Abstract
Aim:
This study aimed to evaluate the effect of supplementation with ground flaxseed (GF) on the concentrations of adiponectin, resistin, and visfatin in patients with ulcerative colitis (UC).
Background:
Inflammatory bowel disease (IBD) is one of the most common gastrointestinal diseases affecting people of all ages. Adipokines secreted from adipose tissue have been shown to play an essential role in the pathogenesis of UC.
Methods:
This trial is an open-labeled randomized controlled trial conducted on 70 patients with UC. The patients were randomly divided into two groups: flaxseed and control. The patients in the intervention received 30 g/day flaxseed powder for 12 weeks. Patients' anthropometric, nutritional, and biochemical factors were evaluated at the beginning and end of the intervention period.
Results:
Totally, 64 patients (36 men and 28 women) with a mean age of 31.12±9.67 were included in the final analysis. There was no significant difference between the two groups regarding baseline weight and height (P>0.05). After the 12-week intervention, flaxseed supplementation led to a significant reduction in the resistin (-4.85±1.89 vs. -1.10±2.25, P<0.001) and visfatin concentration (-1.33±1.14 vs. -0.53±1.63, P=0.018). Further, we found a significant increase in the adiponectin levels after the GF supplementation (3.49±1.29 vs. -0.35±0.96, P<0.001).
Conclusion:
Flaxseed supplementation could exert beneficial effects on adipokine levels in patients with UC.
Key Words: Adipokine, Adiponectin, Clinical trial, Flaxseed, Resistin, Visfatin
Introduction
Inflammatory bowel diseases (IBD), including Crohn's disease (CD) and ulcerative colitis (UC), are chronic inflammatory disorders of the intestine. IBD is not a simple disease and manifests itself in systemic inflammation with various clinical manifestations (1, 2). Chronic inflammation in the intestines of UC patients can spread to the surrounding adipose tissue, causing mesenteric hypertrophy of adipose tissue (3). The specific etiology of IBD has not been fully elucidated; nevertheless, it may occur from an improper activation of the immune system in genetically susceptible people in response to unknown environmental stimuli (4).
The primary function of adipose tissue is to retain extra nutrients as triacylglycerols, release free fatty acids during fasting, and has been classified into white adipose tissue (WAT) and brown adipose tissue (BAT). In addition, over the past few decades, studies have shown that adipose tissue has a secretory and endocrine role. Evidence suggests that adipose tissue secretes more than 50 hormones and signaling molecules known as adipokines such as adiponectin, resistin, and visfatin, which play biological functions in an autocrine, paracrine, or systemic way, and affect numerous physiological activities related to energy, glucose metabolism, and immunology (5). Some of these adipokines, such as leptin, adiponectin, resistin, and visfatin play an essential role in the pathogenesis of chronic diseases such as UC (6, 7).
Adipokines affect the gastrointestinal tract's immune system, exacerbating UC in some instances by promoting inflammation through the production of proinflammatory interleukins, tumor necrosis factor-alpha (TNF-α), and adhesion factors (8). Although some studies have shown that adiponectin reduces intestinal inflammation by affecting T cell expression, increased differentiation of T cells into T helper cell type 1 (Th1), and reduced Th2-type cytokines production (9), in some other studies, proinflammatory effects of adiponectin have been reported (10).
Flaxseed (Linum usitatissimum L.) has been widely used in traditional and modern medicine of different countries. Flaxseed accumulates many biologically active compounds and elements, including a high concentration of alpha-linolenic acid (ALA), constituting approximately 55% of the total fatty acid content; lignans, a class of phytoestrogens; and dietary fiber (11, 12). Previous studies have shown the anti-inflammatory and antioxidant effects of flaxseed (13-15). Another meta-analysis showed that flaxseed supplementation reduced C-reactive protein (CRP) and vascular cell adhesion protein 1 (VCAM-1) concentrations (16-18).
In addition to these anti-inflammatory and antioxidant effects, various studies have revealed that flaxseed supplementation can regulate the concentration of some adipocytokines. Fukumitsu et al. reported that oral administration of flaxseed in animal models caused a significant improvement in the adiponectin gene expression (19, 20).
Regarding the crucial role of adipose tissue and adipokines in the pathogenesis of IBD, it is suggested that dietary flaxseed may improve adipokine levels, as a preventative agent against UC. The purpose of the present study is thus to examine the effects of 12-week dietary supplementation with flaxseed, the richest dietary source of ALA on circulating levels of adiponectin, resistin, and visfatin.
Methods
Study design and participants
The study was designed as a randomized, open-label clinical trial with a parallel standard control group. The 12-week, single-centered study was conducted at Gastroenterology and Liver Diseases Clinic in Shahid Beheshti University of Medical Sciences, Tehran, Iran. The participants were enrolled from the outpatient treatment facility of the hospital from September 2018 to June 2019. Before initiating the trial, all participants attended a briefing session with a dietician and were provided with a structured program based on the AHA guidelines, including a low-fat diet; increasing consumption of fruits and vegetables, and growing consumption of fish instead of red or processed meat (21). Furthermore, all groups were directed not to change their physical activity during the study period.
Participants
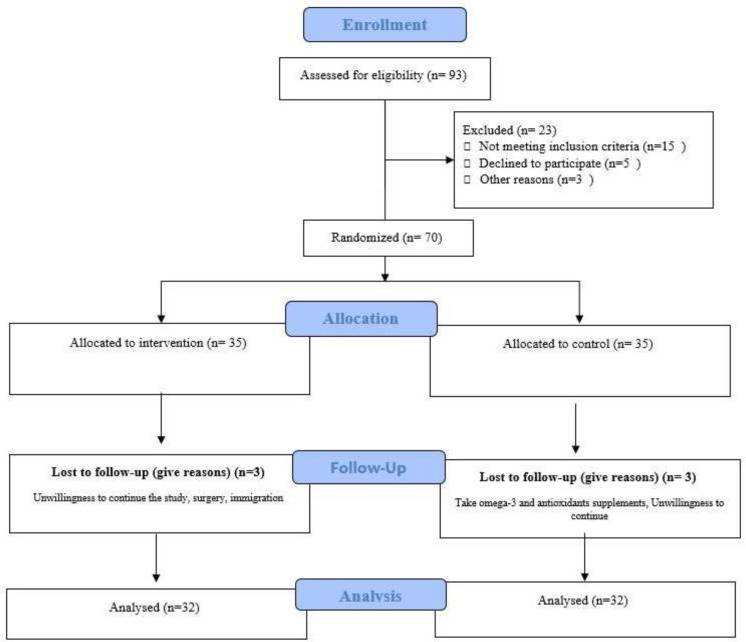
The study participants consisted of adult men and women with active phase of UC referring to the Gastroenterology and Liver Diseases Clinic in Shahid Beheshti University of Medical Sciences, Tehran, Iran. Diagnosis of UC was made by a gastrointestinal specialist, and those who met the criteria were included in the study. The criterion for UC diagnosis by the gastroenterologist was based on histopathological results in the last three months. As displayed in Figure 1, out of 93 volunteers to participate in this trial, 70 patients met the inclusion criteria. The age range of patients participating in this trial was between 18 and 55 years old and BMI > 20 kg/m2 (Table 1). The participants were screened for eligibility using the following inclusion criteria: 1) willingness to participate, 2) age range between 18 and 60, 3) patients with active phase of the disease (mild to moderate) as determined by the gastroenterologist based on laboratory findings and colonoscopy. Patients were excluded from the study if there was evidence of other intestinal diseases, inflammatory diseases, and autoimmune diseases, regular consumption of omega-3, flaxseed, or any supplements with antioxidant and anti-inflammatory properties during the past month, pregnancy and lactation, sensitivity to flaxseed compounds, use of anti-inflammatory drugs such as corticosteroids, immune-modulators (such as azathioprine, 6-mercaptopurine, methotrexate, and cyclosporine A), and anti-TNF-α medications (such as Adalimumab, Certolizumab pegol, and Infliximab) in the baseline or during the study, and unwillingness for participation.
Figure 1.
CONSORT flow diagram
Table 1.
The characteristics of the study participants
| Variables | Groups | P-value | |
|---|---|---|---|
| Intervention (n=32) | Control (n=32) | ||
| Age (year) | 30.35 ± 9.90 | 32.10 ± 10.43 | 0.38 |
| Height (cm) | 165.43 ± 12.22 | 163.34 ± 9.23 | 0.68 |
| Weight (kg) | 65.15± 10.80 | 65.89 ± 8.03 | |
| Sex | 0.54 | ||
| Male | 18 (56.3%) | 19 (59.3%) | |
| Female | 14 (43.8%) | 13 (40.6%) | |
| BMI (kg/m2) | 23.69± 2.83 | 23.79± 2.20 | 0.65 |
Values are means ±SD, BMI, Body Mass Index
P value, mean difference of changes between the two groups (independent t-test).
The sample size was determined based on the study of Morshedzadeh et al., in which the sample size required to compare an intervention group against the control group based on the comparison of the mean severity of the disease and the probability of type 1 error is 5% (α=0.05). Accordingly, the mean difference in disease severity was 6 with a standard deviation of 8.95. the probability of error. The second type was 20% (β=0.2) and the information presented in the above article was equal to 35 people in each group (22).
Randomization
At visit 2 (day 0), a card shuffling method was employed for patients' randomization, and the patients were provided with a unique randomization code. The patients were randomized according to a preexisting list produced by a card shuffling method, and the group assignments were concealed in an opaque sealed envelope. The participants were assigned in a 1:1 ratio to the flaxseed and control groups. Given the open-label design of the study, researchers and participants were aware of the nature of these two groups. However, the unit secretary, nurses in relevant units, technicians, laboratory, and statistics specialists were unaware of that.
Procedures and outcomes
After the baseline measurements, the intervention group was provided with a flaxseed powder package. The duration of intervention in this trial was 12 weeks. At the beginning of the study, after recording the demographic information of all patients, patients in the intervention group were asked to consume 30 grams of flaxseed powder daily, while the control group was advised to follow their routine medication regimen. The same nutritional recommendations were given to all patients to observe ethical issues and encourage participants in both groups to participate in the study. The dose of flaxseed powder was selected based on the results of previous studies (22, 23). Flaxseed was provided from a farm in Khoy, West Azerbaijan province of Iran. The School of Pharmacy analyzed the flaxseed powder, where the composition of macronutrients and micronutrients per 100 g of powder was as follows: energy: 450 kcal; fat: 41 g; ALA: 21.5 g; protein: 20 g; carbohydrate: 29 g and fiber: 28 g. Flaxseed was cleaned, milled, and packed (250 g each pack) with a 15 g measure.
The participants in the flaxseed group were asked to use one serving (15 g) of flaxseed powder mixed in a glass of cold water after breakfast and one serving in the evening with an hour gap of taking medications. To prevent gastrointestinal complications such as cramps, the patients were advised to use the flaxseed powder in two divided doses. They were also notified to take flaxseed powder mixed with 250 ml water two times daily or add flaxseed powder into a salad and eat it at lunch or dinner. The packages were given to the participants at the beginning, 4th and, 8th weeks of the study. The patients were asked not to consume flaxseed products during the 12-week study period. Adherence to the diet was checked at each follow-up visit. The participants were instructed to return the empty and non-empty containers of the supplements, which were weighed/ counted as a measure of study compliance, at weeks 4, 8, and 12. If they had consumed less than 90% of the prescribed flaxseed powder, they were excluded from the analysis. The primary outcome of this trial was to evaluate the effect of flaxseed supplementation on adiponectin concentration. The secondary outcomes in this trial were serum concentrations of resistin and visfatin.
Data collection
Fasting venous blood (10 ml) was collected from each subject after 12 hours of fasting. To separate the serum, blood samples were centrifuged at room temperature at 3000 rpm for 10 min, with the isolated serum stored at -80 °C until the biochemical tests were carried out. Serum concentrations of adiponectin, resistin, and visfatin were measured using ELISA kits (Eastbiopharm Co. Ltd., Hangzhou, PRC, and Diagnostics Biochem Canada (DBS)).
The 3-day 24-hour recall was designed to quantitatively assess the current nutrient intake at baseline and end of the study. Then, each food item was entered into Nutritionist IV software (1997, First DataBank Inc., San Bruno, CA), where the mean intake of energy, micronutrients, and macronutrients was calculated at the baseline and end of the study.
At the beginning and end of the study, the weight and height of patients participating in both groups were assessed using standard equipment. Patients’ weight was assessed using Seca device with 100 g precision, while their height was evaluated using Seca stadiometer in a standing position and without shoes, with an accuracy of 0.1 cm. The standard formula was used to calculate the BMI.
Ethical considerations
The executive protocol of this trial was presented by the Research Council of Shahid Beheshti University of Medical Sciences and approved by the Ethics Committee of this university (Ethics Code: IR.SBMU.ENDOCRINE.REC.1397.115), and carried out based on the Helsinki Declaration. The protocol for this trial was submitted in the protocol records system for the Iranian Clinical Trials Registration (www.irct.ir) (IRCT20180311039043N1). Also, written informed consent was acquired from all study participants in agreement with the principles of the Declaration of Helsinki.
Statistical methods
Quantitative and qualitative data were reported as mean (standard deviation) and frequency (%). Kolmogorov–Smirnov test was used to evaluate the normality of the data. Qualitative variables were compared using the chi-square test. For normal distribution variables, the independent sample t-test and paired sample t-test were employed to compare parameters at the beginning and the end of the study between and within groups, respectively. Also, one-way analysis of variance and LSD post-hoc tests were used to compare groups in terms of quantitative variables.
Furthermore, analysis of covariance (ANCOVA) was used to adjust the effect of confounding variables (dietary intake of energy, protein, fat, polyunsaturated fatty acids, omega 3 polyunsaturated fatty acids, and omega 6 polyunsaturated fatty acids). The P-value<0.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 24 (IBM Corp. IBM SPSS Statistics for Windows, Armonk, NY).
Results
Basic characteristics of the patients
Of the 93 patients who had registered from September 2018 to June 2019 (a time interval of 10 months) to participate in this trial, 70 patients met the study inclusion criteria. Among patients randomized in two groups, three patients discontinued participation in each group during the first week of supplementation (due to unwillingness to continue the study as well as surgery and other diseases). Sixty-four patients (37 men and 27 women) with a mean age of 31.12±9.67 were included in the final analysis. Overall, 57.8% (n=37) were male, 54.6% (n=35) were single, and 65.6 % (n=42) of the participants were employed. The mean duration past the diagnosis in the flaxseed group was 5.21±2.45 years vs. 5.00±3.05 years in the control group, showing no significant differences between the two groups (P=0.73). There was no significant difference either between the two groups regarding baseline weight and height (P>0.05). No differences were found between groups in any dietary variable (P>0.05) (Table 2).
Table 2.
Dietary intakes of subjects at baseline and post intervention
| Variables | Groups | P1-value | |
|---|---|---|---|
| Intervention (n=32) | Control (n=32) | ||
| Energy (kcal) | |||
| Before | 2295 ± 281.02 | 2226.56 ± 268.27 | 0.24 |
| After | 2372.87 ± 259.76 | 2366.97 ± 233.86 | 0.75 |
| P2-value | 0.12 | 0.19 | |
| Carbohydrate (g) | |||
| Before | 326.64 ± 51.18 | 320.65 ± 44.82 | 0.56 |
| After | 334.19 ± 47.85 | 332 ± 45.19 | 0.89 |
| P2-value | 0.378 | 0.298 | |
| Protein (g) | |||
| Before | 70.52 ±18.12 | 70.93 ± 17.93 | 0.29 |
| After | 67.05± 13.46 | 66.19 ± 14.93 | 0.76 |
| P2-value | 0.35 | 0.24 | |
| Fat (g) | |||
| Before | 66.76 ± 14.03 | 63.19 ± 12.95 | 0.35 |
| After | 66.27 ± 13.25 | 63.49 ± 12.63 | 0.85 |
| P2-value | 0.79 | 0.82 | |
| PUFA (g) | |||
| Before | 13.28 ± 2.40 | 13.57 ± 1.94 | 0.56 |
| After | 12.85 ± 2.47 | 13.46 ± 2.02 | 0.45 |
| P2-value | 0.18 | 0.83 | |
| Omega-3 (g) | |||
| Before | 0.65 ± 0.19 | 0.68 ± 0.17 | 0.43 |
| After | 0.66 ± 0.17 | 0.7 ± 0.18 | 0.28 |
| P2-value | 0.25 | 0.23 | |
| Omega-6 (g) | |||
| Before | 1.38 ± 0.52 | 1.58 ± 0.53 | 0.35 |
| After | 1.48 ± 0.51 | 1.64 ± 0.51 | 0.18 |
| P2-value | 0.41 | 0.53 | |
Values are means ± SD. MUFA: Monounsaturated fatty acids; PUFA: Polyunsaturated Fatty Acid, SFA: Saturated fatty acids.
P1 value, mean difference of changes between the two groups (independent t-test).
P2 value, difference compared with the value at the beginning of the study within groups (paired t-test).
Effect of flaxseed on primary and secondary outcomes
Table 3 reports the mean levels of adiponectin, resistin, and visfatin between flaxseed and control groups at the baseline and end of the study. At the beginning of the study, there was no difference between the two groups in terms of mean serum concentrations of resistin, adiponectin, and visfatin (P>0.05). After the intervention, flaxseed supplementation led to a significant reduction in the resistin (-4.85±1.89 vs. -1.10±2.25, P<0.001) and visfatin (-1.33±1.14 vs. -0.53±1.63, P=0.018) concentrations. Further, a significant increase in the adiponectin levels was observed after flaxseed supplementation (3.49±1.29 vs. -0.35±0.96, P<0.001). After adjusting for confounding variables, no significant changes occurred in results.
Table 3.
Means and standard deviations of the adipokine levels
| P Value | Control group (n 32) | Intervention group (n 32) | ||||||
|---|---|---|---|---|---|---|---|---|
| P4 | P3 | P2 | P1 | After | Before | After | Before | Variables |
| <0.001 | <0.001 | 0.01 | <0.001 | 18.23 ± 2.30 | 19.33 ± 1.92 | 15.25 ± 1.14 | 20.13 ± 2.51 | Resistin |
| 0.03 | 0.018 | 0.07 | <0.001 | 15.28 ± 2.09 | 15.82 ± 2.38 | 14.83 ± 1.43 | 16.17 ± 1.87 | Visfatin |
| <0.001 | <0.001 | 0.618 | 0.002 | 13.98 ± 4.22 | 14.33 ± 4.11 | 17.14 ± 3.65 | 13.65 ± 4.19 | Adiponectin (mg/mL) |
Values are expressed as mean ± SD.
P1 and P2 value, difference compared with the value at the beginning of the study within groups (paired t-test).
P3 value, mean difference of changes between the two groups (independent t-test).
P4 value, Based on ANCOVA model regressing change from baseline on the treatment group, baseline value of the outcome, sex, age, MET and energy.
Discussion
The results of the present study indicated that flaxseed supplementation in patients with UC led to a significant reduction in the resistin and visfatin concentration. Also, a significant improvement was found in the adiponectin level in the flaxseed group than in control.
In recent years, various studies have been investigating the role of different adipokines in the pathogenesis of chronic diseases. Adipokines have both pro-and anti-inflammatory effects in IBD patients. Some studies showed a rise in leptin concentration in patients with UC despite weight loss, anorexia, and increased release of TNF-α. Elevated leptin concentration is associated with exacerbation of inflammation in patients with UC (24). Adiponectin is one of the adipokines secreted from adipose tissue, which has shown anti-inflammatory effects in various studies (25, 26).
In the present study, a significant increase in adiponectin levels was observed after flaxseed supplementation. Weigert et al. in a cross-sectional study found that circulating levels of chemerin and adiponectin were higher in ulcerative colitis (27). Also, Karmiris et al. showed that the concentration of adiponectin was higher in patients with UC than in the control group (28). In contrast, the results of Valentini et al.'s study contradicted this study and showed a decline in adiponectin levels in CD and UC patients (7).
There are several reasons for this discrepancy seen in previous studies, including this fact that the concentration of adiponectin is higher in women than in men, and in the studies conducted, the distribution of participants in the studies was not the same in terms of gender (29). Various studies have shown that adiponectin can exert anti-inflammatory effects by promoting the synthesis of interleukin receptor antagonists and reducing the dendritic cell release of interferon-gamma. Adiponectin can also induce macrophages to perform more phagocytosis (30, 31).
In line with the results of the present study, some findings have indicated a rise in the concentration of adiponectin following flaxseed supplementation. Haidari et al. reported an increase in the adiponectin levels after the flaxseed supplementation in women with polycystic ovary syndrome (22). Also, Sekine et al. showed that ALA-rich flaxseed oil (FSO) oral administration in rats led to a significant increase in the adiponectin levels (32).
However, the results of some studies are contradictory, which observed no significant change in adiponectin concentration following flaxseed supplementation (33, 34). ALA, the main component of flaxseed products, acts as a ligand for PPAR-γ, and can increase adiponectin's expression and circulating levels (35). Some researchers have also suggested that weight loss following flaxseed intake may be one of the reasons affecting adiponectin levels. It has been reported that adiponectin's expression and circulating level increased following weight loss (36). The beneficial effects of flaxseed in weight loss are due to its high fiber content and active ingredients (37). However, in our study, no significant change was observed in patients' weight in the flaxseed group compared to the control.
Resistin expression in human monocytes was markedly elevated by endotoxin and proinflammatory cytokines (38, 39). Based on the findings of various studies, the serum level of resistin is strongly associated with the concentration of some inflammatory factors (40, 41). Some previous studies have shown that patients with UC had a significantly higher concentration of resistin, and the level of this adipokine was correlated with disease activity, hs-CRP levels, and fat mass (42, 43). Resistin is important in exacerbating chronic inflammation by inducing nuclear factor-kappa B (NF-κB) inflammatory pathways (44). Bokarewa et al. reported that resistin levels could upregulate IL-6 and TNF-a related genes (45). Also, another study revealed that human resistin significantly promotes the production and secretion of TNF-a and IL-12 by activating the NF-kB transcription factor (46). In the present study, a significant reduction was found in the resistin concentration after the flaxseed supplementation. According to our search, no previous study has evaluated the effect of flaxseed on resistin concentration. However, it seems that these positive effects may be due to the high content of ALA in flaxseed (47).
In the present study, flaxseed supplementation caused a significant reduction in visfatin concentration. Moschen et al. reported that visfatin could encourage production of inflammatory cytokines and be considered a new proinflammatory adipocytokine (48). It has also been reported that visfatin plasma levels and mRNA expression significantly increased in patients with UC (7). Researchers have suggested that visfatin exacerbates IBD through a variety of mechanisms. These mechanisms affect peripheral blood mononuclear cells, direct stimulation of proinflammatory cytokine production, and suppression of neutrophil apoptosis (49, 50). Some researchers have also suggested that visfatin can increase the expression of genes involved in inflammatory pathways such as NF-κB p65 (RelA) DNA-binding activity in human leukocytes by p38 and MEK-1 (51, 52).
Before our study, no study had evaluated the effect of flaxseed supplementation on visfatin concentration. However, some studies have shown that omega-3 supplementation significantly reduces serum visfatin concentrations (53, 54). Our results have shown that flaxseed reduces visfatin and resistin concentration, while the adiponectin increased in the flaxseed group after 12 weeks. It should be due to its n-3 fatty acid content, antioxidant activity, as well as lignan and soluble fiber (11, 55).
Our study was the first clinical trial to evaluate the effect of flaxseed supplementation on adipokine levels in patients with UC. However, our study had some limitations. First, in this study, due to financial problems, serum leptin concentration was not assessed. Due to the strong correlation between leptin concentration and the severity of inflammation, evaluation of this factor could enhance the accuracy of the results. Secondly, measuring the expression of genes related to some inflammatory factors could be helpful. Finally, one of the most important limitations of this study was the type of study design, which due to the lack of a suitable placebo, double blinding in the study was not possible.
Conclusion
In conclusion, our study revealed that flaxseed supplementation led to a significant reduction in the visfatin and resistin concentration and a considerable improvement in the adiponectin levels. However, further studies with a larger sample size and measurement of more adipokines are required to confirm the present results.
Funding
This study was funded by the Shahid Beheshti University of Medical Sciences.
Conflict of interests
The authors have no conflicts of interest to declare.
Acknowledgements
The researchers of this project sincerely thank all the patients and their families as well as the staff of the data collection centers.
References
- 1.Kopp A, Buechler C, Neumeier M, Weigert J, Aslanidis C, Schölmerich J, et al. Innate immunity and adipocyte function: ligand-specific activation of multiple Toll-like receptors modulates cytokine, adipokine, and chemokine secretion in adipocytes. Obesity. 2009;17:648–656. doi: 10.1038/oby.2008.607. [DOI] [PubMed] [Google Scholar]
- 2.Karrasch T, Schaeffler A. Adipokines and the role of visceral adipose tissue in inflammatory bowel disease. Ann Gastroenterol. 2016;29:424–438. doi: 10.20524/aog.2016.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuo L, Ge S, Ge Y, Li J, Zhu B, Zhang Z, et al. The adipokine metrnl ameliorates chronic colitis in Il-10−/− mice by attenuating mesenteric adipose tissue lesions during spontaneous colitis. J Crohn's Colitis. 2019;13:931–941. doi: 10.1093/ecco-jcc/jjz001. [DOI] [PubMed] [Google Scholar]
- 4.Ananthakrishnan AN. Environmental risk factors for inflammatory bowel diseases: a review. Dig Dis Sci. 2015;60:290–298. doi: 10.1007/s10620-014-3350-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 6.Waluga M, Hartleb M, Boryczka G, Kukla M, Żwirska-Korczala K. Serum adipokines in inflammatory bowel disease. World J Gstroenterol. 2014;20:6912. doi: 10.3748/wjg.v20.i22.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valentini L, Wirth EK, Schweizer U, Hengstermann S, Schaper L, Koernicke T, et al. Circulating adipokines and the protective effects of hyperinsulinemia in inflammatory bowel disease. Nutrition. 2009;25:172–181. doi: 10.1016/j.nut.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Morshedzadeh N, Rahimlou M, Asadzadeh Aghdaei H, Shahrokh S, Reza Zali M, Mirmiran P. Association between adipokines levels with inflammatory bowel disease (IBD): systematic reviews. Dig Dis Sci. 2017;62:3280–3286. doi: 10.1007/s10620-017-4806-5. [DOI] [PubMed] [Google Scholar]
- 9.Nishihara T, Matsuda M, Araki H, Oshima K, Kihara S, Funahashi T, et al. Effect of adiponectin on murine colitis induced by dextran sulfate sodium. Gastroenterology. 2006;131:853–861. doi: 10.1053/j.gastro.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Ogunwobi OO, Beales IL. Adiponectin stimulates proliferation and cytokine secretion in colonic epithelial cells. Regul Pept. 2006;134:105–113. doi: 10.1016/j.regpep.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Kajla P, Sharma A, Sood DR. Flaxseed—a potential functional food source. J Food Sci Technol. 2015;52:1857–1871. doi: 10.1007/s13197-014-1293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Touré A, Xueming X. Flaxseed lignans: source, biosynthesis, metabolism, antioxidant activity, bio‐active components, and health benefits. Compr Rev Food Sci Food Saf. 2010;9:261–269. doi: 10.1111/j.1541-4337.2009.00105.x. [DOI] [PubMed] [Google Scholar]
- 13.Parikh M, Maddaford TG, Austria JA, Aliani M, Netticadan T, Pierce GN. Dietary flaxseed as a strategy for improving human health. Nutrients. 2019;11:1171. doi: 10.3390/nu11051171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parikh M, Netticadan T, Pierce GN. Flaxseed: Its bioactive components and their cardiovascular benefits. Am J Physiol Heart Circ Physiol. 2018;314:146–159. doi: 10.1152/ajpheart.00400.2017. [DOI] [PubMed] [Google Scholar]
- 15.Rahimlou M, Jahromi NB, Hasanyani N, Ahmadi AR. Effects of flaxseed interventions on circulating inflammatory biomarkers: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2019;10:1108–1119. doi: 10.1093/advances/nmz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Askarpour M, Karimi M, Hadi A, Ghaedi E, Symonds ME, Miraghajani M, et al. Effect of flaxseed supplementation on markers of inflammation and endothelial function: a systematic review and meta-analysis. Cytokine. 2020;126:154922. doi: 10.1016/j.cyto.2019.154922. [DOI] [PubMed] [Google Scholar]
- 17.Hadi A, Askarpour M, Ziaei R, Venkatakrishnan K, Ghaedi E, Ghavami A. Impact of flaxseed supplementation on plasma lipoprotein (a) concentrations: a systematic review and meta‐analysis of randomized controlled trials. Phytother Res. 2020;34:1599–1608. doi: 10.1002/ptr.6640. [DOI] [PubMed] [Google Scholar]
- 18.Hadi A, Askarpour M, Salamat S, Ghaedi E, Symonds ME, Miraghajani M. Effect of flaxseed supplementation on lipid profile: An updated systematic review and dose-response meta-analysis of sixty-two randomized controlled trials. Pharmacol Res. 2020;152:104622. doi: 10.1016/j.phrs.2019.104622. [DOI] [PubMed] [Google Scholar]
- 19.Fukumitsu S, Aida K, Ueno N, Ozawa S, Takahashi Y, Kobori M. Flaxseed lignan attenuates high-fat diet-induced fat accumulation and induces adiponectin expression in mice. Br J Nutr. 2008;100:669–676. doi: 10.1017/S0007114508911570. [DOI] [PubMed] [Google Scholar]
- 20.Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, et al. PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–2099. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 21.Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, et al. AHA Dietary Guidelines: revision 2000: a statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation. 2000;102:2284–2299. doi: 10.1161/01.cir.102.18.2284. [DOI] [PubMed] [Google Scholar]
- 22.Morshedzadeh N, Shahrokh S, Aghdaei HA, Pourhoseingholi MA, Chaleshi V, Hekmatdoost A, et al. Effects of flaxseed and flaxseed oil supplement on serum levels of inflammatory markers, metabolic parameters and severity of disease in patients with ulcerative colitis. Complement Ther Med. 2019;46:36–43. doi: 10.1016/j.ctim.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Toulabi T, Yarahmadi M, Goudarzi F, Ebrahimzadeh F, Momenizadeh A, Yarahmadi S. Effects of flaxseed on blood pressure, body mass index, and total cholesterol in hypertensive patients: a randomized clinical trial. Explore. 2022;18:438–445. doi: 10.1016/j.explore.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Rivero-Gutierrez B, Aranda CJ, Ocon B, Arredondo M, Martinez-Augustin O, de Medina FS. Exogenous leptin reinforces intestinal barrier function and protects from colitis. Pharmacol Res. 2019;147:104356. doi: 10.1016/j.phrs.2019.104356. [DOI] [PubMed] [Google Scholar]
- 25.Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol. 2008;121:326–330. doi: 10.1016/j.jaci.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Engeli S, Feldpausch M, Gorzelniak K, Hartwig F, Heintze U, Janke J, et al. Association between adiponectin and mediators of inflammation in obese women. Diabetes. 2003;52:942–947. doi: 10.2337/diabetes.52.4.942. [DOI] [PubMed] [Google Scholar]
- 27.Weigert J, Obermeier F, Neumeier M, Wanninger J, Filarsky M, Bauer S, Aslanidis C, et al. Circulating levels of chemerin and adiponectin are higher in ulcerative colitis and chemerin is elevated in Crohn's disease. Inflamm Bowel Dis. 2010;16:630–637. doi: 10.1002/ibd.21091. [DOI] [PubMed] [Google Scholar]
- 28.Karmiris K, Koutroubakis IE, Xidakis C, Polychronaki M, Voudouri T, Kouroumalis EA. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:100–105. doi: 10.1097/01.MIB.0000200345.38837.46. [DOI] [PubMed] [Google Scholar]
- 29.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin: implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 30.Batra A, Zeitz M, Siegmund B. Adipokine signaling in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1897–1905. doi: 10.1002/ibd.20937. [DOI] [PubMed] [Google Scholar]
- 31.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–635. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 32.Sekine S, Sasanuki S, Murano Y, Aoyama T, Takeuchi H. α-linolenic acid-rich flaxseed oil ingestion increases plasma adiponectin level in rats. Int J Vitam Nutr Res. 2008;78:223–229. doi: 10.1024/0300-9831.78.45.223. [DOI] [PubMed] [Google Scholar]
- 33.Paschos GK, Zampelas A, Panagiotakos DB, Katsiougiannis S, Griffin BA, Votteas V, et al. Effects of flaxseed oil supplementation on plasma adiponectin levels in dyslipidemic men. Eur J Nutr. 2007;46:315–320. doi: 10.1007/s00394-007-0668-5. [DOI] [PubMed] [Google Scholar]
- 34.Jalili C, Pezeshki M, Askarpour M, Marx W, Hassani B, Hadi A, et al. The effect of flaxseed supplementation on circulating adiponectin and leptin concentration in adults: a systematic review and meta‐analysis of randomized controlled trials. Phytother Res. 2020;34:1578–1586. doi: 10.1002/ptr.6634. [DOI] [PubMed] [Google Scholar]
- 35.Ishtiaq SM, Rashid H, Hussain Z, Arshad MI, Khan JA. Adiponectin and PPAR: a setup for intricate crosstalk between obesity and non-alcoholic fatty liver disease. Rev Endocr Metab Disord. 2019;20:253–261. doi: 10.1007/s11154-019-09510-2. [DOI] [PubMed] [Google Scholar]
- 36.Ma W, Huang T, Zheng Y, Wang M, Bray GA, Sacks FM, et al. Weight-loss diets, adiponectin, and changes in cardiometabolic risk in the 2-year POUNDS Lost Trial. J Clin Endocr. 2016;101:2415–2422. doi: 10.1210/jc.2016-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohammadi-Sartang M, Mazloom Z, Raeisi-Dehkordi H, Barati-Boldaji R, Bellissimo N, Totosy de Zepetnek JO. The effect of flaxseed supplementation on body weight and body composition: a systematic review and meta-analysis of 45 randomized placebo-controlled trials. Obes Rev. 2017;18:1096–1107. doi: 10.1111/obr.12550. [DOI] [PubMed] [Google Scholar]
- 38.Kaser S, Kaser A, Sandhofer A, Ebenbichler CF, Tilg H, Patsch JR. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem Biophys Res Commun. 2003;309:286–290. doi: 10.1016/j.bbrc.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-α and IL-12 in macrophages by NF-κB-dependent pathway. Biochem Biophys Res Commun. 2005;334:1092–1101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 40.Ramirez JL, Khetani SA, Zahner GJ, Spaulding KA, Schaller MS, Gasper WJ, et al. Serum resistin is associated with impaired endothelial function and a higher rate of adverse cardiac events in patients with peripheral artery disease. J Vasc Surg. 2019;69:497–506. doi: 10.1016/j.jvs.2018.05.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park HK, Kwak MK, Kim HJ, Ahima RS. Linking resistin, inflammation, and cardiometabolic diseases. Korean J Intern Med. 2017;32:239. doi: 10.3904/kjim.2016.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konrad A, Lehrke M, Schachinger V, Seibold F, Stark R, Ochsenkühn T, Parhofer KG, et al. Resistin is an inflammatory marker of inflammatory bowel disease in humans. Eur J Gastroenterol Hepatol. 2007;19:1070–1074. doi: 10.1097/MEG.0b013e3282f16251. [DOI] [PubMed] [Google Scholar]
- 43.Abedimanesh N, Motlagh B, Abedimanesh S, Ostadrahimi A, Somi MH, Jafarabad MA, et al. Circulating resistin in ulcerative colitis, relation with anthropometric, body composition and inflammatory parameters. Prog Nutr. 2018;20:132–136. [Google Scholar]
- 44.Nagaev I, Bokarewa M, Tarkowski A, Smith U. Human resistin is a systemic immune-derived proinflammatory cytokine targeting both leukocytes and adipocytes. PloS One. 2006;1:31. doi: 10.1371/journal.pone.0000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 46.Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-α and IL-12 in macrophages by NF-κB-dependent pathway. Biochem Biophys Res Commun. 2005;334:1092–1101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 47.Domínguez-López I, Yago-Aragón M, Salas-Huetos A, Tresserra-Rimbau A, Hurtado-Barroso S. Effects of dietary phytoestrogens on hormones throughout a human lifespan: a review. Nutrients. 2020;12:2456. doi: 10.3390/nu12082456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, Tilg H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178:1748–1758. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- 49.Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD, et al. Pre–B cell colony–enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Investig. 2004;113:1318–1327. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moschen AR, Geiger S, Gerner R, Tilg H. Pre-B cell colony enhancing factor/NAMPT/visfatin and its role in inflammation-related bone disease. Mutat Res-Fund Mol M. 2010;690:95–101. doi: 10.1016/j.mrfmmm.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 51.Kwon J, Arsenis C, Suessmilch M, McColl A, Cavanagh J, Morris BJ. Differential effects of toll-like receptor activation and differential mediation by map kinases of immune responses in microglial cells. Cell Mol Neurobiol. 2021;42:2655–2671. doi: 10.1007/s10571-021-01127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gross CM, Kellner M, Wang T, Lu Q, Sun X, Zemskov EA, et al. LPS-induced acute lung injury involves NF-κB–mediated downregulation of SOX18. Am. J Respir Cell Mol Biol. 2018;58:614–624. doi: 10.1165/rcmb.2016-0390OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hajianfar H, Hosseinzadeh MJ, Bahonar A, Mohammad K, Askari GR, Entezari MH, et al. The effect of omega-3 on the serum visfatin concentration in patients with type II diabetes. J Res Med Sci. 2011;16:490. [PMC free article] [PubMed] [Google Scholar]
- 54.Hoseinzadeh Attar M, Hajianfar H, Bahonar A, Mohamad K, Keshavarz S, Entezari M, Askari G. The effect of n-fatty acid (omega 3) on serum visfatin concentration in patients with type2 diabetes. Pars Journal of Medical Sciences. 2022;10:26–32. [PMC free article] [PubMed] [Google Scholar]
- 55.Ma Y, Griffith JA, Chasan-Taber L, Olendzki BC, Jackson E, Stanek III EJ, et al. Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr. 2006;83:760–766. doi: 10.1093/ajcn/83.4.760. [DOI] [PMC free article] [PubMed] [Google Scholar]