Abstract
A third of the world’s population suffers from unexplained fatigue, hugely impacting work learning, efficiency, and health. The fatigue development may be a concomitant state of a disease or the side effect of a drug, or muscle fatigue induced by intense exercise. However, there are no authoritative guides or clinical medication recommendations for various fatigue classifications. Traditional Chinese medicines (TCM) are used as dietary supplements or healthcare products with specific anti-fatigue effects. Thus, TCM may be a potential treatment for fatigue. In this review, we outline the pathogenesis of fatigue, awareness of fatigue in Chinese and western medicine, pharmacodynamics mechanism, and substances. Additionally, we offer a comprehensive summary of fatigue and forecast the potential effect of novel herbal-based medicines against fatigue.
Keywords: Active constituents, Fatigue, Herbal medicine, Mechanisms
1. Introduction
Unhealthy diet, lack of rest, physical activity deficiency, unreasonable exercise, high-intensity work, mental tension, sore throat, tender lymph nodes, multi-joint and muscle pain, headaches, and long-term bad mood are increasingly prevalent in a fast-paced living environment (van't Leven, M., et al., 2010, Finsterer and Mahjoub, 2014, Zielinski et al., 2019). Therefore, a large of individuals are in sub-healthy conditions (a special state between health and disease) and experience “unexplained fatigue.” A third of the world’s population suffers chronic fatigue for six months or even longer (Cook and Boore, 1997, Klimas et al., 2012). There are several reasons for fatigue, such as ‘Qi’ and ‘Xue’ deficiency (Peijiang et al., 2001, Xingzhe et al., 2022), postoperative fatigue, ‘Pi Xu,’ depression, poor sleep, energy imbalance, physical disability, aging, and long-term exercise (Luo et al., 2019). Meanwhile, diabetes, hyperthyroidism, anemia, high body fat, and liver disease, among other health conditions, may also cause fatigue (Swain, 2006, Fox et al., 2020, Nocerino et al., 2020, Monahan et al., 2021). Fatigue can be a direct manifestation of disease or the side effect of some medications. Besides, fatigue can cause some illnesses. Fatigue is a complex and comprehensive physiological phenomenon without a clear mechanism and standard treatment.
Traditional Chinese medicine (TCM) has revealed its unique advantages in treating fatigue owing to the characteristics of multiple components, targets, and pathways. Studies have shown that TCM can alleviate fatigue through many pathways, such as antioxidation, decreasing the accumulation of metabolites, anti-inflammation, promotion of exercise endurance, and regulation of the hypothalamic–pituitaryadrenal axis (HPA) function, energy metabolism, gut microbiota, and immune system. Therefore, developing regulators/nutrients from TCMs against fatigue is of utmost importance.
Therefore, this review aims to provide a comprehensive guide for drug development against fatigue by overviewing the classification and cause of fatigue, the mechanism, the composition of natural medicine, and relevant animal model trials.
2. Fatigue in western medicine
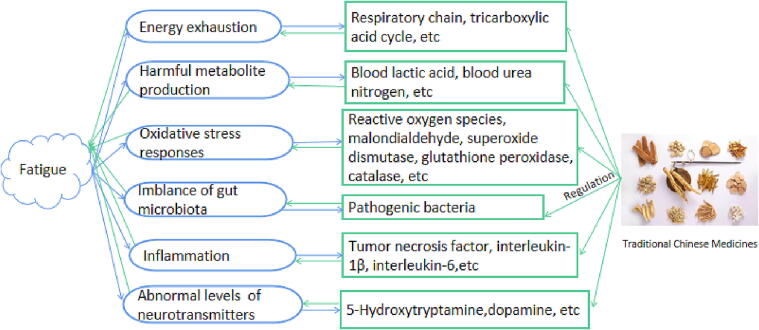
Fatigue is a disabling symptom in which physical and cognitive functions are limited by interactions between performance and perceived fatigability (Enoka and Duchateau 2016). Fatigue is a common phenomenon in the general population. Fatigue generation is complex, with multiple causes and mechanisms (Twomey et al., 2017) (Fig. 1). There are three main classes of fatigue: general, chronic, and disease-related fatigue.
Fig. 1.
Pathogenesis of fatigue and its management through TCM.
2.1. General fatigue
General fatigue, which mainly results from muscle fatigue, is created by intense long-time physical activity and is an effort by central and peripheral interactions (Boyas and Guével, 2011, Twomey et al., 2017, Sadler and Cressman, 2019), which are a classic dichotomy and have long been acknowledged. Peripheral fatigue is a consequence of an overactivity-induced decline in muscle function. There are three theories explaining peripheral fatigue: 1) the exhaustion theory, which entails the exhaustion of muscle and liver glycogen(Zhou and Jiang, 2019); 2) the radical theory, which is the imbalance between oxidation and the antioxidant system (Ding et al., 2011, Qin et al., 2014); and 3) the clogging theory, which is the accumulation of metabolites(Wang et al., 2008). Central fatigue is a sensation mediated by the central nervous system (CNS). It is a consequence of the failure of the CNS to transmit motor impulses or perform voluntary activities.
2.2. Chronic fatigue
Chronic fatigue syndrome (CFS) is unexplained fatigue that lasts six months or longer. This kind of fatigue cannot be alleviated by rest and is accompanied by at least four of eight case-defining symptoms, including sore throat, tender lymphadenopathy, impaired memory or concentration, myalgia, arthralgia, unrefreshing sleep, postexertional malaise, and headaches (Campos et al., 2011, Bower, 2014, Fox et al., 2020). The pathophysiology of CFS is severely understood, and the biomarker is difficult to quantify. Some studies have reported that viral infection, endocrine, neuroendocrine, psychology, inflammation, and immunologic factors mediate the psychosocial response to CFS (Bower, 2014, Thong et al., 2020). Neuroimmunological interactions and chronic immune activation have been implicated as major factors in the pathogenesis of CFS (Bower and Lamkin 2013). Other factors may include inflammation, oxidative stress, and mitochondrial dysfunction.
2.3. Disease-related fatigue
Fatigue is a common symptom of several chronic and mental diseases, such as acquired immune deficiency syndrome, cancer, heart failure, chronic kidney disease, multiple sclerosis, CFS, rheumatoid arthritis, and chronic obstructive pulmonary disease (Klimas et al., 2012, Bruno and Sethares, 2015, Nocerino et al., 2020, Johansson et al., 2021). Additionally, postoperative fatigue syndrome is a universal condition after surgery (Chen et al., 2015, Lu et al., 2016). The pathophysiological mechanism of disease-related fatigue is unclear, although existing studies are mainly related to inflammation (Herlofson et al., 2018). The change of plasma C-reactive protein and interleukin-6 (IL-6) was prospectively associated with new-onset fatigue, which suggests that inflammation has a role in fatigue development (Lee and Giuliani 2019).
3. Fatigue in TCM
Fatigue has a long history in TCM. The word ‘fatigue’ was first recorded in ‘Synopsis of the Golden Chamber’ (xingzhe et al., 2022). Fatigue belongs to the ‘deficiency’ and ‘consumptive disease’ categories. The meaning of central fatigue is the closest to that of mental fatigue. Long-term and large-scale exercises easily led to an imbalance between ‘Yin’and ‘Yang’ in the body, manifested as a deficiency of ‘Qi’ and ‘Xue’ (Jingtao 2014). The etiology and pathogenesis of fatigue are closely related to the dysfunction of ‘Zangfu organs’, mainly involving the dysfunction of ‘Pi’, ‘Gan’, and ‘Shen’, especially ‘Pi’ (Jingtao 2014). The TCM theory considers that the ‘Pi’ regulates water and nutrient metabolism. When the function of ‘Pi’ is normal, nutrients are easily absorbed and utilized by the body, thus providing sufficient energy and nutrition to the body. When the ‘Pi’ is abnormal (e.g., ‘Pi Xu’) (Jun et al., 2021), nutrient absorption and utilization are impaired, resulting in inadequate energy nutrition and supply, leading to fatigue. The ‘Gan’ has the function of storing ‘Xue’ and dredging ‘Qi.’ The un-dredged ‘Qi’ of ‘Gan’ will lead to depression and the dredging of ‘Qi’ of ‘Gan’ may alleviate fatigue (Chen and You 2022). The abnormal life rhythm caused by various stress aspects can easily cause ‘Gan’ dysfunction and further induce muscle weakness and body fatigue. The ‘Shen’ has the function of storing the essence of life, whereas the dysfunction of ‘Shen’ (e.g., ‘Shen xu’) will lead to decreased immunity, night sweats, and poor memory, which are connected with fatigue (Zhang et al., 2022).
4. Fatigue management by TCM
Many TCMs have been found to relieve fatigue. The animal models that evaluate the anti-fatigue efficacy of TCM mainly include forced weight-loaded swimming/running experiments, forelimb grip strength experiments, open field experiments, and tail suspension tests (Chen, et al., 2016a, Chen, et al., 2016b, Baguley, et al., 2017, Baguley et al., 2019). Indicators currently used to evaluate the effect of anti-fatigue mainly include antioxidant indexes (such as reactive oxygen species, malondialdehyde, superoxide dismutase, glutathione peroxidase, and catalase (Chen and Zhang, 2011, Chen et al., 2019, Chen, et al., 2021)), accumulation of harmful metabolites (such as blood lactic acid and blood urea nitrogen(Chen et al., 2011)), energy metabolites (respiratory chain and tricarboxylic acid cycle (Gao et al., 2018)), and inflammation (such as tumor necrosis factor, IL-1β, and IL-6 (Herlofson et al., 2018)). The pharmacological effects and mechanisms of TCM on fatigue management (Table 1, Table 2, Table 3) mainly include inhibition of inflammatory response, regulation of the immune system and HPA axis, remedy of the synthesis and release of neurotransmitters, enhancement of mitochondrial biogenesis, regulation of endogenous antioxidant system, amelioration of metabolite production, and improvement of glycogen storage (Fig. 1). Additionally, the anti-fatigue ability of several TCMs, such as Buqi Shengxue Formula composed of Panacis Quinquefolia Radix and Astragali Radix, and Yangxin tablet composed of Astragali Radix, Ginseng Radix et Rhizoma, and Codonopsis Radix, has been confirmed in clinical tests by evaluating the improvement of fatigue symptoms and several classic physiological and biochemical indexes (He, 2021, Ling et al., 2021), indicating the credible therapy of TCMs.
Table 1.
Anti-fatigue effects of herbal prescriptions.
| Prescriptions | Composition | Mechanisms | Experimental models |
|---|---|---|---|
| Yangqi Shengye Tang (Hao 2010) | Polygonati Rhizoma, Ophiopogonis Radix, and Crataegi Fructus | Reducing interleukin-6 (IL-6) content; increasing the levels of immunoglobulin G and A | Weight-loaded swimming test (LST) |
| Bazhen Soup (Zhao et al., 2020) | Angelicae Sinensis Radix, Ginseng Radix et Rhizoma, Poria, Paeoniae Radix Alba, Atractylodis Macrocephalae Rhizoma, Chuanxiong Rhizoma, Rehmanniae Radix Praeparata, and Glycyrrhizae Radix et Rhizoma Praeparata Cum Melle | Degrading malondialdehyde (MDA) content of skeletal muscle; improving superoxide dismutase (SOD) activity; scavenging free radical | LST |
| Liqi Tiaobu Tang (Wang et al., 2010) | Bupleuri Radix, Aurantii Fructus, Astragali Radix, Ziziphi Spinosae Semen, Schisandrae Chinensis Fructus, Salviae Miltiorrhizae Radix et Rhizoma, Ginseng Radix et Rhizoma, Chuanxiong Rhizoma, and Angelicae Sinensis Radix | Increasing the content of T cell and T/C cell ratio; recovering the function of the hypothalamic–pituitaryadrenal axis | LST |
| Lizhong Pill (Li et al., 2013) | Zingiberis Rhizoma, Ginseng Radix et Rhizoma, Atractylodis Macrocephalae Rhizoma, and Glycyrrizae Radix et Rhizoma Praeparata Cum Melle | Exciting the adrenal cortex system | LST |
| Duoxuekang Capsule (Chen et al., 2021a) | Phyllanthi Fructus, Rhodiolae Crenulatae Radix et Rhizoma, Hippophae Fructus, and Zingiberis Rhizoma Recens | Decreasing lactate dehydrogenase (LDH) activity; increasing the hepatic glycogen (HG) level | LST |
| Huangjing Sanqi Extract (Yang et al., 2020a, Yang et al., 2020b) | Polygonati Rhizoma, and Notoginseng Radix et Rhizoma | Promoting energy metabolism, and antioxidation | LST |
| Yangxin Tablet (ling et al., 2021) | Astragali Radix, Ginseng Radix et Rhizoma, and Codonopsis Radix | Elevating cellular immune function | Officers and soldiers on a long voyage |
| Buqi Shengxue Formula (He 2021) | Panacis Quinquefolia Radix and Astragali Radix | Increasing hemoglobin level | Young athletes |
| Sijunzi Decoction (Hao 2014) | Ginseng Radix et Rhizoma, Poria, Atractylodis Macrocephalae Rhizoma, and Glycyrrhizae Radix et Rhizoma. | Enhancing the organ index of immune organs | Forced swimming test (FST) |
| Danggui Buxue Decoction (Miao et al., 2018) | Angelicae Sinensis Radix, and Astragali Radix |
Promoting hematopoietic function; regulating the immune system | FST |
| Xiyangshen Pill (Zhao and Wang 2005) | Panacis Quinquefolia Radix, and Ginseng Radix et Rhizoma Rubra | Enhancing HG content; decreasing blood urea nitrogen (BUN), and blood lactic acid (LA) levels | FST |
| Hongjingtian Formula (Yi-bo et al., 2021) | Rhodiolae Crenulatae Radix et Rhizoma, Angelicae Dahuricae Radix, Schisandrae Chinensis Fructus, Cistanches Herba, and Lycii Fructus | Decreasing BUN and LA contents; increasing HG level | FST |
| Maca Xiyangshen Capsule (Sun and Zhang 2021) | Lepidium Meyenii, and Panacis Quinquefolii Radix | Decreasing LA and BUN contents; increasing HG content | FST |
| Yinyanghuo Pill (Wang et al., 2020) | Panacis Quinquefolii Radix, and Epimedii Folium | Decreasing LA and BUN content; increasing HG level | LST |
| Renshen Huangqi Tablet (Ren and Li 2021) | Astragali Radix, and Ginseng Radix et Rhizoma | Increasing HG level; reducing BUN level | LST |
Table 2.
Anti-fatigue effects of single herbs.
| Single herbs | Mechanisms | Experimental models |
|---|---|---|
| Chaenomeles speciosa (Sweet) Nakai (Lang 2012) | Elevating maximal oxygen consumption, increasing the blood glucose level, and decreasing the LA level | Volunteers working in thermal environment |
| Pyracantha fortuneana (Maxim.) Li (Hou et al., 2002) | Enhancing LDH and glutathione peroxidase (GSH-Px) activity in the brain; decreasing the MDA content in the brain | LST |
| Gastrodia elata Bl. (Huang 2019) | Decreasing BUN content and increasing HG content | Sleep deprivation model |
| Lycium barbarum L. (Hu et al., 2022) | Increasing HG content | LST and climb-mouse experiment |
| Cistanche deserticolaY.C.Ma (Wang 2019) | Reducing LA content and enhancing adenosine triphosphatase (ATPase) activity | Rotate stick experiment |
| Thamnolia vermicularis (Sw.) Ach.(Maying and Zhang, 2010) | Accelerating recovery rate of blood glucose and LA clearance; increasing HG and decreasing BUN level | LST |
| Hypericum perforatum L. (Ye et al., 2014) | Increasing activity of SOD and GSH-Px; decreasing the content of MDA | LST |
| Lepidium meyenii Walp. (Zhu et al., 2021) | Increasing HG level; decreasing BUN level | FST |
| Dimocarpus longan Lour. (Guo et al., 2019) | Increasing HG content; decreasing LA and BUN levels | LST |
| Acanthopanax senticosus (Rupr. et Maxim.) Harms (Nam et al., 2011) | Increasing tissue glycogen content; reducing LA and BUN level | FST |
| Ophiopogon japonicus (Linn.f) Ker-Gawl. (YinFei et al., 2020) | Alleviating the oxidative effect of free radicals; reducing the decomposition of amino acids; increasing hemoglobin content | LST, climbing test, and rotarod test |
| Sasa borealis (Hack.) Makino et Shibata (Song et al., 2019) | Enhancing endogenous antioxidation | Exhaustive swimming without any load |
| Aegle marmelos (L.) Corrêa (Lalremruta and Prasanna 2012) | Reducing the duration of immobility and anxiety; increasing locomotor activity | Forced swimming every day for 15 min to induce a state of chronic fatigue |
| Areca catechu L. (HeShuang et al., 2009) | Increasing HG content; decreasing BUN and LA contents | LST |
| Salvia miltiorrhiza Bge. (Wang et al., 2021) | Improving energy metabolism; regulating the oxidant-antioxidant balance | LST |
| Vitis vinifera L. (Xianchu et al., 2018) | Anti-inflammation; antioxidation | FST |
| Antrodia cinnamomea (Liu et al., 2017) | Antioxidation | FST |
| Astragalus membranaceus (Fisch.) Bge. (Yeh et al., 2014) | Raising contractibility of skeletal muscle; increasing the activity of SOD and expression of α-action mRNA in skeletal muscle; inhibiting lipid peroxidation in blood and skeletal muscle. | FST and gastrocnemius and soleus muscle contractibility experiment |
| Trigonella foenum-graecum L. (Yan et al., 2022) | Decreasing LA, and BUN levels, increasing muscle glucose content | LST |
| Cordyceps sisensis (Berk.) Sacc. (Kumar et al., 2011) | Increasing HG content; reducing the accumulation of harmful metabolites | Swimming training (without load) |
Table 3.
Anti-fatigue effects of TCM constituents.
| Monomers and ingredient groups | Mechanisms | Experimental models |
|---|---|---|
| Polysaccharides from Lepidium meyenii Walp (Li et al., 2017, Tang et al., 2019) | Increasing HG content; decreasing BUN level; up-regulating mitochondrial biogenesis; antioxidation | FST; mouse leg grip-strength test; rotarod test; LST; treatment with H2O2 in C2C12 skeletal muscle cells |
| Oligosaccharides from Amorphophallus konjac C. K.Koch (Zeng et al., 2018) | Antioxidation; increasing blood glucose content | LST |
| Polysaccharides from Avena sativa Linn. (Chao et al., 2009) | Increasing non-esterified fat acid content, and glycogen storage | Running performance test |
| Polysaccharides from Lycium barbarum L. (Wu and Guo 2015) | Changing glycerophospholipid and tyrosine metabolism; inhibiting lipid peroxidation; elevating catalase and GSH-Px activity; decreasing MDA level |
FST |
| Polysaccharides from Polygonatum sibricum Red. (Cui et al., 2018) | Decreasing LA and BUN contents; increasing HG content | FST |
| Polysaccharides from Polygonatum alte-lobatum Hayata (Horng et al., 2014) | Decreasing BUN and MDA contents; increasing SOD activity; scavenging free radical | Exhaustive treadmill exercise |
| Polysaccharides from Gynostemma pentaphyllum (Thunb.) Makino (Qi and Huang 2014) | Scavenging ROS and increasing glycogen levels in skeletal muscle | FST |
| Polysaccharides from Hericium erinaceus (Bull.) Pers. (Zhang 2015) | Increasing HG content; decreasing BUN and LA content | LST |
| Polysaccharides from Dendrobium officinale Kimura et Migo (Chen et al., 2021b) | Increasing glycogen storage; antioxidation | LST |
| Polysaccharide from Ziziphus jujube Mill. var. spinosa (Bunge) Hu ex H. F. Chow (Chi et al., 2015) | Improving immune function; antioxidation | Mimic the multiple-factor pathogenesis of CFS using electric shock, restraint stress, and cold-water-swim |
| Polysaccharides from Radix Rehmanniae Preparata (Tan et al., 2012) Protein-bound polysaccharides from Epimedium brevicorum Maxim. (Chi et al., 2017) |
Increasing HG content; decreasing BUN and LA contents Improving tyrosine, arginine, and proline metabolism |
LST Mimic the multiple-factor pathogenesis of CFS using restraint-stress, forced exercise, and crowed and noisy environment |
| Polysaccharides from Abelmoschus esculentus (L.) Moench (Gao et al., 2018) | Decreasing BUN and LA contents; increasing HG, muscle glycogen, and adenosine triphosphate levels; enhancing LDH and ATPase activity | LST |
| Polysaccharides from Ganoderma lucidum (Curtis) P. Karst. (Hu et al., 2012) | Increasing antioxidant enzymes activity; decreasing MDA content in skeletal muscle | FST |
| Polysaccharides from Zea mays L. (Yang et al., 2020a, Yang et al., 2020b) | Decreasing BUN and LA content; increasing LDH activity and HG content | LST |
| Polysaccharides from Polygonatum cyrtonema Hua. (Shen et al., 2021) | Decreasing LA, BUN, and MDA levels; increasing HG, muscle glucose, and adenosine triphosphate content in muscle | LST |
| Adventitious root protein from Panax ginseng C.A.Mey. (Wang et al., 2022) | Activating the adenosine 5′-monophosphate-activated protein kinase/ glucose transporter type 4 signaling pathway to promote glucose uptake | LST |
| Polysaccharides from Codonopsis pilosula (Franch.) Nannf. (Cai et al., 2014) | Preventing lipid peroxidation; antioxidation | LST |
| Flavonoids from Saussurea involucrate (Kar. et Kir.) Sch.Bip. (Su et al., 2014) | Increasing SOD and GSH-Px activity; decreasing LA content | FST |
| Flavonoids from Sophopra japonica L.(Tao 2013) | Antioxidation | FST |
| Flavonoids from Wasps drone pupae (Xi et al., 2018) | Eliminating metabolic accumulation | LST |
| Flavonoids from Pueraria lobata (Willd.) Ohwi (Wang et al., 2012) | Antioxidation | FST |
| Flavonoids from Astragalus englerianus Ulbr. (Xiao et al., 2014) | Scavenging free radical | Free radical scavenging capability assay |
| Flavonoids from Taraxacum mongolicum Hand. -Mazz. (Liu et al., 2020) | Delaying LA production | FST |
| Polyphenols from Camellia sinensis (L.) O. Ktze. (Yi 2016) | Anti-inflammation; antioxidation | Athletes |
| Polyphenols from Euryale ferox Salisb. ex DC (Wu et al., 2013) | Antioxidation | FST |
| Polysaccharides from Angelica sinensis (Oliv.) Diels (Choi et al., 2022) | Increasing muscle glucose content | FST |
| Peptide from Hippocampus kelloggi Jordan et Snyder (Guo et al., 2017) | Increasing production of serum glucose, free fatty acid, HG, and adenosine triphosphate; reducing L-lactate, and citrate content | Rotarod test |
| Saponins from Panax notoginseng (Burk.) F.H.Chen (Yong-Xin and Jian-Jun 2013) | Increasing HG content; decreasing LA content | LST |
| Small-molecule oligopeptides from Panax quinquefolium L. (Li et al., 2018) | Antioxidation; improving mitochondrial function | FST |
| Peptide from Pseudosciaena crocea (Zhao et al., 2016) | Inhibiting oxidative reaction | LST |
| Peptide from Sheep Placenta (Wang et al., 2018) | Decreasing MDA and LA levels; enhancing GSH-Px and SOD activity; increasing HG content | LST |
| Polysaccharides from Polygonum multiflorum Thunb. (Kai et al., 2016) |
Improving HG content, and SOD activity; decreasing BUN, LA, and MDA levels | LST |
| Cyanidin-3-glucoside (Matsukawa et al., 2017) | Activating lactate metabolism through skeletal muscle receptor-gamma coactivator-1α (PGC-1α) upregulation | LST |
| Quercetin (Zhang and Iop, 2017, Chen, et al., 2021) | Enhancing muscle function; antioxidation | LST and non-loading swimming tests |
| Anwulignan (Zhang et al., 2019) | Regulating nuclear factor erythroid-2 related factor 2, and PGC-1α signaling pathway | LST, rotarod test, grip strength test, and tail suspension test |
| Securinine (Wang 2012) | Reducing BUN level; increasing glycogen level | LST |
| Betaine (Hoffman et al., 2009) | Decreasing BUN and LA contents; increasing HG content | LST |
| Ginsenoside Rb1 (Tan et al., 2013, Chen et al., 2015, Liu et al., 2021) | Improving energy metabolism; suppressing skeletal muscle oxidative stress | Postoperative fatigue syndrome (POFS) induced by major small intestinal resection model |
| Ginsenoside Compound K (Lan and Liang 2022) | Antioxidation | FST |
| Ginsenoside Rg3 (Yang et al., 2018) | Up-regulating concentration of LDH and SOD; decreasing MDA level | POFS model |
| N-benzyloleamide (Yang et al., 2016) | Increasing HG content; decreasing LA and BUN content | LST |
| Luteolin-6-C-neohesperidoside (Duan et al., 2017) | Decreasing LA and BUN levels; enhancing antioxidant enzymes activity | FST |
| Neoagarotetraose (Zhang et al., 2017) | Modulating gut microbial composition, and function in intense exercise | A forced exercise wheel-track treadmill |
| γ-aminobutyric acid (Chen, et al., 2016a) | Increasing HG content; decrease LA and BUN content | LST |
| Dihydromyricetin (Zou et al., 2014) | Reducing LA and BUN content; decreasing LDH, CK, and GSH-Px activity; increasing glycogen content | LST |
| Hypericin (Sun et al., 2022) | Normalizing changes in LA, BUN, creatine kinase, MDA, and HG level, and LDH activity in the liver; improving GSH-Px and SOD activity, and total antioxidant capacity; decreasing the release of tumor necrosis factor-α, interleukin-6, and interleukin-1β | Mice swimming for 120 min for six weeks (for six consecutive days per week) |
| Salidroside (Ma et al., 2015) | Enhancing antioxidant enzyme activities in mitochondria | LST |
Currently, anti-fatigue bioactive components of TCM mainly include polysaccharides, polyphenols, flavonoids, terpenes, peptides, and proteins. Polysaccharides, a macromolecule, are composed of numerous monosaccharides and are widely distributed in animals, plants, and fungi. Polysaccharides can increase glycogen storage and reduce metabolite accumulation, which may be mainly related to the regulation of the immune systems (Cao, 2013, Cai et al., 2014, Tian Jiajun, Qin Yang ,Wang Nanping, 2021). Polyphenols and flavonoids contain polyhydroxy and have high antioxidant activity (Hu et al., 2010, Li and Zhang, 2013), which may be the main mechanism for their anti-fatigue ability. Terpenes isolated from TCMs are typical components of anti-fatigue substances (Tang et al., 2008, Zu et al., 2016). The reduction of metabolite accumulation may be the main mechanism of terpenes. Peptides and proteins exhibit their anti-fatigue effect mainly via the inhibition of oxidative stress (Qi et al., 2014, Bao, et al., 2016, Feng, et al., 2021).
At present, although most anti-fatigue TCMs are in the procedure of preclinical research, a few drugs are in clinical research, such as modafinil and zaleplon (ChiCTR20000031407), moringa tablet (ChiCTR2100047631), Shen-Mai injection (ChiCTR1800015478), and Korean ginseng (ChiCTR-IPR-17012151), which consists of chemosynthetic drugs and natural TCMs. Generally, chemosynthetic drugs own the single and known constituents and pharmacological mechanism, while TCMs possess the extreme complex active ingredients mechanism and mechanism, which creates a mode of action of the multi-compound, multi-target, and multi-pathway.
5. Conclusion
Fatigue has become a serious threat to human health. Many TCMs exhibit anti-fatigue activity via the action of their constituent compounds on several pathways and targets. However, a few TCMs have been used as nutritional supplements or clinical drugs against fatigue, which may be ascribe to the complex chemical ingredients and action mechanisms, difficult quality control and unstable quality. It is necessary to explore the anti-fatigue effect of TCM and its related mechanisms. In addition, anti-fatigue medicinal substances of TCM are seldom and poorly understood, which instigates the need for more relevant studies in the field. With deep research, it is of utmost significance that more TCMs with an anti-fatigue effect can be found and used in clinical settings.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers: 82060707, 82104381, 82174037, and 81960710).
The Application and Basis Research Project of Yunnan China (grant numbers: 202201AW070016, 202001AZ07001006, 202001AV70007, and 2019IB009), and the Young and Middle-aged Academic and Technological Leader of Yunnan (grant number: 202005AC160059).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Jie Yu, Email: cz.yujie@gmail.com.
Xingxin Yang, Email: yxx78945@163.com.
References
- Baguley B.J., et al. The effect of nutrition therapy and exercise on cancer-related fatigue and quality of life in men with prostate cancer: a systematic review. Nutrients. 2017;9(9) doi: 10.3390/nu9091003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., et al. Anti-Fatigue effects of small molecule oligopeptides isolated from Panax ginseng C. A. Meyer in Mice. Nutrients. 2016;8(12) doi: 10.3390/nu8120807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower J.E. Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014;11(10):597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower J.E., Lamkin D.M. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav. Immun. 2013;30(Suppl(0)):S48–S57. doi: 10.1016/j.bbi.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyas S., Guével A. Neuromuscular fatigue in healthy muscle: underlying factors and adaptation mechanisms. Ann. Phys. Rehabil. Med. 2011;54(2):88–108. doi: 10.1016/j.rehab.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Bruno A.E., Sethares K.A. Fatigue in Parkinson disease: an integrative review. J. Neurosci. Nurs. 2015;47(3):146–153. doi: 10.1097/JNN.0000000000000131. [DOI] [PubMed] [Google Scholar]
- Cai L.M., Luo L., Zeng Y.H. Effects of polysaccharides from the root of Codonopsis pilosula (Dangshen) on physical fatigue induced by forced swimming. Environ. Technol. Resource Utilization. 2014;II:1591–1594. [Google Scholar]
- Campos M.P., et al. Cancer-related fatigue: a review. Rev. Assoc. Med. Bras (1992) 2011;57(2):211–219. doi: 10.1590/s0104-42302011000200021. [DOI] [PubMed] [Google Scholar]
- Cao L. Extraction and anti-fatigue activity of polysaccharides from Cyclocarya paliurus (Batal.) Ilfinskaja. Progress Environ. Protection Process, Res., PTS. 2013;1–4:293–297. [Google Scholar]
- Chao X., Hu X., Luo Q. antifatigue effects of oat on mice. J. Chinese Cereals Oils Assoc. 2009;24(09):36–39. in Chinese. [Google Scholar]
- Chen W.Z., et al. Prevention of postoperative fatigue syndrome in rat model by ginsenoside Rb1 via down-regulation of inflammation along the NMDA receptor pathway in the hippocampus. Biol. Pharm. Bull. 2015;38(2):239–247. doi: 10.1248/bpb.b14-00599. [DOI] [PubMed] [Google Scholar]
- Chen H., et al. Extraction, purification and anti-fatigue activity of γ-aminobutyric acid from mulberry (Morus alba L.) leaves. Sci. Rep. 2016;6:18933. doi: 10.1038/srep18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.W., et al. Extraction, purification and anti-fatigue activity of gamma-aminobutyric acid from mulberry (Morus alba L.) leaves. Sci. Rep. 2016:6. doi: 10.1038/srep18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.-B., et al. Effects of Raphani Semen on anti-fatigue and pharmacokinetics of Panax ginseng. Chinese Herbal Med. 2019;11(3):308–313. doi: 10.1016/j.chmed.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.L., et al. Anti-fatigue effect of quercetin on enhancing muscle function and antioxidant capacity. J. Food Biochem. 2021;45(11) doi: 10.1111/jfbc.13968. [DOI] [PubMed] [Google Scholar]
- Chen K., et al. Tibetan medicine Duoxuekang capsule ameliorates high-altitude polycythemia accompanied by brain injury. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.680636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., et al. Traditional uses, phytochemistry, pharmacology, and quality control of dendrobium officinale Kimura et Migo. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.726528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J., You, Q., 2022. Traditional Chinese medicine understanding of postoperative fatigue syndrome in massage and rehabilitation medicine 13(14) 44-45.
- Chen Y.Z., Lin F., Li P.P. Anti-fatigue Effect of Renshen Yangrong Decoction ((sic)) in Mice. Chinese J. Integrative Med. 2011;17(10):770–774. doi: 10.1007/s11655-011-0879-8. [DOI] [PubMed] [Google Scholar]
- Chen X., Zhang G.H. Scavenging and anti-fatigue activity of Wu-Wei-Zi aqueous extracts. African J. Microbiol. Res. 2011;5(32):5933–5940. [Google Scholar]
- Chi A., et al. Immunomodulating and antioxidant effects of polysaccharide conjugates from the fruits of Ziziphus Jujube on Chronic Fatigue Syndrome rats. Carbohydr. Polym. 2015;122:189–196. doi: 10.1016/j.carbpol.2014.12.082. [DOI] [PubMed] [Google Scholar]
- Chi A., et al. Characterization of a protein-bound polysaccharide from Herba Epimedii and its metabolic mechanism in chronic fatigue syndrome. J. Ethnopharmacol. 2017;203:241–251. doi: 10.1016/j.jep.2017.03.041. [DOI] [PubMed] [Google Scholar]
- Choi T.J., et al. Anti-Inflammatory Activity of Glabralactone, a Coumarin Compound from Angelica sinensis, via Suppression of TRIF-Dependent IRF-3 Signaling and NF-κB Pathways. Mediators Inflamm. 2022;2022:5985255. doi: 10.1155/2022/5985255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N.F., Boore J.R. Managing patients suffering from acute and chronic fatigue. Br. J. Nurs. 1997;6(14):811–815. doi: 10.12968/bjon.1997.6.14.811. [DOI] [PubMed] [Google Scholar]
- Cui X., et al. A review: The bioactivities and pharmacological applications of polygonatum sibiricum polysaccharides. Molecules. 2018;23(5) doi: 10.3390/molecules23051170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J.-F., et al. Study on effect of jellyfish collagen hydrolysate on anti-fatigue and anti-oxidation. Food Hydrocolloids. 2011 [Google Scholar]
- Duan F.F., et al. Antifatigue effect of Luteolin-6-C-Neohesperidoside on oxidative stress injury induced by forced swimming of rats through modulation of Nrf2/ARE signaling pathways. Oxid. Med. Cell Longev. 2017;2017:3159358. doi: 10.1155/2017/3159358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka R.M., Duchateau J. Translating fatigue to human performance. Med. Sci. Sports Exerc. 2016;48(11):2228–2238. doi: 10.1249/MSS.0000000000000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei Y., et al. Effect of ophiopogon japonicus water extract on endurance of exercise mice. Chinese J. Public Health Eng. 2020;19(02):180–182. in Chinese. [Google Scholar]
- Feng T., et al. Anti-fatigue effects of pea (Pisum sativumL.) peptides prepared by compound protease. J. Food Sci. Technol.-Mysore. 2021;58(6):2265–2272. doi: 10.1007/s13197-020-04737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterer J., Mahjoub S.Z. Fatigue in healthy and diseased individuals. Am. J. Hosp. Palliat Care. 2014;31(5):562–575. doi: 10.1177/1049909113494748. [DOI] [PubMed] [Google Scholar]
- Fox R.S., et al. Sleep disturbance and cancer-related fatigue symptom cluster in breast cancer patients undergoing chemotherapy. Support Care Cancer. 2020;28(2):845–855. doi: 10.1007/s00520-019-04834-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., et al. Purification, characterization and anti-fatigue activity of polysaccharide fractions from okra (Abelmoschus esculentus (L.) Moench) Food Function. 2018;9(2):1088–1101. doi: 10.1039/c7fo01821e. [DOI] [PubMed] [Google Scholar]
- Guo Z.B., et al. In vitro antioxidant activity and in vivo anti-fatigue effect of Sea Horse (Hippocampus) peptides. Molecules. 2017;22(3) doi: 10.3390/molecules22030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C., Bing Z., Bao C. Experimental study on anti-fatigue and anti-hypoxia effect of aqueous extract of longan in mice. Asia-Pacific Traditional Med. 2019;15(10):14–16. in Chinese. [Google Scholar]
- Hao H. Effects of sijunzitang on anti-fatigue of mice. Sports Res. Education. 2014;29(02):127–128. in Chinese. [Google Scholar]
- Hao, Y., The effects of traditional Chinese medicine on physical performance and glycometabolism in rats. 2010, Beijing Sport University. in Chinese.
- He J. Chinese traditional medicine formula in adolescent athletes fatigue resistance increase hemoglobin content of effectiveness study. Sci. Technol. Stationery & Sporting Goods. 2021;469 in Chinese. [Google Scholar]
- Herlofson K., et al. Inflammation and fatigue in early, untreated Parkinson's Disease. Acta Neurol. Scand. 2018;138(5):394–399. doi: 10.1111/ane.12977. [DOI] [PubMed] [Google Scholar]
- Hoffman J.R., et al. Effect of betaine supplementation on power performance and fatigue. J. Int. Soc. Sports Nutr. 2009;6:7. doi: 10.1186/1550-2783-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horng C.T., et al. Antioxidant and antifatigue activities of Polygonatum Alte-lobatum Hayata rhizomes in rats. Nutrients. 2014;6(11):5327–5337. doi: 10.3390/nu6115327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J., Liu X., Wu M. The research on the anti-fatigue effect of pyracantha fortuneana. J. Wuhan Textile Univ. 2002;15(05):66–68. in Chinese. [Google Scholar]
- Hu Q.L., et al. Purification and anti-fatigue activity of flavonoids from corn silk. Int. J. Phys. Sci. 2010;5(4):321–326. [Google Scholar]
- Hu J.H., et al. Evaluation of antioxidant and anti-fatigue activities of Ganoderma lucidum polysaccharides. J. Animal Veterinary Adv. 2012;11(21):4040–4044. [Google Scholar]
- Hu Y., Hu K., Chen J. Study of the anti-fatigue effect of extracts from cooked Lycium barbarum. Food Res. Dev. 2022;43(04):29–33. in Chinese. [Google Scholar]
- Huang, H., DNA identification and pharmacological study on Tianma improving memory and anti-fatigue. In: Chinese 2019, Chengdu university of traditional Chinese medicine.
- Jingtao J. Study on the concept of fatigue in traditional Chinese medicine in Chinese. China Medical Herald. 2014;11:28. [Google Scholar]
- Johansson K., Wasling P., Axelsson M. Fatigue, insomnia and daytime sleepiness in multiple sclerosis versus narcolepsy. Acta Neurol. Scand. 2021;144(5):566–575. doi: 10.1111/ane.13497. [DOI] [PubMed] [Google Scholar]
- Jun J., et al. Clinical efficacy of Du Moxibustion combined with Fuzi Lizhong Decoction for chronic fatigue syndrome of spleen-kidney yang deficiency and its effect on fatigue degree and traditional Chinese medicine symptom He Bei traditional. Chinese Med. 2021;43(10):1698–1701. [Google Scholar]
- Kai C., et al. Anti-fatigue effect and mechanism of polygonum multiflorum polysaccharide in mice. Chinese J. Gerontol. 2016;36(24):6054–6055. in Chinese. [Google Scholar]
- Klimas N.G., Broderick G., Fletcher M.A. Biomarkers for chronic fatigue. Brain Behav. Immun. 2012;26(8):1202–1210. doi: 10.1016/j.bbi.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., et al. Cordyceps sinensis promotes exercise endurance capacity of rats by activating skeletal muscle metabolic regulators. J. Ethnopharmacol. 2011;136(1):260–266. doi: 10.1016/j.jep.2011.04.040. [DOI] [PubMed] [Google Scholar]
- Lalremruta V., Prasanna G.S. Evaluation of protective effect of Aegle marmelos Corr. in an animal model of chronic fatigue syndrome. Indian J. Pharmacol. 2012;44(3):351–356. doi: 10.4103/0253-7613.96316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan R., Liang Y. Effects of ginsenoside CK on anti-fatigue and oxidative stress of skeletal muscle in exhaustive swimming rats. J. Yunnan Agric. Univ. (Nat. Sci.) 2022;37(03):491–496. in Chinese. [Google Scholar]
- Lang, H., The anti-fatigue effect of chaebomeles extract and taurine mixture on volunteers working in thermal environment. In: Chinese 2012, Third Military Medical University.
- Lee C.H., Giuliani F. The role of inflammation in depression and fatigue. Front. Immunol. 2019;10:1696. doi: 10.3389/fimmu.2019.01696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., et al. Anti-fatigue activity of polysaccharide fractions from Lepidium meyenii Walp (maca) Int. J. Biol. Macromol. 2017;95:1305–1311. doi: 10.1016/j.ijbiomac.2016.11.031. [DOI] [PubMed] [Google Scholar]
- Li D., et al. Anti-fatigue effects of small-molecule oligopeptides isolated from Panax quinquefolium L. in mice. Food Funct. 2018;9(8):4266–4273. doi: 10.1039/c7fo01658a. [DOI] [PubMed] [Google Scholar]
- Li X., Hong X., Wang K. Research on Lizhong Pill for enhance the cold resistance and fatigue resistance in experimental. J. Harbin Sport Univ. 2013;31(06) in Chinese. [Google Scholar]
- Li C.G., Zhang L.Y. In vivo Anti-fatigue activity of total flavonoids from sweetpotato (Li and Zhang) leaf in mice. Indian J. Biochem. Biophys. 2013;50(4):326–329. [PubMed] [Google Scholar]
- Ling L.Y., et al. Effects of Yangxin Tablet on Fatigue Improvement of Officers and soldiers on long voyage. World Chinese Med. 2021;16:18. [Google Scholar]
- Liu Y., et al. Antifatigue effects of antrodia cinnamomea cultured mycelium via modulation of oxidative stress signaling in a mouse model. Biomed. Res. Int. 2017;2017:9374026. doi: 10.1155/2017/9374026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., et al. The effects of solid-state fermentation on the content, composition and in vitro antioxidant activity of flavonoids from dandelion. PLoS One. 2020;15(9):e0239076. doi: 10.1371/journal.pone.0239076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., et al. Inflammation disturbed the tryptophan catabolites in hippocampus of post-operative fatigue syndrome rats via Indoleamine 2,3-Dioxygenas enzyme and the improvement effect of ginsenoside Rb1. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.652817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., et al. Serum metabolite profiles of postoperative fatigue syndrome in rat following partial hepatectomy. J. Clin. Biochem. Nutr. 2016;58(3):210–215. doi: 10.3164/jcbn.15-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C., et al. Natural medicines for the treatment of fatigue: Bioactive components, pharmacology, and mechanisms. Pharmacol. Res. 2019;148 doi: 10.1016/j.phrs.2019.104409. [DOI] [PubMed] [Google Scholar]
- Ma C.Y., et al. An UPLC-MS-based metabolomics investigation on the anti-fatigue effect of salidroside in. J. Pharm. Biomed. Anal. 2015;105:84–90. doi: 10.1016/j.jpba.2014.11.036. [DOI] [PubMed] [Google Scholar]
- Matsukawa T., et al. Upregulation of skeletal muscle PGC-1alpha through the elevation of cyclic AMP levels by Cyanidin-3-glucoside enhances exercise performance. Sci. Rep. 2017;7:44799. doi: 10.1038/srep44799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maying T., Zhang Y. Study on the anti-fatigue of extract of tebatan medicine ice tea in mice. Acta Chinese Med. Pharmacol. 2010;38(02):29–32. in Chinese. [Google Scholar]
- Miao X., et al. Metabolomics analysis of serum reveals the effect of Danggui Buxue Tang on fatigued mice induced by exhausting physical exercise. J. Pharm. Biomed. Anal. 2018;151:301–309. doi: 10.1016/j.jpba.2018.01.028. [DOI] [PubMed] [Google Scholar]
- Monahan R.C., et al. Fatigue in patients with systemic lupus erythematosus and neuropsychiatric symptoms is associated with anxiety and depression rather than inflammatory disease activity. Lupus. 2021;30(7):1124–1132. doi: 10.1177/09612033211005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam Y., et al. Anti-stress and anti-fatigue effect of Acanthopanax senticosus shoot extract via inhibition of oxidative stress. J. Pharmacol. Sci. 2011;115:281P–P. [Google Scholar]
- Nocerino A., et al. Fatigue in inflammatory bowel diseases: etiologies and management. Adv. Ther. 2020;37(1):97–112. doi: 10.1007/s12325-019-01151-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peijiang, C., et al., 2001. The effects of Chinese traditional herbs on the relieve of sports fatigue of endurance trained mice journal of sports and science 22 22.
- Qi B., et al. Anti-fatigue effects of proteins isolated from Panax quinquefolium. J. Ethnopharmacol. 2014;153(2):430–434. doi: 10.1016/j.jep.2014.02.045. [DOI] [PubMed] [Google Scholar]
- Qi B., Huang H. Anti-fatigue effects of polysaccharides from Gynostemma pentaphyllum Makino by forced swimming test. Chem. Mater. Metall. Eng. III, PTS. 2014;1–3:426–429. [Google Scholar]
- Qin L., et al. In vitro antioxidant activity and in vivo antifatigue effect of layered double hydroxide nanoparticles as delivery vehicles for folic acid. Int. J. Nanomed. 2014;9:5701–5710. doi: 10.2147/IJN.S74306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D., Li S. Effects of Renshenhuangqi tablet on relieving physical fatigue in mice and safety evaluation. Special Wild Econ. Animal Plant Res. 2021;43(6) in Chinese. [Google Scholar]
- Sadler C.M., Cressman E.K. Central fatigue mechanisms are responsible for decreases in hand proprioceptive acuity following shoulder muscle fatigue. Hum. Mov. Sci. 2019;66:220–230. doi: 10.1016/j.humov.2019.04.016. [DOI] [PubMed] [Google Scholar]
- Shen W.D., et al. Polygonatum cyrtonema Hua polysaccharide exhibits anti-fatigue activity via regulating osteocalcin signaling. Int. J. Biol. Macromol. 2021;175:235–241. doi: 10.1016/j.ijbiomac.2021.01.200. [DOI] [PubMed] [Google Scholar]
- Shuang H., Li Z., Xiong G. Study on anti-fatigue effects areca catechu extracts in mice. Food Mach. 2009;25(02):67–70. in Chinese. [Google Scholar]
- Song H., et al. Sasa quelpaertensis leaf extract mitigates fatigue and regulates the transcriptome profile in mice. Genes Genomics. 2019;41(3):317–324. doi: 10.1007/s13258-018-0765-2. [DOI] [PubMed] [Google Scholar]
- Su K.Y., et al. Rutin, a flavonoid and principal component of saussurea involucrata, attenuates physical fatigue in a forced swimming mouse model. Int. J. Med. Sci. 2014;11(5):528–537. doi: 10.7150/ijms.8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., et al. Anti-fatigue effect of hypericin in a chronic forced exercise mouse model. J. Ethnopharmacol. 2022;284 doi: 10.1016/j.jep.2021.114767. [DOI] [PubMed] [Google Scholar]
- Sun P., Zhang X. Maca Ginseng capsule functions in alleviating physical fatigue. Shandong Chem. Ind. 2021;50(15) in Chinese. [Google Scholar]
- Swain M.G. Fatigue in liver disease: pathophysiology and clinical management. Can. J. Gastroenterol. 2006;20(3):181–188. doi: 10.1155/2006/624832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W., et al. Anti-fatigue activity of polysaccharides extract from Radix Rehmanniae Preparata. Int. J. Biol. Macromol. 2012;50(1):59–62. doi: 10.1016/j.ijbiomac.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Tan S.J., et al. Anti-fatigue effect of ginsenoside Rb1 on postoperative fatigue syndrome induced by major small intestinal resection in rat. Biol. Pharm. Bull. 2013;36(10):1634–1639. doi: 10.1248/bpb.b13-00522. [DOI] [PubMed] [Google Scholar]
- Tang W., et al. The Anti-fatigue Effect of 20(R)-Ginsenoside Rg3 in Mice by Intranasally Administration. Biol. Pharm. Bull. 2008;31(11):2024–2027. doi: 10.1248/bpb.31.2024. [DOI] [PubMed] [Google Scholar]
- Tang Y., et al. Structure analysis and anti-fatigue activity of a polysaccharide from Lepidium meyenii Walp. Nat. Product Res. 2019;33(17):2480–2489. doi: 10.1080/14786419.2018.1452017. [DOI] [PubMed] [Google Scholar]
- Tao, W., Experimental study of the anti-fatigue effect of rutin. 2013, Shanxi Normal University (in Chinese).
- Thong M.S.Y., et al. Cancer-Related fatigue: causes and current treatment options. Curr. Treat Options Oncol. 2020;21(2):17. doi: 10.1007/s11864-020-0707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Jiajun, Qin Yang, Wang Nanping Research progress about the anti-fatigue effect and mechanism of polysaccharides from traditional Chinese medicine. Chem. Life. 2021;41(5):1018–1024. [Google Scholar]
- Twomey R., et al. Neuromuscular fatigue during exercise: Methodological considerations, etiology and potential role in chronic fatigue. Neurophysiol. Clin. 2017;47(2):95–110. doi: 10.1016/j.neucli.2017.03.002. [DOI] [PubMed] [Google Scholar]
- van't Leven M., et al. Fatigue and chronic fatigue syndrome-like complaints in the general population. Eur. J. Public Health. 2010;20(3):251–257. doi: 10.1093/eurpub/ckp113. [DOI] [PubMed] [Google Scholar]
- Wang L., et al. The decapeptide CMS001 enhances swimming endurance in mice. Peptides. 2008;29(7):1176–1182. doi: 10.1016/j.peptides.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Wang C., et al. Effect of Liqi Tiaobu tang on hypothalamic-pituitary-adrenal axis in fatigue induced by exercise in rat. J. Experimental Traditional Medical Formulae. 2010;16(05):139–141. in Chinese. [Google Scholar]
- Wang L., et al. Antifatigue effects of peptide isolated from sheep placenta. Chinese Herbal Med. 2018;10(3):279–284. [Google Scholar]
- Wang Y.Y., et al. Metabolomics study on the intervention effect of Radix Salviae Miltiorrhizae extract in exercise-induced exhaustion rat using gas chromatography coupled to mass spectrometry. J. Chromatogr. B-Analytical Technol. Biomed. Life Sci. 2021:1178. doi: 10.1016/j.jchromb.2021.122805. in Chinese. [DOI] [PubMed] [Google Scholar]
- Wang M., Fu B., Xu X. Anti-fatigue effect of ginseng adventitious root protein in mice and its mechanism. J. Jilin Univ. (Med. Ed.) 2022;48(01):18–25. in Chinese. [Google Scholar]
- Wang X.M., Lei L., Zhang J.H. Antioxidant and anti-fatigue activities of flavonoids from Puerariae Radix. African J. Traditional Complementary Alternative Med. 2012;9(2):221–227. doi: 10.4314/ajtcam.v9i2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Liu L., Ren L. Study on the effect of panax quinquefolius-epimedium brevicornu tablets on relieving physical fatigue. Asia-Pacific Traditional Med. 2020;16(06):29–32. in Chinese. [Google Scholar]
- Wang, X., The study of extraction technology and anti-fatigue activity of securinine. 2012, jilin Agricultural University.
- Wang, X., Study on effect component and mechanisms of Cistanche deserticola in relieving physical fatigue. 2019, Peking Union Medical College (in Chinese).
- Wu C., et al. Antioxidant and anti-fatigue activities of phenolic extract from the seed coat of Euryale ferox Salisb. and identification of three phenolic compounds by LC-ESI-MS/MS. Molecules. 2013;18(9):11003–11021. doi: 10.3390/molecules180911003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Guo L.Q. Anti-Fatigue and anti-hypoxic effects of Lycium barbarum polysaccharides. Proc. Int. Conf. Adv. Energy Environ. Chem. Eng. 2015:686–689. [Google Scholar]
- Xi X., et al. Anti-exercise-fatigue and promotion of sexual interest activity of total flavonoids from wasps drone-pupae in male mice. Biomed. Pharmacother. 2018;107:254–261. doi: 10.1016/j.biopha.2018.07.172. [DOI] [PubMed] [Google Scholar]
- Xianchu L., et al. Grape seed proanthocyanidin extract supplementation affects exhaustive exercise-induced fatigue in mice. Food Nutr. Res. 2018;62 doi: 10.29219/fnr.v62.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C.J., et al. Schistosomicidal and antioxidant flavonoids from Astragalus englerianus. Planta Med. 2014;80(18):1727–1731. doi: 10.1055/s-0034-1383219. [DOI] [PubMed] [Google Scholar]
- Xingzhe Y., et al. Research progress on anti-fatigue effect of Chinese medicine world. Chinese Medicine. 2022;17:5. [Google Scholar]
- Yan D., et al. Ameliorating effect of Trigonella foenum-graecum L. (fenugreek) extract tablet on exhaustive exercise-induced fatigue in rats by suppressing mitophagy in skeletal muscle. Eur. Rev. Med. Pharmacol. Sci. 2022;26(20):7321–7332. doi: 10.26355/eurrev_202210_30001. [DOI] [PubMed] [Google Scholar]
- Yang Q., et al. Effects of macamides on endurance capacity and anti-fatigue property in prolonged swimming mice. Pharm. Biol. 2016;54(5):827–834. doi: 10.3109/13880209.2015.1087036. [DOI] [PubMed] [Google Scholar]
- Yang Q.Y., et al. Effects of Ginsenoside Rg3 on fatigue resistance and SIRT1 in aged rats. Toxicology. 2018;409:144–151. doi: 10.1016/j.tox.2018.08.010. [DOI] [PubMed] [Google Scholar]
- Yang Y.Q., et al. Muscle Fatigue-Alleviating Effects of a Prescription Composed of Polygonati Rhizoma and Notoginseng Radix et Rhizoma. Biomed. Res. Int. 2020;2020:3963045. doi: 10.1155/2020/3963045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Wang X., Li Y. Study on the anti-fatigue and mechanism of black glutinous maize polysaccharide on mice. Cereals & Oils. 2020;33(02):88–90. in Chinese. [Google Scholar]
- Ye T., et al. Effect of Hypericum Perforatum L. extracts on anti-stress response and antioxidant activity of mice with exhausting exercise. Chinese J. Appl. Physiol. 2014;30(04):332–334. [PubMed] [Google Scholar]
- Yeh T.S., et al. Astragalus membranaceus improves exercise performance and ameliorates exercise-induced fatigue in trained mice. Molecules. 2014;19(3):2793–2807. doi: 10.3390/molecules19032793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, B.L., 2016. Effect of Tea Polyphenols on Anti Fatigue after Sports. In 2016 5th EEM International Conference on Education Science and Social Science (EEM-ESSS 2016), pp. 3-6.
- Yi-bo W., et al. Large plant Rhodiola capsules improve normobaric hypoxia tolerance and anti-fatigue action in mice. Chinese J. Pharmacol. Toxicol. 2021;6(35) [Google Scholar]
- Yong-Xin X., Jian-Jun Z. Evaluation of anti-fatigue activity of total saponins of Radix notoginseng. Indian J. Med. Res. 2013;137:151–155. [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., et al. Prebiotic, immunomodulating, and antifatigue effects of konjac oligosaccharide. J. Food Sci. 2018;83(12):3110–3117. doi: 10.1111/1750-3841.14376. [DOI] [PubMed] [Google Scholar]
- Zhang N., et al. Neoagarotetraose protects mice against intense exercise-induced fatigue damage by modulating gut microbial composition and function. Mol. Nutr. Food Res. 2017;61(8) doi: 10.1002/mnfr.201600585. [DOI] [PubMed] [Google Scholar]
- Zhang X.Y., et al. Anti-fatigue effect of anwulignan via the NRF2 and PGC-1 alpha signaling pathway in mice. Food Funct. 2019;10(12):7755–7766. doi: 10.1039/c9fo01182j. [DOI] [PubMed] [Google Scholar]
- Zhang L., et al. Clinical research progress in the treatment of chronic fatigue syndrome by differentiation typing in traditional Chinese medicine. Modern J. Integrated Traditional Chinese Western Med. 2022;31(18):2623–2628. [Google Scholar]
- Zhang, W.Q., Iop, 2017. Evaluation of anti-fatigue and immunomodulating effects of quercetin in strenuous exercise mice. In 3rd International Conference on Energy Materials and Environment Engineering.
- Zhang, X., Northeast Hericium polysaccharide extraction and purification classification relieve physical fatigue function, 2015, Changchun University technology (in Chinese).
- Zhao Y.Q., et al. Anti-Fatigue Effect by Peptide fraction from protein hydrolysate of Croceine Croaker (Pseudosciaena crocea) swim bladder through inhibiting the oxidative reactions including DNA damage. Marine Drugs. 2016;14(12) doi: 10.3390/md14120221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Liu S., Li Z. Study on anti-fatigue mechanism of BaZhen decoction in exhausted mice. J. Traditional Chinese Veterinary Med. 2020;39:3. in Chinese. [Google Scholar]
- Zhao W., Wang T. Experimental study of Panax Quiquefolium tablet on the anti-fatigue effect in mice. Food Sci. 2005;26(9) in Chinese. [Google Scholar]
- Zhou S.S., Jiang J.G. Anti-fatigue effects of active ingredients from traditional chinese medicine: a review. Curr. Med. Chem. 2019;26:1833–1848. doi: 10.2174/0929867324666170414164607. [DOI] [PubMed] [Google Scholar]
- Zhu H.K., et al. Anti-fatigue effect of Lepidium meyenii Walp. (Maca) on preventing mitochondria-mediated muscle damage and oxidative stress in vivo and vitro. Food & Funct. 2021;12(7):3132–3141. doi: 10.1039/d1fo00383f. [DOI] [PubMed] [Google Scholar]
- Zielinski M.R., Systrom D.M., Rose N.R. Fatigue, sleep, and autoimmune and related disorders. Front Immunol. 2019;10:1827. doi: 10.3389/fimmu.2019.01827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D., et al. Dihydromyricetin improves physical performance under simulated high altitude. Med. Sci. Sports Exerc. 2014;46(11):2077–2084. doi: 10.1249/MSS.0000000000000336. [DOI] [PubMed] [Google Scholar]
- Zu G., et al. Protective effects of ginsenoside Rg1 on intestinal ischemia/reperfusion injury-induced oxidative stress and apoptosis via activation of the Wnt/beta-catenin pathway. Sci. Rep. 2016;6:38480. doi: 10.1038/srep38480. [DOI] [PMC free article] [PubMed] [Google Scholar]