Abstract
Diabetes Mellitus (DM) is a group of chronic metabolic diseases distinguished by elevated glycemia due to the alterations in insulin metabolism. DM is one of the most relevant diseases of the modern world, with high incidence and prevalence worldwide, associated with severe systemic complications and increased morbidity and mortality rates. Although genetic factors and lifestyle habits are two of the main factors involved in DM onset, viral infections, such as enteroviruses, cytomegalovirus, hepatitis C virus, human immunodeficiency virus, severe acute respiratory syndrome coronavirus 2, among others, have been linked as triggers of type 1 (T1DM) and type 2 (T2DM) diabetes. Over the years, various groups identified different mechanisms as to how viruses can promote these metabolic syndromes. However, this field is still poorly explored and needs further research, as millions of people live with these pathologies. Thus, this review aims to ex-plore the different processes of how viruses can induce DM and their contribution to the prevalence and incidence of DM worldwide.
Keywords: Diabetogenic viruses, diabetes mellitus, metabolic syndrome
1. Introduction
According to the World Health Organization (WHO), over 420 million people worldwide have been diagnosed with diabetes mellitus (DM) (https://www.who.int/health-topics/diabetes#tab=tab_1). This disease is classified as a chronic metabolic syndrome. It is characterized by the inability of cells to internalize glucose, which remains in the extracellular space and leads to high glycemic levels [1]. The mechanism by which cells cannot uptake glucose results from changes in insulin metabolism, either in its production or in its sensitivity to the insulin receptor [1]. Therefore, DM can be classified based on the activity of this hormone. Type 1 DM (T1DM) is an autoimmune disease related to the inaptitude of pancreatic β-cells to synthesize insulin due to its annihilation by T-cells [2]. In type 2 DM (T2DM), pancreatic β-cells can produce insulin, but in smaller amounts than healthy individuals, insufficient to control blood glucose [3]. T2DM patients also have insulin resistance (IR), which is strongly associated with dietary habits [3]. Although the absence of insulin or its resistance are the major features of DM, some viruses can induce it in infected individuals.
Viruses generally hijack the host cellular machinery for their benefits. One of the outcomes is the host cell metabolism reprogramming to increase nutrient consumption to assist viral replication. Interestingly, the upregulation of energy metabolism is a trigger for DM onset [4]. Some viruses have tropism for pancreatic β-cells whereas others trigger β-cell-specific autoimmunity. Somehow, viral infection can lead to β-cell death as a result of cytopathic events [5]. Overproduction of inflammatory cytokines during the infection also contributes for DM development [6]. Hence, both the metabolism reprogramming, and the infection of pancreatic cells are mechanisms by which viruses can trigger DM. Despite the relevance of the theme, this field is still poorly explored, and data are very dispersed in the literature. In the present review, we will compile the current knowledge on the theme, focusing on the mechanisms by which viruses can induce DM and discuss how viruses contribute to incidence of DM in the world whenever these data are available. For this work, PubMed (https://pubmed.ncbi.nlm.nih.gov/) was used as web source for searching and selection of the reference articles. The keywords “viruses and type 1 diabetes”, “viruses and type 2 diabetes”, and “diabetogenic viruses” were used in the search and articles were selected based on title, abstract, research area and keywords.
1.1. Viruses and type 1 diabetes mellitus (T1DM)
T1DM, also known as insulin-dependent diabetes mellitus (IDDM) or juvenile-onset diabetes, is an autoimmune disorder characterized by the destruction of pancreatic β-cells [1]. This self-destructive process results from the infiltration of immune cells, including T and B lymphocytes, macrophages (MØ), dendritic cells (DC) and natural killer (NK) cells, along with the production of β-cells autoantigens, such as glutamic acid decarboxylase (GAD) 65, tyrosine phosphatase-like insulinoma antigen (IA2), islet cell antigen, among others [7]. These autoantigens are recognized by the immune system and lead to cytokine overexpression and activation of β-cell-specific cytotoxic T CD8+ cells, which promote the annihilation of β-cells [8].
Despite β-cell disability, insulitis advances slowly as the pancreatic tissue is gradually being damaged. Individuals at any age can be diagnosed with T1DM, but most of the cases are identified during childhood and adolescence, and genetic factors play an important role in the development of this disorder. More than 60 loci have been associated with T1DM, among them are MHC class II genes HLA-DR and HLA-DQ [9]. Interestingly, identical twins exhibit a concordance rate for T1DM lower than 50% [10], which reinforce the idea that environmental factors also contribute to the pathology onset.
The evidence that viral infection is directly linked to T1DM was originally shown in studies focusing on the isolation of viruses from patients who developed the disease after an episode of flu-like symptoms at colder seasons of the year [11]. Virus-specific antibodies were found in recent diagnosed T1DM patients and in diabetic offspring of infected mothers, demonstrating a potential correlation between viral infections and this metabolic syndrome [12,13]. However, the connection between viruses and T1DM were clearer after description of diabetic ketoacidosis and fulminant type 1 diabetes mellitus (FT1D) occurrence in response to viral infections [14,15]. Currently, many viruses have been associated with T1DM in humans so as the mechanisms involved in this process.
Coxsackie A virus (CAV), coxsackie B virus (CBV), echovirus (ECV), rubella virus (RuV), cytomegalovirus (CMV), mumps (MuV) and rotavirus (RoV) are known to have tropism to β-cell and are therefore classified as diabetogenic viruses. Vuorinen et al. demonstrated that CBV-3 and MuV can infect human fetal pancreatic endocrine cells in in vitro cell models [16]. The study reported that insulin production on MuV-infected cells ended 7 days-post infection, suggesting that infection impaired the pancreatic function. Likewise, RuV infection diminished β-cell population in pancreas and leads to insulin synthesis interruption [17].
Initiation of T1DM is associated with large infiltration of immune cells and production of autoantibodies. Although its production is not enough to initiate the disease, its presence is considered a risk factor for the disease appearance, as they recognize antigens generally located in the endocrine part of the pancreatic tissue [8]. Islet-cell antibodies (ICA), acts against specific islet cell surface and cytoplasmic antigens, being identified in the serum before the complete destruction of β-cell [18]. IA-2A recognizes the membrane of secretory granules, which carries insulin and are also target for insulin autoantibodies (IAA) [19]. On the other hand, GAD65 is located at the cytoplasmic site of the synaptic-like micro-vesicles and can be targeted by the immune system [19]. Like ICA, these antibodies can be detected prior to IDDM onset and can be used as diagnostic serum biomarker. Some diabetogenic viruses can induce the production of these antibodies, as it is the case of the identification of ICA in samples from MuV and ECV-16 infected children, respectively [20,21]. IAA, anti-GAD65, IA-2A and cytotoxic β-cell surface antibodies (CBSA) were also identified in the serum of patients with no background of T1DM, infected by ECV-16, RoV, CMV and RuV infection, confirming that viruses can induce autoantibodies against β-cell [[21], [22], [23], [24]]. Although the ability of some viruses to induce the production of autoantibodies are extremely interesting, further research is needed to help us understand the mechanism behind this induction.
Viruses can also induce T1DM development by a molecular mimicry process. This mechanism consists of a sequence or structure similarity between a foreign antigen and an autoantigen [25], and is one of the main explanations of how viruses trigger autoimmunity. Several viruses have been linked to this mechanism as an inductor of T1DM. Studies with CMV, RoV, and CBV-4 identified sequence similarity between viral proteins and autoantigens. RoV VP7, a glycoprotein localized at the outer layer of the virus capsid, is strongly immunogenic and shares amino acid sequences to host IA2 and GAD65 [26]. This resemblance region in VP7 protein was also able to interact with HLA-DRB1*04 and stimulate T cells expansion in a similar fashion to the self-antigen response [26]. Likewise, CBV-4 structural protein VP1 and non-structural protein 2C share sequence similarity with GAD65 and IA2, respectively, with epitopes in this autoantigen being identified by viral-induced antisera [27,28]. Moreover, Hiemstra et al. identified that GAD65-specific T cell response cross-react with CMV major DNA-binding protein (MDBP) [29]. The group reported that this region can be processed for presentation by HLA-DR3 and therefore being identified by GAD65-responsive T cells, suggesting that autoimmunity might be induced by this mimicry process. In addition, viral proteins may have common regions with other host proteins. Epstein-Barr virus (EBV) nuclear antigen 3C protein (EBVNA3C) possesses a five amino acids-long region with sequence similarity to HLA-DQw8β, which is an important risk factor for T1DM [30].
Cross-reaction between viral antibodies and islet regions have also been investigated as an inductor of T1DM. During viral infection, host immune system is activated and produce specific antibodies against the pathogen, necessary to control the infection. Regardless of its crucial role, studies with RuV and influenza A (IAV) described that antibody against these viruses can recognize pancreatic tissue. RuV have β-cell tropism and replication in this cell leads to its destruction. One monoclonal antibody against RuV capsid protein cross-reacted with the extract from rat and human pancreatic tissue [31]. A virus-like epitope is localized in a 52 kDa protein, which is also recognized by autoantibodies isolated from T1DM patients and non-obese diabetic (NOD) mice, suggesting the mechanism by how RuV triggers this metabolic syndrome [31]. More recently, influenza H1N1-induced anti-IAV antibodies were described to cross-react with pancreatic α-cell. As this cell is the precursor of β-cell, the group postulated that these antibodies, by attacking α-cell, diminish β-cell population and, consequently, triggers T1DM onset [5]. Moreover, β-cells are also target of anti-IAV H1N1 antibodies [5].
Although viruses can promote T1DM initiation, infection by any of diabetogenic viruses does not ascertain the disease onset. In most cases, the immune system solves the infection properly, but in susceptible individuals, especially those with genetic predisposition to T1DM, viruses accelerate the disorder manifestation. Genes encoding for class I HLA-B and II HLA genes have been considered as a risk factor for T1DM [32]. On the other hand, HLA-DR2 gene confers protection against this disease [33]. As note, antibodies against CBV-4 were in higher titers in patients with HLA-DR3 and/or HLA-DR4 genes than in individuals caring HLA-DR2, suggesting that the presence of T1DM-related genes correlates with an exacerbated immune response, what may induce β-cell destruction [34]. Furthermore, T1DM-related genes are required for the progression of glucose sensitivity in patients with congenital rubella [35] and the proteins codified by these genes interact with viral proteins or share similar sequences and/or structures with them, as in the case of RoV, CMV, and EBV [26,29,30].
More recently, with the ongoing pandemic of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), outstanding studies have made the link between this virus infection and T1DM development. Elevation of newly diagnosed T1DM in children and an increase of diabetic ketoacidosis (DKA) after the pandemic have been reported [36,37]. Furthermore, it was identified anti-GAD-65 autoantibody in individuals facing coronavirus disease 19 (COVID-19) recovery [38], but a case of T1DM after SARS-CoV-2 infection without the presence of autoantibodies was also reported [39]. Pancreatitis and pancreatic enlargement were also identified in some COVID-19 patients [40,41]. Wu et al. identified that human pancreatic β-cells cultured ex vivo from fatal cases of COVID-19 were infected with SARS-CoV-2 [42]. The authors also evaluated the expression of SARS-Cov-2 cell receptors and showed that angiotensin converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) were present in β-cells, as well as transferrin receptor (TFRC) and neuropilin 1 (NRP1). Curiously, NRP1 was overexpressed in these cells and is probably the explanation why the pancreatic tissue is infected with SARS-CoV-2. Moreover, it was reported that following infection, insulin expression levels decreased and α- and acinar cell markers, such as glucagon and trypsin1 increased in β-cells, which indicates a transdifferentiation process [43].
Altogether, it is clear that viruses can induce T1DM through different mechanisms (Fig. 1), and susceptible individuals are more prone to develop this disease after infection. A list of viruses that induce T1DM and their main outcomes are summarized in Table 1. However, albeit difficult, extensive studies should be made to calculate the influence of virus infection in new diagnosed T1DM cases.
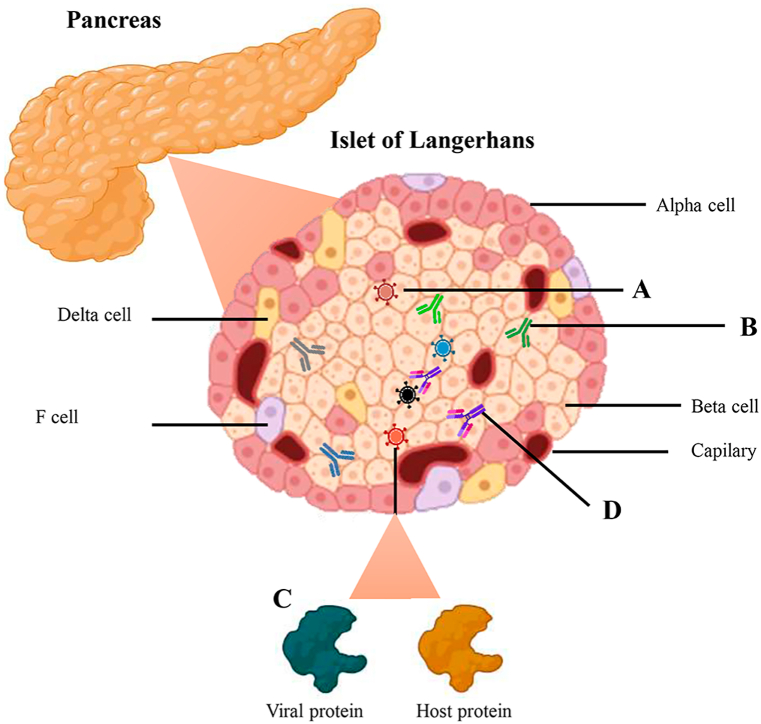
Fig. 1.
- Mechanisms involved in viral-induced T1DM. A. Direct infection of β-cells with diabetogenic viruses. B. Induction of specific T1DM antibodies after viral infection. C. Molecular mimicry process between viral and host proteins. D. Cross-reaction between viral antibodies and islet regions.
Table 1.
Summary of diabetogenic viruses correlated to T1DM.
| Virus | Family | Genus | Genome | Main outcomes | Reference |
|---|---|---|---|---|---|
| Coxsackie A virus | Picornaviridae | Enterovirus | (+)ssRNA | • Associated with fulminant type 1 diabetes mellitus (FT1D) | [12,72] |
| Coxsackie B virus | Picornaviridae | Enterovirus | (+)ssRNA | • Infection in fetal pancreas. | [13,16,27,28,34,[73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84]] |
| • Diabetic ketoacidosis. | |||||
| • Induction of β-cells autoimmunity and hyperglycemia. | |||||
| • Presence of viral RNA in blood samples and PBMCs from adult and children's patients at T1DM onset. | |||||
| • β-cells dysfunction and inflammation. | |||||
| • Islet lesions. | |||||
| • Elevated viral antibodies in patients with HLA-DR3 and/or HLA-DR4 alleles. | |||||
| • Reduction of type 2 insulin-like growth factor 2 (Igf2) transcription. | |||||
| • Cross-reaction between viral capsid protein VP1 and tyrosine phosphatase IA-2/IAR. | |||||
| • Cross-reaction between viral 2C protein and GAD65. | |||||
| • Alteration of miRNAs targeting T1DM-related genes expression. | |||||
| • Manipulation of the UPR in β-cells for viral replication. | |||||
| Echovirus | Picornaviridae | Enterovirus | (+)ssRNA | • Elevated levels of neutralizing antibodies in T1DM patients. | [17,23,31,35,81,85,86] |
| • Presence of ICA, IAA, GAD and IA-2. | |||||
| • Presence of IgM antibodies in the onset of T1DM. | |||||
| • β-cell autoimmunity in offspring of infected mother. | |||||
| Rubella virus | Togaviridae | Rubivirus | (+)ssRNA | • Isolated from pancreas of infants. | [17,23,31,35,81,85,86] |
| • Induction of islet lesions. | |||||
| • Decrease in the number of β-cells and, consequently, insulin synthesis. | |||||
| • Enhanced prevalence of HL-A8 and HLA-DR3 antigen but not HL-A W15 and HLA-DR2 antigen. | |||||
| • Islet cell surface antibodies before onset of T1DM. | |||||
| • Enhanced frequency of anti-thyroid antibodies. | |||||
| • Antibody cross reactivity between a viral antigen and an undetermined islet cell protein. | |||||
| Cytomegalovirus | Herpesviridae | Cytomegalovirus | dsDNA | • Development of autoimmunity by a molecular mimicry mechanism involving T cells cross reactivity with viral antigen and GAD65. | [14,24,29,81,[87], [88], [89]] |
| • Islet lesions. | |||||
| • Associated with fulminant T1DM. | |||||
| • Presence of islet cell antibody (ICA) and cytotoxic β-cell surface antibody (CBSA). | |||||
| • Infection of β-cells associated with immune cells activation, release of pro-inflammatory cytokines and enhanced cellular immunogenicity. | |||||
| • Induction of association between ICA and a cell-specific 38 kd protein from pancreatic islets. | |||||
| Epstein-Barr virus | Herpesviridae | Lymphocryptovirus | dsDNA | • Associated with fulminant type 1 diabetes mellitus (FT1D). | [15,30,90] |
| • Elevated viral antibodies in T1DM patients. | |||||
| • Sequence identity of EBV nuclear antigen 3C (EBNA3C) protein and HLA-DQw8β. | |||||
| Varicella Zoster | Herpesviridae | Varicellovirus | dsDNA | • Islet lesions. | [81,91] |
| • Sequence and structure homology between the viral glycoprotein gE with the human molecule Hsp60. | |||||
| Measles | Paramyxoviridae | Morbillivirus | (−)ssRNA | • Sequence and structure homology between the viral hemagglutinin glycoprotein with the human molecule Hsp60. | [91] |
| Mumps | Paramyxoviridae | Orthorubulavirus | (−)ssRNA | • Infection of fetal pancreas. | [16,20,[92], [93], [94]] |
| • Halt insulin production. | |||||
| • Higher IgA levels in T1DM patients than non-diabetic control group. | |||||
| • Associated with fulminant type 1 diabetes mellitus (FT1D). | |||||
| • Presence of islet cell antibody (ICA) and islet cell surface antibodies (ICSA). | |||||
| Rotavirus | Reoviridae | Rotavirus | dsRNA | • Elevated levels of IgG antibodies in the onset of T1DM. | [22,26,95] |
| • Presence of GAD65 and IA-2 in T1DM onset. | |||||
| • Cross-reactivity between viral VP7 protein and GAD65 and IA-2. | |||||
| • Destruction of infected β-cell via MDA5 pathway. | |||||
| Influenza A virus | Orthomyxoviridae | Alphainfluenzavirus | (−)ssRNA | • Diabetic ketoacidosis. | [5,12,[96], [97], [98], [99], [100]] |
| • Presence of IgM antibodies in the onset of T1DM. | |||||
| • Cross-reaction of prohibitin and antibody against viral hemagglutinin protein. | |||||
| • Cross-reaction of pancreatic α-cells and antibody against the virus. | |||||
| • Envelope protein possesses insulin binding sites. | |||||
| Influenza B virus | Orthomyxoviridae | Betainfluenzavirus | (−)ssRNA | • Presence of IgM antibodies in the onset of T1DM. | [12,101] |
| • Associated with fulminant type 1 diabetes mellitus (FT1D). | |||||
| Parainfluenza | Paramyxoriridae | Respirovirus | (−)ssRNA | • Presence of IgM antibodies in the onset of T1DM. | [12,102] |
| • Diabetic ketoacidosis. | |||||
| Severe acute respiratory syndrome coronavirus 2 | Coronaviridae | Betacoronavirus | (+)ssRNA | • New cases of T1DM in children. | [[103], [104], [105], [106], [107], [108]] |
| • Increase in diabetes ketoacidosis. | |||||
| • Presence of anti-GAD65 antibodies. | |||||
| • Pancreatitis and pancreatic enlargement. | |||||
| • β-cell transdifferentiation. |
1.2. Viruses and type 2 diabetes mellitus (T2DM)
T2DM, also known as non-insulin-dependent DM, is characterized by the inability of the organism to use and synthesize insulin. T2DM individuals are usually insulin-resistant and are unable to control serum glucose levels. According to WHO, approximately 95% of individuals diagnosed with diabetes have T2DM (https://www.who.int/health-topics/diabetes), and prevalence is increasing exponentially, as a result of longer life span, higher body mass, and sedentary lifestyle. Overweight individuals have more adipose tissues, which are associated to elevated production of metabolic hormones and substances that influence the insulin metabolism. Not surprisingly, obesity and sedentarism are the two major risk factors for T2DM [1]. However, it is postulated that the main cause for developing this pathology is the continuous deterioration of insulin secretion by β-cells and a previous condition of IR in multiple organs, such as skeletal muscle, liver, and adipose tissue [44]. Nonetheless, other processes have been linked to the initiation of T2DM such as genetics and environmental factors.
Like T1DM, genetic background plays an important role in T2DM establishment. It is heritable, with off-spring of diagnosed mothers having higher probability of becoming diabetics than children from diabetic fathers [45]. In addition, polymorphisms and mutations in specific genes, as those associated with insulin sensitivity, are important for T2DM onset [46]. Regarding environmental factors, as previously mentioned, dietary and lifestyle habits play an important role in the disease onset. Moreover, viruses have been linked to the initiation of this metabolic syndrome, but the complete relationship between these processes has not fully been characterized.
The research field of viral infection that promotes T2DM is narrow, with few cases reported. The most studied T2DM-related virus is hepatitis C virus (HCV). Other viruses, such as hepatitis B virus (HBV), and human immunodeficiency virus (HIV) have also been associated with T2DM, but literature about their correlation is very scarce. More recently, outstanding studies linking SARS-CoV-2 to T2DM establishment have been published and will be discussed in more detail later.
HCV infects the liver and induces a large local inflammation and fibrosis. Many infected individuals are asymptomatic, but, in some cases, the disease can evolve to a chronic stage, which is characterized by lifelong sequelae. As DM is the most prevalent outcome of liver injury, progression of HCV and the resultant hepatic damage is a clear inductor of DM. Vanni and colleagues reported that HCV induces hepatic and peripheral or muscle IR, by altering the glucose and lipid metabolism [47]. The incidence of T2DM in HCV-infected people is higher than in the overall population [48]; approximately 20% of HCV-infected individuals is also diagnosed with T2DM, contrasting to 8.5% in the HCV-negative group. Ali and coworkers showed that levels of fetuin A and selenoprotein P, hepatokines responsible for glucose and lipid homeostasis, are significantly elevated during HCV infection [49].
It is suggested that AMP-activated protein kinase (AMPK) and Glycogen synthase kinase 3 (GSK3) are two signaling molecules essential to proper HCV replication. AMPK is a kinase in charge of sensing the cell metabolic status through the evaluation of AMP/ATP ratio. After identifying an unbalance in the energy status, AMPK is activated by upstream proteins, which phosphorylate the threonine residue located in the protein kinase domain [50]. Following this event, AMPK phosphorylates important intracellular factors, activating distinct pathways to reinstall cellular homeostasis [50]. Curiously, HCV developed a method to influence AMPK activity. Mankouri and colleagues demonstrated that HCV could reduce AMPK activity [51], as consequence of its phosphorylation at serine 485 by protein kinase B (PKB or AKT), a protein related to glucose metabolism, which results in its inhibition. In addition, restoration of AMPK activity led to reduced HCV replication and lipid accumulation, suggesting an important role for AMPK in the virus replicative process.
GSK3 is another important protein in the cellular metabolism. This ubiquitous protein is a serine/threonine kinase, which is associated with different cellular pathways, including cellular metabolism [52]. GSK3 exists in two isoforms, α and β (GSK-3α and GSK-3β), each one involved in a variety of cellular functions, and responsible to interact with different substrates [52]. As HCV alters carbohydrate metabolism, Saleh et al. identified that only GSK-3β has a role on the virus life cycle. Using synthetic inhibitors and gene silencing targeting this protein, the group described that HCV replication is reduced when GSK-3β is inhibited [53]. Conversely, when overexpressed, GSK-3β increases viral replication. Therefore, the group postulated that the role of GSK-3β in HCV life cycle was because of the expression of microRNA-122 (miR-122), an essential endogenous molecule for virus replicative process, as this kinase promotes the increased levels of this microRNA, identifying other host factor important to HCV life cycle. In addition, in HCV genotype 3a, insulin receptor substrate (IRS-1) is degraded as result of downregulation of peroxisome proliferator-activated receptor ɤ (PPARɤ), which is associated with metabolic syndromes, and upregulation of cytokine signal 7 (SOCS-7) which is related to IRS-1 ubiquitination and, therefore, inducing its degradation [54,55]. On the other hand, HCV genotype 1b decreases IRS-1 levels by inducing the mammalian target of rapamycin (mTOR), a protein implicated in the one of the multiple activities of insulin and insulin-like growth factors (IGFs) axis [56]. mTOR phosphorylates IRS-1 at Ser422, therefore, triggering its ubiquitination [56].
Like HCV, HBV also has tropism for hepatocytes, and infected individuals usually evolve to chronic stage, in which immune cells constantly attack the hepatic tissue to annihilate the virus. As consequence, liver regeneration pathways are activated and, occurs the upregulation of signaling through insulin receptor [57]. HBV, in its turn, hampers this process. In the course of infection, HBV induces the expression of the insulin receptor by stimulating transcription factor NF-E2-related factor 2 (Nrf2) [58], which is responsible for the expression of oxidative stress-responsive genes. Thus, HBV provokes the increase of insulin receptor levels intracellularly and, consequently, its decrease in the cell surface. This mechanism occurs because of increased quantity of α-taxilin, a free syntaxin binding protein associated with intracellular vesicle trafficking, which hampers the translocation of the insulin receptor to the cellular membrane. This explains why HBV infection induces insulin sensitivity and slower liver regeneration. In addition, HBV X protein (HBx) has been characterized as an important factor in the manipulation of host metabolism. Hepatic cells harboring HBx protein have lower levels of IRS-1 compared to non-infected cells, due to the expression of SOCS3, which directly influence the insulin signaling pathways [59]. HBx also interferes in regulation of the glucose metabolism [60], as it is responsible for the upregulation of gluconeogenesis enzymes by activating the inducible nitric oxide synthase (iNOS). Nitric oxide (NO), in turn, promotes gluconeogenesis via the stimulation of HBx transcription. Increased gluconeogenesis results in increased blood glucose levels by de novo glucose synthesis. Despite the augment of blood glucose levels, this mechanism was not correlated to IR.
Apart from direct effect on insulin and glucose metabolism, HBx interacts with liver X receptor α (LXRα), a nuclear receptor responsible for controlling lipid and cholesterol metabolism and induces the expression of LXR-response element (LXRE), culminating in the elevation of sterol-regulatory-element-binding protein 1 (SREBP1), a key regulator of lipogenic genes expression, and fatty acid synthase (FAS) levels [61]. HBx can also increase the activating signal co-integrator 2 (ASC2), a coactivator of many nuclear receptors and transcription factors, such as LXRs, indicating that this protein also interferes in the fatty acid metabolism, which is also altered during T2DM [62].
HIV is another example of potential T2DM inductor. Combination antiretroviral therapy-naïve (cART-naïve) patients possess alterations in glucose and lipid profile, and patients with more advanced disease has the worst lipid status and signs of IR [63]. This lipidic alterations is explained by changes in lipid synthesis, secretion and transportation mediated by HIV replication, which exhorts lipogenesis [64]. In addition, HIV protein R (Vpr) was reported to delay preadipocyte differentiation and to promote lipodystrophy and IR because of its role as PPARγ corepressor [65]. Vpr was also identified as a damper of Foxo-mediated gene transcription, resulting in the blockage of insulin activity. HIV Nef protein can interfere in cellular glucose uptake after exposure to insulin, as this protein interferes with glucose transport 4 (GLUT4) translocation to the plasma membrane in adipocytes [66]. However, further studies on the relationship of HIV and IR are difficult, because of the widely use of cART, which successfully blocks HIV infection and is associated with metabolism alterations.
SARS-CoV-2 was also linked to IR induction. Patients with and without diabetes developed hyperglycemia, insulin sensitivity and IR after SARS-CoV-2 infection [67,68]. The onset of these symptoms is explained by virus-mediated expression of RE1-silencing transcription factor (REST), which controls the transcription of some key metabolic factors, such as myeloperoxidase, apelin, and myostatin [69]. SARS-CoV-2 also have tropism to adipocytes, and infection of these cells leads to alterations in adipokines secretion, including downregulation of adiponectin, a hormone responsible for the glycemic and lipid homeostasis [70,71]. It is believed, therefore, that SARS-CoV-2 induces T2DM by inducting adipose tissue disorder.
Although still poorly explored in the literature, the link between virus infections, and T2DM is a promising research field. A summary of viruses that induces T2DM is presented in Table 2. It is corroborated by the growing number of recently diagnosed patients and the risk of new life-threatening pandemic virus emergence.
Table 2.
Summary of viruses linked to T2DM.
| Virus | Family | Genus | Genome | Main outcomes | Reference |
|---|---|---|---|---|---|
| Hepatitis B | Hepadnaviridae | Orthohepadnavirus | dsDNA | • Suppression of insulin receptor signaling. | [[58], [59], [60], [61], [62],109] |
| • Development of insulin resistance. | |||||
| • Induction of gluconeogenesis genes expression by X protein. | |||||
| • Up-regulation of lipogenic and pro-inflammatory genes induced by X protein overexpression. | |||||
| • Promotion of lipid accumulation in hepatic cells by sterol regulatory element binding protein 1 (SREBP1). | |||||
| Hepatitis C | Flaviviridae | Hepacivirus | (+)ssRNA | • Alterations on glucose and fatty acid metabolism. |
[47,49,51,53,[110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120]] |
| • Development of metabolic syndrome. | |||||
| • Alterations on hepatokines levels. | |||||
| • Core protein promotes the substrate of the insulin receptor degradation through a genotype-specific mechanism. | |||||
| • Alterations of Akt/PKB and GSK3β signaling by E2 protein. | |||||
| • Up-regulation of phosphoenolpyruvate carboxykinase (PEPCK) and related transcription factors. | |||||
| • Viral replication is associated with IR. | |||||
| • Glycogen synthase kinase 3β (GSK-3β) is important for viral replication through its influence on miR-122 expression levels. | |||||
| Ns5A protein induces hepatic gluconeogenesis. | |||||
| Human immunodeficiency virus | Retroviridae | Lentivirus | (+)ssRNA | • Glucose uptake inhibition in adipocytes by Nef protein. | [[63], [64], [65], [66],121,122] |
| • Repression of adipocyte differentiation and inhibition of transcriptional activity of PPAR-γ via Vpr. | |||||
| • Vpr suppress the effect of insulin on the FOXO-mediated gene transcription. | |||||
| • Enhanced lipogenesis. | |||||
| Cytomegalovirus | Herpesviridae | Cytomegalovirus | dsDNA | • Amplified insulin production. | [123,124] |
| • Decreased insulin sensitivity. | |||||
| • Induction of adipocyte-like lipogenesis genes. | |||||
| Severe acute respiratory syndrome coronavirus 2 | Coronaviridae | Betacoronavirus | (+)ssRNA | • Alterations on glucose and lipid metabolism. | [[67], [68], [69], [70], [71],125,126] |
| • Insulin resistance. | |||||
| • Infection of adipocytes. | |||||
| Influenza A | Orthmyxoviridae | Alphainfluenzavirus | (−)ssRNA | • Deficiency of host insulin signaling | [127] |
| • Elevates the expression levels of enzymes that metabolizes fatty acids in the liver. | |||||
| • Development of insulin resistance. |
2. Conclusion
Albeit genetic factors and lifestyle habits are the main predispositions for T1DM and T2DM development, viruses can be associated with the onset of these pathologies, although it is still not possible to estimate the real impact of virus infection in DM incidence in the world. Recently published data indicates, for example, that COVID-19 exhibited an increased risk of diabetes incidence among hospitalized and non-hospitalized individual. Further long-term observational studies started after viral epidemics are essential to measure the direct impact of viruses on DM. Notwithstanding, several mechanisms displayed by viruses have already been linked to DM, but a fully understanding of whole picture needs more consideration. As millions of people are currently living with DM and with a pandemic virus related to these diseases, additional research should be carried out to better understand the connection between viral infections and DM.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Authors’ contributions
Bia Francis Rajsfus: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper. Ronaldo Mohana-Borges: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Diego Allonso: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding
This work was supported by the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) [grant numbers E-26/201.303/2022 and E-26/210.403/2022], Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/PROEX) - Finance code 001, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
- 1.Tan S.Y., Mei Wong J.L., Sim Y.J., Wong S.S., Mohamed Elhassan S.A., Tan S.H., Ling Lim G.P., Rong Tay N.W., Annan N.C., Bhattamisra S.K., Candasamy M. Type 1 and 2 diabetes mellitus: a review on current treatment approach and gene therapy as potential intervention. Diabetes Metabol. Syndr.: Clin. Res. Rev. 2019;13:364–372. doi: 10.1016/j.dsx.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Liu X., Zhang S., Li X., Zheng P., Hu F., Zhou Z. Vaccination with a co-expression DNA plasmid containing GAD65 fragment gene and IL-10 gene induces regulatory CD4 + T cells that prevent experimental autoimmune diabetes. Diabetes/Metab. Res. Rev. 2016;32:522–533. doi: 10.1002/dmrr.2780. [DOI] [PubMed] [Google Scholar]
- 3.Druet C., Tubiana-Rufi N., Chevenne D., Rigal O., Polak M., Levy-Marchal C. Characterization of insulin secretion and resistance in type 2 diabetes of adolescents. J. Clin. Endocrinol. Metab. 2006;91:401–404. doi: 10.1210/jc.2005-1672. [DOI] [PubMed] [Google Scholar]
- 4.Yang Q., Vijayakumar A., Kahn B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018;19:654–672. doi: 10.1038/s41580-018-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi Z., Hu H., Wang Z., Wang G., Li Y., Zhao X., Feng Y., Huo X., Sun J., Feng Q., Liu Y., Wang N., Guo C., Li Y., Wang R., Hu J. Antibodies against H1N1 influenza virus cross-react with α-cells of pancreatic islets. J. Diabetes Invest. 2018;9:265–269. doi: 10.1111/jdi.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nair S., Leung K.-C., Rawlinson W.D., Naing Z., Craig M.E. Enterovirus infection induces cytokine and chemokine expression in insulin-producing cells. J. Med. Virol. 2010;82:1950–1957. doi: 10.1002/jmv.21900. [DOI] [PubMed] [Google Scholar]
- 7.Lampasona V., Liberati D. Islet autoantibodies. Curr. Diabetes Rep. 2016;16:53. doi: 10.1007/s11892-016-0738-2. [DOI] [PubMed] [Google Scholar]
- 8.Yoon J.-W., Jun H.-S. Autoimmune destruction of pancreatic β cells. Am. J. Therapeut. 2005;12:580–591. doi: 10.1097/01.mjt.0000178767.67857.63. [DOI] [PubMed] [Google Scholar]
- 9.Qiu Y., Deng F., Li M., Lei S. Identification of novel risk genes associated with type 1 diabetes mellitus using a genome‐wide gene‐based association analysis. J Diabetes Investig. 2014;5:649–656. doi: 10.1111/jdi.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redondo M.J., Jeffrey J., Fain P.R., Eisenbarth G.S., Orban T. Concordance for islet autoimmunity among monozygotic twins. N. Engl. J. Med. 2008;359:2849–2850. doi: 10.1056/NEJMc0805398. [DOI] [PubMed] [Google Scholar]
- 11.Gamble D.R., Taylor K.W. Seasonal incidence of diabetes mellitus. BMJ. 1969;3:631–633. doi: 10.1136/bmj.3.5671.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karaoglan M., Eksi F. The coincidence of newly diagnosed type 1 diabetes mellitus with IgM antibody positivity to enteroviruses and respiratory tract viruses. J. Diabetes Res. 2018;2018:1–7. doi: 10.1155/2018/8475341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahlquist G.G., Ivarsson S., Lindberg B., Forsgren M. Maternal enteroviral infection during pregnancy as a risk factor for ChildHood IDDM: a population-based case-control study. Diabetes. 1995;44:408–413. doi: 10.2337/diab.44.4.408. [DOI] [PubMed] [Google Scholar]
- 14.Yoneda S., Imagawa A., Fukui K., Uno S., Kozawa J., Sakai M., Yumioka T., Iwahashi H., Shimomura I. A histological study of fulminant type 1 diabetes mellitus related to human cytomegalovirus reactivation. J. Clin. Endocrinol. Metab. 2017;102:2394–2400. doi: 10.1210/jc.2016-4029. [DOI] [PubMed] [Google Scholar]
- 15.Fujiya A., Ochiai H., Mizukoshi T., Kiyota A., Shibata T., Suzuki A., Ohashi N., Sobajima H. Fulminant type 1 diabetes mellitus associated with a reactivation of Epstein–Barr virus that developed in the course of chemotherapy of multiple myeloma. J. Diabetes Invest. 2010;1:286–289. doi: 10.1111/j.2040-1124.2010.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vuorinen T., Nikolakaros G., Smell O., Hyypia T., Vainionpaa R. Mumps and coxsackie B3 virus infection of human fetal pancreatic islet-like cell clusters. Pancreas. 1992;7:460–464. doi: 10.1097/00006676-199207000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Johnson G.M. Diabetes mellitus and congenital rubella infection. Arch. Pediatr. Adolesc. Med. 1970;120:453. doi: 10.1001/archpedi.1970.02100100117014. [DOI] [PubMed] [Google Scholar]
- 18.Wong F.S., Tree T.I. Historical and new insights into pathogenesis of type 1 diabetes. Clin. Exp. Immunol. 2019;198:292–293. doi: 10.1111/cei.13396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lampasona V., Liberati D. Islet autoantibodies. Curr. Diabetes Rep. 2016;16:53. doi: 10.1007/s11892-016-0738-2. [DOI] [PubMed] [Google Scholar]
- 20.Helmke K., Otten A., Willems W.R., Brockhaus R., Mueller-Eckhardt G., Stief T., Bertrams J., Wolf H., Federlin K. Islet cell antibodies and the development of diabetes mellitus in relation to mumps infection and mumps vaccination. Diabetologia. 1986;29:30–33. doi: 10.1007/BF02427277. [DOI] [PubMed] [Google Scholar]
- 21.Cabrera-Rode E., Sarmiento L., Tiberti C., Molina G., Barrios J., Hernández D., Díaz-Horta O., di Mario U. Type 1 diabetes islet associated antibodies in subjects infected by echovirus 16. Diabetologia. 2003;46:1348–1353. doi: 10.1007/s00125-003-1179-4. [DOI] [PubMed] [Google Scholar]
- 22.Ataei-Pirkooh A., Tehrani M., Keyvani H., Esghaei M., Tavakoli A., Nikmanesh B., Farahmand M., Ghaffari H., Monavari S.H. Rotavirus infection enhances levels of autoantibodies against islet cell antigens GAD65 and IA-2 in children with type 1 diabetes. Fetal Pediatr. Pathol. 2019;38:103–111. doi: 10.1080/15513815.2018.1547338. [DOI] [PubMed] [Google Scholar]
- 23.Ginsberg-Fellner F., Witt M.E., Yagihashi S., Dobersen M.J., Taub F., Fedun B., McEvoy R.C., Roman S.H., Davies T.F., Cooper L.Z., Rubinstein P., Notkins A.L. Congenital rubella syndrome as a model for Type 1 (insulin-dependent) diabetes mellitus: increased prevalence of islet cell surface antibodies. Diabetologia. 1984;27:87–89. doi: 10.1007/BF00275655. [DOI] [PubMed] [Google Scholar]
- 24.Pak ChinY., Mcarthur RobertG., Eun H.-M., Yoon J.-W. Association of cytomegalovirus infection with autoimmune type 1 diabetes. Lancet. 1988;332:1–4. doi: 10.1016/s0140-6736(88)92941-8. [DOI] [PubMed] [Google Scholar]
- 25.Rojas M., Restrepo-Jiménez P., Monsalve D.M., Pacheco Y., Acosta-Ampudia Y., Ramírez-Santana C., Leung P.S.C., Ansari A.A., Gershwin M.E., Anaya J.M. Molecular mimicry and autoimmunity. J. Autoimmun. 2018;95:100–123. doi: 10.1016/j.jaut.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Honeyman M.C., Stone N.L., Falk B.A., Nepom G., Harrison L.C. Evidence for molecular mimicry between human T cell epitopes in rotavirus and pancreatic islet autoantigens. J. Immunol. 2010;184:2204–2210. doi: 10.4049/jimmunol.0900709. [DOI] [PubMed] [Google Scholar]
- 27.Härkönen T., Lankinen H., Davydova B., Hovi T., Roivainen M. Enterovirus infection can induce immune responses that cross-react with β-cell autoantigen tyrosine phosphatase IA-2/IAR. J. Med. Virol. 2002;66:340–350. doi: 10.1002/jmv.2151. [DOI] [PubMed] [Google Scholar]
- 28.Lonnrot M., Hyöty H., Knip M., Roivainen M., Kulmala P., Leinikki P., Åkerblom H.K. Antibody cross-reactivity induced by the homologous regions in glutamic acid decarboxylase (GAD65) and 2C protein of coxsackievirus B4. Clin. Exp. Immunol. 1996;104:398–405. doi: 10.1046/j.1365-2249.1996.60771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiemstra H.S., Schloot N.C., van Veelen P.A., Willemen S.J.M., Franken K.L.M.C., van Rood J.J., de Vries R.R.P., Chaudhuri A., Behan P.O., Drijfhout J.W., Roep B.O. Cytomegalovirus in autoimmunity: T cell crossreactivity to viral antigen and autoantigen glutamic acid decarboxylase. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3988–3991. doi: 10.1073/pnas.071050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parkkonen P., Hyöty H., Ilonen J., Reijonen H., Ylä-Herttuala S., Leinikki P. Antibody reactivity to an Epstein-Barr virus BERF4-encoded epitope occurring also in Asp-57 region of HLA-DQ8 β chain. Clin. Exp. Immunol. 2008;95:287–293. doi: 10.1111/j.1365-2249.1994.tb06525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karounos D., Wolinsky J., Thomas J. Monoclonal antibody to rubella virus capsid protein recognizes a beta-cell antigen. J. Immunol. 1993;150:3080–3085. [PubMed] [Google Scholar]
- 32.Noble J.A., Valdes A.M. Genetics of the HLA region in the prediction of type 1 diabetes. Curr. Diabetes Rep. 2011;11:533–542. doi: 10.1007/s11892-011-0223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noble J.A., Valdes A.M., Cook M., Klitz W., Thomson G., Erlich1’ H.A. IDDM; 1996. The Role of HLA Class 11 Genes in Insulin-dependent Diabetes Mellitus: Molecular Analysis of 180 Caucasian, Multiplex Families Summary We Report Here Our Analysis of HLA Class II Alleles in 180 Caucasian Nuclear Families with at Least Two Children with Insulin-dependent Diabetes Mellitus. [PMC free article] [PubMed] [Google Scholar]
- 34.Sadeharju K., Knip M., Hiltunen M., Åkerblom H.K., Hyöty H. The HLA-DR phenotype modulates the humoral immune response to enterovirus antigens. Diabetologia. 2003;46:1100–1105. doi: 10.1007/s00125-003-1157-x. [DOI] [PubMed] [Google Scholar]
- 35.Rubinstein P., Walker M.E., Fedun B., Cooper L.Z., Ginsberg-Fellner F., Rubinstein P., Kimball L.F. The HLA system in congenital rubella patients with and without diabetes. Diabetes. 1982;31:1088–1091. doi: 10.2337/diacare.31.12.1088. [DOI] [PubMed] [Google Scholar]
- 36.Unsworth R., Wallace S., Oliver N.S., Yeung S., Kshirsagar A., Naidu H., Kwong R.M.W., Kumar P., Logan K.M. New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the U.K. Diabetes Care. 2020;43:e170–e171. doi: 10.2337/dc20-1551. [DOI] [PubMed] [Google Scholar]
- 37.Ambati S., Mihic M., Rosario D.C., Sanchez J., Bakar A. New-onset type 1 diabetes in children with SARS-CoV-2 infection. Cureus. 2022;14 doi: 10.7759/cureus.22790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchand L., Pecquet M., Luyton C. Type 1 diabetes onset triggered by COVID-19. Acta Diabetol. 2020;57:1265–1266. doi: 10.1007/s00592-020-01570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollstein T., Schulte D.M., Schulz J., Glück A., Ziegler A.G., Bonifacio E., Wendorff M., Franke A., Schreiber S., Bornstein S.R., Laudes M. Autoantibody-negative insulin-dependent diabetes mellitus after SARS-CoV-2 infection: a case report. Nat. Metab. 2020;2:1021–1024. doi: 10.1038/s42255-020-00281-8. [DOI] [PubMed] [Google Scholar]
- 40.Liu F., Long X., Zhang B., Zhang W., Chen X., Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin. Gastroenterol. Hepatol. 2020;18:2128–2130.e2. doi: 10.1016/j.cgh.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akarsu C., Karabulut M., Aydin H., Sahbaz N.A., Dural A.C., Yegul D., Peker K.D., Ferahman S., Bulut S., Dönmez T., Asar S., Yasar K.K., Adas G.T. Association between acute pancreatitis and COVID-19: could pancreatitis Be the missing piece of the puzzle about increased mortality rates? J. Invest. Surg. 2022;35:119–125. doi: 10.1080/08941939.2020.1833263. [DOI] [PubMed] [Google Scholar]
- 42.Wu C.-T., Lidsky P.v., Xiao Y., Lee I.T., Cheng R., Nakayama T., Jiang S., Demeter J., Bevacqua R.J., Chang C.A., Whitener R.L., Stalder A.K., Zhu B., Chen H., Goltsev Y., Tzankov A., Nayak J.v., Nolan G.P., Matter M.S., Andino R., Jackson P.K. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metabol. 2021;33:1565–1576.e5. doi: 10.1016/j.cmet.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang X., Uhl S., Zhang T., Xue D., Li B., Vandana J.J., Acklin J.A., Bonnycastle L.L., Narisu N., Erdos M.R., Bram Y., Chandar V., Chong A.C.N., Lacko L.A., Min Z., Lim J.K., Borczuk A.C., Xiang J., Naji A., Collins F.S., Evans T., Liu C., tenOever B.R., Schwartz R.E., Chen S. SARS-CoV-2 infection induces beta cell transdifferentiation. Cell Metabol. 2021;33:1577–1591.e7. doi: 10.1016/j.cmet.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Defronzo R.A. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009:773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Groop L., Forsblom C., Lehtovirta M., Tuomi T., Karanko S., Nissén M., Ehrnström B.-O., Forsén B., Isomaa B., Snickars B., Taskinen M.-R. Metabolic consequences of a family history of NIDDM (the botnia study): evidence for sex-specific parental effects. Diabetes. 1996;45:1585–1593. doi: 10.2337/diab.45.11.1585. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y., Luan Y., Feng Q., Chen X., Qin B., Ren K.-D., Luan Y. Epigenetics and beyond: targeting histone methylation to treat type 2 diabetes mellitus. Front. Pharmacol. 2022;12 doi: 10.3389/fphar.2021.807413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanni E., Abate M.L., Gentilcore E., Hickman I., Gambino R., Cassader M., Smedile A., Ferrannini E., Rizzetto M., Marchesini G., Gastaldelli A., Bugianesi E. Sites and mechanisms of insulin resistance in nonobese, nondiabetic patients with chronic hepatitis C. Hepatology. 2009;50:697–706. doi: 10.1002/hep.23031. [DOI] [PubMed] [Google Scholar]
- 48.Ambachew S., Eshetie S., Geremew D., Endalamaw A., Melku M. Prevalence of type 2 diabetes mellitus among hepatitis C virus-infected patients: a systematic review and meta-analysis. Int. J. Diabetes Metabol. 2018:29–37. doi: 10.1186/s13643-019-0976-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ali S.A., Nassif W.M.H., Abdelaziz D.H.A. Alterations in serum levels of fetuin A and selenoprotein P in chronic hepatitis C patients with concomitant type 2 diabetes: a case-control study. Clin. Res. Hepatol. Gastroenterol. 2016;40:465–470. doi: 10.1016/j.clinre.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Steinberg G.R., Carling D. AMP-activated protein kinase: the current landscape for drug development. Nat. Rev. Drug Discov. 2019;18:527–551. doi: 10.1038/s41573-019-0019-2. [DOI] [PubMed] [Google Scholar]
- 51.Mankouri J., Tedbury P.R., Gretton S., Hughes M.E., Griffin S.D.C., Dallas M.L., Green K.A., Hardie D.G., Peers C., Harris M. Enhanced hepatitis C virus genome replication and lipid accumulation mediated by inhibition of AMP-activated protein kinase. Proc. Natl. Acad. Sci. USA. 2010;107:11549–11554. doi: 10.1073/pnas.0912426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beurel E., Grieco S.F., Jope R.S. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol. Ther. 2015;148:114–131. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saleh M., Rüschenbaum S., Welsch C., Zeuzem S., Moradpour D., Gouttenoire J., Lange C.M. Glycogen synthase kinase 3β enhances hepatitis C virus replication by supporting miR-122. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahmadian M., Suh J.M., Hah N., Liddle C., Atkins A.R., Downes M., Evans R.M. PPARγ signaling and metabolism: the good, the bad and the future. Nat. Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banks A.S., Li J., McKeag L., Hribal M.L., Kashiwada M., Accili D., Rothman P.B. Deletion of SOCS7 leads to enhanced insulin action and enlarged islets of Langerhans. J. Clin. Invest. 2005;115:2462–2471. doi: 10.1172/JCI23853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoneyama Y., Inamitsu T., Chida K., Iemura S.-I., Natsume T., Maeda T., Hakuno F., Takahashi S.-I. Serine phosphorylation by mTORC1 promotes IRS-1 degradation through SCFβ-TRCP E3 ubiquitin ligase. iScience. 2018;5:1–18. doi: 10.1016/j.isci.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sasaki Y., Zhang X.F., Nishiyama M., Avruch J., Wands J.R. Expression and phosphorylation of insulin receptor substrate 1 during rat liver regeneration. J. Biol. Chem. 1993;268:3805–3808. [PubMed] [Google Scholar]
- 58.Barthel S.R., Medvedev R., Heinrich T., Büchner S.M., Kettern N., Hildt E. Hepatitis B virus inhibits insulin receptor signaling and impairs liver regeneration via intracellular retention of the insulin receptor. Cell. Mol. Life Sci. 2016;73:4121–4140. doi: 10.1007/s00018-016-2259-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim K., Kim K.H., Cheong J. Hepatitis B virus X protein impairs hepatic insulin signaling through degradation of IRS1 and induction of SOCS3. PLoS One. 2010;5 doi: 10.1371/journal.pone.0008649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shin H.-J., Park Y.-H., Kim S.-U., Moon H.-B., Park D.S., Han Y.-H., Lee C.-H., Lee D.-S., Song I.-S., Lee D.H., Kim M., Kim N.-S., Kim D.-G., Kim J.-M., Kim S.-K., Kim Y.N., Kim S.S., Choi C.S., Kim Y.-B., Yu D.-Y. Hepatitis B virus X protein regulates hepatic glucose homeostasis via activation of inducible nitric oxide synthase. J. Biol. Chem. 2011;286:29872–29881. doi: 10.1074/jbc.M111.259978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim K., Kim K.H., Kim H.H., Cheong J. Hepatitis B virus X protein induces lipogenic transcription factor SREBP1 and fatty acid synthase through the activation of nuclear receptor LXRα. Biochem. J. 2008;416:219–230. doi: 10.1042/BJ20081336. [DOI] [PubMed] [Google Scholar]
- 62.Kim K.H., Shin H., Kim K., Choi H.M., Rhee S.H., Moon H., Kim H.H., Yang U.S., Yu D., Cheong J. Hepatitis B virus X protein induces hepatic steatosis via transcriptional activation of SREBP1 and PPARγ. Gastroenterology. 2007;132:1955–1967. doi: 10.1053/j.gastro.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 63.El-Sadr W., Mullin C., Carr A., Gibert C., Rappoport C., Visnegarwala F., Grunfeld C., Raghavan S. Effects of HIV disease on lipid, glucose and insulin levels: results from a large antiretroviral-naive cohort. HIV Med. 2005;6:114–121. doi: 10.1111/j.1468-1293.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 64.Rasheed S., Yan J.S., Lau A., Chan A.S. HIV replication enhances production of free fatty acids, low density lipoproteins and many key proteins involved in lipid metabolism: a proteomics study. PLoS One. 2008;3:e3003. doi: 10.1371/journal.pone.0003003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shrivastav S., Kino T., Cunningham T., Ichijo T., Schubert U., Heinklein P., Chrousos G.P., Kopp J.B. Human immunodeficiency virus (HIV)-1 viral protein R suppresses transcriptional activity of peroxisome proliferator-activated receptor γ and inhibits adipocyte differentiation: implications for HIV-associated lipodystrophy. Mol. Endocrinol. 2008;22:234–247. doi: 10.1210/me.2007-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheney L., Hou J.C., Morrison S., Pessin J., Steigbigel R.T. Nef inhibits glucose uptake in adipocytes and contributes to insulin resistance in human immunodeficiency virus type I infection. J. Infect. Dis. 2011;203:1824–1831. doi: 10.1093/infdis/jir170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Affinati A.H., Wallia A., Gianchandani R.Y. Severe hyperglycemia and insulin resistance in patients with SARS-CoV-2 infection: a report of two cases. Clin Diabetes Endocrinol. 2021;7:8. doi: 10.1186/s40842-021-00121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montefusco L., ben Nasr M., D'Addio F., Loretelli C., Rossi A., Pastore I., Daniele G., Abdelsalam A., Maestroni A., Dell'Acqua M., Ippolito E., Assi E., Usuelli V., Seelam A.J., Fiorina R.M., Chebat E., Morpurgo P., Lunati M.E., Bolla A.M., Finzi G., Abdi R., Bonventre J.v., Rusconi S., Riva A., Corradi D., Santus P., Nebuloni M., Folli F., Zuccotti G.V., Galli M., Fiorina P. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat. Metab. 2021;3:774–785. doi: 10.1038/s42255-021-00407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He X., Liu C., Peng J., Li Z., Li F., Wang J., Hu A., Peng M., Huang K., Fan D., Li N., Zhang F., Cai W., Tan X., Hu Z., Deng X., Li Y., Mo X., Li L., Shi Y., Yang L., Zhu Y., Wu Y., Liang H., Liao B., Hong W., He R., Li J., Guo P., Zhuo Y., Zhao L., Hu F., Li W., Zhu W., Zhang Z., Guo Z., Zhang W., Hong X., Cai W., Gu L., Du Z., Zhang Y., Xu J., Zuo T., Deng K., Yan L., Chen X., Chen S., Lei C. COVID-19 induces new-onset insulin resistance and lipid metabolic dysregulation via regulation of secreted metabolic factors. Signal Transduct. Targeted Ther. 2021;6:427. doi: 10.1038/s41392-021-00822-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsilingiris D., Dalamaga M., Liu J. SARS-CoV-2 adipose tissue infection and hyperglycemia: a further step towards the understanding of severe COVID-19. Metabol Open. 2022;13 doi: 10.1016/j.metop.2022.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reiterer M., Rajan M., Gómez-Banoy N., Lau J.D., Gomez-Escobar L.G., Ma L., Gilani A., Alvarez-Mulett S., Sholle E.T., Chandar V., Bram Y., Hoffman K., Bhardwaj P., Piloco P., Rubio-Navarro A., Uhl S., Carrau L., Houhgton S., Redmond D., Shukla A.P., Goyal P., Brown K.A., tenOever B.R., Alonso L.C., Schwartz R.E., Schenck E.J., Safford M.M., Lo J.C. Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2. Cell Metabol. 2021;33:2174–2188.e5. doi: 10.1016/j.cmet.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ohara N., Kaneko M., Nishibori T., Sato K., Furukawa T., Koike T., Sone H., Kaneko K., Kamoi K. Fulminant type 1 diabetes mellitus associated with coxsackie virus type A2 infection: a case report and literature review. Intern. Med. 2016;55:643–646. doi: 10.2169/internalmedicine.55.5292. [DOI] [PubMed] [Google Scholar]
- 73.Roivainen M., Rasilainen S., Ylipaasto P., Nissinen R., Ustinov J., Bouwens L., Cio D.É., Eizirik L., Hovi T., Otonkoski T. 2000. Mechanisms of Coxsackievirus-Induced Damage to Human Pancreatic-Cells*. [DOI] [PubMed] [Google Scholar]
- 74.Dotta F., Censini S., van Halteren A.G.S., Marselli L., Masini M., Dionisi S., Mosca F., Boggi U., Muda A.O., del Prato S., Elliott J.F., Covacci A., Rappuoli R., Roep B.O., Marchetti P. 2007. Coxsackie B4 Virus Infection of Cells and Natural Killer Cell Insulitis in Recent-Onset Type 1 Diabetic Patients.www.pnas.org/cgi/content/full/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yin H., Berg A.-K., Tuvemo T., Frisk G. Enterovirus RNA is found in peripheral blood mononuclear cells in a majority of type 1 diabetic children at onset. Diabetes. 2002;51:1964–1971. doi: 10.2337/diabetes.51.6.1964. [DOI] [PubMed] [Google Scholar]
- 76.André L., Hober D., Hober-Vandenberghe C., Belaich S., Vantyghem M.-C., Lefebvre J., Wattré P. 1997. Detection of Coxsackie B Virus RNA Sequences in Whole Blood Samples from Adult Patients at the Onset of Type I Diabetes Mellitus. [DOI] [PubMed] [Google Scholar]
- 77.Lönnrot M., Korpela K., Knip M., Ilonen J., Simell O., Korhonen S., Savola K., Muona P., Simell T., Koskela P., Hyöty H. 2000. Enterovirus Infection as a Risk Factor For-Cell Autoimmunity in a Prospectively Observed Birth Cohort the Finnish Diabetes Prediction and Prevention Study.http://diabetesjournals.org/diabetes/article-pdf/49/8/1314/365044/10923631.pdf [DOI] [PubMed] [Google Scholar]
- 78.Gerling I., Chatterjee K., Nejman C. 1991. Coxsackievirus B4-Induced Development of Antibodies to 64,000-Mr Islet Autoantigen and Hyperglycemia in Mice. [DOI] [PubMed] [Google Scholar]
- 79.Frisk G., Friman G., Tuvemo T., Fohlman J., Diderholln H. 1992. Coxsackie B Virus IgM in Children at Onset of Type 1 (Insulin-dependent) Diabetes Mellitus: Evidence for IgM Induction by a Recent or Current Infection. [DOI] [PubMed] [Google Scholar]
- 80.Yoon J.-W., Austin M., Onodera T., Notkins A.L. Virus-induced diabetes mellitus. N. Engl. J. Med. 1979;300:1173–1179. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]
- 81.Bennettjenson A. Pancreatic islet-cell damage in children with fatal viral infections. Lancet. 1980;316:354–358. [PubMed] [Google Scholar]
- 82.Jaidane H., Caloone D., Lobert P.-E., Sane F., Dardenne O., Naquet P., Gharbi J., Aouni M., Geenen V., Hober D. Persistent infection of thymic epithelial cells with coxsackievirus B4 results in decreased expression of type 2 insulin-like growth factor. J. Virol. 2012;86:11151–11162. doi: 10.1128/JVI.00726-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim K.W., Ho A., Alshabee-Akil A., Hardikar A.A., Kay T.W.H., Rawlinson W.D., Craig M.E. Coxsackievirus B5 infection induces dysregulation of microRNAs predicted to target known type 1 diabetes risk genes in human pancreatic islets. Diabetes. 2016;65:996–1003. doi: 10.2337/db15-0956. [DOI] [PubMed] [Google Scholar]
- 84.Colli M.L., Paula F.M., Marselli L., Marchetti P., Roivainen M., Eizirik D.L., Op de beeck A. Coxsackievirus B tailors the unfolded protein response to favour viral amplification in pancreatic β cells. J. Innate Immun. 2019;11:375–390. doi: 10.1159/000496034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Menser MargaretA., Forrest JillM., Honeyman MargoC., Burgess J.A. Letter: diabetes, HL-A antigens, and congenital rubella. Lancet. 1974;2:1508–1509. doi: 10.1016/s0140-6736(74)90240-2. [DOI] [PubMed] [Google Scholar]
- 86.Cooper L.Z. Neonatal thrombocytopenic purpura and other manifestations of rubella contracted in utero. Arch. Pediatr. Adolesc. Med. 1965;110:416. doi: 10.1001/archpedi.1965.02090030436011. [DOI] [PubMed] [Google Scholar]
- 87.Roep B.O., Hiemstra H.S., Schloot N.C., P De Vries R.R., Chaudhuri A., Behan P.O., Drijfhout J.W. 2002. Molecular Mimicry in Type 1 Diabetes Immune Cross-Reactivity between Islet Autoantigen and Human Cytomegalovirus but Not Coxsackie Virus. [PubMed] [Google Scholar]
- 88.Smelt M.J., Faas M.M., de Haan B.J., Draijer C., Hugenholtz G.C.G., de Haan A., Engelse M.A., de Koning E.J.P., de Vos P. 2012. Susceptibility of Human Pancreatic A Cells for Cytomegalovirus Infection and the Effects on Cellular Immunogenicity.www.pancreasjournal.com39 [DOI] [PubMed] [Google Scholar]
- 89.Pak C.Y., Cha C.Y., v Rajotte R., Mcarthur R.G., Yoon J.W. Human pancreatic islet cell specific 38 kilodalton autoantigen identified by cytomegalovirus-induced monoclonal islet cell autoantibody. Diabetologia. 1990;33:569–572. doi: 10.1007/BF00404146. [DOI] [PubMed] [Google Scholar]
- 90.Bian X., Wallstrom G., Davis A., Wang J., Park J., Throop A., Steel J., Yu X., Wasserfall C., Schatz D., Atkinson M., Qiu J., LaBaer J. Immunoproteomic profiling of antiviral antibodies in new-onset type 1 diabetes using protein arrays. Diabetes. 2016;65:285–296. doi: 10.2337/db15-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meziane F.Z., Dali-Sahi M., Dennouni-Medjati N., Boulenouar H., Kachekouche Y., Benslama Y., Harek Y. Molecular mimicry between varicella, measles virus and Hsp60 in type 1 diabetes associated HLA-DR3/DR4 molecules. Diabetes Metabol. Syndr.: Clin. Res. Rev. 2020;14:1783–1789. doi: 10.1016/j.dsx.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 92.Hyoty H., Huupponent T., Leinikki P. 1985. Humoral Immunity against Viral Antigens in Insulin-dependent Diabetes Mellitus (IDDM): Altered IgA Class Immune Response against Mumps Virus. [PMC free article] [PubMed] [Google Scholar]
- 93.Goto A., Takahashi Y., Kishimoto M., Nakajima Y., Nakanishi K., Kajio H., Noda M. 2008. A Case of Fulminant Type 1 Diabetes Associated with Significant Elevation of Mumps Titers.http://www.cste.org/PS/2007ps/2007psfinal/ID/07- [DOI] [PubMed] [Google Scholar]
- 94.Ratzmann K.P., Strese J., Witt S., Berling H., Keilacker H., Michaelis D. Mumps infection and insulin-dependent diabetes mellitus (IDDM) Diabetes Care. 1984;7:170–173. doi: 10.2337/diacare.7.2.170. [DOI] [PubMed] [Google Scholar]
- 95.Dou Y., Yim H.C., Kirkwood C.D., Williams B.R., Sadler A.J. The innate immune receptor <scp>MDA</scp> 5 limits rotavirus infection but promotes cell death and pancreatic inflammation. EMBO J. 2017;36:2742–2757. doi: 10.15252/embj.201696273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nenna R., Papoff P., Moretti C., Pierangeli A., Sabatino G., Costantino F., Soscia F., Cangiano G., Ferro V., Mennini M., Salvadei S., Scagnolari C., Antonelli G., Midulla F. Detection of respiratory viruses in the 2009 winter season in rome: 2009 influenza a (H1N1) complications in children and concomitant type 1 diabetes onset. Int. J. Immunopathol. Pharmacol. 2011;24:651–659. doi: 10.1177/039463201102400311. [DOI] [PubMed] [Google Scholar]
- 97.Larcombe P.J., Moloney S.E., Schmidt P.A. Pandemic (H1N1) 2009: a clinical spectrum in the general paediatric population. Arch. Dis. Child. 2011;96:96–98. doi: 10.1136/adc.2009.176859. [DOI] [PubMed] [Google Scholar]
- 98.Sun L., Li H., Sun J., Guo C., Feng Y., Li Y., Zhao X., Xie X., Hu J. Antibodies against H1N1 influenza virus hemagglutinin cross-react with prohibitin. Biochem. Biophys. Res. Commun. 2019;513:446–451. doi: 10.1016/j.bbrc.2019.03.188. [DOI] [PubMed] [Google Scholar]
- 99.Tani N., Michiue T., Chen J.-H., Oritani S., Ishikawa T. Usefulness of postmortem biochemistry in identification of ketosis: diagnosis of ketoacidosis at the onset of autoimmune type 1 diabetes in an autopsy case with cold exposure and malnutrition. Leg. Med. 2016;22:23–29. doi: 10.1016/j.legalmed.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 100.Samnotra V., Germinario R.J., Wainberg M.A. Presence of insulin binding sites on viral particles. Antivir. Res. 1988;9:285–293. doi: 10.1016/0166-3542(88)90024-1. [DOI] [PubMed] [Google Scholar]
- 101.Sano H., Terasaki J., Tsutsumi C., Imagawa A., Hanafusa T. A case of fulminant type 1 diabetes mellitus after influenza B infection. Diabetes Res. Clin. Pract. 2008;79:e8–e9. doi: 10.1016/j.diabres.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 102.Silvestri F., Lapa D., Ferrara C., Grisolia D., Costantino F., Castilletti C., Tromba V. Parainfluenza viruses: a trigger for type 1 diabetes new onset? New Microbiol. 2021;44:241–244. [PubMed] [Google Scholar]
- 103.Unsworth R., Wallace S., Oliver N.S., Yeung S., Kshirsagar A., Naidu H., Kwong R.M.W., Kumar P., Logan K.M. New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the U.K. Diabetes Care. 2020;43:e170–e171. doi: 10.2337/dc20-1551. [DOI] [PubMed] [Google Scholar]
- 104.Ambati S., Mihic M., Rosario D.C., Sanchez J., Bakar A. New-onset type 1 diabetes in children with SARS-CoV-2 infection. Cureus. 2022;14 doi: 10.7759/cureus.22790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marchand L., Pecquet M., Luyton C. Type 1 diabetes onset triggered by COVID-19. Acta Diabetol. 2020;57:1265–1266. doi: 10.1007/s00592-020-01570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu F., Long X., Zhang B., Zhang W., Chen X., Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin. Gastroenterol. Hepatol. 2020;18:2128–2130.e2. doi: 10.1016/j.cgh.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Akarsu C., Karabulut M., Aydin H., Sahbaz N.A., Dural A.C., Yegul D., Peker K.D., Ferahman S., Bulut S., Dönmez T., Asar S., Yasar K.K., Adas G.T. Association between acute pancreatitis and COVID-19: could pancreatitis Be the missing piece of the puzzle about increased mortality rates? J. Invest. Surg. 2022;35:119–125. doi: 10.1080/08941939.2020.1833263. [DOI] [PubMed] [Google Scholar]
- 108.Tang X., Uhl S., Zhang T., Xue D., Li B., Vandana J.J., Acklin J.A., Bonnycastle L.L., Narisu N., Erdos M.R., Bram Y., Chandar V., Chong A.C.N., Lacko L.A., Min Z., Lim J.K., Borczuk A.C., Xiang J., Naji A., Collins F.S., Evans T., Liu C., tenOever B.R., Schwartz R.E., Chen S. SARS-CoV-2 infection induces beta cell transdifferentiation. Cell Metabol. 2021;33:1577–1591.e7. doi: 10.1016/j.cmet.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ghenea A.E., Padureanu V., Cioboata R., Udristoiu A.-L., Drocas A.I., Țieranu E., Carsote M., Vasile C.M., Morosanu A., Biciusca V., Salan A.-I., Turculeanu A., Ungureanu A. The study of clinical and biochemical parameters in assessing the response to the antiviral therapy in the chronic viral hepatitis B. Medicina. 2021;57:757. doi: 10.3390/medicina57080757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lonardo A., Adinolfi L.E., Loria P., Carulli N., Ruggiero G., Day C.P. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004;126:586–597. doi: 10.1053/j.gastro.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 111.Pazienza V., Clément S., Pugnale P., Conzelman S., Foti M., Mangia A., Negro F. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology. 2007;45:1164–1171. doi: 10.1002/hep.21634. [DOI] [PubMed] [Google Scholar]
- 112.Romero-Gómez M., del Mar Viloria M., Andrade R.J., Salmerón J., Diago M., Fernández-Rodríguez C.M., Corpas R., Cruz M., Grande L., Vázquez L., Muñoz-de-Rueda P., López-Serrano P., Gila A., Gutiérrez M.L., Pérez C., Ruiz-Extremera A., Suárez E., Castillo J. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636–641. doi: 10.1053/j.gastro.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 113.Li Y., Wang X., Yu G., Sun H., Lv J., Chi X., Wu R., Gao X., Niu J. The association of hepatitis c virus infection status with serum glucose levels. BMC Gastroenterol. 2019;19 doi: 10.1186/s12876-019-1003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lupberger J., Croonenborghs T., Roca Suarez A.A., van Renne N., Jühling F., Oudot M.A., Virzì A., Bandiera S., Jamey C., Meszaros G., Brumaru D., Mukherji A., Durand S.C., Heydmann L., Verrier E.R., el Saghire H., Hamdane N., Bartenschlager R., Fereshetian S., Ramberger E., Sinha R., Nabian M., Everaert C., Jovanovic M., Mertins P., Carr S.A., Chayama K., Dali-Youcef N., Ricci R., Bardeesy N.M., Fujiwara N., Gevaert O., Zeisel M.B., Hoshida Y., Pochet N., Baumert T.F. Combined analysis of metabolomes, proteomes, and transcriptomes of hepatitis C virus–infected cells and liver to identify pathways associated with disease development. Gastroenterology. 2019;157:537–551.e9. doi: 10.1053/j.gastro.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Postic C., Girard J. The role of the lipogenic pathway in the development of hepatic steatosis. Diabetes Metabol. 2008;34:643–648. doi: 10.1016/S1262-3636(08)74599-3. [DOI] [PubMed] [Google Scholar]
- 116.Alaei M., Negro F. Hepatitis C virus and glucose and lipid metabolism. Diabetes Metabol. 2008;34:692–700. doi: 10.1016/S1262-3636(08)74606-8. [DOI] [PubMed] [Google Scholar]
- 117.Hsieh M.-J., Lan K.-P., Liu H.-Y., Zhang X.-Z., Lin Y.-F., Chen T.-Y., Chiou H.-L. Hepatitis C virus E2 protein involve in insulin resistance through an impairment of Akt/PKB and GSK3β signaling in hepatocytes. BMC Gastroenterol. 2012;12:74. doi: 10.1186/1471-230X-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oliveira L., Jesus R., Boulhosa R., Mendes C., Lyra A., Lyra L. Metabolic syndrome in patients with chronic hepatitis C virus genotype 1 infection who do not have obesity or type 2 diabetes. Clinics. 2012;67:219–223. doi: 10.6061/clinics/2012(03)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Deng L., Shoji I., Ogawa W., Kaneda S., Soga T., Jiang D., Ide Y.-H., Hotta H. Hepatitis C virus infection promotes hepatic gluconeogenesis through an NS5A-mediated, FoxO1-dependent pathway. J. Virol. 2011;85:8556–8568. doi: 10.1128/JVI.00146-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Perrin-Cocon L., Kundlacz C., Jacquemin C., Hanoulle X., Aublin-Gex A., Figl M., Manteca J., André P., Vidalain P.-O., Lotteau V., Diaz O. Domain 2 of hepatitis C virus protein NS5A activates glucokinase and induces lipogenesis in hepatocytes. Int. J. Mol. Sci. 2022;23:919. doi: 10.3390/ijms23020919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hellerstein M.K., Grunfeld C., Wu K., Christiansen M., Kaempfer S., Kletke C., Shackleton C.H. Increased de novo hepatic lipogenesis in human immunodeficiency virus infection. J. Clin. Endocrinol. Metabol. 1993;76:559–565. doi: 10.1210/jcem.76.3.8445011. [DOI] [PubMed] [Google Scholar]
- 122.Kino T., de Martino M.U., Charmandari E., Ichijo T., Outas T., Chrousos G.P. HIV-1 accessory protein Vpr inhibits the effect of insulin on the Foxo subfamily of forkhead transcription factors by interfering with their binding to 14-3-3 proteins. Diabetes. 2005;54:23–31. doi: 10.2337/diabetes.54.1.23. [DOI] [PubMed] [Google Scholar]
- 123.Šestan M., Marinović S., Kavazović I., Cekinović Đ., Wueest S., Turk Wensveen T., Brizić I., Jonjić S., Konrad D., Wensveen F.M., Polić B. Virus-induced interferon-γ causes insulin resistance in skeletal muscle and derails glycemic control in obesity. Immunity. 2018;49:164–177.e6. doi: 10.1016/j.immuni.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 124.Yu Y., Maguire T.G., Alwine J.C. Human cytomegalovirus infection induces adipocyte-like lipogenesis through activation of sterol regulatory element binding protein 1. J. Virol. 2012;86:2942–2949. doi: 10.1128/JVI.06467-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ilias I., Diamantopoulos A., Pratikaki M., Botoula E., Jahaj E., Athanasiou N., Tsipilis S., Zacharis A., Vassiliou A.G., Vassiliadi D.A., Kotanidou A., Tsagarakis S., Dimopoulou I. Glycemia, beta-cell function and sensitivity to insulin in mildly to critically ill covid-19 patients. Medicina. 2021;57:68. doi: 10.3390/medicina57010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen M., Zhu B., Chen D., Hu X., Xu X., Shen W.-J., Hu C., Li J., Qu S. COVID-19 may increase the risk of insulin resistance in adult patients without diabetes: a 6-month prospective study. Endocr. Pract. 2021;27:834–841. doi: 10.1016/j.eprac.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ohno M., Sekiya T., Nomura N., ji Daito T., Shingai M., Kida H. Influenza virus infection affects insulin signaling, fatty acid-metabolizing enzyme expressions, and the tricarboxylic acid cycle in mice. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-67879-6. [DOI] [PMC free article] [PubMed] [Google Scholar]