Abstract
Objective
The optimal dose and efficacy of tranexamic acid (TXA) and epsilon‐aminocaproic acid (EACA) in total knee arthroplasty (TKA) were under controversial, and we aimed to make comparisons between different doses of TXA and EACA in intravenous (IV) or intra‐articular (IA) applications in patients undergoing TKA.
Methods
This network meta‐analysis was guided by the Priority Reporting Initiative for Systematic Assessment and Meta‐Analysis (PRISMA). According to the administrations of antifibrinolytic agents, patients in eligible studies were divided into three subgroups: (i) IA applications of TXA and EACA; (ii) IV applications (g) of TXA and EACA; (iii) IV applications (mg/kg) of TXA and EACA. Total blood loss (TBL), hemoglobin (HB) drops and transfusion rates were the primary outcomes, while drainage volume, pulmonary embolism (PE) or deep vein thrombosis (DVT) risk were the secondary outcomes. A multivariate Bayesian random‐effects model was adopted in the network analysis.
Results
A total of 38 eligible trials with different regimens were assessed. Overall inconsistency and heterogeneity were acceptable. Taking all primary outcomes into account, 1.0–3.0 g TXA were most effective in IA applications, 1–6 g TXA and 10–14 g EACA were most effective in IV applications (g), while 30 mg/kg TXA and 150 mg/kg EACA were most effective in IV applications (mg/kg). None of the regimens showed increasing risk for pulmonary embolism (PE) or deep vein thrombosis (DVT) compared with placebo.
Conclusion
0 g IA TXA, 1.0 g IV TXA or 10.0 g IV EACA, as well as 30 mg/kg IV TXA or 150 mg/kg IV EACA were most effective and enough to control bleeding for patients after TKA. TXA was at least 5 times more potent than EACA.
Keywords: Bleeding, Epsilon‐Aminocaproic acid, Total knee arthroplasty, Tranexamic acid, Transfusion
Network plots of treat comparisons. TXA, tranexamic acid; EACA, aminocaproic acid; IV, intravenous; IA, intra‐articular; PE, pulmonary embolism; DVT, deep vein thrombosis; HB, hemoglobin.
Introduction
Total knee arthroplasty (TKA) is a common surgical procedure for the treatment of patients with end‐stage osteoarthritis of the knee, 1 , 2 which was estimated to grow to 1.26 million in 2030. 3 After TKA surgery, the patient will experience a lot of bleeding, so a lot of blood transfusion is needed. 4 Blood transfusion has many adverse clinical risks, including transfusions related infection, intravascular hemolysis, kidney damage, immune incompatibility, and even death. 5 , 6 In addition, massive bleeding in the knee cavity will also aggravate the swelling and pain of the affected limb after surgery, affect the patient's postoperative rehabilitation exercise and satisfaction with the operation. 7
Perioperative bleeding in TKA is associated with postoperative hyperfibrinolysis. 8 Tranexamic acid (TXA) and epsilon‐aminocaproic acid (EACA) are antifibrinolytic agents with similar mechanisms of action. 9 Although the clinical effect of TXA in reducing bleeding has been demonstrated in patients with TKA, 10 , 11 , 12 there are few data on the antifibrinolytic effect of EACA in joint surgery. 13 , 14 , 15 Intravenous (IV) or intra‐articular (IA) administrations of TXA and EACA were most commonly used and studied, but the optimal dosage and efficacy of TXA and EACA to control bleeding for patients after unilateral TKA are still controversial. Although several meta‐analyses have compared the blood‐sparing effects of TXA and EACA, the enrolled patients consisted of total hip arthroplasty and total knee arthroplasty, which may lead to confounding results. 16 , 17 , 18 Another two studies did not take the dosage of antifibrinolytic drugs into account and only focus on the intravenous administration. 19 , 20 Fillingham et al. 21 compared the efficacy of different administrations of TXA regarding the blood loss after total knee arthroplasty but did not make comparison among the EACA. Because of the contradictory evidence and limitations in previous studies, the optimal regimens of antifibrinolytic agents and relative effects of EACA compared with TXA were needed to be further investigated.
To date, there were few clinical randomized controlled trials (RCTs) comparing the efficacy and safety of EACA and TXA in TKA. In addition, the dosage and applications of TXA and EACA ranged among different studies. Through direct and indirect comparison, network meta‐analysis (NMA) allows a comprehensive analysis of the effects of EACA and TXA in different regimens. Taking total blood loss (TBL), hemoglobin (HB) drops, transfusion rates, drainage volume and pulmonary embolism (PE) or deep vein thrombosis (DVT) risk into account, we aimed to: (i) determine the optimal doses of TXA and EACA in IA or IV applications; and (ii) and make comparisons of EACA and TXA through NMA and subgroup analyses.
Method
This network meta‐analysis was reported in line with PRISMA 22 guidelines. After collecting characteristics of included studies, our group members used Cochrane Collaboration's tools and Newcastle‐Ottawa Score to assess the risk of bias for eligible studies (Data S2). This meta‐analysis was registered prospectively in the INPLASY database (registry number 202210094).
Search Strategy
We searched Cochrane, PubMed, Web of Science, Embase, Chinese National Knowledge Infrastructure and CBM with the following mesh terms: (“tranexamic acid” or “aminocaproic acid” or ‘epsilon aminocaproic acid’ or “AMCHA” or “transamin” or “AMCA” or “anvitoff” or “amchafibrin” or “exacyll” or “6 aminohexanoic acid” or “epsikapron” or “capralense” or “capramol” or “caproamin” or “caprocid” or “epsamon”) and “total” and “knee” and (“arthroplasty” or “replacement”).
Inclusion and Exclusion Criteria
According to PICOS guideline, the inclusion criteria were: (i) participants: enrolled patients were 55 years older and without anemia, coagulation disorders, history of anticoagulant drugs preoperatively; (ii) interventions: choosing placebo, EACA or TXA to control bleeding; (iii) control: comparing one antifibrinolytic agent with placebo or another; (iv) outcomes: reported transfusion rates, total blood loss (TBL), HB drop, drainage volume or pulmonary embolism (PE) or deep vein thrombosis (DVT) rates after TKA; and (v) study: randomized controlled trial (RCT) or retrospective studies.
The exclusion criteria were: (i) patients who did not underwent unilateral TKA, such as bilateral TKA and revision knee surgery; (ii) in vitro studies or animal research; (iii) review or meta‐analysis; and (iv) patients with treatments other than TXA and EACA that may affect the outcomes, such as autologous blood transfusion.
Subgroups and Outcomes
Based on three different applications of TXA and EACA, patients in eligible studies were divided into subgroups as follows: (i) IA applications of EACA and TXA; (ii) IV applications of EACA and TXA (g); and (iii) IV applications of EACA and TXA (mg/kg). In the IA administrations subgroup, 0.5 g TXA, 1.0 g TXA, 1.5 g TXA, 2.0 g TXA, 3.0 g TXA and 5.0 g EACA were evaluated. In the IV (g) applications subgroup, 1.0 g TXA, 1.5 g TXA, 2.0 g TXA, 6.0 g TXA, 5.0 g EACA, 10.0 g EACA and 14.0 g EACA were evaluated. 10 mg/kg TXA, 15 mg/kg TXA, 20 mg/kg TXA, 30 mg/kg TXA and 150 mg/kg EACA were evaluated in another intravenous (mg/kg) subgroup. TBL, HB drop and transfusion rates were the primary outcomes, while drainage volume and PE/DVT rates were the secondary outcomes.
Data Extraction and Quality Assessment
Once eligible studies were selected, two investigators (ZC and XJW) independently assessed the quality by Jadad score (all eligible studies scored>3 and showed high quality). Then characteristics of studies including sample size, mean age, antifibrinolytic drugs, administration and dosage of the drugs and proportion of female patients were collected. Finally, primary and secondary outcomes were collected. Any inconsistencies will be adjudicated by a senior researcher (LY).
Statistical Analysis
The effect of continuous variables was evaluated by mean difference (MD) and 95% CIs, while the effect of categorical variables was evaluated by odds ratios (OR) and 95% CI. Statistical significance (α) was set at 0.05. We use gemtc (R‐Project, Vienna, Austria, version 0.8–2) package of R studio (version February 1, 2019) to evaluate data homogeneity, transitivity and consistency of this network meta‐analysis. 23
First, we did pairwise meta‐analysis and used I2 metric to evaluate the heterogeneity of included studies. After sensitivity analysis, we picked out and removed studies with obviously high heterogeneity (I2 > 75%).
Then, Bayesian Markov chain Monte Carlo method with a random‐effect or fixed‐effect model was used and the data were entered into R software in gemtc package. Transitivity covers the validity of the logical inference and should be satisfied for all cases in an NMA. Convergence status reflected the results of Markov chain Monte Carlo simulations. Brocks‐Gelman‐Rubin plots, trace graph and density graph were adopted to evaluate the transitivity and convergence, while a node‐splitting method was to evaluate the consistency of direct and indirect comparisons. The rank probability diagrams and matrix of pairwise comparisons tables were provided by gemtc software package.
Results
A total of 38 12 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 studies, which consisted of 29 RCTs and 9 non‐RCTs, were included in the network analysis. Based on previously described research strategy, we identified 5403 studies. After removing 1792 studies for duplication, 2730 were excluded for meeting the exclusion criteria. Through the screening of the full text, 10 conference articles, 221 articles lacking interested outcomes, 195 articles with inaccessible data, 127 articles lacking access to the full text, 110 articles in oral or combined administrations of TXA and EACA, and 80 non‐English articles were excluded.
The PRISMA flow chart demonstrating the electronic search process is presented in the Fig. 1. The PRISMA Checklist is provided in Data S1. The basic characteristics and regimens of TXA and EACA are summarized in Data S2, Table S1, and the risk of bias for eligible studies is summarized in Data S2.
Fig. 1.
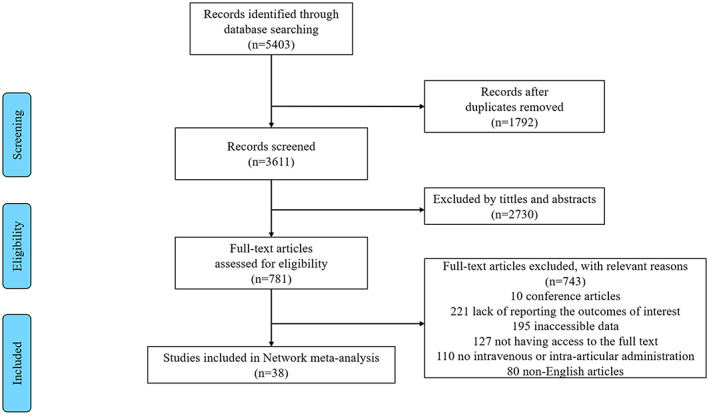
PRISMA flow chart of the study selection
Total Blood Loss
Formulas described by Nadler et al. 61 and Brecher et al. 62 to calculate the TBL were adopted in eligible studies. Twenty‐two eligible studies reported TBL. 12 , 24 , 25 , 26 , 28 , 29 , 32 , 33 , 36 , 37 , 40 , 43 , 44 , 49 , 50 , 51 , 52 , 53 , 55 , 56 , 57 , 59 Network diagrams of comparisons on TBL are presented in Fig. 2A–C. Estimated effects of EACA and TXA were respectively shown in Tables 1, 2, 3.
Fig. 2.
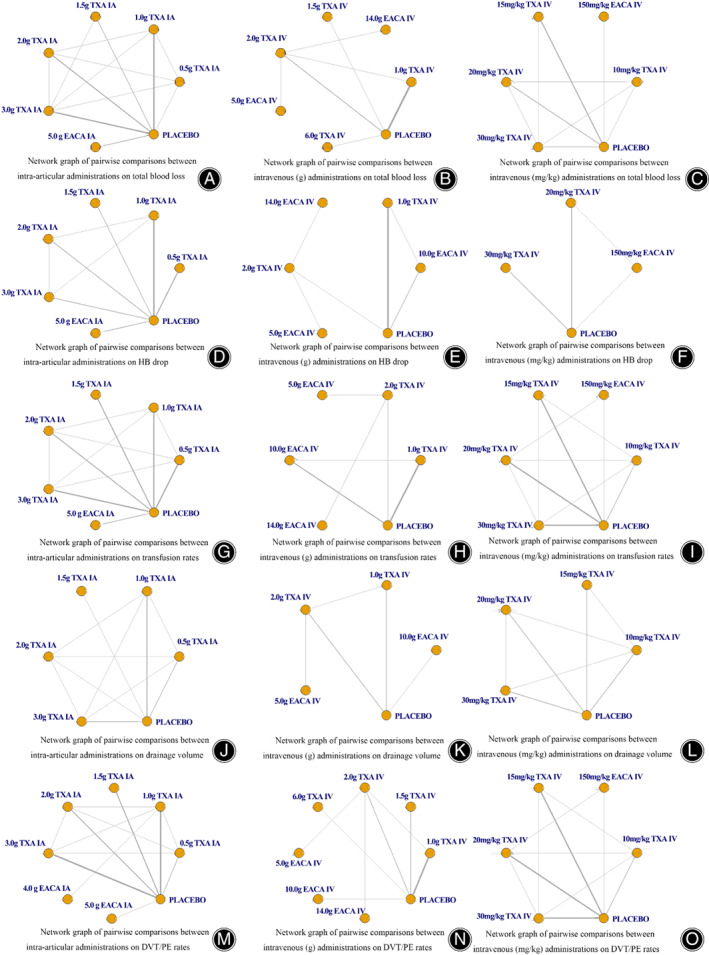
Network plots of treat comparisons. TXA, tranexamic acid; EACA, aminocaproic acid; IV, intravenous; IA, intra‐articular; PE, pulmonary embolism; DVT, deep vein thrombosis; HB, hemoglobin
TABLE 1.
Matrix of pairwise comparisons on total blood loss (L) in intra‐articular administration (shown as mean difference and 95% confidence intervals)
| 0.5 g TXA IA | 1.0 g TXA IA | 1.5 g TXA IA | 2.0 g TXA IA | 3.0 g TXA IA | 5.0 g EACA IA | Placebo | |
|---|---|---|---|---|---|---|---|
| 0.5 g TXA IA | 1 | −0.119 (−0.263, 0.023) | −0.13 (−0.321, 0.065) | −0.237 (−0.394, −0.087) | −0.164 (−0.306, −0.021) | −0.016 (−0.192, 0.17) | 0.193 (0.055, 0.335) |
| 1.0 g TXA IA | 0.119 (−0.023, 0.263) | 1 | −0.011 (−0.166, 0.147) | −0.119 (−0.243, 0.003) | −0.045 (−0.151, 0.064) | 0.103 (−0.03, 0.248) | 0.311 (0.238, 0.391) |
| 1.5 g TXA IA | 0.13 (−0.065, 0.321) | 0.011 (−0.147, 0.166) | 1 | −0.108 (−0.285, 0.065) | −0.034 (−0.185, 0.117) | 0.114 (−0.064, 0.299) | 0.322 (0.183, 0.464) |
| 2.0 g TXA IA | 0.237 (0.087, 0.394) | 0.119 (−0.003, 0.243) | 0.108 (−0.065, 0.285) | 1 | 0.074 (−0.05, 0.203) | 0.222 (0.068, 0.389) | 0.431 (0.322, 0.544) |
| 3.0 g TXA IA | 0.164 (0.021, 0.306) | 0.045 (−0.064, 0.151) | 0.034 (−0.117, 0.185) | −0.074 (−0.203, 0.05) | 1 | 0.148 (0.005, 0.3) | 0.356 (0.266, 0.449) |
| 5.0 g EACA IA | 0.016 (−0.17, 0.192) | −0.103 (−0.248, 0.03) | −0.114 (−0.299, 0.064) | −0.222 (−0.389, −0.068) | −0.148 (−0.3, −0.005) | 1 | 0.208 (0.09, 0.321) |
| Placebo | −0.193 (−0.335, −0.055) | −0.311 (−0.391, −0.238) | −0.322 (−0.464, −0.183) | −0.431 (−0.544, −0.322) | −0.356 (−0.449, −0.266) | −0.208 (−0.321, −0.09) | 1 |
Note: Bold results mean statistically significance.
Abbreviations: IA, intra‐articular; TXA, tranexamic acid; EACA, aminocaproic acid.
TABLE 2.
Matrix of pairwise comparisons on total blood loss (L) in intravenous (g) administration (shown as mean difference and 95% confidence intervals)
| 1.0 g TXA IV | 14.0 g EACA IV | 1.5 g TXA IV | 2.0 g TXA IV | 5.0 g EACA IV | 6.0 g TXA IV | Placebo | |
|---|---|---|---|---|---|---|---|
| 1.0 g TXA IV | 1 | −0.187 (−0.508, 0.139) | −0.062 (−0.363, 0.242) | −0.331 (−0.537, −0.121) | −0.101 (−0.423, 0.224) | −0.248 (−0.504, 0.015) | 0.279 (0.174, 0.389) |
| 14.0 g EACA IV | 0.187 (−0.139, 0.508) | 1 | 0.125 (−0.297, 0.545) | −0.144 (−0.393, 0.104) | 0.086 (−0.263, 0.438) | −0.061 (−0.452, 0.331) | 0.465 (0.154, 0.778) |
| 1.5 g TXA IV | 0.062 (−0.242, 0.363) | −0.125 (−0.545, 0.297) | 1 | −0.27 (−0.609, 0.071) | −0.039 (−0.459, 0.381) | −0.186 (−0.551, 0.181) | 0.341 (0.058, 0.625) |
| 2.0 g TXA IV | 0.331 (0.121, 0.537) | 0.144 (−0.104, 0.393) | 0.27 (−0.071, 0.609) | 1 | 0.23 (−0.018, 0.478) | 0.083 (−0.218, 0.386) | 0.61 (0.421, 0.8) |
| 5.0 g EACA IV | 0.101 (−0.224, 0.423) | −0.086 (−0.438, 0.263) | 0.039 (−0.381, 0.459) | −0.23 (−0.478, 0.018) | 1 | −0.147 (−0.537, 0.244) | 0.38 (0.069, 0.692) |
| 6.0 g TXA IV | 0.248 (−0.015, 0.504) | 0.061 (−0.331, 0.452) | 0.186 (−0.181, 0.551) | −0.083 (−0.386, 0.218) | 0.147 (−0.244, 0.537) | 1 | 0.527 (0.29, 0.763) |
| Placebo | −0.279 (−0.389, −0.174) | −0.465 (−0.778, −0.154) | −0.341 (−0.625, −0.058) | −0.61 (−0.8, −0.421) | −0.38 (−0.692, −0.069) | −0.527 (−0.763, −0.29) | 1 |
Note: Bold results mean statistically significance.
Abbreviations: IA, intra‐articular; TXA, tranexamic acid; EACA, aminocaproic acid.
TABLE 3.
Matrix of pairwise comparisons on total blood loss (L) in intravenous (mg/kg) administration (shown as mean difference and 95% confidence intervals)
| 10 mg/kg TXA IV | 150 mg/kg EACA IV | 15 mg/kg TXA IV | 20 mg/kg TXA IV | 30 mg/kg TXA IV | Placebo | |
|---|---|---|---|---|---|---|
| 10 mg/kg TXA IV | 1 | −0.474 (−0.894, −0.038) | 0.017 (−0.236, 0.32) | −0.024 (−0.264, 0.224) | −0.075 (−0.329, 0.164) | 0.205 (−0.029, 0.466) |
| 150 mg/kg EACA IV | 0.474 (0.038, 0.894) | 1 | 0.493 (0.117, 0.882) | 0.45 (0.05, 0.841) | 0.398 (−0.007, 0.779) | 0.68 (0.326, 1.036) |
| 15 mg/kg TXA IV | −0.017 (−0.32, 0.236) | −0.493 (−0.882, −0.117) | 1 | −0.04 (−0.289, 0.166) | −0.089 (−0.324, 0.073) | 0.189 (0.026, 0.327) |
| 20 mg/kg TXA IV | 0.024 (−0.224, 0.264) | −0.45 (−0.841, −0.05) | 0.04 (−0.166, 0.289) | 1 | −0.05 (−0.271, 0.145) | 0.229 (0.056, 0.422) |
| 30 mg/kg TXA IV | 0.075 (−0.164, 0.329) | −0.398 (−0.779, 0.007) | 0.089 (−0.073, 0.324) | 0.05 (−0.145, 0.271) | 1 | 0.278 (0.127, 0.476) |
| Placebo | −0.205 (−0.466, 0.029) | −0.68 (−1.036, −0.326) | −0.189 (−0.327, −0.026) | −0.229 (−0.422, −0.056) | −0.278 (−0.476, −0.127) | 1 |
Note: Bold results mean statistically significance.
Abbreviations: IA, intra‐articular; TXA, tranexamic acid; EACA, aminocaproic acid.
IA TXA and EACA Subgroup
From the matrix (Table 1), all doses of IA EACA and TXA showed significant efficacy in reducing TBL compared with placebo. 2.0 g IA TXA and 3.0 g IA TXA were significantly superior to 5.0 g IA EACA (MD −0.222, 95%CI −0.389 to −0.068; MD −0.148, 95%CI −0.3 to −0.005) and 0.5 g IA TXA (MD −0.237, 95%CI −0.394 to −0.087; MD −0.164, 95%CI −0.306 to −0.021). Intra‐articular administrations of 1.0 g–3.0 g TXA did not show inferiority to other regimens and were most effective,
Treatment rankings based on direct plot of rank probability for less TBL (Fig. 3A), from largest to smallest, were 2.0 g IA TXA, 3.0 g IA TXA, 1.5 g IA TXA, 1.0 g IA TXA, 5.0 g IA EACA, 0.5 g IA TXA and placebo.
Fig. 3.
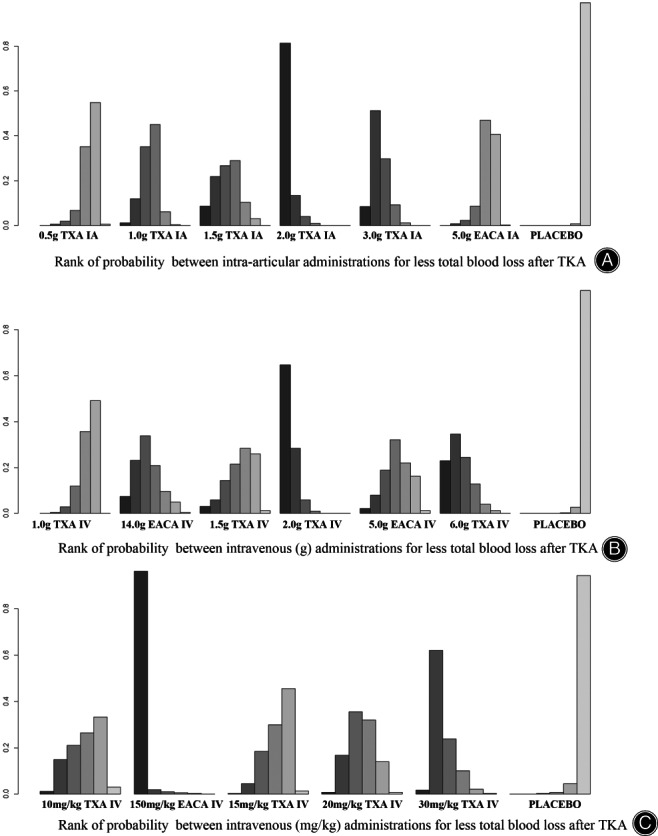
Rank of probability for less total blood loss after TKA
IV TXA and EACA (g) Subgroup
Table 2 showed the six interventions resulted in a significant reduction in TBL after TKA compared with placebo. Intravenous administrations of 1.5 g TXA, 2.0 g TXA, 6.0 g TXA, 5.0 g EACA and 14.0 g EACA were most effective, while 1.0 g IV TXA was significant inferior to 2.0 g IV TXA (MD 0.331, 95%CI 0.121 to 0.537).
Rank of probability for less TBL (Fig. 3B), from largest to smallest, were 2.0 g IV TXA, 6.0 g IV TXA, 14.0 g IV EACA, 1.5 g IV TXA, 5.0 g IV EACA, 1.0 g IV TXA and placebo.
IV TXA and EACA (mg/kg) Subgroup
As for intravenous applications based on patients' weight (Table 3), 15 mg/kg TXA, 20 mg/kg TXA, 30 mg/kg TXA and 150 mg/kg EACA showed significant efficacy compared with placebo, while 10 mg/kg TXA were inefficient. Among the efficient regimens, efficacy of 30 mg/kg IV TXA and 150 mg/kg IV EACA were comparable, while 15 mg/kg IV TXA and 20 mg/kg IV TXA were significant inferior to 150 mg/kg IV EACA (MD 0.493, 95%CI 0.117 to 0.882; MD 0.45, 95%CI 0.05 to 0.841).
Rank of probability for less TBL (Fig. 3C), from largest to smallest, were 150 mg/kg IV EACA, 30 mg/kg IV TXA, 20 mg/kg IV TXA, 10 mg/kg IV TXA, 15 mg/kg IV TXA and placebo.
HB Drop
A total of 23 eligible studies 24 , 26 , 27 , 28 , 30 , 32 , 33 , 34 , 37 , 38 , 39 , 41 , 42 , 43 , 44 , 46 , 48 , 49 , 51 , 52 , 57 , 58 , 59 reported HB drop. Network plots of comparisons on HB drop are shown in Fig. 2D–F.
IA TXA and EACA Subgroup
As the estimated effects table for HB drop presented (Table 4), all six regimens were effective compared with placebo. In addition, 5.0 g IA EACA was significantly inferior to 1.0 g, 1.5 g, 2.0 g and 3.0 g TXA in reducing HB drop (MD 0.61, 95%CI 0.18 to 1.03; MD 0.67, 95%CI 0.1 to 1.24; MD 0.59, 95%CI 0.11 to 1.06; MD 0.53, 95%CI 0.03 to 1.03). The efficacy of 0.5–3.0 g TXA were comparable and did not show inferiority to others.
TABLE 4.
Matrix of pairwise comparisons on HB drop (g/dL) in intra‐articular administration (shown as mean difference and 95% confidence intervals)
| 0.5 g TXA IA | 1.0 g TXA IA | 1.5 g TXA IA | 2.0 g TXA IA | 3.0 g TXA IA | 5.0 g EACA IA | Placebo | |
|---|---|---|---|---|---|---|---|
| 0.5 g TXA IA | 1 | −0.13 (−0.55, 0.31) | −0.19 (−0.76, 0.38) | −0.11 (−0.58, 0.38) | −0.06 (−0.54, 0.45) | 0.47 (−0.01, 0.98) | 0.84 (0.48, 1.2) |
| 1.0 g TXA IA | 0.13 (−0.31, 0.55) | 1 | −0.06 (−0.58, 0.45) | 0.02 (−0.34, 0.38) | 0.08 (−0.3, 0.46) | 0.61 (0.18, 1.03) | 0.97 (0.72, 1.21) |
| 1.5 g TXA IA | 0.19 (−0.38, 0.76) | 0.06 (−0.45, 0.58) | 1 | 0.08 (−0.47, 0.64) | 0.14 (−0.43, 0.71) | 0.67 (0.1, 1.24) | 1.03 (0.58, 1.48) |
| 2.0 g TXA IA | 0.11 (−0.38, 0.58) | −0.02 (−0.38, 0.34) | −0.08 (−0.64, 0.47) | 1 | 0.06 (−0.35, 0.45) | 0.59 (0.11, 1.06) | 0.95 (0.62, 1.27) |
| 3.0 g TXA IA | 0.06 (−0.45, 0.54) | −0.08 (−0.46, 0.3) | −0.14 (−0.71, 0.43) | −0.06 (−0.45, 0.35) | 1 | 0.53 (0.03, 1.03) | 0.89 (0.54, 1.24) |
| 5.0 g EACA IA | −0.47 (−0.98, 0.01) | −0.61 (−1.03, −0.18) | −0.67 (−1.24, −0.1) | −0.59 (−1.06, −0.11) | −0.53 (−1.03, −0.03) | 1 | 0.36 (0.01, 0.71) |
| Placebo | −0.84 (−1.2, −0.48) | −0.97 (−1.21, −0.72) | −1.03 (−1.48, −0.58) | −0.95 (−1.27, −0.62) | −0.89 (−1.24, −0.54) | −0.36 (−0.71, −0.01) | 1 |
Note: Bold results mean statistically significance.
Abbreviations: IA, intra‐articular; TXA, tranexamic acid; EACA, aminocaproic acid.
As for rank of probability for less HB drop in IA applications (Fig. 4A), 1.5 g TXA ranked first while 5.0 g EACA ranked last.
Fig. 4.
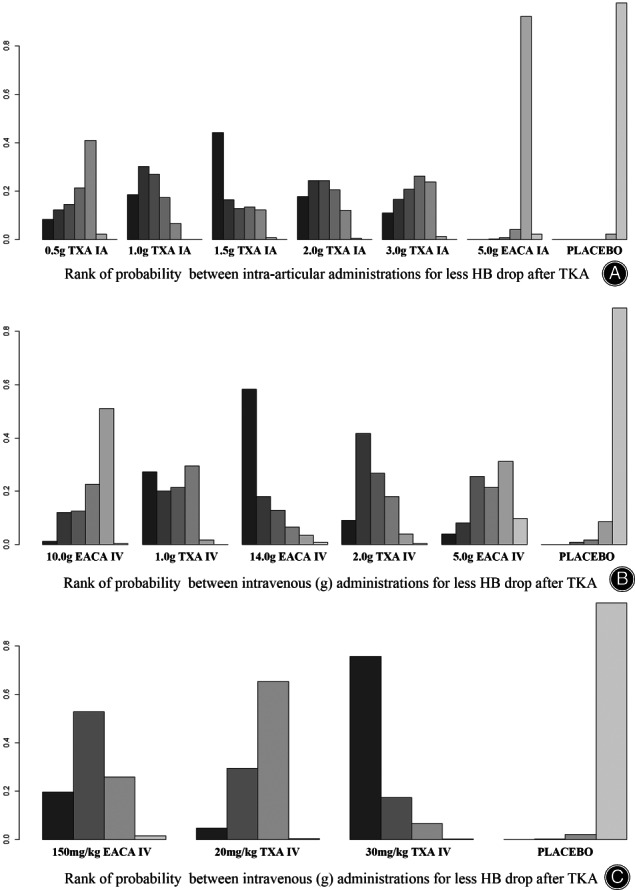
Rank of probability for less HB drop after TKA
IV TXA and EACA (g) Subgroup
1.0 g IV TXA, 2.0 g IV TXA, 10.0 g IV EACA and 14.0 g IV EACA showed significant efficacy in reducing HB drop and their efficacy were comparable (Table 5), while 5.0 g IV EACA was inefficient compared with placebo.
TABLE 5.
Matrix of pairwise comparisons on HB drop (g/dL) in intravenous (g) administration (shown as mean difference and 95% confidence intervals)
| 10.0 g EACA IV | 1.0 g TXA IV | 14.0 g EACA IV | 2.0 g TXA IV | 5.0 g EACA IV | Placebo | |
|---|---|---|---|---|---|---|
| 10.0 g EACA IV | 1 | −0.22 (−0.52, 0.06) | −0.42 (−1.28, 0.46) | −0.25 (−0.97, 0.48) | −0.05 (−0.88, 0.8) | 0.44 (0.2, 0.73) |
| 1.0 g TXA IV | 0.22 (−0.06, 0.52) | 1 | −0.19 (−1.05, 0.67) | −0.02 (−0.73, 0.7) | 0.17 (−0.65, 1.02) | 0.67 (0.48, 0.9) |
| 14.0 g EACA IV | 0.42 (−0.46, 1.28) | 0.19 (−0.67, 1.05) | 1 | 0.17 (−0.3, 0.64) | 0.37 (−0.27, 1.01) | 0.87 (0.04, 1.71) |
| 2.0 g TXA IV | 0.25 (−0.48, 0.97) | 0.02 (−0.7, 0.73) | −0.17 (−0.64, 0.3) | 1 | 0.2 (−0.23, 0.64) | 0.7 (0.01, 1.39) |
| 5.0 g EACA IV | 0.05 (−0.8, 0.88) | −0.17 (−1.02, 0.65) | −0.37 (−1.01, 0.27) | −0.2 (−0.64, 0.23) | 1 | 0.5 (−0.31, 1.31) |
| Placebo | −0.44 (−0.73, −0.2) | −0.67 (−0.9, −0.48) | −0.87 (−1.71, −0.04) | −0.7 (−1.39, −0.01) | −0.5 (−1.31, 0.31) | 1 |
Note: Bold results mean statistically significance.
Abbreviations: IA, intra‐articular; TXA, tranexamic acid; EACA, aminocaproic acid.
Treatment rankings based on direct plot of rank probability for less HB drop (Fig. 4B), from largest to smallest, were 14.0 g IV EACA, 1.0 g IV TXA, 2.0 g IV TXA, 5.0 g IV EACA, 10.0 g IV EACA and placebo.
IV TXA and EACA (mg/kg) Subgroup
Compared with placebo, all three regimens were effective in reducing HB drop and their efficacy were comparable (Table 6). Rank of probability for less HB drop (Fig. 4C), from largest to smallest, were 30 mg/kg IV TXA, 150 mg/kg IV EACA, 20 mg/kg IV TXA and placebo.
TABLE 6.
Matrix of pairwise comparisons on HB drop (g/dL) in intravenous (mg/kg) administration (shown as mean difference and 95% confidence intervals)
| 150 mg/kg EACA IV | 20 mg/kg TXA IV | 30 mg/kg TXA IV | Placebo | |
|---|---|---|---|---|
| 150 mg/kg EACA IV | 1 | 0.18 (−0.74, 1.05) | −0.33 (−1.37, 0.88) | 1.02 (0.16, 1.93) |
| 20 mg/kg TXA IV | −0.18 (−1.05, 0.74) | 1 | −0.51 (−1.29, 0.5) | 0.84 (0.33, 1.46) |
| 30 mg/kg TXA IV | 0.33 (−0.88, 1.37) | 0.51 (−0.5, 1.29) | 1 | 1.36 (0.6, 1.98) |
| Placebo | −1.02 (−1.93, −0.16) | −0.84 (−1.46, −0.33) | −1.36 (−1.98, −0.6) | 1 |
Note: Bold results mean statistically significance.
Abbreviations: IA, intra‐articular; TXA, tranexamic acid; EACA, aminocaproic acid.
Transfusion Rates
Transfusion rates of 31 studies 12 , 24 , 25 , 26 , 27 , 28 , 30 , 32 , 33 , 34 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 46 , 47 , 49 , 50 , 51 , 52 , 53 , 55 , 56 , 57 , 58 , 59 , 60 were evaluated in the network. Network plots of comparisons on transfusion rates are shown in Fig. 2G–I. Estimated effects of EACA and TXA regimens were respectively shown in Tables 7, 8, 9.
TABLE 7.
Matrix of pairwise comparisons on transfusion rates in intra‐articular administration (shown as odds ratios and 95% confidence intervals)
| 0.5 g TXA IA | 1.0 g TXA IA | 1.5 g TXA IA | 2.0 g TXA IA | 3.0 g TXA IA | 5.0 g EACA IA | Placebo | |
|---|---|---|---|---|---|---|---|
| 0.5 g TXA IA | 1 | 1.54 (0.5, 5.5) | 1.21 (0.29, 5.29) | 0.95 (0.27, 3.46) | 1.33 (0.39, 4.87) | 3.13 (0.94, 13.35) | 6.04 (2.64, 16.78) |
| 1.0 g TXA IA | 0.65 (0.18, 2.01) | 1 | 0.79 (0.18, 3.13) | 0.62 (0.18, 2.08) | 0.86 (0.25, 2.83) | 2.04 (0.6, 7.93) | 3.94 (1.68, 10.1) |
| 1.5 g TXA IA | 0.83 (0.19, 3.46) | 1.26 (0.32, 5.5) | 1 | 0.79 (0.18, 3.47) | 1.1 (0.26, 4.81) | 2.59 (0.66, 12.56) | 4.96 (1.76, 16.65) |
| 2.0 g TXA IA | 1.05 (0.29, 3.65) | 1.62 (0.48, 5.7) | 1.27 (0.29, 5.54) | 1 | 1.39 (0.4, 5.07) | 3.34 (0.9, 14.17) | 6.3 (2.56, 18.4) |
| 3.0 g TXA IA | 0.75 (0.21, 2.56) | 1.16 (0.35, 3.97) | 0.91 (0.21, 3.82) | 0.72 (0.2, 2.51) | 1 | 2.39 (0.66, 9.78) | 4.58 (1.83, 12.4) |
| 5.0 g EACA IA | 0.32 (0.07, 1.07) | 0.49 (0.13, 1.68) | 0.39 (0.08, 1.52) | 0.3 (0.07, 1.11) | 0.42 (0.1, 1.52) | 1 | 1.91 (0.72, 4.86) |
| Placebo | 0.17 (0.06, 0.38) | 0.25 (0.1, 0.6) | 0.2 (0.06, 0.57) | 0.16 (0.05, 0.39) | 0.22 (0.08, 0.55) | 0.52 (0.21, 1.39) | 1 |
Note: Bold results mean statistically significance.
Abbreviations: IA, intra‐articular; TXA, tranexamic acid; EACA, aminocaproic acid.
TABLE 8.
Matrix of pairwise comparisons on transfusion rates in intravenous (g) administration (shown as odds ratios and 95% confidence intervals)
| 1.0 g TXA IV | 2.0 g TXA IV | 5.0 g EACA IV | 10.0 g EACA IV | 14.0 g EACA IV | Placebo | |
|---|---|---|---|---|---|---|
| 1.0 g TXA IV | 1 | 0.19 (0, 4.49) | 0.17 (0, 34.84) | 0.58 (0.1, 2.64) | 0.04 (0, 4.1) | 5.01 (1.53, 17.07) |
| 2.0 g TXA IV | 5.29 (0.22, 294.29) | 1 | 0.99 (0.02, 61.65) | 3.02 (0.11, 165.14) | 0.26 (0, 5.89) | 26.44 (1.46, 1255.83) |
| 5.0 g EACA IV | 5.73 (0.03, 1598.32) | 1.01 (0.02, 62.88) | 1 | 3.28 (0.01, 849.81) | 0.25 (0, 45.47) | 28.86 (0.17, 7239.88) |
| 10.0 g EACA IV | 1.72 (0.38, 10.23) | 0.33 (0.01, 9.2) | 0.31 (0, 68.22) | 1 | 0.08 (0, 8.13) | 8.65 (2.5, 40.17) |
| 14.0 g EACA IV | 22.54 (0.24, 5155.59) | 3.89 (0.17, 202.23) | 4.05 (0.02, 1091.86) | 12.99 (0.12, 2905.01) | 1 | 114.61 (1.46, 23256.29) |
| Placebo | 0.2 (0.06, 0.65) | 0.04 (0, 0.69) | 0.03 (0, 6.04) | 0.12 (0.02, 0.4) | 0.01 (0, 0.68) | 1 |
Note: Bold results mean statistically significance.
Abbreviations: IA, intra‐articular; TXA, tranexamic acid; EACA, aminocaproic acid.
TABLE 9.
Matrix of pairwise comparisons on transfusion rates in intravenous (mg/kg) administration (shown as odds ratios and 95% confidence intervals)
| 10 mg/kg TXA IV | 150 mg/kg EACA IV | 15 mg/kg TXA IV | 20 mg/kg TXA IV | 30 mg/kg TXA IV | Placebo | |
|---|---|---|---|---|---|---|
| 10 mg/kg TXA IV | 1 | 0.29 (0.03, 2.55) | 0.91 (0.28, 2.8) | 0.49 (0.14, 1.67) | 0.26 (0.07, 1.1) | 2.25 (0.85, 6.38) |
| 150 mg/kg EACA IV | 3.47 (0.39, 38.79) | 1 | 3.18 (0.36, 32.75) | 1.7 (0.21, 17.12) | 0.91 (0.11, 10.19) | 7.88 (1.13, 73.73) |
| 15 mg/kg TXA IV | 1.1 (0.36, 3.59) | 0.31 (0.03, 2.78) | 1 | 0.54 (0.15, 1.92) | 0.28 (0.08, 1.21) | 2.48 (1.1, 6.32) |
| 20 mg/kg TXA IV | 2.06 (0.6, 7.25) | 0.59 (0.06, 4.73) | 1.85 (0.52, 6.67) | 1 | 0.53 (0.15, 2.18) | 4.61 (1.85, 12.86) |
| 30 mg/kg TXA IV | 3.91 (0.91, 13.86) | 1.1 (0.1, 9.24) | 3.56 (0.83, 12.16) | 1.9 (0.46, 6.5) | 1 | 8.78 (2.95, 23.98) |
| Placebo | 0.44 (0.16, 1.18) | 0.13 (0.01, 0.88) | 0.4 (0.16, 0.91) | 0.22 (0.08, 0.54) | 0.11 (0.04, 0.34) | 1 |
Note: Bold results mean statistically significance.
Abbreviations: IA, intra‐articular; TXA, tranexamic acid; EACA, aminocaproic acid.
IA TXA and EACA Subgroup
As the Table 7 shows, all six regimens except 5.0 g IA EACA showed significant efficacy in reducing transfusion rates compared with placebo. Among the effective regimens, none of them showed superiority to each other.
Treatment rankings based on direct plot of rank probability for less transfusion (Fig. 5A), from largest to smallest, were 0.5 g IA TXA, 2.0 g IA TXA, 1.5 g IA TXA, 3.0 g IA TXA, 1.0 g IA TXA, 5.0 g IA EACA and placebo.
Fig. 5.
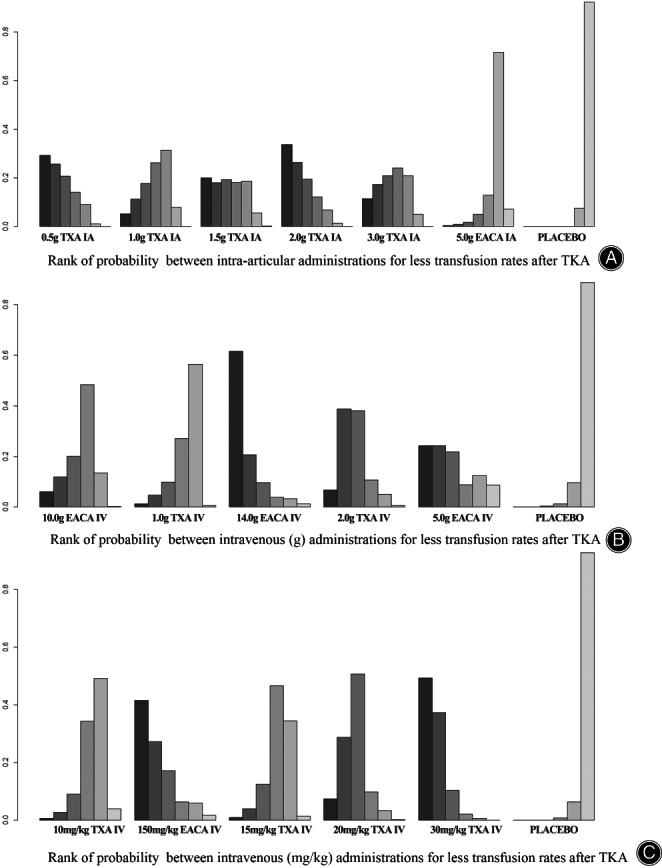
Rank of probability for less transfusion rates after TKA
IV TXA and EACA (g) Subgroup
According to Table 8, in intravenous applications, 1.0 g TXA, 2.0 g TXA, 10.0 g EACA and 14.0 g EACA can reduce transfusion rates compared with placebo, while 5.0 g EACA was not effective enough to control transfusion rates. 14.0 g IV EACA ranked first for probability of less transfusion rates in the network (Fig. 5B).
IV TXA and EACA (mg/kg) Subgroup
As for intravenous applications based on patients' weight (Table 9), 15 mg/kg‐30 mg/kg TXA and 150 mg/kg EACA can reduce transfusion rates compared with placebo and their efficacy were comparable. However, the rest in the network were not effective enough.
Treatment rankings based on direct plot of rank probability for less transfusions (Fig. 5C), from largest to smallest, were 30 mg/kg IV TXA, 150 mg/kg IV EACA, 20 mg/kg IV TXA, 15 mg/kg IV TXA, 10 mg/kg IV TXA and placebo.
Drainage Volume
Thirteen studies 12 , 29 , 30 , 32 , 35 , 39 , 43 , 44 , 47 , 49 , 52 , 55 , 60 reported drainage volume. Network diagrams of comparisons on drainage volume are shown in Fig. 2J–L. Rank of probability for less drainage volume in IA or IV applications are shown in Data S3.
In intra‐articular applications (Table 10), all five regimens can reduce drainage volume compared with placebo and their efficacy was comparable. In intravenous TXA and EACA (g) subgroup (Table 11), 1.0 g TXA and 2.0 g TXA showed significant efficacy compared with placebo, the rest were inefficient. In another subgroup, 20 mg/kg IV TXA and 30 mg/kg IV TXA were most effective, while the rest in the network were inefficient compared with placebo (Table 12).
TABLE 10.
Matrix of pairwise comparisons on drainage volume (L) in intra‐articular administration (shown as mean difference and 95% confidence intervals)
| 0.5 g TXA IA | 1.0 g TXA IA | 1.5 g TXA IA | 2.0 g TXA IA | 3.0 g TXA IA | Placebo | |
|---|---|---|---|---|---|---|
| 0.5 g TXA IA | 1 | −0.068 (−0.222, 0.049) | −0.048 (−0.276, 0.137) | −0.042 (−0.21, 0.104) | −0.054 (−0.211, 0.068) | 0.234 (0.09, 0.338) |
| 1.0 g TXA IA | 0.068 (−0.049, 0.222) | 1 | 0.02 (−0.173, 0.207) | 0.025 (−0.113, 0.181) | 0.014 (−0.109, 0.141) | 0.302 (0.206, 0.392) |
| 1.5 g TXA IA | 0.048 (−0.137, 0.276) | −0.02 (−0.207, 0.173) | 1 | 0.006 (−0.201, 0.234) | −0.006 (−0.2, 0.197) | 0.282 (0.116, 0.447) |
| 2.0 gTXA IA | 0.042 (−0.104, 0.21) | −0.025 (−0.181, 0.113) | −0.006 (−0.234, 0.201) | 1 | −0.012 (−0.169, 0.132) | 0.277 (0.123, 0.407) |
| 3.0 g TXA IA | 0.054 (−0.068, 0.211) | −0.014 (−0.141, 0.109) | 0.006 (−0.197, 0.2) | 0.012 (−0.132, 0.169) | 1 | 0.289 (0.174, 0.391) |
| Placebo | −0.234 (−0.338, −0.09) | −0.302 (−0.392, −0.206) | −0.282 (−0.447, −0.116) | −0.277 (−0.407, −0.123) | −0.289 (−0.391, −0.174) | 1 |
Note: Bold results mean statistically significance.
Abbreviations: IA, intra‐articular; TXA, tranexamic acid; EACA, aminocaproic acid.
TABLE 11.
Matrix of pairwise comparisons on drainage volume (L) in intravenous (g) administration (shown as mean difference and 95% confidence intervals)
| 10.0 g EACA IV | 1.0 g TXA IV | 2.0 g TXA IV | 5.0 g EACA IV | Placebo | |
|---|---|---|---|---|---|
| 10.0 g EACA IV | 1 | 0.007 (−0.463, 0.455) | −0.159 (−0.627, 0.293) | 0.004 (−0.598, 0.588) | 0.256 (−0.123, 0.636) |
| 1.0 g TXA IV | −0.007 (−0.455, 0.463) | 1 | −0.167 (−0.473, 0.146) | −0.005 (−0.487, 0.484) | 0.248 (0.001, 0.518) |
| 2.0 g TXA IV | 0.159 (−0.293, 0.627) | 0.167 (−0.146, 0.473) | 1 | 0.163 (−0.21, 0.533) | 0.415 (0.163, 0.681) |
| 5.0 g EACA IV | −0.004 (−0.588, 0.598) | 0.005 (−0.484, 0.487) | −0.163 (−0.533, 0.21) | 1 | 0.252 (−0.193, 0.714) |
| Placebo | −0.256 (−0.636, 0.123) | −0.248 (−0.518, −0.001) | −0.415 (−0.681, −0.163) | −0.252 (−0.714, 0.193) | 1 |
Note: Bold results mean statistically significance.
Abbreviations: IA, intra‐articular; TXA, tranexamic acid; EACA, aminocaproic acid.
TABLE 12.
Matrix of pairwise comparisons on drainage volume (L) in intravenous(mg/kg) administration (shown as mean difference and 95% confidence intervals)
| 10 mg/kg TXA IV | 15 mg/kg TXA IV | 20 mg/kg TXA IV | 30 mg/kg TXA IV | Placebo | |
|---|---|---|---|---|---|
| 10 mg/kg TXA IV | 1 | −0.015 (−0.147, 0.117) | −0.104 (−0.253, 0.023) | −0.111 (−0.244, 0.03) | 0.061 (−0.045, 0.172) |
| 15 mg/kg TXA IV | 0.015 (−0.117, 0.147) | 1 | −0.089 (−0.255, 0.056) | −0.095 (−0.246, 0.061) | 0.076 (−0.033, 0.19) |
| 20 mg/kg TXA IV | 0.104 (−0.023, 0.253) | 0.089 (−0.056, 0.255) | 1 | −0.006 (−0.133, 0.147) | 0.166 (0.061, 0.294) |
| 30 mg/kg TXA IV | 0.111 (−0.03, 0.244) | 0.095 (−0.061, 0.246) | 0.006 (−0.147, 0.133) | 1 | 0.172 (0.057, 0.285) |
| Placebo | −0.061 (−0.172, 0.045) | −0.076 (−0.19, 0.033) | −0.166 (−0.294, −0.061) | −0.172 (−0.285, −0.057) | 1 |
Note: Bold results mean statistically significance.
Abbreviations: IA, intra‐articular; TXA, tranexamic acid; EACA, aminocaproic acid.
PE/DVT Rates
Twenty‐nine studies 12 , 24 , 25 , 29 , 30 , 31 , 32 , 33 , 36 , 37 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 47 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 reported PE/DVT rates. Network diagrams of comparisons on PE/DVT rates are shown in Fig. 2M–O. From the matrix (Tables 13, 14, 15), none of the antifibrinolytic regimens showed increasing risk for PE/DVT compared with placebo. Treatment rankings based on direct plot of rank probability are shown in Data S3.
TABLE 13.
Matrix of pairwise comparisons on PE/DVT rates in intra‐articular administrations (shown as odds ratios and 95% confidence intervals)
| 0.5 g TXA IA | 1.0 g TXA IA | 1.5 g TXA IA | 2.0 g TXA IA | 3.0 g TXA IA | 4.0 g EACA IA | 5.0 g EACA IA | Placebo | |
|---|---|---|---|---|---|---|---|---|
| 0.5 g TXA IA | 1 | 0.68 (0.17, 2.66) | 0.89 (0.15, 5.99) | 0.74 (0.2, 2.85) | 0.66 (0.17, 2.58) | 0.66 (0.01, 25.58) | 0.38 (0.01, 17.76) | 0.73 (0.24, 2.29) |
| 1.0 g TXA IA | 1.47 (0.38, 5.75) | 1 | 1.31 (0.23, 8.41) | 1.09 (0.29, 4.34) | 0.97 (0.24, 3.83) | 0.98 (0.02, 30.14) | 0.56 (0.01, 25.58) | 1.07 (0.38, 3.25) |
| 1.5 g TXA IA | 1.13 (0.17, 6.87) | 0.76 (0.12, 4.44) | 1 | 0.84 (0.13, 4.88) | 0.74 (0.11, 4.4) | 0.74 (0.01, 32.86) | 0.42 (0.01, 22) | 0.83 (0.18, 3.44) |
| 2.0 g TXA IA | 1.34 (0.35, 5.1) | 0.92 (0.23, 3.47) | 1.19 (0.2, 7.5) | 1 | 0.88 (0.23, 3.45) | 0.88 (0.02, 33.76) | 0.51 (0.01, 22.88) | 0.98 (0.35, 2.84) |
| 3.0 g TXAIA | 1.52 (0.39, 6) | 1.03 (0.26, 4.13) | 1.35 (0.23, 8.86) | 1.14 (0.29, 4.41) | 1 | 1.01 (0.02, 38.89) | 0.57 (0.02, 25.78) | 1.11 (0.39, 3.33) |
| 4.0 g EACA IA | 1.52 (0.04, 74.48) | 1.02 (0.03, 41.66) | 1.36 (0.03, 79.2) | 1.14 (0.03, 54.92) | 0.99 (0.03, 48.57) | 1 | 0.59 (0, 108.35) | 1.11 (0.03, 51.04) |
| 5.0 g EACA IA | 2.66 (0.06, 100.41) | 1.79 (0.04, 67.17) | 2.38 (0.05, 96.65) | 1.96 (0.04, 71.29) | 1.74 (0.04, 64.91) | 1.69 (0.01, 244.42) | 1 | 1.95 (0.05, 61.46) |
| Placebo | 1.37 (0.44, 4.1) | 0.93 (0.31, 2.62) | 1.21 (0.29, 5.59) | 1.02 (0.35, 2.84) | 0.9 (0.3, 2.55) | 0.9 (0.02, 31.7) | 0.51 (0.02, 20.51) | 1 |
Abbreviations: IA, intra‐articular; TXA, tranexamic acid; EACA, aminocaproic acid.
TABLE 14.
Matrix of pairwise comparisons on PE/DVT rates in intravenous (g) administration (shown as odds ratios and 95% confidence intervals)
| 1.0 g TXA IV | 1.5 g TXA IV | 2.0 g TXA IV | 6.0 g TXA IV | 5.0 g EACA IV | 10.0 g EACA IV | 14.0 g EACA IV | Placebo | |
|---|---|---|---|---|---|---|---|---|
| 1.0 g TXA IV | 1 | 0.72 (0.05, 7.95) | 1.16 (0.24, 5.55) | 1.67 (0.03, 90.83) | 0.46 (0.01, 11.91) | 1.87 (0.12, 29.36) | 6.63 (0.36, 272.35) | 1.67 (0.56, 4.98) |
| 1.5 g TXA IV | 1.39 (0.13, 18.4) | 1 | 1.6 (0.12, 25.55) | 2.33 (0.03, 198.74) | 0.62 (0.01, 33.25) | 2.62 (0.09, 76.91) | 9.64 (0.26, 675.3) | 2.3 (0.27, 23.94) |
| 2.0 g TXA IV | 0.86 (0.18, 4.22) | 0.62 (0.04, 8.35) | 1 | 1.44 (0.02, 90.69) | 0.4 (0.01, 6.76) | 1.62 (0.09, 29.99) | 5.58 (0.5, 172.78) | 1.44 (0.34, 6.27) |
| 6.0 g TXA IV | 0.6 (0.01, 30.79) | 0.43 (0.01, 32.8) | 0.69 (0.01, 40.27) | 1 | 0.26 (0, 39.52) | 1.1 (0.01, 104.47) | 4.18 (0.03, 752) | 1.01 (0.02, 43.3) |
| 5.0 g EACA IV | 2.19 (0.08, 118.17) | 1.61 (0.03, 137.46) | 2.49 (0.15, 99.97) | 3.86 (0.03, 837.17) | 1 | 4.21 (0.07, 420.46) | 15.62 (0.33, 2061.22) | 3.64 (0.15, 187.89) |
| 10.0 g EACA IV | 0.53 (0.03, 8.52) | 0.38 (0.01, 10.62) | 0.62 (0.03, 11.24) | 0.91 (0.01, 89.34) | 0.24 (0, 13.81) | 1 | 3.66 (0.08, 293.42) | 0.89 (0.07, 11.12) |
| 14.0 g EACA IV | 0.15 (0, 2.8) | 0.1 (0, 3.89) | 0.18 (0.01, 1.98) | 0.24 (0, 29.02) | 0.06 (0, 3.01) | 0.27 (0, 13.04) | 1 | 0.25 (0.01, 4.26) |
| Placebo | 0.6 (0.2, 1.79) | 0.43 (0.04, 3.7) | 0.69 (0.16, 2.91) | 0.99 (0.02, 48.32) | 0.27 (0.01, 6.75) | 1.12 (0.09, 14.09) | 3.97 (0.23, 155.84) | 1 |
Abbreviations: IA, intra‐articular; TXA, tranexamic acid; EACA, aminocaproic acid.
TABLE 15.
Matrix of pairwise comparisons on PE/DVT rates in intravenous(mg/kg) administration (shown as odds ratios and 95% confidence intervals)
| 10 mg/kg TXA IV | 150 mg/kg EACA IV | 15 mg/kg TXA IV | 20 mg/kg TXA IV | 30 mg/kg TXA IV | Placebo | |
|---|---|---|---|---|---|---|
| 10 mg/kg TXA IV | 1 | 1.48 (0.06, 26.35) | 1.01 (0.38, 2.72) | 0.86 (0.19, 4.17) | 1.35 (0.34, 5.2) | 1.13 (0.43, 2.81) |
| 150 mg/kg EACA IV | 0.68 (0.04, 15.9) | 1 | 0.68 (0.04, 15.73) | 0.58 (0.04, 13.02) | 0.89 (0.05, 24.36) | 0.75 (0.05, 15.78) |
| 15 mg/kg TXA IV | 0.99 (0.37, 2.65) | 1.46 (0.06, 23.41) | 1 | 0.86 (0.19, 3.66) | 1.34 (0.4, 4.18) | 1.12 (0.51, 2.37) |
| 20 mg/kg TXA IV | 1.16 (0.24, 5.2) | 1.73 (0.08, 27.82) | 1.16 (0.27, 5.18) | 1 | 1.58 (0.3, 7.34) | 1.3 (0.37, 4.75) |
| 30 mg/kg TXA IV | 0.74 (0.19, 2.9) | 1.12 (0.04, 18.8) | 0.74 (0.24, 2.48) | 0.63 (0.14, 3.33) | 1 | 0.83 (0.29, 2.49) |
| Placebo | 0.88 (0.36, 2.33) | 1.34 (0.06, 18.63) | 0.89 (0.42, 1.95) | 0.77 (0.21, 2.7) | 1.21 (0.4, 3.43) | 1 |
Abbreviations: IA, intra‐articular; TXA, tranexamic acid; EACA, aminocaproic acid.
Data Homogeneity, Transitivity and Consistency
After removing the studies with obviously high heterogeneity, none of the I2 were > 75%, which is shown in Data S4. The density plots, trace plots as well as Brocks‐Gelman‐Rubin plots (Data S4) showed good transitivity. The relative Bayesian P values of the node‐splitting method in this network are all > 0.05(Data S4), indicating consistency of direct and indirect comparisons in our analysis. Based on these, we concluded that our model was established well.
Discussion
This study identified the optimal dosages of TXA and EACA in IV or IA applications in TKA. In addition, through direct and indirect comparisons, we determined the relative effect of EACA compared to TXA.
Lowest and most Effective Dose of TXA and EACA
Taking all primary outcomes into account, including total blood loss, HB drop and transfusion rates, 1–3 g TXA were most effective in intra‐articular administrations. In the IV TXA and EACA (g) subgroup, 1–6 g TXA and 10–14 g EACA were most effective and their efficacy were comparable. 30 mg/kg IV TXA and 150 mg/kg IV EACA were most effective in another intravenous subgroup. Assuming the weights of patients after TKA were among 60–70kg, the most effective doses in intravenous applications based on patients' weights were 1.8–2.1 g TXA and 9–10.5 g EACA, which was in consistency with the results that 1–6 g IV TXA and 10–14 g IV EACA were most effective.
Two network meta‐analyses 16 , 21 have compared the efficacy of different administrations of TXA, both of them concluded that all regimens of TXA had significantly lower blood loss and transfusion rates compared with control and showed comparable effects. Their findings were in partial consistency with our results that both intravenous and intra‐articular administrations showed significantly efficacy in conserving blood compared with placebo. However, one of the two network meta‐analyses 21 defined high dose TXA as any dose ≥20 mg/kg or > 1 g. No significant differences were found in results of comparisons, even between high dose IV TXA versus low dose IV TXA, as well as high dose IA TXA versus low dose IA TXA. Considering the large range of TXA dosages in eligible studies, our group members believed the dividing line between high and low dose TXA was wrong and yielded potential confounding factors. Therefore, we chose the specific dose of antifibrinolytic agents in the network analysis. In addition, to diminish the influences of patients' weight on dosage, intravenous administrations of TXA and EACA were divided into two subgroups. Another network meta‐analyses 16 enrolled patients after total knee and hip replacements and did not make subgroup analysis. Thus, the conclusion might be less convincing and we only focused on the patients after TKA.
Relative Effect of EACA Compared to TXA in TKA
For the efficacy between TXA and EACA, two conventional meta‐analyses 19 , 20 concluded that TXA was not superior to EACA in controlling bleeding in TKA. Their conclusions were partially consistent with ours, regardless of dosage and administration. According to our results, 1 g IA TXA was most effective and superior to 5 g IA EACA, while 1 g or 30 mg/kg IV TXA was most effective and comparable with 10 g or 150 mg/kg IV EACA. We summarized the blood conserving effects of EACA and TXA in TKA were comparable only when the dose of EACA was at least five times more than TXA.
EACA and TXA share similar mechanisms by interacting with plasminogen. 63 After saturating the lysine binding sites of plasminogen, the two drugs inhibiting plasminogen from binding to the surface of fibrin. 63 , 64 , 65 EACA and TXA had similar elimination half‐life. 64 However, TXA's ability to bind to plasminogen was stronger than EACA. 63 TXA was estimated to be 6–10 times more potent than EACA, which is consistent with our conclusion. 64
Differences between IV and IA Applications
TBL for patients with TKA ranges from 1300 to 1500 ml, 66 which consisted of apparent and hidden blood loss (HBL). 67 , 68 HBL composed half of TBL, 66 leading to the limb swelling and postoperative inflammation. HBL is formed from extravasation of blood into the tissues in significant amounts, residual blood in the joint, and blood loss owing to hemolysis. Topical TXA administration directly targets the bleeding site in a surgical wound and reduces the intraoperative and drainage blood loss, while intravenous administrations could reduce hidden and systemic blood loss. Regarding the safety profile of TXA and EACA regimens, the major concern is about potential risk of deep vein thrombosis or pulmonary embolism. Some hypothesized that topical application of TXA can be as efficacious as IV administration, with lower hidden blood loss and lower risk of venous thromboembolism because of lower systemic absorption. Nonetheless, none of EACA and TXA regimens, including intravenous and intra‐articular, showed increasing risk for PE and DVT according to our results, which is consistent with some studies and meta‐analyses. 16
Strengths and Limitations
There are some strengths in the current study. To our knowledge, this is the first Bayesian network meta‐analysis of TXA and EACA in TKA. Through subgroup analysis and exhaustive literature reviews, we determined the most effective and lowest dosage of TXA and EACA in IA and IV applications, and provided relative efficacy estimates of EACA and TXA in TKA. Our work could offer guidelines for orthopedic surgeons in selecting optimal regimen of antifibrinolytic agents.
Limitations existed in this network analysis. First, we cannot adequately take all treatment regimens into account for lack of original data, especially oral TXA and EACA. Second, a variety of factors other than TXA and EACA that had influence in blood loss still existed, such as supplement of tourniquet and anticoagulant drugs, which may result in unexpected heterogeneity in the network. Third, if there were fewer studies proving direct connections between some nodes, the results of comparisons will rely more heavily on indirect comparison.
Conclusions
In intra‐articular applications, 1.0 g TXA was most effective and enough to control bleeding in TKA. In intravenous applications (g), 1.0 g TXA or 10.0 g EACA were enough in reducing bleeding. In another intravenous subgroup (mg/kg), 30 mg/kg TXA or 150 mg/kg EACA were most effective and enough. None of regimens showed higher risk for PE/DVT, and TXA was at least five times more potent than EACA.
Conflict of Interest Statement
The authors declare that they have no conflict of interests.
Author Contributions
Bin Shen and Che Zheng conceived and designed the study. Jiawen Xu and Yuan Liu contributed to data acquisition. Che Zheng, Mingyang Li, Liming Wu and Yuangang Wu analyzed and interpreted the data. All authors read and approved the final manuscript.
Supporting information
Data S1. Supporting Information.
Data S2. Supporting Information.
Data S3. Supporting Information.
Data S4. Supporting Information.
Acknowledgments
We thank the staff from the Department of Orthopedics of West China Hospital, Sichuan University for technical assistance in data analysis. This study was supported through grants from the Key Project of Sichuan Science & Technology Department (2020YFS0139), and National Clinical Research Center for Geriatrics, West China Hospital Sichuan University (NO. Z2021JC002).
References
- 1. Sultan AA, Cantrell WA, Rose E, Surace P, Samuel LT, Chughtai M, et al. Total knee arthroplasty in the face of a previous tuberculosis infection of the knee: what do we know in 2018? Expert Rev Med Devices. 2018;15(10):717–24. [DOI] [PubMed] [Google Scholar]
- 2. Worland RL, Johnson G, Alemparte J, Jessup DE, Keenan J, Norambuena N. Ten‐to‐fourteen‐year survival and functional analysis of the AGC total knee replacement system. Knee. 2002;9(2):133–7. [DOI] [PubMed] [Google Scholar]
- 3. Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Jt Surg Am. 2018;100(17):1455–60. [DOI] [PubMed] [Google Scholar]
- 4. Sculco TP. Global blood management in orthopaedic surgery. Clin Orthop Relat Res. 1998;357:43–9. [DOI] [PubMed] [Google Scholar]
- 5. Cardone D, Klein AA. Perioperative blood conservation. Eur J Anaesthesiol. 2009;26(9):722–9. [DOI] [PubMed] [Google Scholar]
- 6. Kwak J, Wilkey AL, Abdalla M, Joshi R, Roman PEF, Greilich PE. Perioperative blood conservation: guidelines to practice. Adv Anesth. 2019;37:1–34. [DOI] [PubMed] [Google Scholar]
- 7. Reikerås O, Clementsen T. Time course of thrombosis and fibrinolysis in total knee arthroplasty with tourniquet application. Local versus systemic activations. J Thromb Thrombolysis. 2009;28(4):425–8. [DOI] [PubMed] [Google Scholar]
- 8. Sassoon A, Nam D, Jackups R, Johnson SR, Nunley RM, Barrack RL. Tranexamic acid: optimal blood loss management in surface replacement arthroplasty. Bone Jt J. 2016;98‐b(2):173–8. [DOI] [PubMed] [Google Scholar]
- 9. Huang F, Wu Y, Yin Z, Ma G, Chang J. A systematic review and meta‐analysis of the use of antifibrinolytic agents in total hip arthroplasty. Hip Int. 2015;25(6):502–9. [DOI] [PubMed] [Google Scholar]
- 10. Gandhi R, Evans HMK, Mahomed SR, Mahomed NN. Tranexamic acid and the reduction of blood loss in total knee and hip arthroplasty: a meta‐analysis. BMC Res Notes. 2013;6:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pachauri A, Acharya KK, Tiwari AK. The effect of tranexamic acid on hemoglobin levels during total knee arthroplasty. Am J Ther. 2014;21(5):366–70. [DOI] [PubMed] [Google Scholar]
- 12. Maniar RN, Kumar G, Singhi T, Nayak RM, Maniar PR. Most effective regimen of tranexamic acid in knee arthroplasty: a prospective randomized controlled study in 240 patients. Clin Orthop Relat Res. 2012;470(9):2605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ray M et al. Aprotinin and epsilon aminocaproic acid are effective in reducing blood loss after primary total hip arthroplasty – a prospective randomized double‐blind placebo‐controlled study. J Thromb Haemost. 2005;3(7):1421–7. [DOI] [PubMed] [Google Scholar]
- 14. Oppenheimer AJ, Ranganathan K, Levi B, Strahle JM, Kapurch J, Muraszko KM, et al. Minimizing transfusions in primary cranial vault remodeling: the role of aminocaproic acid. J Craniofac Surg. 2014;25(1):82–6. [DOI] [PubMed] [Google Scholar]
- 15. Martin K, Knorr J, Breuer T, Gertler R, MacGuill M, Lange R, et al. Seizures after open heart surgery: comparison of ε‐aminocaproic acid and tranexamic acid. J Cardiothorac Vasc Anesth. 2011;25(1):20–5. [DOI] [PubMed] [Google Scholar]
- 16. Xu S, Chen JY, Zheng Q, Lo NN, Chia SL, Tay KJD, et al. The safest and most efficacious route of tranexamic acid administration in total joint arthroplasty: a systematic review and network meta‐analysis. Thromb Res. 2019;176:61–6. [DOI] [PubMed] [Google Scholar]
- 17. Sun C, Zhang X, Chen L, Deng J, Ma Q, Cai X, et al. Comparison of oral versus intravenous tranexamic acid in total knee and hip arthroplasty: a GRADE analysis and meta‐analysis. Medicine. 2020;99(44):e22999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ye W, Liu Y, Liu WF, Li XL, Shao J. The optimal regimen of oral tranexamic acid administration for primary total knee/hip replacement: a meta‐analysis and narrative review of a randomized controlled trial. J Orthop Surg Res. 2020;15(1):457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Riaz O, Aqil A, Asmar S, Vanker R, Hahnel J, Brew C, et al. Epsilon‐aminocaproic acid versus tranexamic acid in total knee arthroplasty: a meta‐analysis study. J Orthop Traumatol. 2019;20(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li Z et al. Tranexamic acid versus epsilon‐aminocaproic acid in total knee arthroplasty: a meta‐analysis. J Healthcare Eng. 2021;2021:1758066. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Fillingham YA, Ramkumar DB, Jevsevar DS, Yates AJ, Shores P, Mullen K, et al. The efficacy of tranexamic acid in total knee arthroplasty: a network meta‐analysis. J Arthroplasty. 2018;33(10):3090–3098.e1. [DOI] [PubMed] [Google Scholar]
- 22. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 23. Dias S et al. NICE decision support unit technical support documents. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta‐Analysis of Randomised Controlled Trials. London: National Institute for Health and Care Excellence (NICE); 2014. [PubMed] [Google Scholar]
- 24. Camarasa MA, Ollé G, Serra‐Prat M, Martín A, Sánchez M, Ricós P, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96(5):576–82. [DOI] [PubMed] [Google Scholar]
- 25. Orpen NM, Little C, Walker G, Crawfurd EJP. Tranexamic acid reduces early post‐operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106–10. [DOI] [PubMed] [Google Scholar]
- 26. Wong J, Abrishami A, el Beheiry H, Mahomed NN, Roderick Davey J, Gandhi R, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Jt Surg Am. 2010;92(15):2503–13. [DOI] [PubMed] [Google Scholar]
- 27. Roy SP, Tanki UF, Dutta A, Jain SK, Nagi ON. Efficacy of intra‐articular tranexamic acid in blood loss reduction following primary unilateral total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2494–501. [DOI] [PubMed] [Google Scholar]
- 28. Georgiadis AG, Muh SJ, Silverton CD, Weir RM, Laker MW. A prospective double‐blind placebo‐controlled trial of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 Suppl):78–82. [DOI] [PubMed] [Google Scholar]
- 29. Iwai T, Tsuji S, Tomita T, Sugamoto K, Hideki Y, Hamada M. Repeat‐dose intravenous tranexamic acid further decreases blood loss in total knee arthroplasty. Int Orthop. 2013;37(3):441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sa‐Ngasoongsong P et al. Efficacy of low‐dose intra‐articular tranexamic acid in total knee replacement; a prospective triple‐blinded randomized controlled trial. BMC Musculoskeletal Disord. 2013;14:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seo JG, Moon YW, Park SH, Kim SM, Ko KR. The comparative efficacies of intra‐articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1869–74. [DOI] [PubMed] [Google Scholar]
- 32. Aguilera X, Martínez‐Zapata MJ, Hinarejos P, Jordán M, Leal J, González JC, et al. Topical and intravenous tranexamic acid reduce blood loss compared to routine hemostasis in total knee arthroplasty: a multicenter, randomized, controlled trial. Arch Orthop Trauma Surg. 2015;135(7):1017–25. [DOI] [PubMed] [Google Scholar]
- 33. Lin SY, Chen CH, Fu YC, Huang PJ, Chang JK, Huang HT. The efficacy of combined use of intraarticular and intravenous tranexamic acid on reducing blood loss and transfusion rate in total knee arthroplasty. J Arthroplasty. 2015;30(5):776–80. [DOI] [PubMed] [Google Scholar]
- 34. Wang CG, Sun ZH, Liu J, Cao JG, Li ZJ. Safety and efficacy of intra‐articular tranexamic acid injection without drainage on blood loss in total knee arthroplasty: a randomized clinical trial. Int J Surg. 2015;20:1–7. [DOI] [PubMed] [Google Scholar]
- 35. Akgül T, Büget M, Salduz A, Edipoğlu İS, Ekinci M, Küçükay S, et al. Efficacy of preoperative administration of single high dose intravenous tranexamic acid in reducing blood loss in total knee arthroplasty: a prospective clinical study. Acta Orthop Traumatol Turc. 2016;50(4):429–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tzatzairis TK, Drosos GI, Kotsios SE, Ververidis AN, Vogiatzaki TD, Kazakos KI. Intravenous vs topical tranexamic acid in total knee arthroplasty without tourniquet application: a randomized controlled study. J Arthroplasty. 2016;31(11):2465–70. [DOI] [PubMed] [Google Scholar]
- 37. Boese CK, Centeno L, Walters RW. Blood conservation using tranexamic acid is not superior to epsilon‐aminocaproic acid after total knee arthroplasty. J Bone Jt Surg Am. 2017;99(19):1621–8. [DOI] [PubMed] [Google Scholar]
- 38. Churchill JL et al. Comparing ε‐aminocaproic acid and tranexamic acid in reducing postoperative transfusions in total knee arthroplasty. J Knee Surg. 2017;30(5):460–6. [DOI] [PubMed] [Google Scholar]
- 39. Song EK, Seon JK, Prakash J, Seol YJ, Park YJ, Jin C. Combined administration of IV and topical tranexamic acid is not superior to either individually in primary navigated TKA. J Arthroplasty. 2017;32(1):37–42. [DOI] [PubMed] [Google Scholar]
- 40. Stowers MDJ et al. Tranexamic acid in knee surgery study‐a multicentered, randomized, controlled trial. J Arthroplasty. 2017;32(11):3379–84. [DOI] [PubMed] [Google Scholar]
- 41. Sun Q, Yu X, Wu JZ, Ge W, Cai M, Li S. Efficacy of a single dose and an additional dose of tranexamic acid in reduction of blood loss in total knee arthroplasty. J Arthroplasty. 2017;32(7):2108–12. [DOI] [PubMed] [Google Scholar]
- 42. Uğurlu M, Aksekili M, Çağlar C, Yüksel K, Şahin E, Akyol M. Effect of topical and intravenously applied tranexamic acid compared to control group on bleeding in primary unilateral total knee arthroplasty. J Knee Surg. 2017;30(2):152–7. [DOI] [PubMed] [Google Scholar]
- 43. Wang J, Wang Q, Zhang X, Wang Q. Intra‐articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385–9. [DOI] [PubMed] [Google Scholar]
- 44. Yen SH et al. Topical tranexamic acid reduces blood loss in minimally invasive total knee arthroplasty receiving rivaroxaban. Biomed Res Int. 2017;2017:9105645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yuan X, Li B, Wang Q, Zhang X. Comparison of 3 routes of Administration of Tranexamic acid on primary unilateral total knee arthroplasty: a prospective, randomized, controlled study. J Arthroplasty. 2017;32(9):2738–43. [DOI] [PubMed] [Google Scholar]
- 46. Arslan A, Görmeli G. Using intra‐articular tranexamic acid in total knee replacement surgery with and without bleeding control: a prospective randomized double‐blind study. Eur Rev Med Pharmacol Sci. 2018;22(18):6127–32. [DOI] [PubMed] [Google Scholar]
- 47. Liu W, Yang C, Huang X, Liu R. Tranexamic acid reduces occult blood loss, blood transfusion, and improves recovery of knee function after total knee arthroplasty: a comparative study. J Knee Surg. 2018;31(3):239–46. [DOI] [PubMed] [Google Scholar]
- 48. López‐Hualda Á, Dauder‐Gallego C, Ferreño‐Márquez D, Martínez‐Martín J. Efficacy and safety of topical tranexamic acid in knee arthroplasty. Med Clin. 2018;151(11):431–4. [DOI] [PubMed] [Google Scholar]
- 49. Bradley KE, Ryan SP, Penrose CT, Grant SA, Wellman SS, Attarian DE, et al. Tranexamic acid or epsilon‐aminocaproic acid in total joint arthroplasty? A randomized controlled trial. Bone Jt J. 2019;101:1093–9. [DOI] [PubMed] [Google Scholar]
- 50. Tille E, Mysliwietz J, Beyer F, Postler A, Lützner J. Intraarticular use of tranexamic acid reduces blood loss and transfusion rate after primary total knee arthroplasty. BMC Musculoskeletal Disord. 2019;20(1):341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang S, Xie J, Cao G, Lei Y, Huang Q, Pei F. Six‐dose intravenous tranexamic acid regimen further inhibits postoperative fibrinolysis and reduces hidden blood loss following total knee arthroplasty. J Knee Surg. 2021;34(2):224–32. [DOI] [PubMed] [Google Scholar]
- 52. Kim JK, Park JY, Lee DY, Ro DH, Han HS, Lee MC. Optimal dose of topical tranexamic acid considering efficacy and safety in total knee arthroplasty: a randomized controlled study. Knee Surg Sports Traumatol Arthrosc. 2021;29(10):3409–17. [DOI] [PubMed] [Google Scholar]
- 53. Xue CX, Yao YF, Lv H, Cheng L, Jing JH. Efficacy and safety of postoperative intravenous tranexamic acid in total knee arthroplasty: a prospective randomized controlled study. Orthop Surg. 2021;13(8):2227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guerreiro JPF et al. Intraarticular epsilon aminocaproic acid versus tranexamic acid in total knee arthroplasty. Acta Ortop Bras. 2021;29(6):312–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lei Y, Xie J, Huang Q, Huang W, Pei F. Is there a role of tranexamic acid in rheumatoid arthritis with total knee arthroplasty? Findings from a multicenter prospective cohort study in China. Arch Orthop Trauma Surg. 2021;141(3):489–96. [DOI] [PubMed] [Google Scholar]
- 56. Magill P, Hill JC, Bryce L, Martin U, Dorman A, Hogg R, et al. Oral tranexamic acid for an additional 24 hours postoperatively versus a single preoperative intravenous dose for reducing blood loss in total knee arthroplasty: results of a randomized controlled trial (TRAC‐24). Bone Jt J. 2021;103(10):1595–603. [DOI] [PubMed] [Google Scholar]
- 57. Harper RA, Sucher MG, Giordani M, Nedopil AJ. Topically applied epsilon‐aminocaproic acid reduces blood loss and length of hospital stay after total knee arthroplasty. Orthopedics. 2017;40(6):e1044–9. [DOI] [PubMed] [Google Scholar]
- 58. Hobbs JC, Welsby IJ, Green CL, Dhakal IB, Wellman SS. Epsilon aminocaproic acid to reduce blood loss and transfusion after total hip and total knee arthroplasty. J Arthroplasty. 2018;33(1):55–60. [DOI] [PubMed] [Google Scholar]
- 59. Lum ZC, Manoukian MAC, Pacheco CS, Nedopil AJ, Giordani M, Meehan JP. Intravenous tranexamic acid versus topical aminocaproic acid: which method has the least blood loss and transfusion rates? J Am Acad Orthop Surg Glob Res Rev. 2018;2(11):e072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Churchill JL, Toney VA, Truchan S, Anderson MJ. Using aminocaproic acid to reduce blood loss after primary unilateral total knee arthroplasty. Am J Orthop. 2016;45(5):E245–8. [PubMed] [Google Scholar]
- 61. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224–32. [PubMed] [Google Scholar]
- 62. Brecher ME, Monk T, Goodnough LT. A standardized method for calculating blood loss. Transfusion. 1997;37(10):1070–4. [DOI] [PubMed] [Google Scholar]
- 63. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132–8. [PubMed] [Google Scholar]
- 64. Hardy JF, Desroches J. Natural and synthetic antifibrinolytics in cardiac surgery. Can J Anaesth. 1992;39(4):353–65. [DOI] [PubMed] [Google Scholar]
- 65. Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57(6):1005–32. [DOI] [PubMed] [Google Scholar]
- 66. Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee arthroplasty? Correct blood loss management should take hidden loss into account. Knee. 2000;7(3):151–5. [DOI] [PubMed] [Google Scholar]
- 67. Turan S, Bingöl O. Is tranexamic acid effective on hidden blood loss in patients during total knee arthroplasty? Jt Dis Relat Surg. 2020;31(3):488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Jt Surg Br. 2004;86(4):561–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Data S2. Supporting Information.
Data S3. Supporting Information.
Data S4. Supporting Information.


