Abstract
Background and Objectives
Primary spinal glioblastoma (PsGBM) is extremely rare. The dramatic neurologic deterioration and unresectability of PsGBM makes it a particularly disabling malignant neoplasm. Because it is a rare and heterogeneous disease, the assessment of prognostic factors remains limited.
Methods
PsGBMs were identified from the French Brain Tumor Database and the Club de Neuro-Oncologie of the Société Française de Neurochirurgie retrospectively. Inclusion criteria were age 18 years or older at diagnosis, spinal location, histopathologic diagnosis of newly glioblastoma according to the 2016 World Health Organization classification, and surgical management between 2004 and 2016. Diagnosis was confirmed by a centralized neuropathologic review. The primary outcome was overall survival (OS). Therapeutic interventions and neurologic outcomes were also collected.
Results
Thirty-three patients with a histopathologically confirmed PsGBM (median age 50.9 years) were included (27 centers). The median OS was 13.1 months (range 2.5–23.7), and the median progression-free survival was 5.9 months (range 1.6–10.2). In multivariable analyses using Cox model, Eastern Cooperative Oncology Group (ECOG) performance status at 0–1 was the only independent predictor of longer OS (hazard ratio [HR] 0.13, 95% CI 0.02–0.801; p = 0.02), whereas a Karnofsky performance status (KPS) score <60 (HR 2.89, 95% CI 1.05–7.92; p = 0.03) and a cervical anatomical location (HR 4.14, 95% CI 1.32–12.98; p = 0.01) were independent predictors of shorter OS. The ambulatory status (Frankel D–E) (HR 0.38, 95% CI 0.07–1.985; p = 0.250) was not an independent prognostic factor, while the concomitant standard radiochemotherapy with temozolomide (Stupp protocol) (HR 0.35, 95% CI 0.118–1.05; p = 0.06) was at the limit of significance.
Discussion
Preoperative ECOG performance status, KPS score, and the location are independent predictors of OS of PsGBMs in adults. Further analyses are required to capture the survival benefit of concomitant standard radiochemotherapy with temozolomide.
Primary spinal cord cancers are rare entities, accounting for 2%–4% of all CNS tumors.1,2 Therefore, primary spinal glioblastoma (PsGBM) is extremely rare, accounting for only 1.5% of all spinal cord tumors.3,4 Low-grade histology is predominant, with high-grade tumors accounting for only 10%–15% of pediatric tumors and a slightly higher proportion in adults.5-7 These lesions are highly aggressive and lead to rapid and dramatic neurologic deterioration and death after only a short history of presentation.4,6 The therapeutic management for PsGBM is poorly defined because of the scarcity of cases and usually consists of a biopsy followed by radiotherapy (RT) with chemotherapy (CT), mainly the concomitant standard radiochemotherapy with temozolomide.8,9
Our current knowledge of primary PsGBM is incomplete, and an understanding of epidemiology, diagnosis, and optimal treatment modalities is warranted.1 While survival predictors have been well described for supratentorial hemispheric isocitrate dehydrogenase (IDH) wild-type glioblastomas10 and cerebellar glioblastomas11; prognostic factors for overall survival (OS) and progression-free survival (PFS) cannot be ascertained for PsGBM; so far, only case reports or small retrospective studies have been conducted.5,12 Moreover, most case series evaluating spinal cord astrocytomas usually pool low-grade and high-grade lesions together, children and adults together, limiting the generalizability of the results.2,3,9,13,14
The aim of this study was to assess the natural history and clinicopathologic and therapeutic factors that influence the prognosis of patients with PsGBM. We report the largest multicentric, nationwide cohort with a central histomolecular review of adult patients harboring a primary PsGBM.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
Data collected during the study were stored in a computer file in accordance with the law of the French Data Protection Act of January 6, 1978, amended in 2004. The protocol can be found in the reference methodology MR003 chapter adopted by the CNIL to which conform the different University Hospitals of this project. Ethical approval for this study was obtained from the Ethics Committee in Human Research of the Hospital of Tours (approval number: 2018 005).
Identification of Patients With PsGBM and Data Collection
The French Brain Tumor Database (FBTDB) identifies and records patients with newly diagnosed and histologically confirmed primary CNS tumors in France (hospital based). Its methodology has been previously published.11,15 For this study, the FBTDB and the Club of Neuro-Oncology of the Société Française de Neurochirurgie were screened to identify cases of glioblastoma (GBM) with spinal location. Before inclusion, it was verified by 1 investigator (A.A. or 1 local neurosurgeon specialized in neuro-oncology) that all patients met the following inclusion criteria: (1) age 18 years or older at diagnosis, (2) spinal location, (3) histopathologic diagnosis of newly GBM according to the World Health Organization (WHO) classification, version 2016 (prevailing classification during the study), and (4) surgical management between January 1, 2004, and December 31, 2016. The exclusion criteria were (1) the presence of a supraspinal tumor at diagnosis, (2) recurrent tumor, and (3) another glioma than primary GBM. In each neurosurgical center, data collection was performed by 1 neurosurgeon specialized in neuro-oncology (A.A. or 1 local neurosurgeon). Demographics, clinical data, imaging features, surgical details, postoperative course, type of adjuvant treatment, and follow-up (FU) data including cause of death were locally extracted from medical records using a chart designed for the study.
Detection of Sequence Variations
IDH1 and H3K27M sequence variations were first screened by immunohistochemistry labeling and completed by genomic DNA amplification with Sanger method or Next-Generation Sequencing depending on the pathology center. Telomerase reverse transcriptase (TERT) mutation was searched by DNA amplification with Sanger method or droplet digital PCR. The methylation status of O(6)-methylguanine-DNA methyltransferase (MGMT) promoter was determined by pyrosequencing of cytosine-phosphate-guanine sites from MGMT promoter.
Histopathologic Diagnosis
We included patients whose histomolecular diagnosis of PsGBM had been reviewed by the French neuropathologic network (RENOP, “Réseau de Neuro-oncologie pathologique”) for clinical purposes during the initial diagnosis. Cases that did not benefit from this central neuropathologic review systematically underwent a post hoc central neuropathologic review by the new French neuro-oncology network RENOCLIP (“Réseau de Neuro-Oncologie Clinico Pathologique”) to confirm or exclude the diagnosis.
Frankel Score
The Frankel grade classification provides an assessment of spinal cord function and is used as a tool in spinal cord injury.16
Progression Measures
GBM progression occurring within the initial tumor site was defined as local progression. The progression was defined as an MRI recurrence or progression according to RANO criteria.17 Therefore, progression was defined by at least one of these criteria: (1) increase of 25% or more in the sum of the products of the perpendicular diameters of the contrast-enhancing T1 MRI lesions compared with the examination that measured the smallest tumor dimensions; (2) increase in fluid-attenuated inversion recovery (FLAIR) MRI sequence not related to comorbidity; or (3) any new measurable or nonmeasurable lesion associated with clinical deterioration.
Review of Case Reports
A search was conducted in Medline through PubMed, from 2005 to 2022, using the following keywords: primary/spinal/glioblastoma. With these keywords, 234 articles were found. Inclusion criteria of articles in our review were as follows: adult patients, PsGBM histologic proof, year of diagnosis >2005, and outcome (survival and treatments). With these inclusion criteria, we included 33 case report/series and identified 72 patients with PsGBM.e1-e33
Statistical Analyses
All tests were 2-sided; p values <0.05 were considered statistically significant. Univariate and multivariable Cox proportional hazard regression models were conducted using SPSS software, version 22.0 (SPSS, Chicago, IL). Establishment and verification of nomograms were implemented using the opensource software R, version 3.2.5, with Rms packages (Design, Vienna, Austria). Categorical variables (sex, treatment, histopathology, location, and medical history) were described with frequencies and percentages, whereas continuous variables (age, FU, and survival) were described with mean/median ± SD. OS was measured from the date of histopathologic diagnosis to the date of death. PFS was measured from the date of histopathologic diagnosis to the date of first radiologic evidence of progression, or to the date of death. Surviving patients were censored at last FU. In univariate analyses, categorical variables were assessed using the Pearson χ2 or Fisher exact test. Multivariable analyses were conducted separately for each diagnosis, and the Cox proportional hazards model was used to estimate hazard ratios (HRs) and 95% CIs.18 All potential explanatory variables included in the multivariable analyses were subjected to collinearity analysis with a correlation matrix. Variables associated with one another were not included in the model. The Kaplan-Meier method was used to estimate the OS and the PFS.19
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Population
Fifty patients were enrolled. We excluded 17 patients (34.0%): 14 because what was believed to be a spinal location was in fact a metastasis from a primary cerebral GBM and 3 for which a misclassification was revealed by a simple review of the neuropathologic records (astrocytoma WHO grade 3). Finally, a total of 33 patients with a diagnosis of PsGBM were retained for full analyses (Figure 1). Only 11 of the 33 patients had already benefited from an initial RENOP review. Hence, the remaining 22 patients (66.6%) were independently reviewed by the RENOCLIP, which confirmed the diagnosis in all cases. The 33 PsGBM were WHO grade 4 glioblastomas according to the WHO classification, version 2016.20
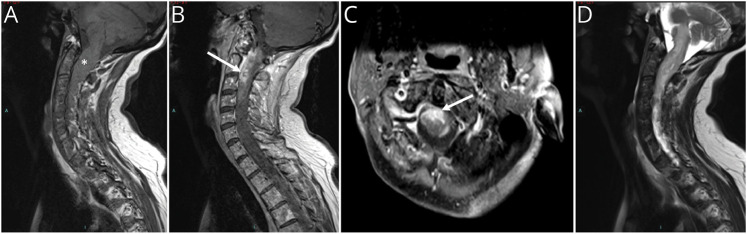
Figure 1. MRI of Cervical PsGBM.
(A) Sagittal T1-weighted MRI showing a thickening of the cervical spinal cord (white star). (B) Sagittal and (C) axial T1-weighted MRI with gadolinium injection demonstrating an enhancement of the anterior cervical spinal cord (white arrow). (D) Sagittal T2-weighted MRI of the same tumor showing peripheral edema in hypersignal. PsGBM = primary spinal glioblastoma
Epidemiologic and Clinical Data
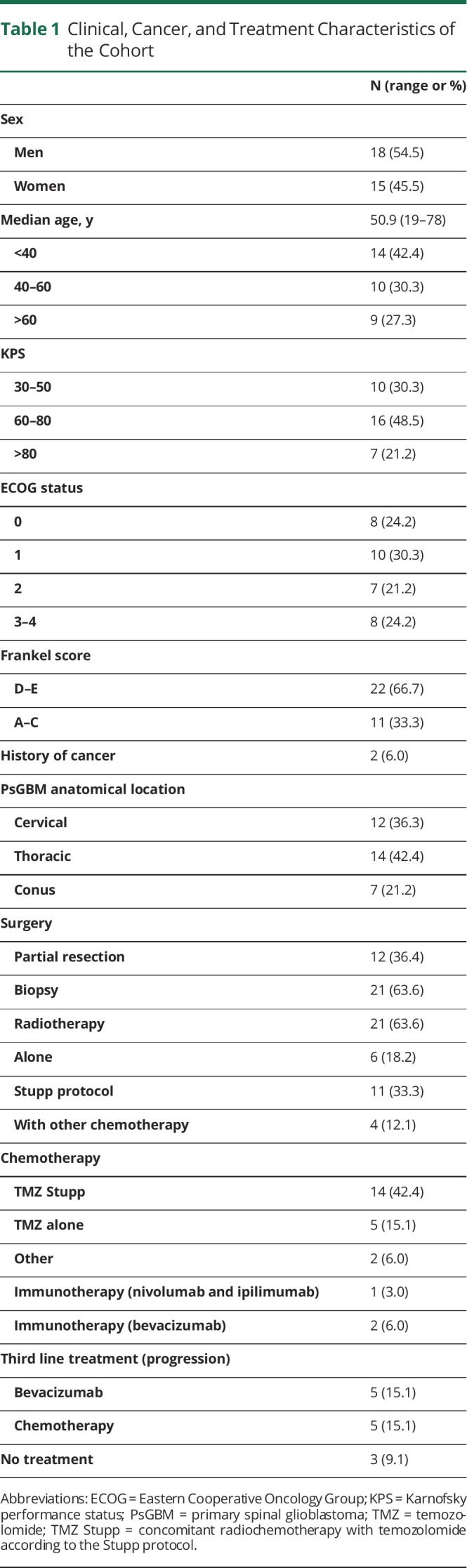
Clinical data are summarized in Table 1 and demonstrated 15 (45.5%) women and 18 (54.5%) men, with a median age of 50.9 years (range 19–78 years). Two patients (6.0%) had a history of cancer of non-neurologic origin. Neurologic symptomatology at the initial discovery was graded according to Frankel score16: 22 (66.6%) were ambulatory (Frankel D–E) and 11 (33.3%) were not (Frankel A–C). At diagnosis, the Karnofsky performance status (KPS) score was between 30 and 50 in 10 patients (30.3%), between 60 and 80 in 16 patients (48.5%), and >80 in 7 patients (21.2%). The ECOG status was at 0 in 8 patients (24.3%), 1 in 10 patients (30.3%), 2 in 7 patients (21.1%), and 3–4 in 8 patients (24.3%). The patients had typical imaging findings of a unifocal mass, extending from 1 to 3 vertebrae with intense annular contrast enhancement surrounding a central necrosis (Figure 1). The medullar anatomical repartition was cervical in 12 cases (36.3%), thoracic in 14 cases (42.4%), and lumbar medullaris in 7 cases (21.2%).
Table 1.
Clinical, Cancer, and Treatment Characteristics of the Cohort
Oncological Treatment
Therapeutic data are summarized in Table 1. Twelve patients (36.4%) underwent a subtotal resection surgery (STR), and 21 patients (63.6%) underwent surgical biopsy. The distribution of STR in cervical, thoracic, and lumbar regions was 2/12 (16.6%), 6/12 (50%), and 4/12 (33.3%), respectively (p = 0.453). Twenty-one patients (63.6%) received spinal RT: alone (6/33 patients; 18.2%), with concomitant adjuvant temozolomide CT according to Stupp et al.21 (11/33, 33.3%), or with another CT (4/33, 12.1%). Five patients (15.1%) received CT with temozolomide alone. Two patients (6.0%) received another CT (lomustine, carmustine), 1 patient (3.0%) was treated with an immunotherapy (nivolumab and ipilimumab), and 2 patients (6.0%) with bevacizumab. Finally, 3 patients (9.1%) did not receive CT nor RT and received supportive care management. The therapeutic strategies at tumor progression were as follows: a second resection for 3 cases and a second-line treatment for 10 cases with continuation of temozolomide (3) and bevacizumab (5), RT alone (1) or concomitant with temozolomide (2), and supportive care in 19 cases.
Neurologic Damages
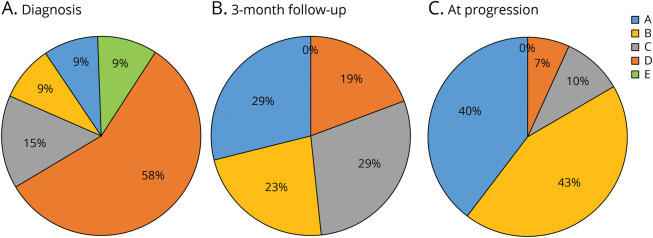
Neurologic condition evolution is presented in Figure 2. At diagnosis, 22 patients (67%) were ambulatory (Frankel D–E) (Figure 2A). In the postoperative period, 6/33 (18.2%) patients (4 cervical and 2 thoracic) developed a neurologic deterioration, 2/6 after biopsy and 4/6 after surgery. For these 6 patients, 3 presented an increase in sensory disorders, and 3 presented a motor deterioration due to hematoma (2 patients: 1 biopsy and 1 surgery) or an increase in medullary edema (1). For the 21 patients (63.6%) who received spinal RT, no side effect related to irradiation was observed. At 3-month FU, neurologic evolution deteriorated, and only 6 patients (19%) remained ambulatory (Figure 2B). Nine of 12 (75%) patients who underwent STR developed a neurologic deterioration at 3-month FU, vs 13/21 (61.9%) for biopsy, p = 0.703. By contrast, the patients who developed neurologic deterioration at 3-month FU had a significantly larger PsGBM lesion at presentation (4.2 cm3, SD 2.9) (p = 0.029). At progression, only 2 patients were ambulatory, and 22 patients (67%) presented a complete disappearance of motor function (Figure 2C).
Figure 2. Evolution of Frankel Scores (Neurologic Function) of Patients With PsGBM During the Follow-up.
(A) At presentation, 67% of patients were ambulatory (Frankel D and E), before early presentation (B) at the 3-month follow-up, a degradation was noted and only 19% of patients remained ambulatory (Frankel D). At lesion progression, 83% of patients developed plegia (Frankel A and B) and only 7% were ambulatory. PsGBM = primary spinal glioblastoma.
Progression Analyses
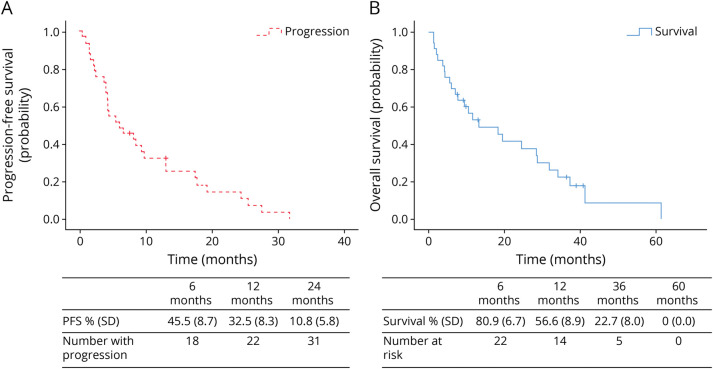
The median FU was 10.5 months (range 1.3–61.4). Thirty-one patients (93.3%) presented a progression during the FU period. The median PFS was 5.9 months (range 1.6–10.2). The 6-month, 12-month, and 24-month PFS estimates were 45.5% (SD 8.7), 32.5% (SD 8.3), and 10.8% (SD 5.8), respectively (Figure 3A). Progression occurred locally in 29 patients (87.9%). Intracranial spreading was observed in 7 patients (21.2%) with a mean delay of 18.4 months (SD 1.9). Four patients (12.1%) developed leptomeningeal progression evaluated on MRI, 2 patients (1 lumbar and 1 thoracic) developed distal C3-C4 and C5 dissemination, and 3 patients with cervical lesions presented a thoracic spreading. We did not perform autopsies in our series.
Figure 3. OS Analysis.
Kaplan-Meier survival analysis. (A) PFS in months, defined by clinical and MRI progression: table demonstrated the progression rates at 6, 12, and 24 months of follow-up. (B) Overall survival (OS) in months for the 33 patients after PsGBM diagnosis: table demonstrated the survival rates at 6, 12, 36, and 60 months of follow-up. OS = overall survival; PFS = progression-free survival; PsGBM = primary spinal glioblastoma.
Survival Analyses
The median OS was 13.1 months (range 2.5–23.7 months). The 6-month, 12-month, 36-month, and 60-month OS estimates were 80.9%, 56.6%, 22.7%, and 0%, respectively (Figure 3B). Twenty-six patients (78.8%) died during the FU period. Five patients (3 thoracic and 2 lumbar) died of a brain dissemination, 2 patients with thoracic localizations died of respiratory failure, 2 patients (1 lumbar and 1 thoracic) died of cervical C3-C4 dissemination with tetraplegia, and 2 patients with thoracic PsGBM died of glial meningitis with no other medullary or cranial localizations. Finally, 5 more fragile patients (median age 72.3 years) died after general deterioration. Five patients are still alive (2 thoracic and 3 lumbar).
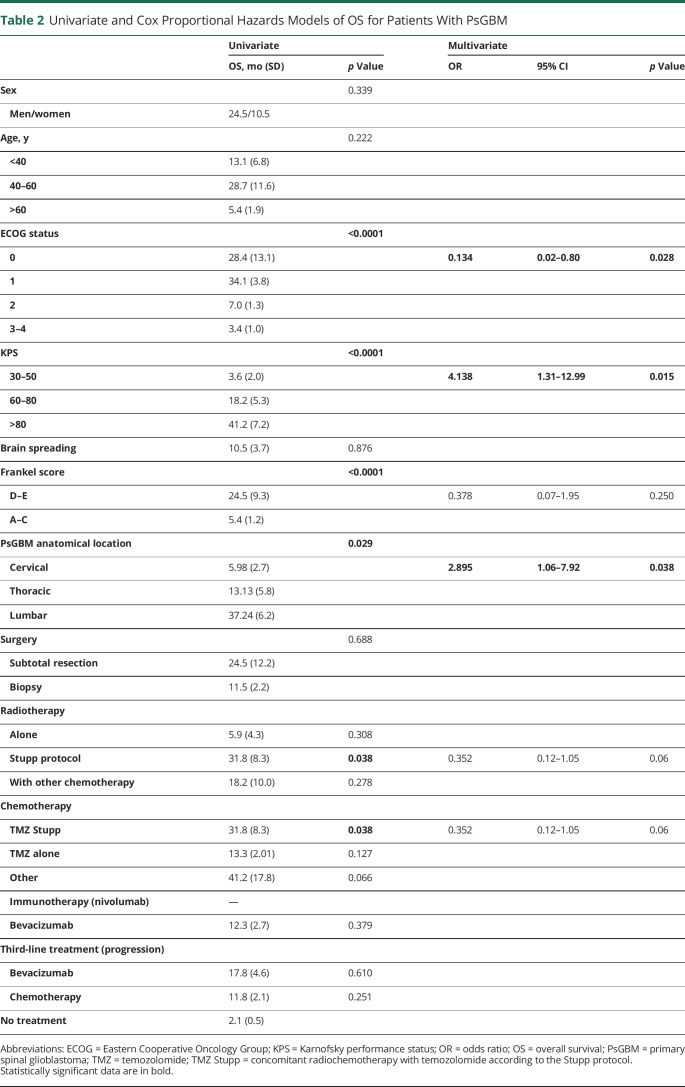
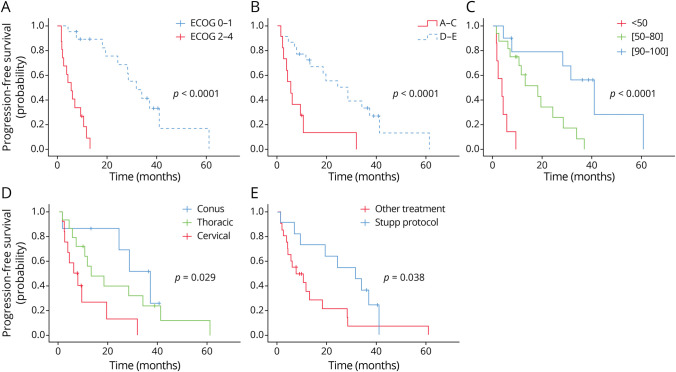
Prognostic factors associated with OS are summarized in Table 2. In univariate analysis, patients with a preserved ECOG performance status 0–1 (OS: 31.8 months, Figure 4A), patients with preserved ambulatory and neurologic functions at diagnosis (OS: 24.4 months, Figure 4B), and patients with a KPS score >80 (OS: 41.2 months, Figure 4C) had significantly longer survival (p < 0.0001). Patients with a cervical location had significantly shorter survival (OS: 5.9 months, Figure 4D) (p = 0.029). Patients who received a standard RT with concomitant temozolomide (Stupp protocol)21 had a significantly longer survival (OS; 31.8 months, Figure 4E) (p = 0.038).
Table 2.
Univariate and Cox Proportional Hazards Models of OS for Patients With PsGBM
Figure 4. OS in Univariate Analyses.
(A) The median OS for good prognosis ECOG (0–1) was 31.8 months (SD 3.6) vs 5.4 months (SD 1.5) for poor ECOG (2–4) (p < 0.0001). (B) The median OS for ambulatory patients (Frankel D and E) was 24.4 months (SD 9.3) vs 5.4 months (SD 1.2) for nonambulatory (Frankel A–C) (p = 0.002). (C) The median OS for patients with a KPS <50 was 3.6 months (SD 2.0) vs 18.2 months (SD 5.3) for KPS (50–80), vs 41.2 months (SD 7.2) for KPS >80 (p < 0.0001). (D) The median OS for patients with a cervical PsGBM was 5.9 months (SD 2.7) vs 13.1 months (SD 5.8) for thoracic lesions, vs 37.2 months (SD 6.2) for lumbar PsGBM (p = 0.029). (E) The median OS for patients with concomitant radiochemotherapy with temozolomide according to the Stupp protocol was 31.8 months (SD 8.1) vs 7.6 months (SD 4.07) for other oncological treatment (p = 0.055). ECOG = Eastern Cooperative Oncology Group; KPS = Karnofsky performance status; OS = overall survival; PsGBM = primary spinal glioblastoma.
In multivariable analysis with the Cox proportional hazard model, only ECOG performance status at 0–1 (HR 0.134, 95% CI 0.02–0.801; p = 0.02) was an independent predictor of a longer OS, whereas KPS score <60 (HR 2.895, 95% CI 1.05–7.92; p = 0.03) and cervical anatomical location (HR 4.138, 95% CI 1.32–12.98; p = 0.01) were independent predictors of a shorter OS. Neither the standard chemoradiotherapy with concomitant temozolomide (HR 0.352, 95% CI 0.118–1.05; p = 0.062) nor the ambulatory status (Frankel D–E) (HR 0.378, 95% CI 0.07–1.985; p = 0.250) were identified as independent predictors of OS.
Molecular Profile
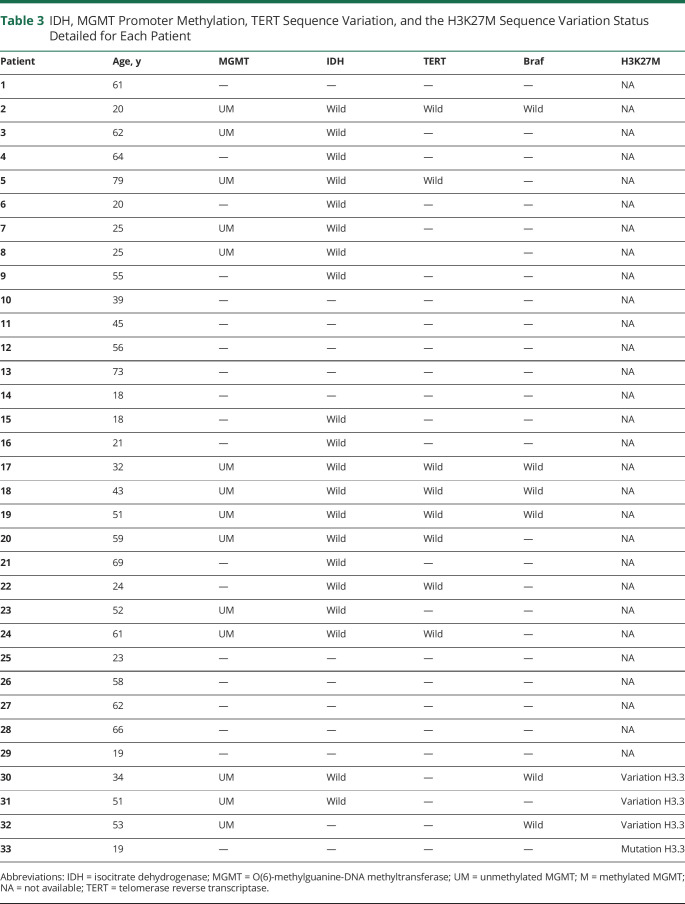
IDH sequence variation was investigated in 20/33 patients (60.6%) (Table 3). All these 20 patients had an IDH wild-type tumor. The MGMT promoter methylation status was investigated in 14/33 patients (51.5%). All these 14 cases were unmethylated. The TERT sequence variation was investigated in 8/33 patients (24.2%), and no case presented a TERT variation. Four patients presented an H3K27M variation (see eTable 1, links.lww.com/WNL/C617).
Table 3.
IDH, MGMT Promoter Methylation, TERT Sequence Variation, and the H3K27M Sequence Variation Status Detailed for Each Patient
Review of Recent Cases Reports (2005–2022)
As summarized in eTable 1 (links.lww.com/WNL/C617), we performed an inventory in the modern literature of PsGBM diagnosed and treated since 2005, date of introduction of radiochemotherapy (temozolomide) according to the Stupp protocol for brain GBM.21
In an integrative survival analysis performed by extracting individual patient data from the 72 cases reported in the recent literature (2005–2022) associated with our 33 patients, we determined for these 105 patients (median age 37 years, SD 16.5) a median OS of 13.1 months (SD 1.6 months) and a median PFS of 8.0 months (SD 0.629). We confirmed that the extent of resection was not associated with better OS for PsGBM: biopsy (11 months, SD 1.8), gross total resection (8 months, SD 1.4), and subtotal resection (15 months, SD 2.7). The anatomical localization (cervical, thoracic, or lumbar) was not associated with a better survival. Furthermore, we noted that complementary treatments, no matter the type, improved the survival of patients compared with the absence of any complementary treatment: CT alone (10.5 months, SD 1.0), RT alone (11 months, SD 4.6), RT + temozolomide (16 months, SD 1.5), RT + temozolomide + immunotherapy/CT (13 months, SD 3.5), and palliative treatment (3.4 months, SD 1.4). Moreover, patients aged younger than 60 presented better survival.
Discussion
This study determines the median PFS and OS of adult PsGBM, which are 5.9 and 13.1 months, respectively. We highlighted 2 independent predictors of survival: the preoperative clinical status (ECOG and KPS) and the anatomical location.
Previous studies concerning PsGBM are limited to small case series, individual case reports, or literature reviewing.2,4,9,13,14,22,23 In addition, previous studies were composed of both adult and pediatric cases, assorted different WHO grades of malignancy, without expert central histomolecular review, and did not detail clinical, imaging, or therapeutic data. In this study, the median age at diagnosis was older than what has previously been reported,3,14,23,24 explained by the exclusion of the pediatric population and the careful selection of PsGBM excluding anaplastic or low-grade astrocytoma or oligodendroglioma. However, the median age at diagnosis seems lower for PsGBM than for supratentorial hemispheric glioblastomas.25
Regarding survival, the previous series of PsGBM reported an OS of approximately 10 months, in contrast to a somewhat better prognosis of 14 months for supratentorial hemispheric glioblastomas.3,13 In this study, we identified a median OS (13.1 months), which is comparable with the one reported for supratentorial hemispheric glioblastomas.
The optimal therapeutic management of PsGBM remains debated. Some studies have litigated for aggressive resections,4,6 but the lack of a clear surgical plane between the infiltrative tumor and the healthy tissue of the spinal cord makes gross total resection quite unachievable. Furthermore, the extent of resection has proved to correlate with better OS,8,24 even if Adams et al.3 identified the extent of resection as an independent prognostic factor including both adults and children and both anaplastic astrocytomas and PsGBM.
To date, there are highlights that MRI diffusion tensor imaging and perfusion-weighted imaging could be useful for differentiating between intramedullary tumors and tumor-like lesions (tumefactive demyelinating lesion, inflammatory/infectious diseases). Nevertheless, the presentation of spinal tumors (i.e., astrocytomas, ependymomas, unspecified gliomas, medulloblastomas, metastases, neurinomas, and rarely teratomas) frequently have similarities in radiologic appearances: occupying a large portion of the spinal cord, intratumor necrosis, cystic degeneration, and massive edema, such that it was difficult to differentiate the various kinds of tumors based solely on morphological and signal characteristics on MRI. Recently, Michalopoulos et al.,26 in a meta-analysis of 39 studies reporting the diagnostic performance and complications of 3,598 medullar biopsies, identified a diagnostic accuracy of 86% for a complication rate of 1%. Despite the possible postbiopsy neurologic complications described in the literature and the findings in our series, we believe that the risk is worth taking if an intramedullary tumor is suspected because with an appropriate treatment, the prognosis is much better for the differential spinal tumor lesions.
In our series, we did not perform CSF sampling analyses that remain debated due to their poor diagnosis efficiency and accuracy.27 Concerning pediatric studies, the previous clinical series did not find conclusive evidence on OS to support the aggressive administration of CT.4,9,28 There exists a paucity of literature regarding the use of CT in the adult population. Our results concerning the absence of OS improvement with CT recall those from Raco et al.22 and Chamberlain et al.29 for high-grade astrocytomas or recurrent low-grade astrocytomas. More recently, Hernández-Durán et al.14 performed a literature review (64 adult patients) and demonstrated no significant therapeutic impact with the adjuvant use of temozolomide and with RT. The survival benefit from the use of RT also remains highly debated.30-32 In 2005, the introduction of adjuvant RT concomitant with temozolomide to the treatment of GBM, the so-called standard chemoradiotherapy, dramatically improved OS and became the gold standard of care for patients harboring a supratentorial hemispheric GBM.21 To date, no series studied the impact of the standard chemoradiotherapy protocol in PsGBM.
In our series, the standard chemoradiotherapy with concomitant temozolomide was not an independent predictor of OS, possibly due to the low rate of MGMT promoter methylation observed in this population. Moreover, for the 17/33 (51.5%) patients of our series in whom we sought the MGMT promoter methylation status, no hypermethylated tumor had been identified. This point may reveal a molecular particularity of PsGBMs because in hemispheric localizations, approximately 40% of primary GBM harbor a MGMT promoter hypermethylation.33,34 Considering other molecular data in our cohort, we found no IDH variation in PsGBM, which is consistent with data from primary brain GBM, in which IDH variation is very rare (<5%).35 Similarly, although it was investigated in only a small sample of our cohort (12/33 (36.4%) patients), none of the 12 cases investigated presented a TERT variation. These data are very different from primary brain GBM, in which TERT variation is found in 67% of cases.36 The cervical spinal cord is the most affected region by PsGBM.24,37 In agreement with our data, Raco et al.22 and Konar et al.23 demonstrated that thoracic and lumbar tumors had a decreased risk of mortality.
These findings should be interpreted with caution, given the retrospective design, exploratory design of statistical analyses, absence of a control group, and lack of an external validation set, all limiting the generalizability of the results. The precise topography of the tumor is not always well specified in the FBTDB. It is possible that we have underestimated the number of cases in some centers. However, the rarity of this condition makes the inclusion of a large number of patients difficult, and a prospective study seems unrealistic. Further confirmatory analysis is required. Moreover, we have to highlight that we were not able to inform all the histomolecular markers, that is, MGMT, TERT, and IDH variation, particularly for the cases with biopsies in which biological material was limited. Finally, it is also important to note that we included 4 cases of H3K27M mutated gliomas in our cohort, although they should be nowadays classified as diffuse midline glioma rather than GBM. We decided not to exclude them not only because the primary diagnosis of GBM of these 4 patients was made on morphological features several years before the availability of histone variation characterization but also because we did not find any OS statistical difference for these 4 patients (p = 0.362) compared with the rest of the cohort, although this result may be the consequence of the small size of the sample (very large SD, data not shown).
We report the largest study on PsGBM for which a central histopathologic review was conducted. Unlike cerebral GBM, PsGBM has received little attention in the literature, and very limited data exist to support treatment guidelines. Although treatments of PsGBM generally mirror those used for intracranial GBM, optimal therapeutic strategies for PsGBM have not been established, and the rarity of the disease precludes the conduct of clinical trials to test for treatment efficacy. To date, the various literature reviews that have studied PsGBM over different periods spread out, up to 2016: 38, 1970–2014: 14, and 1938–2015: 22, did not demonstrate efficacy on OS neither of different surgical treatments (biopsy, subtotal vs total resection) nor of different types of CT. Thanks to our series and our integrative reported literature (2005–2022), we confirmed that for these infiltrative lesions, the extent of resection causes more surgical morbidity and no survival gain. Whatever the implemented complementary treatment (RT alone or with temozolomide/immunotherapy), there is a benefit on survival compared with palliative care. Unfortunately, very little histomolecular results for IDH variation and for the MGMT promoter methylation status were investigated in our series or in the case reports. Therefore, through our recent review of the literature and our series, it remains impossible to establish a molecular profile of “better survival prognosis.” The trend that seems to emerge would be that there is almost no identification of mutated IDH (1/47 cases informed). On the contrary, the MGMT promoter methylation seems to be more commonly found (18/32 informed).
Despite the PsGBM are currently best treated similarly to intracranial GBM with radiation ± temozolomide depending on age/KPS, the poor survival prognosis and the dramatic neurologic decline makes the PsGBM one of the most disabling malignancies. Extensive surgical resection of contrast-enhanced and FLAIR infiltration areas is not possible and does not confer to better survival. Molecular testing is encouraged, if tissue available, to help guide other possible targeted treatment options and/or immunotherapy. Routinely performing molecular testing on these patients in the future and forming an international registry may help better understand this entity and assist in establishing novel treatment options. The management of such patients would have to be conducted “à la carte” with complementary treatment adapted to DNA sequencing (temozolomide, immunotherapy, and third-generation epidermal growth factor receptor tyrosine kinase inhibitor).
Acknowledgment
The authors thank Associations pour la Recherche sur les Tumeurs Cerebrales (ARTC) North and South, Des Etoiles dans la Mer and Ligue contre le Cancer comite 34 for their assistance in identifying medullary glioblastoma cases and patient support. Coauthor Anne Jouvet, MD, PhD, died in December 2018.
Glossary
- CT
chemotherapy
- ECOG
Eastern Cooperative Oncology Group
- FBTDB
French Brain Tumor Database
- FLAIR
fluid-attenuated inversion recovery
- FU
follow-up
- GBM
glioblastoma
- HR
hazard ratio
- IDH
isocitrate dehydrogenase
- KPS
Karnofsky performance status
- MGMT
O(6)-methylguanine-DNA methyltransferase
- OS
overall survival
- PFS
progression-free survival
- PsGBM
primary spinal glioblastoma
- RENOCLIP
Réseau de Neuro-Oncologie CLInico Pathologique
- RENOP
Réseau de Neuro-oncologie pathologique
- RT
radiotherapy
- STR
subtotal resection surgery
- TERT
telomerase reverse transcriptase
- WHO
World Health Organization
Appendix. Authors
Study Funding
No targeted funding reported.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Benes V, Barsa P, Benes V, Suchomel P. Prognostic factors in intramedullary astrocytomas: a literature review. Eur Spine J. 2009;18(10):1397-1422. doi: 10.1007/s00586-009-1076-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker DJ, Weller RO, Garfield JS. Epidemiology of primary tumours of the brain and spinal cord: a regional survey in southern England. J Neurol Neurosurg Psychiatry. 1976;39(3):290-296. doi: 10.1136/jnnp.39.3.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams H, Avendaño J, Raza SM, Gokaslan ZL, Jallo GI, Quiñones-Hinojosa A. Prognostic factors and survival in primary malignant astrocytomas of the spinal cord: a population-based analysis from 1973 to 2007. Spine (Phila Pa 1976). 2012;37(12):E727-E735. doi: 10.1097/BRS.0b013e31824584c0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen AR, Wisoff JH, Allen JC, Epstein F. Malignant astrocytomas of the spinal cord. J Neurosurg. 1989;70(1):50-54. doi: 10.3171/jns.1989.70.1.0050 [DOI] [PubMed] [Google Scholar]
- 5.Ciappetta P, Salvati M, Capoccia G, Artico M, Raco A, Fortuna A. Spinal glioblastomas: report of seven cases and review of the literature. Neurosurgery. 1991;28(2):302-306. [PubMed] [Google Scholar]
- 6.Houten JK, Cooper PR. Spinal cord astrocytomas: presentation, management and outcome. J Neurooncol. 2000;47(3):219-224. doi: 10.1023/a:1006466422143 [DOI] [PubMed] [Google Scholar]
- 7.Miller DC, Hochberg FH, Harris NL, Gruber ML, Louis DN, Cohen H. Pathology with clinical correlations of primary central nervous system non-Hodgkin's lymphoma. The Massachusetts General Hospital experience 1958-1989. Cancer. 1994;74(4):1383-1397. [DOI] [PubMed] [Google Scholar]
- 8.McGirt MJ, Goldstein IM, Chaichana KL, Tobias ME, Kothbauer KF, Jallo GI. Extent of surgical resection of malignant astrocytomas of the spinal cord: outcome analysis of 35 patients. Neurosurgery. 2008;63(1):55-60; discussion 60-61. doi: 10.1227/01.NEU.0000335070.37943.09 [DOI] [PubMed] [Google Scholar]
- 9.Allen JC, Aviner S, Yates AJ, et al. Treatment of high-grade spinal cord astrocytoma of childhood with “8-in-1” chemotherapy and radiotherapy: a pilot study of CCG-945. Children's Cancer Group. J Neurosurg. 1998;88(2):215-220. doi: 10.3171/jns.1998.88.2.0215 [DOI] [PubMed] [Google Scholar]
- 10.Karschnia P, Vogelbaum MA, van den Bent M, et al. Evidence-based recommendations on categories for extent of resection in diffuse glioma. Eur J Cancer. 2021;149:23-33. doi: 10.1016/j.ejca.2021.03.002 [DOI] [PubMed] [Google Scholar]
- 11.Picart T, Meyronet D, Pallud J, et al. Management, functional outcomes and survival in a French multicentric series of 118 adult patients with cerebellar glioblastoma. J Cancer Res Clin Oncol. 2021;147(6):1843-1856. doi: 10.1007/s00432-020-03474-6 [DOI] [PubMed] [Google Scholar]
- 12.Banczerowski P, Simó M, Sipos L, Slowik F, Benoist G, Veres R. Primary intramedullary glioblastoma multiforme of the spinal cord: report of eight cases. Ideggyogyaszati Szle. 2003;56(1-2):28-32. [PubMed] [Google Scholar]
- 13.Santi M, Mena H, Wong K, Koeller K, Olsen C, Rushing EJ. Spinal cord malignant astrocytomas. Clinicopathologic features in 36 cases. Cancer. 2003;98(3):554-561. doi: 10.1002/cncr.11514 [DOI] [PubMed] [Google Scholar]
- 14.Hernández-Durán S, Bregy A, Shah AH, Hanft S, Komotar RJ, Manzano GR. Primary spinal cord glioblastoma multiforme treated with temozolomide. J Clin Neurosci. 2015;22(12):1877-1882. doi: 10.1016/j.jocn.2015.04.017 [DOI] [PubMed] [Google Scholar]
- 15.Zouaoui S, Rigau V, Mathieu-Daudé H, et al. French brain tumor database: general results on 40,000 cases, main current applications and future prospects [in French]. Neurochirurgie. 2012;58(1):4-13. doi: 10.1016/j.neuchi.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 16.Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7(3):179-192. doi: 10.1038/sc.1969.30 [DOI] [PubMed] [Google Scholar]
- 17.Vogelbaum MA, Jost S, Aghi MK, et al. Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) Working Group. Neurosurgery. 2012;70(1):234-243; discussion 243-244. doi: 10.1227/NEU.0b013e318223f5a7 [DOI] [PubMed] [Google Scholar]
- 18.Hidalgo B, Goodman M. Multivariate or multivariable regression? Am J Public Health. 2013;103(1):39-40. doi: 10.2105/AJPH.2012.300897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krewski D, Rao J. Inference from stratified samples: properties of the linearization, jacknife and balanced repeated replication methods. Ann Stat. 1981;9:1010-1019. [Google Scholar]
- 20.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820. doi: 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 21.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. doi: 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 22.Raco A, Piccirilli M, Landi A, Lenzi J, Delfini R, Cantore G. High-grade intramedullary astrocytomas: 30 years' experience at the Neurosurgery Department of the University of Rome “Sapienza.” J Neurosurg Spine. 2010;12(2):144-153. doi: 10.3171/2009.6.SPINE08910 [DOI] [PubMed] [Google Scholar]
- 23.Konar SK, Maiti TK, Bir SC, Kalakoti P, Bollam P, Nanda A. Predictive factors determining the overall outcome of primary spinal glioblastoma multiforme: an integrative survival analysis. World Neurosurg. 2016;86:341-348.e1-3. doi: 10.1016/j.wneu.2015.08.078 [DOI] [PubMed] [Google Scholar]
- 24.Epstein FJ, Farmer JP, Freed D. Adult intramedullary astrocytomas of the spinal cord. J Neurosurg. 1992;77(3):355-359. doi: 10.3171/jns.1992.77.3.0355 [DOI] [PubMed] [Google Scholar]
- 25.Bauchet L, Mathieu-Daude H, Fabbro-Peray P, et al. Oncological patterns of care and outcome for 952 patients with newly diagnosed glioblastoma in 2004. Neuro Oncol. 2010;12(7):725-735. doi: 10.1093/neuonc/noq030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michalopoulos GD, Yolcu YU, Ghaith AK, Alvi MA, Carr CM, Bydon M. Diagnostic yield, accuracy, and complication rate of CT-guided biopsy for spinal lesions: a systematic review and meta-analysis. J Neurointerventional Surg. 2021;13(9):841-847. doi: 10.1136/neurintsurg-2021-017419 [DOI] [PubMed] [Google Scholar]
- 27.Shibahara I, Saito R, Osada Y, et al. Incidence of initial spinal metastasis in glioblastoma patients and the importance of spinal screening using MRI. J Neurooncol. 2019;141(2):337-345. doi: 10.1007/s11060-018-03036-4 [DOI] [PubMed] [Google Scholar]
- 28.Bouffet E, Pierre-Kahn A, Marchal JC, et al. Prognostic factors in pediatric spinal cord astrocytoma. Cancer. 1998;83(11):2391-2399. doi: [DOI] [PubMed] [Google Scholar]
- 29.Chamberlain MC. Temozolomide for recurrent low-grade spinal cord gliomas in adults. Cancer. 2008;113(5):1019-1024. doi: 10.1002/cncr.23677 [DOI] [PubMed] [Google Scholar]
- 30.Jyothirmayi R, Madhavan J, Nair MK, Rajan B. Conservative surgery and radiotherapy in the treatment of spinal cord astrocytoma. J Neurooncol. 1997;33(3):205-211. doi: 10.1023/a:1005758313700 [DOI] [PubMed] [Google Scholar]
- 31.Minehan KJ, Brown PD, Scheithauer BW, Krauss WE, Wright MP. Prognosis and treatment of spinal cord astrocytoma. Int J Radiat Oncol Biol Phys. 2009;73(3):727-733. doi: 10.1016/j.ijrobp.2008.04.060 [DOI] [PubMed] [Google Scholar]
- 32.Isaacson SR. Radiation therapy and the management of intramedullary spinal cord tumors. J Neurooncol. 2000;47(3):231-238. doi: 10.1023/a:1006470523052 [DOI] [PubMed] [Google Scholar]
- 33.Katsigiannis S, Grau S, Krischek B, et al. MGMT-positive vs MGMT-negative patients with glioblastoma: identification of prognostic factors and resection threshold. Neurosurgery. 2021;88(4):E323-E329. doi: 10.1093/neuros/nyaa562 [DOI] [PubMed] [Google Scholar]
- 34.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003. doi: 10.1056/NEJMoa043331 [DOI] [PubMed] [Google Scholar]
- 35.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765-773. doi: 10.1056/NEJMoa0808710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stichel D, Ebrahimi A, Reuss D, et al. Distribution of EGFR amplification, combined chromosome 7 gain and chromosome 10 loss, and TERT promoter mutation in brain tumors and their potential for the reclassification of IDHwt astrocytoma to glioblastoma. Acta Neuropathol (Berl). 2018;136(5):793-803. doi: 10.1007/s00401-018-1905-0 [DOI] [PubMed] [Google Scholar]
- 37.Guidetti B, Mercuri S, Vagnozzi R. Long-term results of the surgical treatment of 129 intramedullary spinal gliomas. J Neurosurg. 1981;54(3):323-330. doi: 10.3171/jns.1981.54.3.0323 [DOI] [PubMed] [Google Scholar]
- eReferences are listed at links.lww.com/WNL/C616.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.