Abstract
Objective
This case–control study aimed to analyze the dynamics of macrophage infiltration in subcutaneous adipose tissue following bariatric surgery or conservative treatment of obesity and to clarify whether these features predict the weight loss outcome after the surgery.
Methods
Subcutaneous tissue samples taken before and 12 months after laparoscopic Roux‐en‐Y gastric bypass surgery (n = 39) or conservative (n = 43) treatment for obesity were analyzed. Fat cell size was determined, and with CD68 immunohistochemistry, crown‐like structures (CLS) were counted and single macrophages were quantitated.
Results
A major decline in CLS density from 4.1 (SD 3.5) to 1.1 (SD 0.8) per 1000 fat cells (p < 0.000) was found, regardless of the degree of weight loss after the surgery. Surgery had no effect on the fraction of infiltrating single‐cell macrophages in subcutaneous adipose tissue. The abundance of these macrophage populations before the intervention did not predict the degree of postsurgery weight loss or suboptimal response to the surgery.
Conclusions
The effect of gastric bypass on adipose tissue inflammatory status associates closely with CLS density even in subjects with suboptimal weight loss. The study suggests that factors related to bypass surgery other than weight loss modify the inflammatory response in adipose tissue.
Study Importance.
What is already known?
Chronic low‐grade inflammation of adipose tissue, characterized by macrophage infiltration, is associated with systemic and metabolic complications of obesity.
Bariatric surgery results in a high rate of remission of metabolic disorders and decline in systemic inflammation.
What does this study add?
We found a major decline in the density of pyroptotic and inflammation‐associated macrophage populations regardless of the degree of weight loss after laparoscopic Roux‐en‐Y gastric bypass. Surgery has no effect on the fraction of infiltrating single‐cell macrophages in subcutaneous adipose tissue.
Baseline macrophage infiltration to subcutaneous adipose tissue does not predict the postsurgery weight loss.
How might these results change the direction of research or the focus of clinical practice?
The study suggests that factors related to bypass surgery other than weight loss modify the inflammatory response in adipose tissue.
The findings emphasize a role of the pyroptotic and inflammation‐associated macrophage populations in obesity‐related adipose tissue inflammation.
INTRODUCTION
Obesity and associated comorbidities have reached epidemic proportions worldwide [1, 2]. Many health issues related to excessive weight gain, such as type 2 diabetes and atherosclerotic vascular disease, are associated with low‐grade inflammation of the adipose tissue [3]. Thus, obesity may be viewed as a chronic, systemic, low‐grade inflammatory disease and malfunctioning adipose tissue as a hormonally active organ producing numerous secretions mediating these effects [4, 5, 6]. Despite intensive research and discoveries in drug development, bariatric surgery remains the most effective and reliable long‐term intervention for severe obesity [7]. The most common methods, laparoscopic Roux‐en‐Y gastric bypass (LRYGB) and vertical sleeve gastrectomy, both result in constant long‐term outcomes [8, 9]. The weight loss after the operation is associated with a high rate of remission of metabolic disorders such as diabetes, hyperlipidemia, and hypertension [10]. Total weight loss (TWL) of at least 20% following the bariatric surgery has been proposed as a standard criterion for a good response [11]. A majority of the patients achieve a good response to the surgery. However, for 11% to 35% of the patients, the intervention may provide suboptimal results, at least in regard to the weight loss [12, 13, 14, 15, 16].
The adipocytes are susceptible to the inflammatory type of programmed cell death resembling pyroptosis [17, 18]. In adipose tissue, this phenomenon is associated with macrophages forming crown‐like structures (CLS) around the dead adipocyte remnants to sequester and remove them [17]. Most macrophages in human and animal adipose tissue with obesity seem to localize at the CLS sites [17, 19]. The macrophage accumulation in the adipose tissue is generally associated with both local and systemic inflammation and metabolic complications of obesity [19, 20]. For instance, the macrophage infiltration and formation of CLSs relate to insulin resistance and elevated levels of inflammation biomarkers in plasma [21, 22].
Weight loss is associated with the improvement of inflammatory status and obesity‐related comorbidities [23, 24]. Cancello et al. [25] found that the LRYGB‐induced weight loss was linked with a reduction of macrophages infiltrating the adipose tissue and disappearance of CLSs among 17 patients with severe obesity who underwent the surgery. In line with this, CLS density in subcutaneous adipose tissue (SAT) declined 1 year after LRYGB in both 13 patients with severe obesity and type 2 diabetes and their 15 matched nondiabetic counterparts [21]. Curiously, very low‐calorie diet‐induced weight loss alone led to a rise in CLS density, at least in the short term [26]. However, these findings are consistent with a study in which caloric restriction in high‐fat diet‐fed mice initially increased adipose tissue macrophage accumulation followed by a decrease of macrophage content after an extended period of time and weight loss [27].
The aim of the present prospective study was to analyze the changes in CLS density and macrophage infiltration in SAT following bariatric surgery (here LRYGB) or conservative treatment of obesity. Furthermore, we strove to clarify whether these features predict the outcome after the surgery and whether they could offer an insight into the mechanisms opposing the weight loss. As far as we are aware, this is the largest prospective study assessing CLS formation and macrophage infiltration in adipose tissue after bariatric surgery and the first study exploring the relationship of these characteristics and the extent of weight loss.
METHODS
Subjects
Initially, 122 patients with severe obesity participated in the study assessing the role of bariatric surgery–induced weight reduction on inflammatory markers and symptoms of osteoarthritis from 2014 to 2019 in Oulu, Finland. Of them, 60 were scheduled for bariatric surgery (LRYGB), following the national guideline criteria for bariatric surgery, i.e., BMI over 40 kg/m2 or BMI over 35 kg/m2 when accompanied by obesity‐related comorbidity or BMI over 30 kg/m2 with type 2 diabetes not manageable by conservative means. The subjects in the surgery group had demonstrated a commitment to lifestyle changes and had achieved at least temporary 5% weight loss during conservative treatment lasting at least 6 months as recommended by the national guidelines. The subjects were evaluated by an internist before referral to the surgeon and they underwent a very low‐calorie diet for 3 to 5 weeks prior to the surgery. The control group consisted of 62 subjects with obesity recruited among patients not interested in bariatric surgery. These patients received conservative treatment (such as scheduled supportive meetings, nutritional guidance, and medication when indicated) for obesity and related comorbidities in local public health care. However, there was no special program for the conservative intervention, and no data about attendance of the patients could be collected. Demographic data and full medical history were recorded. All subjects were monitored for weight and obesity‐related comorbidities for 1 year at a minimum. Study size was based on assuming a difference of 11.6 between the groups in the RAND‐36 Measure of Health‐Related Quality of Life survey physical functioning score observed for total knee arthroplasty and 20% loss of subjects during the first year. The study was authorized by Oulu University Hospital Ethics Committee following the principles of the Declaration of Helsinki. Informed written consent for participation in the study was acquired from all subjects.
Histological specimens and immunohistochemistry
We aimed to collect two fat tissue samples from all subjects, one at surgery and the second one a year after bariatric surgery (surgery group) or before and after receiving conservative treatment for 1 year (conservative group). Ultimately, after dropouts, refusals, and additional data loss, 39 (data missing for 21) and 43 (data missing for 19) paired samples were analyzed in the surgery and conservative groups, respectively. Missing data were omitted in analysis. An SAT biopsy specimen (circa 1 cm3) from the periumbilical area was obtained during the surgery or clinic appointment under local anesthesia. Tissue biopsy specimens were fixed in formalin and embedded in paraffin. The specimens were sectioned at 5 μm. One section was stained with hematoxylin and eosin (H&E) to assess adipocyte density. For CD68, immunohistochemistry was performed with BOND Polymer Refine Detection System (DS9800; Leica Biosystems, Buffalo Grove, Illinois) with 3,3′‐diaminobenzidine as a chromogen. Mouse monoclonal anti‐human CD68 (1:200; Dako; Agilent, Santa Clara, California) was used for 30 minutes with 20 minutes of Tris‐EDTA pretreatment.
Image analysis
H&E and CD68 stained SAT sections were scanned with Leica Aperio AT2 (Leica Biosystems) at 40× magnification and analyzed with QuPath 0.2.3 and/or ImageJ 1.53 open source software [28, 29]. To assess adipocyte density in SAT for each subject, two 10E6 μm2 sample areas from homogeneous adipocyte areas were delineated and sent to ImageJ. ImageJ plugin Adipocyte Tools [30, 31] was used to determine the number and size of the adipocytes. Faulty detections were manually corrected, and the exact area containing the selections was delineated to calculate accurate adipocyte density. Quantification of CD68 staining was performed with QuPath 0.2.3 using the pixel classifier feature. After reviewing the images, 15 representative CD68 images were used to train QuPath's pixel classifier to reliably identify positively stained and other (nonstained) tissue. The classifier was then applied to annotated adipocyte areas on whole‐slide sections to measure areas of CD68‐positive and ‐negative tissue. The number of CLSs found was manually counted in these annotated areas. CLSs were defined as CD68‐positive macrophage aggregate circularly surrounding dead adipocyte or necrotic adipocyte debris and making up at least 50% of its circumference [32]. To avoid distortion caused by variation in adipocyte sizes, for comparative analysis, the results are expressed in relation to 1000 adipocytes.
Statistical analysis
Statistical analyses were performed and graphs built using SPSS Statistics for Windows (version 25; IBM Corp., Armonk, New York). Summary values are shown as mean with standard deviation (SD). Student t test or Welch t test was used for continuous independent variables and Mann–Whitney for nonparametric variables. For repeated measures, paired t test or Wilcoxon signed rank test was used for parametric or nonparametric variables, respectively. Normality of data was assessed with Shapiro–Wilk test. Pearson correlation coefficient (r) or Spearman correlation coefficient (ρ) was calculated, the latter if either variable was non‐normally distributed. Two‐tailed p values are presented. Weight information was missing for 10/122 and 15/122 subjects at 0 and 12 months, respectively. Adipocyte size information could not be acquired for 20/122 and 28/122 subjects at 0 and 12 months, respectively.
RESULTS
Baseline anthropometric data, CLS density, and infiltrating macrophages in fat tissue
Both the surgery and the conservatively treated group showed similar general anthropometric characteristic at baseline. However, mean weight and BMI were, to some extent, higher in the surgery group (117 [SD 19.5] kg and 42.4 [SD 6.5] kg/m2) than in the conservative group (110 [SD 17.3] kg and 40.0 [SD 5.0] kg/m2) (p = 0.037 and p = 0.055 for Mann–Whitney test, respectively, Table 1A). Both groups had a considerable prevalence of obesity‐related comorbidities (Table 1A), with no significant differences between the groups.
TABLE 1.
Basic clinical characteristics (A) and evolution of anthropometric data and counts of different macrophage populations (B) in the study groups after 12 months
| A | Surgery | Conservative |
|---|---|---|
| n | 60 | 62 |
| Sex (F/M) | 51/9 | 51/11 |
| Age (y) | 48.5 (8.2) | 50.9 (8.0) |
| Height (cm) | 166 (6.5) | 166 (7.9) |
| Hypertension | 47.4% | 48.4% |
| Type 2 diabetes | 33.3% | 25.8% |
| Obstructive sleep apnea | 21.7% | 21.0% |
| B | 0 months | 12 months | 0 months | 12 months |
|---|---|---|---|---|
| Weight (kg) | 117 (19.5)* | 91.5 (16.8)*** | 110 (17.3)* | 109.6 (17.2) |
| BMI (kg/m2) | 42.4 (6.5) | 32.9 (5.7)*** | 40.0 (5.0) | 39.8 (4.6) |
| TWL% | 21.9 (6.8)*** | 0.1 (4.1) | ||
| FC density (cells/10E6 μm2) | 182.6 (36.1) | 225.9 (46.3)*** | 169.7 (37.7) | 182.4 (42.4) |
| CLS count/1000 FC | 4.1 (3.6) | 1.1 (0.8) a , b | 3.2 (2.3) | 4.2 (3.3) b |
| CD68 outside of CLS (μm2/1000 FC) | 12.0 (7.2) | 11.0 (5.3) b | 11.9 (6.6) | 16.4 (10.5) a , b |
| Proportion (%) of CLS of all CD68+ cells | 20.2 (12.6) b | 4.1 (3.0) a , b | 13.0 (9.8) b | 12.0 (9.2) b |
Note: Values are mean (SD).
Abbreviations: CLS, crown‐like structure; FC, fat cell; TWL, total weight loss.
Statistically significant difference vs. initial value (see Figure 3).
Statistically significant difference between the groups (see Figure 3).
p = 0.037, for Mann–Whitney test between the groups.
p < 0.001, for paired t test vs. initial value.
CD68 immunohistochemistry markedly facilitated the identification of CLSs compared with H&E staining (Figure 1A,B). At baseline before interventions, the subjects had a mean 3.7 (SD 2.9) CLSs found for every 1000 fat cells (FC). On average, CD68 stained 0.26% (SD 0.19%) of the entire tissue area and 2.4% (SD 1.0%) of all visible tissue (ignoring the empty dissolved fat areas) in the delineated adipocyte areas. Of positive CD68 staining, 16.6% (SD 11.6%) was localized within CLSs, and the remaining 83.4% (SD 11.6%) was dispersed throughout the adipose tissue, presumably in the infiltrating single‐cell macrophage population. The mean CD68 stained area in the adipocyte regions outside any CLSs was 11.9 (SD 6.8) μm2/1000 FC and it correlated to CLS density in bivariate analysis (ρ = 0.53, p < 0.001, R 2 = 0.294, Figure 2).
FIGURE 1.
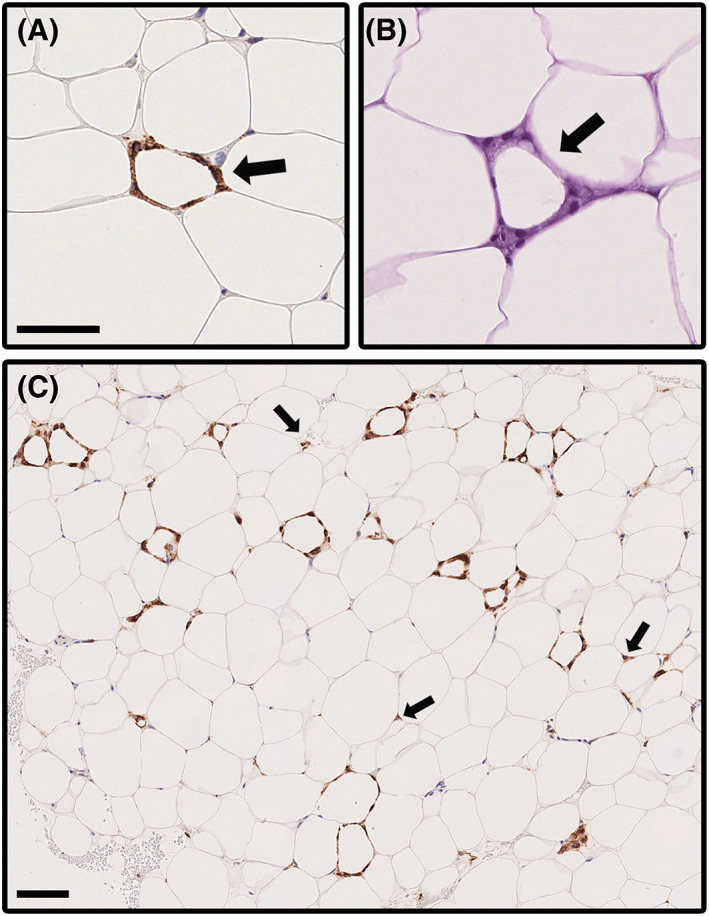
Microphotographs illustrating crown‐like structures (CLS) and dispersed CD68‐positive cells in subcutaneous adipose tissue of participants with overweight. Identification of CLSs is facilitated with (A) CD68 staining (arrow) as compared with (B) H&E staining (arrow). Scale bar = 50 μm. (C) Sometimes CLSs were present at a high density as shown by CD68 staining. A 40‐year‐old woman with BMI 44.9 kg/m2 and no comorbidities had CLS density of 14.33 per 1000 adipocytes, the highest count found among the subjects. A particularly high‐density region is shown in the image. This patient's CLS density had decreased to 1.23 per 1000 adipocytes and BMI decreased to 37.7 kg/m2 at 12 months after the bariatric surgery. Arrows point to some CD68‐positive cells dispersed outside CLSs. Scale bar = 100 μm [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
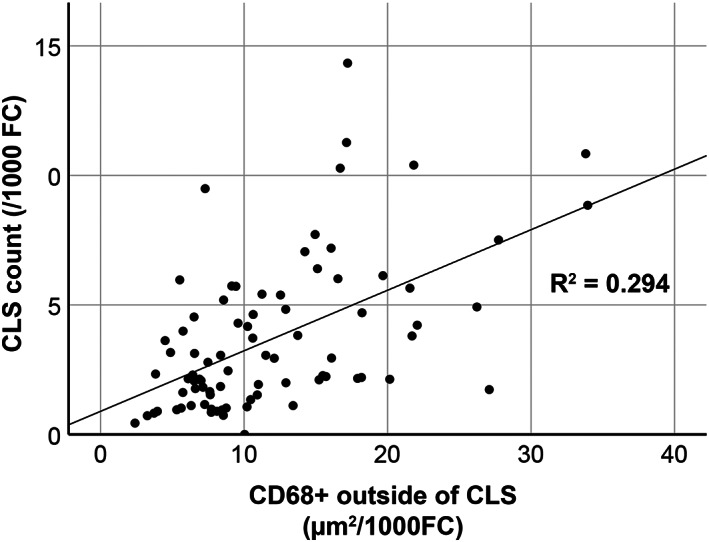
Scatterplot showing correlation of total crown‐like structure (CLS) density (CLS/1000 fat cells [FC]) and dispersed CD68‐positive cells outside CLSs (area of CD68 expression/1000 FC) in subcutaneous adipose tissue samples of subjects with obesity taken before intervention. Correlation is significant (bivariate analysis; ρ = 0.53, p < 0.001, R 2 = 0.294)
There were no significant associations between CLS density or the extent of the dispersed CD68 staining and gender, age, baseline BMI, smoking history, or presence of type 2 diabetes (data not shown). However, at baseline, the subjects with type 2 diabetes had significantly larger mean FC size of 77.2 (SD 9.0) versus 72.3 (SD 8.0) μm and subsequently smaller FC density 163.2 (SD 30.4) versus 180.7 (SD 39.5) cells/10E6 μm2 (p = 0.008 and 0.032 for Student t test, respectively).
The effect of surgical and conservative interventions for obesity, CLS density, and infiltrating macrophages
At 12 months after the intervention, weight, BMI, and TWL% had declined in the surgery group but not in the conservative group (Table 1B). In the surgery group, TWL% was 16.6 (SD 4.6) for subjects with type 2 diabetes and 24.2 (SD 6.5) for nondiabetic counterparts (p < 0.000 for Student t test). The weight loss associated with surgery resulted in a shift of FC size distribution toward smaller adipocyte populations and an increase in adipocyte density after LRYGB, whereas these changes were not seen after conservative treatment (Supporting Information Figure S1, Table 1B).
In the surgery group, the observed CLS density declined by 3.0 (95% C 1.8–4.2) from 4.1 (SD 3.6) to 1.1 (SD 0.8) per 1000 FC (Figure 3A). In contrast, CLS density showed an increasing trend from 3.2 (SD 2.3) to 4.2 (SD 3.3) in the conservative group (p = 0.07 for paired t test, Figure 3A). The surgery or the weight loss associated with it did not affect the amount of adipose tissue CD68 staining outside CLSs. However, the CD68 staining outside CLSs increased significantly in the conservative group (Figure 3B). The relative proportion of CD68 staining found within versus outside CLSs declined significantly after surgery but remained unaffected after conservative treatment (Figure 3C). The surgery group had a significantly higher proportion of CD68 staining found in CLSs at baseline and a significantly lower proportion found in CLSs at 12 months compared with the conservative group (Figure 3C).
FIGURE 3.
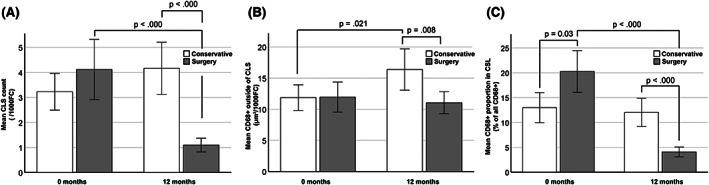
Bariatric surgery (laparoscopic Roux‐en‐Y gastric bypass) decreases human SAT CLS density without affecting macrophage density elsewhere in SAT compared with conservative treatment of obesity at 1 year. (A) Before interventions, the mean CLS count/1000 adipocytes did not differ between the groups (p = 0.209, Welch t test), but after 12 months, the surgery group had a significantly lower CLS density (p < 0.000, Welch t test). In the conservative group, mean CLSs per 1000 adipocytes increased from 3.2 (SD 2.3) to 4.2 (SD 3.3) (p = 0.07, paired t test) after 12 months. In the surgery group, mean CLS density decreased from 4.1 (SD 3.6) to 1.1 (SD 0.8) (p < 0.000, Wilcoxon signed rank test). (B) CD68 positivity outside CLSs did not initially differ between the groups. However, after 12 months, the CD68 staining significantly increased in the conservative group (p = 0.021, paired t test) and was higher than in the surgery group (p = 0.008, Welch t test) in which no change was seen. (C) At baseline, the surgery group had a larger proportion of CD68 expression in CLSs as compared with the conservative group, but after 12 months, the proportion in this group had decreased and was now lower than in the conservative group (Student t test and paired t test). All bars are presented with 95% CI. CLS, crown‐like structure; SAT, subcutaneous adipose tissue
The relationship of weight loss and signs of inflammation and adipocyte characteristics in the subcutaneous fat
Because no significant weight loss was seen in the conservative group and none of subjects in that group achieved TWL of 20% or more, only the surgery group could be considered in these analyses. Of the subjects continuing to 12 months in the surgery group, 64.7% (33/51) achieved a good response based on TWL > 20%, whereas 35.3% (18/51) remained under this threshold (TWL < 20%) at the given time point. These subgroups did not show differences in the measurements of SAT samples prior to surgery, including CLS density (3.88 SD 3.1 vs. 5.3 SD 4.3 for 1000 FC), extent of CD68 positivity outside CLSs (Figure 4A,B), and FC size measurements (data not shown). We could not detect any significant associations between these preoperative fat tissue measurements and the extent of weight loss or change in BMI at 12 months by using correlation tests either. Moreover, the change in CLS count or CD68 positivity outside CLSs from 0 to 1 year did not significantly differ between the good or suboptimal weight loss responders (data not shown). These findings suggest that the current measurements in the pretreatment samples do not provide any information predicting the response to bariatric surgery. Nevertheless, in both subgroups of weight loss, the numbers of CLSs found per 1000 adipocytes declined by 2.7 (95% CI 1.3–4.1) and 4.3 (95% CI 1.4–7.1) for the TWL > 20% and TWL < 20% groups, respectively (Figure 4A). Notably, CLS density also decreased in subjects achieving a lesser degree of weight loss, as depicted in the scatterplot in Figure 5. Reflecting the decline in CLS density, the proportional distribution of CD68 staining shifted from CLSs toward staining in single cells within the adipose tissue at 12 months (Figure 4C).
FIGURE 4.
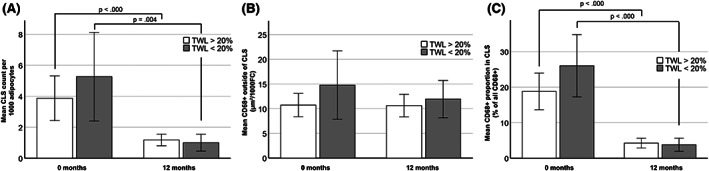
CLS density in SAT decreases independently of weight loss response after bariatric surgery (LRYGB). Total weight loss (TWL) of 20% or more at 12 months after bariatric (laparoscopic Roux‐en‐Y gastric bypass) surgery indicated a good response. (A) No significant difference was observed in the pretreatment CLS density between the subjects with good response (TWL > 20%; 3.88 (SD 3.1); N = 22) and those without such response (TWL < 20%; 5.3 (SD 4.3); N = 11; Student t test). However, along with treatment, CLS density declined in both groups (Wilcoxon signed rank test). In addition, the presence of good treatment response did not associate with (B) the pretreatment extent of CD68 positivity outside CLSs or (C) the distribution of CD68 positivity in and outside CLSs. However, the distribution of CD68 positivity significantly shifted from CLSs to cells outside CLSs, reflecting the decrease of CLS density and constancy of CD68 staining outside CLSs (paired t test). All bars are presented with 95% CI. CLS, crown‐like structure; SAT, subcutaneous adipose tissue
FIGURE 5.
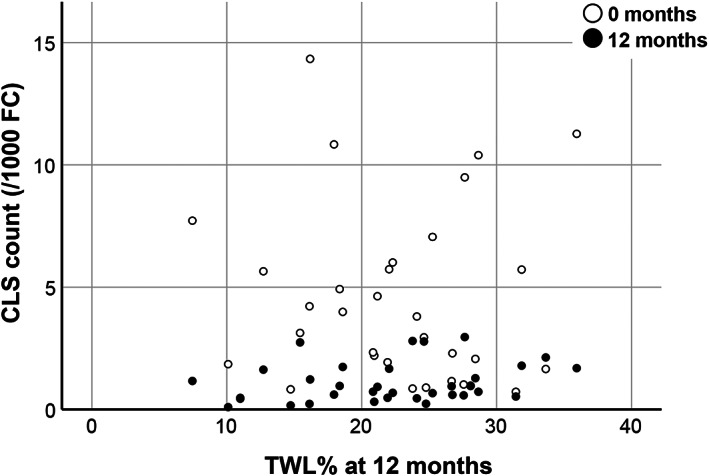
Density of CLSs found in subcutaneous adipose tissue 12 months after bariatric surgery (laparoscopic Roux‐en‐Y gastric bypass) declines in a similar fashion even in subjects with suboptimal weight loss response (TWL < 20%) to the surgery. CLS, crown‐like structure; TWL, total weight loss (kg)
DISCUSSION
To date, our study is the largest tissue‐level follow‐up analysis of 122 subjects with obesity undergoing bariatric surgery or conservative treatment without surgery, of which we were able to include 39 and 43 cases with paired adipose tissue samples, respectively. Our histological analyses confirmed that, as previously shown, CLSs are present in SAT of subjects with obesity [21, 25]. As a novel finding, our data show that LRYGB significantly reduced CLS density independently of weight loss. As a second novel finding, CD68 staining, a marker for macrophages, was predominantly found dispersed in adipose tissue outside CLSs, which extends the view of previous reports indicating that macrophage populations are mainly found in CLSs [17], and the effect of surgery led to dynamic change of macrophage distribution toward less inflammatory positions as CLSs were significantly reduced. Finally, we could not identify any feature of the preoperative macrophage populations in the SAT samples prior to LRYGB that would have provided any predictive information about suboptimal response to surgery if measured by weight loss only.
There are no previous studies separately quantitating CLSs and diffusely infiltrating macrophages in SAT of subjects with obesity. We consider that the number of subjects and samples in our study allowed us to perform this analysis reliably. It is notable that we conducted a software‐based analysis on whole‐slide images, in contrast to an early report by Cinti et al. [17], who analyzed micrographs and did not separately quantify distribution of positive staining in CLSs versus single‐cell macrophages dispersed throughout the fat. In their study, they concluded that macrophages are mainly found in CLSs, whereas our data clearly show that CLSs represent only a fraction of the total number of macrophages residing in adipose tissue. In addition, it is notable that Cinti et al. used MAC‐2 antibody recognizing galectin‐3 in their research, whereas we used CD68 (PGMI). One possibility is that the cells that were recognized in these studies are somewhat different. In addition to macrophages, both antibodies may label several other cell types [33, 34]. Furthermore, in macrophages, their expression may be related to the activation status. MAC‐2 possibly has a suppressing effect on the proinflammatory phenotype [34], whereas upregulation of CD68 may be related with responses to proinflammatory stimuli [33]. It seems obvious that using several complementary macrophage markers would facilitate the quantitation of diffusely infiltrating macrophages in SAT by increasing the cell type specificity of the assay or even recognition of tissue‐specific macrophage activation mechanisms. If such specific recognition could be achieved in further studies, it would be important to recognize the activation status of these cells before and after the treatment of obesity and to get information about the role of the diffuse macrophage population in the pathogenesis of systemic manifestations of SAT inflammation.
The surprising effect that bariatric surgery reduced CLS density and CLS‐associated macrophages in SAT in a weight‐loss–independent manner deserves further attention. After the surgery, the reduction in adipose tissue macrophages occurred specifically in the population of CLS‐associated macrophages because the CD68 staining in adipose tissue outside CLSs was not affected by the intervention. Interestingly, CLS density declined similarly even with suboptimal weight loss after the surgery. Subjects with TWL < 20% had a slightly higher baseline mean CLS density and macrophage infiltration in CLSs. Although these differences were not statistically significant in this relatively small subgroup (11 subjects), it prompts the question whether severe baseline adipose tissue inflammation or an unknown factor either contributing to it or operating independently could hinder weight loss after bariatric surgery. Nevertheless, CLS density declined in a similar fashion, and no difference was seen at 12 months, indicating that even subjects with suboptimal weight loss response may benefit from the anti‐inflammatory effect of the surgery.
The apparent independence of weight loss from CLS density decrease after bariatric surgery may suggest that instead of obesity alone, there are additional mechanisms fueling the adipocyte death and subsequent formation of CLSs. One such mechanism could consist of free fatty acids, which have been identified as a major driver for macrophage recruitment into adipose tissue and which also decrease in concentration after gastric bypass surgery [27, 35]. Another speculative candidate might be the involvement of gut‐derived bacteria in the process, because bariatric surgery profoundly alters the gut microbiota, and translocation of bacteria even to adipose tissue has been confirmed [36, 37]. In obesity, the enlarged adipocytes are more susceptible to gut microbiota‐derived products, such as lipopolysaccharides or their micelles, to trigger cell death [38]. Thus, adipocyte hypertrophy might be a necessary—but not sufficient alone—ingredient of programmed adipocyte death by pyroptosis. This is in line with the notion that CLS density tends to be higher in visceral adipose tissue despite the generally smaller FC size than in SAT [21]. Overall, this study further emphasizes a central or perhaps even a major role for CLSs in obesity‐related adipose tissue inflammation as well as the need to study the details of macrophage dynamics in the whole body in order to better understand the effects of bariatric surgery.
In summary, we show here that CLS density as a marker of adipose tissue low‐grade inflammation decreased dramatically in SAT after bariatric surgery (LRYGB). The effect was apparently independent of weight loss and was observed at the same magnitude even in subjects with suboptimal response to surgery. Macrophage infiltration in SAT declined only in CLSs after the surgery, but the majority of macrophages were found dispersed outside CLSs in all situations. Baseline inflammation status of adipose tissue represented as CLS density or macrophage infiltration to SAT did not predict the postsurgery weight loss.
FUNDING INFORMATION
Government funding (Ministry of Social Affairs and Health, K47867) covered the expenses of writing and publication of this article.
CONFLICT OF INTEREST
The authors declared no or conflict of interest.
Supporting information
Figure S1.
ACKNOWLEDGMENTS
The authors express their gratitude to Eija Tomperi and Miia Vierimaa for technical expertise in cutting, staining, and scanning the original sections and to Anne Kukkonen for her efforts in the research project.
Palomäki VA, Lehenkari P, Meriläinen S, Karttunen TJ, Koivukangas V. Dynamics of adipose tissue macrophage populations after gastric bypass surgery. Obesity (Silver Spring). 2023;31(1):184‐191. doi: 10.1002/oby.23602
Funding information Sosiaali‐ ja Terveysministeriö, Grant/Award Number: K47867
DATA AVAILABILITY STATEMENT
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Abraham A, Ikramuddin S, Jahansouz C, Arafat F, Hevelone N, Leslie D. Trends in bariatric surgery: procedure selection, revisional surgeries, and readmissions. Obes Surg. 2016;26:1371‐1377. [DOI] [PubMed] [Google Scholar]
- 2. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860‐867. [DOI] [PubMed] [Google Scholar]
- 4. Cottam DR, Mattar SG, Barinas‐Mitchell E, et al. The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effect of weight loss. Obes Surg. 2004;14:589‐600. [DOI] [PubMed] [Google Scholar]
- 5. Hauner H. The new concept of adipose tissue function. Physiol Behav. 2004. 30;83:653‐658. [DOI] [PubMed] [Google Scholar]
- 6. Lago F, Dieguez C, Gómez‐Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol. 2007;3:716‐724. [DOI] [PubMed] [Google Scholar]
- 7. Li W, Richard D. Effects of bariatric surgery on energy homeostasis. Can J Diabetes. 2017;41:426‐431. [DOI] [PubMed] [Google Scholar]
- 8. Sjöström L, Narbro K, David S, Karason K. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741‐752. [DOI] [PubMed] [Google Scholar]
- 9. Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux‐en‐Y gastric bypass on weight loss at 5 years among patients with morbid obesity the SLEEVEPASS randomized clinical trial. JAMA. 2018;319:241‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Puzziferri N, Roshek TB, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long‐term follow‐up after bariatric surgery: a systematic review. JAMA. 2014;312:934‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grover BT, Morell MC, Kothari SN, Borgert AJ, Kallies KJ, Baker MT. Defining weight loss after bariatric surgery: a call for standardization. Obes Surg. 2019;29:3493‐3499. [DOI] [PubMed] [Google Scholar]
- 12. Brolin RE, Cody RP. Adding malabsorption for weight loss failure after gastric bypass. Surg Endosc. 2007;21:1924‐1926. [DOI] [PubMed] [Google Scholar]
- 13. Corcelles R, Boules M, Froylich D, et al. Total weight loss as the outcome measure of choice after Roux‐en‐Y gastric bypass. Obes Surg. 2016;26:1794‐1798. [DOI] [PubMed] [Google Scholar]
- 14. Livhits M, Mercado C, Yermilov I, et al. Preoperative predictors of weight loss following bariatric surgery: systematic review. Obes Surg. 2012;22:70‐89. [DOI] [PubMed] [Google Scholar]
- 15. Parikh M, Pomp A, Gagner M. Laparoscopic conversion of failed gastric bypass to duodenal switch: technical considerations and preliminary outcomes. Surg Obes Relat Dis. 2007;3:611‐618. [DOI] [PubMed] [Google Scholar]
- 16. Rawlins ML, Teel D, Hedgcorth K, Maguire JP. Revision of Roux‐en‐Y gastric bypass to distal bypass for failed weight loss. Surg Obes Relat Dis. 2011;7:45‐49. [DOI] [PubMed] [Google Scholar]
- 17. Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347‐2355. [DOI] [PubMed] [Google Scholar]
- 18. Giordano A, Murano I, Mondini E, et al. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J Lipid Res. 2013;54:2423‐2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity‐related insulin resistance. J Clin Invest. 2003;112:1821‐1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Camastra S, Vitali A, Anselmino M, et al. Muscle and adipose tissue morphology, insulin sensitivity and beta‐cell function in diabetic and nondiabetic obese patients: effects of bariatric surgery. Sci Rep. 2017;7:9007. doi: 10.1038/s41598-017-08444-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weyer C, Foley JE, Borgadus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43:1498‐1506. [DOI] [PubMed] [Google Scholar]
- 23. Cummings DE, Overduin J, Foster‐Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608‐2615. [DOI] [PubMed] [Google Scholar]
- 24. Pinkney J, Kerrigan D. Current status of bariatric surgery in the treatment of type 2 diabetes. Obes Rev. 2004;5:69‐78. [DOI] [PubMed] [Google Scholar]
- 25. Cancello R, Henegar C, Viguerie N, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery‐induced weight loss. Diabetes. 2005;54:2277‐2286. [DOI] [PubMed] [Google Scholar]
- 26. Alemán JO, Iyengar NM, Walker JM, et al. Effects of rapid weight loss on systemic and adipose tissue inflammation and metabolism in obese postmenopausal women. J Endocr Soc. 2017;1:625‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kosteli A, Sugaru E, Haemmerle G, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466‐3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bankhead P, Loughrey MB, Fernández JA, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;1:16878. doi: 10.1038/s41598-017-17204-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schneider C, Rasband W, Eliceiri K. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bäcker V. ImageJ macro tool sets for biological image analysis. Paper presented at: 4th ImageJ User and Developer Conference, 24‐26 October 2012, Luxembourg. http://dev.mri.cnrs.fr/attachments/download/947/image_analysis.pdf
- 31. Palomäki VA, Koivukangas V, Meriläinen S, Lehenkari P. A straightforward method for adipocyte size and count analysis using open‐source software QuPath. Adipocyte. 2022;11:99‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bigornia SJ, Farb MG, Mott MM, et al. Relation of depot‐specific adipose inflammation to insulin resistance in human obesity. Nutr Diabetes. 2012;2:e30. doi: 10.1038/nutd.2012.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chistiakov DA, Killingsworth MC, Myasoedova VA, Orekhov AN, Bobryshev YV. CD68/macrosialin: not just a histochemical marker. Lab Invest. 2016;97:4‐13. [DOI] [PubMed] [Google Scholar]
- 34. Di Gregoli K, Somerville M, Bianco R, et al. Galectin‐3 identifies a subset of macrophages with a potential beneficial role in atherosclerosis. Arterioscler Thromb Vasc Biol. 2020;40:1491‐1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liakh I, Proczko‐Stepaniak M, Sledzinski M, Mika A. Serum free fatty acid levels and insulin resistance in patients undergoing one‐anastomosis gastric bypass. Wideochir Inne Tech Maloinwazyjne. 2022;17:194‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Debédat J, Clément K, Aron‐Wisnewsky J. Gut microbiota dysbiosis in human obesity: impact of bariatric surgery. Curr Obes Rep. 2019;8:229‐242. [DOI] [PubMed] [Google Scholar]
- 37. Massier L, Chakaroun R, Tabei S, et al. Adipose tissue derived bacteria are associated with inflammation in obesity and type 2 diabetes. Gut. 2020;69:1796‐1806. [DOI] [PubMed] [Google Scholar]
- 38. Hersoug LG, Møller P, Loft S. Role of microbiota‐derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr Res Rev. 2018;31:153‐163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


