Abstract
Both an increased frequency of chromosome missegregation (chromosomal instability, CIN) and the presence of an abnormal complement of chromosomes (aneuploidy) are hallmarks of cancer. To better understand how cells are able to adapt to high levels of chromosomal instability, we previously examined yeast cells that were deleted of the gene BIR1, a member of the chromosomal passenger complex (CPC). We found bir1Δ cells quickly adapted by acquiring specific combinations of beneficial aneuploidies. In this study, we monitored these yeast strains for longer periods of time to determine how cells adapt to high levels of both CIN and aneuploidy in the long term. We identify suppressor mutations that mitigate the chromosome missegregation phenotype. The mutated proteins fall into four main categories: outer kinetochore subunits, the SCFCdc4 ubiquitin ligase complex, the mitotic kinase Mps1, and the CPC itself. The identified suppressor mutations functioned by reducing chromosomal instability rather than alleviating the negative effects of aneuploidy. Following the accumulation of suppressor point mutations, the number of beneficial aneuploidies decreased. These experiments demonstrate a time line of adaptation to high rates of CIN.
Keywords: aneuploidy, Aurora B, chromosomal instability, kinetochore, SCF complex
Subject Categories: Cell Cycle; DNA Replication, Recombination & Repair
A series of genomic changes including acquisition of specific aneuploidies, karyotype refinement, point mutations, and eventual loss of beneficial copy number alterations allows cells to adapt to high rates of chromosome missegregation.
Introduction
The accurate distribution of chromosomes to daughter cells is a fundamental requirement of cell division. An increase in the frequency of errors in chromosome segregation is called chromosomal instability (CIN). Chromosomal instability leads to abnormal karyotypes through the gain or loss of chromosomes, a state called aneuploidy. Aneuploidy and CIN are both hallmarks of cancer that have causative roles in cancer development, cancer progression, and resistance to chemotherapy (reviewed in Ben‐David & Amon, 2020). Despite the promotion of cell proliferation in cancer, aneuploidy and CIN have consistently been demonstrated to decrease cell growth and division (Gropp et al, 1983; Torres et al, 2007). Cancers therefore likely develop adaptations to ameliorate the negative effects of CIN and aneuploidy.
Different models for how cells adapt to CIN and aneuploidy have been proposed. Cells could adapt via compensatory mutations that decrease the levels of CIN after the accumulation of beneficial aneuploidies (Cahill et al, 1999; Kwon et al, 2008; Sansregret et al, 2017). Alternatively, researchers postulated that cancer cells could adapt to CIN and aneuploidy through mutations that lead to aneuploidy tolerance (Torres et al, 2010). Additionally, it was suggested that CIN and aneuploidy provide for a fast, but transient mechanism of adaptation. In this model, aneuploidy provides a short‐term benefit that outweighs its downsides but would eventually be superseded by more targeted genome alterations (Yona et al, 2012). How aneuploidy and more specific types of mutations affect each other during the course of adaptation is currently unknown.
The molecular mechanisms that lead to CIN in cancer cells have long been elusive (Gordon et al, 2012). However, one relevant phenotype that is frequently observed across many cancer types is the overstabilization of connections between microtubules and kinetochores, which are the binding sites for microtubules at the centromeres of chromosomes (Bakhoum et al, 2009a). These overstabilized attachments lead to the accumulation of misattached chromosomes where both of the sister chromatids are attached to microtubules emanating from the same spindle pole (merotelic and syntelic attachments). One or both of the kinetochores must then be detached from the microtubules in order to properly distribute one sister chromatid to each daughter cell. Although the basis behind this phenotype in cancer cells is currently unknown, the central player in destabilizing erroneous kinetochore–microtubule attachments is the chromosomal passenger complex (CPC). The CPC contains a kinase, Aurora B, that phosphorylates kinetochores to lower their affinity for microtubules (Tanaka et al, 2002; Cheeseman et al, 2006; Sarangapani et al, 2013; Kalantzaki et al, 2015). Inhibition of Aurora B in mammalian cells leads to an increased frequency of lagging chromosomes in anaphase due to the inability to detach microtubules from kinetochores that are attached to both spindle poles (Cimini et al, 2006). This lagging chromosome phenotype is also frequently observed in cancers (Bakhoum et al, 2014).
In addition to Aurora B, the CPC contains the subunits INCENP, Survivin, and Borealin. The C‐terminus of INCENP binds to and activates Aurora B, while the N‐terminus binds to Survivin and Borealin (Ainsztein et al, 1998; Bishop & Schumacher, 2002; Honda et al, 2003; Sessa et al, 2005; Klein et al, 2006; Jeyaprakash et al, 2007). Survivin and Borealin target the complex to centromere‐proximal chromatin through an interaction with Shugoshin. This interaction was shown to promote the activity of the CPC in correcting erroneous kinetochore–microtubule attachments through phosphorylation of substrates at the outer kinetochore (Gassmann et al, 2004; Kawashima et al, 2007, 2010; Vanoosthuyse et al, 2007).
The budding yeast Saccharomyces cerevisiae is a valuable model organism for studying CIN and aneuploidy as it can tolerate high levels of CIN that are similar to what is frequently observed in cancer. We previously determined how cells initially adapt to extremely high rates of chromosome missegregation by growing budding yeast cells carrying mutations that decrease CPC function (Ravichandran et al, 2018). The budding yeast homologs of the CPC subunits Aurora B, INCENP, Survivin, and Borealin are Ipl1, Sli15, Bir1, and Nbl1, respectively (Fig 1A). By sequencing populations of yeast that were grown in the absence of the CPC subunit Survivin/Bir1, we found that the yeast adapted by acquiring specific aneuploidies that decreased the rate of CIN (Ravichandran et al, 2018). The compositions of karyotypes were further refined over time until the cells acquired certain combinations of beneficial aneuploid chromosomes. However, these adapted cells were still substantially less fit than wild‐type due to a combination of the negative effects of the acquired aneuploidies and residual chromosomal instability. It is currently not known how cells with high rates of CIN and aneuploidy further adapt after the initial optimization of their karyotypes.
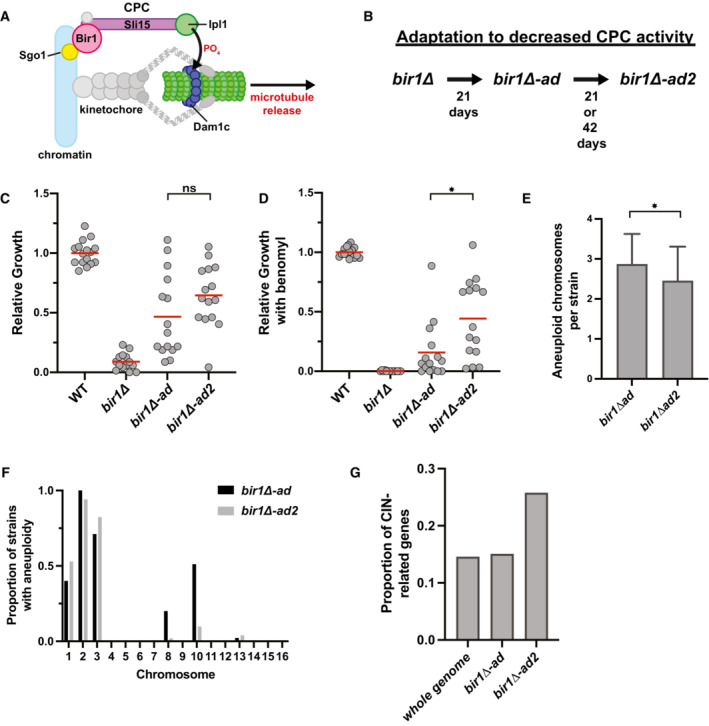
Figure 1. Strains with high levels of CIN and aneuploidy adapt by decreasing CIN.
-
ASchematic summarizing the main localization of the CPC and its role in phosphorylating the Dam1 complex (Dam1c) to destabilize microtubule attachments.
-
BSummary of the adaptation time‐line. bir1∆ strains from tetrads were grown clonally for 21 days to produce bir1∆‐ad strains. Colonies from the bir1∆‐ad strains were then grown in liquid media for 21 or 42 additional days to produce the bir1∆‐ad2 strains.
-
C, DGrowth comparisons of wild‐type (WT, n = 16), unadapted (bir1∆, n = 14), partially adapted (bir1∆‐ad, n = 15), and further adapted (bir1∆‐ad2, n = 15) strains as measured by area of growth after serial dilution. The serial dilutions of all these strains were carried out once in parallel. Examples of dilutions series such as those used in the quantification for bir1∆‐ad and bir1∆‐ad2 strains can be found in Fig EV1C. Ten‐fold serial dilutions were made on YPAD plates containing either 0.1% DMSO (C) or 10 μg/ml of benomyl (D). The mean (red line) is shown. Growth was normalized to WT.
-
EComparison of the mean number of aneuploid chromosomes per strain for the bir1∆‐ad (n = 45 strains) and bir1∆‐ad2 (n = 51 strains) collections. Only data from bir1∆‐ad strains that were subjected to additional adaptation are shown. Means and standard deviations are shown.
-
FHistogram showing the proportion of strains that are aneuploid for specific chromosomes in partially adapted (bir1∆‐ad) and further adapted (bir1∆‐ad2) strains.
-
GEight hundred and seventy‐four genes out of 6,002 total yeast genes (14.5%) have phenotypes related to CIN. The percentage of newly identified mutations in the bir1∆‐ad2 (26 out of 98) and bir1∆‐ad (37 out of 246) strains that are related to CIN are shown.
Data information: (ns) not significant; (*) P < 0.05; unpaired t‐test.
In this study, we have now monitored these CPC‐deficient cells for longer periods of time to determine how cells evolve to cope in the long term with increased levels of both CIN and aneuploidy. We aimed to determine whether they adapt through genetic changes that either (i) allow for better aneuploidy tolerance, (ii) decrease the levels of CIN, or (iii) further optimize their karyotypes. We found that cells evolved through hypomorphic mutations in essential genes that decreased the levels of CIN. The lower levels of CIN then allowed the cells to decrease their levels of aneuploidy, as the beneficial effects of the extra chromosomes no longer outweighed the fitness costs. The identified mutations fall into two broad functional categories. The first category of mutations destabilizes kinetochore–microtubule interactions, counteracting the overstabilization created by decreased CPC activity. These mutations were found in outer kinetochore proteins that directly interact with microtubules. The second category of mutations affect the function of the CPC more directly. These include mutations in the CPC itself, the mitotic kinase Mps1, and the ubiquitin ligase SCFCdc4 complex. SCFCdc4 mutations increase the recruitment of the CPC to key regulatory regions in both yeast and human cells. We conclude that cells generally adapt to high levels of CIN and aneuploidy through mutations that alleviate the CIN phenotype. Furthermore, we have identified specific pathways of adaptation to defects in CPC function.
Results
Adaptation to high levels of CIN and aneuploidy occurs through point mutations that reduce the rate of CIN
To determine how cells adapt to high rates of CIN and aneuploidy, we started with a collection of haploid yeast strains that were previously cultured for 21 days via clonal expansion following BIR1 deletion (Ravichandran et al, 2018). We call these partially adapted strains bir1Δ‐ad. These strains have a high frequency of specific aneuploidies that decrease the rate of CIN. However, they are not enriched for mutations that are related to CIN. We adapted 68 of these strains for an additional 21 or 42 days (bir1Δ‐ad2) in liquid culture to determine how cells continue to evolve after their initial adaptation through aneuploidy (Fig 1B). In comparison with the clonal expansion of the initial adaptation, liquid culture adaptation allows time for rarely occurring beneficial genomic changes to take over the population competitively. This additional adaptation greatly improved the growth of some strains but did not result in a significant level of growth improvement for all of the adapted populations combined (Fig 1C). Cells with impaired chromosome segregation are especially sensitive to microtubule‐depolymerizing drugs. Consistent with this, BIR1 deletion results in a strong sensitivity to moderate amounts of the microtubule‐depolymerizing drug benomyl (Makrantoni & Stark, 2009). Many of the bir1Δ‐ad2 strains have strongly decreased benomyl sensitivity in comparison with the bir1Δ‐ad strains, suggesting that they have acquired additional changes that suppress the CIN phenotype (Fig 1D). To determine whether this additional adaptation occurs through further increased aneuploidy, we measured chromosome copy numbers via read counts from whole‐genome sequencing. Two strains that were observed to have large segmental amplifications of chromosomes 2 and 13 were excluded from this analysis. Overall, the bir1Δ‐ad2 strains have decreased numbers of aneuploid chromosomes, suggesting that these strains do not adapt through further increases in aneuploid chromosomes that attenuate CIN (Fig 1E and F). We conclude that the additional adaptation to CIN in the further adapted CPC‐deficient strains likely comes from specific mutations rather than chromosome copy number alterations.
Adaptation to CIN and aneuploidy could potentially come from mutations that either decrease CIN or increase aneuploidy tolerance. To determine whether mutations in the adapted strains fall into these categories, we searched for nonsynonymous mutations that arose during liquid culture adaptation in the whole‐genome sequencing data. We identified 97 such mutations in 68 strains. These mutations were enriched in genes related to CIN (Fig 1G). Mutations that increase aneuploidy tolerance were previously reported in budding yeast (Torres et al, 2010). However, there was no overlap between the 22 genes identified in the aneuploidy tolerance screen and the genes mutated in the bir1Δ‐ad2 strains. We therefore used the most heavily characterized gene whose loss leads to aneuploidy tolerance, the ubiquitin‐specific protease UBP6 (Torres et al, 2010). We tested whether a mutation in UBP6 that was previously reported to increase aneuploidy tolerance would increase resistance to bir1Δ. The mutation was introduced into a haploid strain whose only copy of BIR1 is on a minichromosome that also contains the URA3 gene. This minichromosome can be selected against using 5‐Fluoroorotic acid (5‐FOA), which is converted into a toxin by the URA3 gene product. The addition of the ubp6(E256X) mutation did not increase viability after BIR1 was deleted (Fig EV1A). This result suggests that aneuploidy tolerance does not lead to CIN tolerance following BIR1 deletion. We note that we used the same strain background as the studies that identified and characterized the role of UBP6 in tolerating aneuploidy (W303), which has been shown to have a mutation in the SSD1 gene that makes them more sensitive to aneuploidy (Hose et al, 2020). We conclude that cells with high levels of CIN and aneuploidy adapt primarily through mutations that mitigate CIN rather than aneuploidy.
Figure EV1. CIN‐related mutations in bir1∆‐ad2 strains are associated with decreased aneuploidy.
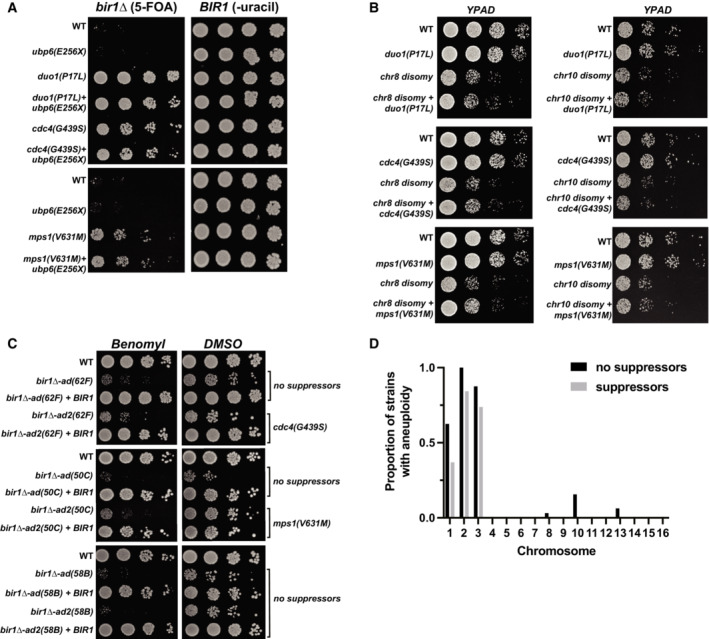
- Ten‐fold serial dilutions on the indicated media are shown. Strains engineered with the ubp6(E256X) aneuploidy tolerance mutation show no bir1∆ suppression. Strains with both the ubp6(E256X) mutation and a bir1∆ suppressor mutant do not additionally rescue the loss of CPC activity.
- Ten‐fold serial dilutions on the indicated media are shown. Strains have disomy of either chromosome 8 (chr8) or chromosome 10 (chr10) with or without the bir1∆ suppressor mutations. The combination of either type of disomy with any of the three suppressors shows no change in growth.
- Ten‐fold serial dilutions of the indicated strains were made on YPAD plates containing either 0.1% DMSO or 10 μg/ml of benomyl. Strains were adapted to persistent loss of CPC function for different amounts of time with or without BIR1 then restored. Those bir1∆‐ad strains that developed suppressor mutations after the extended growth period (bir1∆‐ad2(62F) and bir1∆‐ad2(50C)) were better able to cope with benomyl than one that did not develop suppressors (bir1∆‐ad2(58B)).
- Histogram showing the proportion of strains that are aneuploid for specific chromosomes in bir1∆‐ad2 strains that have identified suppressor mutations, and those that do not.
bir1Δ suppressor mutations fall into four major categories
To determine which categories of mutations are most prevalent in our adapted strains, we searched for the enrichment of functional gene ontology (GO) terms. We found significant enrichment of genes related to “chromosome segregation” (FDR = 3.53 × 10−5) and “SCF‐dependent proteasomal ubiquitin‐dependent protein catabolic process” (FDR = 1.3 × 10−1, Table EV2). Further refinement of these categories revealed that the mutations fall largely into four categories (Table 1).
Table 1.
Candidate mutations identified in bir1∆‐ad2.
| Gene mutated | Residue changes | Tested | Chromosome | Days of liquid adaptation | Essential | Basic function |
|---|---|---|---|---|---|---|
| DUO1 | P17L | Yes | 7 | 21 | Yes | Kinetochore–microtubule attachment |
| DAD1 | N43S | Yes | 4 | 21 | Yes | |
| DAD2 | K11Q | Yes | 11 | 21 | Yes | |
| ASK1 | S216F | Yes | 11 | 21 | Yes | |
| SPC34 | D119A | Yes | 11 | 21 | Yes | |
| NDC80 | K181N | Yes | 9 | 42 | Yes | |
| SPC105 | R583G a | Yes | 7 | 21 | Yes | |
| SLI15 | L71S | Yes | 2 b | 21 | Yes | CPC subunit |
| SLI15 | P109A | No | 2 b | 21 | Yes | |
| SLI15 | G334S | Yes | 2 b | 21 | Yes | |
| CDC53 | K448E | No | 4 | 21 | Yes | S‐phase entry |
| CDC53 | A486P | No | 4 | 21 | Yes | |
| CDC34 | M64T a | Yes | 4 | 21 | Yes | |
| CDC4 | S438G | Yes | 6 | 21 | Yes | |
| CDC4 | G439S | Yes | 6 | 21 | Yes | |
| CDC4 | G652D | No | 6 | 21 | Yes | |
| MPS1 | R596H | Yes | 4 | 21 | Yes | Initiation of SAC |
| MPS1 | W629S | No | 4 | 21 | Yes | |
| MPS1 | V631M | Yes | 4 | 21 | Yes | |
| MIF2 | D241N | Yes | 11 | 42 | Yes | Inner kinetochore |
| RTG2 | G154S | Yes | 7 | 21 | Yes | Mitochondrial sensor |
| RTG2 | H425L | No | 7 | 21 | Yes | |
| RTG2 | A433P | No | 7 | 21 | Yes | |
| SPC97 | S816X | Yes | 8 b | 21 | Yes | Spindle pole body |
Identified in the same strain.
Chromosomes that are commonly aneuploid in bir1∆ strains.
The first category is in proteins that form the outer kinetochore, including mutations in the Dam1 complex, the Ndc80 complex, and Spc105/KNL1. All three of these proteins/protein complexes directly bind to microtubules. The Dam1 complex was the most heavily represented in the data set with mutations in five of the 10 subunits. Mutations in the Dam1 complex subunit Dam1 that mimic Ipl1 phosphorylation were previously shown to suppress CPC mutations (Cheeseman et al, 2002). However, none of the mutations identified in the bir1Δ‐ad2 strains were in the Dam1 protein itself, indicating that they do not directly mimic similar phosphorylation events (Fig 2A). The mutation of lysine 181 in the Ndc80 complex resides in the microtubule binding CHD domain and is similar to the lysine 146 mutation in human Ndc80 that was previously shown to reduce microtubule binding (Ciferri et al, 2008).
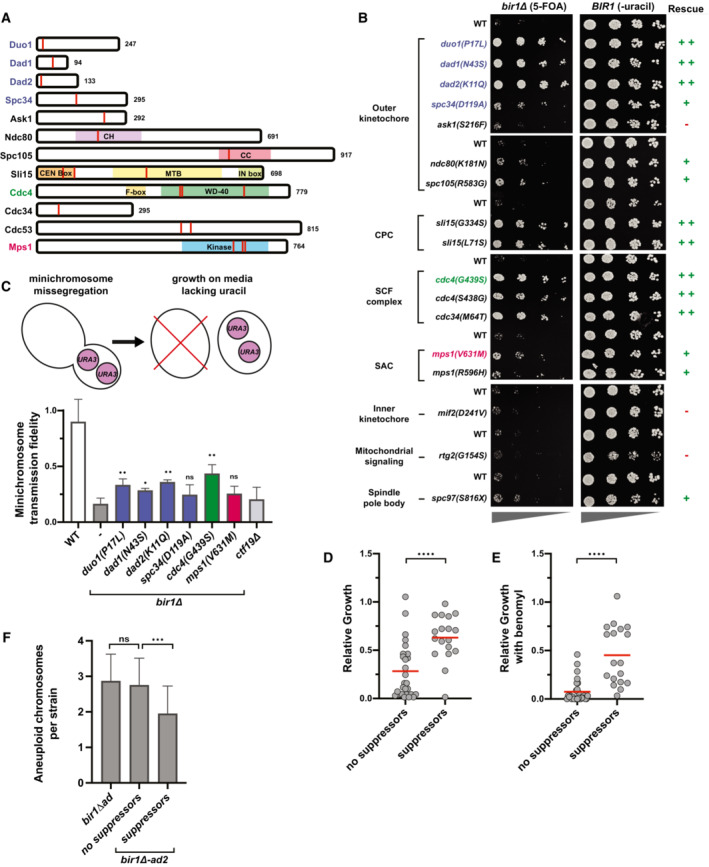
Figure 2. Four main categories of mutations rescue BIR1 deletion.
-
ASchematics showing the location of mutations from Table 1 (red lines). Domains of interest were identified using the Saccharomyces Genome Database.
-
BSerial dilutions of strains engineered with the indicated mutations identified in bir1∆‐ad2 strains were tested for rescue of BIR1 deletion. Ten‐fold serial dilutions on the indicated media are shown. The mutations colored in blue, green, or red were selected for additional characterization.
-
CTop: schematic summarizing how loss of the URA3‐containing plasmid is used to measure relative missegregation rates. Bottom: Proportion of colonies that grow on plates with restrictive (lacking uracil) versus permissive (YPAD) media. Many of the suppressor mutants significantly increase the segregation fidelity of the minichromosome in a bir1∆ background. ctf19∆ serves as a positive‐control for decreased minichromosome transmission fidelity. Statistical significance is relative to bir1∆ alone for three independent experiments. Means and standard deviations are shown.
-
D, EGrowth comparisons of bir1∆‐ad2 strains that contain (18 strains) or do not contain (28 strains) confirmed suppressor mutations as measured by area of colony growth after serial dilution. The mean (red line) is shown. 10‐fold serial dilutions were made on YPAD plates containing either 0.1% DMSO (D) or 10 μg/ml of benomyl (E).
-
FComparison of the mean number of aneuploid chromosomes per strain for bir1∆‐ad2 with and without identified suppressor mutations.
Data information: (ns) not significant; (*) P < 0.05; (**) P < 0.01; (***) P < 0.001; (****) P < 0.0001; unpaired t‐test.
The second category of mutations are in the CPC subunit Sli15. Mutations in Sli15 that disrupt binding to Bir1 or prevent Cdc28 (cyclin‐dependent kinase) phosphorylation were both previously shown to suppress BIR1 deletion (Campbell & Desai, 2013). All three of the observed mutations are consistent with these previous observations, as two were in the CEN box that interacts with Bir1 (L71S and P109H) and one was directly adjacent to the main Cdc28 phosphorylation site (G334S).
The third category of common mutations in the adapted strains is in subunits of the SCF ubiquitin‐protein ligase complex. The SCF is primarily involved in the initiation of S‐phase, but it also has roles in mitosis (Goh & Surana, 1999). We identified mutations in the core SCF subunits Cdc53 and Cdc34 as well as the F‐box adaptor protein Cdc4. F‐box proteins dictate the substrate specificity of the SCF complex (Jonkers & Rep, 2009). Important substrates of SCFCdc4 include the cell cycle kinase inhibitors Sic1 and Far1. No mutations in any other F‐box proteins were identified, suggesting that the ability to suppress BIR1 deletion may be specific to SCFCdc4. All three Cdc4 mutations were in the WD‐40 domain, which directly interacts with SCF complex substrates (Fig 2A). No direct connection between the SCF complex and the CPC has been previously identified.
The final category of potential suppressor mutations was in the mitotic kinase Mps1. All three of the mutations in Mps1 were in the kinase domain (Fig 2A). Mps1 has functions in spindle assembly checkpoint signaling, correction of misattached chromosomes, and spindle pole body duplication (reviewed in Liu & Winey, 2012). We were surprised to identify mutations in Mps1 as the kinase would be expected to promote rather than antagonize CPC activity, and mutations in Mps1 that suppress CPC deficiency have not previously been identified.
Intriguingly, all of the mutations in Table 1 are in essential genes, so they are most likely to be hypomorphic alleles. To determine whether the identified mutations are sufficient to rescue bir1Δ, we introduced mutations from each category prior to BIR1 deletion. In addition, we included mutations identified in the adapted strains in three other potentially interesting proteins: the inner kinetochore protein Mif2, the spindle pole body protein Spc97, and the mitochondrial signaling protein Rtg2. Rtg2 was selected because we identified three independent mutations in this protein. After selection of bir1Δ on 5‐FOA plates, all of the tested mutations at least partially rescued growth with the exception of mif2, rtg2, and ask1 (Fig 2B). We identified multiple mutations that suppress the BIR1 deletion growth phenotype for each of the four major categories. To determine whether the mutants were able to rescue the chromosome missegregation phenotype of BIR1 deletion, we measured the fidelity of minichromosome transmission with representative suppressor mutations from the outer kinetochore, SCF complex, and Mps1 categories. We did not perform any further experiments with the Sli15 mutations, as we previously characterized similar mutations (Campbell & Desai, 2013). For the SCF complex, we analyzed mutations that we identified in Cdc4, as mutations in the F‐box subunit are less likely to have pleiotropic effects. Minichromosome transmission fidelity was higher than the bir1Δ control for mutations in all three categories, although the degree of rescue for the Mps1 mutation was not significant (P = 0.1, Fig 2C). We have therefore identified four categories of mutations—in the outer kinetochore, the CPC, the SCF, and Mps1—that are frequently mutated to suppress the chromosome missegregation phenotype of yeast with impaired CPC activity.
We next wanted to test whether the suppressor mutations could affect aneuploidy tolerance in addition to CIN tolerance. Suppressor mutations in the Dam1 complex, SCF complex, and Mps1 did not suppress the growth defects caused by an extra copy of either chromosome 8 or 10 (Fig EV1B). In addition, a combination of these mutations with ubp6(E256X) did not show any synergistic effects (Fig EV1A). To determine whether the remaining growth impairment in the further adapted strains was primarily due to aneuploidy or CIN, we added wild‐type BIR1 to a subset of the bir1∆‐ad and bir1∆‐ad2 strains and tested their growth. Even in the further adapted strains, re‐addition of BIR1 greatly improved their fitness (Fig EV1C). We conclude that even though the suppressor mutations act exclusively by suppressing the CIN phenotype, the residual CIN is still the primary contributor to the growth defects in the further adapted strains.
We next wanted to determine the degree to which the identified mutations contribute to adaptation. We compared the growth of bir1Δ‐ad2 strains that either do or do not contain identified suppressor mutations. All mutations listed in Table 1 were considered suppressors for this analysis, with the exception of genes with mutations that did not rescue the BIR1 deletion growth phenotype (MIF2, RTG2, and ASK1). On average, adapted strains that contain identified suppressor mutations grew substantially better than those without suppressors, suggesting that we identified most of the impactful mutations and that they contributed greatly to the adaptation (Fig 2D and E). This difference was significant either with or without the addition of benomyl. Other genetic or epigenetic changes that alter protein abundance could also contribute to adaptation under these conditions; however, the strong correlation between the identified suppressor mutations and the growth phenotype indicates that these alterations would have a relatively small contribution. Furthermore, the degree of aneuploidy is significantly reduced in the strains with suppressor mutations, indicating that these mutations decreased the need for aneuploid chromosomes to suppress the bir1Δ phenotype (Fig 2F). This decrease was seen across all of the observed aneuploid chromosomes (Fig EV1D). The aneuploidy burden in the further adapted strains was therefore reduced by decreasing the requirement for aneuploidy rather than decreasing the impact of aneuploidy on cellular fitness. These results provide a time line of events for adaptation to high rates of CIN. First, the cells acquire specific aneuploidies that suppress the CIN phenotype. The cells then acquire optimal combinations of aneuploidies (Ravichandran et al, 2018). Next, point mutations arise that more specifically target the source of the CIN leading to a reduction in CIN. Finally, the level of aneuploidy decreases to relieve the fitness burden placed on the cell.
Suppressor mutations in the Dam1 complex create unattached kinetochores
We next sought to determine where in the chromosome biorientation pathway each of these categories of mutations falls. Defects in CPC activity lead to the overstabilization of kinetochore–microtubule attachments and the failure to correct attachments where both sister chromatids attach to microtubules emanating from the same pole (syntelic attachments; Biggins et al, 2001; Pinsky et al, 2006). Mutations that rescue CPC defects are therefore likely to restore the higher turnover of kinetochore–microtubule attachments. This could occur either by increasing the activity of the CPC or by decreasing the microtubule binding activity of kinetochores. To determine whether the mutations act downstream of the CPC, we tested whether they could rescue a temperature‐sensitive mutation in the CPC kinase Ipl1/Aurora B. Of the mutations tested, only the Dam1c mutants significantly rescued ipl1‐321 at the restrictive temperature (Fig 3A). Although the Dam1c suppressor mutations rescue the temperature‐sensitive IPL1 mutation, they could not rescue a full deletion of SLI15, suggesting that they do not completely bypass the need for CPC activity (Fig EV2A). These results indicate that the Dam1c mutations affect the pathway downstream of the CPC, whereas the SCF complex and Mps1 potentially affect the CPC itself.
Figure 3. Suppressor mutations in the Dam1 complex result in elevated spindle assembly checkpoint activity and minichromosome missegregation.
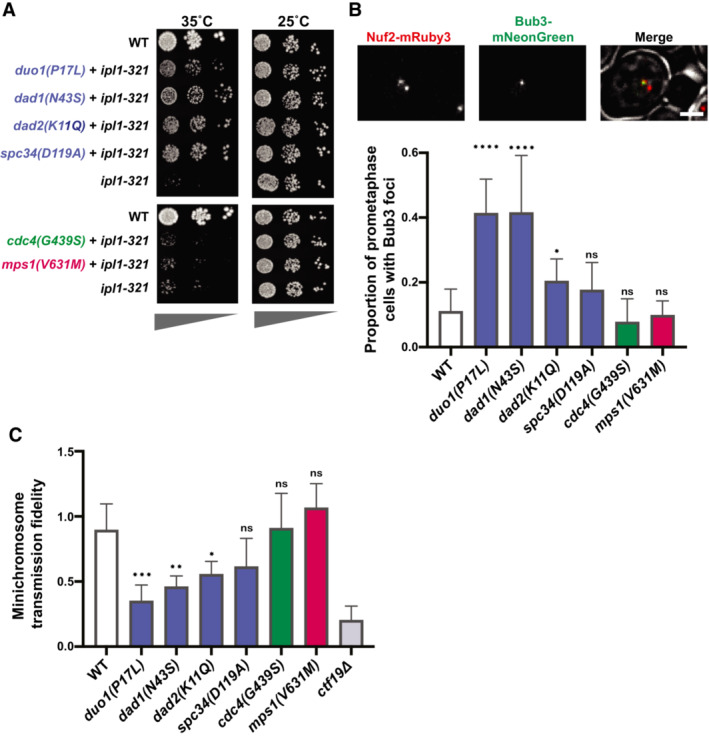
- Serial dilutions on YPAD media at the permissive (25°C) or restrictive (35°C) temperatures for the ipl1‐321 mutation. The suppressor mutations from the Dam1c (blue) were the only mutants that rescued viability at the restrictive temperature.
- Top: representative images showing Bub3‐mNeonGreen localization to kinetochores (Nuf2‐mRuby3), indicating spindle assembly checkpoint activation in wild‐type yeast. Scale bar is 2 μm. Bottom: quantification of the proportion of prometaphase cells with Bub3‐mNeonGreen foci. Only the mutations in Dam1 complex (blue) show a significant increase in SAC activity. Data are from at least five independent experiments. Means and standard deviations are shown.
- Proportion of colonies that grow on plates with restrictive (lacking uracil) versus permissive (YPAD) media after 24 h growth under permissive conditions with a URA3‐containing plasmid. The suppressor mutations in the Dam1c (blue) show a significant reduction of minichromosome segregation fidelity. Data are from three independent experiments. Means and standard deviations are shown.
Data information: (ns) nonsignifcant; (*) P < 0.05; (**) P < 0.01; (***) P < 0.001; (****) P < 0.0001; unpaired t‐test.
Figure EV2. Double mutants of bir1∆ suppressors in the Dam1 complex and the dam1(S20D) phosphomimic have strong synthetic phenotypes.
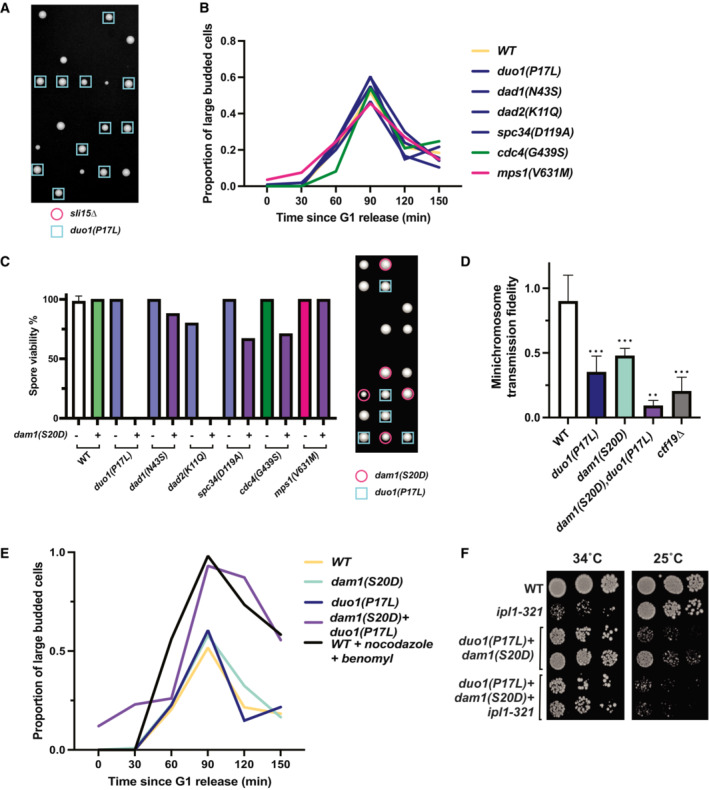
- Tetrad dissections performed on diploids with both sli15∆ and the duo1(P17L) did not produce any viable spores with either sli15∆ or a combination of sli15∆ and duo1(P17L).
- Cell cycle progression as measured by percentage of large budded cells over time after release from G1 arrest. None of the tested suppressor mutants cause a mitotic delay.
- Tetrad dissections of diploids heterozygous for both a bir1∆ suppressor mutant and the dam1(S20D) phosphomimic mutant. Presence of either the suppressor mutant or dam1(S20D) was determined by antibiotic resistance conferred by a gene integrated downstream of the mutations (clonNAT and G418 respectively). Colonies from haploid spores containing either dad2(K11Q), dam1(S20D) or duo1(P17L), dam1(S20D) were extremely rare. Spore viability of each genotype was counted in tetrads whose type (parental ditype, nonparental ditype, or tetratype) could be distinguished. Quantification for each mutant is from a single plate, and the WT numbers are the averages from all six plates. The representative image to the right of the graph depicts six tetrads, demonstrating examples of three tetratypes, two nonparental ditypes, and a single parental ditype. In this example, the expectant duo1(P17L), dam1(S20D) double‐mutant formed a colony from 0 out of seven spores.
- Proportion of colonies that grow on plates with restrictive (lacking uracil) versus permissive (YPAD) media after 24 h growth under permissive conditions with a URA3‐containing plasmid. duo1(P17L), dam1(S20D) double mutants have a strong decrease in transmission fidelity. Data are from three independent experiments Means and standard deviations are shown.
- Cell cycle progression as measured by percentage of large budded cells over time after release from G1 arrest. Rare surviving duo1(P17L), dam1(S20D) double mutants have a strong mitotic delay. Wild‐type (WT) cells treated with nocodazole and benomyl were used as a positive control for mitotic arrest.
- Serial dilution showing growth of strains containing either the double‐mutant (duo1(P17L), dam1(S20D)), the ipl1‐321 temperature‐sensitive allele, or a combination of all three mutations. At the restrictive temperature (34°C), the double‐mutant rescues growth of the ipl1‐321 allele. Ten‐fold serial dilutions were done on YPAD plates at the indicated temperatures and were grown for 48 h.
Data information: (ns) not significant; (*) P < 0.05; (**) P < 0.01; (***) P < 0.001; unpaired t‐test.
If the mutations rescue by globally destabilizing kinetochore–microtubule attachments, then the number of unattached kinetochores should increase even in the presence of Bir1. Since unattached kinetochores trigger the spindle assembly checkpoint, we first determined whether the mutations induce a delay in mitosis. None of the identified mutations showed substantial changes in cell cycle duration, as measured by the sustained accumulation of large budded cells over time after release from synchronization in G1 (Fig EV2B). These mutations therefore do not maintain sustained checkpoint activity. As a more sensitive assay, we monitored the presence of unattached kinetochores by measuring the frequency of cells with foci of the checkpoint protein Bub3 that colocalize with kinetochore clusters. For this assay, we only counted cells that have two kinetochore clusters that are separated yet still in close proximity, indicative of a stage around the prometaphase to metaphase transition. At this stage, three of the four Dam1c mutations resulted in significantly more cells with Bub3‐mNeonGreen foci than the wild‐type control (Fig 3B). By contrast, suppressor mutations in Cdc4 and Mps1 did not increase the frequency of Bub3 foci. We conclude that suppressor mutations in the Dam1 complex transiently increase the number of unattached kinetochores, likely through increased kinetochore–microtubule attachment turnover.
The suppression of the bir1Δ chromosome missegregation phenotype through an increase in unattached kinetochores in Dam1 complex mutants suggests that there is a restoration of the balance between microtubule attachment and detachment when both mutations are combined. If this were the mechanism of rescue, then we would expect that in the absence of BIR1 deletion, the increase in kinetochore–microtubule attachment turnover in the Dam1 mutants would overly destabilize attachments and increase the chromosome missegregation rate. To test this, we measured the rate of minichromosome transmission in strains that have suppressor mutations and wild‐type BIR1. Similar to the results of the Bub3 foci counts, three of the four Dam1c mutants showed a significant decrease in minichromosome transmission fidelity (Fig 3C). The Cdc4 and Mps1 mutations had no measurable effect. The Dam1c mutation that did not show a significant result in either the unattached kinetochore or chromosome segregation assays, spc34(G439S), also had the weakest rescue of growth following BIR1 deletion (Fig 2B). Overall, these results demonstrate that mutations in outer kinetochore proteins rescue deficient CPC activity by destabilizing kinetochore–microtubule attachments. By contrast, Cdc4 and Mps1 suppressor mutations act through an alternative mechanism that does not directly affect the stability of kinetochore–microtubule attachments.
Dam1c suppressor mutations have similar phenotypes to a phosphomimic mutation that decreases Dam1c microtubule binding
Phosphomimics of Ipl1 phosphorylation sites on the Dam1 subunit of the Dam1 complex were previously shown to partially rescue ipl1‐ts mutants (Cheeseman et al, 2002). These phosphosites include serine 20 (dam1(S20D)) near the N‐terminus and three serines (S257, S265, S292, dam1(3D)) closer to the C‐terminus of the protein (Fig 4A). The two phosphomimic mutants change the serines to negatively charged aspartic acid residues. The S20D mutation has been demonstrated to reduce microtubule binding in vitro, whereas the 3D mutant results in activation of the mitotic checkpoint (Jin & Wang, 2013; Sarangapani et al, 2013). To determine whether the identified suppressor mutations function similarly to the phosphomimics, we first determined whether the dam1(S20D) or dam1(3D) mutations rescue BIR1 deletion. Intriguingly, dam1(S20D) rescues BIR1 deletion but dam1(3D) does not, indicating that these mutants are mechanistically distinct (Fig 4B). Both mutations increase the frequency of Bub3 foci in prometaphase cells, similarly to the Dam1c suppressor mutations that we identified (Fig 4C). However, the 3D mutant shows a strong cell cycle delay, further indicating that it differs from the other Dam1c mutants in either mechanism or severity (Fig 4D). Despite the delay, the 3D mutant does not affect minichromosome transmission fidelity (Fig 4E). This mitotic delay without any decrease in chromosome segregation fidelity has been previously reported for the dam1(3D) mutation and may indicate a defect in SAC silencing after chromosome biorientation (Jin & Wang, 2013). The dam1(S20D) mutation, however, does show a decrease in minichromosome transmission in line with the suppressor mutations (Fig 4E). We conclude that the mutations in the Dam1 complex that adapt to suppress BIR1 deletion have a similar phenotype to the dam1(S20D) mutation. Intriguingly, the S20D phosphomimic mutation was shown to disrupt microtubule binding of kinetochores in vitro, whereas the triple serine phosphomimic (3D) mutant maintained wild‐type microtubule binding activity (Sarangapani et al, 2013). The similarity in phenotype between the suppressor mutations and dam1(S20D) suggests that the suppressor mutations may also directly decrease kinetochore–microtubule affinity.
Figure 4. Suppressor mutations in the Dam1 complex have phenotypes similar to the dam1(S20D) phosphomimic mutation.
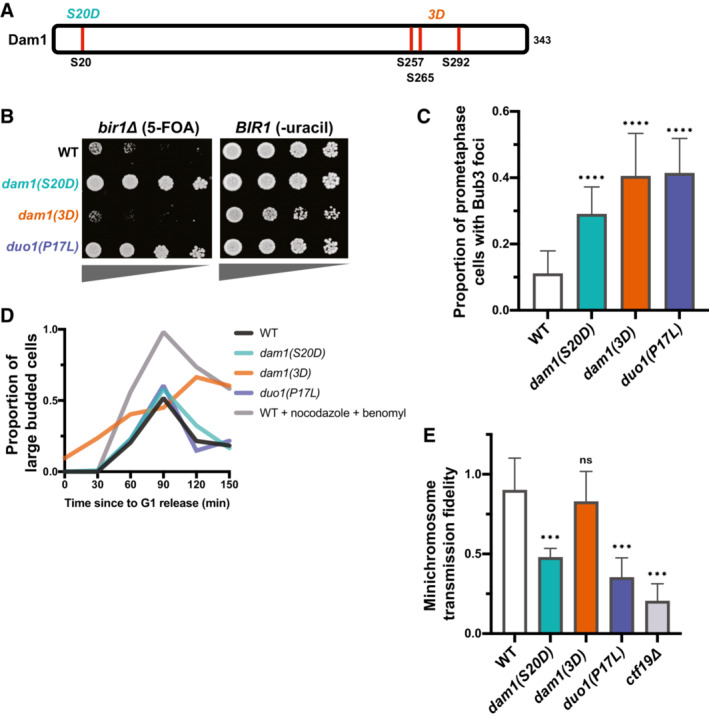
- A schematic showing the relative locations of the Ipl1 phosphosites in Dam1 that are mutated to aspartic acid in the phosphomimics.
- Serial dilutions of strains engineered with the indicated mutations were tested for rescue of BIR1 deletion. Ten‐fold serial dilutions on the indicated media are shown. dam1(S20D) rescues loss of CPC activity to a similar extent as a suppressor mutant in Duo1.
- Quantification of the proportion of prometaphase cells with Bub3‐mNeonGreen foci localized to kinetochores (Nuf2‐mRuby3), indicating SAC activation in all three mutant strains. Data are from at least five independent experiments. Means and standard deviations are shown.
- Degree of cell cycle delay as measured by percentage of large budded cells over time after release from G1 arrest. Wild‐type (WT) cells treated with nocodazole and benomyl were used as a positive control for mitotic arrest.
- Proportion of colonies that grow on plates with restrictive (lacking uracil) versus permissive (YPAD) media after 24 h growth under permissive conditions with a URA3‐containing plasmid. Minichromosome segregation fidelity is reduced in the dam1(S20D) strain, similar to the duo1(P17L) strain. Data are from three independent experiments. Means and standard deviations are shown.
Data information: (ns) nonsignifcant; (***) P < 0.001; (****) P < 0.0001; unpaired t‐test.
If both the Dam1c suppressor mutations and dam1(S20D) act through destabilizing kinetochore–microtubule attachments, we hypothesized that combining the two mutants might destabilize the connections to a greater extent. Indeed, combination of either duo1(P17L) or dad2(K11Q) with dam1(S20D) resulted in zero viable spores after 3 days of growth (Fig EV2C). For the duo1(P17L), dam1(S20D) double mutant, rare colonies grew up after 5 days. These double‐mutant cells displayed severe minichromosome loss and a strong metaphase delay, as expected for high rates of unattached kinetochores (Fig EV2D and E). Intriguingly, the double mutant was able to rescue loss of IPL1 activity to an extent even greater than the single mutants (Fig EV2F). However, the reverse interaction was not observed, as the ipl1‐321 mutation did not improve the growth of the duo1(P17L), dam1(S20D) double mutant at the restrictive or permissive temperature. This could potentially be due to Ipl1 mutations preferentially stabilizing specific kinetochore–microtubule attachment states, whereas mutations in the kinetochore would destabilize all attachments indiscriminately. We conclude that the suppressor mutations in the Dam1 complex and the dam1(S20D) mutant both act by destabilizing kinetochore–microtubule attachments. The combination of the mutants increases the severity of the phenotype to create an extended spindle assembly checkpoint arrest and cell death.
Dam1c suppressor mutations lie proximal to multimerization interfaces and decrease spindle localization in vivo
To determine a potential mechanism of action for the Dam1c suppressor mutations, we mapped them onto the existing crystal structure of the Chaetomium thermophilum version of the complex (Jenni & Harrison, 2018). Three of the mutations are in helices that lie near interfaces of oligomerization between Dam1c decamers. spc34(D119A) is found in a region that corresponds to a short helix that forms part of interface 1. The dad1(N43S) mutation affects a highly conserved residue that lies on the surface of interface 2 (Fig 5A). The dad2(K11Q) mutation affects a highly conserved lysine that is also proximal to interface 2 but is not directly on the surface. The duo1(P17L) mutation lies in a region outside of the crystal structure. However, the affected residue would likely be near the N‐terminal part of Duo1, which resides in interface 1. These interfaces are proposed to be important for the ring formation that allows the complex to encircle microtubules. It has been noted that serine 20 of the Dam1 protein is potentially also located in the vicinity of interface 1 (Jenni & Harrison, 2018). These results suggest that the suppressor mutants could act by limiting higher order oligomerization of the Dam1 complex.
Figure 5. Suppressor mutations in the Dam1 complex reduce spindle localization.
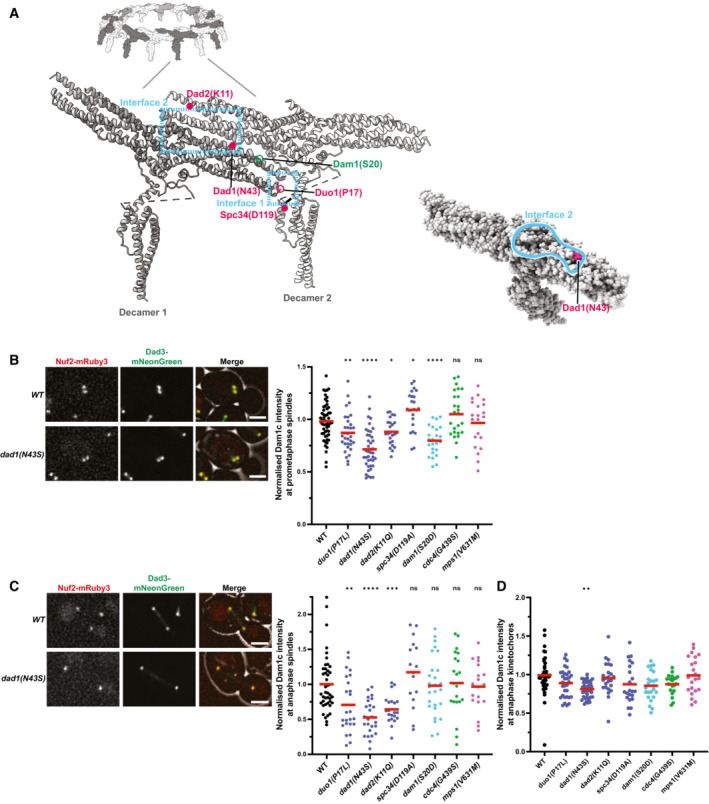
- View of the Chaetomium thermophilum Dam1c‐ring, with focus on two adjacent protomers (labeled decamers 1 and 2). Interfaces 1 and 2 between adjacent decamers are shown with the blue dashed boxes as previously identified by Jenni & Harrison (2018). Mutations in the Saccharomyces cerevisiae proteins have been labeled based on sequence homology with C. thermophilum. Mutations within the structure are depicted as solid circles. Those that are located outside of the structured domains are shown as open‐circles. Suppressor mutations identified in this study are colored magenta, and the residues mutated in the Dam1 phosphomimics are colored green. The marked residues are the closest approximations of the residues mutated in S. cerevisiae. N43 of Dad1 is conserved in C. thermophilum. The Dad2 residue K11 in S. cerevisiae is homologous to K35 in C. thermophilum. D119 of Spc34 maps to a short helix that contains H117 in C. thermophilum. The bottom right shows the location of the Dad1 residue N43 on the surface of interface 2. Images were made using ChimeraX (Pettersen et al, 2021).
- Left: representative images showing the localization of Dad3‐mNeonGreen (Dam1c member) and Nuf2‐mRuby3 (kinetochore marker) in prometaphase cells. Scale bar is 2 μm. Right: quantification of the intensity of Dad3‐mNeonGreen at kinetochores/spindles in prometaphase cells. Each individual point on the graph represents a single measurement of Dad3‐mNeonGreen intensity; up to 10 measurements per replicate. Data are from five independent experiments.
- Left: representative images showing the localization of Dad3‐mNeonGreen (Dam1c member) and Nuf2‐mRuby3 (kinetochore marker) in anaphase cells. Scale bar is 2 μm. Right: quantification of the intensity of Dad3‐mNeonGreen at the spindle in anaphase cells. The intensity was measured in the middle of the spindle using a perpendicular line‐scan. Each individual point on the graph represents a single measurement of Dad3‐mNeonGreen intensity; up to 10 measurements per replicate. Data are from five independent experiments.
- Quantification of the intensity of Dad3‐mNeonGreen at kinetochores in anaphase cells. Kinetochore position was based on Nuf2‐mRuby3 localization. The differences in localization at the anaphase kinetochores are much weaker than those measured along the spindle (Fig 5C). Each individual point on the graph represents a single measurement of Dad3‐mNeonGreen intensity; up to 10 measurements per replicate. Data are from five independent experiments. The mean (red line) is shown.
Data information: (ns) nonsignifcant; (*) P < 0.05; (**) P < 0.01; (***) P < 0.001; (****) P < 0.0001; unpaired t‐test.
The oligomerization of the Dam1 complex into rings is primarily associated with microtubule binding (Miranda et al, 2005; Westermann et al, 2005). We therefore determined whether the Dam1c mutants affect the localization of the complex to microtubules and kinetochores. We measured the amount of the Dam1 complex member Dad3 labeled with the fluorophore mNeonGreen at prometaphase and anaphase spindles in the presence of the suppressor mutants. The intensity of Dad3‐mNeonGreen was significantly reduced at both prometaphase kinetochores/spindles and anaphase spindles for the three Dam1c suppressor mutations with the strongest rescue phenotypes (Fig 5B and C). The dam1(S20D) mutation also has a significant decrease in Dad3 localization in prometaphase. No significant decreases in Dad3 spindle localization were observed for cdc4(G439S) or mps1(V631M). Interestingly, the decrease in localization for the Dam1c mutants was much more pronounced along the anaphase spindle than at anaphase kinetochores (Fig 5D). The maintenance of strong localization at kinetochores suggests that the localization phenotypes could result from defects in microtubule binding rather than kinetochore association. We conclude that the Dam1c suppressor mutations decrease the amount of Dam1 complex at microtubules and this potentially occurs by decreasing the ability of the complex to oligomerize.
Mps1 suppressor mutations do not act through previously established pathways
We next wanted to determine how the Mps1 mutations rescue BIR1 deletion. Mps1 kinase functions in activating the spindle assembly checkpoint at unattached kinetochores and recruiting the CPC to the inner centromere for chromosome biorientation (Weiss & Winey, 1996; van der Waal et al, 2012). In yeast, it also has an essential function in spindle pole body duplication (Winey et al, 1991). Disruption of any of these functions would be expected to decrease, rather than increase, the ability of cells to function with reduced CPC activity. One possible explanation for this would be if the Mps1 suppressor mutations have a gain‐of‐function phenotype that increases the kinase's activity. We therefore tested whether the Mps1, Cdc4, and Dam1c suppressors have gain‐of‐function activity by determining whether they have a dominant phenotype in the heterozygous state. Only the Dam1c mutation duo1(P17L) had any improved growth when heterozygous, indicating that neither the Cdc4 nor the Mps1 mutations are gain of function (Fig EV3A). To determine whether the suppressor mutations affect the ability of Mps1 to activate the spindle assembly checkpoint, we measured the percentage of cells with Bub3‐mNeonGreen foci either with or without the addition of nocodazole to depolymerize microtubules and create unattached kinetochores. The levels of Bub3 foci were unaffected by the mps1(V631M) mutation in either the presence or the absence of nocodazole, demonstrating that the mutant cells are capable of activating the spindle assembly checkpoint (Fig EV3B).
Figure EV3. Suppression of bir1∆ by Mps1 mutations occurs through decreased kinase activity that does not inhibit spindle assembly checkpoint activity.
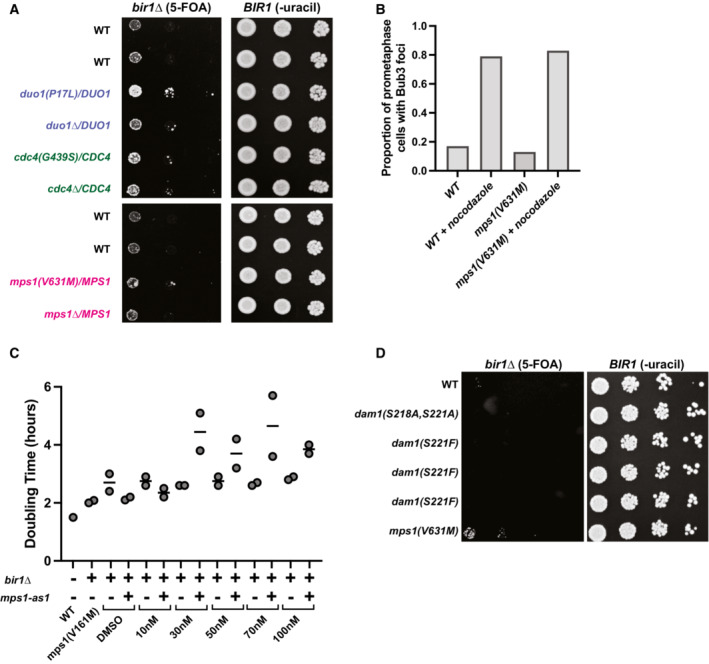
- Ten‐fold serial dilutions on the indicated media are shown. Strains engineered with the indicated mutations were tested for rescue of BIR1 deletion. Diploid strains were heterozygous for either an identified suppressor mutation or a full deletion of the gene.
- Quantification of the proportion of prometaphase cells with Bub3‐mNeonGreen foci with or without 15 μg/ml nocodazole. The mps1(V631M) mutation does not affect the percentage of cells with spindle assembly checkpoint foci. Data are from a single experiment.
- Growth assays conducted with an ATP‐analog (1NM‐PP1) that specifically inhibits the mps1‐as1 allele. Strains with wild‐type Mps1 were not affected by the inhibitor. The WT strain is CCY1905. Data are from two independent experiments.
- Ten‐fold serial dilutions on the indicated media are shown. Strains engineered with the indicated mutations were tested for rescue of BIR1 deletion. Dam1 alleles with nonphosphorylatable mutations in Mps1 phosphosites (S218 and S221) do not rescue growth after bir1∆.
Since the Mps1 suppressor alleles have a recessive, partial loss‐of‐function phenotype and the mutations are located in the kinase domain, we next tested whether a partial loss of Mps1 kinase activity can rescue BIR1 deletion. We used a mutation in the kinase domain that renders it sensitive to ATP analogs (Jones et al, 2005). This mutation, mps1‐as1, decreased the doubling time of bir1Δ cells to a similar extent to the suppressor mutation mps1(V161M) even in the absence of the small molecule inhibitor (Fig EV3C). This result suggests that the analog‐sensitive mutation partially decreases kinase activity on its own, as was previously observed for similar alleles in other kinases (Bishop et al, 2000; Pinsky et al, 2006) and, furthermore, that the Mps1 suppressor mutations affect its kinase activity to a comparable extent. The addition of higher concentrations of the analog inhibitor decreased growth in mps1‐as1,bir1Δ cells, demonstrating that too little Mps1 activity is detrimental to growth even in the absence of Bir1.
We next tested whether the Mps1 mutations are suppressing CPC activity though its phosphorylation of the Dam1 complex subunit Dam1. Mutations that prevent phosphorylation of Dam1 at S218 and S221 by Mps1 kinase decrease kinetochore–microtubule stability and partially rescue temperature‐sensitive Ipl1 mutations (Shimogawa et al, 2006, 2010). However, we did not observe any rescue of BIR1 deletion with these mutants (Fig EV3D). This result agrees with our experiments demonstrating that the Mps1 and Dam1c suppressor mutations act through different mechanisms (Fig 3A–C). We conclude that partial disruption of Mps1 kinase activity results in rescue of BIR1 deletion through a currently unknown mechanism.
The SCFCdc4 complex affects CPC localization to the spindle/kinetochores in prometaphase in yeast and human cells
Finally, we wanted to determine how the SCF mutations rescue BIR1 deletion. We first tested whether a previously identified temperature‐sensitive mutation in CDC4, cdc4‐1, is capable of rescuing loss of Bir1. We also tested a temperature‐sensitive version of another F‐box protein, Met30. Both Cdc4 and Met30 affect the stability of the yeast CENP‐A homolog Cse4 (Au et al, 2020). The cdc4‐1 mutation shows a degree of rescue nearly as strong as the suppressor mutation cdc4(G439S) (Fig 6A). By contrast, a temperature‐sensitive mutation in Met30 failed to show any rescue of BIR1 deletion, demonstrating the specificity of the suppression phenotype for SCFCdc4. We conclude that rescue of bir1Δ results from decreased SCFCdc4 activity and is not unique to the mutations identified in our screen.
Figure 6. Cdc4 limits CPC localization in prometaphase independently of Sgo1.
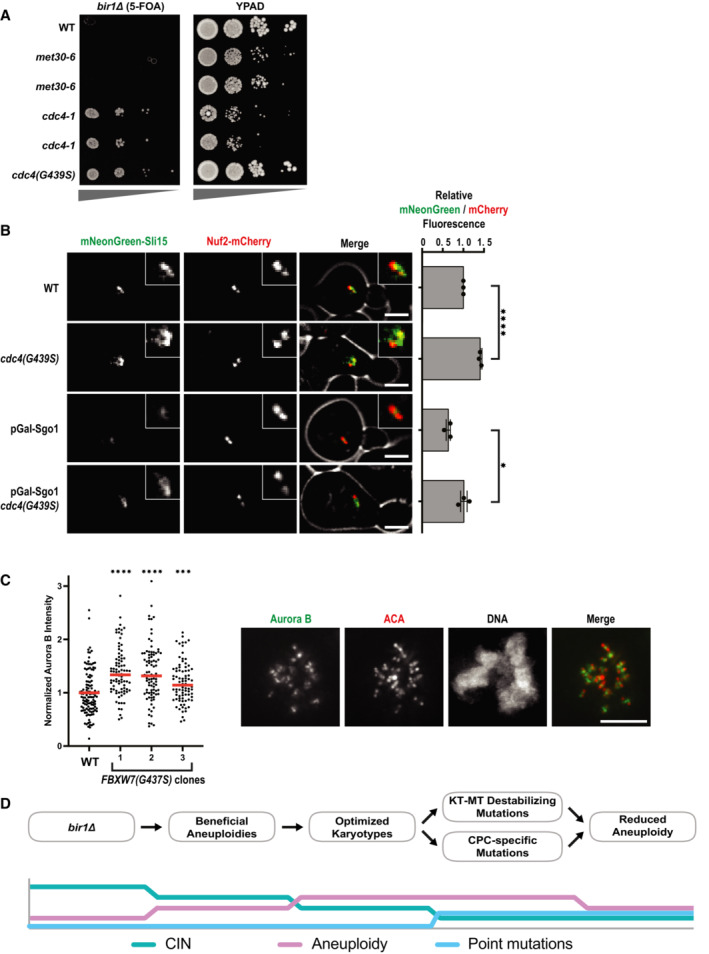
- Serial dilutions of strains engineered with the indicated mutations were tested for rescue of BIR1 deletion. Ten‐fold serial dilutions on the indicated media are shown. The met30‐6 and cdc4‐1 alleles are temperature‐sensitive. Two independent clones of each of these alleles are shown. The serial dilutions were performed at the permissive temperature (20°C).
- The amount of mNeonGreen‐Sli15 (CPC member) located at kinetochores (Nuf2‐mCherry) was measured in prometaphase. Sgo1 was depleted by placing it under a galactose‐inducible promoter and switching to glucose‐containing media (YPAD). Representative images are on the left. Scale bar is 2 μm. Averages from three independent experiments are shown.
- Measurements of Aurora B intensity at the inner centromere of prometaphase kinetochores. Inner centromeres were identified as the regions directly between two centromeres (ACA staining). Images from an example WT cell are shown to the right. Scale bar is 5 μm long. Each data point is an individual chromosome. More than 15 cells for each cell line were measured across two independent experiments. The mean (red line) is shown.
- Time line of adaptation to high levels of CIN through aneuploidy and point mutations.
Data information: (ns) nonsignifcant; (*) P < 0.05; (***) P < 0.001; (****) P < 0.0001; unpaired t‐test.
To determine how Cdc4 activity affects CPC function, we first determined whether the ubiquitin ligase directly degrades the CPC subunits Sli15 or Ipl1. We monitored Sli15 and Ipl1 protein levels in cells that were prevented from expressing new protein by the addition of cycloheximide. Sli15 and Ipl1 protein levels were unchanged by the presence of the cdc4(G439S) suppressor mutation, indicating that this mutation does not rescue BIR1 deletion by increasing the levels of Sli15 or Ipl1 (Fig EV4A). We next tested whether the Cdc4 suppressor mutations change the localization of the CPC. The cdc4(G439S) mutation significantly increased CPC localization to prometaphase spindles/kinetochores by ~50% (Fig 6B). Sli15 expression levels were also unaffected by the mutation at this cell cycle stage, suggesting that the SCF specifically affects CPC localization (Fig EV4B). We wanted to determine whether this mutant also rescues CPC localization in bir1Δ cells. However, bir1Δ greatly reduces Sli15 expression (Campbell & Desai, 2013), so we instead determined whether cdc4(G439S) rescues localization after depletion of Sgo1, the upstream recruiter of Bir1 to the inner centromere (Fig 1A). When compared to wild‐type CDC4, mNeonGreen‐Sli15 localization in cdc4(G439s) mutant cells was significantly increased in prometaphase after Sgo1 depletion, demonstrating that this increase in CPC localization is independent of the Sgo1 recruitment pathway that acts through Bir1 (Fig. 6B). Intriguingly, the effect of cdc4(G439S) on Sli15 localization is only observed prior to anaphase, as the localization differences are no longer observed later in mitosis (Fig EV4C). This suggests that the SCF complex specifically affects CPC localization during chromosome biorientation.
Figure EV4. bir1∆ suppressor mutations in Cdc4 do not affect protein stability of Sli15 or Ipl1.
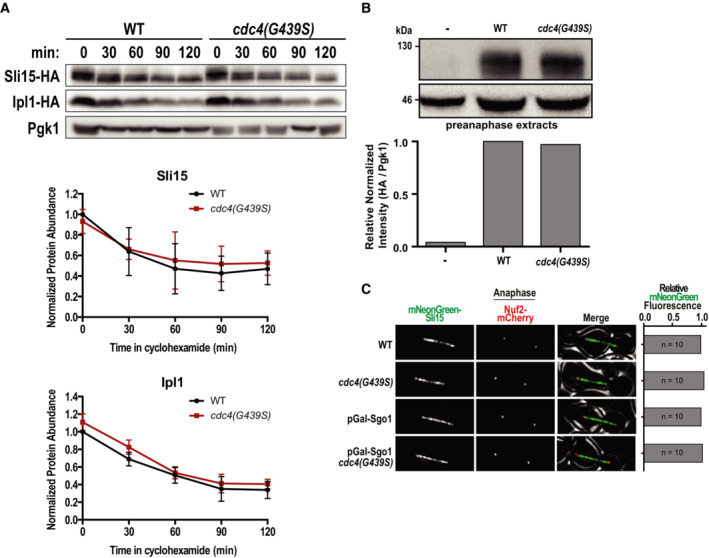
- Western‐blot of a time series of Ipl1 and Sli15 protein levels after inhibition of protein translation with cycloheximide. Cycloheximide was added to cells at time point 0, and cells were harvested and fixed at 30 min intervals. Representative images from one experiment are shown on top and quantification of three independent experiments is shown on bottom. Error bars represent standard deviation of three biological replicates from Western Blot quantifications.
- Western‐blot and quantification of mNeonGreen‐HA‐Sli15 expression levels in cdc4(G439S) expressing cells. Pre‐anaphase protein extracts were prepared as in Fig 6B and probed for Sli15 expression levels via immunoblotting using an HA antibody. Data are from a single experiment.
- The amount of mNeonGreen‐Sli15 spindle localization was measured in anaphase. Sgo1 was depleted by placing the gene under a galactose‐inducible promoter and switching to glucose‐containing media (YPAD). For quantifications, line‐scans perpendicular to the spindle were measured. Representative images are on the left. Scale bar is 2 μm. Data are from a single experiment.
We next tested whether mutations in the human homolog of Cdc4, FBXW7, also affect the accumulation of the CPC at the inner centromere. We therefore engineered the sole copy of FBXW7 in the haploid HAP1 chronic myeloid leukemia cell line with the equivalent of the G439S point mutation in the WD‐40 domain that we identified in adapted bir1Δ cells (Fig EV5A and B). Of note, mutations in this region of the protein are frequently observed in uterine and colon cancer (Yeh et al, 2018). This mutation (G437S in humans) resulted in a slight increase in colony size, consistent with the function of FBXW7 as a suppressor of cell cycle entry (Fig EV5C). Intriguingly, all three cell lines engineered with this mutation showed an increase in Aurora B staining at the inner centromere in prometaphase (Fig 6C). This ~50% increase is similar to what we observe in yeast, indicating that this function of the SCF complex in CPC localization is conserved. We conclude that SCFCdc4/FBXW7 activity limits the recruitment of the CPC to the spindle and/or kinetochores and that reduction of this function partially restores CPC localization when the inner centromere recruitment pathway is disrupted.
Figure EV5. Engineering and characterization of FBXW7 mutant cell lines.
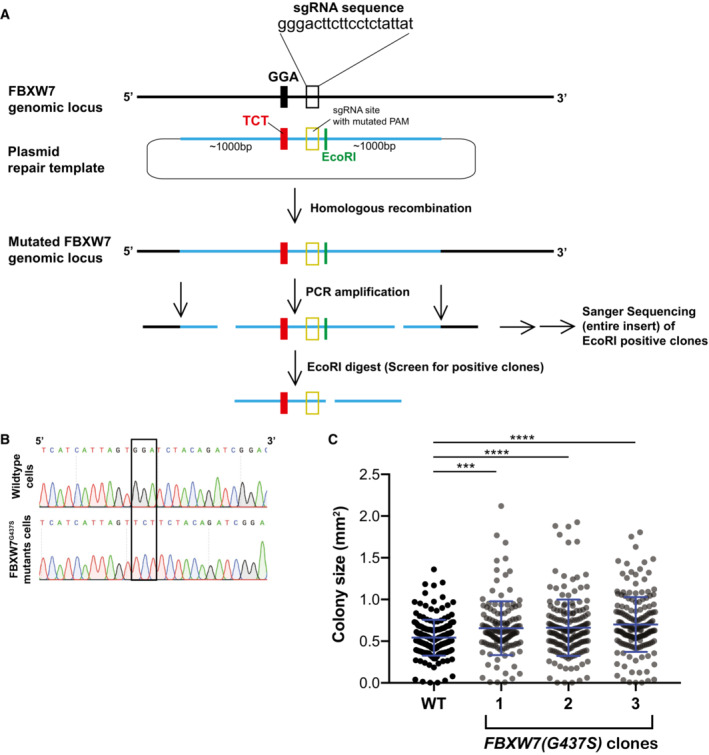
- Strategy implemented to introduce specific point mutations in FBXW7 in HAP1 cells. Transfected cell lines were originally screened for a silent mutation that creates an EcoR1 restriction site.
- Sanger sequencing confirms the presence of the G437S mutation in FBXW7.
- Cell lines mutant for FBXW7 have an increased colony size after 12 days of growth in comparison to a cell line with wild‐type FBXW7. Means and standard deviations are shown. Each individual value point is the size measurement of a single clone. Data are from two independent experiments. (***) P < 0.001; (****) P < 0.0001; unpaired t‐test.
Discussion
In cancers, cells with high levels of CIN and aneuploidy often have overstabilized kinetochore–microtubule connections (Bakhoum et al, 2009a). Although the mechanisms that cause this phenotype are still largely unclear, one way to decrease microtubule turnover at the kinetochore is through decreased CPC activity (Cimini et al, 2006). In this study, we identify multiple mechanisms that cells use to adapt to the overstabilization of microtubules resulting from the deletion of the CPC subunit Bir1/Survivin. We determined that bir1Δ suppressor mutations act through two distinct mechanisms. The first mechanism involves mutations in proteins that directly attach the kinetochore to microtubules; such mutations were identified in five of 10 subunits of the Dam1 complex. The second mechanism results from mutations in genes that appear to affect the CPC more directly, which includes members of the SCF complex, the kinase Mps1, and the CPC member Sli15.
All of the suppressor mutations that we identified decrease the levels of CIN that result from BIR1 deletion. Notably, we did not identify mutations whose function suggests that they allow for the greater tolerance of aneuploidy. Furthermore, a mutation in the gene UBP6 that was previously demonstrated to reduce the negative effects of aneuploidy did not rescue BIR1 deletion (Torres et al, 2010). However, mutation of UBP6 was previously shown to improve the growth of only a subset of aneuploid chromosomes (5, 8, 9, and 11). Of these chromosomes, only aneuploidy of chromosome 8 is frequently observed in strains adapted to bir1Δ. Therefore, aneuploidy‐tolerating mutations could potentially improve the growth of cells with other sources of CIN that adapt through different aneuploid chromosomes. In addition, the dynamics of CIN adaptation and the subsequent underlying mechanisms behind coping with CIN could vary in other strain backgrounds that may be more inherently tolerant of CIN.
In our adaptation experiments, we identified mutations in three different kinetochore complexes that directly bind to microtubules. The majority of these mutations were in the Dam1 complex. These mutations decrease the localization of the complex to microtubules and likely interfere with the complex's ability to oligomerize. The cellular phenotypes that we observe for these mutations are consistent with increased turnover of kinetochore microtubules. In vertebrates, kinetochore–microtubule turnover can be increased through the overactivation of the kinetochore‐localized kinesins MCAK and Kif2b (Walczak et al, 1996; Kline‐Smith & Walczak, 2002; Bakhoum et al, 2009b). Overexpression of either of these kinesins decreases the mitotic errors observed in some cancer cell lines (Bakhoum et al, 2009b). Furthermore, adaptation of human cells to a drug that activates MCAK resulted in cells with decreased Aurora B activity (Orr et al, 2016). Together, these results all point to the importance of a balance between Aurora B activity and other factors that affect the stability of connections between kinetochores and microtubules.
Mps1 is a highly conserved kinase that functions in sensing unattached kinetochores. Mps1 phosphorylates Spc105/Knl1 on MELT repeats, which recruits the spindle assembly checkpoint proteins Bub1 and Bub3/BubR1. CPC mutations have synthetic growth defects with spindle assembly checkpoint mutations, indicating that decreased SAC activity does not generally rescue CPC mutants (Ng et al, 2009). Bub1 binding to Spc105/Knl1 also contributes to the localization of the CPC to the inner centromere, which is one of the key locations for its function in chromosome biorientation. Bir1 is required for the CPC to bind to the inner centromere, so this targeting function of Mps1 should not affect cells with BIR1 deletion (Makrantoni & Stark, 2009; Shimogawa et al, 2009). In budding yeast, Mps1 has an additional essential function in spindle pole body duplication. Interestingly, we would predict that decreased activity of any of these three functions would make the bir1Δ phenotype worse, not better. We conclude that the suppressor mutations partially decrease kinase activity and rescue via an unidentified mechanism. In a large‐scale screen for synthetic interactions between temperature‐sensitive mutations, it was found that some combinations of MPS1 and IPL1 alleles had positive genetic interactions, while others had negative interactions (Costanzo et al, 2016). These results support the idea that a balance between Mps1 activity and CPC activity is required for accurate chromosome segregation. Additional research will be required to determine which specific functions of Mps1 and which of its substrates are responsible for rescuing BIR1 deletion.
The SCF ubiquitin ligase complex is a key regulator of many pathways related to cell cycle entry. It degrades factors that inhibit cyclins, allowing for the activation of cyclin‐dependent kinases. The human homolog of the SCF F‐box protein Cdc4 is FBXW7/hCDC4. FBXW7 is a tumor suppressor that is frequently mutated in many cancer types (Spruck et al, 2002). This function is largely attributed to the ability of FBXW7 to target important oncogenes for degradation, including MYC and Cyclin E (reviewed in Yeh et al, 2018). In addition to its roles in regulating cell cycle initiation, FBXW7/Cdc4 also has functions in mitosis. In yeast, certain Cdc4 mutants arrest in mitosis prior to anaphase (Goh & Surana, 1999). More recently, it was shown that Cdc4 contributes to the degradation of the inner kinetochore protein Ame1 (Böhm et al, 2021). In human cells, FBXW7 mutants are sensitive to inhibitors of the spindle assembly checkpoint, demonstrating a potential function in mitosis (Bailey et al, 2015). However, the mechanisms by which SCFCdc4 regulates mitosis are still unclear. Our screen for bir1Δ suppressors has uncovered a connection between the SCFCdc4 complex and regulation of the CPC. These suppressor mutations in the SCF complex increase the localization of the CPC to the spindle/chromosomes in the absence of inner centromere targeting, which suggests that the SCFCdc4 complex may play a role in regulating alternative mechanisms of CPC localization in early mitosis. One possibility is that the SCF complex targets the degradation of a CPC recruitment factor.
Determining how cells adapt to CIN caused by the overstabilization of kinetochore‐bound microtubules has important implications in cancer. Additionally, Aurora kinase inhibitors are actively being investigated in a variety of combination therapies in both preclinical and clinical trials (reviewed in Du et al, 2021). It is therefore important to know how cells adapt to the overstabilization of kinetochore attachments. Here, we have outlined a time line of events that cells use to adapt to decreased Aurora B activity (Fig 6D). First, cells obtain specific aneuploidies that partially decrease CIN. Next, the aneuploid karyotypes are refined to obtain an optimal complement of aneuploid chromosomes (Ravichandran et al, 2018). Following adaptation through aneuploidy, specific point mutations are acquired that further decrease the rate of CIN. These point mutations can affect the CPC itself or independently increase the turnover of kinetochore microtubules. Finally, point mutations that reduce CIN in a more targeted way allow for a decrease in the number of aneuploid chromosomes.
This time line of adaptation supports the theory that aneuploidy often provides a rapid but temporary form of adaptation, as has previously been observed in yeast adapted to heat stress or a tubulin mutation (Yona et al, 2012; Pavani et al, 2021). In both of those studies, a single chromosome was gained early in the adaptation process and sometimes lost again at later time points. Here, we observe the reversion toward the euploid state for many different chromosomes resulting from a wide variety of mutations. This return to the original copy number could help explain the high prevalence of whole chromosome loss of heterozygosity (LOH) in cancers. In colorectal cancers, for example, whole chromosome LOH is extremely common, even though the chromosomes are often present in multiple copies (Thiagalingam et al, 2001). This theory could help explain why the bulk of LOH events do not contain known tumor suppressor genes, as the LOH itself was not the driver event, but simply a byproduct of temporary adaptation through aneuploidy (Ryland et al, 2015).
Materials and Methods
Yeast strains and media
All yeast strains and plasmids that were used in this study are listed in Table EV1. Strains were grown in yeast extract and peptone supplemented with 40 μg/ml adenine‐HCl (YPA) and sugars (2% glucose: YPAD, 1% galactose and 1% raffinose: YPAGR). All strains used were made in the W303 background. Benomyl (Sigma‐Aldrich, 381586), nocodazole (VWR, 487928), and 5‐FOA (Chempur, 220141‐70‐8) were used at concentrations of 10 μg/ml, 15 μg/ml, and 1 mg/ml, respectively. All cultures were incubated at 30°C unless otherwise stated. Gene deletions were carried out as previously described (Longtine et al, 1998). The engineering of specific mutations at endogenous loci was achieved using the method established by the Boone laboratory (Li et al, 2011).
Tissue culture
All cell lines used in this study are listed in Table EV1. All cell lines tested negatively for mycoplasma contamination. HAP1 cell lines were cultured in a humidified growth chamber at 37°C and 5% CO2 in Iscove's Modified Dulbecco's Medium (IMDM; Sigma‐Aldrich) supplemented with 10% Fetal Bovine Serum (Thermo Fisher Scientific) and 1% (v/v) Penicillin–Streptomycin (Sigma‐Aldrich).
For mutation of the endogenous FBXW7 locus in HAP1 p53− cells, a CRISPR/Cas9 strategy was applied. SgRNAs were cloned into pSpCas9(BB)‐2A‐GFP (PX458, Addgene plasmid #48138). For homologous recombination, a repair template carrying the respective mutation and 1,000 base pair (bp) homology flanks was synthesized as gBlock gene fragment (Integrated DNA Technologies IDT) and inserted into the plasmid pmScarlet_C1 (Addgene plasmid #85042). The plasmid mix of guide RNA plasmid and the repair template was transfected into HAP1 cells using FuGENE HD (Promega). Two days after transfection, cells were sorted for the presence of Cas9 (GFP positive) and repair template (mScarlet positive). Three days later, single cells were sorted into 96‐well plates. Clonal populations were expanded gradually over the course of 3 weeks. The FBXW7 mutations were identified by Sanger Sequencing, and karyotypes of the cell lines were validated by flow cytometry and whole‐genome sequencing.
Flow cytometry
HAP1 cells were trypsinized and stained with 10 μg/ml Hoechst 33342 (Thermo Fisher Scientific) for 30 min at 37°C. Cells were then analyzed for their ploidy state (based on G1 haploid peaks) in PBS supplemented with 5% FBS in a FACSAria III (BD) flow cytometer.
Next‐generation sequencing and data analysis
Overnight cultures of each strain were made for DNA isolation. DNA was purified with the Wizard genomic DNA purification kit (Promega). The DNA was fragmented to ∼500 bp using the Bioruptor Pico sonicator for three cycles (30 s on/off). AMPure XP beads were used for size selection, and DNA libraries were prepared using the NEBNext Ultra II DNA library preparation kit for Illumina (New England Biosciences). Approximately 14 strains per run were multiplexed with NEBNext Multiplex oligos (96 index primers) and mixed at equimolar ratios. The multiplexed samples were sequenced using the 50‐bp paired‐end setting on an Illumina HiSeq 2500 system at the Vienna Biocenter Next‐Generation Sequencing Facility (VBCF). All the raw bam files are accessible in the Sequence Read Archive (SRA—Bioproject accession number: PRJNA870080, Table EV3). The demultiplexed datasets were then aligned to the yeast genome using Bowtie2 (version 2.2.9; http://bowtie‐bio.sourceforge.net/bowtie2) and converted to bed files using SAMtools (version 1.3.1; http://samtools.sourceforge.net) and Bedtools (version 2.14, http://bedtools.readthedocs.io). The subsequent bed files were used to calculate chromosome copy numbers using custom‐made Python scripts. To normalize for differences in chromosome sizes, only the 15 kb closest to the telomeres was used. The value for the second‐lowest quartile chromosomes was used for normalization. After normalization, if the chromosome copy number of a certain chromosome was greater than 1.5, it was counted as disomic (aneuploid in a haploid cell). Larger segmental copy number changes were identified visually by binning the data into 4 kb regions. Mutations in the bir1∆‐ad and bir1∆‐ad2 strains were identified by running the mpileup function with SAMtools using the output from Bowtie2 alignment. These data were filtered by quality score and read depth; the quality needed to be greater than 95 and the read depth cutoff was 300. In the next step, BCFtools (version1.3.1) was implemented to convert the bcf files generated by mpileup (SAMtools) to variant call format (.vcf) files. Subsequently, VCFtools (version0.1.13) was used to compare all mutations found in our test strains with mutations already identified in the parent strain. Last, a custom‐made Python script generated lists containing the strain identity, the coordinates of the mutation (in base pairs), and the type of mutation (coding/noncoding, silent/missense/nonsense etc.; Ravichandran et al, 2018). GOrilla gene ontology tool (http://cbl-gorilla.cs.technion.ac.il) was used for GO enrichment tests. The target gene list was composed of all genes with nonsynonymous mutations in the adapted strains (gene repeats included), and the background list was all 6,002 genes annotated in the S. cerevisiae genome. The list of CIN‐related genes in the yeast genome was assembled from the Saccharomyces Genome Database by searching for six specific GO terms: colony sectoring: increased; chromosome segregation: abnormal; chromosome/plasmid maintenance: decreased rate; chromosome/plasmid maintenance: abnormal; chromosome segregation: premature; and chromosome segregation: decreased. Eight hundred and seventy‐four different genes satisfied these criteria.
Growth measurements
To measure growth from serial dilutions of yeast on agar plates, the images were first binarized using a threshold that distinguished the yeast colonies from the background using ImageJ. Next, a circle was placed around the first spot in the dilution series that was not saturated in the wild‐type strain, and the percentage of the area above the threshold was calculated. The same dilution and circle size were used for the measurements of each other strain on the same plate.
Microscopy
Strains were grown overnight in YPAD or YPAGR, subsequently diluted 1,000‐fold in the morning into fresh media, and grown for 4–6 h to midlog phase. Cells were pelleted by brief centrifugation, washed twice with 1 ml water, and resuspended in 200 μl water. Two microliters of cell suspension was spotted onto 1% agarose pads supplemented with complete synthetic media (SC) and 2% glucose. A coverslip was placed on top and sealed with 1:1:1 mixture by weight of paraffin (Merck), lanolin (Alfa Aesar), and Vaseline (Ferd. Eimermacher). Images were collected on a DeltaVision Ultra Epifluorescence Microscope system (Cytiva) at 30°C and a PlanApo N 60/1.42 Oil objective and a sCMOS sensor, 1,020 × 1,020 pixels, 6.5 μm pixel size camera. For live yeast imaging, 12 z‐sections with step size of 0.5 μm were taken. Images were deconvolved using the softWoRx software (Life Sciences Software). Counting of Bub3 foci was carried out using deconvolved images. Quantification of Dad3 and Sli15 fluorescence intensity in anaphase was performed on nondeconvolved images using ImageJ and a custom‐made Python script (Fink et al, 2017). Line scans perpendicular to the spindle were used for the entire spindle in prometaphase and either at the kinetochore or in between the kinetochores in anaphase. Spindle and kinetochore position were determined using the Nuf2 localization. A Gaussian curve was then fit to the intensity measurements and the area under the curve was calculated. Sli15 localization in preanaphase was quantified by drawing a circle around the Nuf2‐mCherry signal, and measuring the intensities of both mCherry and mNeonGreen signals from the same circle. Background intensities were subtracted using the measurements in a larger circle that extends outside of the spindle. All analyses (except for Fig EV4C) were obtained from images obtained from a minimum of three different days. Microscopy measurements were taken with blinded samples. Representative images are deconvolved and were contrast adjusted identically using ImageJ.
To test the localization of Sli15 to the mitotic spindle, endogenous Sli15 was put under the control of a Gal10‐1 promoter. For synchronization and depletion of endogenous Sli15, overnight cultures grown in YPAGR were diluted 1:100 into YPAGR and shaken for 2 h at 30°C. Cells were resuspended in YPAGR containing α‐factor (10 μg/ml) for 45 min at 30°C. Subsequently, the medium was exchanged with YPAD containing α‐factor (10 μg/ml) and grown for 2 h and 15 min at 30°C. Cells were then released from the G1 arrest into the cell cycle by washing twice with YPAD and resuspending in YPAD containing Pronase E (Merck). Synchronized yeast cells were released into the cell cycle and grown for 45 min at 30°C. The cells were then pelleted, washed with 1 ml sterile water, resuspended in 100 μl sterile water, and 2 μl cell suspension was put onto 1% agarose SC pads.
For the immunofluorescence of human cells, mitotic cells were collected by mechanical shake‐off and immobilized on adhesion slides (Marienfeld) for 30 min at 37°C. Next, cells were washed once in PBS, fixed for 15 min with 4% Formaldehyde in PBS, and subsequently permeabilized using 0.5% Triton‐X‐100 in PBS (0.5% PBST). Cells were blocked for 1 h in 0.01% PBST + 2% Bovine Serum Albumin and co‐stained overnight with rabbit monoclonal anti‐Aurora B antibody (Abcam, ab45145, 1:200) and human anticentromere antibody (Antibodies Incorporated, 15‐234, 1:200). After several washes with 0.01% PBST, cells were co‐stained with goat antirabbit IgG Alexa Fluor 488 (Thermo Fisher Scientific, A32723, 1:500) and goat antihuman IgG Alexa Fluor 647 (Invitrogen, A‐21445, 1:500) for 2 h. After Immunostaining, DNA was stained for 1 min with 1 μg/ml DAPI (Thermo Fisher Scientific).
Minichromosome loss assay
Yeast strains transformed with the minichromosome pRS316 were grown to saturation overnight in SC‐uracil media, diluted to a starting OD600 of 0.05, and grown for 24 h in 2 ml of YPAD media. After 24 h of growth, these strains were diluted 106‐fold in sterile water, and 100 μl of each strain was plated onto an SC‐uracil plate and a YPAD plate. The plates were incubated at 30°C for 48 h. Pictures taken of the subsequent colonies by a Canon EOS Digital Camera. The colony numbers were counted using the “Analyze Particles” function in ImageJ. The number of colonies on the ura‐plates were then divided by the number that grew on the YPAD plate to obtain the measurement of transmission fidelity.
ATP‐analog growth assay
Strains were grown overnight in YPAD and diluted to a starting OD600 of 0.05 in 3 ml YPAD at 25°C. The strains were given up to 4.5 h to reach exponential phase. At time point zero, the indicated amount of ATP‐analog 1NM‐PP1 (Merck) or DMSO was added to the strains. Immediately after drug addition, 0.5 ml of each sample was diluted 2× in a 1 ml cuvette to analyze the OD600 (time point 0). This was repeated every 1.5 h thereafter, for 7.5 h.
Budding index
Strains were grown overnight in YPAD media, and 100 μl of each overnight was added to 2 ml YPAD. After allowing 1 h for recovery, 2 μl of α‐factor was added every 45 min for 2 h. Strains were then washed using fresh YPAD media mixed with Pronase E (Merck; 1:10). The number of large‐budded cells was counted via light‐microscopy every half an hour for 2.5 h. A total of 100 cells were counted per slide per time point.
Colony formation assay
On Day 1, 600 cells were seeded into six‐well plates. The plates were incubated for 12 days. Colonies were then fixed with 4% Paraformaldehyde in PBS (Sigma‐Aldrich) for 20 min, washed with water, stained for 30 min with Crystal Violet, washed with water, and dried.
Protein extraction and Western blotting
For yeast protein extraction in the cycloheximide time course, saturated overnight cultures were diluted in 30 ml YPAD in order to obtain an OD600 of 0.25. Cells were grown at 30°C while shaking until an OD600 of 0.7 was reached. At this point, cycloheximide (50 μg/ml) was added (t = 0). Five milliliters aliquots was taken every 30 min for a 2 h period at 30°C. Immediately after each aliquot was taken, proteins were extracted by pelleting the cells and resuspending them in 100 μl 5% trichloroacetic acid. Following 10‐min incubation at room temperature, the cells were washed once with 1 ml ddH2O and resuspended in 100 μl lysis buffer (50 mM Tris, pH 7.4, 50 mM dithiotreitol, 1 mM EDTA, Complete EDTA‐free protease inhibitor cocktail (Roche) and Phosstop (Roche)). Cells were vortexed for 30 min at 4°C after the addition of glass beads. Subsequently, 33 μl 4× sample buffer was added and incubated at 95°C for 5 min. For protein extractions in prometaphase cells, saturated overnight cultures were diluted in 10 ml YPAGR in order to obtain an OD600 of 0.25. Cells were then synchronized as described as for the microscopy experiments. Sixty minutes after G1 release, proteins were extracted by resuspending cells in 100 μl 0.2 M NaOH. After 5‐min incubation at room temperature, cells were resuspended in 100 μl H2O. Thirty‐three microliters of 4× sample buffer was added and incubated at 95°C for 5 min. Samples were stored at −20°C. For immunoblots, the following antibodies were used: rat anti‐HA‐clone 3F10 (Roche), mouse anti‐PGK1 monoclonal 22C5D8 (Thermo Fisher Scientific). Membranes were probed with the corresponding secondary antibodies: antirat IgG‐HRP‐lined (Cell Signaling Technology) or antimouse IgG‐HRP‐linked (Cell Signaling Technology). Immunoblots were quantified using ImageJ.
Statistics
Statistical analysis was performed using the GraphPad Prism software. Details to the statistical tests used in a particular experiment are reported in the figure legends.
Author contributions
Matthew N Clarke: Conceptualization; data curation; formal analysis; writing – original draft; writing – review and editing. Theodor Marsoner: Data curation; formal analysis; writing – original draft. Manuel Alonso Y Adell: Data curation; formal analysis; writing – original draft. Madhwesh C Ravichandran: Data curation; formal analysis; writing – original draft. Christopher S Campbell: Conceptualization; data curation; formal analysis; supervision; funding acquisition; writing – original draft; writing – review and editing.
Disclosure and competing interests statement
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Table EV1
Table EV2
Table EV3
PDF+
Acknowledgements
The authors would like to thank the Campbell and Dammermann laboratories for helpful discussions and comments. We thank the Basrai and Klein laboratories for gifts of yeast strains and plasmids. The parental HAP1 p53‐null cell line was kindly provided by J.I. Loizou. We acknowledge the service of the Perutz Labs BioOptics Facility, a member of the VLSI. We would also like to acknowledge the services provided to us by the VBC sequencing facility located at the VBCF. This work was supported by Vienna Science and Technology Fund (WWTF) grant VRG14‐001 and Austrian Science Fund (FWF) grants Y944‐B28 and W1238‐B20 to CSC.
The EMBO Journal (2023) 42: e111500
See also: Z Storchová (April 2023)
Data availability
All the raw bam files are accessible in the Sequence Read Archive (SRA‐Bioproject accession number: PRJNA870080). Information on each sample ID can be found in Table EV3.
References
- Ainsztein AM, Kandels‐Lewis SE, Mackay AM, Earnshaw WC (1998) INCENP centromere and spindle targeting: identification of essential conserved motifs and involvement of heterochromatin protein HP1. J Cell Biol 143: 1763–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au W‐C, Zhang T, Mishra PK, Eisenstatt JR, Walker RL, Ocampo J, Dawson A, Warren J, Costanzo M, Baryshnikova A et al (2020) Skp, Cullin, F‐box (SCF)‐Met30 and SCF‐Cdc4‐mediated proteolysis of CENP‐A prevents Mislocalization of CENP‐A for chromosomal stability in budding yeast. PLoS Genet 16: e1008597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey ML, Singh T, Mero P, Moffat J, Hieter P (2015) Dependence of human colorectal cells lacking the FBW7 tumor suppressor on the spindle assembly checkpoint. Genetics 201: 885–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhoum SF, Genovese G, Compton DA (2009a) Deviant kinetochore microtubule dynamics underlie chromosomal instability. Curr Biol 19: 1937–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhoum SF, Thompson SL, Manning AL, Compton DA (2009b) Genome stability is ensured by temporal control of kinetochore–microtubule dynamics. Nat Cell Biol 11: 27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhoum SF, Silkworth WT, Nardi IK, Nicholson JM, Compton DA, Cimini D (2014) The mitotic origin of chromosomal instability. Curr Biol 24: R148–R149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐David U, Amon A (2020) Context is everything: aneuploidy in cancer. Nat Rev Genet 21: 44–62 [DOI] [PubMed] [Google Scholar]
- Biggins S, Bhalla N, Chang A, Smith DL, Murray AW (2001) Genes involved in sister chromatid separation and segregation in the budding yeast Saccharomyces cerevisiae . Genetics 159: 453–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop JD, Schumacher JM (2002) Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B kinase stimulates Aurora B kinase activity. J Biol Chem 277: 27577–27580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, Blethrow J, Shimizu E, Tsien JZ, Schultz PG, Rose MD et al (2000) A chemical switch for inhibitor‐sensitive alleles of any protein kinase. Nature 407: 395–401 [DOI] [PubMed] [Google Scholar]
- Böhm M, Killinger K, Dudziak A, Pant P, Jänen K, Hohoff S, Mechtler K, Örd M, Loog M, Sanchez‐Garcia E et al (2021) Cdc4 phospho‐degrons allow differential regulation of Ame1CENP‐U protein stability across the cell cycle. eLife 10: e67390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill DP, Kinzler KW, Vogelstein B, Lengauer C (1999) Genetic instability and darwinian selection in tumours. Trends Cell Biol 9: M57–M60 [PubMed] [Google Scholar]
- Campbell CS, Desai A (2013) Tension sensing by Aurora B kinase is independent of survivin‐based centromere localization. Nature 497: 118–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman IM, Anderson S, Jwa M, Green EM, Kang JS, Yates JR, Chan CSM, Drubin DG, Barnes G (2002) Phospho‐regulation of kinetochore–microtubule attachments by the Aurora kinase Ipl1p. Cell 111: 163–172 [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Chappie JS, Wilson‐Kubalek EM, Desai A (2006) The conserved KMN network constitutes the core microtubule‐binding site of the kinetochore. Cell 127: 983–997 [DOI] [PubMed] [Google Scholar]
- Ciferri C, Pasqualato S, Screpanti E, Varetti G, Santaguida S, Reis Dos G, Maiolica A, Polka J, De Luca JG, De Wulf P et al (2008) Implications for kinetochore–microtubule attachment from the structure of an engineered Ndc80 complex. Cell 133: 427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimini D, Wan X, Hirel CB, Salmon ED (2006) Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr Biol 16: 1711–1718 [DOI] [PubMed] [Google Scholar]
- Costanzo M, VanderSluis B, Koch EN, Baryshnikova A, Pons C, Tan G, Wang W, Usaj M, Hanchard J, Lee SD et al (2016) A global genetic interaction network maps a wiring diagram of cellular function. Science 353: aaf1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R, Huang C, Liu K, Li X, Dong Z (2021) Targeting AURKA in cancer: Molecular mechanisms and opportunities for cancer therapy. Mol Cancer 20: 15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink S, Turnbull K, Desai A, Campbell CS (2017) An engineered minimal chromosomal passenger complex reveals a role for INCENP/Sli15 spindle association in chromosome biorientation. J Cell Biol 216: 911–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann R, Carvalho A, Henzing AJ, Ruchaud S, Hudson DF, Honda R, Nigg EA, Gerloff DL, Earnshaw WC (2004) Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J Cell Biol 166: 179–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh PY, Surana U (1999) Cdc4, a protein required for the onset of S phase, serves an essential function during G(2)/M transition in Saccharomyces cerevisiae . Mol Cell Biol 19: 5512–5522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DJ, Resio B, Pellman D (2012) Causes and consequences of aneuploidy in cancer. Nat Rev Genet 13: 189–203 [DOI] [PubMed] [Google Scholar]
- Gropp A, Winking H, Herbst EW, Claussen CP (1983) Murine trisomy: developmental profiles of the embryo, and isolation of trisomic cellular systems. J Exp Zool 228: 253–269 [DOI] [PubMed] [Google Scholar]
- Honda R, Körner R, Nigg EA (2003) Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell 14: 3325–3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hose J, Escalante LE, Clowers KJ, Dutcher HA, Robinson D, Bouriakov V, Coon JJ, Shishkova E, Gasch AP (2020) The genetic basis of aneuploidy tolerance in wild yeast. eLife 9: e52063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni S, Harrison SC (2018) Structure of the DASH/Dam1 complex shows its role at the yeast kinetochore–microtubule interface. Science 360: 552–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaprakash AA, Klein UR, Lindner D, Ebert J, Nigg EA, Conti E (2007) Structure of a Survivin‐Borealin‐INCENP core complex reveals how chromosomal passengers travel together. Cell 131: 271–285 [DOI] [PubMed] [Google Scholar]
- Jin F, Wang Y (2013) The signaling network that silences the spindle assembly checkpoint upon the establishment of chromosome bipolar attachment. Proc Natl Acad Sci USA 110: 21036–21041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MH, Huneycutt BJ, Pearson CG, Zhang C, Morgan G, Shokat K, Bloom K, Winey M (2005) Chemical genetics reveals a role for Mps1 kinase in kinetochore attachment during mitosis. Curr Biol 15: 160–165 [DOI] [PubMed] [Google Scholar]
- Jonkers W, Rep M (2009) Lessons from fungal F‐box proteins. Eukaryot Cell 8: 677–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantzaki M, Kitamura E, Zhang T, Mino A, Novak B, Tanaka TU (2015) Kinetochore–microtubule error correction is driven by differentially regulated interaction modes. Nat Cell Biol 17: 421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima SA, Tsukahara T, Langegger M, Hauf S, Kitajima TS, Watanabe Y (2007) Shugoshin enables tension‐generating attachment of kinetochores by loading Aurora to centromeres. Genes Dev 21: 420–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima SA, Yamagishi Y, Honda T, Ishiguro K‐I, Watanabe Y (2010) Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science 327: 172–177 [DOI] [PubMed] [Google Scholar]
- Klein UR, Nigg EA, Gruneberg U (2006) Centromere targeting of the chromosomal passenger complex requires a ternary subcomplex of Borealin, Survivin, and the N‐terminal domain of INCENP. Mol Biol Cell 17: 2547–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline‐Smith SL, Walczak CE (2002) The microtubule‐destabilizing kinesin XKCM1 regulates microtubule dynamic instability in cells. Mol Biol Cell 13: 2718–2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon M, Godinho SA, Chandhok NS, Ganem NJ, Azioune A, Théry M, Pellman D (2008) Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev 22: 2189–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Vizeacoumar FJ, Bahr S, Li J, Warringer J, Vizeacoumar FS, Min R, VanderSluis B, Bellay J, Devit M et al (2011) Systematic exploration of essential yeast gene function with temperature‐sensitive mutants. Nat Biotechnol 29: 361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Winey M (2012) The MPS1 family of protein kinases. Annu Rev Biochem 81: 561–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR (1998) Additional modules for versatile and economical PCR‐based gene deletion and modification in Saccharomyces cerevisiae . Yeast 14: 953–961 [DOI] [PubMed] [Google Scholar]
- Makrantoni V, Stark MJR (2009) Efficient chromosome biorientation and the tension checkpoint in Saccharomyces cerevisiae both require Bir1. Mol Cell Biol 29: 4552–4562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda JJL, De Wulf P, Sorger PK, Harrison SC (2005) The yeast DASH complex forms closed rings on microtubules. Nat Struct Mol Biol 12: 138–143 [DOI] [PubMed] [Google Scholar]
- Ng TM, Waples WG, Lavoie BD, Biggins S (2009) Pericentromeric sister chromatid cohesion promotes kinetochore biorientation. Mol Biol Cell 20: 3818–3827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr B, Talje L, Liu Z, Kwok BH, Compton DA (2016) Adaptive resistance to an inhibitor of chromosomal instability in human cancer cells. Cell Rep 17: 1755–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavani M, Bonaiuti P, Chiroli E, Gross F, Natali F, Macaluso F, Póti Á, Pasqualato S, Farkas Z, Pompei S et al (2021) Epistasis, aneuploidy, and functional mutations underlie evolution of resistance to induced microtubule depolymerization. EMBO J 40: e108225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Meng EC, Couch GS, Croll TI, Morris JH, Ferrin TE (2021) UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci 30: 70–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky BA, Kung C, Shokat KM, Biggins S (2006) The Ipl1‐Aurora protein kinase activates the spindle checkpoint by creating unattached kinetochores. Nat Cell Biol 8: 78–83 [DOI] [PubMed] [Google Scholar]
- Ravichandran MC, Fink S, Clarke MN, Hofer FC, Campbell CS (2018) Genetic interactions between specific chromosome copy number alterations dictate complex aneuploidy patterns. Genes Dev 32: 1485–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryland GL, Doyle MA, Goode D, Boyle SE, Choong DYH, Rowley SM, Li J, Bowtell DD, Tothill RW, Campbell IG et al (2015) Loss of heterozygosity: what is it good for? BMC Med Genomics 8: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansregret L, Patterson JO, Dewhurst S, López‐García C, Koch A, McGranahan N, Chao WCH, Barry DJ, Rowan A, Instrell R et al (2017) APC/C dysfunction limits excessive cancer chromosomal instability. Cancer Discov 7: 218–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarangapani KK, Akiyoshi B, Duggan NM, Biggins S, Asbury CL (2013) Phosphoregulation promotes release of kinetochores from dynamic microtubules via multiple mechanisms. Proc Natl Acad Sci USA 110: 7282–7287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa F, Mapelli M, Ciferri C, Tarricone C, Areces LB, Schneider TR, Stukenberg PT, Musacchio A (2005) Mechanism of Aurora B activation by INCENP and inhibition by hesperadin. Mol Cell 18: 379–391 [DOI] [PubMed] [Google Scholar]
- Shimogawa MM, Graczyk B, Gardner MK, Francis SE, White EA, Ess M, Molk JN, Ruse C, Niessen S, Yates JR et al (2006) Mps1 phosphorylation of Dam1 couples kinetochores to microtubule plus ends at metaphase. Curr Biol 16: 1489–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogawa MM, Widlund PO, Riffle M, Ess M, Davis TN (2009) Bir1 is required for the tension checkpoint. Mol Biol Cell 20: 915–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogawa MM, Wargacki MM, Muller EG, Davis TN (2010) Laterally attached kinetochores recruit the checkpoint protein Bub1, but satisfy the spindle checkpoint. Cell Cycle 9: 3619–3628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruck CH, Strohmaier H, Sangfelt O, Müller HM, Hubalek M, Müller‐Holzner E, Marth C, Widschwendter M, Reed SI (2002) hCDC4 gene mutations in endometrial cancer. Cancer Res 62: 4535–4539 [PubMed] [Google Scholar]
- Tanaka TU, Rachidi N, Janke C, Pereira G, Galova M, Schiebel E, Stark MJR, Nasmyth K (2002) Evidence that the Ipl1‐Sli15 (Aurora kinase‐INCENP) complex promotes chromosome bi‐orientation by altering kinetochore‐spindle pole connections. Cell 108: 317–329 [DOI] [PubMed] [Google Scholar]
- Thiagalingam S, Laken S, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B, Lengauer C (2001) Mechanisms underlying losses of heterozygosity in human colorectal cancers. Proc Natl Acad Sci USA 98: 2698–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres EM, Sokolsky T, Tucker CM, Chan LY, Boselli M, Dunham MJ, Amon A (2007) Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science 317: 916–924 [DOI] [PubMed] [Google Scholar]
- Torres EM, Dephoure N, Panneerselvam A, Tucker CM, Whittaker CA, Gygi SP, Dunham MJ, Amon A (2010) Identification of aneuploidy‐tolerating mutations. Cell 143: 71–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Waal MS, Saurin AT, Vromans MJM, Vleugel M, Wurzenberger C, Gerlich DW, Medema RH, Kops GJPL, Lens SMA (2012) Mps1 promotes rapid centromere accumulation of Aurora B. EMBO Rep 13: 847–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoosthuyse V, Prykhozhij S, Hardwick KG (2007) Shugoshin 2 regulates localization of the chromosomal passenger proteins in fission yeast mitosis. Mol Biol Cell 18: 1657–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak CE, Mitchison TJ, Desai A (1996) XKCM1: a xenopus kinesin‐related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell 84: 37–47 [DOI] [PubMed] [Google Scholar]
- Weiss E, Winey M (1996) The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J Cell Biol 132: 111–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann S, Avila‐Sakar A, Wang H‐W, Niederstrasser H, Wong J, Drubin DG, Nogales E, Barnes G (2005) Formation of a dynamic kinetochore‐microtubule interface through assembly of the Dam1 ring complex. Mol Cell 17: 277–290 [DOI] [PubMed] [Google Scholar]
- Winey M, Goetsch L, Baum P, Byers B (1991) MPS1 and MPS2: novel yeast genes defining distinct steps of spindle pole body duplication. J Cell Biol 114: 745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh C‐H, Bellon M, Nicot C (2018) FBXW7: a critical tumor suppressor of human cancers. Mol Cancer 17: 115–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yona AH, Manor YS, Herbst RH, Romano GH, Mitchell A, Kupiec M, Pilpel Y, Dahan O (2012) Chromosomal duplication is a transient evolutionary solution to stress. Proc Natl Acad Sci USA 109: 21010–21015 [DOI] [PMC free article] [PubMed] [Google Scholar]


