Abstract
Ginseng has been used in China as a superior medicinal material for thousands of years that can nourish the five internal organs, calm the mind and benefit wisdom. Due to its anti-inflammatory, antioxidant and neuroprotective activities, one of the active components of ginseng, ginsenoside Rg1, has been extensively investigated in the remedy of brain disorders, especially dementia and depression. In this review, we summarized the research progress on the action mechanisms of Rg1 ameliorating depression-like behaviors, including inhibition of hyperfunction of hypothalamic-pituitary-adrenal (HPA) axis, regulation of synaptic plasticity and gut flora. Rg1 may alleviate Alzheimer’s disease in the early phase, as well as in the middle-late phases through repairing dendrite, axon and microglia- and astrocyte-related inflammations. We also proposed that Rg1 could regulate memory state (the imbalance of working and aversive memory) caused by distinct stimuli. These laboratory studies would further the clinical trials on Rg1. From the prospective of drug development, we discussed the limitations of the present investigations and proposed our ideas to increase permeability and bioavailability of Rg1. Taken together, Rg1 has the potential to treat neuropsychiatric disorders, but a future in-depth investigation of the mechanisms is still required. In addition, drug development will benefit from the clinical trials in one specific neuropsychiatric disorder.
Keywords: ginsenoside Rg1, neurological disease, depression, Alzheimer’s disease, learning and memory
Introduction
Neurological disorder is a general term for nervous system diseases and psychiatric diseases. It is a series of diseases that occur in the central nervous system (CNS) or peripheral nervous system and are mainly manifested by sensory, cognitive, emotional, behavioral and psychological disorders [1]. Neurological disorders, especially dementia and depression not only bring great pain to patients, but also increase the medical economic burden. Most worryingly, neurological disorders have become a major source of disability globally, with an increasing prevalence [2]. Alzheimer’s disease (AD) and depression are two types of common and highly prevalent neurological disorders. Statistically, the number of AD patients is increasing year by year, and AD has become a fast-growing neurological disorder. From 1990–2015, the growth rate of AD patients was 111.7% and the mortality rate increased by 14.9%, and the number has reached 45,956,000, which may still continue to rise, as predicted from the analysis of population data [3]. Depression is not only a high incidence, but also a leading cause of disability and suicide worldwide. In the last 20 years, the rate of depression-induced suicide in the United States alone has increased by 35%. Treatment for depression will thus have significant medical and economic costs, which are expected to double by 2030 [4]. Of concern, human genetic and epidemiological studies have elucidated that depression may be a risk factor or early symptom of AD, and that 30%–40% of AD patients experience depression during disease progression [5, 6]. The risk of comorbidity of depression and AD is much greater than that of depression or AD. Therefore, it is more important to prevent the occurrence of comorbidity in the treatment of depression and AD. The medical community is still persistently developing chemical drugs that can treat AD, as well as depression, but the success rate is low [7]. Therefore, many researchers began to change their exploratory thinking and seek answers from natural components [8, 9].
Ginseng is derived from the root and rhizome of Panax ginseng C.A. Mey, a plant of Araliaceae. It has been used as a medicine in China for thousands of years. As recorded in Sheng Nong’s herbal classic (the earliest pharmaceutical book in China), ginseng is proposed as a superior medicinal material. It is believed that ginseng can nourish the five internal organs, calm the mind and benefit wisdom, and taking ginseng for a long time has the effect of prolonging life. Because of its unique beneficial effect and the rarity of wild species resources, ginseng has become a valuable traditional Chinese medicine. Nowadays, ginseng is not only widely used in the prevention and treatment of diseases in traditional Chinese medicine, but also plays an important role in ethnic medicine in Republic of Korea, Democratic People’s Republic of Korea, Japan and other countries [10]. Modern pharmacological studies have shown that the main active substances of ginseng are ginsenosides, such as ginsenoside Rb1 (Rb1), ginsenoside Rg1 (Rg1) and ginsenoside Rg3 (Rg3), etc. These steroidal saponins have a variety of pharmacological effects (Table 1). As one of the main components of ginseng, Rg1 has attracted wide attention in the prevention of neurological disorders, especially in dementia and depression. In order to further the development of Rg1, this review focuses on the recent experimental studies of Rg1 in depression, AD, and learning and memory dysfunction, and summarizes the mechanism of Rg1 in the treatment of dementia and depression. At the same time, the clinical potential of Rg1 and challenges are extensively discussed.
Table 1.
Ginsenosides and therapeutic potential.
| Ginsenosides | Molecular formula | Effects | References |
|---|---|---|---|
| Ra1 | C58H98O26 | Immune regulation, treatment of coronary artery disease and cancer | [106, 107] |
| Ra2 | C58H98O26 | Treatment of coronary artery disease and cancer | [107] |
| Ra3 | C59H100O27 | Treatment of coronary artery disease and cancer | [107] |
| Rb1 | C54H92O23 | Anti-angiogenic effect, anti-inflammatory effect, cardioprotective effect, anti-aging effect, anti-diabetic effect, antidepressant effect, neuroprotective effect, preventing alcoholic liver disease, ischemic stroke, AD, PD | [58, 108–117] |
| Rb2 | C53H90O22 | Proangiogenic effect, neuroprotective effect, anti-inflammatory effect, attenuating platelet function, alleviating obesity, treatment of colorectal cancer | [118–123] |
| Rb3 | C53H90O22 | Cardioprotective effect, alleviating lung and kidney injury | [124–126] |
| Rc | C53H90O22 | Cardioprotective effect and anti-inflammatory effect | [127, 128] |
| Rd | C48H82O18 | Alleviating lung injury, attenuating autoimmune neuritis, preventing cardiovascular diseases, ameliorating auditory cortex injury, alleviating anxiety/depression, ameliorating obesity | [129–134] |
| Re | C48H82O18 | Anti-diabetic, inhibiting neointimal thickening, alleviating cognitive deficits, attenuating myocardial injury | [135–138] |
| Rf | C42H72O14 | Anti-melanogenic effect, antidepressant effect, anti-inflammatory effect, antinociceptive effect | [139–142] |
| Rg1 | C42H72O14 | Ameliorating traumatic brain injury, preventing cognitive impairment, cardioprotective effect, AD, PD, treatment of inflammatory bowel disease and diabetes, attenuating depression, preventing cell senescence, alleviating liver injury, ameliorating reproductive function injury, preventing post-traumatic stress disorder (PTSD), Huntington’s disease (HD), ameliorating glomerular fibrosis, promoting sleep | [47, 56, 78, 80, 90, 104, 143–152] |
| Rg2 | C42H72O13 | Ameliorating brain injury, affecting cell growth, improving memory impairment and neuronal death, antiarrhythmic effect, decreasing lipogenesis | [153–156] |
| Rg3 | C42H72O13 | Ameliorating heart failure, anti-inflammatory effect and attenuating skin inflammatory diseases, alleviating bone impairment, attenuating cellular senescence, anti-angiogenic effect, alleviating endothelial dysfunction, preventing atherosclerosis, inhibiting hepatocellular carcinoma and gastric ulcer | [157–165] |
| Rh1 | C36H62O9 | Immune regulation, improving diabetic nephropathy, alleviating cognitive deficits | [106, 166, 167] |
| Rh2 | C36H62O8 | Anti-tumor effect, antibacterial and antiviral activity, anti-inflammatory effect, improving memory deficits, immune regulation, alleviating lung injury | [168–174] |
| Rh3 | C37H62O7 | Anti-inflammatory effect and renoprotective effect | [175, 176] |
The reasons for Rg1 in treatment of depression and Alzheimer’s disease
Although depression and AD belong to two different types of neurological disorders, the presence of comorbidity suggests that the two diseases are related, and the occurrence of depression and AD has the same mechanisms and targets. To support that point of view, antidepressants, like paroxetine could also improve AD [11]. This provided an important basis for Rg1 to effectively treat both depression and AD. In fact, the common pathological features of depression and AD include synaptic damage, oxidative stress and inflammatory response, etc [12–14].
Synaptic deficit is an important factor leading to depression and AD. As an important neurotrophic factor, brain-derived neurotrophic factor (BDNF) could prevent neuronal apoptosis and synaptic deficit by activating downstream tropomyosin receptor kinase B (TrkB) and mitogen-activated protein kinase (MAPK)/ extracellular regulated protein kinase (ERK) signaling pathways [15]. AD studies revealed that the decrease of BDNF in the brain was inversely proportional to the increase of amyloid β-protein (Aβ), and BDNF had an antagonistic effect on Aβ toxicity [16, 17]. In depression, the decrease of BDNF reduced the formation of hippocampal neurons and the activity of downstream signaling molecules, leading to depressive behavior [18]. Excitotoxicity caused by excessive activation of N-methyl-D-aspartate receptor (NMDAR) is considered to be the common pathophysiological basis of depression and AD. In AD, Aβ-induced synaptic inhibition was related to the activation of NMDAR caused by glutamate overrelease, and the activation of extra-synaptic NMDAR induced the overexpression of Tau [19, 20]. Our team also found that depression-like behavior and hippocampal synaptic dysfunctions could be antagonized by inhibiting the overactivation of NMDAR and calpain, which is consistent with the results of many antidepressant drugs [21].
In AD, mitochondrial dysfunction could lead to excessive ROS production, subsequently causing oxidative damage of protein, DNA and RNA, and promote the appearance of clinical symptoms and Aβ pathology of AD [22]. Clinical study also supported the involvement of DNA oxidative damage in the early phase of AD development [23]. In depression, the increase of ROS regulated the activation of hypothalamic-pituitary-adrenal (HPA) axis, thus increasing oxidative stress damage [24]. Therefore, the inhibition of HPA axis is also one of the mechanisms of antidepressants [25]. Clinical study revealed that oxidative stress, especially lipid peroxidation, in elderly patients with depression was significantly higher than that in non-depressed ones [26].
Neuroinflammation is the most common pathogenic factor in neurological diseases and plays a catalytic role in the occurrence of depression and AD. In the pathological state of depression, the activation of microglia resulted in the production and release of inflammatory factors, including interleukin-1beta (IL-1β) and tumor necrosis factor-alpha (TNF-α), which further mediated the depressive symptoms and dysfunction of synaptic plasticity [27]. Therefore, anti-inflammation is an effective way to fight against depression. Similarly, in the early stage of AD, Aβ could activate microglia, promote neuroinflammation, damage synaptic function and cause neuronal death [28]. In our study, it has also been confirmed that improving neuroinflammation is beneficial for the prevention of AD-like phenotypes [29].
In addition to the common pathological mechanisms of synaptic damage, oxidative stress and inflammation, we also analyzed the differences in brain regions, damage degree and reversibility between depression and AD. These similarities of pathogenesis and molecular mechanisms for the two disorders would be beneficial for the understanding of the treatment of depression and AD with Rg1 (Table 2).
Table 2.
Differences in brain regions, damage degree and reversibility between depression and Alzheimer’s disease.
| Depression | Alzheimer’s disease | |
|---|---|---|
| Brain regions | Prefrontal cortex, hippocampus, amygdala, ventral tegmental area-nucleus accumbens, striatum, anterior cingulate cortex, insula [177–180] | Cingulate, cerebellum, parietal, thalamus, hippocampus, prefrontal cortex, frontal, temporal, amygdala [71, 181–183] |
| Damage degree | The pathological damage of depression is mainly manifested by neuronal damage (including neuronal atrophy/apoptosis and synaptic number reduction), and the volume reduction of PFC and hippocampus [184–186] | The pathological damage of AD is characterized by neurofibrillary tangles, senile plaques, neuronal degeneration/loss/apoptosis, and synaptic loss, and atrophy in various brain regions, with the hippocampus being the most severe [187–189] |
| Reversibility | Neuronal damage and reduction of brain region volume could be rescued or relieved [38, 190, 191] | Hippocampal atrophy is irreversible, but neuronal damage could be reduced by drugs [192, 193] |
Rg1 and depression
It is believed that the occurrence of depression is mainly related to the dysfunction of HPA axis [30], impairment of synaptic plasticity [31], disturbance of neuronal regeneration [32], increased release of inflammatory factors and increased level of oxidative stress [27]. According to the meta-analysis of the antidepressant effect of ginsenosides on animal model of depression, Rg1 is one of the most investigated ginsenosides [33]. Although the antidepressant mechanism of Rg1 has not been clearly elucidated, the improvement effect of Rg1 on depression has been verified in several well-recognized pathogenesis of depression. Rg1 may exert its antidepressant effect by inhibiting the hyperfunction of HPA axis, regulating synaptic plasticity, inhibiting neuroinflammation and oxidative stress at the same time (Table 3, Fig. 1).
Table 3.
Main mechanism and effect sites of Rg1 on depression.
| Animal or cell line | Model | Main mechanism | Effect sites (receptor, cell, axon or dendrite) | Reference |
|---|---|---|---|---|
| Male kunming mice and male Sprague-Dawley rats | CUMS | Regulating the HPA and the hypothalamic-pituitary-gonadal (HPG) axis | Glucocorticoid receptor (GR) and androgen receptor (AR) | [36] |
| Male Wistar rats | LPS treatment | Regulation of neuroinflammation by targeting the peripheral immune system | Microglia | [37] |
| Male C57BL/6 J mice | CMS | Activation of the BDNF signaling pathway and up-regulation of hippocampal neurogenesis | Progenitor cells in DG and pyramidal neurons dendrite in CA3 | [9] |
| Male Wistar rats | CUMS | Anti-inflammatory effect, improve neuronal structural changes and anti-apoptosis | Microglia, astrocytes, and dendrite and synapse of vmPFC neurons | [40] |
| Male Wistar rats | CUMS | Regulating the CREB-BDNF pathway within the amygdala | Amygdala neurons | [41] |
| Male C57BL/6 J mice | CUMS | Regulation of hippocampal synaptic proteins and inhibition of inflammatory responses | Microglia and astrocytes | [43] |
| Male C57BL/6 mice, human leukemia cell line THP-1 cells and human astrocytoma cell line U251 MG | LPS treatment or CSDS | Disrupting downstream signaling of CCL2- C-C motif chemokine receptor 2 (CCR2) interaction and inhibiting the pro-inflammatory potential of Ly6Chi monocytes | Ly6Chi monocytes and astrocytes | [45] |
| Male Sprague-Dawley rats | Chronic unpredictable stress (CUS) | Protection of astrocyte gap junctions within the PFC | Astrocytes | [46] |
| Primary prefrontal cortical and hippocampal astrocytes | CORT treatment | Alleviating CORT-induced gap junction dysfunction | Astrocytes | [48] |
| Primary hippocampal astrocytes and Male Sprague-Dawley rats | CORT treatment or CUS | Improving hippocampal astrocyte gap junction dysfunction | Astrocytes | [49] |
| Primary prefrontal cortical astrocytes, CTX TNA2 cell line and male Sprague-Dawley rats | CORT or carbenoxolone (CBX) or Gap26 treatment | Astrocyte gap junctions formed by Cx43 mediate antidepressant effects | Astrocytes | [50] |
| Male Wistar rats and primary rat glial cells | CUS or LPS treatment | Inhibition of Cx43 ubiquitination to improve neuroinflammation | Glial cells | [47] |
| Male Wistar rats | CUMS | Prevent oxidative stress, thereby preventing neuronal degeneration caused by inflammation | Microglia, astrocytes and CA1 neurons | [53] |
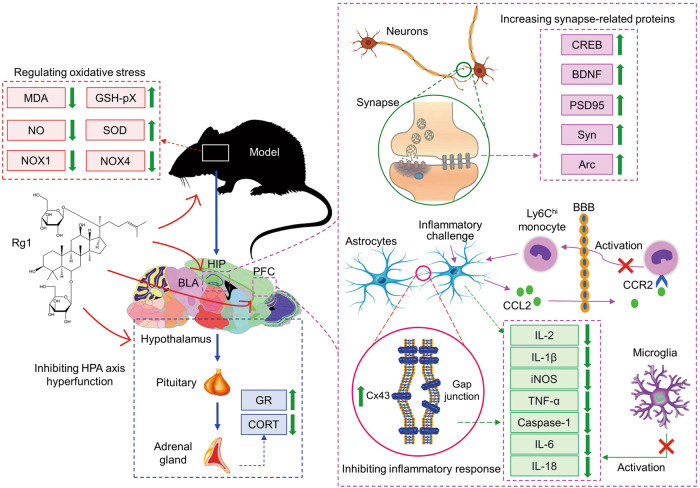
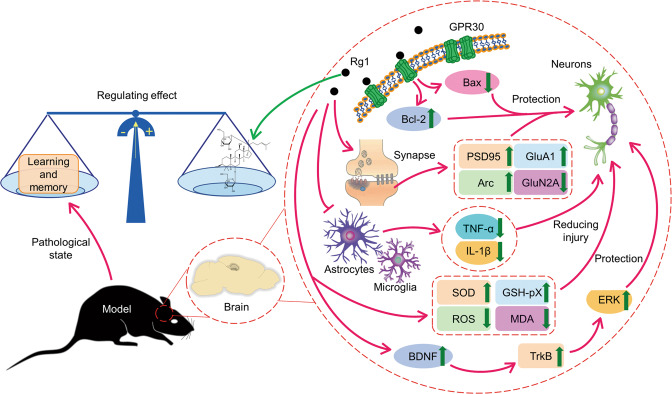
Fig. 1. The mechanism of antidepressant effect of Rg1.
Rg1 inhibits the hyperfunction of HPA axis, increases the expression of GR and decreases the level of CORT. Rg1 increases the expression of synaptic-related proteins in the hippocampus, cortex and amygdala and regulates synaptic plasticity. Rg1 protects the gap junction function of astrocytes and inhibits inflammation by increasing the level of Cx43. Rg1 can also inhibit the activation of Ly6Chi monocytes by CCL2-CCR2 interaction in inflammatory environment, destroy the inflammatory loop and reduce the level of pro-inflammatory factors. At the same time, Rg1 can also inhibit microglia activation and inflammation. Additionally, Rg1 can regulate the level of oxidative stress.
Rg1 inhibits the hyperfunction of HPA axis
As an important neuroendocrine system in the body, HPA axis is involved in the regulation of stress and emotional coordination [34]. Research has shown that the HPA axis feedback loop is closely related to the occurrence of depression, and affects the occurrence and development of depression by acting on the neuroendocrine system and the neuroimmune system [35]. Therefore, the effective regulation of HPA axis has become an important antidepressant stratagem.
Mou et al. used chronic unpredictable mild stress (CUMS) rats to evaluate the antidepressant effect of Rg1. The results showed that the CUMS rats showed obvious depression-like behavior, and the level of serum corticosterone (CORT) increased and the expression of glucocorticoid receptor decreased in the hippocampus. After oral administration of Rg1, the depression-like behaviors were alleviated, the level of serum CORT decreased and the expression of glucocorticoid receptor increased in CUMS rats [36]. Zheng et al. established a rat model of depression induced by lipopolysaccharide (LPS). Sucrose consumption test showed that the modeled rats showed depression-like behaviors, and the levels of IL-1β, IL-6, TNF-α and plasma CORT were significantly higher than those of the control rats. After intraperitoneal injection of Rg1, the depression-like behaviors of the modeled rats were significantly improved, and the contents of pro-inflammatory factors and plasma CORT were down-regulated [37]. Jiang et al. also found that Rg1 could reduce the abnormal increase of serum CORT in chronic mild stress (CMS) depressed mice [9]. This study shows that the mechanism of Rg1 in improving depression-like behavior is not only due to its anti-inflammatory effect, but also related to the fact that Rg1 can block neuroendocrine disorders. Based on those results, we believe that down-regulating the level of CORT in depression model, increasing the expression of glucocorticoid receptor protein and inhibiting the hyperfunction of HPA axis are the important mechanisms underlying antidepressant effect of Rg1.
Rg1 regulates synaptic plasticity
Depression-like behaviors are closely linked to interruption of key signaling pathways in synaptogenesis, and at the cellular and molecular levels, are also characterized by neuronal atrophy and a decrease in the number of synapses in the prefrontal cortex (PFC) or hippocampus [38, 39]. Rg1 has excellent neuroprotective effect and can theoretically prevent synaptic deficit during depression, which is also confirmed by experimental results.
Fan et al. found that CUMS rats exhibited depression-like behaviors, with reduced dendritic spine density and synapse numbers in the ventral medial prefrontal cortex (vmPFC) region, and decreased expression of cAMP response element-binding protein (CREB), BDNF, postsynaptic density protein 95 (PSD95), and synapsin. Intraperitoneal injection of Rg1 could ameliorate the depression-like behaviors and the decreased expression of synaptic-related proteins in CUMS rats [40]. Liu et al. also explored the antidepressant effects and neuroprotective mechanism of Rg1 in CUMS rat model. The results showed that CUMS rats had decreased number and density of synapses in basolateral amygdala (BLA) neurons, as well as low levels of protein kinase A (PKA), CREB and BDNF in the BLA. Five-week intraperitoneal injection of Rg1 improved the number and density of synapses of BLA neurons, and the expression of PKA, CREB and BDNF [41]. Similarly, Rg1 can also reverse the decrease of CREB and BDNF in the PFC of CUMS rats [42].
Rg1 has antidepressant-like effect not only in depressed rats, but also in depressed mice. In the study of the antidepressant effect of Rg1 on CMS mice, Jiang et al. found that CMS mice showed decrease in neurogenesis and dendritic spine density, and a low expression level of BDNF in the hippocampus. Rg1 can increase the density of dendritic spines and the expression of BDNF in the hippocampal neurons of CMS mice, which may be the mechanism of Rg1 improving depression-like behavior in CMS mice [9]. Our group also investigated the antidepressant effect of Rg1 in CUMS mice. The results showed that the expression of PSD95, activity-regulated cytoskeletal-associated protein (Arc) and BDNF in the hippocampus of CUMS mice was lower than that of control mice, while intraperitoneal injection of Rg1 could up-regulate the expression of synaptic-related proteins and improve the depression-like behavior of CUMS mice [43].
Rg1 prevents neuroinflammatory response
When neuroinflammation occurs, glial cells are activated and simultaneously release a series of inflammatory mediators such as cytokines and chemokines, which in turn lead to oxidative stress injury and damage to the CNS, ultimately resulting in depression [44]. Zheng et al. used LPS-induced neuroinflammation mouse model, chronic social defeat stress (CSDS)-induced depression mouse model and in vitro cellular experiment to evaluate the antidepressant effect of Rg1. It was found that peripheral Ly6Chi monocytes (also known as pro-inflammatory monocytes) in LPS-induced depression were activated and migrated into the inflamed brain. In vitro experiments demonstrated that Ly6Chi monocytes could promote astrocytes to secrete C-C motif chemokine ligand 2 (CCL2), a ligand driving the activation and migration of Ly6Chi monocytes, and CCL2 activates P38/MAPK and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathways (the key to controlling monocyte activation and migration), thus forming an inflammatory circuit. Interestingly, the accumulation of Ly6Chi monocytes was also found in the brain of CSDS mice. Intraperitoneal injection of Rg1 can inhibit the migration of peripheral Ly6Chi into the brain, thereby blocking the pro-inflammatory potential of astrocytes and improving depression-like behavior in mice [45]. In recent years, Chen et al. investigated the effects of gap junction of astrocytes on depression-like behaviors, and explored the improving effect and mechanism of Rg1 on depression in a variety of depressive animal models and in vitro cellular experiments. Studies have shown that the dysfunction of gap junction function of astrocytes in the cortex and hippocampus contributes to depression-like behaviors, and connexin 43 (Cx43) is the main protein that constitutes the gap junction of astrocytes, which is closely related to the occurrence of depression [46]. Multiple studies have revealed that Cx43 is involved in the antidepressant effect of Rg1. Rg1 up-regulates the expression of Cx43 mRNA, inhibits the ubiquitin-proteasome and autophagy-lysosome degradation pathway of Cx43, reduces the degradation of Cx43, then increases the level of Cx43, protects the gap junction function of astrocytes in the cortex and hippocampus, reduces neuronal inflammation, and exerts antidepressant effect [47–51].
Rg1 prevents oxidative stress
In addition, there were abnormal changes in cells and molecules in the brain during the occurrence of depression, the gene expression regulating oxidative stress changed, and the content of oxidative stress indicators increased significantly, which indicated that the level of oxidative stress was inseparable from the occurrence of depression [52]. Li et al. demonstrated that the content of oxidative stress products malondialdehyde (MDA) and nitric oxide (NO) in the hippocampal CA1 region of CUMS rats increased, the activities of antioxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase (GSH-pX) decreased, and the expression of nicotinamide adenine dinucleotide phosphate oxidase 1 (NOX1)/NOX4 in oxidative stress pathway was abnormal. Intraperitoneal injection of Rg1 could significantly adjust the levels of oxidative stress products and antioxidant enzymes, and inhibit the increase of NOX1 and NOX4 levels induced by chronic stress. It is suggested that the mechanism of antidepressant effect of Rg1 may be related to the reduction of oxidative stress injury [53].
Potential targets involved in the protection of Rg1 against depression
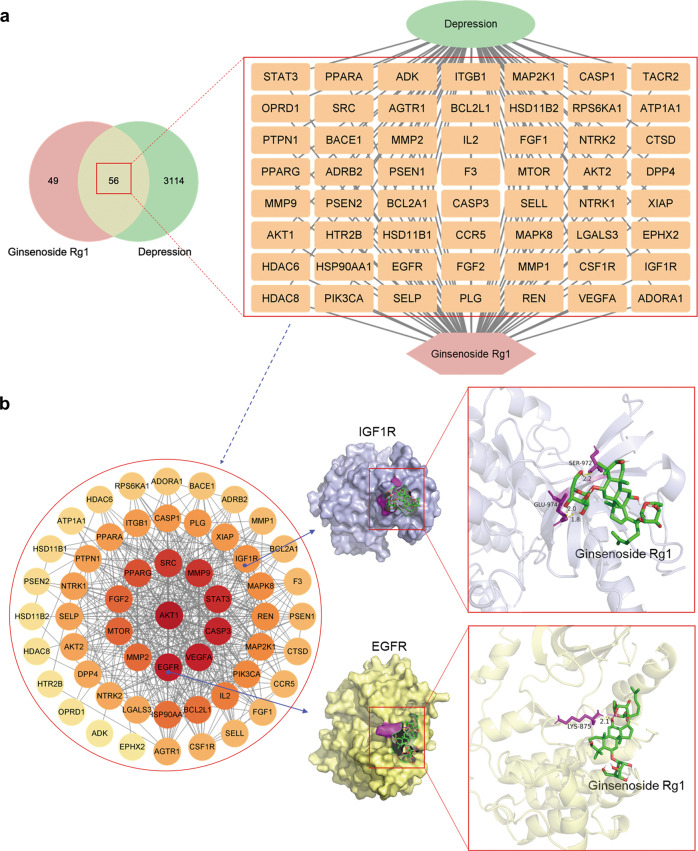
In addition to the above mechanism of action of Rg1 for depression, we screened the targets of Rg1 for depression by network pharmacology. Firstly, all probable action targets of Rg1 were obtained by SwissTargetPrediction (http://www.swisstargetprediction.ch/), and then the GeneCards database (https://www.genecards.org/) was used to screen (score >1) the relevant genes for depression. The obtained targets were imported into Cytoscape 3.8 software to make drug-targets-disease network and combined with String database (https://cn.string-db.org/) to complete protein-protein interaction (PPI) network. Finally, we selected targets that were both present in the PPI network and explored in the experimental evidence described above, and used AutoDockTools 1.5 and PyMOL software in combination with the PDB database (https://www.rcsb.org/) for molecular docking to verify the reliability of the targets obtained by network pharmacology. The results revealed 105 probable action targets of Rg1 and 3170 depression disease-related targets, including 56 intersecting targets. IGF1R (PDB ID: 2OJ9) and EGFR (PDB ID: 3POZ) were selected from the intersecting targets for molecular docking, and both showed high docking effects (Fig. 2).
Fig. 2. Potential targets and molecular docking of Rg1 against depression.
a Venn diagram and drug-targets-disease network of Rg1 action targets and depression-related genes. There are 105 Rg1 action targets and 3170 depression-related genes, 56 of which are common targets. b PPI network and molecular docking of IGF1R and EGFR. The PPI network contains 55 key targets. The binding energy of IGF1R and EGFR were −2.09 kcal/mol and −1.57 kcal/mol, respectively, indicating a good docking effect.
Rg1 and Alzheimer’s disease
Many kinds of ginsenosides have definite neuroprotective effects and have been used in the research of anti-AD, among which Rg1 is one of the most investigated ginsenosides [54]. Rg1 can reduce the activity of inflammatory factors induced by inflammasome activation and inhibit oxidative stress and apoptosis through anti-inflammatory and antioxidant effects. Rg1 improved AD-like symptoms by down-regulating the expression of beta-site APP cleaving enzyme 1 (BACE1) and APP, reducing the production of Aβ and increasing the level of insulin-degrading enzyme (IDE), accelerating the clearance of excess Aβ in the brain, and inhibiting the abnormal phosphorylation and deposition of Tau. In addition, Rg1 can regulate synaptic plasticity, reduce neuronal damage and apoptosis, and protect neurons, which is also helpful for AD treatment (Table 4, Fig. 3).
Table 4.
Main mechanism and effect sites of Rg1 on AD.
| Animal or cell line | Model | Main mechanism | Effect sites (receptor, cell, axon or dendrite) | Reference |
|---|---|---|---|---|
| Male APP/PS1 mice and wild type (WT) mice | APP/PS1 AD model | Inhibition of NOX2 expression and accumulation of ROS to alleviate cognitive impairment and Aβ production | Hippocampal and cortical neurons | [56] |
| Primary hippocampal neurons | Aβ25–35 treatment | Activation of AKT and ERK1/2 signaling pathways | Neurite in hippocampal neurons | [57] |
| Male SAMP8 mice and male SAMR1 mice | SAMP8 AD model | Repair of hippocampal neuronal cell loss and inhibition of astrocyte and microglia activation | Hippocampal neurons, microglia and astrocytes | [58] |
| Male tree shrews | Aβ25–35 treatment | Changing the abundance of gut microbiota | Proteobacteria and Verrucomicrobia | [66] |
| Male tree shrews | Aβ25–35 treatment | Inhibiting the expression of pro-apoptotic proteins, protecting hippocampal and cortical neurons, and reducing the abundance of Bacteroidetes | Hippocampal and cortical neurons and Bacteroidetes | [67] |
| HT22 cells | LPS treatment | Inhibition of NOX2-NLRP1 inflammasome protects against LPS-induced neuronal damage | HT22 cells | [72] |
| Primary hippocampal neurons | H2O2 treatment | Reducing NOX2-mediated ROS production, inhibiting NLRP1 inflammasome activation, and inhibiting neuronal aging and damage | Hippocampal neurons | [73] |
| Male ICR mice | Dexamethasone treatment | Protecting against neuroinflammation and neuronal damage from chronic glucocorticoid exposure and inhibiting the NLRP-1 inflammasome | Hippocampal and frontal cortex neurons | [74] |
| Neuroglial cell line NG108-15 | Aβ25–35 treatment | Inhibiting TLR3 and TLR4 signaling transduction pathways and reducing Aβ25-35-induced cellular inflammatory factors | Neuroglial cell | [75] |
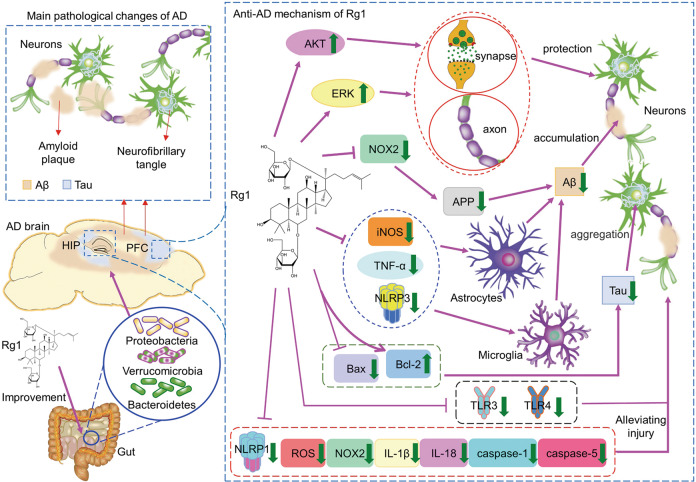
Fig. 3. The mechanisms involved in the effects of Rg1 against Alzheimer’s disease.
The main pathological changes of AD are amyloid plaques formed by Aβ aggregation and neurofibrillary tangles formed by abnormal aggregation of phosphorylated Tau. Rg1 alleviates amyloid plaques and neurofibrillary tangles by improving gut microbiota. Rg1 can improve AD by increasing synaptic protein expression, promoting axonal growth, inhibiting Aβ generation and abnormal increase in the phosphorylation of Tau, as well as improving oxidative stress and inflammatory response and protecting neurons.
Rg1 reduces Aβ accumulation in the brain
The Aβ protein deposited in the form of amyloid plaque in the hippocampus will recruit more Aβ protein to form insoluble aggregates, which will induce mitochondrial damage and lead to synaptic dysfunction [55]. In addition, Aβ aggregation can also activate microglia and astrocytes, induce neuroinflammation, and eventually lead to a series of pathological reactions such as neuronal dysfunction and apoptosis [55].
Zhang et al. explored the anti-AD effect and mechanism of Rg1 in amyloid precursor protein/presenilin 1 (APP/PS1) mice. The results suggest that long-term treatment with Rg1 can improve cognitive and behavioral impairment and neuronal damage in APP/PS1 AD mice, and reduce the expression of amyloid precursor protein (APP) and the production of Aβ. The main mechanism of this effect is that Rg1 inhibits the activity of NOX2 and reduces the damage of neurons, and finally reduces the production of Aβ in APP/PS1 mice [56]. Huang et al. treated primary rat hippocampal neurons with Aβ25–35 to study the effect and mechanism of Rg1 on hippocampal neurons after Aβ25–35 exposure. The results showed that Rg1 could promote the axonal growth of hippocampal neurons and reverse the apoptosis of hippocampal neurons induced by Aβ25–35. Interestingly, this effect of Rg1 can be blocked by API-2 (AKT inhibitor) and PD98059 (MAPK/ERK inhibitor), which means that Rg1 can increase the phosphorylation level of AKT and ERK [57]. The results suggest that Rg1 attenuates Aβ25–35-induced hippocampal neuronal injury and protects hippocampal neurons by activating AKT and ERK signaling pathways. Yang et al. evaluated the ameliorating effect of Rg1 on cognitive impairment in senescence accelerated mice P8 (SAMP8) mice. The results showed that Rg1 can enhance the spatial memory of model mice, and can regulate the nod-like-receptor-protein-3 (NLRP3) inflammasome, reduce the level of TNF-α and the expression of inducible nitric oxide synthase (iNOS), inhibit the activation of astrocytes and microglia, and reduce the accumulation of Aβ in the hippocampus, which may be an important mechanism for Rg1 to improve the cognitive impairment of AD [58]. Interestingly, cyclin-dependent kinase 5 (CDK5) mediated phosphorylation of peroxisome proliferator-activated receptor γ (PPARγ) at Ser273. Quan et al. found that Rg1 inhibited the expression of CDK5 and the level of p-PPARγ, thus decreasing the p-PPARγ/PPARγ ratio [59]. Moreover, the reduction of p-PPARγ could upregulate IDE and downregulate the level of BACE1 [59, 60]. Since BACE1 is an enzyme necessary for Aβ production by APP hydrolysis, and Aβ is considered to be the substrate of IDE [61]. Thus, these results demonstrated that Rg1 not only reduced BACE1 and prevented Aβ production, but also increased IDE to promote Aβ degradation, thus improving AD.
Rg1 reduces abnormal deposition and phosphorylation of Tau
Tau is a microtubule-associated protein involved in regulating the growth and transport of axons in the brain, helping to maintain the stability of axonal microtubules. Overexpression or abnormal phosphorylation of Tau can depolymerize microtubules and impair axonal transport, and phosphorylation and aggregation of Tau also make it the main component of neurofibrillary tangles. Pathological accumulation of Tau further leads to neuronal degeneration, resulting in neuronal apoptosis [62]. Clinical studies have found that the level of Tau in the brain of AD patients with cognitive impairment was higher than that of the normal population, and the level of Tau was correlated with the severity of cognitive impairment [63]. This indicates that Tau has been regarded as a pathological marker of AD.
In the experiment of Rg1 improving cognitive impairment and neuronal damage in APP/PS1 mice, Zhang et al. found that long-term oral administration of Rg1 not only improved the cognitive impairment of AD mice, but also decreased the level of p-Tau, blocked the production of ROS and the expression of NOX2 in the cortex and hippocampus, and increased the expression of PSD95 [56]. The mechanism of this effect is related to the inhibition of NOX2 activation by Rg1. In addition, overexpression of glycogen synthase kinase-3β could induce phosphorylation of Tau. In exploring the mechanism of Rg1 inhibiting the phosphorylation of Tau, Li et al. thought that Rg1 inhibited the phosphorylation of Tau and promoted the clearance of deposited Tau by up-regulating the expression of BDNF to inhibit glycogen synthase kinase-3β activity [64].
Rg1 adjusts the intestinal flora
The relationship between intestinal flora and AD is an interesting topic. Studies have revealed that intestinal flora could affect brain activity through the microbiota-gut-brain axis, and the imbalance of flora could cause brain dysfunction [65]. Wang et al. explored the mechanism of Rg1 improving AD cognitive impairment in AD tree shrews for the first time. The results showed that Rg1 could restore the cognitive function of AD tree shrews and reduce the expression of Tau in the hippocampus and cortex. Meanwhile, Rg1 significantly improved the intestinal flora abundance of AD tree shrews, and Proteobacteria and Verrucomicrobia were identified as two of them key flora. This study suggested that improving intestinal flora abundance may be an effective mechanism for Rg1 to treat AD [66]. Coincidentally, while exploring the improving effect and mechanism of Rg1 on AD tree shrews, Guo et al. found that Rg1 could reduce the levels of Aβ, pS404-Tau and Bcl2-associated X protein (Bax) in the hippocampus and cortex, up-regulate the expression of Bcl-2, inhibit β-secretase1 and increase the expression of microtubule-associated protein 2 (MAP2) and Fox-3. Meanwhile, Rg1 significantly reduced the abundance of Bacteroidetes in AD tree shrews. The findings revealed that Rg1 may inhibit apoptosis and protect neurons by decreasing the abundance of intestinal flora, ultimately improving AD [67].
Rg1 reduces neuroinflammation and oxidative stress
Clinical research has shown that in the early stage of AD, the increase of chitinase-3-likeprotein 1 (YKL-40) was related to the accumulation of Aβ and increased the risk of AD dementia, while the high expression of IL-15 aggravated the cognitive deterioration of AD [68]. IL-17 was involved in the short-term memory and dysfunction of synaptic plasticity in the early stage of the disease in 3xTg-AD mice [69]. Glutathione (GSH) is an important antioxidant in the body. Clinical evidence revealed that the decrease of GSH constituted a potential biomarker for mild cognitive impairment (MIC) and AD diagnosis [70]. It was suggested that neuroinflammation and oxidative stress might play a key role in the early stage of AD, and the brain damage in the development of AD was earlier than Aβ-induced neuronal loss [71]. Therefore, it is considered that AD can be improved by reducing neuroinflammation and oxidative stress, especially in the early stage of AD.
Zhang et al. exposed HT22 cells to LPS to mimic neuronal inflammatory damage in AD and observed the protective effect of Rg1. LPS could induce the activation of NOX2-NLRP1 inflammasome in HT22 cells, resulting in an inflammatory response, and at the same time, excessive ROS were generated and neurons were damaged. Rg1 has neuroprotective effects on LPS-exposed HT22 cells by inhibiting NOX2-NLRP1 inflammasome activation and reducing ROS generation, which may also be an important pathway for Rg1 to alleviate neuronal damage in AD [72]. Xu et al. established an AD model different from the above study by inducing primary rat hippocampal neurons with H2O2, and observed the protective effect on the injury of hippocampal neurons after treatment with Rg1. The results showed that after H2O2 induction, ROS increased in hippocampal neurons, the expressions of NOX2 and NLRP1 increased, and hippocampal neurons appeared senescent and were damaged. Rg1 blocked the up-regulation of H2O2 on the above indicators, and reduced the pathological changes of hippocampal neurons [73]. This study points out that Rg1 exerts a protective effect on hippocampal neurons by reducing NOX2-mediated ROS production and inhibiting NLRP1 inflammasome activation. Zhang et al. also demonstrated that Rg1 could protect neurons by inhibiting NLRP1 inflammasome and reducing the expression of apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC), caspase-1, caspase-5, IL-1β and IL-18 in the hippocampus of AD mice through the AD mouse model of neuronal inflammatory injury induced by dexamethasone [74]. Zhao et al. treated the Aβ25–35-induced glial cell line NG108–15 with Rg1. The results showed that Rg1 could down-regulate the increase of TNF-α in NG108–15 induced by Aβ25–35 and inhibit the expression of toll-like receptor 3 (TLR3) and TLR4 protein. It is suggested that the mechanism of Rg1 in the treatment of AD may be related to the inhibition of TLR3 and TLR4 signaling pathways and the decrease of the activity of inflammatory factors induced by Aβ25–35 [75]. In addition, Kwan et al. compared the antioxidant activities of Rb1, Rd, Re and Rg1, and concluded that Rg1 has the strongest antioxidant capacity, which also suggests that among many ginsenosides, Rg1 may be the most potential therapy for AD [76].
Potential targets involved in the protection of Rg1 against AD
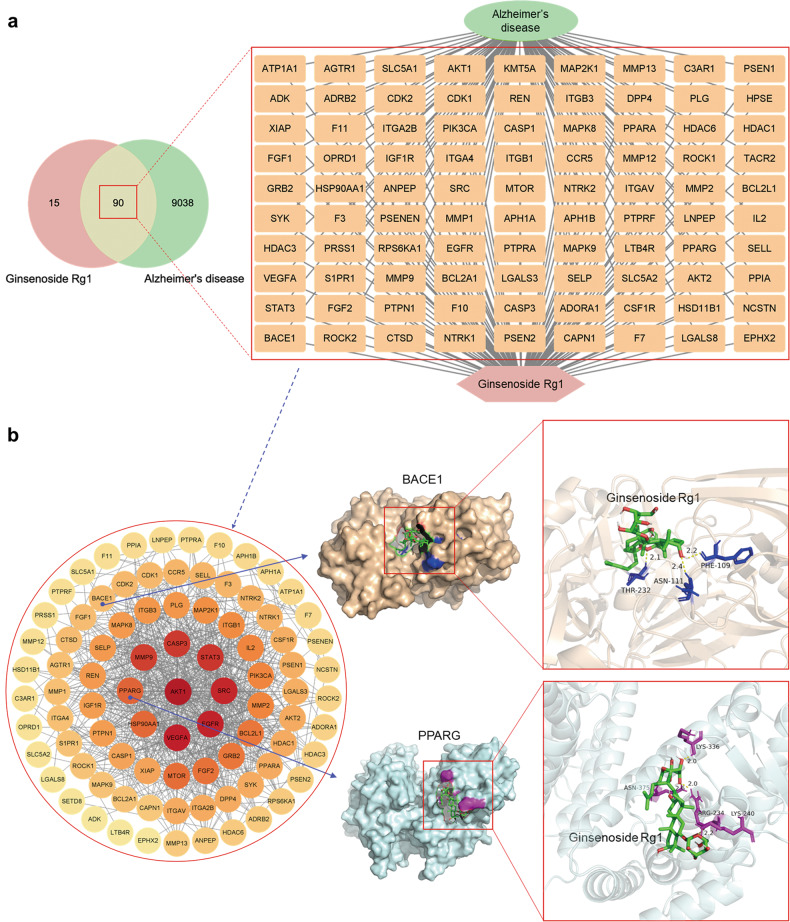
In order to obtain more information on the targets of Rg1 for AD, we performed a network pharmacology screen and molecular docking of targets from experimental evidence to show the reliability of the targets obtained by network pharmacology. The results revealed 105 probable action targets of Rg1 and 9128 AD disease-related targets, including 56 intersecting targets. BACE1 (PDB ID: 4B78) and PPARG (PDB ID: 2YFE) were selected from the intersecting targets for molecular docking, and both showed good docking effects (Fig. 4).
Fig. 4. Potential targets and molecular docking of Rg1 against AD.
a Venn diagram and drug-targets-disease network of Rg1 action targets and AD-related genes. There are 105 Rg1 action targets and 9128 AD-related genes, 90 of which are common targets. b PPI network and molecular docking of BACE1 and PPARG. The PPI network contains 88 key targets. The binding energy of BACE1 and PPARG were −1.93 kcal/mol and −1.91 kcal/mol, respectively, indicating a good docking effect.
Rg1 and learning and memory dysfunction
Besides AD, other types of dementia, especially vascular cognitive disorders were also featured by impairment of learning and memory [77]. Interestingly, our group has previously shown that Rg1 prevented the memory impairment caused by bilateral ligation of common carotid artery [78]. In addition, memory could be divided into declarative and non-declarative memory, and cue-based fear memory is one important type of non-declarative memory. Especially, over-activation of fear memory was related to development of psychiatric disorders, like depression and anxiety [79]. Therefore, we specifically discuss the role and mechanism of Rg1 on learning and memory dysfunction as an independent part of this review.
Our group established a mouse model of vascular dementia (VD) to explore the preventive effect of Rg1 on cognitive impairment. The results of Morris water maze showed that Rg1 could improve the spatial memory of model mice, and the effect was consistent with G1, a G protein-coupled estrogen receptor 1 (GPR30) agonist. In the study of drug mechanism, we confirmed that promoting the expression of GPR30 to prevent cognitive impairment in VD mice is a key way for Rg1 to play a role [78]. At the same time, we also found that the generalization of fear memory in PTSD model mice is severe and difficult to extinct, and administration of Rg1 can promote the extinction of fear memory [80]. In the Barnes maze test, Miao et al. pointed out that Isoflurane/surgery (I/S) could induce hippocampus-dependent learning and memory impairment in mice, increased the escape latency and error times of model mice in Barnes maze, while Rg1 improved the learning and memory impairment induced by I/S. Through the correlation analysis between behaviors and biochemical indexes, it was considered that the improving effect of Rg1 on learning and memory was related to the decrease of ROS level and up-regulation of oxygen consumption rate (OCR) [81]. Wang et al. used reward devaluation test and conditional visual discrimination task to evaluate the effect of Rg1 on cognitive impairment in chronic restraint stress (CRS) rats. The results showed that Rg1 had an improvement effect on the learning and memory of CRS rats. The mechanism of this improvement was related to the activation of BDNF/TrkB/ERK signaling pathway in the PFC by Rg1 [82]. In addition, study has also pointed out that the effect of Rg1 on learning and memory impairment is related to the cholinergic system [83].
In the above studies, no matter which model is used to simulate the learning and memory impairment, Rg1 has a good behavioral improvement effect, although the mechanism involved is different. However, it is not difficult to find that the improvement effect of Rg1 in these studies is still inseparable from its excellent neuroprotective effect, as well as anti-inflammatory and antioxidant functions. Based on the experimental evidences, it is sufficient to support its extensive and in-depth preclinical research in the field of neuropsychiatric disorders. Interestingly, memory is divided into various types (such as working memory and fear memory), and different diseases can induce the impairment of different types of memory. In PTSD, aversive memory is abnormally active and easily reactivate, which leads to the generalization of fear memory and fear extinction disorder [84–86]. In AD, the loss of working memory is the main factor leading to memory impairment and decreased decision-making ability [87, 88]. Therefore, Rg1 has a good effect on memory impairment in different pathological states. In addition, the previous research of our group showed that long-term administration of Rg1 can enhance the long-term memory ability of middle-aged mice under normal conditions [89]. This well explains part of the mechanism of ginseng’s nootropic effect (Table 5, Fig. 5).
Table 5.
Modulating effects, main mechanism and effect sites of Rg1 on learning and memory dysfunction.
| Animal or cell line | Model | Modulating effects on learning and memory | Main mechanism | Effect sites (receptor, cell, axon or dendrite) | Reference |
|---|---|---|---|---|---|
| Male C57BL/6 J mice | Bilateral common carotid artery stenosis (BCAS) model | Spatial memory (↑) | Promoting GPR30 expression to prevent cognitive impairment and hippocampal neuronal apoptosis in VD mice | GPR30 and hippocampal neurons | [78] |
| Male C57BL/6 J mice | Isoflurane plus abdominal surgery treatment | Spatial learning and memory (↑) | Improving learning and memory function, reducing ROS levels and ameliorating the OCR, mitochondrial membrane potential, expression and deacetylation activity of Sirtuin3 | Mitochondria | [81] |
| Male Wistar rats | CRS | Episodic memory (↑) | Activation of the BDNF/TrkB/ERK signaling pathway in the PFC | PFC | [82] |
| Male Wistar rats | LPS treatment | Working and spatial memory (↑) | Regulating acetylcholine levels, acetylcholinesterase activity, and expression of the alpha7 nicotinic acetylcholine receptor (α7 nAChR) | α7 nAChR in PFC and hippocampus | [83] |
| Male Kunming mice | D-galactose and AlCl3 induced aging | Spatial learning and memory (↑) | Up-regulating fibroblast growth factor 2 and BDNF levels, activating TrkB/AKT signaling pathway in hippocampus and prefrontal cortex to inhibit apoptosis | Hippocampus and PFC neurons | [194] |
| Female 3xTg-AD mice | AD model | Spatial learning and memory (↑) | Up-regulating the expression of complexin-2 (CPLX2), synapsin-2 (SYN2) and synaptosoma-associated protein 25 (SNP25) | Hippocampus | [195] |
| Male tree shrews | Aβ25-35 induced AD model | Spatial memory (↑) | Regulating Wnt/GSK-3β/β-catenin signaling pathway, reducing oxidative stress injury, improving neuroinflammation, and protecting neurons | Hippocampus | [196] |
| Male C57BL/6 J mice | Single-prolonged stress model | Fear memory (↓) | Promoting synaptic protein expression and reducing Kir4.1 and TNF-α in hippocampus | Astrocytes and microglia | [80] |
Fig. 5. Effects of Rg1 on learning and memory functions. Rg1 could regulate the learning and memory function in pathological state.
Rg1 protects neurons by activating GPR30, inhibiting apoptosis, increasing the expression of synapse-related proteins and regulating synaptic plasticity. Rg1 can inhibit the activation of astrocytes and microglia, reduce inflammatory factors, and reduce neuronal damage. Rg1 can also protect neurons by regulating oxidative stress and activating the BDNF/TrkB/ERK signaling pathway.
Rg1 has the potential for drug development, but the process faces challenges
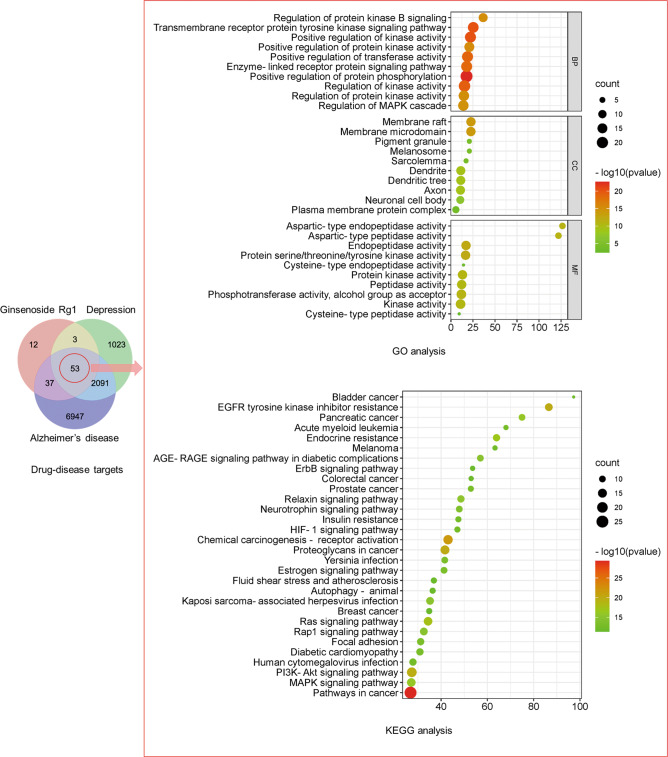
Moreover, we also obtained the “drug-disease” targets of Rg1 and depression and AD through network pharmacology, and did Gene Ontology (GO) (Top 10) and Kyoto Encyclopedia of Genes and Genomes (KEGG) (Top 30) analysis (http://metascape.org/), including neuronal cell body, dendrite, axon, and MAPK signaling pathway. This also confirmed the possible biological pathways of Rg1 in the treatment of depression and AD (Fig. 6). It also suggests that Rg1 has the potential to be developed as a promising drug.
Fig. 6. “Drug-disease” targets of Rg1, depression and AD, and GO (Top 10) and KEGG (Top 30) enrichment analysis of common targets.
The 53 targets include PIK3CA, SELP, STAT3, IL2, FGF2, BCL2L1, VEGFA, FGF1, ATP1A1, HSP90AA1, REN, TACR2, AKT2, RPS6KA1, AKT1, LGALS3, PSEN2, HSD11B1, OPRD1, IGF1R, EGFR, MMP1, MAPK8, MAP2K1, PTPN1, BCL2A1, MTOR, DPP4, EPHX2, ADK, XIAP, BACE1, CSF1R, ADRB2, PPARA, NTRK1, NTRK2, PLG, HDAC6, ITGB1, MMP9, F3, CASP3, PPARG, CTSD, MMP2, ADORA1, CCR5, SELL, CASP1, AGTR1, SRC, PSEN1. GO enrichment analysis includes biological processes (BP), cellular components (CC) and molecular functions (MF).
In addition to the above-mentioned effects of Rg1 on depression, AD, and learning and memory impairment, it has also been extensively investigated in other neuropsychiatric disorders, such as HD [90]. In addition, our group investigated the preventive and therapeutic effect of Rg1 on PTSD. The results showed that Rg1 could exert a protective effect on PTSD-like behaviors by inhibiting the expression of inflammatory factors and promoting synaptic proteins [80]. Findings such as those suggest that Rg1 has the potential to be developed into a neuropsychiatric drug. However, when we searched the Rg1 drug information on the official websites of the drug regulatory agencies of China (National Medical Products Administration), the United States (Food and Drug Administration), Republic of Korea (Ministry of Food and Drug Safety) and Japan (Pharmaceuticals and Medical Devices Agency), we found that only one Rg1 drug, Qishengli tablet (Z20027165) on the National Medical Products Administration official website of China. Therefore, we think, since Rg1 has a potential for prevention and treatment of various neuropsychiatric disorders, what are the factors that hinder the further development of Rg1 drugs? Based on the experimental research of Rg1, we propose that there are three reasons.
The mechanism of Rg1 still needs to be further explored
It is currently believed that the role of Rg1 in neuropsychiatric disorders is mainly related to anti-inflammatory, antioxidant and neuronal protection [91, 92]. However, the treatment of diseases by drugs is often based on the pathogenesis of existing diseases. With the in-depth study of the mechanism of disease, the mechanism of drug action should be updated rapidly. Taking AD research as an example, neurofibrillary tangles formed by the aggregation of Aβ and phosphorylated Tau are considered to be the main factor in AD pathogenesis. Studies have shown that transactive response DNA-binding protein of 43 kDa (TDP-43) (a DNA-binding protein predominantly found in the nucleus) and Aβ or Tau protein co-occur in neurons of neurofibrillary tangles through protein co-localization, and there is a direct interaction with Aβ or Tau protein, which is involved in Aβ fibrosis or the aggregation of Tau protein, and then aggravates AD-like pathology [93–95]. Clinical studies have found that TDP-43 deposition is common in patients with typical and limbic AD, accounting for 59% and 67%, respectively [96]. TDP-43 pathological changes were found in 49.4% of brain samples from autopsy of deceased aging and AD patients [97]. Therefore, TDP-43 is a factor that cannot be ignored in the pathogenic mechanism of AD. As mentioned earlier, Rg1 can reduce the production of Aβ, inhibit the aggregation of Tau protein and improve AD, which has been elucidated in many experimental studies, but there is no direct experimental evidence to support whether this mechanism is related to the regulation of TDP-43 expression. Therefore, we speculate that TDP-43 may also be an important target of Rg1, and may participate in the inhibition of Aβ and Tau protein phosphorylation through this target. In addition, regarding the effects of Rg1 on Aβ, most of the studies focused on the influence of Rg1 against Aβ production. Whether Rg1 could help or assist the clearance of Aβ was rarely investigated. This is also important point that needs to be improved in Rg1 studies. It also suggests that with the continuous study of the mechanism of AD, the prevention and treatment mechanism of Rg1 should be discussed more deeply. The limitation of exploring the mechanism of Rg1 is also applicable to other neuropsychiatric disorders.
Low blood-brain barrier (BBB) permeability and bioavailability of Rg1
The saponins in ginseng extract can be divided into three types: panaxadiol, panaxatriol and oleanolic acid. Rg1 belongs to the type of panaxatriol. Pharmacokinetic experiments showed that Rg1 could not effectively penetrate the BBB and distribute in the brain [98]. In addition, after oral administration, Rg1 and its metabolites in bile, urine and feces of rats were detected by LC-MS/MS. The results showed that the recovery rate of Rg1 and its metabolites was more than 70%, indicating that the bioavailability of Rg1 through oral administration was poor [99]. In pharmaceutical research, for drugs with strong pharmacological activity but low oral bioavailability, chemical structure modification and drug dosage form modification are usually selected to improve bioavailability [8]. For Rg1 in the prevention and treatment of neuropsychiatric disorders, it is equally important to increase BBB permeability and bioavailability.
In terms of increasing the permeability of the BBB, it has been reported that depending on the high expression of transferrin receptor (TfR) in the capillary endothelium of BBB, Rg1 nanoparticles targeting TfR are designed to increase brain exposure of Rg1 through endocytosis penetrating BBB, which has been effectively verified in vivo and in vitro [100]. It has to be mentioned that the design of such brain-targeted drugs is interesting, but the mode of administration used in the study is intravenous injection. We believe that such drug design is more beneficial for the treatment of acute neuropsychiatric disorders. By contrast, diseases like AD, have slow progress, long course and need continuous drug treatment. The design of oral drug preparation is in line with the characteristics of long-term drug treatment. In addition, the experimental results of Liang et al. suggest that extract of ginkgo biloba (EGb) can activate the A1 adenosine receptor signaling pathway and reversibly increase the permeability of BBB, and the use of EGb combined with Rg1 can increase the intracerebral uptake of Rg1 [101]. The design of such a combination drug may be a good research method for increasing brain distribution of Rg1. In terms of improving bioavailability, Baek et al. developed an oral formulation of ginsenoside (containing Rg1 or Rb1) microspheres composed of an enteric-coated polymer and an adhesive polymer. The results of in vitro experiments showed that the oral preparation of microspheres was resistant to acidity, and the release of ginsenosides in the microspheres decreased under the acidic condition of pH=1.2. This means that the degradation of ginsenosides is reduced in the acidic environment of the stomach [102]. Although there are individual novel basic researches on improving the BBB permeability and bioavailability of Rg1, the transformation of Rg1 into a drug is still a long way off.
Insufficient clinical research on Rg1
We searched the information on clinical trials carried out by Rg1 in China, Republic of Korea, Japan, the National Institutes of Health (NIH) of the United States and the WHO international clinical trial registration platform, but did not retrieve the clinical trial information of Rg1 as a single component drug. Rg3, Rd and Re, which are also ginsenosides, all have clinical trial information about the use of single-component drugs to treat diseases including hepatocellular carcinoma, ischemic stroke and diabetes. Additionally, we found that clinical trials of Rg1 in combination with other ginsenosides such as Rb1 and Re have been conducted in vascular dementia and ischemic stroke. It is worth noting that among all ginsenosides, Rg3 is one of the most widely used agents in clinical research and proprietary medicine. At present, the China National Medical Products Administration has approved Rg3 single-component Shenyi capsule (Z20030044) and Rg3 active pharmaceutical ingredient (Z20030043).
In pharmacological study, the effectiveness of Rg1 has been confirmed, and toxicological experiments also indicate that Rg1 has no adverse reactions and potential toxicity [103]. This shows that Rg1 has the characteristics of safety and efficacy of drugs, and meets the basic conditions for entering into clinical trials as therapeutic drugs. Rg1 can fully refer to proprietary drug model of Rg3, that is, focused drug research in the scope of treatment. Although Rg3 has many pharmacological effects, in the research of proprietary medicine, it only focuses on the adjuvant therapy of tumors. Therefore, we believe that Rg1, as a single-component drug to quickly enter clinical research and promote proprietary medicine, should also focus on the therapeutic effect of a single disease. From the results of searching “ginsenoside Rg1” and “ginsenoside Rg3” in PubMed database, the number of experimental studies related to Rg1 is much higher than that of Rg3, so we have more reason to believe that Rg1 has the potential to become a clinical drug.
Prospects for the future development direction of Rg1
Based on the above discussions, we believe that Rg1 needs to carry out more researches in the following aspects in the future. First of all, the exploration of the mechanism of neuropsychiatric disorders by Rg1 should be more in-depth, not in a simple exploration of the anti-inflammatory, antioxidant and neuroprotective effects, but should be combined with the deeper mechanism of neuropsychiatric disorders to trace the target of Rg1. The protective effect of Rg1 on brain neurons has been validated by experiments, and it is also a research fact that it cannot pass through BBB effectively, so how can Rg1 exert its protective effect on brain neurons without passing through BBB? Previous studies have confirmed that Rg1 can improve brain injury through peripheral effects, but there is few similar study [104]. Therefore, the in-depth study of the mechanism is the main direction of future research on Rg1. Secondly, Rg1 is a natural product extracted from ginseng, which has defects in chemical structure. The strong hydrophilicity of Rg1 may be an important reason why it is difficult to pass through the BBB, and the strength of Rg1’s protective effect on brain neurons is related to whether it can pass through the BBB to achieve effective brain exposure [105]. At present, there are only a few drug dosage forms designed by Rg1 in improving the permeability of BBB. We believe that the modification of chemical structure is also the key to solve the problem. The acquisition of a Rg1 modification molecule that can effectively penetrate into the brain means that ordinary drug dosage forms may also complete the task of Rg1 delivery into the brain. Therefore, the modification of the chemical structure of Rg1 is crucial to the exertion of drug action. Finally, the clinical research data of Rg1 should be completed and improved as soon as possible. Several preclinical studies have been done on Rg1, the effects of the drug have been scientifically explained, and clinical research is imminent. From the development experience of Rg3, the research mode focusing on a single disease may be the right choice for the further development of Rg1.
In summary, Rg1 is a natural product that integrates antioxidant, anti-inflammatory and neuroprotective effects, and its effectiveness on neuropsychiatric disorders has been confirmed by a large number of experimental studies, indicating that Rg1 has good application prospects in the future. However, the difficulties and challenges faced by the experiment also imply that there are still limitations in the current researches. Based on the coexistence of development potential and research challenges of Rg1, we prefer to call Rg1 an urgently developed drug for neuropsychiatric disorders. The transition between Rg1 in current research and Rg1 in future applications requires more systematic exploration. Of course, we hope to call Rg1 a widely used clinical drug for neuropsychiatric disorders as soon as possible.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (81673716, 82004481), Anhui Natural Science Foundation (2208085MH282), University Excellent Top Talent Cultivation Foundation of Anhui Province (gxgnfx2020089) and Key Research and Development Plan of Anhui Province (202104j07020004).
Competing interests
The authors declare no competing interests.
Contributor Information
Jing-ji Wang, Email: wjjglacial@163.com.
Guo-qi Zhu, Email: guoqizhu@gmail.com.
References
- 1.Sanacora G, Yan Z, Popoli M. The stressed synapse 2.0: Pathophysiological mechanisms in stress-related neuropsychiatric disorders. Nat Rev Neurosci. 2022;23:86–103. doi: 10.1038/s41583-021-00540-x. [DOI] [PubMed] [Google Scholar]
- 2.Dorsey ER, Sherer T, Okun MS, Bloem BR. The emerging evidence of the Parkinson pandemic. J Parkinsons Dis. 2018;8:S3–8. doi: 10.3233/JPD-181474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Group GBDNDC Global, regional, and national burden of neurological disorders during 1990-2015: A systematic analysis for the global burden of disease study 2015. Lancet Neurol. 2017;16:877–97. doi: 10.1016/S1474-4422(17)30299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarron RM, Shapiro B, Rawles J, Luo J. Depression. Ann Intern Med. 2021;174:ITC65–ITC80. doi: 10.7326/AITC202105180. [DOI] [PubMed] [Google Scholar]
- 5.Yang HS. Human genetics clarifies the relationship between depression and Alzheimer’s disease. Biol Psychiatry. 2022;92:2–4. doi: 10.1016/j.biopsych.2022.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Zhang C, Wang L, Xu Y, Huang Y, Huang J, Zhu J, et al. Discovery of novel dual RAGE/SERT inhibitors for the potential treatment of the comorbidity of Alzheimer’s disease and depression. Eur J Med Chem. 2022;236:114347. doi: 10.1016/j.ejmech.2022.114347. [DOI] [PubMed] [Google Scholar]
- 7.Cummings J, Feldman HH, Scheltens P. The “rights” of precision drug development for Alzheimer’s disease. Alzheimers Res Ther. 2019;11:76. doi: 10.1186/s13195-019-0529-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang S, Zhu G. 7,8-dihydroxyflavone and neuropsychiatric disorders: A translational perspective from the mechanism to drug development. Curr Neuropharmacol. 2022;20:1479–97. doi: 10.2174/1570159X19666210915122820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang B, Xiong Z, Yang J, Wang W, Wang Y, Hu ZL, et al. Antidepressant-like effects of ginsenoside rg1 are due to activation of the bdnf signalling pathway and neurogenesis in the hippocampus. Br J Pharmacol. 2012;166:1872–87. doi: 10.1111/j.1476-5381.2012.01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi YS. New mechanisms of ginseng saponin-mediated anti-inflammatory action via targeting canonical inflammasome signaling pathways. J Ethnopharmacol. 2021;278:114292. doi: 10.1016/j.jep.2021.114292. [DOI] [PubMed] [Google Scholar]
- 11.Ai PH, Chen S, Liu XD, Zhu XN, Pan YB, Feng DF, et al. Paroxetine ameliorates prodromal emotional dysfunction and late-onset memory deficit in Alzheimer’s disease mice. Transl Neurodegener. 2020;9:18. doi: 10.1186/s40035-020-00194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawakami I, Iga JI, Takahashi S, Lin YT, Fujishiro H. Towards an understanding of the pathological basis of senile depression and incident dementia: Implications for treatment. Psychiatry Clin Neurosci. 2022 doi: 10.1111/pcn.13485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juszczyk G, Mikulska J, Kasperek K, Pietrzak D, Mrozek W, Herbet M. Chronic stress and oxidative stress as common factors of the pathogenesis of depression and alzheimer’s disease: The role of antioxidants in prevention and treatment. Antioxidants (Basel) 2021;10:1439. doi: 10.3390/antiox10091439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linnemann C, Lang UE. Pathways connecting late-life depression and dementia. Front Pharmacol. 2020;11:279. doi: 10.3389/fphar.2020.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, Sui C, Wang W, Yan J, Deng N, Du X, et al. Baicalin attenuates oxygen-glucose deprivation/reoxygenation-induced injury by modulating the BDNF-TRKB/PI3K/AKT and MAPK/ERK1/2 signaling axes in neuron-astrocyte cocultures. Front Pharmacol. 2021;12:599543. doi: 10.3389/fphar.2021.599543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang ZH, Xiang J, Liu X, Yu SP, Manfredsson FP, Sandoval IM, et al. Deficiency in BDNF/TrkB neurotrophic activity stimulates delta-secretase by upregulating C/EBPbeta in Alzheimer’s disease. Cell Rep. 2019;28:655–69.e5. doi: 10.1016/j.celrep.2019.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim JY, Reighard CP, Crowther DC. The pro-domains of neurotrophins, including BDNF, are linked to Alzheimer’s disease through a toxic synergy with abeta. Hum Mol Genet. 2015;24:3929–38. doi: 10.1093/hmg/ddv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan W, Xu DW, Ji CH, Wang CN, Liu Y, Tang WQ, et al. Hippocampal miR-206-3p participates in the pathogenesis of depression via regulating the expression of BDNF. Pharmacol Res. 2021;174:105932. doi: 10.1016/j.phrs.2021.105932. [DOI] [PubMed] [Google Scholar]
- 19.Fani G, Mannini B, Vecchi G, Cascella R, Cecchi C, Dobson CM, et al. Abeta oligomers dysregulate calcium homeostasis by mechanosensitive activation of AMPA and NMDA receptors. ACS Chem Neurosci. 2021;12:766–81. doi: 10.1021/acschemneuro.0c00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyamoto T, Stein L, Thomas R, Djukic B, Taneja P, Knox J, et al. Phosphorylation of tau at Y18, but not tau-fyn binding, is required for Tau to modulate nmda receptor-dependent excitotoxicity in primary neuronal culture. Mol Neurodegener. 2017;12:41. doi: 10.1186/s13024-017-0176-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song Z, Bian Z, Zhang Z, Wang X, Zhu A, Zhu G. Astrocytic kir4.1 regulates nmdar/calpain signaling axis in lipopolysaccharide-induced depression-like behaviors in mice. Toxicol Appl Pharmacol. 2021;429:115711. doi: 10.1016/j.taap.2021.115711. [DOI] [PubMed] [Google Scholar]
- 22.Arimon M, Takeda S, Post KL, Svirsky S, Hyman BT, Berezovska O. Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiol Dis. 2015;84:109–19. doi: 10.1016/j.nbd.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pena-Bautista C, Tirle T, Lopez-Nogueroles M, Vento M, Baquero M, Chafer-Pericas C. Oxidative damage of DNA as early marker of Alzheimer’s disease. Int J Mol Sci. 2019;20:6136. doi: 10.3390/ijms20246136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahmoud AM, Alexander MY, Tutar Y, Wilkinson FL, Venditti A. Oxidative stress in metabolic disorders and drug-induced injury: The potential role of Nrf2 and PPARs activators. Oxid Med Cell Longev. 2017;2017:2508909. doi: 10.1155/2017/2508909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song L, Wu X, Wang J, Guan Y, Zhang Y, Gong M, et al. Antidepressant effect of catalpol on corticosterone-induced depressive-like behavior involves the inhibition of HPA axis hyperactivity, central inflammation and oxidative damage probably via dual regulation of NF-kappab and Nrf2. Brain Res Bull. 2021;177:81–91. doi: 10.1016/j.brainresbull.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Diniz BS, Mendes-Silva AP, Silva LB, Bertola L, Vieira MC, Ferreira JD, et al. Oxidative stress markers imbalance in late-life depression. J Psychiatr Res. 2018;102:29–33. doi: 10.1016/j.jpsychires.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Shen F, Song Z, Xie P, Li L, Wang B, Peng D, et al. Polygonatum sibiricum polysaccharide prevents depression-like behaviors by reducing oxidative stress, inflammation, and cellular and synaptic damage. J Ethnopharmacol. 2021;275:114164. doi: 10.1016/j.jep.2021.114164. [DOI] [PubMed] [Google Scholar]
- 28.Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chetelat G, Teunissen CE, et al. Alzheimer’s disease. Lancet. 2021;397:1577–90. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Gao F, Xu W, Cao Y, Wang J, Zhu G. Depichering the effects of Astragaloside IV on AD-like phenotypes: A systematic and experimental investigation. Oxid Med Cell Longev. 2021;2021:1020614. doi: 10.1155/2021/1020614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Min W, Liu C, Yang Y, Sun X, Zhang B, Xu L, et al. Alterations in hypothalamic-pituitary-adrenal/thyroid (HPA/HPT) axes correlated with the clinical manifestations of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:206–11. doi: 10.1016/j.pnpbp.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Song Z, Shen F, Zhang Z, Wu S, Zhu G. Calpain inhibition ameliorates depression-like behaviors by reducing inflammation and promoting synaptic protein expression in the hippocampus. Neuropharmacology. 2020;174:108175. doi: 10.1016/j.neuropharm.2020.108175. [DOI] [PubMed] [Google Scholar]
- 32.Mahar I, Bambico FR, Mechawar N, Nobrega JNStress. serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev. 2014;38:173–92. doi: 10.1016/j.neubiorev.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Kim Y, Cho SH. The effect of ginsenosides on depression in preclinical studies: A systematic review and meta-analysis. J Ginseng Res. 2021;45:420–32. doi: 10.1016/j.jgr.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Connor DB, Thayer JF, Vedhara K. Stress and health: A review of psychobiological processes. Annu Rev Psychol. 2021;72:663–88. doi: 10.1146/annurev-psych-062520-122331. [DOI] [PubMed] [Google Scholar]
- 35.Dwyer JB, Aftab A, Radhakrishnan R, Widge A, Rodriguez CI, Carpenter LL, et al. Hormonal treatments for major depressive disorder: State of the art. Am J Psychiatry. 2020;177:686–705. doi: 10.1176/appi.ajp.2020.19080848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mou Z, Huang Q, Chu SF, Zhang MJ, Hu JF, Chen NH, et al. Antidepressive effects of ginsenoside Rg1 via regulation of HPA and HPG axis. Biomed Pharmacother. 2017;92:962–71. doi: 10.1016/j.biopha.2017.05.119. [DOI] [PubMed] [Google Scholar]
- 37.Zheng X, Liang Y, Kang A, Ma SJ, Xing L, Zhou YY, et al. Peripheral immunomodulation with ginsenoside Rg1 ameliorates neuroinflammation-induced behavioral deficits in rats. Neuroscience. 2014;256:210–22. doi: 10.1016/j.neuroscience.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 38.Wang YT, Wang XL, Feng ST, Chen NH, Wang ZZ, Zhang Y. Novel rapid-acting glutamatergic modulators: Targeting the synaptic plasticity in depression. Pharmacol Res. 2021;171:105761. doi: 10.1016/j.phrs.2021.105761. [DOI] [PubMed] [Google Scholar]
- 39.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–49. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan C, Song Q, Wang P, Li Y, Yang M, Yu SY. Neuroprotective effects of ginsenoside-Rg1 against depression-like behaviors via suppressing glial activation, synaptic deficits, and neuronal apoptosis in rats. Front Immunol. 2018;9:2889. doi: 10.3389/fimmu.2018.02889. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Liu Z, Qi Y, Cheng Z, Zhu X, Fan C, Yu SY. The effects of ginsenoside Rg1 on chronic stress induced depression-like behaviors, BDNF expression and the phosphorylation of PKA and CREB in rats. Neuroscience. 2016;322:358–69. doi: 10.1016/j.neuroscience.2016.02.050. [DOI] [PubMed] [Google Scholar]
- 42.Zhu X, Gao R, Liu Z, Cheng Z, Qi Y, Fan C, et al. Ginsenoside Rg1 reverses stress-induced depression-like behaviours and brain-derived neurotrophic factor expression within the prefrontal cortex. Eur J Neurosci. 2016;44:1878–85. doi: 10.1111/ejn.13255. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Shen F, Zhang Z, Zhu G. Effects of ginsenoside Rg1 on depression-like behaviors, expression of hippocampal synaptic proteins and activation of glial cells in stressed mice. J Biol. 2021;38:26–30. [Google Scholar]
- 44.Kaufmann FN, Menard C. Inflamed astrocytes: A path to depression led by menin. Neuron. 2018;100:511–3. doi: 10.1016/j.neuron.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 45.Zheng X, Ma S, Kang A, Wu M, Wang L, Wang Q, et al. Chemical dampening of Ly6C(hi) monocytes in the periphery produces anti-depressant effects in mice. Sci Rep. 2016;6:19406. doi: 10.1038/srep19406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin C, Wang ZZ, Zhou H, Lou YX, Chen J, Zuo W, et al. Ginsenoside Rg1-induced antidepressant effects involve the protection of astrocyte gap junctions within the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2017;75:183–91. doi: 10.1016/j.pnpbp.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Yang Y, Yang S, Ren S, Feng J, Liu Y, et al. Ginsenoside Rg1 ameliorates neuroinflammation via suppression of connexin43 ubiquitination to attenuate depression. Front Pharmacol. 2021;12:709019. doi: 10.3389/fphar.2021.709019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia CY, Chu SF, Zhang S, Gao Y, Ren Q, Lou YX, et al. Ginsenoside Rg1 alleviates corticosterone-induced dysfunction of gap junctions in astrocytes. J Ethnopharmacol. 2017;208:207–13. doi: 10.1016/j.jep.2017.06.031. [DOI] [PubMed] [Google Scholar]
- 49.Lou YX, Wang ZZ, Xia CY, Mou Z, Ren Q, Liu DD, et al. The protective effect of ginsenoside Rg1 on depression may benefit from the gap junction function in hippocampal astrocytes. Eur J Pharmacol. 2020;882:173309. doi: 10.1016/j.ejphar.2020.173309. [DOI] [PubMed] [Google Scholar]
- 50.Xia CY, Wang ZZ, Wang HQ, Ren SY, Lou YX, Jin C, et al. Connexin 43: A novel ginsenoside Rg1-sensitive target in a rat model of depression. Neuropharmacology. 2020;170:108041. doi: 10.1016/j.neuropharm.2020.108041. [DOI] [PubMed] [Google Scholar]
- 51.Wang HQ, Yang SW, Gao Y, Liu YJ, Li X, Ai QD, et al. Novel antidepressant mechanism of ginsenoside Rg1: Regulating biosynthesis and degradation of connexin43. J Ethnopharmacol. 2021;278:114212. doi: 10.1016/j.jep.2021.114212. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Z, Deng T, Wu M, Zhu A, Zhu G. Botanicals as modulators of depression and mechanisms involved. Chin Med. 2019;14:24. doi: 10.1186/s13020-019-0246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y, Wang L, Wang P, Fan C, Zhang P, Shen J, et al. Ginsenoside-Rg1 rescues stress-induced depression-like behaviors via suppression of oxidative stress and neural inflammation in rats. Oxid Med Cell Longev. 2020;2020:2325391. doi: 10.1155/2020/2325391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li J, Huang Q, Chen J, Qi H, Liu J, Chen Z, et al. Neuroprotective potentials of panax ginseng against Alzheimer’s disease: A review of preclinical and clinical evidences. Front Pharmacol. 2021;12:688490. doi: 10.3389/fphar.2021.688490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hampel H, Hardy J, Blennow K, Chen C, Perry G, Kim SH, et al. The amyloid-beta pathway in Alzheimer’s disease. Mol Psychiatry. 2021;26:5481–503. doi: 10.1038/s41380-021-01249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H, Su Y, Sun Z, Chen M, Han Y, Li Y, et al. Ginsenoside Rg1 alleviates Abeta deposition by inhibiting NADPH oxidase 2 activation in App/PS1 mice. J Ginseng Res. 2021;45:665–75. doi: 10.1016/j.jgr.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang L, Liu LF, Liu J, Dou L, Wang GY, Liu XQ, et al. Ginsenoside Rg1 protects against neurodegeneration by inducing neurite outgrowth in cultured hippocampal neurons. Neural Regen Res. 2016;11:319–25. doi: 10.4103/1673-5374.177741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Y, Li S, Huang H, Lv J, Chen S, Pires Dias AC, et al. Comparison of the protective effects of ginsenosides Rb1 and Rg1 on improving cognitive deficits in SAMP8 mice based on anti-neuroinflammation mechanism. Front Pharmacol. 2020;11:834. doi: 10.3389/fphar.2020.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quan Q, Li X, Feng J, Hou J, Li M, Zhang B. Ginsenoside Rg1 reduces β amyloid levels by inhibiting CDK5 induced PPARγ phosphorylation in a neuron model of Alzheimer’s disease. Mol Med Rep. 2020;22:3277–88. doi: 10.3892/mmr.2020.11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quan Q, Wang J, Li X, Wang Y. Ginsenoside rg1 decreases Abeta(1-42) level by upregulating PPARgamma and IDE expression in the hippocampus of a rat model of Alzheimer’s disease. PLoS One. 2013;8:e59155. doi: 10.1371/journal.pone.0059155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sahoo BR, Panda PK, Liang W, Tang WJ, Ahuja R, Ramamoorthy A. Degradation of Alzheimer’s amyloid-beta by a catalytically inactive insulin-degrading enzyme. J Mol Biol. 2021;433:166993. doi: 10.1016/j.jmb.2021.166993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wegmann S, Biernat J, Mandelkow E. A current view on tau protein phosphorylation in Alzheimer’s disease. Curr Opin Neurobiol. 2021;69:131–8. doi: 10.1016/j.conb.2021.03.003. [DOI] [PubMed] [Google Scholar]
- 63.Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, et al. Tau and abeta imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med. 2016;8:338ra66. doi: 10.1126/scitranslmed.aaf2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X, Li M, Li Y, Quan Q, Wang J. Cellular and molecular mechanisms underlying the action of ginsenoside Rg1 against Alzheimer’s disease. Neural Regen Res. 2012;7:2860–6. doi: 10.3969/j.issn.1673-5374.2012.36.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Angelucci F, Cechova K, Amlerova J, Hort J. Antibiotics, gut microbiota, and Alzheimer’s disease. J Neuroinflammation. 2019;16:108. doi: 10.1186/s12974-019-1494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang L, Lu J, Zeng Y, Guo Y, Wu C, Zhao H, et al. Improving Alzheimer’s disease by altering gut microbiota in tree shrews with ginsenoside Rg1. FEMS Microbiol Lett. 2020;367:fnaa011. doi: 10.1093/femsle/fnaa011. [DOI] [PubMed] [Google Scholar]
- 67.Guo Y, Wang L, Lu J, Jiao J, Yang Y, Zhao H, et al. Ginsenoside Rg1 improves cognitive capability and affects the microbiota of large intestine of tree shrew model for Alzheimer’s disease. Mol Med Rep. 2021;23:291. doi: 10.3892/mmr.2021.11931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Janelidze S, Mattsson N, Stomrud E, Lindberg O, Palmqvist S, Zetterberg H, et al. CSF biomarkers of neuroinflammation and cerebrovascular dysfunction in early Alzheimer disease. Neurology. 2018;91:e867–77. doi: 10.1212/WNL.0000000000006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brigas HC, Ribeiro M, Coelho JE, Gomes R, Gomez-Murcia V, Carvalho K, et al. IL-17 triggers the onset of cognitive and synaptic deficits in early stages of Alzheimer’s disease. Cell Rep. 2021;36:109574. doi: 10.1016/j.celrep.2021.109574. [DOI] [PubMed] [Google Scholar]
- 70.Shukla D, Mandal PK, Tripathi M, Vishwakarma G, Mishra R, Sandal K. Quantitation of in vivo brain glutathione conformers in cingulate cortex among age-matched control, MCI, and AD patients using MEGA-PRESS. Hum Brain Mapp. 2020;41:194–217. doi: 10.1002/hbm.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song T, Song X, Zhu C, Patrick R, Skurla M, Santangelo I, et al. Mitochondrial dysfunction, oxidative stress, neuroinflammation, and metabolic alterations in the progression of Alzheimer’s disease: A meta-analysis of in vivo magnetic resonance spectroscopy studies. Ageing Res Rev. 2021;72:101503. doi: 10.1016/j.arr.2021.101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Ding S, Chen Y, Sun Z, Zhang J, Han Y, et al. Ginsenoside Rg1 alleviates lipopolysaccharide-induced neuronal damage by inhibiting NLRP1 inflammasomes in HT22 cells. Exp Ther Med. 2021;22:782. doi: 10.3892/etm.2021.10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu TZ, Shen XY, Sun LL, Chen YL, Zhang BQ, Huang DK, et al. Ginsenoside Rg1 protects against H2 O2 induced neuronal damage due to inhibition of the NLRP1 inflammasome signalling pathway in hippocampal neurons in vitro. Int J Mol Med. 2019;43:717–26. doi: 10.3892/ijmm.2018.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Hu W, Zhang B, Yin Y, Zhang J, Huang D, et al. Ginsenoside Rg1 protects against neuronal degeneration induced by chronic dexamethasone treatment by inhibiting NLRP-1 inflammasomes in mice. Int J Mol Med. 2017;40:1134–42. doi: 10.3892/ijmm.2017.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao BS, Liu Y, Gao XY, Zhai HQ, Guo JY, Wang XY. Effects of ginsenoside Rg1 on the expression of toll-like receptor 3, 4 and their signalling transduction factors in the NG108-15 murine neuroglial cell line. Molecules. 2014;19:16925–36. doi: 10.3390/molecules191016925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kwan KKL, Yun H, Dong TTX, Tsim KWK. Ginsenosides attenuate bioenergetics and morphology of mitochondria in cultured PC12 cells under the insult of amyloid beta-peptide. J Ginseng Res. 2021;45:473–81. doi: 10.1016/j.jgr.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Du K, Yang S, Wang J, Zhu G. Acupuncture interventions for Alzheimer’s disease and vascular cognitive disorders: A review of mechanisms. Oxid Med Cell Longev. 2022;2022:6080282. doi: 10.1155/2022/6080282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen F, Wang J, Gao F, Wang J, Zhu G. Ginsenoside Rg1 prevents cognitive impairment and hippocampal neuronal apoptosis in experimental vascular dementia mice by promoting GPR30 expression. Neural Plast. 2021;2021:2412220. doi: 10.1155/2021/2412220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maddox SA, Hartmann J, Ross RA, Ressler KJ. Deconstructing the gestalt: Mechanisms of fear, threat, and trauma memory encoding. Neuron. 2019;102:60–74. doi: 10.1016/j.neuron.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Z, Song Z, Shen F, Xie P, Wang J, Zhu AS, et al. Ginsenoside Rg1 prevents ptsd-like behaviors in mice through promoting synaptic proteins, reducing Kir4.1 and TNF-alpha in the hippocampus. Mol Neurobiol. 2021;58:1550–63. doi: 10.1007/s12035-020-02213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miao HH, Wang M, Wang HX, Tian M, Xue FS. Ginsenoside Rg1 attenuates isoflurane/surgery-induced cognitive disorders and sirtuin 3 dysfunction. Biosci Rep. 2019;39:BSR20190069. doi: 10.1042/BSR20190069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kezhu W, Pan X, Cong L, Liming D, Beiyue Z, Jingwei L, et al. Effects of ginsenoside Rg1 on learning and memory in a reward-directed instrumental conditioning task in chronic restraint stressed rats. Phytother Res. 2017;31:81–9. doi: 10.1002/ptr.5733. [DOI] [PubMed] [Google Scholar]
- 83.Jin Y, Peng J, Wang X, Zhang D, Wang T. Ameliorative effect of ginsenoside Rg1 on lipopolysaccharide-induced cognitive impairment: Role of cholinergic system. Neurochem Res. 2017;42:1299–307. doi: 10.1007/s11064-016-2171-y. [DOI] [PubMed] [Google Scholar]
- 84.Rasmusson AM, Pineles SL, Brown KD, Pinna G. A role for deficits in GABAergic neurosteroids and their metabolites with NMDA receptor antagonist activity in the pathophysiology of posttraumatic stress disorder. J Neuroendocrinol. 2022;34:e13062. doi: 10.1111/jne.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raymundi AM, da Silva TR, Sohn JMB, Bertoglio LJ, Stern CA. Effects of (9)-tetrahydrocannabinol on aversive memories and anxiety: A review from human studies. BMC Psychiatry. 2020;20:420. doi: 10.1186/s12888-020-02813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang J, Gao F, Cui S, Yang S, Gao F, Wang X, et al. Utility of 7,8-dihydroxyflavone in preventing astrocytic and synaptic deficits in the hippocampus elicited by ptsd. Pharmacol Res. 2022;176:106079. doi: 10.1016/j.phrs.2022.106079. [DOI] [PubMed] [Google Scholar]
- 87.Gaubert F, Chainay H. Decision-making competence in patients with Alzheimer’s disease: A review of the literature. Neuropsychol Rev. 2021;31:267–87. doi: 10.1007/s11065-020-09472-2. [DOI] [PubMed] [Google Scholar]
- 88.Robbins M, Clayton E, Kaminski, Schierle GS. Synaptic tau: A pathological or physiological phenomenon? Acta Neuropathol Commun. 2021;9:149. doi: 10.1186/s40478-021-01246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu G, Wang Y, Li J, Wang J. Chronic treatment with ginsenoside Rg1 promotes memory and hippocampal long-term potentiation in middle-aged mice. Neuroscience. 2015;292:81–9. doi: 10.1016/j.neuroscience.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 90.Yang X, Chu SF, Wang ZZ, Li FF, Yuan YH, Chen NH. Ginsenoside Rg1 exerts neuroprotective effects in 3-nitropronpionic acid-induced mouse model of Huntington’s disease via suppressing MAPKs and NF-kappaB pathways in the striatum. Acta Pharmacol Sin. 2021;42:1409–21. doi: 10.1038/s41401-020-00558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao Y, Li J, Wang J, Li X, Li J, Chu S, et al. Ginsenoside Rg1 prevent and treat inflammatory diseases: A review. Int Immunopharmacol. 2020;87:106805. doi: 10.1016/j.intimp.2020.106805. [DOI] [PubMed] [Google Scholar]
- 92.Cheng Y, Shen LH, Zhang JT. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin. 2005;26:143–9. doi: 10.1111/j.1745-7254.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- 93.Shih YH, Tu LH, Chang TY, Ganesan K, Chang WW, Chang PS, et al. Tdp-43 interacts with amyloid-beta, inhibits fibrillization, and worsens pathology in a model of Alzheimer’s disease. Nat Commun. 2020;11:5950. doi: 10.1038/s41467-020-19786-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Montalbano M, McAllen S, Cascio FL, Sengupta U, Garcia S, Bhatt N, et al. Tdp-43 and tau oligomers in Alzheimer’s disease, amyotrophic lateral sclerosis, and frontotemporal dementia. Neurobiol Dis. 2020;146:105130. doi: 10.1016/j.nbd.2020.105130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meneses A, Koga S, O’Leary J, Dickson DW, Bu G, Zhao N. Tdp-43 pathology in Alzheimer’s disease. Mol Neurodegener. 2021;16:84. doi: 10.1186/s13024-021-00503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Josephs KA, Whitwell JL, Tosakulwong N, Weigand SD, Murray ME, Liesinger AM, et al. Tar DNA-binding protein 43 and pathological subtype of Alzheimer’s disease impact clinical features. Ann Neurol. 2015;78:697–709. doi: 10.1002/ana.24493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nag S, Yu L, Boyle PA, Leurgans SE, Bennett DA, Schneider JA. Tdp-43 pathology in anterior temporal pole cortex in aging and Alzheimer’s disease. Acta Neuropathol Commun. 2018;6:33. doi: 10.1186/s40478-018-0531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin M, Sun W, Gong W, Ding Y, Zhuang Y, Hou Q. Ginsenoside Rg1 protects against transient focal cerebral ischemic injury and suppresses its systemic metabolic changes in cerabral injury rats. Acta Pharm Sin B. 2015;5:277–84. doi: 10.1016/j.apsb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.He C, Feng R, Sun Y, Chu S, Chen J, Ma C, et al. Simultaneous quantification of ginsenoside Rg1 and its metabolites by HPLC-MS/MS: Rg1 excretion in rat bile, urine and feces. Acta Pharm Sin B. 2016;6:593–9. doi: 10.1016/j.apsb.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shen J, Zhao Z, Shang W, Liu C, Zhang B, Zhao L, et al. Ginsenoside Rg1 nanoparticle penetrating the blood-brain barrier to improve the cerebral function of diabetic rats complicated with cerebral infarction. Int J Nanomed. 2017;12:6477–86. doi: 10.2147/IJN.S139602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liang W, Xu W, Zhu J, Zhu Y, Gu Q, Li Y, et al. Ginkgo biloba extract improves brain uptake of ginsenosides by increasing blood-brain barrier permeability via activating A1 adenosine receptor signaling pathway. J Ethnopharmacol. 2020;246:112243. doi: 10.1016/j.jep.2019.112243. [DOI] [PubMed] [Google Scholar]
- 102.Baek JS, Yeon WG, Lee CA, Hwang SJ, Park JS, Kim DC, et al. Preparation and characterization of mucoadhesive enteric-coating ginsenoside-loaded microparticles. Arch Pharm Res. 2015;38:761–8. doi: 10.1007/s12272-014-0395-4. [DOI] [PubMed] [Google Scholar]
- 103.Park SJ, Lim KH, Noh JH, Jeong EJ, Kim YS, Han BC, et al. Subacute oral toxicity study of korean red ginseng extract in sprague-dawley rats. Toxicol Res. 2013;29:285–92. doi: 10.5487/TR.2013.29.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhai K, Duan H, Wang W, Zhao S, Khan GJ, Wang M, et al. Ginsenoside Rg1 ameliorates blood-brain barrier disruption and traumatic brain injury via attenuating macrophages derived exosomes miR-21 release. Acta Pharm Sin B. 2021;11:3493–507. doi: 10.1016/j.apsb.2021.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martinelli C, Pucci C, Battaglini M, Marino A, Ciofani G. Antioxidants and nanotechnology: Promises and limits of potentially disruptive approaches in the treatment of central nervous system diseases. Adv Health Mater. 2020;9:e1901589. doi: 10.1002/adhm.201901589. [DOI] [PubMed] [Google Scholar]
- 106.Liu J, Nile SH, Xu G, Wang Y, Kai G. Systematic exploration of Astragalus membranaceus and Panax ginseng as immune regulators: Insights from the comparative biological and computational analysis. Phytomedicine. 2021;86:153077. doi: 10.1016/j.phymed.2019.153077. [DOI] [PubMed] [Google Scholar]
- 107.Olaleye OE, Niu W, Du FF, Wang FQ, Xu F, Pintusophon S, et al. Multiple circulating saponins from intravenous ShenMai inhibit OATP1Bs in vitro: Potential joint precipitants of drug interactions. Acta Pharmacol Sin. 2019;40:833–49. doi: 10.1038/s41401-018-0173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang X, Wang L, Zhang Z, Hu J, Liu X, Wen H, et al. Ginsenoside Rb1 enhances plaque stability and inhibits adventitial vasa vasorum via the modulation of miR-33 and PEDF. Front Cardiovasc Med. 2021;8:654670. doi: 10.3389/fcvm.2021.654670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang M, Wang R, Sun H, Sun G, Sun X. Ginsenoside Rb1 ameliorates cardiotoxicity triggered by aconitine via inhibiting calcium overload and pyroptosis. Phytomedicine. 2021;83:153468. doi: 10.1016/j.phymed.2021.153468. [DOI] [PubMed] [Google Scholar]
- 110.Jiang L, Yin X, Chen YH, Chen Y, Jiang W, Zheng H, et al. Proteomic analysis reveals ginsenoside Rb1 attenuates myocardial ischemia/reperfusion injury through inhibiting ROS production from mitochondrial complex I. Theranostics. 2021;11:1703–20. doi: 10.7150/thno.43895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yu S, Xia H, Guo Y, Qian X, Zou X, Yang H, et al. Ginsenoside Rb1 retards aging process by regulating cell cycle, apoptotic pathway and metabolism of aging mice. J Ethnopharmacol. 2020;255:112746. doi: 10.1016/j.jep.2020.112746. [DOI] [PubMed] [Google Scholar]
- 112.Yang X, Dong B, An L, Zhang Q, Chen Y, Wang H, et al. Ginsenoside Rb1 ameliorates glycemic disorder in mice with high fat diet-induced obesity via regulating gut microbiota and amino acid metabolism. Front Pharmacol. 2021;12:756491. doi: 10.3389/fphar.2021.756491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiang N, Huang H, Zhang Y, Lv J, Wang Q, He Q, et al. Ginsenoside Rb1 produces antidepressant-like effects in a chronic social defeat stress model of depression through the BDNF-Trkb signaling pathway. Front Pharmacol. 2021;12:680903. doi: 10.3389/fphar.2021.680903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lai Y, Tan Q, Xv S, Huang S, Wang Y, Li Y, et al. Ginsenoside Rb1 alleviates alcohol-induced liver injury by inhibiting steatosis, oxidative stress, and inflammation. Front Pharmacol. 2021;12:616409. doi: 10.3389/fphar.2021.616409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen H, Shen J, Li H, Zheng X, Kang D, Xu Y, et al. Ginsenoside Rb1 exerts neuroprotective effects through regulation of lactobacillus helveticus abundance and GABAA receptor expression. J Ginseng Res. 2020;44:86–95. doi: 10.1016/j.jgr.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li DW, Zhou FZ, Sun XC, Li SC, Yang JB, Sun HH, et al. Ginsenoside Rb1 protects dopaminergic neurons from inflammatory injury induced by intranigral lipopolysaccharide injection. Neural Regen Res. 2019;14:1814–22. doi: 10.4103/1673-5374.257536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shi YH, Li Y, Wang Y, Xu Z, Fu H, Zheng GQ. Ginsenoside-Rb1 for ischemic stroke: A systematic review and meta-analysis of preclinical evidence and possible mechanisms. Front Pharmacol. 2020;11:285. doi: 10.3389/fphar.2020.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zuo X, Li Q, Ya F, Ma LJ, Tian Z, Zhao M, et al. Ginsenosides Rb2 and Rd2 isolated from panax notoginseng flowers attenuate platelet function through P2Y12-mediated cAMP/PKA and PI3K/AKT/ERK1/2 signaling. Food Funct. 2021;12:5793–805. doi: 10.1039/D1FO00531F. [DOI] [PubMed] [Google Scholar]
- 119.Choi RJ, Mohamad Zobir SZ, Alexander-Dann B, Sharma N, Ma MKL, Lam BYH, et al. Combination of ginsenosides Rb2 and Rg3 promotes angiogenic phenotype of human endothelial cells via PI3K/AKT and MAPK/ERK pathways. Front Pharmacol. 2021;12:618773. doi: 10.3389/fphar.2021.618773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim DH, Kim DW, Jung BH, Lee JH, Lee H, Hwang GS, et al. Ginsenoside Rb2 suppresses the glutamate-mediated oxidative stress and neuronal cell death in HT22 cells. J Ginseng Res. 2019;43:326–34. doi: 10.1016/j.jgr.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Phi LTH, Wijaya YT, Sari IN, Yang YG, Lee YK, Kwon HY. The anti-metastatic effect of ginsenoside Rb2 in colorectal cancer in an EGFR/SOX2-dependent manner. Cancer Med. 2018;7:5621–31. doi: 10.1002/cam4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang Q, Wang T, Wang HY. Ginsenoside Rb2 enhances the anti-inflammatory effect of omega-3 fatty acid in LPS-stimulated RAW264.7 macrophages by upregulating GPR120 expression. Acta Pharmacol Sin. 2017;38:192–200. doi: 10.1038/aps.2016.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dai S, Hong Y, Xu J, Lin Y, Si Q, Gu X. Ginsenoside Rb2 promotes glucose metabolism and attenuates fat accumulation via AKT-dependent mechanisms. Biomed Pharmacother. 2018;100:93–100. doi: 10.1016/j.biopha.2018.01.111. [DOI] [PubMed] [Google Scholar]
- 124.Li H, Cui L, Liu Q, Dou S, Wang W, Xie M, et al. Ginsenoside Rb3 alleviates CSE-induced TROP2 upregulation through p38 MAPK and NF-kappaB pathways in basal cells. Am J Respir Cell Mol Biol. 2021;64:747–59. doi: 10.1165/rcmb.2020-0208OC. [DOI] [PubMed] [Google Scholar]
- 125.Xing JJ, Hou JG, Ma ZN, Wang Z, Ren S, Wang YP, et al. Ginsenoside Rb3 provides protective effects against cisplatininduced nephrotoxicity via regulation of AMPK-/mTOR-mediated autophagy and inhibition of apoptosis in vitro and in vivo. Cell Prolif. 2019;52:e12627. doi: 10.1111/cpr.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang Y, Ji H, Qiao O, Li Z, Pecoraro L, Zhang X, et al. Nanoparticle conjugation of ginsenoside Rb3 inhibits myocardial fibrosis by regulating pparalpha pathway. Biomed Pharmacother. 2021;139:111630. doi: 10.1016/j.biopha.2021.111630. [DOI] [PubMed] [Google Scholar]
- 127.Yu T, Yang Y, Kwak YS, Song GG, Kim MY, Rhee MH, et al. Ginsenoside Rc from Panax ginseng exerts anti-inflammatory activity by targeting TANK-binding kinase 1/interferon regulatory factor-3 and p38/ATF-2. J Ginseng Res. 2017;41:127–33. doi: 10.1016/j.jgr.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huang Q, Su H, Qi B, Wang Y, Yan K, Wang X, et al. A SIRT1 activator, ginsenoside Rc, promotes energy metabolism in cardiomyocytes and neurons. J Am Chem Soc. 2021;143:1416–27. doi: 10.1021/jacs.0c10836. [DOI] [PubMed] [Google Scholar]
- 129.Yang B, Wang R, Ji LL, Li XP, Li XH, Zhou HG, et al. Exploration of the function of ginsenoside Rd attenuates lipopolysaccharide-induced lung injury: A study of network pharmacology and experimental validation. Shock. 2022;57:212–20. doi: 10.1097/SHK.0000000000001824. [DOI] [PubMed] [Google Scholar]
- 130.Ren K, Li S, Ding J, Zhao S, Liang S, Cao X, et al. Ginsenoside Rd attenuates mouse experimental autoimmune neuritis by modulating monocyte subsets conversion. Biomed Pharmacother. 2021;138:111489. doi: 10.1016/j.biopha.2021.111489. [DOI] [PubMed] [Google Scholar]
- 131.Zhang B, Hu X, Wang H, Wang R, Sun Z, Tan X, et al. Effects of a dammarane-type saponin, ginsenoside Rd, in nicotine-induced vascular endothelial injury. Phytomedicine. 2020;79:153325. doi: 10.1016/j.phymed.2020.153325. [DOI] [PubMed] [Google Scholar]
- 132.Chen XM, Ji SF, Liu YH, Xue XM, Xu J, Gu ZH, et al. Ginsenoside Rd ameliorates auditory cortex injury associated with military aviation noise-induced hearing loss by activating SIRT1/PGC-1alpha signaling pathway. Front Physiol. 2020;11:788. doi: 10.3389/fphys.2020.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Han SK, Joo MK, Kim JK, Jeung W, Kang H, Kim DH. Bifidobacteria-fermented red ginseng and its constituents ginsenoside rd and protopanaxatriol alleviate anxiety/depression in mice by the amelioration of gut dysbiosis. Nutrients. 2020;12:901. doi: 10.3390/nu12040901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yao L, Han Z, Zhao G, Xiao Y, Zhou X, Dai R, et al. Ginsenoside Rd ameliorates high fat diet-induced obesity by enhancing adaptive thermogenesis in a cAMP-dependent manner. Obesity (Silver Spring) 2020;28:783–92. doi: 10.1002/oby.22761. [DOI] [PubMed] [Google Scholar]
- 135.Gao C, Zhang K, Liang F, Ma W, Jiang X, Wang H, et al. Inhibition of the Ras/ERK1/2 pathway contributes to the protective effect of ginsenoside Re against intimal hyperplasia. Food Funct. 2021;12:6755–65. doi: 10.1039/D1FO00015B. [DOI] [PubMed] [Google Scholar]
- 136.Wang H, Lv J, Jiang N, Huang H, Wang Q, Liu X. Ginsenoside Re protects against chronic restraint stress-induced cognitive deficits through regulation of NLRP3 and Nrf2 pathways in mice. Phytother Res. 2021 doi: 10.1002/ptr.6947. [DOI] [PubMed] [Google Scholar]
- 137.Sun H, Ling S, Zhao D, Li J, Li Y, Qu H, et al. Ginsenoside Re treatment attenuates myocardial hypoxia/reoxygenation injury by inhibiting HIF-1alpha ubiquitination. Front Pharmacol. 2020;11:532041. doi: 10.3389/fphar.2020.532041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim JM, Park CH, Park SK, Seung TW, Kang JY, Ha JS, et al. Ginsenoside Re ameliorates brain insulin resistance and cognitive dysfunction in high fat diet-induced C57BL/6 mice. J Agric Food Chem. 2017;65:2719–29. doi: 10.1021/acs.jafc.7b00297. [DOI] [PubMed] [Google Scholar]
- 139.Lee HR, Jung JM, Seo JY, Chang SE, Song Y. Anti-melanogenic property of ginsenoside Rf from panax ginseng via inhibition of CREB/MITF pathway in melanocytes and ex vivo human skin. J Ginseng Res. 2021;45:555–64. doi: 10.1016/j.jgr.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim Y, Lee HY, Choi YJ, Cho SH. Antidepressant effects of ginsenoside Rf on behavioral change in the glial degeneration model of depression by reversing glial loss. J Ginseng Res. 2020;44:603–10. doi: 10.1016/j.jgr.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Song H, Park J, Choi K, Lee J, Chen J, Park HJ, et al. Ginsenoside Rf inhibits cyclooxygenase-2 induction via peroxisome proliferator-activated receptor gamma in A549 cells. J Ginseng Res. 2019;43:319–25. doi: 10.1016/j.jgr.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim MK, Kang H, Baek CW, Jung YH, Woo YC, Choi GJ, et al. Antinociceptive and anti-inflammatory effects of ginsenoside Rf in a rat model of incisional pain. J Ginseng Res. 2018;42:183–91. doi: 10.1016/j.jgr.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lu ML, Wang J, Sun Y, Li C, Sun TR, Hou XW, et al. Ginsenoside Rg1 attenuates mechanical stress-induced cardiac injury via calcium sensing receptor-related pathway. J Ginseng Res. 2021;45:683–94. doi: 10.1016/j.jgr.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jin J, Zhong Y, Long J, Wu T, Jiang Q, Wang H, et al. Ginsenoside Rg1 relieves experimental colitis by regulating balanced differentiation of Tfh/Treg cells. Int Immunopharmacol. 2021;100:108133. doi: 10.1016/j.intimp.2021.108133. [DOI] [PubMed] [Google Scholar]
- 145.Wang Z, Wang L, Jiang R, Li C, Chen X, Xiao H, et al. Ginsenoside Rg1 prevents bone marrow mesenchymal stem cell senescence via Nrf2 and PI3K/AKT signaling. Free Radic Biol Med. 2021;174:182–94. doi: 10.1016/j.freeradbiomed.2021.08.007. [DOI] [PubMed] [Google Scholar]
- 146.Xu X, Qu Z, Qian H, Li Z, Sun X, Zhao X, et al. Ginsenoside Rg1 ameliorates reproductive function injury in C57BL/6J mice induced by di-N-butyl-phthalate. Environ Toxicol. 2021;36:789–99. doi: 10.1002/tox.23081. [DOI] [PubMed] [Google Scholar]
- 147.Huang L, Peng Z, Lu C, Chen Y, Lv JW, Qin M, et al. Ginsenoside Rg1 alleviates repeated alcohol exposure-induced psychomotor and cognitive deficits. Chin Med. 2020;15:44. doi: 10.1186/s13020-020-00325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu JQ, Zhao M, Zhang Z, Cui LY, Zhou X, Zhang W, et al. Rg1 improves lps-induced parkinsonian symptoms in mice via inhibition of NF-kappab signaling and modulation of M1/M2 polarization. Acta Pharmacol Sin. 2020;41:523–34. doi: 10.1038/s41401-020-0358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shen X, Dong X, Han Y, Li Y, Ding S, Zhang H, et al. Ginsenoside Rg1 ameliorates glomerular fibrosis during kidney aging by inhibiting NOX4 and NLRP3 inflammasome activation in SAMP8 mice. Int Immunopharmacol. 2020;82:106339. doi: 10.1016/j.intimp.2020.106339. [DOI] [PubMed] [Google Scholar]
- 150.Xu YP, Cui XY, Liu YT, Cui SY, Zhang YH. Ginsenoside Rg1 promotes sleep in rats by modulating the noradrenergic system in the locus coeruleus and serotonergic system in the dorsal raphe nucleus. Biomed Pharmacother. 2019;116:109009. doi: 10.1016/j.biopha.2019.109009. [DOI] [PubMed] [Google Scholar]
- 151.Liu Q, Zhang FG, Zhang WS, Pan A, Yang YL, Liu JF, et al. Ginsenoside Rg1 inhibits glucagon-induced hepatic gluconeogenesis through Akt-FoxO1 interaction. Theranostics. 2017;7:4001–12. doi: 10.7150/thno.18788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lin J, Huang HF, Yang SK, Duan J, Qu SM, Yuan B, et al. The effect of ginsenoside Rg1 in hepatic ischemia reperfusion (I/R) injury ameliorates ischemia-reperfusion-induced liver injury by inhibiting apoptosis. Biomed Pharmacother. 2020;129:110398. doi: 10.1016/j.biopha.2020.110398. [DOI] [PubMed] [Google Scholar]
- 153.Jeon H, Huynh DTN, Baek N, Nguyen TLL, Heo KS. Ginsenoside-Rg2 affects cell growth via regulating ROS-mediated AMPK activation and cell cycle in MCF-7 cells. Phytomedicine. 2021;85:153549. doi: 10.1016/j.phymed.2021.153549. [DOI] [PubMed] [Google Scholar]
- 154.Cui J, Shan R, Cao Y, Zhou Y, Liu C, Fan Y. Protective effects of ginsenoside Rg2 against memory impairment and neuronal death induced by Abeta25-35 in rats. J Ethnopharmacol. 2021;266:113466. doi: 10.1016/j.jep.2020.113466. [DOI] [PubMed] [Google Scholar]
- 155.Gou D, Pei X, Wang J, Wang Y, Hu C, Song C, et al. Antiarrhythmic effects of ginsenoside Rg2 on calcium chloride-induced arrhythmias without oral toxicity. J Ginseng Res. 2020;44:717–24. doi: 10.1016/j.jgr.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cheng B, Gao W, Wu X, Zheng M, Yu Y, Song C, et al. Ginsenoside Rg2 ameliorates high-fat diet-induced metabolic disease through Sirt1. J Agric Food Chem. 2020;68:4215–26. doi: 10.1021/acs.jafc.0c00833. [DOI] [PubMed] [Google Scholar]
- 157.Lai Q, Liu FM, Rao WL, Yuan GY, Fan ZY, Zhang L, et al. Aminoacylase-1 plays a key role in myocardial fibrosis and the therapeutic effects of 20(S)-ginsenoside Rg3 in mouse heart failure. Acta Pharmacol Sin. 2022;43:2003–15. doi: 10.1038/s41401-021-00830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Han NR, Ko SG, Moon PD, Park HJ. Ginsenoside Rg3 attenuates skin disorders via down-regulation of MDM2/HIF1alpha signaling pathway. J Ginseng Res. 2021;45:610–6. doi: 10.1016/j.jgr.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Song M, Cui Y, Wang Q, Zhang X, Zhang J, Liu M, et al. Ginsenoside Rg3 alleviates aluminum chloride-induced bone impairment in rats by activating the TGF-beta1/Smad signaling pathway. J Agric Food Chem. 2021;69:12634–44. doi: 10.1021/acs.jafc.1c04695. [DOI] [PubMed] [Google Scholar]
- 160.Kim D, Yang KE, Kim DW, Hwang HY, Kim J, Choi JS, et al. Activation of Ca2+-ampk-mediated autophagy by ginsenoside Rg3 attenuates cellular senescence in human dermal fibroblasts. Clin Transl Med. 2021;11:e521. doi: 10.1002/ctm2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nakhjavani M, Smith E, Yeo K, Palethorpe HM, Tomita Y, Price TJ, et al. Anti-angiogenic properties of ginsenoside Rg3 epimers: In vitro assessment of single and combination treatments. Cancers (Basel) 2021;13:2223. doi: 10.3390/cancers13092223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Geng J, Fu W, Yu X, Lu Z, Liu Y, Sun M, et al. Ginsenoside Rg3 alleviates ox-LDL induced endothelial dysfunction and prevents atherosclerosis in ApoE-/- mice by regulating PPARgamma/FAK signaling pathway. Front Pharmacol. 2020;11:500. doi: 10.3389/fphar.2020.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shi Y, Wang H, Zheng M, Xu W, Yang Y, Shi F. Ginsenoside Rg3 suppresses the NLRP3 inflammasome activation through inhibition of its assembly. FASEB J. 2020;34:208–21. doi: 10.1096/fj.201901537R. [DOI] [PubMed] [Google Scholar]
- 164.Ren Z, Chen X, Hong L, Zhao X, Cui G, Li A, et al. Nanoparticle conjugation of ginsenoside Rg3 inhibits hepatocellular carcinoma development and metastasis. Small. 2020;16:e1905233. doi: 10.1002/smll.201905233. [DOI] [PubMed] [Google Scholar]
- 165.Zhang K, Liu Y, Wang C, Li J, Xiong L, Wang Z, et al. Evaluation of the gastroprotective effects of 20 (S)-ginsenoside Rg3 on gastric ulcer models in mice. J Ginseng Res. 2019;43:550–61. doi: 10.1016/j.jgr.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Su WY, Li Y, Chen X, Li X, Wei H, Liu Z, et al. Ginsenoside Rh1 improves type 2 diabetic nephropathy through AMPK/PI3K/AKT-mediated inflammation and apoptosis signaling pathway. Am J Chin Med. 2021;49:1215–33. doi: 10.1142/S0192415X21500580. [DOI] [PubMed] [Google Scholar]
- 167.Lu C, Shi Z, Dong L, Lv J, Xu P, Li Y, et al. Exploring the effect of ginsenoside Rh1 in a sleep deprivation-induced mouse memory impairment model. Phytother Res. 2017;31:763–70. doi: 10.1002/ptr.5797. [DOI] [PubMed] [Google Scholar]
- 168.Chen C, Wang YS, Zhang ET, Li GA, Liu WY, Li Y, et al. (20S) ginsenoside Rh2 exerts its anti-tumor effect by disrupting the HSP90A-Cdc37 system in human liver cancer cells. Int J Mol Sci. 2021;22:13170. doi: 10.3390/ijms222313170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen XY, Qian F, Wang YY, Liu Y, Sun Y, Zha WB, et al. Ginsenoside 20(S)-Rh2 promotes cellular pharmacokinetics and intracellular antibacterial activity of levofloxacin against staphylococcus aureus through drug efflux inhibition and subcellular stabilization. Acta Pharmacol Sin. 2021;42:1930–41. doi: 10.1038/s41401-021-00751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen X, Xu T, Lv X, Zhang J, Liu S. Ginsenoside Rh2 alleviates ulcerative colitis by regulating the STAT3/miR-214 signaling pathway. J Ethnopharmacol. 2021;274:113997. doi: 10.1016/j.jep.2021.113997. [DOI] [PubMed] [Google Scholar]
- 171.Lv J, Lu C, Jiang N, Wang H, Huang H, Chen Y, et al. Protective effect of ginsenoside Rh2 on scopolamine-induced memory deficits through regulation of cholinergic transmission, oxidative stress and the ERK-CREB-BDNF signaling pathway. Phytother Res. 2021;35:337–45. doi: 10.1002/ptr.6804. [DOI] [PubMed] [Google Scholar]
- 172.Xia T, Zhang B, Li Y, Fang B, Zhu X, Xu B, et al. New insight into 20(S)-ginsenoside Rh2 against t-cell acute lymphoblastic leukemia associated with the gut microbiota and the immune system. Eur J Med Chem. 2020;203:112582. doi: 10.1016/j.ejmech.2020.112582. [DOI] [PubMed] [Google Scholar]
- 173.Hsieh YH, Deng JS, Chang YS, Huang GJ. Ginsenoside Rh2 ameliorates lipopolysaccharide-induced acute lung injury by regulating the TLR4/PI3K/AKT/mTOR, Raf-1/MEK/ERK, and Keap1/Nrf2/Ho-1 signaling pathways in mice. Nutrients. 2018;10:1208. doi: 10.3390/nu10091208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kang S, Im K, Kim G, Min H. Antiviral activity of 20(R)-ginsenoside Rh2 against murine gammaherpesvirus. J Ginseng Res. 2017;41:496–502. doi: 10.1016/j.jgr.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee YY, Park JS, Lee EJ, Lee SY, Kim DH, Kang JL, et al. Anti-inflammatory mechanism of ginseng saponin metabolite Rh3 in lipopolysaccharide-stimulated microglia: critical role of 5’-adenosine monophosphate-activated protein kinase signaling pathway. J Agric Food Chem. 2015;63:3472–80. doi: 10.1021/jf506110y. [DOI] [PubMed] [Google Scholar]
- 176.Lee HL, Kang KS. Protective effect of ginsenoside Rh3 against anticancer drug-induced apoptosis in LLC-PK1 kidney cells. J Ginseng Res. 2017;41:227–31. doi: 10.1016/j.jgr.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Felger JC. Imaging the role of inflammation in mood and anxiety-related disorders. Curr Neuropharmacol. 2018;16:533–58. doi: 10.2174/1570159X15666171123201142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Price RB, Duman R. Neuroplasticity in cognitive and psychological mechanisms of depression: An integrative model. Mol Psychiatry. 2020;25:530–43. doi: 10.1038/s41380-019-0615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li M, Li C, Yu H, Cai X, Shen X, Sun X, et al. Lentivirus-mediated interleukin-1beta (IL-1beta) knock-down in the hippocampus alleviates lipopolysaccharide (LPS)-induced memory deficits and anxiety- and depression-like behaviors in mice. J Neuroinflammation. 2017;14:190. doi: 10.1186/s12974-017-0964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Palmfeldt J, Henningsen K, Eriksen SA, Muller HK, Wiborg O. Protein biomarkers of susceptibility and resilience to stress in a rat model of depression. Mol Cell Neurosci. 2016;74:87–95. doi: 10.1016/j.mcn.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 181.Bradburn S, Murgatroyd C, Ray N. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Ageing Res Rev. 2019;50:1–8. doi: 10.1016/j.arr.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 182.Chandra A, Dervenoulas G, Politis M. Alzheimer’s disease neuroimaging I. Magnetic resonance imaging in Alzheimer’s disease and mild cognitive impairment. J Neurol. 2019;266:1293–302. doi: 10.1007/s00415-018-9016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lopez-Ortiz S, Pinto-Fraga J, Valenzuela PL, Martin-Hernandez J, Seisdedos MM, Garcia-Lopez O, et al. Physical exercise and Alzheimer’s disease: Effects on pathophysiological molecular pathways of the disease. Int J Mol Sci. 2021;22. [DOI] [PMC free article] [PubMed]
- 184.Wohleb ES, Terwilliger R, Duman CH, Duman RS. Stress-induced neuronal colony stimulating factor 1 provokes microglia-mediated neuronal remodeling and depressive-like behavior. Biol Psychiatry. 2018;83:38–49. doi: 10.1016/j.biopsych.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li Y, Fan C, Wang L, Lan T, Gao R, Wang W, et al. Microrna-26a-3p rescues depression-like behaviors in male rats via preventing hippocampal neuronal anomalies. J Clin Invest. 2021;131. [DOI] [PMC free article] [PubMed]
- 186.Harmer CJ, Duman RS, Cowen PJ. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry. 2017;4:409–18. doi: 10.1016/S2215-0366(17)30015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kamat PK, Kalani A, Rai S, Swarnkar S, Tota S, Nath C, et al. Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease: Understanding the therapeutics strategies. Mol Neurobiol. 2016;53:648–61. doi: 10.1007/s12035-014-9053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sharma VK, Singh TG, Singh S, Garg N, Dhiman S. Apoptotic pathways and Alzheimer’s disease: Probing therapeutic potential. Neurochem Res. 2021;46:3103–22. doi: 10.1007/s11064-021-03418-7. [DOI] [PubMed] [Google Scholar]
- 189.Tank R, Ward J, Flegal KE, Smith DJ, Bailey MES, Cavanagh J, et al. Association between polygenic risk for Alzheimer’s disease, brain structure and cognitive abilities in uk biobank. Neuropsychopharmacology. 2022;47:564–9. doi: 10.1038/s41386-021-01190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gbyl K, Rostrup E, Raghava JM, Andersen C, Rosenberg R, Larsson HBW, et al. Volume of hippocampal subregions and clinical improvement following electroconvulsive therapy in patients with depression. Prog Neuropsychopharmacol Biol Psychiatry. 2021;104:110048. doi: 10.1016/j.pnpbp.2020.110048. [DOI] [PubMed] [Google Scholar]
- 191.Chakraborty S, Tripathi SJ, Srikumar BN, Raju TR, Shankaranarayana, Rao BS. Chronic brain stimulation rewarding experience ameliorates depression-induced cognitive deficits and restores aberrant plasticity in the prefrontal cortex. Brain Stimul. 2019;12:752–66. doi: 10.1016/j.brs.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 192.Li A, Li F, Elahifasaee F, Liu M, Zhang L. Alzheimer’s Disease Neuroimaging Initiative. Hippocampal shape and asymmetry analysis by cascaded convolutional neural networks for Alzheimer’s disease diagnosis. Brain Imaging Behav. 2021;15:2330–9. doi: 10.1007/s11682-020-00427-y. [DOI] [PubMed] [Google Scholar]
- 193.Kabir MT, Uddin MS, Mamun AA, Jeandet P, Aleya L, Mansouri RA, et al. Combination drug therapy for the management of Alzheimer’s disease. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed]
- 194.Zhong SJ, Wang L, Gu RZ, Zhang WH, Lan R, Qin XY. Ginsenoside Rg1 ameliorates the cognitive deficits in D-galactose and AlCl3-induced aging mice by restoring FGF2-Akt and BDNF-TrkB signaling axis to inhibit apoptosis. Int J Med Sci. 2020;17:1048–55. doi: 10.7150/ijms.43979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nie L, Xia J, Li H, Zhang Z, Yang Y, Huang X, et al. Ginsenoside Rg1 ameliorates behavioral abnormalities and modulates the hippocampal proteomic change in triple transgenic mice of Alzheimer’s disease. Oxid Med Cell Longev. 2017;2017:6473506. doi: 10.1155/2017/6473506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yang Y, Wang L, Zhang C, Guo Y, Li J, Wu C, et al. Ginsenoside Rg1 improves Alzheimer’s disease by regulating oxidative stress, apoptosis, and neuroinflammation through Wnt/GSK-3beta/beta-catenin signaling pathway. Chem Biol Drug Des. 2022;99:884–96. doi: 10.1111/cbdd.14041. [DOI] [PubMed] [Google Scholar]