Highlights
-
•
Endoscopic debridement of the Achilles tendon improved pain and function at 12 months in patients with mid-portion tendinopathy.
-
•
The addition of platelet-rich plasma application did not improve outcomes significantly compared to endoscopic debridement alone.
-
•
Nodular tendon thickening and MRI-detected signal alteration persisted after surgery, with no association between imaging and clinical outcome.
Keywords: Achilles tendon, Endoscopic, Non-insertional, PRP, Tendinopathy
Abstract
Background
When non-operative management fails to improve symptoms in patients with non-insertional Achilles tendinopathy, surgery may be required. Various open and endoscopic techniques have been proposed, and platelet-rich plasma (PRP) injections have been proposed as an adjunct to aid tendon healing.
Methods
Thirty-six patients with mid-portion Achilles tendinopathy were randomized to undergo endoscopic debridement alone (n = 19) or in combination with intraoperative PRP application (n = 17). Clinical outcome measures included the Visual Analogue Scale for pain, function, and satisfaction and the Victorian Institute of Sports Assessment–Achilles (VISA-A) questionnaire. Patients were followed-up at 6 weeks, 3 months, 6 months, and 12 months after surgery. An MRI examination at 3 and 12 months was used to assess signal alterations within the tendon.
Results
Both groups showed significant clinical improvement (p < 0.05) after surgery, with no difference between the 2 groups. Tendon diameter increased at 3 months and decreased at 12 months. The tendinopathy area increased at 3 months and decreased at 12 months below baseline level in both groups. There was no significant difference between the groups regarding the MRI parameters. Nodular thickening and MRI-detected signal alteration persisted after surgery, with no association between imaging and clinical outcome. Five minor complications were reported: 2 in the PRP group and 3 in the control group.
Conclusion
Endoscopic debridement of the Achilles tendon improved clinical outcomes in patients with mid-portion tendinopathy. The addition of PRP did not improve outcomes compared to debridement alone. MRI parameters showed no association with clinical outcomes.
Graphical abstract
1. Introduction
Tendinopathy of the main body of the Achilles tendon is common and commonly affects elite and recreational athletes.1, 2, 3, 4, 5, 6 Achilles tendinopathy (AT) is considered a result of tendon overuse combined with a failed healing response. Haphazard proliferation of tenocytes, with disruption of collagen fibers and subsequent increase in non-collagenous matrix, are observed.7 The role of inflammation is unclear and debatable; however, recent evidence, based on tendon biopsies in patients with symptomatic mid-portion AT, or tendon rupture, shows that signs of “complex” chronic inflammation are expressed in tendinopathic cells.8 AT can be disabling, causing pain, stiffness and loss of function.7 The exact origin of symptoms is uncertain. Adhesions between tendon and paratendinous tissue, with concomitant inflammation (paratendinopathy), may be one of the sources of symptoms.9, 10, 11 The presence of neovascularization12, 13 on the ventral aspect into the tendon14, 15, 16, 17 has been associated with pain.18,19 A decreased level of the neoinnervation that accompanies the neovessels has been associated with clinical improvement.19 The issue of pain generation associated with AT is, however, controversial,20 whilst histological studies indicate that tendinopathy is a risk factor for Achilles tendon rupture.4,9,11,21,22 Non-operative management includes rest, local ice therapy, eccentric calf muscle exercises, extracorporeal shockwave therapy, and peritendinous injections. Eccentric exercises are a mainstay of management, and this has also been combined with shock-wave therapy and application of platelet-rich plasma (PRP) into the tendinopathic areas.23,24 Although some have advocated the use of PRP, a recent systematic review of the literature and meta-analysis, which included 5 randomised controlled trials, showed that PRP was more effective than placebo in pain reduction at 6 weeks but not thereafter. The evidence was deemed limited for definitive conclusions to be drawn.25 In approximately 25%–45% of patients, conservative management is not effective, and surgery may be required.9,10,26 One of the surgical options is excision of the tendinopathic tissue within the tendon and release of the paratendinous tissue. This is supposed to result in elimination of the pain-inducing nerve fibers originating from the Kager fat pad; these fibers are located on the ventral aspect of the Achilles tendon. Open surgery results in complication rates of 4.7%–11.6%, with infections and delayed wound healing being the most common,6,27, 28, 29, 30, 31 and minimally invasive endoscopic debridement of the Achilles tendon may help to avoid wound-healing problems.
The combination of PRP with endoscopic debridement could enhance tendon healing. In this way, pain-causing nerve endings are removed endoscopically, and tendon healing can be improved with the application of PRP. Furthermore, with endoscopy, direct visualisation of the tendon allows PRP to be injected precisely into the tendinopathic areas. In the present pilot study, we wished to test the hypothesis that endoscopic debridement of the Achilles tendon coupled with intraoperative application of PRP is more effective than endoscopic debridement alone in improving functional outcomes in patients with tendinopathy of the main body of the Achilles tendon.
2. Methods
2.1. Patients
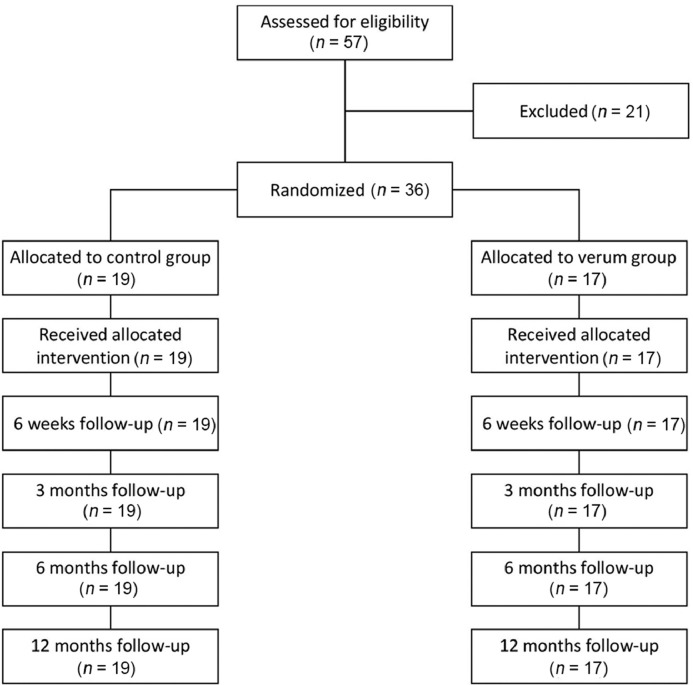
We performed a prospective randomized controlled trial of 36 consecutive patients in 2018. We included patients in whom a clinical diagnosis of mid-portion AT had been confirmed by magnetic resonance imaging (MRI), and conservative management for at least 6 months had been unsuccessful. Exclusion criteria were: presence of a systemic illness, being below 18 years of age or above 70 years of age, other musculoskeletal injuries, previous PRP treatment, and use of fluoroquinolones (Fig. 1).
Fig. 1.
Flow diagram of patients’ induction into the study.
The study participants were recruited at the Center for Knee, Foot and Hip Surgery, Heidelberg, Germany. All patients were secondary referrals to, and were operated on by, the first author. All patients followed the same standardized rehabilitation protocol. To assess the effect of additional PRP injection, the patients were preoperatively and blindly assigned to one of 2 groups. The control group (CON) included patients who underwent endoscopic debridement of the main body of the Achilles tendon only. The study group (PRP) included patients who underwent endoscopic debridement and additional intraoperative application of PRP (Table 1). The trial was approved by the Ethics Committee of the Faculty of Medicine of the University of Hannover review board (#5883), and all participants signed written informed consent to participate in the study.
Table 1.
Patient demographics (mean ± SD or frequencies).
| Variable | CON (n = 19) | PRP (n = 17) | p |
|---|---|---|---|
| Age (year) | 43.2 ± 11.7 | 52.8 ± 9.8 | 0.012 |
| Sex | |||
| Male | 14 | 14 | 0.532 |
| Female | 5 | 3 | |
| Operated side | |||
| Right | 8 | 9 | 0.516 |
| Left | 11 | 8 | |
| BMI (kg/m2) | 27.7 ± 4.2 | 26.0 ± 3.4 | 0.203 |
Abbreviations: BMI = body mass index; CON = control group; PRP = platelet-rich plasma.
2.2. Randomization procedure
For the purposes of this pilot study, no formal power analysis was performed. Patients were randomized preoperatively to one of 2 groups: one receiving endoscopic debridement alone, and the other receiving endoscopic debridement in combination with PRP. We used a random numbers table to allocate subjects. Starting with an arbitrary point in the table, we selected 40 sequential random numbers. The first 20 numbers were assigned to the endoscopic debridement alone, and the next 20 numbers were assigned to the endoscopic debridement in combination with PRP. Patients were blinded regarding the use of PRP. These assignments were then arranged in ascending order. This procedure produced a random sequence of consecutive treatment allocations.
2.3. Surgical technique
The surgical procedure has been previously described.32 With the patient in the prone position and under general anesthesia, the ankles were positioned beyond the edge of the operating table to allow full range of motion during the procedure. Two medial portals were produced through 0.5 cm stab wounds just adjacent to the Achilles tendon. The proximal portal lay 10–12 cm proximally to the calcaneal tuberosity near the musculo-tendinous junction and was used as the viewing portal, through which a standard 4.5 mm arthroscope was introduced in a proximal-to-distal direction. The distal portal was produced just above the calcaneal tuberosity and was used as the working portal through which a 4.5 mm full radius shaver was initially introduced pointing proximal. After the superficial skin incisions had been produced, the subcutaneous tissues dorsally and ventrally to the Achilles tendon were separated by blunt dissection with a mosquito clamp to produce an adequate working space for the arthroscope and the shaver. The Kager space, ventral to the Achilles tendon, was irrigated with normal saline. The whole length of the ventral aspect of the Achilles tendon could then be inspected and released completely from the Kager fat using the shaver. The whole length of the Achilles tendon could be visualized and addressed accordingly. Longitudinal tenotomies were performed by 2 parallel longitudinal incisions along the tendon using a retrograde knife blade, according to the MRI images depicting the site of the lesion (medially, ventrally, or dorsally). Accurate hemostasis was performed using the OPES (Arthrex, Naples, Florida, USA) aspirating ablator.
A blood sample (10 mL) to prepare the PRP was taken during the operation by a dual lumen syringe (Arthrex). To separate the phases, centrifugation was performed for 5 min at 1500 rpm with a Rotofix 32 A centrifuge (Andreas Hettich, Tuttlingen, Germany). The PRP was applied in the study group after the endoscopic debridement had been performed by injecting it under direct endoscopic visualisation in and around the area of tendinopathy. The endoscopy wounds were closed with a single stitch with nonabsorbable monofilament, and a routine bandage was applied.
2.4. Rehabilitation
Postoperatively, foot elevation was encouraged, oral analgesia was administered as required, and patients were encouraged to perform active ankle dorsal and plantar flexion. Mobilisation with crutches and partial weight bearing was allowed on the 1st postoperative day. Full weight bearing was started after removal of sutures around the 14th postoperative day. Proprioceptive training and physical activities with increased loading on the Achilles tendon, such cycling or cross training, were allowed starting at the third week. Running and sport-specific training was allowed after 3 months.
2.5. Outcome assessment
For the purposes of the present study, an orthopaedic fellow who was independent from the surgeon assessed all patients before surgery and after 6 weeks, 3 months, 6 months, and 12 months. Outcome assessment consisted of data collection at each visit, using:
-
•
A visual analog scale (VAS) for pain, function, and satisfaction.
-
•
A VISA-A questionnaire (Victorian Institute of Sports Assessment–Achilles)33 at each time-point except directly postoperative.
-
•
An MRI, preoperatively, at 3 months, and at 12 months. The MRI included a T1w sagittal spin echo (SE) sequence, T1w axial SE sequence, T1w axial turbo 3D gradient echo (GE) sequence and sagittal short-tau inversion recovery (STIR) sequence. Sagittal axes were placed along the longitudinal axis of the Achilles tendon in a coronal scout view. The axial plane was placed in a vertical axis to the sagittal axis. The thickness of the T1w sagittal SE sequence was 2.5 mm, with a gap of 0.3 mm. The thickness of the T1w axial SE sequence was 4.0 mm, with a gap of 0.4 mm. The thickness of the sagittal STIR sequence was 2.5 mm with a gap of 0.2 mm. The images were taken with a 0.3 T imager (O-scan, Esaote, Italy).31
3. Results
Over the calendar year of the study, 36 patients were recruited and randomized. The mean age was significantly different between the 2 groups, whereas gender distribution, treated side, and body mass index were similar (Table 1).
Five minor complications were recorded (13.8%). Two patients (one in each group) developed a superficial infection, and 2 patients (one in each group) reported slight hypoesthesia, one of which also had hematoma formation.
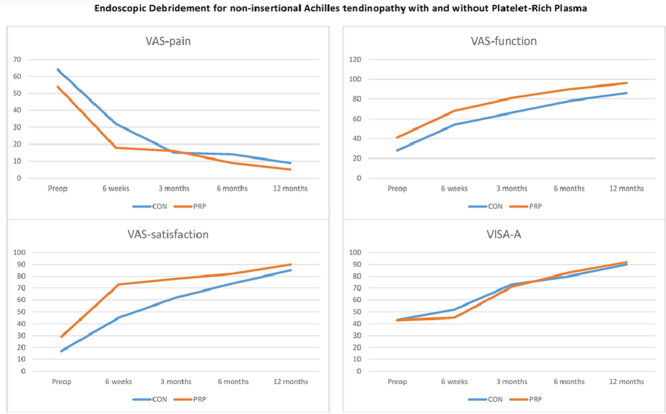
VAS scores for pain, function, and patients’ satisfaction in the entire patient cohort (n = 36), improved significantly (p ≤ 0.001) during all follow-up visits, compared to preoperative (baseline) values. VISA-A showed no significant change at 6 weeks after surgery (p = 0.194), compared to baseline, but was significantly better than at baseline (p ≤ 0.001) at 3 months, 6 months, and 12 months after surgery.
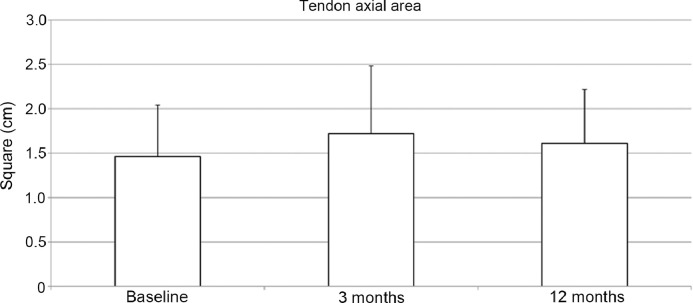
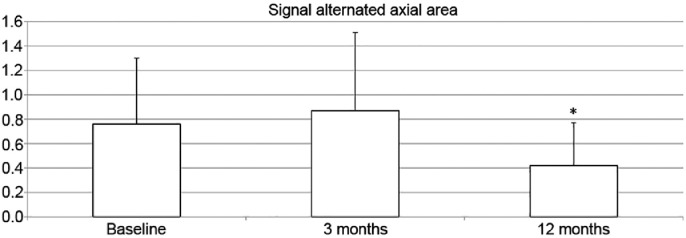
For the entire patient cohort (n = 36), the tendon cross-section (axial) area, as determined by the MRI, increased insignificantly (p = 0.07) at 3 months and decreased to a level above baseline by 12 months after surgery (p = 0.15) (Fig. 2). Maximum tendon diameter moved cranially over time, with high significance in the postoperative examinations. The longitudinal extension of the area of altered tendinopathic signal increased slightly after 3 months (p = 0.24) and subsequently declined significantly by 12 months after surgery (p = 0.004) (Fig. 3).
Fig. 2.
Tendon axial area, measured by magnetic resonance imaging. Mean values with standard deviation, preoperatively (baseline) and postoperatively at 3 and 12 months. Data are presented as mean ± SD.
Fig. 3.
Measure on magnetic resonance imaging. Mean values, with standard deviation, of the entire patients’ cohort (n = 36), preoperatively (baseline) and postoperatively at 3 and 12 months. * p = 0.004, significantly different compared to baseline.
Comparing the 2 groups (CON vs. PRP), VAS-pain showed no difference between the 2 groups at any pre- or postoperative examination (Table 2). VAS-satisfaction was significantly better in the PRP group at 6 weeks and 3 months but showed no difference, compared to the control group, at 6 and 12 months (Table 2). The VISA-A score showed no significant differences between the groups at any point of time (Table 2). None of the MRI parameters showed significant differences between the groups (Table 3).
Table 2.
Clinical outcome parameters (mean ± SD).
| CON | PRP | p | |
|---|---|---|---|
| VAS-pain | |||
| Baseline | 64.4 ± 20.5 | 53.8 ± 25.6 | 0.179 |
| 6 weeks | 32.2 ± 23.9 | 18.3 ± 20.6 | 0.071 |
| 3 months | 15.4 ± 24.0 | 16.4 ± 18.8 | 0.892 |
| 6 months | 13.8 ± 20.4 | 8.8 ± 8.8 | 0.354 |
| 12 months | 9.0 ± 13.1 | 4.5 ± 5.4 | 0.199 |
| VAS-function | |||
| Baseline | 28.0 ± 21.7 | 41.5 ± 19.3 | 0.058 |
| 6 weeks | 54.1 ± 23.5 | 67.8 ± 14.6 | 0.042 |
| 3 months | 66.4 ± 21.7 | 81.1 ± 11.1 | 0.015 |
| 6 months | 78.4 ± 24.5 | 89.3 ± 8.8 | 0.084 |
| 12 months | 86.0 ± 18.9 | 95.6 ± 4.9 | 0.045 |
| VAS-satisfaction | |||
| Baseline | 17.3 ± 25.2 | 28.9 ± 27.6 | 0.194 |
| 6 weeks | 44.6 ± 31.6 | 72.9 ± 19.3 | 0.003* |
| 3 months | 61.7 ± 25.6 | 78.0 ± 16.8 | 0.030* |
| 6 months | 74.2 ± 26.1 | 82.1 ± 13.1 | 0.254 |
| 12 months | 85.1 ± 21.5 | 90.2 ± 9.6 | 0.356 |
| VISA-A | |||
| Baseline | 42.6 ± 17.9 | 43.4 ± 22.5 | 0.916 |
| 6 weeks | 51.6 ± 11.6 | 45.1 ± 19.2 | 0.240 |
| 3 months | 73.4 ± 12.1 | 71.3 ± 18.6 | 0.691 |
| 6 months | 79.6 ± 14.1 | 83.1 ± 11.7 | 0.421 |
| 12 months | 89.5 ± 10.7 | 92.2 ± 8.2 | 0.396 |
p < 0.05, significantly different compared to baseline.
Abbreviations: CON = control group (endoscopic debridement only); PRP = group receiving platelet rich plasma injection; VAS = visual analogue scale; VISA-A = Victorian Institute of Sports Assessment–Achilles.
Table 3.
Tendon thickness and tendinopathic area variation in the 2 groups (mean ± SD).
| CON | PRP | p | |
|---|---|---|---|
| Tendon axial area | |||
| Baseline | 1.34 ± 0.57 | 1.58 ± 0.59 | 0.281 |
| 3 months | 1.66 ± 0.65 | 1.82 ± 0.94 | 0.592 |
| 12 months | 1.57 ± 0.60 | 1.64 ± 0.64 | 0.780 |
| Signal alteration axial area | |||
| Baseline | 0.74 ± 0.59 | 0.79 ± 0.50 | 0.842 |
| 3 months | 0.89 ± 0.60 | 0.85 ± 0.74 | 0.851 |
| 12 months | 0.53 ± 0.37 | 0.30 ± 0.29 | 0.106 |
| Cranio-caudal signal alternation distance | |||
| Baseline | 4.12 ± 2.01 | 3.43 ± 1.70 | 0.334 |
| 3 months | 3.59 ± 1.95 | 4.00 ± 1.68 | 0.571 |
| 12 months | 2.03 ± 1.63 | 1.62 ± 1.28 | 0.497 |
Abbreviations: CON = control group (endoscopic debridement only); PRP = group receiving platelet rich plasma injection.
4. Discussion
The present study confirmed that endoscopic debridement of the Achilles tendon led to clinical improvement in our 36 patients who had mid-portion AT and who had failed previous non-operative management. No major complications were observed. However, the additional intraoperative injections of PRP did not exert any detectable influence on the outcome of the procedure. Imaging findings were not associated with clinical outcome scores. Our findings are consistent with the few published studies from other institutions.
To our knowledge, only 3 studies have been previously published concerning endoscopic procedures for midportion AT.33, 34, 35 They reported major complication rates between 0% and 7.4%. Procedures included debridement of the paratenon, and an additional release of the plantaris tendon was performed in 2 studies and longitudinal tenotomies in 1. The studies showed good results regarding functional outcome and postoperative pain. Only 1 study explicitly mentioned the release of the ventral aspect of the Achilles tendon, where the neovessels are located.36 Postoperative care consisted of weight bearing as tolerated and active range of motion of the ankle in 2 studies,35,36 whilst 1 study (Maquirriain et al.34) adopted a progressive postoperative rehabilitation protocol, using a walker boot until the 4th week and allowing jogging during the 3rd month.32
In other studies, PRP injections showed promising results as part of the nonoperative management of tendinopathies.37, 38, 39 Monto38 reported on 30 patients who had failed conventional conservative therapy for AT and had received PRP monotherapy. At the end of the study, 28 of the 30 patients showed American Orthopaedic Foot & Ankle Society score improvement from 34 points prior to the injection to 88 points 1 year later.38 Owens et al.39 found moderate improvement on the Foot and Ankle Ability Measure scores, which increased from 55.4 to 65.8, Foot and Ankle Ability Measure-Sports scores (FAAMS) which increased from 14.8 to 17.4, and Short Form Health Survey scores (SF-8), which increased from 24.9 to 30.0, 2 years postintervention. MRI results did not show qualitative improvements in the appearance of the tendon. Both studies were case series, with no control groups. On the other hand, a randomised controlled study combining injections therapy (PRP vs. normal saline) with an eccentric exercise program found no significant difference in clinical outcomes (VISA-A) after 1 year23 and no difference in the sonographic appearance of the tendons.40 In our study, despite the high sensitivity of 94%, the specificity of 81%, and a positive predictive value of 90% of MRI scanning, there was no association between tendon appearance and function.
We point out that the patients in the PRP group exhibited a slightly better postoperative function, but these patients had a slightly higher VAS-function score prior to surgery (Table 2). In addition, patients’ satisfaction was slightly better in the PRP group at 6 weeks and 3 months after surgery but was similar to the level of the control group's satisfaction thereafter (Table 2). Given our small sample size, these differences cannot lead to definite conclusions. MRI findings showed that, following surgery, the size of the tendon increased at 3 months and decreased thereafter. The remarkable heterogeneity of the MRI results, in combination with the small sample size, does not allow correlation with clinical outcomes.
The present investigation is a pilot study, and the small sample size in each group obviously could be a source of experimental error. However, in our setting, AT is not often treated operatively, and we used strict inclusion criteria. Hence, the recruitment of a larger number of patients would require a long period of time. On the other hand, our study was a randomized comparative study, where the investigator was blinded to the treatment the patients had received, and all surgeries were performed by the same experienced surgeon. It should be noted that despite the randomization of patients in the 2 treatment groups in our study, patients in the PRP group were significantly (almost 10 years) older than in the non-PRP (control) group. It is uncertain whether this parameter influenced the results, as younger patients may have better healing potential.41 The main finding in our study was that, for the 36 patients in whom a well-described standard surgical technique was used, endoscopic debridement of the noninsertional (tendinopathic) portion of the Achilles tendon resulted in significant clinical improvement. In the present study, the addition of PRP did not exert any significant effect.
5. Conclusion
In our study, endoscopic debridement for tendinopathy of the main body of the Achilles tendon was effective and had a low complication rate, but PRP injection did not improve clinical outcomes, and tendon abnormalities revealed by postoperative MRI scanning did not correlate with clinical or functional results.
Authors’ contributions
HT conceived and supervised the study and operated on all patients; RF took care of the literature search and of the day-to-day running of the study; NG performed the statistical analysis and drafted the manuscript; LC contributed to the statistical analysis and draft of the manuscript; NM advised on the scientific aspect of the study, reviewed the literature, critically interpreted the results, and supervised the writing of the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jshs.2020.06.012.
Appendix. Supplementary materials
References
- 1.Wilder RP, Sethi S. Overuse injuries: Tendinopathies, stress fractures, compartment syndrome, and shin splints. Clin Sports Med. 2004;23:55–81. doi: 10.1016/S0278-5919(03)00085-1. [DOI] [PubMed] [Google Scholar]
- 2.Fahlstrom M, Lorentzon R, Alfredson H. Painful conditions in the Achilles tendon region in elite badminton players. Am J Sports Med. 2002;30:51–54. doi: 10.1177/03635465020300012201. [DOI] [PubMed] [Google Scholar]
- 3.Jarvinen TAH, Kannus P, Maffulli N, Khan KM. Achilles tendon disorders: Etiology and epidemiology. Foot Ankle Clin. 2005;10:255–266. doi: 10.1016/j.fcl.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Knobloch K, Yoon U, Vogt PM. Acute and overuse injuries correlated to hours of training in master running athletes. Foot Ankle Int. 2008;29:671–676. doi: 10.3113/FAI.2008.0671. [DOI] [PubMed] [Google Scholar]
- 5.Kujala UM, Sarna S, Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sports Med. 2005;15:133–135. doi: 10.1097/01.jsm.0000165347.55638.23. [DOI] [PubMed] [Google Scholar]
- 6.Maffulli N, Testa V, Capasso G, Bifulco G, Binfield PM. Results of percutaneous longitudinal tenotomy for Achilles tendinopathy in middle- and long-distance runners. Am J Sports Med. 1997;25:835–840. doi: 10.1177/036354659702500618. [DOI] [PubMed] [Google Scholar]
- 7.Maffulli N, Longo UG, Kadakia A, Spiezia F. Achilles tendinopathy. Foot Ankle Surg. 2020;26:240–249. doi: 10.1016/j.fas.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Dakin SG, Newton J, Martinez FO, et al. Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br J Sports Med. 2018;52:359–367. doi: 10.1136/bjsports-2017-098161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: Time to change a confusing terminology. Arthroscopy. 1998;14:840–843. doi: 10.1016/s0749-8063(98)70021-0. [DOI] [PubMed] [Google Scholar]
- 10.Maffulli N, Sharma P, Luscombe KL. Achilles tendinopathy: Aetiology and management. J R Soc Med. 2004;97:472–476. doi: 10.1258/jrsm.97.10.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kader D, Saxena A, Movin T, Maffulli N. Achilles tendinopathy: Some aspects of basic science and clinical management. Br J Sports Med. 2002;36:239–249. doi: 10.1136/bjsm.36.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lian Ø, Dahl J, Ackermann PW, Frihagen F, Engebretsen L, Bahr R. Pronociceptive and antinociceptive neuromediators in patellar tendinopathy. Am J Sports Med. 2006;34:1801–1808. doi: 10.1177/0363546506289169. [DOI] [PubMed] [Google Scholar]
- 13.Schubert TEO, Weidler C, Lerch K, Hofstadter F, Straub RH. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann Rheum Dis. 2005;64:1083–1086. doi: 10.1136/ard.2004.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alfredson H, Ohberg L, Forsgren S. Is vasculo-neural ingrowth the cause of pain in chronic Achilles tendinosis? An investigation using ultrasonography and colour Doppler, immunohistochemistry, and diagnostic injections. Knee Surg Sports Traumatol Arthros. 2003;11:334–338. doi: 10.1007/s00167-003-0391-6. [DOI] [PubMed] [Google Scholar]
- 15.Ohberg L, Lorentzon R, Alfredson H. Neovascularisation in Achilles tendons with painful tendinosis but not in normal tendons: An ultrasonographic investigation. Knee Surg Sports Traumatol Arthros. 2001;9:233–238. doi: 10.1007/s001670000189. [DOI] [PubMed] [Google Scholar]
- 16.Ohberg L, Alfredson H. Effects on neovascularisation behind the good results with eccentric training in chronic mid-portion Achilles tendinosis? Knee Surg Sports Traumatol Arthros. 2004;12:465–470. doi: 10.1007/s00167-004-0494-8. [DOI] [PubMed] [Google Scholar]
- 17.Peers KHE, Brys PPM, Lysens RJJ. Correlation between power Doppler ultrasonography and clinical severity in Achilles tendinopathy. Int Orthop. 2003;27:180–183. doi: 10.1007/s00264-002-0426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ackermann PW, Ahmed M, Kreicbergs A. Early nerve regeneration after achilles tendon rupture: A prerequisite for healing? A study in the rat. J Orth Res. 2002;20:849–856. doi: 10.1016/S0736-0266(01)00159-0. [DOI] [PubMed] [Google Scholar]
- 19.Ackermann PW, Li J, Lundeberg T, Kreicbergs A. Neuronal plasticity in relation to nociception and healing of rat Achilles tendon. J Orth Res. 2003;21:432–441. doi: 10.1016/S0736-0266(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 20.Knobloch K. The role of tendon microcirculation in Achilles and patellar tendinopathy. J Orthop Surg Res. 2008;3:18. doi: 10.1186/1749-799X-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alfredson H, Forsgren S, Thorsen K, Lorentzon R. In vivo microdialysis and immunohistochemical analyses of tendon tissue demonstrated high amounts of free glutamate and glutamate NMDAR1 receptors, but no signs of inflammation, in Jumper's knee. J Orth Res. 2001;19:881–886. doi: 10.1016/S0736-0266(01)00016-X. [DOI] [PubMed] [Google Scholar]
- 22.Huston KA. Achilles tendinitis and tendon rupture due to fluoroquinolone antibiotics. N Engl J Med. 1994;331:748. doi: 10.1056/NEJM199409153311116. [DOI] [PubMed] [Google Scholar]
- 23.de Jonge S, de Vos RJ, Weir A, et al. One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy: A double-blind randomized placebo-controlled trial. Am J Sports Med. 2011;39:1623–1629. doi: 10.1177/0363546511404877. [DOI] [PubMed] [Google Scholar]
- 24.Rompe JD, Furia J, Maffulli N. Eccentric loading versus eccentric loading plus shock-wave treatment for midportion achilles tendinopathy: A randomized controlled trial. Am J Sports Med. 2009;37:463–470. doi: 10.1177/0363546508326983. [DOI] [PubMed] [Google Scholar]
- 25.Liu CJ, Yu KL, Bai JB, Tian DH, Liu GL. Platelet-rich plasma injection for the treatment of chronic Achilles tendinopathy: A meta-analysis. Medicine (Baltimore) 2019;98:e15278. doi: 10.1097/MD.0000000000015278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolf C, Movin T. Etiology, histopathology, and outcome of surgery in achillodynia. Foot Ankle Int. 1997;18:565–569. doi: 10.1177/107110079701800906. [DOI] [PubMed] [Google Scholar]
- 27.McAllister JL, Thordarson DB. Complications of foot and ankle surgery. Clin Sports Med. 1999;18:927–939. doi: 10.1016/s0278-5919(05)70192-7. [DOI] [PubMed] [Google Scholar]
- 28.Paavola M, Orava S, Leppilahti J, Kannus P, Jarvinen M. Chronic Achilles tendon overuse injury: Complications after surgical treatment: an analysis of 432 consecutive patients. Am J Sports Med. 2000;28:77–82. doi: 10.1177/03635465000280012501. [DOI] [PubMed] [Google Scholar]
- 29.Schepsis AA, Wagner C, Leach RE. Surgical management of Achilles tendon overuse injuries: A long-term follow-up study. Am J Sports Med. 1994;22:611–619. doi: 10.1177/036354659402200508. [DOI] [PubMed] [Google Scholar]
- 30.Iversen JV, Bartels EM, Langberg H. The Victorian Institute of Sports Assessment Achilles questionnaire (VISA-A): A reliable tool for measuring Achilles tendinopathy. Int J Sports Phys Ther. 2012;7:76–84. [PMC free article] [PubMed] [Google Scholar]
- 31.Weber C, Wedegartner U, Maas LC, Buchert R, Adam G, Maas R. MR imaging of the Achilles tendon: Evaluation of criteria for the differentiation of asymptomatic and symptomatic tendons (MR-Tomografie der Achillessehne: Evaluation von Kriterien zur Differenzierung von asymptomatischen und symptomatischen Sehnen) RoFo. 2011;183:631–640. doi: 10.1055/s-0029-1246088. [in German] [DOI] [PubMed] [Google Scholar]
- 32.Thermann H, Benetos IS, Panelli C, Gavriilidis I, Feil S. Endoscopic treatment of chronic mid-portion Achilles tendinopathy: Novel technique with short-term results. Knee Surg Sports Traumatol Arthrosc. 2009;17:1264–1269. doi: 10.1007/s00167-009-0751-y. [DOI] [PubMed] [Google Scholar]
- 33.Wagner P, Wagner E, Oritz C, Zanolli D, Keller A, Maffulli N. Achilles tendoscopy for non insertional Achilles tendinopathy: A case series study. Foot Ankle Surg. 2020;26:421–426. doi: 10.1016/j.fas.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Maquirriain J. Surgical treatment of chronic Achilles tendinopathy: Long-term results of the endoscopic technique. J Foot Ankle Surg. 2013;52:451–455. doi: 10.1053/j.jfas.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 35.Pearce CJ, Carmichael J, Calder JD. Achilles tendinoscopy and plantaris tendon release and division in the treatment of non-insertional Achilles tendinopathy. Foot Ankle Surg. 2012;18:124–127. doi: 10.1016/j.fas.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Steenstra F, van Dijk CN. Achilles tendoscopy. Foot Ankle Clin. 2006;11:429–438. doi: 10.1016/j.fcl.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez M, Anitua E, Orive G, Mujika I, Andia I. Platelet-rich therapies in the treatment of orthopaedic sport injuries. Sports Med. 2009;39:345–354. doi: 10.2165/00007256-200939050-00002. [DOI] [PubMed] [Google Scholar]
- 38.Monto RR. Platelet rich plasma treatment for chronic Achilles tendinosis. Foot Ankle Int. 2012;33:379–385. doi: 10.3113/FAI.2012.0379. [DOI] [PubMed] [Google Scholar]
- 39.Owens Jr RF, Ginnetti J, Conti SF, Latona C. Clinical and magnetic resonance imaging outcomes following platelet rich plasma injection for chronic midsubstance Achilles tendinopathy. Foot Ankle Int. 2011;32:1032–1039. doi: 10.3113/FAI.2011.1032. [DOI] [PubMed] [Google Scholar]
- 40.de Vos RJ, Weir A, Tol JL, Verhaar JAN, Weinans H, van Schie HTM. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. Br J Sports Med. 2011;45:387–392. doi: 10.1136/bjsm.2010.076398. [DOI] [PubMed] [Google Scholar]
- 41.Ruzzini L, Abbruzzese F, Rainer A, Longo UG, Trombetta M, Maffulli N, Denaro V. Characterization of age-related changes of tendon stem cells from adult human tendons. Knee Surg Sports Traumatol Arthrosc. 2014;22:2856–2866. doi: 10.1007/s00167-013-2457-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.