oral Biol. 2022;138:105425. doi: 10.1016/j.archoralbio.2022.105425. [DOI] [PubMed] [Google Scholar]
- 50.Cieplik F, Deng D, Crielaard W, Buchalla W, Hellwig E, Al-Ahmad A, et al. Antimicrobial photodynamic therapy - what we know and what we don't. Crit Rev Microbiol. 2018;44(5):571–589. doi: 10.1080/1040841X.2018.1467876. [DOI] [PubMed] [Google Scholar]
- 51.Cobb CM. Lasers and the treatment of periodontitis: the essence and the noise. Periodontol 2000. 2017;75(1):205–295. doi: 10.1111/prd.12137. [DOI] [PubMed] [Google Scholar]
- 52.Jiang Y, Feng J, Du J, Fu J, Liu Y, Guo L, et al. Clinical and biochemical effect of laser as an adjunct to non-surgical treatment of chronic periodontitis. Oral Dis. 2022;28(4):1042–1057. doi: 10.1111/odi.13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin Z, Strauss FJ, Lang NP, Sculean A, Salvi GE, Stahli A. Efficacy of laser monotherapy or non-surgical mechanical instrumentation in the management of untreated periodontitis patients. A systematic review and meta-analysis. Clin Oral Investig. 2021;25(2):375–391. doi: 10.1007/s00784-020-03584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schar D, Ramseier CA, Eick S, Mettraux G, Salvi GE, Sculean A. Transgingival photodynamic therapy (tg-aPDT) adjunctive to subgingival mechanical instrumentation in supportive periodontal therapy. A randomized controlled clinical study. Photodiagnosis Photodyn Ther. 2020;32:101971. doi: 10.1016/j.pdpdt.2020.101971. [DOI] [PubMed] [Google Scholar]
- 55.Sukumar K, Tadepalli A, Parthasarathy H, Ponnaiyan D. Evaluation of combined efficacy of photodynamic therapy using indocyanine green photosensitizer and non-surgical periodontal therapy on clinical and microbial parameters in the management of chronic periodontitis subjects: a randomized split-mouth design. Photodiagnosis Photodyn Ther. 2020;31:101949. doi: 10.1016/j.pdpdt.2020.101949. [DOI] [PubMed] [Google Scholar]
- 56.Katsikanis F, Strakas D, Vouros I. The application of antimicrobial photodynamic therapy (aPDT, 670 nm) and diode laser (940 nm) as adjunctive approach in the conventional cause-related treatment of chronic periodontal disease: a randomized controlled split-mouth clinical trial. Clin Oral Investig. 2020;24(5):1821–1827. doi: 10.1007/s00784-019-03045-1. [DOI] [PubMed] [Google Scholar]
- 57.Yap KCH, Pulikkotil SJ. Systemic doxycycline as an adjunct to scaling and root planing in diabetic patients with periodontitis: a systematic review and meta-analysis. BMC Oral Health. 2019;19(1):209. doi: 10.1186/s12903-019-0873-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lira Junior R, Santos CMM, Oliveira BH, Fischer RG, Santos APP. Effects on HbA1c in diabetic patients of adjunctive use of systemic antibiotics in nonsurgical periodontal treatment: a systematic review. J Dent. 2017;66:1–7. doi: 10.1016/j.jdent.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Citterio F, Gualini G, Chang M, Piccoli GM, Giraudi M, Manavella V, et al. Pocket closure and residual pockets after non-surgical periodontal therapy: a systematic review and meta-analysis. J Clin Periodontol. 2022;49(1):2–14. doi: 10.1111/jcpe.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loos BG, Needleman I. Endpoints of active periodontal therapy. J Clin Periodontol. 2020;47(Suppl 22):61–71. doi: 10.1111/jcpe.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45(7):769–773. doi: 10.1016/0895-4356(92)90054-q. [DOI] [PubMed] [Google Scholar]
- 62.Abrams KR, Gillies CL, Lambert PC. Meta-analysis of heterogeneously reported trials assessing change from baseline. Stat Med. 2005;24(24):3823–3844. doi: 10.1002/sim.2423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available on request from the authors.