Abstract
Collagen is one of the main components of the extracellular matrix of the dermis and articular cartilage and influences the body’s mechanical, organizational, and tissue formation properties. Produced from food industry by-products, it is considered a nutraceutical product widely used as an ingredient or supplement in food, pharmaceutical, and cosmetic industries. This study aimed to conduct a literature review on the scientific evidence regarding the beneficial effects of collagen consumption in the treatment of skin and orthopedic diseases. Literature data have shown that hydrolyzed collagen supplementation promotes skin changes, such as decreased wrinkle formation; increased skin elasticity; increased hydration; increased collagen content, density, and synthesis, which are factors closely associated with aging-related skin damage. Regarding orthopedic changes, collagen supplementation increases bone strength, density, and mass; improves joint stiffness/mobility, and functionality; and reduces pain. These aspects are associated with bone loss due to aging and damage caused by strenuous physical activity. Thus, this review addresses the economic and health potential of this source of amino acids and bioactive peptides extracted from food industry by-products.
Keywords: Skin, Joint, Bone metabolism, Senescence, Aging
1. Introduction
The development of science, improvements and innovations in the field and industry of food and access to sanitation and education contribute to increase human life expectancy. However, access to these factors is not uniform worldwide, making it a privilege of developed countries, where life expectancy is higher than 80 years old while in some African countries, in 2019, life expectancy ranged from 53 to 76 years old [1]. The increase in life expectancy carries with it senescence, promoting progressive and cumulative functional and structural changes in the human body [2].
Collagen is a very important protein in the aging process, since it influences the structural, mechanical, organizational, and tissue-building properties of the body [3]. This protein interacts with cells by several families of receptors, regulating their proliferation, migration, and differentiation, and some types of collagen have a restricted distribution in tissues and, therefore, a specific biological function [4]. Besides being a source of amino acids, collagen products can perform a biological activity in extracellular matrix cells by their bioactive peptides, which justifies their application in dietary supplements and pharmaceutical preparations [5].
Collagen is extracted from industrial by-products, such as bones, cartilage, tendons, and the skin of cattle, pigs, chickens, fish, or other marine organisms, and may undergo a hydrolysis process to obtain bioactive peptides [[6], [7], [8]]. The variables and methods applied in protein hydrolysis influence the composition of the peptides present in the final product and can impact the molecular weight, the amino acid composition, and the solubility and functionality of the product [6,7,9]. The molecular weight of collagen peptides can range from 0.3 to 8 kDa [10] and low-molecular-weight peptides usually have better bioactivity compared with higher-molecular-weight peptides [11]. According to Sibilla et al. [10], the advantage of using collagen hydrolysates over native collagen is their high digestibility rate, which contributes to increased absorption, distribution, and use in the human body.
The greatest beneficial effects of collagen peptides occur in populations with collagen degradationor at greater risk of developing this type of condition, such as bone and cartilage loss resulting from aging, or even from strenuous physical activity, joint impact, excess weight, hormonal changes, trauma, burns, aggressive cancer therapy, and skin and dental implants [12]. Therefore, collagen can be considered a nutraceutical product, since, besides its nutritional function (amino acid supply), it promotes physiological benefits and protection against some diseases. Notably, collagen is a low-tryptophan protein, an essential amino acid for humans. The study by Bordin and Naves [13] showed that replacing 20%–25% of this protein in the diet of male Wistar rats decreased feed efficiency and the bioavailability of high biological value proteins (casein) existing in their diet. However, Paul, Lesser, and Oesser [14] observed that adding up to 54% of collagen peptides to the standard American diet does not change dietary protein quality (PDCAAS equal to 0.75). The authors also indicate that collagen is a source of conditionally essential amino acids (glycine and proline), which are important in some physiological situations.
Considering the beneficial properties and clinical relevance of using hydrolyzed collagen for health promotion, this study aims to perform a literature review on the beneficial effects of the consumption of collagen peptides on skin and orthopedic changes. We searched articles from the PubMed and ScienceDirect databases using the following keywords: collagen, collagen supplementation, collagen supplement, dermatological alterations, and orthopedic diseases. We selected studies published from 2000 to 2022, including all research that has evaluated the effects of collagen (native or hydrolyzed) supplementation in clinical trials or experimental protocols without the addition of any other nutrient or substance.
1.1. Structure and characterization of collagen
In mammals, 25%–35% of the total protein mass corresponds to collagen [10]. To date, 29 types of collagen [15] have been described so far. In the human body, this protein can be found in bones, tendons, ligaments, hair, skin, and muscles [16]. Types I, II, and III correspond to 80%–90% of the total collagen found in the human body [15] and type I is the most abundant in the skin (80%). Type III collagen corresponds to 15% [10]. In cartilage, type II collagen predominates (90–95%) in the formation of the extracellular matrix. Other types of collagen (I, IV, V, VI, IX, and XI) contribute to the formation and stabilization of the type II collagen fibril network [17]. The sequence of amino acids and covalent structures found in the molecule, as well as post-transcriptional modifications in amino acid side chains by hydroxylation, glycosylation, and cross-linking, characterize the collagen structure [11]. The collagen molecule has a trimetric nature, allowing combinations of pro-α chains. The triple-helix structure is a common feature in collagen, which can vary its incidence from 96% for type I collagen to 10% for type XII collagen [4]. A single type of collagen can have multiple isoforms and supramolecular structures with multiple αchains, which increases the diversity of the existing family [4]. For example, type I collagen is mostly a heterotrimer of two α1 chains and one α2 chain, but it can also be a homotrimer of three α1 chains. Types II, III, and VII are exclusively homotrimers whereas type IV has six different α chains available for combination and production of several isoforms [15].
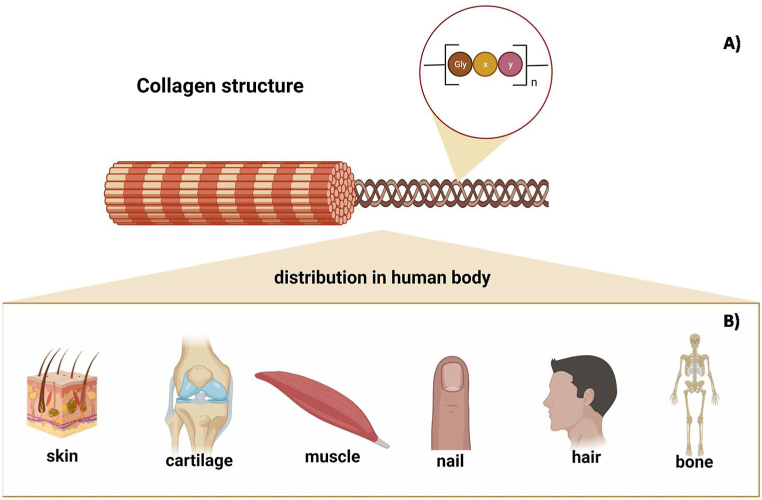
Besides the triple helix, the collagen family has two common characteristics: the repetition of amino acids [Gly – X–Y]n with and without interruptions, with the amino acids proline and its hydroxylated form (hydroxyproline) occupying the X and Y positions (Fig. 1) [15].
Fig. 1.
Chemical structure (A) and distribution of collagen in the body (B). The sequence of amino acids [Gly – X–Y]n with and without interruptions is a characteristic of the collagen family and the amino acids proline and its hydroxylated form (hydroxyproline) occupy the X and Y positions. n: number of times that this sequence appears in the protein structure. Created by the authors using BioRender.com.
2. Digestion and absorption of collagen
Similar to all proteins ingested as food or food supplement, when collagen is digested in the gastrointestinal tract, it releases amino acids and peptides in the small intestine to be absorbed. The degradation of the polymeric structure of collagen is the first step of digestion, and forms peptides, especially dipeptides, tripeptides, or free amino acids. Before the ingested components reach the blood, they cross many barriers, such as enzymatic degradation and affinity for membrane transporters [18]. During digestion, several proteases existing in the small intestine, such as the pancreatic protease, and peptidases are involved in the degradation of the polymeric structure of proteins [10]. Possibly, the presence of proline and hydroxyproline in its structure is responsible for the formation of bioactive peptides, since both provide resistance against the action of proteases, limiting hydrolysis [19]. The bioaccessibility of amino acids and peptides in the intestine, their absorption rate and availability in the bloodstream define their bioavailability for the regulation of metabolic processes in target tissues [20].
According to Daniel [21], protein digestion results in a huge variety and quantity of short-chain peptides (di- and tripeptides), which are absorbed intact by the PEPT1 peptidyl carrier protein. Peptide transport is enantioselective, involving variable proton-substrate stoichiometry for peptides with neutral, mono-, or polyvalent charge [21]. For many years, only amino acids, di-, and tripeptides were believed to be absorbed in the intestine. However, as Urao [22] showed in his study a different pattern of intestinal permeability for particles with different molecular weights, the literature currently presents different absorption mechanisms, showing that oligopeptides can also be absorbed by passive diffusion across cell junctions [23]. According to Sibilla et al. [10], considerable evidence shows that peptides can be absorbed, since Pro-Hyp is the main dipeptide found in human plasma after ingestion of hydrolyzed collagen.
Yazaki et al. [24] identified by chromatography 17 collagen-derived peptides in the serum of adults (31.5 ± 6.5 years), especially high levels of the Gly-Pro-Hyp tripeptide after seven days of daily consumption of 300 mg/kg of hydrolyzed collagen. A similar study by Shigemura et al. [25] identified by chromatography peptides with hydroxyproline (Pro-Hyp) in blood after consumption of hydrolyzed collagen. Moreover, the authors showed the maximum concentration of collagen peptides in blood 2 h after oral ingestion, with a subsequent decrease in serum concentration after this period. The maximum absorption data obtained by Shigemura et al. [25] corroborate the study by Iwaia et al. [26], who found the maximum concentration of peptides one to 2 h after oral ingestion of hydrolyzed collagen, with the concentration decreasing by half after 4 h. These results confirm the resistance of proline and hydroxyproline to the action of proteases in the intestine.
3. Oral supplementation of hydrolyzed collagen in the treatment of skin changes
The skin is the largest organ in the human body and represents the main barrier to the external environment. Collagen, elastin, and hyaluronic acid are the main components of the skin and play an important role in maintaining its structure and hydration. Skin collagen is mainly produced by fibroblasts [10], which produce three components of the extracellular matrix of the dermis: collagen fibers, elastic fibers, and proteoglycans. The collagen-rich extracellular matrix builds and repairs the structure of skin components and collagen is the most abundant connective tissue in the dermis, besides being responsible for its strength and resilience [27,28].
However, individuals live under the constant influence of internal and external factors, which affect their aging process and some factors contribute to damage or loss of skin functionality (Fig. 2). Solar and ultraviolet radiation, air pollution, tobacco smoke, poor nutrition, and the use of cosmetic products are examples of external or environmental factors [29]. In turn, intrinsic changes occur due to genetic determinants that differ between groups and different anatomical sites in the same individual.
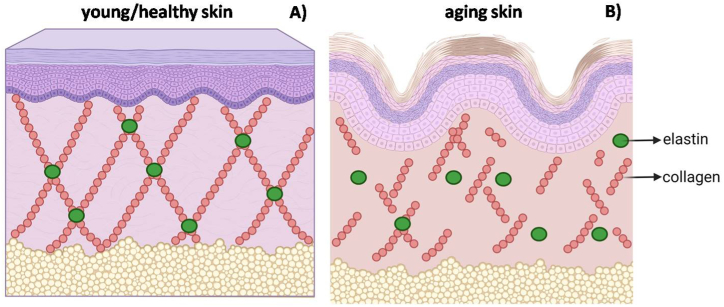
Fig. 2.
Structure of young, health skin (A) and the effect of aging on its structure (B). The interaction between collagen and elastin guarantees skin strength and elasticity, however, with aging or due to exposure to other extrinsic factors (nutrition, sun exposure, infrared radiation, visible light, air pollution, for example), collagen production decreases, destabilizing this interaction and, consequently, reducing strength and skin elasticity. Created by the authors using BioRender.com.
According to Fensk [30], in the different layers of the skin, intrinsic aging causes structural and functional changes, making the skin thinner and, thus, reducing its protective barrier function. Moreover, Rittié and Fisher [31] state that changes in the structure of type I collagen (the most abundant structural protein in the skin) is a sign of a chronologically aged and photo-aged human skin and contributes to a decrease in its resistance and, consequently, wrinkle formation. Aging leads to a decrease in both the number of collagen bundles in the skin and their synthesis [32]. Lupu et al. [8] also suggest that these changes may contribute to increased skin flaccidity, fragility, and dryness. Aging can also interfere with wound healing, skin pigmentation, vasculature, and immunity [31].
As aforementioned, several situations can lead to loss of collagen in the skin. Table 1, shows the beneficial effects of consuming oral hydrolyzed collagen supplements on skin changes based on scientific research. According to Sibilla et al. [10], in the dermis, hydrolyzed collagen has a dual-action mechanism, either providing amino acids for the synthesis of endogenous collagen and elastin fibers or stimulating the production of new collagen, elastin, and hyaluronic acid by bioactive peptides binding to fibroblast membrane receptors. These data can be explained by histological evidence. Czakja et al. [41], in their study on hydrolyzed fish collagen supplementation, showed by histological analysis changes in the skin structure with reduced solar elastosis and improved organization of collagen fibers. Using another source of collagen extracted from fish (Pangasius hypophthalmus), Evans et al. [33] evaluated 85 women aged 45–60 years in a 12-week randomized clinical trial. By administering 10 g of hydrolyzed collagen (Vinh Wellness Collagen, Vinh Hoan Corporation), the authors observed improvement in skin elasticity, hydration, brightness, firmness, and the presence of wrinkles.
Table 1.
Studies that used collagen supplementation in the treatment of dermatological changes.
| Collagen | Design | Population (n) | Period | Dose/day | Result | Reference |
|---|---|---|---|---|---|---|
| HC derived from fish (Pangasius hypophthalmus) | T-RCT | Women 45–60 years (45) | 12 weeks | 10 g | ↓face wrinkles score; ↑ elasticity, hydration, shine, and firmness | [33] |
| HC containing dipeptides (Pro-Hyp and Hyp-Gly) | D-RCT | Chinese healthy women 37–48 years (56) | 8 weeks | 2.5 g | ↑ skin hydration; ↑ facial skin elasticity; ↓ facial skin roughness | [34] |
| HC had a low ratio of dipeptide-to-product content (L-CP) and a high ratio of dipeptide-to-product content (H-CP) | D-RCT | Chinese women 35–55 years (85) | 8 weeks | 5 g | ↑ skin moisture; ↓ wrinkles area; ↓ roughness H-CP intake additionally ↓ number of wrinkles; ↓ wrinkle depth |
[35] |
| CP with 15% tripeptide form | S-RCT | Healthy Korean women and men 30–48 years (32) | 12 weeks | 3 g | ↑ stratum corneum hydration; ↔ TEWL; ↑ skin elasticity; ↔ skin erythema and pigmentation | [36] |
| CP of fish origin (PeptanF) and porcine origin (PeptanP) | D-RCT | Adult and middle-aged Japanese women 40–59 years (33) | 8 weeks | 10 g | ↑ skin hydration | [37] |
| HC with peptides of various sizes of swine-origin of the brand VERISOL@ | D-RCT | Women 35–55 years (69) | 8 weeks | 2.5–5 g | ↔ between the two collagen dosages; ↔ skin moisture and water evaporation; ↔ smoothness of the skin; ↑skin elasticity | [38] |
| HC containing more than 15% tripeptides, including 3% of Gly-Pro-Hyp. | D-RCT | Women 40–60 years (53) | 12 weeks | 1.0 g | ↑ hydration after 6 and 8 weeks of treatment; ↓ visual wrinkle after 12 weeks; ↑ skin elasticity (general elasticity and liquid elasticity) after 12 weeks | [39] |
| CP derived from fish scales (NittaGelatin Inc., India) | D-RCT | Healthy women 40–51 years (71) | 12 weeks | 3.0 g | ↑skin moisture; ↑ elasticity (crude, liquid, and biological elasticity); ↓ roughness. | [40] |
HC: hydrolyzed collagen; CP: collagen peptide; T-RCT: randomized, triple-blind and placebo controlled clinical trial; D-RCT: randomized, double-blind and placebo controlled clinical trial; S-RCT: randomized, single-blind controlled clinical trial; ↑: increase, ↓: decrease, ↔: no difference; TEWL: transepidermal water loss.
Choi et al. [42], after evaluating 11 randomized clinical trials in their systematic review, showed that the consumption of oral hydrolyzed collagen supplement increases skin elasticity, hydration, and collagen density, improves wound healing, and protects the skin against aging. Barati et al. [43], in their systematic review, analyzed 10 randomized clinical trials and concluded that the consumption of both intact and hydrolyzed collagen improves clinical manifestations of skin health by either increasing the synthesis of extracellular matrix or the interaction of regulatory T-cells and type 2 macrophages in maintaining the skin immune response to endogenous collagen. De Miranda [44], in their systematic review and meta-analysis, corroborates the previous results, as the analysis of 19 studies (1124 patients) showed that after 60–90 days of collagen consumption, skin elasticity and density increased and facial wrinkles reduced. However, the authors observed a great heterogeneity between studies, especially regarding the type of collagen used, treatment time, and dose.
Rustad et al. [45] highlights the importance of a critical evaluation of the literature, since the authors relate their results to studies with small samples and a great variation in administered doses, consumption time, and types of products (different hydrolysis processes generate different peptides). Moreover, conflict of interest is an essential issue, as many studies are funded by collagen-producing industries. Collagen, as well as other nutritional supplements, acts as an adjuvant in the treatment. Therefore, considering all the intrinsic and extrinsic factors that contribute to senescence, changes in lifestyle and environment are also important for the prevention and treatment of skin changes.
Among the studies presented in Table 1, Table 2, the results regarding skin changes are associated with the consumption of bioactive collagen peptides, since all studies used hydrolyzed collagen or peptides in their clinical trials and experimental protocols. Although the interest of men in the field of aesthetics has increased in recent years, all studies were carried out only with women, mostly aged 30–60 years, with a variation in treatment time (8–12 weeks) and administered dose (1–10 g/day). Thus, despite evidence that the consumption of hydrolyzed collagen can help minimize skin changes, especially those associated with aging, no study presented follow-up results, such as how long the changes last after 12 weeks of supplementation or the existence of any nutritional or physiological damage to the chronic collagen consumption. These and other questions remain open and unanswered.
Table 2.
Studies on the effect of consumption of hydrolyzed collagen in animal models.
| Collagen | Animal Model | Period | Dose/day | Result | Reference |
|---|---|---|---|---|---|
| Type I - H | Male C57BL/6J mice (OA) | 12 Weeks | Standard feed plus Type I–H bovine (200 Da) 3.8 mg or 38 mg | ↑cartilage area; ↑chondrocytes; ↑proteoglycan matrix; ↓apoptosis; ↓MMP13 protein; ↓synovial hyperplasia and TnfmRNA; ↓inflammation | [46] |
| HC | Three months of age female Wistar rat (OVA) | 8 Weeks | Standard diet formulated according to AIN 93-M plus 5 or 10 times the recommended human consumption of HC calculated according to rat weight, dietary protein content 13.15% and 14.30% | ↑bone mass; ↑osteocalcin; ↑mechanical strength of the vertebrae; ↑vertebrae protein content | [47] |
| HFC | Five-week-old male Wistar rat in growth phase undergoing exercises | 11 Weeks | Diet with 20% protein, 30% HC (1 KDa) or diet with 40% protein, 30% HC (1 KDa) | ↑bone mass; ↔bone strength; ↑ exercise effect | [48] |
| CP | Eight-week-old maleSprague-Dawley rats (OA) | 12 weeks | Gavage 0.8 g/kg.bw or 1.6 g/kg.bw Atlantic salmon (Salmo salar) CP | ↓serum IL-6 and IL-17; ↓ cartilage IL-1βand TNF-α; ↓cartilage degeneration | [49] |
| HC | Nine-week-old female Hos:HR-1 hairless mice exposed to UV-B irradiation | 11 days | Gavage 2.0 g/kg.bw of fish scale HC | ↓ TEWL on days 2, 3, and 4 | [50] |
| 7 weeks | Diet with 2 g of fish scale HC per 100 g of the AIN-93G diet | ↓ TEWL at weeks 4 and 6; ↑ water content at weeks 2, 4, and 6; ↑ skin elasticity at week 6; ↑ dermal hyaluronic acid content | |||
| CTP | Five-week-old female hairless mice (SKH-1) exposed to UV-B irradiation | 14 weeks | Gavage 167 mg/kg/day or 333 mg/kg/day | ↓ wrinkle formation induced by UVB irradiation; ↓ collagenase (MMP3 and -13) and gelatinase (MMP-2 and -9); ↓ skinfold thickness; ↑ skin hydration; ↑ collagen fibers and hydroxyproline; ↑ skin elasticity; ↓ erythema formation | [51] |
4. Oral supplementation of hydrolyzed collagen in the treatment of orthopedic alterations
The loss of collagen in osteoarticular tissues is a physiological phenomenon of multifactorial etiology and its intensification is related to senescence, hormonal profile, adiposity, inflammatory processes, immobility, mechanical overload, and joint damage [52,53]. Physical activity is regarded as a modifiable protective factor for the loss of collagen and many other comorbidities [54]. However, factors inherent to physical exercise, such as contact, impact, repetitive strain, and joint overload, increase the chance of joint injuries in active individuals [53].
Joints can be affected by several pathological conditions. Osteoarthritis is the most prevalent and can be characterized by slow and gradual cartilage degradation and joint space narrowing. This condition can evolve over decades and pain and progressive loss of joint function are the main symptoms [53,55]. Joint discomfort, usually associated with pain, reduces mobility and the ability to perform routine tasks, directly affecting the individual’s quality of life, especially when it occurs in the hips, knees, and lower back [56].
Joint diseases have no effective cure, so current resources focus mainly on reducing pain, inflammation, and joint stiffness [55]. In general, joint damage induces microlesions in connective tissue and reduce the ability of fibroblasts to synthesize new tissue (negative nitrogen balance), creating a deficit in the renewal rate (catabolic processes predominate over anabolic processes) and leading to degeneration of tissue matrix [53]. This deficit, along with joint biomechanical/functional stress, can induce an inflammatory response, which, in turn, can worsen, ultimately evolving into the irreversible loss of joint functional capacity [57].
Collagen is a low biological value protein, since its amino acid composition is poor in essential amino acids. However, it has a positive intrinsic value because its amino acid composition is equivalent to that of human connective tissue. Cartilage consists primarily of the extracellular matrix, a network of proteins, such as type II collagen, interacting with polysaccharides, such as hyaluronic acid and chondroitin sulfate—all synthesized and secreted by chondrocytes. During normal cartilage restoration (metabolism) in healthy joints, the rate of extracellular matrix production balances with its degradation rate, ensuring homeostasis achieved by continued cartilage restoration [58].
C-terminal cross-linked telopeptides of type II collagen (CTX-II) are one of the main cartilage degradation biomarkers. CTX-II levels are high in patients with joint diseases, but also elevated by strenuous exercise, high-impact activities, high in postmenopausal women and individuals with overweight/obesity [[57], [58], [59]].
Ruff et al. [57] found that the treatment with 0.5 g of hydrolyzed collagen from eggshell membrane significantly mitigated CTX-II levels. Moreover, the consumption of hydrolyzed collagen caused a reducing effect on biomarkers of muscle damage, inflammation, and apoptosis [46,60]. However, for Konig et al. [61] and Clifford et al. [62], CTX-I and B-CTX levels remained unchanged after treatment with hydrolyzed collagen.
Despite this, evidence supporting the idea of consuming hydrolyzed collagen to reduce joint pain has been increasing (Table 3). The popularization of studies on pain reduction is mainly related to the easy application and low cost use of the visual pain scale, making its implementation possible in a large number of studies. Furthermore, its self-applied results are similar to those obtained by experts [63].
Table 3.
Studies with functional and biochemical changes induced by consumption of hydrolyzed collagen.
| Collagen | Design | Population (n) | Period | Dose/day | Result | Reference |
|---|---|---|---|---|---|---|
| HC | D-RM | Young men 19–29 years (24) | 1 week | 20 g | ↓pain; ↑ muscle recovery (48 h after exercise); ↔β-CTX; ↔P1NP; ↔bone collagen inflammation and synthesis | [62] |
| BioCP | D-RM | Young athletes 18–30 years with knee discomfort during sport, both gender (139) | 12 weeks | 5 g | ↓pain related to physical activity; ↔ pain at rest; ↔mobility; ↓ other medication options after BCP treatment | [63] |
| UC-II | D-RM | Adults 40–75 years with OA, both gender (186) | 25 weeks + 5 days | 0.4 g | ↓WOMAC; ↓pain; ↓stiffness; ↑mobility; ↓VAS score; ↓ LFI Score,↔ serum markers and knee flexibility | [64] |
| HC (Biocell Collagen®) | D-RCT | Adults 40–70 years with OA, both gender (68) | 10 weeks | 2 g | ↓pain (VAS score); ↓WOMAC; ↑PA | [65] |
| Type II | D-RCT | Adults 18–65 years with RA, both gender (454) | 24 weeks | 0.1 mg | ↓pain; ↓joint stiffness; ↓ number of tender and swollen joints; ↓HAQ | [66] |
| SCP | D-RCT | Athletes 26.9 ± 9.1 years with ankle instability, both gender (50) | 24 weeks | 5 g | ↑ subjective ankle stability; ↓ joint injuries over time; ↔ankle stiffness; ↔feeding behavior | [67] |
| HC | D-RM | Caucasian adults 50 years or over with joint pain, both gender (144) | 24 weeks | 1.2 g | ↔pain; ↑clinical response (VAS score) | [68] |
| PCP and BCP | D-RCT | Adults and middle-aged individuals 30–65 years diagnosed with knee OA, both gender (30) | 13 weeks | 5 g | ↓ WOMAC, VAS and QOL | [69] |
| CP (Peptan B2000®) | D-RCT | Middle-aged to elderly individuals 50–75 years physically active, both gender (167) | 12 weeks | 10 g | ↔ knee joint pain and knee function | [70] |
| HC | D-RCT | Young adults 20.1 ± 1.47 years, physically active, both gender (72) | 24 weeks | 10 g | ↓ joint pain; ↓ use of alternative therapies | [71] |
| HC (Fortigel®) | D-RCT | Middle-aged to elderly individuals 49 years or older with mild to moderate severity knee OA (29) | 48 weeks | 10 g | ↑dGEMRIC score in medial and lateral tibial cartilage regions | [72] |
| HC (Colnatur®) | RCT | Middle-aged to elderly individuals 48–70 years with light symptomatology OA (207) | 6 months | 10 g | ↓ VAS for knee pain; ↓ WOMAC pain score | [73] |
Most interventional clinical studies show significant pain reduction compared with placebo/control groups [57,[62], [63], [64], [65], [66],74,75] and improved joint stiffness/mobility, both immediate [56,57,64,66] and for joint stability and recovery [67], except for the studies by Bruyère et al. [68], who observed no significant differences between groups, and Lopez, Ziegenfuss, and Park [60], who found an increase in pain in the intervention group and a reduction in the circulation of damage markers. Notably, the perception of pain can be hyperactivated by pro-inflammatory cytokines [57]. Furthermore, the reduction of muscle damage corroborates the findings of Clifford et al. [62], who found better muscle recovery resulting from the consumption of hydrolyzed collagen.
Traditionally, delayed onset muscle soreness is explained by myogenic factors, however, the interest in understanding the role of injury for other extramuscular connective tissue elements (such as extracellular matrix, basal lamina, types I, III, IV, and VI collagen, proteoglycans/glycosaminoglycans, muscular layers, and tendons) in delayed onset muscle soreness has been increasing [60]. The perception of musculoskeletal pain limits the performance of repetitive activities, reduces range of motion, and decreases strength production [57,62].
Reduced pain and increased joint functional capacity improve functionality indices (Table 2). Several collagen intervention models showed a significant reduction in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC index) and other indices [64,65,[74], [75], [76]]. However, Hewlings, Kalman, and Schneider [56]found no significant changes in functional tests.
Besides functional aspects, collagen consumption positively influences the entire osteoarticular structure (Table 2; Table 4). Collagen peptides have shown a positive effect on bone strength and mineral density, supporting the idea of effectiveness for diseases that debilitate these structures, such as osteoporosis. Human and animal studies have observed increased bone mineral density [61,81,82], bone mass [47,48], and cartilage volume, besides a higher number of chondrocytes [46]. Collagen consumption in the growth phase can help bone formation [81] and increases muscle mass, strength, and motor control [[77], [78], [79], [80]].
Table 4.
Studies with changes in body composition induced by consumption of hydrolyzed collagen.
| Collagen | Design | Population (n) | Period | Dose/day | Result | Reference |
|---|---|---|---|---|---|---|
| SCP | D-RCT | Postmenopausal women 64.3 ± 7.2 years (102) | 48 weeks | 5 g | ↑BMD of the spine and femoral neck; ↑P1NP | [61] |
| SCP | D-RCT | Pre-menopausal women 29–48 years (77) | 12 weeks | 15 g | ↑muscle mass; ↑ hand pressure strength; ↓FM; ↑ leg strength gain | [77] |
| CP | D-RCT | Recreationally active young men 24 ± 3 years (57) | 12 weeks | 15 g | ↑muscle mass; ↑strength; ↔fCSA | [78] |
| CP | D-RCT | Young men 24.2 ± 2.6 years (25) | 12 weeks | 15 g | ↑muscle mass; ↑strength; ↑body mass; ↑ proteins related to contractile fibers | [79] |
| CP | D-RCT | Elderly people 72.2 ± 4.7 years with sarcopenia (53) | 12 weeks | 15 g | ↑muscle mass; ↑IQS; ↑muscle strength; ↑ loss of FM | [80] |
↑: increase; ↓: decrease; ↔: no difference; D-RCT: randomized, double-blind clinical trial; CP: collagen peptides; SCP: specific collagen peptides; BMD: bone mineral density; P1NP: terminal propeptides of type 1 pro-collagen phosphatase; P1NP: amino-terminal propeptide of type I collagen; FM: Fat mass; IQS: isokinetic quadriceps strength; fCSA: muscle fiber cross-sectional area.
However, the effects of hydrolyzed collagen depend on the concentration, experimental conditions, and characteristics of the hydrolysates tested [63,82]. In the studies analyzed native collagen was used as an oral tolerance protocol, seeking to reduce the inflammatory response to collagen compounds in a scenario where consumption by itself leads to an inflammatory response. The doses of native collagen were small (0.01–0.0001 g/day) but effectively generated positive results in functional and biomolecular aspects (Table 3). Studies with larger amounts of collagen hydrolysates (2–20 g) aimed to induce an anabolic response of osteoarticular tissues (Table 3; Table 4). The anabolic effect can counteract the wear processes of cartilage tissue and, thus, the decreased degradation of the extracellular matrix could reduce pro-inflammatory and pain-stimulating processes. Moreover, a direct anti-inflammatory potential of collagen peptides can decrease joint pain intensity, since collagen peptide supplementation inhibits glycine-mediated cytokine release [63].
Despite the evident benefits of collagen consumption, studies present several relevant differences that deserve further discussion, such as the type of collagen used, dose, and the optimal time for consumption. These variations in experimental protocols (target population, sex, dose, duration of treatment, presence, or absence of orthopedic alterations) contribute to the lack of agreement on the observed results. Furthermore, a better understanding of the physiological mechanisms that promote these benefits is still required [20]. Therefore, considering the positive effect of collagen consumption on joint diseases and physiology and the wide variety of intervention protocols, future clinical studies should better address guidelines regarding the optimal dose for consumption, the type of collagen, and duration of intervention.
5. Conclusion
The loss of collagen can be influenced by intrinsic factors, such as genetics, or extrinsic factors, such as nutrition, sun exposure, infrared radiation, visible light, air pollution, and strenuous physical activity. The loss of collagen in osteoarticular tissues and skin is related to senescence, hormonal profile, adiposity, inflammatory processes, immobility, mechanical overload, and joint damage. The literature shows that collagen supplementation can be beneficial in the treatment of skin changes, reducing wrinkles; increasing skin elasticity, hydration, firmness, and brightness; decreasing pores and solar elastosis; and increased collagen synthesis density and skin content. The use of collagen in the treatment of orthopedic alterations increases bone strength, density, and mineral mass; decreases extracellular matrix degradation; inhibits inflammatory cytokines; improves joint stability, functional capacity, and stiffness/mobility, and muscle recovery; reduces pain; and mitigates markers of joint cartilage degradation. These results show the economic and health-promoting potential of this product extracted from food industry by-products. We emphasize that future studies should address remaining gaps regarding signaling pathways and obtain bioactive peptides from intact collagen for health promotion.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
Dr Cínthia Baú Betim Cazarin was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [Finance Code 001] and Conselho Nacional de Desenvolvimento Científico e Tecnológico [306891/2021-2].
Data availability statement
Data included in article/supplementary material/referenced in article.
↑: increase; ↓: decrease; ↔: no difference; HC: hydrolyzed collagen; type I - H: hydrolyzed type 1 collagen; HFC: hydrolyzed fish collagen; CP: collagen peptides; CTP: collagen tripeptide; OA: osteoarthritis model; OVA: ovariectomy; bw: body weight; Tnf-α mRNA (Tumor Necrosis Factor - Messenger ribonucleic acid); MMP13 protein: matrix metallopeptidase 13 protein; AIN 93-M: American Institute of Nutrition; TEWL: transepidermal water loss; IL-6: interleukin-6. IL-7: interleukin-17; IL-1β: interleukin-1β.
↑: increase; ↓: decrease; ↔: no difference. Type II: type II collagen; HC: hydrolyzed collagen; SCP: specific collagen peptides; UC-II: undenatured type II collagen; BioCP: bioactive collagen peptides; PCP: pork skin collagen peptide; BCP: bovine bone collagen peptide; RCT: randomized clinical trial; S-RCT: randomized; D-RCT: randomized, double-blind clinical trial; D-RM: double-blind randomized multicenter study; RA: rheumatoid arthritis; OA: osteoarthritis; HAQ: health assessment questionnaire; VAS: visual analogical scale; WOMAC: Western Ontario and Mc Master Universities Osteoarthritis Index; LFI: Lequesne Functional Index, PA: physical activities; P1NP: terminal propeptides of type 1 pro-collagen, β-CTX: C-terminal telopeptide of type 1 collagen; QOL: quality of life scores; dGEMRIC: delayed gadolinium enhanced MRI of cartilage.
References
- 1.Roser M., Ortiz-Ospina E., Ritchie H. 2019. Life Expectancy.https://ourworldindata.org/life-expectancy [cited 2022 2nd March]; Available from: [Google Scholar]
- 2.Sgarbieri V.C., Pacheco M.T.B. Healthy human aging: intrinsic and environmental factors. Braz. J. Food Technol. 2017;20:e2017007. [Google Scholar]
- 3.Kavitha O., Thampan R.V. Factors influencing collagen biosynthesis. J. Cell. Biochem. 2008;104(4):1150–1160. doi: 10.1002/jcb.21728. [DOI] [PubMed] [Google Scholar]
- 4.Ricard-Blum S. The collagen family. Cold Spring Harbor Perspect. Biol. 2011;3(1) doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu D., et al. Collagen and gelatin. Annu. Rev. Food Sci. Technol. 2015;6:527–557. doi: 10.1146/annurev-food-031414-111800. [DOI] [PubMed] [Google Scholar]
- 6.Bolke L., et al. A collagen supplement improves skin hydration, elasticity, roughness, and density: results of a randomized, placebo-controlled, blind study. Nutrients. 2019;11(10):2494. doi: 10.3390/nu11102494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz M.A., et al. Collagen from marine sources and skin wound healing in animal experimental studies: a systematic review. Mar. Biotechnol. 2021;23(1):1–11. doi: 10.1007/s10126-020-10011-6. [DOI] [PubMed] [Google Scholar]
- 8.Lupu M.A., et al. Beneficial effects of food supplements based on hydrolyzed collagen for skin care (Review) Exp. Ther. Med. 2020;20(1):12–17. doi: 10.3892/etm.2019.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.León-López A., et al. Hydrolyzed collagen-sources and applications. Molecules (Basel, Switzerland. 2019;24(22):4031. doi: 10.3390/molecules24224031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sibilla S., et al. An overview of the beneficial effects of hydrolysed collagen as a nutraceutical on skin properties: scientific background and clinical studies. Open Nutraceuticals J. 2015;8:29–42. [Google Scholar]
- 11.Hong H., et al. Preparation of low-molecular-weight, collagen hydrolysates (peptides): current progress, challenges, and future perspectives. Food Chem. 2019;301 doi: 10.1016/j.foodchem.2019.125222. [DOI] [PubMed] [Google Scholar]
- 12.Figueres Juher T., Basés Pérez E. An overview of the beneficial effects of hydrolysed collagen intake on joint and bone health and on skin ageing. Nutr. Hosp. 2015;32(Suppl 1):62–66. doi: 10.3305/nh.2015.32.sup1.9482. [DOI] [PubMed] [Google Scholar]
- 13.Bordin C.C.D., Naves M.M.V. Hydrolyzed collagen (gelatin) decreases food efficiency and the bioavailability of high-quality protein in rats. Rev. Nutr. 2015;28(4):421–430. [Google Scholar]
- 14.Paul C., Leser S., Oesser S. Significant amounts of functional collagen peptides can be incorporated in the diet while maintaining indispensable amino acid balance. Nutrients. 2019;11(5) doi: 10.3390/nu11051079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorushanova A., et al. The collagen suprafamily: from biosynthesis to advanced biomaterial development. Adv. Mater. 2019;31(1):e1801651. doi: 10.1002/adma.201801651. [DOI] [PubMed] [Google Scholar]
- 16.Gelse K., Pöschl E., Aigner T. Collagens--structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003;55(12):1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Sophia Fox A.J., Bedi A., Rodeo S.A. The basic science of articular cartilage: structure, composition, and function. Sport Health. 2009;1(6):461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto S., et al. Absorption and urinary excretion of peptides after collagen tripeptide ingestion in humans. Biol. Pharm. Bull. 2016;39(3):428–434. doi: 10.1248/bpb.b15-00624. [DOI] [PubMed] [Google Scholar]
- 19.Udenigwe C.C., et al. Bioaccessibility of bioactive peptides: recent advances and perspectives. Curr. Opin. Food Sci. 2021;39:182–189. [Google Scholar]
- 20.Daneault A., et al. Biological effect of hydrolyzed collagen on bone metabolism. Crit. Rev. Food Sci. Nutr. 2017;57(9):1922–1937. doi: 10.1080/10408398.2015.1038377. [DOI] [PubMed] [Google Scholar]
- 21.Daniel H. Molecular and integrative physiology of intestinal peptide transport. Annu. Rev. Physiol. 2004;66:361–384. doi: 10.1146/annurev.physiol.66.032102.144149. [DOI] [PubMed] [Google Scholar]
- 22.Urao M., et al. Intestinal permeability to small- and large-molecular-weight substances in the newborn rabbit. J. Pediatr. Surg. 1997;32(10):1424–1428. doi: 10.1016/s0022-3468(97)90553-4. [DOI] [PubMed] [Google Scholar]
- 23.Sun X., et al. Considering food matrix and gastrointestinal effects in enhancing bioactive peptide absorption and bioavailability. J. Funct.Foods. 2020;64 [Google Scholar]
- 24.Yazaki M., et al. Oral ingestion of collagen hydrolysate leads to the transportation of highly concentrated Gly-Pro-Hyp and its hydrolyzed form of Pro-Hyp into the bloodstream and skin. J. Agric. Food Chem. 2017;65(11):2315–2322. doi: 10.1021/acs.jafc.6b05679. [DOI] [PubMed] [Google Scholar]
- 25.Shigemura Y., et al. Changes in composition and content of food-derived peptide in human blood after daily ingestion of collagen hydrolysate for 4 weeks. J. Sci. Food Agric. 2018;98(5):1944–1950. doi: 10.1002/jsfa.8677. [DOI] [PubMed] [Google Scholar]
- 26.Iwai K., et al. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J. Agric. Food Chem. 2005;53(16):6531–6536. doi: 10.1021/jf050206p. [DOI] [PubMed] [Google Scholar]
- 27.Khavkin J., Ellis D.A. Aging skin: histology, physiology, and pathology. Facial Plast Surg Clin North Am. 2011;19(2):229–234. doi: 10.1016/j.fsc.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Sparavigna A. Role of the extracellular matrix in skin aging and dedicated treatment - state of the art. Plast Aesthet Res. 2020;7:14. [Google Scholar]
- 29.Krutmann J., et al. The skin aging exposome. J. Dermatol. Sci. 2017;85(3):152–161. doi: 10.1016/j.jdermsci.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Fenske N.A., Lober C.W. Structural and functional changes of normal aging skin. J. Am. Acad. Dermatol. 1986;15(4 Pt 1):571–585. doi: 10.1016/s0190-9622(86)70208-9. [DOI] [PubMed] [Google Scholar]
- 31.Rittié L., Fisher G.J. Natural and sun-induced aging of human skin. Cold Spring Harbor Perspect. Med. 2015;5(1) doi: 10.1101/cshperspect.a015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lovell C.R., et al. Type I and III collagen content and fibre distribution in normal human skin during ageing. Br. J. Dermatol. 1987;117(4):419–428. doi: 10.1111/j.1365-2133.1987.tb04921.x. [DOI] [PubMed] [Google Scholar]
- 33.Evans M., et al. A randomized, triple-blind, placebo-controlled, parallel study to evaluate the efficacy of a freshwater marine collagen on skin wrinkles and elasticity. J. Cosmet. Dermatol. 2021;20(3):825–834. doi: 10.1111/jocd.13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugihara F., Inoue N., Wang X. Clinical effects of ingesting collagen hydrolysate on facial skin properties - a randomized, placebo-controlled, double-blind trial. Jpn. Pharmacol. Ther. 2015;43(1):67–70. [Google Scholar]
- 35.Inoue N., Sugihara F., Wang X. Ingestion of bioactive collagen hydrolysates enhance facial skin moisture and elasticity and reduce facial ageing signs in a randomised double-blind placebo-controlled clinical study. J. Sci. Food Agric. 2016;96(12):4077–4081. doi: 10.1002/jsfa.7606. [DOI] [PubMed] [Google Scholar]
- 36.Choi S.Y., et al. Effects of collagen tripeptide supplement on skin properties: a prospective, randomized, controlled study. J. Cosmet. Laser Ther. 2014;16(3):132–137. doi: 10.3109/14764172.2013.854119. [DOI] [PubMed] [Google Scholar]
- 37.Asserin J., et al. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J. Cosmet. Dermatol. 2015;14(4):291–301. doi: 10.1111/jocd.12174. [DOI] [PubMed] [Google Scholar]
- 38.Proksch E., et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol. Physiol. 2014;27(1):47–55. doi: 10.1159/000351376. [DOI] [PubMed] [Google Scholar]
- 39.Kim D.-U., et al. Oral intake of low-molecular-weight collagen peptide improves hydration, elasticity, and wrinkling in human skin: a randomized, double-blind, placebo-controlled study. Nutrients. 2018;10(7):826. doi: 10.3390/nu10070826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koizumi S., et al. Effects of dietary supplementation with fish scales-derived collagen peptides on skin parameters and condition: a randomized, placebo-controlled, double-blind study. Int. J. Pept. Res. Therapeut. 2018;24(3):397–402. [Google Scholar]
- 41.Czajka A., et al. Daily oral supplementation with collagen peptides combined with vitamins and other bioactive compounds improves skin elasticity and has a beneficial effect on joint and general wellbeing. Nutr. Res. 2018;57:97–108. doi: 10.1016/j.nutres.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Choi F.D., et al. Oral collagen supplementation: a systematic review of dermatological applications. J. Drugs Dermatol. JDD. 2019;18(1):9–16. [PubMed] [Google Scholar]
- 43.Barati M., et al. Collagen supplementation for skin health: a mechanistic systematic review. J. Cosmet. Dermatol. 2020;19(11):2820–2829. doi: 10.1111/jocd.13435. [DOI] [PubMed] [Google Scholar]
- 44.de Miranda R.B., Weimer P., Rossi R.C. Effects of hydrolyzed collagen supplementation on skin aging: a systematic review and meta-analysis. Int. J. Dermatol. 2021;60(12):1449–1461. doi: 10.1111/ijd.15518. [DOI] [PubMed] [Google Scholar]
- 45.Rustad A.M., et al. Myths and media in oral collagen supplementation for the skin, nails, and hair: a review. J. Cosmet. Dermatol. 2022;21(2):438–443. doi: 10.1111/jocd.14567. [DOI] [PubMed] [Google Scholar]
- 46.Dar Q.A., et al. Daily oral consumption of hydrolyzed type 1 collagen is chondroprotective and anti-inflammatory in murine posttraumatic osteoarthritis. PLoS One. 2017;12(4):e0174705. doi: 10.1371/journal.pone.0174705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Almeida Jackix E., et al. A food supplement of hydrolyzed collagen improves compositional and biodynamic characteristics of vertebrae in ovariectomized rats. J. Med. Food. 2010;13(6):1385–1390. doi: 10.1089/jmf.2009.0256. [DOI] [PubMed] [Google Scholar]
- 48.Takeda S., et al. Hydrolyzed collagen intake increases bone mass of growing rats trained with running exercise. J Int Soc Sports Nutr. 2013;10(1):35. doi: 10.1186/1550-2783-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo X., et al. A novel Atlantic salmon (Salmo salar) bone collagen peptide delays osteoarthritis development by inhibiting cartilage matrix degradation and anti-inflammatory. Food Res. Int. 2022;162(Pt B) doi: 10.1016/j.foodres.2022.112148. [DOI] [PubMed] [Google Scholar]
- 50.Oba C., et al. Collagen hydrolysate intake improves the loss of epidermal barrier function and skin elasticity induced by UVB irradiation in hairless mice. Photodermatol. Photoimmunol. Photomed. 2013;29(4):204–211. doi: 10.1111/phpp.12051. [DOI] [PubMed] [Google Scholar]
- 51.Pyun H.B., et al. Effects of collagen tripeptide supplement on photoaging and epidermal skin barrier in UVB-exposed hairless mice. Prev Nutr Food Sci. 2012;17(4):245–253. doi: 10.3746/pnf.2012.17.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lane A.R., et al. Body mass index and type 2 collagen turnover in individuals after anterior cruciate ligament reconstruction. J. Athl. Train. 2019;54(3):270–275. doi: 10.4085/1062-6050-525-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waldron T. In: Ortner's Identification of Pathological Conditions in Human Skeletal Remains. third ed. Buikstra J.E., editor. Academic Press; San Diego: 2019. Chapter 20 - Joint Disease; pp. 719–748. [Google Scholar]
- 54.Anderson E., Durstine J.L. Physical activity, exercise, and chronic diseases: a brief review. Sports Med. Health Sci. 2019;1(1):3–10. doi: 10.1016/j.smhs.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwatra B. Collagen supplementation : therapy for the prevention and treatment of osteoporosis and osteoarthritis : a review. World J. Pharm. Pharmaceut. Sci. 2020;9(5):589–604. [Google Scholar]
- 56.Hewlings S., Kalman D., Schneider L.V. A randomized, double-blind, placebo-controlled, prospective clinical trial evaluating water-soluble chicken eggshell membrane for improvement in joint health in adults with knee osteoarthritis. J. Med. Food. 2019;22(9):875–884. doi: 10.1089/jmf.2019.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruff K.J., et al. Beneficial effects of natural eggshell membrane versus placebo in exercise-induced joint pain, stiffness, and cartilage turnover in healthy, postmenopausal women. Clin. Interv. Aging. 2018;13:285–295. doi: 10.2147/CIA.S153782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carballo C.B., et al. Basic science of articular cartilage. Clin. Sports Med. 2017;36(3):413–425. doi: 10.1016/j.csm.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Xin L., et al. Comparative study of CTX-II, Zn2+, and Ca2+ from the urine for knee osteoarthritis patients and healthy individuals. Medicine (Baltim.) 2017;96(32):e7593. doi: 10.1097/MD.0000000000007593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lopez H.L., Ziegenfuss T.N., Park J. Evaluation of the effects of BioCell collagen, a novel cartilage extract, on connective tissue support and functional recovery from exercise. Integr. Med. (Encinitas) 2015;14(3):30–38. [PMC free article] [PubMed] [Google Scholar]
- 61.König D., et al. Specific collagen peptides improve bone mineral density and bone markers in postmenopausal women- a randomized controlled study. Nutrients. 2018;10(1):97. doi: 10.3390/nu10010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clifford T., et al. The effects of collagen peptides on muscle damage, inflammation and bone turnover following exercise: a randomized, controlled trial. Amino Acids. 2019;51(4):691–704. doi: 10.1007/s00726-019-02706-5. [DOI] [PubMed] [Google Scholar]
- 63.Zdzieblik D., et al. Improvement of activity-related knee joint discomfort following supplementation of specific collagen peptides. Appl. Physiol. Nutr. Metabol. 2017;42(6):588–595. doi: 10.1139/apnm-2016-0390. [DOI] [PubMed] [Google Scholar]
- 64.Lugo J.P., Saiyed Z.M., Lane N.E. Efficacy and tolerability of an undenatured type II collagen supplement in modulating knee osteoarthritis symptoms: a multicenter randomized, double-blind, placebo-controlled study. Nutr. J. 2016;15:14. doi: 10.1186/s12937-016-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schauss A.G., et al. Effect of the novel low molecular weight hydrolyzed chicken sternal cartilage extract, BioCell collagen, on improving osteoarthritis-related symptoms: a randomized, double-blind, placebo-controlled trial. J. Agric. Food Chem. 2012;60(16):4096–4101. doi: 10.1021/jf205295u. [DOI] [PubMed] [Google Scholar]
- 66.Wei W., et al. A multicenter, double-blind, randomized, controlled phase III clinical trial of chicken type II collagen in rheumatoid arthritis. Arthritis Res. Ther. 2009;11(6):R180. doi: 10.1186/ar2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dressler P., et al. Improvement of functional ankle properties following supplementation with specific collagen peptides in athletes with chronic ankle instability. J. Sports Sci. Med. 2018;17(2):298–304. [PMC free article] [PubMed] [Google Scholar]
- 68.Bruyère O., et al. Effect of collagen hydrolysate in articular pain: a 6-month randomized, double-blind, placebo controlled study. Compl. Ther. Med. 2012;20(3):124–130. doi: 10.1016/j.ctim.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 69.Kumar S., et al. A double-blind, placebo-controlled, randomised, clinical study on the effectiveness of collagen peptide on osteoarthritis. J. Sci. Food Agric. 2015;95(4):702–707. doi: 10.1002/jsfa.6752. [DOI] [PubMed] [Google Scholar]
- 70.Bongers C., et al. Effectiveness of collagen supplementation on pain scores in healthy individuals with self-reported knee pain: a randomized controlled trial. Appl. Physiol. Nutr. Metabol. 2020;45(7):793–800. doi: 10.1139/apnm-2019-0654. [DOI] [PubMed] [Google Scholar]
- 71.Clark K.L., et al. 24-Week study on the use of collagen hydrolysate as a dietary supplement in athletes with activity-related joint pain. Curr. Med. Res. Opin. 2008;24(5):1485–1496. doi: 10.1185/030079908x291967. [DOI] [PubMed] [Google Scholar]
- 72.McAlindon T.E., et al. Change in knee osteoarthritis cartilage detected by delayed gadolinium enhanced magnetic resonance imaging following treatment with collagen hydrolysate: a pilot randomized controlled trial. Osteoarthritis Cartilage. 2011;19(4):399–405. doi: 10.1016/j.joca.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 73.Benito-Ruiz P., et al. A randomized controlled trial on the efficacy and safety of a food ingredient, collagen hydrolysate, for improving joint comfort. Int. J. Food Sci. Nutr. 2009;60(Suppl 2):99–113. doi: 10.1080/09637480802498820. [DOI] [PubMed] [Google Scholar]
- 74.Bakilan F., et al. Effects of native type II collagen treatment on knee osteoarthritis: a randomized controlled trial. Eurasian J. Med. 2016;48(2):95–101. doi: 10.5152/eurasianjmed.2015.15030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Puigdellivol J., et al. Effectiveness of a dietary supplement containing hydrolyzed collagen, chondroitin sulfate, and glucosamine in pain reduction and functional capacity in osteoarthritis patients. J. Diet. Suppl. 2019;16(4):379–389. doi: 10.1080/19390211.2018.1461726. [DOI] [PubMed] [Google Scholar]
- 76.Praet S.F.E., et al. Oral supplementation of specific collagen peptides combined with calf-strengthening exercises enhances function and reduces pain in achilles tendinopathy patients. Nutrients. 2019;11(1) doi: 10.3390/nu11010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jendricke P., et al. Specific collagen peptides in combination with resistance training improve body composition and regional muscle strength in premenopausal women: a randomized controlled trial. Nutrients. 2019;11(4) doi: 10.3390/nu11040892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kirmse M., et al. Prolonged collagen peptide supplementation and resistance exercise training affects body composition in recreationally active men. Nutrients. 2019;11(5) doi: 10.3390/nu11051154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oertzen-Hagemann V., et al. Effects of 12 weeks of hypertrophy resistance exercise training combined with collagen peptide supplementation on the skeletal muscle proteome in recreationally active men. Nutrients. 2019;11(5) doi: 10.3390/nu11051072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zdzieblik D., et al. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: a randomised controlled trial. Br. J. Nutr. 2015;114(8):1237–1245. doi: 10.1017/S0007114515002810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin-Bautista E., et al. A nutritional intervention study with hydrolyzed collagen in pre-pubertal Spanish children: influence on bone modeling biomarkers. J. Pediatr. Endocrinol. Metab. 2011;24(3–4):147–153. doi: 10.1515/jpem.2011.009. [DOI] [PubMed] [Google Scholar]
- 82.Noma T., et al. Effects of dietary gelatin hydrolysates on bone mineral density in magnesium-deficient rats. BMC Muscoskel. Disord. 2017;18(1):385. doi: 10.1186/s12891-017-1745-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.
↑: increase; ↓: decrease; ↔: no difference; HC: hydrolyzed collagen; type I - H: hydrolyzed type 1 collagen; HFC: hydrolyzed fish collagen; CP: collagen peptides; CTP: collagen tripeptide; OA: osteoarthritis model; OVA: ovariectomy; bw: body weight; Tnf-α mRNA (Tumor Necrosis Factor - Messenger ribonucleic acid); MMP13 protein: matrix metallopeptidase 13 protein; AIN 93-M: American Institute of Nutrition; TEWL: transepidermal water loss; IL-6: interleukin-6. IL-7: interleukin-17; IL-1β: interleukin-1β.
↑: increase; ↓: decrease; ↔: no difference. Type II: type II collagen; HC: hydrolyzed collagen; SCP: specific collagen peptides; UC-II: undenatured type II collagen; BioCP: bioactive collagen peptides; PCP: pork skin collagen peptide; BCP: bovine bone collagen peptide; RCT: randomized clinical trial; S-RCT: randomized; D-RCT: randomized, double-blind clinical trial; D-RM: double-blind randomized multicenter study; RA: rheumatoid arthritis; OA: osteoarthritis; HAQ: health assessment questionnaire; VAS: visual analogical scale; WOMAC: Western Ontario and Mc Master Universities Osteoarthritis Index; LFI: Lequesne Functional Index, PA: physical activities; P1NP: terminal propeptides of type 1 pro-collagen, β-CTX: C-terminal telopeptide of type 1 collagen; QOL: quality of life scores; dGEMRIC: delayed gadolinium enhanced MRI of cartilage.